Abstract
Green tea epigallocatechin gallate (EGCG) and microRNA (miRNA) molecules modulate obesity. Nevertheless, it is still unknown whether EGCG modulates fat cell growth via miRNA-related signaling. In this study, white preadipocytes were used to examine whether the antimitogenic effect of EGCG on fat cells is regulated by the miR-143/MAPK7 pathway. We showed that EGCG upregulated the levels of miR-143, but not miR-155, in 3T3-L1 preadipocytes. Moreover, EGCG downregulated MAPK7 mRNA and protein levels time- and dose-dependently. MAPK7 expression increased during 3T3-L1 cell proliferation. miR-143 overexpression in the absence of EGCG mimicked the effects of EGCG to suppress preadipocyte growth and MAPK7 expression, whereas knockdown of miR-143 antagonized the EGCG-altered levels of miR-143, MAPK7, and pERK1/2 and reversed the EGCG-inhibited cell growth. These findings suggest that EGCG inhibits 3T3-L1 cell growth via miR-143/MAPK7 pathway.
Keywords: Green tea, EGCG, 3T3-L1, cell growth, microRNA-143, MAPK7
Impact Statement
Epigallocatechin gallate (EGCG) directly modulates the functions of adipocytes. Few studies have examined whether EGCG has distinct microRNA (miR) signaling pathways to act on cellular processes among white fat cells. This study provides convincing evidence that EGCG can upregulate the expression of miR-143 in 3T3-L1 cells. We demonstrated that EGCG downregulated mRNA and protein levels of MAPK7 and miR-143 suppressed 3T3-L1 preadipocyte proliferation by directly targeting MAPK7. We found a novel miR-143/MAPK7 signaling pathway for EGCG regulation of the cell growth of 3T3-L1 preadipocytes.
Introduction
Obesity has turned out to be a global problem.1,2 The progress of obesity is portrayed by either an increase in fat cell number or an increase in fat cell/lipid droplet size. 3 Previous research showed that several important adipokines secreted by adipocytes can modulate insulin sensitivity and energy homeostasis in the muscles and liver.4–6 Thus, investigations on the epigenetic mechanisms of obesity and metabolism of adipocytes may shed light on novel therapeutic strategies for various metabolic disorders.
MicroRNAs (miRNAs) are small regulatory RNAs (18–25 nt in length), which post-transcriptionally modulate the expression levels of particular genes by base-pairing, generally to the 3′-untranslated regions (3′-UTRs) of target gene, to result in a decrement in translation and/or stability. 7 Various miRNAs display numerous biological functions, including effects on cell proliferation and metabolism. 8 In addition, several miRNAs, including miR-103, 9 miR-27, 10 let-7, 11 miR-199a, 12 miR-143, 13 and miR-425, 14 are emerging as new regulators in the modulation of metabolic activity of adipocytes. Although previous studies found that miR-143 can enhance adipocyte differentiation, the role of miR-143 in regulating 3T3-L1 cell growth is not clear.
Epigallocatechin gallate (EGCG) has been suggested as beneficial agents for antiobesity and as regulators of cell proliferation, differentiation, and browning in white adipocytes.15–17 Numerous studies have demonstrated that EGCG can modulate miRNA expression for the regulation of cancer cell growth in cervical, breast, prostate, lung, and liver cancer and melanoma.18–23 Nevertheless, there is little knowledge about the miRNA regulated by EGCG for the modulation of fat cell growth.
This study aims to explore the signal pathway through which EGCG affects miR-143 in relation to growth and to improve our understanding of the utilization of EGCG or miRNA molecules in antiobesity strategies.
Materials and methods
Reagents
EGCG was dissolved in 0.1% dimethyl sulfoxide (DMSO). 24 The miR-143 mimic (sequences for sense strand: UGAGAUGAAGCACUGUAGCUC; sequences for antisense strand: GCUACAGUGCUUCAUCUCAUU) mimic negative control (NC; sequences for sense strand: UUCUCCGAACGUGUCACGUTT; sequences for antisense strand: ACGUGACACGUUCGGAGAATT), miR-143 inhibitor (sequences for sense strand: GAGCUACAGUGCUUCAUCUCA), and inhibitor NC (sequences for sense strand: CAGUACUUUUGUGUAGUACAA) were purchased from Shanghai GenePharma Co., Ltd. MaestrofectinTM transfection reagent was obtained from Omics Bio.
Cell culture
3T3-L1 cells (ATCC-CL-173) were used in this study. The cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. The 3T3-L1 cells were induced for differentiation by using the standard 3T3-L1 cell differentiation protocol described previously. 16
MTT assay
Twenty microliters of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) solutions from the stock (5 mg/mL) was added to the cells (10,000 cells per well, 48-well culture plates) for 1 h. Formazan crystals were dissolved using DMSO. The absorbance was recorded at 570 nm.
Decreased formazan quantification was assayed using a formazan standard.
MiR-143 mimic or inhibitor transfection
3T3-L1 cells were transfected with miRNA mimic (100 nM), miRNA inhibitor (100 nM), and scrambled NCs using the MaestrofectinTM transfection reagent. After transfection for 48 h, miR-143 levels and cell number were examined.
Real-time polymerase chain reaction
Real-time polymerase chain reaction (RT-PCR) with SYBR green (Bio-Rad) was carried out on a 7300 RT-PCR System (Applied Biosystems). The primer sequences for the genes were as follows: MAPK7 forward, 5′-TAGTGAGCCTGTGTGTCCAG-3′ and reverse, 5′-CTGCGCTTCTCTTCTCGTTC-3′, C/EBPα forward, 5′-GTAACCTTGTGCCTTGGATACT-3′ and reverse, 5′-GGAAGCAGGAATCCTCCAAATA-3′, PPARγ forward, 5′-CACAAGAGCTGACCCAATGGT-3′ and reverse, 5′-GATCGCACTTTGGTATTCTTGGA-3′, and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) forward, 5′-CCTCTGGAAAGCTGTGGCGT-3′ and reverse, 5′-TTGGCAGGTTTCTCCAGGCG-3′. For miRNA analysis, complementary DNAs (cDNAs) were synthesized using the TaqMan MicroRNA Reverse Transcription Kit and subjected to RT-PCR using KAPA PROBE FAST qPCR Kit Master Mix (2X) Universal. The primers for mus-miR-143 (00-0377, Thermo Fisher Scientific) were used. Synthetic miRNA U6 was used as a reference gene.
Western blot analysis
Protein concentrations were determined using the Bradford method. The following antibodies and secondary antibodies were used: phospho-ERK1/2 (Cat. No. 9101; 1:1000; Cell Signaling Technology), MAPK7 (Cat. No. 33725; 1:1000; Cell Signaling Technology), actin (Cat. No. 8457; 1:1000; Cell Signaling Technology), and ERK1/2 (Cat. No. sc-93; 1:1000; Santa Cruz Biotechnology).
Statistical analysis
Values are expressed as mean ± standard error of the mean (SEM). Student’s t-test and one-way analysis of variance (ANOVA) and a subsequent post hoc Tukey test were used in this study. P < 0.05 was considered statistically significant.
Results
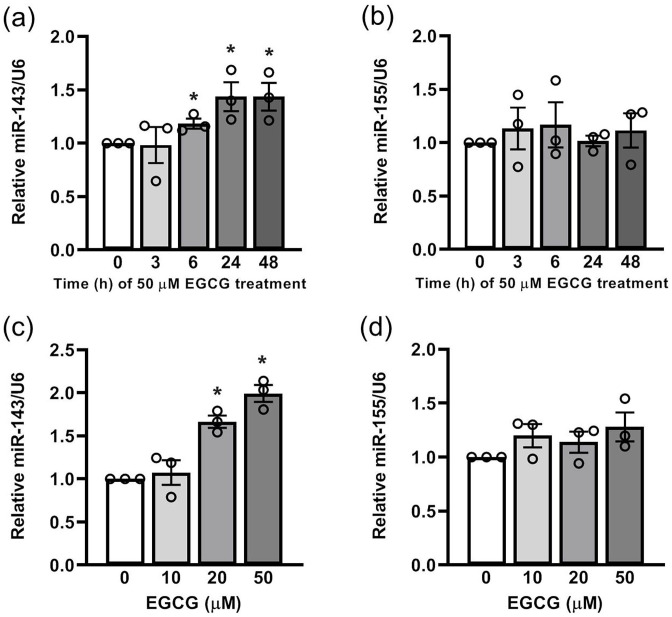
EGCG upregulates the expression levels of miR-143 time- and dose-dependently, but not miR-155, in 3T3-L1 preadipocytes
MiR-143 modulates adipocyte differentiation by directly targeting MAP2K5 signaling, 25 and EGCG is considered a chemopreventive agent for the modulation of cell growth, apoptosis, and differentiation in white adipocytes;15,26–28 however, it is unknown whether miR-143 serves as a molecular target of antimitogenic effects of EGCG in white adipocytes. We first investigated miR-143 levels after EGCG treatment in 3T3-L1 preadipocytes. We found that EGCG time- and dose-dependently upregulated miR-143 expression levels (Figure 1(a) and (c)). In addition, miR-155 secreted from the adipose tissue macrophage-derived exosomes can regulate insulin sensitivity. 29 MiR-155 was significantly upregulated in the adipose tissue of obese subjects. 30 We further found that EGCG did not change miR-155 expression (Figure 1(b) and (d)).
Figure 1.
The effect of EGCG on the levels of miR-143, but not miR-155 in 3T3-L1 cells: For dose-dependent effect, the cells were treated with EGCG for 24 h. (a) and (c) miR-143 expression was analyzed via quantitative PCR (qPCR). (b) and (d) miR-155 expression was analyzed by qPCR. Data are presented as mean ± SEM (n = 3). *P < 0.05, compared with the control group.
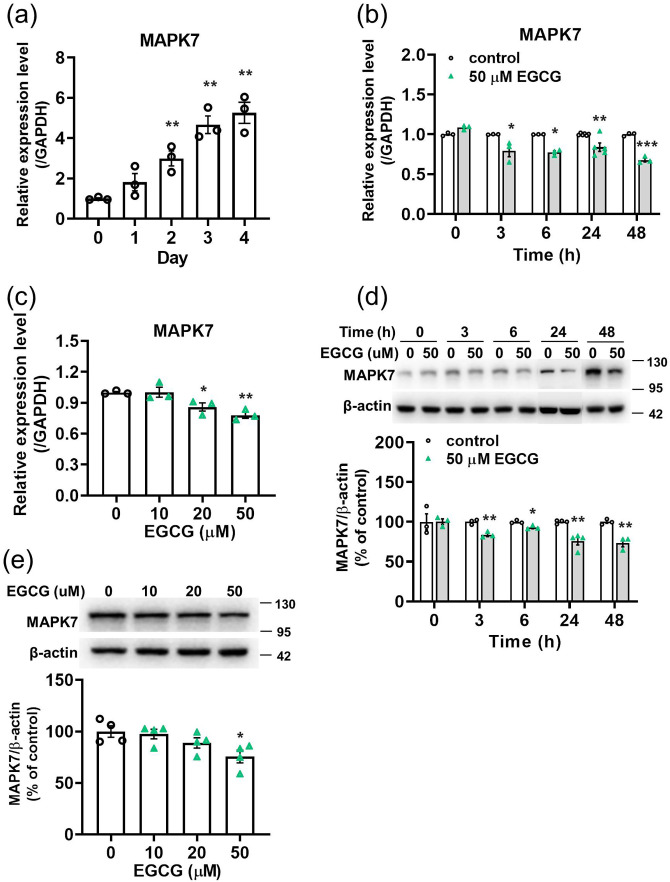
EGCG inhibits the mRNA and protein levels of MAPK7 in 3T3-L1 cells time- and dose-dependently
According to the TargetScan Mouse database for target gene prediction and previous reports, 25 MAPK7 can act as the target gene of miR-143. We showed that MAPK7 was upregulated during the proliferative stages of 3T3-L1 cells (Figures 2(a)) and that EGCG significantly reduced the mRNA (Figure 2(b) and (c)) and protein levels (Figure 2(d) and (e)) of MAPK7 time- and dose-dependently.
Figure 2.
The effect of EGCG on the mRNA and protein levels of MAPK7 in 3T3-L1 preadipocytes. For dose-dependent effect, the cells were treated with EGCG for 24 h. (a), (b), and (c) MAPK7 expression was analyzed via qPCR. (d) and (e) Representative immunoblotting analyses of MAPK7 and quantification of western blot bands. Data are presented as mean ± SEM (n = 3 or 4). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the control group. (A color version of this figure is available in the online journal.)
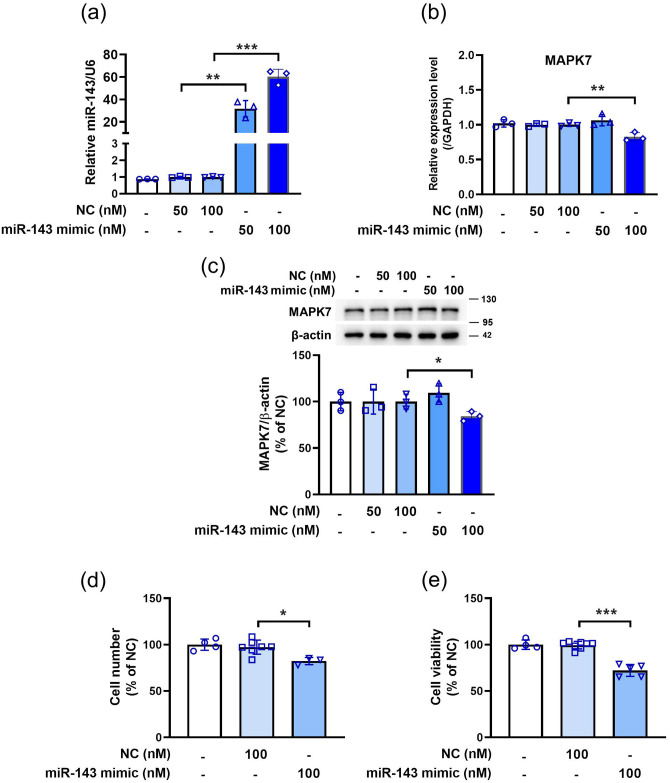
MiR-143 inhibits 3T3-L1 preadipocyte proliferation
We next investigated whether miR-143 regulates the cell growth in 3T3-L1 preadipocytes. Compared with the NC, 50 and 100 nM miRNA-143 mimic upregulated the miR-143 expression (Figure 3(a)). As shown in Figure 3(b) and (c), 100 nM miRNA-143 mimic decreased the mRNA and protein levels of MAPK7 compared to the NC. Moreover, miR-143 mimics inhibited cell proliferation (Figure 3(d)) and viability (Figure 3(e)). These findings demonstrate that miR-143 can suppress 3T3-L1 cell growth.
Figure 3.
MiR-143 mimic inducing significant decreases in MAPK7 mRNA and protein levels and inhibiting cell growth in 3T3-L1 cells. The cells were transfected with miR-143 mimic for 48 h. (a) miR-143 expression was analyzed via qPCR. (b) The expression of MAPK7 was analyzed by qPCR. (c) The protein level of MAPK7 was measured via western blot analysis. (d) Cell number. (e) Cell viability was analyzed by the MTT assay. Data are presented as mean ± SEM (n = 3–8). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the NC group. (A color version of this figure is available in the online journal.)
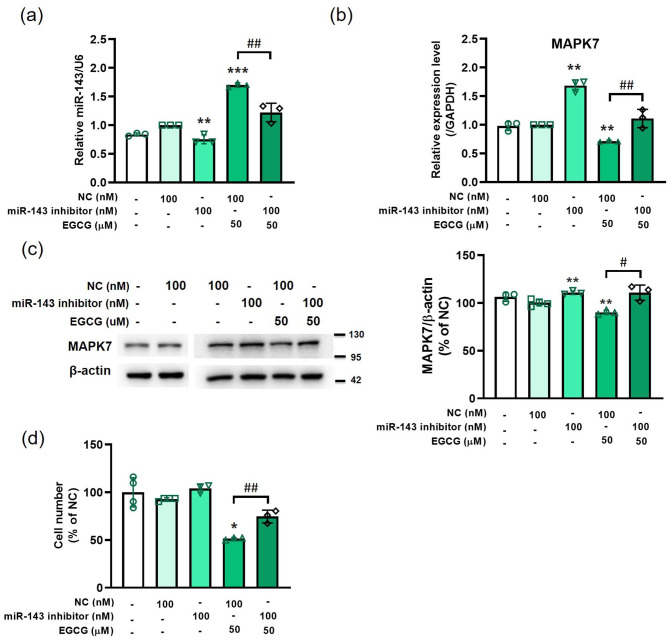
Knockdown of miR-143 antagonizes the EGCG regulation of expression levels of miR-143 and MAPK7 and cell proliferation in 3T3-L1 preadipocytes
The miR-143 inhibitor significantly decreased the miR-143 expression level and antagonized EGCG-induced miR-143 expression (Figure 4(a)). miR-143 knockdown caused by the inhibitor significantly upregulated the mRNA (Figure 4(b)) and protein levels (Figure 4(c)) of MAPK7. In addition, the miR-143 inhibitor counteracted the EGCG-induced decrease in the MAPK7 mRNA (Figure 4(b)) and protein levels (Figure 4(c)). The miR-143 inhibitor also antagonized the EGCG-induced downregulation in cell number (Figure 4(d)).
Figure 4.
Knockdown of miR-143 antagonizing the EGCG regulation of expression levels of miR-143 and MAPK7, as well as cell proliferation in 3T3-L1 preadipocytes. The cells are pretreated with miR-143 inhibitor or negative control (NC) for 24 h and followed by 50 μM EGCG treatment for 48 h. (a) miR-143 expression was analyzed via qPCR. (b) MAPK7 expression was analyzed by qPCR. (c) The protein level of MAPK7 was measured by western blot. (d) Cell number. Data are presented as mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, compared with the NC group. #P < 0.05, ##P < 0.01, EGCG + NC versus EGCG + miR-143 inhibitor. (A color version of this figure is available in the online journal.)
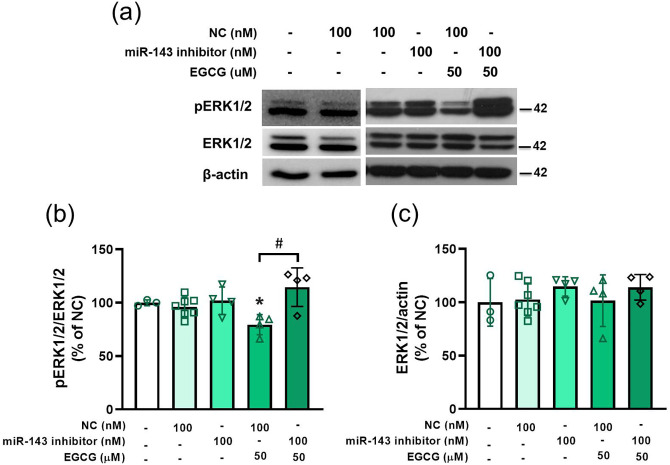
Knockdown of miR-143 antagonizes EGCG-regulated pERK1/2 in 3T3-L1 cells
The miR-143 inhibitor reversed the EGCG downregulation of phosphorylation of ERK1/2 (Figure 5(a) and (b)) but did not alter the total ERK1/2 protein level (Figure 5(a) and (c)). Therefore, miR-143 was involved in EGCG regulation of the ERK1/2 signaling.
Figure 5.
Knockdown of miR-143 antagonizing EGCG-regulated pERK1/2 in 3T3-L1 preadipocytes. The cells are pretreated with miR-143 inhibitor or NC for 24 h, followed by 50 μM EGCG treatment for 48 h. (a) Representative immunoblotting analyses of pERK1/2 and ERK1/2. (b) and (c) Quantification of western blot bands. Data are presented as mean ± SEM (n = 3–7). *P < 0.05, compared with NC group. #P < 0.05, EGCG + NC versus EGCG + miR-143 inhibitor. (A color version of this figure is available in the online journal.)
Discussion
In this study, we demonstrated that EGCG upregulated miR-143 expression in 3T3-L1 cells. We further showed that EGCG significantly decreased the mRNA and protein levels of MAPK7. The miR-143 mimic inhibited 3T3-L1 cell growth, and the MiR-143 inhibitor antagonized the EGCG-induced decrease in the cell number. Furthermore, miR-143 was involved in the EGCG modulation of the ERK1/2 signaling pathway. Our findings demonstrate that EGCG might suppress 3T3-L1 cell proliferation via miR-143/MAPK7 signaling pathways (Figure 6).
Figure 6.
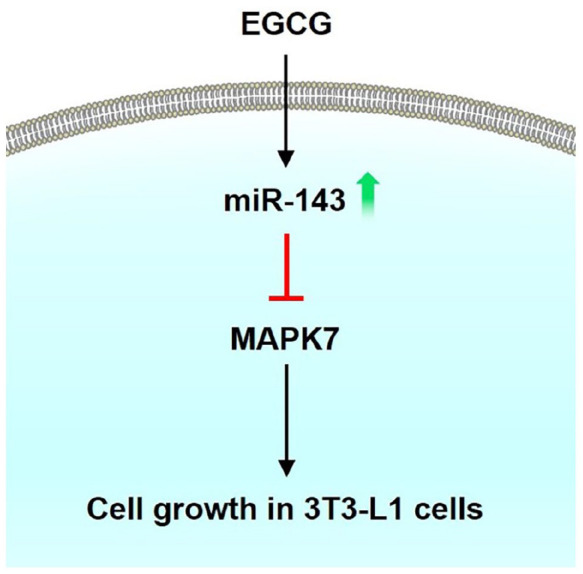
EGCG inhibiting cell growth of 3T3-L1 preadipocytes through the miR-143/ MAPK7 pathways. (A color version of this figure is available in the online journal.)
We found that EGCG could reduce body fat, body weight, blood lipids, adipokines, and food uptake.24,31 Green tea extract and catechin-polyphenols increase energy expenditure and thermogenesis in rats and humans.32,33 Lee et al. showed that EGCG-fed mice had higher body temperature and mitochondrial DNA content in brown adipose tissue. 34 In addition, EGCG suppressed the expression of genes related to the synthesis of de novo fatty acids such as ACC1, CCAAT/enhancer-binding protein beta (C/EBPβ) and PPARγ and increased the expression of hormone-sensitive lipase in the white adipose tissue of mice. 35 To support the in vivo findings, our in vitro studies demonstrated that EGCG suppressed preadipocyte mitogenesis, adipogenic differentiation, and triglyceride biosynthetic enzyme activity.15,16,26,27,36,37 In addition, EGCG stimulates preadipocyte apoptosis, AMPK activity, and reactive oxygen species production by decreasing glutathione levels.15,28,38 Moreover, EGCG decreases triglyceride levels during the differentiation of white adipocytes, suggesting its suppressive actions on terminal differentiation.15,16,39 EGCG also inhibits the activity of various fat metabolism–related enzymes (e.g. ACC, fatty acid synthase, gastric and pancreatic lipases, and squalene epoxidase)40–44 and cell migration, and suppresses lipid deposition. 45 Although EGCG directly modulates the functions of the fat cells and adipose tissues, or indirectly modulates hormone-mediated fat cell activity, few studies have examined whether EGCG has distinct miRNA signaling pathways to act on the cellular processes among white fat cells.
MiRNAs are associated with fat cell function and obesity46–52 and involved in the modulation of lipid and glucose metabolism53,54 A previous study showed the miRNA regulation of fat cell function in Drosophila and demonstrated that miR-14 inhibits fat metabolism by targeting the p38 and MAPK pathways. 55 Of the adipogenesis-related miRNAs, miR-143 was first described as a positive modulator of adipocyte differentiation through ERK5 pathway. 13 However, miR-143 was found to impair mitotic clonal expansion (MCE), 25 suggesting a cell process-dependent effect. miRNA-143 was found to be upregulated after induction of differentiation in preadipocytes and the mesenteric fat of high-fat diet–induced obese mice.13,48,56 A previous study showed that obesity-induced upregulation of miRNA-143 increased insulin resistance by inhibiting AKT signaling and downregulating oxysterol-binding-protein-related protein 8. 57
MAPK7, also known as ERK5, belongs to the MAPK family. The suppression of miR-143 inhibits adipogenesis and upregulates the protein levels of MAPK7, suggesting that miR-143 targets MAPK7. 58 However, it is unknown whether miR-143/MAPK7 pathways are involved in the EGCG modulation of the proliferation stage in 3T3-L1 cells. In our study, we showed that EGCG alone or miR-143 mimic alone could decrease the expression levels of MAPK7 mRNA and protein (Figures 2 and 3(b) and (c)), suggesting that MAPK7 may regulate cell growth in 3T3-L1 cells. We further showed that EGCG alone or miR-143 mimic alone could inhibit the pERK1/2 signaling pathway. Knockdown of miR-143 antagonized EGCG-regulated pERK1/2 in 3T3-L1 preadipocytes (Figure 5).
Although the effects of EGCG and miRNA on 3T3-L1 cell differentiation have been extensively reported,42,48 only a few studies reported that EGCG had distinct miRNA signaling pathways to act on the cellular processes of white fat cells. Because the mitotic clonal expansion of 3T3-L1 preadipocytes is preceded by the process of adipogenic differentiation, an examination of the effects of EGCG and miRNA on the growth of 3T3-L1 preadipocytes will improve the understanding of the mechanism by which the miRNA signaling cascades mediate EGCG action. Our study provides evidence that EGCG can upregulate miR-143 expression to suppress the growth of 3T3-L1 cells. As EGCG and miR-143 were, respectively, found to downregulate and upregulate 3T3-L1 cell differentiation,42,59 it is worthwhile to explore whether EGCG influences the differentiation process of 3T3-L1 cells through modulation of miR-143 expression. We showed that EGCG at 25 µM enhanced miR-143 and miR-let-7a levels and inhibited the mRNA levels of other adipogenesis inducers, such as C/EBPα and PPARγ (Supplemental Figure 1). As transfection of let-7 into 3T3-L1 cells suppressed growth and differentiation 11 and as C/EBPα and PPARγ functioned to stimulate adipogenic differentiation, 60 these data suggest that EGCG may suppress 3T3-L1 cell differentiation via distinct miRNA pathways and/or through inhibition of other adipogenic inducers.
Green tea EGCG possesses multiple biological effects in vitro, and its effective doses generally range from 1 to 100 µM. 16 In vivo, the circulating and tissue levels of EGCG, as generally reported in animals and humans, are in the range of 0.1–24 µM and 0.5–565 µM, respectively. 16 The wide range of EGCG levels is caused by the low bioavailability of administrated EGCG, which depends on the purity, dosage, route of administration, duration of treatment, type of tissue, and species involved. 61 When human subjects receive a single ascending dose of EGCG from 50 to 1600 mg, the maximum plasma concentrations of EGCG ranged from 0.2 to 11.4 µM. 62 When human subjects drink two to three cups of green tea, the saliva levels of EGCG reach peaks of 11–48 µM after a few minutes of consumption. 63 Based on these previous reports, we chose the ranging doses of EGCG from 0 to 50 µM for the study because the dosage range covered at least its physiological levels. Whether the circulating levels of EGCG can be accumulated by long-term tea drinking was not determined in this study. Thus, the concentrations of EGCG used in this study ranged from 10 to 50 µM, in which, part of them corresponds to the higher circulating EGCG levels in human subjects and tissue EGCG levels in animals. However, our findings could not exclude the possibility that the 50 µM of EGCG used in the study may be pharmacological. We did observe that the doses of EGCG at 20 and 50 μM were effective in upregulating the expression of miR-143 and decreasing MAPK7 expression. In addition, we found that significant changes in MAPK7 mRNA and protein levels of 3T3-L1 cells induced by 50 μM EGCG were more consistent than those treated by 20 μM EGCG. This allowed us to choose 50 μM EGCG in the subsequent studies. Unfortunately, the dose of 20 μM EGCG that is more physiologically relevant than 50 μM EGCG was not determined in the subsequent experiments. This is one of our limitations. No matter whatever the physiological or pharmacological effects of EGCG are, these concentrations are compatible with the effective doses (10–100 µM) of EGCG for regulating mitogenesis and adipogenesis in fat cells and body weight in animals.62,64 Most epidemiological studies indicate that an inverse association exists between the consumption of tea and obesity and that miR-143 functions to regulate adipogenesis;13,58 however, further research is required to obtain definite conclusions on whether these epidemiological results can be explained by the effects of EGCG on preadipocyte miR-143 signaling cascade. It is also worthwhile to explore what the physiological and pharmacological levels of EGCG are appropriately and effectively used for respective preventive and therapeutic treatments for fat cell–associated diseases. Whether the use of EGCG as a therapeutic delivered by an intravenous way to get past the bioavailability issue is interesting and testable, but the possibility needs to be examined in future thorough studies.
In conclusion, we showed that EGCG inhibits the cell growth of 3-T3-L1 cells through miR-143/MAPK7 pathways (Figure 6).
Supplemental Material
Supplemental material, sj-docx-1-ebm-10.1177_15353702221108925 for Green tea epigallocatechin gallate suppresses 3T3-L1 cell growth via microRNA-143/MAPK7 pathways by Chia-Pei Chen, Tsung-Chen Su, Meei-Ju Yang, Wen-Ting Chen, An-Ci Siao, Ling-Ru Huang, Yen-Yue Lin, Yow-Chii Kuo, Jia-Fang Chung, Ching-Feng Cheng, Hui-Chen Ku and Yung-Hsi Kao in Experimental Biology and Medicine
Footnotes
Authors’ Contributions: C-PC and T-CS contributed equally to this work. Experiments were conceived and designed by C-FC, Y-YL, M-JY, T-CS, Y-CK, H-CK, and Y-HK and performed by C-PC, W-TC, J-FC, A-CS, L-RH, and H-CK. Experimental data were analyzed by C-PC, W-TC, and J-FC. Reagents/materials/analysis tools were contributed by C-FC, Y-YL, M-JY, T-CS, and Y-CK. The article was written by H-CK and Y-HK. Equal contribution was by C-FC, H-CK, and Y-HK. The final manuscript has the approval of all authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST 109-2320-B-008-001-MY3), National Central University and Landseed Hospital Joint Research (NCU-LSH-109-B-00), Research Fund of the Taoyuan Armed Forces General Hospital (TYAFGH-D-110048; TYAFGH-A-110016), and Research Fund of Tzu Chi General Hospital (TCMMP 111-01-02).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kopelman PG. Obesity as a medical problem. Nature 2000;404:635–43 [DOI] [PubMed] [Google Scholar]
- 3. Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am 2008;37:753–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adeghate E. An update on the biology and physiology of resistin. Cell Mol Life Sci 2004;61:2485–96 [DOI] [PubMed] [Google Scholar]
- 5. Ohashi K, Ouchi N, Sato K, Higuchi A, Ishikawa TO, Herschman HR, Kihara S, Walsh K. Adiponectin promotes revascularization of ischemic muscle through a cyclooxygenase 2-dependent mechanism. Mol Cell Biol 2009;29:3487–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan WW, Myers MG., Jr. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci 2018;19:95–105 [DOI] [PubMed] [Google Scholar]
- 7. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight. Nat Rev Genet 2008;9:102–14 [DOI] [PubMed] [Google Scholar]
- 8. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97 [DOI] [PubMed] [Google Scholar]
- 9. Li M, Liu Z, Zhang Z, Liu G, Sun S, Sun C. miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biol Chem 2015;396:235–44 [DOI] [PubMed] [Google Scholar]
- 10. Lin Q, Gao Z, Alarcon RM, Ye J, Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J 2009;276:2348–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol 2009;23:925–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi XE, Li YF, Jia L, Ji HL, Song ZY, Cheng J, Wu GF, Song CC, Zhang QL, Zhu JY, Yang GS. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int J Mol Sci 2014;15:8526–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 2004;279:52361–5 [DOI] [PubMed] [Google Scholar]
- 14. Qi R, Wang J, Wang Q, Qiu X, Yang F, Liu Z, Huang J. MicroRNA-425 controls lipogenesis and lipolysis in adipocytes. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864:744–55 [DOI] [PubMed] [Google Scholar]
- 15. Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res 2006;50:188–210 [DOI] [PubMed] [Google Scholar]
- 16. Kao Y-H, Ku H-C, Chang H-H, Liu C-W, Shih L-J, Weng J-T, Yeh C-C. Chapter 88: Effect of green tea (−)-epigallocatechin gallate on adipocytes: signaling effects. In: Preedy VR. (ed.) Tea in health and disease prevention. Cambridge, MA: Academic Press, 2013, pp.1053–64 [Google Scholar]
- 17. Mi Y, Liu X, Tian H, Liu H, Li J, Qi G, Liu X. EGCG stimulates the recruitment of brite adipocytes, suppresses adipogenesis and counteracts TNF-α-triggered insulin resistance in adipocytes. Food Funct 2018; 9:3374–86 [DOI] [PubMed] [Google Scholar]
- 18. Zhu Y, Huang Y, Liu M, Yan Q, Zhao W, Yang P, Gao Q, Wei J, Zhao W, Ma L. Epigallocatechin gallate inhibits cell growth and regulates miRNA expression in cervical carcinoma cell lines infected with different high-risk human papillomavirus subtypes. Exp Ther Med 2019;17: 1742–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zan L, Chen Q, Zhang L, Li X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019;10:374–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada S, Tsukamoto S, Huang Y, Makio A, Kumazoe M, Yamashita S, Tachibana H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci Rep 2016;6:19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siddiqui IA, Asim M, Hafeez BB, Adhami VM, Tarapore RS, Mukhtar H. Green tea polyphenol EGCG blunts androgen receptor function in prostate cancer. FASEB J 2011;25:1198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011;32:1881–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem 2010;21:140–6 [DOI] [PubMed] [Google Scholar]
- 24. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000; 141:980–7 [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Hou J, Ye L, Chen Y, Cui J, Tian W, Li C, Liu L. MicroRNA-143 regulates adipogenesis by modulating the MAP2K5-ERK5 signaling. Scientific Reports 2014;4:3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ku HC, Chang HH, Liu HC, Hsiao CH, Lee MJ, Hu YJ, Hung PF, Liu CW, Kao YH. Green tea (-)-epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am J Physiol Cell Physiol 2009;297:C121–32 [DOI] [PubMed] [Google Scholar]
- 27. Ku HC, Liu HS, Hung PF, Chen CL, Liu HC, Chang HH, Tsuei YW, Shih LJ, Lin CL, Lin CM, Kao YH. Green tea (-)-epigallocatechin gallate inhibits IGF-I and IGF-II stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor, but not AMP-activated protein kinase pathway. Molecular Nutrition & Food Research 2012;56:580–92 [DOI] [PubMed] [Google Scholar]
- 28. Wu BT, Hung PF, Chen HC, Huang RN, Chang HH, Kao YH. The apoptotic effect of green tea (-)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the Cdk2 pathway. Journal of Agricultural and Food Chemistry 2005;53:5695–701 [DOI] [PubMed] [Google Scholar]
- 29. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB, Ofrecio JM, Wollam J, Hernandez-Carretero A, Fu W, Li P, Olefsky JM. Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 2017;171:372–412 [DOI] [PubMed] [Google Scholar]
- 30. Karkeni E, Astier J, Tourniaire F, El Abed M, Romier B, Gouranton E, Wan L, Borel P, Salles J, Walrand S, Ye J, Landrier JF. Obesity-associated inflammation induces microRNA-155 expression in adipocytes and adipose tissue: outcome on adipocyte function. J Clin Endocrinol Metab 2016;101:1615–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kao YH, Hiipakka RA, Liao S. Modulation of obesity by a green tea catechin. Am J Clin Nutr 2000;72:1232–4 [DOI] [PubMed] [Google Scholar]
- 32. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, Chantre P, Vandermander J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 1999;70:1040–5 [DOI] [PubMed] [Google Scholar]
- 33. Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord 2000;24:252–8 [DOI] [PubMed] [Google Scholar]
- 34. Lee MS, Shin Y, Jung S, Kim Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr Res 2017;61:1325307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li F, Gao C, Yan P, Zhang M, Wang Y, Hu Y, Wu X, Wang X, Sheng J. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol 2018;9:1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hung PF, Wu BT, Chen HC, Chen YH, Chen CL, Wu MH, Liu HC, Lee MJ, Kao YH. Antimitogenic effect of green tea (-)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. Am J Physiol Cell Physiol 2005;288:C1094–108 [DOI] [PubMed] [Google Scholar]
- 37. Ku HC, Tsuei YW, Kao CC, Weng JT, Shih LJ, Chang HH, Liu CW, Tsai SW, Kuo YC, Kao YH. Green tea (-)-epigallocatechin gallate suppresses IGF-I and IGF-II stimulation of 3T3-L1 adipocyte glucose uptake via the glucose transporter 4, but not glucose transporter 1 pathway. General and Comparative Endocrinology 2014;199:46–55 [DOI] [PubMed] [Google Scholar]
- 38. Wang CT, Chang HH, Hsiao CH, Lee MJ, Ku HC, Hu YJ, Kao YH. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol Nutr Food Res 2009;53:349–60 [DOI] [PubMed] [Google Scholar]
- 39. Lin J, Della-Fera MA, Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res 2005;13:982–90 [DOI] [PubMed] [Google Scholar]
- 40. Watanabe J, Kawabata J, Niki R. Isolation and identification of acetyl-CoA carboxylase inhibitors from green tea (Camellia sinensis). Biosci Biotechnol Biochem 1998;62:532–4 [DOI] [PubMed] [Google Scholar]
- 41. Ho CT, Chen Q, Shi H, Zhang KQ, Rosen RT. Antioxidative effect of polyphenol extract prepared from various Chinese teas. Prev Med 1992;21:520–5 [DOI] [PubMed] [Google Scholar]
- 42. Wu M, Liu D, Zeng R, Xian T, Lu Y, Zeng G, Sun Z, Huang B, Huang Q. Epigallocatechin-3-gallate inhibits adipogenesis through down-regulation of PPARγ and FAS expression mediated by PI3K-AKT signaling in 3T3-L1 cells. European Journal of Pharmacology 2017;795:134–42 [DOI] [PubMed] [Google Scholar]
- 43. Abe I, Seki T, Umehara K, Miyase T, Noguchi H, Sakakibara J, Ono T. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochemical and Biophysical Research Communications 2000;268:767–71 [DOI] [PubMed] [Google Scholar]
- 44. Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem 2007;18:179–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chani B, Puri V, Chander Sobti R, Puri S. Epigallocatechin gallate inhibits mouse mesenchymal stem cell differentiation to adipogenic lineage. J Stem Cells Regen Med 2016;12:16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scheideler M. MicroRNAs in adipocyte formation and obesity. Best Pract Res Clin Endocrinol Metab 2016;30:653–64 [DOI] [PubMed] [Google Scholar]
- 47. Karbiener M, Scheideler M. MicroRNA functions in brite/brown fat: novel perspectives towards anti-obesity strategies. Comput Struct Biotechnol J 2014;11:101–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kajimoto K, Naraba H, Iwai N. MicroRNA and 3T3-L1 pre-adipocyte differentiation. RNA 2006;12:1626–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xie H, Lim B, Lodish HF. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 2009;58:1050–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keller P, Gburcik V, Petrovic N, Gallagher IJ, Nedergaard J, Cannon B, Timmons JA. Gene-chip studies of adipogenesis-regulated microRNAs in mouse primary adipocytes and human obesity. BMC Endocrine Disorders 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim SY, Kim AY, Lee HW, Son YH, Lee GY, Lee JW, Lee YS, Kim JB. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem Biophys Res Commun 2010; 392:323–8 [DOI] [PubMed] [Google Scholar]
- 52. Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernandez C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI, Lindgren CM. Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 2010;53:1099–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arner P, Kulyté A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol 2015;11:276–88 [DOI] [PubMed] [Google Scholar]
- 54. Hartig SM, Hamilton MP, Bader DA, McGuire SE. The miRNA interactome in metabolic homeostasis. Trends Endocrinol Metab 2015;26:733–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xu P, Vernooy SY, Guo M, Hay BA. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr Biol 2003;13:790–5 [DOI] [PubMed] [Google Scholar]
- 56. Takanabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H, Kita T, Satoh N, Shimatsu A, Hasegawa K. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochemical and Biophysical Research Communications 2008;376:728–32 [DOI] [PubMed] [Google Scholar]
- 57. Jordan SD, Krüger M, Willmes DM, Redemann N, Wunderlich FT, Brönneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Böttger T, Braun T, Seibler J, Brüning JC. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 2011;13:434–46 [DOI] [PubMed] [Google Scholar]
- 58. Zhang P, Du J, Wang L, Niu L, Zhao Y, Tang G, Jiang Y, Shuai S, Bai L, Li X, Wang J, Zhang S, Zhu L. MicroRNA-143a-3p modulates preadipocyte proliferation and differentiation by targeting MAPK7. Biomed Pharmacother 2018;108:531–9 [DOI] [PubMed] [Google Scholar]
- 59. Yi C, Xie WD, Li F, Lv Q, He J, Wu J, Gu D, Xu N, Zhang Y. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett 2011;585:3303–9 [DOI] [PubMed] [Google Scholar]
- 60. Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev 2002;16:22–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Legeay S, Rodier M, Fillon L, Faure S, Clere N. Epigallocatechin gallate: a review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015;7:5443–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res 2003;31:88–101 [DOI] [PubMed] [Google Scholar]
- 63. Yang CS, Lee MJ, Chen L. Human salivary tea catechin levels and catechin esterase activities: implication in human cancer prevention studies. Cancer Epidemiol Biomarkers Prev 1999;8:83–9 [PubMed] [Google Scholar]
- 64. Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr 2014;68:1075–87 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ebm-10.1177_15353702221108925 for Green tea epigallocatechin gallate suppresses 3T3-L1 cell growth via microRNA-143/MAPK7 pathways by Chia-Pei Chen, Tsung-Chen Su, Meei-Ju Yang, Wen-Ting Chen, An-Ci Siao, Ling-Ru Huang, Yen-Yue Lin, Yow-Chii Kuo, Jia-Fang Chung, Ching-Feng Cheng, Hui-Chen Ku and Yung-Hsi Kao in Experimental Biology and Medicine