Abstract
Background/Aims
Data of coronavirus disease 2019 (COVID-19) vaccine immunogenicity among chronic liver disease (CLD) and liver transplant (LT) patients are conflicting. We performed meta-analysis to examine vaccine immunogenicity regarding etiology, cirrhosis status, vaccine platform and type of antibody.
Methods
We collected data via three databases from inception to February 16, 2022, and reported pooled seroconversion rate, T cell response and safety data after two vaccine doses.
Results
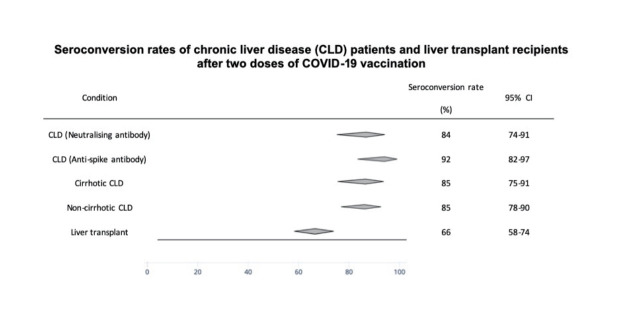
Twenty-eight (CLD only: 5; LT only: 18; both: 2; LT with third dose: 3) observational studies of 3,945 patients were included. For CLD patients, seroconversion rate ranged between 84% (95% confidence interval [CI], 76–90%) and 91% (95% CI, 83–95%), based predominantly on neutralizing antibody and anti-spike antibody, respectively. Seroconversion rate was 81% (95% CI, 76–86%) in chronic hepatitis B, 96% (95% CI, 93–97%) in non-alcoholic fatty liver disease, 85% (95% CI, 75–91%) in cirrhosis and 85% (95% CI, 78–90%) in non-cirrhosis, 86% (95% CI, 78–92%) for inactivated vaccine and 89% (95% CI, 71–96%) for mRNA vaccine. The pooled seroconversion rate of anti-spike antibody was 66% (95% CI, 55–75%) after two doses of mRNA vaccines and 88% (95% CI, 58–98%) after third dose among LT recipients. T cell response rate was 65% (95% CI, 30–89%). Prevalence of adverse events was 27% (95% CI, 18–38%) and 63% (95% CI, 39–82%) among CLD and LT groups, respectively.
Conclusions
CLD patients had good humoral response to COVID-19 vaccine, while LT recipients had lower response.
Keywords: COVID-19, SARS-CoV-2, Liver transplant, Chronic hepatitis B, Non-alcoholic fatty liver disease
Graphical Abstract
INTRODUCTION
Coronavirus disease 2019 (COVID-19) has affected over 400 million people and caused near nearly 6 million deaths globally as of March 2022 [1]. Vaccination has high efficacy profile against COVID-19 infection using different vaccine platforms, such as mRNA (e.g., BNT162b2 [2], mRNA-1273 [3]), adenoviral vector (e.g., ChAdOx1 nCov-19/AZD1222 [4]), and inactivated vaccines (e.g., CoronaVac [5], BBIBP-CorV [6]). However, these trials had limited data on patients with chronic liver disease (CLD).
CLD is associated with higher risk of adverse outcomes following COVID-19 infection, especially those with liver cirrhosis [7,8]. Immunogenicity and safety of COVID-19 vaccine is a concern in this group of patients, as cirrhosis affects innate and adaptive immune response [9]. A study of 581 subjects receiving inactivated vaccines revealed that the seroconversion rates of neutralizing antibody (Nab) were 76.8%, 78.9%, and 76.7% among non-cirrhosis, compensated and decompensated cirrhosis groups respectively, in comparison with healthy subjects (90.3%) [10]. However, other studies reported a higher seroconversion rate of at least 90% among CLD patients [11,12].
Due to use of immunosuppressants, liver transplant (LT) recipients are at higher risk of severe infection [13], and have attenuated response to vaccinations against other diseases [14]. Lower immunogenicity was reported in LT recipients (73.9%) comparing with cirrhotic patients (100%) and controls (100%) [15]. An even lower seroconversion rate of <50% was reported in some studies [16-21].
The conflicting data of COVID-19 vaccine immunogenicity among CLD patients and LT recipients could be related to significant heterogeneity among studies in terms of CLD etiology, cirrhosis status, vaccine platform and type of antibody measured (including Nab, anti-spike receptor binding domain [RBD] antibody and anti-spike antibody). Currently, Nab level is a surrogate marker of vaccine effectiveness [22] and is predictive of protection from symptomatic COVID-19 infection [23,24]. Although levels of anti-spike antibody correlate with Nab, seropositivity is lower upon measurement of Nab than anti-spike antibody [11,25,26].
We therefore performed a systematic review and meta-analysis to summarize data on vaccine immunogenicity and reactogenicity among patients with liver diseases with stratification according to etiology, cirrhosis status, vaccine platform and type of antibody measured.
MATERIALS AND METHODS
Data sources and searches
We searched electronic databases MEDLINE (OVID), EMBASE, and Cochrane Library from inception to February 16, 2022. Keywords include liver disease, LT, organ transplant, COVID-19, vaccination. The search details can be found in Appendix 1. This review was conducted and reported in consonance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Study selection
Two reviewers (KSC, CHM) screened the titles and abstracts independently for inclusion. Full texts were retrieved if they met the inclusion criteria and assessed independently, and dissonance was resolved by WKS and MFY. Inclusion criteria included (1) study population: CLD patients and LT recipients; (2) intervention: COVID-19 vaccines (including CoronaVac, BBIBP-CorV, WIBP-CorV, BNT162b2, mRNA-1273, AZD1222); (3) study design: randomized controlled trials and observational studies; and (4) primary outcome: seroconversion rate of either Nab or anti-spike antibody. Secondary outcomes are T cell immune response and frequency of adverse events.
Exclusion criteria included (i) age <18 years; (ii) history of COVID-19 infection; and (iii) non-original studies, such as systematic reviews, meta-analysis, review articles, or guidelines. A summary of studies identified, included, and excluded is shown in PRISMA flow diagram (Supplementary Fig. 1).
Data extraction and quality assessment
For eligible studies, we recorded the first author, site of study, study duration, sample size, age, sex, causes of CLD and LT, COVID-19 vaccine type administered, antibody type measured, time interval of antibody measurement from second dose of vaccination, method of antibody test, and cut-off of antibody level regarded as seropositive (Table 1).
Table 1.
Background characteristics of the included studies
| Study | Country | Study duration | Participants | Age (years) | Male | Liver disease | Immunosup-pressants used | Vaccine type | Antibody type | Time interval of antibody measurement from second dose of vaccination | Method of antibody test | Cut-off of antibody level regarded as seropositive | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronic liver disease | |||||||||||||
| Ai et al. [10] | China | January to August 2021 | 437 | Median (IQR): 47.0 (38.0–56.0) | 278 (63.6%) | Non-cirrhotic chronic liver disease: HBV, 260 (91.5%); HCV, 8 (2.82%); NAFLD, 9 (3.17%); ALD, 1 (0.35%); AIH/PBC/PSC, 1 (0.35%); others, 5 (1.76%) | NA | CoronaVac: NA | Neutralising Ab | ≥14 days | CLIA | >10.0 AU/mL | |
| BBIBP-CorV: NA | |||||||||||||
| Cirrhosis: HBV, 124 (81.0%); HCV, 12 (7.84%); NAFLD. 3 (1.96%); AIH/PBC/PSC, 7 (4.58%); others, 7 (4.58%) | WIBP-CorV: NA | ||||||||||||
| Bakasis et al. [11] | Greece | March to May 2021 | 87 | Median (range): cirrhosis, 67.0 (27–86); noncirrhotic liver disease, 65.0 (35–81) | 43 (49.4%) | Non-cirrhotic chronic liver disease: HBV, 23 (46.9%); HCV, 1 (2.04%); NAFLD, 7 (14.3%); AIH, 6 (12.2%); PBC, 11 (22.4%); PSC, 1 (2.04%) | MTX: 5 (5.75%) | BNT162b2: 81 (93.1%) | Antispike Ab, neutralising Ab | Cirrhosis: 24 days (IQR, 14–57) | ELISA | Anti-spike Ab: >1.1 OD ratio | |
| AZA: 7 (8.05%) | |||||||||||||
| RTX: 4 (4.60%) | |||||||||||||
| Cirrhosis: HBV, 7 (18.4%); HCV, 1 (2.63%); NAFLD, 9 (23.7%); ALD, 6 (15.8%); AIH, 8 (21.1%); PBC, 1 (2.63%); PSC, 3 (7.89%); HSC, 1 (2.63%); BCS, 1 (2.63%); DILI, 1 (2.63%) | MMF: 6 (6.90%) | mRNA-1273: 6 (6.90%) | Non-cirrhotic liver disease: 25 days (IQR, 15–45) | Neutralising Ab: >30% inhibitory concentration | |||||||||
| TNFI: 4 (4.60%) | |||||||||||||
| S: 14 (16.1%) | |||||||||||||
| He et al. [26] | China | July to August 2021 | 362 | Median (range): 45.0 (19.0–78.0) | 223 (61.6%) | HBV, 362 (100%); cirrhosis, 48 (13.3%) | NA | CoronaVac: NA | Antispike Ab, antispike RBD Ab, neutralising Ab | ≥21 days (range, 21–105) | ELISA | Anti-spike Ab, antispike RBD Ab: ≥2.1 OD ratio | |
| BBIBP-CorV: NA | Neutralising Ab: ≥20% inhibitory concentration | ||||||||||||
| Ruether et al. [15] | Germany | NA | 48 | Mean±SD: 53.8±9.5 | 108 (58.1%) | Cirrhosis: ALD, 23 (47.9%); VH, 3 (6.30%); AIH, 11 (22.9%); NAFLD, 4 (8.30%); CC, 5 (10.4%); ALF, 1 (2.10%); others, 1 (2.10%); HCC, 5 (10.4%)* | NA | mRNA/mRNA: 44 (91.6%) | Antispike RBD Ab, antispike trimer Ab | Median (IQR): 28 days (21–41) | CLIA | Anti-spike RBD Ab: >100 U/mL | |
| BNT162b2: 38 (79.2%) | ECLIA | ||||||||||||
| mRNA-1273: 6 (12.4%) | Anti-spike trimer Ab: >100 BAU/mL | ||||||||||||
| AZD1222/AZD1222: 1 (2.08%) | |||||||||||||
| AZD1222/mRNA: 3 (6.25%) | |||||||||||||
| Thuluvath et al. [21] | USA | NA | 171 | Mean±SD: noncirrhotic chronic liver disease, 60.4±13.9; liver cirrhosis, 63.8±11.1 | Noncirrhotic chronic liver disease: 37 (40.2%) | Non-cirrhotic chronic liver disease: AIH/PBC/PSC, 36 (39.1%); ALD, 2 (2.17%); HBV/HCV, 20 (21.7%); NAFLD, 36 (39.1%); others, 8 (8.70%) | AZA: 25 (14.6%) | BNT162b2: 80 (46.8%) | Antispike Ab | Mean±SD: non-cirrhotic chronic liver disease, 40.8±19.6; liver cirrhosis, 40.9±23.9 | ECLIA | >250 U/mL | |
| S: 19 (11.1%) | mRNA-1273: 77 (45.0%) | ||||||||||||
| Liver cirrhosis: 40 (50.6%) | Cirrhosis: AIH/PBC/PSC, 17 (21.5%); ALD, 17 (21.5%); HBV/HCV, 17 (21.5%); NAFLD, 33 (41.8%); others, 8 (10.1%) | TAC: 0 (0.00%) | |||||||||||
| Others: 11 (6.43%) | JNJ-78436735: 14 (8.19%) | ||||||||||||
| Wang et al. [12] | China | October 2020 to March 2021 | 381 | Median (IQR): 39.0 (33.0–48.0) | 179 (47.0%) | NAFLD: 381 (100%) | NA | BBIBPCorV: 381 (100%) | Neutralising Ab | 14 days | CLIA | NA | |
| Xiang et al. [25] | China | March to September 2021 | 149 | Median (IQR): 41.0 (33.0–49.0) | 108 (72.5%) | HBV: 284 (100%) | NA | CoronaVac: NA | Antispike RBD Ab, neutralising Ab | Median (IQR): 33 days (24–48) | CLIA | Anti-spike RBD Ab: 1 AU/mL | |
| BBIBP-CorV: NA | |||||||||||||
| WIBP-CorV: NA | Neutralising Ab: 0.05 AU/mL | ||||||||||||
| Liver transplant | |||||||||||||
| Rashidi-Alavijeh et al. [44] | Germany | February to March 2021 | 43 | Median (IQR): 47.0 (36.0–54.0) | 26 (60.5%) | HCC: 10 (23.3%); PSC: 7 (16.3%); AC: 6 (14.0%); HCV: 3 (6.98%); ALF: 3 (6.98%); WD: 3 (6.98%); CC: 2 (4.65%); AAD: 2 (4.65%); others: 7 (16.3%) | TAC+EVE: 22 (51.2%) | BNT162b2: 43 (100%) | Antispike Ab | 15 days (IQR: 12–24) | CLIA | ≥13.0 AU/mL | |
| TAC+MMF: 11 (25.6%) | |||||||||||||
| TAC: 7 (16.3%) | |||||||||||||
| CSA: 2 (4.65%) | |||||||||||||
| EVE: 1 (2.33%) | |||||||||||||
| Boyarsky et al. [45] | USA | December 2020 to March 2021 | 129 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | Median (IQR): 29 days (28–31) | ECLIA | ≥0.8 U/mL | |
| mRNA-1273: NA | |||||||||||||
| Cholankeril et al. [16] | USA | January to February 2021 | 69 | Median (IQR): 63.0 (51–68) | 48 (69.6%) | ALD: 24 (34.8%); NAFLD: 13 (18.8%); HCC: 21 (30.4%) | TAC: 64 (92.8%) | BNT162b2: 69 (100%) | Antispike Ab | 30–75 days | ELISA | Titer ≥1 | |
| MMF: 23 (33.3%) | |||||||||||||
| S: 22 (31.9%) | |||||||||||||
| Davidov et al. [46] | Israel | January to May 2021 | 76 | Mean±SD: 59.0±15 | 43 (56.6%) | HBV: 7 (9.30%); HCV: 19 (25.3%); NAFLD: 13 (17.3%); PSC: 11 (14.7%); PBC: 3 (4.00%); others: 23 (30.3%) | CNI (TAC/CSA): 40 (52.6%) | BNT162b2: 76 (100%) | Antispike RBD Ab | Mean±SD: 38±24 days | ELISA | Titer ≥1.1 | |
| CNI+MMF: 12 (15.8%) | |||||||||||||
| CNI+EVE: 10 (13.2%) | |||||||||||||
| CNI+S: 9 (11.8%) | |||||||||||||
| CNI+MMF+S: 4 (5.26%) | |||||||||||||
| SRL: 1 (1.32%) | |||||||||||||
| Erol et al. [47] | Turkey | April to June 2021 | 10 | NA | NA | NA | NA | BNT162b2: 4 (40.0%) | Antispike Ab | 28–42 days | CLIA | ≥50 AU/mL | |
| CoronaVac: 6 (60.0%) | |||||||||||||
| Fernández-Ruiz et al. [37] | Spain | April to June 2021 | 13 | NA | NA | NA | NA | mRNA-1273: 13 (100%) | Antispike Ab | 14 days | ELISA | OD ≥1.1 | |
| Guarino et al. [48] | Italy | May to August 2021 | 444 | Median (IQR): seronegative, 65.6 (59.4-71.0); seropositive, 65.2 (56.9-70.1) | 332 (74.8%) | VH: 342 (77.0%); ALD: 34 (7.66%); NAFLD: 8 (1.80%); AIH: 18 (4.05%); others: 43 (9.68%) | TAC/CSA: 357 (80.4%) | BNT162b2: 444 (100%) | Antispike Ab | Median (IQR): 1st collection, 28 days (28–31); 2nd collection, 88 days (86–91) | CLIA | >25 AU/mL | |
| MMF: 151 (34.0%) | |||||||||||||
| EVE/SRL: 118 (26.6%) | |||||||||||||
| Hall et al. [35] | Canada | March to April 2021 | 11 | NA | NA | NA | NA | mRNA-1273: 11 (100%) | Antispike RBD Ab, neutralising Ab | 28–42 days | ECLIA | Anti-spike RBD Ab: ≥0.8 U/mL | |
| ELISA | Neutralising Ab: >30% inhibitory concentration | ||||||||||||
| Herrera et al. [36] | Spain | NA | 58 | Median (range): 61.5 (18.0–88.0) | 40 (69.0%) | NA | CNI: 53 (91.4%) | mRNA-1273: 58 (100%) | Antispike RBD Ab | ≥28 days | CLIA | NA | |
| MMF: 15 (25.9%) | |||||||||||||
| S: 13 (22.4%) | |||||||||||||
| mTORI: 13 (22.4%) | |||||||||||||
| Holden et al. [17] | Denmark | From January 2021 | 13 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | Median (IQR): 5.6 weeks (5.1–6.3) | ELISA | Titer ≥1.1 | |
| Huang et al. [18] | USA | January to April 2021 | 87 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | ≥14 days | ELISA | Titer >1:50 | |
| mRNA-1273: NA | |||||||||||||
| Marion et al. [19] | France | January to April 2021 | 58 | NA | NA | NA | NA | BNT162b2: NA | Antispike Ab | 28 days | ELISA | NA | |
| mRNA-1273: NA | |||||||||||||
| Mazzola et al. [49] | France | January to April 2021 | 58 | Median (IQR): 64.0 (58.0–68.2) | 43 (74.1%) | NA | S: 15 (25.9%) | BNT162b2: 58 (100%) | Antispike Ab | 28 days | CLIA | ≥50 AU/mL | |
| CNI: 45 (77.6%) | |||||||||||||
| MMF: 33 (56.9%) | |||||||||||||
| mTORI: 13 (22.4%) | |||||||||||||
| Mulder et al. [50] | Netherlands | March to July 2021 | 476 | Median (IQR): BNT162b2, 71.0 (59.0–79.0); mRNA-1273, 59.0 (49.0–66.0); AZD1222, 63.0 (60.0–64.0) | 286 (60.1%) | PSC: 101 (21.2%); HCC: 103 (21.6%); ALF: 46 (9.66%); PBC/SSC/CBD: 37 (7.77%); NAFLD/ALD: 42 (8.82%); CC: 22 (4.62%); VH: 24 (5.04%); DC: 20 (4.20%); others: 50 (10.5%); retransplant: 31 (6.51%) | TAC: 243 (51.1%) | BNT162b2: 25 (5.25%) | Antispike Ab | Median (IQR): BNT162b2, 31 (29.0–40.0); mRNA-1273, 43.0 (33.0–56.3); AZD1222, 31.0 (26.0–38.0) | CLIA | NA | |
| MMF: 27 (5.67%) | |||||||||||||
| CSA: 5 (1.05%) | mRNA-1273: 430 (90.3%) | ||||||||||||
| SRL: 2 (0.42%) | |||||||||||||
| EVE: 1 (0.21%) | AZD1222: 21 (4.41%) | ||||||||||||
| AZA: 1 (0.21%) | |||||||||||||
| S: 39 (8.19%) | |||||||||||||
| TAC+MMF: 113 (23.7%) | |||||||||||||
| TAC+S: 27 (5.67%) | |||||||||||||
| TAC+SRL: 16 (3.36%) | |||||||||||||
| CSA+EVE: 6 (1.26%) | |||||||||||||
| TAC+AZA: 7 (1.46%) | |||||||||||||
| TAC+EVE: 5 (1.05%) | |||||||||||||
| MMF+EVE: 3 (0.63%) | |||||||||||||
| MMF+SRL: 2 (0.42%) | |||||||||||||
| AZA+S: 1 (0.21%) | |||||||||||||
| CSA+AZA: 1 (0.21%) | |||||||||||||
| MMF+S: 1 (0.21%) | |||||||||||||
| TAC+MMF+S: 8 (1.68%) | |||||||||||||
| TAC+SRL+S: 1 (0.21%) | |||||||||||||
| Nazaruk et al. [51] | Poland | January to June 2021 | 55 | Mean±SD: 58.4±13.3 | 44 (80.0%) | NA | S: 20 (36.4%) | BNT162b2: 55 (100%) | Antispike Ab | 28–56 days | CLIA | >50 AU/mL | |
| MMF: 16 (29.1%) | |||||||||||||
| AZA: 5 (9.10%) | |||||||||||||
| CSA: 11 (20.0%) | |||||||||||||
| TAC: 43 (78.2%) | |||||||||||||
| SRL: 2 (3.60%) | |||||||||||||
| EVR: 2 (3.60%) | |||||||||||||
| CNI/MMF: 24 (43.6%) | |||||||||||||
| CNI+S/MMF/AZA/mTORI: 18 (32.7%) | |||||||||||||
| CNI/mTORI+S+MMF/AZA: 13 (23.6%) | |||||||||||||
| Rabinowich et al. [20] | Israel | From December 2020 | 80 | Mean±SD: 60.1±13.3 | 56 (70.0%) | HBV: 13 (16.3%); HCV: 26 (32.5%); NAFLD: 16 (20.0%); ALD: 3 (3.75%); AIH: 6 (7.50%); PBC: 3 (3.75%); PSC: 7 (8.75%); ALF: 2 (2.50%); CC: 3 (3.75%); WD: 1 (1.25%); SSC: 1 (1.25%); CHF: 1 (1.25%); HCC: 26 (32.5%)* | S: 24 (30.0%) | BNT162b2: 80 (100%) | Antispike Ab | 10–20 days | CLIA | ≥15 AU/mL | |
| TAC: 65 (81.3%) | |||||||||||||
| CSA: 10 (12.5%) | |||||||||||||
| EVE: 18 (22.5%) | |||||||||||||
| AZA: 4 (5.00%) | |||||||||||||
| MMF: 40 (50.0%) | |||||||||||||
| Ruether et al. [15] | Germany | NA | 138 | Mean±SD: 55.0±13.2 | 79 (57.2%) | ALD: 28 (20.3%); VH: 17 (12.3%); AIH: 40 (29.0%); NAFLD: 7 (5.10%); PLD: 5 (3.60%); CC: 13 (9.4%); ALF: 5 (3.60%); others: 23 (16.7%); HCC: 25 (18.1%)* | S: 43 (31.2%) | mRNA/mRNA: 121 (87.7%) | Antispike RBD Ab, antispike trimer Ab | Median (IQR): 29 days (25–39) | CLIA | Anti-spike RBD Ab: >100 U/mL | |
| TAC: 95 (68.8%) | ECLIA | ||||||||||||
| CSA: 33 (23.9%) | BNT162b2: 110 (79.7%) | Anti-spike trimer Ab: >100 BAU/mL | |||||||||||
| CNI: 33 (23.9%) | |||||||||||||
| CNI+S: 19 (13.8%) | mRNA-1273: 11 (7.97%) | ||||||||||||
| CNI+mTORI: 17 (12.3%) | |||||||||||||
| CNI+MMF: 48 (34.8%) | AZD1222/AZD1222: 6 (4.35%) | ||||||||||||
| CNI+AZA: 9 (6.50%) | |||||||||||||
| Biologics: 8 (5.8%) | AZD1222/mRNA: 11 (7.97%) | ||||||||||||
| Strauss et al. [52] | USA | January to April 2021 | 161 | Median (IQR): 64.0 (48.0–69.0) | 69 (42.9%) | NA | TAC: 81 (50.3%) | BNT162b2: 85 (52.8%) | Antispike Ab, antispike RBD Ab | Median (IQR): 30 days (28–31) | ECLIA | >250 U/mL | |
| MMF: 35 (21.7%) | |||||||||||||
| S: 22 (13.7%) | mRNA-1273: 76 (47.2%) | ||||||||||||
| SRL: 11 (6.83%) | |||||||||||||
| CSA: 8 (4.97%) | |||||||||||||
| AZA: 6 (3.73%) | |||||||||||||
| ERL: 3 (1.86%) | |||||||||||||
| Thuluvath et al. [21] | USA | NA | 62 | Mean±SD: 65.7±8.7 | 41 (66.1%) | AIH/PBC/PSC: 8 (12.9%); ALD: 13 (21.0%); HBV/HCV: 26 (41.9%); NAFLD: 15 (24.2%); others: 16 (25.8%) | AZA: 2 (3.23%) | BNT162b2: 24 (38.7%) | Antispike Ab | Mean±SD: 38.9±19.6 days | ECLIA | >250 U/mL | |
| S: 8 (12.9%) | |||||||||||||
| TAC: 41 (66.1%) | mRNA-1273: 33 (53.2%) | ||||||||||||
| Others: 29 (46.8%) | JNJ-78436735: 5 (8.06%) | ||||||||||||
| Timmermann et al. [53] | Germany | May to July 2021 | 118 | Mean (range): 66.1 (28.0–89.0) | 75 (63.6%) | ALD: 25 (21.1%); VH: 28 (23.7%); LT: 26 (22.0%); AIH: 18 (15.3%); CC: 4 (3.4%); other: 17 (14.4%) | TAC: 42 (35.6%) | BNT162b2: 114 (96.6%) | Antispike Ab | Mean (range): 44.6 days (21–132) | ELISA | NA | |
| MMF: 16 (13.6%) | |||||||||||||
| TAC+MMF: 24 (20.3%) | mRNA-1273: 3 (2.54%) | ||||||||||||
| TAC+EVE: 15 (12.7%) | |||||||||||||
| EVR: 1 (0.85%) | JNJ-78436735: 1 (0.85%) | ||||||||||||
| CSA+MMF: 3 (2.54%) | |||||||||||||
| CSA: 2 (1.69%) | |||||||||||||
| TAC+AZA: 1 (0.85%) | |||||||||||||
IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, non-alcoholic fatty liver disease; ALD, alcoholic liver disease; AIH, autoimmune hepatitis; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis; NA, not available; Ab, anti-body; CLIA, chemiluminescence immunoassays; HSC, hepatic sarcoidosis; BCS, Budd-Chiari syndrome; DILI, drug induced liver injury; MTX, methotrexate; AZA, azathioprine; RTX, rituximab; MMF, mycophenolate mofetil; TNFI, tumour necrosis factor inhibitor; S, steroid; OD, optical density; RBD, receptor binding domain; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation; VH, viral hepatitis; CC, cryptogenic cirrhosis; ALF, acute liver failure; HCC, hepatocellular carcinoma; ECLIA, electrochemiluminescence immunoassay analyzer; TAC, tacrolimus; AC, alcoholic cirrhosis; WD, Wilson’s disease; AAD, α-1 antitrypsin deficiency; EVE, everolimus; CSA, cyclosporin A; CNI, calcineurin inhibitors; SRL, sirolimus; mTORI, mTOR inhibitors; SSC, secondary sclerosing cholangitis; CBD, congenital biliary disease; DC, dysmetabolic cirrhosis; CHF, congenital hepatic fibrosis; PLD, pediatric liver disease; LT, liver tumour.
Concomitant condition.
The quality of observational studies was assessed using Newcastle-Ottawa scale (NOS). Risk of bias was categorized into three groups: low risk (7–9 points), moderate risk (4–6 points), and high risk (<4 points) [27].
Data analysis
All statistical analyses were conducted in R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) statistical software. Continuous variables were expressed as median (interquartile range [IQR]) or mean±standard deviation). The pooled rate of seroconversion and adverse events were expressed as proportion and 95% confidence interval (95% CI) using random effects model, and was presented as Forest plot. A P-value of <0.05 was used to define statistical significance. We used Cochran Q test to detect heterogeneity among studies, with a P-value <0.10 indicating significant heterogeneity. We calculated I2 statistic to measure proportion of total variation in study estimates attributed to heterogeneity. I2 values of ≥50% and ≥75% indicate substantial and considerable heterogeneity, respectively [28]. Meta-regression analysis was used to examine association between background characteristics of the included studies and pooled seroconversion rates [29].
We assessed publication bias by funnel plot and Egger regression. Publication bias was considered significant if P-value of Egger regression is <0.1 [30]. The trim-and-fill method was used to adjust for publication bias, if present, which re-estimated the effect size after imputing potentially missing studies.
Subgroup analysis was performed according to type of antibody tested, method of antibody test (electrochemiluminescence immunoassay analyzer [ECLIA], enzyme-linked immunosorbent assay [ELISA], and chemiluminescence immunoassays [CLIA]), age (with a cut-off of 60 years), etiology of CLD, cirrhosis status, vaccine platform, individual vaccine type, use of multiple immunosuppressants, and region, where applicable.
RESULTS
Study characteristics of meta-analysis
Supplementary Figure 1 depicts the study selection process. Of the 3,590 studies identified, 28 (CLD only: 5, LT only: 18, both CLD and LT: 2; LT with third dose vaccine: 3) are included in the meta-analysis with 3,945 subjects. The characteristics of included studies are shown in Table 1 (for CLD patients and LT recipients receiving two doses of vaccine) and Supplementary Table 1 (for LT recipients receiving third dose). For CLD patients, the median age was 53.8 years (IQR, 43.0–64.4 years), and 62.1% were male. For LT recipients, the median age was 63.0 years (IQR, 59.0–65.6 years), and 64.0% were male. All studies scored at least six stars in NOS, indicating low to moderate risk of bias with satisfactory quality (Supplementary Table 1).
Meta-analysis for CLD patients
Humoral immune response
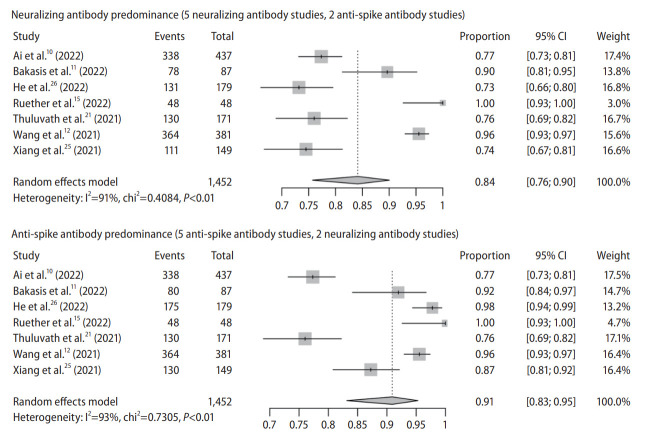
There are seven observational studies with 1,452 subjects (studies reporting both Nab and anti-spike antibody: 3; Nab only: 2; anti-spike antibody only: 2) (Table 1). In Nab predominance forest plot (Nab: 5; anti-spike antibody: 2), pooled seroconversion rate was 84% (95% CI, 76–90%) with considerable heterogeneity among the studies (P<0.01; I2=91%) (Fig. 1). In anti-spike antibody predominance forest plot (anti-spike antibody: 5; Nab: 2), pooled seroconversion rate was 91% (95% CI, 83–95%) with considerable hereogeneity (P<0.01; I2=93%) (Fig. 1).
Figure 1.
Pooled seroconversion rate in chronic liver disease. CI, confidence interval.
The funnel plot appeared to be have some asymmetry for studies with either anti-spike antibody (P=0.038 by Egger test) or neutralizing antibody predominance (P=0.012 by Egger test), indicating publication bias (Supplementary Fig. 2). Trim and fill-method was used to adjust for publication bias, and the pooled seroconversion rate was 83% (95% CI, 71–90%) for Nab predominance analysis and 82% (95% CI, 61–93%) for anti-spike antibody predominance analysis.
Meta-regression analysis showed significant association between seroconversion of Nab and etiology of liver disease (P<0.001) and a trend for method of antibody test (P=0.053 for ECLIA vs. ELISA) but not other factors (Supplementary Fig. 3).
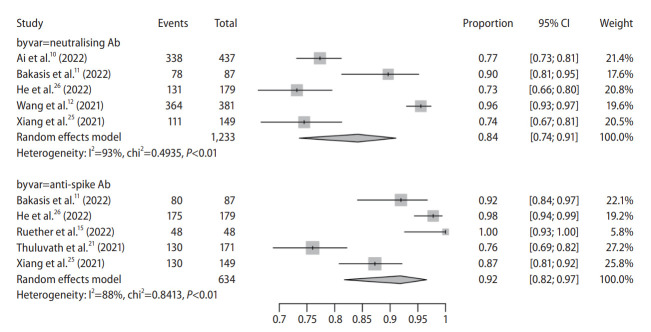
Antibody type
Pooled seroconversion rate of Nab and anti-spike antibody was 84% (95% CI, 74–91%) and 92% (95% CI, 82–97%), respectively (Fig. 2).
Figure 2.
Pooled seroconversion rate in chronic liver disease according to antibody type. CI, confidence interval; Ab, antibody.
Method of antibody test
There were four studies on CLIA, two on ELISA and one on ECLIA. Seroconversion rate was 89% (95% CI, 77–95%), 88% (95% CI, 82–92%), 76% (95% CI, 69–82%) in CLIA, ELISA and ECLIA, respectively (Supplementary Fig. 4).
Age
There were two studies with median age ≥60 years and five studies with median age <60 years. Seroconversion rate was 85% (95% CI, 64–94%) and 88% (95% CI, 79–93%) in the older and younger age group, respectively (Supplementary Fig. 5).
Etiology of liver disease and cirrhosis status
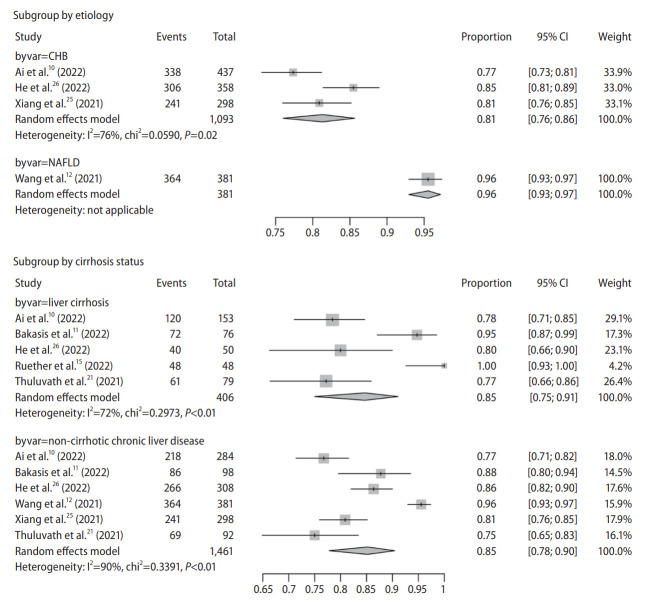
We used 80% as cut-off for classifying the major etiology of a study. There were three studies on chronic hepatitis B (CHB) infection (two studies with 100% CHB [25,26] and one with 87.9% [10]) and one study on non-alcoholic fatty liver disease (NAFLD) (Table 1) [12]. Other studies recruited a heterogeneous population of CLD patients of various etiologies without available individual data, and therefore were excluded from subgroup analysis. Seroconversion rate was 81% (95% CI, 76–86%) and 96% (95% CI, 93–97%) in CHB and NAFLD patients, respectively (Fig. 3).
Figure 3.
Pooled seroconversion rate in chronic liver disease according to etiology and cirrhosis status. CI, confidence interval; CHB, chronic hepatitis B; NAFLD, non-alcoholic fatty liver disease.
There were five studies on cirrhosis and six studies on non-cirrhosis CLD, four of which reported both outcomes. Seroconversion rate was 85% (95% CI, 75–91%) and 85% (95% CI, 78–90%) in patients with cirrhosis and those without cirrhosis, respectively (Fig. 3). Only one study reported seroconversion rate regarding cirrhosis severity (compensated cirrhosis: 78.9%; decompensated cirrhosis: 76.7%) [10].
Vaccine platform
There were four studies on inactivated vaccine and three on mRNA vaccine. Seroconversion rate was 86% (95% CI, 78–92%) and 89% (95% CI, 71–96%) in inactivated and mRNA vaccine, respectively (Supplementary Fig. 6).
Individual vaccine type
We used 80% as cut-off for classifying vaccine type of a study. There were two studies using BNT162b2 [11,15] and one using BBIBP-CorV [12] with 516 subjects. Other studies recruited a heterogeneous population of patients with various vaccine types without available individual data, and therefore were excluded from subgroup analysis. Seroconversion rate was 95% (95% CI, 72–99%) and 96% (95% CI, 93–97%) in BNT162b2 and BBIBP-CorV subgroups, respectively (Supplementary Fig. 7).
Region
There were four studies from the East and three from the West. Seroconversion rate was 86% (95% CI, 78–92%) and 89% (95% CI, 1–96%) in the East and West subgroups, respectively (Supplementary Fig. 8).
Cell-mediated vaccine immunogenicity
Only one study reported T-cell immune response among cirrhosis patients [15]. A T cell response was observed in 65% of cirrhosis patients, 37% of LT recipients and 100% of control subjects, with a strong response being present in 46%, 32%, and 100% in the three groups, respectively.
Adverse events
There were five studies (inactivated vaccine: 4; mRNA vaccine: 1) reporting adverse events with 1,360 subjects. Prevalence of adverse events was 27% (95% CI, 18–38%) with considerable heterogeneity (P<0.01; I2=88%) (Supplementary Fig. 9). Ai et al. [10] reported three subjects having grade 3 laboratory abnormalities with raised alanine transaminase five times above upper limit of normal, one of whom developed trend of acute liver failure requiring hospitalization (grade 4). Ruether et al. [15] reported two subjects with severe systemic side effects or requiring medications (grade 3) and one requiring hospitalization (grade 4). Supplementary Table 2 showed pooled prevalence of local and systemic adverse events among inactivated vaccine recipients. The most common local and systemic adverse event was pain (13%; 95% CI, 7–23%) and fatigue (3%; 95% CI, 2–5%), respectively. The study using mRNA vaccine did not report detailed data on individual adverse events.
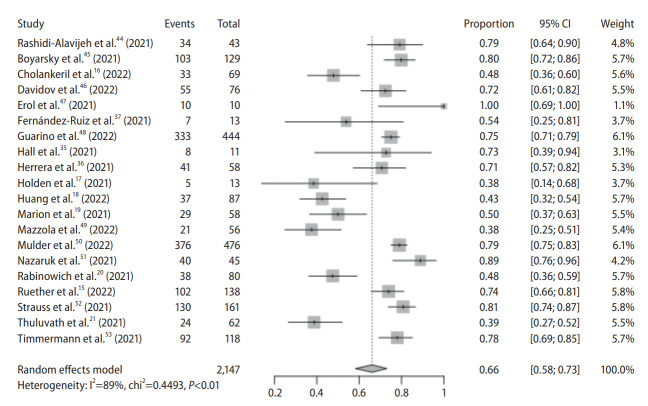
Liver transplant recipients
Humoral immune response
There were 20 observational studies with 2,147 subjects. All studies were conducted in the West. Pooled seroconversion rate was 66% (95% CI, 58–73%) with considerable heterogeneity (P<0.01; I2=89%) (Fig. 4). The funnel plot appeared to be have some asymmetry (P=0.059 by Egger test), indicating publication bias (Supplementary Fig. 10). Trim and fill-method was used to adjust for publication bias, and the pooled seroconversion rate was 69% (95% CI, 61–76%).
Figure 4.
Pooled seroconversion rate in liver transplant recipients. CI, confidence interval.
Meta-regression analysis showed a trend for association between seroconversion of Nab and age (P=0.085) and method of antibody test (P=0.066 for CLIA vs. ECLIA) but not other factors (Supplementary Fig. 11).
Subgroup analysis
Method of antibody test
There were nine studies on CLIA, seven on ELISA, and four on ECLIA. Seroconversion rate was 71% (95% CI, 61–79%), 56% (95% CI, 43–69%), 70% (95% CI, 47–86%) in CLIA, ELISA and ECLIA, respectively (Supplementary Fig. 12).
Age
There were nine studies with median age ≥60 years and four studies with median age <60 years. A total of 1,826 subjects were included. Seroconversion rate was 64% (95% CI, 52–74%) and 77% (95% CI, 70–83%) in the older and younger age groups, respectively (Supplementary Fig. 13).
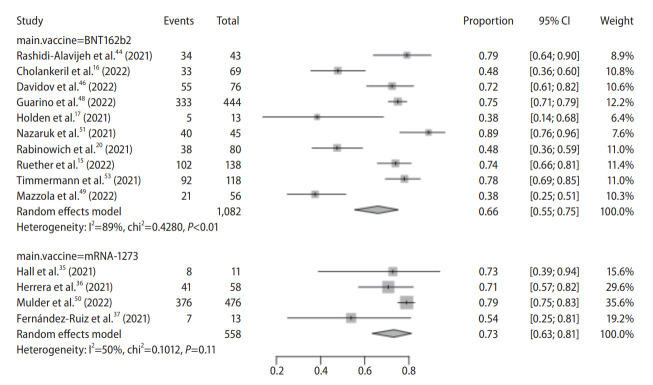
Individual vaccine type
All studies used mRNA vaccines (BNT162b2: 10; mRNA-1273: 4) with 1,640 subjects. We used 80% as cut-off for classifying vaccine type of a study. Seroconversion rate was 66% (95% CI, 55–75%) and 73% (95% CI, 63–81%) in BNT162b2 and mRNA-1273 subgroups, respectively (Fig. 5).
Figure 5.
Pooled seroconversion rate in liver transplant recipients according to individual vaccine type. CI, confidence interval.
Number of immunosuppressants
Four studies reported data on use of ≥2 immunosuppressants with 274 subjects. Seroconversion rate was 62% (95% CI, 43–79%) (Supplementary Fig. 14).
Cell-mediated vaccine immunogenicity
Four studies reported cell-mediated immune response in 157 LT recipients. T cell response rate was 65% (95% CI, 30–89%) with considerable heterogeneity (P<0.01; I2=90%) (Supplementary Fig. 15). Among those with negative humoral response in two studies, T cell response rate was 52% (95% CI, 12–90%) (Supplementary Fig. 15).
Adverse events
Three studies reported adverse events with 251 subjects. Pooled prevalence of adverse events was 63% (95% CI, 39–82%) with considerable heterogeneity (P<0.01; I2=92%) (Supplementary Fig. 16). Ruether et al. [15] reported 17 subjects with severe systemic side effects or requiring medications (grade 3) and one subject requiring hospitalization (grade 4). Data on individual adverse events were not reported in these studies.
Seroconversion rate among LT recipients receiving third dose
Three observational studies with 151 subjects were conducted in the West. Pooled seroconversion rate was 88% (95% CI, 58–98%) with considerable heterogeneity (P<0.01; I2=83%) (Supplementary Fig. 17).
DISCUSSION
This is the first meta-analysis to report COVID-19 vaccine immunogenicity and reactogenicity among CLD patients and LT recipients. Overall seroconversion rate ranges between 84% (based predominantly on Nab) and 91% (based predominantly on anti-spike antibody) among CLD patients; similar immunogenicity is noted regardless of cirrhosis status. Seroconversion rate of anti-spike antibody is 68% after two doses and 88% after third dose among LT recipients.
CLD
CLD patients are at a higher risk of developing severe COVID-19 disease and acute decompensation [31] with mortality reaching 14% [31,32]. Owing to immune dysregulation, CLD patients had lower immunologic response rate to inactivated vaccines like influenza or hepatitis vaccines [33]. CLD and fibrosis hamper production of innate immunity proteins and pattern recognition receptors, and adversely influence B- and Tlymphocytes in terms of absolute counts and functions via various mechanisms. However, the pooled seroconversion rate is good, ranging from 84% to 91% in our meta-analysis. Notably, although seroconversion rate is similar among CLD patients compared with healthy controls, their titer is generally lower [11,15]. A lower seroconversion rate of Nab of 77% was noted in the study by Ai et al. [10] recruiting subjects (87.8% CHB) who received inactivated vaccines. Another study of CHB patients receiving inactivated vaccines also found a seroconversion rate of Nab at 64.0–78.9%, dependent on HBV activity and cirrhosis status [26]. Our meta-analysis showed a numerical difference in seroconversion rate for CHB and NAFLD patients (81% vs. 96%). Nonetheless, a firm conclusion could not be drawn as there were only three studies on CHB (using three different inactivated vaccines) and one on NAFLD (using BBIBP-CorV only), and the difference could be due to different vaccine platforms used in each study. The seroconversion rate appears to be similar among younger and older subjects (85% vs. 88%).
There is also no difference in seroconversion rate between cirrhotic and non-cirrhotic groups (both 85%). There is only one study reporting no difference in vaccine immunogenicity as regards cirrhosis severity (compensated cirrhosis: 78.9%; decompensated cirrhosis: 76.7%) [10]. Subgroup analysis showed no difference in seroconversion rate between inactivated and mRNA vaccines.
Measuring anti-spike immunoglobulin G (IgG) or anti-RBD IgG results in a slightly higher seroconversion rate than Nab (91% vs. 84%). This difference is exemplified by one study showing seroconversion rate of 64.0–78.9% for Nab (dependent on HBV replication and cirrhosis status) and 96–100% for anti-spike IgG or anti-RBD IgG [26]. Nab level is a surrogate marker of vaccine effectiveness against symptomatic infection [22-24]. Anti-spike and anti-RBD IgG levels correlate with Nab [34] but not equate Nab. Using ECLIA to measure antibody level also results in slightly lower seroconversion rate compared with CLIA and ELISA (76% vs. 89% vs. 88%).
LT
Pooled seroconversion rate is less satisfactory (66%) among LT recipients, in particular for older than younger patients (64% vs. 77%). However, when compared with other organ transplant recipients (e.g., kidney, heart), LT recipients have higher seroconversion rate [19,35]. This may be related to stricter and higher levels of immunosuppression in other organ transplant recipients. However, all studies except one [35] reported seroconversion rate of anti-spike antibody but not Nab. Hall et al. [35] noted that 28.5% of organ transplant recipients with anti-RBD did not have Nab. Seroconversion rate of anti-spike IgG varied from 38% to 100%, likely related to variance in immunosuppression regimen. Known risk factors for seronegativity include high-dose steroid, triple immunosuppression, mycophenolate mofetil [20,36], low B-lymphocytes [15], hypogammaglobulinemia [36], vaccination during the first year post-transplantation [36], low estimated glomerular filtration rate [20], old age and alcohol-related liver disease [12]. Our meta-analysis showed that pooled seroconversion rate of patients receiving ≥2 immunosuppressants is slightly lower (62%) than that of whole cohort (66%). Subgroup analysis also shows the seroconversion rate of mRNA-1273 is slightly higher than that of BNT162b2 (73% vs. 66%). Importantly, pooled seroconversion rate increases to 88% after booster dose. Data on immunogenicity of inactivated vaccines in LT recipients are currently lacking. Notably, using ELISA to measure antibody level also results in slightly lower seroconversion rate compared with CLIA and ECLIA (56% vs. 71% vs. 70%).
There are four studies reporting T-cell immune response, with a pooled response rate of 65% [15,35-37]. Similar to the phenomenon observed in humoral response, level of T cell response is higher in LT recipients than other organ transplant recipients, e.g., heart transplant [36]. Our meta-analysis shows 52% have T-cell response despite seronegativity. Vaccine-induced T-cell response may offer protection via suppressing viral replication and supporting long-term memory of the immune system [38], hence protecting against severe infection despite seronegativity [23].
Concerning vaccine reactogenicity, pooled prevalence of adverse reactions is 27% among CLD patients receiving mainly inactivated vaccines, which is similar among healthy subjects (23% in a meta-analysis of randomized controlled trials) [39]. There is only one study reporting no significant difference in frequency of adverse events as regards cirrhosis status (non-cirrhosis: 15.5%; compensated cirrhosis: 16.3%; decompensated cirrhosis: 20.0%) [10]. As for LT recipients receiving mRNA vaccines, pooed prevalence of adverse reactions is 63%, compared with 48% among healthy subjects [39].
Our study findings support current international recommendation on COVID-19 vaccination in CLD patients and LT recipients [13,40]. LT recipients should receive vaccine platforms with more data (e.g., mRNA vaccine) and third-dose booster. Another strategy may be heterologous vaccination [41], in which seroconversion rate of 81.8% was reported for 8% of LT cohort who had heterologous vaccination in the study by Ruether et al. [15].
Limitations of the current study should be acknowledged. First, some studies did not measure Nab level, and the test kits differed among different studies. Second, the optimal antibody thresholds for protection is still unknown. Titers above the cut-off should protect against severe disease for the majority of vaccine recipients, but not against asymptomatic infection [23,42,43]. Third, only three studies reported vaccine immunogenicity among CHB patients and one on NAFLD; others recruited a heterogeneous population of CLD patients without available data for individual disease etiology (e.g., chronic viral hepatitis, NAFLD, autoimmune hepatitis) which may have different vaccine immunogenicity, in particular among autoimmune liver diseases which require immunosuppressants. Similarly, the LT recipients were comprised of a heterogeneous population of various disease etiology and different immunosuppressive regimen. Individual studies did not provide the seroconversion rate according to disease etiology and immunosuppressive regimen, and therefore subgroup analysis could not be performed according to these factors. Fourth, we did not include studies with general population that might enrol CLD recipients for comparison.
While an excellent safety profile is demonstrated in CLD and LT patients, the former group has good humoral response and the latter has lower response. Third-dose booster or heterologous vaccination may be considered in LT recipients, although more studies with larger sample size are warranted before this practice is widely recommended.
Abbreviations
- CHB
chronic hepatitis B
- CI
confidence interval
- CLD
chronic liver disease
- CLIA
chemiluminescence immunoassays
- COVID-19
coronavirus disease 2019
- ECLIA
electrochemiluminescence immunoassay analyzer
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- IQR
interquartile range
- LT
liver transplant
- Nab
neutralising antibody
- NAFLD
non-alcoholic fatty liver disease
- NOS
Newcastle-Ottawa scale
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RBD
receptor binding domain
Study Highlights
• Chronic liver disease patients, either cirrhotic or non-cirrhotic, have good humoral response to inactivated or mRNA COVID-19 vaccine
• Liver transplant recipients have lower humoral response to mRNA vaccine and hence early booster dose should be considered
• COVID-19 vaccine has good safety profile in both chronic liver disease patients and liver transplant recipients
Footnotes
Authors’ contributions
Dr. Ka-Shing Cheung and Mr. Chiu Hang Mok were involved with study concept and design; acquisition of data; analysis and interpretation of data; drafting of manuscript; Mr. Xianhua Mao, Dr. Ricky Zhang and Prof. Ivan FN Hung were involved with analysis and interpretation of data; and critical revision of the manuscript for important intellectual content. Profs. Wai-Kay Seto and Man-Fung Yuen were involved with the study concept and design; analysis and interpretation of data; drafting of manuscript; critical revision of the manuscript for important intellectual content; and study supervision. The corresponding author had full access to all data, and was fully responsible for the data integrity and statistical analysis. All authors revised the manuscript and approved the final version of this article.
Conflicts of Interest
The authors have no conflicts to disclose.
SUPPLEMENTAL MATERIAL
Supplementary material is available at Clinical and Molecular Hepatology website (http://www.e-cmh.org).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. COVID-19, corona-virus disease 2019.
Funnel plot of seropositivity in chronic liver disease.
Meta-regression analysis for seropositivity in chronic liver disease. Bubble plot with fixed meta-regression line of (A) region, (B) participant number, (C) age ≥60, (D) etiology of liver disease, (E) cirrhosis status, (F) vaccine type, (G) vaccine platform, (H) antibody type, (I) time interval of antibody measurement from second dose of vaccination (≥30 days), (J) method of antibody test (ECLIA vs. ELISA), (K) method of antibody test (CLIA vs. ECLIA), (L) method of antibody test (CLIA vs. ELISA). The size of circles is proportional to weight of each study in fitted random effects meta-regression. ECLIA, electrochemiluminescence immunoassay analyzer; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence immunoassays.
Pooled seroconversion rate in chronic liver disease – subgroup by method of antibody test. CI, confidence interval; CLIA, chemiluminescence immunoassays; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay analyzer.
Pooled seroconversion rate in chronic liver disease – subgroup by age ≥60 years. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by vaccine platform. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by individual vaccine type. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by region. CI, confidence interval.
Adverse events of vaccine in chronic liver disease patients. CI, confidence interval.
Funnel plot of seroconversion rate in liver transplant recipients.
Meta-regression analysis for seroconversion rate in liver transplant recipients. Bubble plot with fixed meta-regression line of (A) region (Europe vs. North America), (B) region (Europe vs. Israel), (C) region (Israel vs. North America), (D) participant number, (E) age ≥60, (F) vaccine type, (G) time interval of antibody measurement from second dose of vaccination (≥30 days), (H) method of antibody test (ECLIA vs. ELISA), (I) method of antibody test (CLIA vs. ECLIA), (J) method of antibody test (CLIA vs. ELISA). The size of circles is proportional to weight of each study in fitted random effects meta-regression. ECLIA, electrochemiluminescence immunoassay analyzer; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence immunoassays.
Pooled seroconversion rate in liver transplant recipients – subgroup by method of antibody test. CI, confidence interval; CLIA, chemiluminescence immunoassays; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay analyzer.
Pooled seroconversion rate in liver transplant recipients – subgroup by age ≥60 years. CI, confidence interval.
Pooled seroconversion rate in liver transplant recipients– subgroup by 2 or more immunosuppressants. CI, confidence interval.
Pooled cell-mediated vaccine immunogenicity in liver transplant recipients. CI, confidence interval.
Adverse events of vaccine in liver transplant recipients. CI, confidence interval.
Pooled seroconversion rate in liver transplant recipients with third dose of vaccine. CI, confidence interval.
Quality assessment according to Newcastle-Ottawa quality assessment scale
Background characteristics of the included studies for liver transplant recipients with third dose of vaccine
REFERENCES
- 1. Johns Hopkins University Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Johns Hopkins University web site, < https://coronavirus.jhu.edu/map.html>. Accessed 5 Mar 2022.
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacios R, Patiño EG, de Oliveira Piorelli R, Conde MTRP, Batista AP, Zeng G, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:853. doi: 10.1186/s13063-020-04775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Ai J, Wang J, Liu D, Xiang H, Guo Y, Lv J, et al. Safety and immunogenicity of SARS-CoV-2 vaccines in patients with chronic liver diseases (CHESS-NMCID 2101): a multicenter study. Clin Gastroenterol Hepatol. 2022;20:1516–1524.e2. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakasis AD, Bitzogli K, Mouziouras D, Pouliakis A, Roumpoutsou M, Goules AV, et al. Antibody responses after SARS-CoV-2 vaccination in patients with liver diseases. Viruses. 2022;14:207. doi: 10.3390/v14020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID-19 vaccination in patients with nonalcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. 2021;75:439–441. doi: 10.1016/j.jhep.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong PP, Avery RK. A comprehensive review of immunization practices in solid organ transplant and hematopoietic stem cell transplant recipients. Clin Ther. 2017;39:1581–1598. doi: 10.1016/j.clinthera.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20:162–172.e9. doi: 10.1016/j.cgh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cholankeril G, Al-Hillan A, Tarlow B, Abrams D, Jacobs JS, Flores NP, et al. Clinical factors associated with lack of serological response to SARS-CoV-2 messenger RNA vaccine in liver transplantation recipients. Liver Transpl. 2022;28:123–126. doi: 10.1002/lt.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holden IK, Bistrup C, Nilsson AC, Hansen JF, Abazi R, Davidsen JR, et al. Immunogenicity of SARS-CoV-2 mRNA vaccine in solid organ transplant recipients. J Intern Med. 2021;290:1264–1267. doi: 10.1111/joim.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang HJ, Yi SG, Mobley CM, Saharia A, Bhimaraj A, Moore LW, et al. Early humoral immune response to two doses of severe acute respiratory syndrome coronavirus 2 vaccine in a diverse group of solid organ transplant candidates and recipients. Clin Transplant. 2022;36:e14600. doi: 10.1111/ctr.14600. [DOI] [PubMed] [Google Scholar]
- 19.Marion O, Del Bello A, Abravanel F, Couat C, Faguer S, Esposito L, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med. 2021;174:1336–1338. doi: 10.7326/M21-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434–1439. doi: 10.1016/j.jhep.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earle KA, Ambrosino DM, Fiore-Gartland A, Goldblatt D, Gilbert PB, Siber GR, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 24.Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang T, Liang B, Wang H, Quan X, He S, Zhou H, et al. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. 2021;18:2679–2681. doi: 10.1038/s41423-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He T, Zhou Y, Xu P, Ling N, Chen M, Huang T, et al. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;42:1287–1296. doi: 10.1111/liv.15173. [DOI] [PubMed] [Google Scholar]
- 27.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 28.Deeks JJ, Higgins JPT, Altman DG, Group obotCSM . In: Cochrane Handbook for systematic reviews of interventions. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. London: Cochrane; 2019. Chapter 10: analysing data and undertaking meta-analyses; pp. 241–284. [Google Scholar]
- 29.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alukal JJ, Naqvi HA, Thuluvath PJ. Vaccination in chronic liver disease: an update. J Clin Exp Hepatol. 2022;12:937–947. doi: 10.1016/j.jceh.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zost SJ, Gilchuk P, Case JB, Binshtein E, Chen RE, Nkolola JP, et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. 2020;584:443–449. doi: 10.1038/s41586-020-2548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak-Kita B, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:3980–3989. doi: 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-González E, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21:3971–3979. doi: 10.1111/ajt.16768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-Ruiz M, Almendro-Vázquez P, Carretero O, Ruiz-Merlo T, Laguna-Goya R, San Juan R, et al. Discordance between SARS-CoV-2-specific cell-mediated and antibody responses elicited by mRNA-1273 vaccine in kidney and liver transplant recipients. Transplant Direct. 2021;7:e794. doi: 10.1097/TXD.0000000000001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, et al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10:94. doi: 10.1186/s40249-021-00878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, et al. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbert PB, Montefiori DC, McDermott A, Fong Y, Benkeser D, Deng W, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. 2021 Aug 15; doi: 10.1101/2021.08.09.21261290. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashidi-Alavijeh J, Frey A, Passenberg M, Korth J, Zmudzinski J, Anastasiou OE, et al. Humoral response to SARS-Cov-2 vaccination in liver transplant recipients-a single-center experience. Vaccines (Basel) 2021;9:738. doi: 10.3390/vaccines9070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-Dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidov Y, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, Levy I, et al. Immunogenicity and adverse effects of the 2-dose BNT162b2 messenger RNA vaccine among liver transplantation recipients. Liver Transpl. 2022;28:215–223. doi: 10.1002/lt.26366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erol Ç, Yanık Yalçın T, Sarı N, Bayraktar N, Ayvazoğlu Soy E, Yavuz Çolak M, et al. Differences in antibody responses between an inactivated SARS-CoV-2 vaccine and the BNT162b2 mRNA vaccine in solid-organ transplant recipients. Exp Clin Transplant. 2021;19:1334–1340. doi: 10.6002/ect.2021.0402. [DOI] [PubMed] [Google Scholar]
- 48.Guarino M, Esposito I, Portella G, Cossiga V, Loperto I, Tortora R, et al. Humoral response to 2-dose BNT162b2 mRNA COVID-19 vaccination in liver transplant recipients. Clin Gastroenterol Hepatol. 2022;20:1534–1541.e4. doi: 10.1016/j.cgh.2022.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazzola A, Todesco E, Drouin S, Hazan F, Marot S, Thabut D, et al. Poor antibody response after two doses of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in transplant recipients. Clin Infect Dis. 2022;74:1093–1096. doi: 10.1093/cid/ciab580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulder MB, van der Eijk AA, GeurtsvanKessel CH, Erler NS, de Winter BCM, Polak WG, et al. High antibody response in relation to immunosuppressive blood levels in liver transplant recipients after SARS-CoV-2 vaccination: an observational, cohort study. Gut. 2022 Jan 31; doi: 10.1136/gutjnl-2021-326755. doi: [DOI] [PubMed] [Google Scholar]
- 51.Nazaruk P, Monticolo M, Jędrzejczak AM, Krata N, Moszczuk B, Sańko-Resmer J, et al. Unexpectedly high efficacy of SARS-CoV-2 BNT162b2 vaccine in liver versus kidney transplant recipients-is it related to immunosuppression only? Vaccines (Basel) 2021;9:1454. doi: 10.3390/vaccines9121454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl. 2021;27:1852–1856. doi: 10.1002/lt.26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines (Basel) 2021;9:1422. doi: 10.3390/vaccines9121422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. COVID-19, corona-virus disease 2019.
Funnel plot of seropositivity in chronic liver disease.
Meta-regression analysis for seropositivity in chronic liver disease. Bubble plot with fixed meta-regression line of (A) region, (B) participant number, (C) age ≥60, (D) etiology of liver disease, (E) cirrhosis status, (F) vaccine type, (G) vaccine platform, (H) antibody type, (I) time interval of antibody measurement from second dose of vaccination (≥30 days), (J) method of antibody test (ECLIA vs. ELISA), (K) method of antibody test (CLIA vs. ECLIA), (L) method of antibody test (CLIA vs. ELISA). The size of circles is proportional to weight of each study in fitted random effects meta-regression. ECLIA, electrochemiluminescence immunoassay analyzer; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence immunoassays.
Pooled seroconversion rate in chronic liver disease – subgroup by method of antibody test. CI, confidence interval; CLIA, chemiluminescence immunoassays; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay analyzer.
Pooled seroconversion rate in chronic liver disease – subgroup by age ≥60 years. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by vaccine platform. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by individual vaccine type. CI, confidence interval.
Pooled seroconversion rate in chronic liver disease – subgroup by region. CI, confidence interval.
Adverse events of vaccine in chronic liver disease patients. CI, confidence interval.
Funnel plot of seroconversion rate in liver transplant recipients.
Meta-regression analysis for seroconversion rate in liver transplant recipients. Bubble plot with fixed meta-regression line of (A) region (Europe vs. North America), (B) region (Europe vs. Israel), (C) region (Israel vs. North America), (D) participant number, (E) age ≥60, (F) vaccine type, (G) time interval of antibody measurement from second dose of vaccination (≥30 days), (H) method of antibody test (ECLIA vs. ELISA), (I) method of antibody test (CLIA vs. ECLIA), (J) method of antibody test (CLIA vs. ELISA). The size of circles is proportional to weight of each study in fitted random effects meta-regression. ECLIA, electrochemiluminescence immunoassay analyzer; ELISA, enzyme-linked immunosorbent assay; CLIA, chemiluminescence immunoassays.
Pooled seroconversion rate in liver transplant recipients – subgroup by method of antibody test. CI, confidence interval; CLIA, chemiluminescence immunoassays; ELISA, enzyme-linked immunosorbent assay; ECLIA, electrochemiluminescence immunoassay analyzer.
Pooled seroconversion rate in liver transplant recipients – subgroup by age ≥60 years. CI, confidence interval.
Pooled seroconversion rate in liver transplant recipients– subgroup by 2 or more immunosuppressants. CI, confidence interval.
Pooled cell-mediated vaccine immunogenicity in liver transplant recipients. CI, confidence interval.
Adverse events of vaccine in liver transplant recipients. CI, confidence interval.
Pooled seroconversion rate in liver transplant recipients with third dose of vaccine. CI, confidence interval.
Quality assessment according to Newcastle-Ottawa quality assessment scale
Background characteristics of the included studies for liver transplant recipients with third dose of vaccine