Abstract
Background
The non-saponin fraction (NSF) of Korean Red Ginseng is a powder in which saponin is eliminated from red ginseng concentrate by fractionation. In this study, we examined the effect of NSF on age-associated sarcopenia in old mice.
Methods
NSF (50 or 200 mg/kg/day) was administered orally daily to young (3–6-month-old) and old (20–24-month-old) C57BL/6 J mice for 6 weeks. Body weight and grip strength were assessed once a week during the oral administration period. The gastrocnemius and quadriceps muscle were excised, and the muscle fiber size was compared through hematoxylin and eosin staining. In addition, the effect of NSF on sarcopenia and inflammation/oxidative stress-related factors in hindlimb muscles was investigated by western blotting. Flow cytometry analysis was conducted to investigate the effect of NSF on immune homeostasis. Blood samples were collected by cardiac puncture, and the serum levels of insulin-like growth factor 1, pro-inflammatory cytokines, and glutathione were evaluated.
Results
NSF significantly alleviated muscle strength, mass, and also fiber size in old mice. Age-associated impairment of immune homeostasis was recovered by NSF through retaining CD11b+F4/80+ macrophages and regulating inflammatory biomarkers. NSF also decreased the age-induced expression of oxidative stress factors. Taken together, NSF showed the effect of improving sarcopenia by inhibiting low-grade chronic inflammatory/oxidative stress factors.
Conclusion
NSF exhibited anti-sarcopenia effects by regulating chronic inflammation and oxidative stress in old mice. Thus, we suggest that NSF is a promising restorative agent that can be used to improve sarcopenia in the elderly as well as maintain immune homeostasis.
Keywords: Sarcopenia, Non-saponin fraction, Korean Red Ginseng, Immune homeostasis
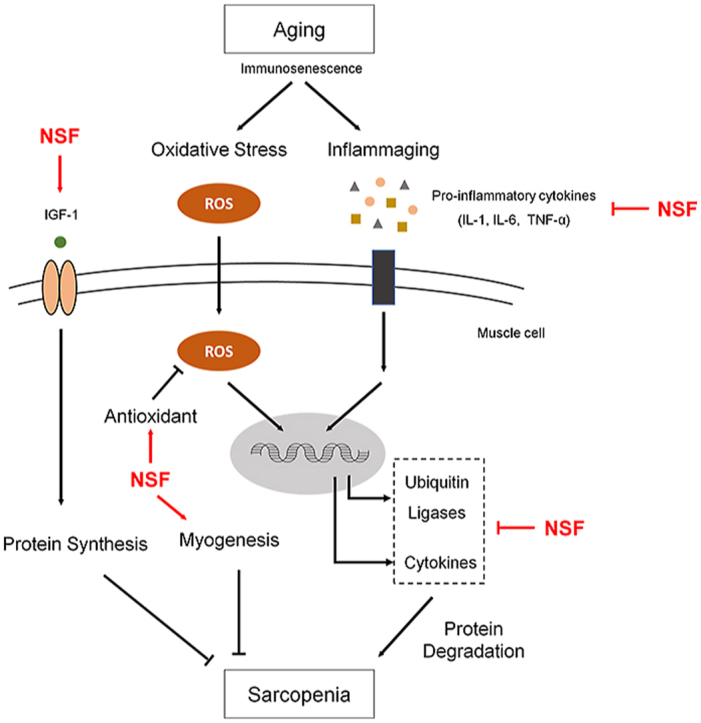
Graphical abstract
1. Introduction
As the world's population ages, it is becoming gradually more essential to extend a healthy lifespan by maintaining a healthy lifestyle and improving a person's quality of life. Aging is characterized by complex physiological processes that increase the risk of developing disease. As the body ages, the functions of all organs, including muscles, gradually deteriorate. The loss of muscle function due to aging, termed sarcopenia, includes a progressive loss of muscle strength and mass [1,2]. Age-related sarcopenia significantly contributes to the deterioration of physical activity, and therefore preventing or slowing sarcopenia is considered an important factor in maintaining a healthy quality of life in the elderly. Although interest in age-related sarcopenia is increasing, no definitive treatments are available. Several conventional strategies to increase muscle strength have been proposed, but these are either unsuitable for the elderly who have poor physical activity at the required level (such as exercise) [3,4] or are focused on the treatment of acute muscle injury [5]. Therefore, considering the seriousness of sarcopenia in an aging population and the consequent increase in social and economic welfare problems, there is a need for effective and fundamental treatment methods for the elderly suffering from sarcopenia.
The development of sarcopenia is initiated by an imbalance in muscle metabolism accompanied by increased muscle protein breakdown and decreased synthesis [6,7]. Various factors such as changes in hormone levels, neuronal changes, and nutrient intake have been investigated as the underlying causes of dysregulation of muscle metabolism [[8], [9], [10], [11]]. However, our work focuses on age-related immune dysfunction, called immunosenescence, as the underlying cause of sarcopenia, and is based on the fact that muscle metabolism is a highly coordinated procedure involving crosstalk between immune cells and muscle cells [[12], [13], [14]].
Immunosenescence is characterized by the breakdown of immune homeostasis due to the gradual deterioration of the immune system during the natural aging procedure [15]. One of the most representative causes of immunosenescence is inflammaging [16,17]. Inflammaging refers to a low-grade chronic inflammatory state in the elderly that not only suppresses the immune response to vaccination [18,19], but can also cause the advancement of diseases such as sarcopenia [20,21]. The existence of a low-grade chronic inflammatory state is supported by the fact that the serum levels of pro-inflammatory cytokines, interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNF-α), are higher in the elderly than in the young [22,23]. The innate immune system, particularly a network centered on cytokine-secreting macrophages, is thought to be central to inflammaging [24]. Increased circulating pro-inflammatory cytokines are a major cause of sarcopenia because they promote the breakdown of myofibrillar proteins and decrease protein synthesis, directly leading to muscle wasting [25].
The efficiency of oxidative metabolism, that is, the capability to counteract the generation of reactive oxygen species physiologically generated by cellular metabolic procedures, also reduces with age and contributes to the development of age-related inflammatory diseases [26]. Oxidative stress states resulting from an imbalance of oxidative and antioxidant levels underlie the pathogenesis of chronic diseases accompanied by sarcopenia [27]. This is because oxidative stress dulls the anabolic signals of muscle metabolism and increases the catabolic signal. In addition, oxidative stress and low-grade chronic inflammation are closely related to aging and are implicated in the development of sarcopenia, where inflammatory and oxidative stress pathways intersect. Therefore, in this study, we focused on modulating the key features of inflammatory and oxidative stress-related changes that are likely to contribute to sarcopenia.
Korean Red Ginseng (RG, Panax ginseng Meyer) is a long-established herb that is widely used in Asian countries for preventing and treating various medical indications. It has great pharmacologic effects in conditions such as metabolic syndrome, cancer, diabetes, and immune diseases [[28], [29], [30], [31]]. The components of RG consist of saponin and non-saponin. There are already many studies that have reported pharmacological activity of Korean Red Ginseng extract (RGE) or saponin, which is an active ingredient of RG. However, the pharmacological effects of the non-saponin fraction (NSF) are less well understood. The component that accounts for most of RG is NSF, and it is a promising material that can be used as a functional health food. Furthermore, due to the protein component of NSF, we thought NSF would have a potential promising effect on muscle strength improvement. Therefore, we focused on better understanding of the bioactive effect of NSF. We evaluated the impacts of NSF on age-associated sarcopenia and immunosenescence in 20–24-month-old C57BL/6 J mice. Our results suggest that the NSF of Korean Red Ginseng could be used as a novel aging-specific food supplement.
2. Materials and methods
2.1. Preparation and analysis of the NSF of Korean Red Ginseng
The NSF of Korean Red Ginseng used in this study was a powder prepared by removing saponin from red ginseng concentrate as previously described [32]. NSF was gained from the Korea Ginseng Corporation Research Institute (Daejeon, Korea) and was prepared from red ginseng extract by adsorption chromatography detailed in the methods below using a Diaion HP20 ion exchange resin (Mitsubishi Chemical Industries, Ltd.). The red ginseng extract was diluted to 10% in purified water and filtered. The solution was applied to HP20 resin for adsorption and then eluted using water and 30% ethanol in water. The two fractions were mixed, concentrated, and spray-dried to prepare NSF. The general composition of NSF is presented in Table S1, and the calorie content of NSF was 364.11 kcal/100 g (dry basis). Analysis of the ginsenoside components (Rg1, Re, Rf, Rh1, Rg2s, Rb1, Rc, Rb2, Rd, Rg3s, and Rg3r) of NSF using the previously described method [33] showed that these ginsenosides were not detected in NSF. High-performance liquid chromatography (HPLC) analysis of NSF and the arginine–fructose–glucose content of NSF was assessed as previously described [32,33] (Table S2). The simple sugar composition of NSF is presented in Table S3.
2.2. Reagents
Antibodies against myoblast determination protein 1 (MyoD), F-box protein (Fbx32, also known as atrogin), muscle ring-finger 1 (MuRF1), and myostatin were bought from Abcam (Cambridge, UK). Antibodies against myogenin, myocyte enhancer factor 2 (MEF-2), superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1), catalase, and α-tubulin were bought from Santa Cruz Biotechnology (Dallas, TX, USA). FITC anti-mouse CD11b and APC anti-mouse F4/80 were bought from BioLegend (San Diego, CA, USA).
2.3. Animals
Female C57BL/6 J mice (aged 3–6 or 20–24 months) were obtained from the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). Mice were housed at 20 ± 3 °C in a room kept under a 12-h light/dark cycle. Mice had a period to adapt to experimental facilities for 1 week prior to oral administration of NSF. Mice aged 3–6 months (young mice; YM) were randomly grouped into two groups (n = 8 per group) as follows: (I) YM control and (II) YM NSF200. Mice aged 20–24 months (old mice; OM) were randomly grouped into three groups (n = 8 per group) as follows: (I) OM control, (II) OM NSF50, and (III) OM NSF200. NSF was liquefied in distilled water for oral administration and NSF-treated young and old mice received NSF (50 or 200 mg/kg/day) every day for 6 weeks. The daily dosage of NSF was derived from the human dosage (0.25 and 1 g/60 kg/day) from a mathematical table detailed in Ref. [34]. Throughout the treatment period, body weight and grip strength were assessed once per week. Mice were sacrificed by CO2 and blood samples were collected through cardiac puncture. After sacrifice, the spleen and hindlimb muscles were cut out, weighed, and stored at −80 °C for further analysis.
3. Ethics statement
All mice were handled according to the guidelines outlined in the “Guide for the Care and Use of Laboratory Animals” written by the US National Academy of Sciences and published by the US National Institutes of Health. All experiments were authorized by the Institutional Animal Care and Use Committee (IACUC 200156) of CHA University (Seongnam, Kyunggi, Korea).
3.1. Grip strength measurement
A Chatillon force measurement system (Columbus Instruments, Columbus, OH, USA) was used to determine all-limb grip strength in parallel. Mice were put on a mesh grid so that they could grasp the mesh with their four feet, and the tail was pulled softly 3–5 times. Peak tension in the limbs at the moment the mice let go of the mesh was documented.
3.2. Histological analysis
Samples of gastrocnemius and quadriceps muscle were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 10 μm sections. Sections were dyed with hematoxylin and eosin, mounted on slides. All muscle sections were photographed under a Nikon E600 microscope (Nikon, Tokyo, Japan).
3.3. Western blotting
Muscle samples were lysed for 25 min in lysis buffer (iNtRON Biotechnology, Seoul, Korea) supplemented with protease and phosphatase inhibitors. The amount of lysate protein was calculated using a BCA protein assay (Pierce, Rockford, IL, USA), and the same amounts of proteins were divided by SDS-PAGE and transferred to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked for 1 h in 5% skimmed milk, washed, and then incubated with certain primary antibodies overnight at 4 °C. The membranes were washed again, and blocked for 1 h in 5% skimmed milk including peroxidase-conjugated anti-rabbit or anti-mouse antibodies. Protein expressions were observed by an EZ-Western Lumi Femto kit (DoGenBio, Seoul, Korea) and imaged by a LAS-4000 apparatus (GE Healthcare Life Sciences, Marlborough, MA, USA).
3.4. Flow cytometry analysis
Spleen samples were homogenized, and a suspension of single cells was obtained by passing the homogenate through a 40 μm strainer. The red blood cells were lysed in ammonium–chloride–potassium lysis buffer (Lonza, Basel, Switzerland). The single cells were washed, stained with the specific antibodies on ice for 20 min, and then washed. Flow cytometry was executed using a CytoFlex flow cytometer (Beckman Coulter, CA, USA) and data were evaluated using FlowJo software (Ashland, OR, USA).
3.5. Serum analysis
Blood was collected by cardiac puncture at the moment of euthanasia. Blood samples were centrifuged at 800×g for 20 min at 4 °C, and serum was kept at −80 °C. The serum levels of IL-1, IL-6, TNF-α, and C-reactive protein (CRP) were assessed using a mouse Th17 magnetic bead panel (Merck Millipore, MA, USA). The serum level of insulin-like growth factor (IGF)-1 was evaluated using an IGF-1 ELISA kit (Thermo Fisher Scientific, MA, USA). The serum level of glutathione (GSH) was evaluated using a glutathione assay kit (Cayman, MI, USA). All analyses were carried out following the manufacturers’ instructions. Absorbances were assessed at appropriate wavelengths using a Luminex 100 analyzer (Luminex, Austin, TX, USA). All samples were evaluated in duplicate.
3.6. Statistical analysis
All parameters are presented as the mean ± standard error of the mean (SEM), and statistically differences were estimated using a one-way analysis of variance (ANOVA) followed by Tukey's post-hoc tests. P < 0.05 was considered significant, and values with different letters are meaningly different (a > ab > b > bc > c > d).
4. Results
4.1. The effect of NSF on muscle strength, size, and mass in old mice
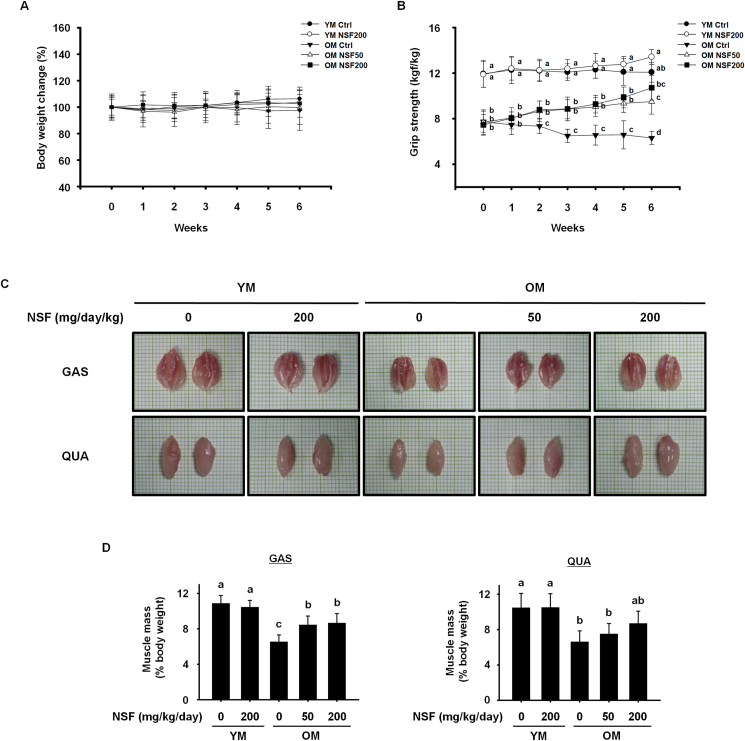
To evaluate the effect of NSF on the gradual loss of muscle strength and mass, and sarcopenia, young (3–6-month-old) and old (20–24-month-old) mice were treated with NSF (50 or 200 mg/kg/day) for 6 weeks. Body weight and grip strength were assessed once per week. The body weight of the control groups and the OM and YM groups receiving NSF did not change during the administration period (Fig. 1A). The grip strength of the OM control group was considerably lower than that of the YM control group, and gradually decreased during the experimental period. Treatment with NSF statistically significantly strengthened the grip strength of OM mice steadily over 6 weeks (Fig. 1B). After sacrifice, gastrocnemius and quadriceps muscles from the hindlimb were cut out, photographed, and weighed. As shown in Fig. 1C, the muscles from the OM control group were tinier than those from the YM groups, and treatment with NSF slightly increased muscle size in the OM NSF50 and OM NSF200 groups (Fig. 1C). To analyze muscle size quantitatively, muscle mass was standardized to the body weight obtained on the last day. The muscle mass of the OM control group was considerably lower than the muscle mass of the YM control and YM NSF200 groups, indicating that aging induced severe loss of skeletal muscle mass, and NSF administration improved this effect (Fig. 1D). Overall, these data indicate that NSF administration for 6 weeks improves muscle strength and muscle mass in old mice, and ameliorates severe sarcopenia.
Fig. 1.
NSF increased muscle strength, size, and mass in old mice. Measurements of (A) body weight and (B) grip strength of mice given NSF for 6 weeks. (C) Representative images of the gastrocnemius (GAS, top) and quadriceps (QUA, bottom) muscles. (D) The muscle mass of GAS (left) and QUA (right). Muscle mass was standardized to the body weight obtained on the last day. Ctrl, control; YM, young mice. The comparisons were performed using one-way ANOVA followed by Tukey's post-hoc test. Distinct letters present statistically considerable differences; p < 0.05, a > ab > b > bc > c > d.
4.2. The effect of NSF on muscle fiber size and myogenic transcription factors in old mice
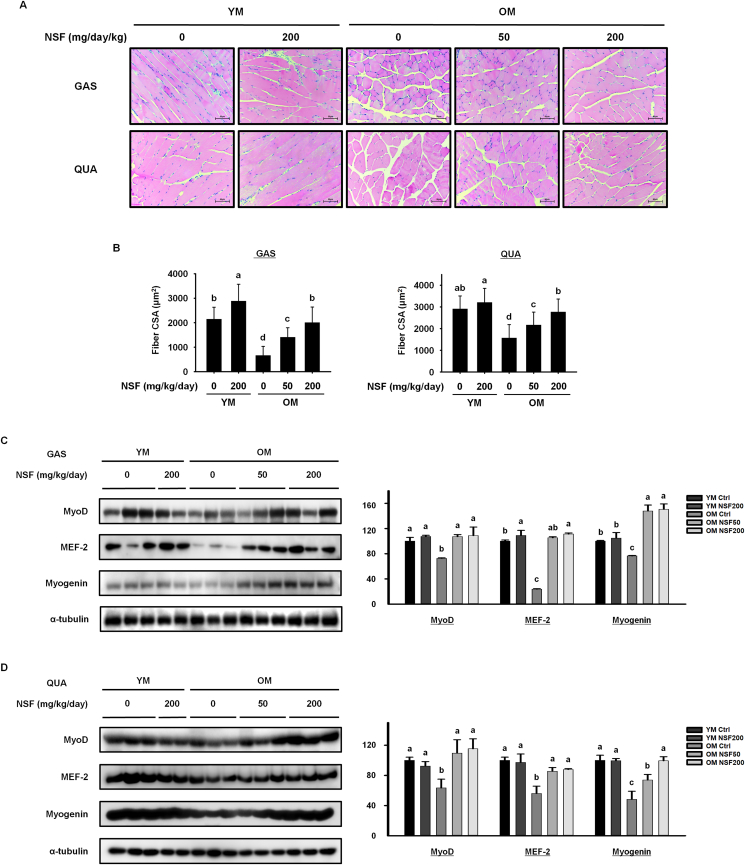
Based on the above results showing that NSF improved muscle strength, we assessed the effect of NSF on muscle fiber size. Muscles with a large cross-sectional area of muscle fibers can produce a larger force [35]. The gastrocnemius and quadriceps muscle histology demonstrated that the muscle fiber size was smaller in the OM control group than in the YM control group, and that NSF statistically significantly increased the muscle fiber size in the OM NSF50 and OM NSF200 groups. NSF also increased muscle fiber size in the YM NSF200 group (Fig. 2A and B). Furthermore, we estimated the effect of NSF on the expression of myogenic transcription factors (Fig. 2C and D). The levels of MyoD, MEF-2, and myogenin in gastrocnemius and quadriceps muscles were lower in the OM control group than in the YM control and YM NSF200 groups. NSF administration increased the expression of MyoD, MEF-2, and myogenin in gastrocnemius and quadriceps muscles in the OM NSF50 and OM NSF200 groups. These data indicate that NSF induces myogenesis by promoting the expression of myogenic transcription factors, which subsequently increases the muscle fiber size in old mice.
Fig. 2.
NSF increased size of muscle fiber and the expression of myogenic transcription factors in old mice. (A) Histological analysis of hematoxylin and eosin staining of the gastrocnemius (GAS, top) and quadriceps (QUA, bottom) muscles. (B) Quantification of the cross-sectional area (CSA) of muscle fibers. The CSA of each muscle fiber was measured using the Image J program. The protein expression levels of myogenic transcription factors were assessed by western blotting in (C) GAS and (D) QUA muscles. Ctrl, control; YM, young mice. The comparisons were performed using one-way ANOVA followed by Tukey's post-hoc test. Distinct letters present statistically considerable differences; p < 0.05, a > ab > b > c.
4.3. The effect of NSF on the expression of sarcopenia-related protein in old mice
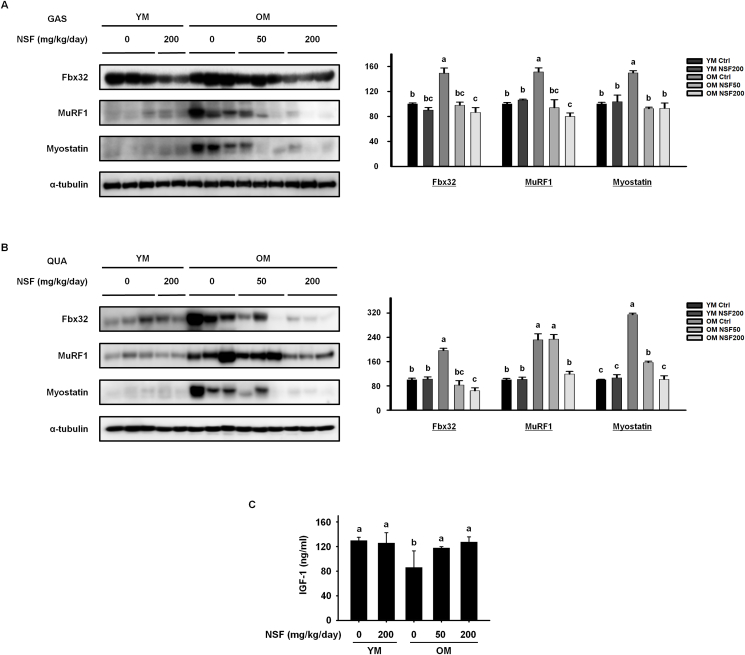
Age-related sarcopenia results from dysregulated muscle metabolism, where there is a higher rate of muscle protein degradation than muscle protein synthesis [36]. The protein expression of regulatory elements involved in muscle protein breakdown in the gastrocnemius and quadriceps muscles was observed (Fig. 3A and B). The OM control group had considerably elevated protein levels of muscle-specific ubiquitin ligases, Fbx32 and MuRF1, and the muscle cell growth inhibitor myostatin, than the YM control group. NSF drastically reduced the expression of all three proteins in the OM NSF50 and OM NSF200 groups. Next, we assessed the serum levels of IGF-1, which activates the signaling system that stimulates muscle protein synthesis (Fig. 3C). The serum level of IGF-1 was lower in the OM control group than in the YM control and YM NSF200 groups, and NSF increased the IGF-1 level in the OM NSF50 and OM NSF200 groups. Taken together, this data demonstrates that NSF suppresses the breakdown of muscle protein and increases muscle protein synthesis in old mice.
Fig. 3.
NSF decreased the expression of sarcopenia-related proteins in old mice. The expression levels of sarcopenic proteins were assessed by western blotting in (A) gastrocnemius (GAS) and (B) quadriceps (QUA) muscles. (C) Serum levels of insulin-like growth factor 1 (IGF-1). Ctrl, control; YM, young mice. The comparisons were performed using one-way ANOVA followed by Tukey's post-hoc test. Distinct letters present statistically considerable differences; p < 0.05, a > b > bc > c.
4.4. The effect of NSF on inflammatory responses that affect sarcopenia in old mice
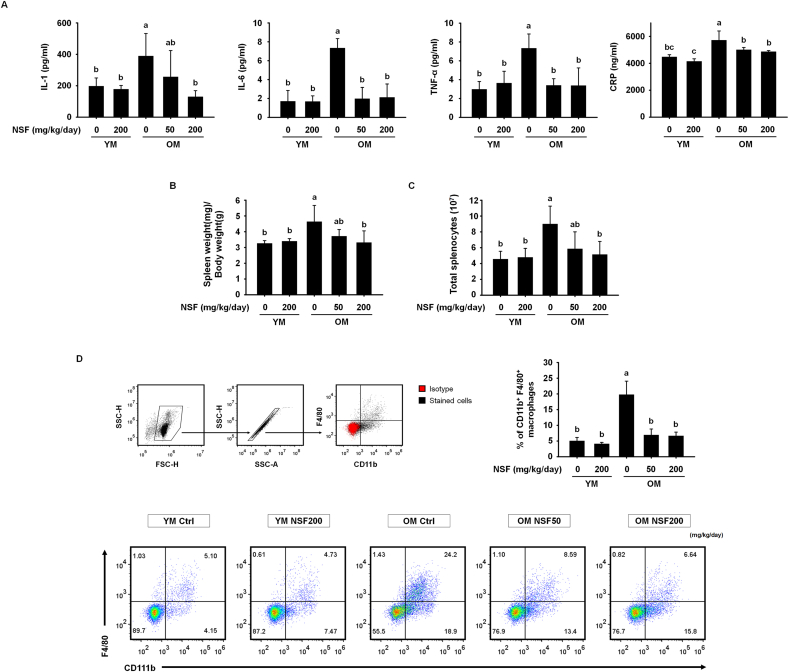
The pathology of sarcopenia is highly correlated with the presence of inflammation, and therefore it is very important to control age-related low-grade chronic inflammatory responses in order to inhibit muscle loss [25]. In particular, the pro-inflammatory cytokines IL-1, IL-6, and TNF-α directly cause muscle loss by facilitating the degradation of myofibrillar proteins [37]. Therefore, we investigated whether NSF affected the release of CRP (which is a marker of chronic inflammation) and pro-inflammatory cytokines (Fig. 4A). The blood serum levels of IL-1, IL-6, and TNF-α were significantly higher in the OM control group than in the YM control and YM NSF200 groups. Also, the serum level of CRP was slightly elevated in the OM control group than in the YM control group, showing that old mice were in a state of low-grade chronic inflammation. NSF administration substantially lowered the serum levels of all three pro-inflammatory cytokines and CRP in the OM NSF50 and OM NSF200 groups. To investigate the protecting effects of NSF on immunosenescence in old mice, the spleen weight and total splenocyte numbers were measured (Fig. 4B and C). The spleen weight and total splenocyte number were greater in the OM control group than in the YM control and YM NSF200 groups. NSF treatment significantly reduced spleen weight and splenocyte number in old mice. Furthermore, we investigated changes in the proportion of macrophages that release pro-inflammatory cytokines following NSF administration using flow cytometry (Fig. 4D). Interestingly, spleens from the OM control group contained a higher percentage of CD11b+F4/80+ macrophages than spleens from the YM control and YM NSF200 groups, and treatment of old mice with NSF recovered CD11b+F4/80+ macrophage levels to those observed in the YM groups. Together, these results suggest that NSF improved sarcopenia by rescuing age-related disturbance of immune homeostasis and inhibiting age-related increases in pro-inflammatory cytokine levels.
Fig. 4.
NSF regulated inflammatory responses that affect sarcopenia in old mice. (A) Serum levels of pro-inflammatory cytokines (interleukin (IL)-1, IL-6, and tumor necrosis factor α (TNF-α) and C-reactive protein (CRP). (B) Weights of the spleen. Spleen weights were standardized to the body weight obtained on the last day. (C) Total number of splenocytes. (D) Gating scheme for macrophages (top left). The red particles present the isotype controls and black particles display the cells dyed with particular antibodies. The bar graph shows the mean percentage of CD11b+F4/80+ macrophages in splenocytes (top right). Representative dot plots of CD11b+F4/80+ macrophages (bottom). YM, young mice. The comparisons were performed using one-way ANOVA followed by Tukey's post-hoc test. Distinct letters present statistically considerable differences; p < 0.05, a > ab > b > c.
4.5. The effect of NSF on age-associated oxidative stress in old mice
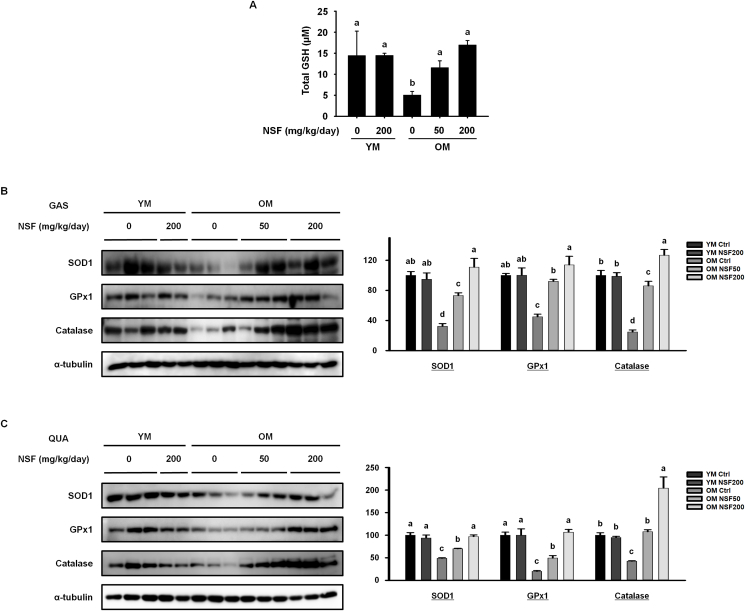
In addition to the role of age-related low-grade chronic inflammation in the loss of muscle function, age-induced oxidative stress works a vital role in the breakdown of muscle proteins and the loss of muscle mass [27]. We evaluated serum levels of GSH, a strong antioxidant that is involved in the response to oxidative stress (Fig. 5A). The serum level of GSH was substantially lower in the OM control group than in the YM control and YM NSF200 groups. NSF increased serum levels of GSH in the OM NSF50 and OM NSF200 groups. Furthermore, we investigated the effect of NSF on antioxidant enzymes that respond to muscle cell damage caused by free radicals and prevent the accumulation of H2O2 in muscle cells (Fig. 5B and C). In both the gastrocnemius and quadriceps muscles, the protein expression of SOD1, GPx1, and catalase was lower in the OM control group than in the YM control and YM NSF200 groups. This result implies that there is increased oxidative stress in the muscles of old mice, causing muscle loss. The expression of antioxidant enzymes significantly increased after NSF treatment. Overall, these data suggest that NSF suppressed age-induced oxidative stress, which is a major cause of muscle loss, by promoting the expression of antioxidant enzymes.
Fig. 5.
NSF enhanced the expression of antioxidants in old mice. (A) The serum level of glutathione (GSH). The protein expression of antioxidant enzymes was evaluated using western blotting in (B) gastrocnemius (GAS) and (C) quadriceps (QUA) muscles. Ctrl, control; YM, young mice. The comparisons were performed using one-way ANOVA followed by Tukey's post-hoc test. Distinct letters present statistically considerable differences; p < 0.05, a > ab > b > c > d.
5. Discussion
Sarcopenia results in negative health outcomes, and therefore it is becoming increasingly important to find ways of inhibiting sarcopenia and improving muscle function as the world's population ages. In this study, we used naturally aged mice suffering from sarcopenia. A mouse aged 20–24 months corresponds to >65 years of age in a human [38], and is therefore a suitable mouse model for sarcopenia studies. Indeed, our data showed that the OM group had symptoms of sarcopenia, including loss of muscle mass and smaller muscle fibers, that were not present in young mice. Daily administration with NSF for 6 weeks recovered all the tested parameters in old mice.
The upkeep of muscle mass relys on the balance between muscle protein anabolic and catabolic pathways [36]. During the aging process, muscle protein degradation is accelerated, and the rate of muscle protein breakdown is higher than the rate of muscle protein synthesis, resulting in sarcopenia [39]. Treatments for sarcopenia have yet to be identified, but recent studies suggest that several pathways related to protein synthesis and metabolism such as the phosphatidylinositol 3-kinases (PI3K)/Akt pathway, which is regulated by IGF-1 [3,40], are potential targets. We found that the serum level of IGF-1 was lower in old mice than in young mice, and that NSF treatment increased the level of IGF-1 in old mice, showing that NSF modulates muscle protein synthesis pathways. However, further studies are needed to determine if NSF also affects signaling downstream of the PI3K/Akt pathway.
This study focused on the effectiveness of NSF for restoring correct skeletal muscle structure and function by inducing myogenesis and inhibiting the breakdown of muscle protein. A better understanding of how NSF contributes to myogenesis could provide insight into effective strategies to improve sarcopenia. The primary muscle synthesis regulator, MyoD, regulates skeletal muscle differentiation, which induces expression of the secondary muscle synthesis regulators, MEF-2 and myogenin [[41], [42], [43], [44]]. This study showed that in old mice, NSF increased the expression of genes encoding the myogenic transcription factors MyoD, MEF-2, and myogenin, suggesting that NSF improves muscle development. Suppressing the age-induced increase in muscle protein degradation is an important strategy to improve sarcopenia. The muscle-specific proteins Fbx32, MuRF1, and myostatin are part of the ubiquitin–proteasome pathway, and induce the progression of muscle loss [45,46]. Aging increased the expression of these muscle wasting-related proteins in skeletal muscles, and NSF treatment reduced this effect. Collectively, our results showed that administration of NSF was involved in balancing muscle protein metabolism by inducing myogenesis and preventing muscle protein breakdown in old mice. This effect of NSF resulted in increased muscle strength, mass, and fiber size.
Low-grade chronic inflammation due to aging is an immune response triggered by disruption of immune homeostasis, and is characterized by high levels of inflammatory cytokines [25,47,48]. Numerous studies have demonstrated that inflammation activates pathways associated with skeletal muscle wasting, resulting in an imbalance between protein synthesis and degradation, and in particular, high levels of pro-inflammatory cytokines are directly related to muscle wasting [37,49]. Therefore, maintaining immune homeostasis by suppressing inflammation could be a strategy to inhibit aging-related sarcopenia. Indeed, our data showed that serum levels of pro-inflammatory cytokines were much higher in old mice than in young mice, and that NSF treatment lowered the levels of pro-inflammatory cytokines in old mice. Moreover, we showed that the proportion of inflammatory cytokine-secreting macrophages was greater in old mice than in young mice, and that NSF recovered the percentage of CD11b+F4/80+ macrophages to that seen in young mice. These results indicate that treatment of old mice with NSF effectively eliminates the cause of sarcopenia by restoring immune homeostasis.
To further extend our hypothesis that sarcopenia results from dysregulation of immune homeostasis, we showed that NSF reduces age-related oxidative stress, which is inextricably linked with immunosenescence. Increasing evidence shows that oxidative stress is critically responsible for the development of sarcopenia [27,50,51]. GSH is a powerful antioxidant that protects against oxidative damage [52], and our data showed that the serum level of GSH was lower in old mice than in young mice. As a further consequence of oxidative stress, the expression of antioxidant enzymes, SOD1, GPx1, and catalase, which prevent the accumulation of intracellular H2O2 that destroys muscle cells [52], were lower in old mice. Considering our data along with previous studies, aging reduces the ability of mice to defend against oxidative stress, which activates a pathway leading to the development of sarcopenia. The administration of NSF increased the serum level of GSH and the expression of antioxidant enzymes in old mice, indicating that NSF enabled old mice to be protected against oxidative stress, thereby preventing muscle cell damage and inhibiting sarcopenia.
In summary, this study showed that NSF enhanced muscle strength, mass, and fiber size by inducing myogenesis and inhibiting the breakdown of muscle protein in old mice. We concentrated on the effect of NSF on immunosenescence, which is the main reason of sarcopenia. NSF not only effectively regulated low-grade chronic inflammation, but also suppressed age-induced oxidative stress, thereby restoring immune homeostasis in old mice. We propose that NSF suppressed the development of severe sarcopenia by delaying some procedures that cause chronic inflammation and oxidative stress, and by regulating immunosenescence. Thus, NSF can be used in the development of a functional health food customized for the elderly to prevent sarcopenia by maintaining immune homeostasis.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This work was partially supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A2C2006180), and by a grant from the Korean Society of Ginseng, funded by the Korean Ginseng Corporation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2022.05.007.
Contributor Information
Hyun-Ji Oh, Email: guswl264@naver.com.
Heegu Jin, Email: heegu94@hanmail.net.
Boo-Yong Lee, Email: bylee@cha.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Santilli V., Bernetti A., Mangone M., Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177–180. [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson L., Degens H., Li M., Salviati L., Lee Y.I., Thompson W., et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99(1):427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziaaldini M.M., Marzetti E., Picca A., Murlasits Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med. 2017;4:167. doi: 10.3389/fmed.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen L., Meng X., Zhang Z., Wang T. Physical exercise for muscle atrophy. Adv Exp Med Biol. 2018;1088:529–545. doi: 10.1007/978-981-13-1435-3_24. [DOI] [PubMed] [Google Scholar]
- 5.Baoge L., Van Den Steen E., Rimbaut S., Philips N., Witvrouw E., Almqvist K.F., et al. Treatment of skeletal muscle injury: a review. ISRN Orthop. 2012;2012:689012. doi: 10.5402/2012/689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirks-Naylor A.J., Lennon-Edwards S. Cellular and molecular mechanisms of apoptosis in age-related muscle atrophy. Curr Aging Sci. 2011;4(3):269–278. [PubMed] [Google Scholar]
- 7.Fry C.S., Rasmussen B.B. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 2011;4(3):260–268. doi: 10.2174/1874609811104030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96(3):183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 9.Robinson S., Cooper C., Aihie Sayer A. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abiri B., Vafa M. Nutrition and sarcopenia: a review of the evidence of nutritional influences. Crit Rev Food Sci Nutr. 2019;59(9):1456–1466. doi: 10.1080/10408398.2017.1412940. [DOI] [PubMed] [Google Scholar]
- 11.Kwon Y.N., Yoon S.S. Sarcopenia: neurological point of view. J Bone Metab. 2017;24(2):83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelke C., Dziewas R., Minnerup J., Meuth S.G., Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingues-Faria C., Vasson M.P., Goncalves-Mendes N., Boirie Y., Walrand S. Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev. 2016;26:22–36. doi: 10.1016/j.arr.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Ligibel J.A., Schmitz K.H., Berger N.A. Sarcopenia in aging, obesity, and cancer. Transl Cancer Res. 2020;9(9):5760–5771. doi: 10.21037/tcr-2019-eaoc-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chei S., Oh H.J., Lee K., Jin H., Lee J.Y., Lee B.Y. Dysfunction of B Cell leading to failure of immunoglobulin response is ameliorated by dietary silk peptide in 14-month-old C57bl/6 mice. Front Nutr. 2020;7:583186. doi: 10.3389/fnut.2020.583186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santoro A., Bientinesi E., Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. 2021;71:101422. doi: 10.1016/j.arr.2021.101422. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi C., Santoro A., Capri M. The complex relationship between immunosenescence and inflammaging: special issue on the new biomedical perspectives. Semin Immunopathol. 2020;42(5):517–520. doi: 10.1007/s00281-020-00823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira B., Xu X.N., Akbar A.N. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front Immunol. 2020;11:583019. doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livshits G., Kalinkovich A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev. 2019;56:100980. doi: 10.1016/j.arr.2019.100980. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa S., Yakabe M., Akishita M. Age-related sarcopenia and its pathophysiological bases. Inflamm Regen. 2016;36:17. doi: 10.1186/s41232-016-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanada F., Taniyama Y., Muratsu J., Otsu R., Shimizu H., Rakugi H., et al. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruunsgaard H., Pedersen M., Pedersen B.K. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 24.van Beek A.A., Van den Bossche J., Mastroberardino P.G., de Winther M.P.J., Leenen P.J.M. Metabolic alterations in aging macrophages: ingredients for inflammaging? Trends Immunol. 2019;40(2):113–127. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Dalle S., Rossmeislova L., Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. doi: 10.3389/fphys.2017.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen K.S., Smith C. Ageing-associated oxidative stress and inflammation are alleviated by Products from grapes. Oxid Med Cell Longev. 2016;2016:6236309. doi: 10.1155/2016/6236309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010;11(4):1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chei S., Oh H.J., Jang H., Lee K., Jin H., Choi Y., et al. Korean red ginseng suppresses the expression of oxidative stress response and NLRP3 inflammasome genes in aged C57bl/6 mouse ovaries. Foods. 2020;9(4) doi: 10.3390/foods9040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chei S., Song J.H., Oh H.J., Lee K., Jin H., Choi S.H., et al. Gintonin-Enriched fraction suppresses heat stress-induced inflammation through LPA receptor. Molecules. 2020;25(5) doi: 10.3390/molecules25051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C., et al. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43(2):209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K., Jin H., Chei S., Oh H.J., Choi S.H., Nah S.Y., et al. The gintonin-enriched fraction of ginseng regulates lipid metabolism and browning via the cAMP-protein kinase a signaling pathway in mice white adipocytes. Biomolecules. 2020;10(7) doi: 10.3390/biom10071048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park S.J., Lee D., Kim D., Lee M., In G., Han S.T., et al. The non-saponin fraction of Korean Red Ginseng (KGC05P0) decreases glucose uptake and transport in vitro and modulates glucose production via down-regulation of the PI3K/AKT pathway in vivo. J Ginseng Res. 2020;44(2):362–372. doi: 10.1016/j.jgr.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.In G., Ahn N.G., Bae B.S., Lee M.W., Park H.W., Jang K.H., et al. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J Ginseng Res. 2017;41(3):361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakkinen K., Hakkinen A. Muscle cross-sectional area, force production and relaxation characteristics in women at different ages. Eur J Appl Physiol Occup Physiol. 1991;62(6):410–414. doi: 10.1007/BF00626612. [DOI] [PubMed] [Google Scholar]
- 36.Anthony T.G. Mechanisms of protein balance in skeletal muscle. Domest Anim Endocrinol. 2016;56(Suppl):S23–S32. doi: 10.1016/j.domaniend.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma B., Dabur R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: a systematic review. Curr Med Chem. 2020;27(13):2161–2188. doi: 10.2174/0929867326666181129095309. [DOI] [PubMed] [Google Scholar]
- 38.Dutta S., Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 39.Short K.R., Nair K.S. Muscle protein metabolism and the sarcopenia of aging. Int J Sport Nutr Exerc Metabol. 2001;11(Suppl):S119–S127. doi: 10.1123/ijsnem.11.s1.s119. [DOI] [PubMed] [Google Scholar]
- 40.McKinnell I.W., Rudnicki M.A. Molecular mechanisms of muscle atrophy. Cell. 2004;119(7):907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee K., Jin H., Chei S., Oh H.J., Lee J.Y., Lee B.Y. Effect of dietary silk peptide on obesity, hyperglycemia, and skeletal muscle regeneration in high-fat diet-fed mice. Cells. 2020;9(2) doi: 10.3390/cells9020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J., McKinsey T.A., Zhang C.L., Olson E.N. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000;6(2):233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- 43.Charge S.B., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 44.Faralli H., Dilworth F.J. Turning on myogenin in muscle: a paradigm for understanding mechanisms of tissue-specific gene expression. Comp Funct Genom. 2012;2012:836374. doi: 10.1155/2012/836374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumucio J.P., Mendias C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43(1):12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quan-Jun Y., Yan H., Yong-Long H., Li-Li W., Jie L., Jin-Lu H., et al. Selumetinib attenuates skeletal muscle wasting in murine cachexia model through ERK inhibition and AKT activation. Mol Cancer Therapeut. 2017;16(2):334–343. doi: 10.1158/1535-7163.MCT-16-0324. [DOI] [PubMed] [Google Scholar]
- 47.Chhetri J.K., de Souto Barreto P., Fougere B., Rolland Y., Vellas B., Cesari M. Chronic inflammation and sarcopenia: a regenerative cell therapy perspective. Exp Gerontol. 2018;103:115–123. doi: 10.1016/j.exger.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Beyer I., Mets T., Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15(1):12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 49.Spate U., Schulze P.C. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7(3):265–269. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Brioche T., Lemoine-Morel S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: molecular aspects. Curr Pharmaceut Des. 2016;22(18):2664–2678. doi: 10.2174/1381612822666160219120531. [DOI] [PubMed] [Google Scholar]
- 51.Rosa C.G.S., Colares J.R., da Fonseca S.R.B., Martins G.D.S., Miguel F.M., Dias A.S., et al. Sarcopenia, oxidative stress and inflammatory process in muscle of cirrhotic rats - action of melatonin and physical exercise. Exp Mol Pathol. 2021;121:104662. doi: 10.1016/j.yexmp.2021.104662. [DOI] [PubMed] [Google Scholar]
- 52.Cerullo F., Gambassi G., Cesari M. Rationale for antioxidant supplementation in sarcopenia. J Aging Res. 2012;2012:316943. doi: 10.1155/2012/316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.