Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most successful human pathogens with the potential to cause significant morbidity and mortality. MRSA has acquired resistance to almost all β-lactam antibiotics, including the new-generation cephalosporins, and is often also resistant to multiple other antibiotic classes. The expression of penicillin-binding protein 2a (PBP2a) is the primary basis for β-lactams resistance by MRSA, but it is coupled with other resistance mechanisms, conferring resistance to non-β-lactam antibiotics. The multiplicity of resistance mechanisms includes target modification, enzymatic drug inactivation, and decreased antibiotic uptake or efflux. This review highlights the molecular basis of resistance to non-β-lactam antibiotics recommended to treat MRSA infections such as macrolides, lincosamides, aminoglycosides, glycopeptides, oxazolidinones, lipopeptides, and others. A thorough understanding of the molecular and biochemical basis of antibiotic resistance in clinical isolates could help in developing promising therapies and molecular detection methods of antibiotic resistance.
Keywords: Staphylococcus aureus, MRSA, antimicrobial resistance, molecular basis, macrolides, glycopeptides, lipopeptides
1. Antibiotic Resistance and Human Health Risk
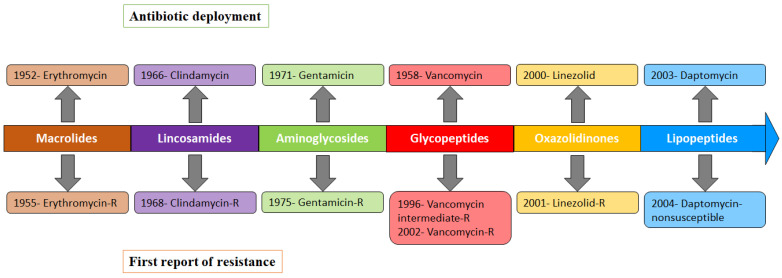
The discovery of penicillin by Alexander Fleming in 1928 was one of the largest triumphs of biomedical research [1], and its introduction for clinical use in 1943 began a new era in the treatment of bacterial infection. Alexander Fleming won a Nobel Prize for the discovery of penicillin in 1945, and during his lecture, he warned that overuse of antibiotics could result in selection for resistant bacteria. True to this prediction, the extensive use of antibiotics has led to the selection and expansion of penicillin-resistant bacteria. In 1940, even before the introduction of penicillin for clinical practice, Abraham and Chain identified an enzyme (penicillinase) from Escherichia coli able to destroy penicillin [2]. Following the development of penicillin, multiple classes of antibiotics were developed and launched to treat bacterial infections: macrolides, e.g., erythromycin; lincosamides, e.g., clindamycin; aminoglycosides, e.g., gentamicin; glycopeptides, e.g., vancomycin; oxazolidinones, e.g., linezolid; lipopeptides, e.g., daptomycin; tetracyclines, e.g., tetracycline; fluoroquinolones, e.g., ciprofloxacin; pyrimidines/sulfonamides, e.g., trimethoprim–sulfamethoxazole, and others (Figure 1). Unfortunately, many bacterial pathogens associated with epidemics of human diseases have evolved resistance to almost every sequential antibiotic introduced to target it. Here, the emergence of non-β-lactam antibiotics resistance is exemplified in the bacterial pathogen Staphylococcus aureus, which causes a wide range of infectious diseases.
Figure 1.
Timeline of the key non-β-lactam antibiotics deployment and the first reported cases of S. aureus resistance identified.
Antimicrobial resistance (AMR) has been observed in most bacteria but is particularly problematic in hospital-acquired infections from multidrug-resistant ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens [3,4]. The ESKAPE pathogens are capable of escaping the bactericidal action of antibiotics and represent the paradigms for resistance, pathogenesis, and disease transmission in both hospital and community settings [3,5]. The emergence of antibiotic-resistant bacteria causing infectious diseases is a serious public health concern [6]. The World Health Organization (WHO) has described antibiotic resistance as one of the serious threats to global public health, food security, and development today [7]. A 2019 joint report by the United Nations (UN), World Health Organization (WHO), and World Organization for Animal Health (WOAH) states that if no action is taken, drug-resistant diseases could cause 10 million deaths worldwide each year by 2050 with more than $100 trillion economic output loss [8]. The U.S. Centers for Disease Control and Prevention (CDC) estimates that more than 2.8 million infections and 35,000 deaths occur due to antimicrobial-resistant pathogens every year in the United States, with this number expected to rise as more antimicrobial-resistant strains evolve [9]. The rise in antibiotic resistance is of concern in S. aureus, which has acquired resistance to almost every sequential antibiotic introduced to target it. For instance, an estimated 323,700 cases of methicillin-resistant S. aureus (MRSA) infections in hospitalized patients with 10,600 death were reported in the United States in 2017 [10]. The spread of MRSA has emerged as a global health concern because infections with MRSA are associated with significant morbidity and mortality.
2. Emergence of Methicillin-Resistant Staphylococcus aureus (MRSA)
The infectious diseases caused by S. aureus were well-treated by penicillin in the 1940s, but with the widespread use of this agent, penicillin-resistant S. aureus began to appear in the clinic. The first penicillin-resistant S. aureus infection was reported in 1942 [11], and a penicillinase from S. aureus that destroys penicillin was identified in 1944 by Kirby [12]. To combat penicillin-resistant S. aureus infection, methicillin (celbenin), semisynthetic β-lactamase-resistant penicillin was introduced to clinical practice in the United Kingdom in 1959 [13]. In 1961, soon after the introduction of methicillin, MRSA strains were identified among clinical isolates from patients hospitalized in the United Kingdom by Patricia Jevons [14]. Between the first reports of MRSA observed in 1961 and the 1990s, infection was common in healthcare settings (HA-MRSA) [15]. However, by the 1990s, MRSA infections has rapidly spread in the community (CA-MRSA) [16]. MRSA is one of the major causes of hospital-acquired infection globally and also occurs outside and independent of hospitals by CA-MRSA [17] and, since the mid-2000s, by livestock-associated MRSA (LA-MRSA) [18]. Several S. aureus clones (strains indistinguishable from each other by a variety of genetic tests) have developed into MRSA, which confer resistance to most β-lactam antibiotics. Furthermore, the prevalence of MRSA strains resistant to multiple non-β-lactam antibiotics has steadily increased and now become a major human health threat in infectious diseases [19].
Methicillin resistance is mediated by mecA gene [20], acquired by horizontal transfer of a mobile genetic element staphylococcal cassette chromosome mec (SCCmec) [21]. The mecA gene encodes an alternative penicillin-binding protein 2a that has a low affinity for β-lactam antibiotics [22,23], resulting in resistance to this entire class. PBP2a enables S. aureus to maintain cell wall synthesis when other PBPs are inhibited by β-lactams [24]. MRSA strains, besides being resistant to nearly all β-lactams, are often resistant to antibiotics of other classes such as macrolides, lincosamides, aminoglycosides, glycopeptides, oxazolidinones, and lipopeptides [25,26]. For example, complicated urinary tract infections (cUTIs) caused by MRSA are commonly treated with vancomycin [27], but strains with decreased susceptibility, designated as vancomycin-intermediate S. aureus (VISA)m emerged in 1996 [28]. Furthermore, clindamycin (discovered in 1966) has been generally used to treat skin and soft-tissue infection (SSTI) caused by CA-MRSA [29], and strains with clindamycin resistance were reported in 1968 [30].
3. Molecular Basis of Non-β-Lactams Resistance
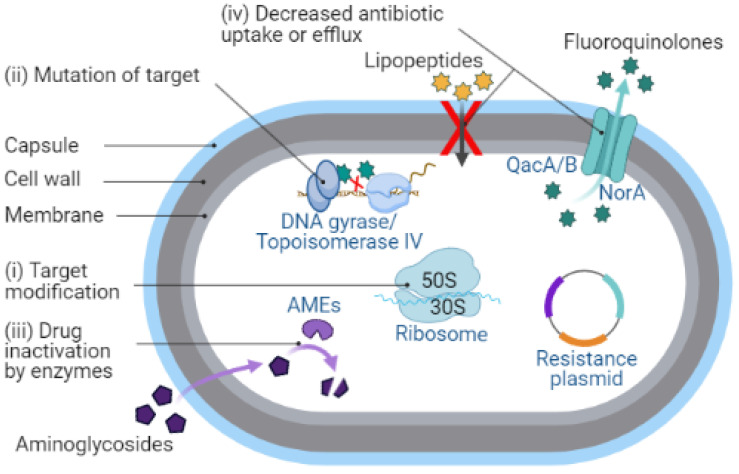
S. aureus has developed numerous mechanisms to neutralize the effect of antibiotics. Antibiotic resistance is commonly associated with the acquisition of resistance genes or mutations affecting central biochemical processes. MRSA confers resistance to non-β-lactam antibiotics by various mechanisms, such as (i) target modification, (ii) mutation of target, (iii) drug inactivation by enzymes, and (iv) decreased antibiotic uptake or efflux (Figure 2).
Figure 2.
Schematic representation of the mechanisms of antibiotic resistance in S. aureus. (i) Target modification: change in the structure or composition of the target site in a bacterial cell can stop the antibiotic to bind, thus shielding it from the antibiotic. Modification of the bacterial ribosome by 23S ribosomal RNA (rRNA) methyltransferase (encoded by erm genes) leads to a conformational change in the ribosome target [31,32], thereby preventing the binding of erythromycin to the ribosomal target. (ii) Mutation of target: mutations in the target can prevent the antibiotic from binding, or if it binds, preventing it from inhibiting the target. Mutation in the DNA topoisomerase IV subunit A (encoded by grlA gene) and an essential enzyme DNA gyrase subunit A (encoded by gyrA gene) is the main contributor to fluoroquinolone resistance in S. aureus [33,34,35]. (iii) Drug inactivation by enzymes: several S. aureus enzymes modify the structure of antibiotics or break them down to make them inactive. The bifunctional aminoglycoside-modifying enzyme (AME) AAC(6′)/APH(2″) (encoded by aac(6′)/aph(2″) genes) confers resistance to aminoglycosides via acetyltransferase and phosphotransferase activities [36,37]. (iv) Decreased antibiotic uptake or efflux: decrease in the permeability of cell membrane to drugs makes it more difficult to pass through or activation of an efflux pump that removes antibiotics from the bacterial cell. The norA, qacA/B, and smr (qacC/D) genes encoding multidrug efflux pump proteins are found mainly in S. aureus clinical isolates and mediate resistance to fluoroquinolones, tetracyclines, and reduced susceptibility to certain antiseptics [38,39].
3.1. Target Modification
Antibiotics work by binding to a cellular target so that an essential biochemical process is blocked. An alteration of the target structure prevents antibiotic binding, or it binds weakly, and thus acts as a self-resistance mechanism. The ribosome is a complex molecular machine associated with protein synthesis, and alteration of the drug-binding site through modification of rRNA results in resistance to ribosome-targeting antibiotics. For example, modification of the bacterial ribosome by 23S rRNA methyltransferase [40] prevents the binding of macrolides to ribosomal targets [31,32]. Methylation of 23S rRNA by chloramphenicol-florfenicol resistance (cfr) gene encoded rRNA methyltransferase alters the drug-binging site 50S ribosomal subunit [41], thus reducing the ability of chloramphenicol and clindamycin antibiotics to inhibit the ribosomes translational activity. RNA methyltransferase, the gene product of cfr from S. sciuri [42] targets nucleotide A2503 of 23S rRNA and inhibits ribose methylation at nucleotide C2498, thereby causing resistance to chloramphenicol, florfenicol, and clindamycin in S. aureus laboratory strain [42].
3.2. Mutation of Target
Chromosomal mutations that drive antibiotic resistance often arise within the genes that encode targets. Mutation of the target plays a major role in the development of resistance across distinct antibiotics such as mutations in the DNA topoisomerase IV and DNA gyrase with fluoroquinolones [33,34,35], alterations to RNA polymerase with high-level resistance to rifampicin [43], and ribosomal mutations (tetM and tetO) with tetracyclines [44,45]. Mutations in the chromosomal genes grlA (referred to as parC in other bacteria) (encoding DNA topoisomerase IV subunit A) [33,34,35,46] and gyrA (encoding an essential enzyme DNA gyrase subunit A) [33,34,35] are the primary mechanisms of fluoroquinolones resistance in S. aureus. The two enzymes are associated with the overlapping and opening of the double-stranded DNA during replication. Mutations of the grlA gene affect the amino acid codons Ser80, Glu84, and Ala116 of GrlA enzyme subunit [35,46], whereas gyrA gene mutations affect the amino acid codons Ser84, Ser85, and Glu88 of GyrA enzyme subunit [34,47] in the quinolone-resistance-determining region (QRDR). The changes in amino acids, particularly those in certain regions of each GrlA and GyrA enzyme subunit called the QRDR, decrease the binding affinity of enzymes and make them less sensitive to inhibition by fluoroquinolones. Nearly all quinolone-resistant S. aureus strains substitute Ser84 of GyrA with Leu or, in some other strains, Ser80 of GrlA with Phe [48,49].
3.3. Drug Inactivation by Enzymes
The enzymatic modification that renders antibiotics of decreased affinity for their main target 16S rRNA is the most prevalent mechanism of aminoglycosides resistance in S. aureus [50]. AMEs catalyze the modification at –OH or –NH2 groups of the 2-deoxystreptamine nucleus or the sugar moieties of aminoglycosides [51]. Resistance to the aminoglycoside antibiotics gentamicin, tobramycin, and kanamycin is generally mediated by a bifunctional AME AAC(6′)/APH(2″) encoded by aac(6′)/aph(2″) gene that specifies 6′-acetyltransferase [AAC(6′)] and 2″-phosphotransferase [APH(2″)] aminoglycoside-modifying activities [36,37]. Esterases encoded by ere genes [31,32] and phosphotransferases encoded by mph genes [52] confer resistance to erythromycin and other 14-, 15-, and 16-membered macrolides in S. aureus. Thiol-S-transferase (encoded by fosB gene) catalyzes the inactivation of fosfomycin [53,54], which is used to treat UTIs.
3.4. Decreased Antibiotic Uptake or Efflux
Resistance can develop either by decreasing the permeability of the cell membrane or by increasing the efflux of antibiotics from the cell through changes in membrane permeability [55]. NorA, QacA/B, and Smr (Staphylococcal multidrug resistance, also known as QacC/D) are multidrug efflux membrane proteins found mainly in S. aureus clinical isolates [56,57]. NorA (encoded by norA gene) is a chromosomally encoded multidrug efflux pump protein of the core genome of S. aureus [58] associated with resistance to fluoroquinolones, tetracyclines, and several antiseptics (chlorhexidine digluconate, cetrimide, benzalkonium chloride) [59]. The qacA and qacB genes encode an efflux pump protein that has been associated with increased resistance to fluoroquinolones and chlorhexidine tolerance [60,61,62]. Furthermore, mutations in genes encoding efflux pumps make the antibiotic export more efficient. For example, mutations in the bacterial DNA can lead the bacteria to produce more of a certain efflux pump. In staphylococci, the tetracyclines resistance is mediated by Tet efflux pumps TetA(K) and TetA(L), which are members of the major facilitator superfamily (MFS) transporters with 14 transmembrane domains. TetK is encoded by the small multicopy plasmid pT181 and is integrated within the chromosomal SCCmecIII cassette of MRSA strains [63].
4. MRSA Resistance to Non-β-Lactams
Since 1961, the incidence of MRSA resistance to β-lactam antibiotics including new-generation cephalosporins is increasing worldwide [64]. MRSA strains become additionally resistant to antibiotics of multiple non-β-lactam classes such as macrolides, aminoglycosides, glycopeptides, oxazolidinone, lipopeptide, pyrimidine/sulfonamide, and others. This is because MRSA strains often harbor genes that convey resistance to antibiotics of multiple non-β-lactam classes. Antibiotic resistance is mediated through several distinct mechanisms, most of which are quite well-understood [65]. Depending on the antimicrobial class, S. aureus can utilize different mechanisms to resist the antibiotic effect. In this review, we included the approved non-β-lactam antibiotics currently used for the management of patients with MRSA infections, which are suggested as per the evidence-based guidelines prepared by an Expert Panel of the Infectious Diseases Society of America (IDSA) [29] and United Kingdom (UK) guidelines produced following a review of the published literature (2007–2018) [27]. A summary of the currently used non-β-lactam antibiotic mechanisms of action and molecular bases of resistance in S. aureus is presented in Table 1. A better understanding of the molecular basis of antibiotic resistance could help in the development of novel drugs that suppress MRSA in multiple ways and molecular detection methods of antibiotic resistance.
Table 1.
Mechanisms of action of non-β-lactam antibiotics active against S. aureus and molecular basis of antibiotic resistance.
| Antibiotic Class/ Primary Agent |
Approve Year and Use | Primary Target and Mechanisms of Action |
Resistance Genes | Mechanism(s) of Resistance | Comments |
|---|---|---|---|---|---|
| Macrolides | Protein synthesis | ||||
| Erythromycin | 1952 [66]. SSTI (Resistance 1955) [67] |
Erythromycin binds to bacterial 23S rRNA in the 50S ribosomal subunit and stops protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and assembly of the 50S ribosomal subunit [68,69]. The target site for macrolides is nucleotides A2058 and A2059 located in the V region of 23S rRNA and, rarely, nucleotide A752 located in domain II [70]. |
ermA [31], ermB, ermC [32], ermY [52], msr(F) [71], msrA [72], msrB, ereA, ereB, mphB, mphC [52] |
(i) Modification of the bacterial ribosome by 23S rRNA methyltransferase (encoded by erm genes) prevents the binding of erythromycin to ribosomal target [31,32]. (ii) Active efflux of macrolides from cells by ATP-binding-cassette family (ABC-F) transporters (encoded by msrA and msrB genes) protects ribosomes from inhibition [72,73]. (iii) Enzymatic hydrolysis of 14- and 15-membered lactone ring of macrolides by esterase (encoded by ere genes) prevents its binding to the antibiotic target site [74]. (iv) Phosphotransferases (encoded by mph genes) introduce phosphate to the 2′-hydroxyl group of the 14-, 15-, and 16-membered lactone rings of macrolides amino sugar, which interferes with the interaction of the antibiotic with nucleotide A2058 [52]. |
Modification of the bacterial ribosome and active efflux from the bacterial cell are important mechanisms of macrolide resistance in S. aureus. |
| Lincosamides | Protein synthesis | ||||
| Clindamycin | Discovered in 1966. SSTI caused by CA-MRSA [29] (Resistance 1968) [30] |
Clindamycin binds to bacterial 23S rRNA in the 50S ribosomal subunit and impedes both the assembly of ribosomes and the translation process [75]. |
ermA, ermB, ermC [76] cfr [41,42] |
(i) The rRNA methylase (encoded by erm genes) methylates an adenosine nucleotide within the peptidyl transferase center, resulting in the C-8 methylation of A2503 (m8A2503) [77]. (ii) The acquired cfr gene encoded rRNA methyltransferase methylates an adenine residue of the 23S rRNA in the 50S ribosomal subunit [41], resulting in altered antibiotic binding sites within the ribosome. |
|
| Aminoglycosides | Protein synthesis | ||||
| Gentamicin | U.S. FDA 1971. Bacterial meningitis, sepsis of newborns, septicemia, UTI (Resistance 1975) [78,79] |
Gentamicin binds to the A-site on the 16S rRNA helix at the mRNA-tRNA decoding center of bacterial 30S ribosome subunit [80,81], causing the inhibition and inaccurate induction of translation, disrupting protein synthesis [82,83,84]. |
aac(6′)/aph(2″) aadD (AG O-adenyltransferase) [85] ant(4′) (AG O-nucleotidyltransferase(4′)) ant(9) (AG O-nucleotidyltransferase(9)) |
The bifunctional AMEs inactivate aminoglycosides by acetylating, phosphorylating, or adenylating amino or hydroxyl groups [51,85] Gentamicin, tobramycin and kanamycin resistance is generally mediated by a bifunctional AME AAC(6′)-APH(2″) (encoded by aac(6′)/aph(2″) gene) that specifies 6′-acetyltransferase [AAC(6′)] and/or 2″-phosphotransferase [APH(2″)] aminoglycoside modifying activities [36,37]. |
The aac(6′)/aph(2″) gene is most prevalent in aminoglycoside resistant S. aureus [86]. |
| Arbekacin (not used clinically in the U.S.) |
Japanese PMDA 1990 [87]. Pneumonia and sepsis due to MRSA. (Resistance 1979) [88] |
Arbekacin binds to both 50S and the 30S ribosomal subunits, resulting in codon misreading and inhibition of translation [89]. | aac(6′)-aph(2″) [88,90] | (i) A single base alteration (G1126A) of aac(6′)/aph(2″) gene resulted in one amino acid substitution S376N in AAC(6′)/APH(2″), which leads to arbekacin resistance in MRSA strain PRC104 [90]. (ii) β-lactam-inducible arbekacin resistance was reported in MRSA strain by the integration of Tn4001-IS257 hybrid structure containing aac(6′)/aph(2″) gene cointegrated into a region downstream of blaZ gene [91].(iii) The AAC(6′)/APH(2″) modify arbekacin by 6′-N-acetylation and/or 2″-O-phosphorylation of AGs that contain 6′-NH2 and/or 2″-OH [37,92]. |
Arbekacin is not inactivated by AMEs (3′)(APH), (4′)(AAD), or AAD(2″) and has a weak affinity to (6′-IV) (AAC) [93]. |
| Glycopeptides | Cell wall synthesis | ||||
| Vancomycin | 1958. Bacteremia, infective endocarditis, osteomyelitis, meningitis, pneumonia, sepsis, and complicated SSTI due to HA-MRSA and CA-MRSA [29]. (Resistance VISA in 1996 [28] and VRSA in 2002 [94]) |
Vancomycin bind to D-Ala-D-Ala termini moieties of Lipid II precursor of peptidoglycan through a series of hydrogen bonds, leading to conformational alteration that prevents incorporation of NAM- and NAG-peptide subunits to the growing peptidoglycan chain and consequent transpeptidation [95,96,97]. This alters membrane integrity and increases permeability, leading to bacterial death. |
vanA [97,98] Mutations in walKR, vraSR, graSR, and clpP Mutation in rpoB [99,100] SNPs in capB (E58K) and lytN (I16V) gene [101] |
(i) VRSA: The Tn1546-borne vanA gene cluster encodes 9 proteins (D-Ala:D-Lac ligases) that modify D-Ala-D-Ala termini of peptidoglycan chains to D-Ala-D-Lactate, thereby inhibiting target binding by vancomycin [102,103]. (ii) VISA: Mutations in TCSs like essential WalKR [104,105,106,107], VraSR [108,109,110], and GraSR [107,109,110,111,112] affect cell wall biosynthesis, resulting in reduced susceptibility to vancomycin. (iii) Mutation in rpoB (encoding RNA polymerase subunit B) [99,100]. (iv) Mutation in TCS walKR and proteolytic regulatory gene clpP leads to raised vancomycin resistance in laboratory VISA strain N315LR5P1 [113]. (v) SNPs in capB (E58K) gene (encoding tyrosine kinase) and lytN (I16V) gene (encoding N-acetylmuramyl-L-alanine amidase) cause increased S. aureus resistance to vancomycin in the absence of van genes [101]. |
VRSA is mediated by the vanA gene cluster, which is transferred from vancomycin-resistant Enterococcus [114]. |
| Teicoplanin (formerly known as teichomycin A2) |
1988. Approved in Europe for SSTI, pneumonia, and sepsis [115]. Never approved for use in the U.S. (Resistance 2000) [116] |
Teicoplanin inhibits peptidoglycan polymerization, leading to the inhibition of bacterial cell-wall synthesis. | tcaRAB [117,118], tcaA [119] | (i) The tcaRAB operon may be involved in increased teicoplanin resistance in S. aureus [118]. (ii) Mutation in tcaRAB may influence the transcription of the cell wall biosynthesis gene and may contribute to increasing teicoplanin resistance [117]. (iii) The tcaA gene within tcaRAB plays a relevant role in teicoplanin resistance in S. aureus clinical isolates [119]. |
BSAC recommended breakpoint for teicoplanin are susceptible (MIC ≤ 2 mg/L) and resistant (MIC > 2 mg/L). |
| Oxazolidinones | Protein synthesis | ||||
| Linezolid | U.S. FDA 2000. ABSSSI, pneumonia, BJI, catheter- related bacteremia [120] (Resistance 2001) [121] |
Linezolid binds to bacterial 23S rRNA in the 50S ribosome subunit, thereby preventing the formation of functional 70S ribosomal initiation complex with 30S subunit, mRNA, initiation factors, and N-formylmethionyl-tRNA (tRNAfMet) [122]. |
cfr [123] Mutations in 23S rRNA [121,124] |
(i) Acquisition of cfr gene encoding 23S rRNA methyltransferase [125], which modifies adenosine at position 2503 in 23S rRNA in the large ribosomal subunit [126]. (ii) The T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA [127]. (iii) Mutations G2576T, G2576T, G2447T in domain V of 23S rRNA [121,124] and amino acid changes in ribosomal proteins L3 and L4 [128] lead to conformational changes in the ribosome. |
|
| Tedizolid | U.S. FDA 2014; E.U. EMA 2015.ABSSSI and pneumonia | Tedizolid binds to 23S rRNA in the 50S ribosome subunit and prevents the formation of 70S ribosomal initial complex, resulting in inhibition of bacterial protein synthesis [129,130]. |
cfr rplC, rplD, rplV, rpoB [131] |
(i) Mutations in domain V region of 23S rRNA target confer resistance to tedizolid. (ii) Mutations in ribosomal proteins L3, L4, and L22 (encoded by rplC, rplD, and rplV genes, respectively) and the 23S rRNA target [132]. (iii) Mutation in rpoB corresponding to amino acid substitution D449N [131]. |
Mutation in L3, L4, and L22 also mediate PhLOPSa (phenicol, lincosamide, oxazolidinone, pleuromutilin, and streptogramin A) resistance. |
| Contezolid | NMPA of China 2021 [133]. Complicated SSTI, ABSSSI (Resistance 2021) [134]. |
Contezolid binds to the 23S rRNA region adjacent to the peptidyl transferase center of the 50S ribosomal subunit and prevents the formation of a functional 70S initiation complex, thereby interfering with bacterial protein synthesis. | cfr, optrA | Contezolid exhibited limited activity against strains with linezolid resistance genes cfr and optrA [134]. | Contezolid has reduced hematologic toxicity compared to linezolid |
| Lipopeptides | Cell wall synthesis Cell membrane |
||||
| Daptomycin | U.S. FDA 2003. Bacteremia, ABSSSI (Nonsusceptible 2004) [135] |
Daptomycin complexes with Ca2+ to form oligomers that insert into bacterial membranes, resulting in depolarization, permeabilization, leakage of ions, and ultimately bacterial death [136]. Daptomycin disrupts the localization of cell wall synthesis enzymes such as MurG, further interfering with cell wall synthesis [137,138]. |
mprF, dltA [139,140], yycH, yycI [141], rpoB [99], walKR, vraSR, graSR [142,143] | (i) Alteration of the surface charge of cells due to mutation in mprF gene (encoding phosphatidylglycerol lysyltransferase) which leads to lysinylation of PG and translocation of lysyl-PG [144]. (ii) Mutation in TCSs walKR, vraSR, and graSR which are involved in cell wall synthesis and permeability are associated with daptomycin susceptibility in S. aureus [142,143]. (iii) Mutation in rpoB gene (encoding RNA polymerase) confers dual heteroresistance to daptomycin and vancomycin [99]. (iv) Mutations in yycH and yycI genes lead to the loss of protein functions essential for cell wall synthesis [141]. (v) dltA gene overexpression leads to electrostatic repulsion and indirectly reduces autolysin, resulting in daptomycin nonsusceptibility [139,140]. |
S. aureus strains with MIC ≤ 1 μg/mL are referred as daptomycin-susceptible (DAP-S) [145] and MIC >1 μg/mL as daptomycin-non susceptible [146]. |
| Lipoglycopeptides | Cell wall synthesis | ||||
| Telavancin (derivative of vancomycin. Addition of the hydrophobic side chain and hydrophilic group results in enhanced activity [147]. |
U.S. FDA 2009 and 2013 [148]. Complicated SSTI, pneumonia, BJI, ABSSSI, bacteremia [149]. |
Telavancin inhibits cell wall biosynthesis by binding to late-stage peptidoglycan synthesis, like vancomycin. Additionally, it depolarizes the bacterial cell membrane and disrupts its functional integrity [150]. | - | The vanA-mediated telavancin resistance is rare in MRSA [151]. |
|
| Tetracyclines | Protein synthesis | ||||
| Tetracycline | 1948 [152] SSTI (Resistance 1953) [44] |
Tetracycline binds to bacterial 30S ribosomal subunit and prevents the aminoacyl tRNA from binding to A site of the rRNA, resulting in inhibition of translation. To some extent, it also binds to the bacterial 50S ribosomal subunit [44,153,154]. | tetM, tetO, tetK [155], tetS/M, tetA | (i) Ribosomal protection: the tetM and tetO genes encode enzymes that destabilize the interaction between tetracyclines and their cellular target ribosome [44,45]. (ii) Active efflux: the tetK gene encodes efflux protein that couples the tetracycline with proton motive force to pump it out from the cell against the concentration gradient [44,155]. |
The tetK gene is normally found in S. aureus. |
| Doxycycline | U.S. FDA 1967 [156,157]. UTI, SSTI [27] |
Doxycycline inhibits bacterial protein synthesis by preventing the association of aminoacyl tRNA with the ribosome, an MoA similar to tetracycline. | tetK [158,159] | Active efflux by tetK encoded efflux [158,159]. | |
| Tigecycline | U.S. FDA 2005.ABSSSI, pneumonia | Tigecycline inhibits protein synthesis, an MoA similar to tetracycline but with enhanced binding. | tetM, tetO, tetX | The oxygen-dependent destruction of tigecycline is catalyzed by the enzyme TetX [160,161,162]. |
Tigecycline retains activity against both tetM and tetO. |
| Omadacycline (derived from tetracycline) [163] |
U.S. FDA 2018.ABSSSI, SSTI [164], pneumonia (CA-associated) | Omadacycline binds to bacterial 30S ribosomal subunit and inhibits protein synthesis, an MoA similar to tetracycline with enhanced binding like tigecycline [165]. |
- | Resistance mechanism not reported. | Unaffected by the presence of tetK active efflux gene and ribosomal protection tetM or tetO gene [166,167]. |
| Fusidane | Protein synthesis | ||||
| Fusidic acid | 1962. ABSSSI |
Fusidic acid binds to elongation factor G (EF-G) on the ribosome, thereby preventing the release of EF-G-guanosine diphosphate complex and delaying bacterial protein synthesis by inhibiting the next stage in translation [168,169]. | fusA [170], fusB [171,172], fusc, fusD | (i) Mutations in chromosomal fusA (encoding ribosomal translocase and translation elongation factor EF-G) [170] or fusE genes confer high-level resistance to fusidic acid. (ii) Mutation in acquired genes fusB (encoding an inducible protein that protects an in vitro translation) [171,172] and fusD genes mediate low-level resistance. These mutations affect the elongation factor EF-6. |
The fusc and fusD are homologs of fusB [173]. |
| Pleuromutilin | Protein synthesis | ||||
| Retapamulin |
U.S. FDA 2007. Impetigo [174] |
Retapamulin binds to domain V of 23S rRNA on the 50S ribosome subunit, thereby blocking peptide formation directly by interfering with substrate binding. | 23S rRNA | Resistance to retapamulin occurs due to mutations in the genes encoding 23S rRNA methyltransferase. | Retapamulin is a semisynthetic derivative of pleuromutilin |
| Fluoroquinolones | DNA replication | [46,175] | |||
| Ciprofloxacin (2nd-generation fluoroquinolone) |
U.S. FDA 1987.UTI | Ciprofloxacin target bacterial DNA topoisomerase IV and DNA gyrase, thus preventing it from supercoiling the bacterial DNA [176], which leads to inhibition of DNA replication [177,178]. |
gyrA [33], grlA [33], flqA (formerly ofx/cfx) [35], norA [58,179] | (i) Mutation in the genes grlA (encoding DNA topoisomerase IV subunit A) [33,34,35,46], gyrA (encoding DNA gyrase subunit A) [33,34,35], and flqA (linked to DNA topoisomerase IV) [35]. (ii) Mutations in the gene norA (encoding a membrane-associated active efflux pump NorA) [58,180]. |
Elevated norA expression potentiates evolution by increasing the fitness benefit provided by a mutation in DNA topoisomerase [179]. |
| Levofloxacin | U.S. FDA 1996. RTI, UTI, SSTI |
Levofloxacin inhibits bacterial DNA replication, an MoA similar to ciprofloxacin. | gyrA, grlA | (i) Mutation in the genes grlA and gyrA [181]. (ii) Mutations in the gene norA [180]. |
|
| Delafloxacin (previously referred to as ABT-492) [182] |
U.S. FDA 2017 [183]; E.U. EMA 2019. SSTI, ABSSSI (Resistance 2017) [184] |
Delafloxacin inhibits bacterial DNA replication by blocking both DNA topoisomerase IV and DNA gyrase, an MoA similar to ciprofloxacin [182]. | grlA | Point mutations in the grlA [185,186]. | Delafloxacin is not active substrate for S. aureus efflux pumps [185]. |
| Quinolones | DNA replication | ||||
| Ozenoxacin (topical quinolone without fluorine at C6-position) |
U.S. FDA 2017.Japanese PMDA 2016 [187]. SSTI (impetigo) caused by MRSA |
Ozenoxacin inhibits bacterial DNA replication by dual-targeting activity against DNA topoisomerase IV and DNA gyrase [35]. | grlA, grlB | Mutations in QRDR regions of grlA and gyrA are the primary cause of decreased susceptibility to ozenoxacin [35]. | Low MIC of ozenoxacin was observed for MSSA and MRSA strains with reduced susceptibility to nadifloxacin [187]. |
| Pyrimidine/ Sulfonamide |
Folate synthesis (DNA synthesis and protein synthesis) | ||||
| Trimethoprim–Sulfamethoxazole (TMP-SMX) | UTI, SSTI, and BJI due to CA-MRSA [29] | TMP binds and inhibits the dihydrofolate reductase, thereby preventing the conversion of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF) [188]. THF is an essential precursor of the thymidine synthesis pathway and interference with this pathway results in inhibition of bacterial DNA synthesis. SMX inhibits bacterial dihydropteroate synthase, an enzyme involved upstream in the thymidine synthesis pathway, resulting in the inhibition of folic acid biosynthesis [188]. |
dfrA, dfrB [189], dfrD [189], dfrG [190], dfrK, dfrS1 [191,192] | (i) The acquisition of dfrA gene (encoding DHFR) and mutation of the chromosomal dfrB gene (encoding SaDHFR) are considered key determinants of TMP-SMX resistance [189,193,194,195]. (ii) Point mutation in the dfrB gene resulted in a single amino acid substitution Phe98Tyr of SaDHFR, which was associated with TMP-SMX resistance in S. aureus [189]. (iii) Transposon-located dfrA gene mediates TMP resistance [194,196]. (iv) The dfrG gene (encoding DHFR) mainly mediates the TMP resistance in S. aureus clinical isolates [190,195]. |
|
| Other classes | |||||
| Mupirocin (previously pseudomonic acid) |
Discovered in 1971 [197] while marketed for clinical use in the UK in 1985 and US in 1988 [198]. SSTI, nasal carriage of S. aureus (Resistance 1987) [199,200]. |
Mupirocin binds to bacterial isoleucyl transfer RNA (tRNA) synthetase, leading to depletion of isoleucyl–tRNA and accumulation of the corresponding uncharged tRNA. This results in the inhibition of protein and RNA synthesis [201]. | ileS [202,203,204], mupA [205,206], and mupB [207] | (i) Mutations in the chromosomal ileS gene (encoding native isoleucyl t-RNA synthetase) result in V588F or V631F alterations [202,203,204], which lead to low-level mupirocin resistance [205]. (ii) Acquisition of the plasmid-encoded mupA gene (encoding eukaryotic-like isoleucyl–tRNA synthetase variant) [208] confers high-level resistance to mupirocin [205,206]. (iii) Acquisition of the plasmid-encoded mupB gene (encoding eukaryotic-like isoleucyl–tRNA synthetase variant) confers high-level resistance to mupirocin [207]. |
Low-level mupirocin resistance (MIC 8–256 μg/mL) and high-level resistance (MIC ≥ 512 μg/mL) [209]. |
| Fosfomycin | Discovered in 1969 [210]. UTI |
Fosfomycin deactivates the enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) and catalyzes the addition of phosphoenolpyruvate to UDP-N-acetylglucosamine (UDP-GlcNAc) to form UDP-N-acetylmuramic acid (UDP-MurNAc), thereby inhibiting bacterial cell-wall synthesis [211]. | fosB [54], glpT and uhpT [212,213,214], murA [213,215] tet38 [216], fosY [217] | (i) Thiol-S-transferase (encoded by fosB gene) catalyzes the inactivation of fosfomycin [53,54]. (ii) Mutations in fosfomycin uptake transporter proteins GlpT (Trp137/Arg) (encoded by glpT gene) [213] and UhpT (encoded by uhpT genes) [214] reduce the permeability and subsequently prevent fosfomycin from invading the bacterium [212,213]. (iii) Mutation in target enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (encoded by murA gene) reduces its affinity for fosfomycin [215]. (iv) The major facilitator superfamily efflux transporter Tet38 (encoded by tet38 gene) contributes to fosfomycin resistance [216]. (v) FosY protein, a putative bacillithiol transferase enzyme (encoded by fosY gene) confers resistance to fosfomycin in CC1 S. aureus [217]. |
|
| Rifampin | Discovered in 1965, introduced for therapy in Italy in 1968, and approved in the United States in 1971 [218]. Endocarditis; BJI [27]. |
Rifampin inhibits transcription (RNA synthesis) by binding to the β-subunit of the bacterial DNA-dependent RNA polymerase [219,220]. | rpoB [43,221] | (i) Mutations in the RRDR region of rpoB gene (encoding RNA polymerase) resulted in amino acid substitutions of Gln468/Arg, His481/Tyr, and Arg484/His and are associated with high-level resistance to rifampicin [43]. (ii) Mutation in the rpoB (N967I) gene causes the substitution Asn967/Ile in the β-subunit of RNA polymerase [221]. |
CLSI breakpoint of rifampicin susceptibility is ≤1 μg/mL [146]. |
–: not studied or reported, AAC: aminoglycoside acetyltransferase, ABSSSI: acute bacterial skin and skin structure infection, AG: aminoglycoside, AMEs: aminoglycoside-modifying enzymes, ANT: aminoglycoside nucleotidyltransferase, APH: aminoglycoside phosphotransferase, BJI: bone and joint infections, BSAC: British Society for Antimicrobial Chemotherapy, CLSI: Clinical and Laboratory Standards Institute, E.U. EMA: European Union European Medicine Agency, Japanese PMDA: Japanese Pharmaceutical and Medical Devices Agency, MRSA: methicillin-resistant S. aureus, MSSA: methicillin-sensitive S. aureus, PG: peptidoglycan, QRDR: quinolone-resistance-determining region, RRDR: rifampin-resistance-determining region, rRNA: ribosomal RNA, SaPI: S. aureus pathogenicity island, SMX: sulfamethoxazole, SSTI: skin and soft tissue infections, TCSs: two-component regulatory systems, TMP: trimethoprim, U.S. FDA: U.S. Food and Drug Administration, UTI: urinary tract infection, VISA: vancomycin intermediate-resistant S. aureus, VRSA: vancomycin-resistant S. aureus.
4.1. Macrolides (Erythromycin)
Erythromycin, a macrolide antibiotic discovered in 1952, has been used for the treatment of SSTIs caused by MRSA [66]. Macrolide antibiotics inhibit protein synthesis by targeting the bacterial ribosome. They bind to bacterial 23S rRNA on the 50S ribosome subunit and stop protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and assembly of the 50S ribosomal subunit [68,69]. Macrolide molecules may also affect the functional properties of the catalytic center of the ribosome, leading to inhibition of translation or a change in the reading frame, resulting in the abnormal synthesis of the polypeptide chain [222]. Macrolides have a broad spectrum of activity against both gram-positive and gram-negative bacteria.
S. aureus resistance to erythromycin was first observed in 1955, following its introduction in 1952 [67]. In S. aureus, the resistance to macrolides, lincosamides, and streptogramin B (MLSB) antibiotics correlates with the resistance to methicillin [223]. MRSA strains showing resistance to MLSB are usually determined by the presence of 23S rRNA methyltransferase encoding ermA or ermC genes, whereas MSSA strains show resistance by ermC, followed by ermB, genes [70]. The MLSB resistance can be inducible or constitutive and is not related to the type of erm genes.
The main mechanisms leading to macrolide resistance in S. aureus are (i) modification of the bacterial ribosome by erm-gene-encoded 23S rRNA methyltransferase [70], which leads to a conformational change in the ribosome, thus preventing the binding of macrolides to ribosomal target [31,32]; (ii) active efflux of macrolides from the cell by ATP-binding cassette family (ABC-F) transporters encoded by msrA and msrB genes, protecting the bacterial ribosome from antibiotic-mediated inhibition [72,73]; (iii) enzymatic inactivation of the macrolides by phosphotransferases (encoded by mph genes) [74] and esterase (encoded by ere genes), which prevent binding to target site [74]. Indeed, the major genes associated with macrolides resistance in S. aureus include ermA [31], ermB, ermC [32], ermY [52], msr(F) [71], msrA [52,72], msrB, ereA, ereB, mphB, and mphC [52].
4.2. Lincosamide (Clindamycin)
Clindamycin, a lincosamide antibiotic approved in 1966, has been used for the treatment of SSTIs caused by CA-MRSA [29]. Clindamycin in MLSB family of antibiotics serves as an alternative to treat both MSSA and MRSA infections due to its excellent pharmacokinetic properties. It inhibits bacterial protein synthesis by binding to the 23S rRNA of the 50S ribosomal subunit and impedes both the assembly of ribosomes and the translation process [75]. It impairs peptide chain initiation and stimulates the dissociation of peptidyl-tRNA from ribosomes.
The widespread use of clindamycin has led to the emergence of resistant strains; the first report of clindamycin resistance in S. aureus appeared in 1968 [30]. Resistance to clindamycin generally occurs through ribosomal target site modification mediated by erm genes [70], which can be expressed either constitutively (cMLSB phenotype) or inducibly (iMLSB phenotype) [40,76]. The ermB, ermC, and ermA genes code for rRNA methylase, which methylates and alters the drug target site 23S rRNA, thus preventing the binding of MLSB antibiotics [77]. Furthermore, resistance to clindamycin occurs through target site modification by cfr-gene-encoded rRNA methyltransferase [41], which methylates an adenine residue of the 23S rRNA in the 50S ribosomal subunit, resulting in altered antibiotic binding sites within the ribosome. The emergence of multidrug-resistant CA-MRSA strains carrying plasmid pUSA03 (codes for resistance to clindamycin and mupirocin), predominant among isolates from men who have sex with men (MSM), is on the rise in Boston and San Francisco [224]. Transformation of a large plasmid pSCFS3 that carried the two chloramphenicol- and florfenicol-resistance genes cfr and fexA from porcine S. aureus strain into S. aureus RN4220 mediated high-level clindamycin resistance (MIC 256 μg/mL) in addition to chloramphenicol–florfenicol resistance (MICs for both ≥ 128 μg/mL) [42].
4.3. Aminoglycosides (Gentamicin)
Gentamicin, an aminoglycoside antibiotic, was approved by the U.S. FDA in 1971 for the treatment of sepsis in newborns, septicemia, and UTI caused by MRSA. Gentamicin acts by binding to the 16S rRNA helix at the mRNA–tRNA decoding center of bacterial 30S ribosome subunit [80,81], thereby causing inaccurate induction and inhibition of translation, disrupting bacterial protein synthesis [82,83,84].
Resistance to aminoglycosides is usually mediated by aminoglycoside-modifying enzyme (AME) AAC(6′)/APH(2″) in S. aureus [85]. The AMEs inactivate aminoglycosides by N-acetylation (N-acetyl transferases, AAC), O-phosphorylation (aminoglycoside phosphotransferases, APH), or O-adenylation (aminoglycoside nucleotidinyl transferases, ANT) of amino or hydroxyl groups of antibiotics [51]. Gentamicin-resistant S. aureus was first reported in 1975 [78,79]. The aac(6′)/aph(2″)-gene-encoded bifunctional AME AAC(6′)/APH(2″) specifies both 6′-acetyltransferase (AAC(6′))- and 2″-phosphotransferase (APH(2″))-aminoglycoside-modifying activities [36,37]. The aac(6′)/aph(2″) gene is the most prevalent in aminoglycoside-resistant S. aureus isolates [86,225].
Arbekacin is a semisynthetic aminoglycoside antibiotic derived from kanamycin. Japanese Pharmaceuticals and Medical Devices Agency (PMDA) approved arbekacin for the treatment of pneumonia caused by MRSA in 1990 [87]. It is not used clinically in the USA. Arbekacin binds to the four nucleotides of the 16S rRNA and one amino acid of protein S12 to interfere with the decoding site around nucleotide 1400 in the 16S rRNA of the 30S ribosome subunit [89]. This site interacts with the wobble base of tRNA, which leads to the misreading of mRNA, such that incorrect amino acids are inserted into the proteins. These error-filled proteins are nonfunctional or even toxic. A single base alteration at site G1126A of the aac(6′)/aph(2″) gene, resulting in one amino acid substitution S376N in the phosphorylation catalytic motif of AAC(6′)/APH(2″), has been reported to be associated with arbekacin resistance in MRSA strain PRC104 [90]. Moreover, MRSA strain KU5801, which has additional β-lactam-inducible arbekacin resistance, was reported in Japan due to an antagonistic mechanism [91] by which the integration of Tn4001-IS257 hybrid structure that contained the aac(6′)/aph(2″) gene cointegrated into a region downstream of the blaZ gene. A previous study found that all 17 MRSA strains belonging to coa-RFLP type M22 possessed the aac(6′)/aph(2″) gene, but 70.1% were resistant to arbekacin [88], whereas of the 363 MRSA type L21 strains, 5.5% were arbekacin-resistant, despite about half (41.9%) of them carrying the aac(6′)/aph(2″) gene. This suggests that AAC(6′)/APH(2″) mediate arbekacin resistance, but there is not a single mechanism among MRSA type L21 strains [88]. AAC(6′)/APH(2″) has the capability of 6′-N-acetylation and/or 2″-O-phosphorylation of arbekacin that contains 6′-NH2 and/or 2″-OH [37,92].
4.4. Glycopeptides (Vancomycin)
Vancomycin, a glycopeptide antibiotic, was introduced in 1958 for the treatment of severe gram-positive bacterial infections, including MRSA [226]. Vancomycin has long been considered the last-line antibiotic to treat serious infections, such as bacteremia, infective endocarditis, osteomyelitis, meningitis, pneumonia, sepsis, and severe SSTI due to both HA-MRSA and CA-MRSA [29]. Vancomycin inhibits bacterial cell wall synthesis by targeting the D-alanyl-D-alanine (D-Ala-D-Ala) terminus of peptidoglycan [227,228]. It forms hydrogen bonds with D-Ala-D-Ala termini moieties of the peptidoglycan precursor lipid II, resulting in conformational alteration that inhibits the incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits into the growing peptidoglycan chain, thereby inhibiting bacterial cell wall biosynthesis [95,229]. This alters bacterial membrane integrity and increases its permeability, which ultimately leads to bacterial death [229]. The Clinical and Laboratory Standards Institute (CLSI) has classified S. aureus isolates as vancomycin-susceptible S. aureus (VSSA; MIC ≤ 2 μg/mL), vancomycin-intermediate S. aureus (VISA; MIC of 4–8 μg/mL), and vancomycin-resistant S. aureus (VRSA; MIC ≥ 16 μg/mL) [146].
While human infections with MRSA are commonly treated with vancomycin, VISA started appearing in the 1990s [28]. The first S. aureus clinical strain with reduced vancomycin susceptibility (MIC 8 μg/mL) was reported in Japan in 1996 [28]. The gradual mutations within genes encoding two-component regulatory systems (TCSs) such as WalKR [104,105,106,107], VraSR [108,109,110], or GraSR [107,109,110,111,112] are predominantly involved in cell wall biosynthesis and are associated with VISA. WalKR is essential for the regulation of cell wall metabolism-associated genes and particularly as a regulator of peptidoglycan synthesis at the time of cross-bridge hydrolysis [230,231,232]. GraSR system is involved in cell envelope modifications through regulation of the dlt operon and mprF/fmtC genes that are linked to teichoic acid alanylation and alteration of cell wall charge [97]. Mutations within the graSR are associated with modified expression of global regulators Rot (repressor of toxins) [233] and accessory gene regulator (Agr) [234], which lead to VISA. Regulator mutation in TCS walKR and truncating mutation in proteolytic regulatory gene clpP in laboratory-derived VISA strain N315LR5P1 leads to 1 to 2 mg/L raised vancomycin resistance [113]. Furthermore, the mutation in rpoB gene encoding the DNA-dependent RNA polymerase β-subunit results in increased cell wall thickness and thereby increased resistance to vancomycin [99,100]. Recently, single-nucleotide polymorphisms (SNPs) in the capB (E58K) gene (encoding tyrosine kinase) and lytN (I16V) gene (encoding N-acetylmuramyl-L-alanine amidase) have been shown to cause increased S. aureus resistance to vancomycin in the absence of van genes [101].
The occurrence of VRSA infections in clinical settings remains rare [235], which could be due to the antagonistic effects of mecA and vanA resistance determinants [236]. The development of VRSA strains occurred through horizontal gene transfer (HGT) of the vanA gene by transposon Tn1546 from vancomycin-resistant Enterococcus faecalis [237]. The vanA gene cluster that encodes D-Ala:D-Lac ligases alters the dipeptide terminus of peptidoglycan precursors from D-Ala-D-Ala to D-Ala-D-lactate (D-Ala-D-Lac) [95,238], which has substantially lower binding affinity for vancomycin and thus fails to inhibit cell wall synthesis in S. aureus [102]. The first vanA-mediated high-level VRSA (MIC ≥ 32 µg/mL) clinical strain was recovered in Michigan, the USA in 2002 [239]. Furthermore, the first vanA-mediated methicillin-resistant VRSA (MIC > 256 µg/mL) strain in Europe was isolated from a patient in Portugal in 2013 [240].
4.5. Oxazolidinones (Linezolid)
Linezolid is the first fully synthetic oxazolidinone antibiotic approved by the U.S. FDA in 2000 for the treatment of acute bacterial skin and skin structure infections (ABSSSI), pneumonia, bone and joint infections (BJI), and catheter-related bacteremia caused by gram-positive bacteria with activity against MRSA [120,241]. Linezolid inhibits bacterial protein synthesis by binding to the domain V region of the 23S rRNA of the 50S ribosome subunit and preventing the complex formation with N-formyl methionyl–tRNA (tRNAfMet), mRNA, and the 30S ribosome subunit [120,122]. The clinical breakpoint of linezolid for MRSA is 8 μg/mL.
MRSA clinical isolates with resistance to linezolid were first reported in 2001 [121]. Further, new oxazolidinone antibiotics such as tedizolid, radezolid, and contezolid with superior efficacy were developed, but MRSA clones with resistance to these antibiotics also evolved [242]. Linezolid resistance in MRSA was due to the acquisition of cfr gene, encoding 23S rRNA methyltransferase enzyme [125], which alters adenosine at position 2503 in 23S rRNA in the large ribosomal subunit [126]. A T2500A mutation in the 23S rRNA gene and loss of a single copy of rRNA has been reported to be associated with linezolid resistance in sequential S. aureus isolates [127]. Furthermore, the mutation of domain V of the 23S rRNA [121] and mutation of the ribosomal proteins near the linezolid binding site in the ribosomal peptidyl transferase center [128] results in linezolid resistance in MRSA. Multiple MRSA clones with two mutations in the ribosomal protein uL3 exhibited resistance to linezolid, with a two-fold higher MIC than the clinical breakpoint [243].
4.6. Lipopeptides (Daptomycin)
Daptomycin, a cyclic lipopeptide antibiotic approved by the U.S. FDA in 2003, has in vitro bactericidal activity against many gram-positive bacteria. It was approved for the treatment of bacteremia and endocarditis caused by S. aureus [244] and has now become the main alternative to vancomycin for serious MRSA infections [245]. Daptomycin exhibits bactericidal activity by targeting membrane phospholipid phosphatidylglycerol as well as bactoprenyl-coupled cell wall precursors such as lipid II in a calcium-dependent manner [246]. In addition, daptomycin affects the localization of cell wall synthesis enzymes like MurG, further interfering with cell wall synthesis [137,138].
Since no resistant breakpoint for daptomycin has been officially established, the term nonsusceptible is used by some researchers over resistant. S. aureus strains with MIC ≤ 1 μg/mL are referred as daptomycin-susceptible (DAP-S) [145] and strains with MIC >1 μg/mL as daptomycin-nonsusceptible [146]. The first daptomycin-nonsusceptible S. aureus isolates have been reported from a patient treated with daptomycin for bacteremia in Boston in 2004 [135]. Even before the daptomycin approval, the daptomycin-nonsusceptible S. aureus mutants were observed after passage through increasing concentrations of daptomycin [247]. Although the development of daptomycin-nonsusceptible S. aureus remains rare, there have been steady reports of the emergence of daptomycin-nonsusceptible MRSA strains (MIC of >1 μg/mL) during treatment with daptomycin [248,249,250,251].
The development of daptomycin-nonsusceptible S. aureus occurs from the stepwise and multifactorial process that involves cell membrane and cell wall alterations [136,142,252]. The most common resistance mechanism includes the alteration of the surface charge of cells which results in the repulsion of anionic daptomycin molecules [142]. This primarily occurs due to the acquisition of gain-of-function mutations in mprF gene encoding a membrane-bound protein MprF called lysyl–phosphatidyl glycerol synthetase [253]. MprF is a bifunctional protein that facilitates both the lysinylation of phosphatidylglycerol, i.e., transfer of negatively charged phosphatidylglycerol to positively charged lysyl–phosphatidylglycerol and the translocation of lysyl–phosphatidylglycerol to the outer leaflet of the membrane [136,144,253,254]. Furthermore, the dlt operon regulates the alanylation of wall teichoic acid, and mutation in the dlt operon leads to an increase in cell surface positive charge, which reduces the daptomycin susceptibility through charge-mediated repulsion [233,255]. Mutations in various genes including those associated with the cell membrane (mprF), cell wall (dltABCD), and RNA polymerase subunits (rpoC and rpoB) [99] have been described to play an important role in daptomycin susceptibility [143,252,256,257]. Daptomycin resistance also occurs due to mutations in yycH and yycI genes, leading to loss of protein functions and downregulated the WalKR and the downstream players Atl and amidase Sle1, including the autolysin Atl and amidase Sle1 which are essential for cell wall synthesis [141]. Mutations of the TCSs like WalKR, VraSR, or GraSR that directly or indirectly control the transcription of several genes encoding proteins involved in cell wall synthesis and permeability have been also associated with daptomycin susceptibility in S. aureus [142,143]. GraSR regulates the expression of genes encoding peptidoglycan hydrolases lysyl–phosphatidylglycerol synthase and flippase, MprF, and the DltABCD system, which modifies teichoic acids with D-alanine [233,258,259].
4.7. Fluoroquinolone (Ciprofloxacin)
Ciprofloxacin, a second-generation synthetic antibiotic of the fluoroquinolone class is active against a broad range of gram-positive and gram-negative bacteria. It was approved by the U.S. FDA in 1987 to treat UTIs caused by both MRSA and MSSA. Ciprofloxacin targets bacterial DNA topoisomerase IV and DNA gyrase enzymes which contributes to the relaxation of positive supercoils during DNA replication [176], thus preventing DNA replication and eventually bacterial death [177,178].
The ciprofloxacin-resistant S. aureus isolates were described soon after the introduction of the agent into clinical practice [260]. Resistance to fluoroquinolones including ciprofloxacin typically arises as a result of the (i) point mutations in the grlA/grlB genes encoding the subunits of DNA topoisomerase IV and gyrA/gyrB genes encoding the subunits of DNA gyrase [33,261], and (ii) decreased intracellular accumulation and/or active efflux of the drug by membrane-integrated transporter proteins (e.g., NorA) [262]. Challenging the S. aureus isolates with the ciprofloxacin elevated the norA expression, which potentiates the evolution by increasing the fitness benefit provided by DNA topoisomerase mutations [179]. The initial target mutations occur more frequently in grlA gene, whereas additional mutations are found in gyrA gene in highly fluoroquinolone-resistant S. aureus strains [35]. Mutational changes result in amino acid substitutions in the QRDR of GrlA and GyrA proteins. The GyrA Ser84Leu and GrlA Ser80Phe mutation caused a two-fold increase in minimum bactericidal concentration (MBC) of fluoroquinolone antibiotic DW286 than its corresponding MIC [49,263].
Efflux pump-mediated fluoroquinolone resistance is due to the extrusion of an intracellular drug into the external environment [57,264]. Efflux pumps are usually expressed at low or nondetectable levels but upregulated upon exposure to certain antimicrobials including fluoroquinolones [57,264]. Several efflux pumps have been identified in S. aureus, including chromosomally encoded NorA, NorB, NorC, MdeA, MepA, SepA, and SdrM and plasmid-encoded QacA/B, QacG, QacH, QacJ, and Smr [265]. However, the NorA, QacA, and Smr proteins are considered major players in multidrug resistance in S. aureus [56,57,61,62]. The chromosomal gene norA was first identified in fluoroquinolone-resistant S. aureus isolate in Japan in 1986 [266]. NorA is a 388 amino acid protein with 12 transmembrane segments (TMS) which belongs to the Major Facilitator Superfamily (MFS) of secondary transporters [59]. NorA is also involved in resistance to several structurally different compounds including ethidium bromide dye, quaternary ammonium compounds (disinfectants), and other antimicrobials [262,267,268].
4.8. Pyrimidines/Sulfonamides (Trimethoprim-Sulfamethoxazole)
Trimethoprim (TMP; 2,4-diamino-5-(3′,4′,5′-trimethoxybenzyl)pyrimidine)/sulfamethoxazole (SMX; 3-(p-aminophenyl sulfonamido)-5-methylisoxazole), also known as co-trimoxazole, is a combination of trimethoprim and sulfamethoxazole class drugs that have been used to treat UTIs, uncomplicated SSTIs, and BJIs caused by CA-MRSA [29,269,270,271]. TMP inhibits bacterial dihydrofolate reductase (DHFR), an enzyme that catalyzes nicotinamide adenine dinucleotide phosphate (NADPH)-dependent conversion of dihydrofolate (DHF) to tetrahydrofolate (THF) [272,273]. TMP is more specific to S. aureus DHFR (SaDHFR) than to human DHFR, which particularly inhibits bacterial folic acid synthesis [189]. SMX is a structural analog of para-aminobenzoic acid (PABA), a substrate important for bacterial folic acid synthesis [274]. SMX binds to dihydropteroate synthase (DHPS) which catalyzes the conversion of PABA to dihydropteroate (DHP) during the THF formation [269]. The inhibition of DHPS leads to defective thymidine biosynthesis and thus reduces or inhibits bacterial folic acid synthesis [275]. In general, TMP–SMX has a greater effect as a combination because synergistically they inhibit two consecutive steps in nucleic acid and protein synthesis which are critical for the growth and cell division of bacteria [276].
The widespread use of TMP–SMX for the treatment of staphylococcal infections leads to the emergence of resistance in both MSSA and MRSA [277,278,279]. S. aureus shows resistance to TMP–SMX due to the mutation of chromosomal gene dfrB encoding SaDHFR and resistance genes that encode variant DHFRs [189,193,194,195], which is the target of TMP [189]. A single amino acid substitution at position 98 (Phe98 to Tyr98) in SaDHFR encoded by dfrB led to the intermediate-level TMP resistance (MIC ≤256 mg/L) in S. aureus [189]. In contrast, acquired plasmid-borne dfrA (also known as dfrS1) encoding type S1 DHFR mediates high-level TMP resistance (MIC ≥512 mg/L). The dfrA, dfrB, dfrG, and dfrK are important TMP resistance genes known to occur in staphylococci including MRSA [191,192]. S. aureus exposed to sub-MIC of TMP-SMX for 14 days resulted in resistant strains due to the F98Y mutation in DHFR encoded by the dfrB gene [280]. Mutations in the dfrB and dfrA have been reported as major determinants of TMP resistance in S. aureus clinical isolates [281,282]. The dfrG gene encoding the TMP-resistant DHFR enzyme was rarely identified in S. aureus clinical isolates [190,283] but mediates TMP resistance [190,195].
4.9. Mupirocin
Mupirocin was discovered in 1971 [197] but marketed for clinical use in the UK in 1985 and US in 1988 [198]. It was widely used as a decolonizing agent during the emergence of the CA-MRSA epidemic in the United States in the 1990s. Currently, mupirocin remains the best option for the treatment of MRSA nasal decolonization and SSTI [27,29]. Mupirocin competitively inhibits bacterial isoleucyl t-RNA synthetase, an enzyme encoded by the chromosomal ileS gene that promotes the conversion of isoleucine and tRNA to isoleucyl–tRNA, leading to the inhibition of protein and RNA synthesis [201].
Resistance to mupirocin among S. aureus clinical isolates was first reported in 1987 [199,200]. The high-level mupirocin resistance (MIC > 500 μg/mL) by S. aureus is generally mediated by the expression of plasmid-encoded mupA gene [205,206], which encodes an alternate isoleucyl–tRNA synthetase enzyme [208]. Moreover, the mupB gene (3102 bp) is also associated with high-level mupirocin resistance in S. aureus, which shares 65.5% sequence identity with mupA and 45.5% with ileS gene [207]. The low-level mupirocin resistance (MIC 8–256 μg/mL) is usually associated with point mutations in the chromosomally encoded ileS gene [205], which result in V588F or V631F alterations in the native isoleucyl–tRNA synthetase [202,203,204]. In addition, low-level mupirocin resistance was confirmed by the chromosomal location of mupA gene [284] in some S. aureus strains from different geographic areas [285].
4.10. Fosfomycin
Fosfomycin discovered in 1969 [210], is a phosphonic acid derivative from cultures of Streptomyces spp. It is a broad-spectrum antibiotic used primarily for the treatment of UTIs caused by multidrug-resistant pathogens including MRSA. Fosfomycin interferes with bacterial cell wall synthesis via irreversibly inhibiting the cytosolic enzyme UDP-N-acetylglucosamine enolpyruvyl transferase (MurA), which catalyzes the addition of phosphoenolpyruvate to UDP-N-acetylglucosamine (UDP-GlcNAc) to form UDP-N-acetylmuramic acid (UDP-MurNAc) [211].
Resistance to fosfomycin has been occurring among MRSA clinical isolates either by chromosome-associated defective transport proteins or plasmid-mediated fosfomycin-inactivating enzymes [213]. GlpT and UhpT transporter proteins mediated the uptake of fosfomycin into bacterial cells. Mutations in GlpT (Trp137/Arg) (encoded by glpT gene) [213] and UhpT (encoded by uhpT gene) [214] reduce the permeability and subsequently prevent fosfomycin from invading the bacterium [212,213]. In addition, the mutation in murA gene encoding UDP-N-acetylglucosamine enolpyruvyl transferase reduces affinity for fosfomycin [215], conferring various degrees of drug resistance. The fosfomycin-inactivating enzyme thiol-S-transferase (encoded by fosB gene) [53,54] catalyzes the inactivation of fosfomycin antibiotic in S. aureus [53,54]. FosY protein, a putative bacillithiol transferase (encoded by fosY gene present on a genomic island) which shares 65.9–77.5% amino acid identity with FosB and FosD, respectively, confers resistance to fosfomycin in clonal complex 1 (CC1) MRSA isolate from China [217]. The chromosomally encoded major facilitator superfamily efflux transporter Tet38 (encoded by tet38 gene) of S. aureus acts as an efflux transporter of fosfomycin, which is affected by glycerol-3-phosphate (G3P) [216].
4.11. Rifampin
Rifampin was discovered in 1965, introduced for clinical therapy in Italy in 1968, and approved in the United States in 1971 [218]. It is used in combination therapy (adjunctive with vancomycin) for the treatment of serious S. aureus infections such as endocarditis and BJI [27]. Rifampicin inhibits transcription via binding to the β-subunit of bacterial DNA-dependent RNA polymerase (encoded by rpoB gene), leading to suppression of RNA synthesis and subsequent cell death [219,220].
Unfortunately, the frequency of rifampicin-resistant S. aureus isolates has increased in recent times [286]. The high-level resistance to rifampin in S. aureus is associated with mutations in an extremely conserved region of the RNA polymerase β-subunit called the rifampin resistance-determining region (RRDR) that resulted in an amino acid substitution Gln468/Arg, His481/Tyr, and Arg484/His [43]. Furthermore, the mutation in rpoB gene (N967I) causes the amino acid substitution Asn967/Ile in the β-subunit of RNA polymerase [221]. It is also demonstrated that mutations in rpoB gene of VISA strain Mu50 are associated with the alteration of vancomycin susceptibility [100]. Mutations within the rpoB gene of clinical S. aureus isolates are associated with a decrease in daptomycin susceptibility, thus giving a daptomycin non-susceptible (DNS) phenotype [252,287].
5. Conclusions
The evolution and spread of MRSA has become a major concern for public health. MRSA strains are intrinsically resistant to almost all β-lactam antibiotics by an acquired mecA encoded PBP2a, which can continue peptidoglycan crosslinking in the face of a challenge by β-lactams. Furthermore, MRSA strains are often also resistant to currently used multiple non-β-lactam antibiotics such as erythromycin, clindamycin, gentamicin, linezolid, tetracycline, fusidic acid, ciprofloxacin, ozenoxacin, TMX–SMX, and others. The selective pressure exerted by antibiotics use has led S. aureus to develop resistance against one or more antibiotics simultaneously. MRSA can become resistant to non-β-lactam antibiotics through different mechanisms including modification of the antibiotic target, enzymatic inactivation of antibiotics, and/or decreased antibiotic uptake or efflux. This is mainly directed by the acquisition of resistant genes by HGT and genetic mutations owing to the selective pressure of antibiotics. Therefore, revealing molecular determinants that confer resistance to antibiotics in clinical isolates as well as laboratory strains is important for the development of molecular detection methods of antibiotic resistance and designing novel strategies to control MRSA infections.
Antibiotic combination therapy is currently used for treating some MRSA infections, such as ceftaroline plus daptomycin for refractory bacteremia and daptomycin plus rifampicin for biofilm-related infections. However, the continuous emergence of antibiotic-resistant bacteria has highlighted the need for the development of new antibiotics and the identification of novel drug targets to tackle AMR and optimal management of MRSA infections.
Author Contributions
Conceptualization, H.L., H.-S.J. and J.-S.K.; writing—original draft preparation, H.L.; writing—review and editing, H.L., H.-S.J. and J.-S.K.; funding acquisition, J.-S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Bio and Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government, Ministry of Science and ICT (MSIT) 2017M3A9E4077232. This study was also supported by the Hallym University research fund.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fleming A. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. injluenzae. Br. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. [DOI] [Google Scholar]
- 2.Abraham E.P., Chain E. An enzyme from bacteria able to destroy penicillin. Nature. 1940;146:837. doi: 10.1038/146837a0. [DOI] [PubMed] [Google Scholar]
- 3.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Pendleton J.N., Gorman S.P., Gilmore B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti. Infect. Ther. 2013;11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 6.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Antimicrobial Resistance. WHO; Geneva, Switzerlnad: 2021. [Google Scholar]
- 8.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. Wellcome Trust; London, UK: 2016. [Google Scholar]
- 9.Centers for Disease Control and Prevention (U.S.) Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); Atlanta, GA, USA: 2019. [Google Scholar]
- 10.Centers for Disease Control and Prevention (U.S.) Methicillin-Resistant Staphylococcus Aureus, 2019. Centers for Disease Control and Prevention (U.S.); Atlanta, GA, USA: 2019. [Google Scholar]
- 11.Rammelkamp C.H., Maxon T. Resistance of Staphylococcus aureus to the Action of Penicillin. Exp. Biol. Med. 1942;51:386–389. doi: 10.3181/00379727-51-13986. [DOI] [Google Scholar]
- 12.Kirby W.M.M. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 13.Knox R. A New Penicillin (BRL 1241) Active Against Penicillin-resistant. Staphylococci. BMJ. 1960;2:690–693. doi: 10.1136/bmj.2.5200.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jevons M.P. “Celbenin”—Resistant. Staphylococci. Br. Med. J. 1961;1:124. doi: 10.1136/bmj.1.5219.124-a. [DOI] [Google Scholar]
- 15.Turner N.A., Sharma-Kuinkel B.K., Maskarinec S.A., Eichenberger E.M., Shah P.P., Carugati M., Holland T.L., Fowler V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenover F.C., McDougal L.K., Goering R.V., Killgore G., Projan S.J., Patel J.B., Dunman P.M. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J. Clin. Microbiol. 2006;44:108–118. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.David M.Z., Daum R.S. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakhundi S., Zhang K. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin. Microbiol. Rev. 2018;31:e00020-18. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers H.F., DeLeo F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuhashi M., Song M.D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J. Bacteriol. 1986;167:975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katayama Y., Ito T., Hiramatsu K. A New Class of Genetic Element, Staphylococcus Cassette Chromosome mec, Encodes Methicillin Resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2000;44:1549–1555. doi: 10.1128/AAC.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartman B.J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1985;28:397–403. doi: 10.1128/AAC.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuda C., Suvorov M., Vakulenko S.B., Mobashery S. The Basis for Resistance to β-Lactam Antibiotics by Penicillin-binding Protein 2a of Methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004;279:40802–40806. doi: 10.1074/jbc.M403589200. [DOI] [PubMed] [Google Scholar]
- 25.Watkins R.R., Holubar M., David M.Z. Antimicrobial resistance in methicillin-resistant Staphylococcus aureus to newer antimicrobial agents. Antimicrob. Agents Chemother. 2019;63:e01216-19. doi: 10.1128/AAC.01216-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lade H., Kim J.-S. Bacterial Targets of Antibiotics in Methicillin-Resistant Staphylococcus aureus. Antibiotics. 2021;10:398. doi: 10.3390/antibiotics10040398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown N.M., Goodman A.L., Horner C., Jenkins A., Brown E.M. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC Antimicrob. Resist. 2021;3:dlaa114. doi: 10.1093/jacamr/dlaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiramatsu K. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J., Kaplan S.L., Karchmer A.W., Levine D.P., Murray B.E., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 30.McGehee R.F.R., Barre F.F., Finland M. Resistance of Staphylococcus aureus to lincomycin, clinimycin, and erythromycin. Antimicrob. Agents Chemother. 1968;8:392–397. [PubMed] [Google Scholar]
- 31.Roberts M.C., Sutcliffe J., Courvalin P., Jensen L.B., Rood J., Seppala H. Nomenclature for Macrolide and Macrolide-Lincosamide-Streptogramin B Resistance Determinants. Antimicrob. Agents Chemother. 1999;43:2823–2830. doi: 10.1128/AAC.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz F.-J., Jones M.E., Hofmann B., Hansen B., Scheuring S., Lückefahr M., Fluit A., Verhoef J., Hadding U., Heinz H.-P., et al. Characterization of grlA, grlB, gyrA, and gyrB Mutations in 116 Unrelated Isolates of Staphylococcus aureus and Effects of Mutations on Ciprofloxacin MIC. Antimicrob. Agents Chemother. 1998;42:1249–1252. doi: 10.1128/AAC.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrero L., Cameron B., Crouzet J. Analysis of gyrA and grlA mutations in stepwise-selected ciprofloxacin-resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 1995;39:1554–1558. doi: 10.1128/AAC.39.7.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng E.Y., Trucksis M., Hooper D.C. Quinolone resistance mutations in topoisomerase IV: Relationship to the flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob. Agents Chemother. 1996;40:1881–1888. doi: 10.1128/AAC.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaffe H.W., Sweeney H.M., Weinstein R.A., Kabins S.A., Nathan C., Cohen S. Structural and phenotypic varieties of gentamicin resistance plasmids in hospital strains of Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob. Agents Chemother. 1982;21:773–779. doi: 10.1128/AAC.21.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ubukata K., Yamashita N., Gotoh A., Konno M. Purification and characterization of aminoglycoside-modifying enzymes from Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1984;25:754–759. doi: 10.1128/AAC.25.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasanvand A., Ghafourian S., Taherikalani M., Jalilian F., Sadeghifard N., Pakzad I. Antiseptic Resistance in Methicillin Sensitive and Methicillin Resistant Staphylococcus aureus Isolates from Some Major Hospitals, Iran. Recent Pat. Antiinfect. Drug Discov. 2015;10:105–112. doi: 10.2174/1574891X10666150623093259. [DOI] [PubMed] [Google Scholar]
- 39.Taheri N., Ardebili A., Amouzandeh-Nobaveh A., Ghaznavi-Rad E. Frequency of Antiseptic Resistance Among Staphylococcus aureus and Coagulase-Negative Staphylococci Isolated From a University Hospital in Central Iran. Oman Med. J. 2016;31:426–432. doi: 10.5001/omj.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mlynarczyk-Bonikowska B., Kowalewski C., Krolak-Ulinska A., Marusza W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022;23:8088. doi: 10.3390/ijms23158088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai K., Stojković V., Noda-Garcia L., Young I.D., Myasnikov A.G., Kleinman J., Palla A., Floor S.N., Frost A., Fraser J.S., et al. Directed evolution of the rRNA methylating enzyme Cfr reveals molecular basis of antibiotic resistance. Elife. 2022;11:e70017. doi: 10.7554/eLife.70017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kehrenberg C., Schwarz S., Jacobsen L., Hansen L.H., Vester B. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: Methylation of 23S ribosomal RNA at A2503. Mol. Microbiol. 2005;57:1064–1073. doi: 10.1111/j.1365-2958.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- 43.Aubry-Damon H., Soussy C.J., Courvalin P. Characterization of mutations in the rpoB gene that confer rifampin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1998;42:2590–2594. doi: 10.1128/AAC.42.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chopra I., Roberts M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts M.C. Tetracycline resistance determinants: Mechanisms of action, regulation of expression, genetic mobility, and distribution. FEMS Microbiol. Rev. 1996;19:1–24. doi: 10.1111/j.1574-6976.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 46.Ferrero L., Cameron B., Manse B., Lagneaux D., Crouzet J., Famechon A., Blanche F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: A primary target of fluoroquinolones. Mol. Microbiol. 1994;13:641–653. doi: 10.1111/j.1365-2958.1994.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 47.Ito H., Yoshida H., Bogaki-Shonai M., Niga T., Hattori H., Nakamura S. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob. Agents Chemother. 1994;38:2014–2023. doi: 10.1128/AAC.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hiramatsu K., Igarashi M., Morimoto Y., Baba T., Umekita M., Akamatsu Y. Curing bacteria of antibiotic resistance: Reverse antibiotics, a novel class of antibiotics in nature. Int. J. Antimicrob. Agents. 2012;39:478–485. doi: 10.1016/j.ijantimicag.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Yun H.-J., Min Y.-H., Jo Y.W., Shim M.-J., Choi E.-C. Increased antibacterial activity of DW286, a novel fluoronaphthyridone antibiotic, against Staphylococcus aureus strains with defined mutations in DNA gyrase and topoisomerase IV. Int. J. Antimicrob. Agents. 2005;25:334–337. doi: 10.1016/j.ijantimicag.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 50.Shaw K.J., Rather P.N., Hare R.S., Miller G.H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol. Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez M.S., Tolmasky M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010;13:151–171. doi: 10.1016/j.drup.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuoka M., Inoue M., Endo Y., Nakajima Y. Characteristic expression of three genes, msr (A), mph (C) and erm (Y), that confer resistance to macrolide antibiotics on Staphylococcus aureus. FEMS Microbiol. Lett. 2003;220:287–293. doi: 10.1016/S0378-1097(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 53.Thompson M.K., Keithly M.E., Goodman M.C., Hammer N.D., Cook P.D., Jagessar K.L., Harp J., Skaar E.P., Armstrong R.N. Structure and function of the genomically encoded fosfomycin resistance enzyme, FosB, from Staphylococcus aureus. Biochemistry. 2014;53:755–765. doi: 10.1021/bi4015852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu Z., Liu Y., Chen C., Guo Y., Ma Y., Yang Y., Hu F., Xu X., Wang M. Characterization of Fosfomycin Resistance Gene, fosB, in Methicillin-Resistant Staphylococcus aureus Isolates. PLoS ONE. 2016;11:e0154829. doi: 10.1371/journal.pone.0154829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kapoor G., Saigal S., Elongavan A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017;33:300. doi: 10.4103/joacp.JOACP_349_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster T.J. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1923–1931. doi: 10.1007/s10096-016-2763-0. [DOI] [PubMed] [Google Scholar]
- 57.Jang S. Multidrug efflux pumps in Staphylococcus aureus and their clinical implications. J. Microbiol. 2016;54:1–8. doi: 10.1007/s12275-016-5159-z. [DOI] [PubMed] [Google Scholar]
- 58.Kaatz G.W., Seo S.M. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1995;39:2650–2655. doi: 10.1128/AAC.39.12.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa S.S., Sobkowiak B., Parreira R., Edgeworth J.D., Viveiros M., Clark T.G., Couto I. Genetic diversity of norA, coding for a main efflux pump of Staphylococcus aureus. Front. Genet. 2019;10:710. doi: 10.3389/fgene.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong S.I., Lee Y.-M., Park K.-H., Ryu B.-H., Hong K.-W., Kim S., Bae I.-G., Cho O.-H. Clinical and Molecular Characteristics of qacA- and qacB-Positive Methicillin-Resistant Staphylococcus aureus Causing Bloodstream Infections. Antimicrob. Agents Chemother. 2019;63:e02157-18. doi: 10.1128/AAC.02157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noguchi N., Suwa J., Narui K., Sasatsu M., Ito T., Hiramatsu K., Song J.H. Susceptibilities to antiseptic agents and distribution of antiseptic-resistance genes qacA/B and smr of methicillin-resistant Staphylococcus aureus isolated in Asia during 1998 and 1999. J. Med. Microbiol. 2005;54:557–565. doi: 10.1099/jmm.0.45902-0. [DOI] [PubMed] [Google Scholar]
- 62.Nakaminami H., Takadama S., Okita M., Sasaki M., Noguchi N. Fast-acting bactericidal activity of olanexidine gluconate against qacA/B-positive methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2019;68:957–960. doi: 10.1099/jmm.0.000979. [DOI] [PubMed] [Google Scholar]
- 63.Guay G.G., Khan S.A., Rothstein D.M. The tet(K) Gene of Plasmid pT181 of Staphylococcus aureus Encodes an Efflux Protein That Contains 14 Transmembrane Helices. Plasmid. 1993;30:163–166. doi: 10.1006/plas.1993.1045. [DOI] [PubMed] [Google Scholar]
- 64.Lee A.S., de Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 65.Wright G.D. Q&A: Antibiotic resistance: Where does it come from and what can we do about it? BMC Biol. 2010;8:123. doi: 10.1186/1741-7007-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGuire J.M., Bunch R.L., Anderson R.C., Boaz H.E., Flynn E.H., Powell H.M., Smith J.W. Ilotycin, a new antibiotic. Antibiot. Chemother. 1952;2:281–283. [PubMed] [Google Scholar]
- 67.MacCabe A.F., Gould J.C. The Epidemiology of an Erythromycin Resistant Staphylococcus. Scott. Med. J. 1956;1:223–226. doi: 10.1177/003693305600100701. [DOI] [PubMed] [Google Scholar]
- 68.Champney W.S., Burdine R. Macrolide antibiotics inhibit 50S ribosomal subunit assembly in Bacillus subtilis and Staphylococcus aureus. Antimicrob. Agents Chemother. 1995;39:2141–2144. doi: 10.1128/AAC.39.9.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinos G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017;174:2967–2983. doi: 10.1111/bph.13936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miklasińska-Majdanik M. Mechanisms of Resistance to Macrolide Antibiotics among Staphylococcus aureus. Antibiotics. 2021;10:1406. doi: 10.3390/antibiotics10111406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwendener S., Donà V., Perreten V. The Novel Macrolide Resistance Genes mef (D), msr (F), and msr (H) Are Present on Resistance Islands in Macrococcus canis, Macrococcus caseolyticus, and Staphylococcus aureus. Antimicrob. Agents Chemother. 2020;64:e00160-20. doi: 10.1128/AAC.00160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ross J.I., Eady E.A., Cove J.H., Cunliffe W.J., Baumberg S., Wootton J.C. Inducible erythromycin resistance in staphlyococci is encoded by a member of the ATP-binding transport super-gene family. Mol. Microbiol. 1990;4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 73.Feßler A.T., Wang Y., Wu C., Schwarz S. Mobile macrolide resistance genes in staphylococci. Plasmid. 2018;99:2–10. doi: 10.1016/j.plasmid.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Leclercq R. Mechanisms of Resistance to Macrolides and Lincosamides: Nature of the Resistance Elements and Their Clinical Implications. Clin. Infect. Dis. 2002;34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 75.Spížek J., Řezanka T. Lincosamides: Chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharmacol. 2017;133:20–28. doi: 10.1016/j.bcp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Wang H., Zhuang H., Ji S., Sun L., Zhao F., Wu D., Shen P., Jiang Y., Yu Y., Chen Y. Distribution of erm genes among MRSA isolates with resistance to clindamycin in a Chinese teaching hospital. Infect. Genet. Evol. 2021;96:105127. doi: 10.1016/j.meegid.2021.105127. [DOI] [PubMed] [Google Scholar]
- 77.Blair J.M.A., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 78.Soussy C.J., Bouanchaud D.H., Fouace J., Dublanchet A., Duval J. A gentamycin resistance plasmid in Staphylococcus aureus. Ann. Microbiol. 1975;126:91–94. [PubMed] [Google Scholar]
- 79.Porthouse A., Brown D.F., Smith R.G., Rogers T. Gentamicin resistance in Staphylococcus aureus. Lancet. 1976;307:20–21. doi: 10.1016/S0140-6736(76)92912-3. [DOI] [PubMed] [Google Scholar]
- 80.Schluenzen F., Tocilj A., Zarivach R., Harms J., Gluehmann M., Janell D., Bashan A., Bartels H., Agmon I., Franceschi F., et al. Structure of Functionally Activated Small Ribosomal Subunit at 3.3 Å Resolution. Cell. 2000;102:615–623. doi: 10.1016/S0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 81.Wimberly B.T., Brodersen D.E., Clemons W.M., Morgan-Warren R.J., Carter A.P., Vonrhein C., Hartsch T., Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 82.Carter A.P., Clemons W.M., Brodersen D.E., Morgan-Warren R.J., Wimberly B.T., Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 83.Magnet S., Blanchard J.S. Molecular Insights into Aminoglycoside Action and Resistance. Chem. Rev. 2005;105:477–498. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 84.Kotra L.P., Haddad J., Mobashery S. Aminoglycosides: Perspectives on Mechanisms of Action and Resistance and Strategies to Counter Resistance. Antimicrob. Agents Chemother. 2000;44:3249–3256. doi: 10.1128/AAC.44.12.3249-3256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rouch D.A., Byrne M.E., Kong Y.C., Skurray R.A. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: Expression and nucleotide sequence analysis. J. Gen. Microbiol. 1987;133:3039–3052. doi: 10.1099/00221287-133-11-3039. [DOI] [PubMed] [Google Scholar]
- 86.Ida T., Okamoto R., Shimauchi C., Okubo T., Kuga A., Inoue M. Identification of aminoglycoside-modifying enzymes by susceptibility testing: Epidemiology of methicillin-resistant Staphylococcus aureus in Japan. J. Clin. Microbiol. 2001;39:3115–3121. doi: 10.1128/JCM.39.9.3115-3121.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inoue M., Nonoyama M., Okamoto R.I.T. Antimicrobial activity of arbekacin, a new aminoglycoside antibiotic, against methicillin-resistant Staphylococcus aureus—PubMed. Drugs Exp. Clin. Res. 1994;20:233–239. [PubMed] [Google Scholar]
- 88.Tsuchizaki N., Ishino K., Saito F., Ishikawa J., Nakajima M., Hotta K. Trends of Arbekacin-resistant MRSA Strains in Japanese Hospitals (1979 to 2000) J. Antibiot. 2006;59:229–233. doi: 10.1038/ja.2006.32. [DOI] [PubMed] [Google Scholar]
- 89.Tanaka N., Matsunaga K., Hirata A., Matsuhisa Y., Nishimura T. Mechanism of action of habekacin, a novel amino acid-containing aminoglycoside antibiotic. Antimicrob. Agents Chemother. 1983;24:797–802. doi: 10.1128/AAC.24.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishino K., Ishikawa J., Ikeda Y., Hotta K. Characterization of a Bifunctional Aminoglycoside-Modifying Enzyme with Novel Substrate Specificity and Its Gene from a Clinical Isolate of Methicillin-Resistant Staphylococcus aureus with High Arbekacin Resistance. J. Antibiot. 2004;57:679–686. doi: 10.7164/antibiotics.57.679. [DOI] [PubMed] [Google Scholar]
- 91.Ida T., Okamoto R., Nonoyama M., Irinoda K., Kurazono M., Inoue M. Antagonism between Aminoglycosides and β-Lactams in a Methicillin-Resistant Staphylococcus aureus Isolate Involves Induction of an Aminoglycoside-Modifying Enzyme. Antimicrob. Agents Chemother. 2002;46:1516–1521. doi: 10.1128/AAC.46.5.1516-1521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kondo S., Tamura A., Gomi S., Ikeda Y., Takeuchi T., Mitsuhashi S. Structures of enzymatically modified products of arbekacin by methicillin-resistant Staphylococcus aureus. J. Antibiot. 1993;46:310–315. doi: 10.7164/antibiotics.46.310. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto T. Arbekacin: Another novel agent for treating infections due to methicillin-resistant Staphylococcus aureus and multidrug-resistant Gram-negative pathogens. Clin. Pharmacol. Adv. Appl. 2014;6:139. doi: 10.2147/CPAA.S44377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Centers for Disease Control and Prevention (CDC) Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb. Mortal Wkly. Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- 95.Reynolds P.E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 96.Parenti F. Structure and mechanism of action of teicoplanin. J. Hosp. Infect. 1986;7:79–83. doi: 10.1016/0195-6701(86)90011-3. [DOI] [PubMed] [Google Scholar]
- 97.McGuinness W.A., Malachowa N., DeLeo F.R. The Year in Infection. Volume 90. CRC Press; Boca Raton, FL, USA: 2005. Vancomycin resistance in Staphylococcus aureus; pp. 133–152. [Google Scholar]
- 98.Chang S., Sievert D.M., Hageman J.C., Boulton M.L., Tenover F.C., Downes F.P., Shah S., Rudrik J.T., Pupp G.R., Brown W.J., et al. Infection with Vancomycin-Resistant Staphylococcus aureus Containing the vanA Resistance Gene. N. Engl. J. Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 99.Cui L., Isii T., Fukuda M., Ochiai T., Neoh H.M., Da Cunha Camargo I.L.B., Watanabe Y., Shoji M., Hishinuma T., Hiramatsu K. An RpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2010;54:5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watanabe Y., Cui L., Katayama Y., Kozue K., Hiramatsu K. Impact of rpoB mutations on reduced vancomycin susceptibility in Staphylococcus aureus. J. Clin. Microbiol. 2011;49:2680–2684. doi: 10.1128/JCM.02144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamaguchi T., Suzuki S., Okamura S., Miura Y., Tsukimori A., Nakamura I., Ito N., Masuya A., Shiina T., Matsumoto T. Evolution and single-nucleotide polymorphisms in methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to vancomycin and daptomycin, based on determination of the complete genome. Antimicrob. Agents Chemother. 2015;59:3585–3587. doi: 10.1128/AAC.05159-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gardete S., Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Investig. 2014;124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arthur M., Courvalin P. Genetics and mechanisms of glycopeptide resistance in Enterococci. Antimicrob. Agents Chemother. 1993;37:1563–1571. doi: 10.1128/AAC.37.8.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peng H., Hu Q., Shang W., Yuan J., Zhang X., Liu H., Zheng Y., Hu Z., Yang Y., Tan L., et al. WalK(S221P), a naturally occurring mutation, confers vancomycin resistance in VISA strain XN108. J. Antimicrob. Chemother. 2016;72:dkw518. doi: 10.1093/jac/dkw518. [DOI] [PubMed] [Google Scholar]
- 105.Hu J., Zhang X., Liu X., Chen C., Suna B. Mechanism of reduced vancomycin susceptibility conferred by walK mutation in community-acquired methicillin-resistant Staphylococcus aureus strain mw2. Antimicrob. Agents Chemother. 2015;59:1352–1355. doi: 10.1128/AAC.04290-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howden B.P., McEvoy C.R.E., Allen D.L., Chua K., Gao W., Harrison P.F., Bell J., Coombs G., Bennett-Wood V., Porter J.L., et al. Evolution of Multidrug Resistance during Staphylococcus aureus Infection Involves Mutation of the Essential Two Component Regulator WalKR. PLoS Pathog. 2011;7:e1002359. doi: 10.1371/journal.ppat.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hafer C., Lin Y., Kornblum J., Lowy F.D., Uhlemann A.C. Contribution of selected gene mutations to resistance in clinical isolates of vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2012;56:5845–5851. doi: 10.1128/AAC.01139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gardete S., Kim C., Hartmann B.M., Mwangi M., Roux C.M., Dunman P.M., Chambers H.F., Tomasz A. Genetic Pathway in Acquisition and Loss of Vancomycin Resistance in a Methicillin Resistant Staphylococcus aureus (MRSA) Strain of Clonal Type USA300. PLoS Pathog. 2012;8:e1002505. doi: 10.1371/journal.ppat.1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doddangoudar V.C., O’Donoghue M.M., Chong E.Y.C., Tsang D.N.C., Boost M.V. Role of stop codons in development and loss of vancomycin non-susceptibility in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2012;67:2101–2106. doi: 10.1093/jac/dks171. [DOI] [PubMed] [Google Scholar]
- 110.Cui L., Neoh H.M., Shoji M., Hiramatsu K. Contribution of vraSR and graSR point mutations to vancomycin resistance in vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2009;53:1231–1234. doi: 10.1128/AAC.01173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Neoh H.M., Cui L., Yuzawa H., Takeuchi F., Matsuo M., Hiramatsu K. Mutated response regulator graR is responsible for phenotypic conversion of Staphylococcus aureus from heterogeneous vancomycin-intermediate resistance to vancomycin-intermediate resistance. Antimicrob. Agents Chemother. 2008;52:45–53. doi: 10.1128/AAC.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howden B.P., Stinear T.P., Allen D.L., Johnson P.D.R., Ward P.B., Davies J.K. Genomic analysis reveals a point mutation in the two-component sensor gene graS that leads to intermediate vancomycin resistance in clinical Staphylococcus aureus. Antimicrob. Agents Chemother. 2008;52:3755–3762. doi: 10.1128/AAC.01613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shoji M., Cui L., Iizuka R., Komoto A., Neoh H.M., Watanabe Y., Hishinuma T., Hiramatsu K. walK and clpP mutations confer reduced vancomycin susceptibility in Staphylococcus aureus. Antimicrob. Agents Chemother. 2011;55:3870–3881. doi: 10.1128/AAC.01563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ito T., Okuma K., Ma X.X., Yuzawa H., Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist. Update. 2003;6:41–52. doi: 10.1016/S1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 115.Glupczynski Y., Lagast H., Van der Auwera P., Thys J.P., Crokaert F., Yourassowsky E., Meunier-Carpentier F., Klastersky J., Kains J.P., Serruys-Schoutens E. Clinical evaluation of teicoplanin for therapy of severe infections caused by gram-positive bacteria. Antimicrob. Agents Chemother. 1986;29:52–57. doi: 10.1128/AAC.29.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Manuel R.J. Detection of teicoplanin resistance in UK EMRSA-17 strains. J. Antimicrob. Chemother. 2002;50:1089–1090. doi: 10.1093/jac/dkf213. [DOI] [PubMed] [Google Scholar]
- 117.Bakthavatchalam Y.D., Babu P., Munusamy E., Dwarakanathan H.T., Rupali P., Zervos M., Victor P.J., Veeraraghavan B. Genomic insights on heterogeneous resistance to vancomycin and teicoplanin in Methicillin-resistant Staphylococcus aureus: A first report from South India. PLoS ONE. 2019;14:e0227009. doi: 10.1371/journal.pone.0227009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brandenberger M., Tschierske M., Giachino P., Wada A., Berger-Bächi B. Inactivation of a novel three-cistronic operon tcaR-tcaA-tcaB increases teicoplanin resistance in Staphylococcus aureus. Biochim. Biophys. Acta Gen. Subj. 2000;1523:135–139. doi: 10.1016/S0304-4165(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 119.Maki H., McCallum N., Bischoff M., Wada A., Berger-Bächi B. tcaA inactivation increases glycopeptide resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004;48:1953–1959. doi: 10.1128/AAC.48.6.1953-1959.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moellering R.C. Linezolid: The first oxazolidinone antimicrobial. Ann. Intern. Med. 2003;138:135–142. doi: 10.7326/0003-4819-138-2-200301210-00015. [DOI] [PubMed] [Google Scholar]
- 121.Tsiodras S., Gold H.S., Sakoulas G., Eliopoulos G.M., Wennersten C., Venkataraman L., Moellering R.C., Ferraro M.J. Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet. 2001;358:207–208. doi: 10.1016/S0140-6736(01)05410-1. [DOI] [PubMed] [Google Scholar]
- 122.Swaney S.M., Aoki H., Ganoza M.C., Shinabarger D.L. The oxazolidinone linezolid inhibits initiation of protein synthesis in bacteria. Antimicrob. Agents Chemother. 1998;42:3251–3255. doi: 10.1128/AAC.42.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arias C.A., Vallejo M., Reyes J., Panesso D., Moreno J., Castañeda E., Villegas M.V., Murray B.E., Quinn J.P. Clinical and microbiological aspects of linezolid resistance mediated by the cfr gene encoding a 23S rRNA methyltransferase. J. Clin. Microbiol. 2008;46:892–896. doi: 10.1128/JCM.01886-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Endimiani A., Blackford M., Dasenbrook E.C., Reed M.D., Bajaksouszian S., Hujer A.M., Rudin S.D., Hujer K.M., Perreten V., Rice L.B., et al. Emergence of linezolid-resistant Staphylococcus aureus after prolonged treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob. Agents Chemother. 2011;55:1684–1692. doi: 10.1128/AAC.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iguchi S., Mizutani T., Hiramatsu K., Kikuchi K. Rapid Acquisition of Linezolid Resistance in Methicillin-Resistant Staphylococcus aureus: Role of Hypermutation and Homologous Recombination. PLoS ONE. 2016;11:e0155512. doi: 10.1371/journal.pone.0155512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Toh S.-M., Xiong L., Arias C.A., Villegas M.V., Lolans K., Quinn J., Mankin A.S. Acquisition of a natural resistance gene renders a clinical strain of methicillin-resistant Staphylococcus aureus resistant to the synthetic antibiotic linezolid. Mol. Microbiol. 2007;64:1506–1514. doi: 10.1111/j.1365-2958.2007.05744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Meka V.G., Pillai S.K., Sakoulas G., Wennersten C., Venkataraman L., DeGirolami P.C., Eliopoulos G.M., Moellering R.C., Jr., Gold H.S. Linezolid Resistance in Sequential Staphylococcus aureus Isolates Associated with a T2500A Mutation in the 23S rRNA Gene and Loss of a Single Copy of rRNA. J. Infect. Dis. 2004;190:311–317. doi: 10.1086/421471. [DOI] [PubMed] [Google Scholar]
- 128.Locke J.B., Hilgers M., Shaw K.J. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob. Agents Chemother. 2009;53:5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhanel G.G., Love R., Adam H., Golden A., Zelenitsky S., Schweizer F., Gorityala B., Lagacé-Wiens P.R.S., Rubinstein E., Walkty A., et al. Tedizolid: A Novel Oxazolidinone with Potent Activity Against Multidrug-Resistant Gram-Positive Pathogens. Drugs. 2015;75:253–270. doi: 10.1007/s40265-015-0352-7. [DOI] [PubMed] [Google Scholar]
- 130.Kisgen J.J., Mansour H., Unger N.R., Childs L.M. Tedizolid: A new oxazolidinone antimicrobial. Am. J. Heal. Pharm. 2014;71:621–633. doi: 10.2146/ajhp130482. [DOI] [PubMed] [Google Scholar]
- 131.Shen T., Penewit K., Waalkes A., Xu L., Salipante S.J., Nath A., Werth B.J. Identification of a novel tedizolid resistance mutation in rpoB of MRSA after in vitro serial passage. J. Antimicrob. Chemother. 2021;76:292–296. doi: 10.1093/jac/dkaa422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Freitas A.R., Dilek A.R., Peixe L., Novais C. Dissemination of Staphylococcus epidermidis ST22 With Stable, High-Level Resistance to Linezolid and Tedizolid in the Greek-Turkish Region (2008–2016) Infect. Control Hosp. Epidemiol. 2018;39:492–494. doi: 10.1017/ice.2018.5. [DOI] [PubMed] [Google Scholar]
- 133.Hoy S.M. Contezolid: First Approval. Drugs. 2021;81:1587–1591. doi: 10.1007/s40265-021-01576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang S., Cai C., Shen Y., Sun C., Shi Q., Wu N., Zheng S., Qian J., Zhang R., Zhou H. In vitro Activity of Contezolid Against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococcus, and Strains With Linezolid Resistance Genes From China. Front. Microbiol. 2021;12:2408. doi: 10.3389/fmicb.2021.729900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mangili A., Bica I., Snydman D.R., Hamer D.H. Daptomycin-Resistant, Methicillin-Resistant Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2005;40:1058–1060. doi: 10.1086/428616. [DOI] [PubMed] [Google Scholar]
- 136.Bayer A.S., Schneider T., Sahl H.G. Mechanisms of daptomycin resistance in Staphylococcus aureus: Role of the cell membrane and cell wall. Ann. N. Y. Acad. Sci. 2013;1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Müller A., Wenzel M., Strahl H., Grein F., Saaki T.N.V., Kohl B., Siersma T., Bandow J.E., Sahl H.G., Schneider T., et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA. 2016;113:E7077–E7086. doi: 10.1073/pnas.1611173113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pogliano J., Pogliano N., Silverman J.A. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J. Bacteriol. 2012;194:4494–4504. doi: 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang S., Kreiswirth B.N., Sakoulas G., Yeaman M.R., Xiong Y.Q., Sawa A., Bayer A.S. Enhanced Expression of dltABCD Is Associated with the Development of Daptomycin Nonsusceptibility in a Clinical Endocarditis Isolate of Staphylococcus aureus. J. Infect. Dis. 2009;200:1916–1920. doi: 10.1086/648473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cafiso V., Bertuccio T., Purrello S., Campanile F., Mammina C., Sartor A., Raglio A., Stefani S. dltA overexpression: A strain-independent keystone of daptomycin resistance in methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2014;43:26–31. doi: 10.1016/j.ijantimicag.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Sulaiman J.E., Wu L., Lam H. Mutation in the Two-Component System Regulator YycH Leads to Daptomycin Tolerance in Methicillin-Resistant Staphylococcus aureus upon Evolution with a Population Bottleneck. Microbiol. Spectr. 2022;10:e01687-22. doi: 10.1128/spectrum.01687-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tran T.T., Munita J.M., Arias C.A. Mechanisms of drug resistance: Daptomycin resistance. Ann. N. Y. Acad. Sci. 2015;1354:32–53. doi: 10.1111/nyas.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mehta S., Cuirolo A.X., Plata K.B., Riosa S., Silverman J.A., Rubio A., Rosato R.R., Rosato A.E. VraSR two-component regulatory system contributes to mprF-mediated decreased susceptibility to daptomycin in in vivo-selected clinical strains of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2012;56:92–102. doi: 10.1128/AAC.00432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Miller W.R., Bayer A.S., Arias C.A. Mechanism of Action and Resistance to Daptomycin in Staphylococcus aureus and Enterococci. Cold Spring Harb. Perspect. Med. 2016;6:a026997. doi: 10.1101/cshperspect.a026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. CLSI supplement M100. [Google Scholar]
- 146.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. CLSI supplement M100. [Google Scholar]
- 147.Leadbetter M.R., Adams S.M., Bazzini B., Fatheree P.R., Karr D.E., Krause K.M., Lam B.M.T., Linsell M.S., Nodwell M.B., Pace J.L., et al. Hydrophobic Vancomycin Derivatives with Improved ADME Properties: Discovery of Telavancin (TD-6424) J. Antibiot. 2004;57:326–336. doi: 10.7164/antibiotics.57.326. [DOI] [PubMed] [Google Scholar]
- 148.Hindy J., Haddad S.F., Kanj S.S. New drugs for methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Curr. Opin. Infect. Dis. 2021;35:1–8. doi: 10.1097/QCO.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 149.Chaftari A.M., Hachem R., Jordan M., Garoge K., Al Hamal Z., El Zakhem A., Viola G.M., Granwehr B., Mulanovich V., Gagel A., et al. Case-control study of telavancin as an alternative treatment for gram-positive bloodstream infections in patients with cancer. Antimicrob. Agents Chemother. 2016;60:239–244. doi: 10.1128/AAC.00617-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bassetti M., Mikulska M., Righi E., Nicolini L., Viscoli C. The role of telavancin in the treatment of MRSA infections in hospital. Expert Opin. Investig. Drugs. 2009;18:521–529. doi: 10.1517/13543780902845630. [DOI] [PubMed] [Google Scholar]
- 151.Karlowsky J.A., Nichol K., Zhanel G.G. Telavancin: Mechanisms of Action, In Vitro Activity, and Mechanisms of Resistance. Clin. Infect. Dis. 2015;61:S58–S68. doi: 10.1093/cid/civ534. [DOI] [PubMed] [Google Scholar]
- 152.Duggar B.M. Aureomycin: A product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 1948;51:177–181. doi: 10.1111/j.1749-6632.1948.tb27262.x. [DOI] [PubMed] [Google Scholar]
- 153.Gale E.F., Folkes J.P. The assimilation of amino-acids by bacteria. 15. Actions of antibiotics on nucleic acid and protein synthesis in Staphylococcus aureus. Biochem. J. 1953;53:493–498. doi: 10.1042/bj0530493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Epe B., Woolley P. The binding of 6-demethylchlortetracycline to 70S, 50S and 30S ribosomal particles: A quantitative study by fluorescence anisotropy. EMBO J. 1984;3:121–126. doi: 10.1002/j.1460-2075.1984.tb01771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schwarz S., Roberts M.C., Werckenthin C., Pang Y., Lange C. Tetracycline resistance in Staphylococcus spp. from domestic animals. Vet. Microbiol. 1998;63:217–227. doi: 10.1016/S0378-1135(98)00234-X. [DOI] [PubMed] [Google Scholar]
- 156.Stephens C.R., Beereboom J.J., Rennhard H.H., Gordon P.N., Murai K., Blackwood R.K., von Wittenau M.S. 6-Deoxytetracyclines. IV. 1,2 Preparation, C-6 Stereochemistry, and Reactions. J. Am. Chem. Soc. 1963;85:2643–2652. doi: 10.1021/ja00900a027. [DOI] [Google Scholar]
- 157.Nelson M.L., Levy S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011;1241:17–32. doi: 10.1111/j.1749-6632.2011.06354.x. [DOI] [PubMed] [Google Scholar]
- 158.Schwartz B.S., Graber C.J., Diep B.A., Basuino L., Perdreau-Remington F., Chambers H.F. doxycycline, not minocycline, induces its own resistance in multidrug-resistant, community-associated methicillin-Resistant Staphylococcus aureus clone usa300. Clin. Infect. Dis. 2009;48:1483–1484. doi: 10.1086/598510. [DOI] [PubMed] [Google Scholar]
- 159.Trzcinski K., Cooper B.S., Hryniewicz W., Dowson C.G. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000;45:763–770. doi: 10.1093/jac/45.6.763. [DOI] [PubMed] [Google Scholar]
- 160.Vaudaux P., Fleury B., Gjinovci A., Huggler E., Tangomo-Bento M., Lew D.P. Comparison of tigecycline and vancomycin for treatment of experimental foreign-body infection due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009;53:3150–3152. doi: 10.1128/AAC.01612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Draghi D.C., Tench S., Dowzicky M.J., Sahm D.F. Baseline in vitro Activity of Tigecycline among Key Bacterial Pathogens Exhibiting Multidrug Resistance. Chemotherapy. 2008;54:91–100. doi: 10.1159/000118660. [DOI] [PubMed] [Google Scholar]
- 162.Nguyen F., Starosta A.L., Arenz S., Sohmen D., Dönhöfer A., Wilson D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014;395:559–575. doi: 10.1515/hsz-2013-0292. [DOI] [PubMed] [Google Scholar]
- 163.Honeyman L., Ismail M., Nelson M.L., Bhatia B., Bowser T.E., Chen J., Mechiche R., Ohemeng K., Verma A.K., Cannon E.P., et al. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob. Agents Chemother. 2015;59:7044–7053. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.O’Riordan W., Green S., Overcash J.S., Puljiz I., Metallidis S., Gardovskis J., Garrity-Ryan L., Das A.F., Tzanis E., Eckburg P.B., et al. Omadacycline for Acute Bacterial Skin and Skin-Structure Infections. N. Engl. J. Med. 2019;380:528–538. doi: 10.1056/NEJMoa1800170. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka S.K., Steenbergen J., Villano S. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg. Med. Chem. 2016;24:6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 166.Draper M.P., Weir S., Macone A., Donatelli J., Trieber C.A., Tanaka S.K., Levya S.B. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob. Agents Chemother. 2014;58:1279–1283. doi: 10.1128/AAC.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Macone A.B., Caruso B.K., Leahy R.G., Donatelli J., Weir S., Draper M.P., Tanaka S.K., Levy S.B. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob. Agents Chemother. 2014;58:1127–1135. doi: 10.1128/AAC.01242-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bodley J.W., Zieve F.J., Lin L., Zieve S.T. Formation of the ribosome-G factor-GDP complex in the presence of fusidic acid. Biochem. Biophys. Res. Commun. 1969;37:437–443. doi: 10.1016/0006-291X(69)90934-6. [DOI] [PubMed] [Google Scholar]
- 169.Tanaka N., Kinoshita T., Masukawa H. Mechanism of protein synthesis inhibition by fusidic acid and related antibiotics. Biochem. Biophys. Res. Commun. 1968;30:278–283. doi: 10.1016/0006-291X(68)90447-6. [DOI] [PubMed] [Google Scholar]
- 170.Nagaev I., Bjorkman J., Andersson D.I., Hughes D. Biological cost and compensatory evolution in fusidic acid-resistant Staphylococcus aureus. Mol. Microbiol. 2001;40:433–439. doi: 10.1046/j.1365-2958.2001.02389.x. [DOI] [PubMed] [Google Scholar]
- 171.O’Neill A.J., Larsen A.R., Henriksen A.S., Chopra I. A fusidic acid-resistant epidemic strain of Staphylococcus aureus carries the fusB determinant, whereas fusA mutations are prevalent in other resistant isolates. Antimicrob. Agents Chemother. 2004;48:3594–3597. doi: 10.1128/AAC.48.9.3594-3597.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.O’Brien F.G. Genetic characterization of the fusidic acid and cadmium resistance determinants of Staphylococcus aureus plasmid pUB101. J. Antimicrob. Chemother. 2002;50:313–321. doi: 10.1093/jac/dkf153. [DOI] [PubMed] [Google Scholar]
- 173.O’Neill A.J., McLaws F., Kahlmeter G., Henriksen A.S., Chopra I. Genetic basis of resistance to fusidic acid in Staphylococci. Antimicrob. Agents Chemother. 2007;51:1737–1740. doi: 10.1128/AAC.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tanus T., Scangarella-Oman N.E., Dalessandro M., Li G., Breton J.J., Tomayko J.F. A Randomized, Double-blind, Comparative Study to Assess the Safety and Efficacy of Topical Retapamulin Ointment 1% Versus Oral Linezolid in the Treatment of Secondarily Infected Traumatic Lesions and Impetigo Due to Methicillin-Resistant Staphylococcus au. Adv. Skin Wound Care. 2014;27:548–559. doi: 10.1097/01.ASW.0000456631.20389.ae. [DOI] [PubMed] [Google Scholar]
- 175.Hooper D.C. Mechanisms of fluoroquinolone resistance. Drug Resist. Updates. 1999;2:38–55. doi: 10.1054/drup.1998.0068. [DOI] [PubMed] [Google Scholar]
- 176.LeBel M. Ciprofloxacin: Chemistry, Mechanism of Action, Resistance, Antimicrobial Spectrum, Pharmacokinetics, Clinical Trials, and Adverse Reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988;8:3–30. doi: 10.1002/j.1875-9114.1988.tb04058.x. [DOI] [PubMed] [Google Scholar]
- 177.Hooper D.C. Mode of Action of Fluoroquinolones. Drugs. 1999;58:6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- 178.Drlica K. Mechanism of fluoroquinolone action. Curr. Opin. Microbiol. 1999;2:504–508. doi: 10.1016/S1369-5274(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 179.Papkou A., Hedge J., Kapel N., Young B., MacLean R.C. Efflux pump activity potentiates the evolution of antibiotic resistance across S. aureus isolates. Nat. Commun. 2020;11:3970. doi: 10.1038/s41467-020-17735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Aeschlimann J.R., Dresser L.D., Kaatz G.W., Rybak M.J. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999;43:335–340. doi: 10.1128/AAC.43.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schmitz F. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 1998;41:481–484. doi: 10.1093/jac/41.4.481. [DOI] [PubMed] [Google Scholar]
- 182.Nilius A.M., Shen L.L., Hensey-Rudloff D., Almer L.S., Beyer J.M., Balli D.J., Cai Y., Flamm R.K. In vitro antibacterial potency and spectrum of ABT-492, a new fluoroquinolone. Antimicrob. Agents Chemother. 2003;47:3260–3269. doi: 10.1128/AAC.47.10.3260-3269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saravolatz L.D., Stein G.E. Delafloxacin: A New Anti–methicillin-resistant Staphylococcus aureus Fluoroquinolone. Clin. Infect. Dis. 2019;68:1058–1062. doi: 10.1093/cid/ciy600. [DOI] [PubMed] [Google Scholar]
- 184.Iregui A., Khan Z., Malik S., Landman D., Quale J. Emergence of Delafloxacin-Resistant Staphylococcus aureus in Brooklyn, New York. Clin. Infect. Dis. 2020;70:1758–1760. doi: 10.1093/cid/ciz787. [DOI] [PubMed] [Google Scholar]
- 185.Remy J.M., Tow-Keogh C.A., McConnell T.S., Dalton J.M., DeVito J.A. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: Resistance selection and characterization. J. Antimicrob. Chemother. 2012;67:2814–2820. doi: 10.1093/jac/dks307. [DOI] [PubMed] [Google Scholar]
- 186.McCurdy S., Lawrence L., Quintas M., Woosley L., Flamm R., Tseng C., Cammarata S. In vitro activity of delafloxacin and microbiological response against fluoroquinolone-susceptible and nonsusceptible Staphylococcus aureus isolates from two phase 3 studies of acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2017;61:2017. doi: 10.1128/AAC.00772-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kurokawa I., Kanayama S., Yamasaki O. Antimicrobial activity of ozenoxacin and other antimicrobials against Staphylococcus aureus strains isolated from clinical skin specimens in Japan in 2019 and 2020. J. Infect. Chemother. 2022;28:1693–1696. doi: 10.1016/j.jiac.2022.08.014. [DOI] [PubMed] [Google Scholar]
- 188.Brogden R.N., Carmine A.A., Heel R.C., Speight T.M., Avery G.S. Trimethoprim: A Review of its Antibacterial Activity, Pharmacokinetics and Therapeutic Use in Urinary Tract Infections. Drugs. 1982;23:405–430. doi: 10.2165/00003495-198223060-00001. [DOI] [PubMed] [Google Scholar]
- 189.Dale G.E., Broger C., D’Arcy A., Hartman P.G., DeHoogt R., Jolidon S., Kompis I., Labhardt A.M., Langen H., Locher H., et al. A single amino acid substitution in Staphylococcus aureus dihydrofolate reductase determines trimethoprim resistance 1 1 Edited by T.Richmond. J. Mol. Biol. 1997;266:23–30. doi: 10.1006/jmbi.1996.0770. [DOI] [PubMed] [Google Scholar]
- 190.Nurjadi D., Olalekan A.O., Layer F., Shittu A.O., Alabi A., Ghebremedhin B., Schaumburg F., Hofmann-Eifler J., Van Genderen P.J.J., Caumes E., et al. Emergence of trimethoprim resistance gene dfrG in Staphylococcus aureus causing human infection and colonization in sub-Saharan Africa and its import to Europe. J. Antimicrob. Chemother. 2014;69:2361–2368. doi: 10.1093/jac/dku174. [DOI] [PubMed] [Google Scholar]
- 191.Perreten V., Kadlec K., Schwarz S., Gronlund Andersson U., Finn M., Greko C., Moodley A., Kania S.A., Frank L.A., Bemis D.A., et al. Clonal spread of methicillin-resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. J. Antimicrob. Chemother. 2010;65:1145–1154. doi: 10.1093/jac/dkq078. [DOI] [PubMed] [Google Scholar]
- 192.Nurjadi D., Schäfer J., Friedrich-Jänicke B., Mueller A., Neumayr A., Calvo-Cano A., Goorhuis A., Molhoek N., Lagler H., Kantele A., et al. Predominance of dfrG as determinant of trimethoprim resistance in imported Staphylococcus aureus. Clin. Microbiol. Infect. 2015;21:1095.e5–1095.e9. doi: 10.1016/j.cmi.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 193.Kadlec K., Schwarz S. Identification of a novel trimethoprim resistance gene, dfrK, in a methicillin-resistant Staphylococcus aureus ST398 strain and its physical linkage to the tetracycline resistance gene tet (L) Antimicrob. Agents Chemother. 2009;53:776–778. doi: 10.1128/AAC.01128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rouch D.A., Messerotti L.J., Loo L.S.L., Jackson C.A., Skurray R.A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol. Microbiol. 1989;3:161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 195.Sekiguchi J.I., Tharavichitkul P., Miyoshi-Akiyama T., Chupia V., Fujino T., Araake M., Irie A., Morita K., Kuratsuji T., Kirikae T. Cloning and characterization of a novel trimethoprim-resistant dihydrofolate reductase from a nosocomial isolate of Staphylococcus aureus CM.S2 (IMCJ1454) Antimicrob. Agents Chemother. 2005;49:3948–3951. doi: 10.1128/AAC.49.9.3948-3951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kadlec K., Schwarz S. Novel ABC Transporter Gene, vga (C), Located on a Multiresistance Plasmid from a Porcine Methicillin-Resistant Staphylococcus aureus ST398 Strain. Antimicrob. Agents Chemother. 2009;53:3589–3591. doi: 10.1128/AAC.00570-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chain E.B., Mellows G. Structure of pseudomonic acid, an antibiotic from Pseudomonas fluorescens. J. Chem. Soc. Chem. Commun. 1974;20:847. doi: 10.1039/c39740000847. [DOI] [PubMed] [Google Scholar]
- 198.Mehtar S. New strategies for the use of mupirocin for the prevention of serious infection. J. Hosp. Infect. 1998;40:S39–S44. doi: 10.1016/S0195-6701(98)90203-1. [DOI] [PubMed] [Google Scholar]
- 199.Rahman M., Noble W.C., Cookson B., Baird D., Coia J. Mupirocin-Resistant Staphylococcus aureus. Lancet. 1987;330:387–388. doi: 10.1016/S0140-6736(87)92398-1. [DOI] [Google Scholar]
- 200.Kavi J., Andrews J.M., Wise R., Smith M.D., Sanghrajka M., Lock S. Mupirocin-Resistant Staphylococcus aureus. Lancet. 1987;330:1472–1473. doi: 10.1016/S0140-6736(87)91179-2. [DOI] [Google Scholar]
- 201.Hughes J., Mellows G. On the mode of action of pseudomonic acid: Inhibition of protein synthesis in Staphylococcus aureus. J. Antibiot. 1978;31:330–335. doi: 10.7164/antibiotics.31.330. [DOI] [PubMed] [Google Scholar]
- 202.Hurdle J.G. The isoleucyl-tRNA synthetase mutation V588F conferring mupirocin resistance in glycopeptide-intermediate Staphylococcus aureus is not associated with a significant fitness burden. J. Antimicrob. Chemother. 2003;53:102–104. doi: 10.1093/jac/dkh020. [DOI] [PubMed] [Google Scholar]
- 203.Antonio M., McFerran N., Pallen M.J. Mutations affecting the Rossman fold of isoleucyl-tRNA synthetase are correlated with low-level mupirocin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2002;46:438–442. doi: 10.1128/AAC.46.2.438-442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hurdle J.G., O’Neill A.J., Ingham E., Fishwick C., Chopra I. Analysis of mupirocin resistance and fitness in Staphylococcus aureus by molecular genetic and structural modeling techniques. Antimicrob. Agents Chemother. 2004;48:4366–4376. doi: 10.1128/AAC.48.11.4366-4376.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fujimura S., Tokue Y., Watanabe A. Isoleucyl-tRNA Synthetase Mutations in Staphylococcus aureus Clinical Isolates and In Vitro Selection of Low-Level Mupirocin-Resistant Strains. Antimicrob. Agents Chemother. 2003;47:3373–3374. doi: 10.1128/AAC.47.10.3373-3374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Udo E.E., Jacob L.E., Mathew B. Genetic analysis of methicillin-resistant Staphylococcus aureus expressing high- and low-level mupirocin resistance. J. Med. Microbiol. 2001;50:909–915. doi: 10.1099/0022-1317-50-10-909. [DOI] [PubMed] [Google Scholar]
- 207.Seah C., Alexander D.C., Louie L., Simor A., Low D.E., Longtin J., Melano R.G. MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob. Agents Chemother. 2012;56:1916–1920. doi: 10.1128/AAC.05325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hodgson J.E., Curnock S.P., Dyke K.G.H., Morris R., Sylvester D.R., Gross M.S. Molecular characterization of the gene encoding high-level mupirocin resistance in Staphylococcus aureus J2870. Antimicrob. Agents Chemother. 1994;38:1205–1208. doi: 10.1128/AAC.38.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. CLSI supplement M100. [Google Scholar]
- 210.Hendlin D., Stapley E.O., Jackson M., Wallick H., Miller A.K., Wolf F.J., Miller T.W., Chaiet L., Kahan F.M., Foltz E.L., et al. Phosphonomycin, a new antibiotic produced by strains of Streptomyces. Science. 1969;166:122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 211.Kahan F.M., Kahan J.S., Cassidy P.J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin) Ann. N. Y. Acad. Sci. 1974;235:364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- 212.Xu S., Fu Z., Zhou Y., Liu Y., Xu X., Wang M. Mutations of the Transporter Proteins GlpT and UhpT Confer Fosfomycin Resistance in Staphylococcus aureus. Front. Microbiol. 2017;8:914. doi: 10.3389/fmicb.2017.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fu Z., Ma Y., Chen C., Guo Y., Hu F., Liu Y., Xu X., Wang M. Prevalence of Fosfomycin Resistance and Mutations in murA, glpT, and uhpT in Methicillin-Resistant Staphylococcus aureus Strains Isolated from Blood and Cerebrospinal Fluid Samples. Front. Microbiol. 2016;6:1544. doi: 10.3389/fmicb.2015.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen T., Zhao L., Liu Y., Wang Y., Jian Y., Zhao N., Yang Z., Wang X., Liu Q., Li M. Mechanisms of high-level fosfomycin resistance in Staphylococcus aureus epidemic lineage ST5. J. Antimicrob. Chemother. 2022;77:2816–2826. doi: 10.1093/jac/dkac236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu W., Chen T., Wang H., Zeng W., Wu Q., Yu K., Xu Y., Zhang X., Zhou T. Molecular Mechanisms and Epidemiology of Fosfomycin Resistance in Staphylococcus aureus Isolated From Patients at a Teaching Hospital in China. Front. Microbiol. 2020;11:1290. doi: 10.3389/fmicb.2020.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Truong-Bolduc Q.C., Wang Y., Hooper D.C. Tet38 Efflux pump contributes to fosfomycin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2018;62:e00927-18. doi: 10.1128/AAC.00927-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen Y., Ji S., Sun L., Wang H., Zhu F., Chen M., Zhuang H., Wang Z., Jiang S., Yu Y., et al. The novel fosfomycin resistance gene fosY is present on a genomic island in CC1 methicillin-resistant Staphylococcus aureus. Emerg. Microbes Infect. 2022;11:1166–1173. doi: 10.1080/22221751.2022.2058421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sensi P. History of the Development of Rifampin. Clin. Infect. Dis. 1983;5:S402–S406. doi: 10.1093/clinids/5.Supplement_3.S402. [DOI] [PubMed] [Google Scholar]
- 219.Adams R.A., Leon G., Miller N.M., Reyes S.P., Thantrong C.H., Thokkadam A.M., Lemma A.S., Sivaloganathan D.M., Wan X., Brynildsen M.P. Rifamycin antibiotics and the mechanisms of their failure. J. Antibiot. 2021;74:786–798. doi: 10.1038/s41429-021-00462-x. [DOI] [PubMed] [Google Scholar]
- 220.Hartmann G., Honikel K.O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim. Biophys. Acta Nucleic Acids Protein Synth. 1967;145:843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- 221.Aiba Y., Katayama Y., Hishinuma T., Murakami-Kuroda H., Cui L., Hiramatsu K. Mutation of RNA polymerase β-subunit gene promotes heterogeneous-to-homogeneous conversion of β-lactam resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013;57:4861–4871. doi: 10.1128/AAC.00720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vázquez-Laslop N., Mankin A.S. How Macrolide Antibiotics Work. Trends Biochem. Sci. 2018;43:668–684. doi: 10.1016/j.tibs.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lim J.-A. Prevalence of resistance to macrolide, lincosamide and streptogramin antibiotics in Gram-positive cocci isolated in a Korean hospital. J. Antimicrob. Chemother. 2002;49:489–495. doi: 10.1093/jac/49.3.489. [DOI] [PubMed] [Google Scholar]
- 224.Diep B.A., Chambers H.F., Graber C.J., Szumowski J.D., Miller L.G., Han L.L., Chen J.H., Lin F., Lin J., Phan T.H., et al. Emergence of Multidrug-Resistant, Community-Associated, Methicillin-Resistant Staphylococcus aureus Clone USA300 in Men Who Have Sex with Men. Ann. Intern. Med. 2008;148:249. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 225.Martineau F., Picard F.J., Lansac N., Ménard C., Roy P.H., Ouellette M., Bergeron M.G. Correlation between the Resistance Genotype Determined by Multiplex PCR Assays and the Antibiotic Susceptibility Patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000;44:231–238. doi: 10.1128/AAC.44.2.231-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rubinstein E., Keynan Y. Vancomycin Revisited—60 Years Later. Front. Public Health. 2014;2:217. doi: 10.3389/fpubh.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Barna J.C.J., Williams D.H. The Structure and Mode of Action of Glycopeptide Antibiotics of the Vancomycin Group. Annu. Rev. Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- 228.Reynolds P.E. Studies on the mode of action of vancomycin. BBA Biochim. Biophys. Acta. 1961;52:403–405. doi: 10.1016/0006-3002(61)90698-9. [DOI] [PubMed] [Google Scholar]
- 229.Typas A., Banzhaf M., Gross C.A., Vollmer W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 2012;10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dubrac S., Boneca I.G., Poupel O., Msadek T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 2007;189:8257–8269. doi: 10.1128/JB.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dubrac S., Bisicchia P., Devine K.M., Msadek T. A matter of life and death: Cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol. Microbiol. 2008;70:1307–1322. doi: 10.1111/j.1365-2958.2008.06483.x. [DOI] [PubMed] [Google Scholar]
- 232.Delaune A., Poupel O., Mallet A., Coic Y.-M., Msadek T., Dubrac S. Peptidoglycan Crosslinking Relaxation Plays an Important Role in Staphylococcus aureus WalKR-Dependent Cell Viability. PLoS ONE. 2011;6:e17054. doi: 10.1371/journal.pone.0017054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Herbert S., Bera A., Nerz C., Kraus D., Peschel A., Goerke C., Meehl M., Cheung A., Götz F. Molecular Basis of Resistance to Muramidase and Cationic Antimicrobial Peptide Activity of Lysozyme in Staphylococci. PLoS Pathog. 2007;3:e102. doi: 10.1371/journal.ppat.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sakoulas G., Eliopoulos G.M., Moellering R.C., Wennersten C., Venkataraman L., Novick R.P., Gold H.S. Accessory gene regulator (agr) locus in geographically diverse Staphylococcus aureus isolates with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 2002;46:1492–1502. doi: 10.1128/AAC.46.5.1492-1502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Howden B.P., Davies J.K., Johnson P.D.R., Stinear T.P., Grayson M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Münch D., Engels I., Müller A., Reder-Christ K., Falkenstein-Paul H., Bierbaum G., Grein F., Bendas G., Sahl H.G., Schneider T. Structural variations of the cell wall precursor lipid II and their influence on binding and activity of the lipoglycopeptide antibiotic oritavancin. Antimicrob. Agents Chemother. 2015;59:772–781. doi: 10.1128/AAC.02663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Arthur M., Molinas C., Depardieu F., Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 1993;175:117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lambert P. Bacterial resistance to antibiotics: Modified target sites. Adv. Drug Deliv. Rev. 2005;57:1471–1485. doi: 10.1016/j.addr.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 239.Sievert D.M., Rudrik J.T., Patel J.B., McDonald L.C., Wilkins M.J., Hageman J.C. Vancomycin-Resistant Staphylococcus aureus in the United States, 2002–2006. Clin. Infect. Dis. 2008;46:668–674. doi: 10.1086/527392. [DOI] [PubMed] [Google Scholar]
- 240.Melo-Cristino J., Resina C., Manuel V., Lito L., Ramirez M. First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. Lancet. 2013;382:205. doi: 10.1016/S0140-6736(13)61219-2. [DOI] [PubMed] [Google Scholar]
- 241.Birmingham M.C., Rayner C.R., Meagher A.K., Flavin S.M., Batts D.H., Schentag J.J. Linezolid for the treatment of multidrug-resistant, gram-positive infections: Experience from a compassionate-use program. Clin. Infect. Dis. 2003;36:159–168. doi: 10.1086/345744. [DOI] [PubMed] [Google Scholar]
- 242.Barber K.E., Smith J.R., Raut A., Rybak M.J. Evaluation of tedizolid against Staphylococcus aureus and Enterococci with reduced susceptibility to vancomycin, daptomycin or linezolid. J. Antimicrob. Chemother. 2016;71:152–155. doi: 10.1093/jac/dkv302. [DOI] [PubMed] [Google Scholar]
- 243.Perlaza-Jiménez L., Tan K.-S., Piper S.J., Johnson R.M., Bamert R.S., Stubenrauch C.J., Wright A., Lupton D., Lithgow T., Belousoff M.J. A Structurally Characterized Staphylococcus aureus Evolutionary Escape Route from Treatment with the Antibiotic Linezolid. Microbiol. Spectr. 2022;10:e00583-22. doi: 10.1128/spectrum.00583-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Humphries R.M., Pollett S., Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin. Microbiol. Rev. 2013;26:759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Arbeit R.D., Maki D., Tally F.P., Campanaro E., Eisenstein B.I. The Safety and Efficacy of Daptomycin for the Treatment of Complicated Skin and Skin-Structure Infections. Clin. Infect. Dis. 2004;38:1673–1681. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 246.Grein F., Müller A., Scherer K.M., Liu X., Ludwig K.C., Klöckner A., Strach M., Sahl H., Kubitscheck U., Schneider T. Ca2+-Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 2020;11:1455. doi: 10.1038/s41467-020-15257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Silverman J.A., Oliver N., Andrew T., Tongchuan L.I. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 2001;45:1799–1802. doi: 10.1128/AAC.45.6.1799-1802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sabat A.J., Tinelli M., Grundmann H., Akkerboom V., Monaco M., Del Grosso M., Errico G., Pantosti A., Friedrich A.W. Daptomycin resistant Staphylococcus aureus clinical strain with novel non-synonymous mutations in the mprF and vraS genes: A new insight into daptomycin resistance. Front. Microbiol. 2018;9:2705. doi: 10.3389/fmicb.2018.02705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Jones T., Yeaman M.R., Sakoulas G., Yang S.-J., Proctor R.A., Sahl H.-G., Schrenzel J., Xiong Y.Q., Bayer A.S. Failures in Clinical Treatment of Staphylococcus aureus Infection with Daptomycin Are Associated with Alterations in Surface Charge, Membrane Phospholipid Asymmetry, and Drug Binding. Antimicrob. Agents Chemother. 2008;52:269–278. doi: 10.1128/AAC.00719-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Van Hal S.J., Paterson D.L., Gosbell I.B. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:603–610. doi: 10.1007/s10096-010-1128-3. [DOI] [PubMed] [Google Scholar]
- 251.Capone A., Cafiso V., Campanile F., Parisi G., Mariani B., Petrosillo N., Stefani S. In vivo development of daptomycin resistance in vancomycin-susceptible methicillin-resistant Staphylococcus aureus severe infections previously treated with glycopeptides. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:625–631. doi: 10.1007/s10096-016-2581-4. [DOI] [PubMed] [Google Scholar]
- 252.Bæk K.T., Thøgersen L., Mogenssen R.G., Mellergaard M., Thomsen L.E., Petersen A., Skov S., Cameron D.R., Peleg A.Y., Frees D. Stepwise decrease in daptomycin susceptibility in clinical Staphylococcus aureus isolates associated with an initial mutation in rpoB and a Compensatory Inactivation of the clpX Gene. Antimicrob. Agents Chemother. 2015;59:6983–6991. doi: 10.1128/AAC.01303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ernst C.M., Slavetinsky C.J., Kuhn S., Hauser J.N., Nega M., Mishra N.N., Gekeler C., Bayer A.S., Peschel A. Gain-of-function mutations in the phospholipid flippase mprf confer specific daptomycin resistance. MBio. 2018;9:1–12. doi: 10.1128/mBio.01659-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ernst C.M., Staubitz P., Mishra N.N., Yang S.-J., Hornig G., Kalbacher H., Bayer A.S., Kraus D., Peschel A. The Bacterial Defensin Resistance Protein MprF Consists of Separable Domains for Lipid Lysinylation and Antimicrobial Peptide Repulsion. PLoS Pathog. 2009;5:e1000660. doi: 10.1371/journal.ppat.1000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bertsche U., Yang S.J., Kuehner D., Wanner S., Mishra N.N., Roth T., Nega M., Schneider A., Mayer C., Grau T., et al. Increased Cell Wall Teichoic Acid Production and D-alanylation Are Common Phenotypes among Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus (MRSA) Clinical Isolates. PLoS ONE. 2013;8:e67398. doi: 10.1371/journal.pone.0067398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Friedman L., Alder J.D., Silverman J.A. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 2006;50:2137–2145. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mishra N.N., Bayer A.S., Weidenmaier C., Grau T., Wanner S., Stefani S., Cafiso V., Bertuccio T., Yeaman M.R., Nast C.C., et al. Phenotypic and Genotypic Characterization of Daptomycin-Resistant Methicillin-Resistant Staphylococcus aureus Strains: Relative Roles of mprF and dlt Operons. PLoS ONE. 2014;9:e107426. doi: 10.1371/journal.pone.0107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li M., Cha D.J., Lai Y., Villaruz A.E., Sturdevant D.E., Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 259.Meehl M., Herbert S., Götz F., Cheung A. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007;51:2679–2689. doi: 10.1128/AAC.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Diekema D.J., Pfaller M.A., Schmitz F.J., Smayevsky J., Bell J., Jones R.N., Beach M. Survey of Infections Due to Staphylococcus Species: Frequency of Occurrence and Antimicrobial Susceptibility of Isolates Collected in the United States, Canada, Latin America, Europe, and the Western Pacific Region for the SENTRY Antimicrobial Surveillanc. Clin. Infect. Dis. 2001;32:S114–S132. doi: 10.1086/320184. [DOI] [PubMed] [Google Scholar]
- 261.Burgold-Voigt S., Monecke S., Simbeck A., Holzmann T., Kieninger B., Liebler-Tenorio E.M., Braun S.D., Collatz M., Diezel C., Müller E., et al. Characterisation and Molecular Analysis of an Unusual Chimeric Methicillin Resistant Staphylococcus Aureus Strain and its Bacteriophages. Front. Genet. 2021;12:1823. doi: 10.3389/fgene.2021.723958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kaatz G.W., Seo S.M., Ruble C.A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1993;37:1086–1094. doi: 10.1128/AAC.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yun H.J., Min Y.H., Lim J.A., Kang J.W., Kim S.Y., Kim M.J., Jeong J.H., Choi Y.J., Kwon H.J., Jung Y.H., et al. In vitro and in vivo antibacterial activities of DW286, a new fluoronaphthyridone antibiotic. Antimicrob. Agents Chemother. 2002;46:3071–3074. doi: 10.1128/AAC.46.9.3071-3074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Schindler B.D., Kaatz G.W. Multidrug efflux pumps of Gram-positive bacteria. Drug Resist. Updates. 2016;27:1–13. doi: 10.1016/j.drup.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 265.Poole K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007;39:162–176. doi: 10.1080/07853890701195262. [DOI] [PubMed] [Google Scholar]
- 266.Ubukata K., Itoh-Yamashita N., Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 1989;33:1535–1539. doi: 10.1128/AAC.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Neyfakh A.A., Borsch C.M., Kaatz G.W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob. Agents Chemother. 1993;37:128–129. doi: 10.1128/AAC.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J. Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Paul M., Bishara J., Yahav D., Goldberg E., Neuberger A., Ghanem-Zoubi N., Dickstein Y., Nseir W., Dan M., Leibovici L. Trimethoprim-sulfamethoxazole versus vancomycin for severe infections caused by meticillin resistant Staphylococcus aureus: Randomised controlled trial. BMJ. 2015;350:h2219. doi: 10.1136/bmj.h2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Elwell L.P., Wilson H.R., Knick V.B., Keith B.R. In vitro and in vivo efficacy of the combination trimethoprim-sulfamethoxazole against clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1986;29:1092–1094. doi: 10.1128/AAC.29.6.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Frei C.R., Miller M.L., Lewis J.S., Lawson K.A., Hunter J.M., Oramasionwu C.U., Talbert R.L. Trimethoprim-Sulfamethoxazole or Clindamycin for Community-Associated MRSA (CA-MRSA) Skin Infections. J. Am. Board Fam. Med. 2010;23:714–719. doi: 10.3122/jabfm.2010.06.090270. [DOI] [PubMed] [Google Scholar]
- 272.Woods D.D. The Relation of p-Aminobenzoic Acid to the Mechanism of the Action of Sulphanilamide. Volume 21 Wiley-Blackwell; Hoboken, NJ, USA: 1940. [Google Scholar]
- 273.Hitchings G.H. Mechanism of Action of Trimethoprim-Sulfamethoxazole—I. J. Infect. Dis. 1973;128:S433–S436. doi: 10.1093/infdis/128.Supplement_3.S433. [DOI] [PubMed] [Google Scholar]
- 274.Kalkut G. Sulfonamides and Trimethoprim. Cancer Invest. 1998;16:612–615. doi: 10.3109/07357909809032892. [DOI] [PubMed] [Google Scholar]
- 275.Chernyshev A., Fleischmann T., Kohen A. Thymidyl biosynthesis enzymes as antibiotic targets. Appl. Microbiol. Biotechnol. 2007;74:282–289. doi: 10.1007/s00253-006-0763-1. [DOI] [PubMed] [Google Scholar]
- 276.Proctor R.A. Role of Folate Antagonists in the Treatment of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008;46:584–593. doi: 10.1086/525536. [DOI] [PubMed] [Google Scholar]
- 277.Khamash D.F., Voskertchian A., Tamma P.D., Akinboyo I.C., Carroll K.C., Milstone A.M. Increasing Clindamycin and Trimethoprim-Sulfamethoxazole Resistance in Pediatric Staphylococcus aureus Infections. J. Pediatric Infect. Dis. Soc. 2019;8:351–353. doi: 10.1093/jpids/piy062. [DOI] [PubMed] [Google Scholar]
- 278.Acree M.E., Morgan E., David M.Z. S. aureus infections in chicago, 2006–2014: Increase in CA MSSA and decrease in MRSA incidence. Infect. Control Hosp. Epidemiol. 2017;38:1226–1234. doi: 10.1017/ice.2017.177. [DOI] [PubMed] [Google Scholar]
- 279.Harris T., Bowen A., Holt D., Sarovich D., Stevens K., Currie B., Howden B., Carapetis J., Giffard P., Tong S. Investigation of trimethoprim/sulfamethoxazole resistance in an emerging sequence type 5 methicillin-resistant Staphylococcus Aureus clone reveals discrepant resistance reporting. Clin. Microbiol. Infect. 2018;24:1027–1029. doi: 10.1016/j.cmi.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 280.Sato T., Ito R., Kawamura M., Fujimura S. The Risk of Emerging Resistance to Trimethoprim/Sulfamethoxazole in Staphylococcus aureus. Infect. Drug Resist. 2022;15:4779–4784. doi: 10.2147/IDR.S375588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jensen S.O., Lyon B.R. Genetics of antimicrobial resistance in Staphylococcus aureus. Future Microbiol. 2009;4:565–582. doi: 10.2217/fmb.09.30. [DOI] [PubMed] [Google Scholar]
- 282.Frey K.M., Lombardo M.N., Wright D.L., Anderson A.C. Towards the understanding of resistance mechanisms in clinically isolated trimethoprim-resistant, methicillin-resistant Staphylococcus aureus dihydrofolate reductase. J. Struct. Biol. 2010;170:93–97. doi: 10.1016/j.jsb.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Oefner C., Bandera M., Haldimann A., Laue H., Schulz H., Mukhija S., Parisi S., Weiss L., Lociuro S., Dale G.E. Increased hydrophobic interactions of iclaprim with Staphylococcus aureus dihydrofolate reductase are responsible for the increase in affinity and antibacterial activity. J. Antimicrob. Chemother. 2009;63:687–698. doi: 10.1093/jac/dkp024. [DOI] [PubMed] [Google Scholar]
- 284.Ramsey M.A., Bradley S.F., Kauffman C.A., Morton T.M. Identification of chromosomal location of mupA gene, encoding low-level mupirocin resistance in Staphylococcal isolates. Antimicrob. Agents Chemother. 1996;40:2820–2823. doi: 10.1128/AAC.40.12.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ramsey M.A., Bradley S.F., Kauffman C.A., Morton T.M., Patterson J.E., Reagan D.R. Characterization of Mupirocin-Resistant Staphylococcus aureus from Different Geographic Areas. Antimicrob. Agents Chemother. 1998;42:1305. doi: 10.1128/AAC.42.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bongiorno D., Mongelli G., Stefani S., Campanile F. Burden of Rifampicin- and Methicillin-Resistant Staphylococcus aureus in Italy. Microb. Drug Resist. 2018;24:732–738. doi: 10.1089/mdr.2017.0299. [DOI] [PubMed] [Google Scholar]
- 287.Peleg A.Y., Miyakis S., Ward D.V., Earl A.M., Rubio A., Cameron D.R., Pillai S., Moellering R.C., Eliopoulos G.M. Whole Genome Characterization of the Mechanisms of Daptomycin Resistance in Clinical and Laboratory Derived Isolates of Staphylococcus aureus. PLoS ONE. 2012;7:e28316. doi: 10.1371/journal.pone.0028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.