Abstract
Poxviruses (PXVs) are mostly known for the variola virus, being the cause of smallpox; however, re-emerging PXVs have also shown a great capacity to develop outbreaks of pox-like infections in humans. The situation is alarming; PXV outbreaks have been involving both endemic and non-endemic areas in recent decades. Stopped smallpox vaccination is a reason offered mainly for this changing epidemiology that implies the protective role of immunity in the pathology of PXV infections. The immune system recognizes PXVs and elicits responses, but PXVs can antagonize these responses. Here, we briefly review the immunology of PXV infections, with emphasis on the role of pattern-recognition receptors, macrophages, and natural killer cells in the early response to PXV infections and PXVs’ strategies influencing these responses, as well as taking a glance at other immune cells, which discussion over them mainly occurs in association with PXV immunization rather than PXV infection. Throughout the review, numerous evasion mechanisms are highlighted, which might have implications for designing specific immunotherapies for PXV in the future.
Keywords: Immunity, Infection, Poxvirus
1. Introduction
Monkeypox virus (MPXV) has been a potentially threatening poxvirus (PXV); it directed several outbreaks during the two recent decades, with the most recent one being now ongoing and has been involving both endemic and non-endemic areas [1]. The first cases of MPXV infections were reported in the 1970s, involving West Africa and Central Africa, mainly the Democratic Republic of the Congo (DRC). This DRC-dominant concentration of MPXV cases remains to date; however, two important events happened during the 2000s: the total number of cases increased sharply, and cases residing outside Africa were documented. In 2003, the outbreak in the US occurred in association with exposure to pet prairie dogs, which, in turn, were infected after exposure to Ghana-imported MPXV-infected animals. Between 2010 and 2019, the UK, Singapore, and Israel also reported a few cases of MPXV infection [1]. Recently, between May 13, 2022, and June 24, 2022, MPXV cases have been reported to the World Health Organization (WHO) from 47 locations worldwide, including the Americas, Eastern Mediterranean, Europe, and Western Pacific, as well as the African region.1 As of June 24, 2022, the total number of confirmed cases exceeds 4100, which concerns health authorities about a new outbreak of zoonotic viral infection [2].
Long before MPXV was discovered, people had to deal with the variola virus (VARV), the causative agent of smallpox infection. The early history of VARV is not clearly determined, with evidence of its observation in China, Egypt, and India thousands of years ago. There have definitely been subsequent movements between countries, regions, and states that have made smallpox spread globally [3]. Since 1900, smallpox has been the cause of death in more than 300 million people—eradication programs by the WHO could eradicate smallpox in 1980. Ectromelia virus (ECTV) is another PXV that has been observed in Chinese children with erythromelalgia and pharyngitis. It remains, however, obscure whether it might have caused outbreaks in humans [4].
Poxviruses (PXVs) that affect vertebrates belong to the family Poxviridae, subfamily Chordopoxvirinae [5]. There are numerous genera ranked as Chordopoxvirinae, including more than 50 species. Orthopoxvirus is a widely known genus, particularly for the variola virus and the vaccinia virus (VACV), the live virus used for smallpox vaccination. The genus includes other viruses, such as monkeypox, camelpox, cowpox, ectromelia, raccoon pox, skunkpox, taterapox, volepox, Abatino macacapox, and Akhmeta. Three other genera of PXVs can also affect humans, including parapoxvirus, yatapoxvirus, and molluscum contagiosum virus (MCV). Of 83 discovered PXVs, only two infect humans as the primary host: VARV and MCV–commonly present with skin lesions in infants and immunocompromised people. The others are zoonotic and transmit from animals to humans. Among all PXVs that can infect humans, VARV and MPXV are the most important in severity. They can cause a generalized infection involving the skin and internal organs, particularly the lungs. MPXV generally tends to cause a less severe infection–MPXV infection resembles a mild form of smallpox; however, it has features that make it less eradicable than VARV, especially the presence of animal reservoirs and lack of a specific vaccine [6].
PXVs are enveloped double-stranded DNA (dsDNA) viruses ranging between 130 and 290 kbp, known as the largest viruses with a diameter between 200 and 400 nm. PXVs genome contains more than 200 open-reading frames (ORFs), each of which has more than 50 amino acids; non-coding regions that separate ORFs; and terminal inverted repeat (TIR) sequences that occur at the two ends of the genome, which might, in turn, comprise up to 12 ORFs [7]. Many DNA viruses need the nucleus and relevant enzymes, metabolites, and factors for replication, but PXVs can complete the replication cycle totally in the cytoplasm [8]. This accomplishment lies in virions that express different proteins that make PXVs replicable in the cytoplasm [9]. PXV virions are complex structures of two main forms. Naturally occurring through the cytoplasmic membrane, PXV virion is fundamentally comprised of a core containing the DNA genome, two lateral bodies, and an inner membrane. When released artificially, it acquires an outer membrane, as well. The former is also known as an intracellular mature virion (IMV) or simply mature virion (MV), and the latter is known as an extracellular enveloped virion (EEV) or simply enveloped virion (EV). MV membrane is more stable than EV membrane; around 20 proteins participate in the synthesis of MV membrane, whereas six proteins are relevant to the synthesis of EV membrane. Both MV and EV contribute to the replication cycle [10].
PXVs virion not only synthesizes proteins essential for DNA replication and viral assembly, but it also encodes for non-essential, host-modulating proteins that boost virulence. The essential proteins are encoded by centrally located, highly conserved PXVs’ genes that participate in DNA processing, replication, and packaging. Essential proteins contributing to DNA replication include DNA polymerase, helicase-primase, uracil DNA glycosylase, processivity factor, protein kinase, single-stranded (ss) DNA-binding protein, and DNA ligase. DNA processing also essentially involves Holliday junction resolvase, and DNA packaging crucially employs ATPase and telomerase-binding protein 1 [9]. Various host-modulating proteins have been identified to be expressed by terminally located, highly variable PXVs’ genes. The expression of these proteins is species-dependent and determines the severity of PXV infection [5]. Some PXV infections might be self-limited and localized to the skin, while there are PXVs highly potent to spread into the body and cause a systemic infection characterized by two (primary and secondary) stages that begin with the skin lesions and the virus’ introduction into the blood, followed by the involvement of internal organs and re-releasing the virus into the blood, and end with the explosion of ulcerative lesions. This way, systemic PXV infection becomes terrible and fatal unless the host can effectively eliminate it; host resistance/susceptibility affects the pathogenesis of PXV infection. E.g., European rabbits are extremely susceptible to myxoma virus than South and North American rabbits. Similarly, BALB/c mice are susceptible compared to C57BL mice against ECTV [5]. It has been shown that STAT1 and STAT3 pathways that have been associated with infection outcomes are activated in resistant C57BL mice but not in susceptible BALB/c mice [11]. Therefore, the host genetics’ background crucially determines PXV susceptibility.
Histopathological studies of smallpox in humans highlight the role of virus-mediated cytopathic effects as well as inflammation both during early infection and second viremia [12]. Edema and lymphocyte infiltration are evident in the skin during the second viremia, and polymorphonuclear (PMN) leukocyte migration into vesicles causes pustules to form. The lungs usually represent a viral interstitial pneumonitis-like picture, including bronchitis, hyperemia, and degeneration of the alveolar epithelium. The kidneys and testes have also shown interstitial edema and lymphocyte infiltration. Moreover, cell degeneration and necrosis, which trigger inflammation, might be seen in the lungs, testes, liver, kidneys, and lymph nodes.
Direct evidence of the immunology of important PXVs, i.e., VARV and MPXV, in humans is lacking. Our present knowledge is, currently, based mainly on animal experiments conducted using VACV immunization and infection. These experimentations have shown that, like other DNA viruses, the immune system recognizes PXVs and activates immune responses. Upon infection with PXVs, the host employs the innate arms by inducing natural killer (NK) cells, interferons (IFNs), the complement system, and inflammatory cells. During days, cell-mediated and humoral immunity come for viral clearance and antibody production for viral neutralization and prevention of re-infection. On the other hand, PXVs can mediate different immune evasion mechanisms, increasing the severity of pathogenic infection or reducing the effectiveness of immunization. Below, we review the immune responses to PXVs as well as PXV strategies influencing the host defense.
2. Host-Cell entry for enveloped viruses
MVs’ binding might depend on or be independent of cellular attachment-related proteins [13]. The former involves surface molecules, like glycosaminoglycans (GAGs), while the latter is mediated through direct interaction with liposomes. EVs require cellular attachment proteins for binding; however, less is known about proteins that play a role in this binding. When viruses bind to the host cells through their MVs/EVs, they undergo two possible processes for entering the host cell: fusion and endocytosis. In the fusion, viral particles are fused at the plasma membrane and later will be uncoated for replication. In endocytosis, the role of the intracellular transport system is crucial. After the internalization of the virus, endocytic vesicles carry the virus to a place suitable for fusion. Then, as mentioned in the fusion path of entry, uncoating and viral replication occur. Interestingly, viruses benefit from entering by endocytic vesicles more than simply fusing at the plasma membrane. The endocytic vesicles serve as guards for viral particles, crossing them from cytoskeleton barriers and protecting them against immune recognition.
Accordingly, the PXV-specific replication cycle is, in brief, as follows. IMV binds to cell surface molecules, i.e., GAGs, and enters the target cell through fusion or endocytosis [14]. Core uncoating leads to DNA replication, followed by assembly and morphogenesis to prepare IMV for packaging by Golgi membranes. The Golgi-packaged IMV is then called the intracellular enveloped virus (IEV), which again undergoes fusion at the membrane, resulting in the cell-associated enveloped virus (CEV). Two possible fates of the CEV are: to be removed or released from the cell. The cell undergoing the former event is known as the extruded cell, while free EEV is what undergoes the latter event. Noteworthy to mention is that IMV might bud to release EEV directly, which, this way, IEV formation is eliminated.
In summary, the host cell-virus interactions occurring at the binding/entry and intracellular levels are essential to mediate PXV cell tropism. In turn, cellular tropism would then direct tropism at the tissue and organ level, determining the extent to which PXV can replicate and disseminate.
3. Innate immunity
3.1. Pattern recognition receptors
3.1.1. Toll-like receptors
Toll-like receptors (TLRs) are receptors occurring in the plasma membrane (membrane-bound) and endosome (intracellular) that mediate the recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) [15]. To date, ten TLRs have been identified in humans, including six membrane-bound TLRs (TLR-1, 2, 4, 5, 6, 10) and four intracellular TLRs (TLR-3, 7, 8, 9). Through interacting with MyD88, an adaptor molecule, all TLRs, but TLR3, can activate mitogen-associated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB) to produce pro-inflammatory cytokines and chemokines. TLR3 interacts with another adaptor, TRIF, inducing NF-κB and IRF3 to generate type I IFNs (T1IFNs) [16]. TLR-7, 8, and 9 also lead to T1IFN response by inducing IRF7. TLR/Interleukin-1 receptor is another pathway to which TLRs signal to induce the expression of inflammatory cytokines. This pathway involves such Toll/IL-1 receptor (TIR) domain-containing adapter proteins (TIRAP) as MyD88, MAL, TRIF, TRAM, and SARM [17], and the IL-1 receptor (IL-1R)-associated kinases (IRAKs), including IRAK1-4. Tumor necrosis factor (TNF) receptor-associated factors (TRAFs), particularly TRAF3 and TRAF6, also participate in the regulation of TLRs signaling by linking TLRs and relevant adaptors to the downstream signaling molecules [18].
PXV infection induces innate signaling pathways both dependent on and independent of TLRs. It might, in turn, depend on viral proteins whether TLRs-dependent pathways, TLRs-independent pathways, or both are triggered. E.g., the TLR3-dependent pathway is downregulated by VACV ORF A52R [19], while both TLR2-dependent and TLR2-independent pathways could occur in vivo in VACV infection; the former directs the production of the pro-inflammatory cytokines and the latter leads to IFN responses [20]. Upon infection with PXVs, TLRs-dependent pathways are triggered by different TLRs in dendritic cells (DCs), T cells, mast cells, monocytes, and macrophages [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33].
Different PXV species engage different TLRs; VACV could interact with TLR-2, 3, 4, 7, 8, and 9; ECTV with TLR2; MCV with TLR4; MYXV with TLR2, 6, 7, and 9; and parapoxvirus ovis with TLR9. Most studies have been conducted using VACV and shown that different VACV-associated proteins, e.g., A52, A46, N1L, E3, and K7, antagonize TLRs in different points of signaling to IL-1R, NF-κB, and IRFs [28], [33], [34], [35], [36], [37], [38], [39], [40]. Particularly, studies elucidate the ability of VACV and its attenuated strain, MVA, to inhibit activation of MAL and MyD88 by TLR2; TRIF, TRAM, MAL, and MyD88 by TLR4; MyD88 by TLR7; and IRAK2 and TRAF6 by TLR3. Later, VACV can also inhibit NF-κB-related proximal kinases, IKKa and IKKb [41]. From a structural perspective, VACV proteins, such as A46, A52, B15, K7, N2, C1, C6, and C16/B22, possess sequences similar to Bcl2 (B cell lymphoma 2, an apoptosis regulator) [42]. Also, biophysical analyses reveal that VACV could directly bind the TIR domain of adaptors, including MyD88, Mal/TIRAP, and TRIF [43]. These observations, in addition to the fact that Bcl2 can negatively regulate innate immune pathways, might explain the ability of PXVs to inhibit TLRs-dependent innate responses [44].
TLRs triggered or targeted by PXV act as double-edged swords, with both beneficial and detrimental effects for the host (Fig. 1). TLR3 has been associated with increased inflammatory responses in both periphery and lungs, viral replication, and mortality and morbidity following infection with VACV [45]. In contrast, TLR2, 4, and 9 increase resistance against ECTV and VACV infection [46], [47], [48], [49]. In ECTV infection, TLR9 seems essential for CD11c(+) cells to induce inflammatory gene expression [48] and for DCs to recruit NK cells to the site of infection [49]. In VACV infection, TLR2 is crucial for CD8 + T cells to expand and differentiate into memory cells [50], for NK cells to become activated [51], for mast cells to increase the production of cathelicidin (LL-37), and therefore antiviral activities [29], and also for macrophages to induce TRAF3 and undergo reprogramming [30]. Unexpectedly, when VACV was intradermally administered, the role of TLR2 was no longer crucial to viral control, but it was MyD88 that induced antiviral immunity in an effective, TLR2-independent manner [52]. Totally, the engagement of TLRs-dependent pathways by PXVs depends at least on the PXV and animal species/strains and route of infection. When all these potential factors are controlled, using TLR antagonists/agonists might confer benefits in treating and preventing PXV infections. For example, TLR7 agonists, imiquimod and resiquimod, could improve immune responses following smallpox vaccination [53].
Fig. 1.
Toll-like receptors (TLR), the immune system, and poxviruses.
3.1.2. RIG-I like receptors
RIG-I-like receptors (RLRs) are another group of PRRs that serves to sense viral RNA-associated PAMPs in the cytosol. There are three RLRs: RIG-I, MDA5, and LGP2, that signal to adaptor proteins, called MAVs, residing on the peroxisomes, mitochondrion, and mitochondrial-associated membranes (MAMs). Upon viral RNA recognition by RLRs, subsequent activation of MAVs leads to the induction of IRFs and NF-κB [54]. RLRs could detect major viral infections. RLRs might be implicated in PXV infection in different cells, such as DCs, fibroblasts, peripheral blood mononuclear cells (PBMCs), monocytes, and macrophages [19], [55], [56], [57], [58], [59], [60], [61], [62], [63].
Evidence points to the potential engagement of all three RLRs with VACV; RIG-I and MDA5 with MVA and ALVAC; and RIG-I with MYXV, Orf virus, and sheeppox virus. RIG-I and MDA5 mainly contribute to the T1IFN responses and the induction of pro-apoptotic proteins, e.g., Noxa, while LPG is particularly involved in NF-κB activation [23]. VACV protein, E3, is highly antagonizing with domains that can bind both DNA and dsRNA, which the latter, i.e., the dsRNA-binding domain, can, in turn, interfere with both dsRNA- and ssRNA-mediated IFN activation [64]. Inhibitory effects of E3 on RIG-I involve MAVs, importantly IFN-β promoter stimulator (IPS)-1, and p38 and JNK kinase, therefore enabling VACV to inhibit IFN-β responses and the production of the pro-inflammatory cytokines [65], [66].
Due to contradictory findings, drawing a sharp line between the pro-viral and antiviral effects of RLRs activation is not possible. For example, the aforementioned antiviral effects of RIG-I activation include IFN responses; on the other hand, RIG-I seems to promote viral replication in cancer cells [67]. Further studies are required to investigate this issue.
3.1.3. Nucleotide-binding and oligomerization domain (NOD)-like receptors
In addition to TLRs and RLRs, nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) detect intracellular PAMPs and DAMPs. NLRs comprise an N-terminal domain that interacts with the ligands, a central nucleotide-binding domain, and a C-terminal domain known as LRR [68]. According to the N-terminal domain, NLRs structurally fall into NLRA, NLRB, NLRC, and NLRP. Functionally, NLRs respond by triggering inflammasomes that, in turn, interact with the adaptor, ASC, to induce caspase 1 that converts the pro-form of cytokine, IL-1β, into its mature form; signaling pathways NF-κB and MAPK; transcription activation; and autophagy. Different NLRs have been implicated in infections; NOD2, NLRP1, and NLRP3 play roles in antiviral responses [69]. Particularly, NLRP1 and NLRP3 are involved in PXV infection.
Canarypox virus-based vector (ALVAC), MVA, and VACV-based vaccine vector lead to inflammasome activation. ALVAC, using cGAS-IFI16-STING-T1IFNs, primes AIM2 to interact with ASC and direct caspase 1 to activate inflammasomes [70], [71]. MVA-induced IL-1β production appears to depend on caspase 1-activating inflammasome signaling [72]. In the local infection model, MVA-activated inflammasome in macrophages is very conducive to inflammation, cell death, and T cells mobilization and effector function [73]. VACV-based vaccine vector has also been shown to cause inflammasome activation in the injection site in the first 12 h post-administration [74]. These lines indicate that PXV-based vectors and attenuated PXVs can engage inflammasome activation in stimulating the immune system. However, MYXV and VACV evade NLRP3 and NLRP1 inflammasome activation by respectively having proteins M013 and F1L that can directly bind ASC-1 and NLRP1 [27], [75], [76], [77]. Moreover, PXVs are among rare pathogens that encode Pyrin domain-only proteins (POPs) that again bind ASC interfering with inflammasome activation [78].
3.1.4. Cytoplasmic DNA receptors
There are numerous DNA sensors in the cytoplasm, among which ZBP1, AIM2, IFI16, RNA Poll III, DNA-PK, DDX41, DDX50, and cGAS play a role in sensing poxviral DNA [21], [22]. The pathways mainly include: DHX9 interacts with MyD88 to activate NF-κB; AIM2 stimulates caspase-1-activating inflammasome to induce IL-1β; and most trigger STING-TBK1 signaling to induce NF-κB, IRFs, and STAT6 resulting in the expression of pro-inflammatory cytokines (IL-1β and IL-6), type I (IFN-α and IFN-β) and III (IFN-λ1) IFNs, and chemokines (CXCL10) [79].
Cytosolic DNA is a stimulant for cGAS, and its activation is regulated by carboxypeptidases (CCP) 5 and 6 [80] to produce cGAMP. cGAMP is then taken by viruses to form extracellular vesicles or viral particles [81], which, in turn, regulate the gap junctions-mediated transfer of cGAMP from producing cells to other cells [82]. In the target cells, cGAMP activates the endoplasmic reticulum (ER) STING to move to the Golgi apparatus (GA), where synthesized sulfated glycosaminoglycans (sGAGs) mediate STING polymerization and TBK1 activation [83]. In the GA, STING is co-localized with RAB2B, which recruits GARIL5 to form the complex RAB2B-GARIL5 [84]. In association with this complex, STING induces ISGs. IFNs not only on their own are antiviral products, but also, they can induce CCL4 to induce monocytes recruitment and direct the differentiation of inflammatory monocytes (CCR2 + ) into engulfing macrophages (Lyve1-) in ACEIIs [63] to help with VACV elimination. In FPXV infection, cGAS-STING-induced macrophages are enhanced in effector functioning to mediate MHC II transcription and related antigen presentation to T cells [85]. Thus, cGAS and STING are crucial to resistance against PXVs [86]. Particularly, animals lacking STING are highly susceptible to subcutaneous, respiratory, and intravenous involvement by ECTV infection [87]. Also, those lacking cGAS in the bone marrow display severe ECTV infection [88]. Unlike replication-defective PXVs, such as MAV [89] and ALVAC [71], which have shown no significant inhibitory effect on DNA-induced STING activation, replication-competent PXVs, such as CPXV, ECTV, and VACV (strains Copenhagen and Western reserve), could interfere with STING activation [90]. Different mechanisms handle this interference, involving both virus-mediated and host-mediated proteins. VACV F17 protein can impair mTOR signaling by binding regulators of mTOR complexes 1 and 2 (Raptor and Rictor) and thereby inhibit STING activation [91], [92]. PXVs also possess immune nucleases, called poxins, that degrade 2′3′ cGAMP and thereby inhibit cGAS-STING signaling in infected cells [93]. Poxins have been identified in human PXVs, such as MPXV and cowpox virus, as well as their homologs being related to insect DNA viruses, Alphabaculovirus [93]. This way, poxins might be a mechanism by which not only can PXVs evade the immune system and spread into the body, but they can also transmit between humans and insects. The host caspases (3 in humans and 7 in mice) by cleaving cGAS impede cGAS-STING activation [94].
DNA-PK, another sensor of viral DNA, leads to STING, IRF3, and TBK1 activation, and its deficiency causes severe combined immunodeficiency in humans. VACV proteins, C4 and C16, prevent DNA-PK activation [95], [96], [97].
DHX9, an RNA helicase, triggers the production of cytokines via TLR2-dependent-induced IL-6 production, TLR8-dependent inflammatory cytokines production, and also TLRs-independent activation of NF-κB to produce IL-6 [33]. In addition to the production of inflammatory cytokines, DHX9 can mediate granules' formation, inhibiting viral replication [98]. VACV E3 protein inhibits DHX9 in monocytes [33].
ZBP1 is a sensor of Z-RNA. Upon activation, ZBP1 activates RIPK3 to mediate necroptosis and viral control. VACV E3, capable of binding Z conformation [99] and evasion of T1IFNs [100], disrupts ZBP1-induced necroptosis [100], [101], [102], [103].
3.1.5. C-type lectin-like receptors
C-type lectin-like receptors (CTLRs) constitute a large family of receptors (greater than1000). The best-known one is the dendritic cell-associated CTLR, called dectin-2, which leads to cytokine and chemokine production by stimulating the axis of Syk, PKCδ, and signalosome CARD9/BCL10/MALT1 (CBM) complex [104]. CARD9 is involved in anti-PXV immunity [23]. MER11, a cytoplasmic DNA sensor, undertakes viral DNA sensing associated with the DNA repair protein 50 (Rad50) [105]. Then, Rad50 links to CARD9, which induces Bcl-10 and NF-κB proximal kinases [23].
3.2. Macrophages
There has been an argument over the origin of tissue-resident macrophages that they are a separate lineage developed and maintained on their own, i.e., through self-renewal, or blood monocytes are responsible for their replacement and maintenance in the tissues during development [106], [107]. Anyway, the availability of tissue-resident macrophages in the various tissue, e.g., the CNS, skin, lungs, and heart, make them effector cells of the innate immune system that can participate in the detection of pathogens and their removal through phagocytizing. Particularly in HIV infection, not only monocytes and macrophages are affected by the virus with diminished capacity to induce cytokines and phagocytosis, but also, they help with the spread and dissemination of the virus to, e.g., the CNS [108].
Different macrophage subsets play different, opposing roles in infection with different PXVs. Upon VACV infection, murine macrophages undergo apoptosis that crucially involves Bcl-xL and facilitates viral pathogenesis [109]. In the skin, VACV binds to MARCO (macrophage receptor with collagenous structure) expressed by keratinocytes to enhance virulence [110]. However, murine bone marrow-derived macrophages are activated by VACV to induce IFNAR and ISG15, which, in addition to IFN production, trigger the STAT1 phosphorylation, macrophage polarization, and mitochondrial function by increasing ATP, NO, and ROS; all are potent antiviral responses [111], [112]. In peritoneal infection, VACV induces PARP1 to activate macrophages, which, in turn, stimulate ISG15, AKT, and NF-κB to produce CC chemokine, CCL2 [113]. The axis CCL2-CCR2 then mediates NK cell recruitment. Moreover, alveolar macrophages are vital to surviving respiratory infection with VACV [114]. In ACEIIs, VACV infection prompts CCR2 + inflammatory monocytes recruitment into the lungs and their differentiation into Lyve1- macrophages that engulf viral particles [63]. In intradermal infection with VACV, macrophages, especially residing on the subscapular sinus and the draining lymph node, have been shown to mediate a systemic, innate response early, and this response crucially contains the disease spread in mice [115]. After VACV infection, human monocytes under the regulation of GM-CSF and M−CSF drive into M1 and M2 macrophages, but these macrophages mostly contain EEVs that, through cell–cell contact, contribute to viral dissemination [116].
Like VACV, MVA also directs apoptosis and necroptosis in murine macrophages [117], possibly by inflammasome activation [73]. Macrophages are among the immune cells early responding to intradermal administration of MVA in non-human primates [118]. Again, similar to VACV, alveolar macrophages are necessarily involved in respiratory infection with MVA, along with other antigen-presenting cells, i.e., DCs [119]. In murine alveolar macrophages, MVA induces T1IFNs [120] to produce chemokines [121], including CXCR2, CXCL2, CCL2, CCR1, CCL3, CCL5, and CCL9, to use chemotaxis to recruit NK cells and T cells into the lung. In murine bone-marrow-derived macrophages, the axis CCR4-CCR2 could lead to the chemotactic recruitment of neutrophils into the lung [122]. Moreover, splenic CD169 + macrophages in the marginal zone capture MVA-related antigens from the blood [123]. These macrophages transfer antigens to dendritic cells, which act as APCs for CD8 + T cells to prime CD8 + T cells' effector function. In human cells, MVA protein N2L can bind macrophage receptors, interfering with IRF3 and contributing to pathogenesis [124]. Moreover, PXV virion encodes for a CC chemokine inhibitor (CCI), which has been shown to bind different chemokines [125].
PXVs-induced macrophages offer opportunities. To combat ECTV infection, murine macrophages mediate T1IFN production in a cGAS-STING-dependent pathway [86] and are considered a good model for the study of ECTV immunopathogenesis [126]. MYXV also encodes for M−T7 and Serp-1, which do fine in regulating macrophages in different contexts, e.g., by decreasing macrophages infiltration in the cavity of injury in animals after spinal cord injury and attenuating inflammation [127], [128], and also by increasing arginase-expressing macrophages and enhancing wound healing [129]. Recombinant VACV could be planned to prime macrophages to elicit such functions as antitumor immune responses [130].
3.3. Natural killer cells
Natural killer (NK) cells are lymphocytes with innate, adaptive, and regulatory properties [131], [132]. As innate immune cells, they occur widely over lymphoid and non-lymphoid tissues directing immune responses quickly and generally. Under the influence of a wide array of inhibitory and activating receptors, they can signal to different cells, i.e., DCs, B cells, T cells, and macrophages, and regulate different functions. And as adaptive players, they can be driven in an antigen-specific manner and undergo clonal expansion mediating long-term memory. NK cells contribute to immune responses against DNA and RNA viruses, from inducing the adaptive immune responses and killing the virus-infected cells by producing cytokines and IFN-γ to generating antigen-specific memory [133].
Studies confirm that NK cells are infected with ECTV, VACV, MAV, MYXV, and MPXV, which is often associated with a Th1 response, NK-cell activation, IFN-γ production, and T-cell activation. PXVs lead to the production of T1IFNs to induce NK cells [134], which, upon activation, secrete cytokines [135], particularly IFN-γ and TNF-α, to proliferate and mediate their cytolytic function and also to induce cytotoxic T cells effector function [136], [137]. Cytolysis by these cells is accompanied by the formation of granules and the release of granzyme b and perforin to kill the infected target cells. PXVs have been shown to induce factors to prompt NK cells’ accumulation. ECTV, in a TLR9-MyD88-dependent manner, induces NKG2D, an activating factor for NK cells, to produce CCL9, which in association with CXCR3, recruits NK cells [49]. ECTV also engages Seprinb9, an inhibitor of granzyme B, to interfere with and balance the granzyme b-induced killing of mature NK and T cells [138], [139]. MVA induces SST, which inhibits ghrelin and prevents its down-regulatory effect on NK cells, and CCL2, which recruits NK cells [120], [140].
However, different mechanisms have been proposed to enable PXVs to evade NK cells. In general, VACV infection downregulates the expression of cellular proteins, including NK cell ligands [141], and is also correlated with JNK2, causing iNKT cell loss [142]. When compared with LCMV-induced NK cells, VACV-induced NK cells appear less effective, with an increased frequency of immature NK cells (CD11-CD27 + cells) and decreased frequency of activated NK cells (CD69 + cells) [143]. Also, VACV-induced NK cells relatively express more NKG2A, an inhibitory regulator for NK cells, and are relatively impaired in function, both cytolysis (lower production of perforin and granzyme B) and IFN production (lower IRF4 expression) [143]. VACV protein N1 inhibits NK cells’ accumulation, too [144]. MPXV increases NK cells in number but decreases them in cytokine production (IFNγ and TNFα), cell degranulation, and chemotactic function early in infection [145]. ECTV encodes for CrmD, which contains SECRET and TNF BD [146]. The binding to chemokines and TNFα decreases NK cell accumulation, corresponding to increased susceptibility to ECTV infection [146]. Also, PXVs possess HA, which upregulates NK ligands, such as NKp30 and NKp46 [147]. By influencing NKp30- and NKp46-triggered NK-cell activation, VACV and ECTV hinder the killing of infected cells by NK cells, facilitating virulence [147].
The transfer of NK cells could improve viral control in VACV infection [148], supporting that NK cells are essential to the host's resistance against PXVs.
3.4. Neutrophils
Linking to cytokines, proteases, antimicrobial proteins, and factors, neutrophils can affect and be affected by other immune cells in the extravascular tissue, such as monocytes, immature or plasmacytoid DCs, B cells, T cells, and macrophages [149]. Neutrophils’ role is from early in viral infection and continues to the killing of pathogens. Particularly, neutrophils internalize and inactivate viruses, transport viruses, and are recruited to infection sites to induce antiviral immune responses [150]. The discussion over neutrophils in PXVs largely occurs in the context of immunization, not infection with PXVs. It shows even when highly attenuated, PXVs could prime neutrophils [151]. Also, in mice, PXV infection could direct neutrophils’ recruitment and neutrophil extracellular traps (NETs) formation, a mechanism of viral inactivation, in the liver [152]. However, PXV semaphorin has been shown to inhibit DCs and neutrophils-mediated viral phagocytosis [153].
3.5. Complement system
Also known as the immune surveillance system, it takes place in the first and fast line of defense, i.e., innate immunity [154]. Three intricate pathways defined by activating factors, e.g., classical (IgG and IgM containing antigen–antibody complexes), lectin (bacterial surfaces), and alternative (pathogen surfaces), involve different complement proteins and enzymes; however, they intersect at C3 and associated products C3a, C3b, and C5a, and the formation of C5b-C9 membrane attack complex [154]. In humans, various complement receptors and regulators have been identified with different roles in chemotaxis, cytokine production, phagocytosis, and antibody production that justifies the complement system’s role from early recognition to the removal of pathogens [155]. Complement deficiency has been associated with increased susceptibility to infections as well as syndromic and autoimmune conditions due to deregulated immune responses [154]. The complement system is activated early in PXV infection in mice, and its activation is crucial for viral control [156]. Again, PXVs have learned to evade the complement system by secreting the virokines, which are viral proteins serving as complement regulators’ mimics. Such a virokine has been primarily identified in VACV (VV complement control protein (VCP)), with orthologues and homologs isolated from MPXV, VARV, and CPV. VCP has been shown to interfere with classical and alternative complement activation. SPICE is a VCP homolog with a higher potency that might account for VARV virulence in humans [157].
4. Innate-Adaptive immune interface
4.1. Dendritic cells
The fine regulation of activities on the innate-adaptive immune interface is the key to pathogen eradication; otherwise, abnormal activation of each of innate and adaptive arms would contribute to reactions that favor the pathogen and ensue pathogen proliferation and dissemination [158]. All of the aforementioned elements of the innate arm are essential to programming to the innate-adaptive immune interface; however, at the center of this interface are dendritic cells (DCs). DCs are antigen-presenting cells (APCs) serving different functions in different phenotypes [159], [160], [161]. Upon antigen capturing in the peripheral tissues, immature DCs undergo maturation to express stimulatory molecules for T cells and subsequently migrate to lymphoid tissues and bone marrow. DCs tune T cells’ function; they induce T cells to act against the non-self, and also, DCs inhibit T cells from reacting against the self. DCs affect B cells’ growth, differentiation, and functions, such as antibody production and immunoglobulin switching. During viral infections, DCs undergo switches to initiate such immune responses as type I IFNs. In PXV infections, DCs are activated with migration to the sites of viral replication and inflammation, e.g., the skin and lungs, to activate immune cells, for example, CD8 + T cells, to combat the infection [162], [163]. However, like other viruses, PXVs have also evolved mechanisms of DCs evasion to facilitate immunopathogenesis by inhibiting DCs-mediated phagocytosis [153].
5. Adaptive immunity
5.1. CD4 + T cells
These cells recognize antigens presented by MHC class II molecules. Upon activation, Th cells fall into different functional categories by cytokines they induce [164], including but not limited to Th1 (IL-12 and IFN-γ), Th2 (IL2 and IL4), Th17 (IL6, IL21, IL23, and TGFβ), induced regulatory T (IL-2 and TGFβ), and follicular Th (IL6 and IL21) cells [165]. Th1 cells play a role in controlling intracellular microbes; Th2 cells in extracellular parasites; Th17 cells in extracellular bacteria and fungi; iTregs in pro-inflammatory responses, inflammation, and allergies; and follicular Th cells in B cell-mediated humoral immunity by inducing the synthesis of immunoglobulins (HgG1, IgG2a, IgE, and IgA) [165]. In combatting viral infections, CD4 + T cells are complex with roles from recruiting CD8 + T cells and DCs to the sites of viral replication and priming CD8 + T cells to differentiate into effector and memory T cells to promote B cells-dependent antibody responses [166]. Human cytotoxic CD4 + T cells could recognize PXVs-related antigens conserved among many PXVs, e.g., VACV, VARV, MPXV, cowpox virus, and ECTV [167]. In mice, cytotoxic CD4 + T cells kill the PXV-infected cells by inducing perforin [168]. Many PXVs, including VACV, have been shown to interfere with the IFN-γ-Jak-Stat pathway. This observation, in addition to the fact that this pathway is involved in MHC class II antigen presentation, concludes with PXVs’ evasion from CD4 + T cells [169].
5.2. CD8 + T cells
CTLs recognize MHC class I molecules-presented antigens. These cells are essential mentors that, upon detection of such changes as microbial pathogens and cellular transformation, trigger the killing of target cells through either indirect induction of killing cytokines, e.g., IFN-γ and TNF-α, or directly through cell–cell contact [164]. The latter is, in turn, possible either via FasL-Fas binding or via pro-apoptotic proteins, e.g., perforin and granzymes. The problem with the cytotoxic activity of CTLs is due to their overactivation; therefore, many infected cells are destroyed. So, although CTLs are required for viral clearance, there should be a mechanism of resistance to regulate the apoptosis of infected cells carefully. NKG2A is a receptor expressed in NK cells with an inhibitory function. It occurs in CD8 + T cells, too, and has been shown to regulate CD8 + T-cell responses in PXV infection [170]. For mice to induce effector CD8 + T cells after VACV infection, DCs appear essential, as well as Batf3, a transcription factor [171]. Recombinant PXVs are highly able in specific CD8 + T cells’ priming effector and memory T cells [172], which might explain their high success in being used as vectors for immune interventions, e.g., antiviral immunization and antitumor immunotherapy. Interestingly, in mice, CD8 + T-cell responses against VACV, ECTV, and MPXV infection appear highly similar, raising the hope that VACV immunization might induce CD8 + T-cell cross-reactivity against other PXVs [173]. This needs further investigations into human populations.
5.3. B cells and antibodies
Several B-cell subsets have been defined by the core markers with different properties and abilities. Among them, some are of most interest to the present discussion: memory B cells that can induce immunological memory in a T-cell-independent manner; antibody-secreting B cells that secrete antibodies; regulatory B cells (Bregs) and regulatory plasma cells (PCregs) that modulate immune responses [174]. When the B-cell receptor (BCR) is bound with a pathogen or apoptotic debris, B cells induce T-bet + cells that mediate IgG2 production and T-bet- cells that mediate IgG1 and IgA production. T-bet + cells, in turn, can undergo self-renewal and differentiation into plasma cells and other lineages that, like other B cells, can mediate viral control and immune protection against intracellular pathogens and, on the other hand, overproduce autoantibodies and cause inflammatory and autoimmune diseases and transplant rejection [175]. During HIV infection, different B-cell subsets are dysregulated in function and frequencies that result in B-cell exhaustion, diminishing B cells’ effector functions [176]. B cells are as important as CD8 + T cells in the recovery from PXV infection; however, CD8 + T-cell responses mainly occur early in infection, whereas B cells later become crucial for antibody production, viral control, and disease prevention [177]. PXVS could evade B cells; Orthopoxvirus MHC class I-like protein (OMCP) binds to the FcR-like 5 (FCRL5) on B cells, interfering with their function [178].
6. Smallpox vaccination
PXVs can be used not only for immunization against PXVs but also in the recombinant form to express the glycoproteins related to other viruses and tumor-associated antigens, thereby contributing to the prevention and treatment of other infectious diseases and cancer. E.g., a recombinant VACV-rabies vaccine was proposed to eradicate rabies in wildlife [179]. Also, variola vaccinia, canarypox, fowlpox, myxoma, tanapox, and orf are species of PXVs applied to cancer research and therapy [180]. Despite the promises of PXVs in the so-called oncolytic virotherapy, studies show that smallpox-vaccinated subjects might not benefit from PXVs-based immunotherapy due to their immunity to PXVs. These lines of evidence pose a challenge that could be resolved with immunosuppressive therapy while supporting the long-lived effectiveness of smallpox immunization [181].
VACV’s origin is not clear, going back to either VARV or cowpox virus or both. Low rates of side effects while providing long-term immunity to smallpox have made VACV successful in eradicating smallpox. To date, three generations of VACV vaccines have been developed, according to the platform they are prepared, from lymph-derived live VACV to tissue-cultured VACV to highly attenuated VACV. Clearly, they have been improved in order to minimize the side effects due to immunization with live, highly virulent VACV. In terms of efficacy, searching for immune correlates of VACV immunization has been a subject of human studies, showing the role of both T cells and B cells in immune protection [182], [183]. Yet, efforts are ongoing to establish subunit vaccines that can enhance smallpox vaccines in time and place, increasing the longevity of immunization and decreasing the occurrence of side effects in the systems beyond immunity, i.e., the nervous system [184].
Cross-reactivity is, in general, a poorly understood immunological phenomenon; however, its promises are attractive, so one might hypothesize about having a broad-spectrum anti-PXV agent. Fortunately, this hypothesis could be proved correct by the presence of cross-neutralizing antibodies for VACV, VARV, CPXV, and MPXV in humans [185]. Whether vaccination against smallpox infection offers a strategy to protect against other poxvirus infections remains to be further investigated, and if so, then it is to measure the amount of protection. The measurement is important for determining how to manage the likelihood of resurging outbreaks, i.e., the case of an ongoing MPXV outbreak, which is influenced by different factors, including at least waned protection and evolved evasion mechanisms.
There have been several vaccines developed for the prevention of smallpox. Studies show that these vaccines are potent to protect against monkeypox to a great extent, as well. For example, the study [186] calculated the efficacy of smallpox vaccination to be as high as 89 % for MPXV protection. Per the Centers for Disease Control and Prevention’s report last updated on August 20, 2022, two vaccines are approved during the current monkeypox outbreak: JYNNEOS and ACAM2000. JYNNEOS is a replication-deficient MVA, third-generation vaccine, while ACAM2000 is a replication-competent VACV, second-generation vaccine [187]. As it is replication-defective, JYNNEOS is associated with a narrower range of and also less severe complications than ACAM2000. These vaccines are, in general, not recommended for the general population, and there have been indicated conditions for the administration of these vaccines. Such conditions are classified as high-risk, and include laboratory personnel, who work in research settings and deal with orthopoxviruses, laboratory or healthcare personnel who undertake diagnostic tests for orthopoxviruses or ACAM2000 administration, healthcare providers who help orthopoxviruses-infected people, and designated members of response team. ACAM2000 is contraindicated for administering to people with severe immunodeficiency, while JYNNEOS can be considered for such people with some precautions.
What concerns us goes beyond the current MPXV outbreak; suppose the re-emergence of smallpox and the emergence of new PXVs. The generations born in the last four decades have not received smallpox vaccination. This means people 0 to 40 years old have low to zero immunity to VARV. In addition to the need to revisit the smallpox vaccination, specific therapeutics are required. With our discussion above, why not immunotherapies?
7. Conclusion
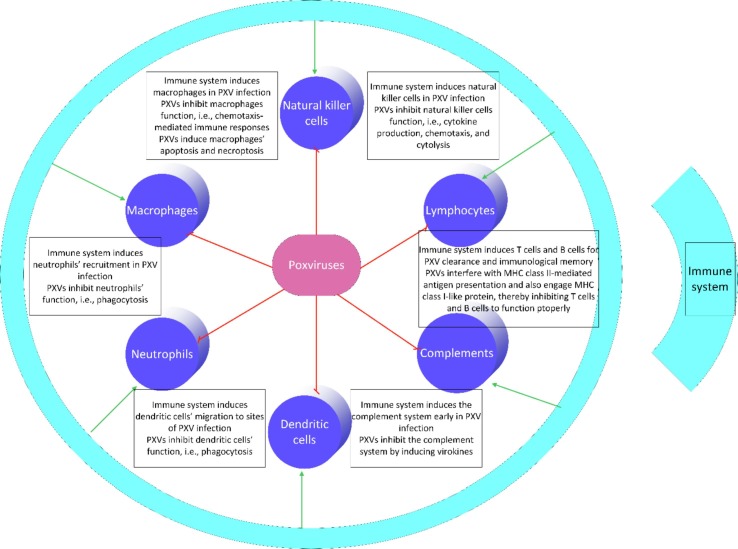
This review concludes that although the immune system engages receptors and cells of all the three levels of innate, innate-adaptive, and adaptive, PXVs are able to adopt mechanisms to interfere with all the immune military (Fig. 2 ). These mechanisms have implications for designing targeted immunotherapy approaches.
Fig. 2.
Immune cells and poxviruses (PXVs). The immune system induces all cells of the innate arm, innate-adaptive interface, and adaptive arm in PXV infection. On the other hand, PXVs can inhibit these cells to function properly through different mechanisms as listed throughout the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Data availability
No data was used for the research described in the article.
References
- 1.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl.Trop. Dis. 2022;16(2):e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.S.D. Edouard Mathieu, Hannah Ritchie and Max Roser (2022) - “Monkeypox”. Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/monkeypox' [Online Resource], 2022. https://ourworldindata.org/monkeypox.
- 3.Geddes A.M. The history of smallpox. Clin. Dermatol. 2006;24(3):152–157. doi: 10.1016/j.clindermatol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z., Gray M., Winter L. Why do poxviruses still matter? Cell & bioscience. 2021;11(1):1–8. doi: 10.1186/s13578-021-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith S.A., Kotwa G.J. Immune response to poxvirus infections in various animals. Crit. Rev. Microbiol. 2002;28(3):149–185. doi: 10.1080/1040-840291046722. [DOI] [PubMed] [Google Scholar]
- 6.e.M.M.t.e.G.T.U.o.T.M.B.a.G.C.A.f. Baxby D. Poxviruses. In: Baron S. [PubMed]
- 7.Seet B.T., Johnston J.B., Brunetti C.R., Barrett J.W., Everett H., Cameron C., Sypula J., Nazarian S.H., Lucas A., McFadden G. Poxviruses and immune evasion. Annu. Rev. Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 8.Lane R.K., Xiang Y. In: Encyclopedia of Infection and Immunity. Rezaei N., editor. Elsevier; Oxford: 2022. Poxvirus; pp. 146–153. [Google Scholar]
- 9.Moss B. Poxvirus DNA replication. Cold Spring Harbor Perspect. Biol. 2013;5(9) doi: 10.1101/cshperspect.a010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4(5):688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Gorman W.E., Sampath P., Simonds E.F., Sikorski R., O'Malley M., Krutzik P.O., Chen H., Panchanathan V., Chaudhri G., Karupiah G. Alternate mechanisms of initial pattern recognition drive differential immune responses to related poxviruses. Cell Host Microbe. 2010;8(2):174–185. doi: 10.1016/j.chom.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin D.B. The cause of death in smallpox: an examination of the pathology record. Mil. Med. 2002;167(7):546–551. [PubMed] [Google Scholar]
- 13.Schmidt F.I., Bleck C.K.E., Mercer J. Poxvirus host cell entry. Current opinion in virology. 2012;2(1):20–27. doi: 10.1016/j.coviro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 14.McFadden G. Poxvirus tropism. Nat. Rev. Microbiol. 2005;3(3):201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saghazadeh A., Rezaei N. Introductory chapter: toll-like receptors. Toll-like Receptors. 2020 [Google Scholar]
- 16.Saghazadeh A., Rezaei N. Implications of Toll-like receptors in Ebola infection. Expert Opinion on Therapeutic Targets. 2017;21(4):415–425. doi: 10.1080/14728222.2017.1299128. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan K.B., Park H.H. Toll/interleukin-1 receptor (TIR) domain-mediated cellular signaling pathways. Apoptosis. 2015;20(2):196–209. doi: 10.1007/s10495-014-1073-1. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon B., Aleithan F., Abdul-Sater Z., Abdul-Sater A.A. The Evolving Role of TRAFs in Mediating Inflammatory Responses. Front. Immunol. 2019;10:104. doi: 10.3389/fimmu.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra S., Nájera J.L., González J.M., López-Fernández L.A., Climent N., Gatell J.M., Gallart T., Esteban M. Distinct gene expression profiling after infection of immature human monocyte-derived dendritic cells by the attenuated poxvirus vectors MVA and NYVAC. J Virol. 2007;81(16):8707–8721. doi: 10.1128/JVI.00444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Martinez J., Huang X., Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109(2):619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H., Bruneau R.C., Brennan G., Rothenburg S. Battle royale: innate recognition of poxviruses and viral immune evasion. Biomedicines. 2021;9(7):765. doi: 10.3390/biomedicines9070765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y., Zhang L. DNA-sensing antiviral innate immunity in poxvirus infection. Front. Immunol. 2020;11:1637. doi: 10.3389/fimmu.2020.01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady G., Bowie A.G. Innate immune activation of NFκB and its antagonism by poxviruses. Cytokine Growth Factor Rev. 2014;25(5):611–620. doi: 10.1016/j.cytogfr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Martinez J., Huang X., Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. PNAS. 2010;107(14):6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Challa S., Woelfel M., Guildford M., Moquin D., Chan F.K. Viral cell death inhibitor MC159 enhances innate immunity against vaccinia virus infection. J Virol. 2010;84(20):10467–10476. doi: 10.1128/JVI.00983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Gorman W.E., Sampath P., Simonds E.F., Sikorski R., O'Malley M., Krutzik P.O., Chen H., Panchanathan V., Chaudhri G., Karupiah G., Lewis D.B., Thorne S.H., Nolan G.P. Alternate mechanisms of initial pattern recognition drive differential immune responses to related poxviruses. Cell Host Microbe. 2010;8(2):174–185. doi: 10.1016/j.chom.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman M.M., McFadden G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways. J Virol. 2011;85(23):12505–12517. doi: 10.1128/JVI.00410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao H., Dai P., Wang W., Li H., Yuan J., Wang F., Fang C.M., Pitha P.M., Liu J., Condit R.C., McFadden G., Merghoub T., Houghton A.N., Young J.W., Shuman S., Deng L. Innate immune response of human plasmacytoid dendritic cells to poxvirus infection is subverted by vaccinia E3 via its Z-DNA/RNA binding domain. PLoS ONE. 2012;7(5):e36823. doi: 10.1371/journal.pone.0036823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Z. Wang, D.T. MacLeod, A. Di Nardo, Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses, Journal of immunology (Baltimore, Md. : 1950) 189(4) (2012) 1551-8. [DOI] [PMC free article] [PubMed]
- 30.Perkins D.J., Polumuri S.K., Pennini M.E., Lai W., Xie P., Vogel S.N. Reprogramming of murine macrophages through TLR2 confers viral resistance via TRAF3-mediated, enhanced interferon production. PLoS Pathog. 2013;9(7):e1003479. doi: 10.1371/journal.ppat.1003479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Buttlar H., Siegemund S., Büttner M., Alber G. Identification of Toll-like receptor 9 as parapoxvirus ovis-sensing receptor in plasmacytoid dendritic cells. PLoS ONE. 2014;9(8):e106188. doi: 10.1371/journal.pone.0106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struzik J., Szulc-Dąbrowska L., Papiernik D., Winnicka A., Niemiałtowski M. Modulation of proinflammatory NF-κB signaling by ectromelia virus in RAW 264.7 murine macrophages. Arch Virol. 2015;160(9):2301–2314. doi: 10.1007/s00705-015-2507-y. [DOI] [PubMed] [Google Scholar]
- 33.Dempsey A., Keating S.E., Carty M., Bowie A.G. Poxviral protein E3-altered cytokine production reveals that DExD/H-box helicase 9 controls Toll-like receptor-stimulated immune responses. The Journal of biological chemistry. 2018;293(39):14989–15001. doi: 10.1074/jbc.RA118.005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.(!!! INVALID CITATION !!! [10-13]).
- 35.Bowie A., Kiss-Toth E., Symons J.A., Smith G.L., Dower S.K., O'Neill L.A. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. PNAS. 2000;97(18):10162–10167. doi: 10.1073/pnas.160027697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröder M., Baran M., Bowie A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. The EMBO journal. 2008;27(15):2147–2157. doi: 10.1038/emboj.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.T. Lysakova-Devine, B. Keogh, B. Harrington, K. Nagpal, A. Halle, D.T. Golenbock, T. Monie, A.G. Bowie, Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule, Journal of immunology (Baltimore, Md. : 1950) 185(7) (2010) 4261-71. [DOI] [PubMed]
- 38.Harte M.T., Haga I.R., Maloney G., Gray P., Reading P.C., Bartlett N.W., Smith G.L., Bowie A., O'Neill L.A. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med. 2003;197(3):343–351. doi: 10.1084/jem.20021652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiPerna G., Stack J., Bowie A.G., Boyd A., Kotwal G., Zhang Z., Arvikar S., Latz E., Fitzgerald K.A., Marshall W.L. Poxvirus protein N1L targets the I-kappaB kinase complex, inhibits signaling to NF-kappaB by the tumor necrosis factor superfamily of receptors, and inhibits NF-kappaB and IRF3 signaling by toll-like receptors. The Journal of biological chemistry. 2004;279(35):36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- 40.S. Fedosyuk, I. Grishkovskaya, E. de Almeida Ribeiro, Jr., T. Skern, Characterization and structure of the vaccinia virus NF-κB antagonist A46, The Journal of biological chemistry 289(6) (2014) 3749-62. [DOI] [PMC free article] [PubMed]
- 41.DiPerna G., Stack J., Bowie A.G., Boyd A., Kotwal G., Zhang Z., Arvikar S., Latz E., Fitzgerald K.A., Marshall W.L. Poxvirus protein N1L targets the I-κB kinase complex, inhibits signaling to NF-κB by the tumor necrosis factor superfamily of receptors, and inhibits NF-κB and IRF3 signaling by Toll-like receptors. J. Biol. Chem. 2004;279(35):36570–36578. doi: 10.1074/jbc.M400567200. [DOI] [PubMed] [Google Scholar]
- 42.González J.M., Esteban M. A poxvirus Bcl-2-like gene family involved in regulation of host immune response: sequence similarity and evolutionary history. Virology journal. 2010;7:59. doi: 10.1186/1743-422X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oda S.-I., Franklin E., Khan A.R. Poxvirus A46 protein binds to TIR domain-containing Mal/TIRAP via an α-helical sub-domain. Mol. Immunol. 2011;48(15–16):2144–2150. doi: 10.1016/j.molimm.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Franklin E., Khan A.R. Poxvirus antagonism of innate immunity by Bcl-2 fold proteins. J Struct Biol. 2013;181(1):1–10. doi: 10.1016/j.jsb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 45.M. Hutchens, K.E. Luker, P. Sottile, J. Sonstein, N.W. Lukacs, G. Núñez, J.L. Curtis, G.D. Luker, TLR3 increases disease morbidity and mortality from vaccinia infection, Journal of immunology (Baltimore, Md. : 1950) 180(1) (2008) 483-91. [DOI] [PMC free article] [PubMed]
- 46.Samuelsson C., Hausmann J., Lauterbach H., Schmidt M., Akira S., Wagner H., Chaplin P., Suter M., O'Keeffe M., Hochrein H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J. Clin. Investig. 2008;118(5):1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hutchens M.A., Luker K.E., Sonstein J., Núñez G., Curtis J.L., Luker G.D. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4(9):e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu R.H., Wong E.B., Rubio D., Roscoe F., Ma X., Nair S., Remakus S., Schwendener R., John S., Shlomchik M., Sigal L.J. Sequential Activation of Two Pathogen-Sensing Pathways Required for Type I Interferon Expression and Resistance to an Acute DNA Virus Infection. Immunity. 2015;43(6):1148–1159. doi: 10.1016/j.immuni.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong E., Xu R.H., Rubio D., Lev A., Stotesbury C., Fang M., Sigal L.J. Migratory Dendritic Cells, Group 1 Innate Lymphoid Cells, and Inflammatory Monocytes Collaborate to Recruit NK Cells to the Virus-Infected Lymph Node. Cell reports. 2018;24(1):142–154. doi: 10.1016/j.celrep.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quigley M., Martinez J., Huang X., Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113(10):2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martinez J., Huang X., Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6(3):e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies M.L., Sei J.J., Siciliano N.A., Xu R.H., Roscoe F., Sigal L.J., Eisenlohr L.C., Norbury C.C. MyD88-dependent immunity to a natural model of vaccinia virus infection does not involve Toll-like receptor 2. J Virol. 2014;88(6):3557–3567. doi: 10.1128/JVI.02776-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martínez O., Miranda E., Ramírez M., Santos S., Rivera C., Vázquez L., Sánchez T., Tremblay R.L., Ríos-Olivares E., Otero M. Immunomodulator-based enhancement of anti smallpox immune responses. PLoS ONE. 2015;10(4):e0123113. doi: 10.1371/journal.pone.0123113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rehwinkel J., Gack M.U. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020;20(9):537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F., Gao X., Barrett J.W., Shao Q., Bartee E., Mohamed M.R., Rahman M., Werden S., Irvine T., Cao J., Dekaban G.A., McFadden G. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 2008;4(7):e1000099. doi: 10.1371/journal.ppat.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harenberg A., Guillaume F., Ryan E.J., Burdin N., Spada F. Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells. Vaccine. 2008;26(39):5004–5013. doi: 10.1016/j.vaccine.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myskiw C., Arsenio J., Booy E.P., Hammett C., Deschambault Y., Gibson S.B., Cao J. RNA species generated in vaccinia virus infected cells activate cell type-specific MDA5 or RIG-I dependent interferon gene transcription and PKR dependent apoptosis. Virology. 2011;413(2):183–193. doi: 10.1016/j.virol.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 58.Pollpeter D., Komuro A., Barber G.N., Horvath C.M. Impaired cellular responses to cytosolic DNA or infection with Listeria monocytogenes and vaccinia virus in the absence of the murine LGP2 protein. PLoS ONE. 2011;6(4):e18842. doi: 10.1371/journal.pone.0018842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eitz Ferrer P., Potthoff S., Kirschnek S., Gasteiger G., Kastenmüller W., Ludwig H., Paschen S.A., Villunger A., Sutter G., Drexler I., Häcker G. Induction of Noxa-mediated apoptosis by modified vaccinia virus Ankara depends on viral recognition by cytosolic helicases, leading to IRF-3/IFN-β-dependent induction of pro-apoptotic Noxa. PLoS Pathog. 2011;7(6):e1002083. doi: 10.1371/journal.ppat.1002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham A.M., Santa Maria F.G., Lahiri T., Friedman E., Marié I.J., Levy D.E. PKR Transduces MDA5-Dependent Signals for Type I IFN Induction. PLoS Pathog. 2016;12(3):e1005489. doi: 10.1371/journal.ppat.1005489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chibssa T.R., Kangethe R.T., Berguido F.J., Settypalli T.B.K., Liu Y., Grabherr R., Loitsch A., Sassu E.L., Pichler R., Cattoli G., Diallo A., Wijewardana V., Lamien C.E. Innate Immune Responses to Wildtype and Attenuated Sheeppox Virus Mediated Through RIG-1 Sensing in PBMC In-Vitro. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.666543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.AlDaif B.A., Mercer A.A., Fleming S.B. The parapoxvirus Orf virus inhibits IFN-β expression induced by dsRNA. Virus Res. 2022;307 doi: 10.1016/j.virusres.2021.198619. [DOI] [PubMed] [Google Scholar]
- 63.Yang N., Luna J.M., Dai P., Wang Y., Rice C.M., Deng L. Lung type II alveolar epithelial cells collaborate with CCR2(+) inflammatory monocytes in host defense against poxvirus infection. Nat. Commun. 2022;13(1):1671. doi: 10.1038/s41467-022-29308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marq J.B., Hausmann S., Luban J., Kolakofsky D., Garcin D. The double-stranded RNA binding domain of the vaccinia virus E3L protein inhibits both RNA- and DNA-induced activation of interferon beta. The Journal of biological chemistry. 2009;284(38):25471–25478. doi: 10.1074/jbc.M109.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deng L., Dai P., Parikh T., Cao H., Bhoj V., Sun Q., Chen Z., Merghoub T., Houghton A., Shuman S. Vaccinia virus subverts a mitochondrial antiviral signaling protein-dependent innate immune response in keratinocytes through its double-stranded RNA binding protein, E3. J Virol. 2008;82(21):10735–10746. doi: 10.1128/JVI.01305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang P., Langland J.O., Jacobs B.L., Samuel C.E. Protein kinase PKR-dependent activation of mitogen-activated protein kinases occurs through mitochondrial adapter IPS-1 and is antagonized by vaccinia virus E3L. J Virol. 2009;83(11):5718–5725. doi: 10.1128/JVI.00224-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahman M.M., Bagdassarian E., Ali M.A.M., McFadden G. Identification of host DEAD-box RNA helicases that regulate cellular tropism of oncolytic Myxoma virus in human cancer cells. Sci. Rep. 2017;7(1):15710. doi: 10.1038/s41598-017-15941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y.K., Shin J.S., Nahm M.H. NOD-Like Receptors in Infection, Immunity, and Diseases. Yonsei Med J. 2016;57(1):5–14. doi: 10.3349/ymj.2016.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.F. Liu, Q. Niu, X. Fan, C. Liu, J. Zhang, Z. Wei, W. Hou, T.D. Kanneganti, M.L. Robb, J.H. Kim, N.L. Michael, J. Sun, L. Soong, H. Hu, Priming and Activation of Inflammasome by Canarypox Virus Vector ALVAC via the cGAS/IFI16-STING-Type I IFN Pathway and AIM2 Sensor, Journal of immunology (Baltimore, Md. : 1950) 199(9) (2017) 3293-3305. [DOI] [PMC free article] [PubMed]
- 72.Zimmerling S., Waibler Z., Resch T., Sutter G., Schwantes A. Interleukin-1β receptor expressed by modified vaccinia virus Ankara interferes with interleukin-1β activity produced in various virus-infected antigen-presenting cells. Virology journal. 2013;10:34. doi: 10.1186/1743-422X-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sagoo P., Garcia Z., Breart B., Lemaître F., Michonneau D., Albert M.L., Levy Y., Bousso P. In vivo imaging of inflammasome activation reveals a subcapsular macrophage burst response that mobilizes innate and adaptive immunity. Nat. Med. 2016;22(1):64–71. doi: 10.1038/nm.4016. [DOI] [PubMed] [Google Scholar]
- 74.Hazlewood J.E., Dumenil T., Le T.T., Slonchak A., Kazakoff S.H., Patch A.M., Gray L.A., Howley P.M., Liu L., Hayball J.D., Yan K., Rawle D.J., Prow N.A., Suhrbier A. Injection site vaccinology of a recombinant vaccinia-based vector reveals diverse innate immune signatures. PLoS Pathog. 2021;17(1):e1009215. doi: 10.1371/journal.ppat.1009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garg R.R., Jackson C.B., Rahman M.M., Khan A.R., Lewin A.S., McFadden G. Myxoma virus M013 protein antagonizes NF-κB and inflammasome pathways via distinct structural motifs. The Journal of biological chemistry. 2019;294(21):8480–8489. doi: 10.1074/jbc.RA118.006040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rahman M.M., Mohamed M.R., Kim M., Smallwood S., McFadden G. Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 2009;5(10):e1000635. doi: 10.1371/journal.ppat.1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerlic M., Faustin B., Postigo A., Yu E.C., Proell M., Gombosuren N., Krajewska M., Flynn R., Croft M., Way M., Satterthwait A., Liddington R.C., Salek-Ardakani S., Matsuzawa S., Reed J.C. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. PNAS. 2013;110(19):7808–7813. doi: 10.1073/pnas.1215995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnston J.B., Barrett J.W., Nazarian S.H., Goodwin M., Ricciuto D., Wang G., McFadden G. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity. 2005;23(6):587–598. doi: 10.1016/j.immuni.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 79.Paludan S.R., Bowie A.G. Immune sensing of DNA. Immunity. 2013;38(5):870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia P., Ye B., Wang S., Zhu X., Du Y., Xiong Z., Tian Y., Fan Z. Glutamylation of the DNA sensor cGAS regulates its binding and synthase activity in antiviral immunity. Nat. Immunol. 2016;17(4):369–378. doi: 10.1038/ni.3356. [DOI] [PubMed] [Google Scholar]
- 81.Gentili M., Kowal J., Tkach M., Satoh T., Lahaye X., Conrad C., Boyron M., Lombard B., Durand S., Kroemer G., Loew D., Dalod M., Théry C., Manel N. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science. 2015;349(6253):1232–1236. doi: 10.1126/science.aab3628. [DOI] [PubMed] [Google Scholar]
- 82.Ablasser A., Schmid-Burgk J.L., Hemmerling I., Horvath G.L., Schmidt T., Latz E., Hornung V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503(7477):530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang R., Jiang Q., Guan Y., Gao P., Zhang R., Zhao Z., Jiang Z. Golgi apparatus-synthesized sulfated glycosaminoglycans mediate polymerization and activation of the cGAMP sensor STING. Immunity. 2021;54(5):962–975.e8. doi: 10.1016/j.immuni.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 84.Takahama M., Fukuda M., Ohbayashi N., Kozaki T., Misawa T., Okamoto T., Matsuura Y., Akira S., Saitoh T. The RAB2B-GARIL5 Complex Promotes Cytosolic DNA-Induced Innate Immune Responses. Cell reports. 2017;20(12):2944–2954. doi: 10.1016/j.celrep.2017.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliveira M., Rodrigues D.R., Guillory V., Kut E., Giotis E.S., Skinner M.A., Guabiraba R., Bryant C.E., Ferguson B.J. Chicken cGAS Senses Fowlpox Virus Infection and Regulates Macrophage Effector Functions. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.613079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng W.Y., He X.B., Jia H.J., Chen G.H., Jin Q.W., Long Z.L., Jing Z.Z. The cGas-Sting Signaling Pathway Is Required for the Innate Immune Response Against Ectromelia Virus. Front Immunol. 2018;9:1297. doi: 10.3389/fimmu.2018.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernáez B., Alonso G., Georgana I., El-Jesr M., Martín R., Shair K.H.Y., Fischer C., Sauer S., Maluquer de Motes C., Alcamí A. Viral cGAMP nuclease reveals the essential role of DNA sensing in protection against acute lethal virus infection. Sci. Adv. 2020;6(38) doi: 10.1126/sciadv.abb4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong E.B., Montoya B., Ferez M., Stotesbury C., Sigal L.J. Resistance to ectromelia virus infection requires cGAS in bone marrow-derived cells which can be bypassed with cGAMP therapy. PLoS Pathog. 2019;15(12):e1008239. doi: 10.1371/journal.ppat.1008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hood A.J.M., Sumner R.P., Maluquer de Motes C. Disruption of the cGAS/STING axis does not impair sensing of MVA in BHK21 cells. The Journal of general virology. 2022;103(5) doi: 10.1099/jgv.0.001755. [DOI] [PubMed] [Google Scholar]
- 90.Georgana I., Sumner R.P., Towers G.J., Maluquer de Motes C. Virulent Poxviruses Inhibit DNA Sensing by Preventing STING Activation. J Virol. 2018;92(10) doi: 10.1128/JVI.02145-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meade N., King M., Munger J., Walsh D. mTOR Dysregulation by Vaccinia Virus F17 Controls Multiple Processes with Varying Roles in Infection. J Virol. 2019;93(15) doi: 10.1128/JVI.00784-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meade N., Furey C., Li H., Verma R., Chai Q., Rollins M.G., DiGiuseppe S., Naghavi M.H., Walsh D. Poxviruses Evade Cytosolic Sensing through Disruption of an mTORC1-mTORC2 Regulatory Circuit. Cell. 2018;174(5):1143–1157.e17. doi: 10.1016/j.cell.2018.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eaglesham J.B., Pan Y., Kupper T.S., Kranzusch P.J. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature. 2019;566(7743):259–263. doi: 10.1038/s41586-019-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ning X., Wang Y., Jing M., Sha M., Lv M., Gao P., Zhang R., Huang X., Feng J.M., Jiang Z. Apoptotic Caspases Suppress Type I Interferon Production via the Cleavage of cGAS, MAVS, and IRF3. Mol. Cell. 2019;74(1):19–31.e7. doi: 10.1016/j.molcel.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 95.Ferguson B.J., Mansur D.S., Peters N.E., Ren H., Smith G.L. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. eLife. 2012;1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scutts S.R., Ember S.W., Ren H., Ye C., Lovejoy C.A., Mazzon M., Veyer D.L., Sumner R.P., Smith G.L. DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing. Cell reports. 2018;25(7):1953–1965.e4. doi: 10.1016/j.celrep.2018.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peters N.E., Ferguson B.J., Mazzon M., Fahy A.S., Krysztofinska E., Arribas-Bosacoma R., Pearl L.H., Ren H., Smith G.L. A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus. PLoS Pathog. 2013;9(10):e1003649. doi: 10.1371/journal.ppat.1003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rahman M.M., Gutierrez-Jensen A.D., Glenn H.L., Abrantes M., Moussatche N., McFadden G. RNA Helicase A/DHX9 Forms Unique Cytoplasmic Antiviral Granules That Restrict Oncolytic Myxoma Virus Replication in Human Cancer Cells. J Virol. 2021;95(14):e0015121. doi: 10.1128/JVI.00151-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim Y.G., Muralinath M., Brandt T., Pearcy M., Hauns K., Lowenhaupt K., Jacobs B.L., Rich A. A role for Z-DNA binding in vaccinia virus pathogenesis. PNAS. 2003;100(12):6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Szczerba M., Subramanian S., Trainor K., McCaughan M., Kibler K.V., Jacobs B.L. Small Hero with Great Powers: Vaccinia Virus E3 Protein and Evasion of the Type I IFN Response. Biomedicines. 2022;10(2) doi: 10.3390/biomedicines10020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.H. Koehler, S. Cotsmire, J. Langland, K.V. Kibler, D. Kalman, J.W. Upton, E.S. Mocarski, B.L. Jacobs, Inhibition of DAI-dependent necroptosis by the Z-DNA binding domain of the vaccinia virus innate immune evasion protein, E3, Proceedings of the National Academy of Sciences of the United States of America 114(43) (2017) 11506-11511. [DOI] [PMC free article] [PubMed]
- 102.Koehler H., Cotsmire S., Zhang T., Balachandran S., Upton J.W., Langland J., Kalman D., Jacobs B.L., Mocarski E.S. Vaccinia virus E3 prevents sensing of Z-RNA to block ZBP1-dependent necroptosis. Cell Host Microbe. 2021;29(8):1266–1276.e5. doi: 10.1016/j.chom.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Balachandran S., Mocarski E.S. Viral Z-RNA triggers ZBP1-dependent cell death. Curr Opin Virol. 2021;51:134–140. doi: 10.1016/j.coviro.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerscher B., Willment J.A., Brown G.D. The Dectin-2 family of C-type lectin-like receptors: an update. Int. Immunol. 2013;25(5):271–277. doi: 10.1093/intimm/dxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahla R., Reddy C., Prasad D., Kumar H. Sweeten PAMPs: Role of sugar complexed PAMPs in innate immunity and vaccine biology. Front. Immunol. 2013;4 doi: 10.3389/fimmu.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ginhoux F., Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14(6):392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 107.Hume D.A., Irvine K.M., Pridans C. The mononuclear phagocyte system: the relationship between monocytes and macrophages. Trends Immunol. 2019;40(2):98–112. doi: 10.1016/j.it.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 108.Kedzierska K., Crowe S.M. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr. Med. Chem. 2002;9(21):1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- 109.Ohmer M., Weber A., Sutter G., Ehrhardt K., Zimmermann A., Häcker G. Anti-apoptotic Bcl-XL but not Mcl-1 contributes to protection against virus-induced apoptosis. Cell Death Dis. 2016;7(8):e2340. doi: 10.1038/cddis.2016.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.MacLeod D.T., Nakatsuji T., Wang Z., di Nardo A., Gallo R.L. Vaccinia virus binds to the scavenger receptor MARCO on the surface of keratinocytes. J. Invest. Dermatol. 2015;135(1):142–150. doi: 10.1038/jid.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kasani S.K., Cheng H.Y., Yeh K.H., Chang S.J., Hsu P.W., Tung S.Y., Liang C.T., Chang W. Differential Innate Immune Signaling in Macrophages by Wild-Type Vaccinia Mature Virus and a Mutant Virus with a Deletion of the A26 Protein. J Virol. 2017;91(18) doi: 10.1128/JVI.00767-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baldanta S., Fernández-Escobar M., Acín-Perez R., Albert M., Camafeita E., Jorge I., Vázquez J., Enríquez J.A., Guerra S. ISG15 governs mitochondrial function in macrophages following vaccinia virus infection. PLoS Pathog. 2017;13(10):e1006651. doi: 10.1371/journal.ppat.1006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yángüez E., García-Culebras A., Frau A., Llompart C., Knobeloch K.P., Gutierrez-Erlandsson S., García-Sastre A., Esteban M., Nieto A., Guerra S. ISG15 regulates peritoneal macrophages functionality against viral infection. PLoS Pathog. 2013;9(10):e1003632. doi: 10.1371/journal.ppat.1003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schneider C., Nobs S.P., Heer A.K., Kurrer M., Klinke G., van Rooijen N., Vogel J., Kopf M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10(4):e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davies M.L., Parekh N.J., Kaminsky L.W., Soni C., Reider I.E., Krouse T.E., Fischer M.A., van Rooijen N., Rahman Z.S.M., Norbury C.C. A systemic macrophage response is required to contain a peripheral poxvirus infection. PLoS Pathog. 2017;13(6):e1006435. doi: 10.1371/journal.ppat.1006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Byrd D., Shepherd N., Lan J., Hu N., Amet T., Yang K., Desai M., Yu Q. Primary human macrophages serve as vehicles for vaccinia virus replication and dissemination. J Virol. 2014;88(12):6819–6831. doi: 10.1128/JVI.03726-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klaas L., Vier J., Gentle I.E., Häcker G., Kirschnek S. Diversity of cell death signaling pathways in macrophages upon infection with modified vaccinia virus Ankara (MVA) Cell Death Dis. 2021;12(11):1011. doi: 10.1038/s41419-021-04286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosenbaum P., Tchitchek N., Joly C., Stimmer L., Hocini H., Dereuddre-Bosquet N., Beignon A.S., Chapon C., Levy Y., Le Grand R., Martinon F. Molecular and Cellular Dynamics in the Skin, the Lymph Nodes, and the Blood of the Immune Response to Intradermal Injection of Modified Vaccinia Ankara Vaccine. Front Immunol. 2018;9:870. doi: 10.3389/fimmu.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Altenburg A.F., van de Sandt C.E., Li B.W.S., MacLoughlin R.J., Fouchier R.A.M., van Amerongen G., Volz A., Hendriks R.W., de Swart R.L., Sutter G., Rimmelzwaan G.F., de Vries R.D. Modified Vaccinia Virus Ankara Preferentially Targets Antigen Presenting Cells In Vitro, Ex Vivo and In Vivo. Sci. Rep. 2017;7(1):8580. doi: 10.1038/s41598-017-08719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lehmann M.H., Torres-Domínguez L.E., Price P.J., Brandmüller C., Kirschning C.J., Sutter G. CCL2 expression is mediated by type I IFN receptor and recruits NK and T cells to the lung during MVA infection. J. Leukoc. Biol. 2016;99(6):1057–1064. doi: 10.1189/jlb.4MA0815-376RR. [DOI] [PubMed] [Google Scholar]
- 121.Lehmann M.H., Price P.J., Brandmüller C., Sutter G. Modified Vaccinia virus Ankara but not vaccinia virus induces chemokine expression in cells of the monocyte/macrophage lineage. Virology journal. 2015;12:21. doi: 10.1186/s12985-015-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Price P.J., Luckow B., Torres-Domínguez L.E., Brandmüller C., Zorn J., Kirschning C.J., Sutter G., Lehmann M.H. Chemokine (C-C Motif) receptor 1 is required for efficient recruitment of neutrophils during respiratory infection with modified vaccinia virus Ankara. J Virol. 2014;88(18):10840–10850. doi: 10.1128/JVI.01524-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.van Dinther D., Veninga H., Iborra S., Borg E.G.F., Hoogterp L., Olesek K., Beijer M.R., Schetters S.T.T., Kalay H., Garcia-Vallejo J.J., Franken K.L., Cham L.B., Lang K.S., van Kooyk Y., Sancho D., Crocker P.R., den Haan J.M.M. Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8(+) T Cell Cross-Priming. Cell reports. 2018;22(6):1484–1495. doi: 10.1016/j.celrep.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 124.García-Arriaza J., Gómez C.E., Sorzano C., Esteban M. Deletion of the vaccinia virus N2L gene encoding an inhibitor of IRF3 improves the immunogenicity of modified vaccinia virus Ankara expressing HIV-1 antigens. J Virol. 2014;88(6):3392–3410. doi: 10.1128/JVI.02723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kuo N.W., Gao Y.G., Schill M.S., Isern N., Dupureur C.M., LiWang P.J. Structural insights into the interaction between a potent anti-inflammatory protein, viral CC chemokine inhibitor (vCCI), and the human CC chemokine, Eotaxin-1. The Journal of biological chemistry. 2014;289(10):6592–6603. doi: 10.1074/jbc.M113.538991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wong P.S., Sutejo R., Chen H., Ng S.H., Sugrue R.J., Tan B.H. A System Based-Approach to Examine Cytokine Response in Poxvirus-Infected Macrophages. Viruses. 2018;10(12) doi: 10.3390/v10120692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kwiecien J.M., Dabrowski W., Kwiecien-Delaney B.J., Kwiecien-Delaney C.J., Siwicka-Gieroba D., Yaron J.R., Zhang L., Delaney K.H., Lucas A.R. Neuroprotective Effect of Subdural Infusion of Serp-1 in Spinal Cord Trauma. Biomedicines. 2020;8(10) doi: 10.3390/biomedicines8100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kwiecien J.M., Dabrowski W., Marzec-Kotarska B., Kwiecien-Delaney C.J., Yaron J.R., Zhang L., Schutz L., Lucas A.R. Myxoma virus derived immune modulating proteins, M-T7 and Serp-1, reduce early inflammation after spinal cord injury in the rat model. Folia Neuropathol. 2019;57(1):41–50. doi: 10.5114/fn.2019.83830. [DOI] [PubMed] [Google Scholar]
- 129.Yaron J.R., Zhang L., Guo Q., Awo E.A., Burgin M., Schutz L.N., Zhang N., Kilbourne J., Daggett-Vondras J., Lowe K.M., Lucas A.R. Recombinant Myxoma Virus-Derived Immune Modulator M-T7 Accelerates Cutaneous Wound Healing and Improves Tissue Remodeling. Pharmaceutics. 2020;12(11) doi: 10.3390/pharmaceutics12111003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Governa V., Brittoli A., Mele V., Pinamonti M., Terracciano L., Muenst S., Iezzi G., Spagnoli G.C., Zajac P., Trella E. A replication-incompetent CD154/40L recombinant vaccinia virus induces direct and macrophage-mediated antitumor effects in vitro and in vivo. Oncoimmunology. 2019;8(5):e1568162. doi: 10.1080/2162402X.2019.1568162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells, science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mujal A.M., Delconte R.B., Sun J.C. Natural killer cells: From innate to adaptive features. Annu. Rev. Immunol. 2021;39:417–447. doi: 10.1146/annurev-immunol-101819-074948. [DOI] [PubMed] [Google Scholar]
- 133.Waggoner S.N., Reighard S.D., Gyurova I.E., Cranert S.A., Mahl S.E., Karmele E.P., McNally J.P., Moran M.T., Brooks T.R., Yaqoob F., Rydyznski C.E. Roles of natural killer cells in antiviral immunity. Current Opinion in Virology. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Abboud G., Tahiliani V., Desai P., Varkoly K., Driver J., Hutchinson T.E., Salek-Ardakani S. Natural Killer Cells and Innate Interferon Gamma Participate in the Host Defense against Respiratory Vaccinia Virus Infection. J Virol. 2016;90(1):129–141. doi: 10.1128/JVI.01894-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daussy C., Faure F., Mayol K., Viel S., Gasteiger G., Charrier E., Bienvenu J., Henry T., Debien E., Hasan U.A., Marvel J., Yoh K., Takahashi S., Prinz I., de Bernard S., Buffat L., Walzer T. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J Exp Med. 2014;211(3):563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alves-Peixoto P., Férez M., Knudson C.J., Stotesbury C., Melo-Silva C.R., Wong E.B., Correia-Neves M., Sigal L.J. Chronic Lymphocytic Choriomeningitis Infection Causes Susceptibility to Mousepox and Impairs Natural Killer Cell Maturation and Function. J Virol. 2020;94(5) doi: 10.1128/JVI.01831-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Melo-Silva C.R., Alves-Peixoto P., Heath N., Tang L., Montoya B., Knudson C.J., Stotesbury C., Ferez M., Wong E., Sigal L.J. Resistance to lethal ectromelia virus infection requires Type I interferon receptor in natural killer cells and monocytes but not in adaptive immune or parenchymal cells. PLoS Pathog. 2021;17(5):e1009593. doi: 10.1371/journal.ppat.1009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mangan M.S., Melo-Silva C.R., Luu J., Bird C.H., Koskinen A., Rizzitelli A., Prakash M., Scarff K.L., Müllbacher A., Regner M., Bird P.I. A pro-survival role for the intracellular granzyme B inhibitor Serpinb9 in natural killer cells during poxvirus infection. Immunol. Cell Biol. 2017;95(10):884–894. doi: 10.1038/icb.2017.59. [DOI] [PubMed] [Google Scholar]
- 139.Bird C.H., Christensen M.E., Mangan M.S., Prakash M.D., Sedelies K.A., Smyth M.J., Harper I., Waterhouse N.J., Bird P.I. The granzyme B-Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death Differ. 2014;21(6):876–887. doi: 10.1038/cdd.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stutte S., Ruf J., Kugler I., Ishikawa-Ankerhold H., Parzefall A., Marconi P., Maeda T., Kaisho T., Krug A., Popper B., Lauterbach H., Colonna M., von Andrian U., Brocker T. Type I interferon mediated induction of somatostatin leads to suppression of ghrelin and appetite thereby promoting viral immunity in mice. Brain Behav. Immun. 2021;95:429–443. doi: 10.1016/j.bbi.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 141.Soday L., Lu Y., Albarnaz J.D., Davies C.T.R., Antrobus R., Smith G.L., Weekes M.P. Quantitative Temporal Proteomic Analysis of Vaccinia Virus Infection Reveals Regulation of Histone Deacetylases by an Interferon Antagonist. Cell reports. 2019;27(6):1920–1933.e7. doi: 10.1016/j.celrep.2019.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu J., Gallo R.M., Khan M.A., Iyer A.K., Kratzke I.M., Brutkiewicz R.R. JNK2 modulates the CD1d-dependent and -independent activation of iNKT cells. Eur J Immunol. 2019;49(2):255–265. doi: 10.1002/eji.201847755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hatfield S.D., Daniels K.A., O'Donnell C.L., Waggoner S.N., Welsh R.M. Weak vaccinia virus-induced NK cell regulation of CD4 T cells is associated with reduced NK cell differentiation and cytolytic activity. Virology. 2018;519:131–144. doi: 10.1016/j.virol.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jacobs N., Bartlett N.W., Clark R.H., Smith G.L. Vaccinia virus lacking the Bcl-2-like protein N1 induces a stronger natural killer cell response to infection. The Journal of general virology. 2008;89(Pt 11):2877–2881. doi: 10.1099/vir.0.2008/004119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song H., Josleyn N., Janosko K., Skinner J., Reeves R.K., Cohen M., Jett C., Johnson R., Blaney J.E., Bollinger L., Jennings G., Jahrling P.B. Monkeypox virus infection of rhesus macaques induces massive expansion of natural killer cells but suppresses natural killer cell functions. PLoS ONE. 2013;8(10):e77804. doi: 10.1371/journal.pone.0077804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alejo A., Ruiz-Argüello M.B., Pontejo S.M., Fernández de Marco M.D.M., Saraiva M., Hernáez B., Alcamí A. Chemokines cooperate with TNF to provide protective anti-viral immunity and to enhance inflammation. Nat. Commun. 2018;9(1):1790. doi: 10.1038/s41467-018-04098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jarahian M., Fiedler M., Cohnen A., Djandji D., Hämmerling G.J., Gati C., Cerwenka A., Turner P.C., Moyer R.W., Watzl C., Hengel H., Momburg F. Modulation of NKp30- and NKp46-mediated natural killer cell responses by poxviral hemagglutinin. PLoS Pathog. 2011;7(8):e1002195. doi: 10.1371/journal.ppat.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Earl P.L., Americo J.L., Moss B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020;16(4):e1008505. doi: 10.1371/journal.ppat.1008505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6(3):173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 150.Naumenko V., Turk M., Jenne C.N., Kim S.-J. Neutrophils in viral infection. Cell Tissue Res. 2018;371(3):505–516. doi: 10.1007/s00441-017-2763-0. [DOI] [PubMed] [Google Scholar]
- 151.Förster R., Wolf G., Mayr A. Highly attenuated poxviruses induce functional priming of neutrophils in vitro. Arch. Virol. 1994;136(1):219–226. doi: 10.1007/BF01538831. [DOI] [PubMed] [Google Scholar]
- 152.Jenne C.N., Wong C.H.Y., Zemp F.J., McDonald B., Rahman M.M., Forsyth P.A., McFadden G., Kubes P. Neutrophils Recruited to Sites of Infection Protect from Virus Challenge by Releasing Neutrophil Extracellular Traps. Cell Host Microbe. 2013;13(2):169–180. doi: 10.1016/j.chom.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 153.Walzer T., Galibert L., Smedt T.D. Poxvirus semaphorin A39R inhibits phagocytosis by dendritic cells and neutrophils. Eur. J. Immunol. 2005;35(2):391–398. doi: 10.1002/eji.200425669. [DOI] [PubMed] [Google Scholar]
- 154.Sarma J.V., Ward P.A. The complement system. Cell Tissue Res. 2011;343(1):227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mellors J., Tipton T., Longet S., Carroll M. Viral evasion of the complement system and its importance for vaccines and therapeutics. Front. Immunol. 2020;11:1450. doi: 10.3389/fimmu.2020.01450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moulton E.A., Atkinson J.P., Buller R.M.L. Surviving mousepox infection requires the complement system. PLoS Pathog. 2008;4(12):e1000249. doi: 10.1371/journal.ppat.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Johnston J.B., McFadden G. Poxvirus immunomodulatory strategies: current perspectives. J. Virol. 2003;77(11):6093–6100. doi: 10.1128/JVI.77.11.6093-6100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hoebe K., Janssen E., Beutler B. The interface between innate and adaptive immunity. Nat. Immunol. 2004;5(10):971–974. doi: 10.1038/ni1004-971. [DOI] [PubMed] [Google Scholar]
- 159.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 160.Steinman R.M., Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 161.Steinman R.M., Hemmi H. Dendritic cells: translating innate to adaptive immunity. From innate immunity to immunological memory. 2006:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 162.Lateef Z., Baird M.A., Wise L.M., Young S., Mercer A.A., Fleming S.B. The chemokine-binding protein encoded by the poxvirus orf virus inhibits recruitment of dendritic cells to sites of skin inflammation and migration to peripheral lymph nodes. Cell. Microbiol. 2010;12(5):665–676. doi: 10.1111/j.1462-5822.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- 163.Beauchamp N.M., Busick R.Y., Alexander-Miller M.A. Functional divergence among CD103+ dendritic cell subpopulations following pulmonary poxvirus infection. J. Virol. 2010;84(19):10191–10199. doi: 10.1128/JVI.00892-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.M.H. Andersen, D. Schrama, P. thor Straten, J.C. Becker, Cytotoxic T cells, Journal of Investigative Dermatology 126(1) (2006) 32-41. [DOI] [PubMed]
- 165.Luckheeram R.V., Zhou R., Verma A.D., Xia B. CD4+ T cells: differentiation and functions. Clinical and developmental immunology. 2012;2012 doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sant A.J., McMichael A. Revealing the role of CD4+ T cells in viral immunity. J. Exp. Med. 2012;209(8):1391–1395. doi: 10.1084/jem.20121517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mitra-Kaushik S., Cruz J., Stern L.J., Ennis F.A., Terajima M. Human cytotoxic CD4+ T cells recognize HLA-DR1-restricted epitopes on vaccinia virus proteins A24R and D1R conserved among poxviruses. J. Immunol. 2007;179(2):1303–1312. doi: 10.4049/jimmunol.179.2.1303. [DOI] [PubMed] [Google Scholar]
- 168.Fang M., Siciliano N.A., Hersperger A.R., Roscoe F., Hu A., Ma X., Shamsedeen A.R., Eisenlohr L.C., Sigal L.J. Perforin-dependent CD4+ T-cell cytotoxicity contributes to control a murine poxvirus infection. Proc. Natl. Acad. Sci. 2012;109(25):9983–9988. doi: 10.1073/pnas.1202143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Forsyth K.S., Eisenlohr L.C. Giving CD4+ T cells the slip: viral interference with MHC class II-restricted antigen processing and presentation. Curr. Opin. Immunol. 2016;40:123–129. doi: 10.1016/j.coi.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rapaport A.S., Schriewer J., Gilfillan S., Hembrador E., Crump R., Plougastel B.F., Wang Y., Le Friec G., Gao J., Cella M. The inhibitory receptor NKG2A sustains virus-specific CD8+ T cells in response to a lethal poxvirus infection. Immunity. 2015;43(6):1112–1124. doi: 10.1016/j.immuni.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Desai P., Tahiliani V., Abboud G., Stanfield J., Salek-Ardakani S. Batf3-dependent dendritic cells promote optimal CD8 T cell responses against respiratory poxvirus infection. J. Virol. 2018;92(16):e00495–e00518. doi: 10.1128/JVI.00495-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zavala F., Rodrigues M., Rodriguez D., Rodriguez J.R., Nussenzweig R.S., Esteban M. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology. 2001;280(2):155–159. doi: 10.1006/viro.2000.0792. [DOI] [PubMed] [Google Scholar]
- 173.Tscharke D.C., Woo W.-P., Sakala I.G., Sidney J., Sette A., Moss D.J., Bennink J.R., Karupiah G., Yewdell J.W. Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J. Virol. 2006;80(13):6318–6323. doi: 10.1128/JVI.00427-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sanz I., Wei C., Jenks S.A., Cashman K.S., Tipton C., Woodruff M.C., Hom J., Lee F.-E.-H. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Knox J.J., Myles A., Cancro M.P. T-bet+ memory B cells: generation, function, and fate. Immunol. Rev. 2019;288(1):149–160. doi: 10.1111/imr.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Moir S., Fauci A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chaudhri G., Panchanathan V., Bluethmann H., Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J. Virol. 2006;80(13):6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Campbell J.A., Davis R.S., Lilly L.M., Fremont D.H., French A.R., Carayannopoulos L.N. Cutting edge: FcR-like 5 on innate B cells is targeted by a poxvirus MHC class I-like immunoevasin. J. Immunol. 2010;185(1):28–32. doi: 10.4049/jimmunol.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brochier B., Kieny M.P., Costy F., Coppens P., Bauduin B., Lecocq J.P., Languet B., Chappuis G., Desmettre P., Afiademanyo K. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991;354(6354):520–522. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- 180.Sharp D.W., Lattime E.C. Recombinant poxvirus and the tumor microenvironment: oncolysis, immune regulation and immunization. Biomedicines. 2016;4(3):19. doi: 10.3390/biomedicines4030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guo Z.S., Parimi V., O'Malley M.E., Thirunavukarasu P., Sathaiah M., Austin F., Bartlett D.L. The combination of immunosuppression and carrier cells significantly enhances the efficacy of oncolytic poxvirus in the pre-immunized host. Gene Ther. 2010;17(12):1465–1475. doi: 10.1038/gt.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moss B. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 2011;239(1):8–26. doi: 10.1111/j.1600-065X.2010.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reynolds M.G., Carroll D.S., Karem K.L. Factors affecting the likelihood of monkeypox's emergence and spread in the post-smallpox era. Current Opinion in Virology. 2012;2(3):335–343. doi: 10.1016/j.coviro.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kennedy R.B., Ovsyannikova I.G., Jacobson R.M., Poland G.A. The immunology of smallpox vaccines. Curr. Opin. Immunol. 2009;21(3):314–320. doi: 10.1016/j.coi.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gilchuk I., Gilchuk P., Sapparapu G., Lampley R., Singh V., Kose N., Blum D.L., Hughes L.J., Satheshkumar P.S., Townsend M.B., Kondas A.V., Reed Z., Weiner Z., Olson V.A., Hammarlund E., Raue H.-P., Slifka M.K., Slaughter J.C., Graham B.S., Edwards K.M., Eisenberg R.J., Cohen G.H., Joyce S., Crowe J.E. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell. 2016;167(3):684–694.e9. doi: 10.1016/j.cell.2016.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fine P.E.M., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int. J. Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 187.Poland G.A., Kennedy R.B., Tosh P.K. Prevention of monkeypox with vaccines: a rapid review. Lancet. Infect. Dis. 2022 doi: 10.1016/S1473-3099(22)00574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.