Abstract
Aging constitutes progressive physiological changes in an organism. These changes alter the normal biological functions, such as the ability to manage metabolic stress, and eventually lead to cellular senescence. The process itself is characterized by nine hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. These hallmarks are risk factors for pathologies, such as cardiovascular diseases, neurodegenerative diseases, and cancer. Emerging evidence has been focused on examining the genetic pathways and biological processes in organisms surrounding these nine hallmarks. From here, the therapeutic approaches can be addressed in hopes of slowing the progression of aging. In this review, data have been collected on the hallmarks and their relative contributions to aging and supplemented with in vitro and in vivo antiaging research experiments. It is the intention of this article to highlight the most important antiaging strategies that researchers have proposed, including preventive measures, systemic therapeutic agents, and invasive procedures, that will promote healthy aging and increase human life expectancy with decreased side effects.
Keywords: aging, hallmarks, risk factors, therapeutic agent, antiaging strategies
1. Introduction
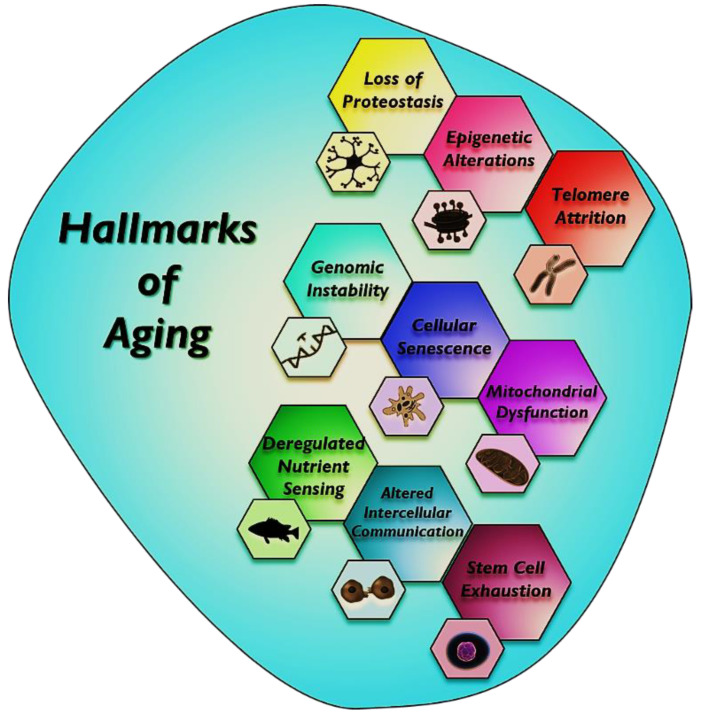
Aging is depicted by a gradual functional decline causing the progressive deterioration that occurs heterogeneously across multiple organs, leading to organ dysfunction in mammals. It is a major risk factor for common chronic diseases, such as heart disease, cancer, diabetes, and Alzheimer’s diseases [1]. In recent years, aging research has been geared toward advancing upon knowledge established on the nine hallmarks of aging at the cellular and molecular levels [2]. Several hallmarks of aging are as follows: (i) genomic instability. high frequency of mutations within the genome of the cell; (ii) telomere attrition, i.e., protective caps going to end with each subsequent cell division; (iii) epigenetic alterations, i.e., DNA methylation, histone modification, and chromatin remodeling that change in gene expression; (iv) loss of proteostasis, i.e., folding, chaperoning, and maintenance of protein function collapses; (v) deregulation of nutrient sensing, i.e., reduction in the function of IGF-1, mTOR, sitrulins, and AMPK that regulate the metabolism; (vi) mitochondrial dysfunction, i.e., reduced efficiency of oxidative phosphorylation and reduction in the production of ATP; (vii) cellular senescence, i.e., ended the activation of p53 and the cyclin-dependent kinase (CDK) inhibitor p16 due to a variety of mechanisms, such as telomere shortening, forms of genotoxic stress, mitogens, and inflammatory cytokines; (viii) stem cell exhaustion, i.e., reduction in stem cell activity and altered intercellular communication, and (ix) the burden of senescent cells (Figure 1).
Figure 1.
Hallmarks of aging. This scheme identifies the nine hallmarks briefly described in this review: loss of proteostasis, epigenetic alterations, telomere attrition, genomic instability, cellular senescence, mitochondrial dysfunction, deregulated nutrient sensing, altered intercellular communication, and stem cell exhaustion.
Recently, scientific achievements have been focused on producing effective antiaging therapeutics that have dramatically improved human life expectancy. Many studies on animal models looking at genetics and dietary and pharmacological interventions have shown an enhanced lifespan [3,4,5]. Other studies have examined antiaging strategies, such as enhancement of autophagy, elimination of senescent cells, transfusion of young blood, intermittent fasting, stem cell therapy, physical exercise, adult neurogenesis boost, and antioxidant and herbal intakes, which have emerged in recent years [6]. In humans, evidence has been accumulated on the styles of life in increasing the lifespan [7].
In this review, we have attempted to collect various in vitro and in vivo research experiments for antiaging approaches based on the major hallmarks of aging. Here, we describe antiaging strategies such as telomere reactivation, epigenetic drugs, activation of chaperons and proteolytic systems, and dietary restriction. Mitophagy and the clearance of senescent cells, etc. will be beneficial in understanding the unique approaches for successful aging and to extend healthy lifespans.
2. Anti-Inflammatory Drugs Used as an Antiaging Approach
Chronic inflammation is one of the major contributors to age-associated diseases and aging and disrupts the normal functioning of tissues [1,8,9]. Increased activity of proinflammatory pathways accompanies inflammation with age [10,11]. Serum concentrations of proinflammatory cytokines (IL-1, IL-2, IL-6, IL-8, IL-12, IL-15, IL-17, IL-18, IL-22, IL-23, tumor necrosis factor-α, and interferon-γ) are significantly increased in normal process aging compared with younger individuals in a normal stage [12,13,14]. A chronic proinflammatory status is a pervasive feature of aging, and increased systemic inflammation is closely associated with aging and age-related diseases [15,16]. The term “inflammaging” is used to describe aging induced by chronic and persistent inflammation. Anti-inflammatory agents block certain substances in the body that cause inflammation, and various studies have shown that anti-inflammatory agents are linked to antiaging [17]. The most important drivers of age-dependent inflammation are derived at the cellular and molecular levels. In a cell, the proinflammatory senescence-associated secretory phenotype (SASP) is associated with cellular senescence that is triggered by agents such as radiation and viruses and by continuous exposure to cellular debris [18] and cellular senescence [19]. In a molecule, ROS (reactive oxygen species) and other agents can trigger inflammatory DNA damage responses that affect DNA and telomeres [20] and activate the inflammasomes and NF-kB pathway [21]. Inhibition of the inflammatory processes by genetic and pharmacological intervention is considered an effective and verified antiaging strategy [2]. Nonsteroidal anti-inflammatory drugs (NSAIDs) not only prevent certain age-associated features but also increase the lifespan in various model organisms, such as yeasts [22], nematodes [23], mice [24,25], and flies [22]; however, their effectiveness for neurodegenerative disorders (Alzheimer’s disease and Huntington’s disease [26]) is not clear, and there is a search for anti-inflammatory bioactive compounds [27,28,29,30,31]. Anti-inflammatory drugs might be considered to have great potential for extending the lifespan. In some studies, spermidine (polyamines) and their action on the expression of pro- and anti-inflammatory cytokines can directly reduce inflammation and indirectly alter inflammation and cell growth by the action of autophagy [32]. Spermidine has been reported to slow down aging due to its antiaging effects [33]. Aspirin, a potent anti-inflammatory and antioxidant compound, may affect oxidant production and cytokine responses and block glycoxidation reactions that protect against oxidative stress, as well as extend the lifespan of Caenorhabditis elegans and mice [34,35,36]. Ibuprofen (NSAID) has been shown to reduce the risk of age-related pathologies and increase the lifespan of Saccharomyces cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster [37,38]. A novel NSAID, M2000, could modify oxidative stress pathways by lowering the expression levels of the SOD2, GST, iNOS, and MPO genes and reduce the risk of inflammatory diseases through its immunosuppressive effects, with no adverse side effects on the enzymatic and nonenzymatic determinants [39]. This can be recommended as an antiaging drug. MAAs (mycosporine-like amino acids), such as M2G (mycosporine-2-glycine), exhibit antioxidant, anti-inflammatory, anti-protein-glycation, and collagenase inhibition activities and show the ability to protect DNA against UV damage [40,41]. Many nutraceuticals (apigenin, quercetin, kaempferol, naringenin, catechins, epigallocatechin, genistein, cyanidin, resveratrol, etc.) and functional foods possess antioxidant activity that might play an important role in delaying aging and be effective in various human neurodegenerative diseases [30,42,43,44,45].
3. Antioxidant Activity
Phytochemicals such as phenolic acids and flavonoids have antioxidant activity, which acts by scavenging free radicals and increasing the levels of antioxidant enzymes in plasma [46]. The function of a primary antioxidant enzyme is to protect organisms from the damaging effects of superoxide radicals, which are quickened by their dismutation into hydrogen peroxide and oxygen [47]. Several studies have confirmed that quercetin is a strong antioxidant that accumulates in nematodes and displays reactive oxygen species (ROS) scavenging activity and has been demonstrated to have a positive effect on longevity and stress resistance in various animal models [48,49,50,51]. Many studies have demonstrated that NSAIDs have antioxidant activity that is mediated by free radical scavenging and antioxidant enzyme activation [52]. The antioxidant activity of NSAIDs has been witnessed in membranes, cells, and at the organismal level [52,53,54].
4. Telomere Reactivation
Telomeres are conserved microsatellite repeats TTAGGG that protect the ends of chromosomes from DNA breakage and prevent DNA end-joining, recombination, and DNA repair [55]. DNA polymerases are incapable of fully replicating the linear chromosomes owing to end replication in somatic cells, and telomeres become gradually shortened after each cell division [56]. This shortening of telomeres is usually fulfilled by the telomerase enzyme, but most somatic cells and adult stem cells do not express enough telomerase to compensate for the telomere length that leads to entering ‘replicative senescence’, which might be followed by cell death [57,58,59]. Telomere shortening occurs during normal aging and is an important biomarker of aging and longevity that is influenced by several factors, such as genetics, epigenetics, and environments [60,61,62,63]. It is also associated with many age-related diseases, such as osteoarthritis, atherosclerosis, coronary heart disease, and atrial fibrillation [64,65,66]. Several studies have reported that aging can be inhibited by the overexpression of telomerase; however, it can enhance tumorigenesis [67,68,69]. A telomerase activator, telomerase expression activator, and telomerase gene therapy have been developed as telomerase-based antiaging strategies in recent years. TA-65 is an extract of a Chinese plant (Astragalus membranaceus), a telomerase activator that can restore telomere length without cancer occurrence and improve age-related indicators, including glucose tolerance, bone health, and skin quality [70]. Additionally, some studies found that TERT transcription activator and sex hormones are directly involved in activating telomerase, which rescued telomere shortening and enhanced the lifespan [71,72]. Evidence has suggested that the reactivation of telomerase expression by using a gene therapy approach is the best example of the lifespan extension of mice and delay aging without cancer occurrence [73]. A recent study has found that Metadichol, a telomerase activator, is used to overcome organ failure by enriching cells with telomerase and is a safer alternative [74]. Another study explained that natural compounds such as 08AGTLF (Centella asiatica), Nutrient 4 (Astragalus), TA-65 (Astragalus membranaceus), OA (oleanolic acid), and MA (maslinic acid); and Nutrients 1, 2, and 3 have telomerase activation, and among all, 08AGTLF has the greatest potential to activate telomerase [75]. The impact of telomeric length on humans has been evidenced by the fact that the expression of telomerase in normal cells may extend a healthy lifespan; however, inhibition of telomerase in cancer cells may be a viable target for anticancer therapeutics [76].
5. Antiaging Approaches Using Epigenetic Drugs
The effects of chromatin on aging are probably complex and bidirectional. Chromatin remodeling appears to counter aging and age-associated diseases and extend organismal lifespan [77]. Chromatin is intensely altered during aging owing to the decreased level of histone proteins and adequate changes in histone modification that were found in recent studies in budding yeast and human fibroblast cells [78]. Elevating histone expression, reducing H4 K16 acetylation, reducing H3 N-terminal acetylation, inactivating the HDAC Rpd3, and inactivating the H3K4 methylase might be capable of extending lifespan or reverting the aged phenotype to a more youthful state of chromatin [79]. These epigenetic factors are influenced by diet, lifestyle and exogenous stress, which raises the possibility of enhancing age-related cellular dysfunction [80,81]. Several studies revealed age-associated changes in DNA methylation patterns leading to a global reduction in DNA methylation [82] (Figure 2); site-specific hypermethylation, specifically at CpG islands and polycomb target sites [83]; site-specific hypomethylation specifically at gene-poor regions, tissue-specific promoters, and polycomb protein regions; and hypermethylation in different tissues but hypomethylation, to be more tissue specific [84,85]. These changes accumulate gradually; such changes are indicative of the aging process and strongly associated with epigenetic changes such as replicative senescence [86]. Diet, lifestyle, environmental interventions, and inhibitors of epigenetic enzymes have proven to be effective in promoting longevity, as seen in various experiments [87]. Natural substances such as spermidine and resveratrol have been found to lead to deacetylation of chromatin, indicating that it has the potential to extend the lifespan in humans [88]. Many studies report that HDAC (histone deacetylase) inhibitors show evidence as an antiaging strategy [89]. HDAC inhibitors have the potential to reverse the aging process that allows healthy aging [90].
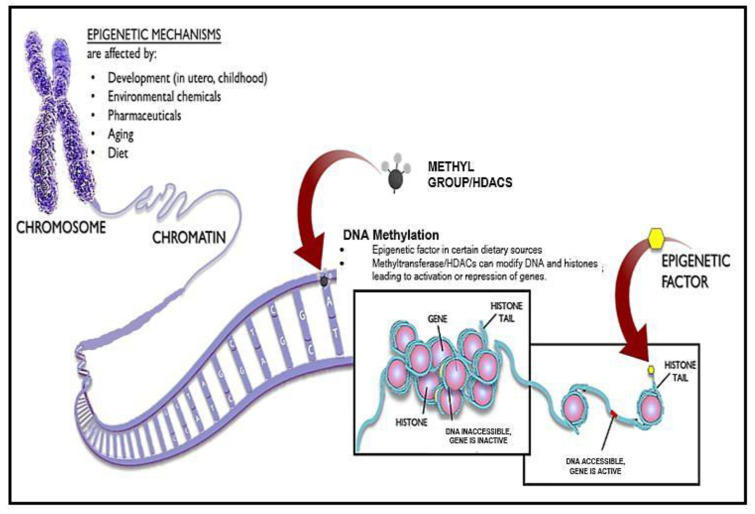
Figure 2.
Epigenetic Mechanisms via DNA Methylation and histone modification. Epigenetics can be altered via developmental mechanisms, environmental chemicals, pharmaceuticals, aging, and diet. Some dietary sources can lead to a direct production of DNA methylation, allowing for the overexpression or repression of genes, thereby increasing the aging process.
6. Activation of Chaperons and the Proteolytic System against Aging
Loss of proteostasis is a common feature of aging that leads to protein aggregation, unfolding, oxidative damage, posttranslational modification, and an altered rate of protein turnover and, ultimately, to cellular dysfunction [91,92,93,94,95]. Two proteolytic systems—the ubiquitin–proteasome system (UPS) and autophagy–lysosome system (ALS)—and chaperones play a major role in maintaining proteostasis [96]. The alteration or deterioration of these pathways impairs normal cell functioning and cell physiology, causing aging [2]. Many studies have found proteostasis changes with age owing to reduced activity of heat-shocked protein chaperones [97,98]. To compensate for this decline, increasing the chaperone protein level has been shown to beneficially impact longevity in worms and flies [99,100,101,102]. A study reported that the aggregation of Hsp104, a chaperone, has been associated with aging and has increased the lifespan in Saccharomyces cerevisiae [103,104,105]. The UPS and ALS systems are the main proteolytic systems that influence the cellular fate and aging process [106,107]. The availability of the chaperone is extremely compromised in aged cells in which the proteostasis collapses by decreased G1-cyclin function that causes an irreversible arrest in G1, configuring a molecular pathway claiming proteostasis deterioration leads to cell senescence [108]. Promoting proteasomal activity via overexpression of the proteasomal β5 subunit either in Caenorhabditis elegans [109] or in human fibroblast [110] and the overexpression of Rpn11 in Drosophila melanogaster [111] increases the lifespan and stress resistance. The compound 18α-glycyrrhetinic acid and loss of IGF (insulin-like growth factor) signaling due to mutated daf-2 induce proteasomal activation and extend the lifespan of Caenorhabditis elegans [112]. Spermidine, metformin, rapamycin, and resveratrol are pharmaceutical approaches well-known to activate the autophagy system [113]. In a recent study, it was found that minocycline, JZL184, monorden, and paxilline directly targeted the 18S rRNA/ribosome, FAAH-4, Hsp90, and the SLO-1 BK channel, significantly increasing the lifespan of Caenorhabditis elegans [114]. The proteostatic system governs the synthesis and conformation of target proteins, and the ubiquitin—proteasome system and autophagy act as the main scavengers of misfolded or excessive proteins. The main cause of Alzheimer’s disease is the accumulation of misfolded proteins as Aβ plaques and tau aggregates owing to dysregulation of proteostasis, which contributes to the accumulation of proteotoxins in Alzheimer’s disease [115]. It has been reported that a decrease in the efficiency of the autophagy and ubiquitin—proteasome systems might lead to aging and neurodegenerative diseases such as AD, PD, and ALS [116]. The prion diseases in mammals are related to altered versions of PrPc (cellular), which is a key component of the infectious agent responsible for transmission, and the disease-associated version of PrPc can be partially resistant to the protease–digestion system, designated PrPsc (scrapie) [117]. Various cellular components, predominantly chaperones such as Hsp104, Hsp40s, HSP42, and HSP70s, can lead to the curing of yeast prions by their deficiency or overproduction. These studies have revealed the requirements for prion propagation and conditions that affect prion stability, which have led to the discovery of anti-prion systems. Btn2p, a component of the yeast anti-prion system, has the aggregate-sequestering abilities; it can cure an artificial and natural yeast prion, and works on a variety of non-prion aggregates as well [118]. This system might be advantageous for neurodegenerative diseases that result from aggregates of proteo-toxins.
7. Mitophagy Activators as an Antiaging Approach
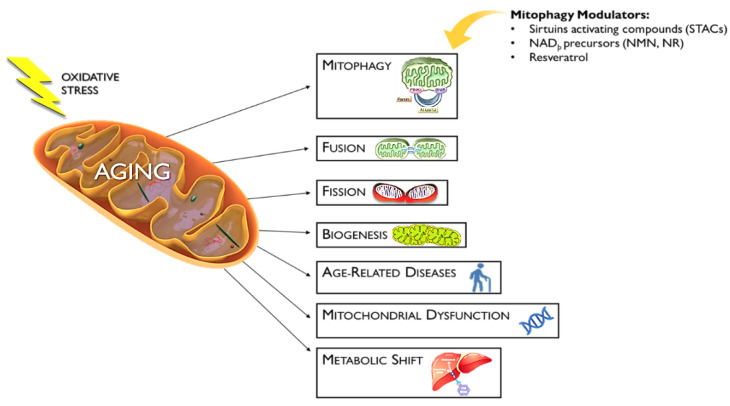
Mitochondrial dysfunction is one of the hallmarks of aging [2] that is associated with aging and is also involved in the development of many neurodegenerative diseases [119]. Mitochondrial dysfunction is caused by a defect in mitophagy that has been associated with aging and other diseases such as metabolic disorders, cancer, senescence, inflammation, and genomic instability [120]. Mitochondrial health plays an important role in the aging process that can assist in the therapeutic approach toward longevity [121]. Mitophagy could turn to a pro-death pathway due to excessive superoxide stress that leads to accelerated aging [122]. Parkin is a key protein in mitophagy [123] and is overexpressed, leading to a decreased lifespan in Drosophila [124,125]. Mitochondrial health depends on inflammation, because many inflammatory pathologies have been associated with mitochondrial defects [126]. Studies report that many natural products have the potential to act as antiaging strategies [127]. Many inducers, such as sirtuin-activating compounds (STACs), NADþ precursors (NMN and NR), and resveratrol, have been shown to modulate mitophagy and repair mitochondrial functions [128,129,130] (Figure 3).
Figure 3.
Mitophagy Modulators Against Mitochondrial Dysfunction. Several factors contribute to the aging process via the mitochondria, including mitophagy, mitochondrial fusion and fission, biogenesis, age-related diseases (i.e., AD and PD), mitochondrial dysfunction in general, and metabolic shifts. STACs, NMN, NR, and resveratrol are compounds that act against the aging process, directly targeting mitophagy and preventing the selective degradation of healthy mitochondria.
Resveratrol (3,5,4-trihydroxystilbene) is a natural polyphenol that can induce autophagy and mitophagy by different mechanisms, such as activation of AMPK and SIRT1; induction of P38 MAPK; suppression of mTOR and p70S6K; and upregulation of eNOS, GABARAP, LC3B, and ATG3 genes, which is a beneficial compound for extending the lifespan [131,132,133,134,135,136,137,138].
Some natural compounds, such as spermidine and urolithin, preserve mitochondrial function and extend longevity through mitophagy induction, which has been demonstrated in several model organisms, such as yeast, nematodes, flies, and mice [113,139,140,141]. Moreover, tomatidine is a natural compound that stimulates the elimination of defective mitochondria, leading to increased longevity and enhanced muscular function in nematodes and mice [142]. Some studies reveal that antibiotics severely affect mitochondrial homeostasis [143]. Actinomycin and doxycycline interrupt energy metabolism and facilitate mitophagy in mammalian cells [144,145]. The mitochondria generates most cellular ROS, but mtDNA is comparatively unprotected from ROS damage due to a lack of histone proteins and a lack of robust mtDNA repair mechanisms. A decline in mitochondrial energy metabolism and accumulation of mtDNA mutations in tissue cells are important contributors to human aging, and some type of mtDNA mutations are leading to the development of cancer [146]. A study showed that deficiency and/or dysfunction in many of the nuclear-encoded mtDNA replicative proteins, such as Pol γ, PolG2, Twinkle, TFAM, MGME1, and RNase H1, are responsible for the development of age-related diseases and/or phenotypes both in vitro and in vivo [147].
8. Inhibition of mTOR and Insulin/IGF-1 Signaling (IIS) as an Antiaging Approach
mTOR promotes cell growth by either promoting protein synthesis or inhibiting autophagy activity. It is involved in senescence-associated phenotypes. The mTOR pathway acts as a nutrient sensor through regulation of mitochondrial biogenesis [148], regulation of mitophagy [149], promotion of the secretory phenotype of senescent cells [150], and inhibition of stem cell senescence [151,152]. Many studies have demonstrated that a reduction of mTOR signaling is associated with extension of the lifespan in Caenorhabditis elegans [153], Drosophila melanogaster [154,155], Saccharomyces cerevisiae [156,157], and Mus musculus [158]. Thus, it is the most observed target for the study of pharmacological treatments. Pharmacological inhibition of mTOR by rapamycin confirms that it can be observed to slow down aging in yeast (S. cerevisiae) [159], Drosophila melanogaster [154,160,161,162,163,164], worm (C. elegans) [165], and mice (M. musculus) [166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187]. It has also been seen to delay age-related diseases in different species including humans [188,189,190,191,192,193,194,195,196,197,198,199,200]. mTORC1 has two substrates, S6 kinase and 4E-BP1, and both are linked to longevity through the reduction of S6 kinase, which promotes longevity [201]. Another nutrient-sensing pathway is the insulin/insulin-like growth factor 1 (IGF-1) signaling (IIS) network, which is a major determinant of longevity important to regulate the lifespan in various species, such as C. elegans [202], D. melanogaster [203,204], and M. musculus [205,206]. It has been shown that suppression of the IIS/mTOR pathways is involved in prolonging the lifespan but also delays the onset of age-related pathologies in numerous organisms, such as yeast, fruit flies, nematodes, mice, rats, and primates [207,208,209]. In recent years, studies have reported that celecoxib (a nonsteroidal anti-inflammatory drug), recombinant BTI (rBTI-mimicking restriction), and the ethyl acetate fraction of Ribes fasciculatum (ERF) downregulate the insulin/IGF-1 signaling cascade and extend the lifespan in Caenorhabditis elegans [23,210]. Recently, a study demonstrated that hypo-taurine, an antioxidant, induces the lifespan in Caenorhabditis elegans by regulating the insulin/IGF-1 signaling (IIS) pathway [211,212].
9. Activation of AMPK and Sirtuin Signaling as an Antiaging Approach
5′adenosine monophosphate-activated protein kinase (AMPK) is a master regulator of energy metabolism in the cell that plays a critical role in regulating health and longevity [213]. AMPK is a serine/threonine protein kinase that is a highly conserved sensor that increases the levels of AMP and ADP [214,215]. Sirtuins (SIRT1 to SIRT7), also known as histone deacetylases (HDACs), delay cellular senescence and promote longevity in various species through increased expression [216,217,218,219,220,221]. Many FDA-approved drugs, such as biguanides, thiazolidinediones, glucagon-like peptide-1 receptor agonists, salicylates and resveratrol, and 5-aminoimidazole-4-carboxamide riboside (AICAR), have AMPK-activating properties [222,223]. Resveratrol (3,5,40-trihydroxystilbene) is a natural activator that increases the production of synthetic SIRT1 activators, which are more potent, soluble and bioavailable [136,224]. Some synthetic activators, such as SRT1720 (imidazothiazoles), thiazolopyridine, benzimidazole, bridged ureas, cilostazol, paeonol, statins, hydrogen sulfide, persimmon, and SRT2104 also extend the lifespan in mice and protect cells against age-related changes [225,226,227,228,229,230,231,232]. Moreover, natural compounds such as quercetin, proanthocyanidins, fisetin, catechins, kaempferol, and butein have been reported to have antiaging properties [233]. Metformin activates AAK-2/AMPK and induces autophagy through AMPK, which is pro-longevity in nematodes, Drosophila, rats, and mice [234,235,236,237,238,239,240,241]. Oligonol is an antioxidant polyphenolic compound with an anti-inflammatory property that activates the autophagy pathway and phosphorylation of AMPK in C. elegans [242].
10. Clearance of Senescent Cells
Cellular senescence is a central component of aging that leads to cell cycle arrest in a damaged cell by preventing the cell from promulgating further damaged tissues [243,244,245]. Senescent cells accumulate with age and promote aging and age-associated pathologies [246,247,248,249]. To extend the lifespan, senescent cells need to be decreased or suppressed through the senolytics approach. There are many senolytics that have been reported to induce apoptosis of senescent cells and reduce the expression of senescence markers, including a combination of dasatinib and quercetin, BCL2 family inhibitors, SCAPs, FOXO4, and piperlongumine [250,251,252,253,254,255,256,257]. Moreover, HSP90 has been identified as a novel senolytics that can induce apoptosis of senescent cells to improve the health span [258]. There are several senomorphic drugs that suppress markers of senescence or their secretory phenotype, including inhibitors of IkB kinase (IKK) and nuclear factor (NF), inhibitors of free radical scavengers and the Janus kinase (JAK) pathway. Rapamycin acts as a senomorphic agent to reduce the SASP, and ABT263 inhibits the antiapoptotic proteins BCL-2 and BCL-XL [259,260,261]. In recent years, two well-known antibiotics, azithromycin and roxithromycin, had senolytic activity to target senescent cells [262]. Curcumin is an ayurvedic formulation that has antisenescence properties and can modulate cellular senescence by activating the sirtuins and AMPK pathways [263].
11. Stem Cell-Based Therapies
Stem cell exhaustion (decline in stem cell number and function) is one of the causes of aging that is observed in all tissues and organs and is maintained by adult stem cells through repair and regeneration during life [264,265]. There is a loss of adult stem cells such as hematopoietic stem cells (HPSCs), which respond to stress and differentiation, and HPSCs are decreased by the hyperactivation of the mechanistic target of rapamycin complex 1 and the disruption of 5′ adenosine monophosphate-activated protein kinase (AMPK) [266,267]. It has been reported that the accumulation of DNA damage and increased levels of p16INK4a have been closely linked to a decline in stem cell populations with aging [268]. Age-associated phenotypes could be restored by the induction of stem cell rejuvenation [269,270,271]. Reprogramming aged hematopoietic stem cells (HPSCs) to a pluripotent state and inducing pluripotent stem cells (iPSCs) from aged human fibroblasts into NSCs are alternate options [272,273]. Metformin is an FDA-approved drug that can induce stem cell rejuvenation in adult stem cell therapy [274]. Studies have proposed that using AMPK activators such as 5-aminoimidazole-4-carboxamide ribonucleotide, A769662, metformin, and oxidized nicotinamide adenine dinucleotide (NAD+) can be used in stem cell-based transplantation therapies with better results [275]. A study revealed that resveratrol-induced SIRT1 activation promotes the self-renewal of human embryonic stem cells (hESCs) [276]. A recent study reported that the administration of caloric restriction (CR) could enhance stem cell proliferation by regulating niche cells and reducing the major energy metabolic pathways that prevent stem cell aging [277].
12. Role of the Microbiome in Aging and Potential Antiaging Therapeutics
The evidence highlighted that microbiome composition may affect the rate of aging [278,279], although no evidence has been found that microbiota composition harshly changes the chronological threshold or age; rather, these changes proceed gradually with time [280]. Between the host and intestinal microbiota, the rate of age-related deterioration is strongly influenced by particular factors such as age-associated alterations in lifestyle, nutrition, frailty, and inflammation [281]. A study reported that the microbial composition is highly similar in young adults and seventy-year-old people but significantly differs in centenarians [280]. This difference was associated with the enrichment in opportunistic proinflammatory bacteria known as pathobionts, which are generally present in adult gut ecosystems in low numbers. The microbiota may favorably affect the host health and aging processes using prebiotics and probiotics that have been shown to be efficient in preventing particular pathological conditions, such as the suppression of inappropriate chronic inflammation in elderly populations. It includes a decrease in the synthesis of proinflammatory cytokines such as interleukins (IL-6, IL-8, IL-10, and TNF) and, thus, an increase in the levels of activated lymphocytes and natural killer cells and phagocytic activity [282]. Some other effects of microbiota, including the regulation of host fat deposition and metabolism [283], prevention of insulin resistance [284], degradation of nondigestible carbohydrates, enhancement of antioxidant activity, production of vitamin B and conjugated linoleic acids [285], and improved maintenance of mucosal barrier integrity and immune homeostasis [286]. Several research findings suggest that direct modulation of the gut microbiome might be beneficial to be applied in treating age-related disorders [287] and considered to be a novel potential therapeutic for healthy aging [288]. Some studies suggest that the intake of functional foods such as prebiotics, probiotics, or synbiotics might be an effective strategy to counter natural aging through modifying the gut microbiota of the elderly population [289].
13. Role of Noncoding RNAs in Aging and Their Potential Therapeutics
Long noncoding RNAs (lncRNAs) can affect key cellular processes, such as proliferation, differentiation, quiescence, senescence, the cellular response to stress and immune agents, and other cellular functions related to the biology of aging. LncRNAs have the ability to modulate gene expression patterns at the transcriptional, posttranscriptional, and posttranslational levels [290,291,292]. The changes in the subsets of expressed proteins are responsible for the aging traits. LncRNAs can modulate protein expression patterns by controlling gene transcription, mRNA stability, and protein abundance; thus, they can modulate key molecular events underlying the aging process, such as the control of telomere length, epigenetic gene expression, proteostasis, stem cell function, intercellular communication, cell proliferation, and cellular senescence [293].
14. Conclusions
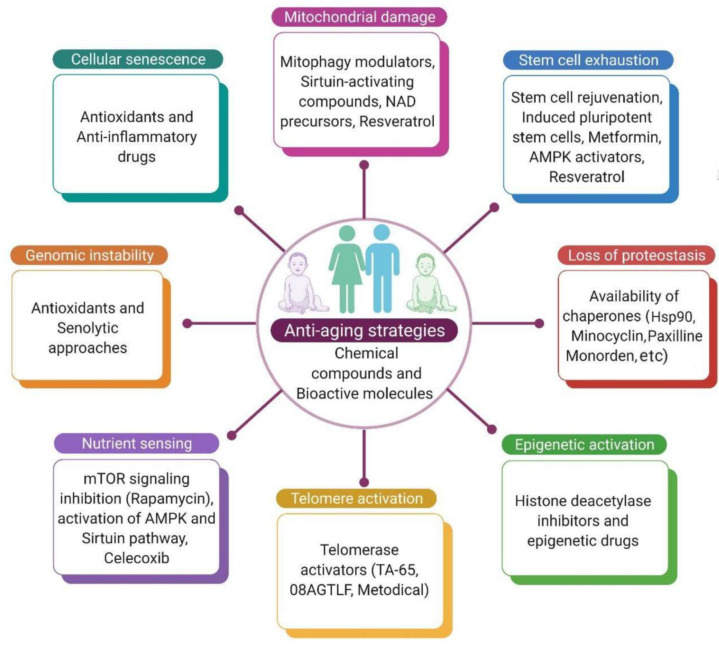
This review has attempted to explain the various antiaging strategies used based on the major hallmarks of aging. Data have been collected from scientific evidence, such as studies conducted on organisms and species targeting aging. These approaches would not only postpone chronic diseases but also prevent many age-related disorders and extend healthy lifespans. Aging and age-related diseases are due to abnormalities in normal biological pathways, such as metabolism, inflammation, growth, and protein synthesis, which also alter the rate of aging. Interestingly, modifications in the normal epigenetic pathway appear to be a major cause of chronic disorders. This review presents various protective interventions as antiaging strategies that are valuable to extend the human healthy lifespan. These include telomere reactivation, epigenetic drugs, activation of chaperons and the proteolytic system, caloric restriction, mitophagy activation, clearance of senescent cells, antioxidant and anti-inflammatory drugs, inhibition of mTOR and insulin/IGF-1 signaling (IIS), and stem cell-based therapy. Targeting senescent cells and inflammation plays a prominent role in aging, such as employing anti-inflammatory bioactive molecules in recent studies or, in some cases, nonsteroidal anti-inflammatory drugs (NSAIDs), senolytics, and SASP suppressors. To achieve adequate telomere length through telomerase activators, HDAC (histone deacetylase) inhibitors may be considered as antiaging agents. To rescue mitochondrial function and promote proteasomal activity, SIRT1 and AMPK activators and mTOR suppressors may be an effective way to combat aging. Reprogramming technology such as iPSCs and stem cell therapy, are emerging pieces of evidence for supplementing stem cells to rejuvenate tissues and organ functions. Thus, exploring the aging process at the molecular level is still a challenge, but the possibility of developing novel therapeutics by using nutraceuticals, molecular medicine, and pharmacogenomics approaches may be options for successful aging (Figure 4).
Figure 4.
Antiaging strategies to counter aging that might extend the human lifespan based on the use of chemical compounds and bioactive molecules and their use in various approaches mentioned in the figure.
Expert Opinion
Various scientific achievements have been focused on producing effective antiaging therapeutics that have dramatically improved human life expectancy. Many studies on animal models looking at genetics and dietary and pharmacological intervention have shown an enhanced lifespan. Thus, in this article, we have highlighted various antiaging strategies that have targeted approach-based hallmarks of aging. Researchers have proposed various strategies, such as preventive measurements, systemic therapeutic agents, and invasive procedures, that will promote healthy aging and increase human life expectancy with decreased side effects.
Author Contributions
Conceptualization, S.K.M., R.B.M., M.P.S., E.V., S.K.S., and M.A.-S.; writing—original draft preparation, S.K.M., V.B., J.E., G.P., E.V., and S.K.S.; and writing—review and editing, S.K.M., V.B., J.E., A.A.O., N.K.J., R.B.M., M.M., M.A.-S., A.A.K., and S.K.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was performed with resources from the Non-Government/Nonprofit Organization Indian Scientific Education and Technology Foundation, Lucknow, India, and Project ID19I-10301 from ANID, Chile. Authors from King Khalid University are highly acknowledge funding support from the Deanship of Scientific Research at King Khalid University, Saudi Arabia, Grant No. (R.G.P.. 2/192/43).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rojo L.E., Fernández J.A., Maccioni A.A., Jimenez J.M., Maccioni R.B. Neuroin flammation: Implications for the Pathogenesis and Molecular Diagnosis of Alzheimer’s Disease. Arch. Med. Res. 2008;39:1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 2.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Magalhães J.P. Programmatic features of aging originating in development: Aging mechanisms beyond molecular damage? FASEB J. 2012;26:4821–4826. doi: 10.1096/fj.12-210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 5.Tacutu R., Craig T., Budovsky A., Wuttke D., Lehmann G., Taranukha D., Costa J., Fraifeld V.E., de Magalhaes J.P. Human Ageing Genomic Resources: Integrated Databases and Tools for the Biology and Genetics Of Ageing. Nucleic Acids Res. 2012;41:D1027–D1033. doi: 10.1093/nar/gks1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty A.K., Kodali M., Upadhya R., Madhu L.N. Emerging Anti-Aging Strategies—Scientific Basis and Efficacy. Aging Dis. 2018;9:1165–1184. doi: 10.14336/AD.2018.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helfand S.L., de Cabo R. Evidence that overnight fasting could extend healthy lifespan. Nature. 2021;598:265–266. doi: 10.1038/d41586-021-01578-8. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi C., Campisi J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. Ser. A. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 9.Howcroft T.K., Campisi J., Louis G.B., Smith M.T., Wise B., Wyss-Coray T., Augustine A.D., McElhaney J.E., Kohanski R., Sierra F. The role of inflammation in age-related disease. Aging. 2013;5:84–93. doi: 10.18632/aging.100531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Magalhães J.P., Curado J., Church G. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25:875–881. doi: 10.1093/bioinformatics/btp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriete A., Mayo K.L., Yalamanchili N., Beggs W., Bender P., Kari C., Rodeck U. Cell autonomous expression of inflammatory genes in biologically aged fibroblasts associated with elevated NF-kappaB activity. Immun. Ageing. 2008;5:5. doi: 10.1186/1742-4933-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minciullo P.L., Catalano A., Mandraffino G., Casciaro M., Crucitti A., Maltese G., Morabito N., Lasco A., Gangemi S., Basile G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016;64:111–126. doi: 10.1007/s00005-015-0377-3. [DOI] [PubMed] [Google Scholar]
- 13.Ventura M.T., Casciaro M., Gangemi S., Buquicchio R. Immunosenescence in aging: Between immune cells depletion and cytokines up-regulation. Clin. Mol. Allergy. 2017;15:21. doi: 10.1186/s12948-017-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rea I.M., Gibson D.S., McGilligan V., McNerlan S.E., Alexander H.D., Ross O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanada F., Taniyama Y., Muratsu J., Otsu R., Shimizu H., Rakugi H., Morishita R. Source of Chronic Inflammation in Aging. Front. Cardiovasc. Med. 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung H.Y., Ki W.C., Lee E.K., Chung K.W., Chung S., Lee B., Seo A.Y., Chung J.H., Jung Y.S., Im E., et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019;10:367–382. doi: 10.14336/AD.2018.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neves J.M., Sousa-Victor P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020;287:43–52. doi: 10.1111/febs.15061. [DOI] [PubMed] [Google Scholar]
- 18.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurk D., Wilson C., Passos J.F., Oakley F., Correia-Melo C., Greaves L., Saretzki G., Fox C., Lawless C., Anderson R., et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014;5:4172. doi: 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitale G., Salvioli S., Franceschi C. Oxidative stress and the ageing endocrine system. Nat. Rev. Endocrinol. 2013;9:228–240. doi: 10.1038/nrendo.2013.29. [DOI] [PubMed] [Google Scholar]
- 21.Youm Y.-H., Grant R.W., McCabe L.R., Albarado D.C., Nguyen K.Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A., et al. Canonical Nlrp3 Inflammasome Links Systemic Low-Grade Inflammation to Functional Decline in Aging. Cell Metab. 2013;18:519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He C., Tsuchiyama S.K., Nguyen Q.T., Plyusnina E.N., Terrill S.R., Sahibzada S., Patel B., Faulkner A.R., Shaposhnikov M., Tian R., et al. Enhanced Longevity by Ibuprofen, Conserved in Multiple Species, Occurs in Yeast through Inhibition of Tryptophan Import. PLoS Genet. 2014;10:e1004860. doi: 10.1371/journal.pgen.1004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ching T.-T., Chiang W.-C., Chen C.-S., Hsu A.-L. Celecoxib extends C. elegans lifespan via inhibition of insulin-like signaling but not cyclooxygenase-2 activity. Aging Cell. 2011;10:506–519. doi: 10.1111/j.1474-9726.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strong R., Miller R.A., Astle C.M., Floyd R.A., Flurkey K., Hensley K.L., Javors M.A., Leeuwenburgh C., Nelson J.F., Ongini E., et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee M.E., Kim S.R., Lee S., Jung Y.-J., Choi S.S., Kim W.J., Han J.A. Cyclooxygenase-2 inhibitors modulate skin aging in a catalytic activity-independent manner. Exp. Mol. Med. 2012;44:536–544. doi: 10.3858/emm.2012.44.9.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalonia H., Kumar P., Kumar A. Licofelone attenuates quinolinic acid induced Huntington like symptoms: Possible behavioral, biochemical and cellular alterations. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:607–615. doi: 10.1016/j.pnpbp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Asanuma M., Miyazaki I., Diaz-Corrales F.J., Ogawa N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta Medica Okayama. 2004;58:221–233. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 28.Black P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002;16:622–653. doi: 10.1016/S0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 29.Choi S.H., Aid S., Caracciolo L., Sakura Minami S., Niikura T., Matsuoka Y., Turner R.S., Mattson M.P., Bosetti F. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2013;124:59–68. doi: 10.1111/jnc.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calfio C., Gonzalez A., Singh S.K., Rojo L.E., Maccioni R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;77:33–51. doi: 10.3233/JAD-200443. [DOI] [PubMed] [Google Scholar]
- 31.Veurink G., Perry G., Singh S.K. Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol. 2020;10:200084. doi: 10.1098/rsob.200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minois N. Molecular Basis of the ‘Anti-Aging’ Effect of Spermidine and Other Natural Polyamines—A Mini-Review. Gerontology. 2014;60:319–326. doi: 10.1159/000356748. [DOI] [PubMed] [Google Scholar]
- 33.Madeo F., Carmona-Gutierrez D., Kepp O., Kroemer G. Spermidine delays aging in humans. Aging. 2018;10:2209–2211. doi: 10.18632/aging.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips T., Leeuwenburgh C. Lifelong Aspirin Supplementation as a Means to Extending Life Span. Rejuvenation Res. 2004;7:243–252. doi: 10.1089/rej.2004.7.243. [DOI] [PubMed] [Google Scholar]
- 35.Ayyadevara S., Bharill P., Dandapat A., Hu C., Khaidakov M., Mitra S., Shmookler Reis R.J., Mehta J.L. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal. 2013;18:481–490. doi: 10.1089/ars.2011.4151. [DOI] [PubMed] [Google Scholar]
- 36.Nadon N.L., Strong R., Miller R.A., Harrison D.E. NIA Interventions Testing Program: Investigating Putative Aging Intervention Agents in a Genetically Heterogeneous Mouse Model. eBioMedicine. 2017;21:3–4. doi: 10.1016/j.ebiom.2016.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orhan H., Doğruer D., Cakir B., Şahin G., Şahin M. The in vitro effects of new non-steroidal antiinflammatory compounds on antioxidant system of human erythrocytes. Exp. Toxicol. Pathol. 1999;51:397–402. doi: 10.1016/S0940-2993(99)80028-1. [DOI] [PubMed] [Google Scholar]
- 38.Danilov A., Shaposhnikov M., Shevchenko O., Zemskaya N., Zhavoronkov A., Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6:19428–19444. doi: 10.18632/oncotarget.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini S., Abdollahi M., Azizi G., Fattahi M.J., Rastkari N., Zavareh F.T., Aghazadeh Z., Mirshafiey A. Anti-aging effects of M2000 (β-D-mannuronic acid) as a novel immunosuppressive drug on the enzymatic and non-enzymatic oxidative stress parameters in an experimental model. J. Basic Clin. Physiol. Pharmacol. 2017;28:249–255. doi: 10.1515/jbcpp-2016-0092. [DOI] [PubMed] [Google Scholar]
- 40.Waditee-Sirisattha R., Kageyama H. Protective effects of mycosporine-like amino acid-containing emulsions on UV-treated mouse ear tissue from the viewpoints of antioxidation and antiglycation. J. Photochem. Photobiol. B. 2021;223:112296. doi: 10.1016/j.jphotobiol.2021.112296. [DOI] [PubMed] [Google Scholar]
- 41.Kageyama H., Waditee-Sirisattha R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs. 2019;17:222. doi: 10.3390/md17040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar Singh S., Barreto G.E., Aliev G., Echeverria V. Ginkgo biloba as an Alternative Medicine in the Treatment of Anxiety in Dementia and other Psychiatric Disorders. Curr. Drug. Metab. 2017;18:112–119. doi: 10.2174/1389200217666161201112206. [DOI] [PubMed] [Google Scholar]
- 43.Singh S.K., Srikrishna S., Castellani R.J., Perry G. Nutritional Antioxidant Therapies: Treatments and Perspectives. Springer International Publishing; Berlin/Heidelberg, Germany: 2018. Antioxidants in the prevention and treatment of alzheimer’s disease; pp. 523–553. [DOI] [Google Scholar]
- 44.Singh S.K., Srivastav S., Castellani R.J., Plascencia-Villa G., Perry G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics. 2019;16:666–674. doi: 10.1007/s13311-019-00767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra S., Mishra S.K., Singh S.K. Ayurveda and Yoga practices: A synergistic approach for the treatment of Alzheimer’s disease. Eur. J. Biol. Res. 2020;11:65–74. [Google Scholar]
- 46.Pietta P.G. Flavonoids as Antioxidants. J. Nat. Prod. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 47.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kampkötter A., Timpel C., Zurawski R.F., Ruhl S., Chovolou Y., Proksch P., Wätjen W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008;149:314–323. doi: 10.1016/j.cbpb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Sugawara T., Sakamoto K. Quercetin enhances motility in aged and heat-stressed Caenorhabditis elegans nematodes by modulating both HSF-1 activity, and insulin-like and p38-MAPK signalling. PLoS ONE. 2020;15:e0238528. doi: 10.1371/journal.pone.0238528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietsch K., Saul N., Swain S.C., Menzel R., Steinberg C.E., Stürzenbaum S.R. Meta-analysis of global transcriptomics suggests that conserved genetic pathways are responsible for quercetin and tannic acid mediated longevity in C. elegans. Front. Genet. 2012;3:48. doi: 10.3389/fgene.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proshkina E., Lashmanova E., Dobrovolskaya E., Zemskaya N., Kudryavtseva A., Shaposhnikov M., Moskalev A. Geroprotective and Radioprotective Activity of Quercetin, (-)-Epicatechin, and Ibuprofen in Drosophila melanogaster. Front. Pharmacol. 2016;7:505. doi: 10.3389/fphar.2016.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Končič M., Rajić Z., Petrič N., Zorc B. Antioxidant activity of NSAID hydroxamic acids. Acta Pharm. 2009;59:235–242. doi: 10.2478/v10007-009-0017-8. [DOI] [PubMed] [Google Scholar]
- 53.Orhan H., Şahin G. In vitro effects of NSAIDS and paracetamolon oxidative stress-related parameters of human erythrocytes. Exp. Toxicol. Pathol. 2001;53:133–140. doi: 10.1078/0940-2993-00179. [DOI] [PubMed] [Google Scholar]
- 54.Peng C., Wang X., Chen J., Jiao R., Wang L., Li Y.M., Zuo Y., Liu Y., Lei L., Ma K.Y., et al. Biology of Ageing and Role of Dietary Antioxidants. BioMed Res. Int. 2014;2014:831841. doi: 10.1155/2014/831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Sullivan R.J., Karlseder J. Telomeres: Protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lingner J., Cooper J.P., Cech T.R. Telomerase and DNA end replication: No longer a lagging strand problem? Science. 1995;269:1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- 57.Greider C.W. Telomerase is processive. Mol. Cell. Biol. 1991;11:4572–4580. doi: 10.1128/mcb.11.9.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cesare A.J., Reddel R.R. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat. Rev. Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 59.Victorelli S., Passos J.F. Telomeres and Cell Senescence—Size Matters Not. eBioMedicine. 2017;21:14–20. doi: 10.1016/j.ebiom.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dodig S., Čepelak I., Pavić I. Hallmarks of senescence and aging. Biochem. Med. 2019;29:483–497. doi: 10.11613/BM.2019.030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cassidy A., De Vivo I., Liu Y., Han J., Prescott J., Hunter D.J., Rimm E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010;91:1273–1280. doi: 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prescott J., Kraft P., Chasman D.I., Savage S.A., Mirabello L., Berndt S.I., Weissfeld J.L., Han J., Hayes R.B., Chanock S.J., et al. Genome-Wide Association Study of Relative Telomere Length. PLoS ONE. 2011;6:e19635. doi: 10.1371/journal.pone.0019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song S., Johnson F.B. Epigenetic Mechanisms Impacting Aging: A Focus on Histone Levels and Telomeres. Genes. 2018;9:201. doi: 10.3390/genes9040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuszel L., Trzeciak T., Richter M., Czarny-Ratajczak M. Osteoarthritis and telomere shortening. J. Appl. Genet. 2015;56:169–176. doi: 10.1007/s13353-014-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carlquist J.F., Knight S., Cawthon R.M., Le V., Bunch T.J., Horne B.D., Rollo J.S., Huntinghouse J.A., Muhlestein J.B., Anderson J.L. Shortened telomere length is associated with paroxysmal atrial fibrillation among cardiovascular patients enrolled in the Intermountain Heart Collaborative Study. Hear. Rhythm. 2016;13:21–27. doi: 10.1016/j.hrthm.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 66.Hunt S.C., Kimura M., Hopkins P.N., Carr J.J., Heiss G., Province M.A., Aviv A. Leukocyte Telomere Length and Coronary Artery Calcium. Am. J. Cardiol. 2015;116:214–218. doi: 10.1016/j.amjcard.2015.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blasco M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 68.Pereira B., Ferreira M.G. Sowing the seeds of cancer: Telomeres and age-associated tumorigenesis. Curr. Opin. Oncol. 2013;25:93–98. doi: 10.1097/CCO.0b013e32835b6358. [DOI] [PubMed] [Google Scholar]
- 69.Wang S., Madu C.O., Lu Y. Telomere and Its Role in Diseases. Oncomedicine. 2019;4:1–9. doi: 10.7150/oncm.28210. [DOI] [Google Scholar]
- 70.Bernades de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bär C., Huber N., Beier F., Blasco M.A. Therapeutic effect of androgen therapy in a mouse model of aplastic anemia produced by short telomeres. Haematologica. 2015;100:1267–1274. doi: 10.3324/haematol.2015.129239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calado R.T., Yewdell W.T., Wilkerson K.L., Regal J.A., Kajigaya S., Stratakis C.A., Young N.S. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood J. Am. Soc. Hematol. 2009;114:2236–2243. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Jesus B.B., Vera E., Schneeberger K., Tejera A.M., Ayuso E., Bosch F., Blasco M.A. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raghavan P.R. Metadichol®: A Novel Nanolipid Formulation That Inhibits SARS-CoV-2 and a Multitude of Pathological Viruses In Vitro. BioMed Res. Int. 2020 doi: 10.1155/2022/1558860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsoukalas D., Fragkiadaki P., Docea A.O., Alegakis A.K., Sarandi E., Thanasoula M., Spandidos D.A., Tsatsakis A., Razgonova M.P., Calina D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 2019;20:3701–3708. doi: 10.3892/mmr.2019.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shay J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6:584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sedivy J.M., Banumathy G., Adams P.D. Aging by epigenetics—A consequence of chromatin damage? Exp. Cell. Res. 2008;314:1909–1917. doi: 10.1016/j.yexcr.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feser J., Tyler J. Chromatin structure as a mediator of aging. FEBS Lett. 2011;585:2041–2048. doi: 10.1016/j.febslet.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Sullivan R.J., Karlseder J. The great unravelling: Chromatin as a modulator of the aging process. Trends Biochem. Sci. 2012;37:466–476. doi: 10.1016/j.tibs.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imai S.-I., Armstrong C.M., Kaeberlein M., Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 81.Longo V.D. Linking sirtuins, IGF-I signaling, and starvation. Exp. Gerontol. 2009;44:70–74. doi: 10.1016/j.exger.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 82.Zhang L., Xie W.J., Liu S., Meng L., Gu C., Gao Y.Q. DNA Methylation Landscape Reflects the Spatial Organization of Chromatin in Different Cells. Biophys. J. 2017;113:1395–1404. doi: 10.1016/j.bpj.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McClay J.L., Aberg K.A., Clark S.L., Nerella S., Kumar G., Xie L.Y., Hudson A.D., Harada A., Hultman C.M., Magnusson P.K., et al. A methylome-wide study of aging using massively parallel sequencing of the methyl-CpG-enriched genomic fraction from blood in over 700 subjects. Hum. Mol. Genet. 2014;23:1175–1185. doi: 10.1093/hmg/ddt511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teschendorff A.E., Menon U., Gentry-Maharaj A., Ramus S.J., Weisenberger D.J., Shen H., Campan M., Noushmehr H., Bell C.G., Maxwell A.P., et al. Age-dependent DNA methylation of genes that are suppressed in stem cells is a hallmark of cancer. Genome Res. 2010;20:440–446. doi: 10.1101/gr.103606.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pal S., Tyler J.K. Epigenetics and aging. Sci. Adv. 2016;2 doi: 10.1126/sciadv.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jylhava J. Determinants of longevity: Genetics, biomarkers and therapeutic approaches. Current Pharmaceutical Design. 2014;20:6058–6070. doi: 10.2174/1381612820666140314153818. [DOI] [PubMed] [Google Scholar]
- 88.Morselli E., Mariño G., Bennetzen M.V., Eisenberg T., Megalou E., Schroeder S., Cabrera S., Bénit P., Rustin P., Criollo A., et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011;192:615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pasyukova E.G., Vaiserman A.M. HDAC inhibitors: A new promising drug class in anti-aging research. Mech. Ageing Dev. 2017;166:6–15. doi: 10.1016/j.mad.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 90.McIntyre R.L., Daniels E.G., Molenaars M., Houtkooper R.H., Janssens G.E. From molecular promise to preclinical results: HDAC inhibitors in the race for healthy aging drugs. EMBO Mol. Med. 2019;11:e9854. doi: 10.15252/emmm.201809854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hetz C., Chevet E., Oakes S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannizzo E.S., Clement C.C., Morozova K., Valdor R., Kaushik S., Almeida L.N., Follo C., Sahu R., Cuervo A.M., Macian F., et al. Age-Related Oxidative Stress Compromises Endosomal Proteostasis. Cell Rep. 2012;2:136–149. doi: 10.1016/j.celrep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brehm A., Krüger E. Dysfunction in protein clearance by the proteasome: Impact on autoinflammatory diseases. Semin. Immunopathol. 2015;37:323–333. doi: 10.1007/s00281-015-0486-4. [DOI] [PubMed] [Google Scholar]
- 94.Dai D.-F., Karunadharma P.P., Chiao Y.A., Basisty N., Crispin D., Hsieh E.J., Chen T., Gu H., Djukovic D., Raftery D., et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong Z., Tasset I. Humanin enhances the cellular response to stress by activation of chaperone-mediated autophagy. Oncotarget. 2018;9:10832–10833. doi: 10.18632/oncotarget.24396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Amm I., Sommer T., Wolf D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin–proteasome system. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2013;1843:182–196. doi: 10.1016/j.bbamcr.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 97.Koga H., Kaushik S., Cuervo A.M. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 2011;10:205–215. doi: 10.1016/j.arr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calderwood S.K., Murshid A., Prince T. The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging—A Mini-Review. Gerontology. 2009;55:550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morrow G., Samson M., Michaud S., Tanguay R.M. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004;18:598–599. doi: 10.1096/fj.03-0860fje. [DOI] [PubMed] [Google Scholar]
- 100.Walker G.A., Lithgow G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2:131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 101.Chiang W.C., Ching T.T., Lee H.C., Mousigian C., Hsu A.L. HSF-1 Regulators DDL-1/2 Link Insulin-like Signaling to Heat-Shock Responses and Modulation of Longevity. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsu A.-L., Murphy C.T., Kenyon C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 103.Ünal E., Kinde B., Amon A. Gametogenesis Eliminates Age-Induced Cellular Damage and Resets Life Span in Yeast. Science. 2011;332:1554–1557. doi: 10.1126/science.1204349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erjavec N., Larsson L., Grantham J., Nyström T. Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev. 2007;21:2410–2421. doi: 10.1101/gad.439307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaeberlein M., Kirkland K.T., Fields S., Kennedy B.K. Genes determining yeast replicative life span in a long-lived genetic background. Mech. Ageing Dev. 2005;126:491–504. doi: 10.1016/j.mad.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 106.Andersson V., Hanzén S., Liu B., Molin M., Nyström T. Enhancing protein disaggregation restores proteasome activity in aged cells. Aging. 2013;5:802–812. doi: 10.18632/aging.100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Press M., Jung T., König J., Grune T., Höhn A. Protein aggregates and proteostasis in aging: Amylin and β-cell function. Mech. Ageing Dev. 2019;177:46–54. doi: 10.1016/j.mad.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 108.Moreno D., Jenkins K., Morlot S., Charvin G., Csikasz-Nagy A., Aldea M. Proteostasis collapse, a hallmark of aging, hinders the chaperone-Start network and arrests cells in G1. eLife. 2019;8:48240. doi: 10.7554/eLife.48240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chondrogianni N., Georgila K., Kourtis N., Tavernarakis N., Gonos E.S. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J. 2015;29:611–622. doi: 10.1096/fj.14-252189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chondrogianni N., Tzavelas C., Pemberton A.J., Nezis I.P., Rivett A.J., Gonos E.S. Overexpression of Proteasome β5 Assembled Subunit Increases the Amount of Proteasome and Confers Ameliorated Response to Oxidative Stress and Higher Survival Rates*[boxs] J. Biol. Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 111.Tonoki A., Kuranaga E., Tomioka T., Hamazaki J., Murata S., Tanaka K., Miura M. Genetic Evidence Linking Age-Dependent Attenuation of the 26S Proteasome with the Aging Process. Mol. Cell. Biol. 2009;29:1095–1106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Papaevgeniou N., Sakellari M., Jha S., Tavernarakis N., Holmberg C.I., Gonos E.S., Chondrogianni N. 18α-Glycyrrhetinic acid proteasome activator decelerates aging and Alzheimer’s disease progression in Caenorhabditis elegans and neuronal cultures. Antioxid. Redox Signal. 2016;25:855–869. doi: 10.1089/ars.2015.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 114.Kim E.J.E., Lee S.-J.V. Recent progresses on anti-aging compounds and their targets in Caenorhabditis elegans. Transl. Med. Aging. 2019;3:121–124. doi: 10.1016/j.tma.2019.11.003. [DOI] [Google Scholar]
- 115.Cheng J., North B.J., Zhang T., Dai X., Tao K., Guo J., Wei W. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell. 2018;17:e12801. doi: 10.1111/acel.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Höhn A., Tramutola A., Cascella R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxidative Med. Cell. Longev. 2020;2020:5497046. doi: 10.1155/2020/5497046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sigurdson C.J., Bartz J.C., Glatzel M. Cellular and Molecular Mechanisms of Prion Disease. Annu. Rev. Pathol. Mech. Dis. 2019;14:497–516. doi: 10.1146/annurev-pathmechdis-012418-013109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wickner R.B., Bezsonov E.E., Son M., Ducatez M., DeWilde M., Edskes H.K. Anti-Prion Systems in Yeast and Inositol Polyphosphates. Biochemistry. 2018;57:1285–1292. doi: 10.1021/acs.biochem.7b01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 120.Fang E.F., Scheibye-Knudsen M., Chua K.F., Mattson M.P., Croteau D.L., Bohr V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016;17:308–321. doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Babbar M., Basu S., Yang B., Croteau D.L., Bohr V.A. Mitophagy and DNA damage signaling in human aging. Mech. Ageing Dev. 2020;186:111207. doi: 10.1016/j.mad.2020.111207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knuppertz L., Warnsmann V., Hamann A., Grimm C., Osiewacz H.D. Stress-dependent opposing roles for mitophagy in aging of the ascomycete Podospora anserina. Autophagy. 2017;13:1037–1052. doi: 10.1080/15548627.2017.1303021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jin S.M., Youle R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Greene J.C., Whitworth A.J., Kuo I., Andrews L.A., Feany M.B., Pallanck L.J. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rana A., Rera M., Walker D.W. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc. Natl. Acad. Sci. USA. 2013;110:8638–8643. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.López-Armada M.J., Riveiro-Naveira R.R., Vaamonde-García C., Valcárcel-Ares M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013;13:106–118. doi: 10.1016/j.mito.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 127.Hashemzaei M., Heravi R.E., Rezaee R., Roohbakhsh A., Karimi G. Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. Eur. J. Pharmacol. 2017;802:44–51. doi: 10.1016/j.ejphar.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 128.Yoshino J., Mills K.F., Yoon M.J., Imai S.-I. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y.S., Viswanathan M., Schoonjans K., et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonkowski M.S., Sinclair D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feige J.N., Lagouge M., Canto C., Strehle A., Houten S.M., Milne J.C., Lambert P.D., Mataki C., Elliott P.J., Auwerx J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 132.Canto C., Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park D., Jeong H., Lee M.N., Koh A., Kwon O., Yang Y.R., Noh J., Suh P.-G., Park H., Ryu S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016;6:21772. doi: 10.1038/srep21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuno A., Hosoda R., Sebori R., Hayashi T., Sakuragi H., Tanabe M., Horio Y. Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin-deficient mdx Mice. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-33930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cao S., Shen Z., Wang C., Zhang Q., Hong Q., He Y., Hu C. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets 1. Food Funct. 2019;10:344–354. doi: 10.1039/C8FO02091D. [DOI] [PubMed] [Google Scholar]
- 136.Pearson K.J., Baur J.A., Lewis K.N., Peshkin L., Price N.L., Labinskyy N., Swindell W.R., Kamara D., Minor R.K., Perez E., et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zarse K., Schmeisser S., Birringer M., Falk E., Schmoll D., Ristow M. Differential Effects of Resveratrol and SRT1720 on Lifespan of Adult Caenorhabditis elegans. Horm. Metab. Res. 2010;42:837–839. doi: 10.1055/s-0030-1265225. [DOI] [PubMed] [Google Scholar]
- 138.Poulsen M.M., Vestergaard P.F., Clasen B.F., Radko Y., Christensen L.P., Stødkilde-Jørgensen H., Møller N., Jessen N., Pedersen S.B., Jørgensen J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–1195. doi: 10.2337/db12-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Eisenberg T., Abdellatif M., Schroeder S., Primessnig U., Stekovic S., Pendl T., Harger A., Schipke J., Zimmermann A., Schmidt A., et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Félix A.A., Williams E.G., Jha P., Lo Sasso G., Huzard D., et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 141.Andreux P.A., Blanco-Bose W., Ryu D., Burdet F., Ibberson M., Aebischer P., Auwerx J., Singh A., Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019;1:595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 142.Fang E.F., Waltz T.B., Kassahun H., Lu Q., Kerr J.S., Morevati M., Fivenson E.M., Wollman B.N., Marosi K., Wilson M.A., et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017;7:46208. doi: 10.1038/srep46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Richter U., Lahtinen T., Marttinen P., Myöhänen M., Greco D., Cannino G., Jacobs H.T., Lietzén N., Nyman T.A., Battersby B.J. A Mitochondrial Ribosomal and RNA Decay Pathway Blocks Cell Proliferation. Curr. Biol. 2013;23:535–541. doi: 10.1016/j.cub.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 144.Sun N., Yun J., Liu J., Malide D., Liu C., Rovira I.I., Holmström K.M., Fergusson M.M., Yoo Y.H., Combs C.A., et al. Measuring In Vivo Mitophagy. Mol. Cell. 2015;60:685–696. doi: 10.1016/j.molcel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xing Y., Liqi Z., Jian L., Qinghua Y., Qian Y. Doxycycline Induces Mitophagy and Suppresses Production of Interferon-β in IPEC-J2 Cells. Front. Cell. Infect. Microbiol. 2017;7:21. doi: 10.3389/fcimb.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kujoth G.C., Bradshaw P.C., Haroon S., Prolla T.A. The Role of Mitochondrial DNA Mutations in Mammalian Aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.DeBalsi K.L., Hoff K.E., Copeland W.C. Role of the mitochondrial DNA replication machinery in mitochondrial DNA mutagenesis, aging and age-related diseases. Ageing Res. Rev. 2017;33:89–104. doi: 10.1016/j.arr.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Morita M., Gravel S.-P., Chénard V., Sikström K., Zheng L., Alain T., Gandin V., Avizonis D., Arguello M., Zakaria C., et al. mTORC1 Controls Mitochondrial Activity and Biogenesis through 4E-BP-Dependent Translational Regulation. Cell Metab. 2013;18:698–711. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 149.Bartolomé A., García-Aguilar A., Asahara S.-I., Kido Y., Guillén C., Pajvani U.B., Benito M. MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling. Mol. Cell. Biol. 2017;37:e00441-17. doi: 10.1128/MCB.00441-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Narita M., Young A.R.J., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., Hong S., Berry L.S., Reichelt S., Ferreira M., et al. Spatial Coupling of mTOR and Autophagy Augments Secretory Phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Iglesias-Bartolome R., Patel V., Cotrim A., Leelahavanichkul K., Molinolo A.A., Mitchell J.B., Gutkind J.S. mTOR Inhibition Prevents Epithelial Stem Cell Senescence and Protects from Radiation-Induced Mucositis. Cell Stem Cell. 2012;11:401–414. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kolesnichenko M., Hong L., Liao R., Vogt P.K., Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–2401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vellai T., Takacs-Vellai K., Zhang Y., Kovacs A.L., Orosz L., Müller F. Influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- 154.Kapahi P., Zid B.M., Harper T., Koslover D., Sapin V., Benzer S. Regulation of Lifespan in Drosophila by Modulation of Genes in the TOR Signaling Pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bai H., Post S., Kang P., Tatar M. Drosophila Longevity Assurance Conferred by Reduced Insulin Receptor Substrate Chico Partially Requires d4eBP. PLoS ONE. 2015;10:e0134415. doi: 10.1371/journal.pone.0134415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kaeberlein M., Powers R.W., III, Steffen K.K., Westman E.A., Hu D., Dang N., Kerr E.O., Kirkland K.T., Fields S., Kennedy B.K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 157.Deprez M.-A., Eskes E., Winderickx J., Wilms T. The TORC1-Sch9 pathway as a crucial mediator of chronological lifespan in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2018;18:48. doi: 10.1093/femsyr/foy048. [DOI] [PubMed] [Google Scholar]
- 158.Wu J.J., Liu J., Chen E.B., Wang J.J., Cao L., Narayan N., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., et al. Increased Mammalian Lifespan and a Segmental and Tissue-Specific Slowing of Aging after Genetic Reduction of mTOR Expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Powers R.W., Kaeberlein M., Caldwell S.D., Kennedy B.K., Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bjedov I., Toivonen J.M., Kerr F., Slack C., Jacobson J., Foley A., Partridge L. Mechanisms of Life Span Extension by Rapamycin in the Fruit Fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Scialo F., Sriram A., Naudi A., Ayala V., Jové M., Pamplona R., Sanz A. Target of rapamycin activation predicts lifespan in fruit flies. Cell Cycle. 2015;14:2949–2958. doi: 10.1080/15384101.2015.1071745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moskalev A., Shaposhnikov M. Pharmacological Inhibition of Phosphoinositide 3 and TOR Kinases Improves Survival of Drosophila melanogaster. Rejuvenation Res. 2010;13:246–247. doi: 10.1089/rej.2009.0903. [DOI] [PubMed] [Google Scholar]
- 163.Danilov A., Shaposhnikov M., Plyusnina E., Kogan V., Fedichev P., Moskalev A. Selective anticancer agents suppress aging in Drosophila. Oncotarget. 2013;4:1507–1526. doi: 10.18632/oncotarget.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fan X., Liang Q., Lian T., Wu Q., Gaur U., Li D., Yang D., Mao X., Jin Z., Li Y., et al. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget. 2015;6:35274–35283. doi: 10.18632/oncotarget.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Robida-Stubbs S., Glover-Cutter K., Lamming D.W., Mizunuma M., Narasimhan S.D., Neumann-Haefelin E., Sabatini D.M., Blackwell T.K. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anisimov V.N., Zabezhinski M.A., Popovich I.G., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M., Antoch M.P., Blagosklonny M.V. Rapamycin Extends Maximal Lifespan in Cancer-Prone Mice. Am. J. Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Anisimov V.N., Zabezhinski M.A., Popovich I.G., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M., Rosenfeld S.V., Blagosklonny M.V. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 169.Wilkinson J.E., Burmeister L., Brooks S.V., Chan C.-C., Friedline S., Harrison D.E., Hejtmancik J.F., Nadon N., Strong R., Wood L.K., et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Comas M., Toshkov I., Kuropatwinski K.K., Chernova O.B., Polinsky A., Blagosklonny M.V., Gudkov A.V., Antoch M.P. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53−/− mice by delaying carcinogenesis. Aging. 2012;4:715–722. doi: 10.18632/aging.100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Komarova E.A., Antoch M.P., Novototskaya L.R., Chernova O.B., Paszkiewicz G., Leontieva O.V., Blagosklonny M.V., Gudkov A.V. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging. 2012;4:709. doi: 10.18632/aging.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramos F.J., Chen S.C., Garelick M.G., Dai D.F., Liao C.Y., Schreiber K.H., MacKay V.L., An E.H., Strong R., Ladiges W.C., et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C–deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Livi C.B., Hardman R.L., Christy B.A., Dodds S.G., Jones D., Williams C., Strong R., Bokov A., Javors M.A., Ikeno Y., et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging. 2013;5:100. doi: 10.18632/aging.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miller R.A., Harrison D.E., Astle C.M., Fernandez E., Flurkey K., Han M., Javors M.A., Li X., Nadon N.L., Nelson J.F., et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Popovich I.G., Anisimov V.N., Zabezhinski M.A., Semenchenko A.V., Tyndyk M.L., Yurova M.N., Blagosklonny M.V. Lifespan extension and cancer prevention in HER-2/neu transgenic mice treated with low intermittent doses of rapamycin. Cancer Biol. Ther. 2014;15:586–592. doi: 10.4161/cbt.28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Y., Bokov A., Gelfond J., Soto V., Ikeno Y., Hubbard G., Diaz V., Sloane L., Maslin K., Treaster S., et al. Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fok W.C.Y., Chen Y., Bokov A., Zhang Y., Salmon A., Diaz V., Javors M., Wood W.H., Zhang Y., Becker K., et al. Mice Fed Rapamycin Have an Increase in Lifespan Associated with Major Changes in the Liver Transcriptome. PLoS ONE. 2014;9:e83988. doi: 10.1371/journal.pone.0083988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leontieva O.V., Paszkiewicz G.M., Blagosklonny M.V. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014;13:616–622. doi: 10.1111/acel.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hasty P., Livi C.B., Dodds S.G., Jones D., Strong R., Javors M., Fischer K.E., Sloane L., Murthy K., Hubbard G., et al. eRapa Restores a Normal Life Span in a FAP Mouse ModelFAP Mice Can Live a Long Healthy Life. Cancer Prev. Res. 2014;7:169–178. doi: 10.1158/1940-6207.CAPR-13-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischer K.E., Gelfond J.A.L., Soto V.Y., Han C., Someya S., Richardson A., Austad S.N. Health Effects of Long-Term Rapamycin Treatment: The Impact on Mouse Health of Enteric Rapamycin Treatment from Four Months of Age throughout Life. PLoS ONE. 2015;10:e0126644. doi: 10.1371/journal.pone.0126644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ye L., Widlund A.L., Sims C.A., Lamming D.W., Guan Y., Davis J.G., Harrison D.E., Vang O., Baur J.A., Sabatini D. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging. 2013;5:7. doi: 10.18632/aging.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnson S.C., Yanos M.E., Kayser E.-B., Quintana A., Sangesland M., Castanza A., Uhde L., Hui J., Wall V.Z., Gagnidze A., et al. mTOR Inhibition Alleviates Mitochondrial Disease in a Mouse Model of Leigh Syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hurez V., Dao V., Liu A., Pandeswara S., Gelfond J., Sun L., Bergman M., Orihuela C.J., Galvan V., Padrón Á., et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi: 10.1111/acel.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Verlingue L., Dugourd A., Stoll G., Barillot E., Calzone L., Londoño-Vallejo A. A comprehensive approach to the molecular determinants of lifespan using a Boolean model of geroconversion. Aging Cell. 2016;15:1018–1026. doi: 10.1111/acel.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Johnson S.C., Yanos M.E., Bitto A., Castanza A., Gagnidze A., Gonzalez B., Gupta K., Hui J., Jarvie C., Johnson B.M., et al. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front. Genet. 2015;6:247. doi: 10.3389/fgene.2015.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xue Q.-L., Yang H., Li H.-F., Abadir P.M., Burks T.N., Koch L.G., Britton S.L., Carlson J., Chen L., Walston J.D., et al. Rapamycin increases grip strength and attenuates age-related decline in maximal running distance in old low capacity runner rats. Aging. 2016;8:769–776. doi: 10.18632/aging.100929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bitto A., Ito T.K., Pineda V.V., Letexier N.J., Huang H., Sutlief E., Tung H., Vizzini N., Chen B., Smith K., et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife. 2016;5:e16351. doi: 10.7554/eLife.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fang Y., Bartke A. Prolonged Rapamycin treatment led to beneficial metabolic switch. Aging. 2013;5:328–329. doi: 10.18632/aging.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Blagosklonny M.V. Rapamycin extends life- and health span because it slows aging. Aging. 2013;5:592–598. doi: 10.18632/aging.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Stanfel M.N., Shamieh L.S., Kaeberlein M., Kennedy B.K. The TOR pathway comes of age. Biochim. Biophys. Acta BBA—Gen. Subj. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Campistol J.M., Eris J., Oberbauer R., Friend P., Hutchison B., Morales J.M., Claesson K., Stallone G., Russ G., Rostaing L., et al. Sirolimus Therapy after Early Cyclosporine Withdrawal Reduces the Risk for Cancer in Adult Renal Transplantation. J. Am. Soc. Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 192.Kauffman H.M., Cherikh W.S., Cheng Y., Hanto D.W., Kahan B.D. Maintenance Immunosuppression with Target-of-Rapamycin Inhibitors is Associated with a Reduced Incidence of De Novo Malignancies. Transplantation. 2005;80:883–889. doi: 10.1097/01.TP.0000184006.43152.8D. [DOI] [PubMed] [Google Scholar]
- 193.Euvrard S., Morelon E., Rostaing L., Goffin E., Brocard A., Tromme I., Broeders N., del Marmol V., Chatelet V., Dompmartin A., et al. Sirolimus and Secondary Skin-Cancer Prevention in Kidney Transplantation. N. Engl. J. Med. 2012;367:329–339. doi: 10.1056/NEJMoa1204166. [DOI] [PubMed] [Google Scholar]
- 194.Pedro J.M.B.-S., Senovilla L. Immunostimulatory activity of lifespan-extending agents. Aging. 2013;5:793–801. doi: 10.18632/aging.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mannick J.B., Del Giudice G., Lattanzi M., Valiante N.M., Praestgaard J., Huang B., Lonetto M.A., Maecker H.T., Kovarik J., Carson S., et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 196.Kennedy B.K., Pennypacker J.K. Aging interventions get human. Oncotarget. 2015;6:590–591. doi: 10.18632/oncotarget.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Blagosklonny M.V. Rejuvenating immunity: “Anti-aging drug today” eight years later. Oncotarget. 2015;6:19405. doi: 10.18632/oncotarget.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ross C., Salmon A., Strong R., Fernandez E., Javors M., Richardson A., Tardif S. Metabolic consequences of long-term rapamycin exposure on common marmoset monkeys (Callithrix jacchus) Aging. 2015;7:964–973. doi: 10.18632/aging.100843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zaseck L.W., Miller R.A., Brooks S.V. Rapamycin Attenuates Age-associated Changes in Tibialis Anterior Tendon Viscoelastic Properties. J. Gerontol. Ser. A. 2016;71:858–865. doi: 10.1093/gerona/glv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lelegren M., Liu Y., Ross C., Tardif S., Salmon A.B. Pharmaceutical inhibition of mTOR in the common marmoset: Effect of rapamycin on regulators of proteostasis in a non-human primate. Pathobiol. Aging Age-Related Dis. 2016;6:31793. doi: 10.3402/pba.v6.31793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Johnson S., Rabinovitch P.S., Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nat. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lin K., Dorman J.B., Rodan A., Kenyon C. daf-16: An HNF-3/forkhead Family Member That Can Function to Double the Life-Span of Caenorhabditis elegans. Science. 1979;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 203.Hwangbo D.S., Gersham B., Tu M.-P., Palmer M., Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nat. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 204.Tatar M., Kopelman A., Epstein D., Tu M.-P., Yin C.-M., Garofalo R.S. A Mutant Drosophila Insulin Receptor Homolog That Extends Life-Span and Impairs Neuroendocrine Function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 205.Selman C., Lingard S., Choudhury A.I., Batterham R.L., Claret M., Clements M., Ramadani F., Okkenhaug K., Schuster E., Blanc E., et al. Evidence for lifespan extension and delayed age–related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 206.Selman C., Partridge L., Withers D. Replication of Extended Lifespan Phenotype in Mice with Deletion of Insulin Receptor Substrate 1. PLoS ONE. 2011;6:e16144. doi: 10.1371/journal.pone.0016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Anderson R.M., le Couteur D.G., de Cabo R. Caloric Restriction Research: New Perspectives on the Biology of Aging. J. Gerontol. Ser. A. 2018;73:1–3. doi: 10.1093/gerona/glx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gems D., Partridge L. Genetics of Longevity in Model Organisms: Debates and Paradigm Shifts. Annu. Rev. Physiol. 2013;75:621–644. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- 209.Mattison J.A., Colman R.J., Beasley T.M., Allison D.B., Kemnitz J.W., Roth G.S., Ingram D.K., Weindruch R., De Cabo R., Anderson R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li J., Cui X., Wang Z., Li Y. rBTI extends Caenorhabditis elegans lifespan by mimicking calorie restriction. Exp. Gerontol. 2015;67:62–71. doi: 10.1016/j.exger.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 211.Jeon H., Cha D.S. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chin. J. Nat. Med. 2016;14:335–342. doi: 10.3724/sp.j.1009.2016.00335. [DOI] [PubMed] [Google Scholar]
- 212.Wan Q.L., Fu X., Meng X., Luo Z., Dai W., Yang J., Wang C., Wang H., Zhou Q. Hypotaurine promotes longevity and stress tolerance via the stress response factors DAF-16/FOXO and SKN-1/NRF2 in Caenorhabditis elegans. Food Funct. 2020;11:347–357. doi: 10.1039/C9FO02000D. [DOI] [PubMed] [Google Scholar]
- 213.Steinberg G.R., Kemp B.E. AMPK in Health and Disease. Physiol. Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 214.Hardie D.G. AMP-activated protein kinase—An energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Burnett C., Valentini S., Cabreiro F., Goss M., Somogyvári M., Piper M.D., Hoddinott M., Sutphin G.L., Leko V., McElwee J.J., et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 218.Kapahi P., Kaeberlein M., Hansen M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res. Rev. 2017;39:3–14. doi: 10.1016/j.arr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Satoh A., Brace C.S., Rensing N., Cliften P., Wozniak D.F., Herzog E.D., Yamada K.A., Imai S.-I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rogina B., Helfand S.L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Coughlan K.A., Valentine R.J., Ruderman N.B., Saha A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014;7:241. doi: 10.2147/DMSO.S43731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Tissenbaum H.A., Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 224.Wang N., Luo Z., Jin M., Sheng W., Wang H.-T., Long X., Wu Y., Hu P., Xu H., Zhang X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging. 2019;11:3117–3137. doi: 10.18632/aging.101966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Minor R.K., Baur J.A., Gomes A.P., Ward T.M., Csiszar A., Mercken E.M., Abdelmohsen K., Shin Y.-K., Canto C., Scheibye-Knudsen M., et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Milne J.C., Lambert P.D., Schenk S., Carney D.P., Smith J.J., Gagne D.J., Jin L., Boss O., Perni R.B., Vu C.B., et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ota H., Eto M., Kano M.R., Ogawa S., Iijima K., Akishita M., Ouchi Y. Cilostazol Inhibits Oxidative Stress–Induced Premature Senescence Via Upregulation of Sirt1 in Human Endothelial Cells. Arter. Thromb. Vasc. Biol. 2008;28:1634–1639. doi: 10.1161/ATVBAHA.108.164368. [DOI] [PubMed] [Google Scholar]
- 229.Jamal J., Mustafa M.R., Wong P.-F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014;154:428–436. doi: 10.1016/j.jep.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 230.Ota H., Eto M., Kano M.R., Kahyo T., Setou M., Ogawa S., Iijima K., Akishita M., Ouchi Y. Induction of Endothelial Nitric Oxide Synthase, SIRT1, and Catalase by Statins Inhibits Endothelial Senescence Through the Akt Pathway. Arter. Thromb. Vasc. Biol. 2010;30:2205–2211. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- 231.Zheng M., Qiao W., Cui J., Liu L., Liu H., Wang Z., Yan C. Hydrogen sulfide delays nicotinamide-induced premature senescence via upregulation of SIRT1 in human umbilical vein endothelial cells. Mol. Cell. Biochem. 2014;393:59–67. doi: 10.1007/s11010-014-2046-y. [DOI] [PubMed] [Google Scholar]
- 232.Lee Y.A., Cho E.J., Yokozawa T. Protective Effect of Persimmon (Diospyros kaki) Peel Proanthocyanidin against Oxidative Damage under H2O2-Induced Cellular Senescence. Biol. Pharm. Bull. 2008;31:1265–1269. doi: 10.1248/bpb.31.1265. [DOI] [PubMed] [Google Scholar]
- 233.Jayasena T., Poljak A., Smythe G., Braidy N., Muench G., Sachdev P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer’s disease. Ageing Res. Rev. 2013;12:867–883. doi: 10.1016/j.arr.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 234.Apfeld J., O’Connor G., McDonagh T., DiStefano P.S., Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Onken B., Driscoll M. Metformin Induces a Dietary Restriction–Like State and the Oxidative Stress Response to Extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS ONE. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Anisimov V.N. Metformin for aging and cancer prevention. Aging. 2010;2:760–774. doi: 10.18632/aging.100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Funakoshi M., Tsuda M., Muramatsu K., Hatsuda H., Morishita S., Aigaki T. A gain-of-function screen identifies wdb and lkb1 as lifespan-extending genes in Drosophila. Biochem. Biophys. Res. Commun. 2011;405:667–672. doi: 10.1016/j.bbrc.2011.01.090. [DOI] [PubMed] [Google Scholar]
- 238.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.-J., et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cabreiro F., Au C., Leung K.-Y., Vergara-Irigaray N., Cochemé H.M., Noori T., Weinkove D., Schuster E., Greene N.D., Gems D. Metformin Retards Aging in C. elegans by Altering Microbial Folate and Methionine Metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Piskovatska V., Stefanyshyn N., Storey K.B., Vaiserman A.M., Lushchak O. Metformin as a geroprotector: Experimental and clinical evidence. Biogerontology. 2019;20:33–48. doi: 10.1007/s10522-018-9773-5. [DOI] [PubMed] [Google Scholar]
- 241.Curtis R., O’Connor G., DiStefano P.S. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5:119–126. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- 242.Park S.-K., Seong R.-K., Kim J.-A., Son S.-J., Kim Y., Yokozawa T., Shin O.S. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway. Nutr. Res. Pract. 2016;10:3–10. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Campisi J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013;75:685. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.van Deursen J.M. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ogrodnik M., Miwa S., Tchkonia T., Tiniakos D., Wilson C.L., Lahat A., Day C.P., Burt A., Palmer A., Anstee Q.M., et al. Cellular senescence drives age-dependent hepatic steatosis. Nat. Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Campisi J., Robert L. Cell Senescence: Role in Aging and Age-Related Diseases. Aging Facts Theor. 2014;39:45–61. doi: 10.1159/000358899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Muñoz-Espín D., Serrano M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 248.Farr J.N., Xu M., Weivoda M.M., Monroe D.G., Fraser D.G., Onken J.L., Negley B.A., Sfeir J.G., Ogrodnik M.B., Hachfeld C.M., et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Childs B.G., Baker D.J., Wijshake T., Conover C.A., Campisi J., Van Deursen J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Baker D.J., Wijshake T., Tchkonia T., Lebrasseur N.K., Childs B.G., Van De Sluis B., Kirkland J.L., Van Deursen J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sagiv A., Bar-Shai A., Levi N., Hatzav M., Zada L., Ovadya Y., Roitman L., Manella G., Regev O., Majewska J., et al. p53 in Bronchial Club Cells Facilitates Chronic Lung Inflammation by Promoting Senescence. Cell Rep. 2018;22:3468–3479. doi: 10.1016/j.celrep.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 253.Childs B.G., Gluscevic M., Baker D.J., Laberge R.-M., Marquess D., Dananberg J., Van Deursen J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.von Kobbe C. Targeting senescent cells: Approaches, opportunities, challenges. Aging. 2019;11:12844. doi: 10.18632/aging.102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhu Y.I., Tchkonia T., Pirtskhalava T., Gower A.C., Ding H., Giorgadze N., Palmer A.K., Ikeno Y., Hubbard G.B., Lenburg M., et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhu Y., Doornebal E.J., Pirtskhalava T., Giorgadze N., Wentworth M., Fuhrmann-Stroissnigg H., Niedernhofer L.J., Robbins P.D., Tchkonia T., Kirkland J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955–963. doi: 10.18632/aging.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Baar M.P., Brandt R.M.C., Putavet D.A., Klein J.D.D., Derks K.W.J., Bourgeois B.R.M., Stryeck S., Rijksen Y., Van Willigenburg H., Feijtel D.A., et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Chang J., Wang Y., Shao L., Laberge R.-M., DeMaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W., et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2017;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chung C.L., Lawrence I., Hoffman M., Elgindi D., Nadhan K., Potnis M., Jin A., Sershon C., Binnebose R., Lorenzini A., et al. Topical rapamycin reduces markers of senescence and aging in human skin: An exploratory, prospective, randomized trial. GeroScience. 2019;41:861–869. doi: 10.1007/s11357-019-00113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Xu M., Tchkonia T., Ding H., Ogrodnik M., Lubbers E.R., Pirtskhalava T., White T.A., Johnson K.O., Stout M.B., Mezera V., et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc. Natl. Acad. Sci. USA. 2015;112:E6301–E6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tilstra J.S., Robinson A.R., Wang J., Gregg S.Q., Clauson C.L., Reay D.P., Nasto L.A., St Croix C.M., Usas A., Vo N., et al. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J. Clin. Investig. 2012;122:2601–2612. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ozsvari B., Nuttall J.R., Sotgia F., Lisanti M.P. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging. 2018;10:3294–3307. doi: 10.18632/aging.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Bielak-Zmijewska A., Grabowska W., Ciolko A., Bojko A., Mosieniak G., Bijoch Ł., Sikora E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019;20:1239. doi: 10.3390/ijms20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Conboy I.M., Rando T.A. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle. 2012;11:2260–2267. doi: 10.4161/cc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gruber R., Koch H., Doll B.A., Tegtmeier F., Einhorn T.A., Hollinger J.O. Fracture healing in the elderly patient. Exp. Gerontol. 2006;41:1080–1093. doi: 10.1016/j.exger.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 266.Goodell M.A., Rando T.A. Stem cells and healthy aging. Science. 2015;350:1199–1204. doi: 10.1126/science.aab3388. [DOI] [PubMed] [Google Scholar]
- 267.Rando T.A. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 268.Molofsky A.V., Slutsky S.G., Joseph N.M., He S., Pardal R., Krishnamurthy J., Sharpless N., Morrison S.J. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lavasani M., Robinson A.R., Lu A., Song M., Feduska J.M., Ahani B., Tilstra J.S., Feldman C.H., Robbins P.D., Niedernhofer L.J., et al. Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat. Commun. 2012;3:608. doi: 10.1038/ncomms1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rando T.A., Chang H.Y. Aging, Rejuvenation, and Epigenetic Reprogramming: Resetting the Aging Clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Rossi D.J., Bryder D., Seita J., Nussenzweig A., Hoeijmakers J., Weissman I.L. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 272.Mertens J., Paquola A.C., Ku M., Hatch E., Böhnke L., Ladjevardi S., McGrath S., Campbell B., Lee H., Herdy J.R., et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell. 2015;17:705–718. doi: 10.1016/j.stem.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wahlestedt M., Norddahl G.L., Sten G., Ugale A., Frisk M.-A.M., Mattsson R., Deierborg T., Sigvardsson M., Bryder D. An epigenetic component of hematopoietic stem cell aging amenable to reprogramming into a young state. Blood. 2013;121:4257–4264. doi: 10.1182/blood-2012-11-469080. [DOI] [PubMed] [Google Scholar]
- 274.Fatt M., Hsu K., He L., Wondisford F., Miller F.D., Kaplan D.R., Wang J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem Cell Rep. 2015;5:988–995. doi: 10.1016/j.stemcr.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Khorraminejad-Shirazi M., Farahmandnia M., Kardeh B., Estedlal A., Kardeh S., Monabati A. Aging and stem cell therapy: AMPK as an applicable pharmacological target for rejuvenation of aged stem cells and achieving higher efficacy in stem cell therapy. Hematol. Stem Cell Ther. 2017;11:189–194. doi: 10.1016/j.hemonc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 276.Safaeinejad Z., Nabiuni M., Peymani M., Ghaedi K., Nasr-Esfahani M.-H., Baharvand H. Resveratrol promotes human embryonic stem cells self-renewal by targeting SIRT1-ERK signaling pathway. Eur. J. Cell Biol. 2017;96:665–672. doi: 10.1016/j.ejcb.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 277.Candela M., Biagi E., Brigidi P., O’Toole P.W., De Vos W.M. Maintenance of a healthy trajectory of the intestinal microbiome during aging: A dietary approach. Mech. Ageing Dev. 2014;136-137:70–75. doi: 10.1016/j.mad.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 278.Saraswati S., Sitaraman R. Aging and the human gut microbiota—From correlation to causality. Front. Microbiol. 2014;5:764. doi: 10.3389/fmicb.2014.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.O’Toole P.W., Jeffery I.B. Gut microbiota and aging. Science. 2015;350:1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 280.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pérez Martínez G., Bäuerl C., Collado M.C. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes. 2014;5:235–246. doi: 10.3920/BM2013.0079. [DOI] [PubMed] [Google Scholar]
- 282.Lowry C.A., Smith D.G., Siebler P.H., Schmidt D., Stamper C.E., Hassell J.E., Yamashita P.S., Fox J.H., Reber S.O., Brenner L.A., et al. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Curr. Environ. Health Rep. 2016;3:270–286. doi: 10.1007/s40572-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nehra V., Allen J.M., Mailing L.J., Kashyap P.C., Woods J.A. Gut Microbiota: Modulation of Host Physiology in Obesity. Physiology. 2016;31:327–335. doi: 10.1152/physiol.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhang X., Zheng X., Yuan Y. Treatment of insulin resistance: Straight from the gut. Drug Discov. Today. 2016;21:1284–1290. doi: 10.1016/j.drudis.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 285.Rivière A., Selak M., Lantin D., Leroy F., De Vuyst L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Patel R., Dupont H.L. New Approaches for Bacteriotherapy: Prebiotics, New-Generation Probiotics, and Synbiotics. Clin. Infect. Dis. 2015;60:S108–S121. doi: 10.1093/cid/civ177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vaiserman A.M., Koliada A.K., Marotta F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017;35:36–45. doi: 10.1016/j.arr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 288.Wu M., Luo Q., Nie R., Yang X., Tang Z., Chen H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2020;61:2175–2193. doi: 10.1080/10408398.2020.1773390. [DOI] [PubMed] [Google Scholar]
- 289.Du Y., Gao Y., Zeng B., Fan X., Yang D., Yang M. Effects of anti-aging interventions on intestinal microbiota. Gut Microbes. 2021;13:1994835. doi: 10.1080/19490976.2021.1994835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Yoon J.-H., Abdelmohsen K., Kim J., Yang X., Martindale J.L., Tominaga-Yamanaka K., White E.J., Orjalo A.V., Rinn J.L., Kreft S.G., et al. Scaffold function of long non-coding RNA HOTAIR in protein ubiquitination. Nat. Commun. 2013;4:2939. doi: 10.1038/ncomms3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Singh D.K., Prasanth K.V. Functional insights into the role of nuclear-retained long noncoding RNAs in gene expression control in mammalian cells. Chromosom. Res. 2013;21:695–711. doi: 10.1007/s10577-013-9391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yoon J.-H., Abdelmohsen K., Gorospe M. Posttranscriptional Gene Regulation by Long Noncoding RNA. J. Mol. Biol. 2013;425:3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Grammatikakis I., Panda A.C., Abdelmohsen K., Gorospe M. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging. 2014;6:992–1009. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.