Abstract
Simple Summary
Surgical resection is the primary curative treatment option for colorectal cancer. However, colorectal resections remain associated with significant postoperative morbidity and mortality. Furthermore, most rectal cancer patients and some patients with locally advanced colon cancer may need preoperative neoadjuvant therapy. It improves long-term outcomes but impairs patients’ physical fitness and thus further increases surgical risk. Prehabilitation is a novel approach, aiming to improve patients’ physical and psychological capacity to reduce postoperative morbidity and improve treatment outcomes. This study aims to comprehensively overview current knowledge on colorectal cancer surgery’s prehabilitation.
Abstract
Colorectal cancer remains the third most prevalent cancer worldwide, exceeding 1.9 million new cases annually. Surgery continues to be the gold standard treatment option. Unfortunately, colorectal cancer surgery carries significant postoperative morbidity and mortality. Moreover, most rectal cancer patients and some patients with locally advanced colon cancer require preoperative neoadjuvant therapy. It improves long-term outcomes but impairs patients’ physical fitness and thus further increases surgical risk. Recently, prehabilitation has gained interest as a novel strategy to reduce treatment-related morbidity for patients undergoing colorectal cancer surgery. However, the concept is still in its infancy, and the role of prehabilitation remains controversial. In this comprehensive review, we sum up present evidence on prehabilitation before colorectal cancer surgery. Available studies are very heterogenous in interventions and investigated outcomes. Nonetheless, all trials show at least some positive effects of prehabilitation on patients’ physical, nutritional, or psychological status or even reduced postoperative morbidity. Unfortunately, the optimal prehabilitation program remains undetermined; therefore, this concept cannot be widely implemented. Future studies investigating optimal prehabilitation regimens for patients undergoing surgery for colorectal cancer are necessary.
Keywords: colon cancer, rectal cancer, colorectal cancer, prehabilitation, exercise, surgical outcomes
1. Introduction
Colorectal cancer (CRC) remains the third most prevalent cancer worldwide, exceeding 1.9 million new cases annually [1]. Surgery continues to be the gold standard treatment option. However, colorectal cancer surgery carries significant postoperative morbidity and mortality [2]. Moreover, the standard advanced rectal cancer management regimen includes neoadjuvant chemoradiotherapy (CRT) with or without neoadjuvant chemotherapy [3,4,5]. Similarly, some locally advanced colon cancer cases may also benefit from neoadjuvant chemotherapy [6]. Despite neoadjuvant cytotoxic therapy improving long-term oncological outcomes, it significantly decreases physiological reserve by deteriorating patients’ physical condition and nutritional status and promoting sarcopenia [7]. Thus, it may be very challenging for patients. Despite the modern CRC treatment achieving good oncological outcomes, the patients are at significant risk of suffering various treatment-related adverse events and complications through the treatment journey [2].
Physical fitness has been linked not only with a lower risk of developing CRC but also with enhanced recovery and better treatment outcomes in the case of the disease [8,9,10,11,12]. Further exercise interventions are considered to be safe and feasible in oncologic patients, including CRC patients before the surgery and afterward, at the time of adjuvant chemotherapy. Additionally, preoperative exercise-based interventions have positive impact on various health-related outcomes, including improved physical fitness [13,14,15]. Therefore, prehabilitation has gained interest as a novel strategy to reduce treatment-related morbidity [16,17,18,19]. The definition of prehabilitation is not yet standardized. Currently, it may be defined as any interventions initiated preoperatively, aiming to strengthen patients’ physical, nutritional, medical, and mental condition to increase patients’ capacity for resisting surgical trauma and facilitating postoperative return to preoperative conditions [20]. However, this is a relatively new concept and still an investigational treatment option. Present studies on prehabilitation in CRC patients are very heterogenous in studied interventions and outcomes. Thus, the role of prehabilitation in modern CRC treatment remains controversial. This comprehensive review summarizes today’s evidence on prehabilitation’s role in modern CRC surgery.
2. Materials and Methods
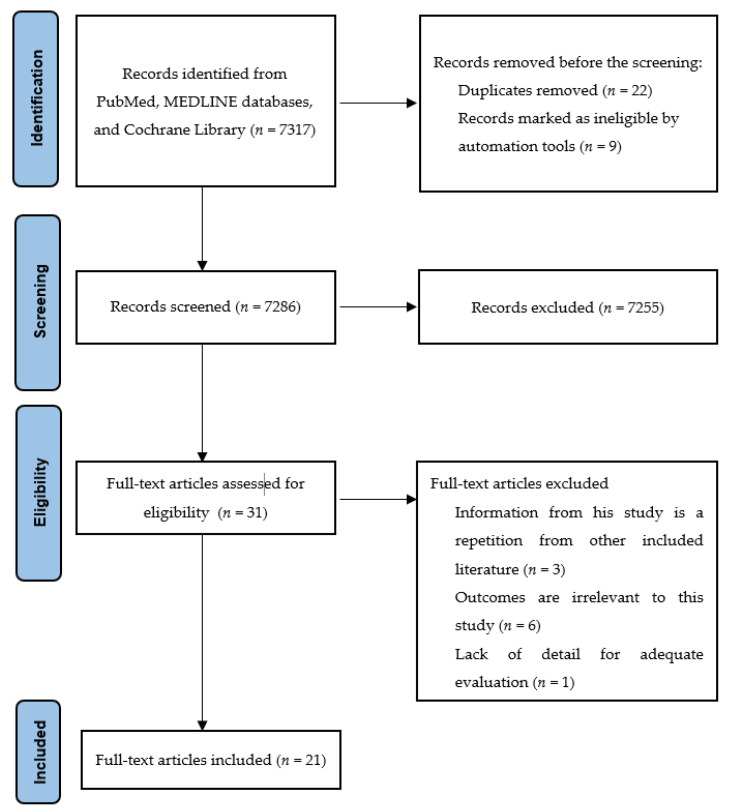
An extensive literature search was performed utilizing the PubMed, MEDLINE databases, and Cochrane Central Register of Controlled Trials on 1 May 2022. The following Medical Subject Heading (MeSH) terms were used during the search process: (prehabilitation OR exercise OR physical therapy OR physical fitness OR nutritional support OR psychological support) AND (colon cancer OR rectal cancer OR colorectal cancer). Only English language manuscripts were considered. As a first step, articles were included based on their title. Next, two independent experienced reviewers (V.A. and A.E.D.) reviewed the titles and identified the appropriate abstracts. After all original studies investigating prehabilitation for CRC were identified and included in this comprehensive review. Full-text manuscripts were extracted for confirmation of inclusion (Figure 1). Jadad [21] and the Newcastle–Ottawa [20] scales for randomized and non-randomized studies were used to determine each study’s quality of evidence. Institutional review board approval was not required.
Figure 1.
Literature search flow diagram.
3. The Present Concept of Prehabilitation in Surgical Management of Colorectal Cancer
The definition of prehabilitation is not yet standardized. Currently, it may be defined as any interventions initiated preoperatively, aiming to strengthen patients’ physical, nutritional, medical, and mental condition to increase patients’ capacity for resisting surgical trauma and facilitating a postoperative return to preoperative conditions [16]. There is a clear emphasis on the time-sensitive component [22]. The preoperative period provides a unique window to condition patients for the upcoming physiological and psychological stress, because most are willing to modify behavior for improved outcomes [23,24]. Today, prehabilitation remains an experimental treatment modality, and there is no general agreement on the optimal design of such programs. Available protocols may include one (unimodal) or several (multimodal) interventions to improve patients’ physical fitness and capacity, optimize nutritional status, and promote psychological resilience. The real benefits of prehabilitation also remain the topic for discussion because today’s evidence is very contradictory. Some studies report minimal benefits regarding the decreased length of hospital stay (LOS) [25]. At the same time, others show larger-scale benefits such as improved nutritional status and physical performance as well as better quality of life (QoL) or even up to 50% lower postoperative morbidity [23,26,27,28]. The variety of interventions, differences in measured outcomes, and heterogeneity of results challenge standardization and wide adoption of this approach. The different prehabilitation programs investigated and their interventions are summarized in Table 1.
Table 1.
Details on interventions in colorectal cancer surgery prehabilitation programs.
| Author; Year | Prehabilitation Group | Control Group | ||
|---|---|---|---|---|
| Type of Intervention (Unimodal vs. Multimodal) |
Timing | Interventions Used for Prehabilitation | ||
| Berkel et al. [26]; 2022 | Unimodal | Start three weeks before surgery. |
|
Standard of care |
| Alejo et al. [29]; 2019 | Unimodal | Start during five weeks of CRT and continuation for additional 6–8 weeks before surgery. |
|
N/A |
| Morielli et al. [30]; 2021 | Unimodal | Start during neoadjuvant CRT. |
|
Standard of care |
| West et al. [16]; 2015 | Unimodal | Start 6 weeks before surgery during neoadjuvant CRT. |
|
Standard of care |
| Moug et al. [31]; 2019 | Unimodal | Start before neoadjuvant CRT; a minimum of 13 weeks duration: 5 weeks during neoadjuvant CRT followed by a minimum of 8 weeks of exercises before surgery. |
|
Standard of care |
| Moug et al. [32]; 2020 | Unimodal | Start before neoadjuvant CRT; a minimum of 13 weeks duration: 5 weeks during neoadjuvant CRT followed by a minimum of 8 weeks of exercises before surgery. |
|
Standard of care |
| Singh et al. [33]; 2017 | Unimodal | Start over a period of 16 weeks before surgery. |
|
N/A |
| Singh et al. [34]; 2018 | Unimodal | Start over a period of 10 weeks during neoadjuvant CRT. |
|
N/A |
| Heldens et al. [35]; 2016 | Unimodal | Start during neoadjuvant CRT, and the exact duration depending on the individual decision for surgery timing. |
|
N/A |
| Loughney et al. [36]; 2017 | Unimodal | Start after completion of neoadjuvant CRT; 6-week duration. |
|
Standard of care |
| Gillis et al. [37]; 2019 | Multimodal | Started 4 weeks before surgery; continued 8 weeks after surgery. |
|
Patients receiving rehabilitation |
| Gillis et al. [38]; 2016 | Unimodal | Start 4 weeks before surgery; continued for 4 weeks after surgery. |
|
Individualized nutrition counseling with a non-nutritive placebo |
| Furyk et al. [39]; 2021 | Multimodal | Start 4 weeks before surgery. |
|
Standard of care |
| Bousquet-Dion et al. [40]; 2018 | Multimodal | Start 4 weeks before surgery. |
|
Standard of care |
| Tweed et al. [41]; 2021 | Multimodal | Start 4 weeks before surgery. |
|
N/A |
| Klerk et al. [42]; 2021 | Multimodal | Start at least 4 weeks before surgery; duration was adjusted based on the date of surgery. |
|
Standard of care |
| Arias et al. [27]; 2021 | Multimodal | Start 30 days before surgery; continued for 30 days after hospital discharge. |
|
Standard of care |
| Karlsson et al. [43]; 2019 | Unimodal | Start at least 2 weeks before surgery. |
|
Standard of care |
| West et al. [44]; 2019 | Unimodal | Start 6 weeks before surgery. |
|
Standard of care |
| Li et al. [17]; 2013 | Multimodal | The start date was predetermined by the time remaining until surgery alone. |
|
Standard of care |
N/A—not applicable; CRT—chemoradiotherapy; HIIT—high-intensity interval training; CaHMB—calcium-β-hydroxy-β-methylbutyrate.
Despite all studies investigating prehabilitation for CRC surgery, they were very different in interventions, timing, and measured outcomes. Table 2 provides more details on study design and measured outcomes as well as each study’s quality of evidence.
Table 2.
Characteristics of prehabilitation studies of surgical management for colorectal cancer.
| Author Year | Design | Description and Number of Participants (n) |
Measured Outcomes | N-O Score | Jadad Score |
|---|---|---|---|---|---|
| Berkel et al. [26]; 2022 | RCT | Colorectal cancer patients undergoing colorectal resection (n = 57) | Primary outcome:
Secondary outcomes:
|
N/A | 3 |
| Alejo et al. [29]; 2019 | Non-randomized pilot study | Colorectal cancer patients undergoing neoadjuvant treatment (n = 12) | Primary outcome:
Secondary outcomes:
|
7 | N/A |
| Morielli et al. [30]; 2021 | RCT | Rectal cancer patients to be treated with neoadjuvant CRT (n = 36) | Primary outcome:
Secondary outcomes:
|
N/A | 3 |
| West et al. [16]; 2015 | Non-randomized, blinded pilot study | Rectal cancer patients to be treated with neoadjuvant CRT (n = 39) | Primary outcome:
Secondary outcomes:
|
8 | N/A |
| Moug et al. [31]; 2019 | RCT | Rectal cancer patients to be treated with neoadjuvant CRT (n = 48) | Primary outcome:
Secondary outcomes:
|
N/A | 3 |
| Moug et al. [32]; 2020 | RCT | Rectal cancer patients to be treated with neoadjuvant CRT (n = 44) | Primary outcome:
|
N/A | 3 |
| Singh et al. [33]; 2017 | Non-randomized pilot study | Rectal cancer patients planned for rectal resection (n = 12) | Primary outcome:
Secondary outcomes:
|
6 | N/A |
| Singh et al. [34]; 2018 | Non-randomized pilot study | Rectal cancer patients to be treated with neoadjuvant CRT (n = 10) | Primary outcomes:
|
6 | N/A |
| Heldens et al. [35]; 2016 | Non-randomized pilot study | Rectal cancer patients to be treated with neoadjuvant CRT (n = 13) | Primary outcome:
Secondary outcomes:
|
6 | N/A |
| Loughney et al. [36]; 2017 | Non-randomized pilot study | Rectal cancer patients to be treated with neoadjuvant CRT (n = 39) | Primary outcome:
|
7 | N/A |
| Gillis et al. [37]; 2019 | RCT | Colorectal cancer patients planned for colorectal resection (n = 139) | Primary outcomes:
|
N/A | 3 |
| Gillis et al. [38]; 2016 | RCT | Colorectal cancer patients planned for colorectal resection (n = 48) | Primary outcome:
Secondary outcomes:
|
N/A | 3 |
| Furyk et al. [39]; 2021 | RCT | Frail colorectal cancer patients planned for colorectal resection (n = 106) | Primary outcome:
Secondary outcome:
|
N/A | 3 |
| Bousquet-Dion et al. [40]; 2018 | RCT | Colorectal cancer patients planned for colorectal resection (n = 80) | Primary outcomes:
Secondary outcomes:
|
N/A | 3 |
| Tweed et al. [41]; 2021 | Non-randomized pilot study | Colorectal cancer patients planned for colorectal resection (n = 9) | Primary outcome:
Secondary outcomes:
|
6 | N/A |
| Klerk et al. [42]; 2021 | Retrospective cohort study | Colorectal cancer patients planned for colorectal resection (n = 351) | Primary outcome:
Secondary outcomes:
|
8 | N/A |
| Arias et al. [27]; 2021 | RCT | Colorectal cancer patients planned for colorectal resection (n = 20) | Primary outcomes:
|
N/A | 3 |
| Karlsson et al. [43]; 2019 | RCT | Colorectal cancer patients planned for colorectal resection (n = 23) | Primary outcome:
Secondary outcomes:
|
N/A | 3 |
| West et al. [44]; 2019 | Non-randomized pilot study | Colorectal cancer patients to be treated with neoadjuvant CRT (n = 35) | Primary outcomes:
|
7 | N/A |
| Li et al. [17]; 2013 | Non-randomized pilot study | Colorectal cancer patients planned for colorectal resection (n = 87) | Primary outcomes:
Secondary outcomes:
|
8 | N/A |
RCT—randomized controlled trial; N/A—not applicable; VAT—ventilatory anaerobic threshold; QoL—quality of life; EORTC - European Organization for Research and Treatment of Cancer; BMI—body mass index; CRT—chemoradiotherapy; 6 MWT—6-minute walking test; SF-36—36-Item Short-Form Health Survey; CCI—comprehensive complication index; PA—physical activity; CHAMPS—Community Healthy Activities Model Program for Seniors; CPET—cardiopulmonary exercise testing; LBM—lean body mass; MF-BIA- multi-frequency bioelectrical impedance analysis; FBM—fat body mass; ypTRG—postoperative pathological tumor regression stage; ypT-stage—postoperative tumor stage pathology; 1-RM—1 repetition maximum.
Among 20 available studies on prehabilitation for CRC patients, there are 10 randomized controlled trials (RCTs) [26,27,30,31,32,37,38,39,40,43], 9 pilot studies [16,17,29,33,34,35,36,41,44] and 1 retrospective cohort study [42]. Table 3 summarizes the reported outcomes of selected studies.
Table 3.
Reported outcomes of prehabilitation for colorectal cancer surgery.
| Author; Year | Impact on Physical Status | Impact on Postoperative Outcomes | Other Effects |
|---|---|---|---|
| Berkel et al. [26]; 2022 | Improved VO2 at the VAT and VO2peak. Quadriceps strength also increased in the prehabilitation group. | Significantly lower complication rate vs. the usual care group. | N/A |
| Alejo et al. [29]; 2019 | Improved VO2peak; after the exercise program, a tendency for increased mean levels of moderate to vigorous PA was observed. | N/A | Adherence to the program was 89% (primary outcome). The scores for the depression and the “emotional function” QoL domain were reduced in the prehabilitation group. |
| Morielli et al. [30]; 2021 | Improved VO2peak while VO2peak decreased in the control group. | Prehabilitation increased rates of pCR/near pCR compared to the control group. | No significant differences were observed between groups for grade ¾ toxicities or treatment completion. |
| West et al. [16]; 2015 | Improved VO2 at the VAT and VO2peak. | N/A | N/A |
| Moug et al. [31]; 2019 | A reduction in step count was observed in both groups, with the prehabilitation group experiencing a lesser decline (non-significant). Prehabilitation increased 6 MWT scores (non-significant). | N/A | The prehabilitation group achieved high levels of satisfaction. |
| Moug et al. [32]; 2020 | A reduction in daily step count was observed in both groups, with a more considerable reduction recorded in the control group. More patients in the intervention group achieved step count improvements at week 12. Prehabilitation increased muscle mass as determined by TPI. | N/A | N/A |
| Singh et al. [33]; 2017 | Prehabilitation increased muscle strength, endurance and preserved lean body mass and ASM. | N/A | No significant changes in any QoL measure or fatigue determined by MFSI scores were reported. There were no significant changes in general well-being at any point in time (assessed using the SF-36 questionnaire) and no adverse effects or health problems related to the exercise program during the training period. |
| Singh et al. [34]; 2018 | Prehabilitation significantly improved muscle strength for the lower limb exercises. While leg press endurance improved, there was no significant change in chest press muscle endurance. Physical performance as measured by 6 m fast walk and 6 m backwards walk improved in the Prehabilitation group. There was no significant change in 400-meter walk time; however, there was a substantial reduction in heart rate immediately after the completion of the test. | N/A | There were significant changes in 3 measures of QoL (emotional function, financial difficulties, diarrhea), with patients also reporting having less constipation. The exercise program did not cause any adverse events. |
| Heldens et al. [35]; 2016 | Prehabilitation increased patient walking distance as determined by 6 MWT and functional exercise capacity (not significant) as well as both leg and arm muscle strength (significantly). | N/A | The feasibility and safety of the program were observed, with a very high attendance rate (95.7%). |
| Loughney et al. [36]; 2017 | Significant improvements in lying down time, sleep efficiency, and duration were reported in the prehabilitation group compared to the control group.In all participants, there was a significant reduction in daily step count, EE, and MET. The apparent improvement in daily step count and overall PAL in the prehabilitation group was not statistically significant compared to the control group. | N/A | N/A |
| Gillis et al. [37]; 2019 | Prehabilitation did not significantly alter body mass compared to rehabilitation. The prehabilitation group had substantially more relative and absolute LBM and less FBM than the control group. | N/A | N/A |
| Gillis et al. [38]; 2016 | The prehabilitation group experienced a clinically meaningful improvement in 6 MWT scores. Recovery rates were similar between groups. No significant differences in self-reported outcomes were observed between the groups. | No significant differences were observed between the groups in an overall 30-day complications rate and severity, emergency department visits and readmission, and median length of stay. | N/A |
| Furyk et al. [39]; 2021 | N/A | N/A | Poor feasibility of an RCT for preoperative prehabilitation in frail colorectal patients was reported. |
| Bousquet-Dion et al. [40]; 2018 | No significant changes in 6 MWD were found between the groups; however, there was a significant correlation between physical activity, energy expenditure, and 6 MWD in the prehabilitation group. | There were no significant differences in the length of stay, emergency department visits, and complications rate between the groups. | Program compliance was 98%. |
| Tweed et al. [41]; 2021 | Prehabilitation improved handgrip strength and exercise capacity. No difference was observed in VO2max and VO2 at VAT before and after prehabilitation. | N/A | No adverse effects were reported. Organizational feasibility was achieved. Overall acceptability of interventions was positive. |
| Klerk et al. [42]; 2021 | Prehabilitation improved 6 MWT and 1-RM. | Compared to the standard care group, rehabilitation reduced complication rate, shortened the median stay, and patients had fewer unplanned readmissions. There was no significant difference in mortality between the groups. | N/A |
| Arias et al. [27]; 2021 | Reduced the deterioration of body composition as compared to the control group 45 days after surgery. These differences, however, were attenuated at 90 days. | Prehabilitation reduced hospital stay duration and postoperative complications. | N/A |
| Karlsson et al. [43]; 2019 | Prehabilitation significantly increased inspiratory muscle strength. | No significant increase in complications was observed in the prehabilitation group. The intervention group showed a shorter median length of stay and better recovery, although not statistically significant. | The recruitment rate was low, at only 35%. Compliance was much higher, at 97%. The overall intervention achieved a high level of acceptability. |
| West et al. [44]; 2019 | Prehabilitation reversed the fall in VO2 at VAT due to NACRT. | The prehabilitation group had significantly greater ypTRG at the time of surgery, which did not result in a significant difference in the ypT-stage. | N/A |
| Li et al. [17]; 2013 | Postoperative walking capacity improved significantly in the prehabilitation group at weeks 4 and 8. A higher share of patients recovered in the prehabilitation group compared to the standard of care at week 8. In addition, higher levels of physical activity before and after surgery were reported in the intervention group. | Similar postoperative complication rates and length of stay were observed in both groups. | Prehabilitated patients immediately before surgery had significantly decreased anxiety and depression symptoms. No clinically or statistically significant increases in any domains of HRQOL were reported for the prehabilitation group. |
VAT—ventilatory anaerobic threshold; N/A—not applicable; PA—physical activity; pCR—pathologic complete response; 6 MWT—6-minute walking test; TPI—total psoas index; MFSI—Multidimensional Fatigue Symptom Inventory; SF-36—36-Item Short Form Health Survey; CCI—comprehensive complication index; EE—energy expenditure; MET—metabolic equivalent; PAL—physical activity level; LBM—lean body mass; FBM—fat body mass; 1-RM—1 repetition maximum; 6MWD—6-minute walking distance; RCT—randomized controlled trial; ypTRG—postoperative pathological tumor regression stage; ypT-stage—postoperative tumor stage pathology; HRQOL—health-related quality of life.
3.1. Exercise Programs Used in Unimodal and Multimodal Prehabilitation
It is well known that exercise in the perioperative period is safe and has many benefits for patients’ health. Exercise has been shown to improve physical fitness, enhance the quality of life, alleviate depression and anxiety symptoms, and reduce cancer-related fatigue [17,29,34]. Thus, it is unsurprising that most studies on prehabilitation in CRC patients investigated unimodal exercise-based programs [16,26,29,30,31,32,33,34,35,36,43,44]. Today, there is no consensus on the best exercise program for CRC patients. This fact explains the heterogeneity of interventions throughout the available studies. Different interventions may include aerobic, resistance, and other training options or combinations. These different types of exercise have various benefits for human health. Even a short intervention with aerobic training (2–3 weeks) was shown to elicit improvements in physical fitness, cardiac, respiratory, and musculoskeletal function [10]. Resistance training is known to stimulate muscle hypertrophy and increase muscle mass, strength, and function. Crucially, it is effective in any age group, including frail elderly patients, who have the highest risk for postoperative complications following CRC surgery [45].
Studies included in this review showed that unimodal exercise prehabilitation consisting of aerobic and/or resistance exercises is a safe and viable option for CRC patients [29,30,31,33,34,35,40,41,43]. Additionally, it positively impacts fitness level (improved VO2peak, 6-minute walking distance scores, functional walking capacity), leg (e.g., quadriceps), arm, and inspiratory muscle strength. Different tools and outcomes were used to objectify exercise’s impact on a patient’s physical condition. Five studies [16,26,29,30,41,44] measured VO2peak and VO2 at the ventilatory anaerobic threshold (VAT). All, except one [41], of these studies showed improved VO2 after prehabilitation. Three studies by Moug and Loughney [31,32,36] investigated the effects of prehabilitation on daily step count and showed a positive impact on the parameter. Previous knowledge indicates that lower physical activity levels, determined by daily step count, are associated with increased rehospitalization rates and poor adherence to neoadjuvant treatment protocols [46]. Thus, prehabilitation may be considered to have the potency to improve postoperative outcomes and patients’ ability to tolerate neoadjuvant treatment [40,42,46]. Another common parameter investigated in a series of studies [17,31,34,35,38,40,42] is the 6-minute walking test (6 MWT) results. All available studies except one [40] show that prehabilitation improves 6 MWT outcomes. This improvement of objective functional reserves representing parameters indicates that prehabilitation improves CRC patients’ physical condition before surgical trauma and that intervention may have therapeutic benefits [26,27,42]. Moreover, seven studies [26,32,33,34,35,41,42] presented the prehabilitation effect on different skeletal muscle functions representing parameters. Every trial showed at least a slight improvement in muscle strength and endurance after prehabilitation. Such impact is relevant because lower muscle mass is associated with impaired postoperative outcomes in cancer patients [9]. Additionally, one study [43] showed that prehabilitation also improves inspiratory muscle strength, and this improvement was linked with a minor decrease in postoperative hospital stay and improved recovery. Besides positively impacting physical capacity, exercise interventions enhanced the quality of life (reduced depression and anxiety symptoms scores) [16,17,26,27,29,30,31,32,33,34,35,36,38,41,42,43,44]. The exercise interventions were effective in both unimodal and multimodal prehabilitation settings.
3.2. Nutritional and Psychological Interventions Used in Multimodal Prehabilitation
Malnutrition is the most common comorbidity in cancer patients [47], affecting 30% to 60% of patients with CRC [48]. This is mainly due to systemic inflammation caused by cancer cells. Cancer expansion triggers the release of proinflammatory cytokines such as IL-1, IL-6, and TNF-α, which in turn increase lipolysis, muscle breakdown, and insulin resistance. All these effects lead to muscle wasting with or without loss of adipose tissue [49]. The oncological patients frequently have impaired physical status and decreased quality of life, preventing adherence to therapy, reducing efficacy and tolerability, and worsening the prognosis. Cancer cachexia remains a decisive, independent prognostic factor for poor treatment outcomes [50,51]. Timely nutritional intervention can improve prognosis as well as decrease rates of morbidity and mortality among cancer patients [52]. Thus, dietary interventions appear to be a good part of multimodal prehabilitation programs in CRC management [17,27,37,39,40,41,42].
Currently, eight studies [17,27,37,38,39,40,41,42] investigated the effect of different nutritional interventions. They included personalized dietary counseling, whey protein supplementation, or a complete diet by providing daily meals. All but one [39] trial found at least some beneficial effect of nutritional support resulting in increased muscle mass and reduced fat mass. These studies indicate that multimodal prehabilitation, which includes nutritional support, could be superior to unimodal prehabilitation in terms of functional status improvement. However, randomized trials are necessary to confirm this hypothesis.
In addition to all the physiological challenges affecting CRC patients, psychological and emotional distress cannot be forgotten. The prevalence of depression among cancer survivors can be as high as 49% [53]. Even for patients with no history of psychological disorders, the extreme burden of cancer diagnosis increases the risk of mental disorders, which can harm patients’ adherence to treatment, postoperative recovery, and quality of life [54]. Personal risk factors for depression include such demographic factors as gender, age, and socioeconomic factors - unemployment and lack of social support [55,56,57]. A biological explanation for increased psychological stress in cancer patients includes a hyperactive hypothalamic-pituitary-adrenal axis, glutamate excitotoxicity, and inflammation [58]. Because psychological stress is a modifiable risk factor for poor CRC treatment outcomes, there is a rationale to address it via specific prehabilitation.
Four studies in CRC patients included psychological support as a part of multimodal prehabilitation [17,27,37,40]. All of them incorporated breathing and relaxation exercises as anxiety-reducing techniques. However, only one trial [17] measured psychological well-being as an outcome. This trial determined that prehabilitation reduced symptoms of anxiety and depression before surgery. However, it had no impact on any domain of health-related quality of life (HRQOL). It is challenging to evaluate the effect of psychological prehabilitation because current studies lack appropriate outcomes.
4. Discussion
4.1. Considerations for the Wider Use of Prehabilitation Programs in Colorectal Cancer Surgery Patients and Existing Gaps in Prehabilitation Research
This review provided an overview of available evidence of prehabilitation use in the management of CRC. Current trials are very heterogeneous in design, used interventions, and evaluated outcomes. Nonetheless, all trials show at least some positive effects of prehabilitation on patients’ physical, nutritional, or psychological status or even reduced postoperative morbidity [16,17,26,27,29,30,31,32,33,34,35,38,42,43,44]. The differences in available trials preclude the broad implementation of prehabilitation in CRC management despite a sufficient amount of evidence encouraging the use. Physicians seeking to implement prehabilitation in CRC management will have a number of questions, with some having no answer because of the gaps in present knowledge.
4.1.1. Question 1: Which Prehabilitation Should Be Used: Multimodal or Unimodal?
The best modality of prehabilitation remains unknown. Both unimodal and multimodal prehabilitation programs can be used for CRC management [59], with comparable effects on clinical outcomes, fitness, and quality of life. Considering that CRC patients suffer physical, nutritional, and psychological burdens [60], it appears that multimodal prehabilitation may be superior [61]. The downside of multimodal prehabilitation mainly lies in the additional resources (both financial and human) needed for adequate care. Ongoing clinical trials investigating multimodal prehabilitation in CRC patients will shed more light on this topic [61].
4.1.2. Question 2: Is Supervised Prehabilitation Superior to the Home-Based Programs?
Prehabilitation is usually implemented either supervised by a medical professional in a health care facility or, after introductory training, in-home setting. Each modality carries its respective advantages and disadvantages. Supervised prehabilitation allows for the monitoring of adherence and swift implementation of any necessary changes. Supervised exercises in patients with chronic low back pain [62], intermittent claudication [63], or after anterior cruciate ligament reconstruction [64] were shown to improve the outcomes. However, the supervised prehabilitations protocols carries significant logistical challenges for patients and healthcare providers. During the COVID-19 pandemic, a new way to provide supervised prehabilitation appeared. This can be achieved via tele-prehabilitation, where patients are supervised utilizing video conferencing applications. This eliminates the logistical challenges of supervised prehabilitation while maintaining all the advantages. Current studies indicate that patients prefer home-based prehabilitation; thus, a higher level of adherence could be achieved [27]. Prehabilitation in a home setting with or without telemonitoring may seem the most effective and rational approach for most patients with CRC. On the other hand, there is a great discussion regarding this topic in the current literature. Most scepsis and criticism for the home-based approach are given for unclear safety and efficacy of this approach. Additionally, the rates of non-compliance and attrition for facility-based prehabilitation may be overestimated [65,66,67,68]. Thus, there is a place for studies that would directly compare home-based vs. supervised prehabilitation for CRC cancer patients.
4.1.3. Question 3: How to Make Sure Patients Comply with Prehabilitation?
Poor compliance remains one of the significant obstacles in current prehabilitation regimens and results in worse-than-expected outcomes [69]. Therefore, finding ways to increase compliance remains the most important. Direct supervision by healthcare professionals could boost patients’ motivation and readiness to adhere to the prehabilitation regimen [70]. Although, as highlighted before, hospital-based prehabilitation has some significant logistical challenges. These challenges could be overcome by a hybrid approach or switching to tele-prehabilitation. In addition, psychological support could be implemented as it may improve motivation for adherence [71]. In our review, only four studies included some psychological prehabilitation [17,27,37,40]. Further research in this field is necessary to delineate ways to ensure maximal adherence.
4.1.4. Question 4: When Should the Prehabilitation Be Started?
As the time window between diagnosis and surgery in CRC patients is relatively short, the prehabilitation in patients undergoing only surgery should begin without delay. For patients receiving neoadjuvant treatment, several options are available. One possibility would be the window between the end of neoadjuvant therapy and surgery, which should ideally last 3–5 weeks [72]. Many studies in this review, however, utilized prehabilitation in conjunction with neoadjuvant CRT, where the feasibility of prehabilitation has already been established [31]. Prehabilitation has been proven to alleviate some negative impacts of neoadjuvant therapy, including declining physical fitness, increased depression and anxiety rates, and reduced HRQOL [10]. Therefore, prehabilitation should be started without delay to minimize the significant declines in function from NACRT and improve surgical outcomes.
4.1.5. Question 5: What Benefits Could Prehabilitation Bring to CRC Patients?
Current evidence on prehabilitation’s impact on postoperative outcomes is controversial. Five studies investigating prehabilitation’s impact on postoperative morbidity showed a positive effect [11,26,27,30,42]. Similarly, only 2 of 6 studies that investigated prehabilitation’s impact on the length of hospital stay showed a positive effect [17,27,38,40,42,43]. Additionally, 1 study demonstrated that prehabilitation promoted neoadjuvant therapy-induced tumor regression [30], but these findings were not confirmed in another study [44]. Taken together, it is likely that prehabilitation has a positive effect on clinical outcomes in CRC patients. Still, currently, there is a lack of studies designed to confirm this.
4.2. Limitations of the Current Knowledge
There are many limitations in the present comprehensive review and current knowledge on prehabilitation for CRC surgery. First, this study is a comprehensive, but not a systematic review. Second, the analysis is limited by significant heterogeneity of available studies in terms of patients and oncologic treatment pathways (upfront surgery vs. surgery after neoadjuvant treatment), different interventions (unimodal exercise prehabilitation vs. multimodal prehabilitation), and measured outcomes. Such limitation rises from the lack of prehabilitation standardization and consequently the nature of the current literature on this topic. Third, there is only limited data from large-scale randomized studies. Therefore, current knowledge on prehabilitation for CRC has to be addressed with caution, and further studies are needed to elucidate remaining unclarities which were highlighted through this review.
5. Conclusions
Prehabilitation is a new approach to improve patients’ physical, and nutritional status and psychological well-being before surgery. This comprehensive review summarized the currently available data on the prehabilitation in the management of CRC. Even though the majority of studies were not homogenous in their design and interventions, the majority showed at least some benefits: improved physical performance and nutritional status, reduced length of hospital stay and postoperative complication rate, as well as improved quality of life. However, more research on optimal prehabilitation techniques is needed to establish the best prehabilitation strategy for managing colorectal cancer patients.
Author Contributions
Conceptualization, M.K., and A.B.; methodology, T.P.; validation, R.B., K.S.; formal analysis, V.A., and A.E.D.; investigation, V.A., and A.E.D.; resources, R.B.; data curation, K.S.; writing—original draft preparation V.A. and A.E.D.; writing—review and editing, R.B., A.B., and T.P.; visualization, V.A.; supervision, T.P.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society|Cancer Facts & Statistics. [(accessed on 28 April 2020)]. Available online: http://cancerstatisticscenter.cancer.org/
- 2.Dulskas A., Kuliavas J., Sirvys A., Bausys A., Kryzauskas M., Bickaite K., Abeciunas V., Kaminskas T., Poskus T., Strupas K. Anastomotic Leak Impact on Long-Term Survival after Right Colectomy for Cancer: A Propensity-Score-Matched Analysis. J. Clin. Med. 2022;11:4375. doi: 10.3390/jcm11154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahadoer R.R., Dijkstra E.A., van Etten B., Marijnen C.A.M., Putter H., Kranenbarg E.M.-K., Roodvoets A.G.H., Nagtegaal I.D., Beets-Tan R.G.H., Blomqvist L.K., et al. Short-Course Radiotherapy Followed by Chemotherapy before Total Mesorectal Excision (TME) versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021;22:29–42. doi: 10.1016/S1470-2045(20)30555-6. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T., Bosset J.-F., Etienne P.-L., Rio E., François É., Mesgouez-Nebout N., Vendrely V., Artignan X., Bouché O., Gargot D., et al. Neoadjuvant Chemotherapy with FOLFIRINOX and Preoperative Chemoradiotherapy for Patients with Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021;22:702–715. doi: 10.1016/S1470-2045(21)00079-6. [DOI] [PubMed] [Google Scholar]
- 5.Kryzauskas M., Bausys A., Degutyte A.E., Abeciunas V., Poskus E., Bausys R., Dulskas A., Strupas K., Poskus T. Risk Factors for Anastomotic Leakage and Its Impact on Long-Term Survival in Left-Sided Colorectal Cancer Surgery. World J. Surg. Oncol. 2020;18:205. doi: 10.1186/s12957-020-01968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laursen M., Dohrn N., Gögenur I., Klein M.F. Neoadjuvant Chemotherapy in Patients Undergoing Colonic Resection for Locally Advanced Nonmetastatic Colon Cancer: A Nationwide Propensity Score Matched Cohort Study. Colorectal Dis. 2022;24:954–964. doi: 10.1111/codi.16116. [DOI] [PubMed] [Google Scholar]
- 7.Swellengrebel H.A.M., Marijnen C.A.M., Verwaal V.J., Vincent A., Heuff G., Gerhards M.F., van Geloven A.A.W., van Tets W.F., Verheij M., Cats A. Toxicity and Complications of Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer. Br. J. Surg. 2011;98:418–426. doi: 10.1002/bjs.7315. [DOI] [PubMed] [Google Scholar]
- 8.Harriss D.J., Cable N.T., George K., Reilly T., Renehan A.G., Haboubi N. Physical Activity before and after Diagnosis of Colorectal Cancer: Disease Risk, Clinical Outcomes, Response Pathways and Biomarkers. Sports Med. 2007;37:947–960. doi: 10.2165/00007256-200737110-00003. [DOI] [PubMed] [Google Scholar]
- 9.Haydon A.M.M., Macinnis R.J., English D.R., Giles G.G. Effect of Physical Activity and Body Size on Survival after Diagnosis with Colorectal Cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Northgraves M.J. Ph.D. Thesis. The University of Hull; Cottingham, UK: 2016. Exercise Prehabilitation in Colorectal Cancer Surgery Patients: The Effects on Physical Functioning, Health Related Quality of Life and Markers of Cellular Protection, Department of Sport, Health and Exercise Science. [Google Scholar]
- 11.Onerup A., Angenete E., Bock D., Haglind E. Association between Self-Assessed Preoperative Level of Physical Activity and Postoperative Complications—An Observational Cohort Analysis within a Randomized Controlled Trial (PHYSSURG-C) Eur. J. Surg. Oncol. 2022;48:883–889. doi: 10.1016/j.ejso.2021.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Slattery M.L. Physical Activity and Colorectal Cancer. Sports Med. 2004;34:239–252. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 13.Adamsen L., Quist M., Andersen C., Møller T., Herrstedt J., Kronborg D., Baadsgaard M.T., Vistisen K., Midtgaard J., Christiansen B., et al. Effect of a Multimodal High Intensity Exercise Intervention in Cancer Patients Undergoing Chemotherapy: Randomised Controlled Trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B., Hayes S.C., Spence R.R., Steele M.L., Millet G.Y., Gergele L. Exercise and Colorectal Cancer: A Systematic Review and Meta-Analysis of Exercise Safety, Feasibility and Effectiveness. Int. J. Behav. Nutr. Phys. Act. 2020;17:122. doi: 10.1186/s12966-020-01021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraemer M.B., Priolli D.G., Reis I.G.M., Pelosi A.C., Garbuio A.L.P., Messias L.H.D. Home-Based, Supervised, and Mixed Exercise Intervention on Functional Capacity and Quality of Life of Colorectal Cancer Patients: A Meta-Analysis. Sci. Rep. 2022;12:2471. doi: 10.1038/s41598-022-06165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West M.A., Loughney L., Lythgoe D., Barben C.P., Sripadam R., Kemp G.J., Grocott M.P.W., Jack S. Effect of Prehabilitation on Objectively Measured Physical Fitness after Neoadjuvant Treatment in Preoperative Rectal Cancer Patients: A Blinded Interventional Pilot Study. Br. J. Anaesth. 2015;114:244–251. doi: 10.1093/bja/aeu318. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Carli F., Lee L., Charlebois P., Stein B., Liberman A.S., Kaneva P., Augustin B., Wongyingsinn M., Gamsa A., et al. Impact of a Trimodal Prehabilitation Program on Functional Recovery after Colorectal Cancer Surgery: A Pilot Study. Surg. Endosc. 2013;27:1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 18.Carli F., Charlebois P., Stein B., Feldman L., Zavorsky G., Kim D.J., Scott S., Mayo N.E. Randomized Clinical Trial of Prehabilitation in Colorectal Surgery. Br. J. Surg. 2010;97:1187–1197. doi: 10.1002/bjs.7102. [DOI] [PubMed] [Google Scholar]
- 19.Milder D.A., Pillinger N.L., Kam P.C.A. The Role of Prehabilitation in Frail Surgical Patients: A Systematic Review. Acta Anaesthesiol. Scand. 2018;62:1356–1366. doi: 10.1111/aas.13239. [DOI] [PubMed] [Google Scholar]
- 20.Ottawa Hospital Research Institute. [(accessed on 17 May 2022)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 21.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Silver J.K., Baima J. Cancer Prehabilitation: An Opportunity to Decrease Treatment-Related Morbidity, Increase Cancer Treatment Options, and Improve Physical and Psychological Health Outcomes. Am. J. Phys. Med. Rehabil. 2013;92:715–727. doi: 10.1097/PHM.0b013e31829b4afe. [DOI] [PubMed] [Google Scholar]
- 23.Bausys A., Mazeikaite M., Bickaite K., Bausys B., Bausys R., Strupas K. The Role of Prehabilitation in Modern Esophagogastric Cancer Surgery: A Comprehensive Review. Cancers. 2022;14:2096. doi: 10.3390/cancers14092096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durrand J., Singh S.J., Danjoux G. Prehabilitation. Clin. Med. 2019;19:458–464. doi: 10.7861/clinmed.2019-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert J.E., Hayes L.D., Keegan T.J., Subar D.A., Gaffney C.J. The Impact of Prehabilitation on Patient Outcomes in Hepatobiliary, Colorectal, and Upper Gastrointestinal Cancer Surgery: A PRISMA-Accordant Meta-Analysis. Ann. Surg. 2021;274:70–77. doi: 10.1097/SLA.0000000000004527. [DOI] [PubMed] [Google Scholar]
- 26.Berkel A.E.M., Bongers B.C., Kotte H., Weltevreden P., de Jongh F.H.C., Eijsvogel M.M.M., Wymenga M., Bigirwamungu-Bargeman M., van der Palen J., van Det M.J., et al. Effects of Community-Based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery With High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022;275:e299–e306. doi: 10.1097/SLA.0000000000004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Rodríguez-Arias F., Sánchez-Guillén L., Aranaz-Ostáriz V., Triguero-Cánovas D., Lario-Pérez S., Barber-Valles X., Lacueva F.J., Ramirez J.M., Arroyo A. Effect of Home-Based Prehabilitation in an Enhanced Recovery after Surgery Program for Patients Undergoing Colorectal Cancer Surgery during the COVID-19 Pandemic. Support. Care Cancer. 2021;29:7785–7791. doi: 10.1007/s00520-021-06343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barberan-Garcia A., Ubré M., Roca J., Lacy A.M., Burgos F., Risco R., Momblán D., Balust J., Blanco I., Martínez-Pallí G. Personalised Prehabilitation in High-Risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018;267:50–56. doi: 10.1097/SLA.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 29.Alejo L.B., Pagola-Aldazabal I., Fiuza-Luces C., Huerga D., de Torres M.V., Verdugo A.S., Ortega Solano M.J., Felipe J.L., Lucia A., Ruiz-Casado A. Exercise Prehabilitation Program for Patients under Neoadjuvant Treatment for Rectal Cancer: A Pilot Study. J. Cancer Res. Ther. 2019;15:20–25. doi: 10.4103/jcrt.JCRT_30_17. [DOI] [PubMed] [Google Scholar]
- 30.Morielli A.R., Usmani N., Boulé N.G., Severin D., Tankel K., Joseph K., Nijjar T., Fairchild A., Courneya K.S. Feasibility, Safety, and Preliminary Efficacy of Exercise During and After Neoadjuvant Rectal Cancer Treatment: A Phase II Randomized Controlled Trial. Clin. Colorectal Cancer. 2021;20:216–226. doi: 10.1016/j.clcc.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Moug S.J., Mutrie N., Barry S.J.E., Mackay G., Steele R.J.C., Boachie C., Buchan C., Anderson A.S. Prehabilitation Is Feasible in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy and May Minimize Physical Deterioration: Results from the REx Trial. Colorectal Dis. 2019;21:548–562. doi: 10.1111/codi.14560. [DOI] [PubMed] [Google Scholar]
- 32.Moug S.J., Barry S.J.E., Maguire S., Johns N., Dolan D., Steele R.J.C., Buchan C., Mackay G., Anderson A.S., Mutrie N. Does Prehabilitation Modify Muscle Mass in Patients with Rectal Cancer Undergoing Neoadjuvant Therapy? A Subanalysis from the REx Randomised Controlled Trial. Tech. Coloproctol. 2020;24:959–964. doi: 10.1007/s10151-020-02262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh F., Newton R.U., Baker M.K., Spry N.A., Taaffe D.R., Galvão D.A. Feasibility and Efficacy of Presurgical Exercise in Survivors of Rectal Cancer Scheduled to Receive Curative Resection. Clin. Colorectal Cancer. 2017;16:358–365. doi: 10.1016/j.clcc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Singh F., Galvão D.A., Newton R.U., Spry N.A., Baker M.K., Taaffe D.R. Feasibility and Preliminary Efficacy of a 10-Week Resistance and Aerobic Exercise Intervention During Neoadjuvant Chemoradiation Treatment in Rectal Cancer Patients. Integr. Cancer Ther. 2018;17:952–959. doi: 10.1177/1534735418781736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heldens A.F.J.M., Bongers B.C., de Vos-Geelen J., van Meeteren N.L.U., Lenssen A.F. Feasibility and Preliminary Effectiveness of a Physical Exercise Training Program during Neoadjuvant Chemoradiotherapy in Individual Patients with Rectal Cancer Prior to Major Elective Surgery. Eur. J. Surg. Oncol. 2016;42:1322–1330. doi: 10.1016/j.ejso.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Loughney L., West M.A., Dimitrov B.D., Kemp G.J., Grocott M.P., Jack S. Physical Activity Levels in Locally Advanced Rectal Cancer Patients Following Neoadjuvant Chemoradiotherapy and an Exercise Training Programme before Surgery: A Pilot Study. Perioper. Med. Lond. Engl. 2017;6:3. doi: 10.1186/s13741-017-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillis C., Fenton T.R., Sajobi T.T., Minnella E.M., Awasthi R., Loiselle S.-È., Liberman A.S., Stein B., Charlebois P., Carli F. Trimodal Prehabilitation for Colorectal Surgery Attenuates Post-Surgical Losses in Lean Body Mass: A Pooled Analysis of Randomized Controlled Trials. Clin. Nutr. Edinb. Scotl. 2019;38:1053–1060. doi: 10.1016/j.clnu.2018.06.982. [DOI] [PubMed] [Google Scholar]
- 38.Gillis C., Loiselle S.-E., Fiore J.F., Awasthi R., Wykes L., Liberman A.S., Stein B., Charlebois P., Carli F. Prehabilitation with Whey Protein Supplementation on Perioperative Functional Exercise Capacity in Patients Undergoing Colorectal Resection for Cancer: A Pilot Double-Blinded Randomized Placebo-Controlled Trial. J. Acad. Nutr. Diet. 2016;116:802–812. doi: 10.1016/j.jand.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Furyk C., Senthuran S., Nye D., Ho Y.H., Leicht A.S. Prehabilitation for Frail Patients Undergoing Colorectal Surgery: Lessons Learnt From a Randomised Feasibility Study. Front. Rehabil. Sci. 2021;2:650835. doi: 10.3389/fresc.2021.650835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bousquet-Dion G., Awasthi R., Loiselle S.-È., Minnella E.M., Agnihotram R.V., Bergdahl A., Carli F., Scheede-Bergdahl C. Evaluation of Supervised Multimodal Prehabilitation Programme in Cancer Patients Undergoing Colorectal Resection: A Randomized Control Trial. Acta Oncol. Stockh. 2018;57:849–859. doi: 10.1080/0284186X.2017.1423180. [DOI] [PubMed] [Google Scholar]
- 41.Tweed T.T.T., Sier M.A.T., Van Bodegraven A.A., Van Nie N.C., Sipers W.M.W.H., Boerma E.-J.G., Stoot J.H.M.B. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients. 2021;13:3493. doi: 10.3390/nu13103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Klerk M., van Dalen D.H., Nahar-van Venrooij L.M.W., Meijerink W.J.H.J., Verdaasdonk E.G.G. A Multimodal Prehabilitation Program in High-Risk Patients Undergoing Elective Resection for Colorectal Cancer: A Retrospective Cohort Study. Eur. J. Surg. Oncol. 2021;47:2849–2856. doi: 10.1016/j.ejso.2021.05.033. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson E., Farahnak P., Franzén E., Nygren-Bonnier M., Dronkers J., van Meeteren N., Rydwik E. Feasibility of Preoperative Supervised Home-Based Exercise in Older Adults Undergoing Colorectal Cancer Surgery—A Randomized Controlled Design. PLoS ONE. 2019;14:e0219158. doi: 10.1371/journal.pone.0219158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West M.A., Astin R., Moyses H.E., Cave J., White D., Levett D.Z.H., Bates A., Brown G., Grocott M.P.W., Jack S. Exercise Prehabilitation May Lead to Augmented Tumor Regression Following Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Acta Oncol. Stockh. 2019;58:588–595. doi: 10.1080/0284186X.2019.1566775. [DOI] [PubMed] [Google Scholar]
- 45.González-Senac N.M., Mayordomo-Cava J., Macías-Valle A., Aldama-Marín P., Majuelos González S., Cruz Arnés M.L., Jiménez-Gómez L.M., Vidán-Astiz M.T., Serra-Rexach J.A. Colorectal Cancer in Elderly Patients with Surgical Indication: State of the Art, Current Management, Role of Frailty and Benefits of a Geriatric Liaison. Int. J. Environ. Res. Public. Health. 2021;18:6072. doi: 10.3390/ijerph18116072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohri N., Halmos B., Bodner W.R., Cheng H., Guha C., Kalnicki S., Garg M.K. Daily Step Counts Predict Outcomes for Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Concurrent Chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2019;105:E524–E525. doi: 10.1016/j.ijrobp.2019.06.2423. [DOI] [PubMed] [Google Scholar]
- 47.Kumar N.B. Nutritional Management of Cancer Treatment Effects. Springer; Berlin/Heidelberg, Germany: 2012. [Google Scholar]
- 48.Lopes J.P., Pereira P.M.D.C.C., Vicente A.F.D.R.B., Bernardo A., de Mesquita M.F. Nutritional Status Assessment in Colorectal Cancer Patients. Nutr. Hosp. 2013;28:412–418. doi: 10.3305/nh.2013.28.2.6173. [DOI] [PubMed] [Google Scholar]
- 49.Aoyagi T., Terracina K.P., Raza A., Matsubara H., Takabe K. Cancer Cachexia, Mechanism and Treatment. World J. Gastrointest. Oncol. 2015;7:17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziętarska M., Krawczyk-Lipiec J., Kraj L., Zaucha R., Małgorzewicz S. Nutritional Status Assessment in Colorectal Cancer Patients Qualified to Systemic Treatment. Contemp. Oncol. Poznan Pol. 2017;21:157–161. doi: 10.5114/wo.2017.68625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrera S., Demark-Wahnefried W. Nutrition During and After Cancer Therapy. Oncology. 2009;23:15–21. [PMC free article] [PubMed] [Google Scholar]
- 52.Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable—PubMed. [(accessed on 18 May 2022)]; doi: 10.1249/MSS.0000000000002116. Available online: https://pubmed.ncbi.nlm.nih.gov/31626055/ [DOI] [PMC free article] [PubMed]
- 53.Krebber A.M.H., Buffart L.M., Kleijn G., Riepma I.C., de Bree R., Leemans C.R., Becker A., Brug J., van Straten A., Cuijpers P., et al. Prevalence of Depression in Cancer Patients: A Meta-Analysis of Diagnostic Interviews and Self-Report Instruments. Psychooncology. 2014;23:121–130. doi: 10.1002/pon.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J., Fang F., Sjölander A., Fall K., Adami H.O., Valdimarsdóttir U. First-Onset Mental Disorders after Cancer Diagnosis and Cancer-Specific Mortality: A Nationwide Cohort Study. Ann. Oncol. 2017;28:1964–1969. doi: 10.1093/annonc/mdx265. [DOI] [PubMed] [Google Scholar]
- 55.Gilligan A.M., Alberts D.S., Roe D.J., Skrepnek G.H. Death or Debt? National Estimates of Financial Toxicity in Persons with Newly-Diagnosed Cancer. Am. J. Med. 2018;131:1187–1199. doi: 10.1016/j.amjmed.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 56.Lu L., O’Sullivan E., Sharp L. Cancer-Related Financial Hardship among Head and Neck Cancer Survivors: Risk Factors and Associations with Health-Related Quality of Life. Psychooncology. 2019;28:863–871. doi: 10.1002/pon.5034. [DOI] [PubMed] [Google Scholar]
- 57.Wen S., Xiao H., Yang Y. The Risk Factors for Depression in Cancer Patients Undergoing Chemotherapy: A Systematic Review. Support. Care Cancer. 2019;27:57–67. doi: 10.1007/s00520-018-4466-9. [DOI] [PubMed] [Google Scholar]
- 58.Young K., Singh G. Biological Mechanisms of Cancer-Induced Depression. Front. Psychiatry. 2018;9:299. doi: 10.3389/fpsyt.2018.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waterland J.L., McCourt O., Edbrooke L., Granger C.L., Ismail H., Riedel B., Denehy L. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front. Surg. 2021;8:628848. doi: 10.3389/fsurg.2021.628848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Husebø A.M.L., Karlsen B., Husebø S.E. Health Professionals’ Perceptions of Colorectal Cancer Patients’ Treatment Burden and Their Supportive Work to Ameliorate the Burden—A Qualitative Study. BMC Health Serv. Res. 2020;20:661. doi: 10.1186/s12913-020-05520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Rooijen S., Carli F., Dalton S., Thomas G., Bojesen R., Le Guen M., Barizien N., Awasthi R., Minnella E., Beijer S., et al. Multimodal Prehabilitation in Colorectal Cancer Patients to Improve Functional Capacity and Reduce Postoperative Complications: The First International Randomized Controlled Trial for Multimodal Prehabilitation. BMC Cancer. 2019;19:98. doi: 10.1186/s12885-018-5232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maul I., Läubli T., Oliveri M., Krueger H. Long-Term Effects of Supervised Physical Training in Secondary Prevention of Low Back Pain. Eur. Spine J. 2005;14:599–611. doi: 10.1007/s00586-004-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keo H., Grob E., Guggisberg F., Widmer J., Baumgartner I., Schmid J.-P., Kalka C., Saner H. Long-Term Effects of Supervised Exercise Training on Walking Capacity and Quality of Life in Patients with Intermittent Claudication. Vasa Z. Gefasskrankheiten. 2008;37:250–256. doi: 10.1024/0301-1526.37.3.250. [DOI] [PubMed] [Google Scholar]
- 64.Rhim H.C., Lee J.H., Lee S.J., Jeon J.S., Kim G., Lee K.Y., Jang K.-M. Supervised Rehabilitation May Lead to Better Outcome than Home-Based Rehabilitation Up to 1 Year after Anterior Cruciate Ligament Reconstruction. Med. Kaunas Lith. 2020;57:19. doi: 10.3390/medicina57010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newton R.U., Taaffe D.R., Chambers S.K., Spry N., Galvão D.A. Effective Exercise Interventions for Patients and Survivors of Cancer Should Be Supervised, Targeted, and Prescribed With Referrals From Oncologists and General Physicians. J. Clin. Oncol. 2018;36:927–928. doi: 10.1200/JCO.2017.76.7400. [DOI] [PubMed] [Google Scholar]
- 66.Hardcastle S.J., Cohen P.A. Reply to S.C. Adams et al, C. Lopez et al, and R.U. Newton et al. J. Clin. Oncol. 2018;36:928–930. doi: 10.1200/JCO.2017.76.8218. [DOI] [PubMed] [Google Scholar]
- 67.Adams S.C., Iyengar N.M., Scott J.M., Jones L.W. Exercise Implementation in Oncology: One Size Does Not Fit All. J. Clin. Oncol. 2018;36:925–926. doi: 10.1200/JCO.2017.76.2906. [DOI] [PubMed] [Google Scholar]
- 68.Hardcastle S.J., Cohen P.A. Effective Physical Activity Promotion to Survivors of Cancer Is Likely to Be Home Based and to Require Oncologist Participation. J. Clin. Oncol. 2017;35:3635–3637. doi: 10.1200/JCO.2017.74.6032. [DOI] [PubMed] [Google Scholar]
- 69.Martin L.R., Williams S.L., Haskard K.B., Dimatteo M.R. The Challenge of Patient Adherence. Ther. Clin. Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 70.Francis-Coad J., Edgar D., Bulsara C., Barrett-Lennard A., Owen K., Fletcher D., Wood F., Hill A.-M. Partnering with Patients to Design a Prehabilitation Program for Optimizing the Patient Experience through General Surgery. Patient Exp. J. 2021;8:135–147. doi: 10.35680/2372-0247.1544. [DOI] [Google Scholar]
- 71.Grimmett C., Bradbury K., Dalton S.O., Fecher-Jones I., Hoedjes M., Varkonyi-Sepp J., Short C.E. The Role of Behavioral Science in Personalized Multimodal Prehabilitation in Cancer. Front. Psychol. 2021;12:634223. doi: 10.3389/fpsyg.2021.634223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y., Liu Z., Shan F., Ying X., Zhang Y., Li S., Jia Y., Li Z., Ji J. Optimal Timing to Surgery After Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer. Front. Oncol. 2020;10:613988. doi: 10.3389/fonc.2020.613988. [DOI] [PMC free article] [PubMed] [Google Scholar]