Abstract
Biotic and abiotic stresses severely affect agriculture by affecting crop productivity, soil fertility, and health. These stresses may have significant financial repercussions, necessitating a practical, cost-effective, and ecologically friendly approach to lessen their negative impacts on plants. Several agrochemicals, such as fertilizers, pesticides, and insecticides, are used to improve plant health and protection; however, these chemical supplements have serious implications for human health. Plants being sessile cannot move or escape to avoid stress. Therefore, they have evolved to develop highly beneficial interactions with endophytes. The targeted use of beneficial plant endophytes and their role in combating biotic and abiotic stresses are gaining attention. Therefore, it is important to experimentally validate these interactions and determine how they affect plant fitness. This review highlights research that sheds light on how endophytes help plants tolerate biotic and abiotic stresses through plant–symbiont and plant–microbiota interactions. There is a great need to focus research efforts on this vital area to achieve a system-level understanding of plant–microbe interactions that occur naturally.
Keywords: endophytes, plant defense, drought, salinity, temperature, crop improvement
1. Major Challenges to Plant’s Growth and Production
The world needs more food to feed the growing population. It is a harsh reality that the world population is increasing rapidly, with an increase of 81 million people (1.05%) every year [1]. The growth is more severe in the developing world, such as Pakistan, where the annual increase is 2.0% [2]. The population spiked in 1962–63, when the world population grew at a rate of 2.2% [3], alerting agronomists and plant scientists to accommodate the demand. Thanks to science, the development of high-stress-resistant new varieties and plant breeding-based enhanced production has increased global food production. According to an estimate by the Food and Agriculture Organization (FAO) [4], primary crop production increased by almost 50% compared with 2000, whereas vegetable oil production increased by 108%. However, this increase in food production comes with a cost. According to the same report, pesticide use went up by one-third between 2000 and 2018, and the use of nitrogen fertilizers increased by 58%. In quantitative terms, 190 million tons of fertilizer were added to the soil during this period [5]. Since then, the uses of fertilizers and other agrochemicals have increased, as they are known to increase yield without much effort [6]. According to research, these agrochemicals have severe consequences on human health, including immune system disorders, hormonal problems, kidney disorders, reproductive problems, and cancer [7]. A detailed account of these agrochemicals and their impact on human health was provided by Srivastav [8].
Therefore, sustainable agriculture is key to coping with increasing food demand, with less detrimental or no effects on human health. In this context, plant microbiomes play a vital role in plant growth and maintenance of soil fertility for sustainable agriculture/food production. Soil microbes are important regulators of global nutrient balance in ecosystems [9]. Plant and microbial associations, such as rhizospheric, epiphytic, and endophytic associations with growth-promoting properties, are critical for sustainable agriculture. These microbes, either directly in the form of phytohormones or indirectly, such as nitrogen fixation, can promote plant growth and therefore can be a reasonable alternative to chemical fertilizers. To that end, this review focuses on the core concerns surrounding the potential for developing innovative microbial-assisted selection of plants, maximizing rhizosphere/root microbiome beneficial connections, with a special focus on endophytes.
2. Endophytes: Friends or Foes?
Endophytes are microorganisms that inhabit plants. Endophytes are known to promote the growth of host plants in several ways, including detoxification of toxic compounds, defense against pathogens, and production of plant growth-promoting hormones [10]. Endophytes are also known to produce biotechnologically important metabolites having anticancer and antimicrobial properties [9]. They also help in the recycling of nutrients and act as bio transformers of different chemicals [9]. Endophytes are diverse and relatively less explored; however, new endophytes with diverse functions are being added to the list with the development of research on plant-microbe interactions.
Colonization and Distribution
Endophytes can be bacterial or fungal. Bacterial endophytes are a class of endosymbiotic microorganisms that live in intercellular or intracellular compartments, apoplasts, water-conducting xylem vessels of shoots and roots, intercellular spaces of cell walls, flowers [11], fruits [12], and seeds [13]. Unlike bacterial phytopathogens, endophytes do not cause disease or morphological abnormalities in plants [14]. The number of bacterial endophytes in plants varies across species, and the type of tissue ranges from 100 to 9 × 109 bacteria per gram of plant tissue [15]. Generally, endophyte density is higher in roots and belowground parts than in aboveground parts [14]. However, this number may vary, as migration of endophytes from roots to leaves has been demonstrated in rice [15], suggesting that endophytes enter the host plant through the roots [14]. Although they live inside plants, the population density and distribution of bacterial endophytes are influenced by both the biotic and abiotic factors faced by plants, including microbe–microbe interactions, plant–microbe interactions, and environmental stresses [16].
Over a century of research suggests that in natural ecosystems, most plants are symbiotic with mycorrhizal fungi or fungal endophytes [17]. This fungal partner can profoundly impact plant evolution, fitness, and distribution [18], defining plant communities [19]. Plant and fungal mycorrhizal and endophytic associations date back more than 400 million years as per fossil records [20,21] and were likely associated at the time of initial footprints of plants on land, thus playing a key role in the evolution of life on land [22]. Similar to their bacterial counterparts, fungal endophytes reside entirely within the plant host, where they may grow within underground or aboveground parts and sporulate during plant or host tissue senescence [23]. Fungal endophytes are classified into four classes based on their host range and mode of transmission [22]. Class 1 endophytes represent Clavicipitaceous fungi, characterized by both vertical and horizontal transmission with their seeds, and can be found in some grasses in cold habitats, while the remaining three classes are non-claviciptaceous endophytes. Class 2 endophytes represent endophytes that grow in both above- and below-ground tissues. These can transmit vertically and horizontally and can be recovered from asymptomatic tissues of non-vascular plants, ferns, conifers, and some angiosperms. Class 3 endophytes generally have a wide host range and colonize only the upper plant parts, and their mode of transmission is horizontal. Class 4 endophytes colonize roots and are transmitted horizontally.
3. Endophytes Modulate Plant Defense Responses
Plants generally respond to pathogenic infections, either by pathogen-associated molecular pattern (PAMPs)-triggered immunity (PTI) or effector-triggered immunity (ETI). A detailed explanation of how plants prioritize resistance responses was given by Jones and Dangl [24]. The fundamental question is how endophytes overcome/avoid such resistance responses by plants while infecting their host. To answer this question, we need to understand the mechanism of inconspicuous entry of endophytes into the host plant and their management to thrive there through a mutually symbiotic relationship.
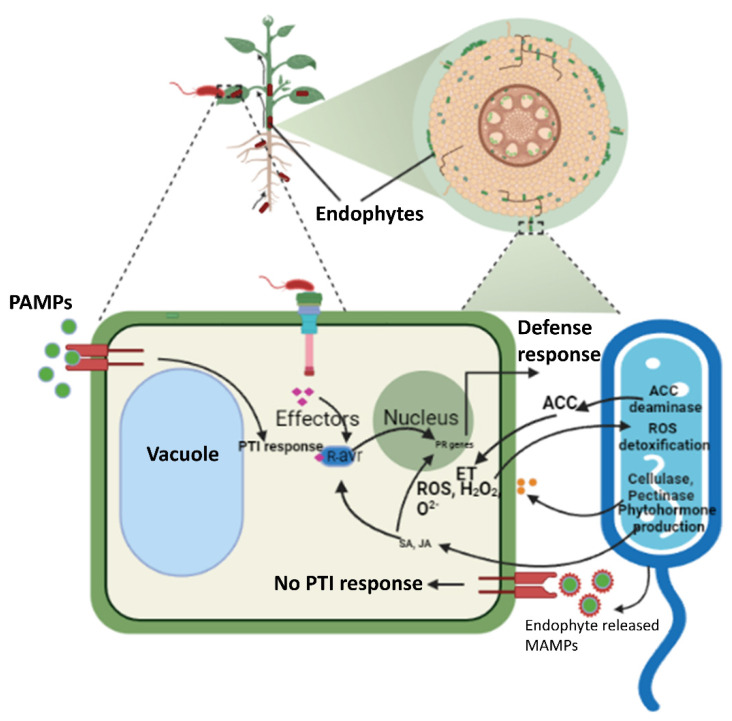
Some endophytes are seed-borne, and they are present in germinated plants [25]. In plants with vegetative propagation, endophyte colonization is relatively easier because they can transmit their endophytic microbiota to the next generation. In others, it starts with root exudates, which are rich in biomolecules and chemo-attractants such as flavonoids, sugars, amino acids, organic acids, and phenolic compounds. Root exudates are also rich in nutrients and water and therefore attract all types of other microbes [26]. Effective endophyte colonization entails compatible plant–microbe interactions. Potential points of entry for endophytes are cracks formed at the emergence of lateral roots or zones of root elongation. To make an entry, some bacteria must find their way to these apertures for successful colonization. Dong et al. [27] showed that Klebsiella sp/strain Kp342 colonizes the lateral root junctions in wheat and alfalfa. Similarly, Herbaspirillum seropedicae and Gluconacetobacter diazotrophicus dominate colonization at later-root junctions [28]. Some endophytes enter through infection colonization, where cellulolytic and pectinolytic enzymes produced by endophytes come into play [14], such as pectate lyase, which has been implicated in the colonization of Klebsiella strains [29]. Figure 1 summarizes the potential entry and colonization of endophytes and their interactions with the host plant tissues. For entry into the host plant, fungal endophytes produce chitin deacetylase enzymes that deacetylate chitosan oligomers and are camouflaged by plant pattern recognition receptors (PRRs) [30]. Similarly, other evidence suggests that some endophytic bacteria release their microbe-associated molecular patterns (MAMPs), which either leads to misrecognition by plant PRRs or induces a comparatively weak and transient defense response [31]. Similarly, cell wall–degrading enzymes such as endoglucanases and polygalacturonase are used by Burkholderia sp. for infection in Vitis vinifera [11].
Figure 1.
Host plant and endophyte crosstalk. The colonization of endophytes and their interaction with the host plant depends on the type of endophytes. Bacterial endophytes mostly make their entry through apertures formed either through the emergence of lateral roots or the zones of elongation. Fungal endophytes can either be transmitted vertically, i.e., transmission from maternal plants to the progeny seeds, or through their entry as fungal spores through herbivory or insects. Some endophytes also release cell wall–degrading enzymes to make their entry possible. Since these endophytes have specific MAMps, they are not recognized by the host plant PRRs. Endophytes help the plant in stress responses by producing phytohormones and reactive oxygen species (ROS) detoxification. ACC, aminocyclopropane-1-carboxylic acid; ROS, reactive oxygen species; MAMPs, microbe-associated molecular patterns; PR, pathogenesi-related; ET, ethylene; SA, salicylic acid; JA, jasmonic acid; PAMPs, pathogen-associated molecular patterns; PTI, PAMP-triggered immunity; R-avr, resistance gene a virulence effector.
Endophytes help in protection against pathogens by being directly involved in the production and release of secondary metabolites or compounds with antimicrobial properties, such as siderophores, antibiotics, and hydrolytic enzymes, which can help in culminating or reducing the invading pathogens or through indirect mechanisms by competing with the pathogen for space and available nutrients [32]. Endophytes are known to play a role in systemic acquired resistance (SAR) against plant pathogens [33]. The phytohormones salicylic acid (SA) and jasmonic acid (JA) are vital for plant defense responses. Some endophytes have increased levels of SA and JA, thereby triggering plant defense responses against pathogens. For example, Waqas et al. [34] reported that the gibberellin-producing endophytes Penicillium citrinum strain LWL4 and Aspergillus terreus strain LWL5 trigger SAR against stem rot in sunflowers by inducing the SA and JA pathways. Similarly, Fusarium solani induces SAR against Septoria lycopersici by inducing pathogenesis-related (PR) gene expression [35]. Table 1 summarizes a list of some of the endophytes that are reported to have defensive role against phyto-pathogens. Foliar application of endophytes also affects plant defense pathways [36,37,38]. For example, foliar application of the endophytic fungus Colletotrichum tropicale reduced Phytophthora infection in the Cacao tree (Theobroma cacao) [39]. The reduction in Phytophthora infection could be attributed to the induction of defense-related pathways, as inoculation with C. tropicale triggered several components of the ethylene (ET) defense pathway and induced several disease-related genes in T. cacao and Arabidopsis thaliana [40,41].
Table 1.
List of endophytes associated with defense against various plant pathogens.
| Endophyte | Host Plant | Pathogen | Target Pathway | Reference |
|---|---|---|---|---|
| Bacillus spp. | Oryza sativa | Pyricularia oryzae | Induce systemic resistance | [50] |
| Rhizobium etli | Phaseolus vulgaris | Pseudomonas syringae pv.phaseolicola | Callose deposition, SA, and JA-dependent gene induction | [50] |
| Bacillus spp. | Nicotiana tabacum | Pseudomonas syringae pv.tobaco | Induce systemic resistance | [50] |
| Paenibacillus | Triticum aestivum | Mycosphaerella graminicola | Defense pathway | [51] |
| Bacillus spp. | Oryza sativa | Pyricularia oryzae | Antioxidant defense activities | [52] |
| Phomopis cassiae | Cassia spectabilis | Cladosporium sphaerospermum | unknown | [53] |
| Streptomyces strain, DEF09 | Triticum aestivum | Fusarium graminearum | Chitinase production | [54] |
| Daldinia eschscholtzii | Helianthus tuberosus, Zingiber officinale, Stemona tuberosa | Colletotrichum acutatum, Sclerotium rolfsii | Production of antimicrobial compounds | [55] |
| Paraburkholderia | Beta vulgaris | Rhizoctonia solani | Systemic acquired resistance | [56] |
| Penicillium citrinum | Helianthus annus L | Sclerotium rolfsii | SA, JA pathway | [34] |
| Fusarium solani | Solanum lycopersicum | Septoria lycopersici | SA-dependent PR gene pathway | [35] |
| Paenibacillus sp. strain B2 | Triticum aestivum | Zymoseptoria tritici | Induce flavonoid and phytohormonal pathways | [57] |
| Paraconiothyrium sp. | Fraxinus excelsior | Hymenoscyphus fraxineus | Unknown | [58] |
| Cladosporium spp. | Pinus monticola | Cronartium Ribicola | Induced resistance | [59] |
| Venturia fraxini | Fraxinus excelsior | Hymenoscyphus fraxineus | Production of antifungal compounds | [60] |
| Flavobacterium | Beta vulgaris | Rhizoctonia solani | Chitinase, nonribosomal peptide synthetases (NRPSs), and polyketide synthases (PKSc) | [61] |
| Gluconacetobacter diazotrophicus | Arabidopsis thaliana | Ralstonia solanacearum | Callose deposition | [62] |
| Piriformospora indica | A. thaliana | Golovinomyces orontii | JA-dependent defense pathway | [63] |
| Pseudomonas simiae | A. thaliana | Mamestra brassicae | JA and ET | [64] |
| Bacillus pumilus strain | A. thaliana | Cucumber mosaic virus | Ethylene pathway | [65] |
| Bacillus subtilis | A. thaliana | P. syringae pv. Tomato DC3000 | SA, ET pathway | [66] |
| Bacillus aryabhattai | Nicotiana tabacum, A. thaliana | Botrytus cinerea | SA, JA | [67] |
Endophytic bacteria are well known for producing compounds with antimicrobial properties, such as lipopeptides. The most studied lipopeptides are those isolated from Bacillus and Paenibacillus species [42]. Similarly, another endosymbiont, Pseudomonas viridiflava, was also reported to produce ecomycins, a lipopeptide containing unique amino acids, such as β-hydroxy aspartic acid and homoserine [43]. Endophytic Pseudomonas putida strain BP25 inhibits several phytopathogens, including Pythium myriotylum, Gibberella moniliformis, Phytophthora capsica, Thizoctonia solani, Colletotrichum gloeosporioides, and the plant-parasitic nematode Radopholus similis through the production of several volatile substances [44]. Similarly, Rhizobium is known for siderophore production and is used as a biocontrol agent for pathogenic Macrophomina phaseolina, which causes charcoal rot in several crops [45]. In addition to lipopeptides, several other endophytes have been reported to produce other antimicrobial compounds, such as mycosubtilin, surfactin, lichenysin, bacillomycin, iturin, fengycin, and plipastaitin [46]. A detailed list of different antimicrobial compounds produced by various endophytes was reviewed in ref. [47].
Plants also produce some low molecular weight antimicrobial molecules known as phytoalexins, including some other groups of secondary metabolites, such as terpenoids [48]. Endophytes can also increase the production of secondary metabolites. Reports have suggested an increase in terpenoid content by the endophytic fungus Fusarium spp. in Euphorbia pekinensis [49]. Similarly, plants inhabited by endophytes have been reported to exhibit increased cellulose content, lamina density, and leaf stiffness, thereby reducing herbivory rates, particularly against leaf-cutting ants [36,37]. Thus, resistance against phytopathogens is achieved by eliciting host responses or producing specific metabolites by endophytes working in close coordination to cope with pathogenic ingress.
4. Endophytes and Abiotic Stresses
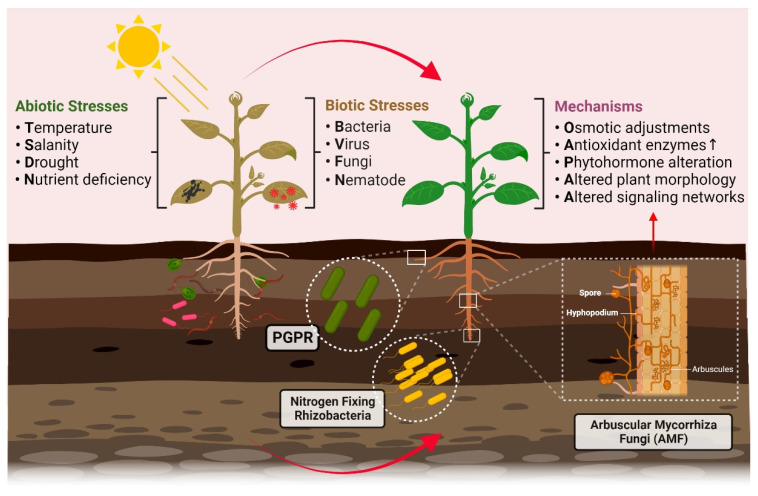
Abiotic stress is a rising challenge in crop production worldwide. The most common abiotic stressors include temperature, drought, salinity, and nutrient deficiency (Figure 2). These stresses may cause alterations in metabolomics and transcriptomics, which change the exudates from the roots and leaves and, in turn, influence the microbial community associated with plants [68]. In the soil ecosystem, plant-associated microbes play a central role, working as natural partners that facilitate local and systemic mechanisms in plants to defend against adverse environmental conditions. This section highlights the underlying mechanisms and how plant–microbe interactions modulate responses under major abiotic stresses, such as temperature, drought, salinity, and nutrition stress.
Figure 2.
An overview diagram showing major environmental stresses and mechanisms of plant–microbe interactions that influence plant fitness during these stresses. Tolerance against these stresses is mainly mediated by enhancement in antioxidant defense enzymes, osmotic adjustment and production of phytohormones. PGPR: plant growth-promoting rhizobacteria.
4.1. Temperature
Temperature is the most common stress experienced by plants. The average global temperature and the frequency of extreme weather events have increased considerably over the past several decades owing to climate change. According to the results of several climate model simulations, the average temperature of the world is predicted to be between 1.1 and 5.4 °C warmer in 2100 than it is today [69]. The main effects of temperature stress include alterations in plasma membrane activity, transpiration, reduced photosynthesis, enzymatic dysfunction, cell division, plant growth, and development [70]. Previous studies have shown that plant response to heat stress may be influenced by microbe interactions [71,72,73].
Reports suggest that the endophytic bacteria Burkholderia phytofirmans strain PsJN can alter photosynthesis and the carbohydrate metabolism involved in chilling stress, thereby enhancing the cold tolerance of grapevine plants [11,74]. Bacterial endophytes induce adaptation to chilling, resulting in reduced cell damage, increased photosynthesis, and accumulation of chilling stress-associated metabolites, such as proline, starch, and phenolic compounds [14].
Some microbes can protect themselves from extreme temperature conditions by enhancing the production of heat- and cold-tolerant proteins. Molecular chaperones have been reported to play essential roles in heat stress tolerance in microbes [75,76]. For example, the DnaK gene encoding heat shock protein (Hsp70), a well-known chaperone in heat stress tolerance, was found to be highly expressed in Alicyclobacillus acidocaldarius during heat and cold stress [77]. Microbes adapted to low or high temperatures could alleviate the negative impacts of extreme temperatures and expand the host plant’s ability to survive these conditions. Khan et al. reported that thermotolerant Bacillus cereus strain SA1 alleviated heat stress in soybean plants by enhancing antioxidant defense enzymes and altering hormonal profiles [78]. Several other studies have reported a similar mechanism of heat stress tolerance in host plants mediated by thermotolerant endophytes and rhizobacteria, such as Bacillus amyloliquefaciens in rice [79], Pseudomonas putida strain AKMP7 in wheat [80], and Pseudomonas sp. strain AKMP6 in sorghum [81]. In some cases, the relationship between plants and microbes is important for both partners. The symbiosis between the fungus Curvularia protuberata and panic grass is interesting, as this relationship allows both organisms to survive high soil temperatures. However, neither the host plant nor the fungus can individually tolerate high-temperature conditions [82]. Furthermore, C. protuberata is required to be infected by Curvularia, a thermal-tolerant virus (CThTV), to confer heat tolerance to the host plant [82]. Moreover, C. protuberate-mediated heat tolerance has been observed in tomatoes [83], indicating that the underlying process may have a wide range of applications to help plants survive at elevated temperatures [84].
4.2. Salinity
Salinity is a global issue that affects agricultural productivity. The regions affected by salinity are gradually expanding owing to insufficient precipitation, poor irrigation systems, salt deposition, water pollution, and other environmental factors [85]. Although the development of salt-tolerant crops has been an important scientific aim, there has not been much progress in this direction because not many of the key genetic traits that affect salt tolerance have been identified [86,87,88]. Introducing salt-tolerant microorganisms that promote plant growth is a potential alternative to increase crop tolerance to salinity stress [89].
Plants exhibit a two-stage response to salinity stress. During the first phase, growth slows drastically, and stomata close in response to decreased water potential. Sodium ions are built up in the second phase, increasing oxidative stress, which in turn damages photosynthetic components [87]. In addition to harmful stress by-products, reactive oxygen species (ROS) may also function as signal molecules to initiate defense mechanisms against salt and other stressors [90].
Multiple mechanisms have been implicated in endophyte-mediated salinity tolerance. One approach is to produce large quantities of organic osmolytes such as trehalose, proline, glycine, and betaine, which offer an adaptive response to salinity stress [85]. Some endophytes help plants tolerate salinity stress by reprogramming the antioxidant defense system and hormonal profiles. For example, Khan et al. [91] reported that bacterial endophytes Bacillus cereus, Micrococcus yunnanensis, Enterobacter tabaci, Curtobacterium oceanosedimentum, Curtobacterium luteum, and Enterobacter ludwigii isolated from halotolerant plants enhanced the salt stress tolerance of rice by altering defense enzymes, indole-3-acetic acid, abscisic acid (ABA), and gibberellin levels. Similar mechanisms have been observed for soybean [92], tomato [93], cucumber [94], and maize [95].
Some endophytes enhance the ability of the host plant to alleviate salt stress by improving key transcripts that are required for salinity tolerance. For example, the bacterial endophyte Bacillus subtilis has been reported to enhance salinity tolerance in Arabidopsis by simultaneously downregulating high-affinity K+ transporter 1 (HKT1) expression in roots and upregulating it in shoots. The induction of HKT1-dependent shoot-to-root recirculation was likely to result in a 50% reduction in plant-wide Na accumulation [96]. Another study reported that auxins produced by Bacillus amyloliquefaciens alleviate salt stress by upregulating the expression of ethylene-responsive element binding protein (EREBP), betaine aldehyde dehydrogenase (BADH), salt overly sensitive 1 (SOS1), and catalase genes in rice [97]. Another endophytic bacterium, Pseudomonas pseudoalcaligenes, was shown to enhance salt tolerance in rice by accumulating high concentrations of glycine betaine-like compounds [98]. Some endophytes secrete exopolysaccharides that bind cations in the roots (particularly Na+) and inhibit their transport to leaves, thereby reducing salt stress [97]. They proposed that roots from inoculated seedlings had a larger proportion of soil sheaths covering them, which could slow down the passage of apoplastic Na+ into the stele.
4.3. Drought
Without water, life on Earth is not possible. Too little water (drought) or too much water (flood) can have a considerable influence on several aspects of plant and microbial life. A drought is described as a period when there is insufficient water available for an organism or environment to function at its best [99,100,101]. Drought stress may occur at any plant developmental stage and can affect growth and development to varying degrees based on the onset time, duration, and severity. At the reproductive stage of the plant, drought stress may directly reduce more than 50% of the yield [79,102]. Plant breeders have made remarkable progress in creating drought-tolerant crops, but despite this, they are still unable to satisfy the needs of food security in the face of a rising global population, climate change, and water scarcity [101]. Traditional breeding, however, requires much work from the breeder, takes a long time, and may result in the loss of other desirable features [103,104]. Endophytic microorganisms have been reported to alter plant responses to drought and can be used as an alternative and rapid way to enhance crop productivity.
Plants alter phytohormone abscisic acid (ABA) production in response to drought stress. When ABA concentration rises, it sets off a chain reaction that causes the plant to undergo extensive physiological and transcriptional changes, such as the closing of stomata to decrease water loss via transpiration [105]. Furthermore, cell size and membrane integrity are reduced, ROS are produced, and leaf senescence is promoted, all of which contribute to a reduction in agricultural productivity [106].
Under water-deficient conditions, endophytic microorganisms colonize the rhizosphere and promote plant growth and development through a variety of direct and indirect mechanisms. Plant endophytes can produce plant hormones that promote plant growth and development. Mahest et al. reported that the endophytic actinobacteria S. olivaceus strain DE10 enhanced wheat growth via auxin production under drought conditions [107]. Azospirillum brasilense improves drought tolerance in Arabidopsis thaliana by increasing ABA levels [108]. Endophyte-facilitated drought tolerance via alteration of hormonal profiles has been reported in soybean [109], wheat [110], Lavandula dentata [111], cucumber [112], sugar cane [113], and soybean [114].
Additionally, in certain instances, Rhizophagus irregularis reduced drought stress in tomatoes and lettuce by colonizing the roots [115]. Interestingly, both drought and colonization by R. irregularis increased the synthesis of the phytohormone strigolactone. Since strigolactones are known as host identification signals for arbuscular mycorrhiza (AM) fungi, this implies that they operate as a “call for help” signal and start a positive feedback loop of R. irregularis colonization that increases host plant tolerance to drought conditions [116]. A similar positive effect of bacterial endophytes has also been reported in wheat, where endophytes played a key role in metabolite balance and the reduced effects of drought stress [117].
Some endophytes help the host plant survive drought-induced oxidative stress by enhancing antioxidant defense enzymes. Moghaddam et al. [118] reported that endophytic fungi Neocamarosporium goegapense, Neocamarosporium chichastianum, and Periconia macrospinosa isolated from a desert plant alleviated drought stress in wheat and cucumber by boosting antioxidant defense enzymes. The endophytic bacteria Bacillus safensis and Ochrobactrum pseudogregnonense increased antioxidant activity and enhanced wheat growth under drought conditions [119]. Similar mechanisms of endophyte-mediated drought tolerance have been reported in maize [120,121], wheat [110], Chinese cabbage [122], and other popular crops [123].
Drought also affects the microbial diversity in the host plant rhizosphere. Xu et al. [124] showed that drought drastically lowered bacterial diversity in the rhizosphere and root endosphere, whereas the bacterial community diversity in the surrounding soil remained mostly unchanged. Furthermore, the authors reported that drought enhanced the abundance of actinobacteria, which in turn resulted in the enrichment of metabolites required for drought tolerance. However, it is still unclear how these drought-enhanced metabolites reconfigure the root microbiome makeup to improve plant stress responses. Nonetheless, this intriguing association raises the possibility that plants, and their associated microbiomes engage in molecular dialogues during drought to change the root microbiota to better tolerate drought stress. Understanding this molecular exchange will help us obtain foundational information regarding the use of microbiota to improve agricultural productivity under drought conditions [84].
4.4. Nutrient Deficiency
Nutrient stress, characterized as either suboptimal availability of necessary nutrients or excessive and toxic amounts of essential and non-essential macronutrients, is considered a major stress that has significantly affected crop productivity worldwide. Extracting insoluble nutrients from soils is one of the biggest challenges in the evolution of terrestrial plants; as a result, they may have formed symbiotic relationships with mobile microbes and have flagella or hyphae that can extend into the soil to provide plants with nutrients [125]. Many studies have shown that endophytes can assist plants by transforming and solubilizing nutrients such as N, P, K, and other microminerals, thus making them conveniently accessible to plants [126,127]. For example, Pseudomonas sp., as described by Choi et al., mediates phosphate solubilization in rice and wheat by altering gibberellic acid production [128]. Abadi and Sepehri [129] reported that the ability of wheat to absorb mineral nutrients, particularly zinc, was aided by the presence of the endophytes Azotobacter chroococcum and Piriformospora indica. The bacterial endophyte Enterobacter sp. has been reported to help plants adapt to various environmental conditions by improving nutrient acquisition, including nitrogen fixation [130]. Yamaji et al. [131] reported that Phialocephala fortinii, Rhizodermea veluwensis, and Rhizoscyphus sp. isolated from mining site soil enhanced the growth of C. barbinervis seedlings by increasing K uptake in shoots and reducing the concentrations of Cu, Ni, Zn, Cd, and Pb in roots. This suggests that isolating and characterizing endophytes from mining sites may help discover novel endophyte species that can assist in crop improvement under heavy metal stress. Some other examples in Table 2 represent endophytes, their host sand, and their role in various abiotic stresses.
Table 2.
List of endophytes mediating abiotic stress tolerance in plants.
| Endophyte | Host Plant | Stress | Mechanism | Reference |
|---|---|---|---|---|
| Mucilaginibacter stain K | Arabidopsis thaliana | Salinity | Increase in anti-oxidative defense machinery | [132] |
| Pantoea agglomerans | Zea mays | Salinity | Upregulation of aquaporins | [133] |
|
Arthrobacter endophyticus, Nocardiopsis alba |
A. thaliana | Salinity | Enhancing the expression of genes responsible for water, potassium ion uptake, carotenoid biosynthesis, phenylalanine metabolism, phenylpropanoid biosynthesis, glycerolipid and nitrogen metabolism | [134] |
| Penicillium brevicompactum and P. chrysogenum | Solanum lycopersicum Lactuca sativa | Salinity | Enhanced energy production and Na+ sequestration | [135] |
| Alternaria chlamydospora | Triticum aestivum | Salinity | Inducing the physiological and biochemical responses | [136] |
| Trichoderma harzianum TH-56 | Oryza sativa | Drought | Upregulation of aquaporin, dehydrin, and malonialdehyde genes | [137] |
| Pseudomonas putida | Cicer arietinum | Drought | miRNAs and their target genes indicated the involvement in the stress regulatory pathways, which control/regulate drought stress response | [138] |
| Bacillus cereus, Pseudomonas otitidis and Pseudomonas sp. | Glycine max | Drought | Improving plant growth, membrane integrity, water status, accumulation of compatible solutes, and osmolytes | [139] |
| Gluconacetobacter diazotrophicus | Saccharum officinarum | Drought | IAA and proline production | [113] |
| Paraphoma sp., Embellisia chlamydospora, and Cladosporium oxysporum | Zea mays | Drought | Altered root development | [140] |
| Pseudomonas aeruginosa strain 2CpS1 | T. aestivum | Heat | Reducing heat and drought–induced oxidative stress | [141] |
| Bacillus amyloliquefaciens subsp. plantarum UCMB5113 | T. aestivum | Heat | by molecular modifications in wheat leaf transcript patterns | [142] |
| Bacillus velezensis | T. aestivum | Heat | (1) express several proteins related to stress defense and energy supply (2) modulate several metabolic pathways of amino acids (3) increase the accumulation of GABA in the leaves |
[143] |
| Burkholderia phytofirmans PsJN | A. thaliana | Cold | Pigment accumulation and cold response pathway induction | [144] |
| Bacillus sp. and Lysinibacillus sp. | Argentine screwbean | Nutrient deficiency | Increase in antioxidant defense enzyme, photosynthetic pigments and low lipid peroxidation siderophore, and alteration in IAA, gibberellic acid content | [145] |
| Bacillus amyloliquefaciens | Oryza sativa | Nutrient deficiency | Modulates carbohydrate metabolism | [146] |
| Pseudomonas sp. | Pisum sativum | Nutrient deficiency | Gluconic acid used by bacteria to solubilize phosphate | [146] |
4.5. Negative Effects of Endophytes: the Other Side of the Picture
Although endophytes promote the host plants’ growth in several ways [10], these endorsing effects are sensitive to changes in the abiotic environment and may shift from positive to negative when the tolerance range of either symbiont is compromised [147]. The shift in this unstable symbiotic relationship between endophytes and host plants to pathogenicity may be due to variations in abiotic environments, such as nutrient shortages or prolonged extreme weather. We expect this because a fungal species may be endophytic and beneficial to one host species under certain abiotic limits and become pathogenic to the same or another host under different environmental conditions [148]. Despite a good understanding of the behavior and mechanism of endophyte-host plants using high-throughput techniques, there are still substantial gaps regarding microbial lifestyle and their function in host plants [32]. It is therefore inferred that endophytic status cannot be assumed to be certain, as these co-evolved relationships are plastic/dynamic and can be anticipated to destabilize under severe environmental change scenarios [149].
In addition, one should consider the compatibility of endophytes, especially when used as a consortium of endophytes in field conditions. Reports suggested that co-inoculation of chickpea with compatible endophytes such as Mesorhizobium, Serratia marcescence, and Serratia spp. have synergetic effects on plant growth in terms of nodule number and dry weight, the number of pods per plant, etc. [150]. Similar results were reported by Oliveira et al. [151], where inoculation with a consortium of five diazotrophic bacteria (Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, Herbaspirillum rubrisubalbicans, Azospirillum amazonense, and Paraburkholderia tropica) resulted in higher stem production in sugarcane as compared to single endophyte inoculated plants. On the contrary, applying a consortium of incompatible bacteria may have damaging effects on crop growth and production. Co-inoculation of nitrogen-fixing bacteria Herbaspirillum seropedicae and H. rubrisubalbicans with sugarcane did not increase the overall yield of sugarcane [151]. Therefore, it is highly recommended to do a compatibility test for the endophytes before their application [152].
5. Conclusions and Future Recommendations
Our understanding of how to plant endophytes can influence plant physiological responses to biotic and abiotic stresses (see Figure 2). Each year, an increasing number of articles indicate the critical role of plant–microbe interactions in plant fitness during extreme environmental conditions. These breakthroughs have provided new insights into crop protection and disease management. Under unpredictable climates, food crops are more vulnerable to increased biotic and abiotic stresses, putting them at higher risk and jeopardizing the security of the world’s food supply.
Despite significant advancements over the past few decades, there is still a knowledge gap regarding the possibility that environmental stresses can shift plant–microbe interactions from commensal to mutualistic or parasitic relationships. They might compete with each other for survival under adverse conditions, for example, by competing for crucial nutrients such as nitrogen and phosphate [153]. Hence, a better understanding of the factors that determine whether a plant–microbe interaction is harmful or beneficial is required. Transcriptome analysis of perturbed microbial associations in grasses, in which the plant–microbe interaction switches from reciprocal to pathogenic, has identified plant and fungal gene candidates that play a central role [154]. Therefore, we suggest that detailed transcriptomic and metabolomic analyses can be used to comprehensively understand the complex interactions between plants and their microbiome in biotic and abiotic environments. This may provide unprecedented opportunities for enhancing agricultural productivity in extreme environments. Despite the well-established reports on the plant-endophytic symbiotic relationship and the effects on improving plant growth and health, there are no examples of this being used in agriculture. This could be a lack of broadcasting the information from the scientific community to the farmers, a view worth considering.
Author Contributions
Conceptualization, M.K. and Q.M.I.; writing—original draft preparation, M.K., Q.M.I., N.F. and A.K.; writing—review and editing, M.K. and B.-W.Y.; visualization, M.B.A.; supervision, M.K. and B.-W.Y.; funding acquisition, B.-W.Y. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is partly supported by the Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (NRF-2021R1A6C101A416).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Worldometer. World Population. [(accessed on 15 August 2022)]. Available online: https://www.worldometers.info/
- 2.Bank T.W. Population Growth (Annual %) [(accessed on 15 August 2022)]. Available online: https://data.worldbank.org/indicator/SP.POP.GROW.
- 3.Roser M., Ritchie H., Ortiz-Ospina E. World population growth. [(accessed on 15 August 2022)];Our World Data. 2013 Available online: https://ourworldindata.org/world-population-growth. [Google Scholar]
- 4.FAO . World Food and Agriculture–Statistical Yearbook 2018. FAO; Rome, Italy: 2018. [Google Scholar]
- 5.FAO . World Food and Agriculture–Statistical Yearbook 2020. FAO; Rome, Italy: 2020. [Google Scholar]
- 6.Alix A., Capri E. Advances in Chemical Pollution, Environmental Management and Protection. Volume 2. Elsevier; Amsterdam, The Netherlands: 2018. Modern agriculture in Europe and the role of pesticides; pp. 1–22. [Google Scholar]
- 7.Abhilash P., Singh N. Pesticide use and application: An Indian scenario. J. Hazard. Mater. 2009;165:1–12. doi: 10.1016/j.jhazmat.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 8.Srivastav A.L. Agrochemicals Detection, Treatment and Remediation. Elsevier; Amsterdam, The Netherlands: 2020. Chemical fertilizers and pesticides: Role in groundwater contamination; pp. 143–159. [Google Scholar]
- 9.Yadav A.N. Plant microbiomes for sustainable agriculture: Current research and future challenges. Plant. Microb. Sus. Agri. 2020:475–482. [Google Scholar]
- 10.Rana K.L., Kour D., Sheikh I., Yadav N., Yadav A.N., Kumar V., Singh B.P., Dhaliwal H.S., Saxena A.K. Biodiversity of Endophytic Fungi from Diverse Niches and Their Biotechnological Applications. In: Singh B.P.., editor. Advances in Endophytic Fungal Research: Present Status and Future Challenges. Springer International Publishing; Cham, Germany: 2019. pp. 105–144. [Google Scholar]
- 11.Compant S., Reiter B., Sessitsch A., Nowak J., Clement C., Ait Barka E. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005;71:1685–1693. doi: 10.1128/AEM.71.4.1685-1693.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Melo Pereira G.V., Magalhães K.T., Lorenzetii E.R., Souza T.P., Schwan R.F. A multiphasic approach for the identification of endophytic bacterial in strawberry fruit and their potential for plant growth promotion. Microb. Ecol. 2012;63:405–417. doi: 10.1007/s00248-011-9919-3. [DOI] [PubMed] [Google Scholar]
- 13.Truyens S., Weyens N., Cuypers A., Vangronsveld J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015;7:40–50. doi: 10.1111/1758-2229.12181. [DOI] [Google Scholar]
- 14.Miliute I., Buzaite O., Baniulis D., Stanys V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste-Agriculture. 2015;102:465–478. doi: 10.13080/z-a.2015.102.060. [DOI] [Google Scholar]
- 15.Chi F., Shen S.H., Cheng H.P., Jing Y.X., Yanni Y.G., Dazzo F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005;71:7271–7278. doi: 10.1128/AEM.71.11.7271-7278.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan R.P., Germaine K., Franks A., Ryan D.J., Dowling D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008;278:1–9. doi: 10.1111/j.1574-6968.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 17.Petrini O. Microbiology of the Phyllosphere. Cambridge Univeristy Press; Cambridge, UK: 1986. Taxonomy of endophytic fungi of aerial plant tissues. [Google Scholar]
- 18.Brundrett M.C. Microbial Root Endophytes. Springer; Berlin/Heidelberg, Germany: 2006. Understanding the roles of multifunctional mycorrhizal and endophytic fungi; pp. 281–298. [Google Scholar]
- 19.Clay K., Holah J. Fungal endophyte symbiosis and plant diversity in successional fields. Science. 1999;285:1742–1745. doi: 10.1126/science.285.5434.1742. [DOI] [PubMed] [Google Scholar]
- 20.Redecker D., Kodner R., Graham L.E. Glomalean fungi from the Ordovician. Science. 2000;289:1920–1921. doi: 10.1126/science.289.5486.1920. [DOI] [PubMed] [Google Scholar]
- 21.Krings M., Taylor T.N., Hass H., Kerp H., Dotzler N., Hermsen E.J. Fungal endophytes in a 400-million-yr-old land plant: Infection pathways, spatial distribution, and host responses. New Phytol. 2007;174:648–657. doi: 10.1111/j.1469-8137.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez R.J., White J.F., Jr., Arnold A.E., Redman R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 23.Mueller G.M. Biodiversity of Fungi: Inventory and Monitoring Methods. Elsevier; Amsterdam, The Netherlands: 2011. [Google Scholar]
- 24.Jones J.D., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 25.Coombs J.T., Franco C.M. Visualization of an endophytic Streptomyces species in wheat seed. Appl. Environ. Microbiol. 2003;69:4260–4262. doi: 10.1128/AEM.69.7.4260-4262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khare E., Mishra J., Arora N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018;9:2732. doi: 10.3389/fmicb.2018.02732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong Y., Iniguez A.L., Triplett E.W. Quantitative assessments of the host range and strain specificity of endophytic colonization by Klebsiella pneumoniae 342. Plant Soil. 2003;257:49–59. doi: 10.1023/A:1026242814060. [DOI] [Google Scholar]
- 28.James E.K., Olivares F.L. Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. CRC Crit. Rev. Plant Sci. 1998;17:77–119. doi: 10.1080/07352689891304195. [DOI] [Google Scholar]
- 29.Kovtunovych G., Lar O., Kamalova S., Kordyum V., Kleiner D., Kozyrovska N. Correlation between pectate lyase activity and ability of diazotrophic Klebsiella oxytoca VN 13 to penetrate into plant tissues. Plant Soil. 1999;215:1–6. doi: 10.1023/A:1004790122353. [DOI] [Google Scholar]
- 30.Cord-Landwehr S., Melcher R.L., Kolkenbrock S., Moerschbacher B.M. A chitin deacetylase from the endophytic fungus Pestalotiopsis sp. efficiently inactivates the elicitor activity of chitin oligomers in rice cells. Sci. Rep. 2016;6:38018. doi: 10.1038/srep38018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenkoornhuyse P., Quaiser A., Duhamel M., Le Van A., Dufresne A. The importance of the microbiome of the plant holobiont. New Phytol. 2015;206:1196–1206. doi: 10.1111/nph.13312. [DOI] [PubMed] [Google Scholar]
- 32.Santoyo G., Moreno-Hagelsieb G., del Orozco-Mosqueda M.C., Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Kloepper J.W., Ryu C.-M. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz B.J.E., Boyle C.J.C., Sieber T.N., editors. Microbial Root Endophytes. Springer; Berlin/Heidelberg, Germany: 2006. pp. 33–52. [Google Scholar]
- 34.Waqas M., Khan A.L., Hamayun M., Shahzad R., Kang S.-M., Kim J.-G., Lee I.-J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015;10:280–287. doi: 10.1080/17429145.2015.1079743. [DOI] [Google Scholar]
- 35.Kavroulakis N., Ntougias S., Zervakis G.I., Ehaliotis C., Haralampidis K., Papadopoulou K.K. Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J. Exp. Bot. 2007;58:3853–3864. doi: 10.1093/jxb/erm230. [DOI] [PubMed] [Google Scholar]
- 36.Van Bael S.A., Seid M.A., Wcislo W.T. Endophytic fungi increase the processing rate of leaves by leaf–cutting ants (Atta) Ecol. Entomol. 2012;37:318–321. doi: 10.1111/j.1365-2311.2012.01364.x. [DOI] [Google Scholar]
- 37.Estrada C., Wcislo W.T., Van Bael S.A. Symbiotic fungi alter plant chemistry that discourages leaf–cutting ants. New Phytol. 2013;198:241–251. doi: 10.1111/nph.12140. [DOI] [PubMed] [Google Scholar]
- 38.Salam N., Khieu T.N., Liu M.J., Vu T.T., Chu-Ky S., Quach N.T., Phi Q.T., Narsing Rao M.P., Fontana A., Sarter S., et al. Endophytic actinobacteria ssociated with Dracaena cochinchinensis Lour.: Isolation, Diversity, and their cytotoxic activities. Biomed. Res. Int. 2017;2017:1308563. doi: 10.1155/2017/1308563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mejía L.C., Rojas E.I., Maynard Z., Van Bael S., Arnold A.E., Hebbar P., Samuels G.J., Robbins N., Herre E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control. 2008;46:4–14. doi: 10.1016/j.biocontrol.2008.01.012. [DOI] [Google Scholar]
- 40.Mejia L.C., Herre E.A., Sparks J.P., Winter K., Garcia M.N., Van Bael S.A., Stitt J., Shi Z., Zhang Y., Guiltinan M.J., et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front Microbiol. 2014;5:479. doi: 10.3389/fmicb.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosaka A., Suemoto H., Singkaravanit-Ogawa S., Takano Y. Plant defensin expression triggered by fungal pathogen invasion depends on EDR1 protein kinase and ORA59 transcription factor in Arabidopsis thaliana. Plant Signal Behav. 2020;15:1823120. doi: 10.1080/15592324.2020.1823120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villarreal-Delgado M.F., Villa-Rodríguez E.D., Cira-Chávez L.A., Estrada-Alvarado M.I., Parra-Cota F.I., Santos-Villalobos S.D.L. The genus Bacillus as a biological control agent and its implications in the agricultural biosecurity. Rev. Mex. Fitopatol. 2018;36:95–130. [Google Scholar]
- 43.Miller C.M., Miller R.V., Garton-Kenny D., Redgrave B., Sears J., Condron M.M., Teplow D.B., Strobel G.A. Ecomycins, unique antimycotics from Pseudomonas viridiflava. J. Appl. Microbiol. 1998;84:937–944. doi: 10.1046/j.1365-2672.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 44.Sheoran N., Valiya Nadakkakath A., Munjal V., Kundu A., Subaharan K., Venugopal V., Rajamma S., Eapen S.J., Kumar A. Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol. Res. 2015;173:66–78. doi: 10.1016/j.micres.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Arora N., Kang S., Maheshwari D. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci. 2001;81:673–677. [Google Scholar]
- 46.Stein T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 47.Oukala N., Aissat K., Pastor V. Bacterial Endophytes: The hidden actor in plant immune responses against biotic stress. Plants. 2021;10:1012. doi: 10.3390/plants10051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu-kang G., Chuan-chao D., Xiao-zhen L. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010;4:1346–1351. [Google Scholar]
- 49.Yong Y., Dai C., Gao F., Yang Q., Zhao M. Effects of endophytic fungi on growth and two kinds of terpenoids for Euphorbia pekinensis. Chin. Tradit. Herb. Drugs. 1994;24:579810. [Google Scholar]
- 50.Morelli M., Bahar O., Papadopoulou K.K., Hopkins D.L., Obradovic A. Editorial: Role of endophytes in plant health and defense against pathogens. Front. Plant Sci. 2020;11:1312. doi: 10.3389/fpls.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samain E., Aussenac T., Selim S. The Effect of Plant Genotype, Growth Stage, and Mycosphaerella graminicola Strains on the Efficiency and Durability of Wheat-Induced Resistance by Paenibacillus sp. Strain B2. Front. Plant Sci. 2019;10:587. doi: 10.3389/fpls.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rais A., Jabeen Z., Shair F., Hafeez F.Y., Hassan M.N. Bacillus spp., a bio-control agent enhances the activity of antioxidant defense enzymes in rice against Pyricularia oryzae. PLoS ONE. 2017;12:e0187412. doi: 10.1371/journal.pone.0187412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva G.H., Teles H.L., Trevisan H.C., Bolzani V.D., Young M.C.M., Pfenning L.H., Eberlin M.N., Haddad R., Costa-Neto C.M., Araujo A.R. New bioactive metabolites produced by Phomopsis cassiae, an endophytic fungus in Cassia spectabilis. J. Braz. Chem. Soc. 2005;16:1463–1466. doi: 10.1590/S0103-50532005000800029. [DOI] [Google Scholar]
- 54.Colombo E.M., Kunova A., Pizzatti C., Saracchi M., Cortesi P., Pasquali M. Selection of an endophytic Streptomyces sp. Strain def09 from wheat roots as a biocontrol agent against Fusarium graminearum. Front. Microbiol. 2019;10:2356. doi: 10.3389/fmicb.2019.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suebrasri T., Somteds A., Harada H., Kanokmedhakul S., Jogloy S., Ekprasert J., Lumyong S., Boonlue S. Novel endophytic fungi with fungicidal metabolites suppress sclerotium disease. Rhizosphere. 2020;16:100250. doi: 10.1016/j.rhisph.2020.100250. [DOI] [Google Scholar]
- 56.Mendes R., Kruijt M., De Bruijn I., Dekkers E., Van Der Voort M., Schneider J.H., Piceno Y.M., DeSantis T.Z., Andersen G.L., Bakker P.A. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science. 2011;332:1097–1100. doi: 10.1126/science.1203980. [DOI] [PubMed] [Google Scholar]
- 57.Samain E., van Tuinen D., Jeandet P., Aussenac T., Selim S. Biological control of septoria leaf blotch and growth promotion in wheat by Paenibacillus sp strain B2 and Curtobacterium plantarum strain EDS. Biol. Control. 2017;114:87–96. doi: 10.1016/j.biocontrol.2017.07.012. [DOI] [Google Scholar]
- 58.Schlegel M., Dubach V., von Buol L., Sieber T.N. Effects of endophytic fungi on the ash dieback pathogen. Fems Microbiol. Ecol. 2016;92:fiw142. doi: 10.1093/femsec/fiw142. [DOI] [PubMed] [Google Scholar]
- 59.Ganley R.J., Sniezko R.A., Newcombe G. Endophyte-mediated resistance against white pine blister rust in Pinus monticola. For. Ecol. Manag. 2008;255:2751–2760. doi: 10.1016/j.foreco.2008.01.052. [DOI] [Google Scholar]
- 60.Bilański P., Kowalski T. Fungal endophytes in Fraxinus excelsior petioles and their in vitro antagonistic potential against the ash dieback pathogen Hymenoscyphus fraxineus. Microbiol. Res. 2022;257:126961. doi: 10.1016/j.micres.2022.126961. [DOI] [PubMed] [Google Scholar]
- 61.Carrión V.J., Perez-Jaramillo J., Cordovez V., Tracanna V., De Hollander M., Ruiz-Buck D., Mendes L.W., van Ijcken W.F., Gomez-Exposito R., Elsayed S.S. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366:606–612. doi: 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez M.V., Tano J., Ansaldi N., Carrau A., Srebot M.S., Ferreira V., Martinez M.L., Cortadi A.A., Siri M.I., Orellano E.G. Anatomical and biochemical changes induced by Gluconacetobacter diazotrophicus stand up for Arabidopsis thaliana seedlings from Ralstonia solanacearum infection. Front. Plant Sci. 2019;10:1618. doi: 10.3389/fpls.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stein E., Molitor A., Kogel K.H., Waller F. Systemic resistance in arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol. 2008;49:1747–1751. doi: 10.1093/pcp/pcn147. [DOI] [PubMed] [Google Scholar]
- 64.Pangesti N., Reichelt M., van de Mortel J.E., Kapsomenou E., Gershenzon J., van Loon J.J., Dicke M., Pineda A. Jasmonic acid and ethylene signaling pathways regulate glucosinolate levels in plants during rhizobacteria-induced systemic resistance against a leaf-chewing herbivore. J. Chem. Ecol. 2016;42:1212–1225. doi: 10.1007/s10886-016-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ryu C.M., Farag M.A., Hu C.H., Reddy M.S., Kloepper J.W., Pare P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudrappa T., Biedrzycki M.L., Kunjeti S.G., Donofrio N.M., Czymmek K.J., Pare P.W., Bais H.P. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 2010;3:130–138. doi: 10.4161/cib.3.2.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Portieles R., Xu H., Yue Q., Zhao L., Zhang D., Du L., Gao X., Gao J., Portal Gonzalez N., Santos Bermudez R., et al. Heat-killed endophytic bacterium induces robust plant defense responses against important pathogens. Sci. Rep. 2021;11:12182. doi: 10.1038/s41598-021-91837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu H., Brettell L.E., Qiu Z., Singh B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020;25:733–743. doi: 10.1016/j.tplants.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 69.Djalante R. Key assessments from the IPCC special report on global warming of 1.5 C and the implications for the Sendai framework for disaster risk reduction. Prog. Disast. Sci. 2019;1:100001. doi: 10.1016/j.pdisas.2019.100001. [DOI] [Google Scholar]
- 70.Kumar A., Verma J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018;207:41–52. doi: 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Li X., Zhao C., Zhang T., Wang G., Amombo E., Xie Y., Fu J. Exogenous Aspergillus aculeatus enhances drought and heat tolerance of perennial ryegrass. Front. Microbiol. 2021;12:593722. doi: 10.3389/fmicb.2021.593722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ismail, Hamayun M., Hussain A., Iqbal A., Khan S.A., Lee I.-J. Aspergillus niger boosted heat stress tolerance in sunflower and soybean via regulating their metabolic and antioxidant system. J. Plant Interact. 2020;15:223–232. doi: 10.1080/17429145.2020.1771444. [DOI] [Google Scholar]
- 73.Khan A.L., Waqas M., Lee I.J. Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J. Plant Res. 2015;128:259–268. doi: 10.1007/s10265-014-0688-1. [DOI] [PubMed] [Google Scholar]
- 74.Fernandez O., Theocharis A., Bordiec S., Feil R., Jacquens L., Clement C., Fontaine F., Barka E.A. Burkholderia phytofirmans PsJN acclimates grapevine to cold by modulating carbohydrate metabolism. Mol. Plant Microbe Interact. 2012;25:496–504. doi: 10.1094/MPMI-09-11-0245. [DOI] [PubMed] [Google Scholar]
- 75.Tomoyasu T., Tabata A., Imaki H., Tsuruno K., Miyazaki A., Sonomoto K., Whiley R.A., Nagamune H. Role of Streptococcus intermedius DnaK chaperone system in stress tolerance and pathogenicity. Cell Stress Chaperones. 2012;17:41–55. doi: 10.1007/s12192-011-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Genevaux P., Georgopoulos C., Kelley W.L. The Hsp70 chaperone machines of Escherichia coli: A paradigm for the repartition of chaperone functions. Mol. Microbiol. 2007;66:840–857. doi: 10.1111/j.1365-2958.2007.05961.x. [DOI] [PubMed] [Google Scholar]
- 77.Jiao L., Ran J., Xu X., Wang J. Heat, acid and cold stresses enhance the expression of DnaK gene in Alicyclobacillus acidoterrestris. Food Res. Int. 2015;67:183–192. doi: 10.1016/j.foodres.2014.11.023. [DOI] [Google Scholar]
- 78.Khan M.A., Asaf S., Khan A.L., Jan R., Kang S.M., Kim K.M., Lee I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020;20:175. doi: 10.1186/s12866-020-01822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tiwari S., Prasad V., Chauhan P.S., Lata C. Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front. Plant Sci. 2017;8:1510. doi: 10.3389/fpls.2017.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali S.Z., Sandhya V., Grover M., Linga V.R., Bandi V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant. Interact. 2011;6:239–246. doi: 10.1080/17429145.2010.545147. [DOI] [Google Scholar]
- 81.Ali S.Z., Sandhya V., Grover M., Kishore N., Rao L.V., Venkateswarlu B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils. 2009;46:45–55. doi: 10.1007/s00374-009-0404-9. [DOI] [Google Scholar]
- 82.Márquez L.M., Redman R.S., Rodriguez R.J., Roossinck M.J. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science. 2007;315:513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- 83.Rodriguez R.J., Henson J., Van Volkenburgh E., Hoy M., Wright L., Beckwith F., Kim Y.O., Redman R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 84.Cheng Y.T., Zhang L., He S.Y. Plant-microbe interactions facing environmental challenge. Cell Host Microbe. 2019;26:183–192. doi: 10.1016/j.chom.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lalit L.K., Nandanwar S.K., Arti S.S., Yogesh M., Yele P.N., Sivalingam N., Kaushal P., Kumar J. Microbe-mediated Salinity Tolerance in Plants. [(accessed on 16 August 2022)]. Available online: https://microbiologycommunity.nature.com/posts/31111-microbe-mediated-salinity-tolerance-in-plants.
- 86.Flowers T.J. Improving crop salt tolerance. J. Exp. Bot. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- 87.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 88.Schubert S., Neubert A., Schierholt A., Sümer A., Zörb C. Development of salt-resistant maize hybrids: The combination of physiological strategies using conventional breeding methods. Plant Sci. 2009;177:196–202. doi: 10.1016/j.plantsci.2009.05.011. [DOI] [Google Scholar]
- 89.Dodd I.C., Perez-Alfocea F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012;63:3415–3428. doi: 10.1093/jxb/ers033. [DOI] [PubMed] [Google Scholar]
- 90.Kumar V., Khare T., Sharma M., Wani S.H. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress. Springer; Berlin/Heidelberg, Germany: 2017. ROS-induced signaling and gene expression in crops under salinity stress; pp. 159–184. [Google Scholar]
- 91.Khan M., Asaf S., Khan A., Adhikari A., Jan R., Ali S., Imran M., Kim K.M., Lee I.J. Plant growth–promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020;22:850–862. doi: 10.1111/plb.13124. [DOI] [PubMed] [Google Scholar]
- 92.Khan A.L., Hamayun M., Khan S.A., Kang S.M., Shinwari Z.K., Kamran M., Ur Rehman S., Kim J.G., Lee I.J. Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J. Microbiol. Biotechnol. 2012;28:1483–1494. doi: 10.1007/s11274-011-0950-9. [DOI] [PubMed] [Google Scholar]
- 93.Khan A.L., Waqas M., Asaf S., Kamran M., Shahzad R., Bilal S., Khan M.A., Kang S.-M., Kim Y.-H., Yun B.-W. Plant growth-promoting endophyte Sphingomonas sp. LK11 alleviates salinity stress in Solanum pimpinellifolium. Environ. Exp. Bot. 2017;133:58–69. doi: 10.1016/j.envexpbot.2016.09.009. [DOI] [Google Scholar]
- 94.Kang S.-M., Khan A.L., Waqas M., You Y.-H., Kim J.-H., Kim J.-G., Hamayun M., Lee I.-J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014;9:673–682. doi: 10.1080/17429145.2014.894587. [DOI] [Google Scholar]
- 95.Chen L., Liu Y., Wu G., Veronican Njeri K., Shen Q., Zhang N., Zhang R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant. 2016;158:34–44. doi: 10.1111/ppl.12441. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H., Kim M.S., Sun Y., Dowd S.E., Shi H., Pare P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol. Plant Microbe. Interact. 2008;21:737–744. doi: 10.1094/MPMI-21-6-0737. [DOI] [PubMed] [Google Scholar]
- 97.Nautiyal C.S., Srivastava S., Chauhan P.S., Seem K., Mishra A., Sopory S.K. Plant growth-promoting bacteria Bacillus amyloliquefaciens NBRISN13 modulates gene expression profile of leaf and rhizosphere community in rice during salt stress. Plant Physiol. Biochem. 2013;66:1–9. doi: 10.1016/j.plaphy.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 98.Jha Y., Subramanian R., Patel S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2011;33:797–802. doi: 10.1007/s11738-010-0604-9. [DOI] [Google Scholar]
- 99.Roodposhti M.S., Safarrad T., Shahabi H. Drought sensitivity mapping using two one-class support vector machine algorithms. Atmos. Res. 2017;193:73–82. doi: 10.1016/j.atmosres.2017.04.017. [DOI] [Google Scholar]
- 100.Lindersson S., Brandimarte L., Mård J., Di Baldassarre G. A review of freely accessible global datasets for the study of floods, droughts and their interactions with human societies. Wiley Interdiscip. Rev. Water. 2020;7:e1424. doi: 10.1002/wat2.1424. [DOI] [Google Scholar]
- 101.Nanzad L., Zhang J., Tuvdendorj B., Nabil M., Zhang S., Bai Y. NDVI anomaly for drought monitoring and its correlation with climate factors over Mongolia from 2000 to 2016. J. Arid Environ. 2019;164:69–77. doi: 10.1016/j.jaridenv.2019.01.019. [DOI] [Google Scholar]
- 102.Venuprasad R., Lafitte H.R., Atlin G.N. Response to direct selection for grain yield under drought stress in rice. Crop. Sci. 2007;47:285–293. doi: 10.2135/cropsci2006.03.0181. [DOI] [Google Scholar]
- 103.Ashraf M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010;28:169–183. doi: 10.1016/j.biotechadv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Philippot L., Raaijmakers J.M., Lemanceau P., van der Putten W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013;11:789–799. doi: 10.1038/nrmicro3109. [DOI] [PubMed] [Google Scholar]
- 105.Zhu J.K. Abiotic Stress Signaling and Responses in Plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tiwari S., Lata C., Chauhan P.S., Nautiyal C.S. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016;99:108–117. doi: 10.1016/j.plaphy.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Yandigeri M.S., Meena K.K., Singh D., Malviya N., Singh D.P., Solanki M.K., Yadav A.K., Arora D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012;68:411–420. doi: 10.1007/s10725-012-9730-2. [DOI] [Google Scholar]
- 108.Cohen A.C., Bottini R., Pontin M., Berli F.J., Moreno D., Boccanlandro H., Travaglia C.N., Piccoli P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015;153:79–90. doi: 10.1111/ppl.12221. [DOI] [PubMed] [Google Scholar]
- 109.Kang S.-M., Radhakrishnan R., Khan A.L., Kim M.-J., Park J.-M., Kim B.-R., Shin D.-H., Lee I.-J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 110.Hussain M.B., Zahir Z.A., Asghar H.N., Asgher M. Can catalase and exopolysaccharides producing rhizobia ameliorate drought stress in wheat? Int. J. Agric. Biol. 2014;16:3–13. [Google Scholar]
- 111.Armada E., Roldan A., Azcon R. Differential activity of autochthonous bacteria in controlling drought stress in native Lavandula and Salvia plants species under drought conditions in natural arid soil. Microb. Ecol. 2014;67:410–420. doi: 10.1007/s00248-013-0326-9. [DOI] [PubMed] [Google Scholar]
- 112.Waqas M., Khan A.L., Kamran M., Hamayun M., Kang S.M., Kim Y.H., Lee I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vargas L., Santa Brigida A.B., Mota Filho J.P., de Carvalho T.G., Rojas C.A., Vaneechoutte D., Van Bel M., Farrinelli L., Ferreira P.C., Vandepoele K., et al. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE. 2014;9:e114744. doi: 10.1371/journal.pone.0114744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Asaf S., Khan A.L., Khan M.A., Imran Q.M., Yun B.W., Lee I.J. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiol. Res. 2017;205:135–145. doi: 10.1016/j.micres.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 115.Ruiz-Lozano J.M., Aroca R., Zamarreño Á.M., Molina S., Andreo-Jiménez B., Porcel R., García Mina J.M., Ruyter-Spira C., López-Ráez J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016;39:441–452. doi: 10.1111/pce.12631. [DOI] [PubMed] [Google Scholar]
- 116.Omae N., Tsuda K. Plant-microbiota interactions in abiotic stress environments. Mol. Plant Microbe Interact. 2022;35:511–526. doi: 10.1094/MPMI-11-21-0281-FI. [DOI] [PubMed] [Google Scholar]
- 117.Naveed M., Hussain M.B., Zahir Z.A., Mitter B., Sessitsch A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014;73:121–131. doi: 10.1007/s10725-013-9874-8. [DOI] [Google Scholar]
- 118.Moghaddam M.S.H., Safaie N., Soltani J., Hagh-Doust N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021;160:225–238. doi: 10.1016/j.plaphy.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 119.Chakraborty U., Chakraborty B.N., Chakraborty A.P., Dey P.L. Water stress amelioration and plant growth promotion in wheat plants by osmotic stress tolerant bacteria. World J. Microbiol. Biotechnol. 2013;29:789–803. doi: 10.1007/s11274-012-1234-8. [DOI] [PubMed] [Google Scholar]
- 120.Vardharajula S., Zulfikar Ali S., Grover M., Reddy G., Bandi V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011;6:1–14. doi: 10.1080/17429145.2010.535178. [DOI] [Google Scholar]
- 121.Naseem H., Bano A. Role of plant growth-promoting rhizobacteria and their exopolysaccharide in drought tolerance of maize. J. Plant Interact. 2014;9:689–701. doi: 10.1080/17429145.2014.902125. [DOI] [Google Scholar]
- 122.Sun C., Johnson J.M., Cai D., Sherameti I., Oelmüller R., Lou B. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 2010;167:1009–1017. doi: 10.1016/j.jplph.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Khan G. Enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016;6:38–47. doi: 10.1016/j.cpb.2016.08.001. [DOI] [Google Scholar]
- 124.Xu L., Naylor D., Dong Z., Simmons T., Pierroz G., Hixson K.K., Kim Y.M., Zink E.M., Engbrecht K.M., Wang Y., et al. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad. Sci. USA. 2018;115:E4284–E4293. doi: 10.1073/pnas.1717308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.White J.F., Kingsley K.I., Kowalski K.P., Irizarry I., Micci A., Soares M.A., Bergen M.S. Disease protection and allelopathic interactions of seed-transmitted endophytic pseudomonads of invasive reed grass (Phragmites australis) Plant Soil. 2018;422:195–208. doi: 10.1007/s11104-016-3169-6. [DOI] [Google Scholar]
- 126.White J.F., Kingsley K.L., Zhang Q., Verma R., Obi N., Dvinskikh S., Elmore M.T., Verma S.K., Gond S.K., Kowalski K.P. Endophytic microbes and their potential applications in crop management. Pest. Manag. Sci. 2019;75:2558–2565. doi: 10.1002/ps.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Soares M.A., Li H.Y., Kowalski K.P., Bergen M., Torres M.S., White J.F. Functional Role of Bacteria from Invasive Phragmites australis in Promotion of Host Growth. Microb. Ecol. 2016;72:407–417. doi: 10.1007/s00248-016-0793-x. [DOI] [PubMed] [Google Scholar]
- 128.Choi O., Kim J., Kim J.-G., Jeong Y., Moon J.S., Park C.S., Hwang I. Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol. 2008;146:657. doi: 10.1104/pp.107.112748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abadi V.A.J.M., Sepehri M. Effect of Piriformospora indica and Azotobacter chroococcum on mitigation of zinc deficiency stress in wheat (Triticum aestivum L.) Symbiosis. 2016;69:9–19. doi: 10.1007/s13199-015-0361-z. [DOI] [Google Scholar]
- 130.Sajjad Mirza M., Ahmad W., Latif F., Haurat J., Bally R., Normand P., Malik K.A. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil. 2001;237:47–54. doi: 10.1023/A:1013388619231. [DOI] [Google Scholar]
- 131.Yamaji K., Watanabe Y., Masuya H., Shigeto A., Yui H., Haruma T. Root fungal endophytes enhance heavy-metal stress tolerance of Cethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS ONE. 2016;11:e0169089. doi: 10.1371/journal.pone.0169089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fan D., Subramanian S., Smith D.L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 2020;10:1–18. doi: 10.1038/s41598-020-69713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gond S., Torres M., Bergen M., Helsel Z., White J., Jr. Induction of salt tolerance and up-regulation of aquaporin genes in tropical corn by rhizobacterium Pantoea agglomerans. Lett. Appl. Microbiol. 2015;60:392–399. doi: 10.1111/lam.12385. [DOI] [PubMed] [Google Scholar]
- 134.Dong Z.Y., Narsing Rao M.P., Wang H.F., Fang B.Z., Liu Y.H., Li L., Xiao M., Li W.J. Transcriptomic analysis of two endophytes involved in enhancing salt stress ability of Arabidopsis thaliana. Sci. Total Environ. 2019;686:107–117. doi: 10.1016/j.scitotenv.2019.05.483. [DOI] [PubMed] [Google Scholar]
- 135.Molina-Montenegro M.A., Acuna-Rodriguez I.S., Torres-Diaz C., Gundel P.E., Dreyer I. Antarctic root endophytes improve physiological performance and yield in crops under salt stress by enhanced energy production and Na(+) sequestration. Sci. Rep. 2020;10:5819. doi: 10.1038/s41598-020-62544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouzouina M., Kouadria R., Lotmani B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2021;130:913–925. doi: 10.1111/jam.14804. [DOI] [PubMed] [Google Scholar]
- 137.Pandey V., Ansari M.W., Tula S., Yadav S., Sahoo R.K., Shukla N., Bains G., Badal S., Chandra S., Gaur A.K., et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta. 2016;243:1251–1264. doi: 10.1007/s00425-016-2482-x. [DOI] [PubMed] [Google Scholar]
- 138.Jatan R., Tiwari S., Asif M.H., Lata C. Genome-wide profiling reveals extensive alterations in Pseudomonas putida-mediated miRNAs expression during drought stress in chickpea (Cicer arietinum L.) Environ. Exp. Bot. 2019;157:217–227. doi: 10.1016/j.envexpbot.2018.10.003. [DOI] [Google Scholar]
- 139.Dubey A., Saiyam D., Kumar A., Hashem A., Abd_Allah E.F., Khan M.L. Bacterial root endophytes: Characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress. Int. J. Environ. Res. Public Health. 2021;18:931. doi: 10.3390/ijerph18030931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li X., He C., He X., Su F., Hou L., Ren Y., Hou Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil. 2019;439:259–272. doi: 10.1007/s11104-019-04057-2. [DOI] [Google Scholar]
- 141.Meena H., Ahmed M.A., Prakash P. Amelioration of heat stress in wheat, Triticum aestivum by PGPR (Pseudomonas aeruginosa strain 2CpS1) Biosci. Biotechnol. Res. 2015;8:171–174. [Google Scholar]
- 142.Abd El-Daim I.A., Bejai S., Fridborg I., Meijer J. Identifying potential molecular factors involved in Bacillus amyloliquefaciens 5113 mediated abiotic stress tolerance in wheat. Plant Biol. 2018;20:271–279. doi: 10.1111/plb.12680. [DOI] [PubMed] [Google Scholar]
- 143.Abd El-Daim I.A., Bejai S., Meijer J. Bacillus velezensis 5113 Induced Metabolic and Molecular Reprogramming during Abiotic Stress Tolerance in Wheat. Sci. Rep. 2019;9:16282. doi: 10.1038/s41598-019-52567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Su F., Jacquard C., Villaume S., Michel J., Rabenoelina F., Clement C., Barka E.A., Dhondt-Cordelier S., Vaillant-Gaveau N. Burkholderia phytofirmans PsJN reduces impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2015;6:810. doi: 10.3389/fpls.2015.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sgroy V., Cassan F., Masciarelli O., Del Papa M.F., Lagares A., Luna V. Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009;85:371–381. doi: 10.1007/s00253-009-2116-3. [DOI] [PubMed] [Google Scholar]
- 146.Oteino N., Lally R.D., Kiwanuka S., Lloyd A., Ryan D., Germaine K.J., Dowling D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015;6:745. doi: 10.3389/fmicb.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saikkonen K., Faeth S.H., Helander M., Sullivan T. Fungal endophytes: A continuum of interactions with host plants. Annu. Rev. Ecol. Syst. 1998;29:319–343. doi: 10.1146/annurev.ecolsys.29.1.319. [DOI] [Google Scholar]
- 148.González-Coloma A., Cosoveanu A., Cabrera R., Giménez C., Kaushik N. Fungi. CRC Press; Boca Raton, FL, USA: 2018. Endophytic fungi and their bioprospection; pp. 14–31. [Google Scholar]
- 149.Stone J.K., Coop L.B., Manter D.K. Predicting effects of climate change on Swiss needle cast disease severity in Pacific Northwest forests. Can. J. Plant Pathol. 2008;30:169–176. doi: 10.1080/07060661.2008.10540533. [DOI] [Google Scholar]
- 150.Shahzad S.M., Khalid A., Arif M.S., Riaz M., Ashraf M., Iqbal Z., Yasmeen T. Co-inoculation integrated with P-enriched compost improved nodulation and growth of Chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol. Fertil. Soils. 2014;50:1–12. doi: 10.1007/s00374-013-0826-2. [DOI] [Google Scholar]
- 151.Oliveira A., Stoffels M., Schmid M., Reis V., Baldani J., Hartmann A. Colonization of sugarcane plantlets by mixed inoculations with diazotrophic bacteria. Eur. J. Soil Biol. 2009;45:106–113. doi: 10.1016/j.ejsobi.2008.09.004. [DOI] [Google Scholar]
- 152.Batra P., Barkodia M., Ahlawat U., Sansanwal R., Wati L. Effect of compatible and incompatible endophytic bacteria on growth of chickpea plant. Def. Life Sci. J. 2020;5:45–48. doi: 10.14429/dlsj.5.15119. [DOI] [Google Scholar]
- 153.Hacquard S., Spaepen S., Garrido-Oter R., Schulze-Lefert P. Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 2017;55:565–589. doi: 10.1146/annurev-phyto-080516-035623. [DOI] [PubMed] [Google Scholar]
- 154.Eaton C.J., Cox M.P., Scott B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011;180:190–195. doi: 10.1016/j.plantsci.2010.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.