Abstract
The De Ritis ratio is widely used to differentiate various causes of liver disease and serves as an independent prognostic predictor for different malignancies and non-malignant illnesses. This retrospective study aimed to identify the association between the De Ritis ratio on admission and mortality outcomes in adult thoracoabdominal trauma patients. A total of 2248 hospitalized adult trauma patients with thoracoabdominal injury, defined as an abbreviated injury scale (AIS) score ≥ 1 in the thoracic and abdominal regions, between 1 January 2009, and 31 December 2019, were included. They were categorized into three tertile groups according to the De Ritis ratio. A 1:1 propensity score-matched study group was established to attenuate the confounding effect of patient characteristics on the mortality outcome assessment. The AST levels of the tertile 1, 2, and 3 groups were 115.8 ± 174.9, 115.7 ± 262.0, and 140.5 ± 209.7 U/L, respectively. Patients in the tertile 3 group had a significantly higher level of AST than those in the tertile 1 group (p = 0.032). In addition, patients in the tertile 1 group had a significantly higher level of ALT than those in the tertile 2 and 3 groups (115.9 ± 158.1 U/L vs. 74.5 ± 107.0 U/L and 61.9 ± 86.0 U/L, p < 0.001). The increased De Ritis ratio in trauma patients with thoracoabdominal injuries was mainly attributed to elevated AST levels. The propensity score-matched patient cohorts revealed that the patients in the tertile 3 group presented a 3.89-fold higher risk of mortality than the patients in the tertile 2 group. In contrast, the patients in the tertile 1 group did not have a significantly different mortality rate than those in the tertile 2 group. This study suggests that a De Ritis ratio > 1.64 may be a useful biomarker to identify patients with a higher risk for mortality.
Keywords: mortality, thoracoabdominal trauma, aspartate aminotransferase (AST), alanine aminotransferase (ALT), De Ritis ratio
1. Introduction
Thoracoabdominal trauma is one of the main causes of mortality in trauma patients, and abdominal and thoracic trauma injuries are the second and third most common causes, respectively, of mortality in polytrauma patients [1]. For those patients with thoracoabdominal trauma, older age, higher Injury Severity Score (ISS), lower Glasgow Coma Scale (GCS) score, massive blood transfusion, initial hypotension, injuries to the liver, heart, and abdominal great vessels were identified as independent risk factors for mortality [2]. In a study of 1661 patients with thoracoabdominal trauma, the mortality rate after excluding patients with severe head trauma was 4.5% [2]. However, when patients sustained severe thoracoabdominal trauma, the 30-day mortality rate increased to 42.5% [3]. Therefore, the identification of patients at a high risk of mortality among those with thoracoabdominal trauma is important.
Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are well-known liver enzymes and blood-based circulating biomarkers [4,5] that are widely used to find out liver function in clinical settings and identify liver diseases such as viral hepatitis and alcohol addiction [4,5,6,7]. While ALT is predominantly detected only in the liver, AST is widely noticed in many organs, such as the liver, heart, kidney, brain, and skeletal muscle [8]; therefore, the contributions of these two enzymes may differ in various illnesses and can be used to distinguish among organ disorders, as ALT specifically indicates liver disease, whereas AST is associated with other organs affected in many illnesses [8]. The AST/ALT ratio, also called the De Ritis ratio [9], was first proposed in 1957 to differentiate various causes of liver disease [8,10,11]. Furthermore, the De Ritis ratio has been demonstrated to be an independent prognostic predictor for many kinds of malignancies [12,13,14,15] and non-malignant diseases, such as nonalcoholic fatty liver disease (NAFLD) [8,16], heart diseases [17,18,19], acute kidney injury [19,20,21], sepsis [22], and even in patients with COVID-19 [23,24,25,26].
Although the ratio of ALT to some other liver-related enzymes, such as gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), and glutamate dehydrogenase activity (GDH) had been widely investigated, the only enzyme ratio that has proven acceptable and is still widely used is the De Ritis ratio [8]. However, the De Ritis ratio has not yet been undertaken in the trauma population, except for two studies demonstrating that the De Ritis ratio is useful for predicting survival in patients with major burn injuries [27] and following burn surgery [28]. Therefore, this retrospective study was designed to identify the association between the De Ritis ratio on admission and mortality outcomes in adult thoracoabdominal trauma patients.
2. Materials and Methods
2.1. Study Population and Data Collection
The Institutional Review Board (IRB) of Chang Gung Memorial Hospital had approved the study with the approval number 202100842B0 before the implementation of the study. Due to the retrospective design of this study, the requirement for informed consent was waived by the IRB. In this study, only hospitalized adult trauma patients with thoracoabdominal injury, defined as an abbreviated injury scale (AIS) ≥ 1 in the thoracic and abdominal regions between 1 January 2009 and 31 December 2019, were included. To attenuate the potentially lethal outcome associated with concurrent traumatic brain injury, those with head AIS ≥ 3 and patients with burns, and those lacking AST or ALT data were not included in the study. In this study, of the 39,135 trauma patients hospitalized for treatment, 4683 had thoracoabdominal injuries (Figure 1). Among these, 4286 adult patients with age ≥ 20 years were included. After excluding patients with head AIS ≥ 3 (n = 1338), burn injuries (n = 5), and incomplete AST or ALT data (n = 695), 2248 adult patients with thoracoabdominal injuries were included in the study population. The study population was grouped into three tertile groups, an approach commonly used in the literature [17,23,29,30,31], according to the De Ritis ratio (tertile 1 group, <1.20, n = 749; tertile 2 group, 1.20–1.64, n = 749; and tertile 3 group, > 1.64, n = 750). The medical information of the patients was collected from the registered trauma database of the hospital [32,33,34]. These included sex, age, preexisting comorbidities, ISS, serum AST and ALT levels (U/L) on admission, De Ritis ratio, and in-hospital mortality. The patients’ illnesses of diabetes mellitus (DM), hypertension (HTN), coronary artery disease (CAD), cerebrovascular accident (CVA), congested heart failure (CHF), and end-stage renal disease (ESRD) were considered as those preexisting comorbidities.
Figure 1.
Flowchart illustrating the inclusion of hospitalized adult thoracoabdominal injury patients from the registered trauma database, with the assignment of the study patient populations into three groups according to the De Ritis ratio.
2.2. Statistical Analysis
In this study, the statistical software of Windows SPSS version 23.0 (IBM Inc., Chicago, IL, USA) was used for all statistical analyses. Two-sided Fisher’s exact test or Pearson’s χ2 test was used to analyze the categorical data. The normalization of the distributed continuous was evaluated by the Kolmogorov–Smirnov test. For continuous data with a normal distribution, the analysis of variance was performed with Bonferroni post hoc correction, while the Mann–Whitney U test was used to analyze the non-normally distributed continuous data. The continuous and noncontinuous data are expressed as mean ± standard deviation or median with interquartile range (IQR; Q1–Q3), respectively. To attenuate the confounding effect of patients’ characteristics, such as sex, age, pre-existing comorbidities, and injury severity, on the mortality outcome assessment, a 1:1 propensity score-matched study group using the Greedy method with a 0.2 caliper width, was created for the comparison of the patients who had a De Ritis ratio > 1.64 (tertile 3 group) or De Ritis ratio < 1.2 (tertile 1 group) with the patients with a De Ritis ratio of 1.2–1.64 (tertile 2 group). In this study, the primary outcome of this study was in-hospital mortality. The cut-off value of the De Ritis ratio that could predict the mortality risk of the studied population was calculated by plotting the receiver operating characteristic (ROC) curves, and the predictive performance was determined according to the area under the receiver operating characteristic curve (AUC). The maximal Youden index (defined as sensitivity + specificity − 1) was performed to determine the accuracy of the parameter in predicting mortality outcomes. In the condition of a two-tailed p-value < 0.05, the analyses are considered significant.
3. Results
3.1. Patient and Injury Characteristics
As shown in Table 1, there were significantly more male patients in the tertile 1 and tertile 3 groups than in the tertile 2 group. More patients with a significantly younger age were found in the tertile 1 group than in the other two groups (p < 0.001). Regarding the liver enzymes, there was no significant difference in the AST level between the patients in the tertile 1 group (115.8 ± 174.9 U/L), tertile 3 group (140.5 ± 209.7 U/L), and those in the tertile 2 group (115.7 ± 262.0 U/L); however, patients in the tertile 3 group had a significantly higher level of AST than those in the tertile 1 group (140.5 ± 209.7 vs. 115.8 ± 174.9 U/L, p = 0.032). In addition, patients in the tertile 1 group had significantly higher levels of ALT than patients in the tertile 2 and tertile 3 groups (115.9 ± 158.1 U/L vs. 74.5 ± 107.0 U/L and 61.9 ± 86.0 U/L, p < 0.001); however, there was no significant difference in the ALT levels between the patients in the tertile 3 group (61.9 ± 86.0 U/L) and tertile 2 group (74.5 ± 107.0 U/L). These results implied that an increased De Ritis ratio could be attributed to a higher AST level. No significant differences in preexisting comorbidities among the three groups of patients were observed. A significantly higher ISS was observed in patients in the tertile 3 group than in the tertile 2 group (median [IQR, Q3–Q3]: 13 [9,10,11,12,13,14,15,16,17,18] vs. 13 [8,9,10,11,12,13,14,15,16,17,18], respectively; p < 0.001). In addition, a significantly higher ISS was observed in patients in the tertile 2 group than in the tertile 1 group (median [IQR]: 13 [8,9,10,11,12,13,14,15,16,17,18] vs. 10 [8,9,10,11,12,13,14,15,16], respectively; p < 0.001). When the injury severity of patients was stratified by an ISS of 16–24 or an ISS ≥ 25, there were no significant differences in patient proportions among these groups; however, significantly more patients in the tertile 1 group had an ISS of 1–15, but fewer had an ISS of 16–24 than those in the tertile 2 group. The patients in the tertile 3 group had a significantly higher mortality rate than those in the tertile 2 group (3.7% vs. 1.3%, p = 0.004), but there was no difference in mortality between patients in the tertile 1 and tertile 2 groups (1.6% vs. 1.3%, p = 0.988).
Table 1.
Patient and injury characteristics of the study population according to the De Ritis ratio.
| De Ritis Ratio | ||||
|---|---|---|---|---|
| Variables | <1.20 (Tertile 1) n = 749 |
1.20–1.64 (Tertile 2) n = 749 |
>1.64 (Tertile 3) n = 750 |
p |
| Gender | 0.001 | |||
| Male, n (%) | 558 (69.8) * | 474 (61.2) | 456 (67.7) * | |
| Female, n (%) | 242 (30.2) * | 300 (38.8) | 218 (32.3) * | |
| Age (years) | 47.0 ± 16.4 * | 50.4 ± 17.6 | 49.9 ± 18.1 | <0.001 |
| AST (U/L) | 115.8 ± 174.9 | 115.7 ± 262.0 | 140.5 ± 209.7 | 0.049 |
| ALT (U/L) | 115.9 ± 158.1 * | 74.5 ± 107.0 | 61.9 ± 86.0 | <0.001 |
| Comorbidities | ||||
| DM, n (%) | 104 (13.0) | 88 (11.4) | 79 (11.7) | 0.580 |
| HTN, n (%) | 181 (22.6) | 160 (20.7) | 160 (23.7) | 0.361 |
| CAD, n (%) | 16 (2.0) | 20 (2.6) | 13 (1.9) | 0.633 |
| CVA, n (%) | 7 (0.9) | 12 (1.6) | 16 (2.4) | 0.068 |
| CHF, n (%) | 2 (0.2) | 4 (0.5) | 5 (0.7) | 0.399 |
| ESRD, n (%) | 10 (1.2) | 4 (0.5) | 6 (0.9) | 0.301 |
| ISS, median (IQR) | 10 (8–16) * | 13 (8–18) | 13 (9–18) * | <0.001 |
| 1–15, n (%) | 581 (72.6) * | 508 (65.6) | 413 (61.3) | <0.001 |
| 16–24, n (%) | 161 (20.1) * | 196 (25.3) | 189 (28.0) | 0.001 |
| ≥25, n (%) | 58 (7.2) | 70 (9.0) | 72 (10.7) | 0.069 |
| Mortality, n (%) | 13 (1.6) | 10 (1.3) | 25 (3.7) * | 0.003 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CVA, cerebral vascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; GCS, Glasgow Coma Scale; HTN, hypertension; IQR, interquartile range; ISS, injury severity score; OR, odds ratio. * indicated p < 0.05 when compared with patients with a De Ritis ratio between 1.20–1.64 (Tertile 2 group).
3.2. Adjusted Outcomes of the Propensity Score-Matched Patients
After 1:1 propensity score-matched analysis, 647 and 604 well-balanced pairs of patients were selected from the tertile 3 and tertile 1 groups, respectively, versus the tertile 2 group (Table 2 and Table 3, respectively). Both propensity score-matched patient cohorts revealed no significant differences in sex, age, comorbidities, and ISS. The patients in the tertile 3 group presented with a 3.89-fold higher risk of mortality (95% confidence interval [CI] 1.44–10.50; p = 0.004) than those in the tertile 2 group. In contrast, the patients in the tertile 1 group did not have a significantly different mortality rate from those in the tertile 2 group (odds ratio [OR], 1.85; 95% CI 0.68–5.03, p = 0.222).
Table 2.
Propensity score matched-cohort of patients in the tertile 3 vs. tertile 2 groups.
| Propensity Score Matched-Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| De Ritis Ratio | OR (95% CI) | p | Standardized Difference | |||||
| >1.64 (Tertile 3) n = 604 |
1.20–1.64 (Tertile 2) n = 604 |
|||||||
| Male, n (%) | 403 | (66.7) | 403 | (66.7) | 1.00 | (0.79–1.27) | 1.000 | 0.00% |
| Age (years) | 48.9 | ±17.9 | 48.7 | ±18.0 | - | 0.839 | 1.17% | |
| DM, n (%) | 59 | (9.8) | 59 | (9.8) | 1.00 | (0.68–1.46) | 1.000 | 0.00% |
| HTN, n (%) | 126 | (20.9) | 126 | (20.9) | 1.00 | (0.76–1.32) | 1.000 | 0.00% |
| CAD, n (%) | 8 | (1.3) | 8 | (1.3) | 1.00 | (0.37–2.68) | 1.000 | 0.00% |
| CVA, n (%) | 6 | (1.0) | 6 | (1.0) | 1.00 | (0.32–3.12) | 1.000 | 0.00% |
| CHF, n (%) | 1 | (0.2) | 1 | (0.2) | 1.00 | (0.06–16.02) | 1.000 | 0.00% |
| ESRD, n (%) | 0 | (0.0) | 0 | (0.0) | - | - | - | |
| GCS | 15 | (15–15) | 15 | (15–15) | - | 0.479 | −4.07% | |
| ISS | 13 | (9–18) | 13 | (9–18) | - | 0.966 | −0.24% | |
| Mortality | 19 | (3.1) | 5 | (0.8) | 3.89 | (1.44–10.50) | 0.004 | - |
CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CVA, cerebral vascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; HTN, hypertension; IQR, interquartile range; ISS, injury severity score; OR, odds ratio.
Table 3.
Propensity score matched-cohort of patients in the tertile 1 vs. tertile 2 groups.
| Propensity Score Matched-Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|
| De Ritis Ratio | OR (95% CI) | p | Standardized Difference | |||||
| <1.20 (Tertile 1) n = 647 |
1.20–1.64 (Tertile 2) n = 647 |
|||||||
| Male, n (%) | 426 | (65.8) | 426 | (65.8) | 1.00 | (0.80–1.26) | 1.000 | 0.00% |
| Age (years) | 48.0 | ±16.2 | 48.6 | ±16.7 | - | 0.539 | −3.42% | |
| DM, n (%) | 71 | (11.0) | 71 | (11.0) | 1.00 | (0.71–1.42) | 1.000 | 0.00% |
| HTN, n (%) | 136 | (21.0) | 136 | (21.0) | 1.00 | (0.77–1.31) | 1.000 | 0.00% |
| CAD, n (%) | 9 | (1.4) | 9 | (1.4) | 1.00 | (0.39–2.54) | 1.000 | 0.00% |
| CVA, n (%) | 4 | (0.6) | 4 | (0.6) | 1.00 | (0.25–4.02) | 1.000 | 0.00% |
| CHF, n (%) | 0 | (0.0) | 0 | (0.0) | - | - | - | |
| ESRD, n (%) | 2 | (0.3) | 2 | (0.3) | 1.00 | (0.14–7.12) | 1.000 | 0.00% |
| GCS | 15 | (15–15) | 15 | (15–15) | - | 0.655 | −2.48% | |
| ISS | 11 | (8–17) | 12 | (8–17) | - | 0.703 | −2.12% | |
| Mortality | 11 | (1.7) | 6 | (0.9) | 1.85 | (0.68–5.03) | 0.222 | - |
CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CVA, cerebral vascular accident; DM, diabetes mellitus; ESRD, end-stage renal disease; GCS, Glasgow Coma Scale; HTN, hypertension; ISS, injury severity score; OR, odds ratio.
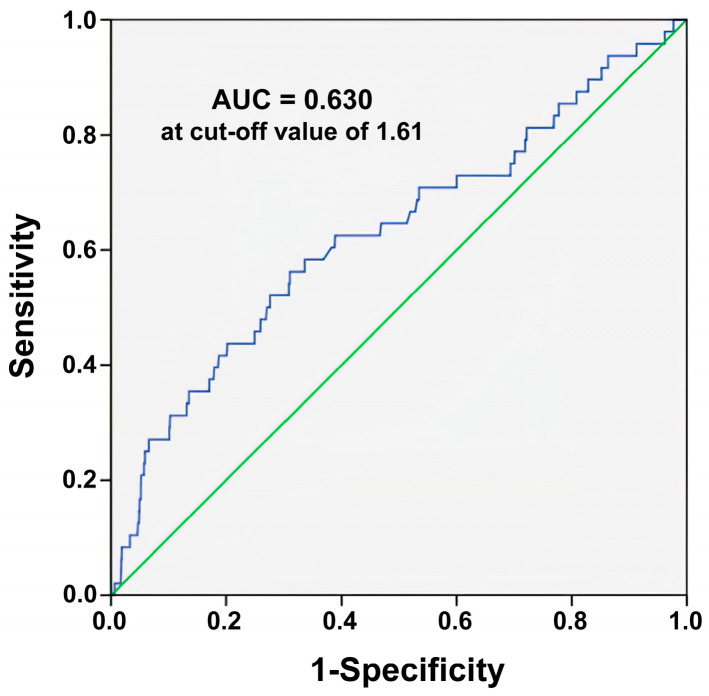
3.3. ROC Curve Analysis
The ROC curve analysis (Figure 2) determined a De Ritis ratio of 1.61 is the best cut-off value for predicting mortality outcomes, with AUCs of 0.630, 68.51% accuracy, 56.3% sensitivity, and 69.0% specificity. The best cut-off value of 1.61, identified from the ROC curve, is close to the cut-off value of 1.64, which defines the patients assigned to the tertile 3 group. Although the mortality prediction relying solely on the De Ritis ratio is not good, it is better than that with an uninformative classifier yielding 0.5.
Figure 2.
Identification of the cut-off values for predicting mortality based on the De Ritis ratio using the receiver operating characteristic curve analysis. Blue line is the plotted line of the De Ritis ratio; Green line indicates the reference line when AUC is equal to 0.5.
4. Discussion
Although the simple limit of the De Ritis ratio has been determined for clinical decisions in some illnesses (e.g., >2.0 for alcoholic hepatitis [35,36]), there are no commonly accepted reference intervals for the De Ritis ratio. In addition, the healthy limits for this ratio are yet to be ascertained. The useful cutoff value of the De Ritis ratio may depend on the studied illness. This study demonstrated that thoracoabdominal trauma patients with a De Ritis ratio > 1.64 were associated with a 3.89-fold higher risk of mortality than the propensity score-matched patient cohort in the tertile 2 group, while there was no significant difference in mortality rate between the patients in the tertile 1 and tertile 2 groups. Similar reports have been found in those articles published by Yin et al., who studied the mortality rate for adult patients with secondary hemophagocytic lymphohistiocytosis [29], by Zinellu et al., who investigated the survival probability of patients with COVID-19 during hospitalization [23], and by Lu et al., who surveyed the ICU mortality and unfavorable neurological outcome of patients with cardiac arrest [17]. Furthermore, although the patients in the tertile 1 group had significantly higher levels of ALT than those patients in the tertile 2 and tertile 3 groups, the patients in the tertile 1 group did not have a significantly different mortality rate from those in the tertile 2 group, implying that an increased AST/ALT ratio but not elevated ALT level was associated with worse patient outcomes. Therefore, for patients with thoracoabdominal trauma, a De Ritis ratio > 1.64 may be a useful biomarker to identify patients with a higher risk of sustaining mortality.
In thoracoabdominal trauma, the De Ritis ratio relies on changes in the AST and ALT levels during injury. Notably, ALT is present only in the cytoplasm of hepatocytes, whereas AST is found in both the cytoplasm and mitochondria of the hepatocytes [8]. Although the functions of both transaminases speak for important metabolic links between proteins and carbohydrates, ALT is involved in the glucose-alanine cycle to produce glucose to encounter glucose consumption, while AST plays a vital role in aerobic glycolysis [8]. For liver injury, when the death of hepatocytes is increased beyond the usual background level, the serum level of AST would indicate the cellular condition where AST is more than twice that of ALT [37]. However, only a few patients with thoracoabdominal injuries may have sustained liver injury. In a study of 1661 patients with thoracoabdominal trauma, the overall incidence of solid organ injury within the abdomen was 59.7% [2]. Considering that ALT is detected predominantly only in the liver and AST is broadly released from many organs such as the liver, heart, kidney, brain, and skeletal muscle [8], the increased level of AST may be attributed to injury to other organs or to the response of these organs against the stress associated with the trauma injury.
In this study, we used propensity score-matched patient cohorts to attenuate the effect of differences in sex, age, comorbidities, and ISS on the mortality of the patients; however, the matched patients in the tertile 3 group still presented with an around four-fold higher risk of mortality than those in the tertile 2 group, indicating that the De Ritis ratio helps to stratify the thoracoabdominal injuries in patients with a high risk for mortality. Nonetheless, this study had some limitations that should be mentioned. First, some selection bias may exist in the retrospective nature of the study. In addition, a selection bias may exist in the outcome measurement since this study only assessed in-hospital mortality but not long-term mortality, and some patients were excluded without data for AST and ALT levels. Furthermore, the presence of undetected liver disease may exist in the study population cohort, leading to a change in the De Ritis ratio, regardless of thoracoabdominal trauma. Moreover, some interventions such as damage control, resuscitation, and operation may result in different outcomes for the patients and were not controlled for further analysis. However, we can only assume that the outcomes of these interventions were uniform across the studied patient population. Finally, the results of this study were limited to a single urban trauma center and may not be generalizable to other areas.
5. Conclusions
This study demonstrated that the De Ritis ratio may be a useful tool to recognize the high mortality risk in adult patients with thoracoabdominal trauma. The investigation of the mechanism behind the AST and ALT changes upon trauma injury would help a more precise application of the De Ritis ratio in the clinical setting. In addition, it would be interesting to study whether this ratio could be used in the stratification of major complications other than mortality and used for patients with trauma injuries other than thoracoabdominal trauma.
Acknowledgments
We appreciate the assistance of the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital, for statistical analyses.
Author Contributions
Conceptualization, C.-H.H.; validation, H.-T.L.; formal analysis, S.-Y.H.; resources, S.-E.C.; data curation, C.-H.T.; writing—original draft preparation, W.-T.S. and C.-S.R.; writing—review and editing, W.-T.S.; supervision, C.-H.H.; funding acquisition, W.-T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chang Gung Memorial Hospital (protocol code 202100842B0 and date of approval 9 June 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of this study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Chang Gung Memorial Hospital, grant number CMRPG8L1251.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lecky B.O., Alexandrescu R., O’Brien S.J. Changing Epidemiology of Polytrauma. Volume 3. Springer; New York, NY, USA: 2017. pp. 27–32. [Google Scholar]
- 2.Berg R.J., Okoye O., Teixeira P.G., Inaba K., Demetriades D. The double jeopardy of blunt thoracoabdominal trauma. Arch. Surg. 2012;147:498–504. doi: 10.1001/archsurg.2011.2289. [DOI] [PubMed] [Google Scholar]
- 3.Demir B., Şaşmaz M., Saglam Gurmen E., Bilge A. Prognostic value of lactate to hematocrit ratio score in patients with severe thoracoabdominal trauma. Ulus. Travma Acil Cerrahi Derg. 2022;28:927–932. doi: 10.14744/tjtes.2021.51189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriles K.E., Azer S.A. StatPearls. StatPearls Publishing, StatPearls Publishing LLC.; Treasure Island, FL, USA: 2022. Alanine Amino Transferase. [Google Scholar]
- 5.Otto-Ślusarczyk D., Graboń W., Mielczarek-Puta M. Aspartate aminotransferase--key enzyme in the human systemic metabolism. Postepy Hig. Med. Dosw. 2016;70:219–230. doi: 10.5604/17322693.1197373. [DOI] [PubMed] [Google Scholar]
- 6.Suciu A., Abenavoli L., Pellicano R., Luzza F., Dumitrascu D.L. Transaminases: Oldies but goldies. A narrative review. Minerva Gastroenterol. Dietol. 2020;66:246–251. doi: 10.23736/S1121-421X.20.02660-4. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe P.C. Biochemical detection and monitoring of alcohol abuse and abstinence. Ann. Clin. Biochem. 2001;38:652–664. doi: 10.1258/0004563011901064. [DOI] [PubMed] [Google Scholar]
- 8.Botros M., Sikaris K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013;34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 9.De Ritis F., Coltorti M., Giusti G. An enzymic test for the diagnosis of viral hepatitis: The transaminase serum activities. 1957. Clin. Chim. Acta. 2006;369:148–152. doi: 10.1016/j.cca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Mo Q., Liu Y., Zhou Z., Li R., Gong W., Xiang B., Tang W., Yu H. Prognostic Value of Aspartate Transaminase/Alanine Transaminase Ratio in Patients With Hepatitis B Virus-Related Hepatocellular Carcinoma Undergoing Hepatectomy. Front. Oncol. 2022;12:876900. doi: 10.3389/fonc.2022.876900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darstein F., Häuser F., Straub B.K., Wenzel J.J., Conradi R., Mittler J., Lang H., Galle P.R., Zimmermann T. Hepatitis E virus genotype 3 is a common finding in liver-transplanted patients undergoing liver biopsy for elevated liver enzymes with a low De Ritis ratio and suspected acute rejection: A real-world cohort. Clin. Transpl. 2018;32:e13411. doi: 10.1111/ctr.13411. [DOI] [PubMed] [Google Scholar]
- 12.Ghahari M., Salari A., Ghafoori Yazdi M., Nowroozi A., Fotovat A., Momeni S.A., Nowroozi M.R., Amini E. Association Between Preoperative De Ritis (AST/ALT) Ratio and Oncological Outcomes Following Radical Cystectomy in Patients With Urothelial Bladder Cancer. Clin. Genitourin Cancer. 2022;20:e89–e93. doi: 10.1016/j.clgc.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Fukui-Kawaura S., Kawahara T., Araki Y., Nishimura R., Uemura K., Namura K., Mizuno N., Yao M., Uemura H., Ikeda I. A higher De Ritis ratio (AST/ALT) is a risk factor for progression in high-risk non-muscle invasive bladder cancer. Oncotarget. 2021;12:917–922. doi: 10.18632/oncotarget.27944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janisch F., Klotzbücher T., Marks P., Kienapfel C., Meyer C.P., Yu H., Fühner C., Hillemacher T., Mori K., Mostafei H., et al. Predictive value of De Ritis ratio in metastatic renal cell carcinoma treated with tyrosine-kinase inhibitors. World J. Urol. 2021;39:2977–2985. doi: 10.1007/s00345-021-03628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olcucu M.T., Karamik K., Yilmaz K., Okuducu Y., Cakir S., Ates M. Preoperative Inflammation Markers and De Ritis Ratio in Predicting Clinical Presentation and Prognosis of Patients with Testicular Germ Cell Tumors. J. Coll. Physicians Surg. Pak. 2020;30:1041–1046. doi: 10.29271/jcpsp.2020.10.1041. [DOI] [PubMed] [Google Scholar]
- 16.Lazo M., Clark J.M. The epidemiology of nonalcoholic fatty liver disease: A global perspective. Semin. Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z., Ma G., Chen L. De-Ritis Ratio Is Associated with Mortality after Cardiac Arrest. Dis. Markers. 2020;2020:8826318. doi: 10.1155/2020/8826318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucukseymen S., Cekin A.H., Bayar N., Arslan S., Uygur Kucukseymen E., Mercan T., Ozdemir S. A novel biomarker for prediction of atrial fibrillation susceptibility in patients with celiac disease. PLoS ONE. 2018;13:e0190382. doi: 10.1371/journal.pone.0190382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H.M., He C., Zhang S.C., You Z.B., Lin X.Q., Luo M.Q., Lin M.Q., Guo Y.S., Zheng W.P., Lin K.Y. Predictive value of aspartate aminotransferase-to-alanine aminotransferase ratio for contrast-associated acute kidney injury in patients undergoing elective percutaneous coronary intervention. J. Cardiol. 2022;79:618–625. doi: 10.1016/j.jjcc.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Pilarczyk K., Carstens H., Heckmann J., Canbay A., Koch A., Pizanis N., Jakob H., Kamler M. The aspartate transaminase/alanine transaminase (DeRitis) ratio predicts mid-term mortality and renal and respiratory dysfunction after left ventricular assist device implantation. Eur. J. Cardiothorac. Surg. 2017;52:781–788. doi: 10.1093/ejcts/ezx247. [DOI] [PubMed] [Google Scholar]
- 21.Park J.Y., Yu J., Hong J.H., Lim B., Kim Y., Hwang J.H., Kim Y.K. Elevated De Ritis Ratio as a Predictor for Acute Kidney Injury after Radical Retropubic Prostatectomy. J. Pers. Med. 2021;11:836. doi: 10.3390/jpm11090836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao P.Y., Yao R.Q., Ren C., Li S.Y., Li Y.X., Zhu S.Y., Yao Y.M., Du X.H. De Ritis Ratio as a Significant Prognostic Factor in Patients with Sepsis: A Retrospective Analysis. J. Surg. Res. 2021;264:375–385. doi: 10.1016/j.jss.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Zinellu A., Arru F., De Vito A., Sassu A., Valdes G., Scano V., Zinellu E., Perra R., Madeddu G., Carru C., et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur. J. Clin. Investig. 2021;51:e13427. doi: 10.1111/eci.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Lukito A.A., Nasution S.A., Siswanto B.B., Kuswardhani R.A.T. Elevated De Ritis Ratio Is Associated With Poor Prognosis in COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2021;8:676581. doi: 10.3389/fmed.2021.676581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzey-Aras Y., Yazar H., Acar T., Kayacan Y., Acar B.A., Boncuk S., Eryilmaz H.A. The Role of De Ritis Ratio as a Clinical Prognostic Parameter in COVID 19 Patients. Clin. Lab. 2021;67 doi: 10.7754/Clin.Lab.2021.210119. [DOI] [PubMed] [Google Scholar]
- 26.Yashashwini A., Vedavathi R. The Study of De Ritis (Ast/Alt) Ratio in Comparision with Other Parameters for Predicting Poor Prognosis in Covid 19 Patients. J. Assoc. Physicians India. 2022;70:11–12. [Google Scholar]
- 27.Wang B., Hu L., Chen Y., Zhu B., Kong W., Zhu Z., Wang K., Yu Q., Zhang W., Wu G., et al. Aspartate transaminase/alanine transaminase (De Ritis ratio) predicts survival in major burn patients. Burns. 2022;48:872–879. doi: 10.1016/j.burns.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Yu J., Kim H.Y., Kong Y.G., Park J.H., Seo Y.J., Kim Y.K. De Ritis ratio as a predictor of 1-year mortality after burn surgery. Burns. 2021;47:1865–1872. doi: 10.1016/j.burns.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Yin G., Man C., Liao S., Qiu H. The Prognosis Role of AST/ALT (De Ritis) Ratio in Patients with Adult Secondary Hemophagocytic Lymphohistiocytosis. Mediat. Inflamm. 2020;2020:5719751. doi: 10.1155/2020/5719751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steininger M., Winter M.P., Reiberger T., Koller L., El-Hamid F., Forster S., Schnaubelt S., Hengstenberg C., Distelmaier K., Goliasch G., et al. De-Ritis Ratio Improves Long-Term Risk Prediction after Acute Myocardial Infarction. J. Clin. Med. 2018;7:474. doi: 10.3390/jcm7120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rief P., Pichler M., Raggam R., Hafner F., Gerger A., Eller P., Brodmann M., Gary T. The AST/ALT (De-Ritis) ratio: A novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. Medicine. 2016;95:e3843. doi: 10.1097/MD.0000000000003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh C.H., Hsu S.Y., Hsieh H.Y., Chen Y.C. Differences between the sexes in motorcycle-related injuries and fatalities at a Taiwanese level I trauma center. Biomed. J. 2017;40:113–120. doi: 10.1016/j.bj.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh C.H., Liu H.T., Hsu S.Y., Hsieh H.Y., Chen Y.C. Motorcycle-related hospitalizations of the elderly. Biomed. J. 2017;40:121–128. doi: 10.1016/j.bj.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh C.H., Chen Y.C., Hsu S.Y., Hsieh H.Y., Chien P.C. Defining polytrauma by abbreviated injury scale >/= 3 for a least two body regions is insufficient in terms of short-term outcome: A cross-sectional study at a level I trauma center. Biomed. J. 2018;41:321–327. doi: 10.1016/j.bj.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Opio C.K., Seremba E., Ocama P., Lalitha R., Kagimu M., Lee W.M. Diagnosis of alcohol misuse and alcoholic liver disease among patients in the medical emergency admission service of a large urban hospital in Sub-Saharan Africa; a cross sectional study. Pan. Afr. Med. J. 2013;15:23. doi: 10.11604/pamj.2013.15.23.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torkadi P.P., Apte I.C., Bhute A.K. Biochemical Evaluation of Patients of Alcoholic Liver Disease and Non-alcoholic Liver Disease. Indian J. Clin. Biochem. 2014;29:79–83. doi: 10.1007/s12291-013-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldstein A.E., Canbay A., Angulo P., Taniai M., Burgart L.J., Lindor K.D., Gores G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.