Keywords: asthma, 3-bromotyrosine, eosinophil, exacerbation
Abstract
Asthma is an inflammatory disease of the airways characterized by eosinophil recruitment, eosinophil peroxidase release, and protein oxidation through bromination, which following tissue remodeling results in excretion of 3-bromotyrosine. Predicting exacerbations and reducing their frequency is critical for the treatment of severe asthma. In this study, we aimed to investigate whether urinary total conjugated bromotyrosine can discriminate asthma severity and predict asthma exacerbations. We collected urine from participants with severe (n = 253) and nonsevere (n = 178) asthma, and the number of adjudicated exacerbations in 1-yr longitudinal follow-up was determined among subjects enrolled in the Severe Asthma Research Program, a large-scale National Institutes of Health (NIH)-funded consortium. Urine glucuronidated bromotyrosine and total conjugated forms were quantified by hydrolysis with either glucuronidase or methanesulfonic acid, respectively, followed by liquid chromatography-tandem mass spectrometry analyses of free 3-bromotyrosine. Blood and sputum eosinophils were also counted. The majority of 3-bromotyrosine in urine was found to exist in conjugated forms, with glucuronidated bromotyrosine representing approximately a third, and free bromotyrosine less than 1% of total conjugated bromotyrosine. Total conjugated bromotyrosine was poorly correlated with blood (r2 = 0.038) or sputum eosinophils (r2 = 0.0069). Compared with participants with nonsevere asthma, participants with severe asthma had significantly higher urinary total conjugated bromotyrosine levels. Urinary total conjugated bromotyrosine was independently associated with asthma severity, correlated with the number of asthma exacerbations, and served as a predictor of asthma exacerbation risk over 1-yr of follow-up.
INTRODUCTION
Asthma is characterized by chronic inflammation of the airways and is one of the most common chronic diseases in the United States. Although some therapies are effective for nonsevere asthma, severe asthma is very difficult to treat.
Elevated levels of reactive oxygen and nitrogen species (ROS and RNS) are prevalent in asthmatic airways (1–5). Driven and dominated by type 2 cytokines, eosinophilic lung inflammation plays an important role in ROS production and tissue damage. Under physiological conditions, eosinophil peroxidase (EPO) can convert respiratory burst-generated hydrogen peroxide (H2O2) into hypobromous acid (HOBr), a reactive brominating oxidant that modifies protein tyrosine residues to form 3-bromotyrosine (BrTyr) (6). Previous studies have shown that eosinophils are increased in the sputum and peripheral blood of participants with asthma and can therefore serve as markers of active atopic inflammation (7, 8). Urinary levels of free BrTyr in turn have also been used as a biomarker of eosinophil activation and protein oxidation to predict asthma exacerbations in children (6, 9), and as a biomarker for asthma severity and corticosteroid responsiveness in general (10). Urine-free BrTyr is present at low levels, and its measurement consequently is difficult. Here, we initially investigated whether alternative conjugated forms of BrTyr exist in urine, and after discovering free BrTyr represents less than 1% of the total conjugated pool of BrTyr forms in urine, we tested whether total conjugated bromotyrosine (TCB) can serve as a sensitive and specific predictor of asthma exacerbation in adults or discriminate severe asthmatics from nonsevere asthmatics.
METHODS
Study Population
The study population consisted of 431 patients with asthma (aged > 18 yr) with available measures of urinary free BrTyr who were enrolled in SARP III. The Severe Asthma Research Program (SARP), funded by the National Heart, Lung, and Blood Institute, is a long-term cohort study on severe asthma (11–14). SARP III recruited participants between November 2012 and October 2015. Exclusion criteria included the following: current smokers (more than 5–10 pack years depending on age), other respiratory diseases, premature birth before 35 wk, gastroesophageal reflux, recurrent sinopulmonary infections or obstructive sleep apnea, and cancer in the past 5 years. The clinical characteristics of the study population are shown in Table 1.
Table 1.
Demographics of the subset of study participants who provided urine for TCB analyses by asthma severity
| Characteristics | Nonsevere Asthma |
Severe Asthma |
P Value | ||
|---|---|---|---|---|---|
| No. | Mean ± SD | No. | Mean ± SD | ||
| Age at enrollment, yr | 178 | 45.4 ± 14.6 | 253 | 50.0 ± 13.3 | 0.001 |
| Age when first diagnosed, yr | 178 | 19.2 ± 15.4 | 253 | 20.1 ± 16.6 | 0.56 |
| Female sex, % | 122/178 | 68.5 | 169/253 | 66.8 | 0.70 |
| Race, % | |||||
| European ancestry | 121/178 | 68.0 | 161/253 | 63.6 | 0.29 |
| African ancestry | 37/178 | 20.8 | 65/253 | 25.7 | 0.24 |
| Body mass index, kg/m2 | 178 | 30.8 ± 7.7 | 253 | 33.2 ± 8.7 | 0.004 |
| Blood pressure, mmHg | |||||
| Systolic | 178 | 120 ± 13 | 253 | 127 ± 14 | <0.001 |
| Diastolic | 178 | 75 ± 11 | 253 | 78 ± 10 | 0.005 |
| Lung functions | |||||
| Forced expiratory volume in 1 s, %predicted | 178 | 81.8 ± 16.9 | 253 | 67.5 ± 19.6 | <0.001 |
| Forced vital capacity, %predicted | 178 | 92.1 ± 15.8 | 253 | 80.0 ± 17.1 | <0.001 |
| FEV1/FVC | 178 | 0.72 ± 0.09 | 253 | 0.67 ± 0.10 | <0.001 |
| Provocative concentration of methacholine causing a 20% fall in FEV1, mg/mL | 63 | 3.4 ± 4.1 | 52 | 3.4 ± 4.2 | 0.94 |
| White blood cells, ×109/L | 178 | 6.7 ± 2.1 | 252 | 8.0 ± 2.8 | <0.001 |
| Neutrophils | 178 | 3.9 ± 1.8 | 252 | 4.8 ± 2.4 | <0.001 |
| Eosinophils | 178 | 0.25 ± 0.21 | 252 | 0.31 ± 0.30 | 0.008 |
| Platelets, ×109/L | 176 | 250 ± 69 | 250 | 261 ± 63 | 0.08 |
| IgE, IU/mL | 177 | 348 ± 655 | 251 | 346 ± 600 | 0.8 |
| Sputum eosinophils, ×106/L | 136 | 59 ± 248 | 193 | 100 ± 263 | 0.15 |
| Asthma quality of life questionnaire | 178 | 5.8 ± 1.0 | 253 | 4.8 ± 1.3 | <0.001 |
| Asthma control test score | 178 | 19.1 ± 3.8 | 253 | 15.7 ± 4.6 | <0.001 |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TCB, total conjugated bromotyrosine.
Asthma was verified based on American Thoracic Society (ATS) guidelines, which include positive methacholine challenge test and/or reversible airflow obstruction. Asthma was classified as severe or nonsevere based on ATS and European Respiratory Society (ERS) guidelines (15). Of the 431 recruited participants with asthma, 59% had severe asthma as defined by the American Thoracic Society (ATS) and European Respiratory Society (ERS), which includes treatment with high-dose inhaled corticosteroids plus a second controller and/or systemic corticosteroids to prevent asthma from becoming uncontrolled, or asthma that remains uncontrolled despite therapy (15). The remaining 41% of participants with asthma did not meet these criteria for severe asthma and were classified as nonsevere asthma. Asthma quality of life questionnaire was designed as described in Juniper et al. (16). Asthma control was assessed with the standardized and validated Asthma Control Test (ACT). A score of 19 or higher indicates well-controlled asthma. Asthma exacerbation is defined as an event of worsening asthma requiring treatment with systemic corticosteroids for 3 or more days to prevent a serious outcome (17, 18). The number of exacerbations was collected using standardized questionnaires at in-person visits during 1-yr longitudinal follow-up of asthmatics and also at 6-mo phone calls. The study protocol and procedures were approved by the Institutional Review Board (IRB) at each participating center and by an independent Data Safety Monitoring Board. All subjects provided written informed consent and/or assent. The study was registered on https://clinicaltrials.gov (NCT01750411).
Urine BrTyr Determination
Urine samples were collected in the morning, and all measurements of urine samples were normalized by concurrently measured urine creatinine concentrations to account for differences in hydration. Urine creatinine was measured by enzymatic method using automated chemical analyzer COBAS Integra 400 plus (Roche Diagnostics USA, Indianapolis, IN). For all BrTyr analyses, synthetic [13C6]-BrTyr (6) was added to samples as internal standard before initiation of sample preparations. Free urinary BrTyr was assayed using stable isotope dilution High-Performance Liquid Chromatography (HPLC) with online electrospray ionization tandem mass spectrometry as previously described (9).
Glucuronidated 3-bromotyrosine (Glc-BrTyr) was measured by first treating urine samples with 150 units/mL glucuronidase (Sigma, Cat. No. G0751) in 0.3 M ammonium acetate buffer, pH 5.0, at 45°C for 16 h. Total conjugated bromotyrosine (TCB; Fig. 1A) was measured by releasing BrTyr from its acid labile conjugated forms by incubation with 6.6 M methanesulfonic acid (Sigma, Cat. No. 471356) in the presence of 1% phenol and 0.1% benzoic acid at 140°C for 4 h. Glc-BrTyr and TCB were then measured in the enzyme and acid hydrolysates, respectively, followed by solid phase extraction as described previously (6). In all studies, in addition to heavy isotope-labeled [13C6]BrTyr internal standard, universally heavy isotope-labeled [13C9,15N1]Tyrosine (Cambridge Isotope Laboratories, Inc., Cat. No. CNLM-439-H) was added at the start of sample preparations to enable simultaneous monitoring for potential artifactual bromination of endogenous urine tyrosine (detected as [13C9,15N1]BrTyr) and confirmed to represent <5% of endogenous BrTyr. The eluent from the solid phase extraction column was dried under SpeedVacuum and resuspended in 100-µL water for LC/MS/MS.
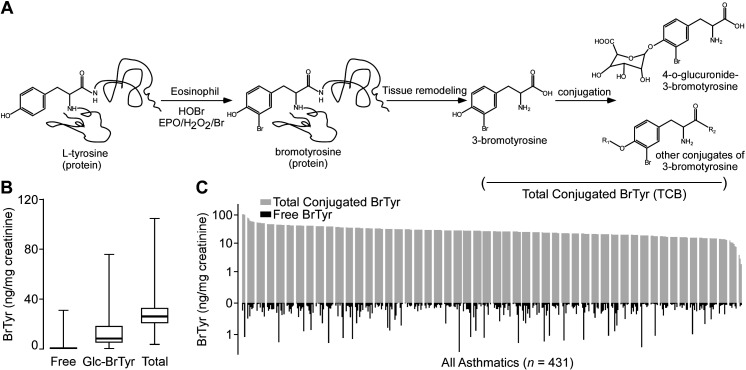
Figure 1.
3-Bromotyrosine (BrTyr) exists mainly in conjugated form in urine, which can be released by hydrolysis. A: protein-bound tyrosine can be oxidized to 3-bromotyrosine by activated eosinophils, which produce HOBr, a product of EPO in the presence of cosubstrates H2O2 and Br−. Protein-bound BrTyr is released during tissue remodeling and excreted in urine either as free form (<1%), 4-o-glucuronide conjugated form (Glc-BrTyr), or as a conjugate with other molecules (R1-, R2-conjugate). The total pool of conjugated BrTyr forms can be measured following release of BrTyr after acid hydrolysis. B: comparison of free, Glc-BrTyr, and TCB levels in subjects. Whiskers indicate minimum and maximum, line inside the Box indicates the median value, Box encompasses 25%–75%. n = 300 for each group. C: urinary levels of free and total (=free + conjugated) BrTyr in 431 participants with asthma (67.5% female). Total conjugated BrTyr was acquired by hydrolysis with methanesulfonic acid. BrTyr was determined by LC/MS/MS with [13C6] BrTyr added as internal standard. EPO, eosinophil peroxidase; HOBr, hypobromous acid; TCB, total conjugated bromotyrosine.
Supernatants (5 μL) were analyzed by injection onto a Titan C18 UHPLC Column (1.9 µm particle size, L × I.D. 10 cm × 2.1 mm, Supelco, Cat. No. 577124-U) at a flow rate of 0.4 mL/min using a 2 Shimadzu LC-20AD Nexera CL pump system, SIL-30AC MP CL autosampler interfaced with an Shimadzu 8050 mass spectrometer. A discontinuous gradient was generated to resolve the analytes by mixing solvent A (0.2% formic acid in water) with solvent B (0.2% formic acid in methanol) at different ratios starting from 0% B for 3 min, then linearly to 100% B over 3.5 min, then hold for 3 min, and then back to 0% B. Tyr, BrTyr, u-[13C9,15N1]-BrTyr, and the internal standard u-[13C9,15N1]Tyr, [13C6]-BrTyr were monitored using electrospray ionization in positive-ion mode with multiple reaction monitoring (MRM) of precursor and characteristic product-ion transitions of m/z 182 →77, 262 →135, 272→ 144, 192 →83, 268→ 141 amu, respectively. The parameters for the ion monitoring were optimized automatically. Nitrogen (99.95% purity) was used as the nebulizing gas and heating gas, and argon was used as collision gas. Various concentrations of nonisotopically labeled Tyr and BrTyr mix were loaded to 96-well plate undergoing the same procedure to prepare the calibration curves for quantification of Tyr and BrTyr, u-[13C9,15N1]-BrTyr, respectively. The internal standard [13C6]-BrTyr was used for quantification as well as to calculate recovery rate of BrTyr (which was >80% based on separate control studies). The artificial production of BrTyr was calculated as concentration of u-[13C9, 15N1]-BrTyr x concentration of Tyrosine/final concentration of u-[13C9, 15N1]Tyr spiked to urine, and can be deducted from the calculated BrTyr concentration (< 5%). The urine levels of total conjugated BrTyr forms, also defined as TCB, are presented as ratio relative to quantified urine creatinine.
Statistical Analysis
Data are presented as median (first quartile–third quartile) or means ± SD for continuous measures and as number (percentage) for categorical measures unless otherwise indicated. Comparisons of continuous measures between two independent groups were done using two-tailed Wilcoxon rank-sum tests (Mann–Whitney test) owing to the nonsymmetrical distribution of many of the measures considered. Comparison of categorical measures between independent groups was done using χ2 tests. Odds ratios were calculated with SPSS (version 11.0.1) using logistic regression with case status as the dependent variable and urine-free BrTyr or TCB quartiles as independent variable, and the adjusted factors include age, sex, blood, and sputum eosinophil number. The correlation analyses were done using Spearman’s Rho (2-tailed) and presented as a band with regression line and 95% confidence interval lines. For all statistical tests, P < 0.05 was considered significant.
RESULTS
3-Bromotyrosine Exists Mainly in Conjugated Forms in Urine
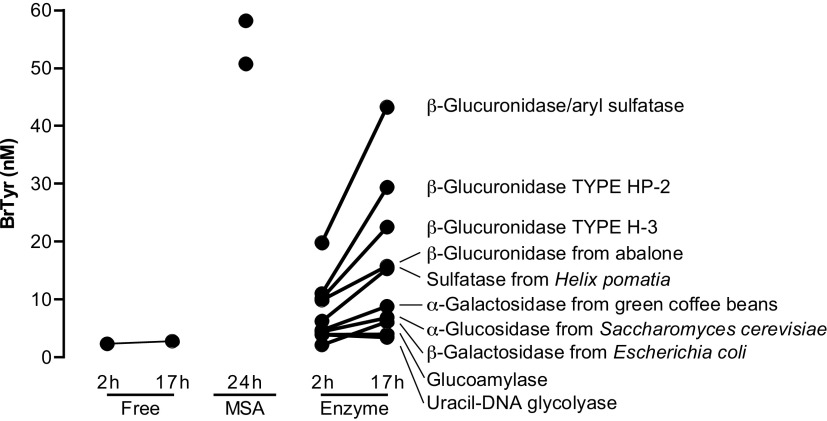
In initial studies, we hypothesized that BrTyr exists in free and conjugated forms released to urine after protein oxidative modification through bromination by reaction with HOBr, or catalyzed by EPO in the presence of cosubstrates H2O2 and Br- (19) (Fig. 1A), and we sought to test whether the free BrTyr detected in urine only represented a fraction of the total potential conjugated forms that might exist, and thus an underestimate of eosinophilic oxidant stress by eosinophil peroxidase-catalyzed bromination. We first incubated a pooled urine sample with different enzymes, glucoamylase, α-glucosidase, α-galactosidase, β-galactosidase, sulfatase, and β-glucuronidase, to test the other possible BrTyr conjugates, and each enzymatic reaction was carried out at different pH and different temperatures, to allow for selection of the maximum BrTyr released, representing free BrTyr released from the corresponding specific conjugate (Fig. 2). From these studies, we observed glucuronidated BrTyr may be a major conjugated form of BrTyr, with other conjugates including gluco-BrTyr, galacto-BrTyr, and possibly, BrTyr-sulfate since treatments with each of these enzyme activities resulted in significant increases in measurable free BrTyr. Then we incubated a second pooled urine sample with glucuronidase and observed a dramatic increase in free BrTyr released with incubation time (Fig. 3A). However, without enzymatic incubation, BrTyr looks stable during incubation (Fig. 3B). Those results suggest that BrTyr in urine may exist as glucuronidated conjugate, 4-o-glucuronide-3-bromotyrosine (Glc-BrTyr), to a large extent. We then recognized all the BrTyr conjugates could in theory be hydrolyzed to release free BrTyr under strong acid conditions—taking careful attention to avoiding conditions wherein trace levels of Br− might exist, leading to artificial bromination. We therefore developed acid hydrolysis methods that did not generate artificial bromination by use of a nonhalide containing strong acid, methanesulfonic acid (MSA; 4 M MSA, 110°C for 24 h) (6, 20, 21). Under these conditions, we noted even more free BrTyr was released, representing total conjugated BrTyr forms (TCB). We noted that with optimized glucuronidase conditions and MSA hydrolysis methods the original (pre-enzyme or acid hydrolysis) free BrTyr detected represented only <1% of TCB (Fig. 1, B and C), and that Glc-BrTyr represented approximately one-third of the TCB (Fig. 1B). We also observed that urine free BrTyr was poorly correlated with TCB (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.5281/zenodo.6914574).
Figure 2.
Urine BrTyr released after incubation with different enzymes or methanesulfonic acid (MSA). Each enzymatic reaction was carried out in 0.3 M ammonium acetate buffer at different pH, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, and 7.4, at different temperatures, 37°C, 45°C, 55°C, and 60°C, for 2 and 17 h. The highest BrTyr released for each source of enzyme reacted among different pH values and temperatures at each time point was shown. The BrTyr in urine sample without enzymatic incubation (free) was carried out in 0.3 M ammonium acetate, pH 7.0 at 37°C. BrTyr released by MSA hydrolysis was carried out by adding MSA to a final concentration of 4 M and incubated at 110°C for 24 h. Stable isotope labeled internal standard mix was added before hydrolysis reaction either by enzyme or MSA. After reaction at the indicated time, the urine sample was diluted with 0.1% formic acid followed by solid phase extraction. BrTyr, 3-bromotyrosine.
Figure 3.
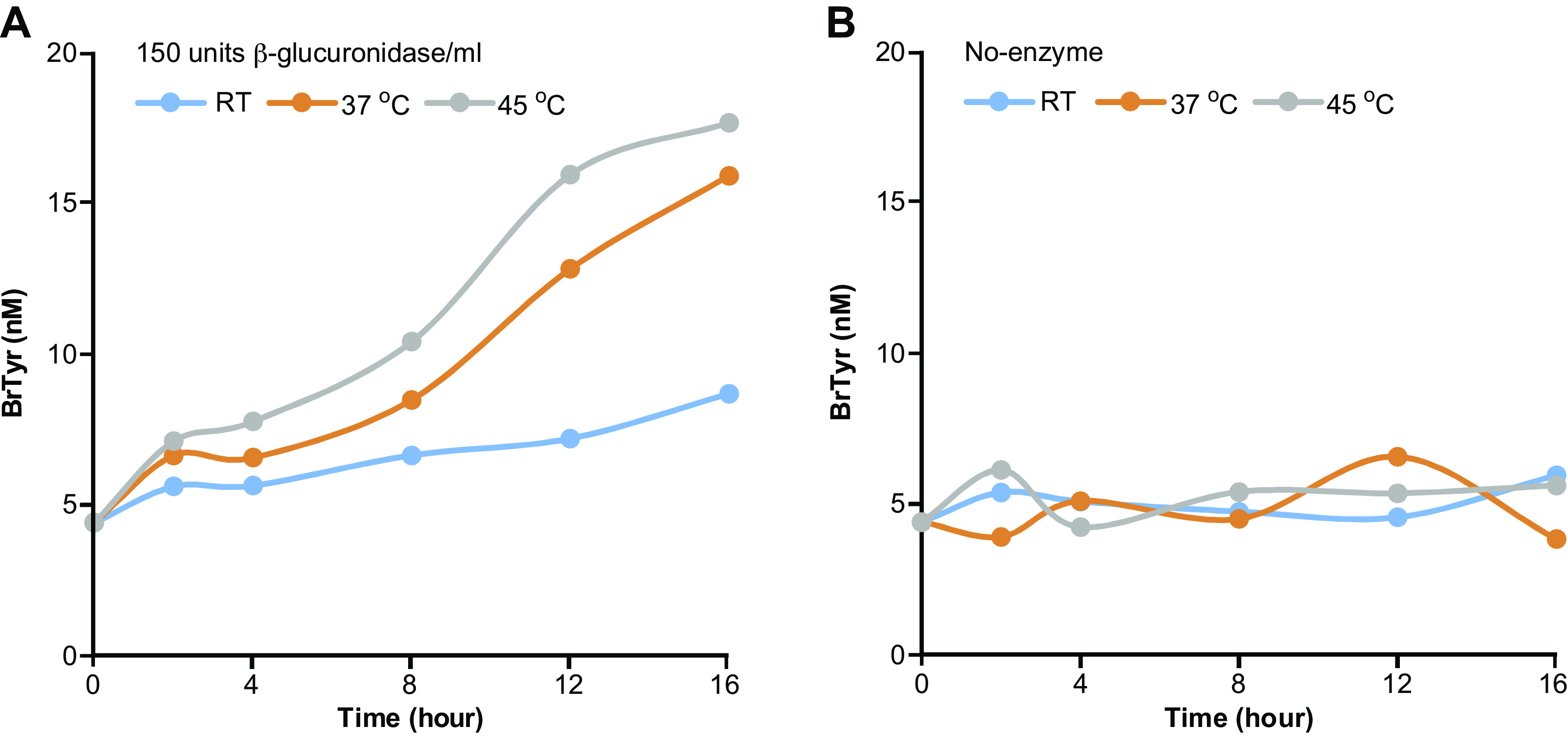
BrTyr released from a pooled urine after incubation with or without β-glucuronidase. A: urine BrTyr released after incubation with 150 units β-glucuronidase/mL at RT, 37°C, and 45°C for different time points. Urine was buffered with 0.3 M ammonium acetate buffer, pH 5.0. The released BrTyr was mixed with internal standard mix followed by solid phase extraction as described in methods. B: without enzymatic incubation, urine BrTyr released after exposure to RT, 37°C, and 45°C for different time points. BrTyr, 3-bromotyrosine; RT, room temperature.
Urinary TCB Levels Correlate with Asthma Severity
We hypothesized that TCB should represent a better global estimate for eosinophilic oxidative tissue injury, and thus sought to determine if urinary TCB is associated with asthma severity. Urine from participants with severe and nonsevere asthma included in the Severe Asthma Research Program (SARP) were therefore examined for TCB levels. The clinical characteristics of the subset of study participants who had urine samples available for analyses are displayed in Table 1. Among 431 individuals, 178 (41%) were participants with nonsevere asthma and 253 (59%) were participants with severe asthma. Participants with severe asthma were significantly older, had higher body mass index (BMI), systolic pressure, and diastolic pressure, and had lower expiratory volume and forced vital capacity. They also showed higher levels of white blood cells, neutrophils, and eosinophils, and had lower asthma control test scores compared with participants with nonsevere asthma. There was no significant difference in sputum eosinophil levels between participants with severe and nonsevere asthma.
Compared with participants with nonsevere asthma, participants with severe asthma showed significantly higher levels of urinary BrTyr after acid hydrolysis (TCB; P = 0.003; Fig. 4A). Urinary TCB levels were also positively associated with age (r = 0.244, P < 0.0001, n = 431; Fig. 4B). To further study the association between urinary TCB and asthma severity, multivariate logistic regression analysis was used to calculate an odds ratio of severe asthma based on quartiles of urine TCB, unadjusted and adjusted for sex, age, and race, blood eosinophils, and adjusted for both blood eosinophils and sputum blood eosinophils. In both the adjusted (sex, age, and race, blood eosinophils, sputum blood eosinophils) and unadjusted models, subjects whose urinary TCB levels were within the 4th quartile showed a two to three times greater risk than subjects in the 1st quartile, indicating that urinary TCB is associated with asthma severity independent of traditional risk factors (Fig. 4C, Supplemental Table S1). In parallel, we also tested the prognostic value of urine-free BrTyr and its association with asthma severity. As shown in Supplemental Table S2, we did not find that patients in the top quartile will be more likely to suffer from severe asthma, compared with patients in the bottom quartile (i.e., the prognostic value of free BrTyr was low as compared with TCB for prediction of severe asthma risk). The Asthma Control Test (ACT) score is used to identify those with poorly controlled asthma. ACT score and urinary TCB showed an inverse relationship, with higher urinary TCB associated with less asthma control (one-way ANOVA P = 0.006). Patients with ACT scores ranging from 17 to 19 and from 20 to 25 had significantly lower urinary TCB than patients with ACT scores ranging from 5 to 12 (P = 0.0008 and P = 0.03, respectively, Wilcoxon rank-sum test). Patients with ACT scores 20–25 seemed to have higher TCB than those with ACT scores 17–19 but did not reach statistical significance (P = 0.11, Wilcoxon rank-sum test; Fig. 4D). Although TCB was regarded as an even better surrogate for eosinophilic oxidative stress, it was poorly correlated with blood (r2 = 0.038) or sputum eosinophils (r2 = 0.0069).
Figure 4.
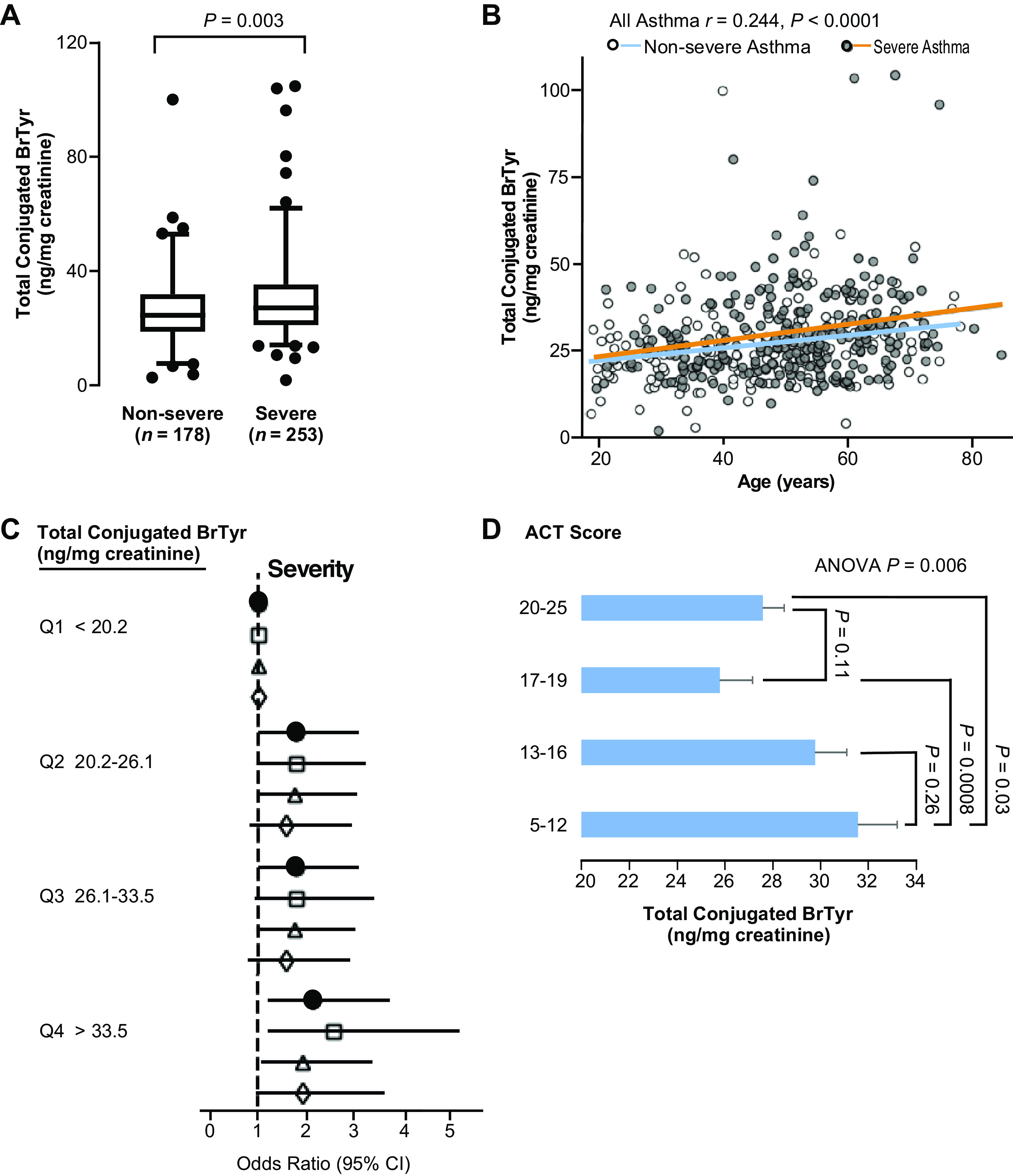
Urinary TCB levels are correlated with asthma severity. A: participants with severe asthma (n = 253, 66.8% female) have significantly higher urinary TCB levels than participants with nonsevere asthma (n = 178, 68.5% female). Whiskers indicate 2.5 and 97.5 percentile, line inside the Box indicates the median value, Box encompasses 25%–75%. B: urinary TCB levels are associated with age. C: odds ratio of asthma severity based on quartiles of urine TCB, unadjusted (closed circles) and adjusted for sex, age, and race (open squares), blood eosinophils (open triangles), and adjusted for both blood eosinophils and sputum blood eosinophils (open diamonds). D: urine TCB levels are related to asthma control test (ACT) score in participants with asthma. Data are presented as means ± SE from, n = 77, 101, 90, and 163 patients for ACT scores, 5–12, 13–16, 17–19, and 20–25 groups, respectively. TCB, total conjugated bromotyrosine.
Urinary TCB Levels Predict Risk for Future Asthma Exacerbation
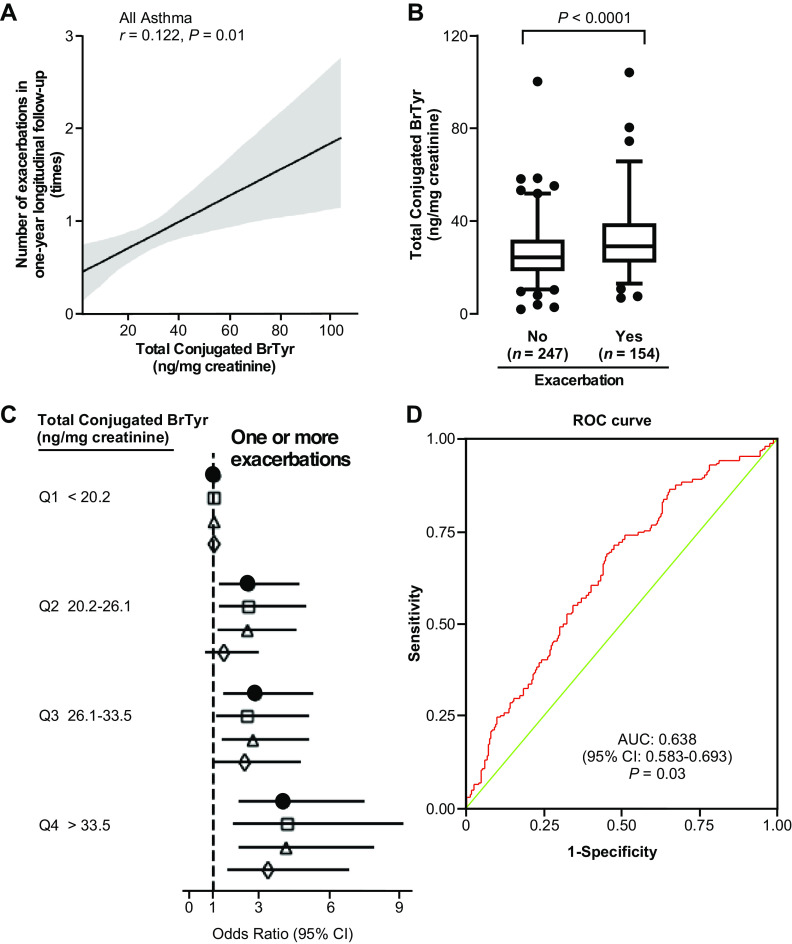
To identify a biomarker for asthma exacerbation, we next analyzed the subset of the above 431 participants in SARP for whom the number of exacerbations within the 1-yr follow-up were recorded (n = 401). Here, participants with asthma were classified into two groups by their number of exacerbations (0, or 1) within the 1-yr longitudinal period (Table 2). Of the 401 participants, 154 (38%) had one or more exacerbations in the 1-yr longitudinal follow-up, whereas 247 (62%) had no exacerbations (Table 2). Compared with those that did not experience an exacerbation, participants with asthma with exacerbation were significantly older and more likely to have European ancestry. They had lower forced expiratory volume in 1 s and lower forced vital capacity, more white blood cells, and showed significant reductions in IgE, asthma quality of life questionnaire scores, and asthma control test scores (18). Notably, urinary TCB levels were positively correlated with the number of exacerbations in 1-yr longitudinal follow-up (r = 0.122, P = 0.01; Fig. 5A), and participants with asthma with exacerbation showed significantly higher urinary TCB than those without exacerbation (P < 0.0001; Fig. 5B).
Table 2.
Demographics of the subset of study participants who provided urine for TCB analyses by asthma exacerbation
| Characteristics | No Exacerbation, n = 247 | Exacerbation, n = 154 | P Value |
|---|---|---|---|
| Age at enrollment, yr | 47.2 ± 14.2 | 50.5 ± 13.4 | 0.02 |
| Age when first diagnosed, yr | 19.1 ± 15.9 | 21.5 ± 16.5 | 0.14 |
| Female sex, % | 64.8 | 68.4 | 0.23 |
| Race, % | |||
| European ancestry | 62.3 | 72.1 | 0.04 |
| African ancestry | 25.8 | 18.2 | 0.08 |
| Body mass index, kg/m2 | 31.4 ± 7.8 | 33.0 ± 8.5 | 0.05 |
| Blood pressure, mmHg | |||
| Systolic | 123 ± 14 | 125 ± 15 | 0.36 |
| Diastolic | 77 ± 10 | 76 ± 10 | 0.19 |
| Lung functions | |||
| Forced expiratory volume in 1 s, %predicted | 75.7 ± 18.8 | 70.4 ± 20.7 | 0.008 |
| Forced vital capacity, %predicted | 87.7 ± 16.7 | 81.5 ± 17.5 | <0.001 |
| FEV1/FVC | 0.69 ± 0.09 | 0.69 ± 0.11 | 0.48 |
| Provocative concentration of methacholine causing a 20% fall in FEV1, mg/mL | 3.0 ± 4.0 | 3.7 ± 4.3 | 0.37 |
| White blood cells, ×109/L | 7.0 ± 2.3 | 8.0 ± 2.8 | <0.001 |
| Neutrophils | 4.1 ± 2.0 | 4.9 ± 2.5 | <0.001 |
| Eosinophils | 0.27 ± 0.26 | 0.32 ± 0.29 | 0.09 |
| Platelets, ×109/L | 253 ± 69 | 265 ± 63 | 0.10 |
| IgE, IU/mL | 359 ± 602 | 280 ± 473 | 0.01 |
| Sputum eosinophils, ×103/L | 94 ± 303 | 71 ± 180 | 0.47 |
| Asthma quality of life questionnaire | 5.5 ± 1.2 | 4.9 ± 1.4 | <0.001 |
| Asthma control test score | 18.3 ± 4.0 | 15.8 ± 4.9 | <0.001 |
Values are represented as means ± SD. FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; TCB, total conjugated bromotyrosine.
Figure 5.
Urinary TCB levels predict risk for future asthma exacerbation. A: urinary TCB level is correlated with the number of exacerbations in 1-yr longitudinal follow-up using a linear model fit with a 95% confidence interval. B: urinary TCB levels are significantly higher in participants with asthma with exacerbation (n = 154, 68.4% female) than those without (n = 247, 64.8% female). Whiskers indicate 2.5 and 97.5 percentile, line inside the Box indicates the median value, Box encompasses 25%–75%. C: odds ratio of asthma exacerbation based on quartiles of urine TCB, unadjusted (closed circles) and adjusted for sex, age, and race (open squares), blood eosinophils (open triangles), and adjusted for both blood eosinophils and sputum blood eosinophils (open diamonds). D: Receiver Operating Characteristic (ROC) curve for urine TCB comparing participants with asthma with exacerbation vs. participants with asthma without exacerbation in 1-yr longitudinal follow-up. The cutoff level of urine TCB was set as 26.68 ng/mg creatinine. TCB, total conjugated bromotyrosine.
To further study the association between urinary TCB and the likelihood of asthma exacerbation, multivariate logistic regression was used to calculate the odds ratio of asthma exacerbation based on quartiles of urinary TCB, unadjusted and adjusted for sex, age, race, blood eosinophils, and sputum blood eosinophils. Subjects with urinary TCB levels in the 4th quartile (>33.5 ng/mg creatinine) had three to five times greater risk of asthma exacerbation compared with subjects with TCB levels in the 1st quartile (<20.2 ng/mg creatinine), and the higher risk remained significant even after adjusting for traditional risk factors (Fig. 5C, Supplemental Table S3). Collectively, these results show that higher urinary TCB levels are dose-dependently associated with a significantly higher risk of having exacerbations (Fig. 5C). In a final analysis, we used a receiver operating characteristic (ROC) analysis to further investigate the ability of urinary TCB to predict asthma exacerbation. As shown in Fig. 5D, we found an area under the curve (AUC) of 0.638 (95% CI: 0.583–0.693, P = 0.03). A good cutoff level of 26.68 ng/mg creatinine urine TCB showed a sensitivity and specificity of 0.60 (Fig. 5D).
DISCUSSION
Asthma has been mechanistically linked to increased reactive oxygen and nitrogen species for many years (1–5). Eosinophils commonly increased in the sputum and peripheral blood of patients with asthma are long known to play a primary role in the reactive species production that leads to the pathology of asthma. On activation, eosinophil peroxidase produces highly oxidizing brominating species that modify protein tyrosine residues and form BrTyr (6–8). Urinary levels of free BrTyr are independent biomarkers of eosinophil activation and inflammation in children but are not related to eosinophils in the sputum and peripheral blood (6, 9). This disconnect between eosinophil numbers and their bromination products is because urinary levels of BrTyr reflect the resolution phase of the protein remodeling caused by eosinophilic tissue injury. As oxidized tissue undergoes remodeling, the nonphysiological amino acids are removed and excreted in urine. Thus, BrTyr levels are a quantitative measure of eosinophilic tissue oxidative damage. The lack of association of eosinophil numbers to BrTyr also suggests that the measure of eosinophils in sputum or blood is inadequate to assess the oxidative damage and remodeling caused by eosinophil peroxidase in lung tissues. Unfortunately, the low levels of urine-free BrTyr, particularly in adults with asthma, have limited the utility of the measure. However, in this study, we show that the majority of urinary BrTyr exists in conjugated forms whose total levels can be quantified following release by acid hydrolysis (TCB), and we subsequently developed a sensitive and specific measure of TCB. Participants with severe asthma had significantly higher levels of TCB when compared with participants with nonsevere asthma, indicating there is more oxidation and tissue remodeling in severe asthma. Even after adjusting for multiple factors, including eosinophils, participants with asthma with TCB levels at the highest quartile were two- to threefold more likely to be severe than their counterparts with TCB levels at the lowest quartile. Similar to the association with severity, TCB levels also predicted risk for one or more asthma exacerbations over the ensuing 1-yr period. Participants with asthma with TCB level at the highest quartile had three- to fivefold higher risk of exacerbation compared with their counterparts with TCB levels at the lowest quartile. These findings suggest that higher oxidative tissue injury is mechanistically related to exacerbations. Overall, TCB is quantitatively associated with higher risk of severe asthma and exacerbations.
Prior evaluations of individuals in SARP have identified an exacerbation-prone asthma phenotype characterized by high blood eosinophils (22) and showed that blood IL-6 and eosinophils can predict exacerbations (18). Furthermore, high sputum cell percentages of eosinophils and neutrophils appear to be associated with a greater number of exacerbations (23). Collectively these prior studies and this current study identify eosinophilic mechanisms in the underlying pathophysiology of asthma severity and exacerbations. Other studies have also identified novel biomarkers for predicting asthma exacerbation, including serum vascular endothelial growth factor, serum eosinophil cationic protein and elevated soluble interleukin–2 receptor (24–26). However, many of these candidate biomarkers are also associated with other disease states, and mechanistically, their link to monitoring an anti-eosinophilic pharmacological intervention is less clear. Serum vascular endothelial growth factor is used to predict various cancers, including colorectal cancer, ovarian cancer, renal cell carcinoma, and pancreatic cancer (27–30), and elevated soluble interleukin–2 receptor can also be found in patients with cancer including breast cancer, hepatocellular cancer, and coronary microvascular dysfunction (31–33). As asthma is a very common disease that causes significant morbidity and healthcare costs, there is a great need for effective and specific biomarkers for asthma severity, exacerbations, and response to therapies. Although results are significant, there is high degree of variability among participants with nonsevere and severe asthma. This biological variability may limit use for diagnosis of asthma, but in an individual with known asthma, higher levels are strongly related to exacerbations, making it a useful biomarker.
TCB is a cumulative biomarker of both eosinophilic inflammation and the respiratory burst of other inflammatory cells in asthma. Thus, it represents a measure of total oxidative stress. Other biomarkers, such as blood eosinophil count, IgE, and nitric oxide, have been used to monitor asthma severity and predict asthma exacerbations (34–37); however, none of these biomarkers reflect total oxidative stress. Furthermore, eosinophil counts are not informative of eosinophil activation and/or eosinophil peroxidase activity. Perhaps most important for clinical applications, collection of a urine sample is noninvasive and simple. Possible clinical applications of TCB could include stepping up therapy to treat airway oxidative stress and lower the risk of subsequent exacerbations. In this context, TCB might be useful to monitor efficacy of medications and adherence to therapy. However, TCB has limitation in sensitivity. Further studies are required to ascertain variance of repeat longitudinal measurements over time and to determine if TCB changes with administration of anti-inflammatory medications. Overall, TCB provides a simple and specific noninvasive approach for monitoring eosinophil activation and oxidative stress and may serve as a useful biomarker for identification of patients who are suffering greater oxidative tissue remodeling.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Fig. S1 and Supplemental Tables S1–S3: https://doi.org/10.5281/zenodo.6914574.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL081064, HL103453, HL109250, and HL130819 and in part by the National Center for Advancing Translational Sciences (ULTR000439). SARP was supported by awards from National Heart, Lung, and Blood Institute (U10 HL109172, U10 HL109168, U10 HL109152, U10 HL109257, U10 HL109046, U10 HL109250, U10 HL109164, and U10 HL109086). The following companies provided financial support for study activities at the Coordinating and Clinical Centers beyond the third year of patient follow-up: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA.
DISCLAIMERS
The following companies provided financial support for study activities but had no role in study design or data analysis, and the only restriction on the funds was that they should be used to support the SARP initiative: AstraZeneca, Boehringer-Ingelheim, Genentech, GlaxoSmithKline, Sanofi–Genzyme–Regeneron, and TEVA. Cleveland Clinic did not receive any of these industry supports for the SARP study or any of the work in the manuscript.
DISCLOSURES
Z. Wang and S. L. Hazen report being named as coinventors on pending and issued patents held by the Cleveland Clinic relating to inflammation and cardiovascular diagnostics and therapeutics and having received royalty payments for inventions or discoveries related to diagnostics or therapeutics from Procter & Gamble and Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics. Hazen reports having been a paid consultant for Procter & Gamble, being a paid consultant for Zehna Therapeutics, and having received research funds from Proctor & Gamble, Zehna Therapeutics, Pfizer Inc., and Roche Diagnostics. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
Z.W., S.A.A.C., E.R.B., M.C., L.C.D., J.V.F., E.I., B.D.L., N.N.J., W.C.M., S.E.W., D.T.M., B.G., and S.L.H. conceived and designed research; Z.W., S.A.A.C., X.F., Z.S., R.B., and J.G.Z. performed experiments; Z.W., W.X., and S.C.E. analyzed data; Z.W., W.X., and S.L.H. interpreted results of experiments; Z.W. and W.X. prepared figures; Z.W., W.X., and S.L.H. drafted manuscript; Z.W., W.X., S.L.H., and S.C.E. edited and revised manuscript; Z.W., W.X., S.A.A.C., X.F., Z.S., R.B., J.G.Z., E.R.B., M.C., L.C.D., J.V.F., E.I., B.D.L., N.N.J., W.C.M., S.E.W., D.T.M., B.G., and S.L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The members of the National Heart, Lung, and Blood Institute Severe Asthma Research Program include Suzy A. A. Comhair, Joe G. Zein, Eugene R. Bleecker, Mario Castro, Loren C. Denlinger, John V. Fahy, Elliot Israel, Bruce D. Levy, Nizar N. Jarjour, Wendy C. Moore, Sally E. Wenzel, David T. Mauger, Benjamin Gaston, and Serpil C. Erzurum. We thank David Schumick for artwork.
REFERENCES
- 1. Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 12: 93–124, 2010. [Erratum in Antioxid Redox Signal 12: 321, 2010]. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. No chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc Natl Acad Sci USA 98: 2622–2627, 2001. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaston B, Drazen JM, Loscalzo J, Stamler JS. The biology of nitrogen oxides in the airways. Am J Respir Crit Care Med 149: 538–551, 1994. doi: 10.1164/ajrccm.149.2.7508323. [DOI] [PubMed] [Google Scholar]
- 4. Haahtela T. Airway remodelling takes place in asthma–what are the clinical implications? Clin Exp Allergy 27: 351–353, 1997. doi: 10.1111/j.1365-2222.1997.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 5. MacPherson JC, Comhair SA, Erzurum SC, Klein DF, Lipscomb MF, Kavuru MS, Samoszuk MK, Hazen SL. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol 166: 5763–5772, 2001. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 6. Wu W, Samoszuk MK, Comhair SA, Thomassen MJ, Farver CF, Dweik RA, Kavuru MS, Erzurum SC, Hazen SL. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest 105: 1455–1463, 2000. doi: 10.1172/JCI9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 161: 1720–1745, 2000. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 8. Erzurum SC. New insights in oxidant biology in asthma. Ann Am Thorac Soc 13, Suppl 1: S35–39, 2016. doi: 10.1513/AnnalsATS.201506-385MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wedes SH, Wu W, Comhair SA, McDowell KM, DiDonato JA, Erzurum SC, Hazen SL. Urinary bromotyrosine measures asthma control and predicts asthma exacerbations in children. J Pediatr 159: 248–55.e1, 2011. doi: 10.1016/j.jpeds.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Comhair SAA, Bochenek G, Baicker-McKee S, Wang Z, Stachura T, Sanak M, Hammel JP, Hazen SL, Erzurum SC, Nizankowska-Mogilnicka E. The utility of biomarkers in diagnosis of aspirin exacerbated respiratory disease. Respir Res 19: 210, 2018. doi: 10.1186/s12931-018-0909-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, Fitzpatrick AM, Gaston BM, Israel E, Hastie A, Hoffman EA, Holguin F, Levy BD, Meyers DA, Moore WC, Peters SP, Sorkness RL, Teague WG, Wenzel SE, Busse WW; NHLBI Severe Asthma Research Program (SARP). Severe asthma: lessons learned from the national heart, lung, and blood institute severe asthma research program. Am J Respir Crit Care Med 185: 356–362, 2012. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE; National Heart, Lung, Blood Institute's Severe Asthma Research Program. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 119: 405–413, 2007. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract 6: 545–554.e4, 2018. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zein J, Gaston B, Bazeley P, DeBoer MD, Igo RP Jr, Bleecker ER, et al. HSD3B1 genotype identifies glucocorticoid responsiveness in severe asthma. Proc Natl Acad Sci USA 117: 2187–2193, 2020. doi: 10.1073/pnas.1918819117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, Adcock IM, Bateman ED, Bel EH, Bleecker ER, Boulet LP, Brightling C, Chanez P, Dahlen SE, Djukanovic R, Frey U, Gaga M, Gibson P, Hamid Q, Jajour NN, Mauad T, Sorkness RL, Teague WG. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 43: 343–373, 2014. [Erratum in Eur Respir J 43: 1216, 2014]. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 16. Juniper EF, Svensson K, Mörk AC, Ståhl E. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 3: 58, 2005. doi: 10.1186/1477-7525-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA Jr, Gern J, Heymann PW, Martinez FD, Mauger D, Teague WG, Blaisdell C. Asthma outcomes: exacerbations. J Allergy Clin Immunol 129: S34–S48, 2012. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, Israel E, Levy BD, Phipatanakul W, Jarjour NN, Castro M, Wenzel SE, Hastie A, Moore W, Bleecker E, Fahy JV, Denlinger LC. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med 202: 973–982, 2020. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu W, Chen Y, d'Avignon A, Hazen SL. 3-Bromotyrosine and 3,5-Dibromotyrosine are major products of protein oxidation by eosinophil peroxidase: potential markers for eosinophil-dependent tissue injury in vivo. Biochemistry 38: 3538–3548, 1999. doi: 10.1021/bi982401l. [DOI] [PubMed] [Google Scholar]
- 20. Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, Ischiropoulos H, Smith JD, Kinter M, Hazen SL. Apolipoprotein a-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest 114: 529–541, 2004. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buss IH, Senthilmohan R, Darlow BA, Mogridge N, Kettle AJ, Winterbourn CC. 3-Chlorotyrosine as a marker of protein damage by myeloperoxidase in tracheal aspirates from preterm infants: association with adverse respiratory outcome. Pediatr Res 53: 455–462, 2003. [Erratum in Pediatr Res 53: 868, 2003]. doi: 10.1203/01.PDR.0000050655.25689.CE. [DOI] [PubMed] [Google Scholar]
- 22. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med 195: 302–313, 2017. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hastie AT, Mauger DT, Denlinger LC, Coverstone A, Castro M, Erzurum S, Jarjour N, Levy BD, Meyers DA, Moore WC, Phillips B, Wenzel SE, Fahy JV, Israel E, Bleecker ER; NHLBI SARP 3 Investigators. Baseline sputum eosinophil + neutrophil subgroups' clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (Sarp 3) cohort. J Allergy Clin Immunol 146: 222–226, 2020. doi: 10.1016/j.jaci.2020.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim JH. Serum vascular endothelial growth factor as a marker of asthma exacerbation. Korean J Intern Med 32: 258–260, 2017. doi: 10.3904/kjim.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wojsyk-Banaszak I, Mikoś M, Szczepankiewicz A, Wielebska A, Sobkowiak P, Kamińska A, Bręborowicz A. Evaluation of exhaled breath temperature (EBT) as a marker and predictor of asthma exacerbation in children and adolescents. J Asthma 54: 699–705, 2017. doi: 10.1080/02770903.2017.1290104. [DOI] [PubMed] [Google Scholar]
- 26. Sorkness C, McGill K, Busse WW. Evaluation of serum eosinophil cationic protein as a predictive marker for asthma exacerbation in patients with persistent disease. Clin Exp Allergy 32: 1355–1359, 2002. doi: 10.1046/j.1365-2222.2002.01471.x. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe T, Shibata M, Nishiyama H, Soeda S, Furukawa S, Gonda K, Takenoshita S, Fujimori K. Elevated serum levels of vascular endothelial growth factor is effective as a marker for malnutrition and inflammation in patients with ovarian cancer. Biomed Rep 1: 197–201, 2013. doi: 10.3892/br.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broll R, Erdmann H, Duchrow M, Oevermann E, Schwandner O, Markert U, Bruch HP, Windhövel U. Vascular endothelial growth factor (VEGF)–a valuable serum tumour marker in patients with colorectal cancer? Eur J Surg Oncol 27: 37–42, 2001. doi: 10.1053/ejso.2000.1052. [DOI] [PubMed] [Google Scholar]
- 29. Guðbrandsdottir G, Hjelle KM, Frugård J, Bostad L, Aarstad HJ, Beisland C. Preoperative high levels of serum vascular endothelial growth factor are a prognostic marker for poor outcome after surgical treatment of renal cell carcinoma. Scand J Urol 49: 388–394, 2015. doi: 10.3109/21681805.2015.1021833. [DOI] [PubMed] [Google Scholar]
- 30. Chang YT, Chang MC, Wei SC, Tien YW, Hsu C, Liang PC, Tsao PN, Jan IS, Wong JM. Serum vascular endothelial growth factor/soluble vascular endothelial growth factor receptor 1 ratio is an independent prognostic marker in pancreatic cancer. Pancreas 37: 145–150, 2008. doi: 10.1097/MPA.0b013e318164548a. [DOI] [PubMed] [Google Scholar]
- 31. Gonda K, Horita S, Maejima Y, Takenoshita S, Shimomura K. Soluble interleukin-2 receptor as a predictive and prognostic marker for patients with familial breast cancer. Sci Prog 104: 368504211039590, 2021. doi: 10.1177/00368504211039590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weis M, Hartmann A, Scheuermann EH, Olbrich HG. Soluble interleukin-2-receptor levels as a marker of coronary microvascular dysfunction after heart transplantation. J Heart Lung Transplant 17: 294–298, 1998. [PubMed] [Google Scholar]
- 33. Izzo F, Cremona F, Delrio P, Leonardi E, Castello G, Pignata S, Daniele B, Curley SA. Soluble interleukin-2 receptor levels in hepatocellular cancer: a more sensitive marker than alfa fetoprotein. Ann Surg Oncol 6: 178–185, 1999. doi: 10.1007/s10434-999-0178-1. [DOI] [PubMed] [Google Scholar]
- 34. Ahmad Al Obaidi AH, Mohamed Al Samarai AG, Yahya Al Samarai AK, Al Janabi JM. The predictive value of IgE as biomarker in asthma. J Asthma 45: 654–663, 2008. doi: 10.1080/02770900802126958. [DOI] [PubMed] [Google Scholar]
- 35. Escamilla-Gil JM, Fernandez-Nieto M, Acevedo N. Understanding the cellular sources of the fractional exhaled nitric oxide (FeNo) and its role as a biomarker of type 2 inflammation in asthma. Biomed Res Int 2022: 5753524, 2022. doi: 10.1155/2022/5753524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc 11: 531–536, 2014. doi: 10.1513/AnnalsATS.201310-354OC. [DOI] [PubMed] [Google Scholar]
- 37. Mathur SK, Fichtinger PS, Evans MD, Schwantes EA, Jarjour NN. Variability of blood eosinophil count as an asthma biomarker. Ann Allergy Asthma Immunol 117: 551–553, 2016. doi: 10.1016/j.anai.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Tables S1–S3: https://doi.org/10.5281/zenodo.6914574.
Data Availability Statement
Data will be made available upon reasonable request.