Abstract
Indoleamine 2,3 dioxygenase-1 (IDO1) catalyzes tryptophan-kynurenine metabolism in many inflammatory and cancer diseases. Of note, acute inflammation that occurs immediately after heart injury is essential for neonatal cardiomyocyte proliferation and heart regeneration. However, the IDO1-catalyzed tryptophan metabolism during heart regeneration is largely unexplored. Here, we find that apical neonatal mouse heart resection surgery led to rapid and consistent increases in cardiac IDO1 expression and kynurenine accumulation. Cardiac deletion of Ido1 gene or chemical inhibition of IDO1 impairs heart regeneration. Mechanistically, elevated kynurenine triggers cardiomyocyte proliferation by activating the cytoplasmic aryl hydrocarbon receptor-SRC-YAP/ERK pathway. In addition, cardiomyocyte-derived kynurenine transports to endothelial cells and stimulates cardiac angiogenesis by promoting aryl hydrocarbon receptor nuclear translocation and enhancing vascular endothelial growth factor A expression. Notably, Ahr deletion prevents indoleamine 2,3 dioxygenase -kynurenine–associated heart regeneration. In summary, increasing indoleamine 2,3 dioxygenase-derived kynurenine level promotes cardiac regeneration by functioning as an endogenous regulator of cardiomyocyte proliferation and cardiac angiogenesis.
Subject terms: Heart failure, Cardiac regeneration, Inflammation
Failed cardiac regeneration to repair adult acute myocardial ischemia is the leading cause of heart failure. Here, the authors show that IDO1-derived kynurenine metabolism promotes cardiomyocyte proliferation and cardiac angiogenesis via cytoplasmic aryl hydrocarbon receptor (AhR) and nucleic AhR translocation signalling.
Introduction
Myocardial infarction (MI) is one of the leading causes of death in the developed world1. Acute MI often results in irreparable heart failure in adult humans, because the adult human heart fails to regenerate following injury2,3. In contrast, case reports of myocardial scarring in infants4 and functional recovery after MI in newborns5 suggest that the human neonatal heart has regenerative capacity. Direct evidence for the robust regenerative capacity in the neonatal heart has been reported in mouse models of apical resection6, MI7, and cryoinfarction8. However, this ability rapidly diminishes at 1 week after birth, when the cardiomyocytes (CMs) exit the cell cycle6,9. Therefore, reactivation of CM proliferation becomes an appealing strategy to repair a damaged heart.
The metabolism of CMs shifts from anaerobic glycolysis to mitochondrial oxidative phosphorylation after birth, coinciding with cell-cycle arrest10. The kynurenine (Kyn) pathway (KP) plays a critical role in generating cellular energy in the form of nicotinamide adenine dinucleotide. Disturbed Kyn metabolism has been linked to the inflammatory response in carcinogenesis and neurological and cardiovascular diseases11,12. Kyn is converted from tryptophan (Trp) by indoleamine 2,3 dioxygenase-1 (IDO1), a well-known rate-limiting enzyme in KP. Kyn may then be further catalyzed by Kyn 3-monooxygenase (KMO), kynureninase (KYNU), and Kyn aminotransferase (KAT) to form various metabolites. Kyn binds to cytoplasmic aryl hydrocarbon receptor (AHR) and promotes its translocation to the nucleus, suppressing effector T-cell proliferation and enhancing T-cell generation13. Moreover, AHR induces transcriptional activation of phase I metabolic enzymes14 and stimulates estrogen receptor destruction and Jak2-Src interaction-independent transcription15,16.
Recent studies show that increased levels of IDO1 and Kyn directly promotes colon tumorigenesis by triggering the major processes of the cell cycle13,17,18. Moreover, Kyn-activated AHR promotes vascular development and angiogenesis by upregulating vascular endothelial growth factor A (VEGFA)19–23, which is critical for heart regeneration24. Therefore, activation of KP may be essential for the extensive activation of CM proliferation in the neonatal heart regeneration.
Epidemiological data show that decreased Trp and increased Kyn levels in serum serve as predictors for cardiovascular disease (CVD), including ischemic heart disease25. Our previous work indicates that Ido1 deletion attenuates angiotensin II-induced abdominal aortic aneurysm, which suggests a causal relationship between activation of KP metabolism and the development of abdominal aortic aneurysm26. In addition, we found that infusion of angiotensin II activates the Kyn metabolic pathway by activating interferon γ (IFNγ) signaling in the mouse aortic endothelium27. Notably, administration of a high-fat diet in atherosclerosis-prone low-density lipoprotein receptor-knockout mice increases IDO1 activity in aortic macrophages and vascular smooth muscle cells28. However, whether IDO1-derived Kyn is involved in heart regeneration remains unknown.
In this work, we demonstrated that neonatal apical resection increased the IDO1 expression in CMs and Kyn production, which further promoted cardiac regeneration. Reduction of Kyn level by genetic silencing of Ido1 or pharmacological inhibition both impaired heart regeneration. Administration of exogenous Kyn promoted cardiac regeneration in neonatal mice. Mechanistically we found that, elevated Kyn level in CMs can activate their proliferation by stimulating the cytoplasmic AHR-SRC-YAP pathway. In addition, CM-produced Kyn, translocated into endothelium, and promoted angiogenesis by increasing AHR nuclear translocation and VEGFA gene transcription. Collectively, our results suggest that IDO1-derived Kyn functions as a critical endogenous regulator of CM proliferation and cardiac angiogenesis in mouse heart regeneration.
Results
Cardiac IDO1-derived Kyn promotes neonatal heart regeneration
We first studied the expression profiles of major enzymes regulating Trp-Kyn metabolism during heart regeneration in mouse neonates subjected to apical resection (AR) at postnatal day 1 (P1). Protein levels of IDO1 were significantly elevated during the whole process of neonatal cardiac regeneration and peaked at P7, but there was no significant change in the levels of KYNU, KMO, and KAT1 during the process (Fig. 1a). Co-immunofluorescence (IF) staining showed that the upregulated IDO1 mainly localized in CMs but not in endothelial cells (EC) or vascular smooth muscle cells (VSMC) (Supplementary Fig. 1). HPLC measurement showed a rapid accumulation of cardiac Kyn, concurrent with a significant reduction in Trp level (Fig. 1b). Thus, the accumulation of IDO1-derived Kyn might be associated with neonatal mouse heart regeneration.
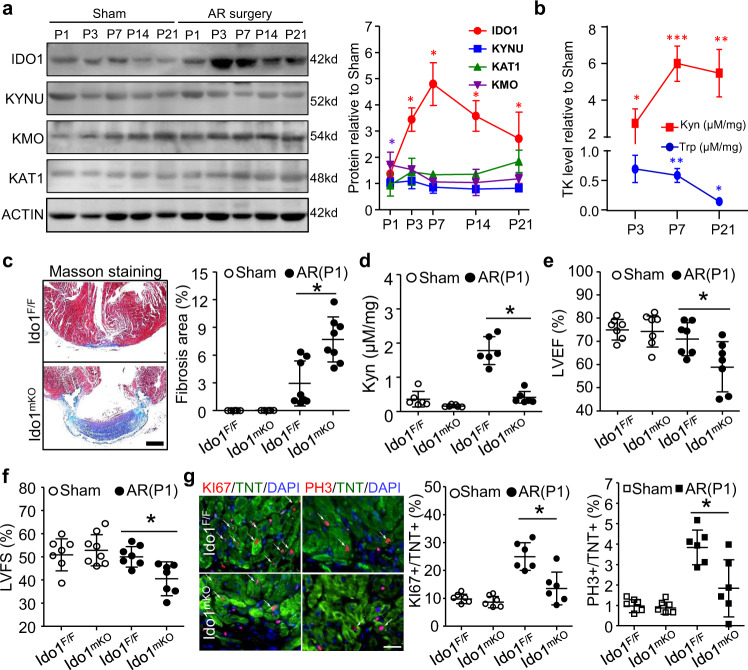
Fig. 1. IDO1-derived Kyn promotes neonatal heart regeneration.
a, b Postnatal 1 day (P1) wild-type mice underwent heart apical resection (AR) or Sham surgery. The regenerating hearts were collected at P3, P7, P14, and P21. a Western blot assay and quantification showing the expression profiles of tryptophan-kynurenine (Trp-Kyn, TK) metabolic mediators during heart regeneration (n = 6/stage). b Kyn and Trp levels measured by HPLC. *P < 0.05 vs. the Sham group at the indicated times (n = 6/stage). c–f Ido1 F/F (Ido1 Flox/Flox) and Ido1 mKO (Tnt Cre; Ido1 Flox/Flox) mice underwent AR or Sham surgery at P1 and heart was collected at P21 for HPLC, Masson staining and echocardiography assay. c Representative images and quantitation of fibrosis area by Masson staining (n = 6 for Sham Ido1 F/F and Sham Ido1 mKO; n = 8 for AP Ido1 F/F and AP Ido1 mKO). Bar = 500 µm. d Cardiac Kyn level measured by HPLC assay (n = 6/group). e, f left ventricular ejection fraction (LVEF, e) and fractional shortening (LVFS, f) analyzed by echocardiography (n = 7/group). g Cardiomyocyte (CM) proliferation assessed by co-staining of KI67 or PH3 with Troponin T (TNT) at P7 (n = 6/group). Bar = 20 µm. Data are mean ± SD. *P < 0.05 by two-way ANOVA followed by Tukey’s multiple comparisons test in (a, b), one-way ANOVA followed by Tukey’s multiple comparisons test in c (P = 0.0002), d (P < 0.0001), e (P = 0.178), f (P = 0.042), and g (P = 0.0005 for KI67 and P = 0.0029 for PH3).
To determine the causal role of IDO1-derived Kyn in heart regeneration, we generated myocardial-specific Ido1 (Troponin T (Tnt)-Cre; Ido1 Flox/Flox, Ido1 mKO) knockout mice (Supplementary Fig. 2a). These mice developed normally, with normal heart function, although the CMs exhibited a slight decrease in proliferating capacity (Supplementary Fig. 2b–f). We performed AR surgery on Ido1 F/F (littermate controls) and Ido1 mKO mice (P1). At 3 weeks after surgery, the hearts of Ido1 F/F mice had almost completely regenerated. Conversely, Ido1 mKO hearts failed to regenerate, but exhibited significant fibrosis (Fig. 1c). Ido1 F/F mice showed higher cardiac Kyn levels than Ido1 mKO mice (Fig. 1d). Cardiac function was impaired in Ido1 mKO mice (Fig. 1e, f). Correspondingly, levels of the CM proliferation markers KI67 and PH3 were significantly lower in Ido1 mKO than in Ido1 F/F resected hearts (Fig. 1g). However, CM size and apoptosis were comparable between Ido1mKO and Ido1 F/F mice (Supplementary Fig. 3a, b). Notably, deletion of Ido1 in neither VSMC nor EC affected cardiac function and remodeling in the resected heart, compared to their wild-type littermates (Ido1 F/F), suggesting that the Ido1 in both VSMCs and ECs were not involved in the neonatal heart regeneration (Supplementary Fig. 4a–g). These results demonstrate that cardiomyocyte IDO1-derived Kyn is required for cardiac regeneration in neonatal mice but is not strictly required for cardiac growth or maintenance after birth.
Kyn directly regulates neonatal heart regeneration
To determine whether accumulated Kyn could directly regulate neonatal heart regeneration, we first inhibited Kyn synthesis by administration of IDO1 inhibitor (1-methyl tryptophan, 1MT) at P1 and then performed AR surgery at P2. The cardiac fibrosis, function, and Kyn concentration were detected at P28 (Supplementary Fig. 5a). Similar to Ido1 mKO mouse hearts, 1MT-treated hearts also showed impaired heart regeneration and repressed Kyn concentration, as assessed by Masson staining, HPLC and echocardiography at P28 (Fig. 2a–d). As excepted, the AR surgery stimulated KI67, and PH3 positive CMs were significantly prevented by 1MT treatment (Fig. 2e–g). We next examined if the administration of Kyn could prevent the impairment of neonatal heart regeneration when AR surgery was performed at P6 (Supplementary Fig. 5b). At P28, Kyn-treated mice exhibited a smaller fibrosis area, higher level of cardiac Kyn, improved cardiac function (increases in LVEF and LVFS), and more CMs re-entering the cell cycle when compared with the mice treated with PBS (Fig. 2h–n). These data indicate that increase in Kyn level in the resected neonatal heart could directly promote regeneration.
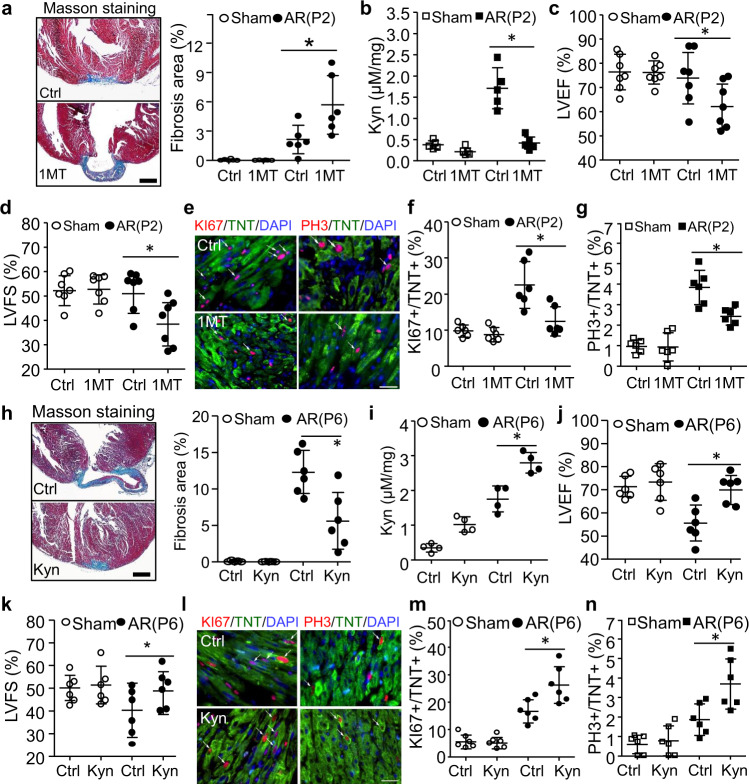
Fig. 2. The effects of Kyn on neonatal heart regeneration.
a–g Wild-type mice were intraperitoneally injected with IDO1 inhibitor (1-methyl tryptophan, 1MT, 100 mg/kg) or PBS (Control, Ctrl) every other day from P1 up to P28 and underwent heart AR surgery at P2. Cardiac fibrosis, function, and Kyn concentration were analyzed at P28. a Masson staining was used for detection and quantitation of fibrosis area (n = 6/group, *P = 0.0074). b Cardiac Kyn concentration was measured by HPLC (n = 5/group, *P < 0.0001). c, d Left ventricular ejection fraction (LVEF, *P = 0.0363) and left ventricular fraction section (LVFS, *P = 0.0156) were determined by echocardiogram (n = 7/group). e–g The cardiomyocyte proliferation was determined and quantified by co-staining of KI67 (*P = 0.0017) or PH3 (*P = 0.0031) with Troponin T (TNT) at P7 (n = 6/group). h–n Wild-type mice were intraperitoneally injected with PBS (Control, Ctrl) or Kyn (100 mg/kg) every other day from P1 up to P28 and underwent heart AR surgery at P6. Cardiac fibrosis, function, and Kyn concentration were analyzed at P28 (n = 6/group). The quantification of fibrosis area (h, n = 6/group, *P = 0.0007), cardiac Kyn concentration (i, n = 4/group, *P = 0.0007), Left ventricular ejection fraction (LVEF, j, *P = 0.0045) and left ventricular fraction section (LVFS, k, *P = 0.087) (n = 6/group), and cardiomyocyte proliferation (P10, l–n, n = 6/group, *P = 0.0048 for KI67, *P = 0.0095 for PH3) were analyzed. Bar = 500 µm for (a, h). Bar = 50 µm for e and l. Values are presented as means ± SD. *P < 0.05. Statistical analysis: one-way ANOVA followed by Tukey’s multiple comparisons test in a–h.
The cytoplasmic IDO1-Kyn-AHR-YAP/ERK pathway promotes CM proliferation
We next identified the downstream signaling pathways of IDO1-derived Kyn during cardiac regeneration. We isolated CMs from the P3 Ido1 F/F and Ido1 mKO resected hearts and performed the Cignal finder multi-pathway reporter array to measure the activities of 45 common signaling pathways. Ido1-deleted CMs exhibited the suppression of cell proliferation signaling, including the activities of the cell cycle, MYC, YAP, ERK, JNK, and GATA, which have been shown to be sufficient to reactivate CM proliferation in regenerating hearts2,3,6. Meanwhile, the inflammatory pathways such as IFN regulation, IFNγ, NFκB, and DNA damage were activated in Ido1-deleted CMs (Fig. 3a). Above changed signaling pathways were confirmed by qRT-PCR in Ido1-deleted CMs (Supplementary Fig. 6).
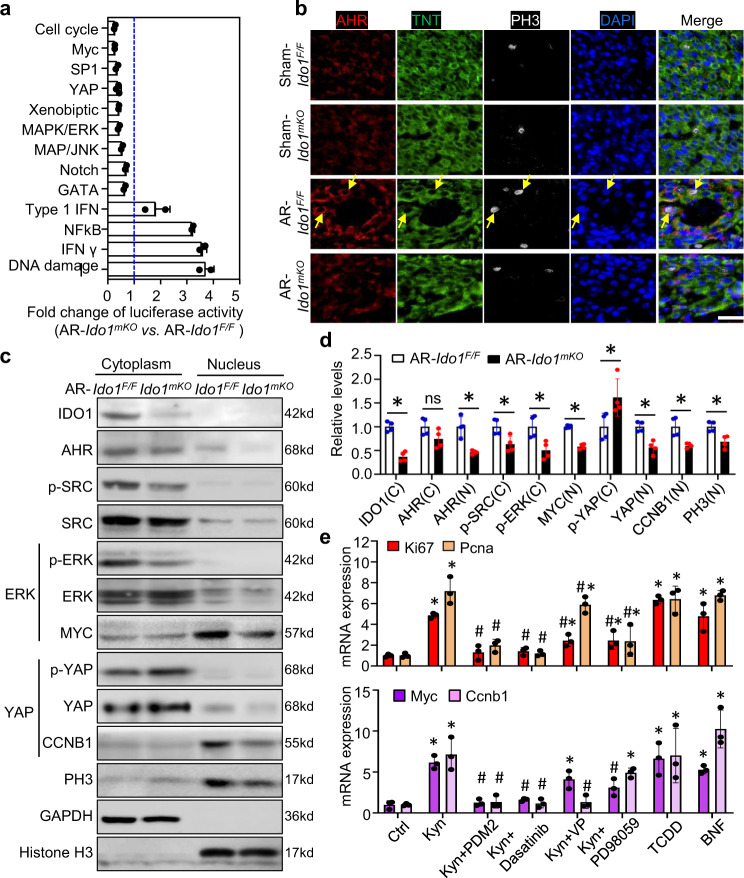
Fig. 3. The cytoplasmic IDO1-Kyn-AHR-YAP/ERK pathway promotes cardiomyocyte (CM) proliferation.
Ido1 F/F (Ido1 Flox/Flox) and Ido1 mKO (Tnt Cre; Ido1 Flox/Flox) mice underwent heart AR or Sham surgery at P1 and were collected at P3 for primary CM isolation and analysis. a A 45 Cignal finder multi-pathway reporter array showed significant changes in signaling activities in primary AR-Ido1 mKO CMs versus AR-Ido1 F/F CMs. The statistical test used the unpaired two-tailed t test between two groups. n = 3 biologically independent samples for the YPA pathway and n = 2 for other pathways. b Co-immunofluorescence staining showed the cytoplasmic location of AHR in CMs (yellow arrow), and PH3 expression in AHR-positive cells in regenerating hearts (n = 4/group). Bar = 40 µm. c, d Western blot analysis of the location and expression of IDO1, AHR, SRC, ERK, YAP, and PCNA in isolated CMs from resected Ido1 mKO and Ido1 F/F mice (n = 4/group). *P < 0.05 by the Student t test (two-tailed). ns not significant. e CMs were treated with PDM2 (AhR inhibitor), Dasatinib (SRC inhibitor), verteporfin (VP, YAP inhibitor), PD98059 (ERK inhibitor), and AhR ligands: Kyn, tetrachlorodibenzo-p-dioxin (TCDD), and β-naphthoflavone (β-NF). mRNA expression was analyzed by qRT-PCR (n = 3/group). *P < 0.05 vs Ctrl, # P < 0.05 vs. Kyn treatment by Student t test (two-tailed) for (d, e). Data are mean ± SD.
Cytoplasmic AHR was reported to bind with and phosphorylate Src kinase, promoting cancer cell survival by activating ERK and YAP signaling16. Using co-IF staining, we also found AHR mainly localized in CM cytoplasm of regenerating hearts. AHR-positive CMs have high level of PH3 protein in Ido1 F/F resected hearts, and the co-localization of AHR and PH3 has decreased in Ido1 mKO resected hearts (Fig. 3b). Western blot analysis showed decreased cytoplasmic p-SRC and p-ERK, and nuclear YAP levels, but increased p-YAP level in CMs from Ido1 mKO resected hearts as compared with that of CMs from Ido1 F/F resected hearts. Similarly, Ido1-deletion CMs also showed reduced levels of MYC and CCNB1, the targets of ERK and YAP, as well as PH3 level, a marker for cell entry into meiosis (Fig. 3c, d). qRT-PCR assay of cultured neonatal CMs further confirmed that Kyn treatment upregulated the mRNA expression of Ki67, Pcna, Myc and Ccnb1 (Fig. 3e). The increases in Ki67 and Pcna were abolished by pre-treating cells with an AHR inhibitor (PDM2) or SRC inhibitor (Dasatinib), but were partially prevented by the inhibitor of YAP (verteporfin, VP) or ERK (PD98059). Similar to Kyn, activation of AHR by its other ligands, tetrachlorodibenzo-p-dioxin (TCDD) or β-naphthoflavone (β-NF), upregulated the target genes of YAP and ERK (Fig. 3e). These results strongly suggest that cytoplasmic AHR is crucial for IDO1-Kyn-activated YAP and ERK signaling to activate CM proliferation, independent of its canonical transcriptional activity during heart regeneration14.
Kyn-activated AHR promotes cardiac angiogenesis in cardiac regeneration
Because Kyn could be transported between cells and Kyn-activated AHR is associated with angiogenesis20, we tested whether the secretion of Kyn from CMs regulates angiogenesis during heart regeneration. We measured the number of cardiac vessels in Ido1 mKO and Ido1 F/F hearts at P7 after apical resection (P1). Immunostaining for both CD31 (endothelial cell-specific marker) and α-SMA (smooth muscle cell-specific marker) was increased in Ido1 F/F resected hearts as compared with the Sham-operated hearts, and both stainings were reduced in Ido1 mKO resected hearts (Fig. 4a). These results suggest that loss of Ido1 in CMs impairs arteriogenesis in the damaged heart. In addition, in CD31-positive ECs of Ido1 F/F hearts, AHR translocated from the cytoplasm to the nucleus on P7 after resection; notably, Ido1 knockout completely prevented this effect (Fig. 4b). To determine the underlying molecular mechanism, we used the Gene Ontology (GO) enrichment analysis of 32 genes that related to cardiac vasculature morphogenesis and measured their mRNA levels in Ido1 F/F and Ido1 mKO hearts. Analysis of qRT-PCR showed that 68.75% (22 of 32) of pro-angiogenic genes were upregulated in ECs isolated from regenerating hearts relative to Sham-operated heart, and the increases in 72.73% (16 of 22) of the genes were prevented by Ido1 knockout (Fig. 4c).
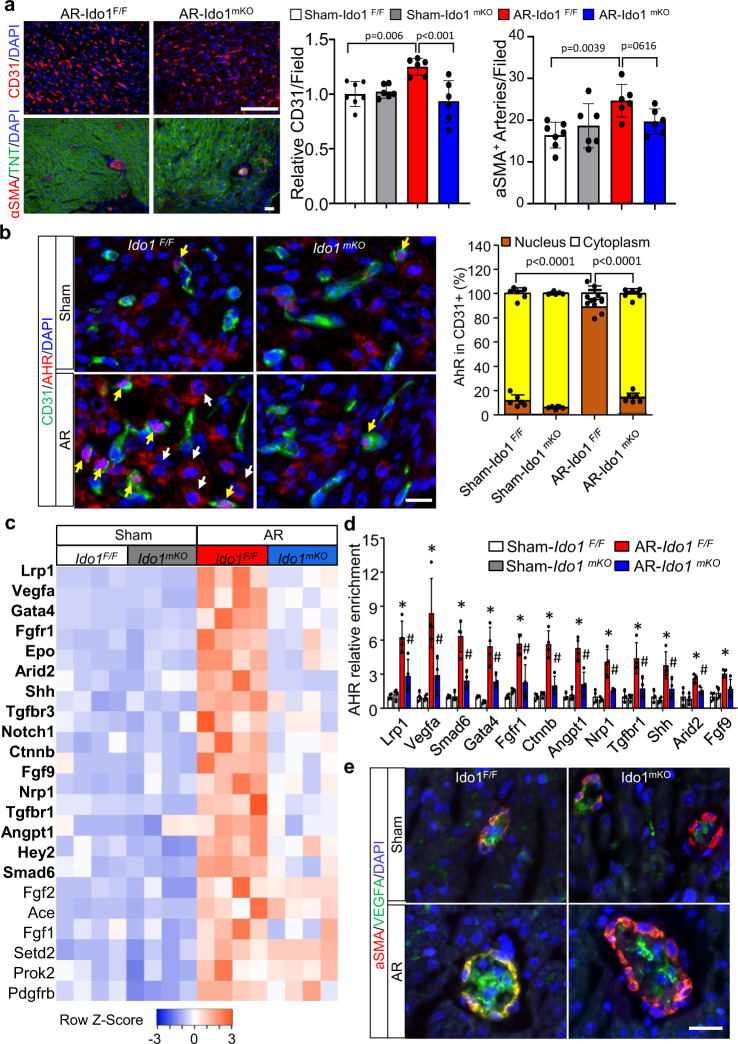
Fig. 4. Kyn-activated AhR promotes cardiac angiogenesis in cardiac regeneration.
Ido1 F/F (Ido1 Flox/Flox) and Ido1 mKO (Tnt Cre; Ido1 Flox/Flox) mice underwent heart AR or Sham surgery at P1 and were collected at P7 for cardiac angiogenesis study. a Representative images and quantification of CD31 (endothelial cell-specific marker) and α-SMA (smooth muscle cell-specific marker) immunofluorescence (IF) staining (n = 7 for Sham-Ido1 F/F group; n = 6 for other three groups). Bar = 100 µm. b Co-IF staining and quantification of the location of AHR at cytoplasm (white arrow) and nucleus (yellow arrow) in CD31-positive cells (n = 7 for Sham-Ido1 F/F group; n = 6 for other three groups). Bar = 20 µm. Two-way ANOVA followed by the Tukey’s test was used for statistical analysis. c Heatmap showing all significant changes (22/32) of the gene related to coronary vasculature morphogenesis in endothelial cells (ECs) isolated from apical resected heart compared with Sham hearts in Ido1 F/F mice. Genes with bold font indicate a significant decrease in resected Ido1 mKO vs. Ido1 F/F mice (n = 4/group). d Chromatin immunoprecipitation quantitative PCR (ChIP-qPCR) analysis of the enrichment of AHR for the indicated genes in ECs isolated from resected or Sham hearts (n = 4/group). e Representative co-IF staining of α-SMA and VEGFA for six mice of each group and five images of each mouse were collected. Bar = 20 µm. Data are mean ± SD. *P < 0.05 vs. Sham-Ido1 F/F group, # P < 0.05 vs. AR-Ido1 F/F group in (d). The Student t test (two-tailed) was used in (a, c, d).
Kyn-activated AHR could be transported to the nucleus, where the activated AHR binds to gene promoters, thus promoting gene transcription in immune cells29. To determine whether AHR could bind to gene promoters and promote pro-angiogenic gene expression in regenerated hearts, we performed the chromatin immunoprecipitation assay followed by quantitative PCR (ChIP-qPCR) in ECs isolated from regenerating hearts. AHR directly bound to 12 gene promoters, including Vegfa, Gata4, Smad6, Nrp1, and Lrp1, et al., and 11 of their bindings were repressed by Ido1 deletion (Fig. 4d and Supplementary Fig. 7). Furthermore, co-immunostaining of α-SMA and VEGFA revealed that the fluorescence intensity for VEGFA staining was stronger in Ido1 F/F apical resected hearts than in Sham-operated hearts and the increased staining was reduced in Ido1-deleted hearts (Fig. 4e). Taken together, our results suggest that accumulated Kyn promotes endothelial AHR to translocate to the nucleus, where AHR binds to the promoter of pro-angiogenic gene (such as Vegfa) and increases its expression, thus leading to cardiac angiogenesis during neonatal heart regeneration.
AHR deletion impairs IDO1-Kyn-related heart regeneration
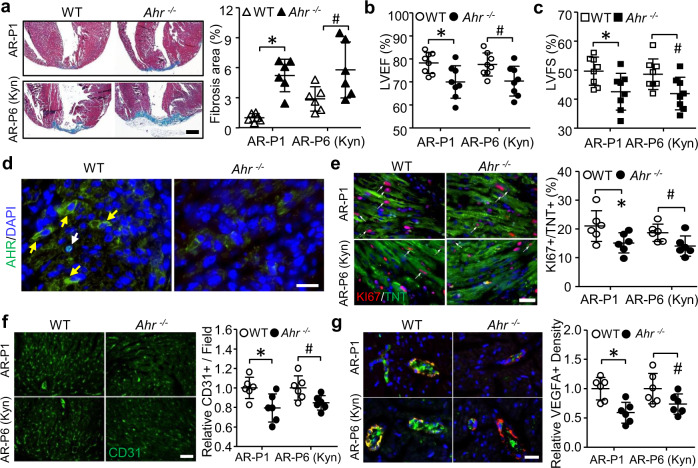
Because AHR activity is the key point of IDO1-Kyn–promoted CM proliferation and cardiac angiogenesis in neonatal heart regeneration. We used the global Ahr-knockout mice to investigate whether AHR was required for Kyn to promote cardiac regeneration in neonates. As we expected, Ahr-null hearts failed to regenerate at P28 when AR surgery was performed at P1 or P6 by Kyn-stimulated, but showed significantly increased cardiac fibrosis (Fig. 5a). This feature was accompanied by impaired cardiac function, as evidenced by reduced LVEF and LVFS as compared with wild-type mice (Fig. 5b, c). In agreement with reduced AHR expression in Ahr-knockout hearts (Fig. 5d), both KI67-positive CMs and CD31-positive ECs were also decreased in resected Ahr-knockout hearts (Fig. 5e, f). In addition, VEGFA staining in cardiac endothelium was also reduced in resected Ahr-knockout hearts (Fig. 5g). Therefore, the global deletion of Ahr mirrors Ido1 mKO impairing cardiac regeneration by inhibiting CM proliferation and cardiac angiogenesis.
Fig. 5. Ahr deletion impairs neonatal heart regeneration.
In one set of experiments, Ahr −/− and wild-type mice underwent apical resection (AR) surgery at P1. In another set of experiments, Ahr −/− and wild-type mice were intraperitoneally injected with Kyn at 100 mg/kg every other day from P1 to P28 and underwent AR surgery at P6. a–c Mice were collected at P28 for the cardiac fibrosis formation and function analysis. a Representative images and quantitation of fibrosis area by Masson staining (n = 6/group. *P = 0.0001, # P = 0.0422). b, c Echocardiography analysis of LVEF (b, *P = 0.0140, # P = 0.0258) and LVFS (c, *P = 0.0253, # P = 0.0283) (n = 8/group). d–g, on day 4 after the surgery, the expressions of AHR, KI67, CD31, and VEGFA were evaluated by immunofluorescence staining. d Representative images of immunostaining of AHR in the cytoplasm (yellow arrows) and nuclear (white arrows) for six mice of each group and five images of each mouse were collected. e CM proliferation was analyzed by co-staining of KI67 with TNT (n = 6/group. *P = 0.0475, # P = 0.0353). f Immunostaining of CD31 showing angiogenesis (n = 6/group. *P = 0.0189, # P = 0.0265). g Co-staining of VEGFA and α-SMC in coronary endothelium (n = 6/group. *P = 0.0033, # P = 0.0659). Bar = 500 µm for (a). Bar = 20 µm for (d–g). Data are mean ± SD. *P < 0.05 by one-way ANOVA followed by Tukey’s multiple comparisons.
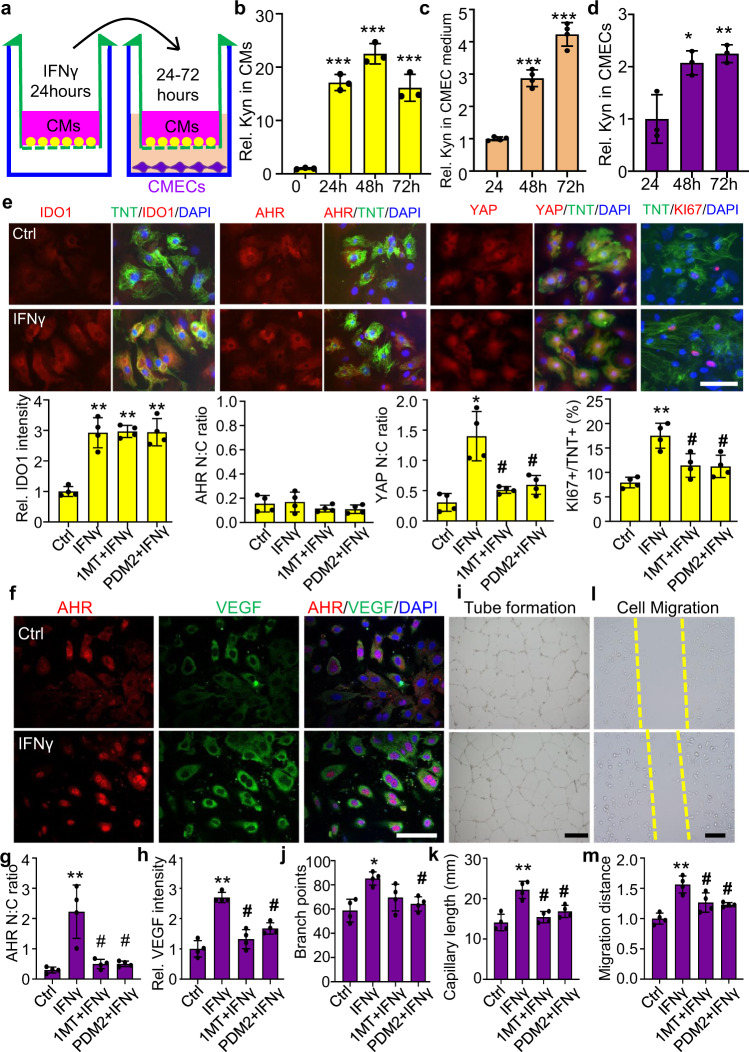
Co-culturing CMs and cardiac microvascular endothelial cells (CMECs) reveals that Kyn promotes CM proliferation and cardiac angiogenesis in vitro
We further verified that Kyn promotes CM proliferation and cardiac angiogenesis in vitro by co-culturing primary mouse neonatal CMs with CMECs. CMs were first treated with IFNγ for 24 h to elevate IDO1 expression and then co-cultured with CMECs for another 48 h (Fig. 6a). Kyn level was increased by 16.6-fold in CMs at 24 h after IFNγ treatment, and the increase was sustained for 72 h. Secreted Kyn level in the medium of CMECs was increased by four to six fold after 24 to 48 h of co-culture. Concurrently, the Kyn level in the CMECs increased by two to three fold (Fig. 6b–d), which suggests free transportation of Kyn between CMs and CMECs. Next, we examined the role of Kyn-AHR in CM proliferation and CMEC angiogenesis. Immunostaining showed upregulation of cytoplasmic IDO1, nuclear translocation of YAP, and increase of KI67-positive CM number after IFNγ treatment (Fig. 6e). The translocation of YAP and increase in KI67 level were significantly reversed by pre-treating cells with 1MT (IDO1 inhibitor) or PDM2 (AHR inhibitor). However, both 1MT and PDM2 did not affect the expression and location of IDO1 or AHR in CMs. Furthermore, IFNγ increased AHR in CMECs, accompanied by an increase in nuclear AHR and cytoplasmic VEGFA level. Concomitantly, IFNγ enhanced tube formation and cell migration. This EC angiogenesis was also significantly inhibited by pre-treatment with IDO1 or PDM2 inhibitor (Fig. 6f–m). Collectively, IDO1-derived Kyn in CMs triggered CM proliferation by activating the cytoplasmic AHR-YAP signaling. In the meantime, Kyn was transported from CMs to the CMECs, where it stimulated AHR nuclear translocation, transcriptionally upregulating VEGFA expression, and promoting cardiac angiogenesis.
Fig. 6. Kyn promotes CM proliferation and cardiac microvascular endothelial cell (CMEC) angiogenesis in vitro on co-culture of CMs and CMECs.
a The experimental procedure for co-culturing primary CMs and CMECs. b–d Kyn concentrations in CMs, CMEC medium, and CMECs detected by HPLC. Relative (Rel.) Kyn concentrations was set as 1 at 0-h group in b (n = 3/group. ***P < 0.001 compared to 0-h group by one-way ANOVA followed by Tukey’s multiple comparisons), and at 24 h group in c, d (n = 4/group in c; n = 3/group in d). *P < 0.05, **P < 0.01, ***P < 0.001 compared to 24-h group by one-way ANOVA followed by Tukey’s multiple comparisons. e Co-immunostaining and quantification of the location and expression of IDO1, AHR, YAP, and KI67 in CMs (TNT staining) treated with IFNγ (50 µM) for 24 h. Bar = 20 µm in e. f–h Representative images and quantification of co-immunostaining of AHR and VEGFA. Rel. IDO1 immunostaining intensity was set as 1 in Control (Ctrl) group. Bar = 50 µm in f, 500 µm in i, and 200 µm in l. i–k Tube formation of CMECs co-cultured with CMs and pretreated with IFNγ. l–m Migration of CMECs co-cultured with CMs and pretreated with IFNγ. *P < 0.05, **P < 0.01, ***P < 0.001, # P < 0.05 compared to Ctrl by one-way ANOVA followed by Tukey’s multiple comparisons in e–m (n = 4/group). Data are mean ± SD.
Discussion
Our study has revealed a role of Trp-Kyn metabolism in CM proliferation and cardiac angiogenesis during cardiac regeneration. Rapid elevation of the Trp-catalyzing enzyme IDO1 expression and its product Kyn are required for neonatal heart regeneration. The accumulated Kyn binds to AHR, activating the Src-YAP/ERK pathway to increase CM proliferation and triggering the nuclear translocation pathway in EC to promote cardiac angiogenesis (Fig. 7). These results highlight the importance of Kyn-AHR signaling in cardiac regeneration.
Fig. 7. Kynurenine promotes cardiac regeneration by activating cardiomyocyte proliferation and endothelial angiogenesis.
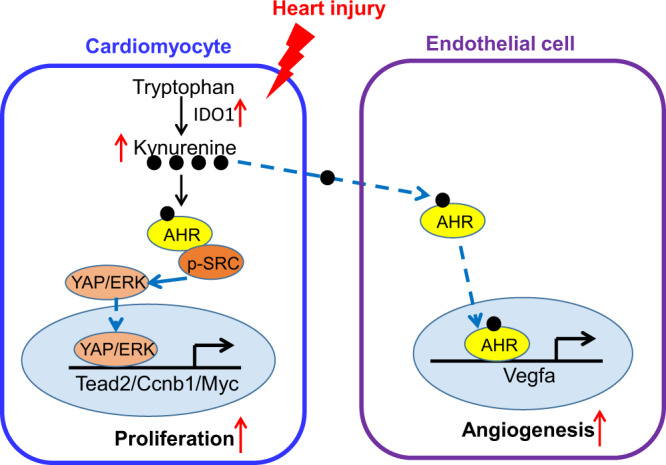
Rapid elevation of IDO1 catalyzes the conversion of tryptophan to Kyn in neonatal and adult heart regeneration models. The accumulated Kyn binds to AhR and triggers the cardiac cytoplasm SRC-YAP/ERK pathway to activate CM proliferation. On the other hand, the accumulated Kyn transports to endothelial cells and binds to AHR, which resulting in translocation to the nucleus, transcriptional upregulation of VEGFA, and promotion the cardiac angiogenesis.
The IDO1-mediated Kyn pathway is usually considered as an immunosuppressive modulator in inflammation-related diseases, including atherosclerosis, cancer, arthritis, and nervous system disease30. Notably, IDO1 is strongly induced by proinflammatory stimuli or T-helper cell-derived cytokines, such as tumor necrosis factor α, interleukin 6, and IFNγ31. This observation is consistent with our finding that the IDO1 level rapidly increased after acute inflammation induced by apical resection in neonatal mouse hearts32,33. Meanwhile, Kyn generation through IDO1 is markedly induced after MI in adult mice34,34. It is further confirmed by epidemiological investigation that decreased Trp and increased Kyn level in serum predict an increased risk of acute MI11. Although Ido1 deletion resulted in a slight reduction in heart weight and CM proliferation 3 days after birth, the heart structure and function remained normal, which suggests that IDO1 is indispensable for heart development. In heart regeneration models of apical resection, elevated IDO1-derived Kyn level extends the CM cell-cycle window. Similarly, IDO1-mediated Kyn production significantly promotes cancer cell proliferation and inhibits apoptosis but has less effect on normal tissues adjacent to the tumor18,35. In addition, the administration of an IDO1 inhibitor decreases the proliferation, adhesion, and invasion of the colon, breast, and endometrium tumors, independent of adaptive immunity17,36–38. Thus, elevated IDO1 level is required for heart regeneration.
Cardiac regeneration involves complex cellular processes, including CM proliferation, neovessel formation, fibrosis dissolution, inflammation, metabolic switching, and cardiac progenitor cell activation2. New technologies and approaches modulating the above processes may provide therapeutic strategies to activate heart regeneration. Of note, elevated Kyn level in hypoxic hearts reactivates CM proliferation and adult heart regeneration10. Kyn exerts its biological role by activating its endogenous ligand AHR39. The activation of AHR via membrane and nuclear pathways is required for Kyn to prevent immune suppression, tumor progression, atherosclerosis, and stem cell self-renewal40–42. c-Src-stimulated AHR43 interacts with other membrane receptors, including insulin-like growth factor-1 receptor, epidermal growth factor receptor19, and transforming growth factor beta 1 receptor44, to regulate cell survival, proliferation, migration, and vessel formation.
In this study, we provided evidence supporting the importance of Kyn in cardiac regeneration. By screening 45 pathways, we identified that the cytoplasmic Kyn-Src-activated YAP and ERK pathways promoted CM proliferation and heart regeneration45–47. Consistently, AHR regulated these two pathways in mouse mammary glands and lung cancer cells16,48. Because of a high level of system L transporter in both CMs and endothelial cells, Kyn could freely transport between these two cells in vivo and in vitro. Our study showed that in cardiac endothelium, Kyn bound to AHR, promoted its nuclear translocation, upregulated VEGFA, and promoted angiogenesis. Furthermore, Kyn-promoted heart regeneration was largely prevented by inhibiting CM proliferation and angiogenesis in Ahr-null mice. Similar to Ido1 CM-specific knockout mice, the three lines of AHR-deficient mice developed slower within the first week after birth49. Thus, AHR is the key point in IDO1-Kyn-mediated heart regeneration.
Furthermore, AHR has been reported to induce VEGFA-mediated angiogenesis in aortic endothelial, human microvascular endothelial, hepatocellular carcinoma, and bronchial epithelial cells19–23. By contrast, enhanced VEGFA expression and angiogenesis were found in ischemic hindlimb and choroid of Ahr-null mice:50,51 Ahr deletion-upregulated VEGFA promoted the hypoxia-induced factor 1/aryl hydrocarbon receptor nuclear translocator complex binding to the Vegf promoter, thus enhancing VEGF expression. Hence, the consequences of increased AHR may vary depending on pathological conditions.
Previous evidence showed that inflammation immediately occurs and stimulates a regenerative response after myocardial injury in neonatal and adult mice32,33. Macrophage-related inflammation is an essential component of heart regeneration in lower vertebrates and mouse neonates52. However, the excessive inflammatory response in the adult heart facilitates scar formation to repair the damaged myocardium but compromises cardiac function. Indeed, clinical studies have shown that traditional anti-inflammatory strategies are ineffective to improve cardiac function in adults after MI53,54. IDO level is increased in response to inflammation and has an immunosuppressive function because of its ability to limit T-cell function and induce immune tolerance. Our observations support this theory in mouse models of apical resection in neonatal hearts. Activating the IDO1-Kyn-AHR pathway by acute inflammation might prevent later excessive inflammatory response, maintain CM proliferation, and promote angiogenesis. A recent study showed that EC-derived IDO1 promoting cardiac injury and cardiac dysfunction after MI in adult heart34. It might be related to the switching of expression and location of IDO1, the micro-environment of inflammation and the cardiac regeneration activity between neonatal and adult under injury stimulation. IDO-Kyn target DNA damage, NFKB and IFN regulation might become much stronger, which would damage adult heart regeneration. Whereas other IDO-Kyn target pathways, such as cell cycle, Myc, Yap, Notch, and GATA become very weak even after MI55,56. Thus, appropriate inflammatory response control may provide an important therapeutic strategy to repair the damaged heart.
In summary, activation of the IDO1-Kyn-AHR pathway is a new mechanism to stimulate neonatal cardiac regeneration. The accumulated IDO1-derived Kyn precisely calibrates the immunological environment, which triggers CM proliferation and promotes cardiac angiogenesis. Thus, activation of the IDO1-derived Kyn pathway may be a potential therapeutic strategy to repair damaged hearts after MI.
Methods
Mice
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Georgia State University (GSU). Cardiomyocyte (CM)-specific knockout Ido1 (Ido1 mKO) mice were developed by crossing floxed Ido1 (Ido1 F/F) mice with Troponin T (Tnt)-Cre mice. In addition, deficiency of Ido1 in endothelial cell (EC) or vascular smooth muscle cell (VSMC) was obtained by crossbreeding Ido1 F/F with Cdh5-Cre or Myh11-Cre and generated Cdh5-Cre; Ido1 F/F (Ido1 ecKO) or Myh11-Cre; Ido1 F/F (Ido1 vsmcKO) transgenic mice. Their respective littermate of Ido1 F/F were used as controls. The transgenic mice and wild-type mice were in a C57BL/6 background and purchased from Jackson Laboratory (Bar Harbor, ME, USA). Tail biopsy DNA was extracted for genotyping according to the Jackson protocol. All mice were housed in temperature-controlled cages under a 12-h light–dark cycle and given free access to water and food at Georgia State University.
Neonatal cardiac apex resection surgery
The neonatal mouse heart regeneration model was generated by heart apical resection (AR) surgery for 3–4 weeks as described9. Briefly, postnatal day 1 (P1) or P6 mice were subjected to anesthesia by freezing for 3–5 min. A curved forcep was extended into the chest to pull the heart out. Then, the left ventricular apex was truncated with microsurgical scissors until the ventricular chamber was exposed. The mouse chest was sewn up with 8-0 sutures, and the mice were warmed until recovery. The entire procedure required approximately about 10 min. Sham procedures excluded apex amputation. To detect the role of 1-methyl-D-tryptophan (1MT) and kynurenine (Kyn) in heart regeneration, 1MT (100 mg/kg) and Kyn (100 mg/kg) were intraperitoneally injected into wild-type mice every other day from P1 and were subjected to AR surgery at P2 and P6, respectively. The equal volume of PBS was used as vehicle control.
Echocardiography analysis
Mouse cardiac functions were evaluated by transthoracic echocardiography. Transthoracic 2D M-mode echocardiogram and pulsed wave Doppler spectral tracings were obtained by using a Vevo 3100 High-Resolution Micro-Ultrasound System (FUJIFILM Visual Sonics Inc., Canada) in mice anesthetized using a mixture of 1.5% isoflurane and 0.5 L/min oxygen. All left ventricular (LV) measurements were taken in M-mode short-axis at the level of papillary muscles. Fractional shortening (FS) and ejection fraction (EF) were calculated from the LV dimensions in the 2D short-axis view. The echocardiography measurements were performed with blinding.
Primary cell isolation, co-culture, and gene silencing transfection
The primary neonatal (P1) mouse CMs were isolated and cultured according to the protocol of the Primary Cardiomyocyte Isolation Kit (Thermo Fisher Scientific). In brief, hearts were freshly minced and incubated with isolation enzymes-I and -II mixture, then pipetted into a single-cell suspension. The isolated CMs were confirmed by Troponin T immunostaining, and cell viability was assessed by Cell Counting Kit-8 (CCK-8) assay. The isolated CMs were maintained in Dulbecco-modified Eagle medium with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 μg/mL) for 1 day, then cardiomyocyte growth supplement (provided by the manufacturer) was added to the medium.
The isolation of primary neonatal (P1) mouse cardiac microvascular endothelial cells (MCECs) was performed as described57. In brief, the whole heart was dissected, minced into small pieces, and digested with dispase/collagenase-II. Then, cells were washed and incubated with magnetic beads, which were conjugated with anti-CD31 antibody. The isolated cells were cultured in M199 Medium with heparin, d-valine, IFCS, EGS, penicillin/streptomycin, and 10% FBS. The purified MCECs were co-stained with lectin and CD31, two EC markers. Primary cells were used after 5 days’ culture.
CMs and MCECs were co-cultured as we previously described58 with modification. Briefly, CMs were cultured in full M199 medium in the upper chamber of a trans-well system with 0.4-μm aperture (Corning, New York, USA). The CMs were cultured in the medium containing IFNγ and treated with or without IDO1 and AHR inhibitor for 24 h. The CMs were transferred to co-culture with MCECs, which were cultured in the lower chamber. At 48 h after the co-culture, the cells were harvested for further experiments. Transient transfection of siRNA in cultured cells involved using Lipofectamine RNAiMAX (Life Technologies) according to the manufacturer’s instructions.
Histology and immunostaining
Isolated hearts at indicated stages were fixed in 4% paraformaldehyde in PBS, embedded in paraffin, and sectioned at 5-μm intervals. H&E staining and Masson’s trichrome staining followed standard procedures as we previously reported9. For immunofluorescent staining, slides were deparaffinized, rehydrated, permeabilized, and blocked. Then, sections were incubated with specific antibodies at 4 °C overnight. After washing, the sections were incubated with a secondary antibody at room temperature for 1 h. At last, the sections were rinsed and sealed with a mounting medium containing DAPI (Vector Laboratories). Terminal deoxynucleotidyl transferase dUTP nick-end label (TUNEL) and TNT fluorescent double staining were performed with the TUNEL Enzyme and TUNEL Label Mix (Roche). Images of five fields per section were obtained by confocal microscopy (LSM800, Carl Zeiss Microscopy) and quantified with LSM510 image analysis software.
Antibodies used in this study included rabbit anti-PH3 (Cell Signaling), rabbit anti-KI67 (Abcam), rabbit anti-VEGFA (Abcam), mouse anti-smooth muscle α-actin (Sigma-Aldrich), rabbit anti-CD31 (Cell Signaling), mouse anti-cardiac troponin T (cTnT, Thermo Scientific), rat anti-IDO1 (Novus), mouse anti-IDO1 (Millipore) and mouse anti-AHR (Santa Cruz Biotechnology).
Western blot analysis
Mouse tissues were homogenized and lysed by using RIPA buffer (Santa Cruz Biotechnology). Protein contents were measured by using the Bradford assay (Pierce Biotechnology). Detailed steps for western blot analysis were as previously described59. The primary antibodies included goat anti-KYNU, rabbit anti-KMO, mouse anti-KAT1, mouse anti-Cyclin B1, mouse anti-GAPDH (all above antibodies from Santa Cruz Biotechnology); mouse anti-YAP1, mouse anti-phospho-YAP (ser 127), rabbit anti-Erk1/2 (Thr202/Tyr204), rabbit anti-Erk1/2, rabbit anti-c-Myc, rabbit anti-phospho-Src (Tyr527) and rabbit anti-Src (all seven antibodies from Cell Signaling). The relative protein levels were calculated by normalizing to GAPDH protein as a loading reference by using ImageJ.
Reverse-transcription PCR (RT-PCR)
Total RNA was isolated by using Trizol (Invitrogen) and reversely transcribed to complementary DNA by using the iScriptTM cDNA Synthesis Kit (Bio-Rad Laboratories). All samples were amplified in triplicate by using the iQTM SYBR Green Supermix kit (Bio-Rad Laboratories) and CFX96 Real-Time System (Bio-Rad Laboratories). All data were analyzed by the 2-ΔΔCT method. The specific primer sequences are in Supplementary Table 1.
Measurement of tryptophan (Trp), Kyn, and 3-hydroxyanthranilic acid (3-HAA)
HPLC assay was used for measuring Trp, Kyn, and 3-HAA concentrations in heart tissues and cultured cells, in conditioned medium. Tissues or cells were homogenized with RIPA lysis buffer, and total protein was quantified by using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The culture medium was centrifuged and filtered. An amount of 100 μl of medium or cell lysis was mixed with100 μl of 12% perchloric acid and vortex and centrifuged at 10,000×g for 5 min. The protein-free supernatant was determined by HPLC assay as we described previously26,27.
ChIP-qPCR
Mouse MCECs were isolated from heart tissues and cross-linked with 1% formaldehyde and quenched with glycine as we previously described9,60. ChIP protocol was performed with the Simple ChIP® Enzymatic Chromatin IP Kit (CST) following the manufacturer’s instructions. We used AHR antibody or anti-IgG (Santa Cruz Biotechnology) to immune-precipitate genomic DNA. Then, we quantified the enrichment of gene DNA fragments by qPCR and normalized with 5% input genomic DNA. The specific primers used for qPCR were designed according to previous AHR ChIP sequencing assay61,62 and are listed in Supplementary Table 1.
Tube-formation assay
Tube-formation assay was used as a model of MCEC angiogenesis. The primary MCECs (5 × 104 cells) were seeded in 24-well plates pre-coated with Matrigel (BD Biosciences). The cells were co-cultured with treated CMs for 24 h. Cells were observed to form capillary structures under light microscopy (Olympus Pittsburgh) at ×20 magnification. Tube formation was quantified as the average of tube numbers and branch points by using ImageJ. Four fields of view/experiments were examined, for a total of three biological replicates.
Scratch wound assay
Cell migration was determined by scratch wound assay. The cell monolayer was scratched in a direction perpendicular to a horizontal line by using a 200-μl pipette tip. Human recombinant VEGF (Sigma; 100 ng/ml)-induced cell migration was used as a positive control. At 24 h after the treatment, the average distance for cells migrating into the wound was measured by using ImageJ and normalized to the vehicle control. Data were generated from four fields of view/experiment in a total of three biological replicates.
Cignal 45-signaling pathway reporter assay
45-signaling and YAP pathway activity involved using Cignal Reporter Arrays (Qiagen, Germany) following the manufacturer’s instructions. The 100-µl complex of transfection reagent with individual nucleic acids was prepared in 96 wells. A 50-µl suspension of primary regenerating CMs (5 × 104/well) from wild-type or IDO1-deletion mice were added and incubated for 24 hr. Luciferase activity was measured by using the Dual-Luciferase Assay system (Promega) on the Infinite 200 PRO (TECAN). Firefly luciferase was used as the experimental reporter, and Renilla luciferase was used as the normalizing reporter. We performed experiments for each vector as biological duplicates with two technical repeats.
Statistical analysis
All statistical analyses were performed by using SPSS 20.0. The data were plotted as column with scattered points using GraphPad Prism 8.3.0. Quantitative results are expressed as mean ± SD. Normality was assumed and variance was compared between or among groups by the Shapiro–Wilk test. The unpaired Student t test with a two-tailed distribution was used to compare the two groups. The nonparametric Mann–Whitney U test was applied to compare two groups when the normally distributed data were not met. One or two-way ANOVA analysis following Tukey’s multiple comparison was used to compare more than two groups. P < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This study was funded by the US National Heart, Lung, and Blood Institute (HL079584, HL089920, and HL110488 to M.-H.Z., HL128014, HL132500, HL137371, and HL142287 to M.-H.Z. and Z.X.). Dr. Zou is the Eminent Scholar in Molecular and Translational Medicine of the Georgia Research Alliance.
Author contributions
M.-H.Z. conceived this project. D.Z., J.N., R.T., C.Y., and X.Z. managed all mouse lines and performed the surgery, data acquisition, analysis, and interpretation. D.Z., Z.X., P.S., and M.-H.Z. wrote, edited, and revised the paper. All authors approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Paolo Puccetti and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All relevant data are provided as a Source data file, or available within the article and its Supplementary Information files or upon request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41467-025-57248-0
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/24/2023
Editors Note: Readers are alerted that the reliability of the data reported in this article is currently in question. Appropriate editorial action will be taken once this matter is resolved.
Change history
3/3/2025
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41467-025-57248-0
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33734-7.
References
- 1.Anderson, J. L. & Morrow, D. A. Acute myocardial infarction. N. Engl. J. Med.376, 2053–2064 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Cahill, T. J., Choudhury, R. P. & Riley, P. R. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat. Rev. Drug Discov.16, 699–717 (2017). [DOI] [PubMed] [Google Scholar]
- 3.He, L., Nguyen, N. B., Ardehali, R. & Zhou, B. Heart regeneration by endogenous stem cells and cardiomyocyte proliferation: controversy, fallacy, and progress. Circulation142, 275–291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fratz, S. et al. Long-term myocardial scarring after operation for anomalous left coronary artery from the pulmonary artery. Ann. Thorac. Surg.92, 1761–1765 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Haubner, B. J. et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res.118, 216–221 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science331, 1078–1080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haubner, B. J. et al. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging4, 966–977 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strungs, E. G. et al. Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regeneration. Methods Mol. Biol.1037, 343–353 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Zhang, D. et al. REST regulates the cell cycle for cardiac development and regeneration. Nat. Commun.8, 1979 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Nakada, Y. et al. Hypoxia induces heart regeneration in adult mice. Nature541, 222–227 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Song, P., Ramprasath, T., Wang, H. & Zou, M. H. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol. Life Sci.74, 2899–2916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramprasath, T., Han, Y. M., Zhang, D., Yu, C. J. & Zou, M. H. Tryptophan catabolism and inflammation: a novel therapeutic target for aortic diseases. Front. Immunol.12, 731701 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladomersky, E. et al. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin. Cancer Res.24, 2559–2573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolson, A. H. & Wang, H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv. Drug Deliv. Rev.62, 1238–1249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohtake, F. et al. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature446, 562–566 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ye, M. et al. Activation of the aryl hydrocarbon receptor leads to resistance to EGFR TKIs in non-small cell lung cancer by activating Src-mediated bypass signaling. Clin. Cancer Res.24, 1227–1239 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Liu, X. et al. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. Int. J. Cancer143, 1516–1529 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Bishnupuri, K. S. et al. IDO1 and kynurenine pathway metabolites activate PI3K-Akt signaling in the neoplastic colon epithelium to promote cancer cell proliferation and inhibit apoptosis. Cancer Res.79, 1138–1150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pontillo, C. et al. Hexachlorobenzene promotes angiogenesis in vivo, in a breast cancer model and neovasculogenesis in vitro, in the human microvascular endothelial cell line HMEC-1. Toxicol. Lett.239, 53–64 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Roman, A. C., Carvajal-Gonzalez, J. M., Rico-Leo, E. M. & Fernandez-Salguero, P. M. Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J. Biol. Chem.284, 25135–25148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terashima, J., Tachikawa, C., Kudo, K., Habano, W. & Ozawa, S. An aryl hydrocarbon receptor induces VEGF expression through ATF4 under glucose deprivation in HepG2. BMC Mol. Biol.14, 27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai, C. F. et al. Benzyl butyl phthalate induces migration, invasion, and angiogenesis of Huh7 hepatocellular carcinoma cells through nongenomic AhR/G-protein signaling. BMC Cancer14, 556 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai, M. J. et al. Aryl hydrocarbon receptor agonists upregulate VEGF secretion from bronchial epithelial cells. J. Mol. Med.93, 1257–1269 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Ingason, A. B. et al. Angiogenesis precedes cardiomyocyte migration in regenerating mammalian hearts. J. Thorac. Cardiovasc Surg.155, 1118–1127 e1111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polyzos, K. A. & Ketelhuth, D. F. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie35, 128–136 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Wang, Q. et al. Tryptophan-derived 3-hydroxyanthranilic acid contributes to angiotensin II-induced abdominal aortic aneurysm formation in mice in vivo. Circulation136, 2271–2283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, Q. et al. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ. Res.114, 480–492 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metghalchi, S. et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab.22, 460–471 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Gutierrez-Vazquez, C. & Quintana, F. J. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity48, 19–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorgdrager, F. J. H., Naude, P. J. W., Kema, I. P., Nollen, E. A. & Deyn, P. P. Tryptophan metabolism in inflammaging: from biomarker to therapeutic target. Front. Immunol.10, 2565 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, S., Miller, B. J., Stefanek, M. E. & Miller, A. H. Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: relevance to cancer-related fatigue. Cancer121, 2129–2136 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Han, C. et al. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res.25, 1137–1151 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frangogiannis, N. G. Regulation of the inflammatory response in cardiac repair. Circ. Res.110, 159–173 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melhem, N. J. et al. Endothelial cell indoleamine 2, 3-dioxygenase 1 alters cardiac function after myocardial infarction through kynurenine. Circulation143, 566–580 (2021). [DOI] [PubMed] [Google Scholar]
- 35.Du, L. et al. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway. Sig. Transduct. Target Ther.5, 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei, L. et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front. Immunol.9, 724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thaker, A. I. et al. IDO1 metabolites activate beta-catenin signaling to promote cancer cell proliferation and colon tumorigenesis in mice. Gastroenterology145, 416–425 e411-414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mei, J. et al. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol. Hum. Reprod.18, 467–476 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Opitz, C. A. et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature478, 197–203 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto, T. et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal12, eaaw3306 (2019). [DOI] [PubMed]
- 41.Bock, K. W. Human AHR functions in vascular tissue: pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem Pharm.159, 116–120 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Labadie, B. W., Bao, R. & Luke, J. J. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the tryptophan-kynurenine-aryl hydrocarbon axis. Clin. Cancer Res.25, 1462–1471 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park, S., Dong, B. & Matsumura, F. Rapid activation of c-Src kinase by dioxin is mediated by the Cdc37-HSP90 complex as part of Ah receptor signaling in MCF10A cells. Biochemistry46, 899–908 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Gomez-Duran, A. et al. Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFbeta signaling. Biochem. Pharm.77, 700–712 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol.20, 211–226 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Chen, Y. et al. Long non-coding RNA ECRAR triggers post-natal myocardial regeneration by activating ERK1/2 signaling. Mol. Ther.27, 29–45 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morikawa, Y., Heallen, T., Leach, J., Xiao, Y. & Martin, J. F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature547, 227–231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miret, N. et al. A dioxin-like compound induces hyperplasia and branching morphogenesis in mouse mammary gland, through alterations in TGF-beta1 and aryl hydrocarbon receptor signaling. Toxicol. Appl. Pharm.334, 192–206 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Mulero-Navarro, S. & Fernandez-Salguero, P. M. New trends in aryl hydrocarbon receptor biology. Front. Cell Dev. Biol.4, 45 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choudhary, M. et al. Aryl hydrocarbon receptor knock-out exacerbates choroidal neovascularization via multiple pathogenic pathways. J. Pathol.235, 101–112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichihara, S. et al. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb. Vasc. Biol.27, 1297–1304 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Investig.124, 1382–1392 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng, B., Chen, H. C., Chou, I. W., Tang, T. W. & Hsieh, P. C. Harnessing the early post-injury inflammatory responses for cardiac regeneration. J. Biomed. Sci.24, 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Investigators, S. et al. Darapladib for preventing ischemic events in stable coronary heart disease. N. Engl. J. Med.370, 1702–1711 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Gong, R., Jiang, Z., Zagidullin, N., Liu, T. & Cai, B. Regulation of cardiomyocyte fate plasticity: a key strategy for cardiac regeneration. Signal Transduct. Target Ther.6, 31 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siebel, C. & Lendahl, U. Notch signaling in development, tissue homeostasis, and disease. Physiol. Rev.97, 1235–1294 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Luo, S., Truong, A. H. & Makino, A. Isolation of mouse coronary endothelial cells. J. Vis. Exp. 113, e53985 (2016). [DOI] [PMC free article] [PubMed]
- 58.Zhang, D. et al. Homocysteine activates vascular smooth muscle cells by DNA demethylation of platelet-derived growth factor in endothelial cells. J. Mol. Cell Cardiol.53, 487–496 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Wu, S. et al. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation139, 1913–1936 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, D. et al. Non-CpG methylation by DNMT3B facilitates REST binding and gene silencing in developing mouse hearts. Nucleic Acids Res.45, 3102–3115 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nault, R. et al. Pyruvate kinase isoform switching and hepatic metabolic reprogramming by the environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci.149, 358–371 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang, S. Y., Ahmed, S., Satheesh, S. V. & Matthews, J. Genome-wide mapping and analysis of aryl hydrocarbon receptor (AHR)- and aryl hydrocarbon receptor repressor (AHRR)-binding sites in human breast cancer cells. Arch. Toxicol.92, 225–240 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are provided as a Source data file, or available within the article and its Supplementary Information files or upon request. Source data are provided with this paper.