Abstract
In recent years, activation of thermal transient receptor potential (TRP) ion channels at a range of temperatures has received widespread attention as a target for traditional Chinese medicine (TCM) to regulate body temperature and relieve pain. Discovery of transient receptor potential vanilloid 1 (TRPV1) was awarded a Nobel Prize, reflecting the importance of these channels. Here, the regulatory effects of TCMs and their active ingredients on TRP ion channels are reviewed, and future directions for research on the cold, hot, warm, cool, and neutral natures of TCMs are considered. In herbs with cold, hot, warm, cool, and neutral natures, we found 29 TCMs with regulatory effects on TRP ion channels, including Cinnamomi Cortex, Capsici Fructus, Rhei Radix et Rhizoma, Macleayae cordatae Herba, Menthae Haplocalycis Herba, and Rhodiolae Crenulatae Radix et Rhizoma. Although some progress has been made in understanding the regulation of TRP ion channels by TCMs and their ingredients, the molecular mechanism by which TCMs have this effect remains to be further studied. We hope this review will provide a reference for further research on the cold, hot, warm, cool, and neutral natures of TCMs.
Keywords: traditional Chinese medicine, Chinese herbal medicinal properties, transient receptor potential channels, natural compounds, pain relief
1 Introduction
Traditional Chinese Medicine (TCM) is a common clinical treatment in traditional Chinese Medicine, and it has demonstrated efficacy in the treatment of complex diseases and major epidemics. Each TCM is a complex medicine with synergy between multiple ingredients, targets, and pathways, and each ingredient has the potential to develop into a new drug. The property theory of TCM is an accumulation of long-term clinical application of TCM, and is an important basis for TCM use. In addition, the four qi theory has important guiding significance for rational use of TCM in clinic (Ma et al., 2020; Zhu et al., 2021) The transient receptor potential (TRP) family mediates the sensations of heat, cold and pain and an understanding of its function can in turn enhance understanding of the pathophysiological regulation of body temperature or pain relief. It is also one of the molecular bases for an in-depth understanding of the cold-hot theory of TCM, and it provides a basis for studying the cold-hot natures of TCM (Gao et al., 2014; Hung and Tan, 2018; Viana, 2018; Isaacson and Hoon, 2021).
Currently, there is a lack of reviews on the regulation of TRP channels by various types of TCMs, and an updated review may help to demonstrate gaps in research, which may also provide new ideas for more in-depth studies in the future. This review focuses on TCMs with regulatory effects on TRP ion channels to systematically explore the molecular mechanism of TCM exerting cold, hot, warm, cool and neutral nature. It will provide a reference for the more scientific use of various types of traditional Chinese medicine in the future.
2 Transient receptor potential channels
Transient receptor potential channels, a class of ion channel that can be activated within a specific temperature range, were discovered in Drosophila melanogaster. TRP ion channels are found in many tissues and have a wide range of physiological functions related to vision, smell, taste, hearing, touch, and body temperature (Uchida et al., 2017). Seven families of TRPs have been identified in mammals, including the vanilloid receptor family (TRPV), the TRP melastatin (TRPM), the TRP ankyrin (TRPA), the TRP canonical (TRPC), the TRP polycystin (TRPP), the TRP mucolipin (TRPML), the TRP NomPC-like (TRPN) (Li, 2017).
TRPC1-7 are activated in a G-protein-coupled receptor-phospholipase C-dependent manner, which is similar to the TRPs of Drosophila (Chen et al., 2020). Mutations in TRPPs cause autosomal dominant polycystic kidney disease and are also involved in taste perception and fertilization processes (Su et al., 2018). TRPML1-3 mediate endo-lysosomal functions such as autophagy, pH regulation and lysosomal exocytosis that are closely related to cancer development (Jaślan et al., 2020; Xu and Dong, 2021). TRPN contributes to the adaption of mechanosensory transduction currents such as tactile sense, auditory, and proprioception, independent of their ion-conduction function (Jin et al., 2017; Li et al., 2021). Three of these families, the TRP vanilloid (TRPV), the TRP melastatin (TRPM), and TRP ankyrin (TRPA), have received a great deal of attention due to their association with cold and heat sensation. The different TRP channel families share common structural features, such as six putative transmembrane (TM) spanning domains with intracellular C and N termini, and a pore lining between the fifth (S5) and sixth (S6) TM. TRP channels function as cellular sensors, regulation of intracellular calcium signalling/free calcium concentration in many cases, and play an important role in muscle contraction, cell differentiation and proliferation, gene transcription, and many other cellular functions as well as cell death (Clapham et al., 2005; Bradshaw et al., 2013; Li, 2017). Jaffal et al. proposed that TRP channels may be involved in the pain, inflammation, fever, and other complications associated with COVID-19, and that blocking or inhibiting TRP channels may be a means of preventing and treating this disease (Jaffal and Abbas, 2021). The relevant functional information of TRP channels that can be activated by different temperatures are comprehensively summarized in Figure 1 and Table 1.
FIGURE 1.
Different TRP channels activated at different temperatures.
TABLE 1.
Introduction to TRP channels
| Channel | Temperature sensitivity | Main tissue distribution | Functions | References |
|---|---|---|---|---|
| TRPV1 | ≥43°C | Sensory neurons, brain, skin. | Noxious thermoreceptors; also involved in inflammatory pain, thermal nociceptive hypersensitivity, hippocampal chronic depression, obesity, diabetes, bladder function, hypertension, hypothermia, gastroenteritis, renal excretory function | (Vay et al., 2012; Uchida et al., 2017; Wu, 2019) |
| TRPV2 | ≥52°C | Sensory neurons, brain, spinal cord, lung, liver, spleen, colon, heart, immunocyte. | Extreme temperature sensor; innate immune system | (Kochukov et al., 2006; Vay et al., 2012; Naticchioni et al., 2015; Uchida et al., 2017) |
| TRPV3 | 34-38°C | Sensory neurons, skin, brain, spinal cord, stomach, colon. | Warmth receptors; may be associated with detection of harmful heat levels | (Kochukov et al., 2006; Vay et al., 2012; Uchida et al., 2017; Neuberger et al., 2021a) |
| TRPV4 | 28-35°C | Sensory neurons, skin, brain, kidneys, lungs, inner ear, bladder. | Thermoreceptors; may be associated with noxious mechanical pain and thermal nociceptive sensitization | (Vay et al., 2012; Uchida et al., 2017; Huang et al., 2019) |
| TRPM2 | >36°C | Brain, immunocyte, pancreas. | Thermoreceptors; associated with pain due to inflammation, diabetes, tumors, cardiovascular system | (Kahya et al., 2017; Uchida et al., 2017) |
| TRPM4 | 15-35°C | Heart, liver, immunocyte, pancreas. | Thermoreceptors; associated with inflammation-induced pain, tumors, cardiovascular system | (Talavera et al., 2005; Uchida et al., 2017; Dutta Banik et al., 2018) |
| TRPM5 | 15-35°C | Taste cells, pancreas. | Warmth receptors; may be related to insulin secretion, obesity, diabetes | (Talavera et al., 2005; Uchida et al., 2017; Dutta Banik et al., 2018) |
| TRPM8 | ≤28°C | Sensory neurons, bladder. | Nontoxic cold sensory receptors, behavioral thermoregulation, cold-mediated analgesia; cold injurious sensation in some neurons | (Vay et al., 2012; Uchida et al., 2017; Yin et al., 2018) |
| TRPA1 | ≤17°C | Sensory neurons, heart, lungs, brain, pancreas, gastrointestinal tract, bladder. | Cold, mechanically- and chemically-induced injuries, cold nociceptive sensitization | (Vay et al., 2012; Uchida et al., 2017 |
2.1 Classification of transient receptor potential channels
2.1.1 Transient receptor potential vanilloid 1
TRPV1 is a non-selective ligand-gated cation channel, which in humans functions as a pain sensor under thermal stimulation (Caterina et al., 1997). The normal activation threshold of TRPV1 was found to be ≥43°C, while conditions such as acidic environment (pH<5.0), voltage stimulation, capsaicin, osmotic concentration, and endogenous cannabinoids can also activate the channel. In response, the body may produce a combination of sensations such as acidity, heat, burning, pain, and itching (Liu et al., 2017b; Wu, 2019). Julius et al. found a loss of sensitivity to capsaicin and resiniferatoxin in TRPV1−/− mice via various cellular and mouse behavioral analyses (Caterina et al., 2000). Lubova et al. found that K688G as a part of the gating apparatus of the TRPV1 channel may uncouple the TRP structural domain from its pore, thus leading to spontaneous activation and desensitization of the channel, which provides a basis for further study of this channel (Lubova et al., 2020).
2.1.2 Transient receptor potential vanilloid 2
TRPV2 is a calcium-permeable, non-selective cation channel that is essential for normal cardiac contractility and is widely distributed in central and peripheral nerves, including the spinal cord, brain, dorsal root ganglia, and trigeminal ganglion (Naticchioni et al., 2015; Xue et al., 2021). Using immunohistochemistry, Nedungadi et al. found that TRPV2 channels are abundantly expressed in the giant cell division of the anterior brain, supraoptic nucleus, and paraventricular nucleus of the hypothalamus (Nedungadi et al., 2012). The thermal activation threshold of TRPV2 is as high as 52°C (Kochukov et al., 2006).
2.1.3 Transient receptor potential vanilloid 3
TRPV3 is activated in the temperature range from 34°C to 38°C and is involved in a variety of physiological processes, such as temperature perception, nociception, pruritus, and wound healing (Kochukov et al., 2006; Neuberger et al., 2021a). Deng et al. found that S100A4 (S100 calcium-binding protein family) interacts with the TRPV3 channel protein, thereby significantly enhancing the latter’s ability to regulate calcium ions, with implications for the treatment of neurological diseases (Deng et al., 2021).
2.1.4 Transient receptor potential vanilloid 4
TRPV4 is activated in the temperature range from 28°C to 35°C (Huang et al., 2019) and can directly cause hereditary neurodegenerative syndromes. Woolums et al. found that TRPV4-mediated neurotoxicity is regulated by the Ca2+-binding mitochondrial GTPase Miro, which demonstrates that TRPV4 antagonists are effective in the treatment of TRPV4-mediated neurodegenerative diseases (Woolums et al., 2020).
2.1.5 Transient receptor potential melastatin 2
TRPM2 plays an important role in the sensation of warmth and helps to control body temperature. TRPM2−/− results in a loss of thermal detection capability (Paricio-Montesinos et al., 2020). Tan and McNaughton. (2016) found that at temperatures above 23°C TRPM2 expressed in somatosensory neurons triggers signals which generate a sensation of warmth in mice, causing them to look for cooler environments. TRPM2−/− mice were unable to distinguish between cool and warmth. Kashio et al. (2012) used the [Ca2+]i test to analyze the temperature threshold for activation of TRPM2 and found that temperature of about 47°C can also activate TRPM2. Gattkowski et al. discovered a novel CaM-binding site in the NudT9H domain of TRPM2, which may have a role in the temperature sensitivity of TRPM2 channels. The discovery of this site laid the foundation for the study of temperature-activated TRPM2 channels (Gattkowski et al., 2019).
2.1.6 Transient receptor potential melastatin 4 and transient receptor potential melastatin 5
TRMP4 and TRPM5 are thermally activated ion channels which are highly sensitive to temperatures ranging from 15°C to 35°C (Talavera et al., 2005). Using live-cell imaging and behavioral analysis of knockout mice, Dutta Banik et al. found that TRPM4 and TRPM5 are required for transduction of gustatory stimulation and loss of either channel severely affects taste perception (Dutta Banik et al., 2018).
2.1.7 Transient receptor potential melastatin 8
TRPM8 is the body’s primary sensor of cold and menthol perception, can be activated by temperatures below 28°C, and is a relevant target for the treatment of pain, such as chronic pain and migraine (Yin et al., 2018). Huang et al. demonstrated that the K423 in the N-terminal of TRPM8 was the major ubiquitination residue for the regulation of TRPM8 by tripartite motif-containing 4 (TRIM4), laying the foundation for study of the interaction between TRIM4 and TRPM8 (Huang et al., 2021).
2.1.8 Transient receptor potential ankyrin 1
TRPA1, a tetrameric, nonselective cation channel in dorsal root sensory neurons, is a key factor in mediating visceral pain. TRPA1 channel agonists can treat obesity and other related diseases, such as type 2 diabetes, by regulating the secretion of insulin, providing a new avenue for study on the treatment of diabetes (Liu et al., 2019; Sahin et al., 2019). TRPA1 is a low threshold sensor (≤17°C). Zhao et al. investigated a highly conserved calcium-binding pocket which can explain all aspects of calcium-dependent TRPA1 regulation, including activation, enhancement, and desensitization of metabolic receptors (Zhao et al., 2020).
3 Studies on regulation of transient receptor potential channels by hot traditional Chinese medicines and their components
3.1 Capsici Fructus
Capsici Fructus is the dried fruit of Capsicum annuum L, pertaining to Solanaceae, which is acrid and hot in nature (State Pharmacopoeia Commission, 2020). Julius et al. investigated the phenomenon of pain desensitization due to the long-term use of capsaicin (CAP). They isolated a functional cDNA encoding capsaicin receptor from sensory neurons using an expression cloning approach based on calcium influx. Using this approach, they found that VR1 (an early name for TRPV1) can be activated by a range of toxic temperatures, and TRPV1 became known as the capsaicin receptor (Caterina et al., 1997). CAP can induce intracellular kinases, including PLC, PKCε, PKA, and TrkA, to lower the thermal threshold of TRPV1 and thus search for novel analgesics through phosphorylated the TRPV1 channels (Szolcsányi, 2015). In addition, CAP is thermogenic in nature and activates brown adipose tissue (BAT) for fat loss by acting on TRPV1 channels (Saito, 2015). Through a series of animal model experiments, Liang et al. concluded that CAP regulates the expression levels of genes related to glucose metabolism, such as PPARα, GLUTs, IRS-1&2, PI3K, AKT, UCPs, and PDX-1, by activating TRPV1 channels. This finding demonstrated that CAP may activate the TRPV1 channel to direct insulin signaling in the brain and treat diabetes and obesity by lowering blood sugar and improving insulin sensitivity (Liang et al., 2021).
3.2 Cinnamomi Cortex
Cinnamomi Cortex is the bark of Cinnamomum cassia Presl, pertaining to Lauraceae, which is acrid, sweet, and hot in nature (Ranasinghe et al., 2013; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Bhave et al. demonstrated that cAMP-dependent protein kinase (PKA) directly phosphorylates VR1 (TRPV1) and increased VR1 sensitivity (Bhave et al., 2002). Li et al. found that Cinnamomi Cortex can increase the levels of cAMP and cGMP, and can significantly increase the ratio of cAMP/cGMP. Its ingredient cinnamaldehyde (CA) can significantly affect the TRP channel of dorsal root ganglion (DRG) neurons after cold load, and can thereby up-regulate the expression of TRPV1 mRNA and down-regulate the expression of TRPM8 mRNA (Sui et al., 2010; Li et al., 2016). Experiments by Hynkova et al. (2016) showed that CA can be used to study the molecular mechanisms regulating TRPA1 channels, such as the N-terminal tetrapeptide T/SPLH motifs. Kang et al. also demonstrated that the concentration of Ca2+ in the cytoplasm was reduced under cold stress, while the cold-sensitive channel TRPA1 was opened. CA was found to promote the excitation of cold-sensitive channel TRPA1 and return Ca2+ to the cytoplasm, thereby increasing the concentration of Ca2+ and activating cell function, thus demonstrating the warming effect of Cinnamomi Cortex (Kang et al., 2017).
3.3 Evodiae/Euodiae Fructus
Evodiae Fructus is the almost ripe fruit of Evodia rutaecarpa (Juss.) Benth, Evodia rutaecarpa (Juss.) Benth. var. officinalis (Dode) Huang or Evodia rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang, pertaining to Rutaceae, which is acrid, bitter, hot, and mildly toxic in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Iwaoka et al. used calcium imaging, whole-cell patch-clamp technology, and behavioral analysis in rats to find that in vivo treatment with evodiamine could inhibit the thermal hyperalgesia induced by plantar injection of capsaicin, and that capsazepine, a competitive antagonist of TRPV1, can completely block the effect of evodiamine (Iwaoka et al., 2016). In contrast, Liu et al. (2022) verified that evodiamine could mediate reactive oxygen species-dependent cytotoxicity associated with the TRPV1/Ca2+ pathway. A study by Wang et al. provides evidence that evodiamine and rutaecarpine may be partial agonists (antagonists) of TRPV1 since they may share binding sites with capsaicin (Wang et al., 2016). Through experiments in rats, Li et al. (2018) demonstrated that evodiamine could agonize the TRPA1/CGRP signaling pathway, thereby inhibiting the gastric mucosal damage caused by cold-binding stress (Li et al., (2020). Through TRPV1, Liu demonstrated that the combination of Evodiae Fructus and Zingiberis Rhizoma Recens can exceed the maximum effect that can be achieved when they are used alone (Liu, 2017).
3.4 Zingiberis Rhizoma
Zingiberis Rhizoma is the rhizoma of Zingiber officinale Rosc, pertaining to Zingiberaceae, which is acrid and hot in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Li et al. (2018) found that Zingiberis Rhizoma may increase the expression of TRPV1 gene and protein and decrease the expression of TRPM8 gene and protein by up-regulating the cAMP/PKA pathway in primary rat dorsal root ganglion cells, thus exhibiting the hot nature of Zingiberis Rhizoma (Li et al., 2018). T551 and E571 are key sites for the binding of 6-gingerol, 6-Shogaol and zingerone to the TRPV1 channel (Yin et al., 2019a). Yang indicated that the hot nature of Zingiberis Rhizoma is achieved by activating the TRPV1 channels, and its ingredients 6- and 10-gingerol can release catecholamines to produce a systemic warm sensation, but this process can be counteracted by TRPV1 receptor antagonists (Yang, 2015).
3.5 Aconiti Radix
Aconiti Radix is the dry root of Aconitum carmichaelii Debx, pertaining to Ranunculaceae, which is acrid, bitter and hot in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Aconiti Radix mainly contains alkaloids such as aconitine, hypaconitine, and mesaconitine. Yu et al. (2020a) found that hypaconitine could induce TRPV4-mediated calcium influx, causing an increase in cell temperature, and speculated that hypaconitine may have the ability to activate TRPV4 channels. They suggested that the “hot/cold” properties of TCM can be verified from the perspectives of cellular and molecular biology, providing a method by which to study the theory of TCM properties.
4 Studies on regulation of transient receptor potential channels by warm traditional Chinese medicines and their components
4.1 Asari Radix et rhizoma
Asari Radix et Rhizoma is the dry root and rhizoma of Asarum heterotropoldes Fr.Schmidt var. mandshuricum (Maxim.) Kitag, Asarum sieboldii Miq. var. seoulense Nakai or Asarum sieboldii Miq, pertaining to Aristolochiaceae, which is acrid and warm in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Yu detected the main ingredients in the water extract of Asarum (WA), such as higenamine and caulesnarinside, via the UHPLC-Q-TOF/MS method, and in a capsaicin-induced pain experiment, observed that WA inhibited the number of foot lifts and the time of foot licking in mice. This suggests that WA may inhibit pain induced by acute pain experiments using TRPV1 (Yu, 2019). The volatile oil is the main active ingredient of Asari Radix et Rhizoma, and is largely retained by the ethanol extract of TCM. Using an electrophysiological whole-cell patch-clamp technique and mouse hot and cold plate experiments, Liu et al. found that the transmembrane currents induced by ethanol extract of Asari Radix et Rhizoma were highly similar to those induced by TRPV1 agonist Caps in terms of current density and current-voltage relationships. They also found that ethanol extract of Asari Radix et Rhizoma can prolong the behavioral latency of cold and hot pain in mice, depending on dose. These results suggest that the volatile oil contained in the ethanol extract of Asari Radix et Rhizoma may activate TRPV1 channels (Liu et al., 2021).
4.2 Zingiberis Rhizoma Recens
Zingiberis Rhizoma Recens is the fresh rhizoma of Zingiber officinale Rosc, pertaining to Zingiberaceae, which is acrid and slightly warm in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Yin showed that the three pungent compounds from Zingiberis Rhizoma Recens were mTRPV1 agonists, with an order of activation intensity of 6-shogaol > 6-gingerol > zingerone, while the molecular structure of 6-shogaol was most similar to that of capsaicin (Yin et al., 2019b). Yue et al. conducted experiments using the rat spine and showed that allyl isothiocyanate, an agonist of TRPA1, inhibited the action of zingerone, but not capsaicin. Zingerone, like TRPA1 agonist, can reduce the amplitude of the excitatory postsynaptic current induced by a single synapse. This indicates that the effect of zingerone enhances glutamatergic spontaneous excitatory transmission in rat substantia gelatinosa neurons is achieved by activating the TRPA1 channels, but not the TRPV1 channels (Yue et al., 2013).
4.3 Angelicae Dahuricae Radix
Angelicae Dahuricae Radix is the dry root of Angelica dahurica (Fisch.ex Hoffm.) Benth.et Hook.f. or Angelica dahurica (Fisch. ex Hoffm.) Benth.et Hook.f.var. formosana (Boiss.) State Pharmacopoeia Commission, (2020), Zhang and Ye, (2020), pertaining to Umbelliferae, which is acrid and warm in nature. Furanocoumarin imperatorin is one of the active ingredients of Angelicae Dahuricae Radix extracts. Chen et al. (2014) found that imperatorin could inhibit formalin and capsaicin-induced nociceptive responses, and confirmed that imperatorin could act as an agonist of TRPV1. This indicates that furanocoumarin imperatorin represents a novel class of TRPV1 regulators.
4.4 Caryophylli Flos
Caryophylli Flos is the dried flower bud of Eugenia caryophllata Thunb, pertaining to Myrtaceae, which is acrid and warm in nature (State Pharmacopoeia Commission, 2020). Inoue et al. used the blind whole-cell patch-clamp technique to demonstrate that eugenol activates TRPA1 channels in the nerve terminals, resulting in an increased spontaneous release of L-glutamate to substantia gelatinosa (SG) neurons. Eugenol therefore has the pharmacological effects of sedation and reduced threshold for convulsions (Inoue et al., 2012).
4.5 ChuanXiong rhizoma
ChuanXiong Rhizoma is the dried rhizoma of Ligusticum chuanxiong Hort, pertaining to Umbelliferae, which is acrid and warm in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Tetramethylpyrazine is an alkaloid monomer extracted from ChuanXiong Rhizoma. In the study of the mechanism of the treatment of traumatic brain injury, Yu et al. (2020b) found that tetramethylpyrazine may regulate TRPM4 channels, aquaporin 4, and mitogen-activated protein kinase p38 by regulating the expression level of brain sulfonylurea receptor 1, with therapeutic effect.
4.6 Genkwa Flos
Genkwa Flos is the dried flower bud of Daphne genkwa Sieb. et Zucc, pertaining to Thymelaeaceae, which is bitter, acrid, warm, and toxic in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Yin et al. (2019c) used 75% ethanol extract from Genkwa Flos to conduct animal behavioral experiments and transmembrane current detection in male C57BL/6 mice and HEK293 cells that expressed human TRPV1. The results showed that the latency of responses to cold or hot pain was prolonged in mice treated with Genkwa Flos, and hTRPV11/HEK293 cells could be activated by 10 g L−1 ethanol extract of Genkwa Flos to induce significant transmembrane current (p < 0.01). These findings demonstrated that the warm, anti-inflammatory and analgesic effects of Genkwa Flos may reflect a series of effects induced by the activation of TRPV1.
4.7 Artemisiae Argyi Folium
Artemisiae Argyi Folium is the leaf of Artemisia argyi Lévl.et Vant, pertaining to Compositae, which is acrid, bitter, warm, and slightly toxic in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Guo et al. (2019) proposed that the ethanol extract of Artemisiae Argyi Folium contains ingredients that can activate TRPV1 channels. Using a whole-cell patch-clamp experiment, they showed that 10 mg/ml ethanol extract of Artemisiae Argyi Folium could activate TRPV1 to exceed activation generated by 10 μM capsaicin. It is suggested that the high capacity of Artemisiae Argyi Folium to activate TRPV1 channels, warm the meridian and dissipate cold to relieve pain effects of Artemisiae Argyi Folium may be related to the activation of TRPV1 channels.
4.8 Notopterygii Rhizoma et radix
Notopterygii Rhizoma et Radix is the dry rhizome and root of Notopterygium incisum Ting ex H.T.Chang or Notopterygium franchetii H. de Boiss, pertaining to Apiaceae, which is acrid, bitter, and warm in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Liu et al. used the whole-cell voltage-clamp technique to record the effect of water extract of Notopterygii Rhizoma et Radix on transmembrane current. They found that the maximum transmembrane current produced by the water extract was at a concentration of 100 mg/ml, and that the current was completely abolished by capsaicin, a specific TRPV1 antagonist. Thus, they hypothesized that Notopterygii Rhizoma et Radix may underlie effects of pain relief, releasing exterior, and dissipating cold after activating TRPV1 channels (Liu et al., 2017a). Wang et al. studied the effect of water extract of Notopterygii Rhizoma et Radix on TRPV1 channels through a neuropathic pain model induced by acute pain and chronic constriction injury. They found that the extract significantly inhibited the mRNA and protein expression of TRPV1, thereby alleviating neuropathic pain (Wang et al., 2021).
4.9 Cnidii Fructus
Cnidii Fructus is the mature dry fruit of Cnidium monieri (L.) Cuss, pertaining to Umbelliferae, which is acrid, bitter, warm, and slightly toxic in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Osthole is the bioactive ingredient of Cnidii Fructus. Neuberger et al. found that two types of osthole binding sites in the transmembrane region of TRPV3 were consistent with the binding sites of agonist 2-APB, indicating that osthole may be used to treat a variety of skin-related diseases (Neuberger et al., 2021b). Sun et al. found that osthole could significantly reduce scratching behavior induced by acetone-ether-water or histamine in mice, whereas mice with TRPV3 gene deletion showed no apparent response. This suggests that osthole can inhibit the function of TRPV3 and provide a therapeutic option for itch-related diseases (Sun et al., 2018). It has also been reported that limonene, an ingredient in the volatile oil of Cnidii Fructus, can activate the TRPA1 channels and exert an analgesic effect (Kaimoto et al., 2016). An et al. (2018) used the water and ethanol extract of Cnidii Fructus to detect the transmembrane currents of hTRPV1/HEK293 cells. The results showed that both the water and ethanol extracts of Cnidii Fructus could produce transmembrane currents in such cells, which suggests that it may exert its acrid and warm natures by activating the TRPV1 channels.
4.10 Allii Sativi Bulbus
Allii Sativi Bulbus is the bulb of Allium sativum L, pertaining to Liliaceae, which is acrid and warm in nature (State Pharmacopoeia Commission, 2020). Allicin from Allii Sativi Bulbus is an antioxidant. Tsuchiya et al. studied the regulatory effect of allicin on electrogenic ion transport in rat intestine via the transmural potential difference, and found that AP-18, an inhibitor of TRPA1 channels, significantly reduced the transmural potential difference induced by allicin. Allicin leads to electrogenic chloride and bicarbonate secretion via TRPA1. In contrast, in the absence of chloride and bicarbonate, the transmural potential difference in the colon is reduced. This may be related to the contractile function of the intestine (Tsuchiya and Kawamata, 2019).
4.11 Cinnamomi Ramulus
Cinnamomi Ramulus is the tender twig of Cinnamomum cassia Presl, pertaining to Lauraceae, which is acrid, sweet, and warm in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Cinnamomi Ramulus contains cinnamaldehyde, which can desensitize both TRPV1 channels and cross-desensitize TRPA1 channels. Hu et al. (2014) indicated that the use of Cinnamomi Ramulus can block overactive bladder syndrome caused by abnormal activation of TRPV1 channels.
4.12 Murrayae Folium et cacumen
Murrayae Folium et Cacumen is the leaf and tender twig with leaves of Murraya exotica L. or Murraya paniculate (L.) Jack, pertaining to Rutaceae, which is acrid, slightly bitter, warm, and slightly toxic in nature (State Pharmacopoeia Commission, 2020). Two new natural coumarin enantiomers, namely B304-1 and B304-2, were extracted and isolated from the root of Murrayae Folium et Cacumen by Zhou, who verified that TRPV2 channels were highly expressed in brown adipocytes. Zhou et al. (2020) performed whole-cell patch-clamp experiments, the results of which showed that both B304-1 and B304-2 could inhibit endogenous TRPV2 currents in differentiated brown adipocytes, with potential for the treatment of energy imbalance or metabolic diseases.
5 Studies on regulation of transient receptor potential channels by cold traditional Chinese medicines and their components
5.1 Rhei Radix et rhizoma
Rhei Radix et Rhizoma is the root and rhizoma of Rheum palmatum L, Rheum tanguticum Maxin.ex Balf. or Rheum officinale Baill, pertaining to Polygonaceae, which is bitter and cold in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Wan et al. found that Rhei Radix et Rhizoma significantly decreased the expression levels of TRPV1 and significantly increased those of TRPM8 in the hypothalamus and dorsal root ganglia using immunohistochemistry and Western blot methods. As a result, the yeast-induced body temperature rise in rats was significantly reduced (Wan et al., 2014).
5.2 Coptidis Rhizoma
Coptidis Rhizoma is the rhizoma of Coptis chinensis Franch, C. deltoidea C. Y. Cheng et Hsiao or C. teeta Wall, pertaining to Ranunculaceae, which is bitter and cold in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Wan et al. found that after administration of Coptidis Rhizoma in rats, body temperature reduced, the expression level of TRPV1 mRNA decreased and that of TRPM8 mRNA in the hypothalamus and dorsal root ganglia was effectively increased. These results show that Coptidis Rhizoma can effectively improve the fever symptoms in an animal model of heat syndrome (Wan et al., 2014).
5.3 Macleayae cordatae herba
Macleayae cordatae Herba is the root and whole plant of Macleaya cordata (Willd.) R. Br. or M. cordata (Maxim.) Fedde, pertaining to Papaveraceae, which is bitter and cold in nature (Xu et al., 2018). The main chemical ingredients of Macleayae cordatae Herba are alkaloids. Sanguinarine is a benzylisoquinoline alkaloid extracted from Macleayae cordatae Herba, which has a wide range of pharmacological activities, such as anti-microbial, anti-tumor, anti-platelet, and anti-hypertensive (Li et al., 2021; Xu et al., 2021). Chi et al. used electrophysiological methods to demonstrate potent activation of TRPA1 channels by sanguinarine with EC500.09 (0.04–0.13) μM, providing evidence that sanguinarine is an agonist of TRPA1 channels (Chi et al., 2021). This may lay the foundation for development of targeted drugs that modulate TRPA1 channel.
5.4 Paeoniae Radix Alba
Paeoniae Radix Alba is the dried root of Paeonia lactiflora pall, pertaining to Ranunculaceae, which is bitter, sour and slightly cold in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Using Western blot, Zhang showed the effect of Paeoniae Radix Alba on TRP in DRG neurons and found that Paeoniae Radix Alba could restore the expression levels of TRPV1 and TRPM8 to normal levels, thus relieving chronic constriction injury of the sciatic nerve (Zhang, 2020).
5.5 Kansui Radix
Kansui Radix is the root tuber of Euphorbia kansui T.N.Liou ex T.P.Wang, pertaining to Euphorbiaceae, which is bitter, cold and poisonous in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Han et al. found that ethanol extract of Kansui Radix could activate hTRPV1/HEK293 cells and induce clear transmembrane currents using an electrophysiological whole-cell patch-clamp technique. This indicates that Kansui Radix contains at least one clear TRPV1 agonist, and its anti-inflammatory and analgesic effects may be related to the activation of TRPV1 ion channels (Han et al., 2020).
5.6 Polygoni Cuspidati Rhizoma et radix
Polygoni Cuspidati Rhizoma et Radix is the root and rhizoma of Polygonum cuspidatum Sieb. et Zucc, pertaining to Polygonaceae, which is slightly bitter and slightly cold in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Resveratrol (RESV) is abundant in Polygoni Cuspidati Rhizoma et Radix, and is a potent antioxidant with protective effects. Çiğ et al. found that TRPM2 channels play an important role in RESV protection of cells from bisphenol A (BisPH-A)-induced oxidative damage. RESV can also effectively reduce the cytotoxicity induced by BisPH-A, such as intracellular Ca2+ overload, reactive oxygen species generation, and caspase activations (Çiğ and Yildizhan, 2020; Dai et al., 2022).
6 Studies on regulation of transient receptor potential channels by cool traditional Chinese medicine and their components
6.1 Menthae Haplocalycis Herba
Menthae Haplocalycis Herba is the dry aboveground part of Mentha haplocalyx Briq, pertaining to Labiatae, which is acrid and cool in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). The monoterpenes menthol is an agonist or antagonist of TRPV1, TRPV3, TRPM8, and TRPA1 channels. Nguyen et al. found that the conserved arginine and glycine residues in the S4-S5 linker of rTRPV1 and mTRPV3 are required for menthol-evoked activation, which may provide a new avenue of research on the pain-relieving properties of menthol (Nguyen et al., 2021). Wang et al. found that carvacrol, an ingredient of peppermint oil, stimulates cell proliferation through TRPV3-mediated calcium influx. In addition, inhibition of TRPV3 expression can eliminate the promotion of carvacrol on the release of TGFα, as well as increasing the phosphorylation levels of EGFR, PI3K, and NF-κB. They also found that inhibition of TRPV3 channels can abolish carvacrol-induced skin diseases such as epidermal hyperplasia, providing a potential form of treatment for such diseases (Wang et al., 2021). Using patch clamp experiments, Niu et al. found that carvacrol can bind to and activate TRPV3 via the S2-S3 linker, providing evidence of chemical stimulation of the TRPV3 channel via this linker (Niu et al., 2022). Nazıroğlu found that carvacrol is a blocker of TRPM2 and TRPV4, and its blocking effect can be achieved by regulating oxidative stress and apoptosis in SH-SY5Y neuronal cells (Nazıroğlu, 2022).
7 Studies on regulation of transient receptor potential channels by neutral traditional Chinese medicine and their components
7.1 Sinomenii Caulis
Sinomenii Caulis is the dried ratan of Sinomenium acutum (Thunb.) Rehd. et Wils. or Sinomenium acutum (Thunb.) Rehd. et Wils. var. cinereum Rehd. et Wils, pertaining to Menispermaceae, which is bitter, acrid, and neutral in nature (State Pharmacopoeia Commission, 2020). Ma et al. found that sinomenine, a constituent of Sinomenii Caulis, significantly reduced capsaicin-induced cough in guinea pigs by reducing the calcium expression of SOX5 and TRPV1 and reducing the secretion of substance P (SP) and neurokinin A (Ma et al., 2021).
7.2 Rhodiolae Crenulatae Radix et rhizoma
Rhodiolae Crenulatae Radix et Rhizoma is the dried root and rhizoma of Rhodiola crenulate (Hook. f. et Thoms.) H. Ohba, pertaining to Crassulaceae, which is bitter, sweet, and neutral in nature (State Pharmacopoeia Commission, 2020). Salidroside is an effective ingredient of Rhodiolae Crenulatae Radix et Rhizoma. Li et al. found that salidroside was able to inhibit the expression level of TRPM8 by reducing the activity of cAMP response element-binding protein, thereby affecting the production of cold-induced mucin and protecting individuals from cold attacks (Li et al., 2013).
7.3 Folium Steviae
Folium Steviae is the leaf of Stevia rebaudiana (Bertoni) Hemsl, pertaining to Compositae, which is sweet and neutral in nature (Li, 2017). Steviol glycosides are natural, zero-calorie, high-intensity sweeteners derived from Folium Steviae. Philippaert et al. (2017) found that these glycosides potentiate the activity of TRPM5 channels and enhance glucose-induced insulin secretion, thus indicating that they can prevent and treat type 2 diabetes by regulating TRPM5 channels.
7.4 Scorpio
Scorpio is the dry bulk of Buthus martensii Karsch, pertaining to Buthidae, which is acrid, neutral and toxic in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Hakim et al. isolated a peptide (BmP01) which can induce pain in mice by activating TRPV1 channels, from the venoms of Scorpio in TRPV1 gene knockout mice. The discovery of BmP01 provides possibilities for the treatment of pain caused by scorpion envenomation (Hakim et al., 2015; Jiang, 2015).
7.5 Cannabis Fructus
Cannabis Fructus is the ripe fruit of Cannabis sativa L, pertaining to Moraceae, which is sweet and neutral in nature (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020). Cannabidiol (CBD) is the active ingredient isolated from Cannabis sativa. It has been reported that CBD is associated with TRPV1, TRPV2, and TRPA1 channels (Iannotti et al., 2014). Kowalski et al. found that the excitatory effect of CBD on vagal afferent neurons may be related to TRPA1 (Kowalski et al., 2020). Pumroy et al. found that CBD interacts with TRPV2 channels through a hydrophobic pocket that is located between S5-S6 helices of adjacent subunits, providing a reference for the study of treatment options for glioblastoma multiforme (Pumroy et al., 2019). Inspired by the fact that TRPV2 and TRPV4 have very similar gene sequences, Huang et al. found that CBD interacts with residues on the S6 helix of TRPV4, thus suggesting that TRPV4 plays an important role in CBD inhibition of glioma (Huang et al., 2021). Maggi et al. confirmed using cell growth and other assays that CBD can inhibit the proliferation and cycle of chronic myeloid leukemia cells through the TRPV2 channel (Maggi et al., 2022).
8 Conclusion and perspectives
In this review, the effects of TCMs and their active ingredients on TRP ion channels are discussed including anti-inflammatory, analgesic, antihypertensive, hypoglycemic, and other pharmacological effects. Despite continued research progress on the protective effects of these TCMs by regulating TRP ion channels, the main active ingredients and mechanisms of their efficacy by activating or inhibiting TRP ion channels remain to be further studied. The information related to these TCMs and their components acting on different TRP channels are comprehensively summarized in Figure 2 and Tables 2, 3.
FIGURE 2.

TCM modulates TRP channels to produce different pharmacological effects.
TABLE 2.
List of Chinese herbal medicines that act on TRP channels
| Name of the medicine | Medicinal properties | Effect | Clinical application | Channels of action | References |
|---|---|---|---|---|---|
| Capsici Fructus | Hot | Warm the center and dissipate cold, promote appetite, and digestion | Cold stagnation and abdominal pain, vomiting, diarrhea, pernio | TRPV1 | (Caterina et al., 1997; State Pharmacopoeia Commission, 2020) |
| Cinnamomi Cortex | Hot | Supplement fire and assist Yang, dissipate cold and relieve pain, warm and unblock the channels, return fire to its source | Yang deficiency syndrome, pain syndromes due to congealing cold, cold congealing, and blood stasis | TRPV1, TRPM8, TRPA1 | (Bhave et al., 2002; Ranasinghe et al., 2013; Hynkova et al., 2016; Li et al., 2016; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Evodiae Fructus | Hot | Dissipate cold and relieve pain, direct counterflow downward and arrest vomiting, assist Yang and arrest diarrhea | All pains due to cold congealing in the liver meridian, vomiting and acid regurgitation, diarrhea due to deficiency-cold | TRPV1, TRPA1 | (Iwaoka et al., 2016; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020; Li et al., 2016) |
| Zingiberis Rhizoma | Hot | Warm the center and dissipate cold, restore Yang to unblock the vessels, warm the lung, and dissolve rheum | Chills and pain of stomach cavity and abdomen, vomiting and diarrhea, Yang collapse syndrome, cough and panting due to cold fluid-retention | TRPV1, TRPM8 | (Li et al., 2018; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Aconiti Radix | Hot | Dispel wind and eliminate dampness, warm the meridians and relieve pain | Bì syndrome, cold pain in the heart and abdomen, and pain due to cold hernia | TRPV4 | (Yu et al., 2020a; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Asari Radix et Rhizoma | Warm | Release exterior and dissipate cold, expel wind and relieve pain, unblock the orifices, warm the lung and dissolve fluid retention | Wind-cold exterior syndrome, headache, toothache, wind-dampness impediment pain, allergic, rhinitis, sinusitis, blocked nose with discharge | TRPV1 | (Yu, 2019; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Zingiberis Rhizoma Recens | Slightly warm | Release exterior and dissipate cold, warm the middle and arrest vomiting, dissolve phlegm and relieve cough, remove toxic of fish and crab | Wind-cold exterior syndrome, vomiting due to stomach cold, cold-phlegm cough, intoxication by eating fish or crab | TRPV1, TRPA1 | (Yue et al., 2013; Yin et al., 2019b; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Angelicae Dahuricae Radix | Warm | Release exterior and dissipate cold, dispel wind and relieve pain, diffuse and unblock the nasal orifices, dry dampness and arrest vaginal discharge, resolve swelling and expel pus | Wind-cold exterior syndrome, headache, pain in supra-orbital bone, toothache, and wind-damp impediment pain, allergic rhinitis, sinusitis, blocked nose with discharge, leukorrhea, sores, and ulcers with swelling and pain | TRPV1 | (Chen et al., 2014; State Pharmacopoeia Commission, 2020) |
| Caryophylli Flos | Warm | Warm the center and direct counterflow downward, nourishing kidney and assist Yang | Deficient cold of spleen and stomach, hiccup vomiting, poor appetite, vomiting and diarrhea, cold pain in the heart and abdomen, impotence due to kidney deficiency | TRPA1 | (Inoue et al., 2012; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| ChuanXiong Rhizoma | Warm | Invigorate blood and move qi, dispel wind and alleviate pain | Pains due to qi stagnation and blood stasis, headache, Bì syndrome | TRPM4 | (Yu et al., 2020b; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020; Zhao et al., 2021) |
| Genkwa Flos | Warm | Expel fluid retention by drastic purgation, kill worms and cure sores (for external use) | Edema, accumulated water in the chest and abdomen, and accumulation of phlegm, scabies, tinea, favus, swollen carbuncle, and pernio | TRPV1 | (Yin et al., 2019c; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020 |
| Artemisiae Argyi Folium | Warm | Warm channels and staunch bleeding, dissipate cold to relieve pain, dispel dampness and relieve itching for external use | Bleeding due to deficiency-cold, irregular menstruation, painful menstruation and threatened miscarriage, itchy skin | TRPV1 | (Guo et al., 2019; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Notopterygii Rhizoma et Radix | Warm | Release exterior and dissipate cold, dispel wind and dampness, relieve pain | External-contraction of wind-cold complicated by dampness, wind-cold-damp impediment, and pain in shoulders and back | TRPV1 | (Liu et al., 2017a; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Cnidii Fructus | Warm | Kill worms and relieve itching, dry dampness and dispel wind, warm the kidney and strengthen Yang | Pudendum itching, eczema and pruritus, as well as acariasis, impotence due to kidney deficiency and sterility due to uterus-cold, leukorrhea due to cold dampness, and lumbago caused by the damp-bì syndrome | TRPV1, TRPV3, TRPA1 | (Kaimoto et al., 2016; An et al., 2018; State Pharmacopoeia Commission, 2020; Neuberger et al., 2021b) |
| Allii Sativi Bulbus | Warm | Resolve toxins and relieve edema, kill worms, arrest dysentery | Carbuncle, swelling, sore and toxic, scabies, phthisis, paroxysmal cough, diarrhea, dysentery | TRPA1 | (Tsuchiya and Kawamata, 2019; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Cinnamomi Ramulus | Warm | Induce sweating to release muscles, warm and unblock meridians, reinforce Yang and promote qi transformation, lower downflow of reversed qi | Wind-cold exterior syndrome, pains induced by wind-cold-damp arthralgia, palpitation phlegm-fluid retention, edema, up-rushing of qi (running piglet qi) | TRPV1, TRPA1 | (Hu et al., 2014; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Murrayae Folium et Cacumen | Warm | Activate qi and relieve pain, invigorate blood and disperse stasis | Stomach-ache, pains of rheumatism and arthralgia, toothache, swelling and pain from falls, insect or snake bite | TRPV2 | (State Pharmacopoeia Commission, 2020; Zhou et al., 2020) |
| Rhei Radix et Rhizoma | Cold | Attack accumulation by purgation, clear heat and drain fire, cool blood and resolve toxic, expel stasis and promote menstruation flow, drain dampness and relieve jaundice | Constipation due to stagnation of excess heat, red eyes and swollen throat, gum pain, hematemesis due to blood heat, pyocutaneous diseases, intestinal abscess with abdominal pain, burn and scald, blood stasis, jaundice, and stranguria | TRPV1, TRPM8 | (Wan et al., 2014; SState Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Coptidis Rhizoma | Cold | Clear heat and dry damp, reduce fire and remove toxic | Stuffiness and fullness due to damp-heat, vomiting, diarrhea, and dysentery, the exuberance of heart-fire syndrome, exuberance of stomach fire syndrome, carbuncle, abscess, furuncle, sores, swollen and painful eyes, eczema, purulent discharge of an auditory canal | TRPV1, TRPM8 | (Wan et al., 2014; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Macleayae cordatae Herba | Cold | Disperse stasis and relieve edema, dispel wind and resolve toxins, kill worms and relieve itching | Thyroid neoplasm, cutaneous tumor, carbuncle, swelling, furuncle, injuries from falls, pains of rheumatism and arthralgia, neurodermatitis, poisonous insect bite | TRPA1 | (Xu et al., 2018; Chi et al., 2021) |
| Paeoniae Radix Alba | Slightly cold | Nourish blood and regulate menstruation, astring yin and arrest sweating, soften the liver and relieve pain, calm and subdue liver Yang | Blood deficiency, a syndrome caused by hyperactivity of liver Yang, rib-side and abdominal pain, spastic pain in four limbs, night sweating, and spontaneous sweating | TRPV1, TRPM8 | (State Pharmacopoeia Commission, 2020; Zhang, 2020; Zhang and Ye, 2020) |
| Kansui Radix | Cold | Expel fluid retention by drastic purgation, relieve swelling and dissipate masses | Edema, accumulated water in the chest and abdomen, and accumulation of phlegm rheum, epilepsy with wind-phlegm, sores and carbuncles with swelling and toxic | TRPV1 | (Han et al., 2020; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Polygoni Cuspidati Rhizoma et Radix | Slightly cold | Remove dampness and jaundice, clear heat and remove toxic, remove blood stasis and relieve pain, remove phlegm and stop cough | Blood stasis syndrome, dampness-heat jaundice, turbid stranguria, leukorrhagia, carbuncle, swelling, sore, scald and snake bite, cough due to lung heat | TRPM2 | (State Pharmacopoeia Commission, 2020; ÇÇiğ and Yildizhan, 2020; Zhang and Ye, 2020) |
| Menthae Haplocalycis Herba | Cool | Scatter and dissipate wind-heat, clear head and eyes, soothe throat, promote eruption, soothe liver and move qi | Wind-heat exterior syndrome and warm diseases at the early stage, wind-heat headache and red-eye with profuse tears, sort throat and pain, measles failing to erupt, itching rubella, syndrome of liver depression, and qi stagnation | TRPV1, TRPV3, TRPV4, TRPM2, TRPM8, TRPA1 | (State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020; Nguyen et al., 2021; Nazıroğlu, 2022; Niu et al., 2022) |
| Sinomenii Caulis | Neutral | Dispel wind and dampness, unblock the meridians and collaterals, promote diuresis | Pains of rheumatism and arthralgia, joint swelling, palsy itching | TRPV1 | (State Pharmacopoeia Commission, 2020; Ma et al., 2021) |
| Rhodiolae Crenulatae Radix et Rhizoma | Neutral | Supplement qi and invigorate blood circulation, unblock the collaterals and relieve panting | Qi deficiency and blood stasis, chest pain, wind-stroke to hemiplegia, lassitude and asthma | TRPM8 | (Li et al., 2013; State Pharmacopoeia Commission, 2020) |
| Folium Steviae | Neutral | Promoting fluid and relieving thirst, promote diuresis for antihypertensive effect | Diabetes, hypertension | TRPM5 | (Li, 2017a; Philippaert et al., 2017) |
| Scorpio | Neutral | Extinguish wind and suppress convulsion, unblock the collaterals and relieve pain, reduce toxicity and dissipate masses | Spasm and convulsion, wind-damp obstinate impediment, hemilateral and overall headache, sores and pyogenic infections, scrofula and phlegm node | TRPV1 | (Hakim et al., 2015; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020) |
| Cannabis Fructus | Neutral | Moisten the intestines to promote defecation | Constipation due to dryness of intestine | TRPV1, TRPV2, TRPV4, TRPA1 | (Iannotti et al., 2014; State Pharmacopoeia Commission, 2020; Zhang and Ye, 2020; Huang et al., 2021a) |
TABLE 3.
List of compounds from Chinese herbal medicine that can affect TRP channels
| Compounds | Structural formula | Origins | References |
|---|---|---|---|
| Capsaicin (CAP) |
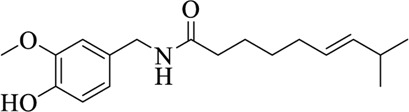
|
Capsici Fructus | Caterina et al. (1997) |
| Cinnamaldehyde (CA) |
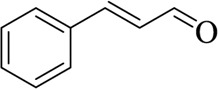
|
Cinnamomi Cortex | Li et al. (2016) |
| Evodiamine |
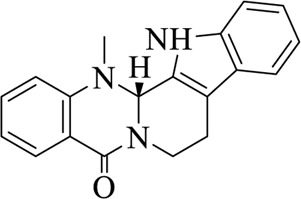
|
Evodiae Fructus | Iwaoka et al. (2016) |
| Rutaecarpine (Rut) |
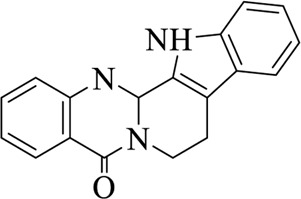
|
Evodiae Fructus | Wang et al. (2016) |
| 6-gingerol |
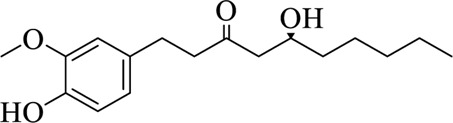
|
Zingiberis Rhizoma, Zingiberis Rhizoma Recens | Yang, (2015) |
| 10-gingerol |
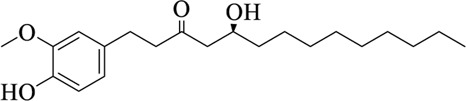
|
Zingiberis Rhizoma, Zingiberis Rhizoma Recens | Yang, (2015) |
| Hypaconitine |
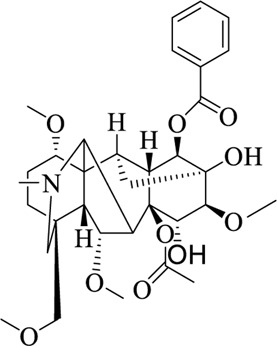
|
Aconiti Radix | Yu et al. (2020a) |
| Higenamine |
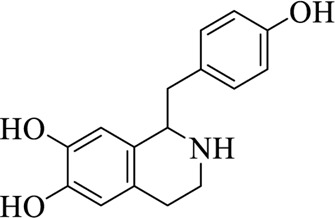
|
Asari Radix et Rhizoma | Yu, (2019) |
| 6-shogaol |
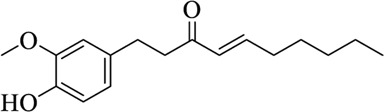
|
Zingiberis Rhizoma, Zingiberis Rhizoma Recens | Yin et al. (2019b) |
| Zingerone |
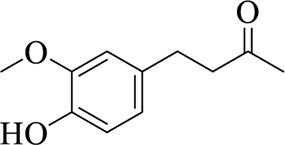
|
Zingiberis Rhizoma, Zingiberis Rhizoma Recens | Yin et al. (2019b) |
| Imperatorin |
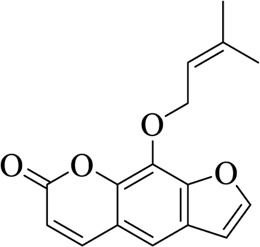
|
Angelicae Dahuricae Radix | Inoue et al. (2012) |
| Eugenol |
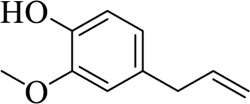
|
Caryophylli Flos | Inoue et al. (2012) |
| Tetramethylpyrazine |
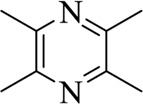
|
ChuanXiong Rhizoma | Yu et al. 2020b |
| Osthole |
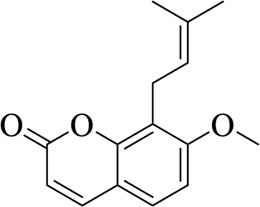
|
Cnidii Fructus | Neuberger et al. 2021b |
| Limonene |
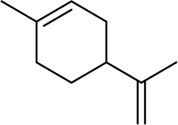
|
Cnidii Fructus | Kaimoto et al. (2016) |
| Allicin |
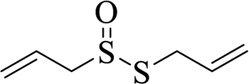
|
Allii Sativi Bulbus | Tsuchiya and Kawamata. (2019) |
| B304-1 |
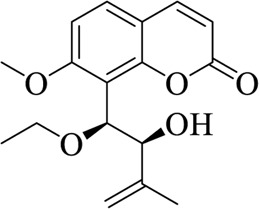
|
Murrayae Folium et Cacumen | Zhou et al. (2020) |
| B304-2 |
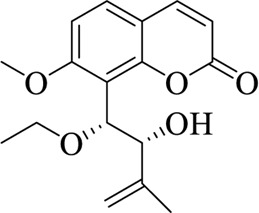
|
Murrayae Folium et Cacumen | Zhou et al. (2020) |
| Sanguinarine |
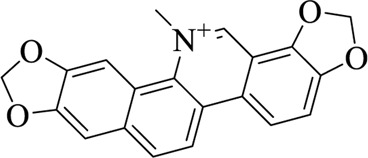
|
Macleayae cordatae Herba | Xu et al. (2021) |
| Resveratrol (RESV) |
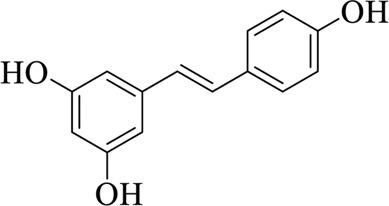
|
Polygoni Cuspidati Rhizoma et Radix | Dai et al. (2022) |
| Menthol |

|
Menthae Haplocalycis Herba | Nguyen et al. (2021) |
| Carvacrol |
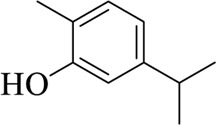
|
Menthae Haplocalycis Herba | Wang et al. (2021b) |
| Sinomenine |
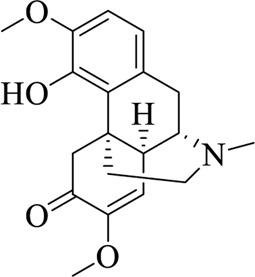
|
Sinomenii Caulis | Ma et al. (2021) |
| Salidroside |
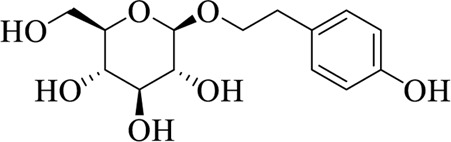
|
Rhodiolae Crenulatae Radix et Rhizoma | Li et al. (2013) |
| Stevioside |
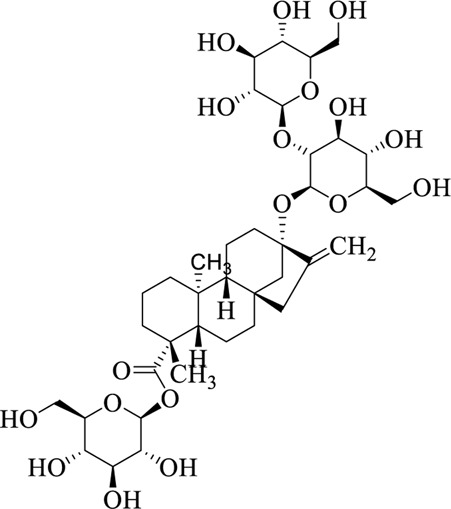
|
Folium Steviae | Philippaert et al. (2017) |
| Cannabidiol (CBD) |
|
Cannabis Fructus | Iannotti et al. (2014) |
For example, some of the TCMs discussed in this review have been investigated using only water or ethanol extracts, and further studies on major active ingredients will be performed in the future. In addition, some TCMs only show effects on TRP ion channels, while the molecular mechanism of regulation needs to be further explored.
In conclusion, we hope that this review will provide a reference for further research on the development and utilization of TRP ion channels in TCM, and provide new research ideas for the study of cold, hot, warm, cool, and neutral natures of TCM.
Author contributions
Conceptualization, SY and EH; writing—original draft preparation, SY and EH; writing—review and editing, SY, EH, and YH; supervision and project administra-tion, ZS, LX, QX, and JX. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (reference: 81960730); Basic and Applied Basic Research Foundation of Guangdong Province (reference: 2020B1515420007) and the Guangxi innovation driven development project (NO. GUIKEAA18242040).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1039412/full#supplementary-material
Abbreviations
BisPH-A, Bisphenol A; CA, Cinnamaldehyde; CAP, Capsaicin; CBD, Cannabidiol; CCI, Chronic constriction injury; PKA, Protein kinase; RESV, Resveratrol; SG, Substantia gelatinosa; SGs, Steviol glycosides; TCM, Traditional Chinese medicine; TM, Transmembrane spanning domains; TRIM4, Tripartite motif-containing 4; TRP, Thermal transient receptor potential; TRPA1, Transient receptor potential ankyrin 1; TRPM2, Transient receptor potential melastatin 2; TRPM4, Transient receptor potential melastatin 4; TRPM5, Transient receptor potential melastatin 5; TRPM8, Transient receptor potential melastatin 8; TRPV1, Transient receptor potential vanilloid 1; TRPV2, Transient receptor potential vanilloid 2; TRPV3, Transient receptor potential vanilloid 3; TRPV4, Transient receptor potential vanilloid 4; WA, Water extract of Asarum; WN, Water extract of Notopterygii Rhizoma et Radix.
References
- An Z., Liu Z., Yu Z., Wang W., Tao X., Gao W., et al. (2018). Study on the mechanism of Cnidii Fructus extract from thermosensitive channel TRPV1. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 24, 1786–1789. [Google Scholar]
- Bhave G., Zhu W., Wang H., Brasier D. J., Oxford G. S., Gereau R. W. (2002). cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721–731. 10.1016/s0896-6273(02)00802-4 [DOI] [PubMed] [Google Scholar]
- Bradshaw H. B., Raboune S., Hollis J. L. (2013). Opportunistic activation of TRP receptors by endogenous lipids: Exploiting lipidomics to understand TRP receptor cellular communication. Life Sci. 92, 404–409. 10.1016/j.lfs.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M. J., Leffler A., Malmberg A. B., Martin W. J., Trafton J., Petersen-Zeitz K. R., et al. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Caterina M. J., Schumacher M. A., Tominaga M., Rosen T. A., Levine J. D., Julius D. (1997). The capsaicin receptor: A heat-activated Ion Channel in the pain pathway. Nature 389, 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chen X., Sooch G., Demaree I. S., White F. A., Obukhov A. G. (2020). Transient receptor potential canonical (TRPC) channels: Then and now. Cells 9, E1983. 10.3390/cells9091983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Sun W., Gianaris N. G., Riley A. M., Cummins T. R., Fehrenbacher J. C., et al. (2014). Furanocoumarins are a novel class of modulators for the transient receptor potential vanilloid type 1 (TRPV1) channel. J. Biol. Chem. 289, 9600–9610. 10.1074/jbc.M113.536862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H., Zhang X., Chen X., Fang S., Ding Q., Gao Z. (2021). Sanguinarine is an agonist of TRPA1 channel. Biochem. Biophys. Res. Commun. 534, 226–232. 10.1016/j.bbrc.2020.11.107 [DOI] [PubMed] [Google Scholar]
- Çiğ B., Yildizhan K. (2020). Resveratrol diminishes bisphenol A-induced oxidative stress through TRPM2 channel in the mouse kidney cortical collecting duct cells. J. Recept. Signal Transduct. Res. 40, 570–583. 10.1080/10799893.2020.1769657 [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Julius D., Montell C., Schultz G. (2005). International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57, 427–450. 10.1124/pr.57.4.6 [DOI] [PubMed] [Google Scholar]
- Dai M., Yuan D., Lei Y., Li J., Ren Y., Zhang Y., et al. (2022). Expression, purification and structural characterization of resveratrol synthase from Polygonum cuspidatum. Protein Expr. Purif. 191, 106024. 10.1016/j.pep.2021.106024 [DOI] [PubMed] [Google Scholar]
- Deng S., Zhang Y., Liao Z., Huang J., Huang R., Li Z. (2021). S100A4 plays a key role in TRPV3 ion channel expression and its electrophysiological function. Neurosci. Lett. 759, 135999. 10.1016/j.neulet.2021.135999 [DOI] [PubMed] [Google Scholar]
- Dutta Banik D., Martin L. E., Freichel M., Torregrossa A.-M., Medler K. F. (2018). TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells. Proc. Natl. Acad. Sci. U. S. A. 115, E772–E781. 10.1073/pnas.1718802115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Zhao H., Yang Z. (2014). A study of the relationship between the thermo-TRPs and the four properties of herbs. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 20, 1687–1690. [Google Scholar]
- Gattkowski E., Johnsen A., Bauche A., Möckl F., Kulow F., Garcia Alai M., et al. (2019). Novel CaM-binding motif in its NudT9H domain contributes to temperature sensitivity of TRPM2. Biochim. Biophys. Acta. Mol. Cell Res. 1866, 1162–1170. 10.1016/j.bbamcr.2018.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Liu Z., Wang W., Gao W., Tao X., Tong H., et al. (2019). Effects of alcohol extracts of Aiye on thermal sensitive channel TRPV1. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 25, 314–316. [Google Scholar]
- Hakim M. A., Jiang W., Luo L., Li B., Yang S., Song Y., et al. (2015). Scorpion toxin, BmP01, induces pain by targeting TRPV1 channel. Toxins (Basel) 7, 3671–3687. 10.3390/toxins7093671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Tong H., Gao W., Ma S., Guo R., Yin Y., et al. (2020). Gansui (Euphorbia kansui) extracts activate the transient receptor potential vanilloid 1. Zhong Hua Zhong Yi Yao Xue Kan. 38, 126–130. 10.13193/j.issn.1673-7717.2020.07.031 [DOI] [Google Scholar]
- Hu H., Gao L., Zhao H., Lu S., Yang Z. (2014). TRPA1, overactive bladder syndrome and warming Yang and promoting flow of qi from Wulingsan. Zhong Yi Yao Xin Xi 31, 36–38. 10.3969/j.issn.1002-2406.2014.05.013 [DOI] [Google Scholar]
- Huang R., Wang F., Yang Y., Ma W., Lin Z., Cheng N., et al. (2019). Recurrent activations of transient receptor potential vanilloid-1 and vanilloid-4 promote cellular proliferation and migration in esophageal squamous cell carcinoma cells. FEBS Open Bio 9, 206–225. 10.1002/2211-5463.12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Xu T., Wang Y., Zhou Y., Yu D., Wang Z., et al. (2021a). Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy 17, 3592–3606. 10.1080/15548627.2021.1885203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Li S., Jia Z., Li S., He W., Zhou C., et al. (2021b). TRIM4 interacts with TRPM8 and regulates its channel function through K423-mediated ubiquitination. J. Cell. Physiol. 236, 2934–2949. 10.1002/jcp.30065 [DOI] [PubMed] [Google Scholar]
- Hung C.-Y., Tan C.-H. (2018). TRP channels in nociception and pathological pain. Adv. Exp. Med. Biol. 1099, 13–27. 10.1007/978-981-13-1756-9_2 [DOI] [PubMed] [Google Scholar]
- Hynkova A., Marsakova L., Vaskova J., Vlachova V. (2016). N-terminal tetrapeptide T/SPLH motifs contribute to multimodal activation of human TRPA1 channel. Sci. Rep. 6, 28700. 10.1038/srep28700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti F. A., Hill C. L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., et al. (2014). Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 5, 1131–1141. 10.1021/cn5000524 [DOI] [PubMed] [Google Scholar]
- Inoue M., Fujita T., Goto M., Kumamoto E. (2012). Presynaptic enhancement by eugenol of spontaneous excitatory transmission in rat spinal substantia gelatinosa neurons is mediated by transient receptor potential A1 channels. Neuroscience 210, 403–415. 10.1016/j.neuroscience.2012.02.040 [DOI] [PubMed] [Google Scholar]
- Isaacson M., Hoon M. A. (2021). An operant temperature sensory assay provides a means to assess thermal discrimination. Mol. Pain 17, 17448069211013633. 10.1177/17448069211013633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaoka E., Wang S., Matsuyoshi N., Kogure Y., Aoki S., Yamamoto S., et al. (2016). Evodiamine suppresses capsaicin-induced thermal hyperalgesia through activation and subsequent desensitization of the transient receptor potential V1 channels. J. Nat. Med. 70, 1–7. 10.1007/s11418-015-0929-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffal S. M., Abbas M. A. (2021). TRP channels in COVID-19 disease: Potential targets for prevention and treatment. Chem. Biol. Interact. 345, 109567. 10.1016/j.cbi.2021.109567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaślan D., Böck J., Krogsaeter E., Grimm C. (2020). Evolutionary aspects of TRPMLs and TPCs. Int. J. Mol. Sci. 21, E4181. 10.3390/ijms21114181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W. (2015). The pain function of toxic BmP01 from scorpion Buthus martensii Karsch. Kunming, China: [D]. Kunming University of Science and Technology. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201601&filename=1015641439.nh . [Google Scholar]
- Jin P., Bulkley D., Guo Y., Zhang W., Guo Z., Huynh W., et al. (2017). Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122. 10.1038/nature22981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahya M. C., Nazıroğlu M., Övey İ. S. (2017). Modulation of diabetes-induced oxidative stress, apoptosis, and Ca2+ entry through TRPM2 and TRPV1 channels in dorsal root ganglion and Hippocampus of diabetic rats by melatonin and selenium. Mol. Neurobiol. 54, 2345–2360. 10.1007/s12035-016-9727-3 [DOI] [PubMed] [Google Scholar]
- Kaimoto T., Hatakeyama Y., Takahashi K., Imagawa T., Tominaga M., Ohta T. (2016). Involvement of transient receptor potential A1 channel in algesic and analgesic actions of the organic compound limonene. Eur. J. Pain 20, 1155–1165. 10.1002/ejp.840 [DOI] [PubMed] [Google Scholar]
- Kang X., Tang Z., Wang L., Wu Z., Liu L. (2017). Effect of cinnamaldehyde on Ca∼(2+) change and TRPA1/TRPM8 mRNA expressions in dorsal root ganglia cells of rats with 0°C cold stress. Zhong Guo Shi Yan Fang. Ji Xue Za Zhi 23, 127–133. [Google Scholar]
- Kashio M., Sokabe T., Shintaku K., Uematsu T., Fukuta N., Kobayashi N., et al. (2012). Redox signal-mediated sensitization of transient receptor potential melastatin 2 (TRPM2) to temperature affects macrophage functions. Proc. Natl. Acad. Sci. U. S. A. 109, 6745–6750. 10.1073/pnas.1114193109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochukov M. Y., McNearney T. A., Fu Y., Westlund K. N. (2006). Thermosensitive TRP ion channels mediate cytosolic calcium response in human synoviocytes. Am. J. Physiol. Cell Physiol. 291, C424–C432. 10.1152/ajpcell.00553.2005 [DOI] [PubMed] [Google Scholar]
- Kowalski C. W., Ragozzino F. J., Lindberg J. E. M., Peterson B., Lugo J. M., McLaughlin R. J., et al. (2020). Cannabidiol activation of vagal afferent neurons requires TRPA1. J. Neurophysiol. 124, 1388–1398. 10.1152/jn.00128.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Li S., Zheng H., Yan Z. (2021a). Nanchung and Inactive define pore properties of the native auditory transduction channel in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 118, e2106459118. 10.1073/pnas.2106459118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Liu S., Xing D., Liu B., Ji Y. (2021b). Chemical components and antimicrobial activity of volatile oil from roots, stems, and leaves of Macleaya cordata. Hua Xue Yu Sheng Wu Gong Cheng 38, 26–33. 10.3969/j.issn.1672-5425.2021.01.006 [DOI] [Google Scholar]
- Li G. (2017a). 800 kinds of Chinese herbal medicine color illustration. Fujian: FuJian Science and Technology Publishing House. [Google Scholar]
- Li H. (2017b). TRP channel classification. Adv. Exp. Med. Biol. 976, 1–8. 10.1007/978-94-024-1088-4_1 [DOI] [PubMed] [Google Scholar]
- Li M., Cheng L., Liu A., Tian M., Wang B. (2018). Effects of warm and hot traditional Chinese medicine on cold and thermal channel TRPM8 and TRPV1 in dorsal root ganglion cells of rats. Zhong Yao Yao Li Yu Lin. Chuang 34, 55–58. In Chinese. [Google Scholar]
- Li M., Wang X., Cheng L., Li J., Wang B., Hou J. (2016). Study on the expression of warm-medicinal properties based on the transient receptor potential vanilloid type-1. Zhong Yao Yao Li Yu Lin. Chuang 32, 49–53. [Google Scholar]
- Li Q., Zhou X.-D., Kolosov V. P., Perelman J. M. (2013). Salidroside reduces cold-induced mucin production by inhibiting TRPM8 activation. Int. J. Mol. Med. 32, 637–646. 10.3892/ijmm.2013.1434 [DOI] [PubMed] [Google Scholar]
- Li Z., Rao X., Li W., Chen F. (2020). Rutaecarpin inhibits the cold stress-induced gastric mucosa injury via activating TRPV1/CGRP signaling. Zhong Nan Yao Xue 18, 910–913. 10.7539/j.issn.1672-2981.2020.06.002 [DOI] [Google Scholar]
- Liang W., Lan Y., Chen C., Song M., Xiao J., Huang Q., et al. (2021). Modulating effects of capsaicin on glucose homeostasis and the underlying mechanism. Crit. Rev. Food Sci. Nutr. 1, 1–19. 10.1080/10408398.2021.1991883 [DOI] [PubMed] [Google Scholar]
- Liu L., Sun X., Guo Y., Ge K. (2022). Evodiamine induces ROS-Dependent cytotoxicity in human gastric cancer cells via TRPV1/Ca2+ pathway. Chem. Biol. Interact. 351, 109756. 10.1016/j.cbi.2021.109756 [DOI] [PubMed] [Google Scholar]
- Liu T., Zhao H., Gao W., Chen X., Tan W., Yang Z., et al. (2021). Intervention effect of Asari Radix ethanol extract on transient receptor potential vanilloid 1 and its mechanism of ameliorating neuropathic pain in mice. Zhong Hua Zhong Yi Yao Za Zhi 36, 4998–5001. [Google Scholar]
- Liu Z. (2017). Based on the thermal channel TRPV1 and TRPA1 to explore the mechanism of Wuzhuyu decoction for dispelling cold and relieving pain. Kunming, China: [D]. Beijing University of Chinese Medicine.. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201702&filename=1017201463.nh . [Google Scholar]
- Liu Z., Gao L., Wang W., Zhao H., Gao W., An Z., et al. (2017a). Effects of Notopterygium incisum extracts on the transient receptor potential vanilloid 1 of thermosensitive channel. Zhong Guo Zhong Yi Ji Chu Yi Xue Za Zhi 23, 553–557. In Chinese. [Google Scholar]
- Liu Z., Gao L., Wang W., Zhao H., Gao W., An Z., et al. (2017b). Study on analgesic effect of Wuzhuyu Tang on acetic acid-induced visceral pain in mice based on transient receptor potential vanilloid 1. Cheng Du. Zhong Yi Yao Da Xue Xue Bao 40, 126. 10.13593/j.cnki.51-1501/r.2017.03.001 [DOI] [Google Scholar]
- Liu Z., Gao W., Guo R., An Z., Yin Y., Han X., et al. (2019). Study of Wuzhuyu decoction, by thermal channel TRPA1, inhibiting formalin-induced visceral pain in mice. Huan Qiu Zhong Yi Yao 12, 1167–1171. 10.3969/j.issn.1674-1749.2019.08.006 [DOI] [Google Scholar]
- Lubova K. I., Chugunov A. O., Volynsky P. E., Trofimov Y., Korolkova Y. V., Mosharova I. V., et al. (2020). Probing temperature and capsaicin-induced activation of TRPV1 channel via computationally guided point mutations in its pore and TRP domains. Int. J. Biol. Macromol. S0141-8130 (20), 1175–1183. 10.1016/j.ijbiomac.2020.04.239 [DOI] [PubMed] [Google Scholar]
- Ma J.-L., Ji K., Shi L.-Q., Li N.-N., Wang L.-Y., Dong S.-J., et al. (2021). Sinomenine attenuated capsaicin-induced increase in cough sensitivity in Guinea pigs by inhibiting SOX5/TRPV1 Axis and inflammatory response. Front. Physiol. 12, 629276. 10.3389/fphys.2021.629276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Huo X.-Q., Chen X., Zhu W.-X., Yao M.-C., Qiao Y.-J., et al. (2020). Study on screening potential traditional Chinese medicines against 2019-nCoV based on Mpro and PLP. Zhongguo Zhong Yao Za Zhi 45, 1219–1224. 10.19540/j.cnki.cjcmm.20200216.401 [DOI] [PubMed] [Google Scholar]
- Maggi F., Morelli M. B., Tomassoni D., Marinelli O., Aguzzi C., Zeppa L., et al. (2022). The effects of cannabidiol via TRPV2 channel in chronic myeloid leukemia cells and its combination with imatinib. Cancer Sci. 113, 1235–1249. 10.1111/cas.15257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naticchioni M., Karani R., Smith M. A., Onusko E., Robbins N., Jiang M., et al. (2015). Transient receptor potential vanilloid 2 regulates myocardial response to exercise. PLoS One 10, e0136901. 10.1371/journal.pone.0136901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazıroğlu M. (2022). A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 37, 711–728. 10.1007/s11011-021-00887-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedungadi T. P., Dutta M., Bathina C. S., Caterina M. J., Cunningham J. T. (2012). Expression and distribution of TRPV2 in rat brain. Exp. Neurol. 237, 223–237. 10.1016/j.expneurol.2012.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Nadezhdin K. D., Sobolevsky A. I. (2021a). TRPV3 expression and purification for structure determination by Cryo-EM. Methods Enzymol. 652, 31–48. 10.1016/bs.mie.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger A., Nadezhdin K. D., Zakharian E., Sobolevsky A. I. (2021b). Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 22, e53233. 10.15252/embr.202153233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. H. D., Itoh S. G., Okumura H., Tominaga M. (2021). Structural basis for promiscuous action of monoterpenes on TRP channels. Commun. Biol. 4, 293. 10.1038/s42003-021-01776-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C., Sun X., Hu F., Tang X., Wang K. (2022). Molecular determinants for the chemical activation of the warmth-sensitive TRPV3 channel by the natural monoterpenoid carvacrol. J. Biol. Chem. 298, 101706. 10.1016/j.jbc.2022.101706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paricio-Montesinos R., Schwaller F., Udhayachandran A., Rau F., Walcher J., Evangelista R., et al. (2020). The sensory coding of warm perception. Neuron 106, 830–841. 10.1016/j.neuron.2020.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippaert K., Pironet A., Mesuere M., Sones W., Vermeiren L., Kerselaers S., et al. (2017). Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity. Nat. Commun. 8, 14733. 10.1038/ncomms14733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumroy R. A., Samanta A., Liu Y., Hughes T. E., Zhao S., Yudin Y., et al. (2019). Molecular mechanism of TRPV2 channel modulation by cannabidiol. Elife 8, e48792. 10.7554/eLife.48792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P., Pigera S., Premakumara G. A. S., Galappaththy P., Constantine G. R., Katulanda P. (2013). Medicinal properties of “true” cinnamon (Cinnamomum zeylanicum): A systematic review. BMC Complement. Altern. Med. 13, 275. 10.1186/1472-6882-13-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin N., Orhan C., Erten F., Tuzcu M., Defo Deeh P. B., Ozercan I. H., et al. (2019). Effects of allyl isothiocyanate on insulin resistance, oxidative stress status, and transcription factors in high-fat diet/streptozotocin-induced type 2 diabetes mellitus in rats. J. Biochem. Mol. Toxicol. 33, e22328. 10.1002/jbt.22328 [DOI] [PubMed] [Google Scholar]
- Saito M. (2015). Capsaicin and related food ingredients reducing body fat through the activation of TRP and Brown fat thermogenesis. Adv. Food Nutr. Res. 76, 1–28. 10.1016/bs.afnr.2015.07.002 [DOI] [PubMed] [Google Scholar]
- State Pharmacopoeia Commission (2020). Pharmacopoeia of the people’s Republic of China. Part 1. Beijing: China Medical Science Press. [Google Scholar]
- Su Q., Hu F., Ge X., Lei J., Yu S., Wang T., et al. (2018). Structure of the human PKD1-PKD2 complex. Science 361, eaat9819. 10.1126/science.aat9819 [DOI] [PubMed] [Google Scholar]
- Sui F., Yang N., Zhang C., Du X., Li L., Weng X., et al. (2010). Effects of ingredients from Chinese herbs with nature of cold or hot on expression of TRPV1 and TRPM8. Zhong Guo Zhong Yao Za Zhi 35, 1594–1598. 10.4268/cjcmm20101220 [DOI] [PubMed] [Google Scholar]
- Sun X.-Y., Sun L.-L., Qi H., Gao Q., Wang G.-X., Wei N.-N., et al. (2018). Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 94, 1164–1173. 10.1124/mol.118.112466 [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. (2015). Effect of capsaicin on thermoregulation: An update with new aspects. Temp. (Austin) 2, 277–296. 10.1080/23328940.2015.1048928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K., Yasumatsu K., Voets T., Droogmans G., Shigemura N., Ninomiya Y., et al. (2005). Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438, 1022–1025. 10.1038/nature04248 [DOI] [PubMed] [Google Scholar]
- Tan C.-H., McNaughton P. A. (2016). The TRPM2 ion channel is required for sensitivity to warmth. Nature 536, 460–463. 10.1038/nature19074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Kawamata K. (2019). Allicin induces electrogenic secretion of chloride and bicarbonate ions in rat colon via the TRPA1 receptor. J. Nutr. Sci. Vitaminol. 65, 258–263. 10.3177/jnsv.65.258 [DOI] [PubMed] [Google Scholar]
- Uchida K., Dezaki K., Yoneshiro T., Watanabe T., Yamazaki J., Saito M., et al. (2017). Involvement of thermosensitive TRP channels in energy metabolism. J. Physiol. Sci. 67, 549–560. 10.1007/s12576-017-0552-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vay L., Gu C., McNaughton P. A. (2012). The thermo-TRP Ion Channel family: Properties and therapeutic implications. Br. J. Pharmacol. 165, 787–801. 10.1111/j.1476-5381.2011.01601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F. (2018). Nociceptors: Thermal allodynia and thermal pain. Handb. Clin. Neurol. 156, 103–119. 10.1016/B978-0-444-63912-7.00006-0 [DOI] [PubMed] [Google Scholar]
- Wan H., Kong X., Li X., Zhu H., Su X., Lin N. (2014). [Effect of traditional Chinese medicines with different properties on thermoregulation and temperature-sensitive transient receptor potentialion channel protein of rats with yeast-induced fever]. Zhong Guo Zhong Yao Za Zhi 39, 3813–3818. 10.4268/cjcmm20141930 [DOI] [PubMed] [Google Scholar]
- Wang D., Ruan Y., Jin X., Ji H., Tang Z., Wang C. (2021a). Water extract of Notopterygium incisum alleviates neuropathic pain by regulating TRPV1. NanJing Zhong Yi Yao Da Xue Xue Bao 37, 508–513. 10.14148/j.issn.1672-0482.2021.0508 [DOI] [Google Scholar]
- Wang S., Yamamoto S., Kogure Y., Zhang W., Noguchi K., Dai Y. (2016). Partial activation and inhibition of TRPV1 channels by evodiamine and rutaecarpine, two major components of the fruits of Evodia rutaecarpa. J. Nat. Prod. 79, 1225–1230. 10.1021/acs.jnatprod.5b00599 [DOI] [PubMed] [Google Scholar]
- Wang Y., Li H., Xue C., Chen H., Xue Y., Zhao F., et al. (2021b). TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 37, 313–330. 10.1007/s10565-020-09536-2 [DOI] [PubMed] [Google Scholar]
- Woolums B. M., McCray B. A., Sung H., Tabuchi M., Sullivan J. M., Ruppell K. T., et al. (2020). TRPV4 disrupts mitochondrial transport and causes axonal degeneration via a CaMKII-dependent elevation of intracellular Ca2. Nat. Commun. 11, 2679. 10.1038/s41467-020-16411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. (2019). Research progress on the effect of some drugs in dog skin ointment on TRPV1 receptor channel expression and its analgesic effect. Ke Xue Ji Shu Chuang Xin (14), 15–17. In Chinese. 10.3969/j.issn.1673-1328.2019.14.008 [DOI] [Google Scholar]
- Xu H., Feng Y., Zhu G. (2018). Anti-cancer Chinese medicines: Modern research and clinical application. Shanghai: Shanghai Scientific and Technical Publishers. [Google Scholar]
- Xu J., Liu M., Zhang M., Liu X., Zeng J. (2021). Research progress on pharmacological study of bioactive components in Macleaya cordata. Si Liao Gong Ye 42, 59–64. In Chinese. 10.13302/j.cnki.fi.2021.14.011 [DOI] [Google Scholar]
- Xu M., Dong X.-P. (2021). Endolysosomal TRPMLs in cancer. Biomolecules 11, 65. 10.3390/biom11010065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Zhang F., Cao Z. (2021). Advances in structure and pharmacological function of temperature-sensitive TRPV2 channel. Yao Xue Xue Bao 56, 2720–2727. 10.16438/j.0513-4870.2021-0308 [DOI] [Google Scholar]
- Yang Z. (2015). Compatibility study of the characteristic ingredients of Li zhong Wan based on the thermal channel TRPV1. Harbin, China: [D]. Heilongjiang University of Chinese Medicine. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2019&filename=1015420543.nh . [Google Scholar]
- Yin Y., Dong Y., Vu S., Yang F., Yarov-Yarovoy V., Tian Y., et al. (2019a). Structural mechanisms underlying activation of TRPV1 channels by pungent compounds in gingers. Br. J. Pharmacol. 176, 3364–3377. 10.1111/bph.14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Dong Y., Vu S., Yang F., Yarov‐Yarovoy V., Tian Y., et al. (2019b). Structural mechanisms underlying activation of TRPV1 channels by pungent compounds in gingers. Br. J. Pharmacol. 176, 3364–3377. 10.1111/bph.14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Liu Z., Gao W., Tong H., Guo R., Han X., et al. (2019c). Effect of Genkwa flos on transient receptor potential vanilloid 1 of thermosensitive channel. Zhong Guo Shi Yan Fang. Ji Xue Za Zhi 25, 56–62. 10.13422/j.cnki.syfjx.20192002 [DOI] [Google Scholar]
- Yin Y., Wu M., Zubcevic L., Borschel W. F., Lander G. C., Lee S.-Y. (2018). Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241. 10.1126/science.aan4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J. (2019). The analgesic mechanism study of Asarum by regulating TRPV1 ion channel. Kunming, China: [D]. Nanjing University of Chinese Medicine. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201902&filename=1019170241.nh . [Google Scholar]
- Yu S., Li C., Ding Y., Huang S., Wang W., Wu Y., et al. (2020a). Exploring the “cold/hot” properties of traditional Chinese medicine by cell temperature measurement. Pharm. Biol. 58, 208–218. 10.1080/13880209.2020.1732429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Ge Y., Zhang Y. (2020b). Mechanism of tetramethylpyrazine treating traumatic brain injury based on SUR1 gene. Shan Xi Yi Ke Da Xue Xue Bao 51, 1226–1231. 10.13753/j.issn.1007-6611.2020.11.015 [DOI] [Google Scholar]
- Yue H.-Y., Jiang C.-Y., Fujita T., Kumamoto E. (2013). Zingerone enhances glutamatergic spontaneous excitatory transmission by activating TRPA1 but not TRPV1 channels in the adult rat substantia gelatinosa. J. Neurophysiol. 110, 658–671. 10.1152/jn.00754.2012 [DOI] [PubMed] [Google Scholar]
- Zhang D. (2020). To explore the analgesic mechanism of Danggui Sini decoction cold medicine and its components based on TRP signal pathway regulated by Artemin/GFRα3. Guangzhou, China: [D]. Jinan University. Available at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname= CMFD202101&filename=1021520496.nh . [Google Scholar]
- Zhang Y., Ye R. (2020). Chinese materia medica. Beijing: China Medical Science Press. [Google Scholar]
- Zhao J., Lin King J. V., Paulsen C. E., Cheng Y., Julius D. (2020). Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature 585, 141–145. 10.1038/s41586-020-2480-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T.-T., Lan R.-R., Liang S.-D., Schmalzing G., Gao H.-W., Verkhratsky A., et al. (2021). An exploration in the potential substance basis and mechanism of chuanxiong rhizoma and Angelicae Dahuricae Radix on analgesia based on network Pharmacology and molecular docking. World J. Traditional Chin. Med. 7, 201–208. 10.4103/wjtcm.wjtcm_81_20 [DOI] [Google Scholar]
- Zhou Q., Shi Y., Qi H., Liu H., Wei N., Jiang Y., et al. (2020). Identification of two natural coumarin enantiomers for selective inhibition of TRPV2 channels. Faseb J. 34, 12338–12353. 10.1096/fj.201901541RRR [DOI] [PubMed] [Google Scholar]
- Zhu T., Sun L., Ji Y. (2021). Advances in modern research on the four - qi theory of traditional Chinese medicine. Xian Dai Zhong Xi Yi Jie He Za Zhi 30, 3188. 10.3969/j.issn.1008-8849.2021.28.026 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



