Abstract
Background
Recurrent Clostridioides difficile infection, associated with dysbiosis of gut microbiota, has substantial disease burden in the USA. RBX2660 is a live biotherapeutic product consisting of a broad consortium of microbes prepared from human stool that is under investigation for the reduction of recurrent C. difficile infection.
Methods
A randomized, double-blind, placebo-controlled, phase III study, with a Bayesian primary analysis integrating data from a previous phase IIb study, was conducted. Adults who had one or more C. difficile infection recurrences with a positive stool assay for C. difficile and who were previously treated with standard-of-care antibiotics were randomly assigned 2:1 to receive a subsequent blinded, single-dose enema of RBX2660 or placebo. The primary endpoint was treatment success, defined as the absence of C. difficile infection diarrhea within 8 weeks of study treatment.
Results
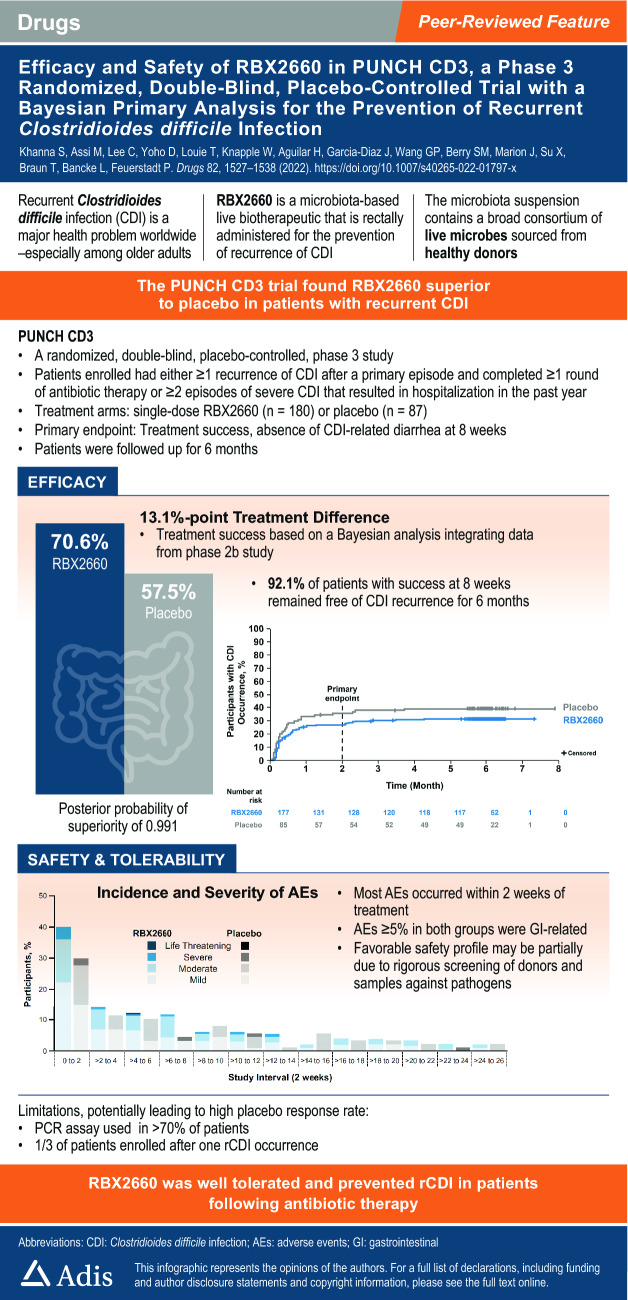
Of the 320 patients screened, 289 were randomly assigned and 267 received blinded treatment (n = 180, RBX2660; n = 87, placebo). Original model estimates of treatment success were 70.4% versus 58.1% with RBX2660 and placebo, respectively. However, after aligning the data to improve the exchangeability and interpretability of the Bayesian analysis, the model-estimated treatment success rate was 70.6% with RBX2660 versus 57.5% with placebo, with an estimated treatment effect of 13.1% and a posterior probability of superiority of 0.991. More than 90% of the participants who achieved treatment success at 8 weeks had sustained response through 6 months in both the RBX2660 and the placebo groups. Overall, RBX2660 was well tolerated, with manageable adverse events. The incidence of treatment-emergent adverse events was higher in RBX2660 recipients compared with placebo and was mostly driven by a higher incidence of mild gastrointestinal events.
Conclusions
RBX2660 is a safe and effective treatment to reduce recurrent C. difficile infection following standard-of-care antibiotics with a sustained response through 6 months.
Clinical Trial Registration
NCT03244644; 9 August, 2017.
Infographic
Video abstract: (MP4 62,291 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01797-x.
Plain Language Summary
Clostridioides difficile is a diarrhea-causing bacterium that is associated with potentially serious and fatal consequences. Antibiotics used to treat or prevent infections have a side effect of damaging the healthy protective gut bacteria (microbiota). Damage to the gut microbiota can allow C. difficile to over-grow and produce toxins that injure the colon. Paradoxically, the standard of care treatment of C. difficile infection (CDI) is antibiotics. Although initially effective for the control of diarrhea, antibiotics can leave a patient at risk for CDI recurrence after antibiotic treatment is stopped. Live biotherapeutic products are microbiota-based treatments used to repair the gut microbiota. These products have been shown to reduce the recurrence of CDI. RBX2660 is an investigational microbiota-based live biotherapeutic. RBX2660 contains a diverse set of microorganisms. RBX2660 has been developed to reduce CDI recurrence in adults following antibiotic treatment for recurrent CDI. This study was conducted to demonstrate that RBX2660 is effective and safe in treating patients with recurrent CDI. Treatment was considered successful in participants who did not experience CDI recurrence within 8 weeks after administration. Overall, statistical modeling demonstrated that 70.6% of participants treated with RBX2660 and 57.5% of participants treated with placebo remained free of CDI recurrence through 8 weeks. A 13.1 percentage point increase in treatment success was observed with RBX2660 treatment compared with placebo. In participants who achieved treatment success at 8 weeks, more than 90% remained free of CDI recurrence through 6 months. The most common side effects with RBX2660 treatment were abdominal pain and diarrhea. No serious treatment-related side effects were reported. The current data from the comprehensive clinical development program support a positive benefit-risk profile for RBX2660 in the reduction of CDI recurrence in adults following antibiotic therapy for recurrent CDI.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01797-x.
| Digital Features for this article can be found at https://doi.org/10.6084/m9.figshare.21225377. |
Key Points
| In this phase III trial, RBX2660 was superior to placebo in reducing recurrent Clostridioides difficile infection after standard-of-care antibiotics. | |
| RBX2660 was well tolerated, with mostly mild-to-moderate adverse events reported. |
Introduction
Clostridioides difficile infection (CDI) is a global healthcare issue. In the USA, CDI is associated with 15,000–30,000 annual deaths and acute inpatient costs exceeding $4.8 billion [1]. There is a substantial risk of recurrent CDI (rCDI), which is associated with significant morbidity and mortality, especially in older patients [2, 3]. While antibiotics are the current standard-of-care treatment, they can disrupt the gut microbiota, increasing the risk for further rCDI. Microbial restoration with live biotherapeutic products may restore the diversity and composition of the gut microbiota to decrease the likelihood of rCDI. Currently, fecal microbiota transplantation (FMT), a process of instilling normal microbiota via donor feces to correct the gut microbiota disruption, is being used under enforcement discretion to reduce rCDI [4–6].
RBX2660 is an investigational live biotherapeutic product, defined in accordance with the US Food and Drug Administration (FDA) [7], and is designed to reduce CDI recurrence following standard-of-care antibiotic treatment in individuals with rCDI. RBX2660 consists of a broad consortium of live microbes prepared from human stool collected from rigorously screened healthy donors. RBX2660 undergoes comprehensive pathogen testing and is processed to a stable cryopreserved liquid suspension.
The efficacy and safety of RBX2660 have been evaluated in a double-blind, randomized, placebo-controlled phase IIb trial (PUNCH CD2; NCT02299570). In this prior study, one and two doses of RBX2660 administered 1 week apart were assessed [8]. An analysis comparing one versus two doses of RBX2660 in the intent-to-treat (ITT) population showed no meaningful difference in efficacy; therefore, a single dose of RBX2660 was selected for subsequent studies. RBX2660 has also been assessed in two open-label phase II clinical trials (PUNCH CD [NCT01925417]; PUNCH CD Open-Label [NCT02589847]). Together, the results of these studies suggested that RBX2660 reduces CDI recurrence with a low risk of adverse events (AEs) related to treatment [8–10] and supported the selection of one dose of RBX2660 for phase III studies, with a second course of RBX2660 treatment allowed for initial treatment failures after the recurrence of symptoms.
Based on the unmet need, RBX2660 was granted Breakthrough Therapy Status, Fast Track, and Orphan Drug designations by the FDA. The present study reports the outcomes from the PUNCH CD3 phase III trial (NCT03244644), comparing the safety and efficacy of RBX2660 with placebo in reducing rates of rCDI.
Methods
Trial Design
PUNCH CD3 was a randomized, double-blind, placebo-controlled, phase III trial (Fig. 1 of the Electronic Supplementary Material [ESM]). This trial was conducted in the USA and Canada according to the ethical principles of the Declaration of Helsinki, Good Clinical Practice guidelines, principles of informed consent, and requirements of publicly registered clinical trials. The protocol received institutional review board approval before its commencement and was conducted under an FDA Investigational New Drug application. Authors had full access to the data and vouch for the accuracy and completeness of the data and for compliance with the protocol and statistical analysis plan.
The development program was originally planned to include two randomized, placebo-controlled, pivotal phase III trials. However, the widespread availability and use of FMT under enforcement discretion made it increasingly difficult to enroll patients into a placebo-controlled trial within this orphan indication. The FDA acknowledged the increasing recruitment difficulties and recommended that innovative designs, such as formal borrowing of data in a Bayesian framework, could be pursued. Therefore, PUNCH CD3 was analyzed using a Bayesian hierarchical model borrowing data from the previous phase IIb trial (PUNCH CD2). In addition, two interim analyses were added for early stopping for futility or efficacy.
Participants
Participants were adults (aged ≥ 18 years) with documentation of rCDI (defined as one or more recurrences after a primary episode) who had completed one or more rounds of standard-of-care antibiotic therapy or had two or more episodes of severe CDI resulting in hospitalization within the past year. Within 30 days before enrollment, participants were required to have a positive stool test for the presence of C. difficile with the capability to produce toxins assessed by polymerase chain reaction (PCR), enzyme immunoassay (EIA), or other assays. Participants must have been taking or just been prescribed antibiotics to control rCDI symptoms. The antibiotic, dose, and regimen for the qualifying event were at the discretion of the treating physician; therefore, taper or taper/pulse regimens were allowed. Participants agreed not to ingest over-the-counter/prescription probiotics for 8 weeks following study treatment. Participants were excluded if they had a known history of refractory CDI, inflammatory bowel disease, irritable bowel syndrome, chronic diarrhea, celiac disease, colostomy, active colitis, continued diarrhea despite antibiotic therapy, required antibiotic therapy for another condition, or had a previous FMT. All key inclusion/exclusion criteria are listed in the Appendix of the ESM, page 5.
Randomization, Interventions, and Masking
Enrolled participants were randomly assigned (2:1) to receive RBX2660 (microbiota suspension) or placebo (normal saline). Randomization was stratified by antibiotics used for the qualifying CDI event (vancomycin alone, vancomycin in combination with another antibiotic, fidaxomicin alone, or other); randomization was not stratified by site.
Both RBX2660 and placebo were administered rectally following the instructions for use and standard site procedures after a full course of antibiotic treatment for rCDI and following a washout period of 24–72 h, and within 14 calendar days of randomization. Enema administration was performed by a qualified and trained healthcare professional not involved in other procedures or assessments during the trial, and product-specific instructions for use were provided to ensure consistency in the procedure. No bowel preparation was required. Study blinding was maintained during administration by covering the infusion bag and tubing in an opaque sleeve. In the event of treatment failure within the first 8 weeks of blinded treatment, participants were offered the opportunity to receive an open-label treatment of RBX2660.
Study Outcomes of PUNCH CD3
The primary endpoint was treatment success, defined as the absence of CDI diarrhea within 8 weeks of study treatment. Sustained clinical response was a secondary endpoint, defined as treatment success of the presenting CDI recurrence and no new CDI episodes for greater than 8 weeks through 6 months after completing a study treatment. Evaluation of safety and tolerability included the incidence and severity of AEs and serious AEs (SAEs), which were collected during follow-up visits, telephone assessments, and diaries through the 6-month follow-up. Although AE collection started at the time of consent, the follow-up for treatment-emergent AEs started at the time of study drug administration.
Statistical Analysis
During PUNCH CD3 enrollment, an agreement was reached with the FDA to implement an adaptive design, with two interim analyses to evaluate the primary endpoint for possible early declaration of study success or futility. Two prespecified sponsor-blinded interim analyses were planned when 160 and 220 participants had completed the week 8 visit; a Pocock α-spending approach was used to determine the stopping criteria at the interim analyses (see ESM, page 8).
Given the recruitment challenges noted above, the FDA agreed with analysis of the primary endpoint using a Bayesian hierarchical model that dynamically borrowed information about the treatment effect from the previous phase IIb trial, PUNCH CD2, taking into account differences in response rates between the two trials. This analysis was considered appropriate, given the similarity of the PUNCH CD2 and CD3 study designs (Table 1 of the ESM). This model incorporated data from the PUNCH CD2 study from the one-dose RBX2660 group and placebo control group (not the two-dose RBX2660 group). The advantage of modeling the data jointly in this manner is that if the treatment effect is similar in both studies, a combined analysis can reduce the amount of uncertainty in the estimate. The Bayesian hierarchical model provides estimates of the treatment success rates for each treatment group in PUNCH CD3 as well as the estimated treatment effect and the associated posterior probability of superiority. The Bayesian hierarchical model (see ESM, pages 7–11) was included in the statistical analysis plan before enrollment completion and unblinding for any interim or final analyses.
This study included two superiority thresholds: (1) posterior probability of superiority > 0.999 selected to control the nominal type I error rate without borrowing at one-sided 0.00125; and (2) posterior probability of superiority > 0.975 selected to control the nominal type I error rate without borrowing at one-sided 0.025. The higher threshold therefore corresponds to a statistically very persuasive finding. The lower threshold provides evidence of a statistically significant phase III trial (see ESM, pages 7–11).
PUNCH CD3 analysis populations included the modified intent-to-treat (mITT), ITT, per-protocol (PP), and safety populations (defined in ESM, page 12). The efficacy results in the mITT population were considered the primary outcome, while the results in the ITT and PP populations were considered supportive. The original intention was to incorporate PUNCH CD2 data from the ITT population only as this was the primary analysis population in that study. During the Biologics License Application review, the FDA recommended increasing exchangeability between the two studies by applying the PUNCH CD3 analysis population definitions to the PUNCH CD2 data and matching the analysis populations when borrowing (i.e., PUNCH CD2 mITT data were borrowed for the PUNCH CD3 mITT primary analysis and PUNCH CD2 ITT data were borrowed for the PUNCH CD3 ITT sensitivity analysis). For completeness, results based on both approaches for borrowing are presented for the primary endpoint. Results for prespecified subgroups are presented based on the FDA-recommended approach only.
Sustained clinical response through 6 months was assessed with frequentist analyses using Pearson’s chi-squared test, with a two-sided α = 0.05. Two-sided 95% confidence intervals for the differences in proportions between treatment arms were calculated. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Houston, TX, USA), R version 3.4.3 (R Core Team 2017), and Stan version 2.17.2 (Stan Development Team 2018).
Data Monitoring
To ensure the safety and well-being of participants throughout the study, an independent data and safety monitoring board reviewed safety data for trends and stopping rules. An endpoint adjudication committee provided independent blinded adjudications of treatment success or failure. An independent statistical analysis committee conducted both interim analyses and the final primary efficacy analysis independent of the sponsor, data and safety monitoring board, or endpoint adjudication committee.
Results
Participants
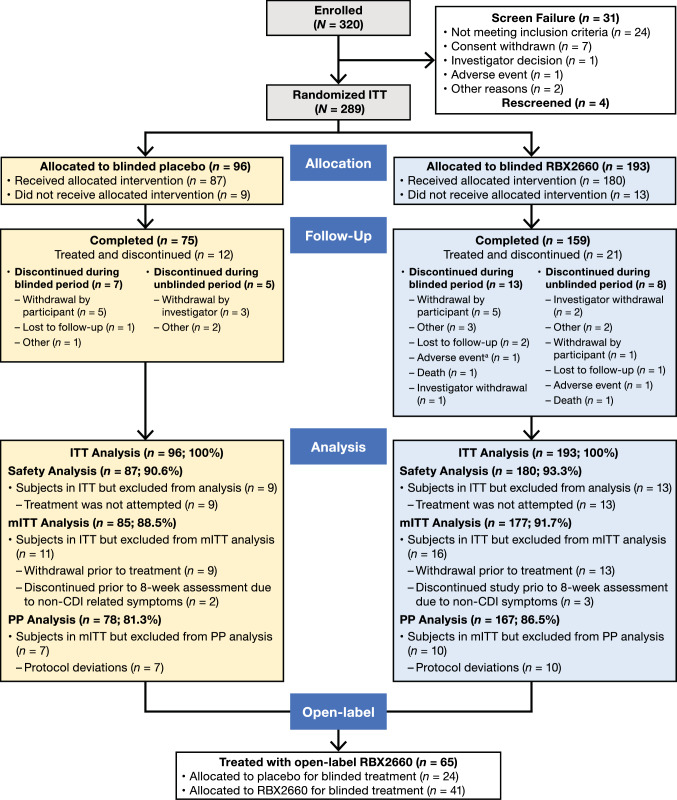
A total of 320 study participants were screened between July 2017 and February 2020 at 44 sites in the USA and Canada; the study was completed in August 2020. Thirty-one participants did not meet inclusion criteria or were withdrawn for various reasons (Fig. 1). Of the 289 participants who were randomized to treatment in PUNCH CD3, 22 did not receive the allocated treatment, and 267 were treated with either blinded RBX2660 (n = 180) or placebo (n = 87) (Fig. 1). Of the 267 treated participants, 33 discontinued the study—21 from the RBX2660 arm and 12 from the placebo arm. Twenty participants withdrew during the 8-week blinded period; 13 withdrew during the 6-month open-label period. Withdrawal by participant (33.3%; 11/33) was the most common reason for study discontinuation, with similar rates across treatment arms. During the 8-week blinded phase, only one participant discontinued because of AEs, the onset of which occurred before treatment administration. Sixty-five participants (n = 41, RBX2660; n = 24, placebo) who did not respond to treatment received a second treatment course with open-label RBX2660.
Fig. 1.
PUNCH CD3 study profile. aOnset of AE occurred prior to treatment administration. AE adverse event, ITT intent-to-treat, mITT modified intent-to-treat, PP per-protocol
The ITT population (i.e., defined as all randomized participants; participants who withdrew from the trial prior to receiving blinded treatment were not included in the analysis) comprised 267 patients; the mITT population (i.e., the primary analysis population, defined as all randomized participants who successfully completed treatment and did not discontinue the trial during the first 8 weeks for reasons unrelated to CDI) comprised 262 participants; and the PP population (i.e., defined as all randomized participants who successfully completed treatment and did not discontinue the trial for reasons not related to CDI or had violations to inclusion/exclusion criteria) comprised 245 patients.
Overall, participants primarily were female (68.5%) and White (92.1%), with a median age of 63 years (range: 19–93); PCR was the most common (73.0%) diagnostic testing method, and vancomycin was the most common antibiotic (88.0%) used to treat the qualifying CDI episode (Table 1). While most baseline characteristics were comparable between groups, the placebo arm had a higher proportion of patients aged < 65 years.
Table 1.
PUNCH CD3 participant demographics and baseline characteristics (safety population, N = 267)
| Placebo (n = 87) | RBX2660 (n = 180) | Total (N = 267) | |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 60.0 (26–86) | 64.0 (19–93) | 63.0 (19–93) |
| Age group, years, n (%) | |||
| < 65 | 54 (62.1) | 91 (50.6) | 145 (54.3) |
| ≥ 65 | 33 (37.9) | 89 (49.4) | 122 (45.7) |
| Sex, n (%) | |||
| Male | 27 (31.0) | 57 (31.7) | 84 (31.5) |
| Female | 60 (69.0) | 123 (68.3) | 183 (68.5) |
| Race, n (%) | |||
| American Indian or Alaska Native | 0 (0.0) | 2 (1.1) | 2 (0.7) |
| Asian | 0 (0.0) | 1 (0.6) | 1 (0.4) |
| Black or African American | 6 (6.9) | 8 (4.4) | 14 (5.2) |
| White | 78 (89.7) | 168 (93.3) | 246 (92.1) |
| Other | 3 (3.4) | 0 (0.0) | 3 (1.1) |
| Multiple | 0 (0.0) | 1 (0.6) | 1 (0.4) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 4 (4.6) | 2 (1.1) | 6 (2.2) |
| Not Hispanic or Latino | 80 (92.0) | 168 (93.3) | 248 (92.9) |
| Not reported | 0 (0.0) | 5 (2.8) | 5 (1.9) |
| Unknown | 3 (3.4) | 5 (2.8) | 8 (3.0) |
| Geography region, n (%) | |||
| Northern USA | 14 (16.1) | 31 (17.2) | 45 (16.9) |
| Eastern USA | 8 (9.2) | 24 (13.3) | 32 (12.0) |
| Southern USA | 28 (32.2) | 48 (26.7) | 76 (28.5) |
| Western USA | 11 (12.6) | 26 (14.4) | 37 (13.9) |
| Outside of USA | 26 (29.9) | 51 (28.3) | 77 (28.8) |
| Randomization strata, n (%) | |||
| Vancomycin alone | 78 (89.7) | 157 (87.2) | 235 (88.0) |
| Vancomycin combination therapya | 2 (2.3) | 5 (2.8) | 7 (2.6) |
| Fidaxomicin | 5 (5.7) | 12 (6.7) | 17 (6.4) |
| Otherb | 2 (2.3) | 6 (3.3) | 8 (3.0) |
| ATLAS score for qualifying CDI episode | |||
| Median (range) | 3.0 (2–8) | 3.0 (2–8) | 3.0 (2–8) |
| Number of CDI episodes before blinded treatment, n (%) | |||
| ≤ 3 | 59 (67.8) | 111 (61.7) | 170 (63.7) |
| > 3 | 28 (32.2) | 69 (38.3) | 97 (36.3) |
| CDI confirmation testing methodc | |||
| PCR positive | 65 (74.7) | 130 (72.2) | 195 (73.0) |
| EIA for toxin A/B positive | 16 (18.3) | 50 (27.8) | 66 (24.7) |
| GDH positive | 10 (11.5) | 36 (20.0) | 46 (17.2) |
| Other | 6 (6.9) | 9 (5.0) | 15 (5.6) |
CDI Clostridioides difficile infection, EIA enzyme immunoassay, GDH glutamate dehydrogenase, PCR polymerase chain reaction
aCombination antibiotics included metronidazole and/or fidaxomicin
bOther included various other antibiotics alone or in combination
cParticipants may have had more than one testing assay to confirm most recent qualifying CDI episode
Primary Outcome
Based on the original borrowing approach, the Bayesian hierarchical model estimates of the treatment success rates for the mITT population were 70.4% (RBX2660) and 58.1% (placebo), representing a 12.3 percentage point treatment difference. The posterior probability of superiority of RBX2660 versus placebo was 0.986 (Fig. 2a of the ESM), which exceeded the lower threshold of 0.975 for demonstrating superiority (Fig. 2b of the ESM). Similar results were observed for the ITT and PP populations (Table 2). Using the FDA-recommended approach to borrowing, the model estimates for the mITT population were 70.6% (RBX2660) and 57.5% (placebo), representing a 13.1 percentage point treatment difference and a posterior probability of success of 0.991. Again, similar results were observed for the ITT and PP populations in the PUNCH CD3 trial (Table 2). Additionally, across subgroups evaluated with the Bayesian model in a post hoc analysis, estimated differences in treatment success rates within 8 weeks of blinded treatment numerically favored RBX2660 compared with placebo (Fig. 3 of the ESM).
Table 2.
Observed and Bayesian model-estimated treatment success (initial planned primary endpoint analysis and matched populations)
| Analysis population | Trial | Observed | Model estimated | ||||
|---|---|---|---|---|---|---|---|
| Treatment success, n/N (%) | Treatment success, % | Treatment effect [95% CI] | Posterior probability | ||||
| Placebo | RBX2660 | Placebo | RBX2660 | ||||
| Planned primary endpoint analysis | |||||||
| ITTa | PUNCH CD2 | 19/44 (43.2) | 25/42 (59.5) | 58.10 | 70.40 | 12.3 [1.4–23.3] | 0.986354 |
| mITTb | PUNCH CD3 | 53/85 (62.4) | 126/177 (71.2) | ||||
| ITTa | PUNCH CD2 | 19/44 (43.2) | 25/42 (59.5) | 56.70 | 69.10 | 12.5 [1.6–23.3] | 0.987292 |
| ITT | PUNCH CD3 | 53/87 (60.9) | 126/180 (70.0) | ||||
| ITTa | PUNCH CD2 | 19/44 (43.2) | 25/42 (59.5) | 57.20 | 70.90 | 13.7 [2.4–25.1] | 0.991208 |
| PP | PUNCH CD3 | 48/78 (61.5) | 120/167 (71.9) | ||||
| Matched populationsc | |||||||
| mITTd | PUNCH CD2 | 19/43 (44.2) | 25/39 (64.1) | 57.50 | 70.60 | 13.1 [2.3–24.0] | 0.99136 |
| PUNCH CD3 | 53/85 (62.4) | 126/177 (71.2) | |||||
| ITTe | PUNCH CD2 | 19/44 (43.2) | 25/43 (58.1) | 56.90 | 69.10 | 12.2 [1.4–23.0] | 0.98637 |
| PUNCH CD3 | 53/87 (60.9) | 126/180 (70.0) | |||||
| PPf | PUNCH CD2 | 19/43 (44.2) | 25/37 (67.6) | 56.20 | 71.50 | 15.3 [4.3–26.3] | 0.99690 |
| PUNCH CD3 | 48/78 (61.5) | 120/167 (71.9) | |||||
CDI Clostridioides difficile infection, CI credible interval, ITT intent-to-treat, mITT modified intent-to-treat, PP per protocol
aThe ITT population in PUNCH CD2 included all randomized participants who successfully completed treatment, which aligns with the ITT population definition in PUNCH CD3 (described in footnote e)
bPrimary analysis population
cPUNCH CD3 analysis population definitions (described in footnotes d–f) were applied to PUNCH CD2, and matched populations (e.g., PUNCH CD2 mITT and PUNCH CD3 mITT) were then used to generate model-estimated treatment success rates
dPrimary analysis population, defined as all randomized participants who successfully completed treatment and did not discontinue the trial during the first 8 weeks for reasons unrelated to CDI
eThe ITT population was defined as all randomized participants. Participants who withdrew from the trial prior to receiving blinded treatment were not included in the primary analysis
fThe PP population was defined as all randomized participants who successfully completed treatment and did not discontinue the trial for reasons not related to CDI or had violations to inclusion/exclusion criteria
Sustained Clinical Response
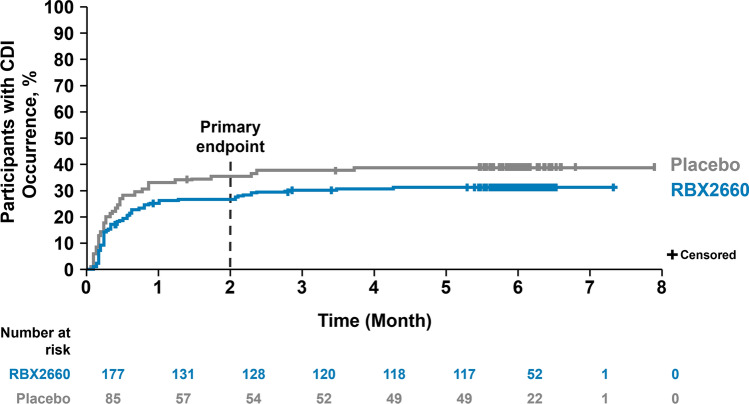
The observed rates of treatment success and treatment failure through 8 weeks and 6 months of follow-up after blinded treatment are shown in Table 2 of the ESM. The observed treatment difference at 8 weeks (range 8.8–10.3) was maintained at 6 months across all analysis populations. The proportion of participants with treatment success at 8 weeks who remained free of CDI recurrence was approximately 90% for both treatment groups across analysis populations (mITT: RBX2660, 92.1% [n = 116/126]; placebo, 90.6% [n = 48/53]). This sustained clinical response is further illustrated in the Kaplan–Meier analysis showing the percentage of participants reporting a CDI event over 6 months of follow-up from the start of blinded treatment (Fig. 2). Compared with placebo, the lower rate of treatment failure observed for RBX2660 at 8 weeks was also maintained through 6 months of follow-up.
Fig. 2.
Kaplan–Meier analysis of time to Clostridioides difficile infection (CDI) recurrence through 6 months (modified intent-to-treat population)
Treatment Success Within 8 Weeks of Open-Label Treatment
Overall, 65 participants received a second treatment course (open-label RBX2660) following confirmed treatment failure. Of the 24 participants treated with blinded placebo who were subsequently treated with open-label RBX2660, 15 (62.5%) achieved treatment success within 8 weeks. All 15 of these participants had sustained response through 6 months. Of the 41 participants treated with blinded RBX2660 who were treated with open-label RBX2660, 22 (53.7%) achieved treatment success within 8 weeks. Of these 22 participants, 19 (86%) had a sustained response through 6 months. In total, 68 of 85 (80%) participants who received blinded placebo and 148 of 177 (83.6%) participants who received blinded RBX2660 achieved treatment success by their second course (i.e., open-label RBX2660).
Safety and Tolerability Profiles of RBX2660 and Placebo
Through 6 months after blinded treatment, a higher rate of AEs was reported in the RBX2660 group (55.6%; n = 100/180) compared with the placebo group (44.8%, n = 39/87), which was driven primarily by participants experiencing a mild event by maximum severity (Table 3). Nine participants experienced one or more SAE; however, no participant experienced an SAE deemed related to study treatment or rectal administration. Rates of AEs through 6 months after open-label RBX2660 treatment were comparable between the treatment groups. Two participants randomly assigned to RBX2660 treatment died during the study: one within the first 8 weeks of blinded treatment and one within the open-label period. Neither death was deemed related to treatment or the administration procedure. Both deaths were related to a pre-existing condition (multiple comorbidities, n = 1; cardiorespiratory arrest, n = 1).
Table 3.
Summary of AEs over the full study period (PUNCH CD3 safety population; N = 267)
| Through 6 months after blinded treatmenta | Through 6 months after open-label treatment | |||
|---|---|---|---|---|
| Blinded placebo (n = 87) | Blinded RBX2660 (n = 180) | Blinded placebo, open-label RBX2660 (n = 24) | Blinded RBX2660, open-label RBX2660 (n = 41) | |
| All AEs, n (%) | 39 (44.8) | 100 (55.6) | 14 (58.3) | 24 (58.5) |
| AEs by maximum severityb | ||||
| Mild | 9 (10.3) | 42 (23.3) | 6 (25.0) | 8 (19.5) |
| Moderate | 25 (28.7) | 47 (26.1) | 6 (25.0) | 10 (24.4) |
| Severe | 5 (5.7) | 10 (5.6) | 1 (4.2) | 5 (12.2) |
| Potentially life threatening | 0 | 1 (0.6)c | 1 (4.2) | 1 (2.4) |
| Discontinued because of AE | 0 | 1 (0.6)c | 0 | 2 (4.9) |
| Serious AEs | 2 (2.3) | 7 (3.9) | 1 (4.2) | 5 (12.2) |
| Deaths | 0 | 1 (0.6)c | 0 | 1 (2.4) |
AE adverse event, CDI Clostridioides difficile infection, IP investigational product
aTreatment failures are censored at the time of CDI recurrence
bAEs reported by maximum severity as assessed by investigator using Common Terminology Criteria for Adverse Events (CTCAE) criteria
cSame participant represented in each category
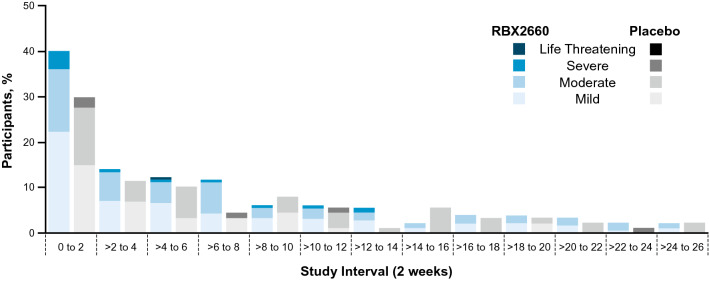
In addition, an analysis of the onset interval of AEs after blinded treatment through 6 months, further delineated by the maximum severity of AEs, was performed (Fig. 3). Most AEs occurred during the first 2 weeks after treatment, and were predominantly mild to moderate in both groups, and the difference between RBX2660 and placebo was primarily attributable to participants experiencing mild events by maximum severity. After the initial 2-week interval, the proportion of participants with AEs declined in subsequent 2-week intervals, with comparable rates of AEs between participants receiving RBX2660 and placebo.
Fig. 3.
Incidence and severity of adverse events (AEs) from baseline through 6 months of blinded treatment. Most AEs occurred during the first 2 weeks and were predominantly mild to moderate in both groups, with differences between RBX2660 and placebo primarily attributable to mild events by maximum severity. Adverse events declined after the initial 2 weeks, with comparable rates of AEs between RBX2660 and placebo. Participants may be represented in more than one interval; treatment failures censored at Clostridioides difficile infection recurrence
Gastrointestinal disorders were the only AEs reported in ≥ 5% of participants in all treatment groups (Table 3 of the ESM). Major complications of new CDI events were reported in two participants after blinded RBX2660. One participant was admitted to the intensive care unit because of the severity of a new CDI event on study day 8. The participant had negative blood and urine cultures but a positive C. difficile stool sample. A second participant experienced septic shock requiring emergency colectomy and admission to the intensive care unit on study day 65. No reported events of death, toxic megacolon, or colonic perforation from the presenting CDI episode were reported after blinded RBX2660 or placebo, and there were no major complications of CDI events after open-label RBX2660 treatment.
Discussion
Uncontrolled and controlled clinical trials report FMT as an effective treatment for rCDI [11]. While these foundational trials have indicated treatment success for microbiota restoration in patients with rCDI [12], they suffer from significant heterogeneity [11, 13]. Tariq et al. conducted a systematic review and meta-analysis of FMT in CDI and found a 67.7% clinical cure rate in randomized trials [12], consistent with the success rates after a single RBX2660 dose using both approaches to borrowing [70.4% and 70.6% Table 2)] and the actual observed treatment success rate from the study (71.2% [see Table 2 of the ESM]).
PUNCH CD3 was a randomized, double-blind, placebo-controlled, phase III study that demonstrated the superiority of RBX2660 compared with placebo in reducing CDI recurrence. After alignment of the data to improve exchangeability and interpretability of the Bayesian analysis, the difference between RBX2660 and placebo was 13.1 percentage points, and the probability that RBX2660 was superior to placebo was 0.991. This positive benefit with RBX2660 was observed across various participant subgroups. Additionally, more than 90% of participants with treatment success during the 8-week blinded period had sustained response through 6 months.
Treatment success rates can be influenced by the diagnostic modality for confirming CDI. While several laboratory tests are available (e.g., PCR, glutamate dehydrogenase with EIA for toxin A/B, EIA for toxin A/B), no single test can reliably diagnose the disease in the absence of clinical symptoms. Clinical guidelines recommend a two-step or three-step testing algorithm to diagnose CDI [5, 6], but clinical practice relies on symptoms and available testing, most frequently PCR in the USA [14]. Within PUNCH CD2 and CD3, toxigenicity was confirmed by PCR in >70% of participants. In the recently published ECOSPOR III trial, a significantly lower rate of CDI recurrence with SER-109, an investigational orally administered FMT, was seen compared with placebo. However, eligibility was rigidly limited to patients with a positive C. difficile stool toxin assay by cell cytotoxicity neutralization assay or EIA [15]. For some clinicians, availability of cell culture cytotoxin neutralization assay may be limited, and PCR more frequently available than other testing options such as EIA. In the ECOSPOR II trial, which used diagnostic methods similar to PUNCH CD3, no significant difference in CDI recurrence rates was observed between SER-109 and placebo [15]; however, other attributes (e.g., suboptimal dosage of SER-109) may have also affected the clinical outcome.
Currently available FMT is well tolerated, with the most common side effects being diarrhea, bloating, and abdominal pain [16, 17]. Consistent with FMT and prior RBX2660 studies, most of the AEs reported with RBX2660 were mild-to-moderate gastrointestinal disorders, with abdominal pain and diarrhea being the most common. Additionally, RBX2660 had a low rate of AEs leading to discontinuation of participation [8–10], no new or unexpected events, no pathogen transfer from donor to recipient, and no product-related or procedure-related SAEs. A documented safety concern for FMT use is the potential transmission of infectious diseases. In 2019, two immunocompromised adults developed serious infections after receiving FMT prepared with stool from the same donor containing a causative pathogen; one died [18]. This underscores the need for consistent and rigorous donor qualification and pathogen screening processes to ensure that donor stool is free of pathogens.
The comprehensive screening program as part of the standardized manufacturing process of RBX2660 has been developed over 10 years under FDA’s Investigational New Drug program with the intent to meet requirements for eventual approval of an FDA-regulated drug product to reduce recurrence of CDI. RBX2660 stool donors undergo rigorous health screening and routine blood and stool testing, before qualifying and throughout their participation in the program. Every qualified stool donation is tested for an extensive list of stool pathogens. Pathogen testing includes but is not limited to HIV, hepatitis A/B/C, syphilis, severe acute respiratory syndrome coronavirus 2, enteropathogenic Escherichia coli, Shiga toxin-producing E. coli, norovirus, rotavirus, adenovirus, vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, other antibiotic-resistant bacterial strains, Vibrio, Listeria, intestinal parasites, and other enteric pathogens. For RBX2660 product from donations after 1 December, 2019, all stool donors are required to undergo frequent testing for the severe acute respiratory syndrome coronavirus 2 virus and screening to assess for symptoms and exposure to the virus. All pathogen tests and test methods were submitted to the FDA, and any future changes to the program will require updated submissions to the FDA for review.
Limitations
While the PUNCH CD3 study population represents the general recurrent C. difficile population, the small number of non-White participants and the lack of participants with irritable bowel syndrome and inflammatory bowel disease, and immunocompromised patients limit the ability to broadly generalize these data. However, an open-label study (PUNCH CD3-OLS) is ongoing, which includes a more diverse rCDI population compared with prior RBX2660 studies and allows enrollment of patients with immunocompromised conditions and chronic conditions such as irritable bowel syndrome or inflammatory bowel disease.
Placebo response in PUNCH CD3 was higher than expected. As previously stated, it has been proposed that treatment success rates can be influenced by the diagnostic modality for confirming CDI. Although the PCR assay is the most commonly used diagnostic tool in clinical practice in the USA [14] and was used in >70% of PUNCH CD3 participants, it can result in a false positive. This may lead to the inclusion of patients who do not actually have CDI and therefore also impact treatment response rates. Another possible explanation for the higher placebo effect is that approximately one-third of PUNCH CD3 participants were enrolled after only one CDI recurrence. As the risk of recurrence increases with each subsequent infection, some PUNCH CD3 placebo participants may have had a lower risk of recurrence because of less severe dysbiosis.
Conclusions
Even with a high placebo response rate, RBX2660 demonstrated superiority as a treatment to reduce CDI recurrence following standard-of-care antibiotic treatment in a Bayesian analysis model. RBX2660 was well tolerated with primarily mild-to-moderate AEs and no treatment-related SAEs. These results further contribute to the totality of clinical evidence for RBX2660 and confirm the positive benefit-risk profile for the reduction of CDI recurrence in adults following antibiotic therapy for rCDI.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors acknowledge all study participants and the study team. Rebiotix Inc. provided financial support for this work. Medical writing assistance was provided by Regina Switzer, PhD, and was funded by Rebiotix Inc. Medical writing assistance was provided by Michelle Boland, PhD, ApotheCom, Yardley, PA, USA, and was funded by Ferring Pharmaceuticals. Editorial assistance was provided by Elizabeth Hermans, PhD, and ApotheCom. All writing and editorial assistance was provided under the authors’ guidance.
Declarations
Funding
Rebiotix Inc., a Ferring Company, provided financial support for this work. The study protocol was developed by Rebiotix Inc., a Ferring Company, in collaboration with the study investigators. Rebiotix Inc. was also involved in data collection, analysis, and interpretation of results. Statistical analyses were performed by statisticians at Rebiotix Inc.
Conflicts of Interest/Competing Interests
Sahil Khanna has received grants or contracts from Rebiotix (a Ferring company), Finch Therapeutics, Seres Therapeutics, and Vedanta BioSciences, consulting fees from Niche Pharmaceuticals and Immuron Limited, participated on advisory or data safety monitoring boards for Ferring Pharmaceuticals, and has stock options with Jetson Probiotics. Christine Lee has received grants or contracts from Rebiotix, Inc., Seres Therapeutics, and Summit Therapeutic and participated on advisory or data safety monitoring boards for Ferring Pharmaceuticals and Pfizer. Thomas Louie has received consulting fees from Crestone, MGB BioPharma, Rebiotix, Inc., and Seres Therapeutics and participated on advisory boards for Seres Therapeutics and Vedanta BioSciences. Scott M. Berry has ownership in Berry Consultants who received fees for trial design and analyses; Tricia Braun, Lindy Bancke, and Xin Su are employees of Rebiotix, Inc. or were during conduct of the study. Paul Feuerstadt has received consulting fees from Rebiotix, Inc. and Takeda Pharmaceuticals, honoraria from Ferring Pharmaceuticals, Seres Therapeutics, and Takeda Pharmaceuticals, and participated on advisory or data safety monitoring boards for Ferring Pharmaceuticals, Seres Therapeutics, and Takeda Pharmaceuticals. Humberto Aguilar, Maha Assi, Julia Garcia-Diaz, Whitfield Knapple, Gary P. Wang, and David Yoho have no conflicts of interest to declare.
Ethics Approval
All procedures performed in studies involving participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are not publicly available because of pending regulatory submission and approval but may be available from the corresponding author on request.
Code Availability
Not applicable.
Authors’ Contributions
SK, LB, and SMB conceptualized and designed the study. SMB, LB, TB, JM, and XS designed and conducted the statistical analyses and vouch for the data. All authors were involved in the acquisition, analysis, or interpretation of the data. Under the guidance from all authors, medical writers Regina Switzer, PhD (paid for by Rebiotix Inc.), and Michelle Boland, PhD (ApotheCom, Yardley, PA, USA; paid for by Ferring Pharmaceuticals) assisted with the drafting of the manuscript. All authors provided critical revision of the manuscript, and all agreed to the submission of the manuscript.
Footnotes
The original article has been updated: Due to Table 3 update.
Change history
11/7/2022
A Correction to this paper has been published: 10.1007/s40265-022-01805-0
References
- 1.McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):e1–48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feuerstadt P, et al. Mortality, health care use, and costs of Clostridioides difficile infections in older adults. J Am Med Dir Assoc. 2022;23:1721–1728.e19. doi: 10.1016/j.jamda.2022.01.075. [DOI] [PubMed] [Google Scholar]
- 3.Feuerstadt P, et al. Clinical burden of recurrent Clostridioides difficile infection in the medicare population: a real-world claims analysis. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e60. doi: 10.1017/ash.2022.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakken JS, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9(12):1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson S, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–e1044. doi: 10.1093/cid/ciab549. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CR, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. 2021;116(6):1124–1147. doi: 10.14309/ajg.0000000000001278. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. Guidance for industry. Early clinical trials with live biotherapeutic products: chemistry, manufacturing, and control information. 2016. https://www.fda.gov/media/82945/download. Accessed 20 Apr 2022.
- 8.Dubberke ER, et al. Results From a randomized, placebo-controlled clinical trial of a RBX2660: a microbiota-based drug for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis. 2018;67(8):1198–1204. doi: 10.1093/cid/ciy259. [DOI] [PubMed] [Google Scholar]
- 9.Orenstein R, et al. Safety and durability of RBX2660 (microbiota suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD Study. Clin Infect Dis. 2016;62(5):596–602. doi: 10.1093/cid/civ938. [DOI] [PubMed] [Google Scholar]
- 10.Orenstein R, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. 2022;22(1):245. doi: 10.1186/s12879-022-07256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuerstadt P, et al. Heterogeneity of randomized controlled trials of fecal microbiota transplantation in recurrent Clostridioides difficile infection. Dig Dis Sci. 2021;67:2763–2770. doi: 10.1007/s10620-021-07141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tariq R, et al. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(8):1351–1358. doi: 10.1093/cid/ciy721. [DOI] [PubMed] [Google Scholar]
- 13.Bafeta A, et al. Methods and reporting studies assessing fecal microbiota transplantation: a systematic review. Ann Intern Med. 2017;167(1):34–39. doi: 10.7326/M16-2810. [DOI] [PubMed] [Google Scholar]
- 14.Guh AY, et al. Trends in U.S. Burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feuerstadt P, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 16.Baxter M, Colville A. Adverse events in faecal microbiota transplant: a review of the literature. J Hosp Infect. 2016;92(2):117–127. doi: 10.1016/j.jhin.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Saha S, et al. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. 2021;160(6):1961–9.e3. doi: 10.1053/j.gastro.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 18.DeFilipp Z, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.