Abstract
Objective
Evidence shows that gene mutation is a significant proportion of genetic factors associated with prostate cancer. The DNA damage response (DDR) is a signal cascade network that aims to maintain genomic integrity in cells. This comprehensive study was performed to determine the link between different DNA damage response gene mutations and prostate cancer.
Materials and methods
A systematic literature search was performed using PubMed, Web of Science, and Embase. Papers published up to February 1, 2022 were retrieved. The DDR gene mutations associated with prostate cancer were identified by referring to relevant research and review articles. Data of prostate cancer patients from multiple PCa cohorts were obtained from cBioPortal. The OR or HR and 95% CIs were calculated using both fixed-effects models (FEMs) and random-effects models (REMs).
Results
Seventy-four studies were included in this research, and the frequency of 13 DDR genes was examined. Through the analysis of 33 articles that focused on the risk estimates of DDR genes between normal people and PCa patients, DDR genes were found to be more common in prostate cancer patients (OR = 3.6293 95% CI [2.4992; 5.2705]). Also, patients in the mutated group had a worse OS and DFS outcome than those in the unmutated group (P < .05). Of the 13 DDR genes, the frequency of 9 DDR genes in prostate cancer was less than 1%, and despite differences in race, BRCA2 was the potential gene with the highest frequency (REM Frequency = .0400, 95% CI .0324 - .0541). The findings suggest that mutations in genes such as ATR, BLM, and MLH1 in PCa patients may increase the sensitivity of Olaparib, a PARP inhibitor.
Conclusion
These results demonstrate that mutation in any DDR pathway results in a poor prognosis for PCa patients. Furthermore, mutations in ATR, BLM, and MLH1 or the expression of POLR2L, PMS1, FANCE, and other genes significantly influence Olaparib sensitivity, which may be underlying therapeutic targets in the future.
Keywords: DNA damage response, prostate cancer, gene mutations, frequency, prognosis
Introduction
Prostate cancer is the most common malignant tumor with the highest number of confirmed cases and the second-highest number of fatal patients after lung cancer in American males. In the United States, an estimated 268 490 new cases will be diagnosed in 2022, with 34 500 men dying as a result of the disease.1 Urologists and academics are focusing on how to detect prostate cancer early and provide accurate and effective treatment. The genetic susceptibility of malignant tumors is receiving increasing attention these days. Cancers such as breast, ovarian, colorectal, and kidney cancer have all been linked to genetic factors. Recent data show that approximately 10% of patients with advanced prostate cancer may have a well-characterized tumor suppressor gene mutation.2 Prostate cancer occurrence may also be linked to genetic factors.3,4 For instance, studies have shown that high-risk genetic factors cause 8% of prostate cancer.5 The proportion of prostate cancer variation by germline genetics is about 58% in prostate cancer patients.6
So far, genome-wide studies have identified more than 100 common mutations in prostate cancer patients, which account for a significant portion of the genetic factors underlying prostate cancer, including mutations in DNA damage repair genes.7-10 Genomic DNA is frequently harmed by a variety of internal and external factors such as double-strand breaks. Cells have evolved a well-coordinated signal cascade network called DNA damage response (DDR) to maintain genomic integrity, which senses and transmits damage signals to effector proteins and induces cell responses such as cell cycle arrest, DNA repair pathway activation, and cell death, and many genes are involved, including BRCA, ATM, and CHEK2.11 Because cancer cells frequently have specific abnormalities in DNA damage response, several treatment strategies based on this discovery have been concerned and developed, for example, in combination with DNA damage drugs to enhance the ability to kill cancer cells, or as a single drug to treat cancer with DNA damage repair defects. One of the most notable examples is the killing effect of poly ADP ribose polymerase (PARP) inhibitor on BRCA1 or BRCA2 deficient tumors, which takes advantage of the defects of DNA repair of cancer cells.12
PARP is an enzyme found in our cells which helps damaged cells to repair themselves. As a targeted cancer drug, PARP inhibitors (PARPi) stop the PARP from doing its repair work in cancer cells. Although PARPi such as Olaparib and Rucaparib has been developed for cancer patients with DDR gene mutation, research on the relationship between prostate cancer and DDR genes mutations is still in infancy. Owing to the variability in research design, target genes, and researches involved in this field, there are only a few systematic reviews on the relationship between the frequency of different subtypes of DDR genes mutations and their prognosis in prostate cancer patients,13,14 and no meta-analysis on the association of different subtypes of DDR gene mutations with prostate cancer risk and frequency. Furthermore, despite a few meta-analyses focusing on the high-incidence mutation genes such as BRCA1/2, there are subtle differences in the results.15,16
Hence, it is imperative to undertake a comprehensive analysis of the relationship between DDR gene mutations and prostate cancer. We examined several genes associated with PCa DDR, including ATM, BRCA1, BRCA2, BRIP1, CHEK2, MUTYH, MSH2, MSH6, NBN, PALB2, PMS2, RAD51D, and TP53. The present study has gathered as many original studies as possible for analysis, which can provide more detailed and credible data support for the incidence of prostate cancer in DDR gene mutation carriers and the frequency of DDR genes mutations in PCa patients.17
Materials and Methods
Literature Search
We consulted relevant research and review articles for the most commonly identified DDR gene mutations in PCa patients. Furthermore, we deleted the DDR genes that few studies focused on.18,19 On February 1, 2022, we conducted searches in PubMed, Web of Science, and Embase using the search string (BRCA1) OR (CHEK2)) OR (BRCA2)) OR (ATM)) OR (BARD1)) OR (BRIP1)) OR (CHEK1)) OR (PALB2)) OR (RAD51D)) OR (RAD51B)) OR (RAD51C)) OR (NBN)) OR (MLH1)) OR (MSH2)) OR (MSH6)) OR (PMS2)) OR (DDR)) OR (DNA damage response) AND (PCa) OR (prostate cancer)). We were left with 2432 potentially relevant articles after removing duplicates. At least 2 of us (Xinglin Chen, Xu Zhang) independently screened the titles and abstracts of retrieved articles. This meta-analysis was conducted in accordance with the guidelines for systematic reviews and meta-analysis preferred reporting items.20 In addition, our study was registered with INPLASY, number INPLASY2021120095.
Study Selection
The following modified PICOS were used to guide study eligibility screening: (1) participants: human adult subjects (age >18) with DDR gene mutations; (2) intervention: none; (3) comparisons: prostate cancer patients vs the general population, prostate cancer patients with DDR gene mutations vs those without DDR gene mutations; (4) outcomes: frequency (number of DDR gene mutation carriers among prostate cancer patients), ratio (with or without DDR genes between prostate cancer patients); (5) study design: observational studies; (6) only prostate cancer were included, other cancers and other prostate diseases were excluded. DDR gene mutations included in our analysis were pathogenic, deleterious (frameshift insertion, deletion, nonsense mutation, or known pathogenic splice-site alteration), truncation, or assumed loss of function (in the pedigree analysis). Studies without explicit mention of clinical significance but with data on the specific nucleotide change were included if the variants were defined as pathogenic in ClinVar (http://www.ncbi.nlm.nih.gov/clinvar/), a public archive of relationships among sequence variation and human phenotype. We also excluded editorials, letters, commentaries, conference abstracts, and review papers, as well as publications reporting on prostate diseases other than prostate cancer, studies with duplicate participants, and studies with insufficient data to allow calculations.
Data Extraction
Four reviewers (Xinglin Chen, Xiaohan Ren, Xu Zhang, and Yuang Wei) independently screened article titles and abstracts for eligibility to reduce bias and improve reliability. Data were extracted using a predeveloped worksheet: author; publication date; study design; mutation type; study location; population; description of cases and, as applicable, controls (eg, number, recruitment method, matching, etc.); age and gender of subjects; estimates of risk, frequency, or survival with corresponding 95% confidence intervals (95% CI) or relevant data to calculate such. If more information was required, the authors were contacted. Disagreement was resolved by consensus.
Quality Assessment
Four reviewers (Xinglin Chen, Xiaohan Ren, Xu Zhang, Guangyao Li) independently assessed the quality of studies using the Newcastle‐Ottawa Scale (NOS), which consists of 8 items covering 3 domains: study group selection, exposure and outcome determination, and group comparability. The ratings are based on a five-star scale, with a maximum score of 9. Studies with 1 to 3 stars are considered low quality, studies with 4 to 6 stars are considered moderate quality, and studies with 7 to 9 stars are considered high quality.
Outcome Measures
We examined several genes associated with PCa DDR, including ATM, BRCA1, BRCA2, BRIP1, CHEK2, MUTYH, MSH2, MSH6, NBN, PALB2, PMS2, RAD51D, and TP53. The mutation frequency of each gene in prostate cancer patients were measured, and the frequency and 95% CI were calculated directly from the data presented in this article.
Open-Access Data Acquisition
cBioPortal (cBio Cancer Genomics Portal, https://www.cbioportal.org/) was used to obtain data of the expression, mutation, and survival data of patients from multiple PCa cohorts. As a public resource project, the cBioProtal integrated multidimensional cancer genomics data from over 5000 tumor samples from 20 cancer studies, which could assist researchers in exploring genomic information in cancers intuitively.17 The drug sensitivity data were obtained from the website of the Genomics of Drug Sensitivity in Cancer (GDSC) project, which is the l most comprehensive open-access resource for drug sensitivity in cancer cells and molecular markers of drug response.18
Data Synthesis and Analysis
All statistical analyses were performed in the R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org) and RevMan v.5.0 software (Cochrane Collaboration, Oxford, UK). Fixed effects models (FEMs) and random effects models (REMs) were both fitted to determine which model types were best suited to the data. Heterogeneity was assessed by the Q test and the I2 statistic. Statistical significance was set at a P-value <.05. Publication bias was assessed using funnel plots for direct comparisons with 10 or more studies. Sensitivity analysis was performed to assess the influence of individual studies on the summary effect estimate.
Results
Search Results
The database search yielded 2208 PubMed results, 104 Cochrane results, 4350 Embase results. We discarded 4094 duplicates and removed 1596 studies based on title and abstract screening. Additionally, 177 studies were systematic reviews or case reports, 374 studies did not provide indicators related to exposure outcomes, and 312 studies focused on the related mechanism of the DDR gene. We were unable to obtain the full text of 35 articles despite efforts to contact the investigators in 74 of included studies.21‐94 The frequency of DDR genes assessment was the main objective of the study. Figure 1 summarizes the study selection procedure and search results.
Figure 1.
Flow diagram of literature search strategy for the meta-analysis.
Description of Studies
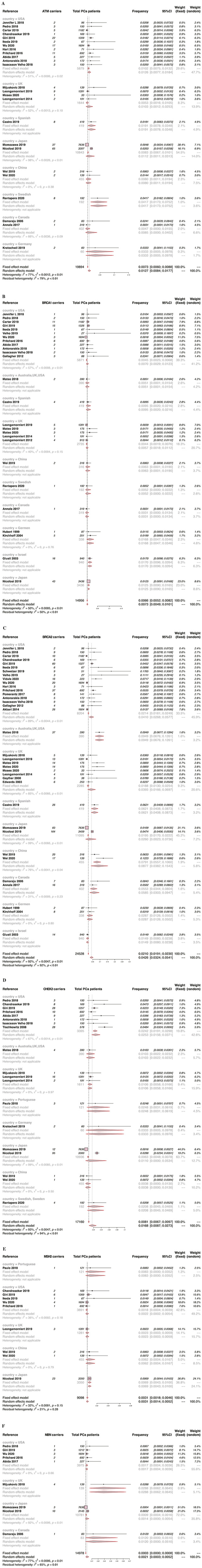
The included studies were published between 1973 and 2021. A total of 74 studies were included in the meta-analysis. According to the DDR gene Classification of outline, we assessed 207655 participants. The DDR gene Classification of outline included base excision repair (BER), Fanconi anemia (FA) pathway, Checkpoint factors, homologous recombination (HR), mismatch excision repair (MMR), nucleotide excision repair (NER), non-homologous end-joining (NHEJ), and translesion DNA synthesis (TLS). In our meta-analysis, we looked at the frequency of 13 DDR genes in prostate cancer gene cells, including ATM, BRCA1, BRCA2, BRIP1, CHEK2, MUTYH, MSH2, MSH6, NBN, PALB2, PMS2, RAD51D, and TP53. 27 studies assessed the frequency of ATM, 28 studies evaluated BRCA1, 38 studies evaluated BRCA2, 6 studies assessed BRIP1, 21 studies assessed CHEK2, 14 studies assessed MSH2, 14 studies assessed MSH6, 6 studies assessed MUTYH, 9 studies assessed NBN, 13 studies assessed PALB2, 10 studies assessed PMS2, 5 studies assessed RAD51D, and 6 studies assessed TP53.
The Frequency of Main DNA Damage Response Gene in Prostate Cancer Patients
BRCA2 gene had the highest possibility of occurrence (REM Frequency = .0400, 95% CI .0299 - .0513), whereas BRIP1 gene had the lowest possibility of occurrence (REM Frequency = .0016, 95% CI .000 - .0046) in PCA patients. The overall results are shown in Figure 2. Furthermore, the mutation frequency of CHEK2, ATM, and MUTYH in prostate cancer patients was greater than 1%.
Figure 2.
Forest plots of the DDR genes mutation rate in patients with prostate cancer (A) BRCA2 (B) CHEK2 (C) ATM (D) MUTYH (E) BRCA1 (F) TP53 (G) PMS2 (H) MSH2 (I) PALB2 (J) NBN (K) MSH6 (L) BRIP1 (M) RAD51D.
We conducted a subgroup analysis of the study and evaluated the frequency of DDR genes in different country PCa patients in order to investigate the causes of heterogeneity; the detailed information is provided below.
The frequency of ATM gene in prostate cancer patients
In articles that explored patients with ATM gene mutation, 12 studies focused on American PCa patients, and the frequency was .0126, heterogeneity estimates were reduced when participants were selected from the USA (I2 = 51%) (Figure 3A), ATM gene mutations occurred in prostate cancer for all different races. Sensitivity analyses demonstrated that the the removal of Momozawa study influenced the observed pooled effect size (Figure 5A). The funnel chart revealed a publication bias (Figure 4A).
Figure 3.
Forest plots of the 6 DNA damage response genes mutation rate in patients with prostate cancer regarding each country (A) ATM (B) BRCA1 (C) BRCA2 (D) CHEK2 (E) MSH2 (F) NBN.
Figure 5.
Forest plots of the key factors in each analysis rate (A) ATM (B) BRCA1 (C) BRCA2 (D) CHEK2 (E) MSH2 (F) NBN.
Figure 4.
Funnel plots of effect estimates on DNA damage response genes mutation rate in patients with prostate cancer (A) ATM (B) BRCA1 (C) BRCA2 (D) CHEK2 (E) MSH2 (F) NBN.
The frequency of BRCA1 gene in prostate cancer patients
The BRCA1 studies demonstrated that the frequency of BRCA1 genes in prostate cancer patients in the USA was .0070 (95CI .0029 to .0123). Heterogeneity reduced when we conducted subgroup analysis and decreased for the USA subgroup (I2 = 57%), for all different races, BRCA1 gene mutations occurred in prostate cancer (Figure 3B). Sensitivity analyses demonstrated that the removal of any of the studies had no material effect on the observed pooled effect size (Figure 5B). The funnel chart showed little publication bias (Figure 4B).
The frequency of BRCA2 gene in prostate cancer patients
BRCA2 gene mutations are also common in prostate cancer patients; the results showed that the frequency of BRCA2 gene in prostate cancer patients in the USA and UK was .041 and .0393, respectively; BRCA2 gene mutations occur in prostate cancer patients of all different races. Heterogeneity remained high for the USA subgroup (I2 = 89%) and UK subgroup (I2= 85%) (Figure 3C). Sensitivity analyses demonstrated that the removal of the Momozawa study influenced the observed pooled effect size (Figure 5C). The funnel chart revealed a publication bias (Figure 4C).
The frequency of CHEK2 gene in prostate cancer patients
The subgroup analysis revealed that the frequency of CHEK2 gene mutations in prostate cancer patients in the USA was .0253, and heterogeneity was modestly reduced (I2 = 67%) across all races (Figure 3D). Sensitivity analyses demonstrated that the removal of the Momozawa study influenced the observed pooled effect size (Figure 5D). The funnel chart showed a publication bias (Figure 4D).
The frequency of MSH2 gene in prostate cancer patients
The frequency of MSH2 genes in prostate cancer patients varied by country, ranging from .0022 to .0083. Subgroup analysis showed that heterogeneity was decreased in the USA subgroup (I2 = 36%), China subgroup (I2 = 0%) (Figure 3E). Sensitivity analyses demonstrated that removing Nicolosi’s study reduced the observed frequency to .0020 (Figure 5E). The funnel chart demonstrated that there is no publication bias (Figure 4E).
The frequency of NBN gene in prostate cancer patients
The subgroup analysis revealed that the frequency of NBN genes in prostate cancer patients from the USA was .0017, and heterogeneity decreased when subgroup analysis was performed (I2 = 0%), and NBN genes mutations were widely observed across all races (Figure 3F). Sensitivity analyses revealed that when the Momozawa study was removed, the observed frequency increased to .0017 (Figure 5F). The funnel chart demonstrated that there is no publication bias (Figure 4F).
The Risk Estimates of DNA Damage Response Genes Between Normal People and PCa Patients
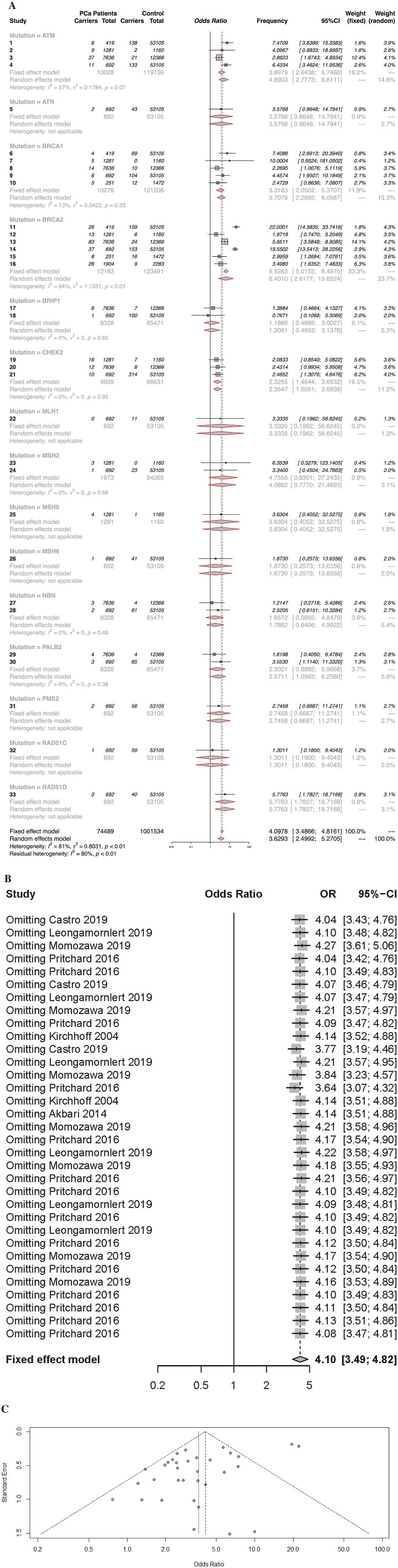
A total of 33 articles compared the risk estimates of DDR genes in healthy subjects and PCa patients; DDR genes are more likely to be found in prostate patients than in healthy subjects (OR = 3.6293 95% CI [2.4992; 5.2705]). Subgroup analysis revealed that the BRCA2 subgroup exhibited high heterogeneity; however, when BRCA2 related research was excluded, the heterogeneity decreased from 74% to 0%. Subgroups analyses also revealed that the incidence of BRCA2 in prostate cancer patients was significantly higher than in healthy subjects (OR = 6.4010 95% CI [2.6177; 15.6524]) (Figure 6A). The funnel chart demonstrates that the results had a certain publication bias (Figure 6C). Sensitivity analyses demonstrated that the removal of any studies had no significant effect on the observed pooled effect size (Figure 6B).
Figure 6.
(A) Forest plots of the DNA damage response genes mutation rate between patients with prostate cancer and normal population (B) Forest plots performed the Key factors (C) Funnel plots performed effect estimates of each study.
The Association of DNA Damage Response With Patient Survival
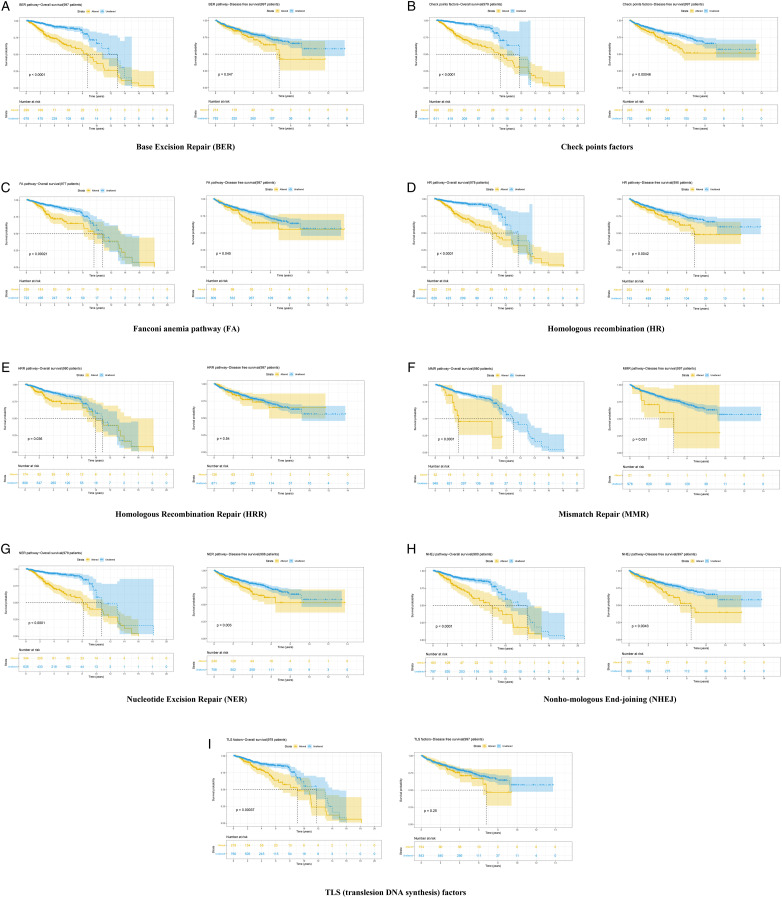
As of October 2020, cBioportal had a total of 22 PCa cohorts, 3 of which are TCGA cohorts (TCGA, Cell 2015; TCGA, Firehose Legacy; TCGA, PanCancer Atlas). Finally, with the exception of TCGA Cell 2015 and PanCancer Atlas, we included 20 PCa cohorts in our survival analysis. The findings showed that patients in the mutated group had a worse prognosis (OS and DFS) than those in the unmutated group in multiple DDR pathways (Figure 7A, Base Excision Repair; Figure 7B, Checkpoints factor; Figure 7C, Fanconi anemia pathway; Figure 7D, Homologous recombination; Figure 7E, Homologous recombination repair; Figure 7F, Mismatch Repair; Figure 7G, Nucleotide Excision Repair; Figure 7H, Non-homologous End-joining; Figure 7I, Translesion DNA synthesis factor).
Figure 7.
Kaplan-Meier curves showing that the patients with DNA damage response mutations may have a worse prognosis (A) BER pathway (B) Checkpoint factors (C) FA pathway (D) HR pathway (E) HRR pathway (F) MMR pathway (G) NER pathway (H) NHEJ pathway (I) TLS pathway.
The Association of DNA Damage Response Genes With Olaparib Sensitivity
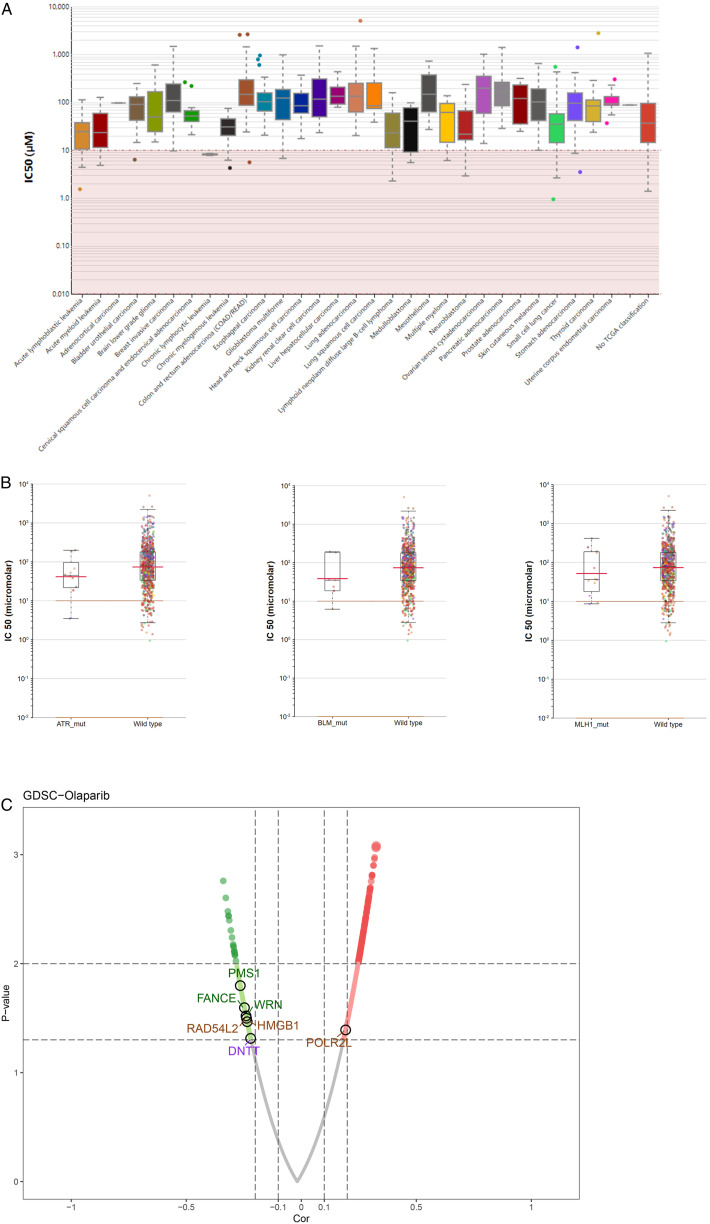
We first investigated the IC50 difference of Olaparib in multiple cancer tissues (Figure 8A) and the relationship between some DDR genes and Olaparib sensitivity by interacting with the website. The findings revealed that the mutation population of ATR, BLM, and MLH1 appears more sensitive to Olaparib (Figure 8B). Moreover, unlike POLR2L, tumor cells with high expression of PMS1, FANCE, WRN, RAD54L2, HMGB1, and DNTT are resistant to Olaparib (Figure 8C).
Figure 8.
(A) The IC50 of Olaparib in multiple cancers (B) The mutations of ATR, BLM, and MLH1 could improve the sensitivity of patients to Olaparib (C) The volcano plot of the association between gene expression with Olaparib sensibility. The blue dot could decrease the sensibility of Olaparib and the red dot could increase the sensibility of Olaparib.
The Association of DNA Damage Response Genes With Rucaparib Sensitivity
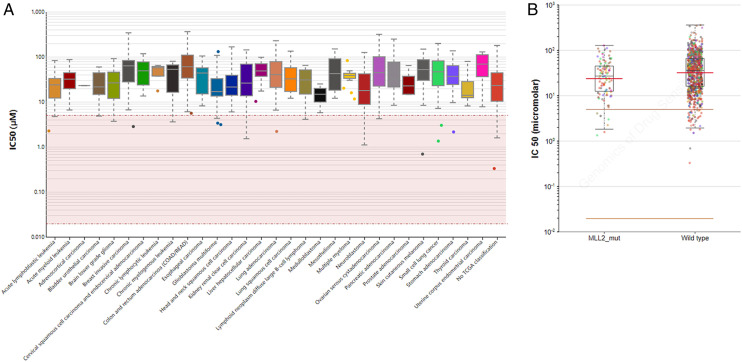
Similarly, the IC50 overview of Rucaparib in multiple cancer tissues was shown in Figure 9A. We found that mutation population of MLL2 appears more sensitive to Rucaparib (Figure 9B).
Figure 9.
(A) The IC50 of rucaparib in multiple cancers (B) The mutations of MLL2 could improve the sensitivity of patients to Olaparib.
Discussion
Despite the high long-term survival of localized prostate cancer, the therapeutic effect of metastatic prostate cancer is still insufficient, even following combined treatment. Recent evidence shows that DDR-related gene mutation is tightly associated with PCa progression, particularly in metastatic castration-resistant prostate cancer (mCRPC).95 Currently, approximately 20-25% of mCRPC patients have germline or somatic DDR gene mutations, and this defect has been shown to influence PCa cell sensitivity to PARP.96 As a result, it is highly imperative to investigate the underlying relationship between DDR genes and PCa patient prognosis.
We systematically investigated the role of DDR genes in PCa progression and prognosis using integrated meta and bioinformatics analysis. In our analysis, we found that DDR gene mutations, particularly BRCA1, were more common in tumor patients than in healthy males and that patients with DDR gene mutations had poorer OS. Moreover, some DDR genes were linked to the sensitivity of Olaparib, a PARP inhibitor approved for treating advanced ovarian cancer patients with BRCA gene deficiency. In addition, Olaparib is now approved for homologous recombination repair mutated mCRPC as well.97
Findings from the present investigation revealed that the frequency of mutations in BRCA2 was the highest of any DDR genes, accounting for approximately 3.98% of all mutations. We hypothesize that the prevalence of BRCA2 mutations in the population is unknown; meanwhile, when compared to healthy males, PCa patients may have a sixfold increase in BRCA2 mutation frequency. This result corroborates the findings of a large body of research on DDR genes. For example, Lecarpentier and colleagues demonstrated that BRCA1/2 mutation could increase the risk of breast and prostate cancer in men based on the genotyping data from 1989 males with a BRCA1/2 mutation. Furthermore, Patel and colleagues analyzed a large sample data of 6333 patients and found that specific BRCA2 mutations may be associated with a higher risk of PCa status.98 Another multicenter study conducted by Bancroft and colleagues found that people with BRCA1/2 mutation have a higher risk of developing PCa in 2481 male cohorts and that this germline mutation could be a useful marker for disease screening.99 It should be noted that the BRCA2 mutation in PCa patients was also closely related to the sensitivity of platinum chemotherapy and PARP inhibitors, which may be an underlying therapeutic target in PCa.100 Aside from BRCA1/2, other DDR genes with high mutation rates in the PCa cohort included ATM, CHEK2, and RAD51D. Southey and colleagues concluded that the CHEK2 mutation serves as reliable evidence for PCa risk in African men based on clinical analysis of 22 301 cases and 22 320 controls.101 Furthermore, in another meta-analysis, researchers assessed the radiation toxicity of PCa using 8 toxicity scores and found that the ATM rs1801516 SNP may be associated with increased toxicity reaction induced by radiation.102
We investigated the impact of mutations in the DDR pathway on PCa prognosis, including OS and DFS, using data from the cBioportal website. To the best of our knowledge, this is the first study that comprehensively examined the role of DDR mutation in PCa survival. Nearly all DDR pathway mutations were associated with a poor prognosis. In contrast to normal cells, cancer cells share the trait of genome instability caused by DDR defects. Meanwhile, men with genome instability, particularly shorter telomere lengths in somatic cells, appeared to have a poor prognosis and were more likely to develop PCa.103 Activation of cancer signaling is thought to increase DNA damage through increased genome instability and cancer progression.104 In the ATM knockout mice model, Liyanage and colleagues demonstrated that tumor tissue developed in mice had an increased copy of chromosome 15, where the c-Myc is located.105 Indeed, c-Myc has been implicated in the development and progression of PCa, and these studies established a link between the DDR gene and c-Myc.106 Undeniably, some DDR genes were rarely found in PCa patients, as such, few studies focused on them. However, based on our findings, any DDR pathway mutation could significantly worsen the prognosis of PCa patients. Subsequently, in clinical practice, it is critical to pay close attention to the disease status of PCa patients with DDR genes mutations.
Two members of the PARP family, PARP1 and PARP2, are known to be the key enzymes in repairing DNA single-strand breaks via the BER pathway. Olaparib, as a PARP inhibitor, can causing strong killing effects in HR-deficient cells by simultaneously blocking these 2 molecules, but not in cells with a normal HR system.107 Our findings suggest that some DDR genes such as POLR2L, PMS1, FANCE, WRN, and others, may influence Olaparib sensitivity in PCa patients. Similarly, our findings also suggest that the DDR gene MLL2 may influence Rucaparib sensitivity in PCa patients. Patients with different levels of expression of these genes may have different sensitivity to Olaparib or Rucaparib, which could be useful for individualized treatment.
Our study has some limitations despite the high-quality data and rigorous analysis process. First, our meta-analysis had a level of heterogeneity that was not significantly reduced after subgroup analysis. Second, the cBioportal only provides data on patient survival in the DDR mutation and wild groups. If clinical information such as TNM classification, age, and so on had been made public and available, the conclusions would have become more believable. Finally, due to a lack of data, the prognosis analysis of a single DDR gene was not completed, which may have resulted in latent bias.
Conclusion
Following the analysis of large sample data from multiple studies, the highest frequency of BRCA2 mutation was found in the PCa cohort. The mutation of ATM, BRCA1, BRCA2, CHEK2 and RAD51D genes was more common in PCa patients than in healthy males. Furthermore, it should be noted that mutations in any DDR pathway have been linked to a poor prognosis in PCa patients. Intriguingly, we discovered that the expression of POLR2L, PMS1, FANCE, WRN, and other genes was closely related to Olaparib sensitivity, suggesting that these genes may be underlying therapeutic targets in clinical practice.
Appendix.
Key of Definitions for Abbreviations
- DDR
DNA damage response
- ATM
ataxia telangiectasia-mutated gene
- BRCA1
breast cancer gene 1
- BRCA2
breast cancer gene 2
- BRIP1
BRCA1 interacting protein C-terminal helicase 1
- CHEK2
checkpoint kinase 2
- MUTYH
MutY DNA glycosylase
- MSH2
MutS homologue 2
- MSH6
MutS homologue 6
- NBN
nibrin
- PALB2
partner and localizer of BRCA2
- PMS2
PMS1 homolog 2
- RAD51D
RAD51 paralog D
- TP53
tumor protein 53
- BER
base excision repair
- CHEK
checkpoints factor
- FA
fanconi anemia pathway
- HR
homologous recombination
- HRR
homologous recombination repair
- MR
mismatch repair
- NER
nucleotide excision repair
- NHEJ
nonho-mologous end-joining
- TLS
translesion DNA synthesis factor
Footnotes
Authors Contribution: DZ, XX and YW collected the data and performed the meta-analysis. XX and DZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81972386, 81672531 to Chao Qin).
Ethics Approval and Consent to Participate: Ethical Approval is not applicable for this article.
Consent for Publication: Yes
Availability of Data and Materials: Yes
ORCID iD
Xinchi Xu https://orcid.org/0000-0002-5638-8804
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Holm NV, Verkasalo K, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland [J]. N Engl J Med. 2000;343(2):78-85. [DOI] [PubMed] [Google Scholar]
- 3.Leongamornlert D, Saunders E, Dadaev T, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease [J]. British journal of cancer. 2014;110(6):1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer [J]. N Engl J Med. 2016;375(5):443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhage BA, Baffoe-Bonnie AB, Baglietto L, et al. Autosomal dominant inheritance of prostate cancer: a confirmatory study [J]. Urology. 2001;57(1):97-101. [DOI] [PubMed] [Google Scholar]
- 6.Hjelmborg JB, Scheike T, Holst K, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer [J]. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research. Cosponsored by the American Society of Preventive Oncology. 2014;23(11):2303-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berndt SI, Wang Z, Yeager M, et al. Two susceptibility loci identified for prostate cancer aggressiveness [J]. Nat Commun. 2015;6:6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfand BT, Roehl KA, Cooper PR, et al. Associations of prostate cancer risk variants with disease aggressiveness: results of the NCI-SPORE genetics working group analysis of 18,343 cases [J]. Hum Genet. 2015;134(4):439-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szulkin R, Karlsson R, Whitington T, et al. Genome-wide association study of prostate cancer-specific survival [J]. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research. Cosponsored by the American Society of Preventive Oncology. 2015;24(11):1796-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin Al Olama A, Kote-Jarai Z, Schumacher FR, et al. A meta-analysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease [J]. Hum Mol Genet. 2013;22(2):408-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives [J]. Molecular cell. 2010;40(2):179-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashworth A. A synthetic lethal therapeutic approach: Poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair [J]. J Clin Oncol : Official Journal of the American Society of Clinical Oncology. 2008;26(22):3785-3790. [DOI] [PubMed] [Google Scholar]
- 13.Swift SL, Lang SH, White H, et al. Effect of DNA damage response mutations on prostate cancer prognosis: a systematic review [J]. Future oncology (London, England). 2019;15(28):3283-3303. [DOI] [PubMed] [Google Scholar]
- 14.Lang SH, Swift SL, White H, et al. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer [J]. Int J Oncol. 2019;55(3):597-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fachal L, Gómez-Caamaño A, Celeiro-Muñoz C, et al. BRCA1 mutations do not increase prostate cancer risk: results from a meta-analysis including new data [J]. Prostate. 2011;71(16):1768-1779. [DOI] [PubMed] [Google Scholar]
- 16.Cui M, Gao XS, Gu X, et al. BRCA2 mutations should be screened early and routinely as markers of poor prognosis: evidence from 8,988 patients with prostate cancer [J]. Oncotarget. 2017;8(25):40222-40232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hjelmborg JB, Scheike T, Holst K, et al. The heritability of prostate cancer in the Nordic Twin Study of Cancer. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2303-2310. doi: 10.1158/1055-9965.EPI-13-0568. Epub 2014 May 8. PMID: 24812039; PMCID: PMC4221420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cancer Genome Atlas Research Network . The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011-1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Wei Y, Pan J, et al. Prevalence of comprehensive DNA damage repair gene germline mutations in Chinese prostate cancer patients. Int J Cancer. 2021;148(3):673-681. doi: 10.1002/ijc.33324 [DOI] [PubMed] [Google Scholar]
- 20.Shamseer L, Moher D, Clarke M, et al. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015:g7647. doi: 10.1136/bmj.g7647. PMID: 25555855. [DOI] [PubMed] [Google Scholar]
- 21.Damaraju S, Murray D, Dufour J, et al. Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer [J]. Clin Cancer Res. 2006;12(8):2545-2554. [DOI] [PubMed] [Google Scholar]
- 22.Pugh T, Keyes M, Barclay L, et al. Sequence variant discovery in DNA repair genes from radiosensitive and radiotolerant prostate brachytherapy patients [J]. Clin Cancer Res. 2009;15(15):5008-5016. [DOI] [PubMed] [Google Scholar]
- 23.Beltran H, Yelensky R, Frampton GM, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity [J]. Eur Urol. 2013;63(5):920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leongamornlert D, Saunders E, Dadaev T, et al. Frequent germline deleterious mutations in DNA repair genes in familial prostate cancer cases are associated with advanced disease [J]. Br J Cancer. 2014;110(6):1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer [J]. N Engl J Med. 2015;373(18):1697-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart SN, Ellingson MS, Schahl K, et al. Determining the frequency of pathogenic germline variants from exome sequencing in patients with castrate-resistant prostate cancer [J]. BMJ Open. 2016;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer [J]. N Engl J Med. 2016;375(5):443-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abida W, Armenia J, Gopalan A, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making [J]. JCO Precision Oncology. 2017;2017(1):1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annala M, Struss WJ, Warner EW, et al. Treatment outcomes and tumor loss of heterozygosity in germline DNA repair–deficient prostate cancer [J]. Eur Urol. 2017;72(1):34-42. [DOI] [PubMed] [Google Scholar]
- 30.Pomerantz MM, Spisák S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer [J]. Cancer. 2017;123(18):3532-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonarakis ES, Lu C, Luber B, et al. Germline DNA-repair gene mutations and outcomes in men with metastatic castration-resistant prostate cancer receiving first-line abiraterone and enzalutamide [J]. Eur Urol. 2018;74(2):218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaacsson Velho P, Silberstein JL, Markowski MC, et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer [J]. Prostate. 2018;78(5):401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubert A, Peretz T, Manor O, et al. The Jewish Ashkenazi founder mutations in the BRCA1/BRCA2 genes are not found at an increased frequency in Ashkenazi patients with prostate cancer [4] [J]. Am J Hum Genet. 1999;65(3):921-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nastiuk KL, Mansukhani M, Terry MB, et al. Common mutations in BRCA1 and BRCA2 do not contribute to early prostate cancer in Jewish men [J]. Prostate. 1999;40(3):172-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazina A, Baniel J, Yaacobi Y, et al. The rate of the founder Jewish mutations in BRCA1 and BRCA2 in prostate cancer patients in Israel [J]. Br J Cancer. 2000;83(4):463-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gayther SA, De Foy KA, Harrington P, et al. The frequency of germ-line mutations in the breast cancer predisposition genes BRCA1 and BRCA2 in familial prostate cancer. The Cancer Research Campaign/British Prostate Group United Kingdom Familial Prostate Cancer Study Collaborators. J Cancer Res. 2000;60(16):4513-4518. [PubMed] [Google Scholar]
- 37.Edwards SM, Kote-Jarai Z, Meitz J, et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene [J]. Am J Hum Genet. 2003;72(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giusti RM, Rutter JL, Duray PH, et al. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology [J]. J Med Genet. 2003;40(10):787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamel N, Kotar K, Foulkes WD. Founder mutations in BRCA1/2 are not frequent in Canadian Ashkenazi Jewish men with prostate cancer [J]. BMC Med Genet. 2003;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirchhoff T, Kauff ND, Mitra N, et al. BRCA mutations and risk of prostate cancer in Ashkenazi Jews [J]. Clin Cancer Res. 2004;10(9):2918-2921. [DOI] [PubMed] [Google Scholar]
- 41.Agalliu I, Karlins E, Kwon EM, et al. Rare germline mutations in the BRCA2 gene are associated with early-onset prostate cancer [J]. Br J Cancer. 2007;97(6):826-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tischkowitz MD, Yilmaz A, Chen LQ, et al. Identification and characterization of novel SNPs in CHEK2 in Ashkenazi Jewish men with prostate cancer [J]. Cancer Lett. 2008;270(1):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agalliu I, Gern R, Leanza S, et al. Associations of high-grade prostate cancer with BRCA1 and BRCA2 founder mutations [J]. Clin Cancer Res. 2009;15(3):1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher DJ, Cronin AM, Milowsky MI, et al. Germline BRCA mutation does not prevent response to taxane-based therapy for the treatment of castration-resistant prostate cancer [J]. BJU Int. 2012;109(5):713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leongamornlert D, Mahmud N, Tymrakiewicz M, et al. Germline BRCA1 mutations increase prostate cancer risk [J]. Br J Cancer. 2012;106(10):1697-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbari MR, Wallis CJD, Toi A, et al. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer [J]. Br J Cancer. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [J]. Cell. 2015;161(5):1215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evans JR, Zhao SG, Chang SL, et al. Patient-Level DNA damage and repair pathway profiles and prognosis after prostatectomy for high-risk prostate cancer [J]. JAMA Oncol. 2016;2(4):471-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweizer MT, Cheng HH, Tretiakova MS, et al. Mismatch repair deficiency may be common in ductal adenocarcinoma of the prostate [J]. Oncotarget. 2016;7(50):82504-82510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mateo J, Cheng HH, Beltran H, et al. Clinical outcome of prostate cancer patients with germline DNA repair mutations: Retrospective analysis from an international study [J]. Eur Urol. 2018;73(5):687-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mijuskovic M, Saunders EJ, Leongamornlert DA, et al. Rare germline variants in DNA repair genes and the angiogenesis pathway predispose prostate cancer patients to develop metastatic disease [J]. British journal of cancer. 2018;119(1):96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paulo P, Maia S, Pinto C, et al. Targeted next generation sequencing identifies functionally deleterious germline mutations in novel genes in early-onset/familial prostate cancer [J]. PLoS Genet. 2018;14(4):e1007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter HB, Helfand B, Mamawala M, et al. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer [J]. Eur Urol. 2019;75(5):743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro E, Lozano Mejorada R, Saez M, et al. Impact of germline mutations in homologous recombination (HR) genes on the response to Radium-223 for metastatic castration resistant prostate cancer (mCRPC) [J]. Ann Oncol. 2019;30:v343. [Google Scholar]
- 55.Chandrasekar T, Gross L, Gomella LG, et al. Prevalence of suspected hereditary cancer syndromes and germline mutations among a diverse cohort of probands reporting a family history of prostate cancer: toward informing cascade testing for men [J]. European urology oncology. 2019;S2588-9311(19):30085-30089. [DOI] [PubMed] [Google Scholar]
- 56.Giri VN, Hegarty SE, Hyatt C, et al. Germline genetic testing for inherited prostate cancer in practice: Implications for genetic testing, precision therapy, and cascade testing [J]. Prostate. 2019;79(4):333-339. [DOI] [PubMed] [Google Scholar]
- 57.Ikeda S, Elkin SK, Tomson BN, et al. Next-generation sequencing of prostate cancer: Genomic and pathway alterations, potential actionability patterns, and relative rate of use of clinical-grade testing [J]. Cancer Biol Ther. 2019;20(2):219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kratochwil C, Giesel FL, Heussel CP, et al. Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA-repair associated genes [J]. J Nucl Med: Official Publication, Society of Nuclear Medicine. 2019. [DOI] [PubMed] [Google Scholar]
- 59.Leongamornlert DA, Saunders EJ, Wakerell S, et al. Germline DNA repair gene mutations in young-onset prostate cancer cases in the UK: evidence for a more extensive genetic panel [J]. Eur Urol. 2019;76(3):329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mateo J, Seed G, Bertan C, et al. Genomics of lethal prostate cancer at diagnosis and castration-resistance [J]. The Journal of clinical investigation. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Momozawa Y, Iwasaki Y, Hirata M, et al. Germline pathogenic variants in 7,636 Japanese patients with prostate cancer and 12,366 controls [J]. Journal of the National Cancer Institute. 2019:djz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolosi P, Ledet E, Yang S, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines [J]. JAMA Oncol. 2019;5(4):523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrovics G, Price DK, Lou H, et al. Increased frequency of germline BRCA2 mutations associates with prostate cancer metastasis in a racially diverse patient population [J]. Prostate Cancer Prostatic Dis. 2019;22(3):406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rusak B, Kluźniak W, Wokołorczykv D, et al. Inherited NBN mutations and prostate cancer risk and survival [J]. Cancer research and treatment. Official Journal of Korean Cancer Association. 2019;51(3):1180-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schweizer MT, Antonarakis ES, Bismar TA, et al. Genomic characterization of prostatic ductal adenocarcinoma identifies a high prevalence of DNA repair gene mutations [J]. JCO precision oncology. 2019;3. doi: 10.1200/PO.18.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Velho PI, Lim D, Wang H, et al. Molecular characterization and clinical outcomes of primary gleason pattern 5 prostate cancer after radical prostatectomy [J]. JCO precision oncology. 2019;3. doi: 10.1200/PO.19.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y, Wu J, Gu W, et al. Germline DNA repair gene mutation landscape in chinese prostate cancer patients [J]. Eur Urol. 2019;76(3):280-283. [DOI] [PubMed] [Google Scholar]
- 68.Zhu J, Tucker M, Marin D, et al. Clinical utility of FoundationOne tissue molecular profiling in men with metastatic prostate cancer [J]. Urol Oncol. 2019;3:813.e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dawson NA, Zibelman M, Lindsay T, et al. An emerging landscape for canonical and actionable molecular alterations in primary and metastatic prostate cancer [J]. Mol Cancer Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 70.Khooshemehri P, Jamaldini SH, Ziaee SAM, et al. Genetic polymorphism of mismatch repair genes and susceptibility to prostate cancer [J]. Urol J. 2020. doi: 10.22037/uj.v0i0.5051 [DOI] [PubMed] [Google Scholar]
- 71.Mateo J, Seed G, Bertan C, et al. Genomics of lethal prostate cancer at diagnosis and castration resistance [J]. The Journal of clinical investigation. 2020:132031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moses M, Koksal U, Ledet E, et al. Evaluation of the genomic alterations in the androgen receptor gene during treatment with high-dose testosterone for metastatic castrate-resistant prostate cancer [J]. Oncotarget. 2020;11(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rantapero T, Wahlfors T, Kähler A, et al. Inherited DNA repair gene mutations in men with lethal prostate cancer [J]. Genes. 2020;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ritch E, Fu SYF, Herberts C, et al. Identification of hypermutation and defective mismatch repair in ctDNA from metastatic prostate cancer [J]. Clin Cancer Res: An Official Journal of the American Association for Cancer Research. 2020;26(5):1114-1125. [DOI] [PubMed] [Google Scholar]
- 75.Vidula N, Rich TA, Sartor O, et al. Routine plasma-based genotyping to comprehensively detect germline, somatic, and reversion BRCA mutations among patients with advanced solid tumors [J]. Clin Cancer Res: An Official Journal of the American Association for Cancer Research. 2020. [DOI] [PubMed] [Google Scholar]
- 76.Wei Y, Wu J, Gu W, et al. Prognostic value of germline DNA repair gene mutations in de novo metastatic and castration-sensitive prostate cancer [J]. Oncologist. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu Y, Yu H, Li S, et al. Rare germline pathogenic mutations of DNA repair genes are most strongly associated with grade group 5 prostate cancer [J]. European urology oncology. 2020;S2588-9311(19):30169-30175. [DOI] [PubMed] [Google Scholar]
- 78.Rusak B, Kluźniak W, Wokołorczyk D, et al. Missense mutations of NBS1 and the risk of breast and prostate cancers [J]. Hered Cancer Clin Pract. 2018;16. [Google Scholar]
- 79.Christenson ES, Antonarakis ES. PARP inhibitors for homologous recombination-deficient prostate cancer [J]. Expet Opin Emerg Drugs. 2018;23(2):123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Y, Yu H, Zheng SL, et al. A comprehensive evaluation of CHEK2 germline mutations in men with prostate cancer [J]. Prostate. 2018;78(8):607-615. [DOI] [PubMed] [Google Scholar]
- 81.Park J-L, Kim S-K, Kim J-H, et al. Generation of whole-genome sequencing data for comparing primary and castration-resistant prostate cancer [J]. Genomics & informatics. 2018;16(3):71-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mano R, Tamir S, Kedar I, et al. Malignant abnormalities in male BRCA mutation carriers: Results from a prospectively screened cohort [J]. JAMA Oncol. 2018;4(6):872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cimadamore A, Lopez-Beltran A, Massari F, et al. Germline and somatic mutations in prostate cancer: focus on defective DNA repair, PARP inhibitors and immunotherapy [J]. Future oncology (London, England). 2020;16(5):75-80. [DOI] [PubMed] [Google Scholar]
- 84.Gupta S, Hovelson DH, Kemeny G, et al. Discordant and heterogeneous clinically relevant genomic alterations in circulating tumor cells vs plasma DNA from men with metastatic castration resistant prostate cancer [J]. Genes, chromosomes & cancer. 2020;59(4):225-239. [DOI] [PubMed] [Google Scholar]
- 85.Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): A multicentre, open-label, randomised, phase 2 trial [J]. Lancet Oncol. 2020;21(1):162-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morova T, Mcneill DR, Lallous N, et al. Androgen receptor-binding sites are highly mutated in prostate cancer [J]. Nat Commun. 2020;11(1):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nyberg T, Frost D, Barrowdale D, et al. Prostate cancer risks for male BRCA1 and BRCA2 mutation carriers: a prospective cohort study [J]. Eur Urol. 2020;77(1):24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel VL, Busch EL, Friebel TM, et al. Association of genomic domains in BRCA1 and BRCA2 with prostate cancer risk and aggressiveness [J]. Cancer Res. 2020;80(3):624-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sztupinszki Z, Diossy M, Krzystanek M, et al. Prevalence of homologous recombination deficiency (HRD)-related signatures indicates that a wider range of prostate cancer patients may benefit from PARP-inhibitor therapy [J]. Ann Oncol. 2019;30(vii14). [Google Scholar]
- 90.Taavitsainen S, Annala M, Ledet E, et al. Evaluation of commercial circulating tumor DNA test in metastatic prostate cancer [J]. JCO Precision Oncology. 2019;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Dessel LF, Van Riet J, Smits M, et al. The genomic landscape of metastatic castration-resistant prostate cancers reveals multiple distinct genotypes with potential clinical impact [J]. Nat Commun. 2019;10(1):5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vanderweele DJ, Finney R, Katayama K, et al. Genomic heterogeneity within individual prostate cancer foci impacts predictive biomarkers of targeted therapy [J]. European urology focus. 2019;5(3):416-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee DJ, Hausler R, Le AN, et al. Association of inherited mutations in DNA repair genes with localized prostate cancer. Eur Urol. 2021. doi: 10.1016/j.eururo.2021.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei J, Yang W, Shi Z, et al. Observed evidence for guideline-recommended genes in predicting prostate cancer risk from a large population-based cohort. Prostate. 2021;81(13):1002-1008. doi: 10.1002/pros.24195 [DOI] [PubMed] [Google Scholar]
- 95.Chakraborty G, Armenia J, Mazzu YZ, et al. Significance of BRCA2 and RB1 co-loss in aggressive prostate cancer progression [J]. Clin Cancer Res: An Official Journal of the American Association for Cancer Research. 2020;26(8):2047-2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Criscuolo D, Morra F, Giannella R, et al. Identification of novel biomarkers of homologous recombination defect in DNA repair to predict sensitivity of prostate cancer cells to PARP-inhibitors [J]. Int J Mol Sci. 2019;20(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102. doi: 10.1056/NEJMoa1911440 [DOI] [PubMed] [Google Scholar]
- 98.Patel VL, Busch EL, Friebel TM, et al. Association of genomic domains in BRCA1 and BRCA2 with prostate cancer risk and aggressiveness [J]. Cancer Res. 2020;80(3):624-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bancroft EK, Page EC, Castro E, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study [J]. Eur Urol. 2014;66(3):489-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheng HH, Sokolova AO, Schaeffer EM, et al. Germline and somatic mutations in prostate cancer for the clinician [J]. J Natl Compr Cancer Netw : J Natl Compr Cancer Netw. 2019;17(5):515-521. [DOI] [PubMed] [Google Scholar]
- 101.Southey MC, Goldgar DE, Winqvist R, et al. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS [J]. J Med Genet. 2016;53(12):800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andreassen CN, Rosenstein BS, Kerns SL, et al. Individual patient data meta-analysis shows a significant association between the ATM rs1801516 SNP and toxicity after radiotherapy in 5456 breast and prostate cancer patients [J]. Radiother Oncol : Journal of the European Society for Therapeutic Radiology and Oncology. 2016;121(3):431-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heaphy CM, Yoon GS, Peskoe SB, et al. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death [J]. Cancer Discov. 2013;3(10):1130-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karanika S, Karantanos T, Li L, et al. DNA damage response and prostate cancer: defects, regulation and therapeutic implications [J]. Oncogene. 2015;34(22):2815-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liyanage M, Weaver Z, Barlow C, et al. Abnormal rearrangement within the alpha/delta T-cell receptor locus in lymphomas from Atm-deficient mice [J]. Blood. 2000;96(5):1940-1946. [PubMed] [Google Scholar]
- 106.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer [J]. Cancer Cell. 2010;18(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bochum S, Berger S, Martens UM. Olaparib [J]. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2018;211:217-233. [DOI] [PubMed] [Google Scholar]