Abstract
Study Design
Systematic review.
Objective
Ehlers-Danlos Syndrome (EDS) comprises a spectrum of connective tissue disorders, which may be associated with cranio-cervical instability (CCI). There is a lack of consensus on diagnostic imaging parameters, indications, and outcomes of surgical treatment.
Methods
This systematic review analyses the literature on diagnostic methods and/or criteria for CCI, screening the databases Ovid Medline, Embase, Cochrane Library, and PubMed. Articles were included based on the PRISMA guidelines and assessed using the Newcastle-Ottawa Quality Assessment Scale (NOS) and according to their evidence level.
Results
Sixteen articles, including 78 surgical patients, met the inclusion criteria. The main diagnostic measures for CCI were dynamic x-rays and CT imaging. Ten different radiographic parameters were reported, of which 4 were the most frequently applied for surgical decision-making: the clivo-axial angle (CXA), the Harris measurement, the Grabb–Mapstone–Oakes measurement, and the angular displacement of C1 to C2. The evidence level ranged between III and V and the article quality between 4 and 8 out of 9 stars on the NOS Scale.
Conclusions
There is a lack of high quality, prospective evidence regarding the evaluation of suspected CCI in patients with EDS. Based on our systematic review, we recommend that the CXA, Harris measurement, Grabb–Mapstone–Oakes measurement, and the angular displacement of C1 to C2 be used to evaluate suspected CCI in EDS patients. Surgical fixation of suspected CCI should only be performed in cases with clear radiographic presence of instability and concordant symptoms/signs. Consensus-based guidelines and care pathways are required.
Keywords: Ehlers-Danlos syndrome, cranio-cervical instability, systematic review, spine, connective tissue disorders
Introduction
Ehlers-Danlos syndrome (EDS) affects 1/5000 individuals and comprises a group of hereditary connective tissue disorders that are directly related to a collagen synthesis deficiency, resulting in skin hyperextensibility, tissue fragility, and joint hyperextensibility.1,2 Initially described as “cutis laxa” by Edvard Ehlers and Henri Danlos in 1904 and 1908, EDS evolved over the decades as a complex syndrome with multiple subtypes. 3 In 2017, the international classification of EDS recognized 13 subtypes that are caused by pathogenic variants in 19 different genes, which affect different tissues and organs to a variable extent. Five subtypes predominantly involve the spine and may cause severe pathological conditions, highly impacting the quality of life of these patients. (Table 1, adapted from Malfait F et al). 4
Table 1.
Overview of the different EDS subtypes, including their individual inheritance pattern, the affected genes, and downstream proteins.
| Clinical EDS Subtype | Abbreviation | IP | Gene | Protein | |
|---|---|---|---|---|---|
| 1 | Classical EDS | cEDS | AD | COL5A1, COL5A1 | Type V collagen |
| COL1A1 | Type I collagen | ||||
| c.934 C>T, p (Arg312Cys) | |||||
| 2 | Classical-like EDS | clEDS | AR | TNXB | Tenascin XB |
| 3 | Cardiac-valvular EDS | cvEDS | AR | COL1A2 | Type I collagen |
| 4 | Vascular EDS | vEDS | AD | COL3A1 | Type III collagen |
| COL1A1 | Type I collagen | ||||
| c.934 C>T, p (Arg312Cys) | |||||
| c.1720 C>T, p (Arg574Cys) | |||||
| c.3227 C>T, p (Arg1093Cys) | |||||
| 5 | Hypermobile EDS | hEDS | AD | Unknown | Unknown |
| 6 | Arthrochalasia EDS | aEDS | AD | COL1A1, COL1A2 | Type I collagen |
| 7 | Dermatoparaxis EDS | dEDS | AR | ADAMTS2 | ADAMTS-2 |
| 8 | Kyphoscoliotic EDS | kEDS | AR | PLOD1 | LH1 |
| FKBP14 | FKBP22 | ||||
| 9 | Brittle cornea syndrome | BCS | AR | ZNF469 | ZNF469 |
| PRDM5 | PRDM5 | ||||
| 10 | Spondyloplastic EDS | spEDS | AR | B4GALT7 | β4GalT7 |
| B3GALT6 | β3GalT6 | ||||
| SLC39A13 | ZIP13 | ||||
| 11 | Musculocontractural EDS | mcEDS | AR | CHST14 | D4ST1 |
| DSE | DSE | ||||
| 12 | Myopathic EDS | mEDS | AD or AR | COL12A1 | Type XII collagen |
| 13 | Periodontal EDS | pEDS | AD | C1R | C1r |
| C1S | C1s |
Abbreviations: IP, inheritance pattern; AD, autosomal dominant; AR, autosomal recessive, NMD, nonsense-mediated mRNA decay.
Spinal manifestations of EDS have been described with higher attention only recently and include cranio-cervical instability (CCI), atlanto-axial subluxation, basilar invagination and various spinal deformities, for example, segmental kyphosis.5-9 CCI arises from ligamentous laxity with hypermobility and represents one of the most pertaining diagnoses for surgical intervention, employing instrumented fusion. 10 Morphologically, it results in cranial settling with clivo-axial kyphosis and ventral brainstem compression.11,12 Clinically, the patients might present with a cervico-medullary syndrome, which is characterized by a variety of disabling symptoms, including autonomous dysregulation, dizziness, sleep apnea, motor weakness and sensory deficits, balance disturbance and vertigo, as well as swallowing difficulties seeking medical attention. 13
Radiological assessment of CCI is conducted to a variable extent in different settings, but often includes dynamic imaging (flexion/extension views), 2D CT imaging and/or upright MRI of the cranio-vertebral junction. It pertains to distinct radiographic metrics being suggestive for potential instability. In this context, various parameters have been proposed in previous reports, however, these measures do not necessarily correspond to the diagnostic criteria for hypermobility and therefore are not accepted internationally as reliable indicators for CCI in EDS patients. 14 Accordingly, the treatment indications for CCI in EDS patients remain a matter of debate resulting in inconsistent surgical care availability among different countries. 14 In view of the of the lack of consensus on the optimal diagnostic criteria and algorithms to diagnose CCI in EDS patients, we sought to undertake a systematic review to critically evaluate and synthesize the available evidence, to assess and summarize the radiographic metrics for CCI, and to identify appropriate surgical treatment criteria.
Materials and Methods
A systematic literature review was performed by two independent reviewers, using the databases Ovid Medline, Embase, Cochrane Library, and PubMed based on the PRISMA guidelines. No language restrictions were applied to the search strategy. Applying the search terms “Ehlers-Danlos” and “spine,” “cervical spine,” “cranio-cervical instability” or “cranio-cervical junction”, 128 records were identified for further assessment. Articles were included if they described the application of diagnostic and/or treatment criteria for CCI in the context of EDS. Reported parameters pertaining to CCI and treatment indication in EDS patients are summarized. Case reports and editorials were equally included for completeness with respect to currently available information on EDS in CCI. The quality of the articles was assessed according to their evidence level and using the Newcastle-Ottawa Quality Assessment (NOS) Scale. 15
Results
The database search identified 128 records. After duplicate removal 97 articles were screened for relevance of titles and abstracts. Fourty-five relevant articles were reviewed for eligibility, which resulted in a full-text review of 18 studies. Two articles were not available as full text and therefore excluded. The remaining 16 articles were included in the final qualitative synthesis, comprising 2 case reports, 2 research articles, 3 editorials as well as 9 original articles (Figure 1). A total of 695 EDS patients were reported in these studies, of which 78 patients were diagnosed with CCI and underwent surgical treatment. The articles were published between November 1995 and May 2021. All articles were published in English. An overview of the included studies is given in Table 2.
Figure 1.
Flowchart illustrating the article selection process according to the PRISMA guidelines.
Table 2.
Table summarizing the current literature on CCI in EDS, including the article type, number of patients reported, as well as the level of evidence and quality.
| Author | Year | Article Type | Number of EDS Patients | Evidence Level | NOS Scale | |||
|---|---|---|---|---|---|---|---|---|
| Selection (★/4) | Comparability (★/2) | Exposure/Outcome (★/3) | Total (9★) | |||||
| Awasthy N et al | 2008 | Case report | 1 | V | n.a | n.a | n.a | n.a |
| Brodbelt AR, flint G | 2017 | Editorial | 0 | V | n.a | n.a | n.a | n.a |
| Halko GJ et al | 1995 | Original article | 26 | IV | 2 (4) | 0 (2) | 1 (3) | 3 (9) |
| Henderson F et al | 2016 | Editorial | 0 | V | n.a | n.a | n.a | n.a |
| Henderson F et al | 2017 | Editorial | 0 | V | n.a | n.a | n.a | n.a |
| Henderson F et al | 2016 | Original article | 10 | IV | 2 (4) | 0 (2) | 3 (3) | 5 (9) |
| Henderson F et al | 2018 | Original article | 22 | IV | 2 (4) | 0 (2) | 3 (3) | 5 (9) |
| Henderson F et al | 2020 | Original article | 20 | IV | 2 (4) | 0 (2) | 2 (3) | 4 (9) |
| Henderson F et al | 2021 | Original article | 20 | IV | 2 (4) | 0 (2) | 3 (3) | 5 (9) |
| Karaa A, stoler JM | 2013 | Case report | 1 | V | n.a | n.a | n.a | n.a |
| Klinge PM et al | 2020 | Original article | 28 | III | 2 (4) | 2 (2) | 3 (3) | 7 (9) |
| Matur AV et al | 2020 | Original article | 279 | III | 4 (4) | 2 (2) | 2 (3) | 8 (9) |
| Milhorat TH et al | 2007 | Original article | 250 | III | 3 (4) | 2 (2) | 2 (3) | 7 (9) |
| Ontario health technology assessment series | 2015 | Research article | 0 | V | n.a | n.a | n.a | n.a |
| Spiessberger A et al | 2020 | Original article | 26 | III | 3 (4) | 2 (0) | 2 (2) | 7 (9) |
| Uehara M et al | 2018 | Research article | 12 | IV | 2 (4) | 0 (2) | 2 (3) | 4 (9) |
Radiographic Parameters
A total of 10 linear and angular morphometric parameters pertaining to the diagnosis of CCI were reported (Table 3). The parameters were mainly established in lateral flexion and extension x-rays of the cervical spine, followed by dynamic CT imaging and MRI. Upright, weight-bearing flexion and extension MRI of the cervical spine was performed in one of the studies. 11 Their application was performed according to the suspected type of instability and the 3-dimensional plane in which they may occur.
Table 3.
Radiographic parameters applied in the reported studies, including their measurement description and normative values.
| Number | Radiographic Parameter | Measurement | Norm Value* |
|---|---|---|---|
| 1 | Clivo-axial angle (CXA) | Angle between a line drawn along the posterior aspect of the lower clivus and the posterior axial line (PAL) | 145° to 160° |
| 2 | Grabb–Mapstone–Oakes measurement (pB-C2 line) | Perpendicular distance to a line drawn between the basion and the most posterior extent of the odontoid process at the dural interface | ≤9 mm |
| 3 | Horizontal Harris measurement | Horizontal distance from the basion to the posterior axial line (PAL) | ≤12 mm |
| 4 | Basion-dens interval (BDI) | Distance from the most inferior portion of the basion and the top of the odontoid measured in the median (midsagittal) plane | <12 mm |
| 5 | Basion-atlas interval (BAI) | Horizontal distance between the basion and a vertical plane of the superior surface of the atlantal anterior arch | 1.8 ± 1.21 mm |
| 6 | Atlanto-dental interval (ADI) | Horizontal distance between the posterior cortex of the anterior arch of the atlas and the anterior cortex of the dens in the median (midsagittal) plane | ≤3 mm |
| 7 | Dens-atlas interval | Distance between the top of the dens and a horizontal line drawn from the lowest point of the atlantal anterior arch to the lowest point of the atlantal posterior arch | 2.3 ± 2.17 mm |
| 8 | Angular displacement between C1/2 | Rotational angle subtended by C1-C2 | ≤41° |
| 9 | Clivus-atlas angle (CAA) | Angle between the superior plane of the clivus and a line drawn between the anterior top of the atlas and the lowest point of its posterior arch | 37.9 ± 7.14° |
| 10 | Atlas-axis angle (AXA) | Angle between a plane of the posterior surface of the dens axis and a line drawn between the anterior top of the atlas and the lowest point of its posterior arch | 56.8 ± 4.71° |
| * Measured in adults |
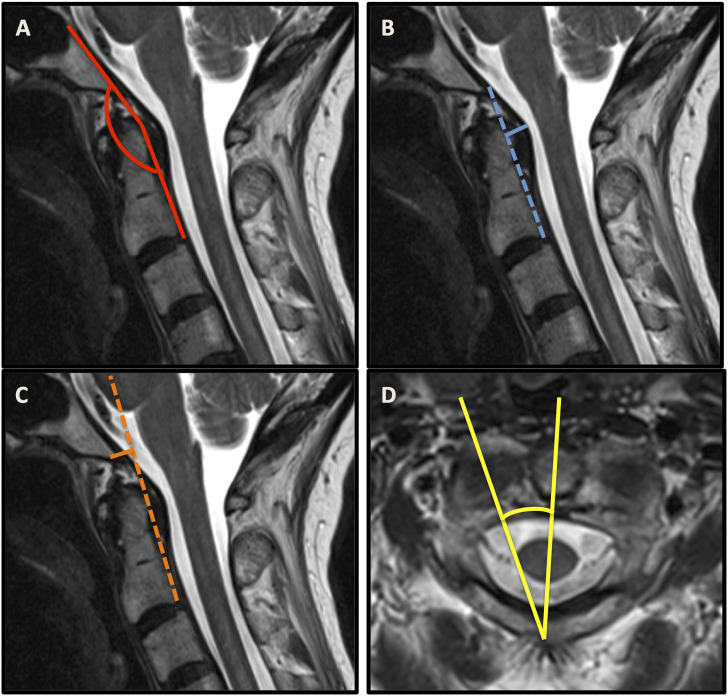
The most applied radiographic metrics to define CCI were the clivo-axial angle (CXA), the Harris measurement, the Grabb-Mapstone-Oakes measurement, as well as the angular displacement of C1 to C2 (Figure 2). The choice of diagnostic parameters was inconsistent in the majority of studies.
Figure 2.
Sagittal MRI sequences containing the clivo-axial angle (CXA) (A), the Harris measurement (B), the Grabb–Mapstone–Oakes measurement (C). Axial MRI sequence illustrating the angular displacement of C1 to C2 (D).
Henderson et al. reported 2 studies using the clivo-axial angle (CXA), the Harris measurement, and the Grabb–Mapstone–Oakes measurement as reliable indicators for CCI.11,13 The CXA was used repetitively to indicate potential brainstem deformity related to CCI. It describes the extent of kyphosis at the cranio-cervical junction being associated with pathological bending of the brainstem, which may result in mechanical injury and chronic damage to the lower brainstem and upper spinal cord.11,16,17 Equally, Spiessberger et al. referred to the CXA when assessing the outcomes of 2 different fusion techniques in EDS patients with CCI and cervico-medullary syndrome. They also used the Grabb–Mapstone–Oakes measurement (pB-C2 line) and confirmed improvement of both parameters regardless of the type of fusion technique, advocating for their value as surgical outcome parameters. 12
Milhorat et al. assessed the relationship between Chiari malformation type I and hereditary disorders of the connective tissue with respect to lower brainstem symptoms attributable to occipito-atlanto-axial hypermobility and cranial settling. Their study included 357 patients diagnosed with Chiari malformation type 1 and associated hereditary disorders of the connective tissue, of which two hundred fifty patients carried the diagnosis of EDS. Investigating motion of the occipito-atlanto-axial complex, they applied new measurements, such as the basion-atlas interval, for evaluating the horizontal relationship of the clivus and anterior arch of the atlas; the dens-atlas interval, for evaluating the vertical relationship of the odontoid and atlas; the atlas-axis angle, for evaluating the angle between the atlas and axis; and the clivus-atlas angle, for evaluating the angle between the clivus and atlas. These new measurements, as well as their derived normative values in healthy controls, are given in Table 3. They were able to identify a reduction of the basion-dens interval (BDI), clivo-axial angle (CXA), clivus-atlas angle and atlas-axis angle, as well as an increase of the basion-atlas interval (BAI) in the upright position, as diagnostic parameters for hypermobility of the occipito-atlantal and atlanto-axial joints in patients with Chiari malformation type 1 and associated hereditary disorders of the connective tissue compared to patients with Chiari malformation type I only or healthy controls. 10
Three articles reported C1/2 dislocations as a subtype of cervical instability in EDS patients using measurements of angular displacement between C1/2.7,18,19
Another study by Uehara et al. assessed spinal manifestations in EDS patients of the musculocontractural subtype. Among 12 patients, 2 suffered from atlanto-axial instability and were diagnosed using the atlanto-dental interval (ADI). Atlanto-axial instability was defined in their cohort if the ADI was >4 mm at flexion, analogous to the criteria for normal populations described by White and Panjabi in 1978.9,20
All parameters, their definitions, as well as reported normative values are described below.
Clivo-Axial Angle
The Clivo-Axial Angle (CXA) describes the angle that is formed between a line drawn along the posterior aspect of the lower clivus and the posterior axial line (PAL). The CXA has a normal range of 145°–160°, and an angle of less than 135° is considered pathological. 17
Grabb–Mapstone–Oakes Measurement (pB-C2 Line)
The pB-C2 line is a measure of ventral canal encroachment. It is drawn perpendicular to a line drawn between the basion and the posterior aspect of the C2 vertebral body, at the most posterior extent of the odontoid process at the dural interface. A pB-C2 measurement exceeding 9 mm is considered pathological and suggestive of a higher risk of ventral brainstem compression. 21
Horizontal Harris Measurement
The Harris measurement is defined as the distance from the basion to the posterior axial line (PAL). In adults, the occipito-vertebral junction can be considered normal when both the basion-axial interval and basion-dental interval are 12 mm or less. In children less than 13 years old, the basion-dental interval is not reliable because of the variable age at which complete ossification and fusion of the dens occur. The normal basion-axial interval in children does not exceed 12 mm. 22 Used in conjunction with flexion-extension, a dynamic translation between the basion and the odontoid of >1 mm may be suggestive of CCI. 13
Basion-Dens Interval
The BDI describes the distance from the most inferior portion of the basion to the top of the odontoid measured in the median (midsagittal) plane. In the normal person, the basion lies over the midpoint of the odontoid process, with a separation of approximately 5 mm. A basion to dental interval >12 mm in adults and >10 mm in children is abnormal.23,24
Basion-Atlas Interval
The BAI comprises the interval between the basion and the plane of the superior surface of the atlantal anterior arch. 10
Atlanto-Dental Interval
The ADI is the horizontal distance between the posterior cortex of the anterior arch of the atlas and the anterior cortex of the dens in the median (midsagittal) plane. Normal radiograph values for adults are <3 mm and <5 mm for children. In CT imaging, the normal value for adults is < 2 mm. 23
Dens-Atlas Interval
The dens-atlas interval corresponds to the distance between the top of the dens and a horizontal line drawn from the lowest point of the atlantal anterior arch to the lowest point of the atlantal posterior arch. 10
Angular Displacement Between C1/2
The angular displacement between C1/2 describes an angle subtended by C1-C2 and is considered pathological when >41° and/or when C1-C2 facet overlap is less than 10%. 25 Furthermore, antlanto-axial rotatory instability is classified according to the Fielding and Hawkins classification. 26
Clivus-Atlas Angle
The CAA comprises the angle between the superior plane of the clivus and a line drawn between the anterior top of the atlas and the lowest point of its posterior arch. 10
Atlas-Axis Angle
The AXA is defined as the angle between the plane of the posterior surface of the dens axis and a line drawn between the anterior top of the atlas and the lowest point of its posterior arch. 10
Surgical Treatment Criteria
Surgical treatment criteria were reported in 4 of the studies, including a total of 78 EDS patients who underwent cranio-cervical or cervical instrumentation for CCI or atlanto-axial instability, respectively.11,12,16,25 Henderson et al. treated 20 patients with cranio-vertebral instability and flexion deformity, showing a kyphotic CXA, cerebellar ectopia (18/20), or ventral brainstem compression in the context of cervico-medullary syndrome. The patients underwent reduction and stabilization via occipital to C1/C2 fusion. Thresholds for surgery were aligned to the following clinical criteria: 1) formal genetic evaluation and diagnosis with a hereditary connective tissue disorder (CF); 2) severe headache and/or neck pain greater than or equal to 7/10 by the visual analog scale for greater than 6 months; 3) symptoms of cervical medullary syndrome according to previous consensus statements27,28; 4) demonstrable neurological deficits; 5) congruent radiological findings including a kyphotic CXA (less than 135°), cranio-cervical instability (the Harris/BAI measurement in flexion minus the Harris measurement in extension >4 mm*), or low lying cerebellar tonsils or Chiari malformation type 1; and 6) failed conservative treatment. 11 A pilot study focusing on the value of CXA included 10 adult patients with brainstem deformity. The applied surgical treatment criteria in this cohort were: 1) moderate to severe headache or suboccipital pain; 2) bulbar symptoms constituting cervical medullary syndrome; 3) neurological findings of myelopathy, and 4) CXA less than 135°. 16
Surgical treatment criteria for atlanto-axial rotatory instability (Fielding type I) were equally described by Henderson et al. in a different subset of EDS patients in 2 unique articles.25,29 They comprised similarly: 1) formal genetic evaluation and diagnosis with a hereditary connective tissue disorder; 2) severe headache and/or neck pain for greater than 6 months; 3) symptoms compatible with atlanto-axial instability30,31; 4) congruent neurological deficits; and 5) radiological findings—an angle subtended by C1-C2 greater than 41°, and/or C1-C2 facet overlap of less than 10%. Another case series by Spiessberger et al. included 26 EDS patients with suspected cervico-medullary syndrome related to CCI. After assessment and confirmation of the diagnosis by an interdisciplinary team, the patients engaged in a 4-6-week trial of hard collar immobilization of the cervical spine. Patients were offered surgical intervention if their symptoms were perceived as unbearable, imaging characteristics confirmed CCI, and the trial of immobilization lead to symptom relief. 12 A summary of all applied surgical treatment criteria is given in Table 4.
Table 4.
Overview of articles reporting surgical treatment for CCI in EDS patients. The clinical assessment measures, criteria for surgery, and imaging methods described in these articles are listed.
| Author | Year | Clinical Assessment and Surgical Criteria | Imaging | Imaging Criteria |
|---|---|---|---|---|
| Henderson F et al | 2016 | Moderate to severe headache or suboccipital pain | Dynamic MRI, CT | CXA <135° |
| Bulbar symptoms constituting the cervical medullary syndrome | ||||
| Neurological findings of myelopathy | ||||
| Henderson F et al | 2018 | Formal genetic evaluation and diagnosis with a hereditary connective tissue disorder | Dynamic MRI or CT | CXA <135° |
| Severe headache and/or neck pain ≥ to7/10 VAS >6 months | Harris/Bai measurement in flexion minus extension >4 mm | |||
| Symptoms of cervical medullary syndrome | ||||
| Demonstrable neurological deficits | ||||
| Failed conservative treatment | ||||
| Henderson F et al | 2020 | Formal genetic evaluation and diagnosis with a hereditary connective tissue disorder | Dynamic CT, x-ray | Angle subtended by C1-C2 > than 41° |
| Severe headache and/or neck pain for greater than 6 months | C1-C2 facet overlap <10% | |||
| Symptoms compatible with atlanto-axial instability | Translation on lateral tilt >3.5 mm on open mouth views | |||
| Congruent neurological deficits | ||||
| Failed conservative treatment | ||||
| Henderson F et al | 2021 | Same as above | Same as above | Same as above |
| Spiessberger A et al | 2020 | Interdisciplinary evaluation confirming CCI-related symptoms | MRI, CT, x-ray (flexion/extension) | Confirmed CCI, not further specified |
| Assessment by EDS specialist | ||||
| Symptoms perceived as unbearable | ||||
| Improvement of symptoms after 4–6-week trial of hard collar immobilization |
Non-surgical therapies were not reported in these studies and therefore remain a separate area of investigation.
Quality Assessment
The quality of the articles was assessed according to their level of evidence and with the Newcastle-Ottawa Quality Assessment (NOS) Scale, when applicable. The majority of the studies provided scientific reporting on an evidence level of IV and V (6 studies each), while 4 studies were classified as level III. The NOS scale was applicable in 10 studies and revealed an overall moderate quality for the included articles. The ratings ranged between 3 and 8, out of a maximum of 9 available stars. A detailed breakdown of the article criteria evaluated, the corresponding quality values, and evidence levels is given in Table 2.
Discussion
In this review we summarized the current literature on diagnostic criteria and therapeutic implications for CCI in EDS patients, aiming to identify knowledge gaps and to provide a scientific overview of the current standard of care in these patients. We analyzed the impact and validity of reported diagnostic radiographic parameters, the applied surgical treatment criteria, as well as the quality of the reported literature. Sixteen articles met the inclusion criteria and were included in this review. The level of evidence was overall low, with variable quality observed in the studies (Table 2).
The diagnostic approaches reported in these studies included a total of 10 distinct radiographic measurements applicable in EDS-related CCI. While the radiographic metrics used deviated between studies, we could derive 6 that were repetitively applied and seem to represent best the methodology of assessing anatomical instability at the cranio-cervical junction. They correspond to the ones listed in the consensus statement on basilar invagination and CCI, including the clivo-axial angle, the Harris measurement, and the Grabb–Mapstone–Oakes measurement, 2 complemented by the BDI and ADI. We also identified the angular displacement between C1 and C2 as being repetitively used for assessment of atlanto-axial rotatory instability.
Most importantly, the choice of imaging methods was variable among the studies. All studies provided dynamic x-rays as well as 2-dimensional CT imaging. An MRI was performed in the majority of cases. Given that EDS patients with CCI do not present with the standard instability we observe in trauma patients but an instability of mainly ligamentous origin, or also defined as ligamentous hypermobility, 14 dynamic imaging, such as flexion/extension x-rays, dynamic CT imaging, as well as dynamic or upright (weight-bearing) MRI should be performed. In the study reported by Milhorat et al., vertical (sitting) MR imaging helped to show the dynamic features of occipito-atlanto-axial hypermobility and functional cranial settling in patients with Chiari malformation type 1 and associated hereditary disorders of the connective tissue. These abnormalities were reducible by traction or a return to the supine position, underlining the value of dynamic imaging. 10 Despite the potential diagnostic gain by extended imaging, choosing the correct metrics and advanced knowledge of the 3-dimensional plane, as well as their pathophysiological and mechanical alterations caused by EDS, are indispensable areas of expertise for each radiologist involved in the diagnostics of EDS-related CCI.32-34 In this context, a cross linkage to patients with Down syndrome, who often present with CCI, could reveal significant knowledge about the anatomical and biomechanical effects of ligamentous laxity and subsequent instability. 35 A recent study assessed predictors for neurological deficits in Down patients with CCI after using dynamic MRI of the cranio-cervical junction. The authors successfully characterized the range of motion seen on dynamic MRI and provided parameters that can be used to distinguish patients at risk for neurologic injury, which could serve as an essential basis for safely providing dynamic MRI in EDS patients. 36 However, while providing significant value diagnostically, dynamic MRI remains a recourse limited to very few centers and therefore can’t be considered as a diagnostic standard yet.
Another important factor in diagnosing CCI in EDS is the correlation between radiographic evidence and clinical symptomatology. The neurological symptoms related to CCI are highly variable, multilayered and sometimes difficult to assign.6,10 The presence of CCI is thought to cause classic Valsalva-related headaches, disturbance of autonomic function, and a diversity of neurological findings significantly affecting their quality of life. 14 The underlying mechanisms are claimed to result from anatomical distortion of the cervico-medullary junction, including stretch of the lower cranial nerves, stretch of the vertebral arteries, and deformative stretching or deformation of the brainstem and upper spinal cord.13,17 In a cohort of EDS patients with atlanto-axial instability (AAI), Henderson et al. observed a significant improvement of syncopal, presyncopal, and autonomic dysfunction after C1-C2 fusion. The authors attribute the symptoms to low grade chronic mechanical stretching and deformity of neural tissue related to the torsional strain on the spinal cord as well as to compromised blood flow in vertebral arteries, suggesting a plausible clinic-morphological correlation in AAI. 36 Distinct observations were made by Klinge et al., who identified laxity of spinal cord suspension ligaments and associated spinal cord motion disorder as possible pathogenic factors for chronic neck pain and headache in patients with EDS, however, without radiologically proven CCI. 37
Even though 4 of the included case series state a clear improvement of the patient’s symptoms after cervical instrumentation, a definite clinico-anatomical association is pending. Furthermore, there has been no method described to date which allows pre-operative assessment of the most probable neurological outcome response after the surgery.11,16,25,36 One of the reviewed studies challenged these processes pre-operatively by submitting their patient to a 4-6 -week duration of hard collar treatment, mimicking stabilization of the cervical spine. The authors offered surgical intervention only to those patients who experienced symptom relief after this trial (n = 26) and defined this response in their surgical treatment criteria. 12 Given that it is the first time that test-immobilization was performed in EDS patients with CCI, this approach may represent a new pre-operative strategy of value during surgical assessment and improve the selection of patients.
Limitations of this review are the paucity of available data and the low to moderate levels of evidence of the included articles. However, we tried to increase its completeness by including case reports and editorials, allowing it to be comprehensive and representative for the current level of evidence. Also, several of the available articles have been published by the same author and partially included repeated sampling. This may incorporate the fact of selection bias and therefore reduce the level of evidence.
Another relevant factor is that the topic concerns a very small subset of the neurosurgical population and therefore may be considered as of low importance. However, given the incidence of EDS of 1/5000, we do think that the actual number of EDS patients with CCI is higher than we experience in our neurosurgical practice. Assuming that this condition is underdiagnosed, most probably due to its complexity and its varying clinical appearance, we hope that this review will shed light on this condition besides raising the awareness for it. Evidently, further studies need to address different aspects of CCI and other spinal conditions in EDS patients, aiming for a more precise characterization of its clinico-radiographical correlations and thresholds for potential surgical and non-surgical care pathways.
Conclusions
Based on the current literature, there is a significant lack of evidence for the evaluation and management of suspected CCI in EDS patients. Based on the available evidence, we recommend that the CXA, the Harris measurement, the Grabb–Mapstone–Oakes measurement, and angular displacement between C1 and C2 be used to assess suspected CCI in EDS patients. However, these parameters warrant further prospective validation in multicentre cohort studies. Surgical treatment of suspected CCI in EDS patients should only be undertaken if clear radiographic evidence of instability is associated with concordant symptoms and signs. As a next step, consensus-based guidelines and care pathways are required.
Acknowledgments
MGF is supported by the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration. The authors wish to acknowledge Nadia Jaber for assistance in data collection and formal manuscript preparation.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Canerector Foundation.
ORCID iDs
Laura-Nanna Lohkamp, MD, MSc https://orcid.org/0000-0001-8027-0569
Nandan Marathe, MD https://orcid.org/0000-0002-8939-2690
Michael G Fehlings, MD, PhD https://orcid.org/0000-0002-5722-6364
References
- 1.Gensemer C, Burks R, Kautz S, Judge DP, Lavallee M, Norris RA. (2021) Hypermobile Ehlers‐Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev Dynam 250:318-344. doi: 10.1002/dvdy.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castori M, Voermans NC. (2014) Neurological manifestations of Ehlers-Danlos syndrome(s): A review. Iran J Neurol 13:190-208 [PMC free article] [PubMed] [Google Scholar]
- 3.Beighton P, de Paepe A, Danks D, et al. (1988) International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986, 1986. Am J Med Genet 29:581-594. doi: 10.1002/ajmg.1320290316 [DOI] [PubMed] [Google Scholar]
- 4.Malfait F, Francomano C, Byers P, et al. (2017) The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 175:8-26. doi: 10.1002/ajmg.c.31552 [DOI] [PubMed] [Google Scholar]
- 5.Nagashima C, Tsuji R, Kubota S, Tajima K. (1981) [Atlanto-axial, Atlanto-occipital dislocations, developmental cervical canal stenosis in the Ehlers-Danlos syndrome (author's transl)]. Noshinkeigeka 9:601-608 [PubMed] [Google Scholar]
- 6.Henderson FC, Austin, Benzel E, et al. (2017) Neurological and spinal manifestations of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet 175:195-211. doi: 10.1002/ajmg.c.31549 [DOI] [PubMed] [Google Scholar]
- 7.Halko GJ, Cobb R, Abeles M. (1995) Patients with type IV Ehlers-Danlos syndrome may be predisposed to atlantoaxial subluxation. J Rheumatol 22:2152-2155 [PubMed] [Google Scholar]
- 8.Matur AV, Nouri A, Huang S, et al. (2020) Complications in children with ehlers-danlos syndrome following spine surgery: Analysis of the pediatric national surgery quality improvement program database. World Neurosurg 133:e473-e478. doi: 10.1016/j.wneu.2019.09.046 [DOI] [PubMed] [Google Scholar]
- 9.Uehara M, Kosho T, Yamamoto N, et al. (2018) Spinal manifestations in 12 patients with musculocontractural Ehlers-Danlos syndrome caused by CHST14/D4ST1 deficiency (mcEDS-CHST14). Am J Med Genet 176:2331-2341. doi: 10.1002/ajmg.a.40507 [DOI] [PubMed] [Google Scholar]
- 10.Milhorat TH, Bolognese PA, Nishikawa M, McDonnell NB, Francomano CA. (2007) Syndrome of occipitoatlantoaxial hypermobility, cranial settling, and chiari malformation type I in patients with hereditary disorders of connective tissue. J Neurosurg Spine 7:601-609. doi: 10.3171/spi-07/12/601 [DOI] [PubMed] [Google Scholar]
- 11.Henderson FCS, Francomano CA, Koby M, Tuchman K, Adcock J, Patel S. (2019) Cervical medullary syndrome secondary to craniocervical instability and ventral brainstem compression in hereditary hypermobility connective tissue disorders: 5-year follow-up after craniocervical reduction, fusion, and stabilization. Neurosurg Rev 42:915-936. doi: 10.1007/s10143-018-01070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiessberger A, Dietz N, Gruter B, Virojanapa J. (2020) Ehlers-Danlos syndrome-associated craniocervical instability with cervicomedullary syndrome: Comparing outcome of craniocervical fusion with occipital bone versus occipital condyle fixation. J Craniovertebral Junction Spine 11:287-292. doi: 10.4103/jcvjs.JCVJS_166_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson FCS. (2016) Cranio-cervical Instability in Patients with Hypermobility Connective Tissue Disorders. J Spine 05(2) doi: 10.4172/2165-7939.1000299 [DOI] [Google Scholar]
- 14.Brodbelt AR, Flint G. (2017) Ehlers Danlos, complex Chiari and cranio-cervical fixation: how best should we treat patients with hypermobility? Br J Neurosurg 31:397-398. doi: 10.1080/02688697.2017.1386282 [DOI] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordhtm
- 16.Henderson FCSr, Henderson FC, Jr., Wilson WA, Mark AS, Koby M. (2018) Utility of the clivo-axial angle in assessing brainstem deformity: pilot study and literature review. Neurosurg Rev 41:149-163. doi: 10.1007/s10143-017-0830-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson FC, Wilson WA, Mott S, et al. (2010) Deformative stress associated with an abnormal clivo-axial angle: A finite element analysis. Surg Neurol Int 1. doi: 10.4103/2152-7806.66461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karaa A, Stoler JM. (2013) Ehlers danlos syndrome: An unusual presentation you need to know about. Case Rep Pediatr, 2013 2013:764659. doi: 10.1155/2013/764659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthy N, Chand K. 2008) Ehler Danlos syndrome with cervical dislocation: An unusual case. J Pediatr Neurosci 3:163-165 [Google Scholar]
- 20.White AA, 3rd, Panjabi MM. (1978) The clinical biomechanics of the occipitoatlantoaxial complex. Orthop Clin N Am 9:867-878 [PubMed] [Google Scholar]
- 21.Grabb PA, Mapstone TB, Oakes WJ. (1999) Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery 44:520-528; discussion 527-528. doi: 10.1097/00006123-199903000-00050 [DOI] [PubMed] [Google Scholar]
- 22.Harris JH, Jr., Carson GC, Wagner LK. (1994) Radiologic diagnosis of traumatic occipitovertebral dissociation: 1. Normal occipitovertebral relationships on lateral radiographs of supine subjects. AJR Am J Roentgenol 162:881-886. doi: 10.2214/ajr.162.4.8141012 [DOI] [PubMed] [Google Scholar]
- 23.Rojas CA, Bertozzi JC, Martinez CR, Whitlow J. (2007) Reassessment of the craniocervical junction: normal values on CT. AJNR Am J Neuroradiol 28:1819-1823. doi: 10.3174/ajnr.A0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riascos R, Bonfante E, Cotes C, Guirguis M, Hakimelahi R, West C. (2015, Imaging of Atlanto-Occipital and Atlantoaxial Traumatic Injuries: What the Radiologist Needs to Know. Radiographics: A Review Publication of the Radiological Society of North America, Inc 35:2121-2134. doi: 10.1148/rg.2015150035 [DOI] [PubMed] [Google Scholar]
- 25.Henderson FC, Rosenbaum, Narayanan M, et al. (2020) Atlanto-axial rotary instability (Fielding type 1): characteristic clinical and radiological findings, and treatment outcomes following alignment, fusion, and stabilization. Neurosurg Rev, 44, 1553-1568. doi: 10.1007/s10143-020-01345-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding JW, Hawkins RJ. (1977) Atlanto-axial rotatory fixation. (Fixed rotatory subluxation of the atlanto-axial joint). J Bone Joint Surg Am;59:37-44 [PubMed] [Google Scholar]
- 27.Batzdorf UHFRDea. (2016) Consensus statement in proceedings of CSF colloquium 2014. In: Batzdorf U. (ed) Comorbidities that complicate the treatment and outcomes of Chiari malformation. Chiari Syringomyelia Foundation, Inc., p 3. [Google Scholar]
- 28.BatzdorfU BEHFea. (2013) Consensus Statement 2013. In: U B. (ed) Proceedings of CSF Colloquium Basilar Impression & Hypermobility at the Craniocervical Junction. Chiari Syringomyelia Foundation. [Google Scholar]
- 29.U B. (2014) Consensus statement. In: Batzdorf U, Henderson F, Rigamonti D. (eds) Co-morbidities that complicate the treatment and outcomes of Chiari malformation. Lulu, pp. 118–122. [Google Scholar]
- 30.Milhorat TH, Chou MW, Trinidad EM, et al. (1999) Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 44:1005-1017. doi: 10.1097/00006123-199905000-00042 [DOI] [PubMed] [Google Scholar]
- 31.Smith FWDJTCSMB, Karger, 2015, pp 48–66. DOI: 10.1159/000365470. [DOI] [Google Scholar]
- 32.Soule E, Fiester P, Rao D, Orallo P, Rahmathulla G. (2021) Anatomic, functional, and radiographic review of the ligaments of the craniocervical junction. J Craniovertebral Junction Spine 12:4-9. doi: 10.4103/jcvjs.JCVJS_209_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phuntsok R, Ellis BJ, Herron MR, Provost CW, Dailey AT, Brockmeyer DL. (2019) The occipitoatlantal capsular ligaments are the primary stabilizers of the occipitoatlantal joint in the craniocervical junction: a finite element analysis. J Neurosurg Spine:1-9. doi: 10.3171/2018.10.spine181102 [DOI] [PubMed] [Google Scholar]
- 34.Astin JH, Wilkerson CG, Dailey AT, Ellis BJ, Brockmeyer DL. (2020) Finite element modeling to compare craniocervical motion in two age-matched pediatric patients without or with Down syndrome: implications for the role of bony geometry in craniocervical junction instability. J Neurosurg Pediatr:1-7. doi: 10.3171/2020.6.peds20453 [DOI] [PubMed] [Google Scholar]
- 35.Tu A, Melamed E, Krieger MD. (2019) Dynamic MRI in the Evaluation of Atlantoaxial Stability in Pediatric Down Syndrome Patients. Pediatr Neurosurg 54:12-20. doi: 10.1159/000495788 [DOI] [PubMed] [Google Scholar]
- 36.Henderson FCS, Rowe PC, Narayanan M, et al. (2021) Refractory Syncope and Presyncope Associated with Atlantoaxial Instability: Preliminary Evidence of Improvement Following Surgical Stabilization. World neurosurgery, 149, e854, e865. doi: 10.1016/j.wneu.2021.01.084 [DOI] [PubMed] [Google Scholar]
- 37.Klinge PM, McElroy A, Donahue JE, Brinker T, Gokaslan ZL, Beland MD. (2021) Abnormal spinal cord motion at the craniocervical junction in hypermobile Ehlers-Danlos patients. J Neurosurg Spine:1-7. doi: 10.3171/2020.10.spine201765 [DOI] [PubMed] [Google Scholar]