Abstract
The bovine beta-lactoglobulin (BLG) is a major cow's milk allergen. Here, we evaluated the immune response against BLG induced in mice, using the organism Lactococcus lactis, which has GRAS (“generally regarded as safe”) status, as a delivery vehicle. The cDNA of the blg gene, encoding BLG, was expressed and engineered for either intra- or extracellular expression in L. lactis. Using a constitutive promoter, the yield of intracellular recombinant BLG (rBLG) was about 20 ng per ml of culture. To increase the quantity of rBLG, the nisin-inducible expression system was used to produce rBLG in the cytoplasmic and extracellular locations. Although the majority of rBLG remained in the cytoplasm, the highest yield (2 μg per ml of culture) was obtained with a secreting strain that encodes a fusion between a lactococcal signal peptide and rBLG. Whatever the expression system, the rBLG is produced mostly in a soluble, intracellular, and denatured form. The BLG-producing strains were then administered either orally or intranasally to mice, and the immune response to BLG was examined. Specific anti-BLG immunoglobulin A (IgA) antibodies were detected 3 weeks after the immunization protocol in the feces of mice immunized with the secreting lactococcal strain. Specific anti-BLG IgA detected in mice immunized with lactococci was higher than that obtained in mice immunized with the same quantity of pure BLG. No specific anti-BLG IgE, IgA, IgG1, or IgG2a was detected in sera of mice. These recombinant lactococcal strains constitute good vehicles to induce a mucosal immune response to a model allergen and to better understand the mechanism of allergy induced by BLG.
The gastrointestinal tract is constantly exposed to substantial amounts of food and bacterial components. In the healthy gut, the immune system is able to create a balance between mucosal immunity and systemic tolerance. In food allergy, this balance is impaired and oral tolerance to dietary antigen is not achieved or maintained (14, 19, 29). Cow's milk allergy is an important problem in infants because it affects 1.9 to 2.8% of infants in the first 2 years of life in various countries of northern Europe (17, 18). Beta-lactoglobulin (BLG) is the most abundant protein of the whey fraction of milk and is regarded as a dominant allergen together with casein (37). In our laboratory, we use BLG as a model protein to evaluate and modulate the immune response to a food allergen in mice, and we have produced both anti-BLG monoclonal antibodies (MAb) (25) and recombinant BLG in Escherichia coli (8). Moreover, the BLG structure is well documented (27); it is a 162-amino-acid globular protein which contains two intramolecular disulfide bonds. In this study, we produced BLG in Lactococcus lactis, a food-grade bacterium, and used these recombinant strains to measure a potential specific immune response after intranasal or oral administration in mice.
L. lactis is a gram-positive lactic acid bacterium that is nonpathogenic, noninvasive, and noncolonizing and that has GRAS (“generally regarded as safe”) status. Its use as a vaccine delivery system (for a review, see reference 39) using tetanus toxin fragment C (TTFC) (40) has been previously reported. Parenteral, intranasal, or oral vaccination of mice with recombinant L. lactis can elicit levels of systemic serum Ab against tetanus toxin which protect against subsequent challenge with otherwise lethal quantities of tetanus toxin (26, 28, 40). Considering its potential as an antigen delivery system for mucosal immunization, we decided to evaluate the immune response of mice after intranasal or oral administration of recombinant L. lactis producing BLG. We thought that such tools would be helpful to study and perhaps modulate the specific immunoglobulin E (IgE) response induced by BLG. BLG was previously expressed in E. coli (4, 7, 8), but this gram-negative bacterium contains lipopolysaccharides which enhance the inflammation process, and some strains are pathogenic for humans and animals.
We constructed strains of L. lactis producing recombinant bovine BLG (rBLG). Both intra- and extracellular locations of rBLG were assessed using the nisin-inducible expression system (9, 10). The highest production was obtained when the mature part of BLG was fused to the signal peptide of the major L. lactis secreted protein Usp45 (34). The recombinant allergen was detected predominantly in a soluble, intracellular, and mostly denatured form in the different constructs. BLG-specific fecal IgA Ab were detected in mice 3 weeks after oral or intranasal administration of the lactococcal BLG-secreting strain. BLG-specific IgE, IgA, IgG1, or IgG2a Ab were not detected in sera of the same mice. Here, we demonstrated that recombinant lactococci constitute good tools to induce a mucosal immune response against BLG after intranasal or oral administration to mice.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are listed in Table 1. L. lactis strains were grown at 30°C in M17 medium containing 0.5% glucose (32). E. coli strains were grown in Luria-Bertani medium at 37°C with vigorous shaking. When required, antibiotics were added at the following concentrations, except when otherwise stated: ampicillin, 50 μg/ml; chloramphenicol, 25 μg/ml for E. coli and 10 μg/ml for L. lactis; and erythromycin, 5 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | Plasmid-free strain | 16 |
| L. lactis | ||
| MG1363 | Plasmid-free strain | 14a |
| NZ9000 | Derivative of MG1363 carrying regulatory genes nisR and nisK | 20 |
| Plasmids | ||
| pBluescript II SK(+) | Apr | Stratagene |
| pVE3556 | Emr; high-copy-number lactococcal vector | 22 |
| pCYT:Nuc | Cmr; carries a DNA fragment encoding the mature Nuc moiety expressed under transcriptional control of the nisin-inducible promoter PnisA | P. Langellaa |
| pSEC:Nuc | Cmr; carries a DNA fragment encoding a fusion of the signal peptide of Usp45 and the mature Nuc moiety expressed under transcriptional control of the nisin-inducible promoter PnisA | P. Langella |
| pTQQ18βlac | Apr; carries a DNA fragment encoding the mature BLG moiety | 3 |
| pIL:BLG | Emr; carries a DNA fragment encoding the mature BLG moiety expressed under transcriptional control of the lactococcal promoter P59 | This work |
| pCYT:BLG | Cmr; pCYT:Nuc where the mature Nuc moiety was replaced by a DNA fragment encoding the mature BLG moiety | This work |
| pSEC:BLG | Cmr; pSEC:Nuc where the mature Nuc moiety was replaced by a DNA fragment encoding the mature BLG moiety | This work |
Unité de Recherches Laitières et Génétique Appliquée, Institut National de la Recherche Agronomique, Jouy en Josas, France.
General DNA techniques, PCR, and transformation.
Plasmid DNA was isolated essentially as described previously (5). For L. lactis, cells were incubated in TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer containing 10 mg of lysozyme per ml at 37°C for 10 min before alkaline lysis. Restriction enzymes and T4 DNA ligase (Gibco BRL or New England Biolabs) were used according to the instructions of the suppliers. PCR was performed with a Perkin-Elmer Cetus (Norwalk, Conn.) thermocycler. Electrotransformation of L. lactis was performed as described previously (21), and transformants were plated on M17–0.5% glucose agar plates containing the required antibiotic.
Construction of expression plasmids carrying the blg gene. (i) Cloning of blg under the control of a constitutive lactococcal promoter.
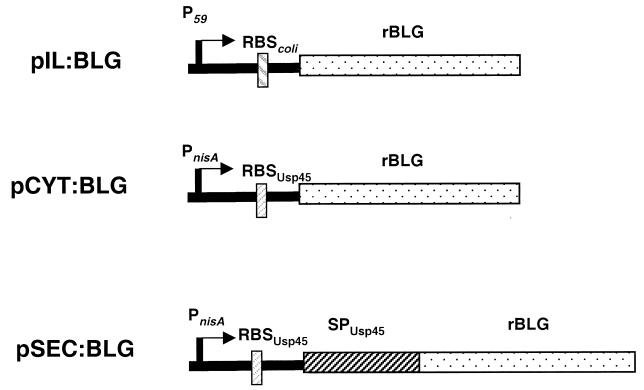
A 550-bp DNA fragment encoding the entire mature BLG under the translational control of an E. coli ribosome-binding site (RBS) was purified after EcoRI digestion of plasmid pTTQ18βlac.7.7.1 (kindly provided by C. Batt) (4). This blg gene was then inserted downstream of the strong L. lactis constitutive promoter P59 (36) by cloning the fragment in an EcoRI-linearized plasmid, pVE3556 (22). The ligation mix was then used to transform L. lactis MG1363. The structure of the resulting plasmid, pIL:BLG (Fig. 1), was confirmed by restriction enzyme digestion and DNA sequencing. pIL:BLG was then introduced into L. lactis NZ9000 (kindly provided by Oscar Kuipers) (20) to be comparable to other inducible constructions (see description below).
FIG. 1.
Schematic representation of the three expression vectors. P59, lactococcal constitutive promoter; PnisA, inducible lactococcal promoter; RBScoli and RBSUsp45, consensual RBSs of E. coli and Usp45, respectively; stippled bars, DNA fragment encoding the mature BLG; hatched bar, DNA fragment encoding the signal peptide of Usp45 (SPUsp45).
(ii) Cloning of blg under the control of a nisin-inducible promoter.
The blg gene was amplified from pTTQ18βlac.7.7.1 (4) using primers BLGLacF (GCCCAATGCATTGGTTCTGATCGTTACCCAGACC) and BLGLacR (GCGCTCGAGGCGCTAGATGTGGCACTGCTC), adding an NsiI site (italicized) at the N terminus of BLG and an XhoI site (italicized) at the C terminus of BLG. This PCR fragment encoded a BLG fragment starting at position Leu17 of the complete protein. The amplified sequence was inserted in SrfI-linearized pPCR-Script Amp SK (+) plasmid as described by the supplier (Stratagene, La Jolla, Calif.). The clone, named BLG-Lacto-pPCR-Script 4.1, was confirmed by DNA sequencing (MWG Biotech, Ebersberg, Germany). The expression plasmids pCYT:Nuc and pSEC:Nuc (P. Langella et al., unpublished data), which allow cloning of the blg gene under the control of the L. lactis inducible promoter PnisA, are described in Table 1. Briefly, pSEC:Nuc and pCYT:Nuc are derivatives of pNZ8010 (kindly provided by Oscar Kuipers) (9, 10) (Table 1), in which the gus gene was under the transcriptional control of the promoter of nisA, PnisA. The gus gene was first deleted by XhoI digestion and replaced by a BamHI-XhoI-cut DNA fragment encoding the RBS, the signal peptide of the usp45 gene product (34) and the mature part of the staphylococcal nuclease protein (22) to obtain the plasmid pSEC:Nuc. This construct allowed us to direct expression of BLG in a secreted form. By using reverse PCR, the DNA fragment encoding the signal peptide of Usp45 was also deleted to obtain pCYT, thus allowing BLG expression in the cytoplasm. An NsiI site was introduced at the 3′ end of the RBS of Usp45 to allow the replacement of part of the nuc gene segment by a DNA fragment encoding a heterologous protein. pCYT:BLG and pSEC:BLG were constructed by inserting the blg gene in pCYT:Nuc and pSEC:Nuc expression vectors (Fig. 1), as follows. In parallel, pCYT:Nuc, pSEC:Nuc, and BLG-Lacto-pPCR-Script 4.1 were digested by NsiI and XhoI. Both double-digested vectors and the insert containing the BLG sequence were purified, and the insert and vector were ligated and electroporated in E. coli strain DH5α (16). Clones containing the BLG sequence were selected by PCR and digestion with restriction enzyme. Plasmids were introduced by electroporation into L. lactis strain NZ9000 containing on its chromosome nisR and nisK, the two regulatory genes of PnisA, which allows induction of the synthesis of foreign proteins in a dose-dependent manner and the attainment of high-level production. Plasmids isolated from the resulting strains, NZ9000(pCYT:BLG) and NZ9000(pSEC:BLG), were confirmed by DNA sequencing.
Purification of BLG from cow's milk.
Natural BLG was purified from the milk of a single cow homozygous for the variant A of BLG as described by Wal et al. (38) and here is considered to be BLG in the native conformation (BLGn). Reduced and carboxymethylated BLG (BLGrcm) was prepared from BLGn as described by Negroni et al. (25) by a method slightly modified from that of McKenzie et al. (24) and here is considered to be BLG in the denatured conformation (BLGd).
Two-site enzyme immunometric assay for BLGn and BLGd.
Anti-BLG MAb production and characterization and development of the two-site enzyme immunometric assays for BLGn and BLGd were described by Negroni et al. (25). Briefly, assays were performed in 96-well microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) coated with a first MAb (capture antibody) specific for either BLGn or BLGrcm (BLGd). Fifty microliters of standard (BLGn or BLGrcm) or sample and 50 μl of tracer, which consists of a second MAb labeled with acetylcholinesterase (AChE) (15), a conjugate recognizing either BLGn or BLGrcm, were then added. Capture and tracer Ab are directed against different complementary epitopes. After 18 h of reaction at 4°C, the plates were washed and solid-phase-bound AChE activity was measured using the method of Ellman et al. (12). Detection limits of 30 and 200 pg/ml were obtained for BLGn and BLGrcm, respectively, with very low or negligible cross-reactivity of the other milk proteins or tryptic fragments of BLG. Cross-reactivity of the BLGn assay with BLGrcm is 0.4%, and cross-reactivity of the BLGrcm assay with BLGn is 0.018%.
SDS-PAGE and immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed using a Tricine buffer as described by Schagger and von Jagow (30). For immunoblot analysis, proteins were separated by SDS–12% PAGE and electroblotted (33) onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). After blotting, nonspecific protein-binding sites were blocked with 1% bovine serum albumin in 50 mM Tris-HCl (pH 8)–150 mM NaCl–0.5% Tween 20. The nylon membranes were incubated overnight with a 1/100,000 dilution of MAb specific for BLGrcm (BLG-74R) (25). After washing, the membranes were incubated for 1 h with alkaline phosphatase-conjugated anti-mouse antibody (1/5,000) (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Color development was obtained according to the supplier's instructions.
L. lactis protein extraction.
Overnight cultures of strains NZ9000(pIL:BLG), NZ9000(pCYT:BLG), and NZ9000(pSEC:BLG) were used to inoculate fresh medium at a dilution of 1:250. For induction of the nisA promoter, strains were grown to an optical density at 600 nm of 0.5, and nisin (Sigma, St. Louis, Mo.) was added to a final concentration of 10 ng/ml. Growth was continued for 1 h. For strain NZ9000(pIL:BLG), growth was continued without addition of nisin until an optical density at 600 nm of 1.0 was reached. To prepare extracts, cells were pelleted by centrifugation at 5,000 × g for 15 min at 4°C and the supernatant (S) was collected. The soluble cytoplasmic protein (Cs) was extracted by disrupting cells with glass beads resuspended in 1/10 of the initial volume of 50 mM Tris-HCl, pH 7.4. Extracts were then centrifuged at 10,000 × g for 15 min at 4°C. The supernatant corresponding to Cs was collected, and the pellet was resuspended in 1/10 of the initial volume of 50 mM Tris-HCl (pH 7.4)–8 M urea–100 mM dithiothreitol for 1 h at room temperature. After centrifugation at 10,000 × g for 15 min at room temperature, the supernatant containing resolubilized insoluble cytoplasmic protein (Ci) was collected and then dialyzed against 50 mM Tris-HCl, pH 7.4. The amounts of BLGn and BLGd in the S, Cs, and Ci preparations were assayed using the two immunoassays described above.
Immunizations.
Recombinant strain NZ9000(pSEC:BLG) and control strain NZ9000(pSEC:Nuc) were grown as described above and induced for 1 h with nisin. Cells were pelleted, resuspended in sterile phosphate-buffered saline (PBS) to obtain the same concentration of cells for each strain, aliquoted, and frozen at −80°C. Yields of rBLGn and rBLGd in one aliquot were determined as described above. Groups of four mice were immunized orally or intranasally with the induced recombinant strain, the induced control strain, and BLGrcm in PBS. Oral doses of 15 μg of BLGrcm, the quantity of cells of NZ9000(pSEC:BLG) corresponding to 15 μg of BLGrcm, and the same quantity of cells of NZ9000(pSEC:Nuc), in 150 μl of suspension, were administered intragastrically on days 1, 2, and 3. Intranasal doses of 1.5 μg of BLGrcm, the quantity of cells from NZ9000(pSEC:BLG) corresponding to 1.5 μg of BLGrcm, or the same quantity of cells from the control strain were slowly administered to mice in 15 μl of suspension on days 1, 2, and 3. Serum samples and fecal pellets were taken on days 0 and 19.
ELISA for the detection of BLG-specific serum antibody.
The enzyme-linked immunosorbent assay (ELISA) was previously described in detail by Adel-Patient et al. (1). Briefly, 96-well microtiter plates (Maxisorp; Nunc) coated with 5 μg of BLGrcm per ml were incubated overnight with serial dilutions of sera from immunized mice. After washing, IgE, IgG1, or IgG2a binding was revealed by incubation with a rat anti-mouse IgE MAb (clone LOME-3) from Serotec (Oxford, England) or a goat anti-mouse IgG1 or goat anti-mouse IgG2a polyclonal affinity-purified Ab (Southern Biotechnology Associates, Birmingham, Ala.) labeled with AChE (15). AChE activity was detected using the method of Ellman et al. (12) by reading absorbance at 414 nm. The minimum detectable concentrations are 8 pg/ml for IgE, 7 pg/ml for IgG1, and 12 pg/ml for IgG2a.
Measurement of fecal and serum IgA response.
Fresh fecal pellets were collected at days 0 and 19 from groups of mice that had been immunized intranasally or orally. Fecal pellet (0.1 g) was added to 1 ml of PBS containing 1% bovine serum albumin, 50 μg of bacitracin per ml, 300 μg of benzamidine per ml, 80 μg of leupeptin per ml, 20 μg of chymostatin per ml, 25 μg of pepstatin per ml, and 200 μM phenylmethylsulfonyl fluoride (Sigma) and incubated on rotary shaker overnight at 4°C. The tubes were vortexed to disrupt all solid material and then centrifuged at 16,000 × g for 5 min at 4°C. The supernatant was removed and tested by ELISA for the presence of BLG-specific IgA as described above. IgA was detected using a goat polyclonal serum anti-mouse IgA (Southern Biotechnology Associates) labeled with AChE (15) and detected as described above. The same assay was used to evaluate specific IgA in serum.
RESULTS
Growth of L. lactis producing BLG depends on the expression system.
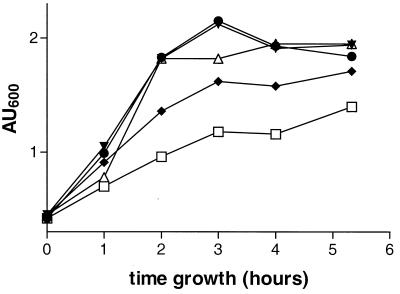
To produce rBLG in L. lactis, the cDNA of the blg gene was cloned in L. lactis MG1363 or NZ9000 under the control of P59, a strong L. lactis constitutive promoter (35), on pIL, a high-copy-number plasmid, resulting in pIL:BLG (Fig. 1). To achieve inducible expression of blg, the nisin-inducible promoter PnisA was used to promote intracellular BLG expression on plasmid pCYT:BLG (Fig. 1). To obtain a secreted form of BLG, the blg gene fragment was also fused in frame with the signal sequence of Usp45, the major L. lactis secreted protein (33), and placed under the control of PnisA, resulting in pSEC:BLG (Fig. 1). These two plasmids were introduced into L. lactis, resulting in strains NZ9000(pCYT:BLG) and NZ9000(pSEC:BLG). Growth of the three strains was monitored without nisin for NZ9000(pIL:BLG) and with or without nisin for the inducible strains (Fig. 2). The growth of strain NZ9000(pIL:BLG) was similar to the growth of the inducible strains in the absence of nisin. Addition of nisin led to a marked decrease in the growth of strains NZ9000(pCYT:BLG) and NZ9000(pSEC:BLG), suggesting a disadvantage due to the induction of the production of rBLG.
FIG. 2.
Growth curves of different strains expressing rBLG. Growth was measured in terms of absorbance units at 600 nm (AU600). Inducible strains were grown with or without nisin. ▵, NZ9000(pIL:BLG); ▾, NZ9000(pCYT:BLG); ●, NZ9000(pSEC:BLG); ♦, NZ9000 (pCYT:BLG) plus nisin; □, NZ9000(pSEC:BLG) plus nisin.
The fusion with a lactococcal signal peptide leads to the highest rBLG production in L. lactis.
For the three strains, rBLG was assayed using specific immunoassays for BLG in the native (rBLGn) or denatured (rBLGd) conformation in the supernatant fraction (S) and the soluble (Cs) and insoluble (Ci) cellular fractions (Table 2). Total production of rBLG was calculated by adding the amounts of rBLGn and rBLGd in S, Cs, and Ci for each strain. The values given in Table 2 represent the means and standard deviations from five independent experiments. Total production was 20.3 ± 3.2 ng/ml, 121 ± 28 ng/ml, and 1,906 ± 355 ng/ml of culture for strain NZ9000 containing pIL:BLG, pCYT:BLG, and pSEC:BLG, respectively (Table 2).
TABLE 2.
Quantitative assay of BLGn and BLGd in different fractions of each straina
| Fraction | BLG | Concn (ng/ml)b produced by strains NZ9000 containing:
|
||
|---|---|---|---|---|
| pIL:BLG | pCYT:BLG | pSEC:BLG | ||
| S | rBLGn | NDc | ND | 3.7 ± 0.1 |
| rBLGd | ND | ND | 46 ± 3 | |
| Cs | rBLGn | 1.8 ± 0.1 | 1.7 ± 0.1 | 0.3 ± 0.1 |
| rBLGd | 17.8 ± 3.2 | 108 ± 25 | 1,510 ± 356 | |
| Ci | rBLGd | 0.7 ± 0.2 | 11.5 ± 3.4 | 346 ± 49 |
| Total | rBLGn + rBLGd | 20.3 ± 3.2 | 121 ± 28 | 1,906 ± 355 |
The limits of detection for BLGn and BLGd are, respectively, 30 and 200 pg/ml. Immunoassays were conducted during exponential growth for the constitutive promoter and after 1 h of induction by nisin for the inducible one.
Values given represent the means ± standard deviations from five independent experiments.
ND, not detectable.
rBLG was found only in the supernatant of NZ9000(pSEC:BLG), which encodes the fusion between the signal peptide of Usp45 and rBLG. We measured 50 ng of rBLG per ml, secreted as 92% rBLGd and 8% rBLGn. Secreted rBLG represented 3% of the synthesized rBLG.
rBLG was found preferentially in soluble form for the three strains. Nevertheless, in the most productive strain, NZ9000(pSEC:BLG), rBLG in Ci reached 20% of the total rBLG.
In Cs, rBLG can be either in a native or in a denatured conformation. rBLG in the denatured conformation represents 90, 98, and more than 99% of rBLG measured in Cs in strains NZ9000(pIL:BLG), NZ9000(pCYT:BLG), and NZ9000(pSEC:BLG), respectively. The level of rBLG in the denatured conformation in Cs increased in parallel to rBLG production.
The precursor preUsp:rBLG is weakly processed.
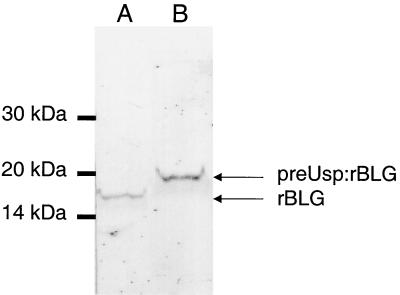
Production of rBLG was induced in strains NZ9000(pCYT:BLG) and NZ9000(pSEC:BLG) as described above. The BLG protein content of total cell extracts was analyzed by Western blot experiments using MAb specific for BLGd. rBLG produced by NZ9000(pCYT:BLG) migrated at the same distance as the native BLG (not shown), corresponding to around 18,000 Da, whereas a larger band was detected in NZ9000(pSEC:BLG) (Fig. 3). The size of this protein form corresponds to around 21,000 Da, which is the size expected for the intracellular precursor form preUsp:rBLG containing the signal peptide of Usp45, suggesting a very weak cleavage of the preUsp:rBLG. The same profile was obtained with different MAb recognizing different linear epitopes of BLG. A degradation band was never observed.
FIG. 3.
Production of rBLG in L. lactis. rBLG production was analyzed by Western blotting of induced cultures of L. lactis NZ9000 strains containing pCYT:BLG (lane A) (encoding the cytoplasmic form) or pSEC:BLG (lane B) (encoding the secreted form). Immunoblotting of total cell extracts of these strains was performed after 1 h of induction as described in Materials and Methods. A MAb specific for BLGd was used as the first antibody. We used different quantities of total extract to obtain comparable signals. Migration positions of the molecular mass markers are given on the left. Migration positions of precursor form (preUsp:rBLG) and mature form (rBLG) are indicated by arrows.
Mucosal antibody response.
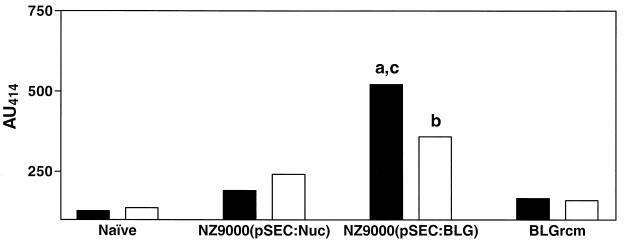
Groups of four mice were inoculated intranasally or orally with recombinant strain NZ9000(pSEC:BLG), strain NZ9000(pSEC:Nuc) as a control strain, or purified denatured BLG. Three weeks after immunization, fresh fecal pellets from mice immunized intranasally or orally with NZ9000(pSEC:BLG) elicited a high BLG-specific mucosal IgA response (Fig. 4). The specific response intensity obtained with strain NZ9000(pSEC:BLG) was higher after oral than after intranasal administration (P < 0.05 [t test]). In contrast, sera from immunized mice did not contain significant levels of BLG-specific IgA, IgG1, IgG2a, or IgE (data not shown). This suggests that the IgA response is located only at the mucosal level. Intranasal or oral administration of BLGrcm elicited a very low BLG-specific IgA level equivalent to that of naive mice, which represents nonspecific background. Though slightly higher, the response obtained with the control strain was of the same order as that in naive mice, although it was higher after intranasal administration than after oral administration (P > 0.05 [t test]).
FIG. 4.
Anti-BLG IgA Ab levels in fecal extracts. Groups of four mice were orally (▪) or intranasally (□) inoculated on days 1, 2, and 3 with the control strain NZ9000(pSEC:Nuc), the strain expressing BLG [NZ9000(pSEC:BLG)], and BLGrcm. Fecal pellets were taken on day 19 and assayed for anti-BLG IgA. Feces from a naive, nonimmunized control group were also taken and assayed. ELISA results are expressed as absorbance units at 414 nm (AU414). a, significant difference between mice orally immunized with NZ9000(pSEC:BLG) and the other groups orally immunized (P < 0.05 [t test]); b, significant difference between mice intranasally immunized with NZ9000(pSEC:BLG) and the other groups intranasally immunized (P < 0.05 [t test]); c, significant difference between mice immunized with NZ9000(pSEC:BLG) orally or intranasally (P < 0.05 [t test]).
DISCUSSION
In this paper, we describe for the first time the induction of a mucosal BLG-specific immune response in mice immunized with recombinant L. lactis BLG producer strains. We determined the type and the localization of immune response elicited in mice after administration of L. lactis producing BLG. Our long-term goal is to study and perhaps modulate immune responses to food allergens with BLG as the protein model and recombinant L. lactis strains as the delivery vehicle.
For this purpose, three rBLG-producing L. lactis strains were constructed to produce rBLG in cytoplasmic and extracellular locations under the control of a constitutive or an inducible promoter. The rates of growth of the three stains were very different and correlated with rBLG production. The lowest and highest rBLG production was obtained with L. lactis strains NZ9000(pIL:BLG) and NZ9000(pSEC:BLG), respectively. In strain NZ9000(pIL:BLG), rBLG was detected intracellularly at a very low level in spite of the use of a high-copy-number cloning vector and of P59, a strong lactococcal constitutive promoter. This low production is probably due to the use of an E. coli RBS that did not allow efficient translation in L. lactis. To increase the production of rBLG, the E. coli RBS was replaced by the RBS of the L. lactis secreted protein Usp45 and the blg gene was placed under the transcriptional control of the nisin-inducible promoter PnisA. In strain NZ9000(pSEC:BLG), the blg gene was fused to the signal peptide of Usp45. These modifications led to a dramatic enhancement of the production of rBLG compared to the constitutive production: 16- and 95-fold compared to inducible and constitutive intracellular production, respectively. Our hypothesis to explain this enhancement is that the precursor preUsp:rBLG could be more efficiently translated in NZ9000(pSEC:BLG) than the intracellular forms of rBLG produced in NZ9000(pIL:BLG) or NZ9000(pCYT:BLG). These forms remain inside the cells as aggregates close to the translation machinery and would congest it. Furthermore, the recognition of preUsp:rBLG by the machinery of secretion of L. lactis could allow it to escape intracellular proteolysis even if no degradation band was observed. The same observations have also been recently made in the case of production in L. lactis of the nonstructural protein NSP4 of bovine rotavirus (13) and of the Brucella abortus ribosomal protein L7/L12 (L. Ribeiro et al., unpublished data).
rBLG is produced in the three strains mostly in the denatured form. This observation could be due to the absence of a homologue of DsbA (3), the protein able to build disulfide bonds in L. lactis (6). It is secreted in very small quantities, i.e., 3% of the total rBLG produced in NZ9000(pSEC:BLG), as in E. coli. In Western blot experiments, we showed that rBLG present in cellular fractions of strain NZ9000(pSEC:BLG) has a molecular weight higher than that in NZ9000(pCYT:BLG). This result indicates that the majority of rBLG is present as the unprocessed intracellular precursor preUsp:rBLG. In E. coli, rBLG is not secreted but is totally processed (7). rBLG found in the supernatant does not result from bacterial lysis, because the proportion of BLGn to BLGd is higher in the supernatant than in the cytoplasm and because no rBLG can be detected in the supernatant of NZ9000(pIL:BLG) or NZ9000(pCYT:BLG). This low maturation of preUsp:rBLG could be due to a low recognition of this hybrid precursor by the machinery of secretion of L. lactis, which leads to the formation of aggregates. At this point, we can note that until now (i) only Usp45, a protein of unknown function, seems to be constitutively secreted efficiently by L. lactis (34), and (ii) only bacterial heterologous proteins have been efficiently secreted in L. lactis (23). Using the staphylococcal nuclease as the secreted model protein, Le Loir et al. (23) showed that a positive net global charge in the first 10 amino acid residues of the N-terminal part of the nuclease resulted in a dramatic decrease in its secretion efficiency in L. lactis. Analysis of the first 10 amino acid residues of the N-terminal part of rBLG revealed the presence of an Arg residue at position +10, resulting in a net global charge of +1. Experiments are currently in progress to investigate the effect of mutation of BLG at this Arg residue and of the insertion of a synthetic propeptide (23) on the secretion efficiency of rBLG.
Few eukaryotic proteins have been produced in L. lactis, with different results. Hen egg white lysozyme could be detected in L. lactis lysates by Western blotting experiments, but no lysozyme activity was observed (35). In contrast, biologically active murine interleukin-2 (IL-2) and IL-6 were secreted by L. lactis (900 ng/ml) (31). A biologically active bovine plasmin was also produced (1,000 ng/ml) and weakly secreted in L. lactis (2).
Expression of BLG in the model gram-negative bacterium E. coli has been previously described (4, 7, 8). One difference between E. coli and L. lactis is in the quantity of rBLG produced. NZ9000(pSEC:BLG) produced around 2 mg/liter of culture medium, 10- to 100-fold less than E. coli (4, 7, 8). Another difference is the proportion of soluble rBLG. In E. coli, soluble rBLG reached 30% at most (7), whereas in L. lactis it was never less than 80%. As in E. coli, the amount of rBLG in the native conformation in L. lactis seems to be correlated with global production of rBLG (7).
After intranasal inoculation of a recombinant inducible strain expressing TTFC, an antigen-specific Ig serum response has been observed, but no antigen-specific fecal IgA was detected (26). Using a constitutive strain expressing TTFC and another immunization protocol, Robinson et al. obtained an antigen-specific Ig serum response and antigen-specific IgA Ab in feces after oral or intranasal administration (28). Immunized mice were protected against further challenge with tetanus toxin in both experiments. Since the biochemical characteristics of rBLG produced in the three strains were the same, we used recombinant strain NZ9000(pSEC:BLG) to immunize mice via the oral or intranasal route because it was the most productive strain. Both types of administration elicited a high BLG-specific enteric response, but no antigen-specific serum Ig response was detected. In our immunization experiment, we administered the same quantity of BLG alone or of BLG expressed by the recombinant strain, e.g.,15 or 1.5 μg. In the two types of administration, the responses elicited by the recombinant strain were on the same order as in naive mice, which represents nonspecific background. Administration of BLG via L. lactis seems to increase the bioavailability of BLG and/or to act as an adjuvant. This result was also found in the case of TTFC (28).
Intranasal or oral administration of the control strain elicited a response that is also on the order of that in naive mice. Nevertheless, the response obtained with the control strain after intranasal administration was greater than that after oral administration. This could be due to cross-reactivity with a protein of L. lactis and BLG, but no significant sequence homology was found between BLG and the L. lactis genome, so the explanation is probably nonspecific background reactivity. Robinson et al. also noted high nonspecific binding in their TTFC-specific fecal IgA responses (28). The intensities of response obtained with their control strain represented between 20 and 60% of that measured with the recombinant strain expressing TTFC.
In our experiments, we used L. lactis that was killed by freezing. This allowed us to quantify the production of rBLG and to control exactly the quantity of rBLG administered to mice. In any case, L. lactis administered by feeding, as in our experiments, died very rapidly and lysed, whereas L. lactis that transits with food survives longer (11). Nevertheless, immunization of mice with live bacteria is under investigation. It seems that in the L. lactis feeding experiment, secretion was not necessary because BLG is released in the digestive tract by lysis. In the case of TTFC, the recombinant protein is also intracellularly accumulated (40). Nevertheless, secretion is not a handicap, because it was demonstrated that immunization of mice with a recombinant strain coexpressing intracellular TTFC and secreting IL-2 or IL-6 increased the anti-TTFC antibody titer (31).
Our results show that addition of the signal peptide of Usp45 to obtain secretion allows a significant increase of expression even if the efficacy of secretion is low. Moreover, we demonstrate that recombinant L. lactis is a good vector to elicit local immune responses even to eukaryotic proteins. Evaluation of the effects of this recombinant strain in a high-IgE-responder mouse model of allergy to BLG is under way.
ACKNOWLEDGMENTS
We thank Oscar P. Kuipers for L. lactis strain NZ9000 and for plasmid pNZ8010. We are grateful to Alexandra Gruss, Yves Le Loir, and Sébastien Nouaille for helpful discussions during the course of this work.
REFERENCES
- 1.Adel-Patient K, Creminon C, Bernard H, Clement G, Negroni L, Frobert Y, Grassi J, Wal J, Chatel J M. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J Immunol Methods. 2000;235:21–32. doi: 10.1016/s0022-1759(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 2.Arnau J, Hjerl-Hansen E, Israelsen H. Heterologous gene expression of bovine plasmin in Lactococcus lactis. Appl Microbiol Biotechnol. 1997;48:331–338. doi: 10.1007/s002530051058. [DOI] [PubMed] [Google Scholar]
- 3.Bardwell J C. Building bridges: disulphide bond formation in the cell. Mol Microbiol. 1994;14:199–205. doi: 10.1111/j.1365-2958.1994.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 4.Batt C A, Rabson L D, Wong D W, Kinsella J E. Expression of recombinant bovine beta-lactoglobulin in Escherichia coli. Agric Biol Chem. 1990;54:949–955. [PubMed] [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolotine A, Mauger S, Malarmé K, Ehrlich S D, Sorokin A. Low redundancy sequencing of entire Lactococcus lactis IL 1403 genome. Antonie Leeuwenhoek. 1999;76:27–76. [PubMed] [Google Scholar]
- 7.Chatel J M, Adel-Patient K, Creminon C, Wal J M. Expression of a lipocalin in prokaryote and eukaryote cells: quantification and structural characterization of recombinant bovine beta-lactoglobulin. Protein Expr Purif. 1999;16:70–75. doi: 10.1006/prep.1999.1055. [DOI] [PubMed] [Google Scholar]
- 8.Chatel J M, Bernard H, Clement G, Frobert Y, Batt C A, Gavalchin J, Peltres G, Wal J M. Expression, purification and immunochemical characterization of recombinant bovine beta-lactoglobulin, a major cow milk allergen. Mol Immunol. 1996;33:1113–1118. doi: 10.1016/s0161-5890(96)00070-3. [DOI] [PubMed] [Google Scholar]
- 9.de Ruyter P G, Kuipers O P, Beerthuyzen M M, Alen-Boerrigter I, de Vos W M. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol. 1996;178:3434–3439. doi: 10.1128/jb.178.12.3434-3439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruyter P G, Kuipers O P, de Vos W M. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol. 1996;62:3662–3667. doi: 10.1128/aem.62.10.3662-3667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drouault S, Corthier G, Ehrlich S D, Renault P. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl Environ Microbiol. 1999;65:4881–4886. doi: 10.1128/aem.65.11.4881-4886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellman G L, Courtney K D, Andres V, Featherstone R M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 13.Enouf V, Langella P, Commissaire J, Cohen J, Corthier G. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl Environ Microbiol. 2001;67:1423–1428. doi: 10.1128/AEM.67.4.1423-1428.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fargeas J M, Theodorou V, More J, Wal J M, Fioramonti J, Bueno L. Boosted systemic immune and local responsiveness after intestinal inflammation in orally sensitized guinea pigs. Gastroenterology. 1995;109:53–62. doi: 10.1016/0016-5085(95)90268-6. [DOI] [PubMed] [Google Scholar]
- 14a.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassi J, Frobert Y, Pradelles P, Chercuitte F, Gruaz D, Dayer J M, Poubelle P E. Production of monoclonal antibodies against interleukin-1 alpha and -1 beta. Development of two enzyme immunometric assays (EIA) using acetylcholinesterase and their application to biological media. J Immunol Methods. 1989;123:193–210. doi: 10.1016/0022-1759(89)90223-8. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 17.Host A, Halken S. Epidemiology and prevention of cow's milk allergy. Allergy. 1998;53:111–113. doi: 10.1111/j.1398-9995.1998.tb04978.x. [DOI] [PubMed] [Google Scholar]
- 18.Host A, Jacobsen H P, Halken S, Holmenlund D. The natural history of cow's milk protein allergy/intolerance. Eur J Clin Nutr. 1995;49(Suppl. 1):S13–S18. [PubMed] [Google Scholar]
- 19.Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105:1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- 20.Kuipers O P, de Ruyter P G, Kleerebezen M, de Vos W M. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21. [Google Scholar]
- 21.Langella P, Le Loir Y, Ehrlich S D, Gruss A. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J Bacteriol. 1993;175:5806–5813. doi: 10.1128/jb.175.18.5806-5813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Loir Y, Gruss A, Ehrlich S D, Langella P. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J Bacteriol. 1994;176:5135–5139. doi: 10.1128/jb.176.16.5135-5139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Loir Y, Gruss A, Ehrlich S D, Langella P. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J Bacteriol. 1998;180:1895–1903. doi: 10.1128/jb.180.7.1895-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenzie H A, Ralston G B, Shaw D C. Location of sulfhydryl and disulfide groups in bovine β-lactoglobulins and effects of urea. Biochemistry. 1972;11:4539–4547. doi: 10.1021/bi00774a017. [DOI] [PubMed] [Google Scholar]
- 25.Negroni L, Bernard H, Clement G, Chatel J M, Brune P, Frobert Y, Wal J M, Grassi J. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J Immunol Methods. 1998;220:25–37. doi: 10.1016/s0022-1759(98)00150-1. [DOI] [PubMed] [Google Scholar]
- 26.Norton P M, Wells J M, Brown H W, Macpherson A M, Le Page R W. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactis expressing tetanus toxin fragment C. Vaccine. 1997;15:616–619. doi: 10.1016/s0264-410x(96)00241-1. [DOI] [PubMed] [Google Scholar]
- 27.Papiz M Z, Sawyer L, Eliopoulos E E, North A C, Findlay J B, Sivaprasadarao R, Jones T A, Newcomer M E, Kraulis P J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature. 1986;324:383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- 28.Robinson K, Chamberlain L M, Schofield K M, Wells J M, Le Page R W. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657. doi: 10.1038/nbt0797-653. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson I R, Walker W A. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update) Gastroenterology. 1993;104:622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- 30.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 31.Steidler L, Robinson K, Chamberlain L, Schofield K M, Remaut E, Le Page R W, Wells J M. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect Immun. 1998;66:3183–3189. doi: 10.1128/iai.66.7.3183-3189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terzaghi B, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Asseldonk M, Rutten G, Oteman M, Siezen R J, de Vos W M, Simons G. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene. 1990;95:155–160. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 35.van de Guchte M, van der Wal F J, Kok J, Venema G. Lysozyme expression in Lactococcus lactis. Appl Microbiol Biotechnol. 1992;37:216–224. doi: 10.1007/BF00178174. [DOI] [PubMed] [Google Scholar]
- 36.van der Vossen J M, van der Lelie D, Venema G. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl Environ Microbiol. 1987;53:2452–2457. doi: 10.1128/aem.53.10.2452-2457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wal J M. Cow's milk allergen. Allergy. 1998;53:1013–1022. doi: 10.1111/j.1398-9995.1998.tb03811.x. [DOI] [PubMed] [Google Scholar]
- 38.Wal J M, Bernard H, Creminon C, Hamberger C, David B, Peltre G. Cow's milk allergy: the humoral immune response to eight purified allergens. Adv Exp Med Biol. 1995;371B:879–881. [PubMed] [Google Scholar]
- 39.Wells J M, Robinson K, Chamberlain L M, Schofield K M, Le Page R W. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek. 1996;70:317–330. doi: 10.1007/BF00395939. [DOI] [PubMed] [Google Scholar]
- 40.Wells J M, Wilson P W, Norton P M, Gasson M J, Le Page R W. Lactococcus lactis: high-level expression of tetanus toxin fragment C and protection against lethal challenge. Mol Microbiol. 1993;8:1155–1162. doi: 10.1111/j.1365-2958.1993.tb01660.x. [DOI] [PubMed] [Google Scholar]