In patients with B-cell lymphoma, neutralizing antibody responses to COVID-19 vaccination after recent anti-CD20 treatment are absent but tend to endure in those who receive anti-CD20 after vaccination.
Abstract
To obtain a deeper understanding of poor responses to COVID-19 vaccination in patients with lymphoma, we assessed blocking antibodies, total anti-spike IgG, and spike-specific memory B cells in the peripheral blood of 126 patients with lymphoma and 20 age-matched healthy controls 1 and 4 months after COVID-19 vaccination. Fifty-five percent of patients developed blocking antibodies postvaccination, compared with 100% of controls. When evaluating patients last treated from days to nearly 18 years prior to vaccination, time since last anti-CD20 was a significant independent predictor of vaccine response. None of 31 patients who had received anti-CD20 treatment within 6 months prior to vaccination developed blocking antibodies. In contrast, patients who initiated anti-CD20 treatment shortly after achieving a vaccine-induced antibody response tended to retain that response during treatment, suggesting a policy of immunizing prior to treatment whenever possible.
Significance:
In a large cohort of patients with B-cell lymphoma, time since anti-CD20 treatment was an independent predictor of neutralizing antibody response to COVID-19 vaccination. Comparing patients who received anti-CD20 treatment before or after vaccination, we demonstrate that vaccinating first can generate an antibody response that endures through anti-CD20–containing treatment.
This article is highlighted in the In This Issue feature, p. 85
INTRODUCTION
Immune suppression in patients with B-cell non-Hodgkin lymphomas (B-NHL) is driven both by the biology of the disease and by the use of B-cell–targeted therapies. Mortality rates from COVID-19 for this population, especially once hospitalized, are high, ranging between 20% and 35% (1–4). Effective SARS-CoV-2 vaccines have reduced risks of hospitalization and death, but patients with B-NHL often have suboptimal responses to immunization (5–9), particularly patients receiving B-cell–targeted therapies, including CD20-targeted antibodies and Bruton tyrosine kinase inhibitors (BTKi). Anticipating the arrival of SARS-CoV-2 vaccines, clinicians began to reevaluate the need and urgency of anti-CD20 treatments for individual patients (10), but if and how the timing of B-cell–targeted therapies may impact response to COVID-19 vaccines was unknown.
We designed a study to examine vaccine-induced immune responses in patients with B-NHL who were either not on treatment or on active treatment with either a BTKi-containing or an anti-CD20–containing regimen, including those receiving vaccination after or just prior to anti-CD20–containing therapy, to study the effect of timing and sequencing of lymphoma treatment and immunization. Neutralizing antibodies are the key measure of immunity against other coronaviruses (11); therefore, we focused on a functional assessment of spike receptor–binding domain (RBD)–ACE2 blocking activity, which correlates strongly with virus neutralization (12), to establish whether patients with B-NHL have significantly impaired humoral responses to SARS-CoV-2 vaccines. We assessed the impact of clinical factors on vaccine response, particularly recent treatment history, the ability of generated immunity to recognize the delta strain of SARS-CoV-2, and the impact of sequencing vaccination prior to treatment on the production and maintenance of blocking antibodies during treatment.
RESULTS
Cohort Characteristics
We enrolled 126 patients with lymphoma and 20 age-matched healthy controls between February 1, 2021, and June 30, 2021 (Supplementary Table S1). Samples were collected from all 146 participants at the 1-month time point after last vaccine dose (median, 28 days) and from 128 participants at the 4-month time point (median, 127 days). Nearly all participants received an mRNA-based SARS-CoV-2 vaccine. All common lymphoma histologies were represented; 70% had either follicular lymphoma or diffuse large B-cell lymphoma. Among the 126 patients with lymphoma, 17 had no prior treatment, 31 had received an anti-CD20 antibody within the prior 6 months, 65 had not been treated for at least 6 months prior to vaccination, 12 were taking a BTKi, and 1 was receiving lenalidomide monotherapy.
Vaccine Responses in Controls and Patients with Lymphoma
We assessed total anti-spike IgG and RBD–ACE2 binding inhibition (ref. 12; “blocking”) in 243 postvaccination samples over two time points. All samples that were negative for total anti-spike IgG were also negative for blocking antibodies. However, 14% of samples positive for total anti-spike IgG did not exhibit blocking antibodies (Fig. 1A). Discordance between total IgG and blocking, which has been observed for multiple anti-spike assays (13), was greater for patients with lymphoma than controls (Supplementary Fig. S1A; P = 0.04 for contribution of participant type), reaffirming our choice of primary measurement.
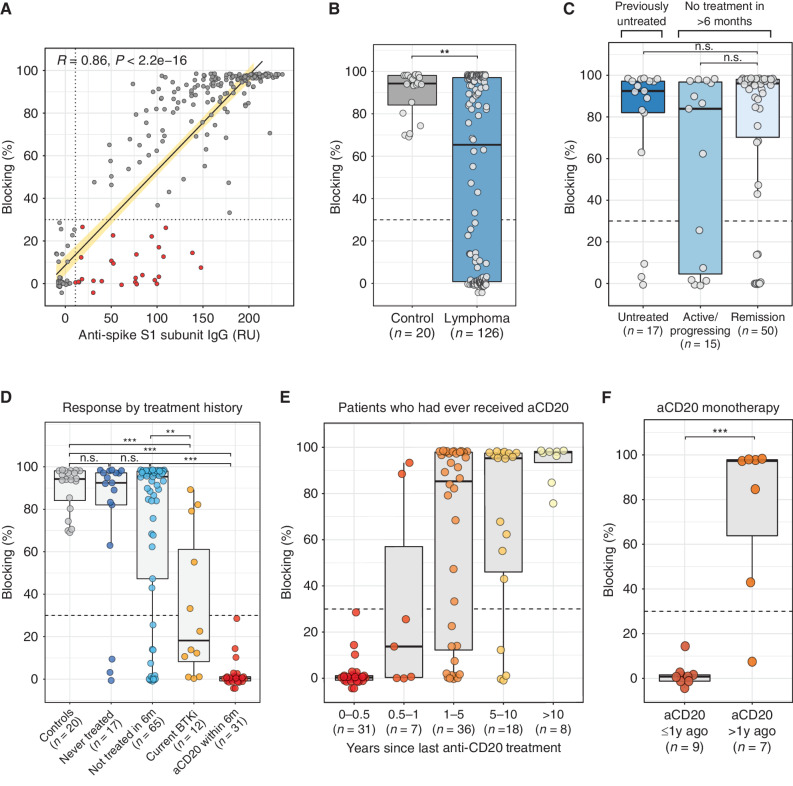
Figure 1.
Treatment history is a major determinant of blocking antibody response to vaccination. A, Comparison of blocking activity versus total anti-spike IgG levels in 243 postvaccination samples. Dashed lines represented validated cutoffs for each assay (see Methods). Data highlighted in red are samples that tested positive for total anti-spike IgG but negative for blocking activity. Blocking antibody responses 1 month after full vaccination in patients with lymphoma (n = 126) versus healthy age-matched controls (n = 20; B), by disease activity among patients not on treatment (C), and by treatment history (D). E, Blocking antibody responses 1 month after full vaccination among patients who had ever received anti-CD20 antibody treatment, binned by time intervals since last anti-CD20 antibody treatment. F, Blocking antibody responses 1 month after full vaccination among patients who received only anti-CD20 monotherapy and had never received chemotherapy, stratified by whether their last anti-CD20 treatment was within 1 year or more than 1 year prior to vaccination. Dashed lines represent validated cutoffs for positivity. Note: All groups within a plot are mutually exclusive, for example, no patients in the “Current BTKi” group received anti-CD20 within the past 6 months. aCD20, anti-CD20 antibody; current BTKi, on a BTKI for ≥3 months; 1y, 1 year; RU, relative units; 6m, 6 months. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
At a median of 28 days after last dose of vaccine, samples from patients with lymphoma had significantly less blocking activity than those from age-matched controls (Fig. 1B). All controls had both anti-spike and blocking antibody responses, while in patients with lymphoma, the proportions were 67% and 55%, respectively (P < 0.001 for each by Fisher exact test; Supplementary Table S2). There was no apparent association between blocking antibody response and age, sex, or vaccine type (Supplementary Fig. S1B–S1D). No statistical difference in response was observed across lymphoma subtypes (Kruskal–Wallis P = 0.06; Supplementary Fig. S1E). We assessed the potential contribution of lymphoma disease activity on vaccine responses within the group that had not been on treatment for at least 6 months. There was no apparent difference in vaccine responses between patients with untreated disease, recurrent progressing disease, or those whose disease was in remission (Fig. 1C).
Recent Treatment History Is a Major Determinant of Vaccine Response
Vaccine responses in previously untreated patients were similar to controls (Fig. 1D; Supplementary Table S2). Seventy-six percent of previously but not recently treated patients developed blocking antibodies. Among patients who were taking a BTKi at the time of vaccination and were neither concurrently receiving nor had recently received anti-CD20 treatment, 5 of 12 developed blocking antibodies. Strikingly, of 31 patients who had received an anti-CD20 antibody within the 6 months prior to vaccination, none developed a blocking antibody response and only 5 had a positive anti-spike IgG result.
To understand the duration of the negative effect of anti-CD20 treatment on vaccine response, we identified all patients in the study who had ever received anti-CD20 treatment. Among these 100 patients, time since last anti-CD20 dose ranged from 1 week to 17.5 years prior to vaccination. We observed a significant correlation between this interval and blocking antibody response (Spearman coefficient 0.59, P < 0.0001; Supplementary Fig. S2A). The likelihood of responding to the vaccine remained low until at least 12 months after last anti-CD20 treatment (Fig. 1E). Similar results were seen in a subset of 16 patients who received anti-CD20 antibody as monotherapy and had never received chemotherapy (Fig. 1F).
Sixteen patients with lymphoma who had last received an anti-CD20 antibody more than a year prior to vaccination did not mount a humoral response after vaccination (Fig. 1E; Supplementary Fig. S2A). Responders and nonresponders among patients who were more than a year out from their last anti-CD20 treatment, appeared relatively balanced in terms of age, sex, vaccine type, or receipt of chemotherapy, and further formal analyses were limited by sample size (Supplementary Fig. S2B–S2G).
Independent Predictors of Vaccine Response
We employed logistic regression to model dichotomous blocking antibody results, with age, sex, vaccine type, receipt of prior chemotherapy, disease status, and time since last anti-CD20 as predictors. Time since last anti-CD20 treatment (Padjusted = 2.1 × 10−4) emerged as a significant independent predictor of vaccine response, controlling for age, sex, vaccine type, disease status, and prior receipt of chemotherapy (Table 1).
Table 1.
Multiple logistic regression model
| Prediction of positive blocking antibody result at 1 month postvaccination | |||
|---|---|---|---|
| Variable | OR (95% CI) | P | P adjusted a |
| Age (years) | 1.0 (0.96–1.0) | 0.97 | 0.97 |
| Sex (M vs. F) | 0.84 (0.34–2.0) | 0.70 | 0.81 |
| Vaccine type (mRNA-1273 vs. BNT162b2) | 1.2 (0.48–3.2) | 0.66 | 0.81 |
| Prior chemotherapy (Y vs. N) | 1.6 (0.57–4.6) | 0.39 | 0.72 |
| Disease status (progressing vs. in remission) | 0.25 (0.08–0.72) | 0.015 | 0.052 |
| Time since last anti-CD20 (years) | 1.3 (1.2–1.4) | 3.3 × 10−7 | 2.3 × 10−6 |
NOTE: Multiple logistic regression model for binary outcome of blocking antibody positivity at 1 month postvaccination among patients with lymphoma. See Methods for details.
Abbreviations: CI, confidence interval; F, female; M, male; N, no; OR, odds ratio; Y, yes.
aUsing Benjamini–Hochberg procedure.
Vaccine-Generated Blocking Antibodies Inhibit Binding of Delta-Variant RBD
Later time points in our study corresponded with a time when the delta variant (B.1.617.2) of SARS-CoV-2 was the predominant circulating strain. To assess whether a blocking response generated against the original (Wuhan-Hu-1, “WT”) strain could inhibit the binding of delta-variant RBD to ACE2, we selected a random subset of 20 patients who had developed blocking antibodies after vaccination and tested blocking against both the WT and delta-variant RBD in parallel. Inhibition of binding to ACE2 was evident against both RBD variants in all samples tested (Fig. 2A).
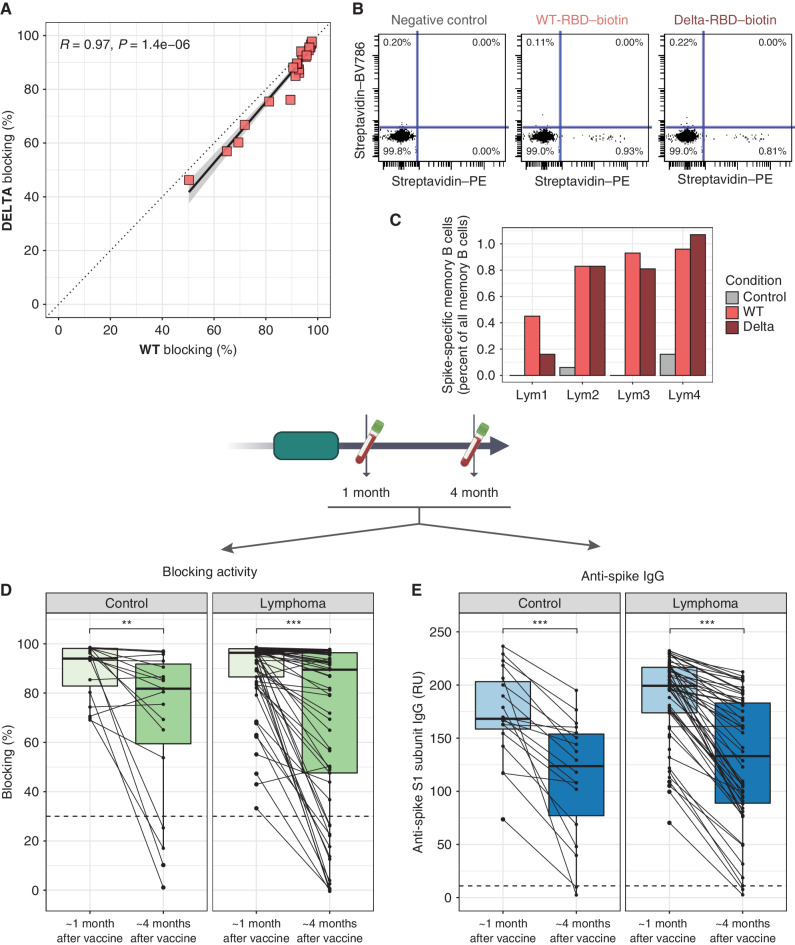
Figure 2.
WT spike-generated vaccine response can recognize B.1.617.2 spike, and most antibody responses wane over time. A, Twenty randomly chosen lymphoma patient samples positive for blocking activity against WT-RBD were tested against B.1.617.2-variant RBD in parallel and RBD–ACE2 binding inhibition against the two variants was compared. B, Representative flow cytometry results from peripheral blood taken 4 months after vaccination from a patient with lymphoma. Memory B cells are pregated on positivity for CD19, CD20, and CD27 and negativity for IgD. Plots show negative control (left), or binding of biotinylated RBD, either WT (middle) or B1.617.2 delta (right), multimerized by streptavidin–PE prior to staining. Streptavidin–BV986 identifies any nonspecific streptavidin binding. C, Summary of results from four patients with lymphoma (Lym1–4) who had circulating memory B cells and were tested for binding to WT- and delta-RBD. Paired data for blocking activity (D; n = 19) and total anti-spike IgG (E; n = 61) from participants who had a positive result at 1-month postvaccination and for whom samples were available from a later time point approximately 4 months postvaccination. Dashed lines represent validated cutoffs for positivity. RU, relative units. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In six patients who had had a positive antibody response to vaccine and for whom we had sufficient peripheral blood mononuclear cells (PBMC) banked, we assessed for WT and delta-variant spike-specific memory B cells (Fig. 2B; Supplementary Fig. S3A). Two had lymphopenia, common among patients with B-NHL, precluding assessment of memory B cells. In three of the remaining four, similar proportions of memory B cells bound WT-RBD and delta-RBD, while in the fourth, detectable but fewer delta-RBD–specific memory B cells were seen (Fig. 2C).
Antibody Responses Decay over Time
To evaluate changes in circulating antibodies over time, we focused on 19 controls and 61 patients with lymphoma who had blocking antibodies at the first postvaccination time point and for whom a second postvaccination sample approximately 4 months after vaccination was available. Both RBD–ACE2 binding inhibition and anti-spike IgG responses decayed over time (Fig. 2D and E). The decay was similar in controls and patients (blocking: mean 90% to 70% in control and 89% to 69% in patients) with no contribution of participant type in relating 4-month responses to 1-month responses (Supplementary Fig. S3B and S3C; blocking: P = 0.27, anti-spike IgG: P = 0.84).
Vaccinating Prior to Therapy Is an Effective Strategy
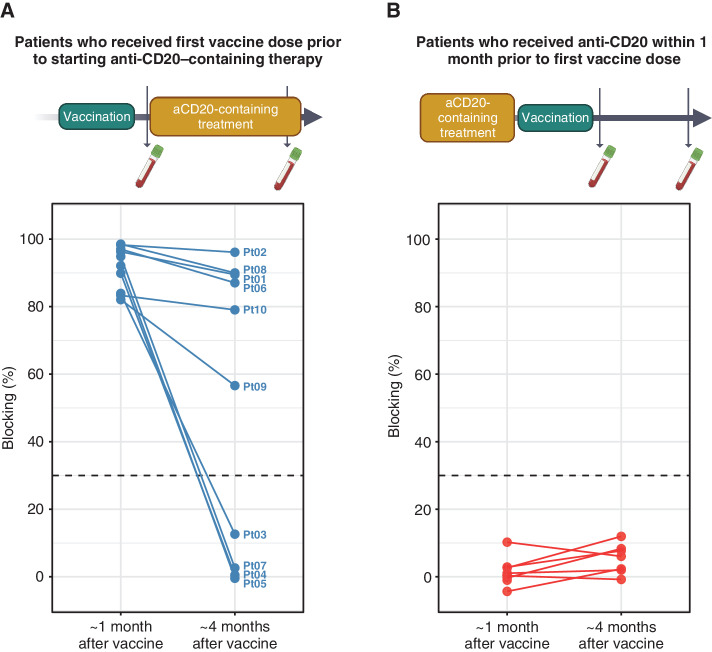
Fifteen patients received a full vaccination series prior to beginning anti-CD20 antibody–containing treatment. Of these, five did not generate a blocking antibody response. Ten patients did, and in six of these, blocking antibody response persisted 4 months after lymphoma treatment initiation (Supplementary Table S3; Fig. 3A). No association was detected between age, sex, diagnosis, vaccine type, prior treatment, current treatment, or time from last vaccine dose to first treatment and persistence of response during therapy. Rates of persistence in this group were comparable to controls (Fig. 2D) despite the intercurrent therapy. In contrast, of nine patients who had been treated with a systemic anti-CD20 antibody within the month prior to vaccination, none responded to vaccination (Fig. 3B).
Figure 3.
Vaccinating prior to anti-CD20 treatment can allow for persistence of antibody responses in the face of therapy. A, Blocking activity for 10 patients who demonstrated positive antibody responses after vaccination and who shortly thereafter received anti-CD20–containing treatment and were followed for persistence of response after 4 months/cycles of treatment had passed. B, For comparison, blocking antibody responses from nine patients who received anti-CD20 antibody therapy shortly prior to vaccination (reversed sequence), assessed 1 and 4 months after vaccination. See Supplementary Table S3 for characteristics of cohort in A. aCD20, anti-CD20 antibody; Pt, patient.
DISCUSSION
This study demonstrates that vaccine responses among patients with B-NHL are largely shaped by the relative timing between anti-CD20 antibody treatment and vaccination. In addition, we show that induced antibodies and memory B cells can recognize the delta variant, and that decay of antibody responses in patients with lymphoma is not different from controls. Importantly, this is the first study to test a strategy of COVID-19 vaccination prior to treatment and demonstrates persistence of pretreatment vaccine-induced immunity in the face of B-cell–targeted therapy, circumventing the negative effects of anti-CD20 treatment on the humoral immune response. In comparison, vaccination after initiation of lymphoma treatment invariably resulted in a failure to generate neutralizing antibodies.
Time from last anti-CD20 treatment to vaccination was a significant independent predictor of vaccine response. Probability of response improved after 1 year, suggesting that immunizations within 1 year of anti-CD20 treatment may generate only limited humoral responses, in line with other recent reports (14–16). In light of these data and the continued pandemic, clinicians should reexamine the risk of using B-cell–targeting therapies in clinical scenarios where their benefit is limited, for example in remission-maintenance strategies that provide no overall survival benefit.
Patients on treatment with a BTKi at the time of vaccination had variable antibody responses. Ibrutinib is known to affect nonmalignant B cells, preferentially decreasing naïve B-cell counts (17), which could impair antibody responses. However, it can also skew Th cells toward phenotypes that could be favorable to generating immune responses (18). These opposing forces may result in different outcomes in different individuals.
Several groups have recently reported poor seroconversion rates in patients with hematologic malignancies [reviewed by Ribas and colleagues (19) and Gagelmann and colleagues (20)]. Importantly, we demonstrate that in patients with B-NHL, antibody positivity does not always predict blocking activity—thus far the best correlate of protection from COVID-19 (21). This discordance has been observed in other settings (13) and also in a recent report of patients with hematologic malignancies (22). Our report adds to these findings and is the first to examine blocking antibody responses in patients with B-NHL in detail in relation to clinical factors, specificity against the delta strain, time-dependent decay, and a pretherapy vaccination strategy. Our study did not include measurements of induced cellular immunity, which we hope to address in subsequent work, or assessment of vaccine effectiveness against infection, which requires larger cohorts.
B-cell–targeting agents are used in a broad array of malignant and nonmalignant conditions, and these findings have immediate implications for clinical care. First, timing of SARS-CoV-2 immunization and anti-CD20 therapy is relevant to many patients globally who have yet to be vaccinated or for whom boosters are recommended. These patients should be prioritized for vaccination, ideally prior to receiving anti-CD20 therapy. Second, these results are likely generalizable to other immunizations. Completing all recommended vaccinations prior to anti-CD20 therapy should be prioritized whenever possible. Third, while the time from final vaccination to start of treatment was a few weeks for most patients in the pretherapy vaccination cohort, the minimum required interval remains unclear. One patient began lymphoma treatment between the first and second vaccine dose and yet had a response that persisted during treatment. If this were found to be generalizable in a larger patient population, a strategy of vaccination initiated before and then continued during therapy could be extended to patients who require treatment more urgently. Finally, this strategy deserves further study with respect to optimal timing, other immunosuppressive agents, and other patient populations.
Patients with B-cell lymphoma can be in double jeopardy from increased susceptibility to infections and poor responses to immunization. Our study suggests strategies for optimizing likelihood of vaccine response by accounting for and managing timing of anti-CD20 therapies. Patients in need of ongoing lymphoma treatment are prime candidates for passive immunization with monoclonal anti–SARS-CoV-2 antibodies, although vaccination should still be attempted, as vaccine benefits may extend beyond neutralizing antibodies. Emerging evidence suggests that vaccinated patients with hematologic malignancies have greater risk of infection, hospitalization, and death compared with vaccinated controls (23, 24). While rates of poor outcomes are much lower than seen in unvaccinated patients with hematologic malignancies, signaling the benefit of vaccination, layering of additional mitigation strategies is clearly needed.
METHODS
Study Design
Participants in this longitudinal observational study were enrolled from the lymphoma clinic at Stanford University (Stanford, CA). Peripheral blood draws were performed at prespecified windows of time: 1 and 4 months after final dose of vaccine. Vaccinations were obtained according to local eligibility rules, which were initially age- and occupation-based. By April 15, 2021, all adults in California were eligible for vaccination.
Patient Population
Eligible patients were at least 18 years of age, had a diagnosis of mature B-cell lymphoma, and planned to receive or had received a complete SARS-CoV-2 vaccination series. Final vaccine dose was either the second dose of BNT162b2 or mRNA-1273 or the single recommended dose of JNJ-78436735. Patients were further classified as either having received anti-CD20 therapy in the past 6 months, currently receiving a BTKi, or having received no anti-lymphoma therapy for at least 6 months, and/or planned to begin therapy for lymphoma after COVID-19 vaccination. Patients classified as currently receiving a BTKi could not have received anti-CD20 treatment in the prior 6 months. Disease activity was assessed on the basis of review of last oncologist note and last available imaging report. Patients with known prior COVID-19 infection, other significant active infection, or immune deficiency unrelated to lymphoma were excluded. Age-matched healthy volunteers without lymphoma were enrolled as control participants. Race/ethnicity data were not available at the time of analysis. All participants provided written informed consent. Study collections were conducted in accordance with the Declaration of Helsinki and were approved by Stanford University's Administrative Panels on Human Subjects in Medical Research.
Sample Collection and Processing
Peripheral blood was collected in heparin-coated phlebotomy tubes and processed within 6 hours of collection. Plasma samples were obtained by centrifugation and stored at −80°C. PBMCs were isolated using a Ficoll gradient and cryopreserved in a liquid nitrogen tank.
Surrogate Virus Neutralization Assay
Blocking activity was assessed in plasma using a surrogate virus neutralization assay (ref. 12; Genscript L00847-A) for either Wuhan-Hu-1 (“WT”) or B.1.617.2 variant (“delta”) strain and reported as a percentage blocking. Briefly, plasma samples were diluted and incubated with horseradish peroxidase–conjugated RBD of the SARS-CoV-2 spike protein, either WT or delta strain, and then added to human ACE2-coated wells, with binding detected via a colorimetric assay. Optical density values from each well were converted to a percentage blocking relative to a plate-specific negative control, which had maximal binding of RBD to plate-bound ACE2. All samples were tested in duplicate, and testing against WT and delta RBD was always done on the same plate. This assay has been well validated against a plaque-based viral neutralization assay. Within our laboratory, the assay was also validated against banked negative prepandemic samples and postvaccination samples that were known to be positive via other assays.
Assessment of SARS-CoV-2–Specific Immunoglobulins
Total anti-spike IgG was measured in plasma samples using the EuroImmun QuantiVac ELISA kit (EI 2606-9601-10 G). Briefly, plasma samples were diluted and incubated in spike S1 subunit-coated plates and binding was quantified via a colorimetric assay. Six calibrators were included on each plate and used to generate a standard curve based on which optical density values for each test well were converted to a relative unit (RU) measurement. All samples were tested in duplicate, and samples from the same patient were tested on the same plate.
Spike-Specific Memory B-cell Detection
PBMCs were thawed at 37°C, washed, and treated with DNase I (Millipore-Sigma) for 10 minutes at room temperature. A total of 1 to 2 × 106 cells were washed and stained with biotinylated SARS-Cov-2 Spike RBD protein (Sino Biological, #40592-V08B-B), multimerized with streptavidin–PE or the multimerized biotinylated delta-variant RBD protein (Sino Biological, #40592-V49H5-B). Streptavidin–BV786 (BD Biosciences, #563858) was added to each to designate nonspecific binding of the streptavidin to the cells (25). Cells were stained with Live/Dead Fixable Blue Stain (Thermo Fisher Scientific, #L34962). Antibodies against CD19 (BD, #612989, Clone SJ25C1, BUV615-conjugated, RRID:AB_2870261), CD20 (Thermo Fisher Scientific, #61-0209-42, Clone 2H7, PE-efluor610–conjugated, RRID:AB_2574540), CD27 (BD, #748704, Clone L128, BUV805-conjugated, RRID:AB_2873108) and IgD (BD, #561303, Clone IA6-2, APC-conjugated, RRID:AB_10642578) were used to designate memory B cells. Flow cytometry was performed using a Cytek Aurora (Cytek Biosciences) and analyzed using Cytobank (Beckman Coulter Life Sciences, RRID:SCR_014043), a cloud-based platform.
Statistical Analyses
Using a two-tailed alpha error rate of 0.05 and a beta error rate of 0.2, this study had >90% power to detect a difference of ≥20% in the proportion of individuals testing positive for blocking antibody in patients with lymphoma versus control participants, assuming a 95% response rate in controls.
Blocking activity was reported as a percentage and analyzed either as a continuous variable or as a binary outcome using the manufacturer recommended cutoff of 30% or higher as a “positive” result. Total anti-spike antibody levels are reported in RU and analyzed either as a continuous variable or as a binary outcome using the manufacturer recommended cutoff of 11 RU or higher as a “positive” result. For categorical variables, Fisher exact test was used to compare two groups and determine statistically significant differences. For continuous variables, Wilcoxon rank-sum test was conducted to evaluate differences between groups. Paired rank-sum tests were utilized to compare samples from the same participant. Nonparametric Spearman correlations were used to assess associations between two continuous variables.
Individual data points are shown along with summarizing visuals such as box plots. For all box plots, the center line represents the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the furthest point within 1.5 times the interquartile range. No outliers were removed; all data are shown. Analyses are univariate and P values unadjusted except where indicated. Adjusted P values are the result of a Benjamini–Hochberg correction.
To assess for influence of participant type (control vs. lymphoma) on associations between total IgG and blocking activity or between results from two time points, we generated linear models predicting blocking activity from anti-spike IgG with and without interaction of participant type using a likelihood ratio test to compare the models.
To understand independent predictors of vaccine response among patients with lymphoma, we constructed a multiple logistic regression model with blocking antibody positivity at 1 month after complete vaccination as the binary outcome and age, sex, vaccine type, receipt of prior chemotherapy, disease status, and time since last anti-CD20 as predictors. Four patients who received JNJ-78436735 were excluded. Patients who had never received anti-CD20 treatment were assigned a value of 18 years, just beyond the maximum time since last anti-CD20 in the dataset (17.5 years). Variance inflation factors were calculated to assess for multicollinearity, and odds ratios with 95% confidence intervals and P values for each predictor are reported. Multiple hypothesis correction was performed using the Benjamini–Hochberg procedure, and adjusted P values are reported.
Data Availability
The datasets included in this study are available from the corresponding author on reasonable request.
Supplementary Material
Acknowledgments
We would like to acknowledge grant support from the Leukemia & Lymphoma Society (IRV-BC0003-22), from Fast Grants, and from the Stanford ChEM-H Innovative Medicines Accelerator program for funding this study. T. Shree is supported by an NIH career development award (K08CA252637). R. Levy is supported by an NIH award (R35CA19735306). Some figure components were generated using BioRender.
Footnotes
Note: Supplementary data for this article are available at Blood Cancer Discovery Online (https://bloodcancerdiscov.aacrjournals.org/).
Authors' Disclosures
T. Shree reports grants from NIH/NCI, Leukemia & Lymphoma Society, and Fast Grants during the conduct of the study. D.M. Kurtz reports personal fees from Roche Molecular Diagnostics and Genentech, and other support from Foresight Diagnostics outside the submitted work, as well as a patent for methods for analysis of cell-free nucleic acids pending and licensed to Foresight Diagnostics. A.A. Alizadeh reports other support from Foresight Diagnostics, FortySeven, and CiberMed; personal fees from Roche, Adaptive Biotechnologies, Genentech, Chugai, and Janssen; grants from Bristol Myers Squibb and Celgene; and personal fees and other support from Gilead outside the submitted work. R. Levy reports grants from Leukemia & Lymphoma Society, NCI, and Fast Grants during the conduct of the study; personal fees from Quaadriga, BeiGene, Teneobio, Nurix, Dragonfly, Apexigen, Viracta, Spotlight, Immunocore, Walking Fish, Kira, Abintus Bio, Khloris, Virsti, and BiolineRx outside the submitted work; and a patent for a three-component vaccine for COVID-19 pending. No disclosures were reported by the other authors.
Authors' Contributions
T. Shree: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, writing–original draft, project administration, writing–review and editing. V. Shankar: Data curation, formal analysis, investigation, writing–review and editing. J.J.K. Lohmeyer: Data curation, investigation. D.K. Czerwinski: Data curation, formal analysis, investigation, project administration, writing–review and editing. J.G. Schroers-Martin: Resources, project administration, writing–review and editing. G.M. Rodriguez: Resources, project administration. S. Beygi: Resources. A.M. Kanegai: Resources, data curation, project administration. K.S. Corbelli: Data curation, project administration. E. Gabriel: Investigation, project administration. D.M. Kurtz: Resources. M.S. Khodadoust: Resources, writing–review and editing. N.K. Gupta: Resources. L.S. Maeda: Resources. R.H. Advani: Resources. A.A. Alizadeh: Conceptualization, resources, supervision, writing–review and editing. R. Levy: Conceptualization, resources, supervision, funding acquisition, project administration, writing–review and editing.
References
- 1. Regalado-Artamendi I, Jiménez-Ubieto A, Hernández-Rivas JÁ, Navarro B, Núñez L, Alaez Cet al. Risk factors and mortality of COVID-19 in patients with lymphoma: a multicenter study. Hema-sphere 2021;5:e538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duléry R, Lamure S, Delord M, Blasi RD, Chauchet A, Hueso Tet al. Prolonged in-hospital stay and higher mortality after Covid-19 among patients with non-Hodgkin lymphoma treated with B-cell depleting immunotherapy. Am J Hematol 2021;96:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Visco C, Marcheselli L, Mina R, Sassone M, Guidetti A, Penna Det al. A prognostic model for patients with lymphoma and COVID-19: a multicentre cohort study. Blood Adv 2022;6:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pagano L, Salmanton-García J, Marchesi F, Busca A, Corradini P, Hoenigl Met al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol 2021;14:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica 2017;102:e397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IEet al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood 2021;137:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilde ND, Offner F. Vaccine response in patients receiving anti-B-cell targeted therapy. Belg J Hematol 2018;9:113–7. [Google Scholar]
- 8. Yri OE, Torfoss D, Hungnes O, Tierens A, Waalen K, Nordøy Tet al. Rituximab blocks protective serologic response to influenza A (H1N1) 2009 vaccination in lymphoma patients during or within 6 months after treatment. Blood 2011;118:6769–71. [DOI] [PubMed] [Google Scholar]
- 9. Vijenthira A, Gong I, Betschel SD, Cheung M, Hicks LK. Vaccine response following anti-CD20 therapy: a systematic review and meta-analysis of 905 patients. Blood Adv 2021;5:2624–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Houot R, Levy R, Cartron G, Armand P. Could anti-CD20 therapy jeopardise the efficacy of a SARS-CoV-2 vaccine? Eur J Cancer 2020;136:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padron-Regalado E. Vaccines for SARS-CoV-2: lessons from other coronavirus strains. Infect Dis Ther 2020;9:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan CW, Chia WN, Qin X, Liu P, Chen MI-C, Tiu Cet al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat Biotechnol 2020;38:1073–8. [DOI] [PubMed] [Google Scholar]
- 13. Szabó Z, Szabó T, Bodó K, Kemenesi G, Földes F, Kristóf Ket al. Comparison of virus neutralization activity and results of 10 different anti-SARS-CoV-2 serological tests in COVID-19 recovered plasma donors. Pract Lab Med 2021;25:e00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon Aet al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 2021;5:3053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gurion R, Rozovski U, Itchaki G, Gafter-Gvili A, Leibovitch C, Raanani Pet al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2021. Jul 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi Ket al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021;27:1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schliffke S, Sivina M, Kim E, von Wenserski L, Thiele B, Akyüz Net al. Dynamic changes of the normal B lymphocyte repertoire in CLL in response to ibrutinib or FCR chemo-immunotherapy. Oncoimmunology 2018;7:e1417720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Yet al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013;122:2539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy Ret al. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov 2021;2:562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gagelmann N, Passamonti F, Wolschke C, Massoud R, Niederwieser C, Klyuchnikov Eet al. Antibody response to COVID-19 vaccination in adults with haematological malignancies: a systematic review and meta-analysis. SSRN 3929967 [Preprint]. 2021. Available from: 10.2139/ssrn.3929967. [DOI] [PMC free article] [PubMed]
- 21. Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JAet al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 22. Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MSet al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2021;2:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader Aet al. Effectiveness of the BNT162b2mRNA covid-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 2021. Oct 18 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, Mesa-Chavez F, Barrientos-Gutiérrez T, Tagliamento Met al. Immunogenicity and risk of SARS-CoV-2 infection after COVID-19 vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer 2022;160:243–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CEet al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021;371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets included in this study are available from the corresponding author on reasonable request.