Abstract
Over the past two years, the SARS-CoV-2 pandemic has highlighted the impact that emerging pathogens can have on global health. The development of new and effective vaccine technologies is vital in the fight against such threats. Viral vectors are a relatively new vaccine platform that relies on recombinant viruses to deliver selected immunogens into the host. In response to the SARS-CoV-2 pandemic, the development and subsequent rollout of adenoviral vector vaccines has shown the utility, impact, scalability and efficacy of this platform. Shown to elicit strong cellular and humoral immune responses in diverse populations, these vaccine vectors will be an important approach against infectious diseases in the future.
Current Opinion in Immunology 2022, 77:102210
This review comes from a themed issue on Vaccines
Edited by Mariagrazia Pizza and Rino Rappuoli
For complete overview of the section, please refer to the article collection, “Vaccines (August 2022)”
Available online 25th May 2022
https://doi.org/10.1016/j.coi.2022.102210
0952-7915/© 2022 Published by Elsevier Ltd.
Introduction
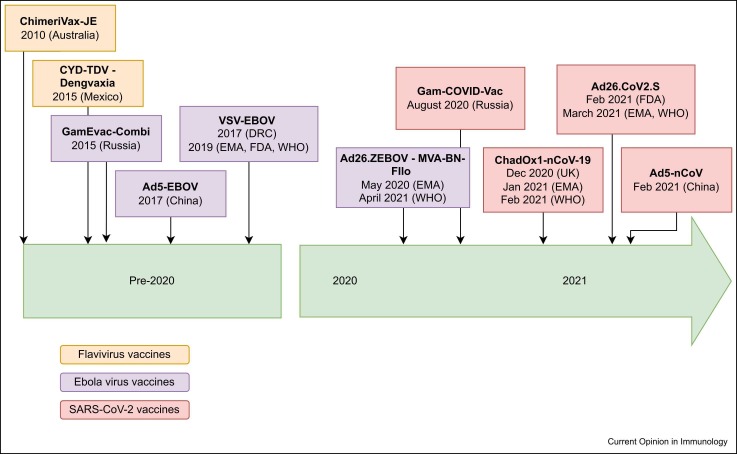
A recombinant viral vector was first used almost forty years ago as a vaccine-delivery system, when the gene for hepatitis-B surface antigen was inserted into a modified vaccinia virus 1, 2. Since then, such vaccine vectors have been widely used in veterinary medicine, but before 2020, only five had progressed through clinical trials to licensure and use in humans ( Figure 1). However, a number of viral vector vaccines have been developed against a wide variety of infectious-disease pathogens and indeed as vaccine vectors against noninfectious diseases, particularly cancer. Owing to the SARS-CoV-2 pandemic over the last two years, there has been an expansion in the use of adenoviral vector vaccines, with doses given to billions of people worldwide. This has given enormous insight into the safety, immunogenicity and efficacy of adenoviral (Ad) vaccine technology, which will be summarised here alongside a discussion of other viral vector vaccines in use today ( Table 1). A more detailed summary of all existing licensed viral vector vaccines can be found in the Supplementary Material.
Figure 1.
Timeline showing licensing of viral vector vaccines approved for use in humans. Before 2020, only five viral vectors were licensed for use in humans, and only one by the World Health Organisation. Since 2020, five further viral vector vaccines (all containing adenoviral vector vaccines) have been licensed for human use, including three which have been licensed by the WHO.
Table 1.
Currently licensed viral vector vaccines for use in humans.
| Vector class | Vector | Vaccine | Target pathogen | Encoded antigen | Developer | Clinical trials |
|---|---|---|---|---|---|---|
| Adenoviruses | Ad5 | Ad5-nCoV (Convidecia) | SARS-CoV-2 | Spike protein | CanSino Biologics (China) | 3, 4 |
| Ad5-EBOV | Ebola virus | Zaire strain (Makona) of glycoprotein | CanSino Biologics Inc | 5, 6, 7 | ||
| Ad26 | Ad26. CoV | SARS-CoV-2 | Pre-fusion- stabilised spike protein | Janssen Pharmaceutical Companies | 8, 9 | |
| Sputnik light | SARS-CoV-2 | Spike protein | Gamaleya Research Institute of Epidemiology and Microbiology (Russia) | [10] | ||
| ChAdOx1 | ChAdOx1- nCoV-19 (Covishield, Vaxzevria) | SARS-CoV-2 | Spike protein with tissue plasminogen leader sequence | University of Oxford/AstraZeneca | 11, 12•, 13 | |
| Rhabdoviruses | VSV | VSV-EBOV (rVSV-ZEBOV, Ervebo) | Ebola virus | Zaire strain (Kikwit 1995) of glycoprotein | Merck | 14, 15 |
| Flaviviruses | YF 17D | ChimeriVax-JE (Imojev) | Japanese encephalitis | Viral envelope (prM and E) of JE strain SA14–14–2 | Sanofi Pasteur | 16, 17, 18 |
| CYD-TDV (Dengvaxia) | Dengue | prM and E genes of DENV 1–4 | Sanofi Pasteur | 19, 20 | ||
| Heterologous regimens | Ad5/Ad26 | Gam-COVID-Vac (Sputnik V) | SARS-CoV-2 | Both spike proteins | Gamaleya Research Institute of Epidemiology and Microbiology (Russia) | 21, 22 |
| VSV/Ad5 | GamEvac-Combi | Ebola virus | Both glycoproteins | Gamaleya Research Institute of Epidemiology and Microbiology (Russia) | [23] | |
| Ad26/MVA | Ad26. ZEBOV
(Zabdeno) MVA-BN-Filo (Mvabea) |
Ebola virus | Ad26 — Zaire
strain MVA — glycoproteins from the Zaire Ebola virus (Mayinga strain), Sudan virus (Gulu strain) and Marburg virus (Musoke strain), and the nucleoprotein from the Tai Forest virus |
Janssen Pharmaceutical Companies | 24, 25, 26, 27•, 28 |
Viral vector vaccines utilise the capacity of viruses to infect cells and induce broad immune responses. Heterologous antigens are expressed by the virus, usually from genes engineered into the viral genome, and induce antigen-specific humoral and cellular immune responses. Viral vectors themselves can be replication-deficient, replication-competent or attenuated. Replication of the virus inside cells allows ongoing amplification of the vaccine antigen and improved immunogenicity, but must be balanced against the risk of increased adverse events or even disease in the host, particularly in the immunocompromised, resulting in some preference for use of replication-incompetent vectors.
Immunogenicity
Innate immune response
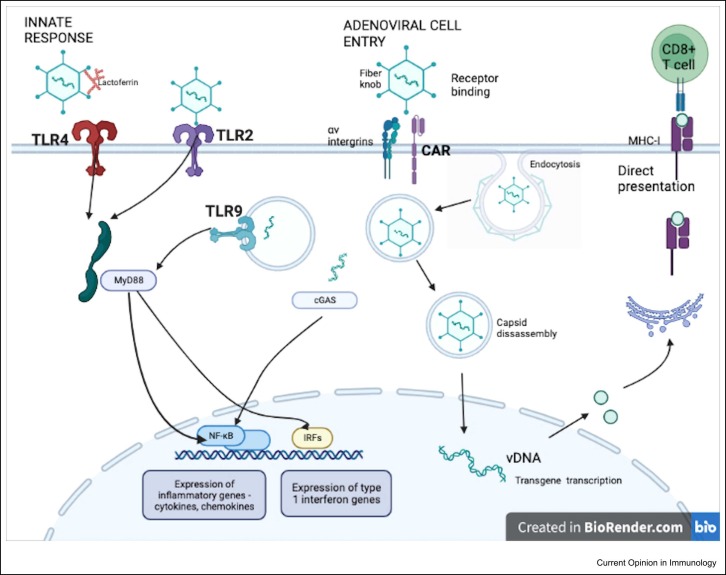
Using viral vectors as vaccine platforms allows induction of an innate immune response without the need for adjuvant. This response is key for stimulating downstream processing and later adaptive immune responses ( Figure 2).
Figure 2.
Induction of innate and adaptive immune responses by adenoviral vector vaccines. Adenoviral binding occurs via the fibre protein of the Ad capsid to infection receptors, such as the coxsackievirus–adenovirus receptor (CAR) and CD46, activating entry to the cell. Secondary attachment is mediated by RGD loops on the penton protein of the Ad capsid to integrins. These binding processes themselves can trigger innate immunity, but it is the pathogen-associated molecular patterns of adenoviruses, which are recognised by cell pattern-recognition receptors (PRRs), for example, toll-like receptors (TLRs). Ad vectors are recognised by TLR2 and TLR4, which are surface receptors, and TLR9, an endosomally located receptor that senses the Ad vector genome in endosomes 29, 30. The binding of lactoferrin, a host defence peptide, to Ad vectors, appears to activate an innate immune response via TLR4-mediated internalisation [31]. Intracellular adaptor proteins, such as MyD88, are vital for TLR signal transduction and induction of antigen-specific T-cell responses via activation of NF-κB transcription factors following Ad vector vaccine [32]. Further, PRRs such as the cytosol DNA sensor cGAS and the receptor RIG-I area are also important for inducing innate immune signals following Ad vector vaccination [33].
The downstream patterns of signalling from Ad vector recognition involve induction of a proinflammatory response, including cytokine and chemokine production, inducing humoral and cellular responses. Importantly, Ad vectors are able to do this without causing host damage and excess cytokine production. However, the excessive induction of type-I interferons (IFNs) by Ad vectors has been associated with dampened transgene expression and reduced antibody and cellular responses 34, 35.
Employing bioinformatic techniques to investigate transcriptional changes induced by viral vectors can give new insight into the activation of innate immune pathways by viral vectors. In a study by Sheerin et al using a mouse immunisation model, Ad vectors induced expression of genes involved in TLR2 stimulation and natural killer (NK) cell activation, whereas modified vaccinia Ankara (MVA) vectors induced expression of type-1 IFN genes [36]. Collingnon et al evaluated cytokine responses and gene expression patterns to characterise innate responses following vaccination with the ChAd155 vector vaccines in preclinical studies. The authors showed that the vaccine induced a bimodal pattern of innate cell-population changes characterised by IFN-associated signatures [37].
Adaptive immune response
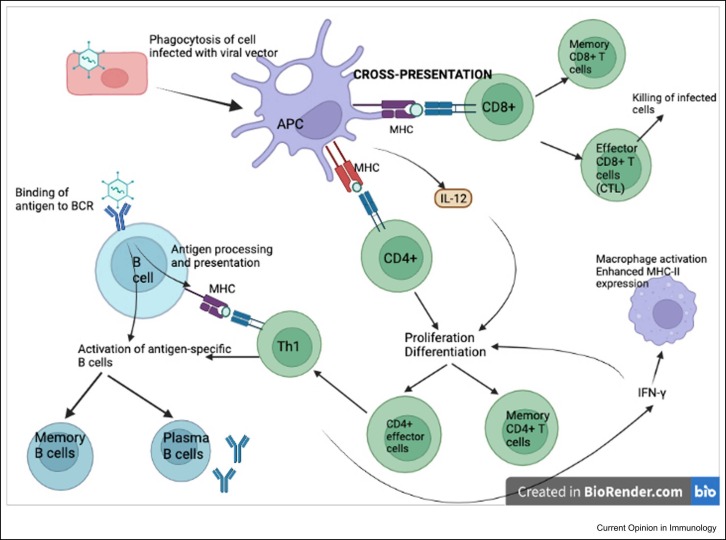
The ability of viral vectors to infect host cells, and express heterologous antigen, allows for antigen presentation and activation of host MHC pathways via direct and cross-presentation, inducing a robust cellular response ( Figure 3). The amount and duration of antigen expression correlate with CD8+ T-cell-protective immunity [35]. This potent T-cell activation has led to prior targeting of viral vector vaccines against intracellular pathogens, for example, HIV and malaria where such responses to such vaccines have correlated with protection [38].
Figure 3.
Adaptive immune response to adenoviral vector vaccines. Cross-presentation of antigen occurs in antigen-presenting cells, for example, dendritic cells following phagocytososis of infected cells. Antigen is presented via MHC molecules to T cells, stimulating proliferation and differentiation of CD4+ and CD8 + T cells. B cells are activated to antigen-specific memory B cells and plasma B cells via T-dependent and T-independent mechanisms. Binding of native antigen to the B-cell receptor (BCR) delivers biochemical signals that initiate B-cell activation independent of T cells. T-cell-dependent activation occurs when the BCR internalises the antigen that is endocytosed and processed into peptides presented by class-II MHC molecules. T-helper cells recognise these and stimulate B-cell activation.
Recent work has confirmed the strong and durable CD4+ and CD8+ antigen-specific T-cell responses that are generated following viral vector vaccines against other pathogens such as SARS-CoV-2, Ebola and RSV 4, 11, 39, 40, 41. The T-cell response following viral vector vaccination appears to be a Th-1-biased response, characterised by IFN-y and TNF-alpha production 27•, 41, 42, 43. Strong transgene expression by Ad vector vaccines also allows robust mono- and polyfunctional CD8+ T-cell responses 42, 44.
These specific T-cell responses may also contribute to protection and reduction in disease severity. For example, when examining these SARS-CoV-2-specific T-cell responses following acute COVID-19 infection, they appear to inversely correlate with COVID-19 disease severity 45, 46. Additionally, SARS-CoV-2 spike-specific follicular helper T cells correlate with neutralising antibody responses [47]. T-cell epitopes also appear to remain relatively preserved in COVID-19 variants of concern (VoC), leading to limited T-cell escape following infection or vaccination 48, 49, 50•. Given that these VoC have significant mutations in spike protein, leading to evasion of the neutralising antibody response 51, 52, 53, the ability of Ad vector vaccines to induce a broad cellular response may be important in sustaining protection from SARS-CoV-2.
As described above, although T-cell-mediated immunity plays a role in reducing disease severity, a neutralising antibody response often correlates with protection against infection. High levels of neutralising antibodies are induced by vesicular stomatitis virus (VSV), MVA and Ad viral vector vaccines against Ebola virus 23, 27•, 54, 55 and following Ad vector vaccines against SARS-CoV-2 12•, 39, 42. When investigating correlates of protection against SARS-CoV-2 following ChAdOx1-nCoV-19 vaccination higher anti-spike IgG, anti-receptor-binding protein IgG and neutralising antibody titres were all associated with lower risk of symptomatic disease [56]. All four Ad viral vector vaccines (Ad26.COV2.S, ChAdOx1-nCoV-19, Gam-COVID-Vac and Ad5-nCoV) are effective in protecting against symptomatic COVID-19 (66.9%, 66.7%, 91.6% and 57.5%, respectively) 11, 12•, 21, 57, 58.
Non-neutralising antibodies are also recognised as important mediators of antipathogen immunity and in preclinical studies Fc-mediated functions were shown to contribute to protection against SARS-CoV-2 59•, 60 and Ebola [61]. Systems' serology work has shown that Ad vector vaccines are able to induce antibody-dependent functional activities, including antibody-dependent neutrophil phagocytosis and antibody-dependent monocyte phagocytosis [8]. In a comparison of vaccine responses from phase-I and phase-II studies in humans using different HIV vaccines, Ad viral vectors induced a more potent IgG1 and IgG3 response than pox-virus vectors, leading to higher levels of functional antibody activity, including antibody-dependent cellular phagocytosis [62].
Induction of a mucosal immune response is likely to play an important role in protection against respiratory pathogens. Provine et al showed that in ChAdOx1-nCoV-19-immunised mice, mucosal-associated invariant T cells were induced, which correlated with vaccine-mediated T-cell responses [63]. Mucosal administration of an Ad vaccine may also induce stronger mucosal immune responses. Lapuente et al showed that mice given an intranasal Ad vector vaccine (either Ad19a or Ad5) boost following an intramuscular plasmid DNA or mRNA prime induced high levels of mucosal IgA and lung-resident tissue-resident memory T cells, in addition to systemic responses, leading to enhanced mucosal neutralisation [64]. Human trials of mucosal Ad vector vaccines against SARS-CoV-2 are underway with phase-I data from an aerosolised Ad5.nCoV vaccine showing two doses elicit a neutralising antibody response similar to one dose of IM injection [65].
Pre-existing immunity
Pre-existing immunity against the Ad vector has the potential to reduce immunogenicity and subsequent protective effect of these vaccine vectors [66]. Multiple studies have shown that existing anti-Ad-neutralising antibodies are inversely correlated with immunological response to vaccine vector 3, 6, 67. This is particularly relevant with Ad5-based vector vaccines, given their high seroprevalence in some populations [68]. However, repeated doses of Ad26 vector vaccination against HIV are able to boost both cellular and humoral immune responses, despite the presence of high Ad26-neutralising antibodies following prime vaccination [69] and following vaccination with ChAdOx1-nCoV-19-neutralising antibodies did not correlate with spike-specific antibody responses or T-cell responses following boost vaccination [12].
To circumvent the issue of antivector immunity less-prevalent adenoviruses, nonhuman adenoviruses or chimeric adenoviruses that express modifications to the hexon major capsid protein have been increasingly used over recent years 9, 70, 71, 72. Higher dosing regimens can also be used to overcome pre-existing vector immunity in the population, but when used with Ad5-nCoV, these higher doses caused increased reactogenicity with limited benefit in immunogenicity [3].
Prime-boost regimens
The use of heterologous prime-boost viral vector regimens may overcome the development of antivector immunity and be more immunogenic than homologous regimens 21, 73, 74, 75, 76. The use of Ad26 encoding the GP of the Zaire strain of Ebola, followed by an MVA boost, was shown to provide 100% protection against lethal Ebola when administered to nonhuman primates [77]. This heterologous prime-boost regimen has now been shown to induce strong and durable immune responses in human trials persisting for at least 1 year in both endemic and nonendemic populations 26, 27•.
Combining viral vectors takes advantage of the differential ability of vectors to prime or boost immune responses. For example, adenoviruses have been shown to prime effective and durable potent B- and T-cell responses, and MVA is able to significantly boost immune responses, but elicits limited humoral responses as a prime 78, 79. However, recent transcriptional data show that an Ad vector boost on an MVA prime appears to augment the molecular response compared with an MVA boost on an Ad prime, including stimulation of preferential TLRs and increased IFN-y signalling [36], suggesting that further exploration of this area is needed for future vaccine development.
Heterologous prime-boost vaccine schedules using different vaccine platforms have also been evaluated. For example, in vaccines against SARS-CoV-2, a prime dose of adenoviral vector vaccine has been boosted with an mRNA vaccine, which appears to increase vaccine efficacy and immunogenicity against symptomatic infection compared with homologous Ad vaccination 80, 81, 82, 83. In preclinical studies, an MVA booster following mRNA vaccine enhanced specific T-cell responses against HIV-1 [84].
Improving immunogenicity
Various methods have been used to further increase the immunogenicity of Ad vectors by enhancing transgene expression and boosting cellular responses. These include the use of endogenous promoters, co-expression of immune-stimulatory molecules and genetic-fusion adjuvants 85, 86 Rollier et al added the Toll-like receptor signalling molecule, TRIF-related adaptor molecule(TRAM), to an adenovirus-based vaccine, showing that co-expression of TRAM and antigen increased the transgene specific CD8+ T-cell responses in mice, but this did not translate into studies in primates [87].
A further way to enhance immunogenicity of viral vectors is to increase immunogen production from vaccine vector. Self-replication via replication-competent vectors allows significant antigen production and may be necessary to induce immunity against some pathogens. The safe use of a replication-competent VSV vector against Ebola virus, VSV-EBOV, in HIV patients, showed that such vectors can be used in immunocompromised patients [88]. An alternative is the use of single-cycle virus vectors, which allow the virus to self-amplify in one additional round of genome replication, circumvent this issue and represent a potential therapy for future viral vector vaccines 89, 90.
Harnessing the specific tropism of certain viruses to deliver antigens to desired cell types is a further potential mechanism of improving immunogenicity against certain pathogens. For example, Viktorova et al used a recombinant Newcastle virus, a virus with mucosal tropism, to express proteins from poliovirus, which stimulated systemic and mucosal responses [91].
Safety
Adenoviral vector vaccines have now been given to billions of people worldwide. Two vaccines (Ad26.COV2.S and ChadOx1-nCoV-19) have been associated with a very rare clotting disorder, thrombosis with thrombocytopaenia syndrome (TTS). This syndrome is characterised by the presence of antiplatelet factor-4 antibodies, although the risk factors for developing TTS and the exact pathogenesis remain unclear [92]. There may be an underlying geographical or genetic link, given variations in the rates of TTS across different populations [93].
Conclusion and future directions
Over the past two years, viral vector vaccines have been used as a cornerstone of the control of SARS-CoV-2 in the pandemic, particularly in low- and middle-income countries. The application of newer techniques such as bioinformatics and systems' serology during this time has provided extensive knowledge on the immunogenicity of the adenoviral vaccine platform.
Although significant advances have been made, further understanding of the spectrum of immune responses stimulated by adenoviral vectors is still needed. Understanding of the mechanism underlying antivector immunity, particularly following repeated dosing, will be vital going forward as vaccines against multiple different pathogens are developed using the same vectors. Evaluating the long-term duration of humoral and cellular responses following widespread Ad vector administration for SARS-CoV-2, and their relationship to vaccine efficacy, will be important in providing invaluable insights into the persistence of immune responses afforded by these vaccine vectors.
Despite the excellent immunogenicity and efficacy of the approved viral vector vaccines, there remains scope to improve immunogenicity. The use of genetic or molecular adjuvants may be a useful strategy, particularly in vaccine vectors that only induce weak or short transgene expression. In addition, the use of heterologous prime-boost regimens, by combining either different viral vectors or different technologies such as mRNA vaccines, has been shown to improve immunogenicity of homologous regimens, and is likely to play an important role in viral vector vaccine regimens going forward. The use of mucosal viral vector vaccines to induce site-specific immune responses may significantly improve protection, particularly against mucosal pathogens, and clinical trial data from such vaccines are eagerly awaited.
Viral vector vaccines have been a major component of the successful response to the SARS-CoV-2 pandemic. Given their safety, immunogenicity and ability to be modified and scaled up at pace, they will remain an important technology for infectious-disease control in the future.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest statement
AJP is chair of the UK Department of Health and Social Care's Joint Committee on Vaccination and Immunisation, but does not participate in policy advice on coronavirus vaccines, and is a member of the WHO Strategic Advisory Group of Experts. AJP is a National Institute for Health Research Senior Investigator. TL is named as an inventor on a patent application covering ChAdOx1-nCoV-19. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1-nCoV-19. All other authors declare no competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.coi.2022.102210.
Supplementary material
Supplementary material
.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
-
•
of special interest
-
••
of outstanding interest
- 1.Smith G.L., MacKett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983;302:490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- 2.Moss B., Smith G.L., Gerin J.L., Purcell R.H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984;311:67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- 3.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu L., Zhang Z., Gao H., Li Y., Hou L., Yao H., et al. Open-label phase I clinical trial of Ad5-EBOV in Africans in China. Hum Vaccines Immunother. 2017;13:2078–2085. doi: 10.1080/21645515.2017.1342021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J.X., Hou L.H., Meng F.Y., Wu S.P., Hu Y.M., Liang Q., et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob Heal. 2017;5:e324–e334. doi: 10.1016/S2214-109X(16)30367-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhu F.C., Hou L.H., Li J.X., Wu S.P., Liu P., Zhang G.R., et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet. 2015;389:621–628. doi: 10.1016/S0140-6736(15)60553-0. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson K.E., Le Gars M., Sadoff J., De Groot A.M., Heerwegh D., Truyers C., et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA J Am Med Assoc. 2021;325:1535–1544. doi: 10.1001/jama.2021.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadoff J., Le Gars M., Shukarev G., Heerwegh D., Truyers C., de Groot A.M., et al. Interim results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tukhvatulin A.I., Dolzhikova I.V., Shcheblyakov D.V., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed ChAdOx1-nCoV-19 induced similar immune response including anti-spike and anti-RBD antibodies in all age groups including older adults. This was an important finding given the risk of more severe disease with older age and other adenoviral vaccines sugggesting reduced immunogenicity in this age group and led to recommendation by Medicines and Healthcare products Regulatory Agency for licensing in UK.
- 13.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henao-Restrepo A.M., Longini I.M., Egger M., Dean N.E., Edmunds W.J., Camacho A., et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;389:P505–P518. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 15.Henao-Restrepo A.M., Camacho A., Longini I.M., Watson C.H., Edmunds W.J., Egger M., et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!) Lancet. 2017;386:P857–P866. doi: 10.1016/S0140-6736(16)32621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torresi J., McCarthy K., Feroldi E., Méric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: randomised controlled phase 3 trials. Vaccine. 2010;28:7993–8000. doi: 10.1016/j.vaccine.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 17.Monath T.P., Guirakhoo F., Nichols R., Yoksan S., Schrader R., Murphy C., et al. Chimeric live, attenuated vaccine against Japanese Encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese Encephalitis antigen. J Infect Dis. 2003;188:1213–1230. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 18.Nasveld P.E., Ebringer A., Elmes N., Bennett S., Yoksan S., Aaskov J., et al. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine: randomized, double-blind, 5-year phase II study in healthy adults. Hum Vaccin. 2010;6:1038–1046. doi: 10.4161/hv.6.12.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capeding M.R., Tran N.H., Hadinegoro S.R.S., Ismail H.I.H.M., Chotpitayasunondh T., Chua M.N., et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 20.Villar L., Dayan G.H., Arredondo-García J.L., Rivera D.M., Cunha R., Deseda C., et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 21.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logunov D.Y., Dolzhikova I.V., Zubkova O.V., Tukhvatullin A.I., Shcheblyakov D.V., Dzharullaeva A.S., et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:P887–P897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolzhikova I.V., Zubkova O.V., Tukhvatulin A.I., Dzharullaeva A.S., Tukhvatulina N.M., Shcheblyakov D.V., et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccines Immunother. 2017;13:613–620. doi: 10.1080/21645515.2016.1238535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan I., Gibani M., Campbell D., Clutterbuck E., Sewell R., Plested E., et al. Heterologous prime-boost schedules of replication-defective adenovirus serotype 26 and modified vaccinia virus ankara vector vaccines expressing ebola virus glycoprotein are immunogenic and well tolerated in healthy adults. Open Forum Infect Dis. 2015;2(1):744. [Google Scholar]
- 25.Milligan I.D., Gibani M.M., Sewell R., Clutterbuck E.A., Campbell D., Plested E., et al. Safety and immunogenicity of novel adenovirus type 26-and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA J Am Med Assoc. 2016;315:1610–1623. doi: 10.1001/jama.2016.4218. [DOI] [PubMed] [Google Scholar]
- 26.Mutua G., Anzala O., Luhn K., Robinson C., Bockstal V., Anumendem D., et al. Safety and immunogenicity of a 2-dose heterologous vaccine regimen with Ad26.ZEBOV and MVA-BN-Filo Ebola vaccines: 12-month data from a phase 1 randomized clinical trial in Nairobi, Kenya. J Infect Dis. 2019;220(1):57–67. doi: 10.1093/infdis/jiz071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Pollard A.J., Launay O., Lelievre J.D., Lacabaratz C., Grande S., Goldstein N., et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2021;21:493–506. doi: 10.1016/S1473-3099(20)30476-X. [DOI] [PubMed] [Google Scholar]; This was first phase 2 study to evaluate this heterologous Ebola vaccine. Authors showed that antibody responses were higher in 28-day and 56-day dosing intervals than in 21-day dosing interval and that both humoral and cellular responses persisted in 98–100% of participants at 1 year, providing further evidence that a heterologous prime-boost regimen is more immunogenic than single dose.
- 28.Ishola D., Manno D., Afolabi M.O., Keshinro B., Bockstal V., Rogers B., et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect Dis. 2021;22:97–109. doi: 10.1016/S1473-3099(21)00125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iacobelli-Martinez M., Nemerow G.R. Preferential activation of toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007;81:1305–1312. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appledorn D.M., Patial S., McBride A., Godbehere S., Van Rooijen N., Parameswaran N., et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 31.Chéneau C., Eichholz K., Tran T.H., Tran T.T.P., Paris O., Henriquet C., et al. Lactoferrin retargets human adenoviruses to TLR4 to induce an abortive NLRP3-associated pyroptotic response in human phagocytes. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.685218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee E.G., Blattman J.N., Kasturi S.P., Kelley R.P., Kaufman D.R., Lynch D.M., et al. Multiple innate immune pathways contribute to the immunogenicity of recombinant adenovirus vaccine vectors. J Virol. 2011;85:315–323. doi: 10.1128/JVI.01597-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coughlan L. Factors which contribute to the immunogenicity of non-replicating adenoviral vectored vaccines. Front Immunol. 2020;11:909. doi: 10.3389/fimmu.2020.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensley S.E., Cun A.S., Giles-Davis W., Li Y., Xiang Z., Lasaro M.O., et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther. 2007;15:393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- 35.Quinn K.M., Zak D.E., Costa A., Yamamoto A., Kastenmuller K., Hill B.J., et al. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J Clin Investig. 2015;125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Sheerin D., Dold C., O’Connor D., Pollard A.J., Rollier C.S. Distinct patterns of whole blood transcriptional responses are induced in mice following immunisation with adenoviral and poxviral vector vaccines encoding the same antigen. BMC Genom. 2021;22:1–12. doi: 10.1186/s12864-021-08061-8. 〈https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-021-08061-8〉 [Internet] (Dec 1) [cited 2022 Feb 16] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use RNA sequencing to establish that different viral vector vaccines induce distinct transcriptional responses and that the sequence of prime-boost vaccines influenced the magnitude of gene expression changes.
- 37•.Collignon C., Bol V., Chalon A., Surendran N., Morel S., van den Berg R.A., et al. Innate immune responses to chimpanzee adenovirus vector 155 vaccination in mice and monkeys. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.579872. (Nov 30) [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors evaluated cytokine responses and gene expression changes following ChAd155 vaccination in mice, showing a bimodal pattern of innate changes characterised by IFN-associated signatures.
- 38.Ewer K.J., O’Hara G.A., Duncan C.J.A., Collins K.A., Sheehy S.H., Reyes-Sandoval A., et al. Protective CD8 + T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun. 2013;4:2836. doi: 10.1038/ncomms3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med. 2021;385:951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winslow R.L., Milligan I.D., Voysey M., Luhn K., Shukarev G., Douoguih M., et al. Immune responses to novel adenovirus type 26 and modified vaccinia virus Ankara-vectored ebola vaccines at 1 year. JAMA J Am Med Assoc. 2017;317:1075–1077. doi: 10.1001/jama.2016.20644. [DOI] [PubMed] [Google Scholar]
- 41.Jordan E., Lawrence S.J., Meyer T.P.H., Schmidt D., Schultz S., Mueller J., et al. Broad antibody and cellular immune response from a phase 2 clinical trial with a novel multivalent poxvirus-based respiratory syncytial virus vaccine. J Infect Dis. 2021;223:1062–1072. doi: 10.1093/infdis/jiaa460. [DOI] [PubMed] [Google Scholar]
- 42.Ewer K.J., Barrett J.R., Belij-Rammerstorfer S., Sharpe H., Makinson R., Morter R., et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 43.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyllie D., Jones H.E., Mulchandani R., Trickey A., Taylor-Phillips S., Brooks T., et al. SARS-CoV-2 responsive T cell numbers and anti-Spike IgG levels are both associated with protection from COVID-19: a prospective cohort study in keyworkers. medRxiv. 2021 [Google Scholar]
- 46.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012. doi: 10.1016/j.cell.2020.09.038. (.e19) (Nov 12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi S.J., Kim D.U., Noh J.Y., et al. T cell epitopes in SARS-CoV-2 proteins are substantially conserved in the Omicron variant. Cell Mol Immunol. 2022;19:447–448. doi: 10.1038/s41423-022-00838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grifoni A., Sidney J., Vita R., Peters B., Crotty S., Weiskopf D., et al. SARS-CoV-2 human T cell epitopes: adaptive immune response against COVID-19. Cell Host Microbe. 2021;29:1076–1092. doi: 10.1016/j.chom.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Keeton R., Tincho M.B., Ngomti A., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. https://www.nature.com/articles/s41586-022-04460-3 [Internet] (2022 Jan 31) [cited 2022 Mar 1] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors showed that CD4+ and CD8+ T cell recognition of the Omicron spike was largely preserved in individuals vaccinated with Ad26.COV2.S, compared with ancestral virus, despite extensive neutralising antibody escape.
- 51.Planas D., Saunders N., Maes P., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. https://www.nature.com/articles/d41586-021-03827-2 [DOI] [PubMed] [Google Scholar]
- 52.Dejnirattisai W., et al. Huo J., Zhou D., Zahradník J., Supasa P., Liu C. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–484. doi: 10.1016/j.cell.2021.12.046. E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:E52–E61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halperin S.A., Das R., Onorato M.T., Liu K., Martin J., Grant-Klein R.J., et al. Immunogenicity, lot consistency, and extended safety of rVSVΔG-ZEBOV-GP vaccine: a phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J Infect Dis. 2019;220:1127–1135. doi: 10.1093/infdis/jiz241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clarke D.K., Xu R., Matassov D., Latham T.E., Ota-Setlik A., Gerardi C.S., et al. Safety and immunogenicity of a highly attenuated rVSVN4CT1-EBOVGP1 Ebola virus vaccine: a randomised, double-blind, placebo-controlled, phase 1 clinical trial. Lancet Infect Dis. 2020;20:455–466. doi: 10.1016/S1473-3099(19)30614-0. [DOI] [PubMed] [Google Scholar]
- 56•.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors used data from the ChAdOx1-nCoV-19 trial to determine antibody levels assocaited with protection against SARS-CoV-2. They showed that higher anti-spike IgG, anti-RBD IgG and neutralising antibody titres are all associated with lower risk of symptomatic disease.
- 57.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halperin S.A., Ye L., MacKinnon-Cameron D., Smith B., Cahn P.E., Ruiz-Palacios G.M., et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet. 2022;399:237–248. doi: 10.1016/S0140-6736(21)02753-7. 〈http://www.thelancet.com/article/S0140673621027537/fulltext〉 [Internet] (Jan 15) [cited 2022 Apr 5] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Barrett J.R., Belij-Rammerstorfer S., Dold C., Ewer K.J., Folegatti P.M., Gilbride C., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2020;27:279–288. doi: 10.1038/s41591-020-01179-4. 〈https://www.nature.com/articles/s41591-020-01179-4〉 (272) [Internet] (2020 Dec 17) [cited 2022 Jan 27] (Available from:) [DOI] [PubMed] [Google Scholar]; The authors used a systems serology approach to characterise vaccine-induced antibodies in humans. They demonstrated that ChAdOx1 booster dose enhanced Fc-mediated functional antibody responses and anti-spike neutralising antibody titres.
- 60.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saphire E.O., Schendel S.L., Gunn B.M., Milligan J.C., Alter G. Antibody-mediated protection against Ebola virus. Nat Immunol. 2018;19:1169–1178. doi: 10.1038/s41590-018-0233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischinger S., Cizmeci D., Deng D., Grant S.P., Frahm N., McElrath J., et al. Sequence and vector shapes vaccine induced antibody effector functions in HIV vaccine trials. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1010016. 〈https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010016〉 [Internet] (Nov 1) [cited 2022 Apr 5] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Provine N.M., Amini A., Garner L.C., Spencer A.J., Dold C., Hutchings C., et al. MAIT cell activation augments adenovirus vector vaccine immunogenicity. Science. 2021;371:521–526. doi: 10.1126/science.aax8819. (80-) [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors showed that MAIT cell-deficient mice displayed impaired CD8+ responses to adenovirus vaccine-encoded antigens and that MAIT cell activation in humans positively correlated with vaccine T-cell responses, suggesting an important role for MAIT cells in adenoviral vector immunogenicity.
- 64•.Lapuente D., Fuchs J., Willar J., et al. Protective mucosal immunity against SARS-CoV-2 after heterologous systemic prime-mucosal boost immunization. Nat Commun. 2021;12:6871. doi: 10.1038/s41467-021-27063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors showed that intranasal adenoviral vector vaccine boost (following an mRNA or DNA prime) induced mucosal IgA and enhanced mucosal neutralisation of virus, suggesting an important role for mucosal adenoviral vaccines.
- 65.Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;21:P1654–P1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barouch D.H., Pau M.G., Custers J.H.H.V., Koudstaal W., Kostense S., Havenga M.J.E., et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 67.Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barouch D.H., Kik S.V., Weverling G.J., Dilan R., King S.L., Maxfield L.F., et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baden L.R., Walsh S.R., Seaman M.S., Tucker R.P., Krause K.H., Patel A., et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–247. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folegatti P.M., Bittaye M., Flaxman A., Lopez F.R., Bellamy D., Kupke A., et al. Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. Lancet Infect Dis. 2020;20:816–826. doi: 10.1016/S1473-3099(20)30160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tapia M.D., Sow S.O., Lyke K.E., Haidara F.C., Diallo F., Doumbia M., et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Excler J.L., Kim J.H. Novel prime-boost vaccine strategies against HIV-1. Expert Rev Vaccines. 2019;18:765–779. doi: 10.1080/14760584.2019.1640117. [DOI] [PubMed] [Google Scholar]
- 73.Kardani K., Bolhassani A., Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34:413–423. doi: 10.1016/j.vaccine.2015.11.062. [DOI] [PubMed] [Google Scholar]
- 74.Parry H., Bruton R., Stephens C., Brown K., Amirthalingam G., Otter A., et al. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun Ageing. 2021;18:1–8. doi: 10.1186/s12979-021-00246-9. 〈https://immunityageing.biomedcentral.com/articles/10.1186/s12979-021-00246-9〉 [Internet] (Dec 1) [cited 2022 Jan 21] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Casimiro D.R., Bett A.J., Fu T., Davies M.-E., Tang A., Wilson K.A., et al. Heterologous human immunodeficiency virus Type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78:11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Venkatraman N., Ndiaye B.P., Bowyer G., Wade D., Sridhar S., Wright D., et al. Safety and immunogenicity of a heterologous prime-boost ebola virus vaccine regimen in healthy adults in the United Kingdom and Senegal. J Infect Dis. 2019;219:1187–1197. doi: 10.1093/infdis/jiy639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Callendret B., Vellinga J., Wunderlich K., Rodriguez A., Steigerwald R., Dirmeier U., et al. A prophylactic multivalent vaccine against different filovirus species is immunogenic and provides protection from lethal infections with Ebolavirus and Marburgvirus species in non-human primates. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanley D.A., Honko A.N., Asiedu C., Trefry J.C., Lau-Kilby A.W., Johnson J.C., et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med. 2014;20:1126–1129. doi: 10.1038/nm.3702. [DOI] [PubMed] [Google Scholar]
- 79.Geiben-Lynn R., Greenland J.R., Frimpong-Boateng K., Letvin N.L. Kinetics of recombinant adenovirus type 5, vaccinia virus, modified vaccinia ankara virus, and DNA antigen expression in vivo and the induction of memory T-lymphocyte responses. Clin Vaccine Immunol. 2008;15:691–696. doi: 10.1128/CVI.00418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:P856–P869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2021;399:P36–P49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:P121–P130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spencer A.J., McKay P.F., Belij-Rammerstorfer S., Ulaszewska M., Bissett C.D., Hu K., et al. Heterologous vaccination regimens with self-amplifying RNA and adenoviral COVID vaccines induce robust immune responses in mice. Nat Commun. 2021;12 doi: 10.1038/s41467-021-23173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gómez C.E., Perdiguero B., Usero L., Marcos-Villar L., Miralles L., Leal L., et al. Sorzano C.O.S., Sánchez-Corzo C., Plana M., García F., Esteban M. Enhancement of the HIV-1-Specific Immune Response Induced by an mRNA Vaccine through Boosting with a Poxvirus MVA Vector Expressing the Same Antigen. Vaccines. 2021;9:959. doi: 10.3390/vaccines9090959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neukirch L., Fougeroux C., Andersson A.M.C., Holst P.J. The potential of adenoviral vaccine vectors with altered antigen presentation capabilities. Expert Rev Vaccines. 2020;19:25–41. doi: 10.1080/14760584.2020.1711054. [DOI] [PubMed] [Google Scholar]
- 86.Matchett W.E., Malewana G.B.R., Mudrick H., Medlyn M.J., Barry M.A. Genetic Adjuvants in Replicating Single-Cycle Adenovirus Vectors Amplify Systemic and Mucosal Immune Responses against HIV-1 Envelope. Vaccines. 2020;8:64. doi: 10.3390/vaccines8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Rollier C.S., Spencer A.J., Sogaard K.C., et al. Modification of Adenovirus vaccine vector-induced immune responses by expression of a signalling molecule. Sci Rep. 2020;10:5716. doi: 10.1038/s41598-020-61730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors added the Toll-like receptors signalling molecule, TRAM, to an Ad vaccine which, when co-expressed with the antigen, increased trangene-specific CD8+ T cell responses in mice.
- 88.Kennedy S.B., Bolay F., Kieh M., Grandits G., Badio M., Ballou R., et al. Phase 2 Placebo-controlled trial of two vaccines to prevent Ebola in liberia. N Engl J Med. 2017;377:1438–1447. doi: 10.1056/NEJMoa1614067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crosby C.M., Nehete P., Sastry K.J., Barry M.A. Amplified and persistent immune responses generated by single-cycle replicating adenovirus vaccines. J Virol. 2015;89:669–675. doi: 10.1128/JVI.02184-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anguiano-Zarate S.S., Matchett W.E., Nehete P.N., Sastry J.K., Marzi A., Barry M.A. A replicating single-cycle adenovirus vaccine against Ebola virus. J Infect Dis. 2018;218:1883–1889. doi: 10.1093/infdis/jiy411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Viktorova E.G., Khattar S.K., Kouiavskaia D., Laassri M., Zagorodnyaya T., Dragunsky E., et al. Newcastle disease virus-based vectored vaccine against poliomyelitis. J Virol. 2018;92 doi: 10.1128/JVI.00976-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baker A.T., Boyd R.J., Sarkar D., Teijeira-Crespo A., Chan C.K., Bates E., et al. ChAdOx1 interacts with CAR and PF4 with implications for thrombosis with thrombocytopenia syndrome. Sci Adv. 2021;7:8213. doi: 10.1126/sciadv.abl8213. 〈https://www.science.org/doi/abs/10.1126/sciadv.abl8213〉 [Internet] (Dec 3) [cited 2021 Dec 16] (Available from:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Soboleva K., Shankar N.K., Yadavalli M., Ferreira C., Foskett N., Putsepp K., et al. Geographical distribution of TTS cases following AZD1222 (ChAdOx1 nCoV-19) vaccination. Lancet Glob Health. 2022;10 doi: 10.1016/S2214-109X(21)00545-3. [Internet] (Jan 1) [cited 2022 Apr 11] (Available from: /pmc/articles/PMC8670752/) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material