Abstract
We report a rare case of Gemella morbillorum endocarditis of the native aortic and mitral valves, and native vertebral osteomyelitis, in a 49-year-old male with HLA-B27 negative ankylosing spondylitis (AKS). G. morbillorum is a rare cause of endocarditis; the incidence of which is unknown. AKS may predispose patients to endocarditis through chronic valvulitis. G. morbillorum bacteremia in patients with AKS should prompt consideration of infective endocarditis and a search for possible portals of entry.
Keywords: commensal organism, Gemella morbillorum, infective endocarditis, osteomyelitis
Résumé
Les auteurs déclarent un rare cas d’endocardite à Gemella morbillorum des valves aortiques et mitrales naturelles et une ostéomyélite des vertèbres naturelles chez un homme de 49 ans atteint de spondylite ankylosante négative au HLA-B27 (SAK). Le G. morbillorum est une rare cause d’endocardite dont on ne connaît pas l’incidence. La SAK peut prédisposer à l’endocardite par une valvulite chronique. La bactériémie à G. morbillorum chez des patients atteints de SAK devrait inciter à envisager rapidement une endocardite infectieuse et à rechercher les voies d’entrée possibles.
Mots clés : endocardite infectieuse, Gemella morbillorum, organisme commensal, ostéomyélite
Introduction
Gemella morbillorum is a facultatively anaerobic gram-positive coccus occurring in pairs or short chains. It is non-motile, non-spore-forming, and is a commensal organism of the oral, gastrointestinal, and genitourinary mucosa. It is a rare cause of endocarditis—a recent review documented 34 cases in the literature (1). The incidence and treatment are not well defined.
Case report
A 49-year-old male, originally from Liberia, with a known history of hypertension and smoking, was admitted with 1 week of orthopnea, paroxysmal nocturnal dyspnea, and New York Heart Association (NYHA) class III symptoms. He reported 6 months of low back pain with prolonged morning stiffness, 1 month of chills and malaise, and an unintentional 22.5 kg (50 pound) weight loss over recent months.
He was followed by a cardiologist for chronic asymptomatic aortic regurgitation (AR) and aortic dilation. Transesophageal echocardiogram (TEE) from a year prior showed a tricuspid aortic valve (AV) with moderate AR, normal mitral valve (MV), ejection fraction (EF) >60%, mild aortic root dilation (4.1 cm), and moderate ascending aorta dilation (4.6 cm). There was no family history of aortic dissection or connective tissue disease. There were no exam findings of Marfan, Loeys–Dietz, or Ehlers–Danlos syndromes.
At one-year follow-up after his TEE, an outpatient transthoracic echocardiogram (TTE) showed severe AR, severe mitral regurgitation (MR), EF >60%, and progressive dilation of the aortic root (4.3 cm) and ascending aorta (4.8 cm) without any vegetations noted. He was referred for inpatient evaluation.
On initial assessment, he was afebrile with a heart rate of 99 beats per minute and blood pressure of 109/72 mm Hg. He had poor dentition with no peripheral stigmata of endocarditis. An S3, grade 2/6 diastolic murmur at the left sternal border, and 3/6 holosystolic murmur at the apex were heard. Breath sounds were clear. The sacroiliac joints were tender to palpation.
Hemoglobin was 92 g/L (reference range 135–180g/L), mean corpuscular volume 85.2 (79.0–99.0 fL), leukocyte count 5.6 × 109/L (4.0–11.0 × 109/L), platelets 207 × 109/L (150–400 × 109/L), creatinine 86 µmol/L (60–104 µmol/L), high-sensitivity troponin-T 15 ng/L (<14.0 ng/L), NT pro BNP 1446 ng/L (0–125 ng/L), and CRP 84 mg/L (0–7 mg/L). HLA-B27 and rheumatoid factor were negative. HIV and syphilis testing were negative.
During his hospitalization, he developed a fever of 39.2°C, which prompted blood cultures to be drawn, followed by initiation of empiric intravenous piperacillin-tazobactam. There were no blood cultures drawn prior to this. Within 48 hours, both bottles from one of the two sites flagged positive, with the Gram stain showing gram-positive cocci. It was further identified as a Gemella species using matrix-assisted laser desorption/ionization time of flight (MALDI–TOF) mass spectrometry (MS). The isolate was sent away for further identification and antimicrobial susceptibility testing. It was speciated as Gemella morbillorum using 16s rDNA sequencing and was found to be susceptible to both penicillin and ceftriaxone with minimum inhibitory concentrations (MICs) of 0.012 and 0.023, respectively. Follow-up blood cultures drawn at 48 hours after the initial set were sterile. Repeat TEE showed large vegetations of the AV and anterior leaflet of the MV, which was flail, perforated, and with multiple diverticula (Figure 1). Computed tomography (CT) aortogram was negative for aortic aneurysm or dissection and showed bilateral sacroiliac joint fusion. This confirmed the diagnosis of AKS. Spinal MRI showed osteopenic cervical compression fractures and native vertebral osteomyelitis and discitis at T10-11 and L4-5.
Figure 1:
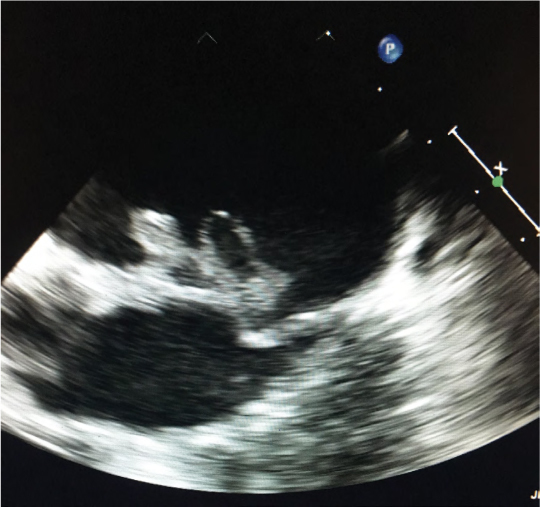
TEE showing large vegetations of the anterior leaflet of the MV
TEE = Transesophageal echocardiogram; AV = Aortic valve; MV = Mitral valve
Antimicrobial therapy was switched to ceftriaxone and gentamicin for 3 weeks with concomitant pre-operative dental care. Dental examination revealed generalized heavy plaque and calculus buildup, exudate present around crowns on teeth 37 and 46, a very mobile tooth 25, and only root tips remaining of tooth 26. These teeth were extracted, and cleaning was performed. He then underwent uneventful mechanical AV and MV replacements. Valve tissue cultures were negative. On surgical pathology, the AV showed patchy chronic inflammation, and the MV had areas of necrosis with signs of acute and chronic inflammation. He was discharged on ceftriaxone monotherapy to complete a 6-week course and was well at his 2-year follow-up.
Discussion
Gemella morbillorum is a facultatively anaerobic gram-positive coccus that can be gram-variable, as it tends to decolourize easily due to its relatively thin walls (2). It is a rare cause of infection in humans and has been known to cause dental abscess, lung abscess, liver abscess, meningitis, brain abscess, mediastinitis, and infective endocarditis (3–6). Being an opportunistic infection, it typically requires the presence of predisposing factors, including poor dentition, colorectal disease, diabetes, immunocompromised state, pre-existing cardiac valve disease, and procedural disruption of colonized mucosa (7). A review of 66 cases of Gemella genus endocarditis found that 34 were related to G. morbillorum, making it the most common cause of Gemella endocarditis (1).
This report also noted that 31/62 cases required surgery. Antimicrobial therapy has not been well defined. In the literature, native valve endocarditis with Gemella has been treated for 4 to 6 weeks using an intravenous β-lactam such as penicillin or ceftriaxone (or vancomycin if intolerant of β-lactams or resistance), with or without an aminoglycoside/rifampin. Reports of resistance to β-lactams, aminoglycosides, and macrolides exist (8,9).
Though most commonly associated with an odontogenic source, Gemella may be linked with colorectal cancer. Our literature review suggested 4 published cases of associated colon cancer and 1 with high-grade dysplastic adenoma (1). Gemella has been found to be more abundant in the stool of patients with colorectal cancer than healthy individuals, with higher amounts found in the cancer tissue (10). A retrospective study of 13,096 patients hospitalized with bacteremia from enteric organisms revealed a possible association between G. morbillorum and incident colorectal cancer (HR 15.2, 95% CI 1.54 to 150, p = 0.020) (11). Colonoscopy should thus be considered where no obvious source can be identified. Our patient is scheduled for an outpatient colonoscopy.
The HLA-B27 allele is present in up to 90% of cases of AKS but is rare in Black Africans (12). We theorize that our patient had previous aortitis and valvulitis due to AKS and poor oral health, predisposing him to G. morbillorum endocarditis. His osteomyelitis–discitis was suspected to be hematogenously spread. As there are few reported cases, the incidence and natural history of infective endocarditis in patients with AKS are unknown. A working diagnosis of AKS alone could have explained the constitutional symptoms, back pain, fever, and chronic compensated AR. However, the acute severe AR our patient presented with mandated the inclusion of infectious endocarditis on the differential.
This case is unique in many ways. In our review of the published literature, this case is the third one describing involvement of multiple native valves by this pathogen. Another unique feature is the associated native vertebral osteomyelitis and discitis. Only a handful of bone and joint infections related to G. morbillorum have been described (13–16); to our knowledge, this is the only case of Gemella native vertebral osteomyelitis–discitis with an associated proven endovascular infection. Furthermore, this is the only case report of Gemella endocarditis predisposition due to the valvular involvement seen in AKS.
Conclusion
G. morbillorum is a rare cause of endocarditis and osteomyelitis. Our patient was predisposed to endovascular infection due to chronic valvulitis from AKS and poor oral health. G. morbillorum bacteremia in patients with AKS should prompt investigation for endocarditis and consideration of colonoscopy to rule out colorectal cancer if no other portal of entry is found.
Ethics Approval:
N/A
Informed Consent:
Informed consent was received from the patient.
Funding:
No funding was received for this work.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
ANIMAL STUDIES:
N/A
References
- 1.Youssef D, Youssef I, Marroush TS, Sharma M. Gemella endocarditis: a case report and a review of the literature. Avicenna J Med. 2019;9(4):164–8. 10.4103/AJM.AJM_3_19. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Facklam R, Elliott JA. Identification, classification, and clinical relevance of catalase-negative, gram-positive cocci, excluding the streptococci and enterococci. Clin Microbiol Rev. 1995;8(4):479–95. 10.1128/CMR.8.4.479-495.1995. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu-Heija AA, Ajam M, Veltman J. Gemella morbillorum cryptogenic brain abscess: a case report and literature review. Cureus. 2018 . Nov;10(11):e3612. 10.7759/cureus.3612. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu CY, Su YC, Wang TL, Chong CF, Chen CC. Gemella morbillorum liver abscess. Scand J Infect Dis. 2007;39(6–7):637–8. 10.1080/00365540601169737. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Savides TJ, Margolis D, Richman KM, Singh V. Gemella morbillorum mediastinitis and osteomyelitis following transesophageal endoscopic ultrasound-guided fine-needle aspiration of a posterior mediastinal lymph node. Endoscopy. 2007. Feb;39(Suppl 1):E123–4. 10.1055/s-2007-966157. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Da Costa CT, Porter C, Parry K, Morris A, Quoraishi AH. Empyema thoracis and lung abscess due to Gemella morbillorum. Eur J Clin Microbiol Infect Dis. 1996;15(1):75–7. 10.1007/BF01586189. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Dupla M, Creus C, Navarro O, Raga X. Association of Gemella morbillorum endocarditis with adenomatous polyps and carcinoma of the colon: case report and review. Clin Infect Dis. 1996;22(2):379–80. 10.1093/clinids/22.2.379. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Kofteridis DP, Anastasopoulos T, Panagiotakis S, Kontopodis E, Samonis G. Endocarditis caused by Gemella morbillorum resistant to β-lactams and aminoglycosides. Scand J Infect Dis. 2006; 38(11–12):1125–7. 10.1080/00365540600740538. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Zolezzi PC, Cepero PG, Ruiz J, Laplana LM, Calvo CR, Gómez-Lus R. Molecular epidemiology of macrolide and tetracycline resistances in commensal Gemella sp. isolates. Antimicrob Agents Chemother. 2007;51(4):1487–90. 10.1128/AAC.01374-06. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Cai G, Qiu Y, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6(2):320–9. 10.1038/ismej.2011.109. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong TN, Wang X, Nakatsu G, et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology. 2018. Aug 1;155(2):383–90. 10.1053/j.gastro.2018.04.028. Medline: [DOI] [PubMed] [Google Scholar]
- 12.Tikly M, Njobvu P, McGill P. Spondyloarthritis in sub-Saharan Africa. Curr Rheumatol Rep. 2014;16(6):421. 10.1007/s11926-014-0421-z. Medline: [DOI] [PubMed] [Google Scholar]
- 13.Sono T, Takemoto M, Shinohara K, Tsuchido Y. An uncommon case of pyogenic spondylodiscitis caused by Gemella morbillorum. Case Rep Orthop. 2018;2018:3127613. 10.1155/2018/3127613. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberger U, Brunkhorst R, Perharic L, Petersen R, Kliem V. Gemella morbillorum spondylodiscitis in a patient with a renal graft. Nephrol Dial Transplant. 1998;13(6):1565–7. 10.1093/ndt/13.6.1565. Medline: [DOI] [PubMed] [Google Scholar]
- 15.Roche M, Smyth E. A case of septic arthritis due to infection with Gemella morbillorum. J Infecti. 2005;51(3):e187–9. 10.1016/j.jinf.2005.01.009. Medline: [DOI] [PubMed] [Google Scholar]
- 16.Gens LM, Benítez AB, Nieto JA. Infection of a total hip arthroplasty due to Gemella morbillorum. Enferm Infecc Microbiol Clín. 2007;25(8):553. 10.1157/13109993. Medline: [DOI] [PubMed] [Google Scholar]


