ABSTRACT
Objective: Analyzing protein kinase C (PKC) alpha, iota, and zeta as well as levels of Mxi-2 and Vim3 in regressive clear cell renal carcinomas (ccRCCs) and urine samples.
Material and methods: Fresh samples of ccRCCs (predominantly pT1a/b) with different degrees of regression (<10%, 30%, 50%, and ≥70%) vs normal renal tissue and oncocytomas were studied by Western blot, using antibodies of different PKC isoforms. Urine samples from these tumors were analyzed by ELISA (PKC isoforms, Mxi-2, and Vim3).
Results: With increasing degree of regression beyond 10%, nuclear Mxi-2 and Vim3 were highly overexpressed in fresh tumor samples. In urine samples, Vim3 was significantly overexpressed in oncocytoma and downregulated in RCCs with 70% regression. Western blot analysis shows that PKC alpha and iota levels were significantly increased in fresh tumor tissue samples (tumors with ≥ 30% regression). PKC zeta was expressed in normal kidney and significantly increased in oncocytoma but not found in ccRCCs. In patients’ urines, Mxi-2 was significantly reduced (regression > 50%), while PKC isoform alpha was significantly increased by advanced regression rate. PKC iota in patients’ urine was overexpressed in oncocytoma and reduced in all ccRCC urines.
Conclusion: Tumor regression in ccRCC tissue shows strong nuclear overexpression of Mxi-2, Vim3, and PKC alpha and iota. In respective urines, PKC alpha was overexpressed; PKC iota was decreased. Mxi-2 and Vim3 decreased with increasing regression rates. These reagents could serve as noninvasive ccRCC markers for regression.
Keywords: Clear cell renal carcinoma, Mxi-2, PKC isoforms, regression, Vim3.
Introduction
According to the WHO in 2020, renal cancer has been reported to cause about 179,000 deaths worldwide (males 115,000; females 64,000). Of these, clear cell renal carcinoma (ccRCC) represents the most prevalent of all malignant primary renal tumors.1 Morphologically, many of these tumors undergo disintegration of their tissue by necrosis, hemorrhage, and scarring. In our biobank, this regression was found in about 68% of all ccRCCs.
We have recently demonstrated a novel mechanism of miRNA regulation by miRNA15a binding on the DNA level. This binding results in two terminally truncated, biologically active proteins including intron sequences2: Mxi-2 and Vim3. Mxi-2,2 Max interacting protein-2, being the product of miRNA15a-induced truncation of MAPKp38 alpha and belonging to the Myc family, is together with the miRNA itself a potential urinary tumor marker in RCC patients.3 Furthermore, in renal carcinoma, a transcription complex, consisting of E-26 transformation-specific transcription factors 1, extracellular signal-regulated kinase-2, and Mxi-2, leads to increased p16INK4a protein expression,4 being involved in a better prognosis of RCC.5 While the expression leads to an improved disease progression,6 downregulation is observed in high degrees of regression.3 Vimentin 3 (Vim3) is the truncated version of the full-length vimentin produced by DNA binding of miR 498.7 The truncation of the C-terminal ending prevents the tetramer formation of intracellular vimentin. The cytoskeleton is disrupted and allows an increased movement and larger number of organelles,6 such as mitochondria. The increased number of mitochondria has been described as morphologic hallmark of the benign kidney tumor, oncocytoma.7 Both, an increased expression of Mxi-2 and Vim3, have already been shown to be detectable in urine samples from kidney tumor patients.8
Analyzing the protein kinase C (PKC) pathway, we found different members of the PKC family9 expressed in ccRCCs versus benign oncocytoma.3 However, PKC alpha (classical), zeta, and iota (atypical) were only expressed in oncocytoma.6 Looking at highly regressed ccRCCs, we observed an unsuspected PKC alpha expression corresponding to decreasing urinary miRNA15a levels.3 Since PKC alpha seemed to play such a pivotal role, we studied whether in ccRCC, different degrees of regression influenced intracellular and urinary levels of PKC alpha versus zeta and iota.
Material and Methods
Patient Collective
The collective originated from the archives of the Department of Urology, University of Cologne (in total 151 tumors, of which 28 were selected: six cases each of oncocytoma, RCC with less than 10% regression, RCC with 30% regression, RCC with or above 50% regression, and four cases of RCC 70% or more regression). Normal control tissue was taken from nephrectomy specimens indicated by + in Table 1. All comparisons made are in reference to the data obtained from these normal controls. The oncocytoma cases were important as subgroup and “positive” control for known strong Vim3 expression.7 All tumors were diagnosed using H&E staining and immune histology,10 being classified according to the current tumor, nodes, and metastases (TNM) classification.11 At the time of operation, tissue samples were acquired from tumors of patients who had presigned the BIOMASOTA agreement, agreeing that their tumor samples being used for research. Fresh tumor tissue collected directly from the OR table was collected and diced, and about 10 mg each of three cryo vials was snap frozen in liquid nitrogen and stored at –80°C to be used for protein extraction. Normal cortical tissues (10 mg each; two cryo vials) were snap frozen from nephrectomy specimens.
Table 1.
Patients’ Data
| # | Age | Gender | Tumor Diag. | Tumor Size | Regression | Tumor Grade | ISUP Grade |
|---|---|---|---|---|---|---|---|
| * | 80 | F | Oncocytoma | 2.5 cm | ≤5% | ||
| 2 | 61 | M | Oncocytoma | 1.8 cm | ≤5% | – | – |
| 3 | 63 | M | Oncocytoma | 1.4 cm | ≤5% | – | – |
| 4 | 58 | M | Oncocytoma | 5.0 cm | ≤5% | − | – |
| 5 | 56 | F | Oncocytoma | 3.1 cm | ≤5% | − | – |
| 6 | 67 | F | Oncocytoma | 1.2 cm | ≤5% | − | – |
| 7 | 50 | F | ccRCC | 3.5 cm | <10% | pT1a | 2 |
| 8 | 58 | M | ccRCC | 1.9 cm | <10% | pT1a | 1 |
| 9 | 55 | F | ccRCC | 5.0 cm | <10% | pT1b | 2 |
| 10 * | 53 | F | ccRCC | 5.0 cm | <10% | pT1b | 1 |
| 11 | 63 | F | ccRCC | 1.0 cm | <10% | pT1a | 1 |
| 12 | 45 | M | ccRCC | 1.8 cm | <10% | pT1a | 1 |
| 13 † | 79 | F | ccRCC | 16.0 cm | 30% | pT2b | 2 |
| 14 | 77 | M | ccRCC | 5.0 cm | 30% | pT1b | 1 |
| 15 * | 53 | M | ccRCC | 4.5 cm | 30% | pT1b | 1 |
| 16 † | 71 | M | ccRCC | 6.8 cm | 30% | pT1b | 2 |
| 17 | 48 | F | ccRCC | 4.5 cm | 30% | pT1b | 1 |
| 18 | 83 | M | ccRCC | 5.5 cm | 30% | pT1b | 1 |
| 19 † | 84 | F | ccRCC | 11.0 cm | ≥50% | pT2b | 1 |
| 20 | 82 | M | ccRCC | 5.0 cm | ≥50% | pT1b | 2 |
| 21 | 66 | M | ccRCC | 3.5 cm | ≥50% | pT1a | 2 |
| 22 | 67 | M | ccRCC | 3.8 cm | ≥50% | pT1a | 1 |
| 23*,† | 86 | M | ccRCC | 7.0 cm | ≥50% | pT3a | 2 |
| 24*,† | 62 | M | ccRCC | 7.0 cm | ≥50% | pT1b | 2 |
| 25 | 55 | M | ccRCC | 1.5 cm | ≥70% | pT1a | 2 |
| 26 † | 57 | M | ccRCC | 8.0 cm | ≥70% | pT2a | 2 |
| 27 * | 62 | M | ccRCC | 5.5 cm | ≥70% | pT1b | 2 |
| 28 | 86 | M | ccRCC | 4.5 cm | ≥70% | pT1b | 2 |
| * WB |
Cases used in Western blot.
Normal control tissue from nephrectomy specimens.
Urine samples were collected before surgery and stored at –80 °C. To determine regression, all tumors of up to 7.0 cm were sliced in less than 2 mm thick sections, fixed in 4% formalin, and completely embedded in paraffin. Regression grade was determined by adding the percentage of each area of disintegration per section divided by the number of total sections taken. In tumors exceeding 7.0 cm, the area of regression was grossly estimated using one tumor sections per centimeter of tumor. This study was preapproved by the ethics committee of the University of Cologne (20-1627).
Since human tissue from patients has been used, we hereby declare that the Declaration of Helsinki was observed, and an informed consent was obtained from any patent prior to his operation in the so-called BIOMASOTA agreement (Biologic Material Sampling for Optimization of Therapeutic Approaches).
Nuclear Extract and Cytoplasm Isolation
Extraction of the nuclear and cytoplasm fraction was done by the Nuclear Extract Kit (Active Motif Rixensart, Germany). Samples were washed with ice-cold PBS containing phosphate inhibitors and ground using a pestle. A 200 mL of 1× hypotonic buffer was added. The manufacturer’s protocol was followed. Protein concentration of both fractions was determined using a Bradford assay.12
Western blot analysis
Western blot analysis from tissue samples was done in triplicate and analyzed using the INTAS Chemostar as previously outlined.13,14 A 25 µg of total protein was used for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading. PKC alpha (sc8393), iota (sc17837), and zeta (sc17787; all from Santa Cruz12) antibodies and ß-actin (as loading control; sc374015; Santa Cruz) were used. In the Western blot, no tissue extracts were mixed. Each was taken from a single biopsy; different patients without any cancer history were used as a control.
Elisa
For PKC detection, ELISA plates were washed with 1×PBS, and 50 µL of patient urine was incubated for 1 h at RT. The primary PKC antibodies used were from Santa Cruz Technology, Heidelberg, Germany, pretested for specificity with designated peptides.3 Antibodies-detecting PKC isoforms (alpha, iota, zeta; dilution 1:500), Mxi-2 (nanoTools, clone 2F2) and Vim3 (Davids Biotechnologie GmbH, Regensburg) were employed overnight (4 °C). After two PBS washes, wells were incubated with the mouse-HRP (horseradish peroxidase) labeled secondary antibody (1 h, RT). Tetramethylbenzidin (TMB) was used according to the manufacturer’s protocols. For ELISA analysis, the FlouroStar Omega reader was utilized.13
Statistical Analysis
Analysis of variance (ANOVA) was performed using the GraphPrism 5 (San Diego, California, USA) program. Significant differences were calculated (*P < .05, **P < .01, ***P < .001). Normal distribution of patient data was calculated. Spearman rank correlation coefficient was performed for Mxi-2 and PKC alpha in nuclear extracts. An ROC analysis was done regarding data of Vim3 and PKC iota in nuclear extracts from oncoctoma versus ccRCC <10% using the GraphPad Prism 9 program (San Diego, CA, USA). Data were analyzed using box plots (***P < .0001, **P < .001, *P < .01; lower whisker: minimal value; upper whisker: maximal value; box = values between 1 and 3 quartile; dividing middle line = mean value; divides quartiles).
Results
Morphologic Regression of Clear Cell Carcinoma
In our biobank, 151 primary renal tumors (excluding urothelial carcinoma of the renal pelvis) were analyzed; of which, 79 are ccRCCs (52.3%). These were classified by their degree of regression. Tumor regression as a morphological term is defined by the destruction of the tumor’s structure under the appearance of necrosis, massive hemorrhage, and inflammation, as well as subsequent restructuring by scaring.3 We found nonregressive tumors (less than 10%; n = 25; 31.6%), minor regression (30%; n = 27; 34.2%), moderate regression (50%; n = 20; 25.3%), and major regression (≥70%; n = 7; 8.8%). Of these, three tumors were beyond 7.0 cm, where the degree of regression was determined macroscopically (one each of ≤30%, 50%, and >75%). All tumor cases used were age-wise normally distributed (ANOVA: F = 0.54 for F0.1/4/23 = 2.206). The cases from our biobank are unique in that they represent predominantly pT1a/b grades of ccRCCs, while tumors with advanced grades (2b, 3a) are the exception. However, the advantage of this setting is the possibility of a histologic analysis of the entire tumor, thus providing an exact degree of regression, which has not been done previously. It shows that even in small tumors, regressive changes without aggressive behavior (no metastases at time of operation or up to 2 years after) are present in two-thirds of cases, more than commonly thought. Thus, OS was 100% at the time; PFS is still at 100%, even in the few cases with larger tumors.
Screening of RCCs with Different Regressions for The Expression of PKC Alpha, Iota, and Zeta in Nuclear Extracts
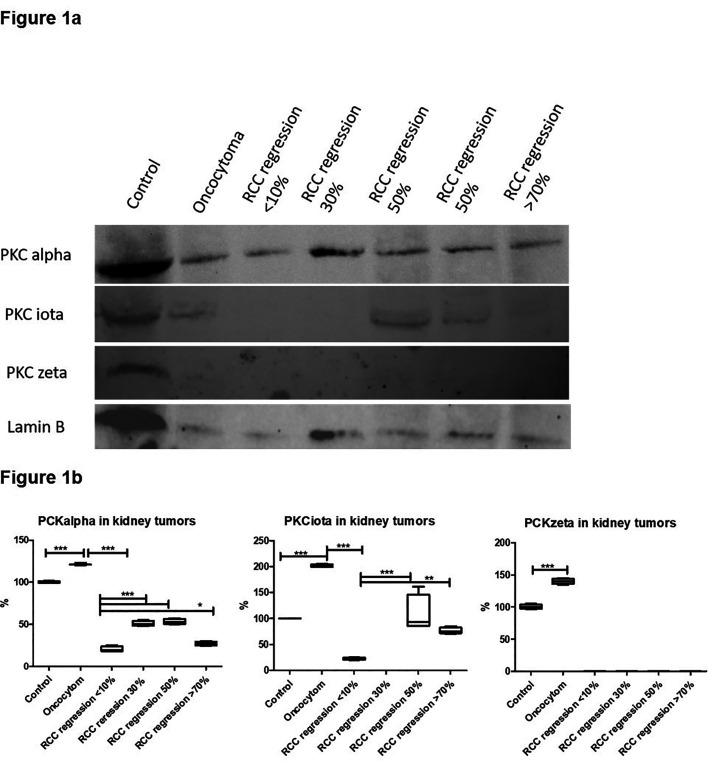
In ccRCCs according to their regression, an initial screening of the expression of different PKCs was determined via Western blot. Normal renal cortical tissue (control) and oncocytoma were served as control. The signal for PKC alpha was maximum at 30% and 50% and statistically highly significant in comparison to the levels of nonregressive RCCs. Otherwise, its levels corresponded to about 50% of normal renal tissue and declined but was still elevated at regression rates of 70% and more. PKC iota was detectable at 50% and higher regression rates, i.e., approximately at control levels. PKC zeta was identified in renal cortical tissue, being significantly increased in oncocytoma but being not detectable in ccRCC samples (Figure 1a and b).
Figure 1.
Western blot and expression in percent of normal tissue in RCCs with varying degrees of regression oncocytoma as controls. (a) Western blot of cytoplasmic extract using RCCs with different degrees of regression, oncocytoma, and control tissue sample showing the expression of PKC isoforms. (b) Box blot showing analysis of PKC isoforms in percent of expression of normal renal tissue being equal to 100%.
Proof of PKC Isoform Expression in Nuclear Extracts via ELISA
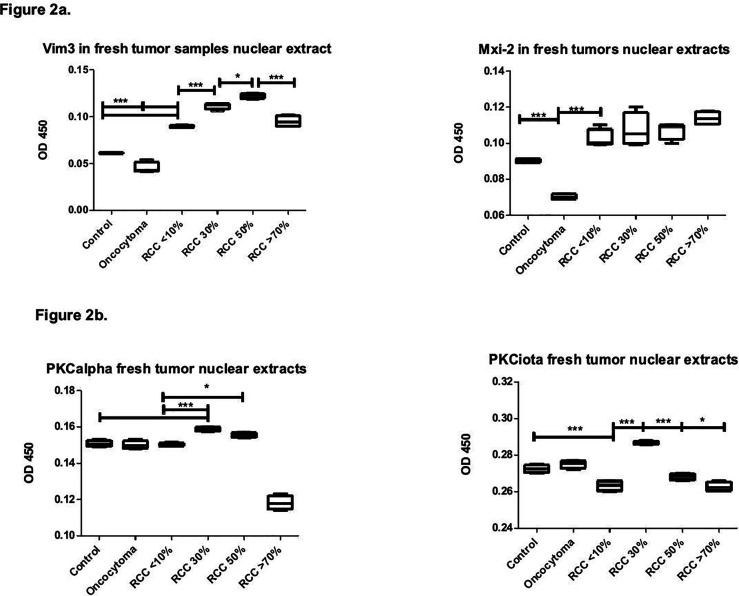
Initial data from the Western blot were confirmed by an ELISA. PKC alpha levels were highly significant beyond 30% regression while higher disintegration of tumor samples showed levels below normal (Figure 2a). PKC iota levels were also upregulated by 30% regression but not significantly upregulated in regressive states equal to or beyond 50%. Nonregressive tumors displayed highly significantly lower levels of PKC iota than tumor regression (30%), oncocytoma, or normal control renal tissue (Figure 2a), while there was no statistical significance versus higher regression levels.
Figure 2.
Fresh tumor nuclear extracts: Box blot showing PKC alpha and iota, Vim 3 and Mxi-2 detection in RCCs with varying degrees of regression vs normal renal tissue as negative controls and oncocytoma as positive controls. (a) PKC alpha and iota in fresh tumor nuclear extracts. (b) Vim3 and Mxi-2 in fresh tumors nuclear extracts.
ELISA of Mxi-2 and Vim3 in Nuclear Extracts from Fresh Tumors
The expression of the novel marker for the differentiation between RCC and oncocytoma showed that Vim3 levels were significantly upregulated in regression rates from 30% to over 50%. In contrast, in all ccRCCs, levels were highly significantly elevated over those of control tissue and in oncocytoma. Mxi-2 was also upregulated in all ccRCCs and clearly above the levels for normal tissue and oncocytoma, with increasing but not statistically significant tendency in higher regression rates (Figure 2b). This is supported by the Spearmann–Rho test with increasing and reverse relation between Mxi-2 and PKC alpha (control cases Rs = –0.135; Onco Rs = –0.245; ccRCC <10% Rs = –0.21; ccRCC 30% Rs = –0.99; ccRCC >50% Rs = –0.40) but no longer in ccRCC >70% (Rs = +0.87). The relationship between Vim3 and PKC iota and between the oncocyzoma and the ccRCC <10% was analyzed by ROC analysis, showing that all data from nuclear extracts from the oncocytoma had higher values for PKC iota and lower values for Vim3 than those from ccRCC <10% (data not shown).
Expression of PKC Isoforms in Patient Urine
To identify a potential marker for ccRCC regression, patient urine from the different groups was analyzed via ELISA. Table 2 shows the comparison of mean values from urines of PKC alpha, iota, Vim3, and Mxi-2 detection.
Table 2.
Comparison of Mean Values from Urines of PKC Alpha, Iota, Vim3, and Mxi-2 Detection
| PKC Alpha | PKC Iota | Vim3 | Mxi-2 | |
|---|---|---|---|---|
| Controls | 1.0 | 1.2 | 1.0 | 1.4 |
| Oncocytoma | 1.2 | 2.0 | 2.3 | 1.0 |
| <10% | 0.8 | 0.9 | 1.1 | 1.4 |
| 30% | 1.8 | 1.2 | 1.1 | 1.35 |
| ≥50% | 1.5 | 1.0 | 1.2 | 1.3 |
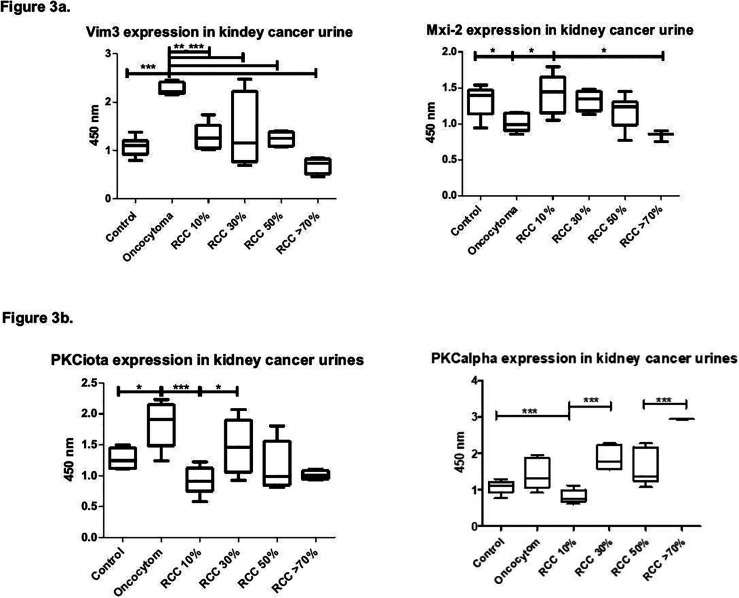
PKC alpha levels increased with tumor regression and were highly significantly upregulated in tumors equal to or beyond 75% regression. PKC iota showed no significant difference between regressive tumors of 30% or more. However, its level was statistically significantly different between normal cortical tissue, nonregressive ccRCCs, and oncocytoma (Figure 3a).
Figure 3.
Kidney urines: Box blot showing PKC alpha and iota, Vim3 and Mix-2 in RCCs with varying degrees of regression vs normal renal tissue and oncocytoma as controls. (a) PKC alpha and iota in kidney cancer urines. (b) Vim3 and Mxi-2 in kidney cancer urines.
ELISA Analysis of Mxi-2 and Vim3 in Urine from Tumor Patients
Since Mxi-2 and Vim3 are noninvasive biomarkers for the differentiation of kidney tumors, ELISAs from urine samples were performed. Levels of Vim3 and Mxi-2 declined slowly with increasing rates of regression (Figure 3b), with Vim3 being lowest in tumors with high regression. As published previously, Vim3 levels were also increased in oncocytoma.8 Mxi-2 slowly declined with an increasing regression rate.
Discussion
This investigation analyzes the expression levels of different PKC isoforms and the truncated proteins Mxi-2 and Vim3, in both nuclear extracts and urine, as potential biomarkers to detect different degrees of tumor regression in ccRCCs.
We predominantly analyzed nuclear extracts from fresh kidney tumors for the expression of the different PKC isoforms. As we have published previously, the nuclear overexpression of PKC alpha15 is involved in miR-15a expression, which, in turn, is responsible for the truncation of MAPK p38 to Mxi-2.2,3 In our opinion, the nuclear presence of PKC isoforms is involved in several different processes.
In nuclear extracts from normal cortical tissue and in oncocytoma, we observed all three isoforms: PKC alpha, iota, and zeta via Western blot (Figure 1a and b) and via ELISA (Figure 2a). The observed levels in the nuclear extract varied considerably compared to those in the control tissue (set as 100%): the nuclear fraction of PKC alpha was about 25% higher; PKC iota 100%, and PKC zeta 50% higher than those in the controls of normal cortical renal tissue (Figure 1b). Via ELISA from the nuclear extracts, there was no significant difference between the amounts of PKC alpha detectable. In the case of PKC iota (Figure 2a, right panel), a decrease was detectable.
This result is in contrast to the one from Engers et al16 analyzing PKC isoforms in four different cell lines established from human ccRCCs. In particular for PKC alpha, we found it upregulated in comparison to normal tissue only in regressive tumors, but not in nonregressive tumors. Here, Engers et al argued that the observed increase in membranous expression meant a special role of PKC alpha in human invasion. However, in cancer urines, while we could show a distinct PKC alpha upregulation, it was clinically not accompanied by more aggressiveness within the last 2 years after tumor resection.
While we have shown that one main function of PKC alpha is present in the cell nucleus, we decided to analyze also urine samples, to get an idea whether the expression is as well detectable in patients’ urines.
Comparing the different PKC isoform values from control and oncocytoma in urine, PKC iota reached the highest standard mean value of all investigated tumors (Figure 3a, right panel). In samples from patients with ccRCC and regression, the levels were significantly downregulated, however, with increasing levels correlating to increasing regression rates.
PKC alpha was only slightly increased in oncocytoma vs control (Figure 3a, left panel). By increasing the regression of ccRCCs above 10%, PKC alpha levels in the urine are clearly increasing above the levels of oncocytoma. This correlates with our previous findings, where, in tumors with a regression above 50%, PKC alpha levels increase and miR-15a levels decrease.3
We know that Mxi-2 expression is dependent on the presence of PKC alpha in the nucleus. Therefore, we analyzed the Mxi-2 and Vim3 levels in the collective. As recently published, both markers can be used for the detection and differentiation of kidney tumors in urine samples.8 Vim3 levels in urine were significantly upregulated in oncocytoma as previously published (Figure 3b).8 In urine samples, Mxi-2 levels declined with increasing regression rates (Figure 3b), the lowest levels at or beyond 70% regression.
PKC iota is believed to be responsible for aggressive tumor behavior by activating the NF-kB signaling cascade through IkB degradation, as was shown in cell culture (prostate carcinoma17 and melanoma).18 Furthermore, in melanoma cells, PKC iota interacted with vimentin, which could be coimmunoprecipitated with vimentin during epithelial-mesenchymal transition.18
By binding the tail to the head region, vimentin phosphorylation at S39 is reported to lead to an increase in cancer cell adhesion and invasion.19 Expecting similar behavior in RCCs, we analyzed PKC iota levels in different regressive stages. We found, however, that these levels were very low and, thus, highly significant in the nuclear extract of nonregressive tumors (Figure 1a and 1b). In nonregressive carcinomas and in higher degrees of regression, the levels were clearly downregulated, also being significantly lower than in control renal tissue or in oncocytomas (Figure 2a). Accordingly, in the urine samples, the PKC iota levels were on average as low as in the control tissue, while being lowest at the highest regression level (Figure 3a). Thus, in ccRCCs, PKC iota levels do not provide a discriminating marker in urine samples.
PKC zeta has been described in the digestive and the respiratory tract,20 being a predictor for poor outcome in breast and lung cancer.21 While we could identify this isoform in renal cortical tissue, we could not detect it in ccRCCs with or without regressive changes.
Potential limitations of this study are that the cases represent predominantly pT1a/b grades of ccRCCs, while tumors with advanced grades (2b and 3a) are the exception. These seem the ones, where there is a major change in the expression of the factors investigated. None of the markers used, however, was exclusively found for any particular tumor subtype/degree of regression. For clinical use, cutoff levels for urinary makers have to be confirmed in a larger study, which will clearly identify oncocytomas (high Vim3 and high PKC iota) and high regression ccRCCs (lowest level for Mxi-2 and highest level for PKC alpha).
In summary, all three PKC isoforms were present in normal cortical renal tissue with highest levels in oncocytoma (Figure 1b). In urines, the highest levels of Vim3 and PKC iota were detected from oncocytoma patients, being markers of choice for differentiation from ccRCCs ± regression and normal renal tissue. High levels for PKC alpha and low values for Vim3 as well as Mxi-2 are indicative of high levels of RCC regression (>70%), while declining Mxi-2 levels go along with increasing PKC alpha levels.
Main Points
To find noninvasive markers for clear cell renal cell carcinoma (ccRCC) with different degrees of regression, defined as tumor disintegration (hemorrhage and necroses scarring), we analyzed PKC alpha, iota, and zeta as well as levels of Mxi-2 and Vim3 in tumors (predominantly pT1a/b) and in corresponding urine samples by Western blot and ELISA.
In tumor tissue, PKC alpha, iota, and zeta high in oncocytoma, low in all ccRCCs. In nuclear extracts in ccRCCs with increasing degree of regression, Mxi-2 and Vim3 were highly overexpressed, low in oncocytoma.
In patients’ urines, Mxi-2, Vim3, and PKC iota were significantly reduced, while PKC isoform alpha was significantly increased by advanced regression rate. In oncocytoma, Vim3 and PKC iota were overexpressed.
PKC zeta only found in normal tissue and overexpressed in oncocytoma.
Conclusion: To diagnose oncocytoma in urines, use Vim 3 and PKC iota. In urines from highly regressive RCCs, PKC alpha is very high; Mxi-2, Vim3 and PKC iota decrease with increasing regression levels.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the University Hospital of Cologne (approval ID: 20-1762).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - B.K., J.W.U.F.; Design - M.V.B., J.W.U.F.; Supervision - A.H.; Materials - M.V.B., A.H.; Data Collection and/or Processing - B.K., M.H., M.V.B., J.W.U.F.; Analysis and/or Interpretation - J.W.U.F.; Literature Search - J.W.U.F.; Writing Manuscript - J.W.U.F.; Critical Review - B.K., M.H., M.V.B., A.H.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The project was funded by the Departmental Funding of the Department of Urology, University Hospital Cologne.
References
- 1.National Cancer Institute, Center for Cancer Research: Clear cell renal carcinoma. Website. March 2020.
- 2. von Brandenstein M, Bernhart SH, Pansky A.et al. Beyond the 3'UTR binding-microRNA-induced protein truncation via DNA binding. Oncotarget. 2018;9:32855–32867.. 10.18632/oncotarget.26023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Brandenstein M, Pandarakalam JJ, Kroon L.et al. MicroRNA 15a, inversely correlated to PKCalpha, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol. 2012;180:1787–1797.. 10.1016/j.ajpath.2012.01.014) [DOI] [PubMed] [Google Scholar]
- 4. von Brandenstein M, Schlosser M, Richter C, Depping R, Fries JW. ETS-dependent p16INK4a and p21waf1/cip1 gene expression upon endothelin-1 stimulation in malignant versus and non-malignant proximal tubule cells. Life Sci. 2012;91:562–571.. 10.1016/j.lfs.2012.04.014) [DOI] [PubMed] [Google Scholar]
- 5. Ikuerowo SO, Kuczyk MA, von Wasielewski R.et al. p16INK4a expression and clinicopathologic parameters in renal cell carcinoma. Eur Urol. 2007;51:732–737.. 10.1016/j.eururo.2006.08.010) [DOI] [PubMed] [Google Scholar]
- 6. Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem. 2015;290:17145–17153.. 10.1074/jbc.R115.640359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. von Brandenstein M, Puetz K, Schlosser M.et al. Vimentin 3, the new hope, differentiating RCC versus oncocytoma. Dis Markers. 2015;2015:368534. 10.1155/2015/368534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Brandenstein M, Herden J, Köditz B.et al. Non-invasive urine markers for the differentiation between RCCs and oncocytoma. J Clin Lab Anal. 2021;35:e23762. 10.1002/jcla.23762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;69:484–496.. 10.1096/fasebj.9.7.7737456) [DOI] [PubMed] [Google Scholar]
- 10. Tickon SK, Vankalakunit M, Reuter VE. Renal cell carcinoma. In Aman MR. (ed.): Diagnostic Pathology: Genitourinary. Salt Lake City, UT: Amirsis Publ., 2010: 1-24–1-46.. [Google Scholar]
- 11. Brierley JD, Gospodarowicz MK, Wittekind CH. (eds.). Classification of Malignant Tumors. 8th ed. Weinheim: Wiley-VCH, 2017. [Google Scholar]
- 12. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.. 10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- 13. Köditz B, Fries JWU, Göbel H.et al. Mxi-2 dependent regulation of p53 in prostate cancer. Anticancer Res. 2020;40:5539–5544.. 10.21873/anticanres.14566) [DOI] [PubMed] [Google Scholar]
- 14. Köditz B, Stog A, Göbel H.et al. Vimentin 3 expression in prostate cancer cells. Anticancer Res. 2021;41:169–174.. 10.21873/anticanres.14762) [DOI] [PubMed] [Google Scholar]
- 15. Gerstung von Brandenstein M, Abety AN, Depping R.et al. A p38-p65 transcription complex induced by endothelin-1 mediates signal transduction in cancer cells. Biochim Biophys Acta. 2008;1783:1613–1622.. 10.1016/j.bbamcr.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 16. Engers R, Mrzyk S, Springer E.et al. Protein kinase C in human renal cell carcinomas: Role in invasion and differential isoenzyme expression. Br J Cancer. 2000;82:1063–1069.. 10.1054/bjoc.1999.1043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Apostolatos AH, Ratnayake WS, Win-Piazza H.et al. Inhibition of atypical protein kinase C‐ι effectively reduces the malignancy of prostate cancer cells by downregulating the NF-κB signaling cascade. Int J Onco. 2018;53:1836–1846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ratnayake WS, Apostolatos CA, Apostolatos AH.et al. Oncogenic PKC-ι activates vimentin during epithelial-mesenchymal transition in melanoma; a study based on PKC-ι and PKC-ζ specific inhibitors. Cell Adh Migr. 2018;12:447–463.. 10.1080/19336918.2018.1471323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eriksson JE, He T, Trejo-Skalli AV.et al. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–932.. 10.1242/jcs.00906) [DOI] [PubMed] [Google Scholar]
- 20. Osada S, Hashimoto Y, Nomura S.et al. Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993;4:167–175.. [PubMed] [Google Scholar]
- 21. Zurgil U, Ben-Ari A, Rotem-Dai N.et al. PKCeta is an anti-apoptotic kinase that predicts poor prognosis in breast and lung cancer. Biochem Soc Trans. 2014;42:1519–1523.. 10.1042/BST20140182) [DOI] [PubMed] [Google Scholar]