Abstract
Adeno-associated viruses (AAVs) are viral vectors that offer an excellent platform for gene therapy due to their safety profile, persistent gene expression in non-dividing cells, target cell specificity, lack of pathogenicity, and low immunogenicity. Recently, gene therapy for genetic hearing loss with AAV transduction has shown promise in animal models. However, AAV transduction for gene silencing or expression to prevent or manage acquired hearing loss is limited. This review provides an overview of AAV as a leading gene delivery vector for treating genetic hearing loss in animal models. We highlight the advantages and shortcomings of AAV for investigating the mechanisms and preventing acquired hearing loss. We predict that AAV-mediated gene manipulation will be able to prevent acquired hearing loss.
Keywords: AAV-mediated gene therapy, Acquired hearing loss, AAV transduction in vivo
INTRODUCTION
Auditory processing in the cochlea depends largely on the integrity of the sensory hair cells, mechanosensors of the inner ear, located on the basilar membrane. Numerous risk factors for hair cell damage can accumulate over a lifetime, such as noise trauma, exposure to ototoxic medications, bacterial or viral ear infections, and the aging process [1]. Damage to sensory hair cells is a common pathological feature of acquired sensorineural hearing loss [2–4]. Since mammalian hair cells do not regenerate, such hearing loss is permanent.
The suffering from and cost of acquired hearing loss highlight its prevention as a high-priority health concern [5]. However, no approved pharmaceutical therapy is currently available in the clinic due partly to the complexity of the cell death pathways induced by noxious challenges and the uncertainty of potential drug targets [3, 6]. The optimal delivery route presents another major challenge as systemic treatment might require large dosages to achieve therapeutic levels in the inner ear and increase the risk of side effects, even when the protective agents can cross the blood-labyrinth barrier to reach the cochlea [7]. A local application can circumvent these problems but might require surgical intervention.
To date, adeno-associated virus (AAV)-based gene therapy has emerged as a method with great potential to treat inherited hearing loss in several animal experiments showing sustained hearing function improvement up to about 10 months of age in mice [8–10]. In particular, AAV-mediated treatments correct the loss of function of genetic defects by complementary expression of recombinant proteins in animal models [9, 11].
AAV is a benign residential virus in humans. Particularly with the second generation of recombinant AAV (rAAV) vector, by designing mutants of the AAV capsid to engineer the best features of the native virus and a deep understanding of the mechanisms of AAV integration, AAV has become one of the leading and most widely adopted gene delivery tools in the laboratory as well as in clinical application [12, 13]. Gene replacement therapy via AAV transfection as a novel strategy for treating genetic deafness has been recently reviewed [14–16]. AAV-based treatment for acquired hearing loss is, however, limited. This review summarizes AAV vectors as a gene therapy platform focusing on acquired hearing loss. Several laboratories, including ours, have successfully delivered naked small interfering RNA (siRNA) onto the round window membrane (RWM) of the middle ear for investigation of the mechanisms of acquired hearing loss in animal models [17–20]. Our previous publication detected fluorescence-conjugated siRNA in the inner ear 24–48 h after intra-tympanic delivery [20]. However, the activity of naked siRNA has a relatively short half-life, lasting about 4–7 days [21]. Combining AAV vector-based expression with RNAi technology can achieve the goal of stable and long-term silencing.
OVERVIEW OF ADENO-ASSOCIATED VIRUS
General Information on AAV
The laboratory of Bob Atchison at the University of Pittsburgh identified AAV as a replication-defective contaminant of adenovirus preparations, hence its name [22]. Wallace Rowe’s laboratory at NIH characterized AAV as a member of the parvovirus family, lacking the ability to replicate independently, and reported its physical, biological, and immunological features [22, 23]. AAV packages a single-stranded (ss) DNA of approximately 4.7 kilobases (kb). The small genome encodes three essential genes: rep (Replication), cap (Capsid), and aap (Assembly) that generate at least nine products by employing three promoters, alternative translation start sites (aTIS), and alternative splicing [24]. The rep gene encodes nonstructural proteins (Rep78, Rep68, Rep52, and Rep40) required for viral DNA replication and packaging. The cap gene encodes the capsid proteins (VP1, VP1/VP2, and VP3), which form the icosahedral capsid [25] that protects the viral genome and facilitates cell surface receptor binding and entry via endocytosis [13]. The cap locus also encodes aap, a nonstructural open reading frame, assembly activating protein (AAP) for protein scaffolding and capsid assembly in several AAV serotypes [26]. However, AAV4, 5, and 11 do not need AAP for capsid assembly [27]. AAP drives the localization of capsid protein to the nucleus in AAV2, but other serotypes localize diffusely in the nucleus [13].
Two inverted terminal repeats (ITRs) of 145 bases flank the coding sequences in AAV, which drives its replication and integration into a specific locus on chromosome 19. Chromosome 19 is one of 23 pairs of chromosomes and has the highest gene density of all human chromosomes, with more than double the genome-wide average [28]. The ITR sequence is an essential region required in cis for packaging the AAV vector, and a second region — cis-acting Rep-dependent element (CARE) — promotes replication in cis [29]. Typical rAAV vectors eliminate the other factors required for DNA replication — rep and capsid formation–cap — as these can function in trans. After removing the original viral DNA, most rAAV vectors are genetically engineered from naturally existing capsids to enhance the transduction, limit integration, and overcome immunity for broader clinical applications [30]. In preclinical studies, extensive testing has established AAV’s safety providing an excellent starting point for further refinement [31].
Serotypes
AAVs mainly interact with carbohydrates to target cells, including sialic acid, galactose, and heparin sulfate [32, 33]. AAV cap is the major determinant of AAV cell tropism, as capsid sequence variants interact with unique receptors on target cells, making AAV serotypes valuable cell-targeting research tools [34]. Recently, a publication has summarized AAV capsid transduction into the mouse and primate inner ear [35]. The capsid proteins include conserved parts that maintain basic structural function and artificially designed elements that enhance transduction efficiency and improve cell targeting [36]. So far, molecular cloning and serologic methods have identified more than 100 different AAV serotypes in humans, primates, and other species. Of these, current gene therapy viral vectors use 12 different AAV serotypes (AAV1-12) and their derivatives. AAV2 was the first isolated virus vector showing natural tropism towards neurons, muscles, and hepatocytes. Similarly, AAV8 effectively transfects the liver of rodents [37], and AAV9 delivers genes of interest to the skeletal and cardiac muscle [38]. To further refine target specificity and immune system avoidance, capsid and other genomic sequences from different AAV pseudotypes are combined to generate new variants. Since AAV2 has been the best-characterized vector, it serves as the basis for most AAV development. For example, the AAV2 plasmid packaged into an Anc80 capsid, defined as (AAV2/Anc80), efficiently targets cardiac and kidney tissue [39] and extends its range to the inner ear and optical structures [40, 41]. Likewise, AAV2 combined with the AAV2 capsid (AAV2/2) preferentially targets the neural system [42].
To efficiently deliver and express genes in specific regions of interest, such as the cochlear sensory hair cells and the outer retina [43], in vivo-directed evolution allows the selection of additional useful variants [44]. One such effort injected three AAV libraries containing 7-amino-acid inserts into the vitreous humor and selected for green fluorescent protein (GFP) reporter expression in the outer retina [45]. The approach identified AAV2.7m8 encoding the sequence LALGETTRP after residue 588 of the AAV2 capsid, which infected Chinese hamster ovary cells 100 times more efficiently, and efficiently expressed GFP in all major cell types of the retina, including retinal ganglion cells, bipolar cells, amacrine cells, Müller cells, horizontal cells, rods, cones, and the retinal pigmented epithelial cells. Moreover, AAV2.7m8 successfully treated mouse models of X-linked retinoschisis and Leber’s congenital amaurosis. Further evaluation of AAV2.7m8 delivery discovered that it infects the inner pillar cells and inner phalangeal cells along with both outer and inner hair cells, making it an excellent vehicle for gene delivery to all inner ear tissues [46].
AAV Plasmids
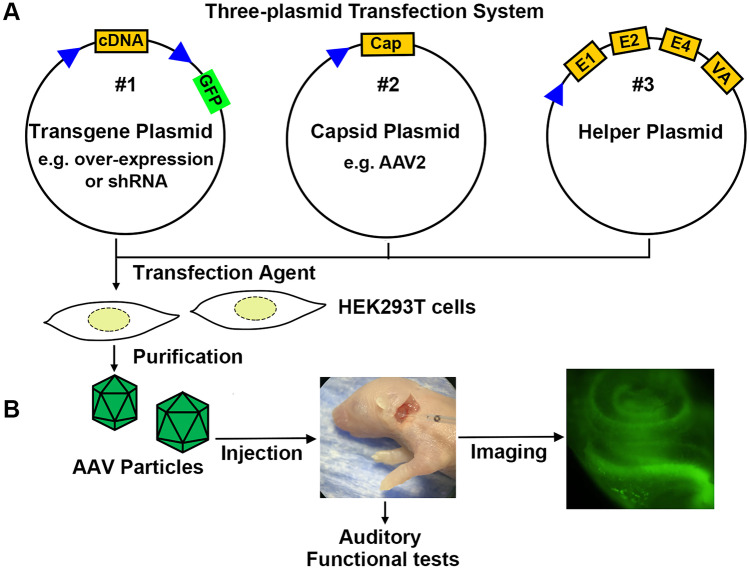
AAV vector systems generally use three plasmids for transfection: (1) a transfer plasmid containing the viral genomic elements, (2) Rep/Cap plasmid containing the structural and packaging genes, and (3) a helper plasmid containing the proteins needed for the virus to replicate. All three plasmids are essential for generating and packaging active AAV vectors [47]. The components of the AAV plasmid include a suitable promoter, a transgene cassette including the gene of interest, such as a short hairpin RNA sequence for silencing or a cDNA sequence for overexpression, a reporter (such as GFP, to follow the transfection), and a termination signal that includes signals for polyadenylation. The vector plasmid backbone contains appropriated cloning sites, such as a polylinker between the promoter and polyadenylation sequence. The Rep/Cap plasmid provides structural (Cap) and nonstructural (Rep) viral protein sequences. The choice of a Rep/Cap plasmid depends on the targeted cells, as discussed in the “Serotypes” section. In addition to Rep and Cap, AAV requires a helper plasmid containing adenovirus genes (E4, E2a, and VA) to support AAV replication. The schematic diagram illustrates the AAV vector (Fig. 1A).
Fig. 1.
Schematic flow diagram of AAV vector use for inner ear therapy. A Packaging of plasmids (#1) encoding the gene of interest and a GFP marker requires co-transfection into HEK293T cells with capsid (#2) and helper (#3) plasmids. The capsid plasmid determines AAV particle structure and subtype, and the helper plasmid encodes the adenoviral genes E1, E2, E4, and VA that facilitate viral packaging. B Purified AAV particles are micro-injected into the inner ear of neonatal mice. Imaging and physiological tests evaluate recombinant gene expression, and its functional consequences once the mice reach designated experimental ages
AAV Promoters
AAV vectors transfected in mammalian cells are comprised of several key elements: (1) an inducible or continuous promoter to provide strong transcriptional activity, (2) a transcriptional terminator to stabilize transcriptional products, such as mRNA or shRNA, that prevents post-transcriptional interference, and (3) a suitable reporter to identify infected cells [48, 49]. Since AAV only allows packaging of small inserts, elimination of the reporter accommodates a larger insert. The promoter contains elements that drive transcription of the desired insert and can be customized as discussed below. Commonly used promoters such as CMV (cytomegalovirus), EF1a (elongation factor 1a), SV40 (simian virus 40), and CAG (CMV enhancer fused to the chicken β-actin, or rabbit β-globin) drive constitutive gene expression in most host cell types. However, some of these constitutive promoters cannot maintain expression due to silence in certain cell types, for example, CMV promoter silencing in the central nervous system [50]. The CAG promoter is the strongest constitutive promoter in most cell types [51]. However, CAG is significantly larger than other promoters, making it unsuitable for packaging larger transgenes [52]. Inducible promoters exhibit low basal transcription levels but can have substantial induction by a non-toxic inducer. Inducible promoters are desirable for limiting the expression of toxic proteins or for experimental analysis of differential expression. Since mutation and the consequent loss of specific gene function lead to sensory hair cell dysfunction in most inherited hearing loss, most studies adopt constitutive promoters to drive continuous gene expression [14–16].
Packaging AAV Vectors
The vast availability of commercial kits and reconstructed packaging cells have greatly aided the laboratory development of AAV plasmids. Briefly, a workflow of production of an AAV vector includes plasmid design and production, cell expansion and transfection into packaging cells, viral particle production, purification, and formulation (Fig. 1A). Many AAV plasmid backbones are freely available from commercial companies, such as Addgene (https://www.addgene.org), further facilitating design and construction. HEK293T is the most common cell line used in the laboratory to produce AAVs [53]. However, other cells such as Sf9, BHK, and HeLa S3 can substitute for increasing safety or virus yield [53–55].
During packaging of AAV particles, a common by-product is empty capsids lacking an ITR-flanked transgene. Such empty AAV particles can significantly reduce infection efficiency [56]. The main approach to removing empty capsids relies on time-consuming density ultracentrifugation, with scale-up challenges for commercial purposes. Another effective method is to adjust the ratio between Rep/Cap and transgene plasmids. AAV-specific resins that target AAV subtypes based on viral binding specificity are now available. Anion exchange chromatography is commonly adopted after resin purification to remove empty capsids [18, 57]. These methods increase the purification efficiency (1013–1014 GC/mL) while reducing cost.
BENEFITS AND LIMITATIONS
Duration and Stability of Transfection
Most of the experimental results in animals show safe and stable AAV transfection. As a platform for gene therapy, AAV transfection of gene expression depends on AAV serotype, dose, and target cell type, and cap reconstruction enables high infection efficiency in specific tissues. AAV without Rep proteins has ITR-flanked transgenes that can form double-stranded (ds) circular episomes from the linear single-stranded virus DNA in the infected cellular nuclei [58]. AAV expresses stably as an episome in non-dividing cells but actively dividing cells lose expression during cell division and the consequent dilution due to the lack of synchronized replication. The stability and expression in non-dividing cells permit its use in the mammalian auditory sensory hair cells since they constitute stable postmitotic cells. Based on the characteristics of sensory hair cells, AAV is an ideal vector for its stability in transgene expression throughout the entire lifespan of the host cell.
AAV Rep proteins (Rep78 and Rep68) are essential for targeted vector integration into chromosomes. They mediate the integration of the virus transgene into human chromosome 19, increasing the stability of the transgene. However, the integration rate is as low as 0.1–0.5 % in animal experiments [59]. Removing the original rep gene blocks the targeted insertion of AAV in chromosome 19 but allows a rare random integration into any chromosome [60]. Such integration poses potential threats to the host genome, including deletion or insertion of genes. For example, experiments using mice detected AAV integration near or into nine oncogenic genes, raising concerns regarding its safety for clinical application [61]. In fact, in the absence of Rep proteins, viral genome integration into the human genome is rare because of the reduced copy number of the viral genome when host cells replicate [62].
Packaging Capacity
AAV is a small virus with a defined icosahedral structure that limits the size of the packaged genome to about 4.7 kb [25]. Thus, the packaging capacity of AAVs needs careful consideration for the expression of large proteins. Aside from the particular cDNA sequence, a single AAV transgene must include a suitable promoter, reporter, polyadenylation sequence, and other regulatory elements. However, this is not a major concern for most hearing-related applications, with a median human protein size of 375 amino acids [63], since the cDNA of about 78 % of the proteins associated with hereditary hearing loss are under the size limit of the AAV packaging capacity [14]. When it becomes necessary to express larger proteins, one may consider variants such as dual-AAV or triple-AAV to achieve gene delivery [64]. For gene silencing, the packaging capacity of AAV is easily sufficient, as described below (“Gene Silencing” section).
Immuno-Compatibility
The body’s reaction to rAAV includes innate and acquired immunity [65, 66]. However, newly developed AAVs have modified open reading frames (ORFs) to trigger a less robust host immune response [67]. Low immunogenicity may allow for long-term therapeutic gene expression after a single administration. Capsid and transgene-translated proteins induce the immune response [68, 69]. An effective strategy to alleviate the immune response is suppressing the viral-capsid-related immune reaction by adopting suitable serotype AAVs and suppressing host immunity. Innate immunity is nonspecific and reacts to the first exposure to AAVs by directly “catching” AAVs, e.g., by macrophages that engulf AAV particles, without the help of antibodies. This type of immune response is the most relevant for research since animals receive a single AAV injection rather than repeated AAV exposures. The mechanism of innate immune reactions to AAV is still unclear. Acquired immunity is associated with multiple exposures to the same serotype of AAV. Interestingly, AAV-specific antibodies occur naturally in 30–40 % of the human population, even without clinical AAV infection history [70]. In 2008, researchers showed that the complement system is essential for innate and acquired immune responses to AAV [71]. One may use several paradigms to limit acquired AAV immunity, such as using single-dose delivery methods or changing the AAV serotype for repeat delivery [72].
In recent decades, several labs have focused on developing suitable AAV serotypes for hearing research. Assessments of auditory function, such as auditory brainstem response (ABR), distortion product otoacoustic emissions (DPOAE), and counts of sensory hair cells, routinely test the safety of the serotype [41, 73, 74]. So far, research on the immune response in the inner ear is very limited. Many studies have used hematoxylin–eosin (HE) staining to identify inflammatory cells, but this may not be very sensitive. Evaluation of inflammatory cell infiltration into the cochlea revealed that AAV8BP2 causes more cochlear inflammation than AAV2.7m8 [46]. Additionally, prolonged overexpression of GFP (5 weeks after Anc80 transfection to adult CBA/CaJ mice) induces inner hair cell (IHC) loss [75]. Although such damage to IHCs may be due to GFP ototoxicity or a GFP-triggered immune response, one cannot rule out the potential immune responses to AAV-mediated treatment.
STRATEGIES FOR TREATMENT OF HEARING LOSS
Surgical Approach for Delivery of AAV into the Inner Ear
The inner ear is a relatively closed space surrounded by bone. It is comprised of two functionally distinct organs: the vestibular system and the cochlea. The vestibular system is responsible for balance and orientation in three-dimensional space, while the cochlea is dedicated to sound. The spiral-shaped structure of the cochlea separates into three fluid-filled cavities. The scala vestibuli and scala tympani contain perilymph fluid, similar to the cerebrospinal fluid. The scala media has endolymph with a composition distinct from the perilymph. There are two windows (the round and oval) between the middle and inner ear, each sealed by a membrane and are involved in sound transmission. The RWM is located at the end of the scala tympani. Except for the stereocilia and cuticular plate, the sensory hair cell surfaces are immersed in perilymph, allowing substances applied to the perilymph to reach all the inner ear cells [76]. Moreover, the bone of the lateral wall of the inner ear, semicircular canal, and the cochlear bulla in neonatal mice is soft. It can easily open with a micro-glass tube without much structural damage. The bone continues to ossify with age [77], which increases the difficulty of AAV delivery at later ages. Researchers have tried several methods to effectively deliver AAVs into targeted sensory hair cells to improve infection rates [15]. In general, there are three main methods to administer AAV vectors into the inner ear: (1) RWM approaches, including direct injection through the RWM [78] and RWM gel foam immersion [79]. However, direct injection of AAV through the RWM may confer a risk of injection-induced hearing loss [80]. (2) Posterior semicircular canal approaches, which may have a lower risk of damage to hearing function but has the potential to disturb vestibular function [73]. (3) A cochleostomy approach requires a skillful surgeon to open a window on the lateral wall of the cochlea without incurring further structural damage. This method also has a high risk of damage to hearing function [80, 81].
AAV Delivery Volume and Titer
A high titer of AAV vectors is required for delivery into the inner ear to achieve an adequate infection rate in cells of interest. Typically, one delivers 109 to 1010 genome copies (GC) in as small a volume as possible (~ 1 µL) into the adult mouse inner ear through different approaches [73, 81, 82]. One should avoid the delivery of large volumes of fluid into the inner ear to avoid increasing the perilymph pressure resulting in damage to sensory hair cells [83]. Tao and colleagues reported that hearing loss appears 2 weeks after injection of just 2 μL of AAV2/Anc80L65 into the adult mouse cochlea via a semicircular canal approach [84]. Additionally, up to 1.5 µL of AAV particles can be inoculated into postnatal day 1–5 mice without causing damage to hearing function [74]. To stay within the safe volume range, we need to use a high virus titer to ensure adequate infection and cell coverage [78]. The tolerance of a relatively high volume of AAV in postnatal pups compared to adult mice may be due to developmental changes in rodents. For example, the blood-labyrinth barrier develops and matures in rats after postnatal day 14 [85, 86].
Targeting Cochlear Sensory Hair Cells
Targeting cochlear sensory hair cells is a key issue for treating hearing loss, including genetic and acquired hearing loss. One of the main common pathological changes in various types of acquired hearing loss, such as noise- and ototoxic-drug-induced hearing loss, is the death of sensory hair cells, with OHCs being more vulnerable to insults than IHCs [3, 6]. Autopsy evaluation of human temporal bones showed that age-related hearing loss is strongly associated with loss of sensory hair cells [4]. Therefore, one basic requirement for using AAV as a vector to transduce genes to prevent and treat acquired hearing loss is to attain a good infection rate in sensory hair cells to achieve functional correction or reverse pathological processes. However, the introduction of AAV vectors into the inner ear shows high infection in IHCs compared to OHCs [78, 87, 88]. By exploring with AAV vectors with new or revised capsids to increase the infection rate in OHCs, a milestone was reached with the application of Anc80L65 into the inner ear, which shows high infection efficiency in both IHCs and OHCs without damaging hearing function [41]. Furthermore, more AAV vectors have high transfection into inner and outer sensory hair cells. For example, AAV9-PHP.B successfully infected a mouse model and the nonhuman primate cochlea [89–91]. Additionally, reports show a high infection rate in neonatal and adult mouse OHC and IHCs by AAV2.7m8 [46] and AAV-ie [74]. These recent discoveries have made AAV vectors a more suitable tool for inner ear research and therapy.
AAV AS A PLATFORM FOR THE TREATMENT OF HEARING LOSS
Mouse Models to Evaluate for Treatment of Acquired Hearing Loss with AAV
Despite the widespread use of mouse models in hearing research [92], no studies assess specific mouse strains resistant to AAV infection. The selection of an animal model for AAV delivery depends on the sensitivity of the mouse strain to hearing damage, the fertility of the mice, and the nursing ability of parental mice. CBA mice are well-characterized for studying noise- and aminoglycoside-induced hearing loss due to their lack of the Ahl mutation in the cadherin 23 gene, which causes accelerated hearing loss. However, CBA mothers abandon their pups once they are returned after handling and manipulation, regardless of how carefully we take care of the pups. In contrast, FVB/NJ mothers reliably accept their pups after experimental manipulations. FVB/NJ mice show normal baseline hearing for at least 10–12 weeks. Our recent results show that FVB/NJ mice have a similar sensitivity to noise-induced loss of OHCs in the basal turn as CBA/J mice [17] in agreement with another report [93]. Since the AAV infection rate is higher in pups than in adults, our lab commonly uses FVB/NJ mice for AAV delivery to mediate gene expression to study NIHL in vivo (Fig. 1B) [94]. After AAV delivery and recovery from cold anesthesia, we routinely put FVB/NJ pups back on their original padding material for a time to make their smell more recognizable by their parents for further nursing [94]. However, we would like to emphasize that CBA mice can be employed for AAV transfection if an FVB/NJ mother nurses the CBA pups.
Gene Silencing
Aside from AAV-mediated gene expression, which requires inserting cDNA of interest combined with a suitable promoter to perform gene replacement, gene silencing relies on RNA interference (RNAi) or post-transcriptional gene silencing. RNAi-based gene silencing reduces targeted protein expression via a viral or bacterial genome (short hairpin RNA, shRNA) or direct siRNA delivery into targeted cells [95]. siRNA (also known as short interfering RNA or silencing RNA) is a class of double-stranded non-coding RNAs about 21 base pairs in length. It is the most commonly used RNAi tool for the short-term silencing of proteins [96]. The research community has employed RNAi as a new therapeutic modality for diseases involving specific gene disorders or targeting pathologic gene/protein expression. The FDA approved the first RNAi drug in 2018 to treat hereditary transthyretin-mediated amyloidosis, a rare and deadly inherited condition [97]. Recently, the FDA approved a siRNA medicine, Inclisiran, which inhibits PCSK9 to treat hyperlipidemia in high-risk cardiovascular patients [98].
Several laboratories, including ours, have successfully delivered naked siRNA onto the round window membrane of the middle ear to investigate the mechanisms of acquired hearing loss [17–20]. However, silencing efficiency and duration of siRNA activity still need improvement for better outcomes. Theoretically, RNAi’s expression in the targeted cell’s genome may achieve long-term silencing by combining RNAi technology with an AAV vector. First, the long-term infection characteristics of AAV vectors enable stable expression of transgenes in targeted cells, making permanent silencing possible in postmitotic cells. In contrast with the long cDNAs, the short hairpin RNA (shRNA) sequence is only about 22nt, making it possible to design a transgene sequence with several shRNA sequences to silence one gene or multiple genes with one AAV vector [95].
Gene Replacement Therapy for Genetic Deafness
Gene replacement via AAV transduction has been well-reviewed as a strategy for the loss-of-function treatment of genetic deafness [14–16]. Briefly, the majority of the genes associated with genetic hearing loss are within the size limits of the AAV packaging capacity, and it is possible to package larger genes through dual-AAV [10] and even triple-AAV systems [14, 15]. In several animal experiments, AAV-based gene replacement therapy has emerged as an important method to treat inherited deafness. However, the efficient rescue of sensory hair cell function and lack of hearing recovery with later-stage treatments remain challenges. Trans-expression of cDNA cannot treat dominant gain-of-function mutant alleles associated with the production of abnormal proteins. Instead, one predicts the need for personalized identification of the defects to restore affected pathways or develop allele-specific attenuators such as mutant-specific shRNA. Prevention of hearing loss needs further development, including the long-term expression of safe constructs targeting the inner ear sensory hair cells to replace the expression of abnormal proteins with normal ones.
AAV-Mediated Gene Therapy in Acquired Hearing Loss
Environmental factors contribute to acquired hearing loss over a lifespan, which is less predictable. Recent developments improved the AAV gene therapy vector to help prevent and treat acquired hearing loss, including noise- and ototoxic-drug-induced and age-related hearing loss. Although some studies reported possible ototoxicity from AAV-mediated gene overexpression, the development of additional strategies, such as inducible temporary overexpression, aims to improve tolerability. We have searched PubMed for AAV-mediated prevention and treatment of acquired hearing loss in preclinical studies using animals before and while writing this short review. The publications reviewed here fall into this category.
Reports indicate that AAV-mediated neurotrophin-3 (NT-3) overexpression serves to alleviate noise-induced hearing loss [75, 99], but NT-3 overexpression may be harmful to IHCs due to cochlear overstimulation [75]. AAV-mediated gene overexpression of glial cell line-derived neurotrophic factor (GDNF) and activity-dependent neurotrophic factor 9 (ADNF-9) showed preventive effects against kanamycin-induced hearing loss [100, 101]. However, like NT-3, GDNF overexpression was reported to damage hair cells and spiral ganglion neurons [102]. Thus, the potential ototoxicity of exogenous proteins in the inner ear needs careful evaluation before further development. Additionally, AAV-mediated X-linked inhibitor of apoptosis protein (XIAP) overexpression also attenuated cisplatin-induced hearing loss [79, 103]. However, AAV-mediated Cav1.3 silencing appeared to aggravate age-related hearing impairment due to the stimulation of calcium-mediated oxidative stress [104].
A new strategy tagged AAV2 with superparamagnetic iron oxide nanoparticles (quad Y-F), which increased AAV-delivered BDNF expression by magnetic targeting via the RWM and protected against noise-induced temporary hearing loss [105]. Furthermore, using the CRISPR/Cas9 gene-editing system via AAV transduction to correct the point mutation of the Htra2 gene reduced neomycin-induced apoptosis in sensory hair cells and prevented neomycin-induced hearing loss [106]. So far, AAV-mediated gene delivery for treating hearing loss, especially for acquired hearing loss in the inner ear, remains in early experimental stages. For example, manipulating AAV-mediated protein expression in sensory hair cells to a physiological state remains elusive. Low expression of AAV encoded proteins may be insufficient to prevent hearing loss, while exaggerated expression may be ototoxic. An ideal trade-off between therapeutic expression and cell toxicity may be achieved by measuring the protein levels under physiological conditions and regulating AAV-mediated protein expression close to this point, either by a controllable promoter or by manipulating delivery. However, manipulating delivery may lead to a problem of only infecting a subset of cells, which may express toxic protein levels.
Additionally, not all types of acquired hearing loss can be prevented or treated by manipulating gene expression. For example, increased protein expression may not improve the function of the sensory mechanoelectrical transduction (MET) channel in cochlear sensory hair cells. Furthermore, the treatment time may be critical with developmental regulation or timing in reaction to toxic exposure.
CONCLUSION
Significant progress in the preclinical treatment of genetic hearing loss has been achieved with the recent development of AAV vectors via capsid engineering, resulting in specific cell and tissue tropism [107, 108]. Several newly designed AAVs are also safe and have a high transfection rate into inner ear cells, including sensory hair cells and supporting cells [10]. In particular, AAV-mediated correction of genetic defects by gain-of-function has shown promise. Consequently, in preclinical studies, AAV-mediated gene therapy has successfully treated genetic hearing loss and prevented acquired hearing loss. However, many challenges remain, such as the lack of detailed data on the fate and impact of empty viral particles, chemicals generated during AAV vector production on hearing function, the need for purification and quality control of AAV vectors, batch-to-batch variations of vector titers, and high cost. Nevertheless, given the complete absence of paradigms to prevent and treat either inherited or acquired hearing loss, we expect that AAV-mediated gain-of-function and loss-of-function hold much promise as the next frontier of therapeutics for the prevention and treatment of genetic and acquired hearing loss.
Acknowledgements
We thank Dr. Jochen Schacht for his valuable comments on the manuscript, and we thank Andra Talaska for proofreading the manuscript.
Funding
R01 DC009222, from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, supports Dr. Sha’s research project.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang J, Puel JL. Toward cochlear therapies. Physiol Rev. 2018;98:2477–2522. doi: 10.1152/physrev.00053.2017. [DOI] [PubMed] [Google Scholar]
- 2.Kros CJ, Steyger PS (2019) Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med 9 [DOI] [PMC free article] [PubMed]
- 3.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26:85–96. doi: 10.1080/13543784.2017.1269171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience. 2019;407:8–20. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CS, Emmett SD, Robler SK, Tucci DL. Global hearing loss prevention. Otolaryngol Clin North Am. 2018;51:575–592. doi: 10.1016/j.otc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Guo J, Chai R, Li H, Sun S. Protection of hair cells from ototoxic drug-induced hearing loss. Adv Exp Med Biol. 2019;1130:17–36. doi: 10.1007/978-981-13-6123-4_2. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A (2019) Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med 11 [DOI] [PMC free article] [PubMed]
- 8.Akil O, Dyka F, Calvet C, Emptoz A, et al. Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc Natl Acad Sci U S A. 2019;116:4496–4501. doi: 10.1073/pnas.1817537116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyorgy B, Nist-Lund C, Pan B, et al. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat Med. 2019;25:1123–1130. doi: 10.1038/s41591-019-0500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Solanes P, Nist-Lund C, Spataro S, Shubina-Oleinik O, Marcovich I, Goldberg H, Schneider BL, Holt JR. Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice. Mol Ther. 2021;29:973–988. doi: 10.1016/j.ymthe.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue Y, Hu X, Wang D, et al. Gene editing in a Myo6 semi-dominant mouse model rescues auditory function. Mol Ther. 2022;30:105–118. doi: 10.1016/j.ymthe.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastie E, Samulski RJ. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success—a personal perspective. Hum Gene Ther. 2015;26:257–265. doi: 10.1089/hum.2015.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naso MF, Tomkowicz B, Perry WL, 3rd, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Askew C, Chien WW. Adeno-associated virus gene replacement for recessive inner ear dysfunction: progress and challenges. Hear Res. 2020;394:107947. doi: 10.1016/j.heares.2020.107947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bankoti K, Generotti C, Hwa T, Wang L, O’Malley BW, Jr, Li D. Advances and challenges in adeno-associated viral inner-ear gene therapy for sensorineural hearing loss. Mol Ther Methods Clin Dev. 2021;21:209–236. doi: 10.1016/j.omtm.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crane R, Conley SM, Al-Ubaidi MR, Naash MI. Gene therapy to the retina and the cochlea. Front Neurosci. 2021;15:652215. doi: 10.3389/fnins.2021.652215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill K, Yuan H, Wang X, Sha SH. Noise-induced loss of hair cells and cochlear synaptopathy are mediated by the activation of AMPK. J Neurosci. 2016;36:7497–7510. doi: 10.1523/JNEUROSCI.0782-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maeda Y, Sheffield AM, Smith RJH. Therapeutic regulation of gene expression in the inner ear using RNA interference. Adv Otorhinolaryngol. 2009;66:13–36. doi: 10.1159/000218205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oishi N, Chen FQ, Zheng HW, Sha SH. Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hear Res. 2013;296:36–41. doi: 10.1016/j.heares.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka M, Asaoka M, Yanagawa Y, Hirashima N. Long-term gene-silencing effects of siRNA introduced by single-cell electroporation into postmitotic CNS neurons. Neurochem Res. 2011;36:1482–1489. doi: 10.1007/s11064-011-0474-6. [DOI] [PubMed] [Google Scholar]
- 22.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 23.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966;55:1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 25.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman MS. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonntag F, Köther K, Schmidt K, et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol. 2011;85:12686–12697. doi: 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earley LF, Powers JM, Adachi K, Baumgart JT, Meyer NL, Xie Q, Chapman MS, Nakai H (2017) Adeno-associated virus (AAV) assembly-activating protein is not an essential requirement for capsid assembly of AAV serotypes 4, 5, and 11. J Virol 91 [DOI] [PMC free article] [PubMed]
- 28.Grimwood J, Gordon LA, Olsen A, et al. The DNA sequence and biology of human chromosome 19. Nature. 2004;428:529–535. doi: 10.1038/nature02399. [DOI] [PubMed] [Google Scholar]
- 29.Tullis GE, Shenk T. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J Virol. 2000;74:11511–11521. doi: 10.1128/jvi.74.24.11511-11521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colon-Thillet R, Jerome KR, Stone D. Optimization of AAV vectors to target persistent viral reservoirs. Virol J. 2021;18:85. doi: 10.1186/s12985-021-01555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Agbandje-McKenna M, Kleinschmidt J. AAV capsid structure and cell interactions. Methods Mol Biol. 2011;807:47–92. doi: 10.1007/978-1-61779-370-7_3. [DOI] [PubMed] [Google Scholar]
- 35.Ivanchenko MV, Hanlon KS, Hathaway DM, Klein AJ, Peters CW, Li Y, Tamvakologos PI, Nammour J, Maguire CA, Corey DP. AAV-S: a versatile capsid variant for transduction of mouse and primate inner ear. Mol Ther Methods Clin Dev. 2021;21:382–398. doi: 10.1016/j.omtm.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EJ, Guenther CM, Suh J. Adeno-associated virus (AAV) vectors: rational design strategies for capsid engineering. Curr Opin Biomed Eng. 2018;7:58–63. doi: 10.1016/j.cobme.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vozenilek AE, Blackburn CMR, Schilke RM, Chandran S, Castore R, Klein RL, Woolard MD. AAV8-mediated overexpression of mPCSK9 in liver differs between male and female mice. Atherosclerosis. 2018;278:66–72. doi: 10.1016/j.atherosclerosis.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moretti A, Fonteyne L, Giesert F, et al. Somatic gene editing ameliorates skeletal and cardiac muscle failure in pig and human models of Duchenne muscular dystrophy. Nat Med. 2020;26:207–214. doi: 10.1038/s41591-019-0738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikeda Y, Sun Z, Ru X, Vandenberghe LH, Humphreys BD. Efficient gene transfer to kidney mesenchymal cells using a synthetic adeno-associated viral vector. J Am Soc Nephrol. 2018;29:2287–2297. doi: 10.1681/ASN.2018040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvalho LS, Xiao R, Wassmer SJ, et al. Synthetic adeno-associated viral vector efficiently targets mouse and nonhuman primate retina in vivo. Hum Gene Ther. 2018;29:771–784. doi: 10.1089/hum.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landegger LD, Pan B, Askew C, et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiha W, Bartlett CA, Petratos S, Fitzgerald M, Harvey AR. Intravitreal application of AAV-BDNF or mutant AAV-CRMP2 protects retinal ganglion cells and stabilizes axons and myelin after partial optic nerve injury. Exp Neurol. 2020;326:113167. doi: 10.1016/j.expneurol.2019.113167. [DOI] [PubMed] [Google Scholar]
- 43.Berry GE, Asokan A. Cellular transduction mechanisms of adeno-associated viral vectors. Curr Opin Virol. 2016;21:54–60. doi: 10.1016/j.coviro.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cronin T, Vandenberghe LH, Hantz P, et al. Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med. 2014;6:1175–1190. doi: 10.15252/emmm.201404077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV (2013) In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5:189ra176 [DOI] [PubMed]
- 46.Isgrig K, McDougald DS, Zhu J, Wang HJ, Bennett J, Chien WW (2019) AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat Commun 10:427 [DOI] [PMC free article] [PubMed]
- 47.Wright JF. Transient transfection methods for clinical adeno-associated viral vector production. Hum Gene Ther. 2009;20:698–706. doi: 10.1089/hum.2009.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Craenenbroeck K, Vanhoenacker P, Haegeman G. Episomal vectors for gene expression in mammalian cells. Eur J Biochem. 2000;267:5665–5678. doi: 10.1046/j.1432-1327.2000.01645.x. [DOI] [PubMed] [Google Scholar]
- 50.Gray SJ, Foti SB, Schwartz JW, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren BZ, Lahn BT. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS ONE. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell SK, Rivera-Soto R, Gray SJ. Viral expression cassette elements to enhance transgene target specificity and expression in gene therapy. Discov Med. 2015;19:49–57. [PMC free article] [PubMed] [Google Scholar]
- 53.Lock M, Alvira M, Vandenberghe LH, Samanta A, Toelen J, Debyser Z, Wilson JM. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cecchini S, Virag T, Kotin RM. Reproducible high yields of recombinant adeno-associated virus produced using invertebrate cells in 0.02- to 200-liter cultures. Hum Gene Ther. 2011;22:1021–1030. doi: 10.1089/hum.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorne BA, Takeya RK, Peluso RW. Manufacturing recombinant adeno-associated viral vectors from producer cell clones. Hum Gene Ther. 2009;20:707–714. doi: 10.1089/hum.2009.070. [DOI] [PubMed] [Google Scholar]
- 56.Wright JF. AAV empty capsids: for better or for worse? Mol Ther. 2014;22:1–2. doi: 10.1038/mt.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Potter M, Lins B, Mietzsch M, Heilbronn R, Van Vliet K, Chipman P, Agbandje-McKenna M, Cleaver BD, Clement N, Byrne BJ, Zolotukhin S. A simplified purification protocol for recombinant adeno-associated virus vectors. Mol Ther Methods Clin Dev. 2014;1:14034. doi: 10.1038/mtm.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi VW, McCarty DM, Samulski RJ. Host cell DNA repair pathways in adeno-associated viral genome processing. J Virol. 2006;80:10346–10356. doi: 10.1128/JVI.00841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalwadi DA, Calabria A, Tiyaboonchai A, Posey J, Naugler WE, Montini E, Grompe M. AAV integration in human hepatocytes. Mol Ther. 2021;29:2898–2909. doi: 10.1016/j.ymthe.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miao CH, Snyder RO, Schowalter DB, Patijn GA, Donahue B, Winther B, Kay MA. The kinetics of rAAV integration in the liver. Nat Genet. 1998;19:13–15. doi: 10.1038/ng0598-13. [DOI] [PubMed] [Google Scholar]
- 61.Nakai H, Wu X, Fuess S, Storm TA, Munroe D, Montini E, Burgess SM, Grompe M, Kay MA. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol. 2005;79:3606–3614. doi: 10.1128/JVI.79.6.3606-3614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hüser D, Gogol-Döring A, Lutter T, Weger S, Winter K, Hammer E-M, Cathomen T, Reinert K, Heilbronn R. Integration preferences of wildtype AAV-2 for consensus rep-binding sites at numerous loci in the human genome. PLoS Pathog. 2010;6:e1000985–e1000985. doi: 10.1371/journal.ppat.1000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chamberlain K, Riyad JM, Weber T. Expressing transgenes that exceed the packaging capacity of adeno-associated virus capsids. Hum Gene Ther Methods. 2016;27:1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Akil O. Dual and triple AAV delivery of large therapeutic gene sequences into the inner ear. Hear Res. 2020;394:107912. doi: 10.1016/j.heares.2020.107912. [DOI] [PubMed] [Google Scholar]
- 65.Muhuri M, Maeda Y, Ma H, Ram S, Fitzgerald KA, Tai PW, Gao G (2021) Overcoming innate immune barriers that impede AAV gene therapy vectors. J Clin Invest 131 [DOI] [PMC free article] [PubMed]
- 66.Ronzitti G, Gross D-A, Mingozzi F (2020) Human immune responses to adeno-associated virus (AAV) vectors. Front Immunol 11 [DOI] [PMC free article] [PubMed]
- 67.Sun JY, Anand-Jawa V, Chatterjee S, Wong KK. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 2003;10:964–976. doi: 10.1038/sj.gt.3302039. [DOI] [PubMed] [Google Scholar]
- 68.Martino AT, Markusic DM. Immune response mechanisms against AAV vectors in animal models. Mol Ther Methods Clin Dev. 2020;17:198–208. doi: 10.1016/j.omtm.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang L, Calcedo R, Bell P, Lin J, Grant RL, Siegel DL, Wilson JM. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaiss AK, Cotter MJ, White LR, Clark SA, Wong NC, Holers VM, Bartlett JS, Muruve DA. Complement is an essential component of the immune response to adeno-associated virus vectors. J Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rabinowitz J, Chan YK, Samulski RJ (2019) Adeno-associated virus (AAV) versus immune response. Viruses 11 [DOI] [PMC free article] [PubMed]
- 73.Suzuki J, Hashimoto K, Xiao R, Vandenberghe LH, Liberman MC. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep. 2017;7:45524. doi: 10.1038/srep45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan F, Chu C, Qi J, et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat Commun. 2019;10:3733. doi: 10.1038/s41467-019-11687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto K, Hickman TT, Suzuki J, Ji L, Kohrman DC, Corfas G, Liberman MC. Protection from noise-induced cochlear synaptopathy by virally mediated overexpression of NT3. Sci Rep. 2019;9:15362. doi: 10.1038/s41598-019-51724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ulfendahl M, Scarfone E, Flock A, Le Calvez S, Conradi P. Perilymphatic fluid compartments and intercellular spaces of the inner ear and the organ of Corti. Neuroimage. 2000;12:307–313. doi: 10.1006/nimg.2000.0617. [DOI] [PubMed] [Google Scholar]
- 77.Richard C, Courbon G, Laroche N, Prades JM, Vico L, Malaval L. Inner ear ossification and mineralization kinetics in human embryonic development — microtomographic and histomorphological study. Sci Rep. 2017;7:4825. doi: 10.1038/s41598-017-05151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshimura H, Shibata SB, Ranum PT, Smith RJH. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci Rep. 2018;8:2980. doi: 10.1038/s41598-018-21233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jie H, Tao S, Liu L, Xia L, Charko A, Yu Z, Bance M, Yin S, Robertson GS, Wang J. Cochlear protection against cisplatin by viral transfection of X-linked inhibitor of apoptosis protein across round window membrane. Gene Ther. 2015;22:546–552. doi: 10.1038/gt.2015.22. [DOI] [PubMed] [Google Scholar]
- 80.Chien WW, McDougald DS, Roy S, Fitzgerald TS, Cunningham LL. Cochlear gene transfer mediated by adeno-associated virus: comparison of two surgical approaches. Laryngoscope. 2015;125:2557–2564. doi: 10.1002/lary.25317. [DOI] [PubMed] [Google Scholar]
- 81.Shu Y, Tao Y, Wang Z, Tang Y, Li H, Dai P, Gao G, Chen ZY. Identification of adeno-associated viral vectors that target neonatal and adult mammalian inner ear cell subtypes. Hum Gene Ther. 2016;27:687–699. doi: 10.1089/hum.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Omichi R, Yoshimura H, Shibata SB, Vandenberghe LH, Smith RJH. Hair cell transduction efficiency of single- and dual-AAV serotypes in adult murine cochleae. Mol Ther Methods Clin Dev. 2020;17:1167–1177. doi: 10.1016/j.omtm.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swan EE, Mescher MJ, Sewell WF, Tao SL, Borenstein JT. Inner ear drug delivery for auditory applications. Adv Drug Deliv Rev. 2008;60:1583–1599. doi: 10.1016/j.addr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao Y, Huang M, Shu Y, et al. Delivery of adeno-associated virus vectors in adult mammalian inner-ear cell subtypes without auditory dysfunction. Hum Gene Ther. 2018;29:492–506. doi: 10.1089/hum.2017.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki M, Kaga K. Development of blood-labyrinth barrier in the semicircular canal ampulla of the rat. Hear Res. 1999;129:27–34. doi: 10.1016/s0378-5955(98)00214-7. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki M, Yamasoba T, Kaga K. Development of the blood-labyrinth barrier in the rat. Hear Res. 1998;116:107–112. doi: 10.1016/s0378-5955(97)00208-6. [DOI] [PubMed] [Google Scholar]
- 87.Shibata SB, Ranum PT, Moteki H, Pan B, Goodwin AT, Goodman SS, Abbas PJ, Holt JR, Smith RJH. RNA interference prevents autosomal-dominant hearing loss. Am J Hum Genet. 2016;98:1101–1113. doi: 10.1016/j.ajhg.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Sun Y, Chang Q, Ahmad S, Zhou B, Kim Y, Li H, Lin X. Early postnatal virus inoculation into the scala media achieved extensive expression of exogenous green fluorescent protein in the inner ear and preserved auditory brainstem response thresholds. J Gene Med. 2013;15:123–133. doi: 10.1002/jgm.2701. [DOI] [PubMed] [Google Scholar]
- 89.Gyorgy B, Meijer EJ, Ivanchenko MV, et al. Gene transfer with AAV9-PHP.B rescues hearing in a mouse model of usher syndrome 3A and transduces hair cells in a non-human primate. Mol Ther Methods Clin Dev. 2019;13:1–13. doi: 10.1016/j.omtm.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ivanchenko MV, Hanlon KS, Devine MK, Tenneson K, Emond F, Lafond JF, Kenna MA, Corey DP, Maguire CA (2020) Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear Res 394:107930 [DOI] [PMC free article] [PubMed]
- 91.Lee J, Nist-Lund C, Solanes P, Goldberg H, Wu J, Pan B, Schneider BL, Holt JR (2020) Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B. Hear Res 394:107882 [DOI] [PubMed]
- 92.Ohlemiller KK. Mouse methods and models for studies in hearing. J Acoust Soc Am. 2019;146:3668. doi: 10.1121/1.5132550. [DOI] [PubMed] [Google Scholar]
- 93.Ho MK, Li X, Wang J, Ohmen JD, Friedman RA. FVB/NJ mice demonstrate a youthful sensitivity to noise-induced hearing loss and provide a useful genetic model for the study of neural hearing loss. Audiol Neurotol Extra. 2014;4:1–11. doi: 10.1159/000357770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu F, Hill K, Fang Q, He Z, Zheng H, Wang X, Xiong H, Sha SH. Traumatic-noise-induced hair cell death and hearing loss is mediated by activation of CaMKKbeta. Cell Mol Life Sci. 2022;79:249. doi: 10.1007/s00018-022-04268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang X, Yuan C, Huang B, et al. Developing a versatile shotgun cloning strategy for single-vector-based multiplex expression of short interfering RNAs (siRNAs) in mammalian cells. ACS Synthe Bio. 2019;8:2092–2105. doi: 10.1021/acssynbio.9b00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol. 2003;67:657–685. doi: 10.1128/MMBR.67.4.657-685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morrison C. Alnylam prepares to land first RNAi drug approval. Nat Rev Drug Discovery. 2018;17:156–157. doi: 10.1038/nrd.2018.20. [DOI] [PubMed] [Google Scholar]
- 98.Bellosta S, Rossi C, Alieva AS, Catapano AL, Corsini A, Baragetti A (2022) Cholesterol lowering biotechnological strategies: from monoclonal antibodies to antisense therapies. a pre-clinical perspective review. Cardiovasc Drugs Ther [DOI] [PubMed]
- 99.Chen H, Xing Y, Xia L, Chen Z, Yin S, Wang J. AAV-mediated NT-3 overexpression protects cochleae against noise-induced synaptopathy. Gene Ther. 2018;25:251–259. doi: 10.1038/s41434-018-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y, Okada T, Shimazaki K, et al. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–480. doi: 10.1038/sj.mt.6300379. [DOI] [PubMed] [Google Scholar]
- 101.Zheng G, Zhu Z, Zhu K, Wei J, Jing Y, Duan M. Therapeutic effect of adeno-associated virus (AAV)-mediated ADNF-9 expression on cochlea of kanamycin-deafened guinea pigs. Acta Otolaryngol. 2013;133:1022–1029. doi: 10.3109/00016489.2013.799777. [DOI] [PubMed] [Google Scholar]
- 102.Akil O, Blits B, Lustig LR, Leake PA. Virally mediated overexpression of glial-derived neurotrophic factor elicits age- and dose-dependent neuronal toxicity and hearing loss. Hum Gene Ther. 2019;30:88–105. doi: 10.1089/hum.2018.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S, Selesnick SH, Kaplitt MG. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol. 2006;27:484–490. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- 104.Qi F, Zhang R, Chen J, et al. Down-regulation of Cav1.3 in auditory pathway promotes age-related hearing loss by enhancing calcium-mediated oxidative stress in male mice. Aging. 2019;11:6490–6502. doi: 10.18632/aging.102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukherjee S, Kuroiwa M, Oakden W et al (2021) Local magnetic delivery of adeno-associated virus AAV2(quad Y-F)-mediated BDNF gene therapy restores hearing after noise injury. Mol Ther [DOI] [PMC free article] [PubMed]
- 106.Gu X, Wang D, Xu Z, Wang J, Guo L, Chai R, Li G, Shu Y, Li H. Prevention of acquired sensorineural hearing loss in mice by in vivo Htra2 gene editing. Genome Biol. 2021;22:86. doi: 10.1186/s13059-021-02311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.El Andari J, Grimm D. Production, processing, and characterization of synthetic AAV gene therapy vectors. Biotechnol J. 2021;16:e2000025. doi: 10.1002/biot.202000025. [DOI] [PubMed] [Google Scholar]
- 108.Penaud-Budloo M, Francois A, Clement N, Ayuso E. Pharmacology of recombinant adeno-associated virus production. Molecular therapy Methods & clinical development. 2018;8:166–180. doi: 10.1016/j.omtm.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]