Abstract
Memory formation in the hippocampus is formed and maintained by circadian clock genes during sleep. Sleep deprivation (SD) can lead to memory impairment and neuroinflammation, and there remains no effective pharmacological treatment for these effects. Myricetin (MYR) is a common natural flavonoid that has various pharmacological activities. In this study, we investigated the effects of MYR on memory impairment, neuroinflammation, and neurotrophic factors in sleep-deprived rats. We analyzed SD-induced cognitive and spatial memory, as well as pro-inflammatory cytokine levels during SD. SD model rats were intraperitoneally injected with 10 and 20 mg/kg/day MYR for 14 days. MYR administration significantly ameliorated SD-induced cognitive and spatial memory deficits; it also attenuated the SD-induced inflammatory response associated with nuclear factor kappa B activation in the hippocampus. In addition, MYR enhanced the mRNA expression of brain-derived neurotropic factor (BDNF) in the hippocampus. Our results showed that MYR improved memory impairment by means of anti-inflammatory activity and appropriate regulation of BDNF expression. Our findings suggest that MYR is a potential functional ingredient that protects cognitive function from SD.
Keywords: Brain-derived neurotrophic factor, Flavonoids, Inflammation, Memory, Sleep deprivation
INTRODUCTION
Sleep deprivation (SD) is linked to various health problems [1]. Sleep physiologically improves the functioning of the nervous, muscular, and immune systems, which are strengthened during sleep. Thus, regular SD increases the risk of metabolic disorders by causing hormonal imbalances. It can also lead to appetite loss, anxiety, stress, depression, and weight loss [2,3]. Important functions of sleep include learning facilitation and memory consolidation [4]. SD damages the hippocampus and cerebral cortex, thereby delaying the speed of information processing; this causes memory loss and impairs cognitive function [5-7].
Rapid eye movement SD increases plasma corticosterone levels and interferes with spatial memory integration in the Morris water maze (MWM) and eight-arm radial maze (8-arm RAM) test, leading to memory impairment in rats [8-11]. In addition, some studies have shown that SD impairs the consolidation of declarative memory and motor-adaptive working memory in rats [12,13]. SD also impairs hippocampal-dependent memory [14] and emotional memory [15], suggesting that hippocampal memory formation is vulnerable to sleep disorders [16,17]. Finally, SD inhibits the induction of long-term potentiation without synthesizing the proteins involved in long-term potentiation stabilization [18,19]. Because SD can impair memory, we selected the 24-h SD model to investigate the effects of SD on cognitive function and memory impairment.
Along with reducing cognitive function, SD leads to high levels of inflammatory cytokines [20]. Cytokines are powerful molecules involved in sleep regulation, and they are stimulated during sleep. For example, in the SD animal model, rats reportedly show decreased memory performance and increased levels of inflammatory cytokines in passive avoidance tasks [21]; the increased levels of inflammatory cytokines caused by SD influence the regulation of synaptic plasticity and consolidation of memory involved in the process of memory formation [22]. Decreased hippocampal volume and densely located cytokine receptors are commonly observed in SD patients [23]. The severity of SD is associated with cognitive dysfunction caused by damage to the hippocampus and enhanced pro-inflammatory cytokine levels [24].
SD may also impair neurotrophin-mediated signaling [25]. Brain-derived neurotrophic factor (BDNF), an important neurotrophin, is associated with the regulation of neuronal development and neuronal survival as well as memory formation via binding interactions with its receptor tropomyosin-related kinase B (TrkB) [26].
Myricetin (MYR, 3,3’,4’,5,5’,7-hexahydroxylflavone) is a natural flavonoid found in berries, vegetables, teas, fruits and wines produced by various plants [27]. This compound has antioxidant activity and anti-apoptotic effects [28]. MYR has various pharmacological activities such as anti-photoaging, anti-cancer, anti-diabetic, and anti-inflammatory effects; it has important roles in the treatment and prevention of some diseases [29]. In particular, MYR has neuroprotective properties in the hippocampus of stressed mice and protects neurons from neurodegenerative diseases (e.g., ischemic stroke, epilepsy, Parkinson’s disease, and Alzheimer’s disease) [30-32]. Some studies have demonstrated that MYR attenuates brain damage in a rat model of cerebral ischemia through activation of the nuclear factor, erythroid 2-like 2 pathway and reduction of oxidative stress [33]. Therefore, MYR may be a potential drug for treatment or supplementary care in various brain diseases, but the mechanisms underlying its effects are unknown.
Therefore, we hypothesized that MYR, a potential flavonoid, would be beneficial for learning and memory in an experimental model of SD-induced memory impairment and that it would improve neuroinflammation caused by SD. We investigated the possible effects of MYR on memory impairment and neuroinflammation in a 72-h SD animal model. The 8-arm RAM and MWM tests were performed to investigate the memory-improving effects of MYR. In addition, neuroinflammatory responses, such as pro- and anti-inflammatory cytokines and neurotrophic pathways, were evaluated to investigate the molecular mechanisms underlying the effects of MYR.
METHODS
Animals and MYR administration
Male Sprague–Dawley rats (7–8 weeks, old, 220–250 g; Samtaco, Seoul, Korea) were used as the experimental model. The rats were reared in groups of three or four in a breeding room maintained at a temperature of 23 ± 2°C and humidity of 55 ± 5%, with a 12:12 light/dark cycle. Sterile drinking water and food were provided to the rats ad libitum. Every effort was made to minimize pain and reduce the number of laboratory animals used. Animal experiments were conducted in accordance with the Code of Ethics and guidelines for the management of laboratory animals by the Animal Care and Use Committee of Kyung Hee University (Seoul, Korea), which also approved the experimental protocol (KHUASP(SE)-21-045). Before behavioral testing, rats were handled daily for at least 1 week by the experimenter to exclude the effects of stress during the experiment.
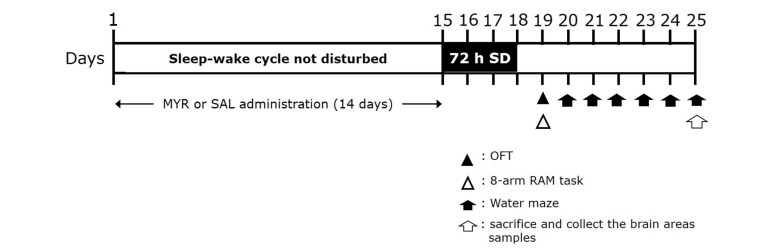
To analyze the inhibitory effects of MYR on cognitive and memory impairment in an animal model of SD. Thirty-nine rats were randomly divided into the following six groups (6–7 rats/group): control, wide platform (WPF), chronic SD, SD with MYR (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), and SD with alprazolam. The MYR group was administered 10 or 20 mg/kg for 14 days. The alprazolam group was administered 0.25 mg/kg alprazolam (ATI; Pfizer, New York, NY, USA), a positive control drug, for 14 days. MYR and ATI were dissolved in 0.9% physiological saline (SAL) before use and administered by intraperitoneal injection. The overall experimental schedule for all drug administrations, behavioral testing and sampling is presented in Fig. 1.
Fig. 1. Experimental protocols for sleep deprivation (SD)-induced memory impairment behaviors and myricetin (MYR) treatment in rats.
Rats were divided into groups (n = 6–7 rats/group), then subjected to the indicated experimental protocols. OFT, open-field test; 8-arm RAM task, eight-arm radial maze task.
SD induction
SD was induced using a modified multi-platform method [34,35], in which rats were deprived of rapid eye movement sleep for 72 h. Briefly, rats were placed in a large round water tank (110 × 50 cm) with 15 columns (platforms) adjusted in three rows. The diameter of each platform was 5 cm, the platform height was 15 cm, and the distance between the two columns (edge to edge) was 7 cm. Rats could move freely in the tank and jump between platforms. The distance between the two columns was the distance that the rat could move freely. Water was filled within 2 cm below the platforms, and the temperature of the water was maintained at approximately 25 ± 2°C. The water in the tank was changed daily. The rats were placed on the platforms. When the rats fell into rapid eye movement sleep, they lost muscle tone and fell off the platforms and into the water. At this point, the rats woke up and immediately climbed back onto the platforms. For the WPF group, wide platforms with a diameter of 12 cm were used to allow the rats to sleep without drowning, thus evaluating possible stress in the water tank environment.
Short-term memory and spatial memory in the rats were evaluated using the 8-arm RAM and MWM tests, respectively. Inflammatory cytokine levels and molecular indicators were measured after behavioral tests. The body weight and food intake of the control and SD group rats were measured regularly at each time point as physiological test markers. Throughout the study, a constant amount (150 g) of food pellets was provided to rats in the control cage and in the SD chamber.
8-Arm RAM task
The 8-arm RAM task was conducted in a device in the form of a central platform made of black wood, with eight arms extending out at a 45 degree angle (radial). The central starting area was a regular octagonal box close to a circle with a diameter of 38 cm and a height of 25 cm. On each side of the starting box were long radial connection arms (70 × 10 × 25 cm). At the ends of the arms, a container (5 × 5 × 2 cm) was installed to hold food that was provided as a reward. SMART software (ver. 2.5; PanLab Co., Barcelona, Spain) was used to track and analyze the behaviors of the rats exploring each arm. Based on signals provided at the ends of the arms, the number of times the rat visited each arm and the number of errors were calculated. Before the experiment began, the rats were deprived of food for 24 h, thus inducing hunger. The rats were placed in the central starting box and allowed to acclimatize for 1 min. The passages to each arm were then opened and the rats were allowed to roam freely through the maze. When the rats visited each arm course and ran to the end, they were allowed to eat food from the reward container. However, if the same route was visited repeatedly, food was not provided beginning in the second visit; these repeat visits were recorded as errors. If the rats did not visit all eight-arms within 5 min, the trial was stopped and considered a failure. When the rats reached the learning criterion, a memory test was performed after 24 h [36].
MWM test
The MWM test was performed in accordance with the Morris method [35]. The test was conducted in a water tank (2 m in diameter and 0.35 m high) filled with water to a depth of 22 cm; the water temperature was 23–25°C. An escape platform (15 cm in diameter and 20 cm in height) was installed in one of the quadrants and slightly submerged (1.5 cm) below the water surface. Sleep-deprived rats were subjected to a training test for 5 consecutive days. This MWM test was conducted at the same time for 5 days, three times per day per. The rats were dunked in water and observed while they escaped to an escape platform; this process was tracked using a video tracking system with the SMART program (ver. 2.5; PanLab). When the rats successfully reached the escape platform, they were allowed to rest for 10 s. However, if the rats did not find an escape platform within 180 s, they were transferred to the escape platform and allowed to rest for 10 s. A spatial memory maintenance test (retention test) was conducted on day 6 of the MWM test. To find the escape platform, the rats in each group were allowed to swim freely for 60 s in the same tank from which the escape platform had been removed; the swimming path was tracked with the video tracker and stored in a computer analysis system. The space in the tank was divided into four zones centered on the location of the escape platform and scores were assigned to each zone; the times spent swimming in each zone during the 60 s swimming times were calculated separately. SMART software was used for this analysis. The MWM test was conducted beginning on day 20 after 3 days of SD, following completion of the eight-arm RAM task.
Open field test
The rats were placed in a black acrylic box (60 × 60 × 30 cm for width, length, and height, respectively); a digital camera was installed on the box. Locomotor activity was measured using a video-tracking system with the SMART program (ver. 2.5; PanLab). Following pre-experiment measurements of rat weight, the rats were individually placed in a measuring box to measure their locomotor activities. After 5 min of adaptation and stabilization, the locomotor activity was measured for 5 min. This test was used as a behavioral indicator of emotional reactivity.
Enzyme-linked immunosorbent assay to assess inflammatory mediator levels
Rats were euthanized immediately after the behavioral test, and pro-inflammatory cytokine levels were measured in the hippocampus using enzyme-linked immunosorbent assays (ELISAs). To prevent the decomposition of unstable inflammatory markers, the hippocampus was separated and stored at –80°C until analysis. Hippocampal brain tissue was homogenized on ice in phosphate-buffered SAL (pH 7.4) containing a protease inhibitor cocktail, using a Polytron homogenizer. The lysate was collected and centrifuged at 10,000 rpm for 10–15 min; the supernatant was then collected and subjected to cytokine analysis. Pro-inflammatory cytokine (interleukin [IL]-1β, IL-4, IL-6, IL-8, IL-12, and tumor necrosis factor [TNF]-α) levels in the hippocampus were quantified using commercially available ELISA kits, in accordance with the manufacturers’ instructions (Abcam, Cambridge, MA, USA and Cell Signaling Technology, Danvers, MA, USA). All samples were evaluated in triplicate. A 50 µl aliquot of the substrate solution was added to each well for 20 min to induce color development, and the quantities of pro-inflammatory cytokines were measured at 450 nm using an ELISA plate reader.
Total RNA isolation and reverse transcription-polymerase chain reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was performed to confirm the changes in BDNF and TrkB mRNA. TRIzol reagent (Sigma-Aldrich) was used to isolate total RNA from the rat brain; the total RNA was then synthesized into cDNA by room temperature (Takara Bio, Otsu, Japan). To analyze the expression levels of BDNF and TrkB in the synthesized cDNA, PCR was performed using 2 μg total RNA, reverse transcriptase and random hexamer primers (QIAGEN, Germantown, MD, USA) at 65°C for 10 min and 42°C for 1 h. For the cDNA amplification process, Taq DNA polymerase (Takara Bio), pre-stained solution, cDNA, and primers were added to a premix (Bioneer, Oakland, CA, USA) containing dNTPs; PCR was performed at 57°C for 27 cycles for BDNF and 58°C for 28 cycles for TrkB using a thermal cycler (MJ Research, Watertown, MA, USA). As an internal standard, glyceraldehyde 3-phosphate dehydrogenase primer was used. BDNF and TrkB expression levels were corrected on the basis of amplification results. The amplified cDNA was subjected to electrophoresis in a 1.5% agarose gel; cDNA expression was observed with an ultraviolet-visible spectrophotometer (Kodak, Rochester, NY, USA) and analyzed using Image TotalLab software (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Western blot analysis
Total protein was extracted from the brain to detect nuclear factor kappa B (NF-κB) protein in the hippocampus. To extract total protein, brain tissue was homogenized with lysis buffer containing a phosphatase inhibitor and a protease inhibitor (CyQUANT; Invitrogen, Carlsbad, CA, USA). To separate proteins from the homogenized sample, the supernatant was collected by centrifugation (12,000 rpm, 15 min, 4°C). The protein concentration was measured using a colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel, electrophoresis at 120 V, then electrotransferred to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). After incubation with a mouse NF-κB antibody (1:500; Cell Signaling, Danvers, MA, USA), the membrane was incubated with horseradish peroxidase conjugated goat anti-mouse IgG secondary antibody (Santa Cruz Biotech, Santa Cruz, CA, USA). A chemiluminescent kit (Super Signal West Pico; Pierce, Rockford, IL, USA) was used to detect NF-κB protein. Protein content was analyzed using an enhanced chemiluminescence detection system (Santa Cruz), and the density was measured using the program Tina 2.1.
Statistical analyses
Data are expressed as means ± standard errors of the mean. Data analyses and graph plotting were conducted using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). To evaluate the change in body weight, Student’s t-test was performed; individual comparisons were made using one-way analysis of variance (ANOVA) with Tukey’s post-hoc test to evaluate changes in behavior and cytokine levels. Learning performance in the MWM test was processed by two-way repeated measures ANOVA; a simple main effect analysis for group comparison and individual comparison of Tukey’s post-hoc test were performed at each training session. p < 0.05 was considered statistically significant.
RESULTS
Rats subjected to SD have reduced body weight and food intake
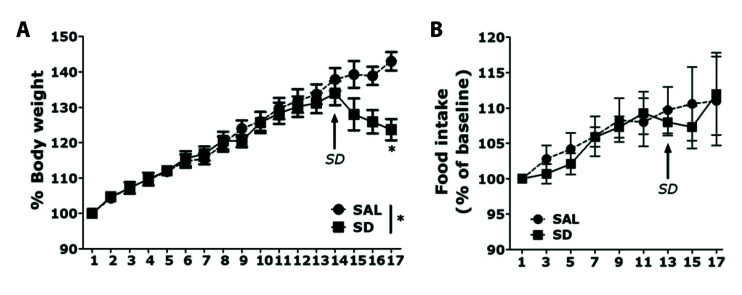
We measured the weights of rats in the SAL-treated control group and SD group for 17 days. Analyses of body weight changes in both groups showed that the rats gained body weight over time (Fig. 2A). However, on the first day after SD induction, the body weight on day 14 was reduced: the SD group showed significant body weight loss on day 17, compared with the SAL group (t = 2.949, p < 0.05). This body weight loss was not associated with SD-induced memory impairment. Nevertheless, body weight loss is an important indicator of rat physiological health; therefore, SD can be used to ensure that rats are sufficiently physiologically stressed. However, rats treated with MYR showed no significant difference in the reduction in body weight gain compared with the SD group (data not shown). We also measured food consumption over 17 days (Fig. 2B). The SD group showed a tendency for decreased food intake after the first day of SD induction, but this was not significantly different from the intake in the SAL group. In particular, the difference in food intake during 3 days after the onset of SD induction did not reach statistical significance between the SAL and SD groups (t = 0.059, p = 0.907). Although this comparison did not reveal a statistically significant interaction between SAL and SD groups, it showed less food consumption by the SD group than by the SAL group during the SD period.
Fig. 2. Results of body weight and food intake analyses of rats subjected to 14 days of sleep deprivation (SD).
(A) Body weight and (B) food intake were significantly lower in SD-exposed rats than in saline (SAL)-treated rats (significant main effect of SD exposure vs. control handling). Data are shown as means ± SEM. *p < 0.05 vs. SAL group.
MYR ameliorates impairments of cognitive and spatial memory in rats subjected to SD
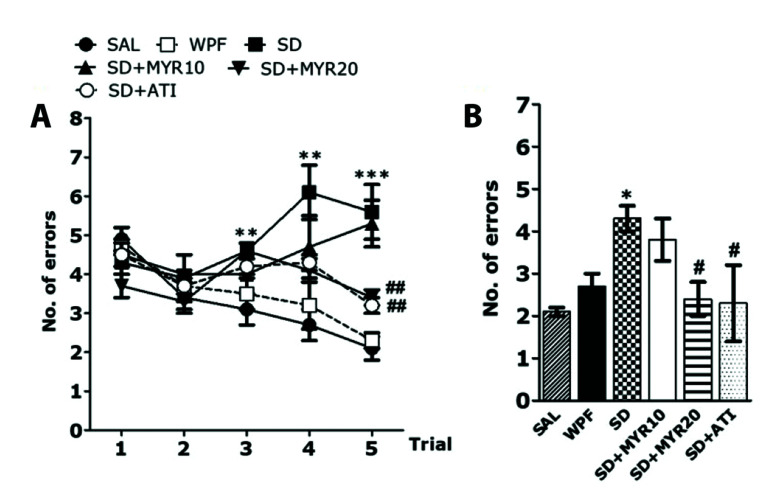
An 8-arm RAM task was used to determine whether SD affected memory performance in rats. In the radial maze task, SAL-treated control normal rats made the correct choice (> 75 % accuracy) to acquire eight foods placed on eight arms. In addition, the WPF group also made the correct choice during food acquisition (> 70 % accuracy) (data not shown). However, rats in the SD group showed less frequently made the correct choice during food acquisition, compared with the SAL and WPF groups. This implied that SD interfered with short-term memory and working memory (Fig. 3A). The SD group showed a slower acquisition curve, compared with the SAL group (p < 0.01 for trials 3 and 4, p < 0.001 for trial 5). The SD group showed more errors, compared with the SAL group. ANOVA (4 × 6, treatment × time) performed on the number of errors indicated a significant difference among groups (F5,33 = 9.091, p < 0.001), and a significant effect of day (F1,33 = 2.633, p = 0.114); it did not reveal a group × day interaction (F5,33 = 8.815; p < 0.001). In post-hoc analyses, the SD + MYR20 group showed significantly fewer errors, compared with the SD group (p < 0.01). This recovery effect of MYR treatment revealed a significant effect during the 5th trial. In the retention test performed 24 h after rats reached the learning criterion, the SD group demonstrated significantly more errors, compared with the SAL group (p < 0.05), and the WPF group showed similar values to the SAL group (Fig. 3B). However, in the retention test, the SD + MYR20 group demonstrated significantly fewer errors, compared with the SD group (p < 0.05). The performance of the 8-arm RAM task was impaired in the SD group, and MYR treatment attenuated SD-induced learning and memory impairment in the 8-arm RAM task.
Fig. 3. Effects of myricetin (MYR) on the number of errors in the 8-arm RAM task in sleep deprivation (SD)-induced rats.
The task was started during the second week after SD, and four trials were performed each day. (A) Comparison of rat performance during the acquisition phase and (B) short-term memory test. 8-arm RAM task, eight-arm radial maze task; WPF, wide platform; SAL, saline; ATI, alprazolam. *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SD group.
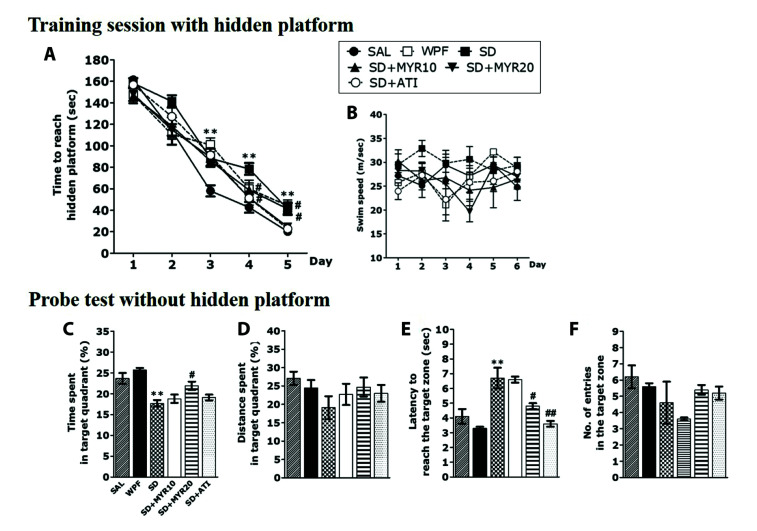
Following MYR treatment in our SD animal model, the MWM test was performed to determine the effects of MYR on learning and memory ability. The MWM is a test that evaluates working memory and long-term memory. Two-way repeated measures ANOVA revealed that the escape time to find the hidden platform was gradually shortened in the SAL group during the 5-day training period (acquisition phase; Fig. 4A), indicating that the normal rats had an improved ability to identify the hidden platform. However, the SD group spent more time finding the hidden platform, compared with the SAL group (p < 0.01 on days 3 to 5). Similar to the normal group, the WPF group had a gradually shortened time to find the hidden platform. In contrast, the MYR group administered a dose of 20 mg/kg had a significantly shorter escape delay, compared with the SD group (p < 0.05). However, there were no differences among groups in the escape latency required to find the escape platform from the first to fourth sessions, indicating that the difference in learning ability among groups increased over time. The SD group exhibited delayed learning from the beginning of the learning phase, indicating that learning was inhibited by MYR treatment. There was no difference in mean swimming speed among groups, implying that the time to reach the platform was mainly influenced by memory impairment (Fig. 4B). In the retention test (probe test) conducted on day 6, we assessed the time spent in the target area (no hidden platform), distance spent in the target area, efficiency of the path to reach the target area and number of entries to the target area. There were significant changes in performance among all rats. In particular, normal rats showed efficiency in all aspects of the spatial memory test. The WPF group showed efficiency in finding the target area with the hidden platform, at a level similar to efficiency in the normal group. However, SD-exposed rats displayed impaired spatial memory (Fig. 4C–F). The MYR group administered a dose of 20 mg/kg showed a significant decrease in the length of time spent in the target area compared with the SD group (p < 0.05); this MYR group also exhibited a significantly efficiency of the path to reach the target area (p < 0.05). These results were similar between the MYR- and alprazolam-treated groups.
Fig. 4. The Morris water maze test was used to assess the effects of myricetin (MYR) on spatial learning and memory.
Time to escape (latency) from the water onto a submerged platform during acquisition trials, (A) a submerged platform during acquisition trials, (B) swimming speed, (C) percentage of time spent in the target quadrant, (D) percentage of distance traversed in the target quadrant, (E) path efficiency to reach the target zone, and (F) number of entries to the target zone outcome measures. SD, sleep deprivation; SAL, saline; WPF, wide platform; ATI, alprazolam. **p < 0.01 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SD group.
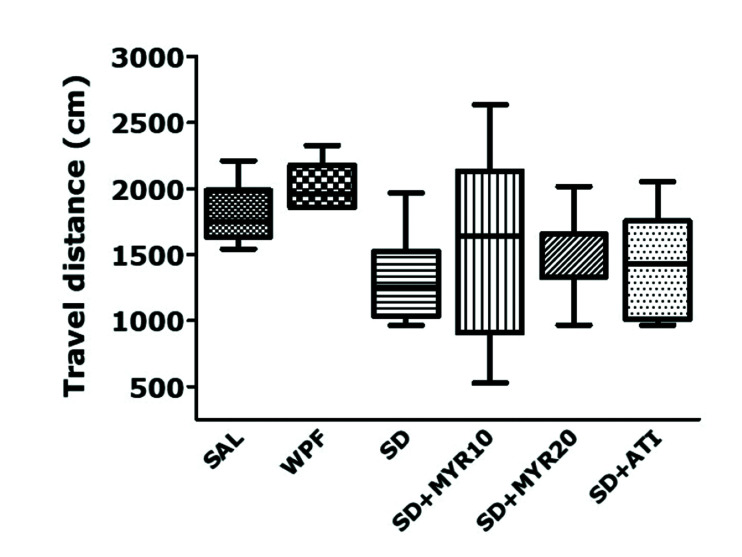
The MWM test showed that impairment of learning and memory occurred in the SD group; these effects were prevented by MYR treatment for 14 days. Because the effect of the SD procedure or drug administration may have been secondary to the change in rat locomotor activity, we examined the locomotor activities of rats in an environment similar to their breeding conditions. The observed behaviors were not affected by traumatic stress or MYR administration (Fig. 5). There was no significant difference among groups in the distance of locomotor activity, but the distance of locomotor activity tended to be higher in the SD group than in the normal group (F5,38 = 0.094, p = 0.993). This implied that the observed behavior in rats was only caused by memory impairment, rather than by other pathological factors or side effects.
Fig. 5. Effects of MYR administration on locomotor activity in the OFT during SD.
MYR, myricetin; OFT, open-field test; SAL, saline; WPF, wide platform; ATI, alprazolam.
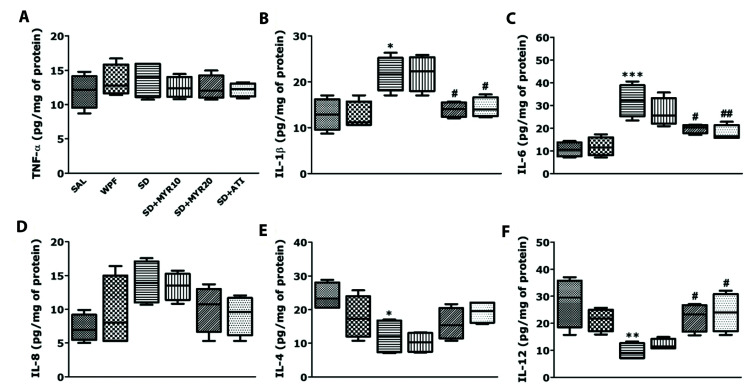
MYR decreases pro-inflammatory cytokine expression levels and increases anti-inflammatory cytokine expression levels in the hippocampus of rats subjected to SD
ELISA was used to evaluate the effects of MYR on the expression levels of inflammatory cytokines in hippocampal tissue. In the SD group, pro-inflammatory cytokine (TNF-α, IL-1β, IL-6, and IL-8) levels were increased in the hippocampus, while anti-inflammatory cytokine (IL-4 and IL-12) levels were decreased (Fig. 6). The SD group showed increases in the expression levels of TNF-α, IL-1β (p < 0.05), IL-6 (p < 0.001), and IL-8 in the hippocampus, compared with the SAL-treated normal group. In addition, the SD group showed decreases in the expression of IL-4 (p < 0.05) and IL-12 (p < 0.01) in the hippocampus, compared with the SAL-treated normal group. However, MYR treatment (20 mg/kg) significantly attenuated the SD-induced increases in the expression levels of IL-1β and IL-6 in the hippocampus (p < 0.05); it also attenuated the decrease in IL-12 expression level (p < 0.05).
Fig. 6. Effects of myricetin (MYR) on tumor necrosis factor (TNF)-α (A), interleukin (IL)-1β (B), IL-6 (C), IL-8 (D), IL-4 (E), and IL-12 (F) concentrations in the hippocampus of rats exposed to sleep deprivation (SD), determined by ELISA analysis.
SAL, saline. *p < 0.05, **p < 0.01, ***p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SD group.
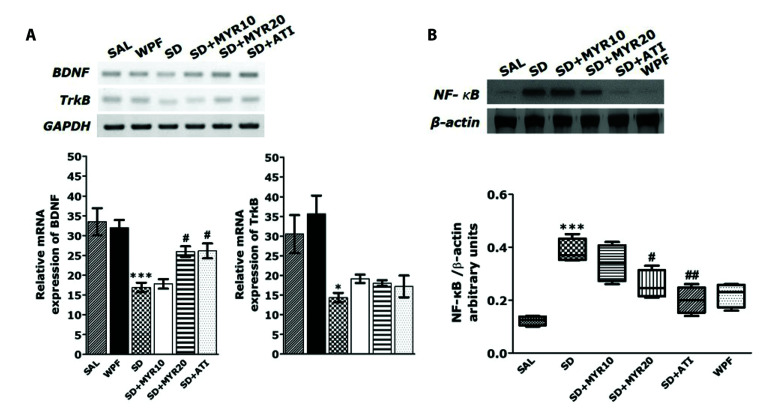
MYR increases mRNA expression levels of BDNF and TrkB in the hippocampus of rats subjected to SD
The mRNA expression levels of BDNF and TrkB upon MYR administration after SD were assessed by PCR analysis. SD markedly reduced the mRNA expression levels of BDNF and TrkB. Two-way ANOVA with MYR administration and SD induction as the main effects showed significant differences among groups in terms of BDNF mRNA (p < 0.001) and TrkB mRNA (p < 0.05; Fig. 7A) expression levels. One-way ANOVA and Tukey’s post-hoc test showed that BDNF mRNA was significantly decreased in the SD group, compared with the SAL-treated normal group. However, in the group subjected to repeated MYR pretreatment, BDNF mRNA expression was significantly increased, compared with the SD group (153.34%, p < 0.05). One-way ANOVA and Tukey’s post-hoc test showed that TrkB mRNA expression was decreased in the SD group, compared with the SAL-treated control group. However, in the group subjected to repeat MYR pretreatment, TrkB mRNA expression was increased compared with the SD group (125.69%, p = 0.953).
Fig. 7. Effects of myricetin (MYR) on the expression of brain-derived neurotrophic factor (BDNF) and tropomyosin-related kinase B (TrkB) mRNA in rats with sleep deprivation (SD)-induced hippocampal impairment.
(A) PCR bands on agarose gels and relative intensities are shown. Expression levels of BDNF and TrkB mRNAs were normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA as the internal control, (B) activation of nuclear factor kappa B (NF-κB) in the hippocampus after MYR treatment. Western blot analysis of protein expression levels of NF-κB. SAL, saline; WPF, wide platform; ATI, alprazolam. *p < 0.05, ***p < 0.001 vs. SAL group; #p < 0.05, ##p < 0.01 vs. SD group.
MYR reduces the activation of NF-κB in the hippocampus of rats subjected to SD
The effects of MYR on the protein expression level of NF-κB in hippocampal tissue were determined by Western blotting. The SD group showed a significant increase in NF-κB protein expression level in the hippocampus, compared with the SAL-treated normal group (p < 0.001; Fig. 7B). However, MYR treatment (20 mg/kg) significantly attenuated the SD-induced increase in NF-κB protein expression level (p < 0.05).
DISCUSSION
Our results showed that SD-induced learning and memory deficits, increased NF-κB-mediated pro-inflammatory cytokine expression levels, and decreased the expression of the neurotrophic factor, BDNF in the hippocampus. Furthermore, sleep-deprived rats showed no change in food intake, although they showed a decrease in body weight. These results suggested that sleep is essential for cognitive function; moreover, sleep disturbances caused physiological and behavioral changes in rats. MYR treatment improved memory function in SD-induced rats, inhibited the production of pro-inflammatory cytokines, inhibited the reduction of anti-inflammatory cytokines, and restored BDNF expression levels in the hippocampus. Thus, MYR treatment significantly alleviated cognitive dysfunction caused by SD through regulation of NF-κB and BDNF; thus, it may serve as an effective anti-inflammatory and potential neuroprotective agent.
In our study, SD led to changes in body weight, suggesting that body weight loss and changes in food intake because of SD induce physiological and psychological stress. This body weight loss can lead to SD-induced neuroinflammation. Several studies have shown that lipopolysaccharide injection and SD increase pro-inflammatory cytokine levels in the hippocampus and decrease body weight in rats [37]. Therefore, chronic stress caused by SD can sufficiently induce neuroinflammation in the hippocampus [38], and MYR treatment can sufficiently suppress neuroinflammation.
In this study, 8-arm RAM (short-term memory) and MWM (long-term memory) tests were performed to measure the effects of MYR administration on the improvement of spatial learning and memory function in rats subjected to SD. Sleep-deprived rats showed significant impairment in working or spatial memory, as evaluated in the 8-arm RAM task. In particular, sleep-deprived rats had significantly more errors when learning the radial arm maze and required more time to complete all tasks in the 8-arm RAM. Thus, the rats did not establish short-term memories of collecting food rewards [39]. However, rats administered MYR performed at a much faster rate (shorter time) than the SD group in the 8-arm RAM task; they also had fewer errors when learning the maze.
Furthermore, sleep-deprived rats showed considerably longer escape latencies to reach the platform in the MWM test, compared with the SAL-treated control group, indicating that sleep-deprived rats had impaired spatial learning ability [40]. Sleep-deprived rats reportedly have impairment in memory consolidation and retrieval in retention tests [40]. Unlike RAM, the MWM task evaluated spatial memory for 5 days and assessed spatial memory learned over a long period of time. However, MYR treatment significantly shortened the escape delay time (latencies) and the distance to the escape zone in the spatial learning test, suggesting that MYR restored the impairment of spatial learning ability that had been caused by SD. In addition, MYR treatment improved the crossing time and target distance around the target quadrant in the spatial probe trail, thus increasing the learning speed and improving memory consolidation. These results showed that MYR administration could improve impairment of spatial ability that had been caused by SD; it also significantly ameliorated severe memory recall and retrieval difficulties. Therefore, our findings suggest that MYR administration can elicit significant improvement in short-term (working) memory and long-term (spatial) memory.
Because no difference in locomotor activity was observed among groups in the open field test, MYR administration did not affect motor function abnormalities or motor performance improvement. Therefore, MYR-related reductions in the number of errors for food reward and in the time required to reach the platform in the 8-arm RAM and MWM tasks were caused by improvements in rat memory deficit, rather than improvement of rat locomotor activity.
We also measured changes in inflammatory cytokine expression levels in the hippocampus to determine whether MYR administration indirectly improved memory performance in sleep-deprived rats through regulation of neuroinflammation. We applied 72-h SD using a multiplatform method, because 72-h SD causes memory impairment in rats with significant decreases in working memory and spatial memory performance [41]. The mechanisms responsible for the amnestic effect induced by SD have not been clearly elucidated; however, some studies have suggested that this effect is related to the regulation of neuroinflammation, which involves mediators of pro-inflammatory cytokines [42]. Neuroinflammation-induced cytokine imbalance (increased pro-inflammatory cytokine expression and decreased anti-inflammatory cytokine expression) in the hippocampus causes memory dysfunction [43]. In addition, pro-inflammatory cytokine expression levels are increased in the hippocampus of SD-induced rats [42]. Some studies have shown that the expression levels of TNF-α, IL-1β, and IL-6 are increased in 72-h sleep deprived rats; moreover, neuroinflammation-related changes in spatial memory cause memory disability [44-46]. Our results also showed that 72-h SD increased the expression levels of pro-inflammatory cytokines and impaired spatial memory. However, MYR administration ameliorated neuroinflammation by inhibiting activation of the NF-κB pathway in the hippocampus. Specifically, MYR administration regulated neuroinflammation (inhibited pro-inflammatory cytokine expression and increased anti-inflammatory cytokine expression) by inhibiting NF-κB pathway activation in SD-induced rats. These data showed that MYR administration can significantly attenuate SD-induced cognitive dysfunction by regulating neuroinflammation via inhibition of inflammatory cytokines and activation of the NF-kB pathway.
BDNF is an important mediator between cognitive impairment and neuroinflammation, and modulation of BDNF signaling may be an important factor in the regulation of neuroinflammation and cognitive function in sleep-deprived rats [47]. In addition, neuroinflammation reduces BDNF expression levels in the hippocampus [48]. Administration of IL-1β receptor antagonists reportedly inhibits the reduction of BDNF, suggesting that pro-inflammatory cytokines have important roles in regulating BDNF expression levels [49-51]. In this study, MYR administration reversed the SD-induced reduction in BDNF mRNA expression, which was likely associated with inhibition of both NF-κB activation and pro-inflammatory cytokine expression (e.g., IL-1β and IL-6). BDNF activates the neuronal signaling pathway of BDNF-TrkB in the brain [52], which participates in the transcription, translation, and trafficking of proteins during synaptic maturation and synaptic plasticity. BDNF also downregulates neuroinflammation-related protein expression [52]. These data suggested that SD-induced neuroinflammation improves impaired memory function by downregulating the BDNF-TrkB pathway. Our results showed that the mRNA expression level of memory-related BDNF was decreased because of SD in rats; MYR treatment markedly inhibited the SD-induced decreases in mRNA expression levels of BDNF and TrkB in the hippocampus. Thus, MYR treatment may activate the BDNF-TrkB signaling pathway in SD-induced memory impairment, thereby regulating neuroinflammation and improving cognitive function. Our findings suggest that the memory-enhancing effect of MYR is related to the inhibition of neuroinflammation via BDNF-TrkB signaling pathway modulation, it is also associated with the inhibition of both pro-inflammatory cytokine expression and NF-κB activation.
This study showed that MYR administration improved cognitive function and abilities (e.g., learning acquisition and memory consolidation) in SD-induced memory impairment in rats, and might be involved in regulating BDNF and NF-kB activation. Therefore, MYR may be a useful alternative therapeutic agent for the treatment of SD-related diseases.
ACKNOWLEDGEMENTS
None.
Footnotes
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (2020R1A2C1100975).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Costa e Silva JA. Sleep disorders in psychiatry. Metabolism. 2006;55(10 Suppl 2):S40–S44. doi: 10.1016/j.metabol.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Alkadhi K, Zagaar M, Alhaider I, Salim S, Aleisa A. Neurobiological consequences of sleep deprivation. Curr Neuropharmacol. 2013;11:231–249. doi: 10.2174/1570159X11311030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14:219–226. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Stickgold R. Sleep: off-line memory reprocessing. Trends Cogn Sci. 1998;2:484–492. doi: 10.1016/S1364-6613(98)01258-3. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Zion M, Shabi A, Levy S, Glasner L, Wiener A. Effects of partial sleep deprivation on information processing speed in adolescence. J Int Neuropsychol Soc. 2016;22:388–398. doi: 10.1017/S1355617716000072. [DOI] [PubMed] [Google Scholar]
- 6.Feng L, Wu HW, Song GQ, Lu C, Li YH, Qu LN, Chen SG, Liu XM, Chang Q. Chronical sleep interruption-induced cognitive decline assessed by a metabolomics method. Behav Brain Res. 2016;302:60–68. doi: 10.1016/j.bbr.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Tripathi S, Jha SK. Short-term total sleep deprivation alters delay-conditioned memory in the rat. Behav Neurosci. 2016;130:325–335. doi: 10.1037/bne0000136. [DOI] [PubMed] [Google Scholar]
- 8.Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem. 2005;12:352–359. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65(12 Pt 2):S244–S252. doi: 10.1301/nr.2007.dec.S244-S252. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 11.Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 12.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–1330. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 13.Hill S, Tononi G, Ghilardi MF. Sleep improves the variability of motor performance. Brain Res Bull. 2008;76:605–611. doi: 10.1016/j.brainresbull.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li XY, Descalzi G, Kim SS, Chen T, Shang YZ, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signalling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes C, Rocha NB, Rocha S, Herrera-Solís A, Salas-Pacheco J, García-García F, Murillo-Rodríguez E, Yuan TF, Machado S, Arias-Carrión O. Detrimental role of prolonged sleep deprivation on adult neurogenesis. Front Cell Neurosci. 2015;9:140. doi: 10.3389/fncel.2015.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeira J, Kolluru SS, Rosenblatt H, Kry J, Strecker RE, McCarley RW. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav Brain Res. 2018;339:124–129. doi: 10.1016/j.bbr.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu J, Li P, Ouyang X, Gu C, Song Z, Gao J, Han L, Feng S, Tian S, Hu B. Rapid eye movement sleep deprivation selectively impairs recall of fear extinction in hippocampus-independent tasks in rats. Neuroscience. 2007;144:1186–1192. doi: 10.1016/j.neuroscience.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 18.Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- 19.Ravassard P, Pachoud B, Comte JC, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi PH, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–374. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- 21.Ma TC, Zhu XZ. Effects of intrahippocampal infusion of interleukin-6 on passive avoidance and nitrite and prostaglandin levels in the hippocampus in rats. Arzneimittelforschung. 2000;50:227–231. doi: 10.1055/s-0031-1300190. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham AJ, Murray CA, O'Neill LA, Lynch MA, O'Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 23.Motivala SJ. Sleep and inflammation: psychoneuroimmunology in the context of cardiovascular disease. Ann Behav Med. 2011;42:141–152. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 24.Wadhwa M, Kumari P, Chauhan G, Roy K, Alam S, Kishore K, Ray K, Panjwani U. Sleep deprivation induces spatial memory impairment by altered hippocampus neuroinflammatory responses and glial cells activation in rats. J Neuroimmunol. 2017;312:38–48. doi: 10.1016/j.jneuroim.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Mahboubi S, Nasehi M, Imani A, Sadat-Shirazi MS, Zarrindast MR, Vousooghi N, Noroozian M. Benefit effect of REM-sleep deprivation on memory impairment induced by intensive exercise in male wistar rats: with respect to hippocampal BDNF and TrkB. Nat Sci Sleep. 2019;11:179–188. doi: 10.2147/NSS.S207339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Xie Y, Niu K, Li K. Electroacupuncture ameliorates post-traumatic stress disorder in rats via a mechanism involving the BDNF-TrkB signaling pathway. Cell Mol Biol (Noisy-le-grand) 2020;66:165–170. doi: 10.14715/cmb/2020.66.3.26. [DOI] [PubMed] [Google Scholar]
- 27.Semwal DK, Semwal RB, Combrinck S, Viljoen A. Myricetin: a dietary molecule with diverse biological activities. Nutrients. 2016;8:90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang KA, Wang ZH, Zhang R, Piao MJ, Kim KC, Kang SS, Kim YW, Lee J, Park D, Hyun JW. Myricetin protects cells against oxidative stress-induced apoptosis via regulation of PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 2010;11:4348–4360. doi: 10.3390/ijms16011482. Erratum in: Int J Mol Sci. 2015;16:1482-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao Y, Lin G, Xie Y, Ma P, Li G, Meng Q, Wu T. Preformulation studies of myricetin: a natural antioxidant flavonoid. Pharmazie. 2014;69:19–26. [PubMed] [Google Scholar]
- 30.Ramezani M, Darbandi N, Khodagholi F, Hashemi A. Myricetin protects hippocampal CA3 pyramidal neurons and improves learning and memory impairments in rats with Alzheimer's disease. Neural Regen Res. 2016;11:1976–1980. doi: 10.4103/1673-5374.197141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L, Xu P, Fu T, Huang X, Song J, Chen M, Tian X, Yin H, Han J. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Mol Med Rep. 2018;17:3274–3280. doi: 10.3892/mmr.2017.8212. [DOI] [PubMed] [Google Scholar]
- 32.Sun ZQ, Meng FH, Tu LX, Sun L. Myricetin attenuates the severity of seizures and neuroapoptosis in pentylenetetrazole kindled mice by regulating the of BDNF-TrkB signaling pathway and modulating matrix metalloproteinase-9 and GABAA. Exp Ther Med. 2019;17:3083–3091. doi: 10.3892/etm.2019.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu S, Yue Y, Peng A, Zhang L, Xiang J, Cao X, Ding H, Yin S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016;7:2624–2634. doi: 10.1039/C6FO00419A. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan G, Ray K, Sahu S, Roy K, Jain V, Wadhwa M, Panjwani U, Kishore K, Singh SB. Adenosine A1 receptor antagonist mitigates deleterious effects of sleep deprivation on adult neurogenesis and spatial reference memory in rats. Neuroscience. 2016;337:107–116. doi: 10.1016/j.neuroscience.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Wolkow A, Ferguson SA, Vincent GE, Larsen B, Aisbett B, Main LC. The impact of sleep restriction and simulated physical firefighting work on acute inflammatory stress responses. PLoS One. 2015;10:e0138128. doi: 10.1371/journal.pone.0138128.52692d0a75d04a9bac8aa4847a6957e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B, Choi Y, Kim H, Kim SY, Hahm DH, Lee HJ, Shim I. Protective effects of methanol extract of Acori graminei rhizoma and Uncariae Ramulus et Uncus on ischemia-induced neuronal death and cognitive impairments in the rat. Life Sci. 2003;74:435–450. doi: 10.1016/j.lfs.2003.06.034. [DOI] [PubMed] [Google Scholar]
- 37.Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur T, Singh H, Mishra R, Manchanda S, Gupta M, Saini V, Sharma A, Kaur G. Withania somnifera as a potential anxiolytic and immunomodulatory agent in acute sleep deprived female Wistar rats. Mol Cell Biochem. 2017;427:91–101. doi: 10.1007/s11010-016-2900-1. [DOI] [PubMed] [Google Scholar]
- 39.Noorafshan A, Karimi F, Karbalay-Doust S, Kamali AM. Using curcumin to prevent structural and behavioral changes of medial prefrontal cortex induced by sleep deprivation in rats. EXCLI J. 2017;16:510–520. doi: 10.17179/excli2017-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou J, Shen Q, Wan X, Zhao B, Wu Y, Xia Z. REM sleep deprivation-induced circadian clock gene abnormalities participate in hippocampal-dependent memory impairment by enhancing inflammation in rats undergoing sevoflurane inhalation. Behav Brain Res. 2019;364:167–176. doi: 10.1016/j.bbr.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 41.Kumar A, Singh A. Protective effect of St. John's wort (Hypericum perforatum) extract on 72-hour sleep deprivation-induced anxiety-like behavior and oxidative damage in mice. Planta Med. 2007;73:1358–1364. doi: 10.1055/s-2007-990234. [DOI] [PubMed] [Google Scholar]
- 42.Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S, Panjwani U. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017;14:222. doi: 10.1186/s12974-017-0998-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Austin PJ, Berglund AM, Siu S, Fiore NT, Gerke-Duncan MB, Ollerenshaw SL, Leigh SJ, Kunjan PA, Kang JW, Keay KA. Evidence for a distinct neuro-immune signature in rats that develop behavioural disability after nerve injury. J Neuroinflammation. 2015;12:96. doi: 10.1186/s12974-015-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham J, Johnson RW. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12:445–453. doi: 10.1089/rej.2009.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adzovic L, Lynn AE, D'Angelo HM, Crockett AM, Kaercher RM, Royer SE, Hopp SC, Wenk GL. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation. 2015;12:63. doi: 10.1186/s12974-015-0321-9. Erratum in: J Neuroinflammation. 2015;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramesh V, Nair D, Zhang SX, Hakim F, Kaushal N, Kayali F, Wang Y, Li RC, Carreras A, Gozal D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Guan Z, Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav Immun. 2006;20:64–71. doi: 10.1016/j.bbi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, Hayashi T, Xia M, Tashiro S, Onodera S, Ikejima T. Protective effect of silibinin on learning and memory impairment in LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res. 2016;41:1662–1672. doi: 10.1007/s11064-016-1881-5. [DOI] [PubMed] [Google Scholar]
- 50.Vasconcelos AR, Yshii LM, Viel TA, Buck HS, Mattson MP, Scavone C, Kawamoto EM. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J Neuroinflammation. 2014;11:85. doi: 10.1186/1742-2094-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 52.Hwang L, Ko IG, Jin JJ, Kim SH, Kim CJ, Chang B, Rho JH, Moon EJ, Yi JW. Dexmedetomidine ameliorates memory impairment in sleep-deprived mice. Anim Cells Syst (Seoul) 2019;23:371–379. doi: 10.1080/19768354.2019.1688185. [DOI] [PMC free article] [PubMed] [Google Scholar]