Highlights
-
•
Orexin neuropeptides roles in hippocampal-dependent memory formation.
-
•
Orexin neuropeptides activate the neural circuits of the basolateral amygdala.
-
•
The power of memorization is modulated by the level of orexin neuropeptides.
Keywords: Orexin neuropeptides, Hippocampus, Basolateral amygdala
Abstract
Orexin neuropeptides have functional roles in hippocampal-dependent memory formation via the consolidation and retrieval of passive avoidance and spatial memories. The effects of these neuropeptides have been confirmed on the induction of long-term potentiation (LTP). The orexinergic system seems to have modulatory effects by sending projection fibers to several brain parts, such as the hippocampus and amygdala. Orexin neuropeptides activate the neural circuits of the basolateral amygdala during different arousal events with various emotional loads. Therefore, this system plays a vital role in creating appropriate behavioral reactions and responses particular to the situation. This review aimed to report new progression and advances in the hippocampus function in memory by focusing on its relationship with the amygdala through the orexinergic system.
1. Introduction
The hippocampal formation is a C-shaped structure distended into the lateral ventricles. This structure consists of different parts such as the dentate gyrus (DG), subiculum, presubiculum, parasubiculum, and entorhinal cortex. In the longitudinal section, the hippocampus is divided into the dorsal, intermediate, and ventral parts, and the transverse axis is divided into CA1, CA3, and the dentate gyrus. The hippocampus's primary cell layout and neuron fibers are the same in all mammals [1]. The entrance to the hippocampus consists of fibers that reach the dentate gyrus via the entorhinal cortex. The axons of the granular cells in the dentate gyrus make mossy fibers that send projections to the dendrites of the pyramidal cells in CA3. The axons of the pyramidal cells in CA3 form Schaffer collateral pathways that connect to CA1 [2]. The unique structure and properties of the hippocampus as an essential area for memory processing are also an interesting target of research. The amygdala is another part of the limbic system consisting of central, basal, and lateral nuclei. The amygdala also has several interconnections with various structures and systems in the brain. The amygdala, the neocortex and the hippocampus are target regions of the various brain system including the adrenergic and orexinergic systems, which have different effects on cognitive functions, such as memory and learning. The amygdala impacts different kinds of memories, such as emotionally charged learning underlying Pavlovian fear conditioning [3]. In addition to mediating emotional memories, the amygdala also has a role in allowing emotional arousal to influence memory formation in other brain regions, such as the hippocampus. The amygdala impacts its target brain regions either directly through glutamatergic projections to or indirectly through activation of the hypothalamic-pituitary-adrenal axis. In line with this mechanism of action, the amygdala is also involved in hippocampal-dependent learning, such as passive avoidance learning and spatial reference memory. Different neurotransmitter systems within the amygdala affect learning and memory. Orexin projections to the amygdala recently has been investigated in several studies. Herein, we intended to report new progression and advances in the hippocampus function in memory by focusing on its relationship with the amygdala through the orexinergic system.
2.1. The orexinergic system
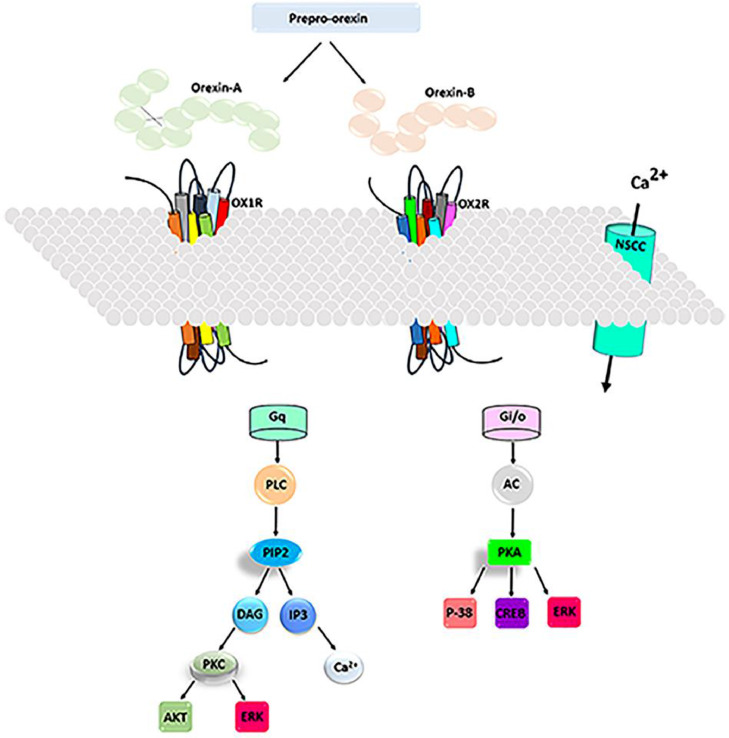
In 1998, two groups of researchers independently discovered two neuropeptides and their receptors while searching for new signaling molecules. One group [4] named these peptides orexin-A and -B because they were initially thought to enhance feeding behaviors in animals. The other group [5] named these peptides hypocretin-1 and −2 because they are produced in the hypothalamus and have similarities to the incretin peptides. This system consists of two endogenous neuropeptides, orexin-A and -B (also known as hypocretin-1 and −2), and their associated G protein-coupled receptors, orexin 1 (OX1) and orexin 2 (OX2) receptors. OX1R is selective for orexin-A, whereas OX2R is a nonselective receptor for both orexin-A and orexin-B. Orexin-A and -B neuropeptides are made by processing the prepro-orexin precursor in an area of the dorsolateral hypothalamus [4]. Neuro-excitatory transduction in orexin receptors can be based on multiple mechanisms. Several studies infer that the major primary signaling transducer for OXR is Gq. These studies have demonstrated a strong coupling to the classically Gq-mediated responses, Ca2+ elevation and phospholipase C (PLC) activation. These mechanisms of action would cause the major response in neurons. The G-protein families Gi/o and Gs have also been implicated in the OXR signaling. Therefore, these mechanisms (the activation of Gq proteins and the stimulation of the phospholipase C-protein kinase C pathway) are shown here (Fig. 1). Orexin neuropeptides also act by increasing the intracellular concentrations of Ca2+. Another mechanism is the regulation of adenylyl cyclase and the activation of the protein kinase A [6,7]. The mechanisms of action of these peptides are briefly demonstrated through the pathways, as shown in Fig. 1. The orexinergic system fibers project to different areas in the brain, such as the thalamus, the hippocampus, dorsal raphe nuclei, the septum, and the amygdala. Orexin neurons are cross-linked to all areas of the brain known to enhance wakefulness and arousal, including the tuberomammillary nucleus, the locus coeruleus, and the dorsal raphe nucleus [8]. These neurons also send projection fibers to the brain nuclei that regulate motivation and emotion [9,10], such as the ventral tegmental area, nucleus accumbens, and the amygdala. Furthermore, orexin neurons innervate many brain regions, such as the septum, the hippocampus, and the amygdala, that regulate spatial learning and memory functions [8].
Fig. 1.
Schematic representation of the orexin signal transduction. OX1R binds orexin-A with high affinity, whereas the orexin receptor type 2 has the same affinity for both neuropeptides. Orexins binding to their receptors stimulates Gq or Gi subtypes of the G proteins, which activates phospholipase C or Adenylyl cyclase, leading to an increase in the cytosolic calcium ion levels and a downstream cascade response. In addition, orexin-A enhances intracellular calcium ion levels by binding to the OX1 receptors through activating nonselective cationic channels (NSCCs). Abbreviations: OX1R; orexin 1 receptor; OX2R: orexin 2 receptor; NSCC: non selective cationic channel; Gi: G protein inhibitory; PLC: phospholipase C; AC: adenylyl cyclase; PKA: protein kinase A; PIP2: Phosphatidylinositol 4, 5-bisphosphate; DAG: diacylglycerol; IP3: Inositol triphosphates; PKA: protein kinas A; AKT: protein kinase B; ERK: extracellular signal-regulated kinase; CREB: cAMP response element-binding protein; P-38: Mitogen-activated protein kinase.
2.2. The effects of the orexinergic system on hippocampal neural circuits
Previous studies have shown that the intracerebroventricular administration of orexin-A results in high-affinity binding of the neuropeptide to its receptors, which are widely expressed throughout memory-related areas in the brain, including the CA1, DG, and CA2 of the hippocampus, the prefrontal cortex, and the retrosplenial cortex [11,12]. Facilitative effects on passive avoidance [13,14] and spatial memory tasks [15] were described for orexin-A administration in rodents. The molecular mechanisms of such involvements were studied in vitro and in vivo. The results showed an increase in the phosphorylation of mitogen-activated protein kinase (MAPK) proteins in hippocampal cells in rodents. MAPK proteins are closely related to plasticity signaling [16]. Also, the central administration of orexin-B could augment the acquisition, consolidation, and recall of the memory process [17]. Further studies in the field revealed that orexin-A elevated neurogenesis in the dentate gyrus, which facilitated memory formation by enhancing the activity of neurotransmission pathways involved in the acquisition and consolidation of implicit memory tasks [18,19]. The orexinergic system may also have a role in aversive and appetitive learning, depending on the situation, and the stimulus context in that orexin signaling can facilitate attentional behavior and some types of learning and memory [20]. Moreover, stressful events have dramatic effects on learning and memory. The orexin system has close contact with regions and pathways involved in stress responses through connections to the hypothalamic-pituitary axis [21]. During stressful events, the hippocampus, which is vulnerable to stress-induced changes, expresses OX1 and OX2 receptors in various sub-regions of the hippocampus. It has been shown that the blockade of OX1 and OX2 receptors in the hippocampus reduced the anxiety and immobility caused by acute stress [22].
2.3. The effects of the orexinergic system on BLA
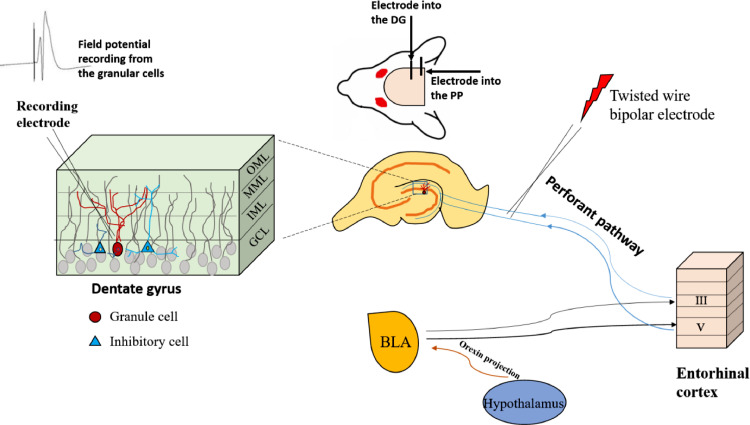
The amygdala is related to memory processing and consists of central (CE), basal (B), and lateral (LA) nuclei. The basolateral complex of the amygdala (BLA) plays a role in translating the emotional charge of events into vivid memories and modulating the emotional memory process in other related brain areas. This part of the amygdala directly or indirectly connects with other parts of the brain structures involved in the memory process, such as the entorhinal cortex, hippocampal formation, and the prefrontal cortex [23]. In fact, the amygdala plays an essential role in hippocampus-dependent and reward-based learning and memory through its basolateral complex. The power of memorization is modulated by a range of neurotransmitters, including norepinephrine, acetylcholine, GABA, opioids, and orexins [24]. Orexin circuits in BLA have proven effects on learning and memory. Different studies have shown that orexin deficiency is related to memory deficits that can be attenuated by orexin agonists. Emotional experiences affect our memory, leading to memory impairment or improvement through a change in synaptic plasticity in a context-dependent manner [25]. Synaptic plasticity is generally regulated by the release of various neurotransmitters from the presynaptic membrane or by varying the density, types, and properties of neurotransmitter receptors at the postsynaptic membrane. NMDA (N-methyl-D-aspartate) receptor-dependent long-term potentiation (LTP) and long-term depression (LTD) of signal transmission in excitatory neurons, such as hippocampal pyramidal neurons, is thought to underlie the formation of learning and memory processes. As mentioned, many neurotransmitter systems within BLA are involved in modulating the hippocampal synaptic plasticity, including LTP and LTD. The greatest density of projections that originate in the BLA, then terminate in layers 3 or 5 of the entorhinal cortex, some of which originating mainly from the layer V are also the main source of fibers that project from the amygdala to the DG. Orexinergic blockade studies using the LTP model reported inhibition of LTP induction in the perforant pathway-dentate gyrus. These studies concluded that the orexinergic system blockade was also involved in modulating (inhibitory) effects on the hippocampal region through BLA [26,27]. The schematic representation of this involvement is depicted in Fig. 2. The blockade of orexin receptors in BLA impairs memory consolidation and the retrieval of inhibitory avoidance learning through the amygdala-hippocampal pathway, showing the vital role of this system in learning and memory. The mechanism of this event was evaluated through LTP, and it was postulated that synaptic transmission efficacy in the hippocampus might be modulated indirectly by manipulating the orexin receptors of BLA.
Fig. 2.
Schematic representation of the BLA projections to the entorhinal cortex, the location of the stimulating electrode in the perforant pathway and recording electrode in the dentate gyrus. There is a monosynaptic pathway between the amygdala and dentate gyrus. These structures communicate together via a relay pathway involving the entorhinal cortex. Abbreviations: GCL; granular cell layer, IML; inner molecular layer, MML; mid molecular layer, OML; outer molecular layer, DG; dentate gyrus, PP; perforant pathway.
Furthermore, some fibers in BLA contain both dopamine beta-hydroxylase and orexin [28]. Therefore, it seems that orexin and norepinephrine efficiently collaborate in this brain area containing overlapping fibers from both systems. Previous studies have confirmed the interaction between the adrenergic and orexinergic systems [28,29]. Arousal experiences by inducing the noradrenergic system increase norepinephrine levels in the amygdala, leading to a change in the memory process by modulating synaptic plasticity in the hippocampus. These behavioral outcomes were confirmed by previous investigations on LTP induction in the hippocampus [30]. Different neurotransmitter systems are involved in emotional memory formation, which depends on interactions of several neuronal circuits within BLA. Previous studies in the field has shown that the orexinergic system can modulate certain cognitive functions and emotional behaviors and the behavior under situations of high motivational relevance, such as during physiological need states, exposure to threats, or reward opportunities [31].
There is some argument about emotional load and its effects on hippocampal-dependent memory. Although the emotional load of an event affects the long-term amplification of hippocampal synapses and improves learning, the formation of memory is not necessarily directly related to the amount of excitement associated with an event. Other studies have shown that high levels of excitement and excessive stress can cause memory impairment and attention deficiency [32,33]. These discrepancies may be due to the animal's emotional state, resulting in the release of a certain amount of norepinephrine at its targets, such as the hippocampus, and other neurotransmitters, such as orexins, which are released simultaneously with norepinephrine.
3. Conclusion
Orexin fibers widely project to several areas of the brain, such as the amygdala, the hippocampus, the cerebral cortex, and the bed nucleus of the stria terminalis [8]. In recent studies, the impressive effects of the orexinergic system antagonists have been shown on sleep disturbances [34] and repetitive and behavioral disorders like obsessive-compulsive disorder [35]. Given that orexins are involved in neural mechanisms mediating emotional memory formation, the manipulation of the orexin receptors can promote learning and memory impairments induced by different pathological situations. In this review, we explained that orexin signal transduction, through OX1 and OX2receptors, improved the formation and consolidation of emotional memory, probably by affecting the interaction between the amygdala and the hippocampus. The findings of this review define the therapeutic potential of orexin receptors as a novel target to impact learning problems associated with disorders caused by orexin deficiency.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
This investigation was funded by a grant at Mazandaran University of Medical Sciences [grant number 8090], Sari, and Iran.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cccb.2021.100035.
Appendix. Supplementary materials
References
- 1.Hevner R.F. Evolution of the mammalian dentate gyrus. J. Compar. Neurol. 2016;524(3):578–594. doi: 10.1002/cne.23851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharani K. In: The Biology of Thought. Dharani K, editor. Academic Press; San Diego: 2015. Chapter 3 - Memory; pp. 53–74. [Google Scholar]
- 3.Dębiec J., LeDoux J.E. In: Memory Reconsolidation. Alberini CM, editor. Academic Press; San Diego: 2013. Chapter four - Reconsolidation of pavlovian conditioned defense responses in the Amygdala; pp. 69–79. [Google Scholar]
- 4.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 5.De Lecea L., Kilduff T., Peyron C., Gao X.-.B., Foye P., Danielson P., et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kukkonen J.P., Leonard C. Orexin/hypocretin receptor signalling cascades. Br. J. Pharmacol. 2014;171(2):314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kukkonen J.P., Turunen P.M. Cellular signaling mechanisms of hypocretin/orexin. The Orexin System Basic Science and Role in Sleep Pathology. 2021;45:91–102. doi: 10.1159/000514962. [DOI] [PubMed] [Google Scholar]
- 8.Peyron C., Tighe D.K., Van Den Pol A.N., De Lecea L., Heller H.C., Sutcliffe J.G., et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakurai T., Mieda M. Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol. Sci. 2011;32(8):451–462. doi: 10.1016/j.tips.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Thompson J.L., Borgland S.L. A role for hypocretin/orexin in motivation. Behav. Brain Res. 2011;217(2):446–453. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Marcus J.N., Aschkenasi C.J., Lee C.E., Chemelli R.M., Saper C.B., Yanagisawa M., et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Compar. Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 12.Hervieu G., Cluderay J., Harrison D., Roberts J., Leslie R. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 13.Telegdy G., Adamik A. The action of orexin A on passive avoidance learning. Involvement of transmitters. Regul. Pept. 2002;104(1):105–110. doi: 10.1016/s0167-0115(01)00341-x. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger L.B., Farr S.A., Banks W.A., Morley J.E. Effects of orexin-A on memory processing. Peptides. 2002;23(9):1683–1688. doi: 10.1016/s0196-9781(02)00110-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X., xue Zhang R., Tang S., yan Ren Y., xia Yang W., min Liu X., et al. Orexin-A-induced ERK1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides. 2014;54:140–147. doi: 10.1016/j.peptides.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Selbach O., Bohla C., Barbara A., Doreulee N., Eriksson K., Sergeeva O., et al. Orexins/hypocretins control bistability of hippocampal long-term synaptic plasticity through co-activation of multiple kinases. Acta Physiol. 2010;198(3):277–285. doi: 10.1111/j.1748-1716.2009.02021.x. [DOI] [PubMed] [Google Scholar]
- 17.Palotai M., Telegdy G., Ekwerike A., Jászberényi M. The action of orexin B on passive avoidance learning. Involvement of neurotransmitters. Behav. Brain Res. 2014;272:1–7. doi: 10.1016/j.bbr.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Ito N., Yabe T., Gamo Y., Nagai T., Oikawa T., Yamada H., et al. Icv administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 2008;157(4):720–732. doi: 10.1016/j.neuroscience.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Yao Y., Li X., Zhang B., Yin C., Liu Y., Chen W., et al. Visual cue-discriminative dopaminergic control of visuomotor transformation and behavior selection. Neuron. 2016;89(3):598–612. doi: 10.1016/j.neuron.2015.12.036. [DOI] [PubMed] [Google Scholar]
- 20.Mahler S.V., Smith R.J., Moorman D.E., Sartor G.C., Aston-Jones G. Progress in Brain Research. 198. Elsevier; 2012. Multiple roles for orexin/hypocretin in addiction; pp. 79–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grafe L.A., Bhatnagar S. Orexins and stress. Front. Neuroendocrinol. 2018;51:132–145. doi: 10.1016/j.yfrne.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahramzadeh Zoeram S., Elahdadi Salmani M., Lashkarbolouki T., Goudarzi I. Hippocampal orexin receptor blocking prevented the stress induced social learning and memory deficits. Neurobiol. Learn. Mem. 2019;157:12–23. doi: 10.1016/j.nlm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Roozendaal B., Barsegyan A., Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog. Brain Res. 2007;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- 24.McDonald A.J. Handbook of Behavioral Neuroscience. 26. Elsevier; 2020. Functional neuroanatomy of the basolateral amygdala: neurons, neurotransmitters, and circuits; pp. 1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold P.E., Hankins L., Edwards R.M., Chester J., McGaugh J.L. Memory interference and facilitation with posttrial amygdala stimulation: effect on memory varies with footshock level. Brain Res. 1975 doi: 10.1016/0006-8993(75)90905-1. [DOI] [PubMed] [Google Scholar]
- 26.Ardeshiri M.R., Hosseinmardi N., Akbari E. Orexin 1 and orexin 2 receptor antagonism in the basolateral amygdala modulate long-term potentiation of the population spike in the perforant path-dentate gyrus-evoked field potential in rats. Neurobiol. Learn. Mem. 2018;149:98–106. doi: 10.1016/j.nlm.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 27.Noorani S.K., Hojati V., Akbari E., Ehsani S., Sakurai T., Ardeshiri M.R. The role of interaction between orexin receptors and β2 adrenergic receptors in basolateral amygdala in dentate gyrus synaptic plasticity in male rats. Brain Res. Bull. 2021;177:164–171. doi: 10.1016/j.brainresbull.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Baldo B.A., Daniel R.A., Berridge C.W., Kelley A.E. Overlapping distributions of orexin/hypocretin-and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J. Compar. Neurol. 2003;464(2):220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson K., Sergeeva O., Haas H., Selbach O. Orexins/hypocretins and aminergic systems. Acta Physiol. 2010;198(3):263–275. doi: 10.1111/j.1748-1716.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre C.K., Miyashita T., Setlow B., Marjon K.D., Steward O., Guzowski J.F., et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. 2005;102(30):10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahler S.V., Moorman D.E., Smith R.J., James M.H., Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci. 2014;17(10):1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lupien S.J., McEwen B.S. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res. Rev. 1997;24(1):1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 33.Nutt D.J. The psychobiology of posttraumatic stress disorder. J. Clin. Psychiatry. 2000 [PubMed] [Google Scholar]
- 34.Recourt K., de Boer P., Zuiker R., Luthringer R., Kent J., van der Ark P., et al. The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl. Psychiatry. 2019;9(1):1–10. doi: 10.1038/s41398-019-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abounoori M., Maddah M.M., Akbari E., Houshmand G., Ardeshiri M.R. The effect of orexin receptor antagonism on quinpirole-induced compulsive-like checking behavior in rats. Neurotox. Res. 2020:1–9. doi: 10.1007/s12640-020-00196-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.