Abstract
Introduction
Since the emergence of the novel coronavirus, herbal medicine has been considered a treatment for COVID-19 patients. This study was done to determine the efficacy of olive leaf extract on the outcomes of COVID-19 patients.
Materials and Methods
This randomized, triple-blinded clinical trial was conducted on hospitalized COVID-19 patients. Using block randomization, eligible patients were allocated to the following groups: intervention A received olive leaf extract (250 mg every 12 hours for five days), intervention B received olive leaf extract (500 mg every 12 hours for five days), and the control group received placebo (every 12 hours for five days). The outcomes (vital signs, laboratory tests, and length of hospitalization) were compared by group.
Results
Of the 150 patients randomized into groups, 141 completed the follow-up and were analyzed. On the fifth day of hospitalization, body temperature (MD=0.34, P<0.001), pulse rate (MD=5.42, P=0.016), respiratory rate (MD=1.66, P=0.001), ESR (MD=13.55, P<0.001), and CRP (MD=15.68, P<0.001) of intervention A were significantly lower than the control group, while oxygen saturation (MD= -1.81, P=0.001) of intervention A was significantly higher than the control group. Furthermore, body temperature (MD=0.30, P=0.001), pulse rate (MD=5.29, P=0.022), respiratory rate (MD=1.41, P=0.006), ESR (MD=14.79, P<0.001), and CRP (MD=16.28, P<0.001) of intervention B were significantly lower than the control group, while oxygen saturation (MD= -2.38, P<0.001) of intervention B was significantly higher than the control group.
Conclusion
Olive leaf extract can improve the clinical status of the patients and decrease the length of hospitalization.
Keywords: COVID-19, Herbal medicine, Oleuropein, Olive extract, SARS-CoV-2
Introduction
In December 2019, many patients with pneumonia were reported in Wuhan, China, the etiology of which was detected as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2).1 The expeditious global spread of SARS-CoV-2 has led to the announcement of a pandemic. It has left millions of infected and expired individuals until now.2 , 3 It causes the coronavirus disease 2019 (COVID-19) with a broad spectrum of manifestations from a flu-like syndrome to multi-organ failure requiring intensive care facilities.4 , 5 Despite many attempts, a definitive drug to treat COVID-19 is undiscovered. Clinicians prescribe symptomatic and supportive therapy for patients.6 , 7
Acceptability, availability, and minimal adverse reactions have increased the consumption of herbal medicines (an element of complementary medicine) in recent decades.8 , 9.Since antiquity, herbal medicine has been pivotal in treating infectious diseases, especially in eastern countries.10 , 11 Since the beginning of the COVID-19 pandemic, herbal medicine has been widely used to treat SARS-CoV-2 infection. Olive is one of the medicinal plants having antiviral properties.12
Olive tree products had traditionally been used in the Mediterranean region for medicinal purposes. Olive leaves are a rich source of phenolic compounds, for instance, oleuropein, verbascoside, and hydroxytyrosol. These compounds have anti-inflammatory, antioxidant, antibacterial, and antiviral activities.13 , 14 As a hypothesis, olive leaf extract contains components that could suppress the inflammatory storm by targeting the receptors of inflammatory cytokines and thus decrease the mortality rate.15 Olive leaf extract can increase the immune response against viruses by stimulating phagocytosis.16 The mechanism by which olive leaf extract fights viruses is not yet fully understood. However, it may interfere with the attachment of the virus to the target cell and its subsequent engulfment by immune cells. Antiviral activity of polyphenols, abundantly found in the olive leaf, has been observed against human papillomavirus (HPV), hepatitis C virus (HCV), hepatitis B virus (HBV), herpes simplex virus (HSV), and influenza virus type A.17 , 18
A previous study revealed that olive leaf extract could impact the human immunodeficiency virus (HIV). It can hinder the transmission of HIV into the target cells.19 In a randomized trial, Somerville et al. investigated oleuropein's antiviral effect (the main olive leaf extract component) for treating respiratory syncytial virus (RSV). The results showed that it could improve patients infected with RSV.20
Nevertheless, there is no clinical trial to assess the efficacy of olive leaf extract on COVID-19 patients. Thus, this study was conducted to determine the effectiveness of olive leaf extract on the outcomes of hospitalized COVID-19 patients.
Materials and methods
Study design and setting
This randomized, triple-blinded clinical trial with three arms was conducted at the infectious diseases ward of Labbafinejad Hospital between July and December 2021. Labafinejad Hospital is an educational and therapeutic medical center in Tehran, Iran. The Iranian Registry approved the study protocol of Clinical Trials (IRCT20201128049520N1).
Participants
The study population was all COVID-19 patients hospitalized in the infectious diseases ward of Labbafinejad Hospital from July to December 2021. The inclusion criteria were: confirmed case of COVID-19 by detecting SARS-CoV-2 genome using the polymerase chain reaction (PCR); admission to the hospital; age between 18 and 72 years; willingness to participate in the study; and Glasgow coma scale (GCS)=15 at the admission. Also, individuals were excluded with the following characteristics: receive oleuropein supplements during the last three months; comorbidities, including chronic respiratory disorders (such as Chronic obstructive pulmonary disease or asthma), diabetes mellitus, hypertension, heart failure, chronic kidney disease, liver failure, malignancies; pregnancy and lactation; immunocompromised patients or consumption of immunosuppressive drugs; hypersensitivity to the drug during the study; blood pressure< 70 mmHg at the admission; require mechanical ventilation, admission in the intensive care unit, or death incomplete follow-up.
Sample size
Based on the study by Susalit et al.21 and considering μ1=19.5; μ2=28.5; δ1=12; δ2=16; α=5%; Z(1-α/2)=1.96; Power=80%, and Z(1-β)=0.84 the sample size was estimated to be 39 patients for each group. Then, considering the 20% drop in the participants, the final sample size was considered equal to 47 patients for each group.
Sampling, randomization, and blinding
Sampling was based on the conventional method. After completing the written informed consent form, the eligible participants were divided into three groups using block randomization: intervention A, intervention B, and Control. We conducted block randomization as follows: to assimilate the distribution of confounding variables, different categories were considered as follows: gender (male/female), age (45≥/45<years), and pulmonary involvement (moderate/severe). The patients were then assigned to the study groups balanced for the confounders.
In this triple-blinded study, patients, physicians who prescribed medication and performed the physical examination, and statistics were blind to the allocation of the patients in groups.
Interventions
The olive leaf extract capsules (extracted from Oleaeuropaea sevillano containing 30% oleuropein) were produced by Adonis Gol Darou Company, Tehran, Iran. The control group received placebo capsules (provided by Adonis Gol Darou company) every 12 hours for five days. Intervention group A and intervention group B received 250 mg and 500 mg capsules of olive leaf extract every 12 hours for five days, respectively.22 In addition, all three groups received standard treatment for COVID-19. According to the national guideline for COVID-19 management, standard treatment in hospitalized patients, who did not require intensive care, including the following: dexamethasone (8 mg daily for five days), remdesivir (200 mg daily for the first day, and 100 mg for the next four days), heparin 5000 IU every 8 hours), and supplemental oxygen with simple mask (6-8 L/minute).23 A trained nurse gave medications based on the allocation of patients.
Outcomes
The primary outcomes were the vital signs (body temperature, mean blood pressure, respiratory rate, pulse rate, and peripheral O2 saturation). They were assessed by an infectious diseases specialist daily (8 AM) from admission to the fifth day of hospitalization. Blood pressure was assessed based on the oscillometric method24 using an aneroid sphygmomanometer, model TY-A02 (made by Brisk Company, Germany). Pulse rate and peripheral O2 saturation were measured using a pulse oximeter, model PO30 (made by Beurer Company, Germany). Also, the respiratory rate was counted by an infectious disease specialist. Secondary outcomes were laboratory tests and length of hospitalization. Laboratory tests included complete blood count (CBC), serum level of C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR), performed daily by a trained nurse.
Statistical analysis
Data were processed in SPSS version 23.0. with a significant level of 0.05. Data were described as frequency, percentage, mean, standard deviation, mean difference (MD), and 95% confidence interval (CI). We also analyzed variables using the Chi-square test and one-way ANOVA, followed by the post hoc tests (Tukey).
Ethical considerations
This study was performed following the Helsinki declaration. It was approved by the research ethics committee of Lorestan University of Medical Sciences, Khorrammabad, Iran on Jan 12, 2021(IR.LUMS.REC.1399.262). All patients completed the informed consent form after explaining the study entirely.
Results
Baseline characteristics of the patients
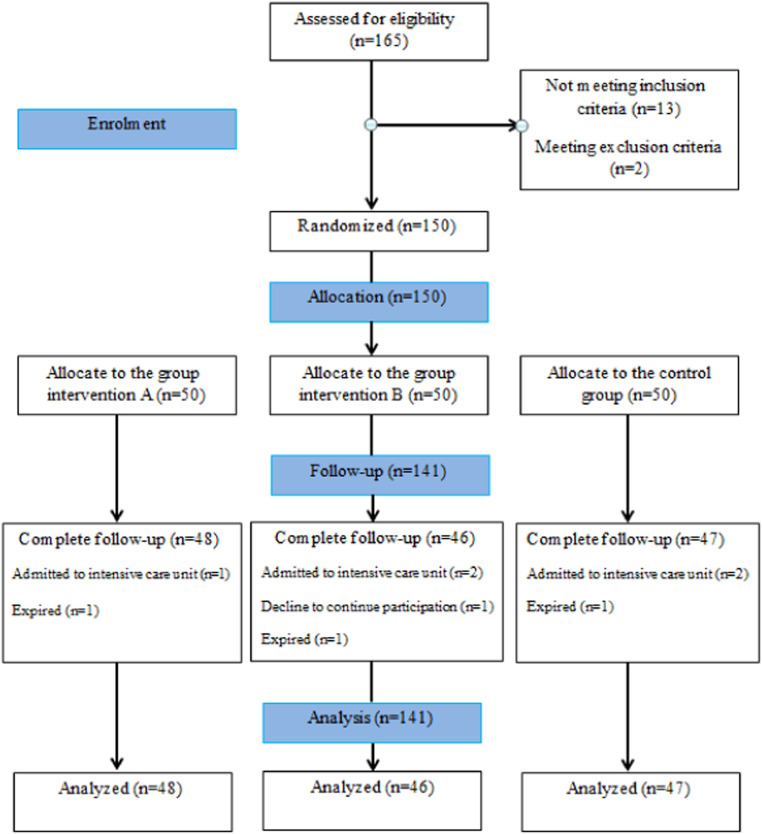
Of 165 individuals who were assessed for eligibility, 150 were randomly selected. Patients were divided into the following groups: intervention A (n=50), intervention B (n=50), and control (n=50). Finally, 141 patients completed the follow-up and were analyzed. Fig. 1 depicts the Consort diagram of the study. The mean age of allocated individuals was 48.88±11.96 years (range: 20-70), and 72 (51.1%) were female. The baseline characteristics of the patients did not differ between groups, except for myalgia (P=0.046). Also, all patients had imaging findings suggestive of COVID-19 pneumonia (48.9% had moderate involvement; 51.1% had severe involvement). Table 1 presents the baseline characteristics of the patients in detail.
Fig. 1.
The study flow diagram.
Table 1.
Baseline characteristics of the patients.
| Variables | Control (n=47) | Intervention A (n=48) | Intervention B (n=46) | Total (n=141) | P-value | |
|---|---|---|---|---|---|---|
| Demographic features | ||||||
| Age | 50.44±11.91 | 46.72±11.19 | 49.54±12.72 | 48.88±11.96 | 0.289 a | |
| Gender | Male | 23 (48.9) | 24 (50.0) | 22 (47.8) | 69 (48.9) | 0.978 b |
| Female | 24 (51.1) | 24 (50.0) | 24 (52.2) | 72 (51.1) | ||
| Body mass index (kg/m2) | <18.5 | 7 (14.9) | 5 (10.4) | 4 (8.7) | 16 (11.3) | 0.182 b |
| 18.5-25.0 | 9 (19.1) | 12 (25.0) | 15 (32.6) | 36 (25.5) | ||
| 25.0-30.0 | 28 (59.6) | 20 (41.7) | 21 (45.7) | 69 (48.9) | ||
| >30.0 | 3 (6.4) | 11 (22.9) | 6 (13.0) | 20 (14.2) | ||
| Clinical features | ||||||
| Dyspnea | 47 (100) | 48 (100) | 46 (100) | 141 (100) | >0.999 b | |
| Cough | 45 (95.7) | 46 (95.8) | 44 (95.7) | 135 (95.7) | >0.999 b | |
| Fatigue | 43 (91.5) | 41 (85.4) | 43 (93.5) | 127 (90.1) | 0.393 b | |
| Anorexia | 41 (87.2) | 37 (77.1) | 41 (89.1) | 119 (84.4) | 0.221 b | |
| Myalgia | 42 (89.4) | 42 (87.5) | 33 (71.7) | 117 (83.0) | 0.046 b | |
| Headache | 39 (83.0) | 30 (62.5) | 36 (78.3) | 105 (74.5) | 0.056 b | |
| Time from symptom onset to randomization (day) | 6.43±2.09 | 7.02±2.32 | 7.57±2.59 | 7.00±2.37 | 0.067 a | |
| Temperature (°c) | 37.68±0.71 | 37.75±0.69 | 37.61±0.65 | 37.68±0.68 | 0.657 a | |
| Mean blood pressure(mmHg) | 112.38±10.76 | 110.05±12.86 | 111.83±9.31 | 111.41±11.06 | 0.565 a | |
| Pulse rate(/minute) | 83.23±10.25 | 81.79±12.64 | 80.47±11.65 | 81.84±11.54 | 0.518 a | |
| Respiratory rate (/minute) | 21.82±2.44 | 22.47±2.01 | 22.50±2.33 | 22.26±2.27 | 0.269 a | |
| O2 Saturation (%) | 90.31±2.86 | 91.00±2.19 | 90.82±2.02 | 90.71±2.39 | 0.358 a | |
| Laboratory tests | ||||||
| White blood cells (× 109cells/L) | 7.02±2.56 | 6.33±2.86 | 6.63±2.90 | 6.66±2.77 | 0.488 a | |
| Neutrophil: Lymphocyte ratio | 5.44±3.55 | 5.01±2.77 | 6.10±4.05 | 5.51±3.49 | 0.317 a | |
| Platelet (× 109 cells/L) | 186.40±58.86 | 182.25±66.31 | 188.30±50.30 | 185.60±58.60 | 0.878 a | |
| CRP (mg/L) | 58.34±18.57 | 57.58±22.73 | 63.08±16.20 | 59.63±19.42 | 0.336 a | |
| ESR (mm/h) | 53.34±19.85 | 49.52±19.19 | 50.50±15.07 | 51.11±18.13 | 0.571 a | |
Values are reported as n (%) or mean± standard deviation
CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate
one-way ANOVA
Chi-square test
Primary outcomes
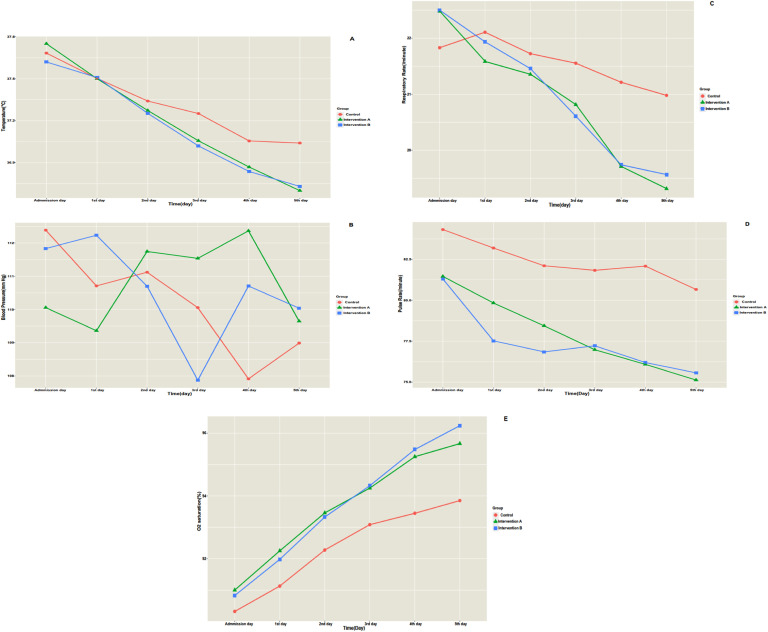
Fig. 2 shows the timeline trend of the patient's vital signs separately by the group. On the fifth day of hospitalization, body temperature (MD=0.34, P<0.001), pulse rate (MD=5.42, P=0.016), and respiratory rate (MD=1.66, P=0.001) of intervention A were significantly lower than the control group, while oxygen saturation (MD= -1.81, P=0.001) of intervention A was significantly higher than the control group. Furthermore, body temperature (MD=0.30, P=0.001), pulse rate (MD=5.29, P=0.022), and respiratory rate (MD=1.41, P=0.006) of intervention B were significantly lower than the control group. In contrast, intervention B's oxygen saturation (MD= -2.38, P<0.001) was significantly higher than the control group. Post hoc tests revealed that in terms of body temperature, pulse rate, respiratory rate, and oxygen saturation, interventions A and B were homogenous groups; they were significantly different from the control group (P<0.001). Table 2 presents multiple comparisons of groups considering vital signs on the fifth day of hospitalization.
Fig. 2.
Timeline trend of vital signs of the patients separately in each group. A, temperature; B, mean blood pressure; C, pulse rate; D, respiratory rate; E, oxygen saturation.
Table 2.
Multiple comparisons of groups on the fifth day of hospitalization considering vital signs and laboratory tests.
| Variables | Reference group | Comparison groups | Mean difference | 95% confidence interval | P-value |
|---|---|---|---|---|---|
| Vital signs | |||||
| Body temperature (°C) | Control | Intervention A | 0.34 | (0.13, 0.54) | <0.001 |
| Intervention B | 0.30 | (0.10, 0.51) | 0.001 | ||
| Mean blood pressure(mmHg) | Control | Intervention A | -0.65 | (-4.38, 3.07) | 0.908 |
| Intervention B | -1.04 | (-4.82, 2.72) | 0.787 | ||
| Pulse rate(/minute) | Control | Intervention A | 5.46 | (0.84, 10.08) | 0.016 |
| Intervention B | 5.29 | (0.62, 9.96) | 0.022 | ||
| Respiratory rate(/minute) | Control | Intervention A | 1.66 | (0.61, 2.71) | 0.001 |
| Intervention B | 1.41 | (0.34, 2.47) | 0.006 | ||
| O2 saturation (%) | Control | Intervention A | -1.81 | (-2.94, -0.68) | 0.001 |
| Intervention B | -2.38 | (-3.52, -1.24) | <0.001 | ||
| Laboratory tests | |||||
| White blood cells (× 109 cells/L) | Control | Intervention A | 0.23 | (-1.15, -1.62) | 0.916 |
| Intervention B | 0.26 | (-1.13, -1.67) | 0.895 | ||
| Neutrophil: Lymphocyte ratio | Control | Intervention A | 0.85 | (-1.00, 2.71) | 0.521 |
| Intervention B | -0.24 | (-2.12, 1.63) | 0.947 | ||
| Platelet (× 109 cells/L) | Control | Intervention A | 25.72 | (-13.09, -64.55) | 0.266 |
| Intervention B | 1.95 | (-37.27, 41.19) | 0.992 | ||
| CRP (mg/L) | Control | Intervention A | 15.68 | (10.07, 21.30) | <0.001 |
| Intervention B | 16.28 | (10.60, 21.95) | <0.001 | ||
| ESR (mm/h) | Control | Intervention A | 13.55 | (7.54, 19.58) | <0.001 |
| Intervention B | 14.79 | (8.71, 20.86) | <0.001 | ||
Data were analyzed by ANOVA, followed by a post hoc test (Tukey).
Secondary outcomes
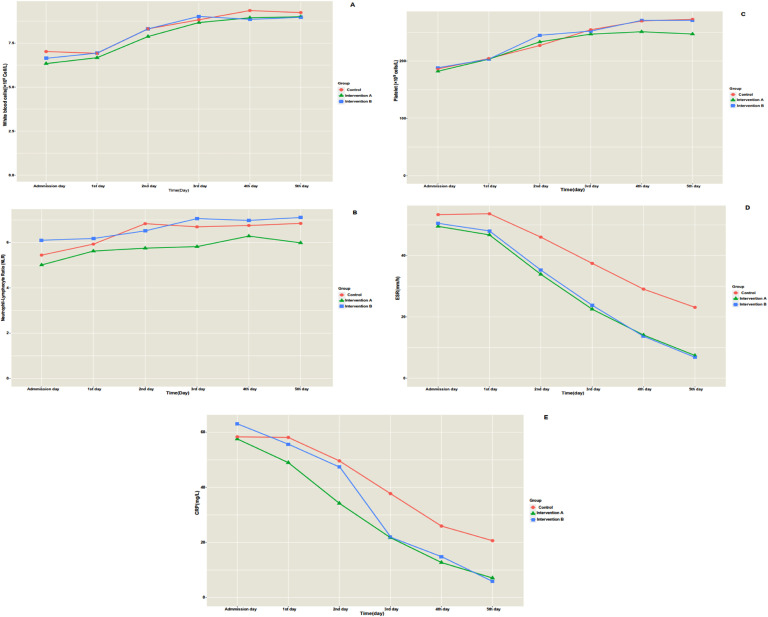
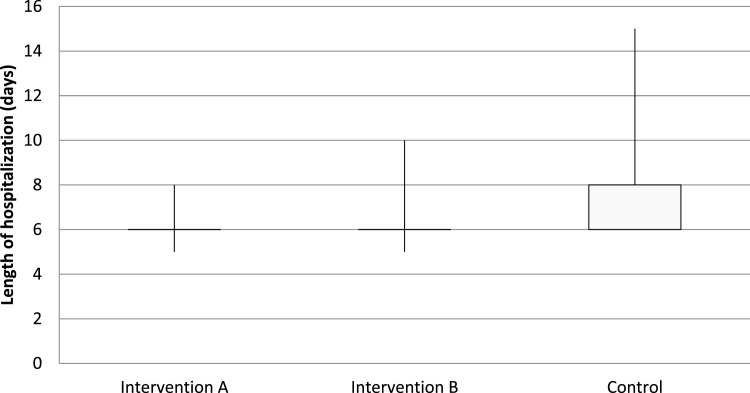
Fig. 3 shows the timeline trend of laboratory tests of the patients separately by the group. On the fifth day of hospitalization, ESR (MD=13.55, P<0.001) and CRP (MD=15.68, P<0.001) of intervention A were significantly lower than the control group; however, other laboratory tests did not differ (P>0.05). Furthermore, ESR (MD=14.79, P<0.001) and CRP (MD=16.28, P<0.001) of intervention B were significantly lower than the control group; however, other laboratory tests did not differ (P>0.05). Post hoc tests revealed that only in terms of ESR and CRP, interventions A and B were homogenous groups; they were significantly different from the control group (P<0.001). Table 2 shows the multiple comparison of groups considering laboratory tests on the fifth day of hospitalization. Additionally, the length of hospitalization for intervention group A (MD=1.75, P<0.001) and intervention group B (MD=1.66, P<0.001) were significantly shorter than the control group (Fig. 4 ). Interestingly, no patient had drug-related adverse events during hospitalization.
Fig. 3.
Timeline trend of laboratory tests of the patients separately in each group. A, white blood cells; B, neutrophil to lymphocyte ratio; C, platelet count; D, ESR; E, CRP.
Fig. 4.
Lengths of hospitalization of the patients (days)
Discussion
Our study was one of the few research projects investigating the efficacy of olive leaf extract on the vital signs and laboratory parameters of COVID-19 patients. Based on the results, olive leaf extract effectively reduced respiratory rate, pulse rate, and body temperature; and increased blood oxygen saturation of COVID-19 patients. It decreased ESR and CRP levels in COVID-19 patients. Also, the findings showed that olive leaf extract can shorten the duration of hospitalization and lead to the early discharge of the patient. Moreover, there was no difference between the two doses of olive leaf extract (250 mg and 500 mg) in terms of efficacy.
Effective treatment for COVID-19 can act in the following ways: 1) to inhibit functional enzymes or proteins required for the virus to survive, 2) to destroy the protein structure of the virus and prevent the virus from forming and interacting with human cells, 3) to stimulate the immune system of the human host, 4) to inhibit virus receptors on the host cells.25 Russo et al. emphasized that polyphenols can act effectively in different stages of the entry and propagation of SARS-CoV-2. They suggested that complementary medicine may be an excellent approach to COVID-19 instead of antiviral drugs.26
Since ancient times, people have used olive extracts for medicinal purposes, such as antipyretic, anti-malarial, and anti-infective agents.27 Ingredients inside olive leaves have antioxidant, antibacterial, antifungal, and antiviral properties.13 , 27 Antiviral activity of polyphenols, abundantly found in the olive leaf, has been observed against human papillomavirus (HPV), hepatitis C virus (HCV), hepatitis B virus (HBV), herpes simplex virus (HSV), and influenza virus type A.17 , 18 Olive leaf extract can increase the immune response against viruses by stimulating phagocytosis.16 During the novel coronavirus pandemic, the question arose about whether olive leaf extract affects patients with COVID-19.
Olive leaves contain secoiridoids, bioactive components with antiviral properties. Previous studies revealed that oleuropein, a secoiridoid, is a potent antiviral agent against HIV and influenza.28 , 29 A molecular study revealed that olive secoiridoids could interfere with the entry and replication of SARS-CoV-2. Olive secoiridoids block the spike protein of SARS-CoC-2, which facilitates virus entry by binding to ACE-2 receptors. ACE-2, the SARS-CoV-2 receptor to enter the target cells, is abundant in the lung and the gastrointestinal system.30 Laboratory studies revealed that the compounds in olive leaf extract could inhibit ACE-2. A clinical trial showed that 500 mg of olive leaf extract has a much better effect than 12.5 mg or 25 mg of Captopril in patients with moderate blood pressure.21
Also, olive secoiridoids inhibit the main protease of SARS-CoV-2, a critical mediator in virus replication.15 , 31 Moreover, olive secoiridoids can suppress inflammation by targeting receptors of pro-inflammatory cytokines (Interleukin-1β, Interleukine-6, and Tumor necrosis factor-α).15 Many of the deaths in COVID-19 patients occurred following a cytokine storm. Physicians administered immunosuppressive drugs (glucocorticoids, tocilizumab, and bariticinib) to prevent severe inflammatory phase in COVID-19 patients. Interestingly, olive leaf extract could act as an anti-inflammatory drug, suppress inflammatory storms, and decrease mortality.32 In our study, olive leaf extract also improved clinical status and laboratory markers of inflammation. It can be explained by the mechanisms mentioned above.
Previous studies revealed that olive leaf extract could improve respiratory diseases. In a study by Somerville et al. the impact of olive leaf extract on respiratory diseases (Influenza virus and respiratory syncytial virus) was compared with the placebo. Olive leaf extract shortened the duration of the disease, which is consistent with our findings. Nevertheless, the occurrence of symptoms did not significantly differ between the two study groups20 , 33, which is inconsistent with our results. This difference may be due to the fact that the presence of symptoms was subjectively expressed. Additionally, the sensitivity of individuals is different for reporting the symptoms.
CRP elevation can be seen in acute infections and inflammatory conditions. Hepatocytes produce serum CRP in response to cytokines (e.g., interleukin-1, interleukin-6, and tumor necrosis factor). CRP production is thought to be primarily controlled by interleukin-6. Therefore, CRP level directly reflects the degree and extent of tissue damage or inflammation. As soon as the stimulation of CRP synthesis stops, its level in the blood decreases rapidly. Thus, measuring the CRP level is applicable in diagnosing and investigating the disease process and response to treatment. The most common causes of ESR elevation are also infections and inflammation. ESR is used in the clinical field as a non-specific marker of systemic inflammation.34 As a result, the reduction of ESR and CRP can be attributed to the anti-inflammatory properties of olive leaf extract.
Based on the study by Susalit et al., olive leaf extract did not affect liver and kidney function, electrolytes, and other blood parameters. So it was safe and tolerable.21 In this study, various drug-related adverse events have been controlled. The patients had no drug-related adverse events. Safety indicators such as adverse reactions, side effects, and frequency of medication withdrawal due to drug complications and liver and kidney dysfunction are included. They should be checked in clinical trials.35
The safety of olive leaf extract can be confirmed through the available data from clinical studies and long-term utilization for more than 30 years in European countries. The results of clinical studies so far showed that most patients tolerate the oral consumption of olive leaf extract. Also, no severe or moderate adverse events have been reported.16
Our study had some limitations. We excluded patients who were admitted to the intensive care unit or expired. It would be better to consider mortality and the requirement for intensive care as an outcome of the study. Individuals without underlying conditions were only included in this study, while many patients have a history of chronic disease. So, our results could not be generalized to the whole population. Few studies were performed on this topic, which made our discussion difficult. Also, other limitations of this study were not checking other inflammatory markers (such as IL-6 and D-dimmer) and not performing PCR tests and CT scan on the last day of the study.
We suggest that further studies with a larger sample size be conducted to discover the efficacy of olive leaf extract in the treatment of COVID-19 patients. It is also necessary to consider the effect of confounding variables such as underlying diseases. This study used two doses of olive leaf extract (250 mg and 500 mg). We recommend evaluating other doses as well.
Conclusion
Olive leaf extract can improve the clinical status of the patients and decrease the length of hospitalization. However, more studies should be performed to evaluate the effectiveness of olive extract on COVID-19 patients.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.explore.2022.10.020.
Appendix. Supplementary materials
References
- 1.Chilamakuri R, Agarwal S. COVID-19: characteristics and therapeutics. Cells. 2021;10(2) doi: 10.3390/cells10020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, et al. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29(1):20–36. [PubMed] [Google Scholar]
- 3.Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.J Gerges Harb, Noureldine HA, Chedid G, Eldine MN, Abdallah DA, Chedid NF, et al. SARS, MERS and COVID-19: clinical manifestations and organ-system complications: a mini review. Pathog Dis. 2020;78(4) doi: 10.1093/femspd/ftaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain A, Kaler J, Tabrez E, Tabrez S, Tabrez SSM. Novel COVID-19: a comprehensive review of transmission, manifestation, and pathogenesis. Cureus. 2020;12(5):e8184. doi: 10.7759/cureus.8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.683296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M, Adil SF, Alkhathlan HZ, Tahir MN, Saif S, Khan M, et al. COVID-19: a global challenge with old history, epidemiology and progress so far. Molecules. 2020;26(1) doi: 10.3390/molecules26010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kütmeç Yilmaz C, Duru Aşiret G, Çetinkaya F. The effect of back massage on physiological parameters, dyspnoea, and anxiety in patients with chronic obstructive pulmonary disease in the intensive care unit: A randomised clinical trial. Intensive Crit Care Nurs. 2021;63 doi: 10.1016/j.iccn.2020.102962. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves PB, Romeiro NC. Multi-target natural products as alternatives against oxidative stress in Chronic Obstructive Pulmonary Disease (COPD) Eur J Med Chem. 2019;163:911–931. doi: 10.1016/j.ejmech.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Ang L, Song E, Lee HW, Lee MS. Herbal medicine for the treatment of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of randomized controlled trials. J Clin Med. 2020;9(5):1583. doi: 10.3390/jcm9051583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, Wang Y, Wu A, Ding Z, Liu X. Influence factors of the pharmacokinetics of herbal resourced compounds in clinical practice. Evid-Based Complement Altern Med. 2019:2019. doi: 10.1155/2019/1983780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunne EF, Friedman A, Datta SD, Markowitz LE, Workowski KA. Updates on human papillomavirus and genital warts and counseling messages from the 2010 sexually transmitted diseases treatment guidelines. Clin Infect Dis. 2011;53(3):S143–S152. doi: 10.1093/cid/cir703. Suppl. [DOI] [PubMed] [Google Scholar]
- 13.Abdelgawad SM, Hassab MAE, Abourehab MAS, Elkaeed EB, Eldehna WM. Olive leaves as a potential phytotherapy in the treatment of COVID-19 disease; a mini-review. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.879118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peršurić Ž, Saftić L, Klisović D, Pavelić SK. Polyphenol-based design of functional olive leaf infusions(§) Food Technol Biotechnol. 2019;57(2):171–182. doi: 10.17113/ftb.57.02.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thangavel N, Al Bratty M, Al Hazmi HA, Najmi A, Ali Alaqi RO. Molecular docking and molecular dynamics aided virtual search of OliveNetTM directory for secoiridoids to combat SARS-CoV-2 infection and associated hyperinflammatory responses. Front Mol Biosci. 2020;7 doi: 10.3389/fmolb.2020.627767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosleh G, Mohagheghzadeh A, Faridi P. Olive leaf: From tradition to clinic. Trends Pharmacol Sci. 2016;2(4):241–252. [Google Scholar]
- 17.Vázquez-Calvo Á, Jiménez de Oya N, Martín-Acebes MA, Garcia-Moruno E, Saiz JC. Antiviral properties of the natural polyphenols delphinidin and epigallocatechin gallate against the flaviviruses west nile virus, zika virus, and dengue virus. Front Microbiol. 2017;8:1314. doi: 10.3389/fmicb.2017.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Toumy SA, Salib JY, El-Kashak WA, Marty C, Bedoux G, Bourgougnon N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Science and Human Wellness. 2018;7(1):91–101. [Google Scholar]
- 19.Salih RH, Odisho SM, Al-Shammari AM, Ibrahim OMS. Antiviral effects of olea europaea leaves extract and interferon-beta on gene expression of newcastle disease virus. Adv Anim Vet Sci. 2017;5(11):436–445. [Google Scholar]
- 20.Somerville V, Moore R, Braakhuis A. The effect of olive leaf extract on upper respiratory illness in high school athletes: a randomised control trial. Nutrients. 2019;11(2):358. doi: 10.3390/nu11020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18(4):251–258. doi: 10.1016/j.phymed.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Hansen K, Adsersen A, Christensen SB, Jensen SR, Nyman U, Smitt UW. Isolation of an angiotensin converting enzyme (ACE) inhibitor from Olea europaea and Olea lancea. Phytomedicine. 1996;2(4):319–325. doi: 10.1016/S0944-7113(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 23.Khoury H, Trinkaus K, Zhang M, Adkins D, Brown R, Vij R, et al. Hydroxychloroquine for the prevention of acute graft-versus-host disease after unrelated donor transplantation. Biol Blood Marrow Transplant. 2003;9(11):714–721. doi: 10.1016/j.bbmt.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28(4):571–586. doi: 10.1016/j.ccl.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu J-M, Cao B, Chen R-C. Prevention and disease control of COVID-19. COVID-19. 2021:75. [Google Scholar]
- 26.Russo M, Moccia S, Spagnuolo C, Tedesco I, Russo GL. Roles of flavonoids against coronavirus infection. Chem Biol Interact. 2020;328 doi: 10.1016/j.cbi.2020.109211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ünlü AE. Green and non-conventional extraction of bioactive compounds from olive leaves: screening of novel natural deep eutectic solvents and investigation of process parameters. Waste Biomass Valorization. 2021;12(10):5329–5346. doi: 10.1007/s12649-021-01411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omar SH. Oleuropein in olive and its pharmacological effects. Sci Pharm. 2010;78(2):133–154. doi: 10.3797/scipharm.0912-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vilaplana-Pérez C, Auñón D, García-Flores LA, Gil-Izquierdo A. Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front Nutr. 2014;1:18. doi: 10.3389/fnut.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laszkowska M, Faye AS, Kim J, Truong H, Silver ER, Ingram M, et al. Disease course and outcomes of COVID-19 among hospitalized patients with gastrointestinal manifestations. Clin Gastroenterol Hepatol. 2021;19(7):1402–1409. doi: 10.1016/j.cgh.2020.09.037. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shawky E, Nada AA, Ibrahim RS. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020;10(47):27961–27983. doi: 10.1039/d0ra05126h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao YM, Xu G, Wang B, Liu BC. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J Intern Med. 2021;289(2):147–161. doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore R. ResearchSpace@; Auckland: 2018. Olive Leaf Extract and its effect on Upper Respiratory Illnesses in an Athletic Cohort. [Google Scholar]
- 34.Calderon AJ, Wener MH. Erythrocyte sedimentation rate and C-reactive protein. Hosp Med Clin. 2012;3(1):e313–ee37. [Google Scholar]
- 35.Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.