Abstract
INTRODUCTION: Red propolis is synthetized from exudates of Dalbergia ecastophyllum (L) Taub. and Symphonia globulifera L.f., presents isoflavones, guttiferone E, xanthochymol, and oblongifolin B and has anti-inflammatory, antioxidant, and antiproliferative activities. OBJECTIVES: This study aimed to evaluate the antigenotoxic and anticarcinogenic potential of red propolis hydroalcoholic extract (RPHE) in rodents. METHODS: The influence of RPHE in doxorubicin (DXR)-induced genotoxicity was investigated through the micronucleus test in Swiss mice. Blood samples were also collected to investigate oxidative stress, hepatotoxicity, and nephrotoxicity. Was investigated the influence of RPHE in 1,2-dimethylhydrazine (DMH)-induced aberrant crypt foci, as well as its influence in proliferating cell nuclear antigen (PCNA) and the cyclooxygenase-2 (COX-2) expression in colon of rats, by immunohistochemistry. RESULTS: The results showed that RPHE (48 mg/kg) reduced DXR-induced genotoxicity. Animals treated with DXR showed significantly lower GSH serum levels in comparison to the negative control. RPHE treatments did not attenuated significantly the DXR-induced GSH depletion. No difference was observed in cytotoxicity parameters of mice hematopoietic tissues between the treatment groups, as well as the biochemical parameters of hepatotoxicity and nephrotoxicity. RPHE (12 mg/kg) reduced the DMH-induced carcinogenicity and toxicity, as well as DMH-induced PCNA and COX-2 expression in colon tissue. CONCLUSION: Therefore, was observed that the RPHE has chemopreventive effect, associated to antiproliferative and anti-inflammatory activities.
Keywords: propolis, chemoprevention, anticarcinogenic, antiproliferative, anti-inflammatory
Introduction
Colorectal cancer is the third most incident cancer and the second most mortal in the world. In countries with high and medium levels of human development index, this cancer ranks first, both in number of cases and in mortality rates. By the year 2018, there were 1.8 million new cases and 880,000 deaths.1 The mechanism that triggers the carcinogenesis is constituted by mutations acquired through genetic inheritance or during life, and considering colorectal cancer, the oxidative stress and inflammatory processes are the main events that cause these mutations, emerging pre-neoplastic lesions and resulting in the development of this cancer.2,3
Considering this, there is a growing interest in the search of nontoxic agent therapies, capable of preventing, interrupting, or reversing the carcinogenesis process in the colon. Natural products have been targeted by researchers that aim to obtaining molecules with chemoprotective potential, influencing in antioxidant, anti-inflammatory, and immunomodulatory signaling pathways.4,5
In this context, the propolis, a bee product, is one of the oldest natural products used by mankind in traditional medicine, due to their antiseptic, antibacterial, and antiparasitic properties. In Brazil, 12 groups of propolis were listed by color and differ by physical and chemical characteristics.6 The red propolis is produced in northeastern Brazil from the collection of exudates from stem of the Dalbergia ecastophyllum (L) Taub., a plant belonging to the Fabaceae Family, and Symphonia globulifera L.f., a tree belonging to the family Clusiaceae.7
The hydroalcoholic extract obtained from the red propolis (RPHE) shows antioxidant, anti-inflammatory, and antiproliferative properties, which are attributed to its chemical composition, rich in flavonoids, especially the isoflavones.7–9 According to the literature, there is a relation between the consumption of isoflavones through the diet and the prevention and reduction of colorectal, stomach, esophagus, breast, and prostate cancer risks.10,11
These data foster interest in explore the chemoprotective potential of the red propolis hydroalcoholic extract (RPHE). Until the moment, there are only a few studies about the influence of RPHE on mutagenesis, only one about its safety in rodents, but there is no study about its protective effect in in-vivo.9,12 Therefore, the present study shows the fists results of the evaluation of the chemopreventive effect of RPHE on induced genomic instability and on colon carcinogenesis in rodents.
Materials and methods
RPHE obtaining
Red propolis (2 kg) was purchased from Cooperativa de Apicultores de Canavieiras (COAPER) in the city of Canavieiras (Bahia state, Brazil) in April of 2018. The obtaining of RPHE as well as the analysis of its main constituents were proceeded as described in Squarisi et al..9 In short, 2 kg of red propolis was prepared by exhaustive maceration [500 g in 1.5 L of ethanol: H2O (7:3 v/v)] for 7 days. The extract was then filtered, concentrated, and lyophilized to result in 75 g of RPHE. The chemical identification of RPHE main compounds was proceeded through HPLC-DAD analysis using a Synergi Polar-RP (150 × 4.60 mm, 4 μm) column as stationary phase and a gradient mobile phase of H2O (A) and acetonitrile (B) starting from 23% up to 100% of (B) in 32 min at 1.2 mL/min. The detection wavelength was 220 nm, and the compounds identification was performed by co-injection of RPHE and the reference standards previously isolated and leaded to the detection of the phenolic compounds liquiritigenin, formononetin, vestitol, neovestitol, medicarpin, 7-O-methylvestitol, and guttiferone E, xanthochymol, and oblongifolin B.
Animals
Male Swiss mice (Mus musculus) weighing ~30 g and 6 weeks old were used in the micronucleus (MN) test. Wistar Hannover rats (Rattus norvegicus) weighing ~120 g and 6 weeks old were employed in the aberrant crypts foci (ACF) assay. These animals were obtained from the Animal House of the School of Pharmaceutical Sciences, University of São Paulo, Ribeirão Preto (São Paulo, Brazil). The animals were kept in plastic boxes in an experimental room with controlled conditions of temperature (23 ± 2 °C) and humidity (50 ± 10%), under a 12 h light–dark cycle, with free access to regular laboratory diet chow and drinking water. The protocols followed the NIH guidelines for care and use of animals and were approved by the Animal Use Ethics Committee of the University of Franca (Approval n° 2,463,240,818).
Experimental design
The treatments were performed after 1 week of acclimation. Three doses of RPHE (12, 24, and 48 mg/kg b.w.) were selected based on the results of antigenotoxic activity of the green propolis hydroalcoholic extract.13 The extract was dissolved in 2.5% dimethyl sulfoxide (DMSO; CAS:67–68-5, Sigma-Aldrich®) and administered to mice (0.2 mL/animal) and rats (1 mL/animal) by gavage. For MN test, the chromosomal damage inducer and positive control used was doxorubicin (DXR; Bergamo®, 0.9 mg/kg b.w.), administered intraperitoneally (ip; 0.3 mL/animal).13
For the ACF assay, the pre-neoplastic lesions inducer and positive control used was the well-established colon carcinogen 1,2-dimethylhydrazine (DMH; CAS:306–37-6, Sigma-Aldrich®, 40 mg/kg b.w.), dissolved immediately before use in 1 mM ethylenediaminetetraacetic acid (EDTA; CAS:60–00-4, Synth®), and administered subcutaneously (sc; 0.5 mL/animal).14 Negative (water), solvent (2.5% DMSO), extract (RPHE 48 mg/kg b.w.), positive (DXR or DMH) control, and solvent plus positive control (2.5% DMSO plus DXR or DMH) were included. Each treatment group consisted of 5 animals.
Evaluation of antigenotoxic activity
MN test
The MN test was performed according to OECD 474 recommendations.15 The treatments were performed twice, at 24 h intervals. The peripheral blood was collected by caudal venipuncture 48 h after the last treatment. The animals were euthanized by ip administration of a single dose of thiopental sodium (840 mg/kg b.w., 1 g Thiopentax, Cristália®). The slides preparation and staining were performed as described by Freitas et al..16 In order to determinate the frequency of micronucleated polychromatic erythrocytes (MNPCEs), 4,000 PCEs per animal were analyzed, totaling 20,000 PCEs per treatment. The cytotoxicity of the treatments was determined based upon the PCE/PCE + NCE (normochromatic erythrocytes) ratio. For this, 2,000 erythrocytes per animal were scored, totaling 10,000 erythrocytes per treatment.
Oxidative stress evaluation
Reduced glutathione (GSH) was quantified according to Ellman, with adaptations.17 Thus, 300 μL of whole blood sample of each mouse were mixed with 200 μL of 10% Triton X-100 (CAS:9002-93-1, Sigma-Aldrich®), using a vortex at 300 G for 30 s, in order to induce blood cells lysis. After, 200 μL of 30% trichloroacetic acid (CAS:76–03-9, Sigma-Aldrich®) was added. The mixture was vortexed again at 300 G for 30 s, resulting in a dark brown mixture. The material was centrifuged at 1400 G for 10 min at 10 °C, obtaining a clear supernatant. In a 96-well plate, 270 μL of potassium triphosphate buffer (pH = 7.4) were pipetted in each well and then added 15 μL of the supernatant and 15 μL of 5,5′-dithio-bis- (2-nitrobenzoic acid) (CAS:69–78-3, Sigma-Aldrich®, 10 mM).
The absorbance of the samples was determined with a multi-plate reader (Asys UVM340, MikroWin 2000 software) at a wavelength of 412 nm. L-Cysteine (CAS:52–90-4, Sigma-Aldrich®) was used in concentrations from 0.01 to 0.15 mM to obtain the standard curve. The experiments were performed in triplicate. The well concentration of mercapturic products (5-mercapto-2-nitrobenzoic acid; yellow) was proportional to the concentration of GSH. The absorbance values were included in the line general equation obtained from the standard curve.
Hepatotoxicity and nephrotoxicity evaluation
Hepatotoxicity and nephrotoxicity of treatments were investigated by measuring the liver enzymes alanine aminotransferase (ASL) and aspartate aminotransferase (AST), and the metabolic products creatinine and urea. To this end, the blood samples collected from the mice were centrifuged for 15 min at 1,100 G, obtaining the plasma. The biochemical assays were carried out in a Mindray® automated analyzer (Mindray Medical Brazil Ltda., São Paulo, Brazil) using enzymatic colorimetric kits (AST, ref. D98616; ALT, ref. D98624; creatinine, ref. D95595; urea, ref. D95704) according to the manufacturer’s recommendations, based upon the principle of absorption.
Evaluation of anticarcinogenic activity
ACF assay
The ACF assay was based on Bird.18 RPHE was administered once a day, 7 days a week, for 2 weeks. In parallel, the carcinogen DMH was administrated twice a week (2nd and 5th day), for 2 weeks. For the next 2 weeks, the animals did not received treatment. Body weight and water consumption data were collected three times a week throughout the whole experimental period. At the end of the 4 experimental weeks, the animals were euthanized by ip administration of a single dose of thiopental sodium (840 mg/kg b.w., 1 g Thiopentax, Cristália®). Colon collection, staining, and analyses procedures were performed as described by Senedese et al..14 A part of the colons was separated and included in paraffin for further immunohistochemical analysis. The frequencies of ACF and aberrant crypts (AC) were observed and counted in the colon tissue by analyzing 15 sequential fields per segment (400× magnification).
PCNA and COX-2 analysis
Colon sections from treated rats were used to investigate the activation of the anti-inflammatory and antiproliferative signaling pathways by immunohistochemistry. The proliferating cell nuclear antigen (PCNA, D3H8P-13110S, Uniscience) and the cyclooxygenase-2 (COX-2, ab15191, Abcam) primary antibodies were analyzed for these purposes. The procedures were conducted as described by Ferreira et al..19 The colon sections were counterstained with hematoxylin and photographed (400× magnification) under a bright-field microscope (Nikon Eclipse E200; Moticam 580INT camera, Nikon). The images were analyzed with the ImageJ software using the IHC Toolbox training plug-in as described by Shu et al..20,21 The results are reported as optical density (OD).
Calculation of percent reduction of damages and statistical analysis
The percent reduction in DXR or DMH-induced damage by RPHE was calculated according to Furtado et al..22 All data obtained were analyzed statistically by analysis of variance (ANOVA) using the GraphPad Prism® 6 software. In cases in which P < 0.05, treatment means were compared by the Tukey’s test and minimum significant difference was calculated for α = 0.05.
Results
Evaluation of antigenotoxic activity
The results obtained from the MN test are shown in Fig. 1. Animals treated with 48 mg/kg b.w. of RPHE plus DXR showed MNPCE frequencies significantly lower than those treated with DXR only, representing 52% reduction in chromosomal damage. The PCE/PCE + NCE ratios did not differ between the treatment groups and the negative control group, revealing absence of cytotoxicity (data not shown).
Fig. 1.

Frequencies of MNPCE in peripheral blood of Swiss mice treated with RPHE and DXR, and their respective controls. NC, negative control (water); DMSO, dimethyl sulfoxide (2.5%); RPHE, red propolis hydroalcoholic extract (12, 24, and 48 mg/kg b.w.); DXR, doxorubicin (10 mg/kg b.w.). Values are mean ± standard deviation (n = 5). aSignificantly different from the NC group (P < 0.05). bSignificantly different from the DXR group (P < 0.05).
Figure 2 shows the GSH levels in peripheral blood of Swiss mice treated with RPHE (48 mg/kg b.w) and DXR. All animals treated with DXR showed significantly lower levels of GSH in comparison to animals from the negative control group. The GSH concentrations observed in animals treated with RPHE plus DXR did not differ from those observed in animals treated with DXR alone.
Fig. 2.
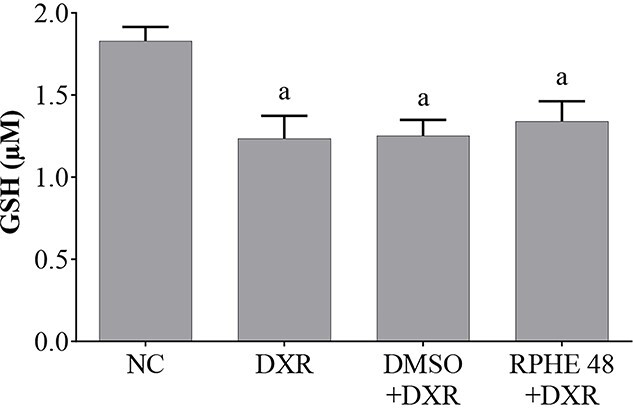
GSH levels in peripheral blood of Swiss mice treated with RPHE and DXR, and their respective controls. NC, negative control (water); DXR, doxorubicin (10 mg/kg b.w.); DMSO, dimethyl sulfoxide (2.5%); RPHE, red propolis hydroalcoholic extract (48 mg/kg b.w.); GSH, reduced glutathione. Values are mean ± standard deviation (n = 5). aSignificantly different from the NC group (P < 0.05).
The results of the biochemical indicators analysis of hepatotoxicity (AST and ALT) and nephrotoxicity (creatinine and urea) are shown in Table 1. The animals submitted to the different treatments presented serum levels of biochemical indicators that did not differ from those in the negative control group, revealing absence of toxicity.
Table 1.
Serum levels (mg/dL) of AST, ALT, creatinine, and urea obtained in peripheral blood of Swiss mice treated with RPHE and DXR, and their respective controls.
| Treatments | ALT | AST | Creatinine | Urea |
|---|---|---|---|---|
| NC | 54.3 ± 12.1 | 96.3 ± 2.4 | 0.4 ± .1 | 68.3 ± 3.1 |
| DMSO | 50.7 ± 9.9 | 86.7 ± 11.7 | 0.4 ± .0 | 67.0 ± 5.0 |
| RPHE 48 | 57.0 ± 9.2 | 87.0 ± 9.5 | 0.5 ± .1 | 65.3 ± 4.2 |
| DXR | 55.0 ± 5.3 | 85.7 ± 14.5 | 0.5 ± .0 | 66.7 ± 4.7 |
| DMSO + DXR | 51.0 ± 4.4 | 80.0 ± 12.1 | 0.5 ± .0 | 67.0 ± 5.0 |
| RPHE 12 + DXR | 46.7 ± 8.5 | 79.7 ± 12.5 | 0.4 ± .1 | 66.3 ± 3.8 |
| RPHE 24 + DXR | 49.3 ± 4.0 | 76.7 ± 8.1 | 0.5 ± .0 | 65.3 ± 3.1 |
| RPHE 48 + DXR | 47.0 ± 7.0 | 75.3 ± 5.5 | 0.5 ± .0 | 66.3 ± 4.2 |
NC, negative control (water); DMSO, dimethyl sulfoxide (2.5%); RPHE, red propolis hydroalcoholic extract (12, 24, and 48 mg/kg b.w.); DXR, doxorubicin (10 mg/kg b.w.); ALT, alanine aminotransferase, AST, aspartate aminotransferase. Values are mean ± standard deviation (n = 5).
Evaluation of anticarcinogenic activity
The ACF assay results are shown in Table 2. Pre-neoplastic lesions were observed in the colon only of animals that received DMH. The RPHE 12 mg/kg b.w. plus DMH treatment group showed significantly lower ACF and AC frequencies than the DMH group, corresponding to a 59% reduction in pre-neoplastic lesions. In addition, immunohistochemical analysis of the of colon showed a significant decrease in the expression of PCNA and COX-2 in this group in compared with DMSO plus DMH (Fig. 3).
Table 2.
ACF and AC frequencies, weight gain (g) and daily water consumption (mL/day) observed in Wistar Hannover rats treated with RPHE and DMH, and their respective controls.
| Treatments | ACF | AC | WG | WC |
|---|---|---|---|---|
| NC DMSO RPHE 48 DMH DMSO + DMH RPHE 12 + DMH RPHE 24 + DMH RPHE 48 + DMH |
- - - 16.0 ± 1.6 15.2 ± 1.9 6.2 ± 1.1b 15.0 ± 1.9 15.8 ± 1.5 |
- - - 25.0 ± 2.5 24.4 ± 5.6 10.6 ± 2.7b 27.4 ± 7.0 23.8 ± 2.2 |
130 ± 16.9 130 ± 11.8 137 ± 16.9 94 ± 8.9a 103 ± 5.3a 142 ± 12.2b 123 ± 11.6 111 ± 8.0 |
45.6 ± 6.3 45.2 ± 9.3 42.8 ± .8 49.8 ± 4.4 45.5 ± 3.1 45.4 ± .8 39.0 ± 1.4 47.1 ± 3.8 |
NC, negative control (water); DMSO, dimethylsulfoxide (2.5%); RPHE, red propolis hydroalcoholic extract (12, 24 and 48 mg/kg b.w.); DMH, 1,2-dimethylhydrazine (40 mg/kg b.w.); ACF, aberrant crypt foci; AC, aberrant crypt; WG, weight gain; WC, watter consumption. Values are mean ± standard deviation (n = 5). aSignificantly different from the NC group (P < 0.05); bSignificantly different from the DMH group (P < 0.05).
Fig. 3.
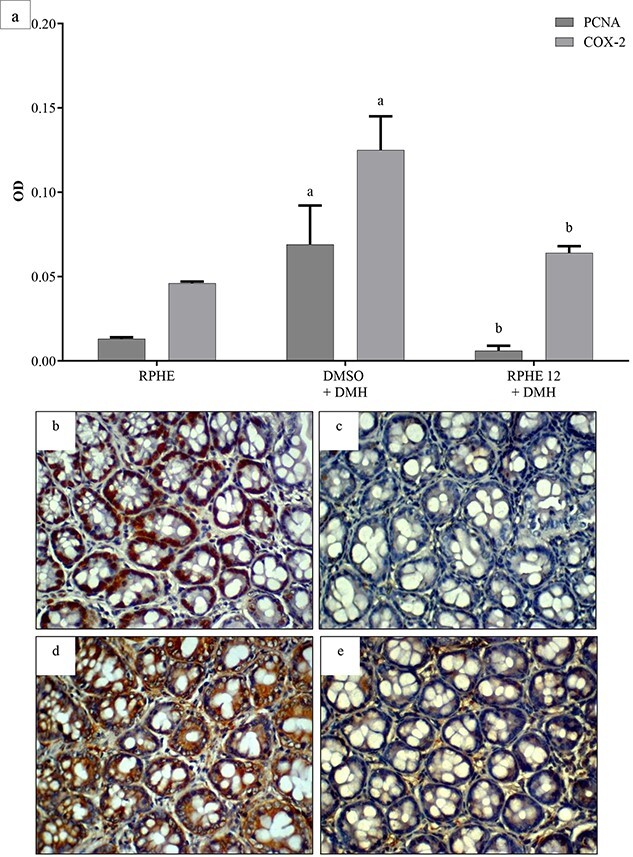
OD values of PCNA and COX-2 immunostaining in colon of Wistar Hannover rats treated with RPHE and DMH (a). Photomicrographs of immunohistochemical staining in colon sections from the DMSO+DMH and RPHE 12 + DMH (PCNA – B and c, respectively; COX-2 – D and e, respectively). (Nikon E200 microscope; 400× magnification; 3,3′- diaminobenzidine immunostaining [brown]; counterstain with hematoxylin [blue]; scale bar: 50 μm). DMSO, dimethyl sulfoxide (2.5%); DMH, 1,2-dimethylhydrazine (40 mg/kg b.w.); RPHE, red propolis hydroalcoholic extract (12 mg/kg b.w.); PCNA, proliferative cell nuclear antigen; COX-2, cyclooxygenase-2. Values are mean ± standard deviation (n = 3). aSignificantly different from the RPHE group (P < 0.05); bSignificantly different from the DMSO+DMH group (P < 0.05).
The weight gain in the DMH and DMH plus DMSO groups was significantly lower than that of the negative control group (Table 2). However, the animals treated with RHPE plus DMH did not show significant differences in relation to the negative control group. In addition, the weight gain of the RHPE 12 mg/kg b.w. plus DMH group was significantly greater than that of the DMH group. Water consumption did not differ between treatment groups.
Discussion
The results obtained in the present study revealed the antigenotoxic effect of red propolis and suggest its anticancer potential. This activity was also observed in Brazilian green propolis experiments, at the same dose, 12 mg/kg b.w.13 Propolis consumers usually take more than a single dose of propolis, which ranges from 0.3 to 1.5 g daily in tablet, extract, or powder form.23 The dose 12 mg/kg b.w. is equivalent to a dose of 0.8 g for human consumption.
Despite this, it is important to highlight that red and green propolis are produced and collected in different regions of the country and have different botanical sources. Dalbergia ecastophyllum and S. globulifera are the botanical sources of red propolis, whereas Baccharis dracunculifolia is the botanical source for green propolis.7,24 These factors imply propolis with different chemical compositions and, consequently, different biological properties. Even so, red and green propolis are rich in phenolic compounds, especially flavonoids, which have been described as chemoprotective agents due to their antioxidant, anti-inflammatory, and immunomodulatory activities.8,10
RPHE reduced DXR-induced genotoxicity in mice. Tavares et al.13 also observed the protective effect of the green propolis hydroalcoholic extract against the chromosomal damage induced by DXR in rats. The anthracycline DXR is a chemotherapeutic agent widely used in the treatment of several types of cancer. Its mechanisms of action include the inhibition of topoisomerase II and/or helicases and the ability to intercalate with DNA by its aglycone portion.25,26 Another mechanism described in the literature for DXR is the reactive oxygen species (ROS) production. The quinone structure from DXR can be reduced to a semiquinone radical, reacting quickly with oxygen, and resulting in superoxide and hydrogen peroxide.25 It is known that ROS react with DNA, changing the deoxyribose chemical structure or interacting with the nitrogenous bases, resulting in damage to genetic material.25,27
Therefore, the antigenotoxic effect of RPHE may be due, at least in part, to the antioxidant action of flavonoids present in its chemical composition. These phenolic compounds can deplete ROS levels, either by scavenging them or by activating antioxidant signaling pathways.28,29 The literature has reported the remarkable and significant properties of flavonoids as anticancer and/or chemopreventive agents, including influencing the immune system, which suggests a positive correlation between a lower cancer risk and a diet rich in flavonoids.3,5,10,29 In this context, strategies are being widely discussed to develop anticancer therapies by the combination of phytochemicals, especially flavonoids, and conventional drugs, such as DXR.10,29
Mendonça et al.,8 using the DPPH chemical test (2,2-diphenyl-1-picryl-hydrazil), observed that RPHE caused the depletion of 86.6% of free radicals at 25 μg/mL, revealing radical capture activity greater than vitamin E (Trolox, Hoffman-LaRoche®; 68.3%). Considering this and in order to clarify the mechanism of action involved in the antigenotoxic action of RHPE observed in the present study, its antioxidant capacity was investigated through the serum levels of GSH. This tripeptide is important for the defense against oxidative stress, being found in high levels under normal conditions in cells of basically all aerobic organisms. GSH act as a scavenger of electrophiles, through conjugation, within the cell, and transport them to the extracellular medium, where these conjugates are cleaved by enzymes present in the outer portion of the cell membrane. The residues are eliminated in later stages and the glutamate and glycine portions are reabsorbed to be used in a new GSH synthesis.30,31
DXR is known to form a conjugate with GSH. DXR detoxification in cells is highly dependent on reactions with GSH to form DXR-specific GS-X conjugates.32 The results of GSH analysis showed that, under the same experimental conditions that the antigenotoxic effect of RPHE was observed, the extract did not influence the DXR-induced GSH depletion. This result indicates that the protective action of RPHE on DXR-induced genotoxicity did not involve GSH activity. However, other mechanisms of antioxidant activity might be involved. According to Huang et al.,32 the isoflavonone liquiritigenin, one of the chemical compounds in the RPHE, promotes nuclear translocation of NRF2 (nuclear factor erythroid 2), leading to increased expression of genes related to antioxidant activity, the thioredoxin 2 and thioredoxin reductase 2 genes.
Additionally, DXR can also cause DNA damage as a result of the induced inflammation. The literature reports that DXR is able to enhance NF-κB expression.33,34 One of the mechanisms related to red propolis anti-inflammatory activity is the inhibition of NF-κB pathway.35 In this regard, RPHE might mitigate the DXR-induced damages in Swiss mice due to its anti-inflammatory potential.
Considering that somatic mutations are involved in cancer initiation and progression, the anticarcinogenic effect of RPHE was evaluated on chemically induced colon carcinogenesis. The results showed the protective activity of the extract against DMH-induced carcinogenicity and toxicity. Colorectal cancer usually starts as small adenomatous polyps in colon, whereas about 50% of cases occur in the distal colon and rectum. Studies show that healthy lifestyle habits, such as the consumption of fiber and foods with antioxidant and anti-inflammatory action, decrease the risk for colorectal cancer, since both oxidative stress and the inflammatory process trigger the events that lead to carcinogenesis.2–4 The mucosa of patients with colon cancer often has putative pre-neoplastic lesions, the AC, being important biomarkers for the colorectal cancer diagnosis. These lesions are also observed in the colon of rats and mice treated with carcinogens and are used as a tool for carcinogenic process evaluation, colon-specific carcinogens investigation, and compounds chemopreventive effect analysis.36–38
DMH is widely used as a carcinogen to induce colon cancer in animal models. DMH is a procarcinogen that requires metabolic activation, which leads to the formation of methyl free radicals. Generation of the hydroxyl radical or hydrogen peroxide can also occur in the presence of metal ions that may contribute to cancer initiation and lipid peroxidation.39 DMH also presents the inflammation inducing as mechanism of action. Animals treated with DMH have shown high expression of COX-2 in colon tissue.40,41 The results of the present study showed that RPHE significantly reduced DMH-induced COX-2 expression, revealing anti-inflammatory activity. COX-2 is a prostaglandin-synthase, converting arachidonic acid to prostaglandins, being an important enzyme for trigger inflammatory and pain responses. This glycoprotein is found mostly in gastrointestinal and endometrial tissues and is activated in response to stimulus. However, COX-2 is also frequently observed in tumor tissues or cells. The COX-2 is overexpressed in colon tumor tissue and is associated to increased tumor aggression and poor prognosis.42,43 Its activation on tumor tissue, mainly in colorectal cancer, is involved to NF-κB signaling pathway. Therefore, this NF-κB–COX-2 pathway can be a promising therapeutic target in the treatment of colon cancer.44–46 In view of the above, the anti-inflammatory activity of RPHE observed in the colon by COX-2 expression reduction should be responsible, at least in part, for its anticarcinogenic effect.
The chemoprotective action of RPHE on pre-neoplastic lesions in the colon was also demonstrated by the reduction of the expression of PCNA, a well-known cell proliferation marker. The PCNA is a critical eukaryotic replication accessory factor, an auxiliary protein of DNA polymerase δ.41,47,48 The RPHE, its fractions and its constituents, have shown to antiproliferative effect in different tumor cell lines.9,12,49,50 This activity of the red propolis is thought to be related mainly to the presence of guttiferone E, xanthochymol, and oblongifolin B. Novak et al.49 observed the antiproliferative effect of a red propolis fraction enriched in xanthochymol and in the isoflavone formononetin in human lung fibroblasts (MCR-5). A red propolis fraction, rich in guttiferone E/xanthochymol, medicarpine, and 7-O-methylvestitol, showed antiproliferative activity in colorectal adenocarcinoma cells (HCT-116 and HT-29).51 According to Lin et al.,52 the guttiferone E/xanthochymol blocked the cell cycle in sub-G0/G1 phase.
The RPHE exerted chemoprotective effect against chromosomal damage in peripheral blood and on colon pre-neoplastic lesions, but the effective doses were different. RPHE showed an antigenotoxic effect at 48 mg/kg b.w., while anticarcinogenic activity was observed at 12 mg/kg b.w. It must be noted that the experimental protocols applied in this study have different schedules, and for each one, a different tissue must be analyzed.15,18 Regarding the ACF assay, whose treatment was carried out for 2 weeks, it is important to consider the RPHE composition, rich in flavonoids. Despite the well-known antioxidant activity of flavonoids, in high concentrations, they can be oxidized in quinones and semiquinones, with molecular oxygen (O2) and a transition metal ion, resulting in ions of O2- and hydrogen peroxide, via auto-oxidation. This mechanism may have enhanced the oxidative stress during the treatment period at highest doses of RPHE in the ACF assay, which can justify the absence of the protective effect of the extract at these doses.53,54
Hepatotoxicity and nephrotoxicity of the treatment with the RPHE were evaluated in the mice used in the MN test. AST and ALT transaminases are produced in adaptive response to the metabolic demand of the liver. ALT is also produced in the heart and kidneys. The quantification of AST and ALT blood levels is useful to detect lesions in the heart and kidneys, but mostly in the liver, resulted of poor diet, alcohol abuse, smoking, viral activity, and drug/medicine toxicity.55–57 Creatinine and urea, in turn, are nitrogenous products from metabolism, which circulate in the blood until being filtered by healthy kidneys. Damage to renal tissues resulting from the toxicity of compounds, for example, leads to increased levels of creatinine and urea.58,59 In the present study, treatments with RPHE did not induce increase in these biochemical marker levels, revealing the absence of hepatotoxicity and nephrotoxicity in the experimental conditions used.
In the ACF test, weight gain and water consumption were used as indicators of treatment toxicity. DMH reduced body weight gain, as already demonstrated in the literature.40,41 On the other hand, RHPE exhibited a protective action against DMH-induced toxicity, since animals treated with RPHE and DMH showed similar weight gain to those untreated.
The presence of several compounds in the chemical constitution of an extract represents a challenge in elucidating the mechanisms of action, since the constituents can exert different biological effects when taken together and when evaluated separately. Despite this, the biological activity of an extract is mostly related to the synergistic effect of the constituents, which influence at different levels, on different targets and pathways at the same time. Therefore, the extracts may have biological properties that the constituents alone would not have.60,61
Conclusions
The present work showed unprecedented results of the red propolis chemopreventive effect, contributing to knowing better the biological activities of this bee product in animal organism. The RPHE exhibited chemoprotective effect in vivo on DXR-induced genomic instability and on DMH-induced colon carcinogenesis. This effect is related to the anti-inflammatory and antiproliferative activities of red propolis. These results were to be expected because of the chemical composition of red propolis and now additional studies should be conducted with the chemical constituents. Therefore, these data contribute to the search of new compounds that present chemopreventive effect and are interesting to the cancer treatment.
Acknowledgments
The authors declare there is no conflict of interest. The authors are grateful to the São Paulo Research Foundation (FAPESP, Brazil), to the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) and to the National Council for Scientific and Technological Development (CNPq, Brazil) for granting fellowships.
Authors’ contributions
Freitas, KS; Bastos, JK and Tavares, DC conceived the study. Bastos, JK furnished the red propolis. Veneziani, RCS and Lemes, DC extracted and provided the RPHE. Freitas, KS; Silva, LHD; Squarisi, IS; Ozelin, SD and Esperandim, TR performed animal treatment, biological samples collection and analysis of the micronucleus test. Freitas, KS performed the oxidative stress evaluation. Freitas, KS; Silva, LHD; Squarisi, IS; Oliveira, LTS; Ribeiro, AB; Alves, BS and Melo, MRS performed animal treatment, biological samples collection and analysis of the aberrant crypt foci assay. Freitas, KS and Silva, LHD performed immunohistochemistry procedures. Freitas, KS processed the data, performed the statistical analysis, produced the tables and figures, wrote and submitted the manuscript, with overall guidance from Tavares, DC.
Contributor Information
Karoline Soares de Freitas, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Lucas Henrique Domingos da Silva, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Iara Silva Squarisi, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Lucas Teixeira de Souza Oliveira, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Arthur Barcelos Ribeiro, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Bianca Silva Alves, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Tábata Rodrigues Esperandim, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Matheus Reis Santos de Melo, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Saulo Duarte Ozelin, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Danieli Cristina Lemes, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Jairo Kenupp Bastos, School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Avenida do Café Ave, Vila Monte Alegre, Ribeirão Preto, São Paulo 14040-900, Brazil.
Rodrigo Cassio Sola Veneziani, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Denise Crispim Tavares, Mutagenesis Laboratory, University of Franca, 201 Dr Armando Salles de Oliveira Ave, Parque Universitário, Franca, São Paulo 14404-600, Brazil.
Funding
This work was supported by the São Paulo Research Foundation (FAPESP, Brazil) [grant numbers #2017/04138–8] and K.S. Freitas was recipient of a Master’s fellowship from FAPESP [grant numbers #2018/02370–3].
Conflict of interest statement. None declared.
References
- 1. Wild CP, Weiderpass E, Stewart BW. World cancer report: cancer research for cancer prevention. France: Lyon; 2020 [Google Scholar]
- 2. Jin K, Ren C, Liu Y, Lan H, Wang Z. An update on colorectal cancer microenvironment, epigenetic and immunotherapy. Int Immunopharmacol. 2020:89(Pt A):1–8. [DOI] [PubMed] [Google Scholar]
- 3. Zhou E, Rifkin S. Colorectal cancer and diet: risk versus prevention, is diet an intervention? Gastroenterol Clin N Am. 2021:50(1):101–111. [DOI] [PubMed] [Google Scholar]
- 4. Huang XM, Yang ZJ, Xie Q, Zhang ZK, Zhang H, Ma JY. Natural products for treating colorectal cancer: a mechanistic review. Biomed Pharmacother. 2019:117:109142. [DOI] [PubMed] [Google Scholar]
- 5. Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front Pharmacol. 2020:10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salatino A, Salatino MLF, Negri G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie. 2021:52(6):1075–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ccana-Ccapatinta GV, Mejía JAA, Tanimoto MH, Groppo M, Carvalho JCAS, Bastos JK. Dalbergia ecastaphyllum (L.) Taub. And Symphonia globulifera L.f.: the botanical sources of isoflavonoids and benzophenones in Brazilian red propolis. Molecules. 2020:25(9):2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendonça ICG, Porto ICCM, Nascimento TG, Souza NS, Oliveira JMS, Arruda RES, Mousinho KC, Santos AF, Basílio-Júnior ID, Parolia A, et al. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement Altern Med. 2015:15:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Squarisi1 IS, Freitas FK, Lemes DC, Ccana-Ccapatinta GV, Aldana-Mejía JA, Bastos JK, Veneziani RCS, Ambrosio SR, Tavares DC. Evaluation of the antiproliferative activity of red propolis hydroalcoholic extract and its fractions obtained by partition. Biofarmasi J Nat Prod Biochem. 2020:18(2):66–69. [Google Scholar]
- 10. Kikuchi H, Yuan B, Hu X, Okazaki M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am J Cancer Res. 2019:9(8):1517–1535. [PMC free article] [PubMed] [Google Scholar]
- 11. Condello M, Meschini S. Role of natural antioxidant products in colorectal cancer disease: a focus on a natural compound derived from Prunus spinosa. Trigno Ecotype Cells. 2021:10(12):3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aldana-Mejía JA, Ccana-Ccapatinta GV, Squarisi IS, Nascimento S, Tanimoto MH, Ribeiro VP, Arruda C, Nicolella H, Esperandim T, Ribeiro AB, et al. Nonclinical toxicological studies of Brazilian red propolis and its primary botanical source Dalbergia ecastaphyllum. Chem Res Toxicol. 2021:34(4):1024–1033. [DOI] [PubMed] [Google Scholar]
- 13. Tavares DC, Lira WM, Santini CB, Takahashi CS, Bastos JK. Effects of propolis crude hydroalcoholic extract on chromosomal aberrations induced by doxorubicin in rats. Planta Med. 2007:73(15):1531–1536. [DOI] [PubMed] [Google Scholar]
- 14. Senedese JM, Alves JM, Lima IM, Andrade EA, Furtado RA, Bastos JK, Tavares DC. Chemopreventive effect of Copaifera langsdorffii leaves hydroalcoholic extract on 1,2-dimethylhydrazine-induced DNA damage and preneoplastic lesions in rat colon. BMC Complement Altern Med. 2013:13 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. OECD . Test no. 474: mammalian erythrocyte micronucleus test, OECD guidelines for the testing of chemicals, section 4. Paris: OECD Publishing; 2016 [Google Scholar]
- 16. Freitas KS, Squarisi IS, Acésio NO, Nicolella HD, Ozelin SD, Melo MRS, Guissone APP, Fernandes G, Silva LM, Silva Filho AA, et al. Licochalcone a, a licorice flavonoid: antioxidant, cytotoxic, genotoxic, and chemopreventive potential. J Toxic Environ Health A. 2020:83(21-22):673–686. [DOI] [PubMed] [Google Scholar]
- 17. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959:82(1):70–sa7. [DOI] [PubMed] [Google Scholar]
- 18. Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987:37(2):147–151. [DOI] [PubMed] [Google Scholar]
- 19. Ferreira NH, Ribeiro AB, Rinaldi-Neto F, Fernandes FS, Do Nascimento S, Braz WR, Nassar EJ, Tavares DC. Anti-melanoma activity of indomethacin incorporated into mesoporous silica nanoparticles. Pharm Res. 2020:37(9):172. [DOI] [PubMed] [Google Scholar]
- 20. ImageJ . Image processing and analysis in java. https://imagej.nih.gov/ij/download.html[accessed 2021 Jan 20].
- 21. Shu J, Dolman GE, Duan J, Qiu G, Ilyas M. Statistical colour models: an automated digital image analysis method for quantification of histological biomarkers. Biomed Eng Online. 2016:15 (46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Furtado RA, Oliveira BR, Silva LR, Cleto SS, Munari CC, Cunha WR, Tavares DC. Chemopreventive effects of rosmarinic acid on rat colon carcinogenesis. Eur J Cancer Prev. 2015:24(9):106–112. [DOI] [PubMed] [Google Scholar]
- 23. Shimizu K, Ashidashida H, Matsuura Y, Kanazawa K. Antioxidative biovailabilitity of artepillin C in Brazilian propolis. Arch Biochem Biophys. 2004:424(2):181–188. [DOI] [PubMed] [Google Scholar]
- 24. Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005:100(2):114–117. [DOI] [PubMed] [Google Scholar]
- 25. Injac R, Strukelj B. Recent advances in protection against doxorubicin-induced toxicity. Technol Cancer Res Treat. 2008:7:497–516. [DOI] [PubMed] [Google Scholar]
- 26. Yang F, Teves SS, Kemp CJ, Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim Biophys Acta. 2014:1845(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saha SK, Lee SB, Won J, Choi HY, Kim K, Yang GM, Dayem AA, Cho S. Correlation between oxidative stress, nutrition, and cancer initiation. Int J Mol Sci. 2017:18(7):1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Razavi-Azarkhiavi K, Iranshahy M, Sahebkar A, Shirani K, Karimi G. The protective role of phenolic compounds against doxorubicin-induced cardiotoxicity: a comprehensive review. Nutr Cancer. 2016:68(6):892–917. [DOI] [PubMed] [Google Scholar]
- 29. Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as anticancer agents. Nutrients. 2020:12(2):457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berndt C, Lillig CH. Glutathione, Glutaredoxins, and iron. Antioxid Redox Signal. 2017:27(15):1235–1251. [DOI] [PubMed] [Google Scholar]
- 31. Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C. Role of glutathione in cancer progression and chemoresistance. Oxidative Med Cell Longev. 2013:2013:972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang Z, Sheng Y, Chen M, Hao Z, Hu F, Ji L. Liquiritigenin and liquiritin alleviated MCT-induced HSOS by activating Nrf2 antioxidative defense system. Toxicol Appl Pharmacol. 2018:355:18–27. [DOI] [PubMed] [Google Scholar]
- 33. Kim JY, Jung HH, Ahn S, Bae S, Lee SK, Kim SW, Lee JE, Nam SJ, Ahn JS, Im YH, et al. The relationship between nuclear factor (NF)-κB family gene expression and prognosis in triple-negative breast cancer (TNBC) patients receiving adjuvant doxorubicin treatment. Sci Rep. 2016:6:31804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhu MM, Wang L, Yang D, Li C, Pang ST, Li XH, Li R, Yang B, Lian YP, Ma L, et al. Wedelolactone alleviates doxorubicin-induced inflammation and oxidative stress damage of podocytes by IκK/IκB/NF-κB pathway. Biomed Pharmacother. 2019:117:1–7. [DOI] [PubMed] [Google Scholar]
- 35. Corrêa FRS, Schanuel FS, Nunesa NM, Costab AMA, Dalepranea JB. Brazilian red propolis improves cutaneous wound healing suppressing inflammation-associated transcription factor NFκB. Biomed Pharmacother. 2017:86:162–171. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi M, Minamoto T, Yamashita N, Yazawa K, Sugimura T, Esumi H. Reduction in formation and growth of 1,2-dimethylhydrazine-induced aberrant crypt foci in rat colon by docosahexaenoic acid. Cancer Res. 1993:53(12):2786–2789. [PubMed] [Google Scholar]
- 37. Bird RP, Good CK. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol Lett. 2000:15:395–402. [DOI] [PubMed] [Google Scholar]
- 38. Schoen RE, Mutch M, Rall C, Dry SM, Seligson D, Umar A, Pinsky P. Natural history of aberrant crypt foci. Gastrointest Endosc. 2008:67(7):1097–1102. [DOI] [PubMed] [Google Scholar]
- 39. Khan R, Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem Biol Interact. 2011:192(3):193–200. [DOI] [PubMed] [Google Scholar]
- 40. Walia S, Kamal R, Kanwar SS, Dhawan DK. Cyclooxygenase as a target in chemoprevention by probiotics during 1,2-dimethylhydrazine induced colon carcinogenesis in rats. Nutr Cancer. 2015:67(4):603–611. [DOI] [PubMed] [Google Scholar]
- 41. Khan R, Rehman MU, Khan AQ, Tahir M, Sultana S. Glycyrrhizic acid suppresses 1,2-dimethylhydrazine-induced colon tumorigenesis in Wistar rats: alleviation of inflammatory, proliferation, angiogenic, and apoptotic markers. Environ Toxicol. 2018:33(12):1272–1283. [DOI] [PubMed] [Google Scholar]
- 42. Goradel NH, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: a review. J Cell Physiol. 2018:234(5):5683–5699. [DOI] [PubMed] [Google Scholar]
- 43. Nagaraju GP, El-Rayes BF. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer. 2019:125(20):1221–1227. [DOI] [PubMed] [Google Scholar]
- 44. Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. 2001. Lab Investig. 2001:81:349–360. [DOI] [PubMed] [Google Scholar]
- 45. Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. Expression of COX-2, NF-kappaB-p65, NF-kappaB-p50 and IKKalpha in malignant and adjacent normal human colorectal tissue. Br J Cancer. 2009:101(1):106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi G, Li D, Fu J, Sun Y, Li Y, Qu R, Jin X, Li D. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am J Transl Res. 2015:7(9):1612–1620. [PMC free article] [PubMed] [Google Scholar]
- 47. Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci. 2008:65(23):3789–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park SY, Jeong MS, Han CW, Yu HS, Jang SB. Structural and functional insight into proliferating cell nuclear antigen. J Microbiol Biotechnol. 2016:26(4):637–647. [DOI] [PubMed] [Google Scholar]
- 49. Novak EM, Silva MSC, Marcucci MC, Sawaya ACHF, López BGC, Fortes MAHZ, Giorgi RR, Marumo KT, Rodrigues RF, Maria DA. Antitumoural activity of Brazilian red propolis fraction enriched with xanthochymol and formononetin: an in vitro and in vivo study. J Funct Foods. 2014:11:91–102. [Google Scholar]
- 50. Frozza CODS, Santos DA, Rufatto LC, Minetto L, Scariot FJ, Echeverrigaray S, Pich CT, Moura S, Padilha FF, Borsuk S, et al. Antitumor activity of Brazilian red propolis fractions against Hep-2 cancer cell line. Biomed Pharmacother. 2017:91:951–963. [DOI] [PubMed] [Google Scholar]
- 51. Santos DA, Munari FM, Frozza COS, Moura S, Barcellos T, Henriques JAP, Roesch-Ely M. Brazilian red propolis extracts: study of chemical composition by ESI-MS/MS (ESI+) and cytotoxic profiles against colon cancer cell lines. Biotechnol Res Int. 2019:3(1):120–130. [Google Scholar]
- 52. Lin X, Tian D, Fu Y, Li Y, Huang L, Gu W, Song J, Li Y, Ben-David Y, Wen M, et al. Synthesis of novel guttiferone E and xanthochymol derivatives with cytotoxicities by inducing cell apoptosis and arresting the cell cycle phase. Eur J Med Chem. 2019:162:765–780. [DOI] [PubMed] [Google Scholar]
- 53. Akagawa M, Shigemitsu T, Suyama K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci Biotechnol Biochem. 2003:67(12):2632–2640. [DOI] [PubMed] [Google Scholar]
- 54. Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc Res. 2007:73(2):341–347. [DOI] [PubMed] [Google Scholar]
- 55. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000:342 (17):1266–1271. [DOI] [PubMed] [Google Scholar]
- 56. Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol. 2012:18(29):3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol. 2015:21(3):711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fuchs TC, Hewit P. Biomarkers for drug-induced renal damage and nephrotoxicity-an overview for applied toxicology. AAPS J. 2011:13(4):615–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gobe GC, Coombes JS, Fassett RG, Endre ZH. Biomarkers of drug-induced acute kidney injury in the adult. Expert Opin Drug Metab Toxicol. 2015:11(11):1683–1694. [DOI] [PubMed] [Google Scholar]
- 60. Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W, Piao G. How can synergism of traditional medicines benefit from network pharmacology? Molecules. 2017:22(7):1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lai X, Wang X, Hu Y, Su S, Li W, Li S. Network pharmacology and traditional medicine. Front Pharmacol. 2020:11:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


