Abstract
Objective:
We aim to assess the technical feasibility of ferumoxytol MRA in vascular mapping prior to transcatheter aortic valve replacement (TAVR) in patients with renal impairment.
Methods:
This is an IRB-approved and HIPAA-compliant study. FE-MRA was performed at 3.0T or 1.5T with an infusion of ferumoxytol. Non-contrast CT images were used to overlay vascular calcification on FE-MRA images as composite fused 3D data. Image quality of the subclavian and aortoiliofemoral arterial tree and confidence in the assessment of calcification were evaluated using a 4-point scale (4=excellent vascular definition or strong confidence, respectively). Signal nonuniformity as reflected by the heterogeneity index (ratio between the mean standard deviation of luminal SI and the mean luminal SI), SNR, and consistency of luminal diameter measurements were quantified. Findings on FE-MRA were compared with pelvic angiograms.
Results:
Twenty-six patients underwent FE-MRA without adverse events. A total of 286 named vascular segments were scored. The image quality score was 4 for 99% (n=283) of the segments (k=0.9). There was moderate to strong confidence in the ability to assess vascular calcific morphology in all studies with complementary non-contrast CT. Steady state luminal heterogeneity index was low and SNR was high. Inter-observer luminal measurements were reliable (ICC=0.98, 95% CI 0.98 to 0.99). FE-MRA findings were consistent with correlative pelvic angiograms in all 16 patients.
Conclusion:
In patients with renal impairment undergoing TAVR, FE-MRA is technically feasible and offers reliable vascular mapping without exposure to iodinated or gadolinium contrast agents. Thus, the total cumulative dose of iodinated contrast is minimized and the risk of acute nephropathy is reduced.
Keywords: Transcatheter aortic valve replacement, TAVR, Magnetic resonance angiography, MRA, ferumoxytol, aortic stenosis
Introduction
Accurate vascular mapping of the subclavian and aortoiliofemoral arterial system is crucial for transcatheter aortic valve replacement (TAVR) risk stratification, device sizing and allocation to vascular access approaches (1–3) (Figure 1). The current reference standard for pre-TAVR vascular mapping is contrast enhanced computed tomographic angiography, which has been shown to be accurate and reproducible for assessment of annular sizing, arterial tortuosity, arterial calcification, atherosclerosis and vessel diameter (2). Although radiation exposure is less of a concern in older than in younger patients, opacification of the perfused arterial lumen requires the use of iodinated contrast agents, with the attendant risk of nephrotoxicity. Acute kidney injury (AKI) occurs in 10% to 30% of patients undergoing TAVR and is associated with a higher mortality risk (4, 5). Furthermore, impaired renal function at baseline is a strong independent predictor of adverse outcome in TAVR (6). Although the mechanisms of AKI following TAVR are multifactorial, there exists a relationship between iodinated contrast media dose and AKI (7, 8). Avoidance of nephrotoxic contrast media for pre-procedure arterial evaluation would therefore be of great potential benefit.
Figure 1.
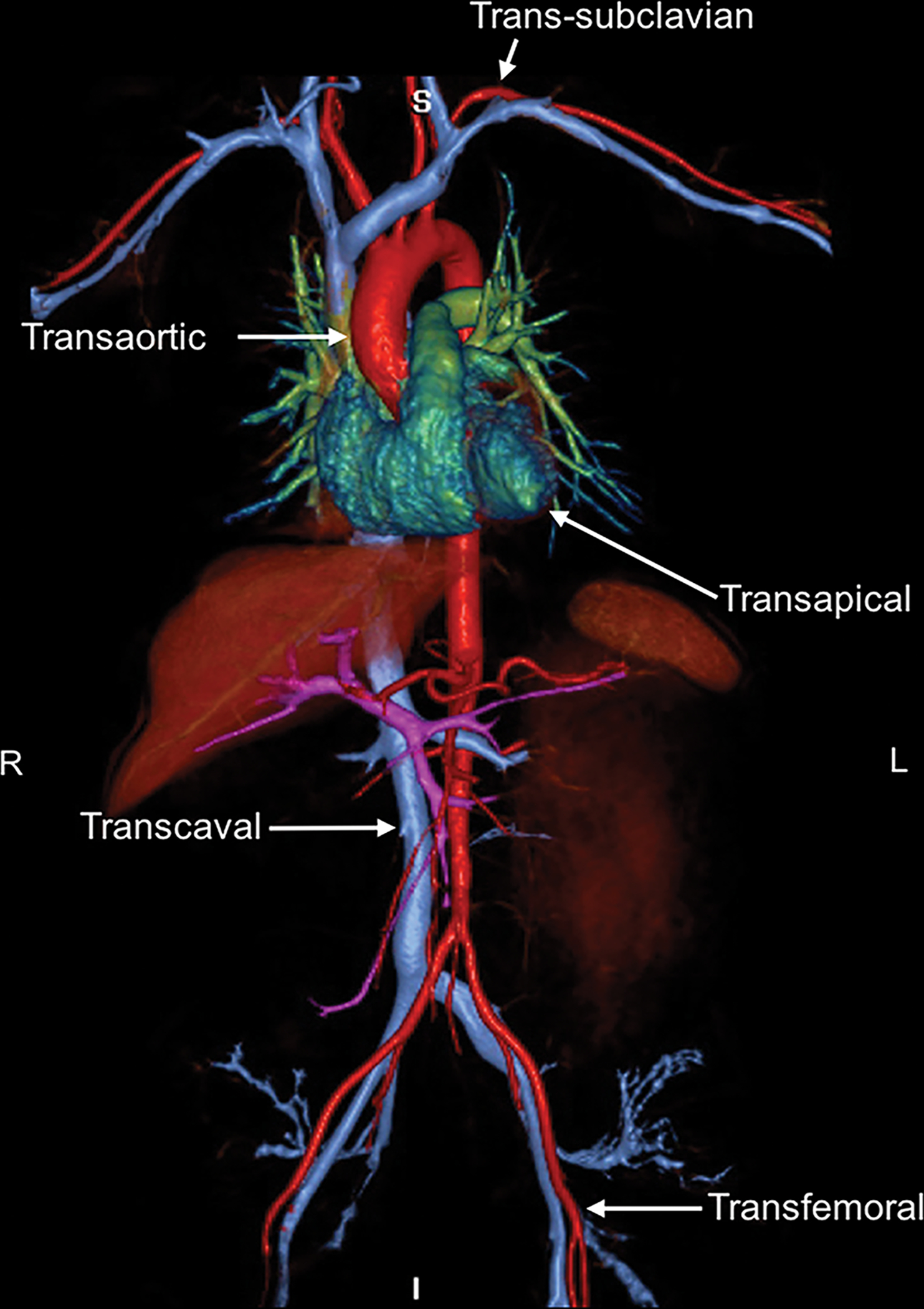
Conventional and emerging alternative vascular access sites for transcatheter aortic valve replacement (TAVR) are depicted in the ferumoxytol enhanced MRA image. Transfemoral and transapical approaches have been most widely used. Emerging alternative vascular access routes include transaortic, trans-subclavian, and transcaval.
Contrast enhanced magnetic resonance angiography (CEMRA) has been shown to provide excellent visualization of both central and peripheral vessels (9) without the confounding effect of dense vascular calcification on luminal assessment, which is often seen on CT. However, in patients with renal impairment, concerns about the risk of nephrogenic systemic fibrosis (NSF) associated with gadolinium based contrast agents (GBCA) has complicated the diagnostic algorithm and has caused many physicians to shy away the use of CEMRA (7, 10).
Recently, ferumoxytol (Feraheme®; AMAG Pharmaceuticals, Inc., Cambridge, MA) has shown promise as a non-gadolinium alternative for CEMRA in patients with renal failure (11). Originally designed as an intravascular contrast agent for MRI, its iron oxide core is embedded in a dextran coat, which diminishes immunogenicity and retards phagocytosis and the release of elemental iron from the core. Ferumoxytol is U.S FDA approved for the treatment of anemia in adults with chronic kidney disease (CKD) and has been used as a vascular MRA contrast agent (12, 13). Because ferumoxytol use has no propensity to cause NSF, it offers immediate promise in the patient with renal impairment. Therefore, we aim to assess the technical feasibility of ferumoxytol MRA in vascular mapping prior to transcatheter aortic valve replacement (TAVR) in patients with renal impairment.
Materials and Methods
Study Population
This study was approved by the local Institutional Review Board and was compliant with the Health Portability and Insurance Accountability Act. All patients provided written informed consent for the FE-MRA study and inclusion into our research protocol. This is a cross-sectional, retrospective, feasibility study based on a review of prospectively collected data. Consecutive patients with significant CKD (defined as having least stage 3a [eGFR ≤ 59 mL/min/1.73m2]) who were referred for FE-MRA TAVR vascular mapping between January 2014 to November 2016 were included (Supplemental Material Figure S1). The risks and benefits of the procedure were explained by an attending physician (JPF), who was present throughout all MRI studies and monitored the vital signs. Follow-up was performed (KLN, TY) via medical record review at the time of data analysis. The patient population described in this manuscript has been included in a prior manuscript focused on the safety profile of ferumoxytol as a diagnostic MR contrast agent (14). There is no overlap in focus.
Image Acquisition
In 21 patients, studies were performed on a whole body 3.0T MRI scanner (Magnetom TIM Trio (n=12) or Magnetom Prisma Fit (n=8) or Magnetom Skyra (n=1), Siemens Medical Solutions, Malvern PA). Five patients were scanned on a 1.5T MRI scanner (Magnetom TIM Avanto, Siemens Medical Solutions, Malvern, PA); three of whom had implantable cardiac devices. Receiver coil configurations were customized to patient size (2 × 8 element body array coils, combined with in-table spine coils, to provide coverage from the thoracic inlet to the mid thighs). Ferumoxytol was administered to a total dose of 4 mg/kg of iron. Continuous monitoring with ECG, pulse oximetry and non-invasive blood pressure was performed prior to ferumoxytol injection and for at least 30 minutes following injection.
Fourteen patients had two-station, first pass imaging and 12 patients had imaging in the steady state distribution of ferumoxytol. For first pass imaging, stock ferumoxytol was diluted 6X and the infusion period was 30 seconds for a two-station acquisition at a flow rate ~1.5 ml/s. A timing bolus using 1 ml of the diluted ferumoxytol was first injected to determine the delay time between injection and the arrival of contrast in the descending aorta, similar to a conventional timing test injection with gadolinium based contrast agents. After the correct timing for the first-pass FE-MRA sequence was obtained, the main contrast bolus was injected at the same rate followed by a 20 ml saline. For each station, a high-resolution 3D acquisition was performed in a breath hold period of 17–21 seconds. Following acquisition of the first station, the table was incremented automatically by 150–200 mm (depending on patient size) and the second station was acquired in a similar breath hold period. The two overlapping 3D datasets had identical spatial resolution (1 mm × 1mm × 1.2 mm) and were processed inline on the MRI system console (Siemens Medical Solutions, Malvern, PA) to produce a composed, fused, extended field-of-view (eFOV) image set. For exams performed after April 2015, no timing acquisition was performed and all images were acquired only during the steady state distribution of ferumoxytol following slow infusion over 8 minutes in compliance with FDA recommendations.
Supplemental ‘black blood’ images were acquired post-contrast using single shot, T2-weighted Half-Fourier Acquisition Single Shot Turbo Spin Echo (HASTE) in coronal and oblique sagittal planes. The HASTE sequence was chosen because it is a commercially available sequence and can be implemented easily at any clinical site. Further, HASTE is not sensitive to motion artifact and can be performed quickly and, without breath holding. In 21 patients who had non-contrast CT scans showing vascular calcification, the calcium was segmented and overlaid on the ferumoxytol enhanced MR angiograms using a commercial 3D modeling software (Mimics, Materalise, Leuven, Belgium), to generate a composite map of perfused lumen and mural calcification (Supplement Material Figure S2).
Qualitative Image Analysis
Image quality and confidence in the assessment of vessel patency, tortuosity, calcific burden, calcific morphology, and aortic atheroma were evaluated. These vascular morphologic features were chosen because they have been shown to be predictive and related to vascular complications during TAVR (1). FE-MRA images, complementary black blood images, and non-contrast CT images were reviewed (MacOsiriX, Pixmeo, Geneva, Switzerland) independently by two board-certified radiologists (JMM, ANP; >5 years of experience in cardiovascular MR/CT) and one board-certified cardiologist (KLN, 5 years of experience in cardiovascular MR/CT). Reviewers had full control to perform additional MIPs and reformats as needed. The right and left subclavian arteries, ascending thoracic aorta, abdominal aorta, and iliofemoral arterial system were assessed (n=286 segments). The abdominal aorta and iliofemoral arterial system were divided into 8 anatomic segments: supra-renal aorta, infra-renal aorta, right and left common Iliac artery, right and left external iliac artery, right and left common femoral artery. The evaluators scored the image quality of FE-MRA images with respect to vascular anatomy using a 4-point scale (Figure 2). Confidence in assessment of vessel tortuosity, calcific burden, calcific morphology, and aortic atheroma was scored using a 4-point scale (Figure 2). For 21 studies with both FE-MRA and non-contrast CT images, both were evaluated during one session. The presence of artifacts including blurring of vessel borders, signal loss and saturation artifacts were also assessed. The findings on the MRA studies were compared with those of standard catheter angiography performed at the time of TAVR which served as the reference standard.
Figure 2.
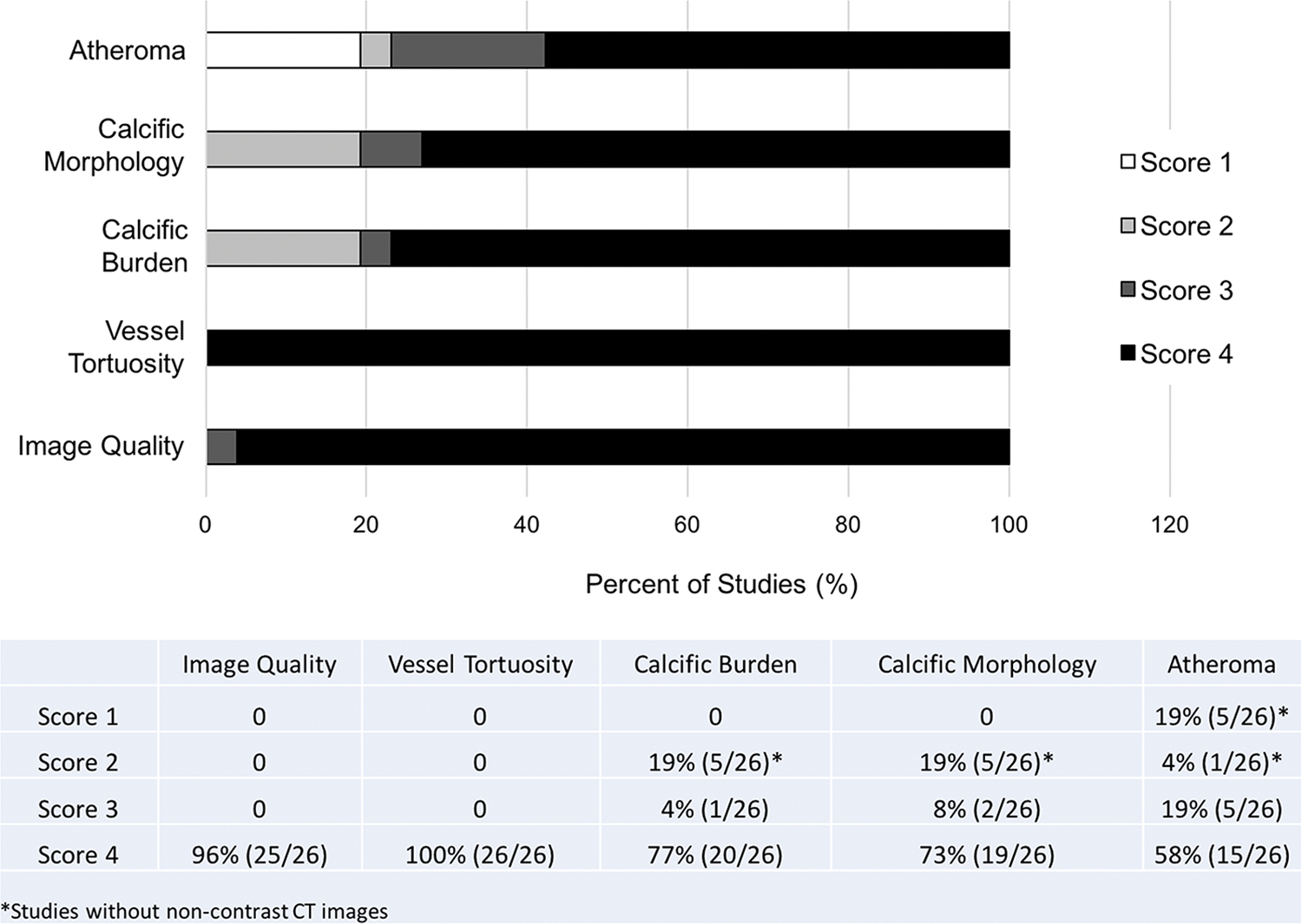
Qualitative assessment of image quality and vascular characteristics. Image quality was evaluated on a 4-point scale (1: non-diagnostic; 2: poor definition such that only gross features such as overall patency are evaluable; 3: good definition such that pathology can be confidently visualized or excluded; 4: excellent definition such that detailed anatomy is clearly visualized with sharp borders). A 4-point scale was also used to assess confidence in vessel tortuosity, calcific burden, calcific morphology, and atheroma (1: no confidence, 2: mild confidence, 3: moderate confidence, 4: strong confidence).
Quantitative Image Analysis
Quantitative measures of luminal heterogeneity (heterogeneity index), signal-to-noise ratio (SNR), and contrast-to-noise (CNR) were evaluated at the level of the abdominal aorta using FE-MRA images. Routine pre-TAVR measurements including aortic root geometry, distance of coronary arteries to the aortic valve annular plane, subclavian arteries, and aortoiliofemoral vessels were measured using FE-MRA images (Figure 3, Online video S1) by two reviewers working independently and who were blinded to all clinical information during quantitative analysis (KLN, TY). Measurements of aortic root geometry were compared to those on complementary cine imaging. As an additional parameter, the minimal luminal diameter at 1.0–1.5 cm above the bifurcation infrarenal abdominal aorta was measured using a manual double oblique method (KLN, TY) for intra- and inter-observer consistency (1). The heterogeneity index (representing signal nonuniformity) was defined as the ratio between the mean standard deviation of the luminal signal intensity and the mean luminal signal intensity (SI), such that higher heterogeneity indices reflect reduced signal uniformity. SNR was calculated as the ratio of the mean luminal SI divided by the standard deviation of noise and was further divided by 1.53 to adjust for Rayleigh noise distribution (15). The CNR was calculated as the difference between the mean SI of the lumen and nearby muscle tissue divided by the standard deviation of the noise. Noise was defined by the standard deviation in regions of interest of air within the imaging FOV.
Figure 3.
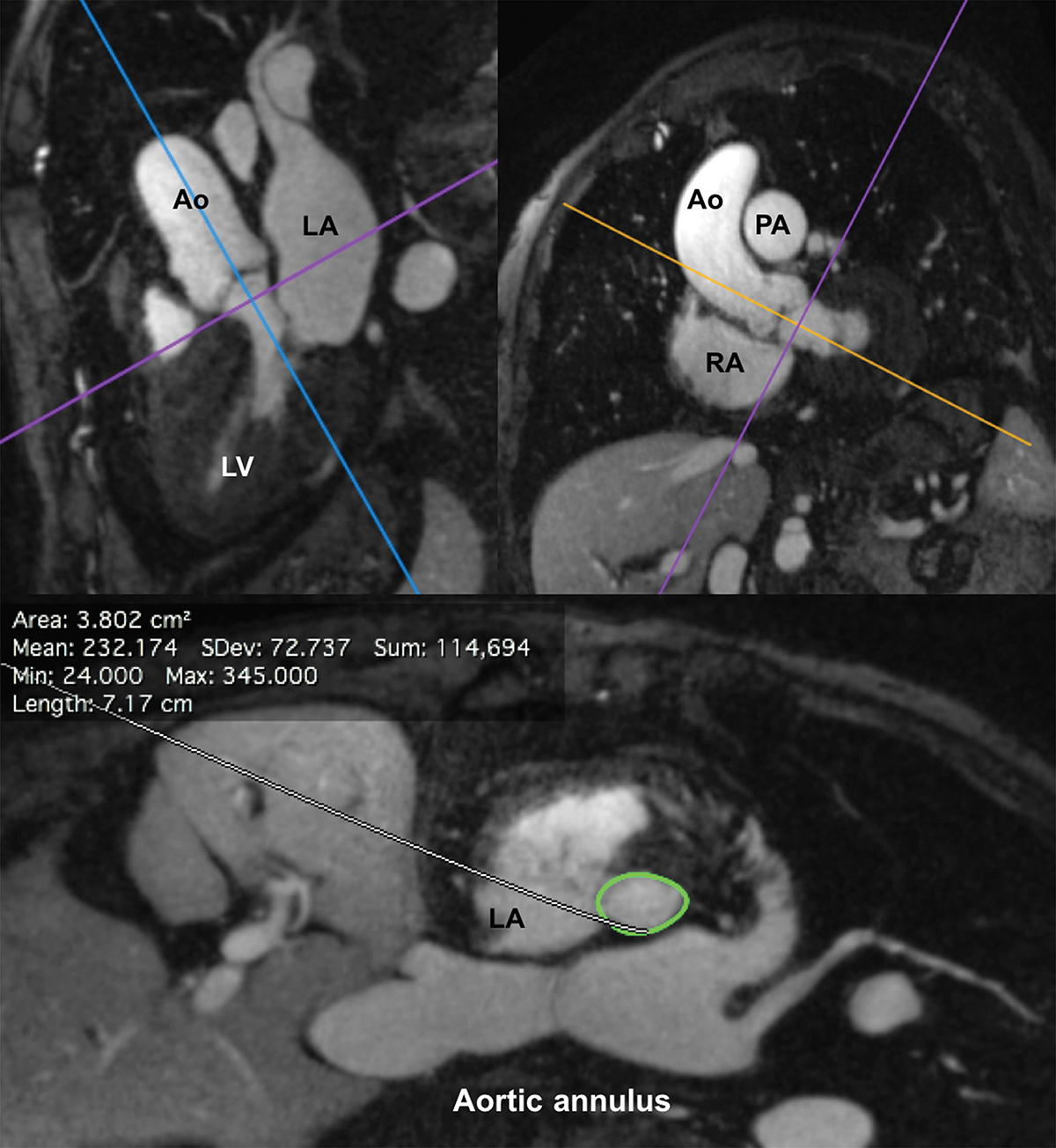
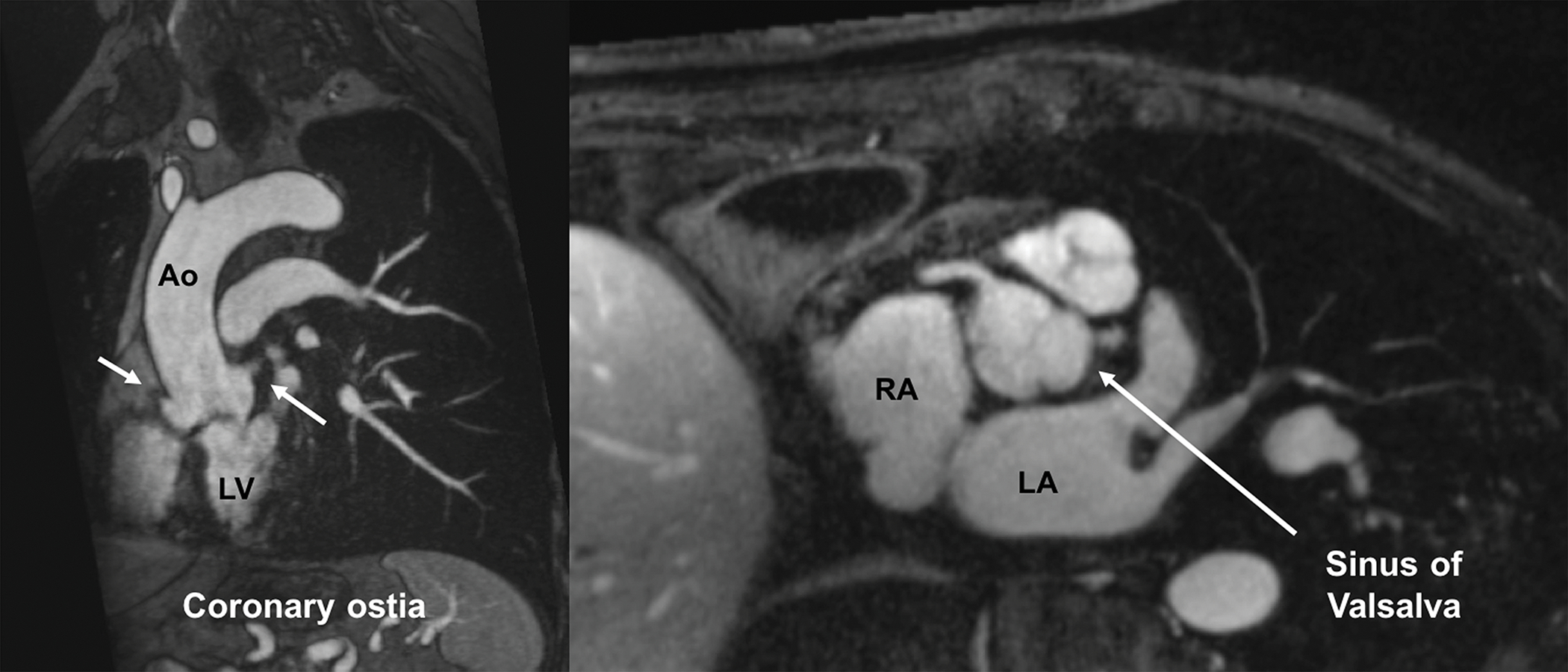
FE-MRI for annular measurements and valve sizing. Multiplanar reformatted FE-MRA images are displayed and can be manipulated using a (a) double oblique technique to estimate transcatheter valve size and other vascular access sites. (b) Distance of the coronary arteries to the annular plane can also be assessed. White arrows show both right and left coronary ostia.
Statistical analysis
Descriptive statistics are expressed as means and standard deviations (SD) or absolutes and percentages. The image quality scores between readers were compared using a Wilcoxon signed-rank test. Qualitative inter-observer agreement was assessed with Kappa statistics. The strength of agreement based on kappa (16) was defined as: poor, k<0.20; fair, 0.21–0.40; moderate, 0.41–0.60; good, 0.61–0.80; very good, 0.81–1.00. A two-tailed t-test was used for measuring differences between pre and post FE-MRA creatinine and eGFR. In patients with both first-pass and steady state FE-MRA (n=14) images, SNR /CNR measurements were compared using a two-tailed Student’s t-test. Inter and intra-observer consistency and agreement of luminal diameter measurements were assessed using intraclass correlation coefficients, Pearson’s rho, and Bland Altman plots. The strength of agreement for correlation coefficients (17) were defined as: poor, <0.90; moderate, 0.90–0.95; substantial, 0.95–0.99; almost perfect, >0.99. Analysis was performed using MedCalc (Version 16.8.4, Belgium). Bonferroni correction was used where appropriate. A P <0.05 was considered statistically significant.
Results
In total, 26 patients (age 82 [SD 11] years, 8 females) underwent an institution-specific FE-MRA pre-TAVR vascular mapping protocol from January 2014 until Feb 2016. All patients underwent FE-MRA safely and without adverse events. There were no changes in heart rate, blood pressure or arterial oxygen saturation related to ferumoxytol injection. Patient characteristics are outlined in Table 1. Routine comprehensive pre-TAVR measurements are reported in Table 2. In all but one case where patients proceeded to TAVR, blinded FE-MRA measurements of the aortic root geometry correctly predicted the transcatheter valve size used by our interventionalists. An illustrative example of annular measurements is provided in Figure 3. Complementary dynamic information about the aortic root and annulus are depicted in Supplemental Material Online video S1.
Table 1.
Patient Characteristics (n=26)*
| Age | 82 (SD 10) y; (range 56–94 y) |
|---|---|
| Gender (female) | 8 |
| Pre MRI eGFR (mL/min/1.73m2) | 32 (SD 11) |
| Pre MRI creatinine | 2.0 (SD 0.8) mg/dL |
| CKD Stage 5 | n=2 (8%) |
| CKD Stage 4 | n=10 (38%) |
| CKD Stage 3b | n=11 (42%) |
| CKD Stage 3a | n=3 (12%) |
| CKD Stage 1–2 | n=0 (0%) |
| Post-TAVR creatinine* | 1.9 (SD 0.8) mg/dL |
| Post-TAVR eGFR* (mL/min/1.73m2) | 36 (SD 13) |
| Hemoglobin | 11 (SD 2) g/dL |
| Weight | 80 (SD 16) kg |
| Total iodinated contrast (n=19)** | 95 (SD 39) mL |
| Total fluoroscopy time (radiation dose) (n=19) | 14 (SD 7) minutes 1643 (SD 1125) mGy |
| Time in cath lab (n=19) | 100 (SD 30) minutes |
Nineteen patients proceeded to TAVR.
Typical institutional range of total contrast for TAVR is 133 (SD 69) mL, p=0.02.
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate
Table 2.
Comprehensive pre-TAVR measurements
| Aortic root geometry | |
| Aortic annulus, area | 501 (SD 119) mm2 |
| Aortic annulus, perimeter | 80 (SD 9) mm |
| Aortic annulus, major | 27 (SD 3) mm |
| Aortic annulus, minor | 23 (SD 3) mm |
| Sinotubular junction | 29 (SD 4) × 28 (SD 4) mm |
| Sinus of Valsalva | 32 (SD 4) × 32 (SD 4) × 32 (SD 4) mm |
| Distance from coronary ostia to AV annular plane | |
| Left main ostium | 14 (SD 2) mm |
| Right coronary ostium | 15 (SD 3) mm |
| Related vasculature | |
| Left subclavian artery | 8 (SD 2) × 7 (SD 1) mm |
| Right subclavian artery | 9 (SD 2) × 8 (SD 1) mm |
| Ascending thoracic aorta | 35 (SD 4) × 33 (SD 4) mm |
| Infrarenal abdominal aorta | 14 (SD 3) × 15 (SD 3) mm |
| Left external iliac artery | 9 (SD 1) × 9 (SD 1) mm |
| Right external iliac artery | 9 (SD 2) × 9 (SD 2) mm |
| Left common femoral artery | 9 (SD 1) × 9 (SD 1) mm |
| Right common femoral artery | 10 (SD 1) × 9 (SD 1) mm |
Nineteen patients proceeded to TAVR. All patients received variations of Edwards SAPIEN valves (Edwards Lifesciences, Irvine, CA). Minimum access vessel diameter for specific valves: SAPIEN (7.0–8.0mm, n=4), SAPIEN XT (6.0–7.0mm, n=12), SAPIEN 3 (5.5–6.0mm, n=3). For aortic root geometry, interobserver precision, accuracy, and reproducibility were: Pearson’s rho 0.99, 95%CI 0.98–0.99; bias correction factor C0 0.99; intraclass correlation coefficient 0.99, 95% CI 0.98–0.99. R2 was 0.97 for all annular and vascular measures (ICC 0.98, 95% CI 0.98 to 0.99).
In those who had FE-MRA, there was no statistical difference in the creatinine (p =0.57) and eGFR (p =0.63) pre and post FE-MRA. Of the 26 patients, 19 patients subsequently underwent TAVR whereas 7 patients did not proceed with TAVR (n=3 had surgical AVR, n=3 died before TAVR, n=1 aborted TAVR due to downsizing of the degree of AS by intra-operative transesophageal echocardiography). Of the 19 patients who underwent TAVR, two patients had a creatinine increase of 0.3 mg/dL from baseline within one week of the procedure. No patients developed AKI in our FE-MRA group (AKI was defined as a change in serum creatinine of >0.3 mg/dL within 48 hours, or 2-fold increase in baseline serum creatinine). Compared to typical institutional range of total contrast for TAVR, those who underwent FE-MRA received less contrast (p=0.02). Twelve patients had trans-femoral access whereas seven had trans-apical access. FE-MRA findings were confirmed in all 16 patients with correlative catheter pelvic angiograms. In all 19 patients that proceeded to TAVR, no additional pre-operative vascular imaging was necessary. In one patient, intra-operative vascular ultrasound showed focal stenosis of the right external iliac artery and confirmed FE-MRA finding of a small right femoral artery; the left femoral artery was used as the TAVR access site. There were no major vascular complications based on modified VARC-2 (Valve Academic Research Consortium 2) criteria (18). One minor vascular complication (defined by modified VARC-2) occurred in a patient with diminutive iliac vessels; the patient had dissection of the left external iliac artery with a mild to moderate stenosis at the repair site without further complications. One patient who underwent TAVR via a trans-apical approach developed septic shock complicated by acute respiratory failure and aspiration pneumonia requiring intubation. During the hospital course, she developed subsequent acute renal failure and required dialysis. She was eventually extubated and transitioned to hospice care. At an average follow-up time of 1.9 (SD 0.5) years), four of the 19 patients who had successful TAVR died from coexisting medical conditions.
Image Quality and Qualitative Assessment of Vessel Morphology
A total of 286 named vascular segments including the subclavian arteries were evaluated. A summary of image quality scores and qualitative assessment of vessel morphology is provided in Figure 2. Depiction of subclavian and aortoiliofemoral arterial vessel conspicuity by FE-MRA was excellent. On a scale of 1–4, the image quality was 4 for 283 (99%) segments (n=3 subclavian segments received an image quality of 3; all were from one study). The inter-observer agreement was very good (κ=0.9). In all patients (n=26), there was strong confidence in the assessment of vessel tortuosity. In patients where non-contrast CT was available (21 of 26), there was moderate to strong confidence in the grading of vascular calcific burden, calcific morphology, and presence of atheromas (Figure 2). For complex atheromas, the combination of FE-MRA and supplemental black-blood images facilitated more confident characterization of the atheromas. Figures 4–5 and Supplementary Figures S3–S5 provide illustrative study examples of FE-MRA image quality and the ability of FE-MRA to depict luminal features and sharp aortiliofemoral arterial wall morphology. Correlative catheter-based angiograms obtained during TAVR are also presented (Figures 4, Supplemental Material Figure S3–S5). Figures 4 and Figure S5 highlight excellent delineation of sharp vascular borders and well-defined luminal characteristics including complex, calcific atheromas. In 21 patients with fused FE-MRA and non-contrast CT images, the availability of fusion images (Figure 4, Supplemental Material Figure S2) facilitated visual confirmation of vascular calcific burden and morphology in addition to their location and relationship to vessel tortuosity, which is reflected in the scoring. Figure 5 compares first pass vs steady state FE-MRA and demonstrates the complementarity of post-contrast black blood imaging to depict atheromatous plaque characteristics. The inherent value of having a 3D volume rendered vascular roadmap for assessment of conventional and alternative access points during TAVR planning is demonstrated in Supplemental Material Online video S2.
Figure 4.

FE-MRA at 3.0T. 89-year-old male with severe AS and chronic kidney disease (creatinine 1.7 mg/dL, stage 3b) presented for TAVR evaluation. 3D volume rendered and multiplanar reformat FE-MRA images demonstrate significant tortuosity of the iliofemoral vessels and areas of atheromatous changes (white arrows) near the bifurcation of the infrarenal abdominal aorta and bilateral iliac arteries. Fused 3D volume rendered and non-contrast CT image provide additional information on calcific morphology, location, and burden. Catheter angiogram of the right common iliac confirmed the diagnostic accuracy of the FE-MRA. A 26mm Edwards Sapien XT valve was successfully deployed via the transfemoral approach without complications.
Figure 5.
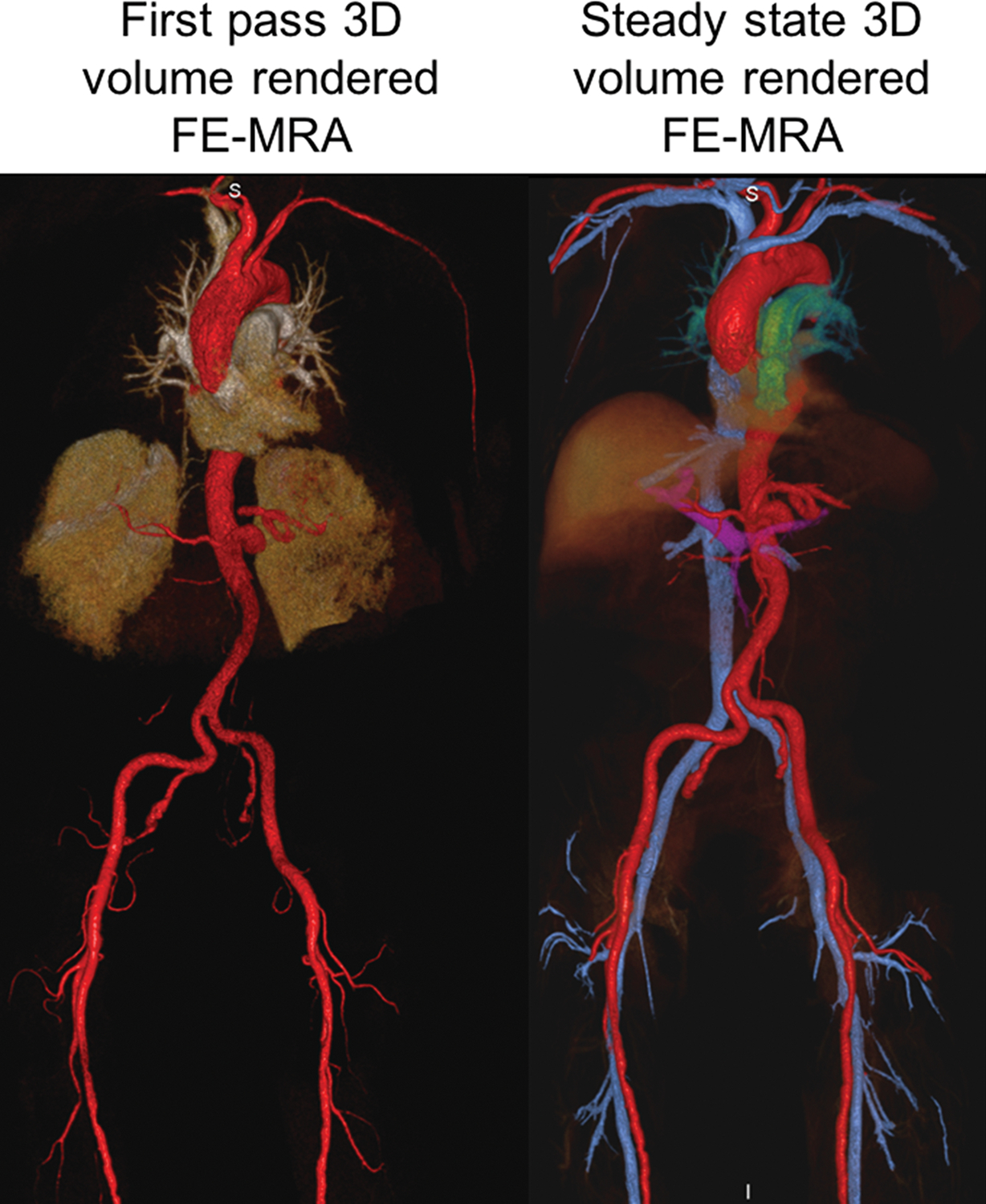
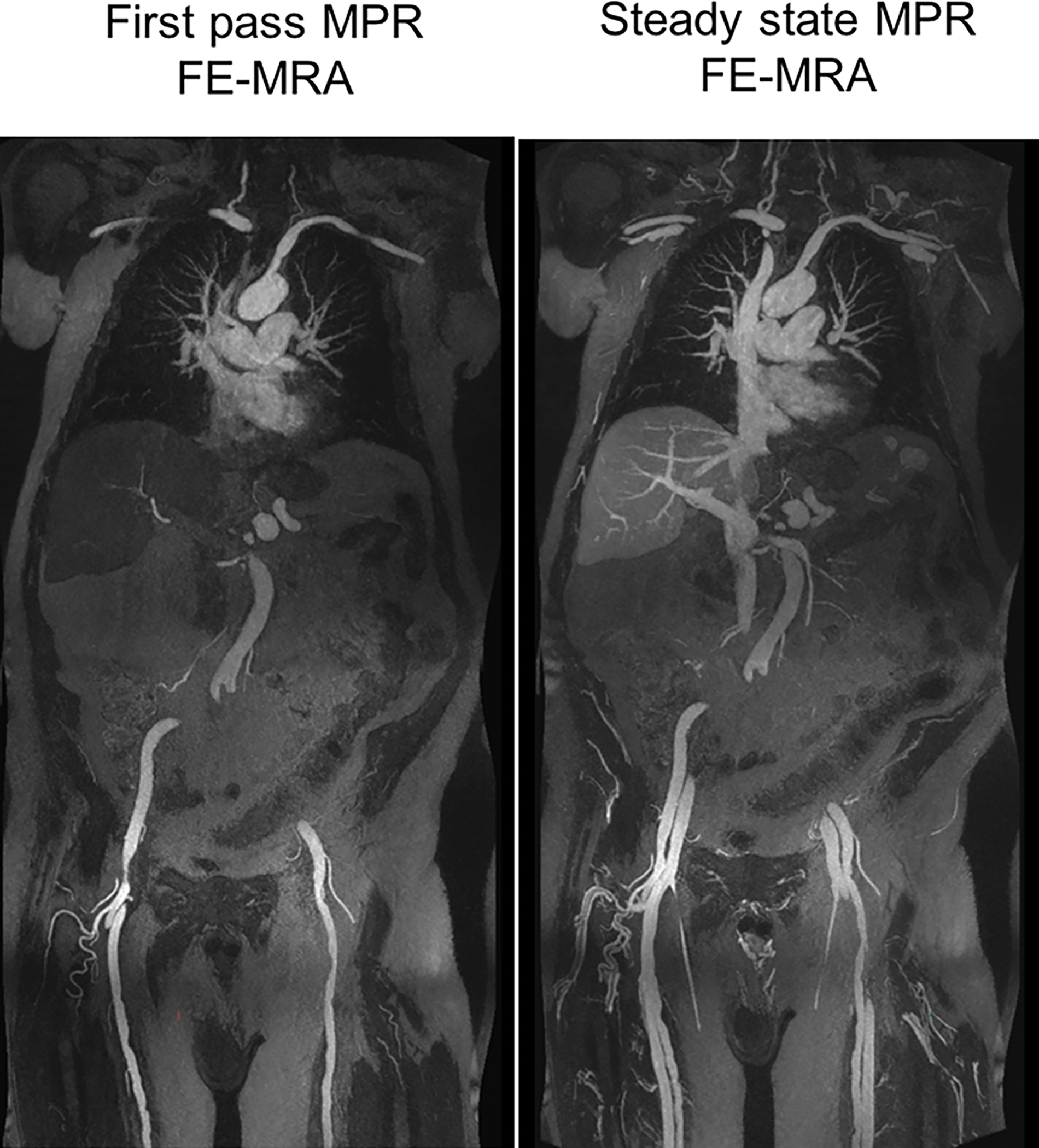
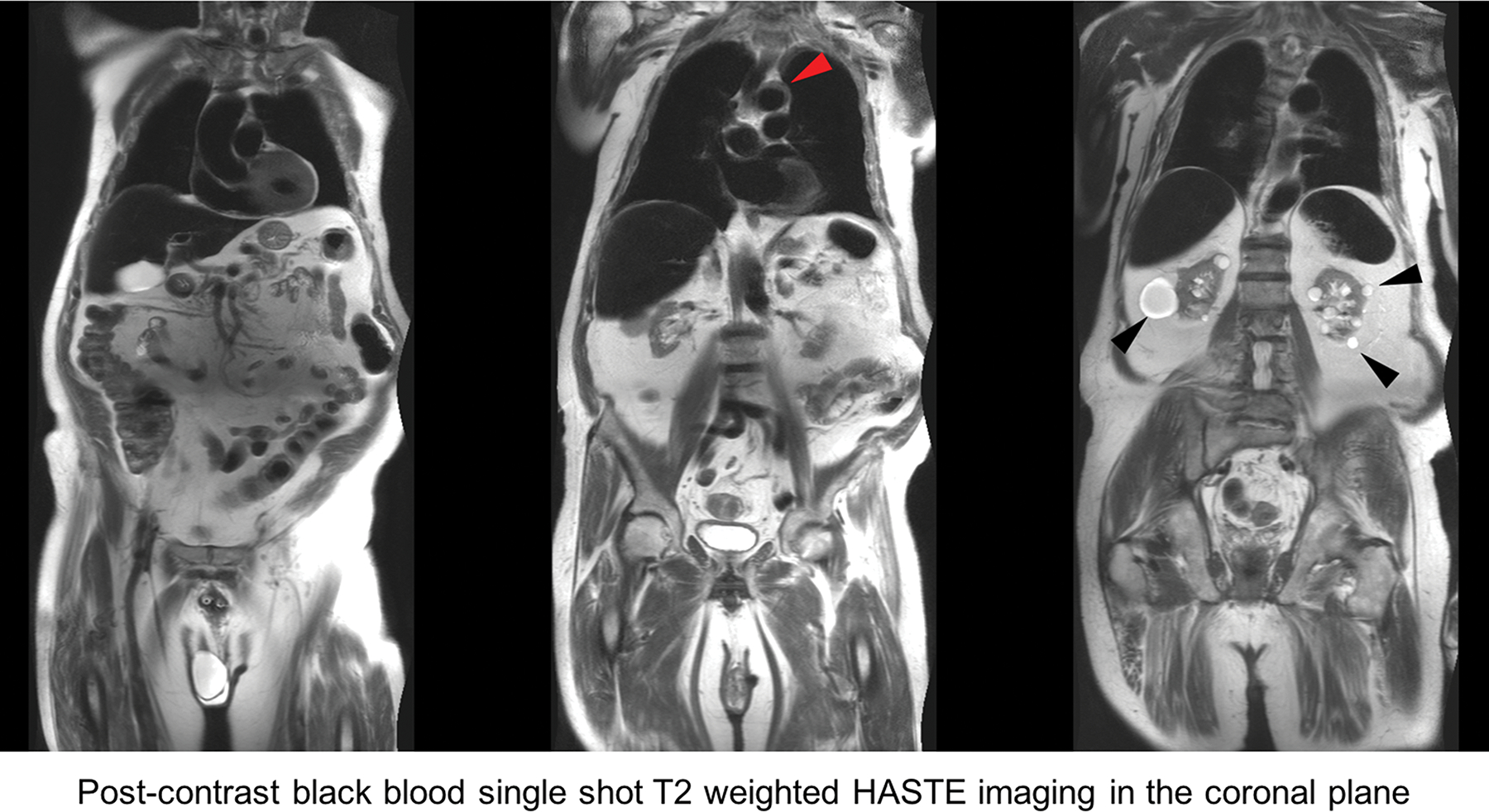
FE-MRA at 3.0T. 84-year-old male with severe AS and moderate renal impairment (creatinine of 1.7 mg/dL, stage 3b) presented for TAVR evaluation. First-pass and steady state (a) 3D volume rendered and (b) multiplanar reformat (MPR) FE-MRA of the entire aorta and pelvic access vessels show significant tortuosity. There is comparable luminal vascular enhancement between first-pass vs steady state imaging. (c) Post-contrast black blood HASTE imaging depicts atheromatous plaque morphology (red arrowhead) at the transverse aortic arch and incidental multiple renal cysts (black arrowhead). This patient had successful transfemoral placement of a 26mm Edwards Sapien valve.
In no studies did minor artifacts affect the diagnostic quality. In the named segments assessed, no vessel blurring was noted. Flow-related signal inhomogeneity was present in 1 of 26 studies (4%).
Quantitative Assessment of Heterogeneity, Signal-to-Noise, and Interobserver Agreement.
The heterogeneity index for steady state FE-MRA imaging was 0.09 (SD 0.05), reflecting a high degree of luminal signal uniformity. There was no difference in the SNR at steady state imaging vs first-pass imaging (61 [SD 38] vs 50 [SD 25], p=0.30) or CNR (50 [SD 33] vs 45 [SD 19], p=0.38). The overall interobserver agreement for FE-MRA derived measurements of the subclavian arteries, aortic root geometry, and the aortoiliofemoral arterial system was substantial (ICC 0.98, 95% CI 0.98 to 0.99). FE-MRA measurements of aortic root geometry and subclavian vessels had substantial interobserver precision (Pearson’s rho 0.99, 95%CI 0.98–0.99), accuracy (bias correction factor C0 0.99), and reproducibility (ICC 0.99, 95% CI 0.98–0.99). There was substantial consistency in interobserver and intraobserver measurements of luminal diameter for pelvic vessels (ICC 0.98 [95% CI 0.96–0.99] and 0.99 [95% CI 0.98–0.99], respectively). Concordance and precision of pelvic vessel measurements between readers were also substantial (Pearson’s rho 0.96, 95% CI 0.90–0.98; R2=0.93). The limits of agreement between readers were narrow (Supplemental Material Figure S6).
Discussion
Ferumoxytol enhanced MR angiography provides reliable arterial vascular access mapping and accurate depiction of aortic root geometry in in TAVR candidates with CKD, without concerns for iodine or gadolinium toxicity. In those undergoing FE-MRA for TAVR vascular access evaluation, the total contrast dose was significantly less than historical institution standard and no AKI occurred. Appropriate treatment planning was instituted without the need for supplemental nephrotoxic contrast agents or additional pre-operative vascular imaging.
Ferumoxytol has unique properties as an MR angiographic contrast agent. The T1 relaxivity is high (r1=15.7 mM−1second−1 at 1.5 tesla) and intravascular residence time is very stable (half-life of ~ 15 hours) when compared to conventional extracellular gadolinium based contrast agents (12, 19). Extended vascular steady state imaging (19) is facilitated by ferumoxytol’s stable blood pool distribution; qualitatively, it is similar to the gadolinium-based blood pool agent gadofosveset (20), which has a blood pool half-life of about 30 minutes but is no longer available.
Because renal impairment portends an adverse prognosis in patients undergoing TAVR, limiting the amount of iodinated contrast in pre-procedural work-up in such high-risk patients is paramount to reduce overall morbidity and mortality. A strong association exists between AS and renal impairment. Up to 75% of patients with aortic valvular pathology have mild (47.7%), moderate (26.7%) or severe (2.5%) renal impairment (21, 22). In patients with end-stage renal disease, dystrophic calcification forms on aortic leaflets and aortic annulus, with the onset occurring 10 to 20 years earlier than the general population (23). Bagur et al (5) found that AKI occurs in 11.7% of 213 patients with chronic kidney disease who underwent TAVR, which was associated with a greater than four-fold increase in the risk of post-operative mortality, independent of baseline comorbidities and peri-procedural complications. Aregger et al (4) reported an even higher incidence of AKI after TAVR (up to 28%), with 7.4% of the patients requiring dialysis during index hospitalization. Thus, FE-MRA for vascular mapping offers a potentially transformative role.
Gadolinium based contrast agents used in MRA raise concerns for NSF in the setting of severe chronic or acute renal impairment. NSF is a debilitating disease involving fibrosis and the skin, musculoskeletal system and visceral organs and is associated with increased mortality (24). Recent reports have also noted deposition of gadolinium in the brains and bones of patients with normal renal function; although these observations are of as yet unknown clinical significance (25, 26). Ferumoxytol, conversely, has no association with NSF and as an intravenous iron supplement, may even offer a therapeutic benefit in this population.
While ferumoxytol may have both off-label diagnostic and on-label therapeutic applications as a theranostic agent, the FDA has warned of potential rare, adverse hypersensitivity allergic reactions (27). The FDA cites a 0.2% risk of serious hypersensitivity reactions in patients with renal disease (27). Release of labile free iron may be the mechanism for variations in blood pressure during injection (28) and thus the FDA recommends slow infusion to reduce the risk of hypotension and monitoring of vital signs up to 30 minutes post-administration. Pooled analysis of post-marketing data for the therapeutic use of ferumoxytol demonstrates an aggregate risk of 0.03% for serious adverse events (29). Recent analyses relating to the diagnostic use of ferumoxytol as an MR contrast agent have shown no severe adverse events (14)(30, 31) or significant changes in vital signs during and post injection. In the largest study to date (14) involving 217 patients of all ages and renal function who had FE-MRI for a variety of clinical indications, there were no serious adverse events.
At our institution, the benefit-to-risk ratio is weighed and close discussion between the treating and imaging physicians occur when deciding the best route for vascular mapping of high risk patients. While iron overload is a theoretical concern, far more patients are anemic (32) and in TAVR candidates, the association with gastrointestinal bleeds (Heyde’s syndrome) and CKD-related anemia, significantly lower the threshold for concern. Lastly, MR effects of ferumoxytol can last up to 3 months (33) and it is important for imagers to document ferumoxytol use on MRI screening forms and the MR report.
The results of our study confirm the feasibility of FE-MRA vascular mapping of the subclavian, aortoiliofemoral arterial anatomy, and aortic root geometry in TAVR candidates, obviating the need for gadolinium or iodinated contrast agents. Moreover, the success of imaging during the steady state (blood pool) distribution phase of ferumoxytol obviates the need to perform bolus timing, or even to perform pre-contrast imaging, thereby simplifying workflow. However, MRI and MRA are insensitive to vascular calcification. Whereas this is an advantage for unencumbered visualization of the perfused arterial lumen, which can be degraded by beam hardening on CT, it is important to know the degree of vascular calcification prior to TAVR. Greater than semi-circumferential calcification is felt to limit arterial distensibility and this has implications in device sizing. In this regard however, we demonstrated that pre-procedural non-contrast CT can be used to overlay vascular calcification on the luminal anatomy defined by FE-MRA, providing confident assessment of combined luminal dimensions and vessel wall calcification. Prior work has demonstrated that the strongest predictor of vascular complications is an outer sheath diameter to minimal access vessel lumen diameter ratio >1.00 with calcification and >1.10 without calcification (1). Thus, accurate depiction of the vascular lumen by ferumoxytol enhancement can offer insight into the potential risk for vascular complications. While other black blood techniques are available (34), complementary T2-weighted HASTE in our case provided complete, flow-independent blood suppression and accurate depiction of atheroma, intramural hematoma, thrombus, and dissection. Although vascular complications are also highly dependent on institutional and operator experience, the combination of FE-MRA and non-contrast enhanced CT images, provided necessary information to evaluate measures that predict vascular complications (vessel tortuosity, lumen diameter, calcifications) with moderate to strong confidence. In the hands of our imagers and interventionalists, those who have undergone FE-MRA scans for vascular mapping in TAVR have not required additional pre-operative imaging.
Our study has limitations. While vascular mapping using non-contrast MRA is an alternative in patients with renal impairment, our work is focused on CEMRA in TAVR. The sample size in our study was relatively modest, as the purpose of the study was to assess feasibility and report our initial experience with FE-MRA in TAVR evaluation. The study as such is reflective of a single center experience. Although the effectiveness of using MRI with gated CEMRA for aortic valve sizing in TAVR has been demonstrated (35), our FE-MRA images in conjunction with complementary cine imaging and non-contrast CT provided accurate assessment of aortic root and related vascular measurements as well as vessel tortuosity and calcific burden. Selection bias may also be present because the study population reflected local referring patterns of our physicians. Compared to other patients undergoing TAVR at our institution, those who underwent FE-MRA tended to have greater comorbidities and vascular disease as reflected by higher surgical risk scores. Despite these limitations, direct comparison of FE-MRA with angiograms and implanted aortic valve size confirmed diagnostic accuracy.
Conclusion
Early results suggest that ferumoxytol enhanced MRA provides accurate assessment of aortoiliofemoral arterial anatomy and reproducible measurements of aortic root geometry in pre-TAVR evaluation without the need for gadolinium or iodinated contrast. This is of particular importance given the high prevalence of renal impairment in the patient population undergoing TAVR. Furthermore, in this patient population who frequently have baseline iron deficiency anemia, ferumoxytol may serve both diagnostic and therapeutic roles.
Supplementary Material
Flowchart of patients included in the study.
Segmentation of vascular calcification and fused FE-MRA /CT images. FE-MRA images and non-contrast CT images were fused using a commercial software (Mimics, Materialise, MN). Vascular calcification burden and morphology were segmented and 3D volume rendered images of the entire aortoiliofemoral vasculature were displayed. Vessel tortuosity, calcific burden and morphology, as well as the presence of complex calcific atheroma were assessed.
FE-MRA at 1.5T. 84-year-old female patient with severe AS and moderate renal impairment (creatinine 1.7 mg/dL, stage 3b) presented for TAVR evaluation. 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels show minimal tortuosity. Pelvic angiogram during TAVR confirmed the diagnostic accuracy of the FE-MRA. This patient successful transfemoral placement of a 23mm Edwards Sapien valve.
FE-MRA at 3.0T. 93-year-old male patient with severe AS and severe renal impairment (creatinine 2.3, stage 4) presented for TAVR evaluation. 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels demonstrate significant tortuosity. Catheter angiogram of the left common femoral artery during TAVR confirmed the diagnostic accuracy of the FE-MRA. A 26mm Edwards Sapien XT valve was successfully deployed via the transfemoral approach without complications despite significant tortuosity.
FE-MRA at 1.5T. 93-year-old female patient with severe AS and moderate renal impairment (creatinine 1.5 mg/dL, stage 3b) presented for TAVR evaluation. Multiplanar reformats and 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels show multiple regions of complex, calcific atheromatous changes (white arrows). Catheter angiogram of the right common femoral artery during TAVR confirmed the diagnostic accuracy of the FE-MRA. This patient had successful transfemoral placement of a 23mm Edwards Sapien XT valve.
Bland-Altman plot of lumen diameter measured at 1.0–1.5cm above the bifurcation of the of the infrarenal abdominal aorta on FE-MRA images (b). The limit of agreement between readers is narrow (bias −0.46, 95% CI −0.81 to −0.11, p=0.01; upper limit of agreement 1.3 [95% CI 0.7 to 1.9], lower limit of agreement −2.2 [95% CI −2.8 to −1.6]).
Ferumoxytol enhanced cine images of the aortic root geometry, aortic valve, and their dynamic relationship to the coronaries are shown. Images of the beating heart and vessels were acquired using a 3D volumetric, high resolution, cardiac phase-resolved sequence (4D-MUSIC)1.
3D color volume rendered FE-MRA images depict vascular morphology of the subclavian arteries and the aortoiliofemoral arterial and venous system. In patients with renal impairment, 3D rendering of potential vascular access sites provides a comprehensive roadmap for planning of transcatheter aortic valve replacement.
Advances in knowledge
This work demonstrates the feasibility of ferumoxytol enhanced magnetic resonance angiography (FE-MRA) as a single approach for reliable vascular mapping in patients with renal impairment undergoing transcatheter aortic valve replacement (TAVR).
No adverse events relating to the diagnostic use of ferumoxytol occurred in this frail, elderly patient population with multiple-comorbidities and impaired renal function.
Implications for patient care
Ferumoxytol enhanced magnetic resonance angiography (FE-MRA) provides a reliable and non-nephrotoxic, patient-specific approach for vascular mapping in patients with chronic renal impairment undergoing evaluation for TAVR.
The total cumulative dose of iodinated contrast is minimized because the need for an iodinated-contrast enhanced CT angiogram is replaced by FE-MRA. Thus, the risk of acute nephropathy is reduced.
Improved visualization of the vascular lumen without confounding effects from dense calcifications can facilitate accurate screening and vascular access planning.
Summary statement
Ferumoxytol enhanced MR angiography provides reliable arterial vascular access mapping and accurate depiction of aortic root geometry in TAVR candidates with CKD, without concerns for iodine or gadolinium toxicity.
Abbreviations
- AKI
Acute kidney injury
- CKD
Chronic kidney disease
- CNR
Contrast-to-noise ratio
- CTA
Computed tomographic angiography
- eGFR
Estimated glomerular filtration rate
- FE-MRA
Ferumoxytol-enhanced magnetic resonance angiography
- GBCA
Gadolinium-based contrast agent
- ICC
Intraclass correlation coefficient
- MRA
Magnetic resonance angiography
- SNR
Signal-to-noise ratio
- TAVR
Transcatheter aortic valve
Footnotes
Competing interests and disclosures: Dr. J. Paul Finn serves on the Scientific Advisory Board for AMAG, Bracco, and Bayer. Dr. William M. Suh receives honoria from Edwards Lifesciences. The other authors declare they have no relevant competing interests.
Contributor Information
Kim-Lien Nguyen, Diagnostic Cardiovascular Imaging Laboratory and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.; Department of Medicine and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.
John M. Moriarty, Diagnostic Cardiovascular Imaging Laboratory and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.; Department of Radiological Sciences and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System. Department of Medicine and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.
Adam N. Plotnik, Diagnostic Cardiovascular Imaging Laboratory and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.; Department of Radiological Sciences and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.
Olcay Aksoy, Department of Medicine and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System..
Takegawa Yoshida, Diagnostic Cardiovascular Imaging Laboratory and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.; Department of Radiological Sciences and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.
Richard J. Shemin, Department of Cardiothoracic Surgery and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System..
William M. Suh, Department of Medicine and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System..
J. Paul Finn, Diagnostic Cardiovascular Imaging Laboratory and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.; Department of Radiological Sciences and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System. Department of Medicine and David Geffen School of Medicine at UCLA and VA Greater Los Angeles Healthcare System.
References
- 1.Singh SP, Alli O, Melby S, et al. TAVR: Imaging Spectrum of Complications. Journal of thoracic imaging. 2015;30(6):359–77. [DOI] [PubMed] [Google Scholar]
- 2.Holmes DR Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collabration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. The Journal of thoracic and cardiovascular surgery. 2012;144(3):e29–84. [DOI] [PubMed] [Google Scholar]
- 3.Dill KE, George E, Abbara S, et al. ACR appropriateness criteria imaging for transcatheter aortic valve replacement. J Am Coll Radiol. 2013;10(12):957–65. [DOI] [PubMed] [Google Scholar]
- 4.Aregger F, Wenaweser P, Hellige GJ, et al. Risk of acute kidney injury in patients with severe aortic valve stenosis undergoing transcatheter valve replacement. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(7):2175–9. [DOI] [PubMed] [Google Scholar]
- 5.Bagur R, Webb JG, Nietlispach F, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. European heart journal. 2010;31(7):865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinning JM, Ghanem A, Steinhauser H, et al. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovascular interventions. 2010;3(11):1141–9. [DOI] [PubMed] [Google Scholar]
- 7.Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney international. 2005;68(1):14–22. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Hayashida K, Mouillet G, et al. Renal function-based contrast dosing predicts acute kidney injury following transcatheter aortic valve implantation. JACC Cardiovascular interventions. 2013;6(5):479–86. [DOI] [PubMed] [Google Scholar]
- 9.Kramer JH, Grist TM. Peripheral MR Angiography. Magnetic resonance imaging clinics of North America. 2012;20(4):761–76. [DOI] [PubMed] [Google Scholar]
- 10.Grobner T Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21(4):1104–8. [DOI] [PubMed] [Google Scholar]
- 11.Finn JP, Nguyen KL, Han F, et al. Cardiovascular MRI with ferumoxytol. Clin Radiol. 2016;71(8):796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W, Tutton S, Vu AT, et al. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging. 2005;21(1):46–52. [DOI] [PubMed] [Google Scholar]
- 13.Hope MD, Hope TA, Zhu C, et al. Vascular Imaging With Ferumoxytol as a Contrast Agent. AJR American journal of roentgenology. 2015;205(3):W366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen KL, Yoshida T, Han F, et al. MRI with ferumoxytol: A single center experience of safety across the age spectrum. J Magn Reson Imaging. 2017. Mar;45(3):804–812. [Epub 2016 Aug 2]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich O, Raya JG, Reeder SB, Reiser MF, Schoenberg SO. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging. 2007;26(2):375–85. [DOI] [PubMed] [Google Scholar]
- 16.Altman D Practical statistics for medical research. London: Chapman and Hall, 1991. [Google Scholar]
- 17.McBride G A proposal for strength-of-agreement criteria for Lin’s Concordance Correlation Coefficient. 2005. [Google Scholar]
- 18.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012;8(7):782–95. [DOI] [PubMed] [Google Scholar]
- 19.Bashir MR, Jaffe TA, Brennan TV, Patel UD, Ellis MJ. Renal transplant imaging using magnetic resonance angiography with a nonnephrotoxic contrast agent. Transplantation. 2013;96(1):91–6. [DOI] [PubMed] [Google Scholar]
- 20.Bremerich J, Bilecen D, Reimer P. MR angiography with blood pool contrast agents. Eur Radiol. 2007;17(12):3017–24. [DOI] [PubMed] [Google Scholar]
- 21.Thourani VH, Keeling WB, Sarin EL, et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann Thorac Surg. 2011;91(6):1798–806; discussion 806–7. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TC, Babaliaros VC, Razavi SA, et al. Impact of varying degrees of renal dysfunction on transcatheter and surgical aortic valve replacement. The Journal of thoracic and cardiovascular surgery. 2013;146(6):1399–406; discussion 13406–7. [DOI] [PubMed] [Google Scholar]
- 23.London GM, Pannier B, Marchais SJ, Guerin AP. Calcification of the aortic valve in the dialyzed patient. J Am Soc Nephrol. 2000;11(4):778–83. [DOI] [PubMed] [Google Scholar]
- 24.Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69(7):661–8. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). Accessed September 1, 2015. http://www.fda.gov/Drugs/DrugSafety/ucm455386.htm
- 26.Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents-current status. Neuroradiology. 2016;58(5):433–41. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). Accessed March 30, 2015. http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm]
- 28.Van Wyck DB. Labile iron: manifestations and clinical implications. J Am Soc Nephrol. 2004;15 Suppl 2:S107–11. [DOI] [PubMed] [Google Scholar]
- 29.Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic. 2016. May;75(5):2107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ning P, Zucker EJ, Wong P, Vasanawala SS. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magnetic resonance imaging. 2016;34(2):152–8. [DOI] [PubMed] [Google Scholar]
- 31.Muehe AM, Feng D, von Eyben R, et al. Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults. Invest Radiol. 2016;51(4):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016. Feb 27;387(10021):907–16. [DOI] [PubMed] [Google Scholar]
- 33.McCullough BJ, Kolokythas O, Maki JH, Green DE. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging. 2013;37(6):1476–9. [DOI] [PubMed] [Google Scholar]
- 34.Engblom H XC, Mavrogeni SI, Smart SM, Aletras AH. Basic Principles of Cardiovascular MRI Physics and Imaging Techniques. “Black Blood CMR”. New York: Springer, 2015: 161–166. [Google Scholar]
- 35.Pontone G, Andreini D, Bartorelli AL, et al. Comparison of accuracy of aortic root annulus assessment with cardiac magnetic resonance versus echocardiography and multidetector computed tomography in patients referred for transcatheter aortic valve implantation. The American journal of cardiology. 2013;112(11):1790–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of patients included in the study.
Segmentation of vascular calcification and fused FE-MRA /CT images. FE-MRA images and non-contrast CT images were fused using a commercial software (Mimics, Materialise, MN). Vascular calcification burden and morphology were segmented and 3D volume rendered images of the entire aortoiliofemoral vasculature were displayed. Vessel tortuosity, calcific burden and morphology, as well as the presence of complex calcific atheroma were assessed.
FE-MRA at 1.5T. 84-year-old female patient with severe AS and moderate renal impairment (creatinine 1.7 mg/dL, stage 3b) presented for TAVR evaluation. 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels show minimal tortuosity. Pelvic angiogram during TAVR confirmed the diagnostic accuracy of the FE-MRA. This patient successful transfemoral placement of a 23mm Edwards Sapien valve.
FE-MRA at 3.0T. 93-year-old male patient with severe AS and severe renal impairment (creatinine 2.3, stage 4) presented for TAVR evaluation. 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels demonstrate significant tortuosity. Catheter angiogram of the left common femoral artery during TAVR confirmed the diagnostic accuracy of the FE-MRA. A 26mm Edwards Sapien XT valve was successfully deployed via the transfemoral approach without complications despite significant tortuosity.
FE-MRA at 1.5T. 93-year-old female patient with severe AS and moderate renal impairment (creatinine 1.5 mg/dL, stage 3b) presented for TAVR evaluation. Multiplanar reformats and 3D volume rendered FE-MRA of the entire aorta and pelvic access vessels show multiple regions of complex, calcific atheromatous changes (white arrows). Catheter angiogram of the right common femoral artery during TAVR confirmed the diagnostic accuracy of the FE-MRA. This patient had successful transfemoral placement of a 23mm Edwards Sapien XT valve.
Bland-Altman plot of lumen diameter measured at 1.0–1.5cm above the bifurcation of the of the infrarenal abdominal aorta on FE-MRA images (b). The limit of agreement between readers is narrow (bias −0.46, 95% CI −0.81 to −0.11, p=0.01; upper limit of agreement 1.3 [95% CI 0.7 to 1.9], lower limit of agreement −2.2 [95% CI −2.8 to −1.6]).
Ferumoxytol enhanced cine images of the aortic root geometry, aortic valve, and their dynamic relationship to the coronaries are shown. Images of the beating heart and vessels were acquired using a 3D volumetric, high resolution, cardiac phase-resolved sequence (4D-MUSIC)1.
3D color volume rendered FE-MRA images depict vascular morphology of the subclavian arteries and the aortoiliofemoral arterial and venous system. In patients with renal impairment, 3D rendering of potential vascular access sites provides a comprehensive roadmap for planning of transcatheter aortic valve replacement.


