Abstract
Background:
2-hydroxy-3-methylanthraquinone (HMA), an anthraquinone monomer in traditional Chinese medicine Hedyotis diffusa, has been reported to inhibit the growth of several types of cancer, but its effect on lung cancer has not been adequately investigated.
Hypothesis/Purpose:
This study aimed to test the hypothesis that HMA inhibit the growth, migration, and invasion of lung cancer cells in part via downregulation of interleukin (IL)-6-induced JAK2/STAT3 pathway.
Methods:
Growth and apoptosis of lung cancer cells were quantitated by CCK-8 assay and Annexin V-FITC/PI flow cytometric analysis, respectively. Migration and invasion of A549 cells were determined by wound-healing assay and transwell invasion assay, respectively. The effect of HMA on cytokines expression in A549 cells was evaluated by the cytokine antibody array assay. Gene expression and protein levels of related molecular markers were quantitated by real time-PCR and Western blot analysis, respectively.
Results:
HMA significantly inhibited IL-6-stimulated growth and colony formation of A549 cells, increased the number of apoptotic cells, and inhibited invasion associated with downregulation of expression of IL-6-induced MMP-1, MMP-2, and MMP-9 genes. IL-6 increased the levels of tyrosine phosphorylation of JAK2 and STAT3 in A549 cells, which was reversed by HMA treatment. In addition, HMA reduced the expression of a series of inflammation-related cytokines in A549 cells supernatant, including IL-6, G-CSF, IL-6R, IL-8, MCP-1, RANTES, TNF-α.
Conclusion:
These results suggest that HMA may inhibit the growth and invasion of lung cancer cells in part via downregulation of IL-6-induced JAK2/STAT3 pathway.
Keywords: 2-Hydroxy-3-methylanthraquinone, Human lung carcinoma, IL-6, JAK2/STAT3
Introduction
Lung cancer is among the most aggressive malignancies and the leading cause of cancer death in both men and women (Torre et al., 2016). Non-small cell lung cancer (NSCLC) accounts for about 80% of lung cancer cases. Despite advances in surgery, radiotherapy and chemotherapy, the 5-year survival rate for NSCLC patients is less than 20% (Parums, 2014), primarily due to low efficacies and/or high toxicities of current therapies. It is thus of great urgency to identify more efficacious and safe therapeutic agents against lung cancer.
Hedyotis diffusa Willd (also known as Oldenlandia diffusa Willd) of the Rubiaceae family is a traditional Chinese herbal medicine commonly used for cancer treatment (Chen et al., 2016). Up to now, more than 170 compounds have been isolated from Hedyotis diffusa, mainly including terpenoids, flavonoids, sterols, organic acids, polysaccharides, and anthraquinones (Chen et al., 2016). These compounds have shown a variety of pharmacological functions, such as anti-cancer, immune regulation, anti-oxidation, and anti-inflammation, among which the anti-cancer activity is particularly prominent (Hu et al., 2018; Zhang et al., 2016).
2-hydroxy-3-methylanthraquinone (HMA) is one of the major anthraquinone monomer components in Hedyotis diffusa Wild (Wang et al., 2013). It has been shown that HMA inhibits the growth of several types of cancer, including leukemia (Wang et al., 2013), ovarian cancer (Wan et al., 2015), breast cancer (Liu et al., 2010), and liver cancer (Shi et al., 2008) cells. However, its effect on lung cancer has not been studied.
IL-6 is a pro-growth, pro-inflammatory, pro-angiogenic and anti-apoptotic cytokine that has been reported to increase in serum and malignant pleural effusions of patients with lung adenocarcinoma (Yeh et al., 2006; Brooks et al., 2016). Recent studies indicated that the elevated serum IL-6 levels in patients with lung cancer were predictive of poor clinical outcome (Pine et al., 2011). Conversely, a significantly decreased serum IL-6 level was found in patients with NSCLC after chemotherapy and was associated with reduced cancer recurrence and prolonged survival (Su et al., 2011). IL-6, upon binding to its receptor on cell surface, activates the Janus kinases (JAKs) family of tyrosine kinases, which then phosphorylates and activates downstream signaling effectors involving signal transducers and activators of transcription 3 (STAT3). Activated STAT3 translocates to the nucleus, binds to specific DNA response elements and then induces the expression of a series of genes involved in proliferation, invasion, and apoptosis (Fukada et al., 1996; Jr, 1997). It has been demonstrated that IL-6/JAK2/STAT3 signaling pathway plays an essential role in the development and progression of lung cancer (Shi et al., 2017).
In this study, we evaluated the effect of HMA on lung cancer cells in vitro aiming to test the hypothesis that HMA may inhibit the growth, migration and/or invasion of lung cancer cells in part via downregulation of IL-6-induced JAK2/STAT3 pathway. Our study findings provide experimental evidence to support the biological activities of HMA in inhibiting lung cancer cells, and warrant further research on potential application of HMA in prevention and treatment of lung cancer.
Materials and methods
Reagents
2-Hydroxy-3-methylanthraquinone (MW: 238.23, Cat No: H946680) was purchased from Yuanye Biological Technology Co., Ltd. (Shanghai, China). HPLC analysis showed that the purity was above 98% (Fig. S1). AG490 (S1509) and Annexin V/PI apoptosis detection kit (C1062) were provided by Beyotime biotechnology Technology Co., Ltd. (Shanghai, China). Recombinant human IL-6 was obtained from PeproTech (200–06, Rocky Hill, NJ, USA). Dulbecco’s modified eagle medium (DMEM), penicillin-streptomycin solution, and fetal bovine serum (FBS) were purchased from Gibco Laboratories (Waltham, MA, USA). Cell counting kit-8 (CCK-8) was purchased from Dojindo Laboratories (CK04, Kamimashiki-gun, Kumamoto, Japan).
Cell lines and culture
The human lung carcinoma H1299, human adenocarcinoma H23, mouse Lewis lung carcinoma LLC, and human umbilical vein endothelial (HUVEC) cell lines were from the American Type Cell Collection (Bethesda, MD), human lung carcinoma A549 and adenocarcinoma SPCA-1 cell lines were from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM supplemented with 10% heat-inactivated FBS, 100 μg/ml streptomycin and 100 U/ml penicillin. All cells were maintained at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Cell growth assay by cell counting kit-8 (CCK-8) method
Cell growth was determined using CCK-8 method following the described protocols (Jiang et al., 2013) with appropriate modification. In brief, cancer cells or HUVEC cells were treated with HMA at various concentrations (0, 20, 40, 80 μM) for 24 or 48 h and the absorbance was measured at 450 nm. The chemotherapeutic drugs paclitaxel and gemcitabine were used as the positive controls. The treatment effect on cell growth was calculated by the ratio of average OD of the treatment group to average OD of the control group. The experiment was repeated at least three times.
To determine the effect of HMA on IL-6-induced cancer cell growth, cells were treated with HMA at different concentrations (0, 20, 40, 80 μM) for 1 h, followed by stimulation with IL-6 (10 ng/ml), and the cell growth was measured after 24, 36 or 48 h of treatment.
Colony formation assay
A549 cells were seeded into 6-well plates at a density of 5 × 102 cells/well and treated with various concentrations of HMA in duplication for 14 days. Culture media were changed every three days. Colonies (>50 cells) were stained with 0.1% crystal violet (Sangon Biotechnologies Inc., Shanghai, China) and counted under light microscopy. The experiment was repeated at least three times.
Cell apoptosis detection
Cells were seeded into 6-well plates and treated with IL-6 (10 ng/ml) alone or in combination with HMA (20, 40 or 80 μM) in duplicate for 24 or 48 h. Cells in the IL-6+HMA group were pre-treated with HMA for 1 h and then treated with IL-6. Cells were then stained with Annexin V-FITC and PI, and the apoptosis rate was measured by flow cytometry (FACS Calibur, BD Biosciences) followed by data analysis using CellQuest software.
Cell wound-healing assay
Cells in logarithmic phase were seeded in 6-well plates at the density of 1 × 106 per well and cultured to reach 60% of confluence. After a scratch was made to establish cell wound, cells were treated with 10 ng/ml IL-6 and HMA (0, 10, 20, 40 μM) for 12 and 24 h. Five fields (×100) were randomly selected under the optical microscope to measure the widths of scratch. The experiment was repeated three times.
Transwell invasion assay
Cell invasion was determined using transwell invasion assay following the protocol used in the lab (Ni et al., 2012) with appropriate modification. In brief, a 24-well transwell chamber with 8.0 μm pore polycarbonate membrane filter inserts coated with diluted matrigel (1:5; BD Biosciences) was used. Cells were suspended in serum-free medium containing 10 ng/ml IL-6 and HMA (0, 10, 20, 40 μM) and seeded in the upper chambers, and the lower chambers were filled with 500 μl of medium containing 20% of FBS. After 24 h, cells in the top well were removed carefully by swiping the cotton swabs and the cells on the underside of the membrane were stained with Giemsa staining solution. The cell invasion ability was assessed by counting the number of cells invaded through the membrane to the lower surface. The treatment was duplicated and the experiment was repeated at least twice.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Gene expression was quantified using RT-qPCR following the protocols in the lab (Mai et al., 2007) with appropriate modification. Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and was reversed-transcribed to complementary DNA (cDNA) with a reverse transcription kit (Takara Bio, Inc., Otsu, Japan). PCR was performed under the following conditions: predenaturation at 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 45 s. The fluorescence signal was detected by Mx3000P real-time PCR system (Agilent Technologies Stratagene, USA). The sequences of primers are listed in Table 1.
Table 1.
Primers for reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward sequence (5’–3’) | Reverse sequence (5’–3’) |
|---|---|---|
|
| ||
| MMP-1 | GGGGCTTTGATGTACCCTAGC | TGTCACACGCTTTTGGGGTTT |
| MMP-2 | GATACCCCTTTGACGGTAAGGA | CCTTCTCCCAAGGTCCATAGC |
| MMP-9 | GGGACGCAGACATCGTCATC | TCGTCATCGTCGAAATGGGC |
| Caspase-3 | CATGGAAGCGAATCAATGGACT | CTGTACCAGACCGAGATGTCA |
| Caspase-9 | CTTCGTTTCTGCGAACTAACAGG | GCACCACTGGGGTAAGGTTT |
| Bax | CCCGAGAGGTCTTTTTCCGAG | CCAGCCCATGATGGTTCTGAT |
| Bcl-2 | GGTGGGGTCATGTGTGTGG | CGGTTCAGGTACTCAGTCATCC |
| GAPDH | GGTCACCAGGGCTGCTTTTA | GGATCTCGCTCCTGGAAGATG |
Western blotting
Protein levels were determined using Western blot analysis following the protocol in the lab (Ni et al., 2012) with modification. The primary antibodies (Cell Signaling Technology) against STAT3 (1:2000), p-STAT3 (1:2000), JAK-2 (1:2000), p-JAK-2 (1:2000), Cleaved caspase-3(1:2000), Cleaved caspase-9 (1:2000), Bax (1:2000), Bcl-2(1:2000), LC3(1:2000) and β-actin (1:2000) were used, and the HRP-conjugated anti-rabbit/mouse IgG secondary antibody (1:2000, Cell Signaling Technology) was used. Intensities of the bands were measured by Image Lab software (Bio-Rad) to assess relative protein levels.
Cytokine antibody array
Human Cytokine Array (cat. # GSR-CAA-440, RayBiotech, Norcross, GA, USA), which contains 440 cytokines, was used to analyze the soluble cytokines in culture media of cells treated with HMA according to the protocols by the vendor. Briefly, 1 × 106 cells/well were seeded into 6-well plates and treated with HMA (0 and 40 μM) for 24 h, the culture media were then collected and concentrated. The protein levels were quantified and the culture media with the same amount of total proteins were incubated with the array. After extensive washing, the membranes were incubated with a cocktail of biotinylated detection antibodies. After incubation for 1 h with labeled streptavidin, the membranes were analyzed for signals using a LI-COR Odyssey Scanner (LI-COR Biosciences, Lincoln, NE, USA), and the concentrations of cytokines were quantified.
Statistical analysis
The results were expressed as means ± standard deviation (SD). Data comparisons were analyzed by two-tailed Studenťs t-test or one-way analysis of variance followed by Bonferroni’s post hoc test using SPSS software (version, 17.0; SPSS Inc., Chicago, IL, USA), and p<0.05 was considered statistically significant.
Results
HMA inhibits the cell growth of lung cancer cells
We first examined the effects of HMA on the growth of murine Luis lung carcinoma (LLC) (Fig. 1A) and human lung carcinoma H23 (Fig. 1B), H1299 (Fig. 1C), SPCA-1 (Fig. 1D) and A549 (Fig. 1E) cells. HMA significantly inhibited the growth of these cancer cell lines, as measured by cell viability, in dose-and time-dependent manners (Fig. 1). The comparative results of activities between HMA and positive controls (paclitaxel and gemcitabine) are shown in Fig. S2. On the other hand, HMA had limited effect on HUVEC cell growth (Fig. 1F), suggesting that HMA may have a minimal adverse effect.
Fig. 1.
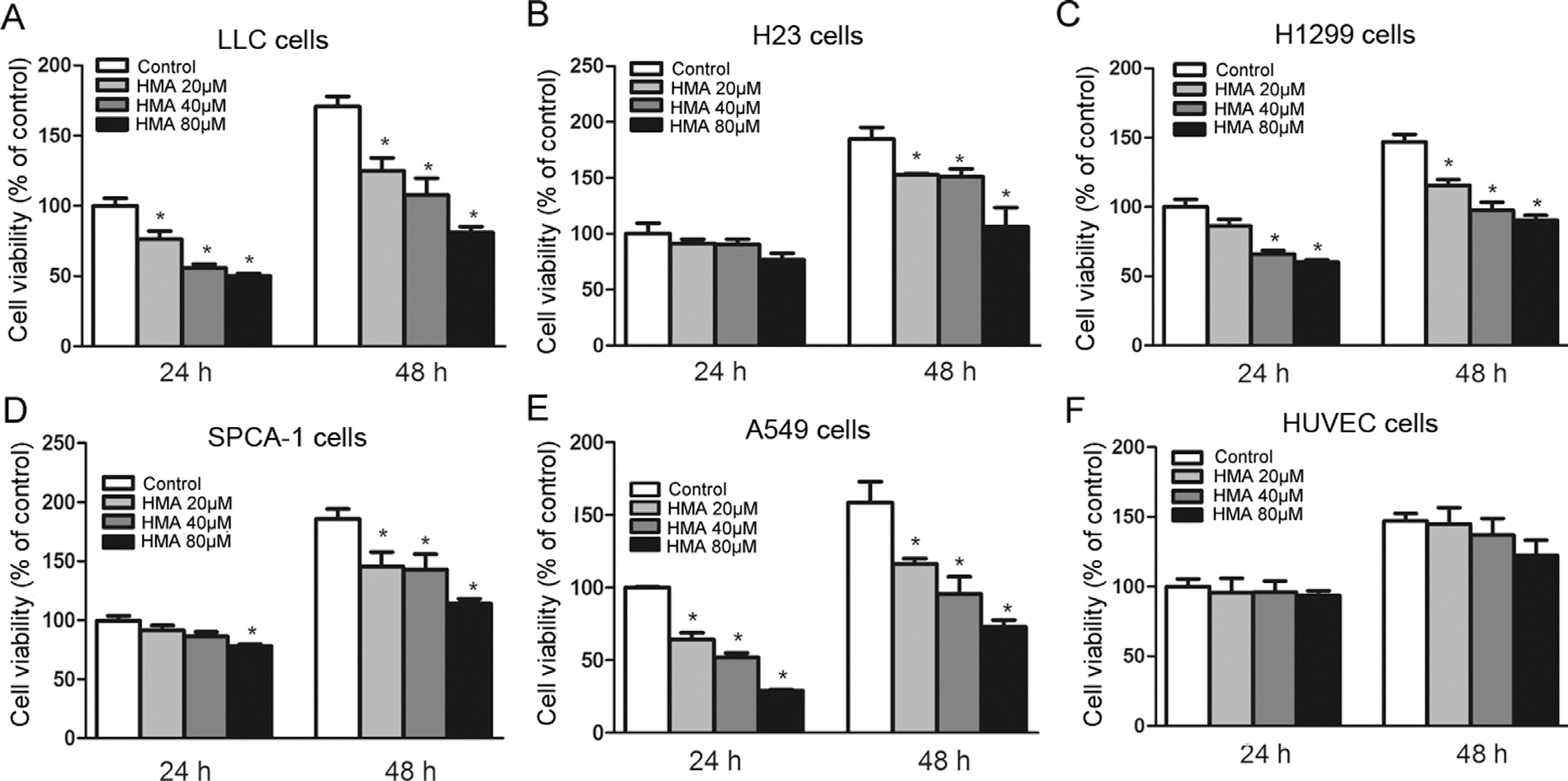
Effects of HMA on viability of lung cancer cells and HUVEC cells. Mouse LLC cells (A), human lung carcinoma H23 (B), H1299 (C), SPCA-1 (D), A549 (E) and HUVEC (F) cells were treated with HMA at various concentrations (0, 20, 40, 80 μM) for 24 and 48 h. *p < 0.05 vs. the control group. n = 6.
Among these cell lines, LLC and A549 cells were more sensitive to HMA treatment. Since LLC cells are murine origin, we used A549 cells in the subsequent experiments.
HMA inhibits IL-6-induced growth and colony formation of A549 cells
We evaluated effect of HMA on IL-6-stimulated lung cancer cell growth. IL-6 significantly increased the growth of A549 cells compared with the control cells (Fig. 2A). By contrast, HMA treatments (20, 40 and 80 μM) significantly reduced the IL-6-stimulated A549 cell growth in time- and dose-dependent manners (p < 0.05 vs. the cells stimulated with IL-6 alone). Similarly, IL-6 significantly promoted colonic formation of A549 cells (p < 0.05), whereas HMA inhibited the IL-6-stimulated colony-forming ability of A549 cells (Fig. 2B and C).
Fig. 2.
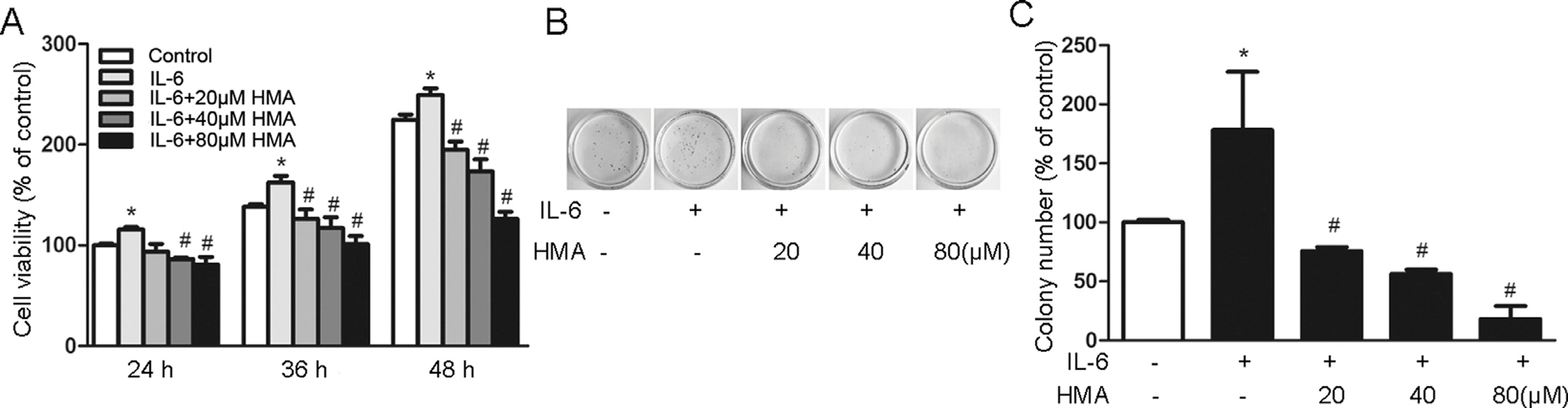
Effects of HMA on IL-6-induced viability and colony formation of A549 cells. (A) A549 cells were exposed to HMA (0, 20, 40, 80 μM) for 1 h prior to 10 ng/ml IL-6 stimulation for 24, 36 and 48 h. (B–C) Colony formation assay of A549 cells. *p < 0.05 vs. the control group; #p < 0.05 vs. the IL-6-stimulated group. N = 3.
HMA induces IL-6-suppressed apoptosis of A549 cells
The apoptosis-suppressing activity of IL-6 is suggested to be an important cancer-promoting mechanism, thus we further determined if HMA could reverse the anti-apoptotic activity of IL-6 in A549 cells. Cells treated with IL-6 (10 ng/ml) for 24 and 48 h significantly suppressed apoptosis by 33.1% (p < 0.05) and 44.7% (p < 0.05) (Fig. 3A and B), respectively. HMA not only reversed the apoptosis-suppressing activity of IL-6, but also significantly enhanced apoptosis of IL-6-treated A549 cells. Compared with the IL-6-treated cells, cells treated with IL-6 and HMA at 20, 40 and 80 μM for 24 and 48 h significantly increased apoptosis by 180.5% (p < 0.001), 259.2% (p < 0.001) and 370.1% (p < 0.001), and 217.8% (p < 0.001), 324.8% (p < 0.001) and 550.5% (p < 0.001) (Fig. 3A and B), respectively.
Fig. 3.
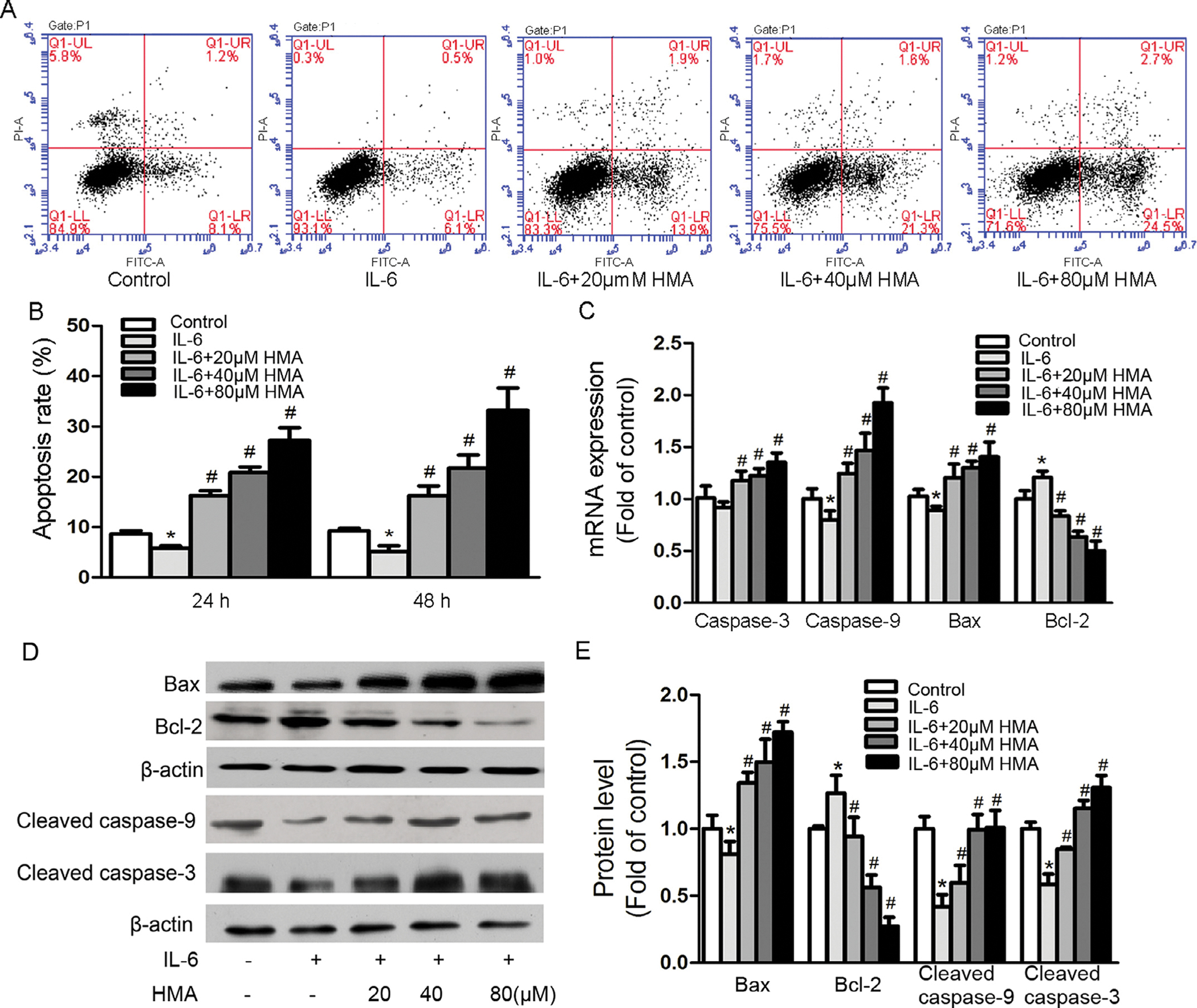
Effects of HMA on IL-6-suppressed apoptosis in A549 cells. Cells were pretreated with HMA (0, 20, 40, 80 μM) for 1 h prior to 10 ng/ml IL-6 stimulation for 24 and 48 h. (A–B) Cell apoptosis was detected by Annexin V/PI staining and FACS analysis. (C) mRNA expression levels were detected by real time RT-PCR. GAPDH served as an internal control. (D–E) protein levels were detected by western blot. β-actin was used as an internal control. *p < 0.05 vs. the control group; #p < 0.05 vs. the IL-6-stimulated group. n = 3.
To determine the possible molecular mechanism by which HMA enhances apoptosis of A549 cells in the presence of IL-6, we measured the expression of some apoptosis related genes, including pro-apoptotic caspase-3, caspase-9 and bax, and anti-apoptotic Bcl-2. IL-6 treatment significantly downregulated the expression of pro-apoptotic genes caspase-9 and bax and significantly upregulated the expression of anti-apoptotic Bcl-2 gene (Fig. 3C). HMA treatments significantly reversed the expression of genes altered by IL-6 (p < 0.05) in a dose-dependent manner. Although IL-6 non-significantly inhibited the pro-apoptotic caspase-3 gene expression, HMA could significantly enhance the expression of caspase-3 gene in a dose-dependent manner (Fig. 3C). In addition, HMA also altered the protein levels of apoptosis related bio-markers. HMA significantly upregulated the protein levels of pro-apoptotic bax, cleaved caspase-3 and cleaved caspase-9 and significantly downregulated the protein levels of anti-apoptotic bcl-2 gene in a dose-dependent manner (Fig. 3D and E).
We also determined if HMA could modulate autophagy by determining the protein levels of LC3-II and LC3-I and calculated the LC3-II/LC3-I ratio, a recognized reliable marker for autophagy. HMA treatment did not significantly upregulate the LC3-II/LC3-I protein ratio (Fig. S3), suggesting that HMA may not significantly induce autophagy.
These results indicate HMA had a potent apoptotic activity and could effectively reverse the anti-apoptotic activity of IL-6 in part via upregulating the expression of pro-apoptotic molecules and downregulating the expression of anti-apoptotic molecules.
HMA suppresses IL-6-stimulated invasion of A549 cells
IL-6 plays an essential role in migration and invasion of lung cancer cells, which is associated with the activation of STAT3 signaling cascades and induction of the expression of various genes, such as matrix metalloproteinases (MMPs) (Yang et al., 2012; Jiang et al., 2015). Therefore, we examined the effect of HMA on migration and invasion of A549 cells and on the expression of MMPs. Since alteration of cell growth influences migration and invasion abilities of cancer cells, it is essential that migration and invasion assays be evaluated at the doses that do not significantly affect cell growth. We thus determined the effect of 10 μM HMA on cell growth and found that 10 μM HMA had minimal effect on cell growth inhibition (about 5%). Therefore, we used HMA at 10 μM to evaluate its effects on migration and invasion. The effects of HMA at higher doses were also included for comparison. HMA at 10 μM did not inhibit cancer cell migration (Fig. 4A), but it significantly inhibited cancer cell invasion by about 25% (p < 0.05, Fig. 4B and C). These results indicate that HMA may have anti-invasion activity. Moreover, IL-6 (10 ng/ml) upregulated the expression of MMP-1, MMP-2 and MMP-9 genes by 104.1% (p < 0.001), 116.9% (p < 0.001), and 77.9% (p < 0.01), compared with untreated controls (Fig. 4D); HMA treatment (40 μM) effectively inhibited the IL-6 stimulated expression of these genes by 67.2% (p < 0.001), 72.2% (p < 0.001), and 58.8% (p < 0.001) (Fig. 4D), compared with the IL-6 treated controls. These results indicate that HMA may suppress A549 cells invasion in part through the inhibition of IL-6-induced MMPs expression.
Fig. 4.

Effects of HMA on IL-6-stimulated migration and invasion of A549 cells. (A) Effects of HMA (10, 20, 40 μM) on A549 cells migration were assessed by wound-healing assays. (B-C) Effects of HMA (10, 20, 40 μM) on A549 cells invasion were measured using transwell invasion assays. (D) Effects of HMA (40 μM) on expression of MMP-1, MMP-2, and MMP-9 genes in A549 cells were measured by real time RT-PCR. *p < 0.05 vs. the control group; #p < 0.05 vs. the IL-6-stimulated group. n = 3.
HMA inhibits IL-6-induced JAK2/STAT3 activation in A549 cells
We further examined if HMA could inhibit IL-6-induced STAT3 activation in A549 cells. A549 cells treated with IL-6 had increased protein levels of phosphorylated STAT3 (p-STAT3) and phosphorylated JAK2 (p-JAK2), which were effectively inhibited by HMA in a dose-dependent manner (Fig. 5A and B). By contrast, non-phosphorylated STAT3 and JAK2 protein levels were not affected by IL-6 and/or HMA treatments.
Fig. 5.
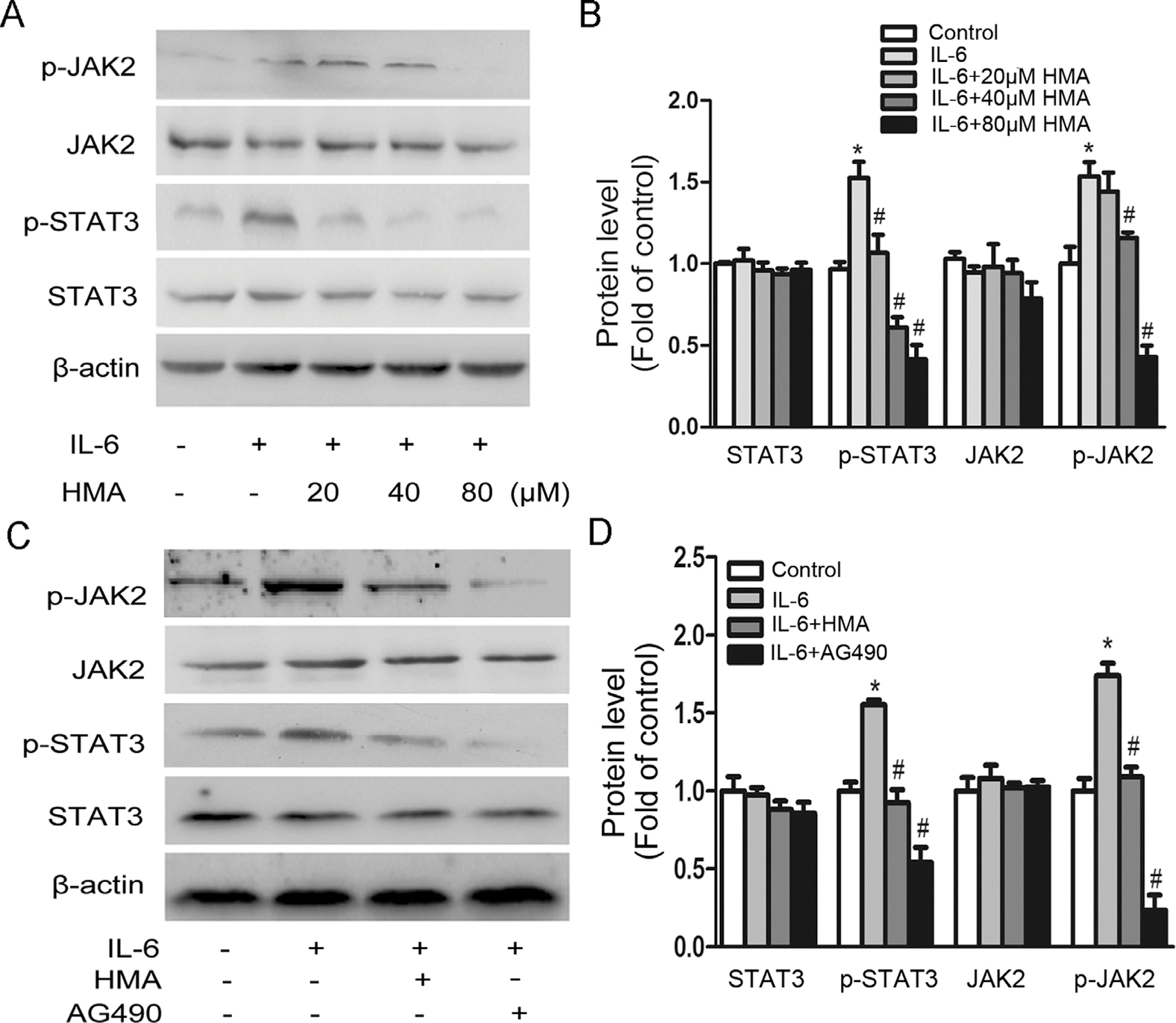
Effects of HMA on IL-6-induced JAK2/STAT3 signaling. (A-B) A549 cells were incubated with 20, 40, 80 μM HMA for 1 h and subsequently stimulated with 10 ng/ml IL-6 for 24 h. (C-D) A549 cells were incubated with 40 μM HMA for 1 h and subsequently stimulated with 10 ng/ml IL-6 and/or 50 μM AG490 for 24 h. *p < 0.05 vs. the control group; #p < 0.05 vs. the IL-6-stimulated group. n = 3.
Since JAK2 is a critical upstream signaling component of IL-6-induced STAT3 activation, we further used AG490, a pan-JAK inhibitor, to investigate whether the inhibition of STAT3 activation by HMA was mediated by upstream signaling. As shown in Fig. 5C and D, both HMA and AG490 significantly inhibited the IL-6-induced tyrosine phosphorylation of STAT3 and JAK2. These results suggest that the inhibition of IL-6-induced STAT3 activation by HMA was associated with the inhibition of its upstream JAK2 signaling.
HMA inhibits secretion of cytokines by A459 cells
Although our primary hypothesis was that HAM inhibited the growth, migration, and invasion of lung cancer cells in part via targeting IL-6 and its induced STAT3/JAK2 signaling pathway, it was possible that HMA also alters the expression/function of other cytokines. Therefore, we performed a protein array assay to investigate the effect of HMA on a panel of cytokines produced/secreted by A549 cells. HMA significantly reduced the protein levels of a series of inflammation-related cytokines in the cell supernatant, such as G-CSF (87.81%), IL-6 (34.94%), IL-6R (63.64%), IL-8 (72.57%), MCP-1 (90.34%), RANTES (89.60%), and TNF-α (21.99%) (Fig. 6). These results indicate that, in addition to inhibiting the expression/function IL-6, HMA may also exert its anti-cancer activities via altering the expression and function of other cytokines.
Fig. 6.
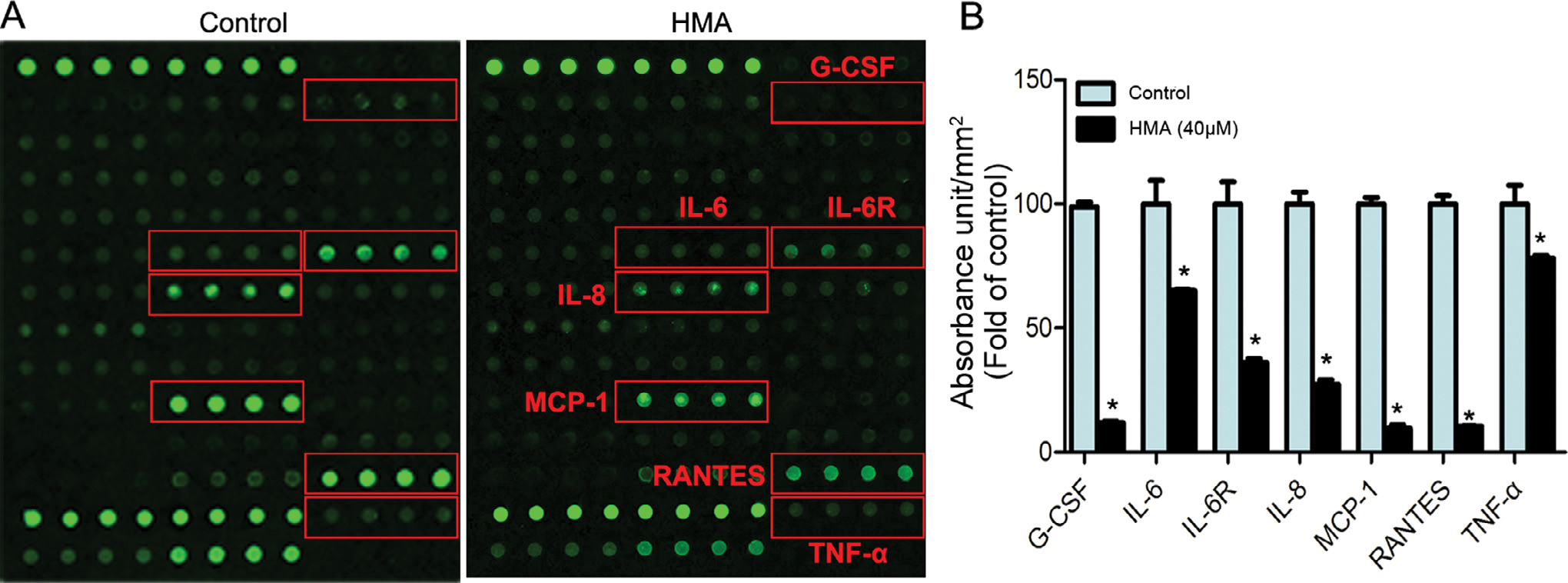
Protein array analysis of cytokines in culture media of A549 cells. (A) A549 cells were cultured with or without 40 μM HMA for 24 h, and the resulting culture supernatants were analyzed by cytokine antibody array. (B) Cytokines significantly altered by HMA treatment. *p < 0.05 vs. the control group. n = 3.
Discussion
In this study, we aimed to investigate if HMA could inhibit the growth, migration, and invasion at least in part via suppressing IL-6 functions. The results showed that IL-6 significantly stimulated the growth and invasion of A549 lung cancer cells, suppressed A549 apoptosis associated with downregulation of gene expression and protein levels of pro-apoptotic caspase-9, caspase-3 and bax genes and upregulation of gene expression and protein level of anti-apoptotic Bcl-2, upregulated the protein levels of MMPs and activated the STAT3/JAK2 signaling pathway. HMA could significantly inhibit the IL-6 stimulated cell growth and invasion of lung cancer cells in part via reversing the IL-6-mediated cellular and molecular alterations. In addition, HMA may also alter the expression and/or function of other inflammatory cytokines.
Hedyotis diffusa Wild is one of the most commonly used Chinese herbal medicines for cancer therapy (Su et al., 2019). HMA, as an anthraquinone monomer component in Hedyotis diffusa Wild, was reported to inhibit the growth of various cancer cells. However, little is known about its effect on lung cancer. Our results in this study showed that HMA could significantly inhibit the growth of lung cancer, and the more aggressive and metastatic A549 cells (and LLC cells) were particularly sensitive to HMA treatment. To the best of our knowledge, this is the first report to demonstrate that HMA is particularly potent against aggressive, metastatic and chemo-resistant NSCLC.
IL-6 is one of the most important inflammatory cytokines that could promote tumor growth and metastasis (Shang et al., 2017). IL-6 may stimulate proliferation, inhibit apoptosis, and promote invasion/metastasis of cancer cells in part via activation of the JAK2/STAT3 signaling pathway (Liu et al., 2014). In this study, we proposed to test the hypothesis that HMA could inhibit the growth, migration, and invasion of lung cancer cells in part via suppression of the IL-6-induced activation of the JAK2/STAT3 signaling pathway. Our study results showed that HMA could effectively alleviate the promoting effect of IL-6 on the growth and invasion of A549 cells associated with downregulation of the JAK2/STAT3 phosphorylation. In addition, we found that HMA had similar inhibitory effect with AG490, a pan-JAK inhibitor, on IL-6-induced tyrosine phosphorylation of JAK2 and STAT3. Although the direct functional role of the JAK2/STAT3 activation in the HMA action remains further elusive, our results provide experimental evidence to support the hypothesis.
Our studies suggest that one of the mechanisms of HMA actions may be via inhibition of the IL-6-stimulated cell growth and enhancement of the IL-6-suppressed apoptosis of A549 cells. IL-6 stimulation inhibited the apoptosis of A549 cells, however, HMA treatment significantly increased the number of apoptotic cells in a dose-dependent manner in part through the induction of the IL-6-inhibited gene expression and protein levels of pro-apoptotic caspase-3, caspase-9 and bax and inhibition of the IL-6-promoted gene expression and protein levels of anti-apoptotic bcl-2 (Fig. 3). Previous studies have shown that IL-6 restores the phosphorylation of STAT3 (Lin et al., 2015), thereby rendering cancer cells resistance to apoptosis, the findings consistent with our results.
Our results also support that one of the mechanisms of HMA actions is via suppression of the IL-6-induced invasion of A549 cells by downregulation of the IL-6-stimulated expression of MMPs (Fig. 4). MMPs play crucial roles in migration and invasion of lung cancer cells (Merchant et al., 2017). Human NSCLC had MMPs overexpression of MMPs (Blanco-Prieto et al., 2017), and IL-6 stimulated the production of MMPs via JAK-STAT signaling pathway (Aida et al., 2012).
Although IL-6 is an important inflammatory cytokine in stimulating the growth and progression/metastasis of lung cancer, it is possible that other pro-inflammatory cytokines may also be the targets for HMA action. Indeed, in addition to IL-6, G-CSF, IL-6R, IL-8, MCP-1, RANTES and TNF-α were also significantly altered/reduced by HMA treatment (Fig. 6). These inflammatory cytokines were also closely related to the development and occurrence of cancer. G-CSF could promote tumor growth and metastasis (Agarwal et al., 2015), MCP-1 was involved in tumor angiogenesis (Liu et al., 2016), RANTES played an important role in cancer metastasis (Khalid et al., 2015). TNF-α, together with other inflammatory factors, could promote tumorigenesis and development (Wu et al., 2017; Dobrzycka et al., 2013). These results strongly suggest that the effects of HMA on lung cancer may also be due to its regulation on other inflammatory cytokines.
Conclusion
In conclusion, our experimental results demonstrated that HMA had potent activity against the growth of highly aggressive and metastatic A549 lung cancer cells and significantly inhibited the IL-6-induced growth and invasion of A549 lung cancer cells associated with induction of apoptosis and inactivation of the IL-6/JAK2/STAT3 signaling pathway, while it had minimal effect on normal cells. Our findings provide supporting evidence to warrant further preclinical investigation for developing HMA as an alternative therapeutic agent against aggressive and metastatic NSCLC.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81774266) to M-H Wu, National Natural Science Funds of China for Young Scholars (81503535) to L Li, and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Abbreviations:
- Bax
Bcl-2 associated × protein
- Bcl-2
B-cell lymphoma-2
- CCK-8
cell counting kit-8
- DMEM
Dulbecco’s modified eagle medium
- FBS
fetal bovine serum
- HMA
2-hydroxy-3-methylanthraquinone
- HUVEC
human umbilical vein endothelial cell
- IL-6
interleukin-6
- JAKs
Janus kinases
- MMP
matrix metalloproteinase
- NSCLC
non-small cell lung cancer
- PCR
polymerase chain reaction
- STAT3
signal transducers and activators of transcription 3
- TBST
Tris-buffered saline with 0.05% Tween-20
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.phymed.2019.152848.
References
- Agarwal S, Lakoma A, Chen Z, Hicks J, Metelitsa LS, Kim ES, Shohet JM, 2015. G-CSF promotes neuroblastoma tumorigenicity and metastasis via STAT3-dependent cancer stem cell activation. Cancer Res. 75, 2566–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, Suzuki N, Shimizu O, Takeichi O, Makimura M, Maeno M, 2012. Il-6 and soluble il-6 receptor stimulate the production of mmps and their inhibitors via jak-stat and erk-mapk signalling in human chondrocytes. Cell Biol. Int. 36, 367–376. [DOI] [PubMed] [Google Scholar]
- Blanco-Prieto S, Barcia-Castro L, Cadena MPDL, Vázquez-Iglesias L, Botana-Rial MI, Fernández-Villar A, Chiara LD, 2017. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. Bmc Cancer 17, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GD, McLeod L, Alhayyani S, Miller A, Russell PA, Ferlin W, Rose-John S, Ruwanpura S, Jenkins BJ, 2016. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res. 15, 866–876. [DOI] [PubMed] [Google Scholar]
- Chen R, He J, Tong X, Tang L, Liu M, 2016. The Hedyotis diffusa Willd.(Rubiaceae): a review on phytochemistry, pharmacology, quality control andpharmacokinetics. Molecules 21, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzycka B, Mackowiak-Matejczyk B, Terlikowska KM, Kulesza-Bronczyk B, Kinalski M, Terlikowski SJ, 2013. Serum levels of IL-6, IL-8 and CRP as prognostic factors in epithelial ovarian cancer. Eur. Cytokine Netw. 24, 106–113. [DOI] [PubMed] [Google Scholar]
- Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T, 1996. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5, 449–460. [DOI] [PubMed] [Google Scholar]
- Hu CJ, He J, Li GZ, Fang PP, Xie JD, Ding YW, Mao YQ, Hu KF, 2018. Analyzing hedyotis diffusa mechanisms of action from the genomics perspective. Comput. Methods Programs Biomed. 17, 30661–30662. [DOI] [PubMed] [Google Scholar]
- Jiang YN, Yan HQ, Huang XB, Wang YN, Li Q, Gao FG, 2015. Interleukin 6 trigged ataxia-telangiectasia mutated activation facilitates lung cancer metastasis via MMP-3/MMP-13 up-regulation. Oncotarget 6, 40719–40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZQ, Yan XJ, Bi L, Chen JP, Zhou Q, Chen WP, 2013. Mechanism for hepato-protective action of Liangxue Huayu Recipe (LHR): blockade of mitochondrial cytochrome c release and caspase activation. J. Ethnopharmacol. 148, 851–860. [DOI] [PubMed] [Google Scholar]
- Jr DJ, 1997. STATs and gene regulation. Science 277, 1630–1635. [DOI] [PubMed] [Google Scholar]
- Khalid A, Wolfram J, Ferrari I, Mu C, Mai J, Yang Z, Zhao Y, Ferrari M, Ma X, Shen H, 2015. Recent advances in discovering the role of CCL5 in metastatic breast cancer. Mini Rev. Med. Chem. 15, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Li Q, Chen H, Lin H, Lai Z, Peng J, 2015. Hedyotis diffusa willd. extract suppresses proliferation and induces apoptosis via il-6-inducible stat3 pathway inactivation in human colorectal cancer cells. Oncol. Lett. 9, 1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT, 2014. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int. J. Oncol. 44, 1643–1651. [DOI] [PubMed] [Google Scholar]
- Liu X, Jing X, Cheng X, Ma D, Jin Z, Yang W, Qiu W, 2016. FGFR3 promotes angiogenesis-dependent metastasis of hepatocellular carcinoma via facilitating MCP-1-mediated vascular formation. Med. Oncol. 33, 1–11. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu M, Liu M, Li J, 2010. Methylanthraquinone from Hedyotis diffusa WILLD induces Ca(2+)-mediated apoptosis in human breast cancer cells. Toxicol. In Vitro 24, 142–147. [DOI] [PubMed] [Google Scholar]
- Mai Z, Blackburn GL, Zhou JR, 2007. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis 28, 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant N, Nagaraju GP, Rajitha B, Lammata S, Jella KK, Buchwald ZS, Lakka SS, Ali AN, 2017. Matrix metalloproteinases: their functional role in lung cancer. Carcinogenesis 38, 766–780. [DOI] [PubMed] [Google Scholar]
- Ni F, Gong Y, Li L, Abdolmaleky HM, Zhou JR, 2012. Flavonoid ampelopsin inhibits the growth and metastasis of prostate cancer in vitro and in mice. Plos One 7, e38802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parums DV, 2014. Current status of targeted therapy in non-small cell lung cancer. Drugs Today (Barc) 50, 503–525. [DOI] [PubMed] [Google Scholar]
- Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, Bowman ED, Engels EA, Caporaso NE, Harris CC, 2011. Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer. J. Natl. Cancer Inst. 103, 1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang GS, Liu L, Qin YW, 2017. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncol. Lett. 13, 4657–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Feng J, Xie J, Mei Z, Shi T, Wang S, Du Y, Yang G, Wu Y, Cheng X, Li S, Zhu L, Yang CS, Tu S, Jie Z, 2017. Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci. Rep. 7, 8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang CH, Gong XG, 2008. Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa WILLD. Biol. Pharm. Bull. 31, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Su C, Zhou C, Zhou S, Xu J, 2011. Serum cytokine levels in patients with advanced non-small cell lung cancer: correlation with treatment response and survival. Med. Oncol. 28, 1453–1457. [DOI] [PubMed] [Google Scholar]
- Su X, Li Y, Jiang M, Zhu J, Zheng C, Chen X, Zhou J, Li Y, Xiao W, Wang Y, 2019. Systems pharmacology uncover the mechanism of anti-non-small cell lung cancer for Hedyotis diffusa Willd. Biomed. Pharmacother. 109, 969–984. [DOI] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Jemal A, 2016. Lung cancer statistics. Adv. Exp. Med. Biol. 93, 1–19. [DOI] [PubMed] [Google Scholar]
- Wan XX, Zhang H, Wang JH, 2015. 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd induces the apoptosis of HO-8910 cells through Fas/FasL signaling pathway. Pract. Pharmacy Clin. Remedies 18, 1405–1409 Chinese. [Google Scholar]
- Wang N, Li DY, Niu HY, Zhang Y, He P, Wang JH, 2013. 2-hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd induces apoptosis in human leukemic U937 cells through modulation of MAPK pathways. Arch. Pharm. Res. 36, 752–7588. [DOI] [PubMed] [Google Scholar]
- Wu GR, Mu TC, Gao ZX, Wang J, Sy MS, Li CY, 2017. Prion protein is required for tumor necrosis factor α (TNFα)-triggered nuclear factor κB (NF-κB) signaling and cytokine production. J. Biol. Chem. 292, 18747–18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, Liu XB, Li Y, Li Z, 2012. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signaling pathway. PLoS One 7, e37960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HH, Lai WW, Chen HH, Liu HS, Su WC, 2006. Autocrine IL-6-induced stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 25, 4300–4309. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang J, Qi B, Jiang G, Liu J, Zhang P, Ma Y, Li W, 2016. The anti-tumor effect and bioactive phytochemicals of Hedyotis diffusa willd on ovarian cancer cells. J. Ethnopharmacol. 192, 132–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


