Abstract
Despite recent major advances in developing effective vaccines against toxoplasmosis, finding new protective vaccination strategies remains a challenging and elusive goal as it is critical to prevent the disease. Over the past few years, various experimental approaches have shown that developing an effective vaccine against T. gondii is achievable. However, more remains unknown due to its complicated life cycle, difficulties in clinical translation, and lack of a standardized platform. This minireview summarizes the recent advances in the development of T. gondii vaccines and the main obstacles to developing a safe, effective and durable T. gondii vaccine. The successes and failures in developing and testing vaccine candidates for the T. gondii vaccine are also discussed, which may facilitate the future development of T. gondii vaccines.
Subject terms: Infectious diseases, Vaccines
Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan from the Phylum Apicomplexa that can infect all warm-blooded animals. Infections in healthy individuals are generally asymptomatic, while they can be severe or even life-threatening among immune-compromised patients. The vertical transmission of T. gondii from mother to child is particularly concerning. In addition, toxoplasmosis has severe effects on animals. It causes certain economic losses to animal husbandry and adversely affects food safety. Chemotherapies are commonly used for the treatment of toxoplasmosis. The combination of pyrimethamine and sulfadiazine is the gold standard in treating toxoplasmosis. However, the treatment success rate remains low, as it can only kill tachyzoites but not bradyzoites. Furthermore, the treatment also causes significant side effects1. It may induce a folate deficiency state, which is probably responsible for hematological side effects and embryopathies. It is also associated with rare severe reactions that may be fatal, including agranulocytosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, and hepatic necrosis. In the case of T. gondii, vaccination against toxoplasmosis could be an effective and appropriate medical prevention, especially for specific populations (pregnant women and HIV patients)2. However, vaccines against T. gondii infection in humans are still under development, despite vaccines for domestic animals and livestock being commercially available. A live attenuated vaccine (Toxovax®, MSD, New Zealand) consisting of a modified T. gondii strain (S48) is approved in some regions (Europe and New Zealand) to reduce losses to the sheep industry due to congenital toxoplasmosis3. The results indicate that the T. gondii vaccine can be successfully developed and commercialized for human immunization. However, developing an ideal vaccine remains a significant challenge due to the complexities of the T. gondii genome, life cycle, and strain diversity.
Anti-T. gondii vaccines
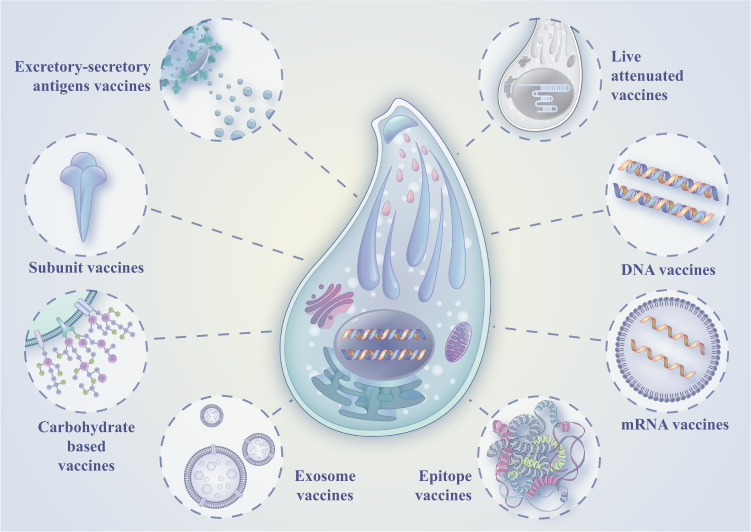
Since the middle of the last century, the research on T. gondii vaccines has gone through different stages, mainly including inactivated vaccines, excretory-secretory antigen vaccines, live attenuated vaccines, subunit vaccines, DNA vaccines, epitope vaccines, and mRNA vaccines (Fig. 1). Although these vaccines can play a particular role in preventing and treating toxoplasmosis, there are also some problems.
Fig. 1.
Anti-T. gondii vaccines.
Vaccines based on excretory-secretory antigens
Excretory-secretory antigens (ESA) produced by tachyzoites account for most circulating antigens in the serum and cerebrospinal fluid of the host. ESA immunization can improve animal survival rates by reducing parasitemia of highly virulent strains and controlling infections4. Subcutaneous injection of ESA reduced the formation of tissue cysts in pigs after T. gondii infection compared to the control group5. However, ESA immunization does not provide complete immune protection and cannot overcome the complex strains and life cycle of T. gondii. An adjuvant mixture of propranolol (PRP), a β-adrenergic receptor antagonist, and aluminium (alum) was used for immunization, significantly improving the protective effect of ESA and extending the survival time of mice6.
Live attenuated vaccines
Gamma irradiation, chemical treatment and multiple passages are commonly used to generate an attenuated T. gondii line incapable of completing its life cycle with reduced virulence. At the same time, the strain’s antigenicity is retained to elicit an immune response in the host, allowing it to produce memory cells and prevent reinfection. Currently, only one live attenuated vaccine, Toxovax®, is commercially available to reduce the damage caused to the sheep industry by congenital toxoplasmosis. The vaccine consists of a modified strain of T. gondii (S48 stain), generated upon years of repeated passages in mice. The vaccine strain has lost the ability to form oocyst7. T-263, a chemical-induced T. gondii mutant, is also immunogenic but incapable of forming oocysts in cats. It has been reported that cats fed live bradyzoites of T-263 do not excrete oocysts after the challenge with oocyst-producing strains8.
With the rapid development of gene-editing technology, CRISPR/Cas9 has become a revolutionary, powerful and precise technology for genome editing in many organisms. The CRISPR/Cas9 system is a powerful tool to generate a specific loss-of-function phenotype by gene knockout. It has become possible to produce live attenuated vaccines by controlling and changing substances required for T. gondii growth and reproduction. With promising results, many gene knockouts and attenuated live vaccines of T. gondii have been studied using the CRISPR/cas9 system9. Chandra et al. showed that the ΔHAP2 parasites could not complete fertilization and meiosis and could only produce a few abnormal oocysts. Inoculation of cats with ΔHAP2 parasites completely prevented wild-type T. gondii oocysts from shedding10. Besides, both Ca2+-dependent protein kinase 2 knocked out (ΔCDPK2) strain of T. gondii and the adenylosuccinate lyase knocked out(ΔADSL) strain of T. gondii Live attenuated vaccines induce anti-T. gondii humoral and cellular immune responses and have 100% immune protection in their experiment11,12.
Oral live attenuated vaccine mimics the natural infection state of T. gondii and induces host cellular and humoral immunity against T. gondii without causing disease. Current T. gondii vaccine research indicates the live attenuated vaccine as the most effective. Nevertheless, live attenuated vaccines also have drawbacks, such as short shelf life, safety issues for handling personnel, ethical reasons, and safety issues, making them unsuitable for humans. Furthermore, because of their unknown genetic background, attenuated vaccines may be counterproductive by reverting to their virulent wild-type and causing the diseases they are designed to prevent3.
Subunit vaccines
Subunit vaccines are created using only the parts of T. gondii required by the immune system. So far, many T. gondii subunit vaccines have been systematically studied. Recombinant heat shock protein 70 (rTgHSP70)-immunized mice induced high and sustained nitric oxide (NO) production in peritoneal macrophages, and rTgHSP70 immunity also enhanced the expression of iNOS in the brain and reduced the occurrence of cerebral cysts. rTgHSP70 immunization reduced parasite numbers13. The subunit vaccines with multiple epitope designs are more valuable for research. Onile et al. used immune informatics tools to design multi-epitope subunit vaccines with different T-cell and B-cell epitopes to fight toxoplasmosis14. Two recombinant proteins, recombinant rhoptry protein 18 (rROP18) and recombinant calcium-dependent protein kinase 6 (rCDPK6), combined with Poly (lactide-co-glycolide) (PLG), a biodegradable and biocompatible polymer, prolong the protein release period, lower protein degradation and induce a long-lasting immune response. The brain cysts load of immunized mice was significantly lower than that of the control group15,16.
The main disadvantage of subunit vaccines is that they provide less immune protection and usually require carrier or adjuvant delivery. However, using nanoparticles as carriers in the design of candidate subunit vaccines can significantly improve immune protection against T. gondii17.
Genetically engineered vaccines
DNA vaccines
Most DNA vaccines are based on parasite virulence-related proteins. These proteins include rhoptry proteins (ROP), dense granular proteins (GRA), microsomal proteins (MIC), and surface antigens (SAG)18. ROP proteins are involved in cell invasion and the formation of parasitic vacuoles. They are critical for T. gondii survival in host cells. DNA vaccination of ROP1, ROP8, ROP13, ROP16, ROP18, and ROP54 antigens induces dominant Th1-mediated immunity, corresponding to the production of cytokines such as IL-22, IL-2, IL-5, and IFN-γ, extending survival time of immunization mice19–24. For example, ROP16 is a key virulence factor in the pathogenesis of T. gondii because it can directly subvert the signal transducer and activator of the transcription 3/6 (STAT3/6) signal and target the host cell nucleus22. ROP16-based DNA vaccines can significantly enhance cytokine IFN-γ, IL-2, and IL-4 production for acute infections and increase survival by 13–27 days22. The GRAs modify the parasitophorous vacuole (PV) and parasitophorous vacuole membrane (PVM) for maintaining intracellular parasitism in host cells and are involved in parasite survival, virulence and replication. Among GRAs, There are many studies on GRA-24, GRA-7, GRA-4, GRA-2, and GRA-1. These antigens induce a mixed Th1/Th2 -mediated immunity, corresponding to the production of cytokines such as IL-4, IL-10, IL-12, IFN-γ, and TNF-α. For example, GRA-24 induced high levels of mixed Th1/Th2 cytokines 6 weeks after immunization, and the survival times were prolonged significantly (24.6 ± 5.5 days)25–27. The MICs are exposed on the tachyzoite surface and bind to host cell surface receptors to promote tachyzoite adhesion and subsequent host cell invasion. Among MICs, MIC2, MIC3, MIC4, MIC8, MIC11, and MIC13 antigens have been evaluated. These can induce the humoral and Th1-type immune responses, significantly enhance IFN-γ, IL-12, and IL-2 production, and higher survival time. The MIC8 DNA vaccine induces strong humoral and cellular immune responses and produces cytokines such as IL-15 and IL-21, which were protective against T. gondii challenge28,29. SAGs are mainly involved in the recognition and adhesion of T. gondii to host cells and the early invasion process. SAG1 (P30), SAG2 (P22), and SAG3 (P43) are the major SAGs. The SAG1 was sufficiently immunogenic to elicit cellular and humoral responses that led to a high degree of protection of animals against T. gondii infection. However, this vaccine has the limitation of being a tachyzoites stage-specific antigen30.
Although many DNA vaccines are inexpensive, easy to administer and induce a strong immune response at low doses, many obstacles still exist. For example, immunity is often weak in large animals, requiring 1000 times as much DNA vaccine as in small animals to be effective31. In addition, potential genome integration of plasmids may activate oncoproteins and produce antibodies in the DNA vaccine itself32.
Epitope vaccines
Because T. gondii has a complex life cycle with different epitopes at different stages, multiple epitopes are frequently employed to design vaccines. The protective effect of the polyvalent epitope vaccine was better than that of the single epitope vaccine. Mcleod’s group has made significant progress in identifying vaccine prototypes entering clinical trials. Using HLA transgenic mice as animal models, they combined epitopes of T. gondii at various stages with self-assembling protein nanoparticles (SAPNs) and induced mice with strong CD8+ T and CD4+ T-cell responses combined with an adjuvant, thus prolonging the survival time of mice33–36. Furthermore, numerous protein epitope vaccines have been thoroughly investigated, making significant progress37. Roozbehani’s group also successfully used SAPNs as scaffolds/platforms, which combined with peptide epitopes derived from SAG1, SAG2C, GRA6, GRA5, and so on for vaccine delivery. In addition, mice vaccinated with the multi-epitope-based vaccine generated more significant Th1 immune responses and had increased survival rates, specific antibody titers, and IFN-γ and IL-2 levels than controls38.
Compared to other types of vaccines, epitope-oriented vaccines have a lower risk of biohazard and the ability to design and optimize epitope structures, enhancing the potential of vaccines and inducing stronger immunity. Due to the lack of secondary and tertiary structure of the natural protein, epitope vaccines have small molecules, poor immunogenicity and a short half-life, which is less potent in stimulating a protective immune response and may cause immune tolerance. Therefore, improving the affinity of vaccine-induced antibodies and increasing the immunogenicity and stability of epitope vaccines has recently been a research hotspot.
Novel vaccines
mRNA vaccines
The mRNA vaccines introduce the mRNA coding T. gondii antigen target into the host through a specific delivery system, where the protein is expressed in the host and stimulates the host to produce a specific immunological response so that the host can obtain protective immunity. Experiments confirmed that an mRNA vaccine based on T. gondii nucleoside triphosphate hydrolase-II (TgNTPase-II), administered to mice for immunization by synthetic lipid nanoparticles (LNP), can stimulate robust humoral and cellular immunity, resulting in high antibodies and IFN-γ39. The results showed that the cyst number in the brain decreased significantly in the experimental group, and the survival time was also significantly extended. Combining mRNA and LNP will contribute to developing safe and long-acting toxoplasmosis vaccines39.
mRNA vaccines do not have to generate a large quantity of live attenuated T. gondii parasites, which may also revert to their virulent wild-type. In contrast to epitope vaccines and subunit vaccines, mRNA vaccines can overcome the challenges of inappropriate folding during the expression of recombinant proteins in vitro. Moreover, mRNA vaccines are more suitable for preventing outbreaks of pathogens than DNA vaccines. mRNA vaccines are relatively safe as they are confined to the cytoplasm, theoretically avoiding the risk of gene recombination and reducing malignant cell conversion. Further, despite an earlier start for research and development, there is still no DNA vaccine approved for human use. In contrast, many mRNA vaccines are already licensed (for example, COVID-19 mRNA vaccine). mRNA vaccine access is through the process of enzyme in vitro transcription production, which does not rely on cell amplification, so the antigen of cell culture, extraction, and purification process are saved, and the production time is shortened. So the time required to design and test new mRNA vaccines is also very short, with significant advantages against outbreaks such as influenza and COVID-1940,41. However, mRNA vaccines have a short intracellular half-life and easily degrade in vivo and during storage. As a result, delivery via substances such as liposomes is typically required42.
Carbohydrate-based vaccines
Carbohydrates on the surface of T. gondii are crucial for the infection of animal and human hosts. Its proteins are tagged with carbohydrates to stabilize and transport them, critical to completing the T. gondii life cycle. Carbohydrate antigens are recognized by the host’s immune system, eliciting carbohydrate-specific antibodies, making carbohydrates an attractive target for vaccine development43. TLR-2 and TLR-4 can recognize T. gondii GPI, inducing an inflammatory response44. However, the immune response induced by carbohydrate vaccines was insufficient to resist the attack of lethal doses of T. gondii tachyzoite44.
Carbohydrate vaccines currently leave much to be desired. Carbohydrates are often less immunogenic than proteins, and less likely to stimulate high-affinity antibodies. Moreover, the structure of carbohydrates tends to be similar to that of the host, which may lead to autoimmunity45.
Exosome vaccines
Exosomes can migrate through the intestinal basement membrane by directly stimulating T cells or being captured by other APCs to amplify the diffusion of MHC molecules, transmitting antigenic information to mucosal and systemic immune cells46,47. Recent studies have shown that exosomes isolated from T. gondii can induce humoral and cellular immune responses and control acute infection in mice48. In addition, experiments have shown that a novel cell-free vaccine composed of DC2.4 cell-derived exosomes can be transferred to the spleen and induce a Th1-mediated T. gondii-specific immune response in vivo, providing excellent anti-infection protection49.
However, exosome vaccines face many significant challenges, such as the lack of quality control, inconsistent purification standards, insufficient manufacturing, storage, management supervision, and poor biocompatibility. These problems must be overcome before they can be used for broad clinical applications and large-scale immunization programs50.
Challenges in T. gondii vaccines development
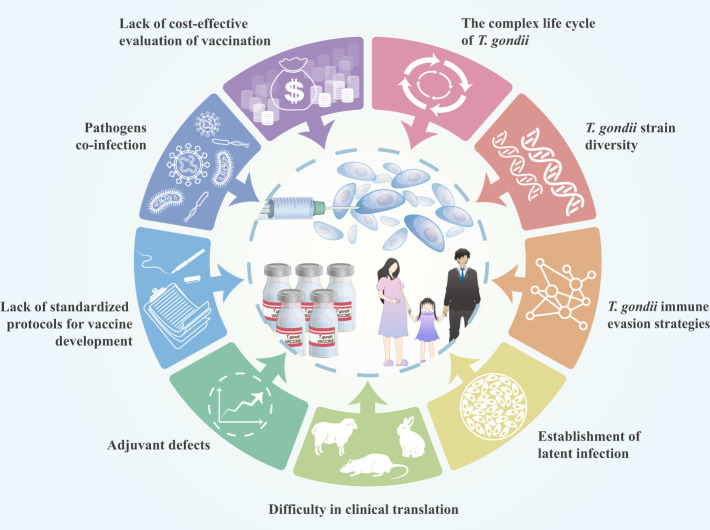
Advances in the post-genomic era, such as bioinformatics, genomics, and proteomics, may assist in identifying and selecting new and effective antigens, thereby reducing future challenges51. However, future studies must address some limitations, such as adjuvants, methods, standardized immunization protocols, evaluation criteria, parasite strains, vaccines construction, and different animal models (Fig. 2).
Fig. 2.
Challenges in T. gondii vaccine development.
The complex life cycle of T. gondii
Due to a complex life cycle involving multiple hosts with diverse protein forms to express various invasion pathways, T. gondii exhibits high antigenic polymorphism and variability. Different states, such as tachyzoites, bradyzoites, or oocysts, exist in the host, and different antigens appear in different stages of T. gondii52. Consequently, vaccines should not be developed with a specific stage in mind. Using the above combination of antigens from various states is preferable, which is much more effective than a single antigen. An ideal immunogen vaccine should adequately encompass CD8+, CD4+, and B-cell epitopes from different stages of T. gondii53.
T. gondii strain diversity
T. gondii strains firstly revealed three major lineages (Type I, II, and III), predominating in North America and Europe, 199554. Although all T. gondii strains share a similar genetic background, their virulence and pathogenesis are strikingly different54. The type I strain is the most virulent and lethal in laboratory mice, infecting doses from a single living organism. The virulence of type II and type III was weaker than that of type I55. A fourth clonal lineage, known as type 12, has been found in North America, commonly in wildlife56. With the development of genetic analysis technology, a system to describe and interpret the evolutionary subdivision of T. gondii has been reported by examining thousands of isolates collected worldwide and genotyping them using three independent sets of polymorphic DNA markers. These cover 30 loci on all chromosomal and cytoplasmic genomes. The 138 unique genotypes revealed by the genetic diversity of markers in this system were grouped into 15 haplogroups that collectively define six major clades using cluster analysis57. Currently, unique genotypic strains recorded in the database ToxoDB (http://toxodb.org/toxo/) are up to ToxoDB#231. Due to the diversity of T. gondii strains, a vaccine based on one T. gondii strain may not be effective against other strains of different genotypes. The infection is not limited to a single strain. Therefore, the development of vaccines against most T. gondii strains is needed58,59. One stretagy could be finding the immunogenic conserved epitopes by analyzing the protein sequences of polymorphic antigens and the host immune responses to design a multi-allele vaccine that induce antibodies to the conserved epitopes and promote antibodies to multiple parasite strains60. Considering that this has been done for malaria, a similar approach can be applied for T. gondii vaccine development60. Finding the protein in T. gondii with limited antigenic diversity like apical membrane antigen 1 (AMA1) in Plasmodium falciparum. It means a vaccine including a small number of alleles might be sufficient to cover most naturally-circulating strains. It supports a multi-allele approach for developing polymorphic antigens for a T. gondii vaccine61.
T. gondii immune evasion strategies
T. gondii invasion of host cells can interfere with the host’s immune system. T. gondii can avoid the damage of NO and hyperoxic substances by making the body’s high level of arginase competes with iNOS for the same substrate, reducing NO production62. T. gondii invasion-secreted inhibitor of STAT1 transcriptional activity (TgIST) translocates to the nucleus via the STAT signaling pathway, resulting in chromatin alteration and signal blocking63,64. T. gondii phosphorylates STAT3 and STAT6 by secreting ROP16 and upregulating M2 macrophage-like anti-inflammatory responses65. ROP5, ROP17, and ROP18 jointly prevent IFN-mediated IRGs from destroying the PVM66. GRA protects PV, helps T. gondii survive in host cells, enhances T. gondii virulence, and increases tissue cysts67–69. In addition, T. gondii effectors inhibit nuclear factor-κB (NF-κB) function by interfering with Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit (EZH2) or tripartite motif-containing 21 (TRIM21)70,71. As a pro-inflammatory molecule, macrophage migration inhibitory factor (MIF) is a crucial mediator of immune responses against various pathogens, including parasites72. T. gondii MIF directly binds to human macrophage MIF receptor CD74 to prevent macrophage apoptosis from obtaining sufficient nutrients and time to reproduce by using this “tent” to disseminate without being killed by the host immune system73. T. gondii successfully evades immune surveillance by manipulating the host immune system through various mechanisms, which has challenged effective vaccine development. However, until now, the interaction between T. gondii and its host must be further studied. Screening the whole T. gondii genome for molecules that may play a key role in immune escape or pathogenesis and further functional identification is critical for the in-depth study of T. gondii genetics, immune escape, and vaccine development.
Establishment of latent infection
When rapidly proliferating T. gondii tachyzoites differentiate into cyst-forming bradyzoites, the parasitic vacuole in which T. gondii replicates is remodeled into a highly glycosylated cyst wall. T. gondii can prolong the infectious period by establishing a latent or chronic state, avoiding immune clearance by slow replication, altering immunogenicity, and reducing the impact on the host. This continuous phase, which can reoccur or contribute to disease transmission, impedes curing and eradicating infectious diseases74. Recently, a Myb-like transcription factor (BFD1) was identified as required for bradyzoite differentiation in T. gondii75. BFD1 accumulates during stress, and its synthetic expression is sufficient to drive differentiation, incorporating promoters of many stage-specific genes. BFD1 provides a genetic switch for studying and controlling T. gondii differentiation and will inform the prevention and treatment of chronic infections75. Therefore, in studying the T. gondii vaccines, we should fully consider how to use the key molecule BFD1 to enhance the immune protective effect of the vaccines.
Difficulty in clinical translation
Laboratory mice are considered the most widely used model for studying the pathogenesis and immunological events involved in controlling or preventing T. gondii infection and have provided important insights for developing vaccines against toxoplasmosis. However, the transfer of experimental studies to clinical practice has been hampered mainly by species differences between experimental mice and humans. First, IRGs, a class of proteins involved in the mice’s early immune response, can aggregate on PVM to form holes, destroying the replication region of T. gondii76. Humans do not have IRG resistance systems77. Second, profilin is an actin-modifying protein that binds and activates TLR11 and TLR12, promotes IL-12 production, inducing NK cells to produce IFN-γ, which plays an important role in eliminating T. gondii tachyzoites78. However, TLR12 is not present in humans, and TLR11 is also a functional pseudogene79. T. gondii antigen can only be presented by ordinary antigen-presenting cells to activate cellular immunity against infection. As a result, antigens found in mice may not protect humans or other animals. Therefore, it is very inappropriate to directly transfer the experimental results obtained using mice as the research object to clinical practice.
Transgenic mice expressing MHC class I and II of human origin have emerged as ideal models for T. gondii vaccine development, which has the potential to facilitate the development of human vaccines33. HLA transgenic mouse model has been widely used in preclinical and experimental studies. However, MHC genes are highly polymorphic in humans and mammals. Furthermore, it has significant geographical and ethnic differences in the distribution of its dominant genotypes. In addition, most of the common international MHC transgenic mouse models are HLA-I or HLA-II single-transgenic mice, which still cannot examine the synergistic immunomodulatory effects of HLA-I and HLA-II class molecules in the organism. Therefore, there is a pressing need to construct HLA-I/II double transgenic mouse models that cover as much of the population as possible with HLA genetic traits, which is a difficult task.
Adjuvant defects
Currently, T. gondii vaccines have evolved from live attenuated vaccines to subunit and genetically engineered vaccines, most of which are weakly immunogenic. As a result, some adjuvants are required to deliver the vaccine and elicit a robust immune response. Adjuvants can reduce antigen dose, improve vaccine efficacy in immunocompromised individuals, and enhance the immune response to protect the vaccines against highly mutagenic pathogens. Currently, adjuvants such as aluminium salts, Toll-like receptor agonists, liposomes, immunostimulating complexes (ISCOM), and CPG motifs synergistically induce a potent immune response80. However, there are some emerging issues with the addition of adjuvants. For example, the uniformity of aluminium salt adjuvant particles is poorly controlled and easily absorbed by other nonimmune cells, resulting in poor immune protection. Toll-like receptor agonist adjuvants help pathogens evade the host’s immune system. Recent experiments have shown that the adjuvant activity of Al-PRP mixtures is stronger than that of single adjuvants (alum or PRP)81. Accordingly, combining adjuvants could be considered to overcome the disadvantages of using different adjuvants alone.
Lack of standardized protocols for vaccines development
It is difficult to compare the efficacy of different candidate vaccines due to the different strains, life cycle stages of T. gondii used in different inoculation routes and doses, and animal models. Different laboratories are not easily comparable. Therefore, establishing standardized protocols for vaccine development is urgently required.
Pathogens co-infection
When humans or other hosts are exposed to the natural environment, infections of multiple pathogens can occur. T. gondii infection induces a Th1-type immune response, while other pathogens, such as the helminth, may induce Th2 immune responses82. This mixed infection also poses a significant challenge for T. gondii vaccine development, which researchers must assess.
Lack of cost-effective evaluation of vaccination
The only commercial vaccine is the attenuated tachyzoite S48 strain (Toxovax®) used in sheep. It is produced to be supplied as a concentrated suspension of tachyzoites and diluent. This vaccinated sheep can reduce abortion and neonatal mortality and improve the birth weight of lambs. Toxovax® vaccine is only used in the UK, New Zealand, France, and Ireland83. However, because it is based on tachyzoite growth in mammalian cell cultures, this vaccine has a very short shelf life and high production costs. Concerns have also been raised about its safety, as live attenuated vaccines risk reverting to wild strains and infecting humans7. In addition, no cost-effectiveness has been reported for this vaccination program, explaining why this vaccine has not been implemented in other countries84. Many different pharmacological treatments are available for T. gondii infection, and farmers may have no incentive to spend money on this vaccine if there is no obvious economic return85.
Another method is the strategic vaccination of cats to reduce environmental contamination by reducing oocyst expulsion. Several studies on T. gondii vaccines for cats have also been conducted86. The T-263 strain completely prevents oocyst-shedding in the tested animals8. Recently, Ramakrishnan et al. modified a T. gondii strain with defective fertilization, reduced fecundity, and the inability to produce normal oocysts. Immunization of cats with this engineered strain completely prevented oocyst excretion following T. gondii infection. These results are very encouraging10. A vaccination program with an adequate cost-benefit analysis would help guide vaccine development and market application87. However, models and conclusions regarding cost-effectiveness analyses of T. gondii vaccination programs for cats have been suboptimal. Sykes et al. created a mathematical model that can be used to predict average vaccination levels in domestic cat populations. They found a critical vaccine cost threshold above which no one would use the vaccine. Vaccine costs slightly below this threshold would result in higher vaccine use, significantly reducing the seropositivity rate in the domestic cat population. Unfortunately, domestic cats can only achieve herd immunity at no vaccine cost88. Another model study showed that the prospects for preventing human toxoplasmosis caused by oocysts by vaccination in large cat populations are not promising due to the extensive vaccination coverage required. This vaccination method is only effective in small cat populations such as those on farms89.
T. gondii infection is common in warm-blooded animals, which provides a constant threat to humans and livestock. Eradication of T. gondii infection is almost impossible due to the wide range of natural reservoirs. In the case of T. gondii infection, a vaccine would be a complement to treatment and other preventive measures rather than a substitute. It is also costly for a parasite that cannot be eradicated and will only clinically affect a small number of people90. To be cost/effective, a vaccination program should prove cheaper than treatment. In addition, a vaccine targeting high-risk groups may be valuable, for example, women of childbearing age or HIV-positive patients. Therefore, it is also important to conduct adequate cost-effectiveness studies of T. gondii vaccination programs for this specific population, either globally or in different countries or regions, in conjunction with experts in economics and statistics.
Current T. gondii vaccine research indicates that live attenuated vaccines are the most effective option. Live attenuated oral vaccines can mimic natural infections. We believe it will be the most promising vaccine if they overcome the abovementioned challenges, especially if the vaccine can overcome their unknown genetic background. Besides, with the wide use of CRISPR technology, generating gene deletion mutants as live vaccines has become feasible and provides a novel approach for controlling toxoplasmosis.
Conclusion
To summarize, T. gondii is an obligate intracellular parasite with global distribution and important medical and veterinary implications. With the identification of neoantigens, adjuvants, and immunization strategies, significant progress has been made in developing T. gondii vaccines. Candidate vaccines include recombinant antigens, multi-epitope antigens, DNA or RNA, and microparticles. The advantages of these vaccines have been extensively studied in various animal models to assess their potential to elicit cellular and humoral immune responses and protect against the T. gondii challenge. Next, a careful selection of highly immunogenic antigens covering different stages of the T. gondii life cycle should be performed to construct a multi-antigen vaccine, combined with appropriate adjuvants and delivery systems, using HLA transgenic mice covering populations with predominant HLA genotypes for vaccines protection evaluation. Furthermore, adequate, cost-effective studies for T. gondii vaccination programs should be conducted, particularly for women of childbearing age and HIV-positive patients. Although there are many challenges in developing a T. gondii vaccine, we remain optimistic that developing an effective vaccine to prevent and treat toxoplasmosis remains possible.
Acknowledgements
This work is supported by Zhejiang Medical and Health Science and Technology Plan (2020KY102), Scientific Research Project of Zhejiang Provincial Department of Education (Y202146047), Special Funding Program in Hangzhou Medical College (YS2021003), and National Natural Science Foundation of China (81871684, 32000293).
Author contributions
Z.Y.Z. and D.L. developed data analysis and drafted the manuscript. S.H.L. and B.Z. edited and reviewed manuscript.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Code availability
Any custom code or mathematical algorithm is not applicable to this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shaohong Lu, Email: llsshh2003@163.com.
Bin Zheng, Email: bin_zheng@foxmail.com.
References
- 1.Dunay, I. R., Gajurel, K., Dhakal, R., Liesenfeld, O. & Montoya, J. G. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev.10.1128/cmr.00057-17 (2018). [DOI] [PMC free article] [PubMed]
- 2.Rémy, V., Zöllner, Y. & Heckmann, U. Vaccination: the cornerstone of an efficient healthcare system. J. Mark. Access. Health Policy10.3402/jmahp.v3.27041 (2015). [DOI] [PMC free article] [PubMed]
- 3.Chu, K. B. & Quan, F. S. Advances in Toxoplasma gondii vaccines: current strategies and challenges for vaccine development. Vaccines (Basel)10.3390/vaccines9050413 (2021). [DOI] [PMC free article] [PubMed]
- 4.Costa-Silva TA, Borges MM, Galhardo CS, Pereira-Chioccola VL. Immunization with excreted/secreted proteins in AS/n mice activating cellular and humoral response against Toxoplasma gondii infection. Acta Trop. 2012;124:203–209. doi: 10.1016/j.actatropica.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang D, Wang G, Yin H, Wang M. Immunization with excreted-secreted antigens reduces tissue cyst formation in pigs. Parasitol. Res. 2013;112:3835–3842. doi: 10.1007/s00436-013-3571-4. [DOI] [PubMed] [Google Scholar]
- 6.Meshkini E, Aminpour A, Hazrati Tappeh K, Seyyedi S, Shokri M. Evaluation of adjuvant effectiveness of alum-propranolol mixture on the immunogenicity of excreted/secreted antigens of Toxoplasma gondii RH strain. Adv. Pharm. Bull. 2021;11:570–577. doi: 10.34172/apb.2021.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubey JP. Toxoplasmosis in sheep-the last 20 years. Vet. Parasitol. 2009;163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Freyre A, Choromanski L, Fishback JL, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J. Parasitol. 1993;79:716–719. doi: 10.2307/3283610. [DOI] [PubMed] [Google Scholar]
- 9.Loh FK, Nathan S, Chow SC, Fang CM. Vaccination challenges and strategies against long-lived Toxoplasma gondii. Vaccine. 2019;37:3989–4000. doi: 10.1016/j.vaccine.2019.05.083. [DOI] [PubMed] [Google Scholar]
- 10.Ramakrishnan C, et al. An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Sci. Rep. 2019;9:1474. doi: 10.1038/s41598-018-37671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JL, et al. Live attenuated Pru:Δcdpk2 strain of toxoplasma gondii protects against acute, chronic, and congenital toxoplasmosis. J. Infect. Dis. 2018;218:768–777. doi: 10.1093/infdis/jiy211. [DOI] [PubMed] [Google Scholar]
- 12.Wang, L., Tang, D., Yang, C., Yang, J. & Fang, R. Toxoplasma gondii ADSL knockout provides excellent immune protection against a variety of strains. Vaccines (Basel)10.3390/vaccines8010016 (2020). [DOI] [PMC free article] [PubMed]
- 13.Czarnewski P, Araújo ECB, Oliveira MC, Mineo TWP, Silva NM. Recombinant TgHSP70 immunization protects against Toxoplasma gondii brain cyst formation by enhancing inducible nitric oxide expression. Front. Cell Infect. Microbiol. 2017;7:142. doi: 10.3389/fcimb.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onile OS, Ojo GJ, Oyeyemi BF, Agbowuro GO, Fadahunsi AI. Development of multiepitope subunit protein vaccines against Toxoplasma gondii using an immunoinformatics approach. NAR Genom. Bioinform. 2020;2:lqaa048. doi: 10.1093/nargab/lqaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foroutan M, Ghaffarifar F. Calcium-dependent protein kinases are potential targets for Toxoplasma gondii vaccine. Clin. Exp. Vaccin. Res. 2018;7:24–36. doi: 10.7774/cevr.2018.7.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majidiani H, et al. In-depth computational analysis of calcium-dependent protein kinase 3 of Toxoplasma gondii provides promising targets for vaccination. Clin. Exp. Vaccin. Res. 2020;9:146–158. doi: 10.7774/cevr.2020.9.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodangeh S, et al. Protective efficacy by a novel multi-epitope vaccine, including MIC3, ROP8, and SAG1, against acute Toxoplasma gondii infection in BALB/c mice. Micro. Pathog. 2021;153:104764. doi: 10.1016/j.micpath.2021.104764. [DOI] [PubMed] [Google Scholar]
- 18.Warner RC, Chapman RC, Davis BN, Davis PH. Review of dna vaccine approaches against the parasite Toxoplasma gondii. J. Parasitol. 2021;107:882–903. doi: 10.1645/20-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiszczyńska-Sawicka E, et al. Modulation of immune response to Toxoplasma gondii in sheep by immunization with a DNA vaccine encoding ROP1 antigen as a fusion protein with ovine CD154. Vet. Parasitol. 2011;183:72–78. doi: 10.1016/j.vetpar.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Parthasarathy S, Fong MY, Ramaswamy K, Lau YL. Protective immune response in BALB/c mice induced by DNA vaccine of the ROP8 gene of Toxoplasma gondii. Am. J. Trop. Med. Hyg. 2013;88:883–887. doi: 10.4269/ajtmh.12-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alizadeh P, et al. IL-17 and IL-22 elicited by a DNA vaccine encoding ROP13 associated with protection against Toxoplasma gondii in BALB/c mice. J. Cell Physiol. 2019;234:10782–10788. doi: 10.1002/jcp.27747. [DOI] [PubMed] [Google Scholar]
- 22.Yuan ZG, et al. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clin. Vaccin. immunol. 2011;18:119–124. doi: 10.1128/CVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan ZG, et al. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine. 2011;29:6614–6619. doi: 10.1016/j.vaccine.2011.06.110. [DOI] [PubMed] [Google Scholar]
- 24.Yang WB, et al. Vaccination with a DNA vaccine encoding Toxoplasma gondii ROP54 induces protective immunity against toxoplasmosis in mice. Acta Trop. 2017;176:427–432. doi: 10.1016/j.actatropica.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Ching XT, Fong MY, Lau YL. Evaluation of the protective effect of deoxyribonucleic acid vaccines encoding granule antigen 2 and 5 against acute toxoplasmosis in BALB/c mice. Am. J. Trop. Med. Hyg. 2017;96:1441–1447. doi: 10.4269/ajtmh.16-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng B, et al. GRA24-based DNA vaccine prolongs survival in mice challenged with a virulent Toxoplasma gondii strain. Front. Immunol. 2019;10:418. doi: 10.3389/fimmu.2019.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaei F, et al. A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization. J. Microbiol. Methods. 2019;165:105696. doi: 10.1016/j.mimet.2019.105696. [DOI] [PubMed] [Google Scholar]
- 28.Li ZY, et al. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine. 2014;32:3058–3065. doi: 10.1016/j.vaccine.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Foroutan M, Zaki L, Ghaffarifar F. Recent progress in microneme-based vaccines development against Toxoplasma gondii. Clin. Exp. Vaccin. Res. 2018;7:93–103. doi: 10.7774/cevr.2018.7.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagheh AS, et al. Toxoplasma gondii surface antigen 1 (SAG1) as a potential candidate to develop vaccine against toxoplasmosis: A systematic review. Comp. Immunol. Microbiol. Infect. Dis. 2020;69:101414. doi: 10.1016/j.cimid.2020.101414. [DOI] [PubMed] [Google Scholar]
- 31.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time. Nat. Rev. Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myhr AI. DNA vaccines: regulatory considerations and safety aspects. Curr. Issues Mol. Biol. 2017;22:79–88. doi: 10.21775/cimb.022.079. [DOI] [PubMed] [Google Scholar]
- 33.El Bissati K, et al. Engineering and characterization of a novel Self Assembling Protein for Toxoplasma peptide vaccine in HLA-A*11:01, HLA-A*02:01 and HLA-B*07:02 transgenic mice. Sci. Rep. 2020;10:16984. doi: 10.1038/s41598-020-73210-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Bissati K, et al. Adjuvanted multi-epitope vaccines protect HLA-A*11:01 transgenic mice against Toxoplasma gondii. JCI Insight. 2016;1:e85955. doi: 10.1172/jci.insight.85955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Bissati K, et al. Effectiveness of a novel immunogenic nanoparticle platform for Toxoplasma peptide vaccine in HLA transgenic mice. Vaccine. 2014;32:3243–3248. doi: 10.1016/j.vaccine.2014.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Bissati K, et al. Protein nanovaccine confers robust immunity against Toxoplasma. npj Vaccines. 2017;2:24. doi: 10.1038/s41541-017-0024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhou H. Moving towards improved vaccines for Toxoplasma gondii. Expert. Opin. Biol. Ther. 2018;18:273–280. doi: 10.1080/14712598.2018.1413086. [DOI] [PubMed] [Google Scholar]
- 38.Roozbehani M, et al. Characterization of a multi-epitope peptide with selective MHC-binding capabilities encapsulated in PLGA nanoparticles as a novel vaccine candidate against Toxoplasma gondii infection. Vaccine. 2018;36:6124–6132. doi: 10.1016/j.vaccine.2018.08.068. [DOI] [PubMed] [Google Scholar]
- 39.Luo, F. et al. Induction of protective immunity against toxoplasma gondii in mice by nucleoside triphosphate hydrolase-II (NTPase-II) self-amplifying RNA vaccine encapsulated in lipid nanoparticle. Front. Microbiol.8, 605 (2017). [DOI] [PMC free article] [PubMed]
- 40.Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front. Immunol. 2020;11:585354. doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilkington EH, et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta Biomater. 2021;131:16–40. doi: 10.1016/j.actbio.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr. Opin. Immunol. 2020;65:14–20. doi: 10.1016/j.coi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Maeda Y, Kinoshita T. Structural remodeling, trafficking and functions of glycosylphosphatidylinositol-anchored proteins. Prog. Lipid Res. 2011;50:411–424. doi: 10.1016/j.plipres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Debierre-Grockiego F, et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 45.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations. Nat. Rev. Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Niel G, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 47.Théry C, et al. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, et al. Characterization of exosomes derived from Toxoplasma gondii and their functions in modulating immune responses. Int. J. Nanomed. 2018;13:467–477. doi: 10.2147/IJN.S151110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect. Immun. 2004;72:4127–4137. doi: 10.1128/IAI.72.7.4127-4137.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santos P, Almeida F. Exosome-based vaccines: history, current state, and clinical trials. Front. Immunol. 2021;12:711565. doi: 10.3389/fimmu.2021.711565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yektaeian N, Malekpour A, Atapour A, Davoodi T, Hatam G. Genetic immunization against toxoplasmosis: a review article. Micro. Pathog. 2021;155:104888. doi: 10.1016/j.micpath.2021.104888. [DOI] [PubMed] [Google Scholar]
- 52.Attias M, et al. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit. Vectors. 2020;13:588. doi: 10.1186/s13071-020-04445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cong H, et al. Human immunome, bioinformatic analyses using HLA supermotifs and the parasite genome, binding assays, studies of human T cell responses, and immunization of HLA-A*1101 transgenic mice including novel adjuvants provide a foundation for HLA-A03 restricted CD8+ T cell epitope based, adjuvanted vaccine protective against Toxoplasma gondii. Immunome Res. 2010;6:12. doi: 10.1186/1745-7580-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J. Infect. Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 55.Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 2005;21:476–481. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Khan A, et al. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int. J. Parasitol. 2011;41:645–655. doi: 10.1016/j.ijpara.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Su C, et al. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc. Natl Acad. Sci. USA. 2012;109:5844–5849. doi: 10.1073/pnas.1203190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jensen KD, et al. Toxoplasma gondii superinfection and virulence during secondary infection correlate with the exact ROP5/ROP18 allelic combination. mBio. 2015;6:e02280. doi: 10.1128/mBio.02280-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dao A, Fortier B, Soete M, Plenat F, Dubremetz JF. Successful reinfection of chronically infected mice by a different Toxoplasma gondii genotype. Int. J. Parasitol. 2001;31:63–65. doi: 10.1016/S0020-7519(00)00151-X. [DOI] [PubMed] [Google Scholar]
- 60.Feng G, et al. Human immunization with a polymorphic malaria vaccine candidate induced antibodies to conserved epitopes that promote functional antibodies to multiple parasite strains. J. Infect. Dis. 2018;218:35–43. doi: 10.1093/infdis/jiy170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terheggen U, et al. Limited antigenic diversity of Plasmodium falciparum apical membrane antigen 1 supports the development of effective multi-allele vaccines. BMC Med. 2014;12:183. doi: 10.1186/s12916-014-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Padrão Jda C, Cabral GR, da Silva Mde F, Seabra SH, DaMatta RA. Toxoplasma gondii infection of activated J774-A1 macrophages causes inducible nitric oxide synthase degradation by the proteasome pathway. Parasitol. Int. 2014;63:659–663. doi: 10.1016/j.parint.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Matta SK, Rinkenberger N, Dunay IR, Sibley LD. Toxoplasma gondii infection and its implications within the central nervous system. Nat. Rev. Microbiol. 2021;19:467–480. doi: 10.1038/s41579-021-00518-7. [DOI] [PubMed] [Google Scholar]
- 64.Olias P, Etheridge RD, Zhang Y, Holtzman MJ, Sibley LD. Toxoplasma effector recruits the Mi-2/NuRD complex to repress STAT1 transcription and block IFN-γ-dependent gene expression. Cell Host Microbe. 2016;20:72–82. doi: 10.1016/j.chom.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen, L. et al. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J. Exp. Med.10.1084/jem.20181757 (2020). [DOI] [PMC free article] [PubMed]
- 66.Etheridge RD, et al. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nadipuram, S. M. et al. In vivo biotinylation of the toxoplasma parasitophorous vacuole reveals novel dense granule proteins important for parasite growth and pathogenesis. mBio10.1128/mBio.00808-16 (2016). [DOI] [PMC free article] [PubMed]
- 68.Gold DA, et al. The toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe. 2015;17:642–652. doi: 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li TT, et al. Effect of deletion of gra17 and gra23 genes on the growth, virulence, and immunogenicity of type II Toxoplasma gondii. Parasitol. Res. 2020;119:2907–2916. doi: 10.1007/s00436-020-06815-z. [DOI] [PubMed] [Google Scholar]
- 70.Yao L, et al. Toxoplasma gondii type-I ROP18 targeting human E3 ligase TRIM21 for immune escape. Front. Cell Dev. Biol. 2021;9:685913. doi: 10.3389/fcell.2021.685913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun L, et al. The Toxoplasma effector TEEGR promotes parasite persistence by modulating NF-κB signalling via EZH2. Nat. Microbiol. 2019;4:1208–1220. doi: 10.1038/s41564-019-0431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernhagen, Jr & Bucala R. Mif Family Cytokines in Innate Immunity and Homeostasis. (Springer International Publishing: Imprint: Springer, 2017).
- 73.Ghosh S, Jiang N, Farr L, Ngobeni R, Moonah S. Parasite-produced MIF cytokine: role in immune evasion, invasion, and pathogenesis. Front. Immunol. 2019;10:1995. doi: 10.3389/fimmu.2019.01995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu, V. et al. Enrichment and proteomic characterization of the cyst wall from in vitro Toxoplasma gondii cysts. mBio10.1128/mBio.00469-19 (2019). [DOI] [PMC free article] [PubMed]
- 75.Waldman BS, et al. Identification of a master regulator of differentiation in toxoplasma. Cell. 2020;180:359–372.e316. doi: 10.1016/j.cell.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasai M, Yamamoto M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 2019;51:1–10. doi: 10.1038/s12276-019-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr. Opin. Microbiol. 2011;14:414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Zhao XY, Ewald SE. The molecular biology and immune control of chronic Toxoplasma gondii infection. J. Clin. Invest. 2020;130:3370–3380. doi: 10.1172/JCI136226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J. Immunol. 2000;165:1498–1505. doi: 10.4049/jimmunol.165.3.1498. [DOI] [PubMed] [Google Scholar]
- 80.Garcia JL, et al. Partial protection against tissue cysts formation in pigs vaccinated with crude rhoptry proteins of Toxoplasma gondii. Vet. Parasitol. 2005;129:209–217. doi: 10.1016/j.vetpar.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Guerrero Manriquez GG, Tuero I. Adjuvants: friends in vaccine formulations against infectious diseases. Hum. Vaccines Immunother. 2021;17:3539–3550. doi: 10.1080/21645515.2021.1934354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Inclan-Rico JM, Siracusa MC. First responders: innate immunity to helminths. Trends Parasitol. 2018;34:861–880. doi: 10.1016/j.pt.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buxton D. Toxoplasmosis: the first commercial vaccine. Parasitol. Today. 1993;9:335–337. doi: 10.1016/0169-4758(93)90236-9. [DOI] [PubMed] [Google Scholar]
- 84.Sander, V., Angel, S. O. & Clemente, M. In Prospects of Plant-Based Vaccines in Veterinary Medicine (ed. MacDonald, J.), 89–120. (Springer International Publishing, 2018).
- 85.Sacks D. L., Peters N. C., Bethony J. M. In The Vaccine Book, 2nd edn. (eds. Bloom, B. R. & Lambert, P.-H.), Ch. 17, 331–360. (Academic Press, 2016).
- 86.Sander VA, et al. Use of veterinary vaccines for livestock as a strategy to control foodborne parasitic diseases. Front. Cell Infect. Microbiol. 2020;10:288. doi: 10.3389/fcimb.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim SY, Goldie SJ. Cost-effectiveness analyses of vaccination programmes: a focused review of modelling approaches. PharmacoEconomics. 2008;26:191–215. doi: 10.2165/00019053-200826030-00004. [DOI] [PubMed] [Google Scholar]
- 88.Sykes D, Rychtář J. A game-theoretic approach to valuating toxoplasmosis vaccination strategies. Theor. Popul. Biol. 2015;105:33–38. doi: 10.1016/j.tpb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 89.Bonačić Marinović AA, et al. Prospects of toxoplasmosis control by cat vaccination. Epidemics. 2019;30:100380. doi: 10.1016/j.epidem.2019.100380. [DOI] [PubMed] [Google Scholar]
- 90.Innes EA, Hamilton C, Garcia JL, Chryssafidis A, Smith D. A one health approach to vaccines against Toxoplasma gondii. Food Waterborne Parasitol. 2019;15:e00053. doi: 10.1016/j.fawpar.2019.e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Any custom code or mathematical algorithm is not applicable to this article.