Abstract
Inhibitory control improves into young adulthood after specialization of relevant brain systems during adolescence. However, the biological mechanisms supporting this unique transition are not well understood. Given that adolescence is defined by puberty, we examined relative contributions of chronological age and pubertal maturation to inhibitory control development. 105 8–19-year-olds completed 1–5 longitudinal visits (227 visits total) in which pubertal development was assessed via self-reported Tanner stage and inhibitory control was assessed with an in-scanner antisaccade task. As expected, percentage and latency of correct antisaccade responses improved with age and pubertal stage. When controlling for pubertal stage, chronological age was distinctly associated with correct response rate. In contrast, pubertal stage was uniquely associated with antisaccade latency even when controlling for age. Chronological age was associated with fMRI task activation in several regions including the right dorsolateral prefrontal cortex, while puberty was associated with right ventrolateral prefrontal cortex (VLPFC) activation. Furthermore, task-related connectivity between VLPFC and cingulate was associated with both pubertal stage and response latency. These results suggest that while age-related developmental processes may support maturation of brain systems underlying the ability to inhibit a response, puberty may play a larger role in the effectiveness of generating cognitive control responses.
Keywords: Adolescence, Puberty, fMRI, PPI, Inhibitory control, Antisaccade
1. Introduction
Cognitive control continues to improve through adolescence in parallel with the maturation of relevant brain systems, leading to its stability in adulthood (Luna et al., 2015). Specifically, while the ability to exert inhibitory control exists as early as infancy (Johnson, 1995), the rate of correct inhibitory responses significantly increases through adolescence, followed by a shift towards stabilization and optimization in adulthood (Bari and Robbins, 2013, Dempster, 1992, Luna, 2009, Luna et al., 2004, Ordaz et al., 2013). These developmental improvements are not linear, but rather, show rapid growth through childhood followed by a deceleration through adolescence, reaching a plateau in young adulthood (Luna et al., 2004, Ordaz et al., 2013). Similarly, prior work examining age-related changes in brain function during inhibitory control has shown that the dorsolateral prefrontal cortex (DLPFC) is predominantly engaged in childhood, but by adolescence, DLPFC activation decreases to adult levels, with engagement of the anterior cingulate cortex (ACC) mediating improvements in performance (Ordaz et al., 2013) as top-down frontal connectivity becomes established (Hwang et al., 2016, Hwang et al., 2010). Thus, both changes in behavior and brain function show that developmental processes occurring uniquely in adolescence underlie the transition to adult-level effective and stable engagement of widely distributed executive systems supporting cognitive control.
Previous studies have examined cognitive development predominantly as a function of chronological age, limiting the ability to assess biological processes unique to the adolescent period and examine variability in developmental trajectories. Characterizing the mechanistic and neurobiological contributions to these neurocognitive processes is critical to understanding the establishment of adult trajectories, including the emergence of psychiatric disorders (e.g., psychosis, mood disorders, substance use disorders) during adolescence and young adulthood (Chambers et al., 2003, Paus et al., 2008, Pfeifer et al., 2011), many of which are associated with cognitive dysfunction (Castellanos-Ryan et al., 2014, Frost et al., 1989, Toren et al., 2000).
The beginning of adolescence is defined by the onset of puberty, when major changes in hormonal levels trigger developmental processes which underlie the transition to adulthood and are believed to play a role in critical period plasticity, which may be present in association cortex during adolescence (Larsen and Luna, 2018). Extensive research shows strong associations between pubertal hormones and cognitive function, underscoring the potential role of puberty in neurocognitive development (Almey et al., 2015, Bimonte and Denenberg, 1999, Colzato et al., 2010, Colzato and Hommel, 2014, Gibbs, 2005, Holmes et al., 2002, Ladouceur, 2012, Sandstrom et al., 2006, Vijayakumar et al., 2018). However, few studies have focused on puberty’s relationship with inhibitory control in particular. One recent study in a sample of 12–14-year-old healthy adolescents found no association between Tanner stage or testosterone levels and inhibitory control error rates, but did find that more advanced Tanner stage was associated with faster response latencies among females (Ordaz et al., 2018). Another study using drift-diffusion modeling found that the interaction of puberty and sex was significantly related to the amount of information considered to make decisions during an inhibitory control task (Castagna and Crowley, 2021). Less, if any, research exists on the associations between pubertal development and changes in brain activation and task-based connectivity during inhibitory control.
A significant recurring issue in examining the relationship between puberty and neurocognitive development is the statistical challenge of disentangling pubertal effects from chronological age, given their significant collinearity. An important consideration when trying to understand puberty- and age-related developmental effects is what biological processes actually underlie development associated with chronological age, since increasing age may encompass a large number of biological processes (including puberty). Indeed, some of this age-related change can simply be attributed to genetic and biological growth programming that drives fundamental human maturational processes. Another key driver that may interact with age-related processes is accumulated experience, which encompasses every day and stressful life events, the practice of skills, social interactions, and many other occurrences that shape how individuals perceive and process the world (Panchanathan and Frankenhuis, 2016). This accumulated experience is particularly important during adolescence, when key developmental processes such as synaptic pruning and myelination occur (Petanjek et al., 2011, Yakovlev et al., 1967). Both rely on repeated experience to determine which neural circuits need to be strengthened and which are less essential -- in the form of experience-dependent plasticity (Dow-Edwards et al., 2019, Wilbrecht et al., 2010). In particular, the need to differentiate from caregivers and develop more independence may lead to a particular sensitivity for social experience, as indicated by rodent studies showing that social isolation in early adolescence leads to reduced spine density in the frontal cortex and changes in cortical dopamine function (Novick et al., 2011, Silva-Gómez et al., 2003, Wright et al., 2008). While these neural processes may also interact with pubertal development, some may be independent – for example, one recent study found that frontal spine pruning in rodents does not depend on the presence of gonadal hormones prior to puberty (Boivin et al., 2018). Further research, especially in animals, is needed to further identify which molecular mechanisms driving adolescent brain development actually rely on the biological changes of puberty.
With these considerations in mind, it may still be worthwhile to examine whether pubertal effects are substantial enough to be statistically separated – and if not, to be able to interpret the effects of puberty accordingly. Previous studies have separated these effects using statistical methods such as Akaike’s Information Criterion (AIC), (Akaike, 1974) to determine which measure produces the best-fitting model when associated with structural brain development or changes in resting state functional connectivity (Goddings et al., 2014, van Duijvenvoorde et al., 2019). Others have included age as a covariate when modeling the effect of puberty on structural brain development to examine the influence of pubertal maturation independent of age (Herting et al., 2017, Vijayakumar et al., 2021). Importantly, these studies incorporated longitudinal data, which is important for modeling both age- and puberty-related trajectories, and spanned wide age ranges incorporating the full span of adolescence (beginning at 7–8 years old and ending at 18–20 years old), so that all pubertal stages could be captured in a substantial number of individuals. Thus, in this study, we examined behavioral and fMRI data during an inhibitory control task using an accelerated longitudinal cohort of 8–19-year-olds, applying similar statistical methods (AIC, effect size, age covariate) to address three aims: 1) to characterize the unique contributions of chronological age and puberty to the development of inhibitory control, 2) to investigate the contributions of chronological age and puberty to the development of brain activity during inhibitory control, and 3) to explore the influence of chronological age and puberty on task-related connectivity during inhibitory control. Based on prior behavioral and resting-state connectivity studies in adolescence (Ordaz et al., 2013), we hypothesized that puberty may contribute to changes in both correct response rate and response latency and thus, may also contribute to developmental change in activity across inhibitory control brain regions broadly. Since task-related connectivity during inhibitory has not been previously investigated with puberty, we took an exploratory approach using a whole-brain voxelwise analysis. However, we hypothesized based on existing resting-state connectivity research (van Duijvenvoorde et al., 2019) that puberty may more specifically relate to the development of cortico-subcortical connectivity, while age may be more strongly associated with cortico-cortical and subcortio-subcortical connections.
2. Methods
2.1. Participants
Data were acquired as part of a large, accelerated longitudinal study, in which neuroimaging and behavioral data were collected on 160 participants (8–30 years old) across 571 visits (1–5 visits per participant). Participants were recruited from the community and were screened for psychiatric and neurological problems in themselves and their first-degree relatives, as well as MRI contraindications such as metal in the body. For all subjects under the age of 18, parental consent and assent from the participant were obtained before beginning data collection. For those over the age of 18, consent was obtained from the participant. Over the course of the study, 24 individuals (14 females) did not complete follow-up visits due to obtaining braces, issues with scheduling or contacting, loss of interest, and change of residence. All experimental procedures were approved by the University of Pittsburgh Institutional Review Board and complied with the Code of Ethics of the World Medical Association (World Medical Association, 2013).
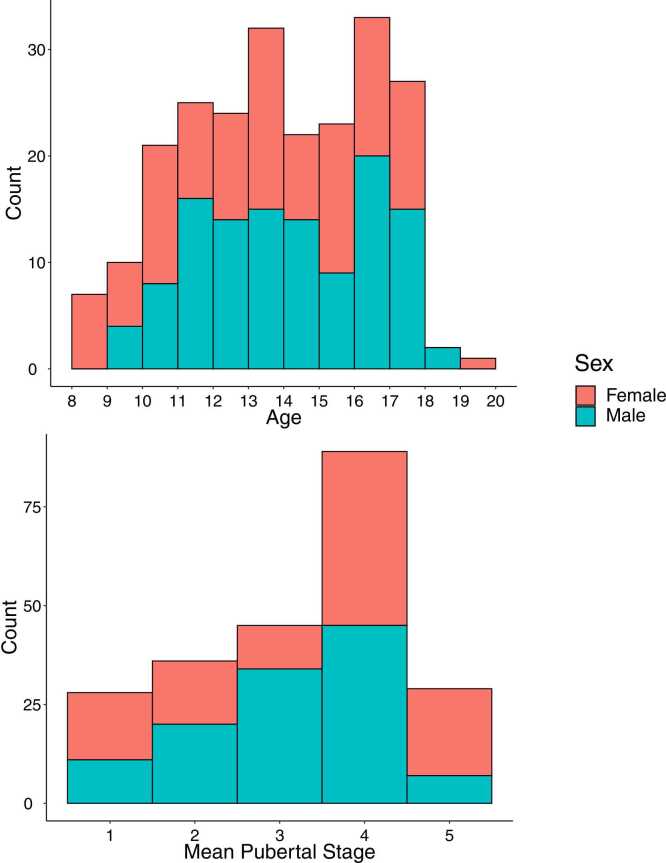
Because this study examines the effects of pubertal development, participants without pubertal data were excluded from all analyses. In order to measure only ongoing pubertal development, we also excluded participants who were already in Stage 5 (completed puberty) at their first study visit. The final sample used in all analyses included behavioral data for 227 study visits (1–5 repeated visits) from 105 participants (53 female, 8.0–19.3 years old, Fig. 1) and fMRI data for 205 study visits from 98 participants (49 female) with additional exclusions due to participant sleepiness, excessive head motion, number of usable functional runs, lack of usable structural images, poor eye tracking quality, scanner inhomogeneities, and issues with data processing and recovery. We note that the subjects excluded due to data quality or other issues did not differ significantly in age from the subjects included in the analyses.
Fig. 1.
Distribution of included participants across age (top) and pubertal stage (bottom), colored by sex.
2.2. Pubertal assessments
Pubertal stage was assessed based on two self-report questionnaires. The first questionnaire was a pictorial Tanner staging questionnaire, in which line drawings of breast development, pubic hair growth, testes/scrotum/penis development, and testicular size were provided for Tanner Stages 1–5 (Morris and Udry, 1980). Participants were asked to select the drawing that best corresponded with their own development at that time, which generated an overall Tanner score for the individual. The second questionnaire was the Pubertal Development Scale (Petersen et al., 1988), in which participants were asked questions about the development of various primary and secondary sexual characteristics, and chose from several options along the lines of “has not yet started growing”, “has barely started growing”, “is definitely underway”, and “seems completed”). The scoring of this questionnaire provides a pubertal stage on a 4-point scale, which was converted to a 5-point scale analogous to Tanner Stage using a previously-created evidence-based coding mechanism (Shirtcliff et al., 2009). For this study, the 5-point PDS score and the Tanner score were then averaged to create a Mean Pubertal Stage measure that was used to represent overall pubertal development in all analyses. We chose to combine these measures due to their high correlation (r = 0.867) and wide use across the literature, indicating no data- or hypothesis-driven reason to choose one over the other. Furthermore, these measures have been combined in previous work (Barendse et al., 2022, Ellis et al., 2011, Ladouceur et al., 2019) and a prior study showed excellent agreement between these measures within one stage (Bond et al., 2006).
2.3. Task design
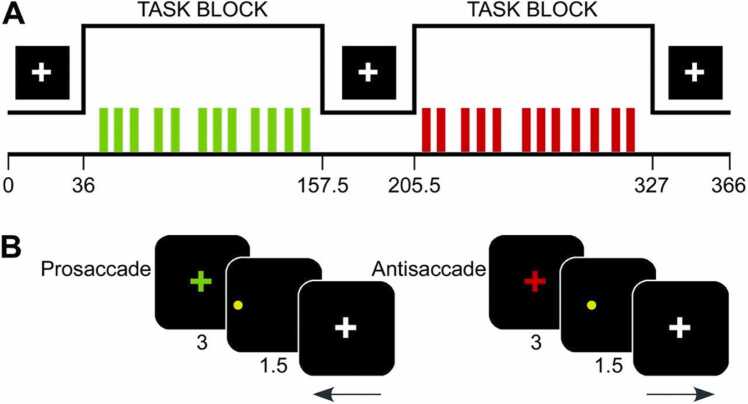
During fMRI scanning, participants completed four runs of an antisaccade (AS) task to assess inhibitory control (Hwang et al., 2010, Ordaz et al., 2013, Velanova et al., 2009, Velanova et al., 2008). Full details of the experimental paradigm (Fig. 2) are described by Velanova et al. (2008). Each run consisted of a block of the AS task and a block of the visually guided saccade (VGS) task (118.5 s each), preceded and separated by three blocked periods of fixation (36, 48, and 39 s each). Task order was counterbalanced across runs and participants. Twelve AS or VGS trials were presented in each task block, for a total of 48 of each trial type. Intertrial intervals (3–9 s) were pseudo-randomly distributed to permit estimation of trial-related activation (Dale, 1999). Each trial began as participants fixated on a colored cross-hair for 3 s instructing them to make a VGS (green) or an AS (red). Next, the saccade target stimulus, a yellow circle, appeared at one of six horizontal eccentricities ( ± 3°, 6°, or 9°) for 1.5 s. For AS trials, participants were instructed to inhibit the reflexive saccade toward the target and to look instead to its horizontal mirror location. Target location order was randomized within each task block and no “gap” was interposed between the instruction cue and saccade target stimulus.
Fig. 2.
Diagram of experimental task paradigm, depicting structure of experimental run (A) and trial type (B). Originally from Velanova et al. (2008) and Ordaz et al. (2013).
2.4. Eye tracking data acquisition and scoring
Eye movement measurements were obtained during fMRI scanning using a long-range optics eye-tracking system (Model R-LRO6, Applied Science Laboratories) with a sampling rate of 60 Hz. Nine-point calibrations were performed at the beginning of the session and between runs as necessary. Correct AS responses were defined as those in which the first eye movement during the saccade epoch with velocity greater than or equal to 30°/sec was made toward the mirror location of the peripheral cue and extended beyond a 2.5°/ visual angle from central fixation. Trials in which no eye movements were generated, gaze tracking was lost, blinks occurred before first onset, and express saccades (saccade starting within the first 4 samples (60 Hz) after trial onset) occurred were excluded from analyses. This scoring system was automated using custom software which has been made publicly available on GitHub (https://github.com/LabNeuroCogDevel/autoeyescore). The two variables of interest used in subsequent analyses were the AS correct response rate (number of correct response trials / total number of usable trials) and mean response latency across correct trials.
2.5. MR data acquisition
Data were acquired using a Siemens 3-Tesla MAGNETOM Allegra fitted with a standard circularity-polarized head coil. Head movement was minimized through use of pillows during scanning to immobilize the head and prior acclimation in an MR simulator. A PC (Dell Dimension 8200, Pentium 4, 2 GHz, Windows XP) running E-Prime (Psychology Software Tools) was used for displaying stimuli. Structural images were acquired using a sagittal magnetization-prepared rapid gradient-echo T1-weighted sequence (TR = 1570 ms; echo time [TE] = 3.04 ms; flip angle = 8°; inversion time [TI] = 800 ms; voxel size = 0.78125 × 0.78125 × 1 mm) and used for functional image alignment. Participants performed four functional runs (6 min 15 s each), during which functional images were acquired using an echoplanar sequence sensitive to blood oxygen level-dependent contrast [T2 * ] with 29 contiguous 4-mm-thick axial images acquired parallel to the anterior–posterior commissure plane (TR = 1500 ms; TE = 25 ms; flip angle = 70°; voxel size = 3.125 × 3.125 mm in-plane resolution).
2.6. fMRI preprocessing and first-level analysis
Structural MRI data were preprocessed to extract the brain from the skull, and warped to the MNI standard brain using both linear (FLIRT) and non-linear (FNIRT) transformations. Preprocessing of task fMRI data followed our in-house standard protocol that incorporates tools from AFNI, NiPy, FSL and BrainWavelet (Cox, 1996, Gorgolewski et al., 2011, Jenkinson et al., 2012, Paulsen et al., 2015). The preprocessing pipeline included 4D slice-timing and head motion correction, skull stripping, intensity thresholding, wavelet despiking (Patel and Bullmore, 2016), co-registration to the structural image and nonlinear warping to MNI space, spatial smoothing using a 5-mm full width at half maximum Gaussian kernel, and intensity normalization. Source code for this pipeline is available online (https://github.com/LabNeuroCogDevel/fmri_processing_scripts).
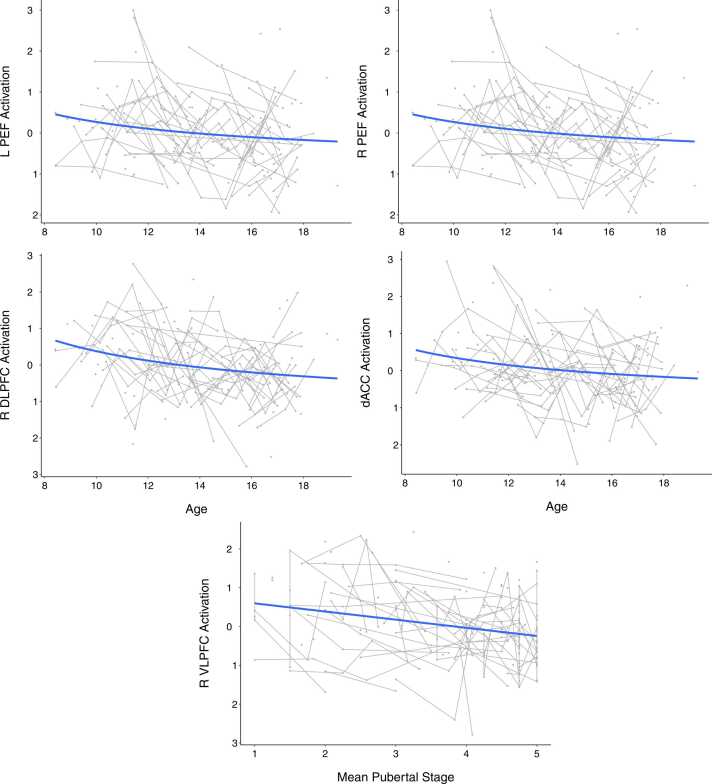
First level analysis for task-related activation was performed by modeling all trial events in AFNI’s 3dDeconvolve (Cox, 1996). Events were defined by condition (visually-guided saccade or antisaccade) and participant’s performance on that trial (correct, corrected error, uncorrected error, or dropped) and modeled as a 4.5-second block. At this stage, nuisance regression was applied for the six head motion parameters, cerebrospinal fluid signal, and white matter signal. Volumes were censored if they contained framewise displacement (FD) estimates > 0.9 mm and subjects with greater than 15% of volumes censored were excluded from group-level analyses, resulting in the exclusion of 5 participants. In the participant sample used for group-level analyses, the mean number of volumes censored was 22 and median was 8 (range: 0–144). To probe task-related brain activity, BOLD activation during correct antisaccade trials was then extracted from 13 inhibitory control regions-of-interest (ROIs), originally derived from Neurosynth (see Ordaz et al., 2013 for additional details of inhibitory control ROI selection), based on prior work showing that these regions are reliably engaged during inhibitory control. These regions encompass both those associated with the executive function component of inhibitory control (bilateral DLPFC, bilateral ventrolateral prefrontal cortex, dorsal ACC), and those associated with the motor functions required for successful inhibitory control (supplementary eye field, pre-supplementary motor area, bilateral frontal eye field, bilateral parietal eye fields, and bilateral putamen; see Ordaz et al., 2013, Fig. 5 for illustration of included ROIs).
Fig. 5.
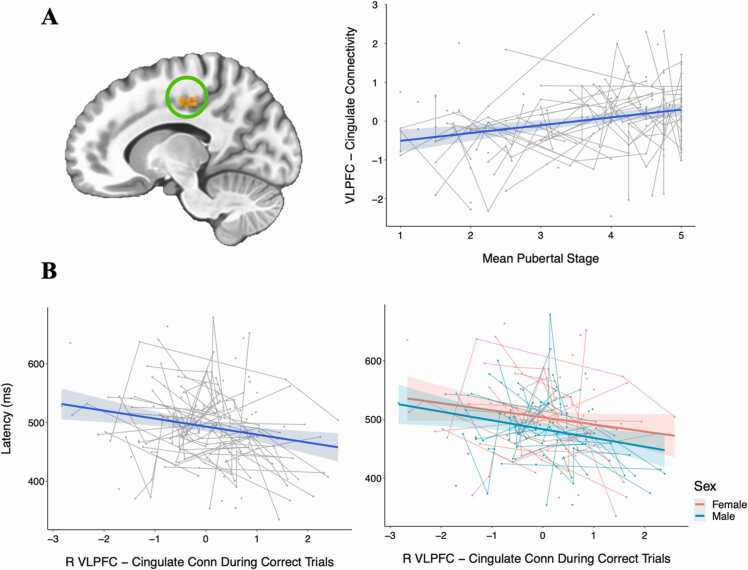
Plots of inhibitory control regions-of-interest (ROIs) in which significant age or puberty effects were observed. Both age and puberty were tested in each ROI, but the measure producing the best fitting model is visualized. Age was the most robust predictor of change in the bilateral parietal eye fields (PEF), right dorsolateral prefrontal cortex (DLPFC) and dorsal anterior cingulate cortex (dACC), while puberty was most associated with the right ventrolateral prefrontal cortex (VLPFC). Individual data points reflect values for each session, and connected lines reflect sessions from the same participant.
2.7. PPI analysis
First level analysis for psychophysiological interactions (PPI) was performed by first generating a GAM-based hemodynamic response function. The BOLD time series for each seed region was then extracted, detrended, transposed, and deconvolved with the hemodynamic response function. An interaction regressor was then created by combining the deconvolved time series with a 1D timing file defining when task conditions occurred throughout the runs. These files were concatenated across runs, and task events were modeled in 3dDeconvolve, with the addition of the seed time series regressor and the interaction regressor.
Seeds for PPI connectivity analyses were selected from among those ROIs in which task-related activation was changing significantly with age or puberty. Only ROIs associated with executive function rather than those previously associated with motor control were considered for inclusion as PPI seeds. To ensure focused and hypothesis-driven analyses, we selected the seed with the greatest effect size (as measured by the conditional R-squared value) for each of age and puberty, among those for which age and puberty respectively were the best fitting model based on AIC/R2. Based on these criteria, the right DLPFC and ventrolateral prefrontal cortex (VLPFC) were selected, and these ROIs were defined based on the original set of ROIs derived from Ordaz et al. (2013).
2.8. Statistical analysis
Behavioral data was analyzed with linear mixed-effects models using the lme4 and lmerTest packages in R (Bates et al., 2014; R Core Team, 2020). Linear-mixed effects models were used to examine the fixed effects of age, puberty, and sex on error rate and latency. Subject was included in the model as a random effect. Outliers in correct response rate and response latency greater than 3 standard deviations from the mean were excluded from respective analyses. Within this model, puberty, linear age, and inverse age were examined and the best fitting model for correct response rate and response latency was determined using both AIC and the conditional R-squared value (variance explained by both fixed and random effects combined). The inverse age term was included here because it has been shown in past studies to best represent the developmental trajectory of cognitive functions across adolescence – rapid and large improvement in performance followed by a plateau in late adolescence or early adulthood (Luna et al., 2004). We also conducted these basic behavioral analyses for response latency variability in a post hoc analysis to explore the potential role of plasticity in these results. The main effects of age and puberty controlling for sex were also tested with Generalized Additive Mixed Models (GAMMs) and AIC values compared to confirm that age or puberty was the best-fitting model even when allowing for non-linearity.
fMRI task activation values extracted from the inhibitory control ROIs were similarly analyzed using linear mixed-effects models to examine puberty, linear age, and inverse age in order to determine which was the best fitting model for each ROI. In this sample, the number of volumes censored for each participant was associated with age (p = 1.84 ×10−10) and puberty (1.45 ×10−10), but not sex. Thus, we ran fMRI analyses with and without number of volumes censored as a covariate, and found that our results did not change, aside from minor variation in effect sizes and p-values. Since the AIC values of these models indicated that the model fit was worse when including number of censored volumes, we present statistics without this covariate.
Statistical analysis for the PPI analyses leveraged AFNI’s 3dLME to test for voxelwise effects of puberty and age separately, controlling for sex, based on whole brain connectivity maps generated for the selected PPI seed regions during correct antisaccade trials. We did not test models including both age and puberty due to known limitations of power in PPI analyses, which likely renders these analyses unable to dissociate age and puberty due to their high collinearity. This analysis was masked to only consider voxels with a 50% or greater probability of being gray matter in the MNI-152 template (Collins et al., 1999, Fonov et al., 2011, Fonov et al., 2009). Based on separate models of age and puberty, significant clusters with main effects of puberty and age were identified and mean parameter estimates were subsequently extracted for these clusters using 3dROIStats, followed by post hoc testing to determine the direction of developmental effects and test for associations with antisaccade performance measures. We tested connectivity values derived from the main effects of puberty analyses for associations with response latency, since this performance measure was uniquely associated with puberty, and similarly, we tested connectivity values from the main effects of age analyses for associations with age. These models were tested alone, controlling for puberty/age or sex, and controlling for both puberty/age and sex. Results were corrected for multiple comparisons using a combination of cluster size and voxel probability, with parameters determined through a Monte Carlo simulation using AFNI’s 3dFWHMx and 3dClustSim program on randomly generated data within the gray matter mask with the same smoothness as the group mean smoothness estimated from first-level residuals for each region. The autocorrelation function (ACF) option was used when running these scripts, which then specified the cluster size threshold applied with a single voxel threshold of p < 0.02 (cluster size: 70 voxels) that was required to achieve a cluster-wise corrected p < 0.05, based on current recommendations to prevent obtaining false positive clusters of connectivity (Chen et al., 2015).
3. Results
3.1. Development of antisaccade performance
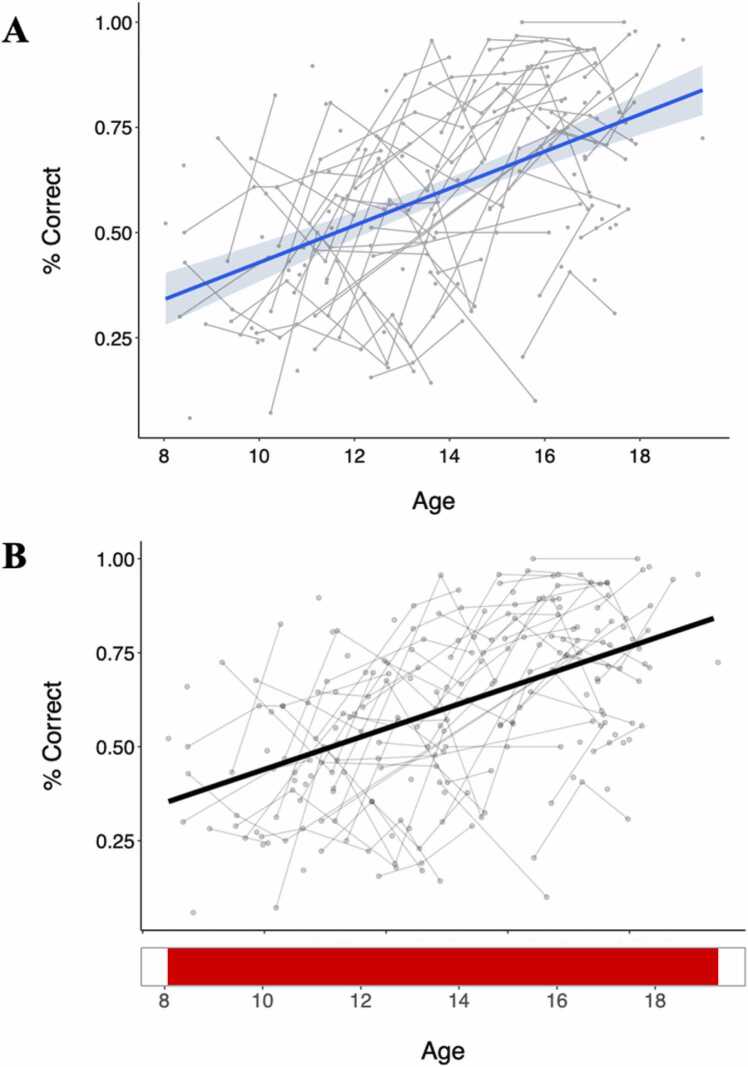
Linear mixed models were used to examine the main and interaction effects of puberty, linear age, and inverse age, controlling for the effect of sex. We found significant effects of puberty (β = 0.38, pFDR < 0.001), linear age (β = 0.48, pFDR < 0.001), and inverse age (β = −0.46, pFDR < 0.001) on antisaccade correct response rate, with AIC and R-squared values indicating that linear age produces the best fitting model in this sample (AIClinearage = 528.42, AICinverseage = 534.48, AICpuberty = 551.78, Fig. 3A, Table 1). When examining models that included additive or interactive effects of age and puberty together, only age was significant across these models (p < 0.001) while puberty was not, indicating that the effects of increasing age drive the change in correct response rate seen across this sample. We confirmed these results with GAMM analyses, which indicated a linear fit as the best model for the effect of age (p < 0.001, Fig. 3B), and the AIC values of the age and puberty GAMMs also indicate that age controlling for sex produced the best fitting model (AICage = −235.59, AICpuberty = −208.16).
Fig. 3.
Plots of correct response rate data across age (years) using both a linear mixed model (A) and generalized additive mixed model (B). Both show a significant positive association, such that increasing age is associated with improvements in correct response rate. Bottom panel in (B) depicts intervals of significant age-related change. Individual datapoints reflect values for each session, and connected lines reflect sessions from the same participant.
Table 1.
Regression statistics for linear mixed models assessing the main, additive, and interactive effects of puberty and age (linear and inverse forms) on correct response rate. All models are controlling for sex and statistics are FDR-corrected.
| % Correct∼Puberty | % Correct∼Linear Age | % Correct∼Inverse Age | % Correct∼Puberty+Age | % Correct∼Puberty*Age | % Correct∼Puberty+Inverse Age | % Correct∼Puberty*Inverse Age | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df |
| Intercept | -0.14 | 0.216 | 222 | -0.08 | 0.658 | 222 | -0.06 | 0.725 | 222 | -0.07 | 0.723 | 221 | -0.11 | 0.811 | 220 | -0.05 | 0.792 | 221 | -0.22 | 0.149 | 220 |
| Puberty | 0.38 *** | < 0.001 | 222 | -0.06 | 0.723 | 221 | -0.03 | 0.811 | 220 | -0.03 | 0.792 | 221 | 0 | 0.976 | 220 | ||||||
| Sex | 0.25 | 0.17 | 222 | 0.07 | 0.658 | 222 | 0.06 | 0.725 | 222 | 0.05 | 0.771 | 221 | 0.07 | 0.811 | 220 | 0.04 | 0.792 | 221 | 0.09 | 0.75 | 220 |
| Age | 0.48 *** | < 0.001 | 222 | 0.54 *** | < 0.001 | 221 | 0.52 *** | < 0.001 | 220 | ||||||||||||
| Inverse Age | -0.46 *** | < 0.001 | 222 | -0.48 *** | < 0.001 | 221 | -0.57 *** | < 0.001 | 220 | ||||||||||||
| Puberty:Age | 0.03 | 0.811 | 220 | ||||||||||||||||||
| Puberty: Inverse Age |
-0.15 * | 0.016 | 220 | ||||||||||||||||||
| Marginal R2 / Conditional R2 | 0.160 / 0.637 | 0.244 / 0.690 | 0.219 / 0.676 | 0.245 / 0.692 | 0.245 / 0.691 | 0.218 / 0.676 | 0.248 / 0.691 | ||||||||||||||
| AIC | 551.777 | 528.421 | 534.484 | 532.744 | 538.073 | 538.969 | 537.396 | ||||||||||||||
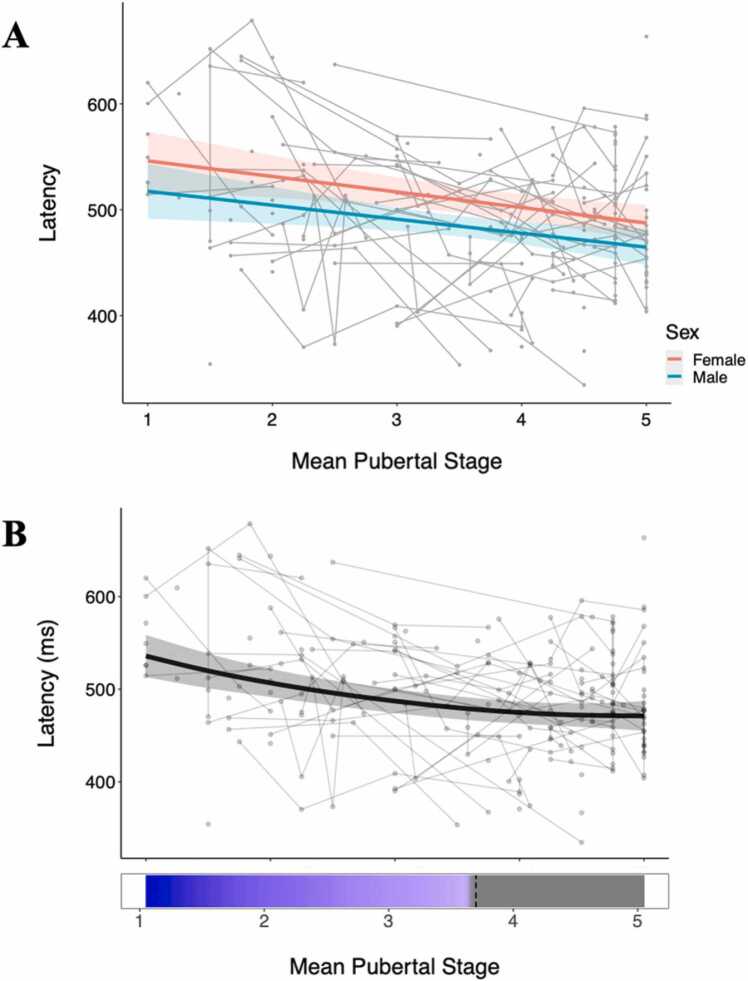
When testing the effects on response latency, we again found significant effects of puberty (β = −0.34, pFDR < 0.001), linear age (β = −0.27, pFDR < 0.001), and inverse age (β = 0.29, pFDR < 0.001), but in this case, AIC and R-squared values indicated that puberty produced the best fitting model (AIClinearage = 603.64, AICinverseage = 600.95, AICpuberty = 594.33, Fig. 4A, Table 2). Furthermore, the main effect of sex was also significant in the puberty model (β = −0.43, puncorrected =0.010, pFDR = 0.030). For response latency, puberty and sex were both significant in additive models of puberty and age (puberty controlling for the effect of age), while age was nonsignificant, indicating that unlike correct response rate, the developmental change in latency appears to be driven to a greater extent by pubertal maturation. We again confirmed these results with GAMM analyses, which indicated that pubertal development provided the best model fit (p < 0.001, Fig. 4B), and the AIC values of the age and puberty GAMMs also indicate that puberty produced the best fitting model (AICage = 2390.12, AICpuberty = 2372.64). Notably, this GAMM model also indicated that the significant change in response latency occurred in pubertal stages 1–3.
Fig. 4.
Plots of response latency data across mean pubertal stage using both a linear mixed model (A) and generalized additive mixed model (B). Both show a significant negative association, such that increasing pubertal stage is associated with decreasing response latency. Bottom panel (B) depicts intervals of significant age-related change. Individual datapoints reflect values for each session, and connected lines reflect sessions from the same participant. In (A), red and blue lines reflect females and males, respectively.
Table 2.
Regression statistics for linear mixed models assessing the main, additive, and interactive effects of puberty and age (linear and inverse forms) on response latency. All models are controlling for sex and statistics are FDR-corrected.
| Latency∼Puberty | Latency∼Linear Age | Latency∼Inverse Age | Latency∼Puberty+Age | Latency∼Puberty*Age | Latency∼Puberty+Inverse Age | Latency∼Puberty*Inverse Age | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df | Estimates | p | df |
| Intercept | 0.23 * | 0.049 | 219 | 0.19 | 0.112 | 219 | 0.17 | 0.144 | 219 | 0.24 | 0.058 | 218 | 0.13 | 0.467 | 217 | 0.23 | 0.071 | 218 | 0.14 | 0.399 | 217 |
| Puberty | -0.34 *** | < 0.001 | 219 | -0.40 ** | 0.007 | 218 | -0.3 | 0.111 | 217 | -0.35 * | 0.029 | 218 | -0.33 | 0.053 | 217 | ||||||
| Sex | -0.43 * | 0.015 | 219 | -0.32 | 0.085 | 219 | -0.29 | 0.111 | 219 | -0.45 * | 0.018 | 218 | -0.4 | 0.111 | 217 | -0.43 * | 0.029 | 218 | -0.41 | 0.053 | 217 |
| Age | -0.27 *** | < 0.001 | 219 | 0.07 | 0.6 | 218 | 0.01 | 0.925 | 217 | ||||||||||||
| Inverse Age | 0.29 *** | < 0.001 | 219 | -0.01 | 0.951 | 218 | -0.06 | 0.688 | 217 | ||||||||||||
| Puberty:Age | 0.09 | 0.418 | 217 | ||||||||||||||||||
| Puberty: Inverse Age |
-0.09 | 0.312 | 217 | ||||||||||||||||||
| Marginal R2 / Conditional R2 | 0.142 / 0.534 | 0.107 / 0.478 | 0.117 / 0.484 | 0.142 / 0.539 | 0.144 / 0.533 | 0.141 / 0.535 | 0.145 / 0.537 | ||||||||||||||
| AIC | 594.33 | 603.64 | 600.953 | 598.345 | 602.225 | 598.528 | 602.332 | ||||||||||||||
* p < 0.05. ** p < 0.01. *** p < 0.001.
We also conducted an exploratory post hoc analysis of performance variability using response latency standard deviation to understand whether the associations between puberty and response latency might relate to a role of puberty in increased adolescent plasticity. As with the other performance measures, we found significant effects of puberty (β = −0.45, pFDR < 0.001), linear age (β = −0.51, pFDR < 0.001), and inverse age (β = 0.54, pFDR < 0.001), with AIC and R-squared values indicating that inverse age produced the best-fitting model (AIClinearage = 569.14, AICinverseage = 560.30, AICpuberty = 583.67, Supplemental Table 12). Additionally, linear and inverse age remained significant throughout additive and interaction models of puberty and age.
3.2. Task activation
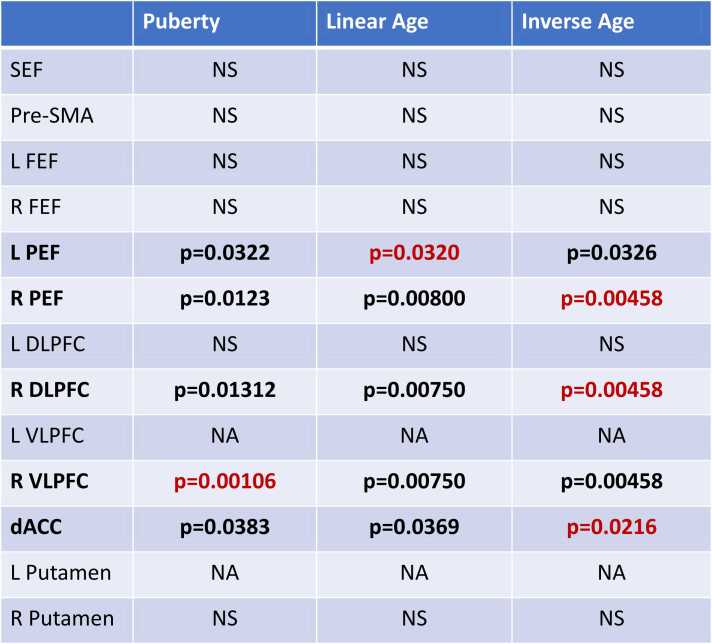
Activation was measured within a priori regions of interest during performance of inhibitory control tasks (Ordaz et al., 2013). During correct trials, activation in the bilateral parietal eye fields, right DLPFC, right VLPFC, and dorsal ACC was significantly associated with both age and puberty (pFDR<0.05, Table 3, Fig. 5) when tested separately. The inverse and/or linear age models were a better fit than puberty for all of these ROIs based on AIC and R2 values, except for the right VLPFC, which was better fit by the puberty model (AIClinearage = 569.67, AICinverseage = 569.55, AICpuberty = 564.68). There was a significant main effect of sex in models including puberty for the right frontal eye field, however, this effect did not survive correction for multiple comparisons. Activation in all ROIs that showed developmental effects was also tested for associations with correct response rate and latency, but all were nonsignificant at a threshold of p < 0.05.
Table 3.
Summary table of significant puberty, linear age, and inverse age associations with task activation extracted from 13 inhibitory control ROIs. All p-values are FDR-corrected for multiple comparisons. Values in red are for the best-fitting model as per AIC and R2. NAs indicate that the linear mixed model did not converge, so p-values could not be obtained.
L=left; R=right; SEF=supplementary eye field; pre-SMA=pre-supplementary motor area; FEF=frontal eye field; PEF=posterior eye fields; DLPFC=dorsolateral prefrontal cortex; VLPFC=ventrolateral prefrontal cortex; dACC=dorsal anterior cingulate cortex.
3.3. Task-related connectivity
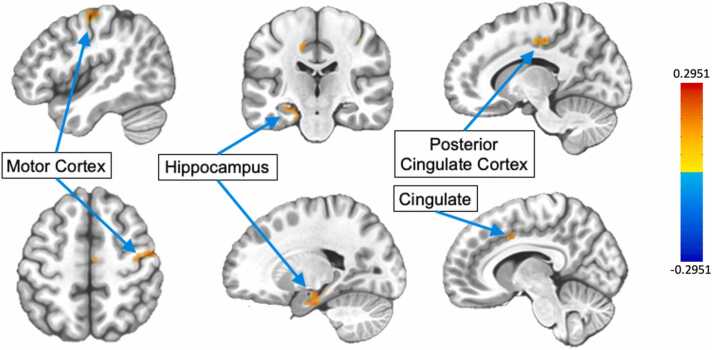
Because the association between pubertal development and task-related connectivity during inhibitory control has not been examined in the literature thus far, we conducted an exploratory PPI analysis based on the results of the behavioral and task activation analyses. Seed regions for the PPI analyses were selected from the developmentally- and task-relevant ROIs as described above. The overall task-related connectivity revealed, first, a widespread network of regions to which the right VLPFC was connected during correct antisaccade trials, including large areas across the frontal and parietal cortices, as well as regions of the striatum (Fig. 6). Because we had identified puberty as the more robust predictor of task activation in the right VLPFC (as compared to age), we then tested for puberty-related developmental change across voxelwise task-related connectivity patterns seeded on the right VLPFC. This analysis was performed across the whole brain with no masking and identified significant clusters (p < 0.05 cluster-corrected) within the dACC, motor cortex, parahippocampal gyrus, and cingulate as areas for which task-related connectivity with the right VLPFC changed with pubertal development (Fig. 7, Table 4). For each of these clusters, we tested for associations between mean connectivity strength across the cluster and response latency, since this was the behavioral measure distinctly associated with pubertal development. We tested these associations alone, including puberty or sex in the model (controlling for puberty or controlling for sex), and including both puberty and sex in the model (controlling for puberty and sex). Notably, connectivity with the cingulate was significantly associated with latency (pFDR = 0.0396), and remained significant when controlling for sex or puberty individually (Fig. 8). When both puberty and connectivity are included in this model, puberty (p = 0.00932) and connectivity (p = 0.0264) are both significant predictors of response latency. While connectivity was no longer a significant predictor of latency when both sex and puberty were included in the model, it should be noted that the effect size of puberty alone as a predictor of latency (β = −0.34) decreased when connectivity was added to the model (β = −0.15), suggesting that the development of this VLPFC-cingulate connectivity may contribute to the effect of puberty on response latency development.
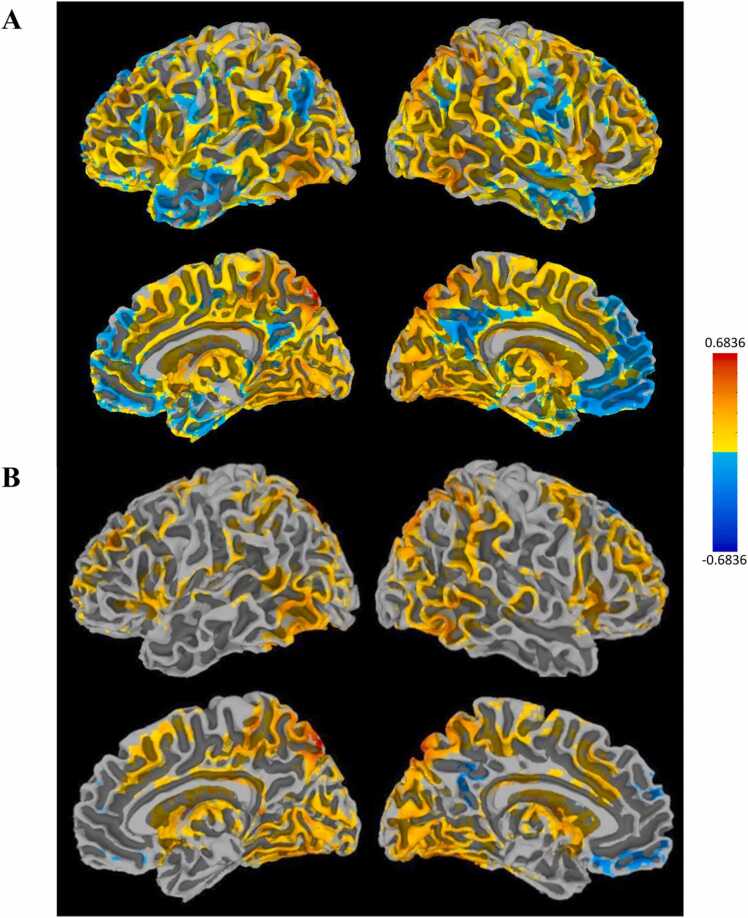
Fig. 6.
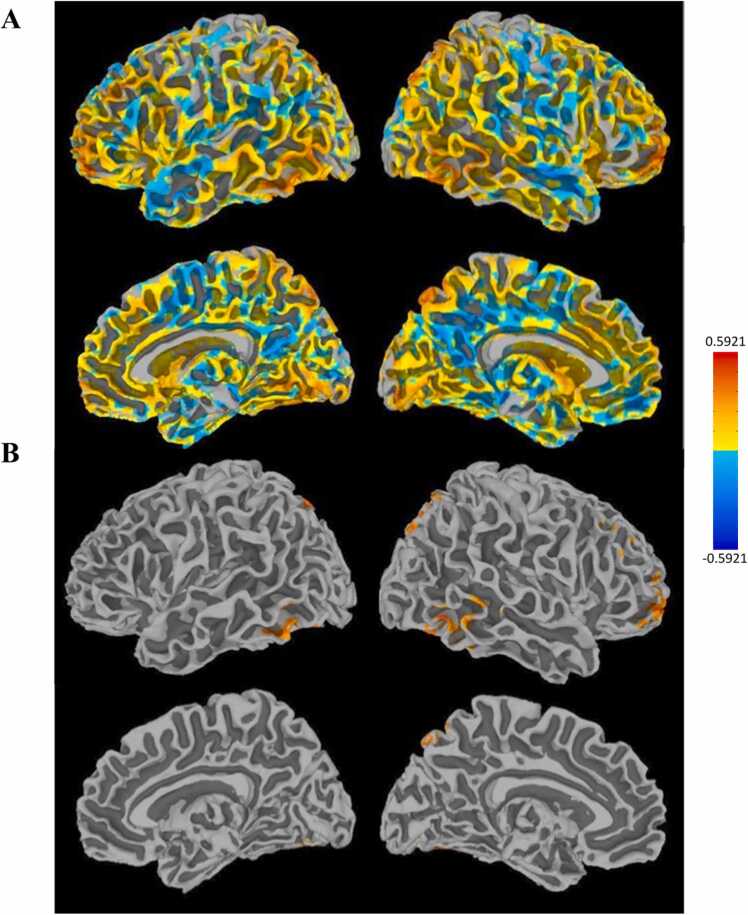
Maps showing R VLPFC PPI connectivity with the rest of the brain during correct trials. The top panel (A) shows unthresholded maps and the bottom (B) shows a cluster-corrected threshold of p < 0.05.
Fig. 7.
Maps showing clusters in R VLPFC PPI connectivity analysis that were significantly associated with pubertal stage when controlling for sex (p < 0.05 cluster-corrected).
Table 4.
Clusters for which right ventrolateral prefrontal cortex (VLPFC) connectivity is associated with pubertal stage at a threshold of p < 0.05 cluster-corrected. R = right; L = left.
| ROI # | Region | X | Y | Z | Cluster Size | Pos/Neg Effect |
|---|---|---|---|---|---|---|
| 1 | R Posterior Cingulate | -5.8 | -8.4 | + 39.8 | 95 vox | Positive |
| 2 | Motor Cortex | -49.4 | + 7.7 | + 53.6 | 94 vox | Positive |
| 3 | L Parahippocampal Gyrus | + 17.2 | + 16.9 | -17.6 | 91 vox | Positive |
| 4 | L Cingulate Motor Zone | + 12.7 | + 23.8 | + 44.4 | 73 vox | Positive |
Fig. 8.
PPI analyses revealed several clusters within the brain in which task-related connectivity with the right VLPFC was significantly associated with pubertal stage, including the cingulate cluster visualized above (p < 0.05 cluster-corrected). Connectivity beta values were z-scored before plotting their association with mean pubertal stage (A). Within this cluster, connectivity was significantly associated with response latency, such that greater VLPFC connectivity was associated with faster response latencies (β = −0.19, pFDR = 0.04). There was also a significant effect of sex such that females had slightly slower response latencies across this sample’s age range (β = - 0.31, p = 0.03).
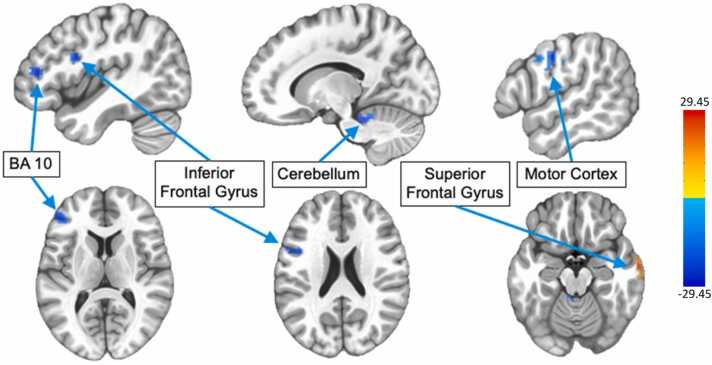
PPI analyses also revealed regions connected to the right DLPFC during correct trials, including areas of the prefrontal cortex, parietal cortex, and smaller portions of the striatum (Fig. 9). Similar to the right VLPFC PPI analysis given above, we tested for connectivity changes with age since task activation in the right DLPFC was mostly strongly associated with chronological age, which revealed clusters in the cerebellum, motor cortex, superior temporal gyrus (STG) and inferior frontal gyrus (IFG) (Fig. 10, Table 5). Among these regions whose DLPFC connectivity was changing with age, we examined associations with correct response rate, to determine if this connectivity might be a factor in the strong age-related component of this behavioral development. However, we did not find any significant associations between connectivity strength and antisaccade accuracy rates. We also did not observe any voxelwise sex differences in the connectivity of either the right VLPFC or right DLPFC.
Fig. 9.
Maps showing R DLPFC PPI connectivity with the rest of the brain during correct trials. The top panel (A) shows unthresholded maps and the bottom (B) shows a cluster-corrected threshold of p < 0.05 (bottom).
Fig. 10.
Maps showing clusters in R DLPFC PPI connectivity analysis that were significantly associated with chronological age when controlling for sex at a cluster-corrected threshold of p < 0.05.
Table 5.
Clusters for which right dorsolateral prefrontal cortex (DLPFC) connectivity is associated with age at a threshold of p < 0.05 cluster-corrected. R = right; L = left; IFG=inferior frontal gyrus; STG=superior temporal gyrus; BA 10 =Brodmann Area.
| ROI # | Region | X | Y | Z | Cluster Size | Pos/Neg Effect |
|---|---|---|---|---|---|---|
| 1 | L Cerebellum | + 19.5 | + 39.9 | -26.8 | 182 vox | Negative |
| 2 | L IFG | + 37.9 | -6.1 | + 26.1 | 112 vox | Negative |
| 3 | Motor Cortex | -56.4 | + 5.4 | + 39.8 | 94 vox | Negative |
| 4 | R STG | -63.2 | + 7.7 | -19.9 | 87 vox | Positive |
| 5 | L BA 10 | + 42.5 | -38.3 | + 12.1 | 71 vox | Negative |
4. Discussion
In this study, we examined unique associations between pubertal development and chronological age and inhibitory control, as well as associated neurobiological measures. We found that while development associated with chronological age drives improvements in correct response rate across adolescence, pubertal development was a more robust predictor of developmental improvement in response latency across this period. Furthermore, activity in the right VLPFC during correct inhibitory trials decreased with pubertal stage, and task-related connectivity of the right VLPFC to a region of the cingulate defined based on its association with pubertal stage was associated with developmental improvements in response latency. In contrast, the right DLPFC during correct inhibitory trials decreased with increasing age, with no particular task-related connectivity contributing to age-related change in inhibitory control performance. Notably, while prior studies have shown that antisaccade correct response rate improves rapidly in adolescence followed by a plateau in adulthood, in line with an inverse function (Ordaz et al., 2013), this study found that linear age was a better predictor of correct response rate than inverse age. This was likely due to the lack of participants above 20 years old, thus, this sample did not capture enough of the age range in which performance plateaus for the inverse age function to fit this data.
Importantly, our findings suggest that the process of pubertal development appears to have a unique influence on improvements in response latency. Thus, puberty may support the optimizing of performance, while other age-related developmental processes may more specifically relate to the refinement of inhibitory control and cognitive abilities in general. In this context, performance optimization refers to improvements in the ability to generate correct task responses quickly, which may reflect more effective exertion of cognitive control. Of note, the aforementioned study showing that dendritic spine pruning did not rely on gonadal hormones also found that if pre-pubertal hormones were present prior to puberty, this had an impact on the morphology of dendritic spines, suggesting a similar optimization role for puberty in dendritic spine maturation (Boivin et al., 2018). This suggests that while puberty may not be critical in establishing neural and cognitive developmental processes, it may play a significant role in adolescent brain plasticity needed for the refinement of these systems to establish adult trajectories of neurocognitive function.
One possible mechanism for the influence of puberty on these developmental decreases in response latency is the hypothesized contribution of pubertal onset to the beginning of critical period plasticity in adolescence (Larsen and Luna, 2018). This increased brain plasticity provides a clear mechanism through which pubertal processes help the brain to specialize and optimize its cognitive performance. Furthermore, animal studies and some correlational human studies have found significant effects of pubertal hormones on brain plasticity, though the nature of this role is still unclear (Laube et al., 2020). Though plasticity is difficult to measure directly in humans, one measure that may speak to the role of plasticity in cognitive development is variability in cognitive performance. An exploratory analysis of response latency variability in this sample found that this measure was better fit by inverse age than linear age or puberty (Supplemental Table 12). This does not preclude a role of adolescent plasticity in puberty-dependent cognitive development, but further studies using animal models and other methodologies will be required to better understand the mechanisms of this development.
While canonical brain regions associated with inhibitory control were tested in this study, only VLPFC activation and connectivity were related to puberty, supporting the pubertally-dependent maturation of VLPFC as contributing to changes in latency-related aspects of behavior. Moreover, initial results on an overlapping cohort from our group looking at effects of frontostriatal connectivity (Ojha et al., 2022) also find that pubertally-dependent maturation of VLPFC connectivity to the putamen is associated with antisaccade response latency. Since much of the literature directly linking pubertal mechanisms to regional brain development has utilized rodents, who do not have a lateral prefrontal cortex, there is a dearth of mechanistic support for how puberty might be directly implicating VLPFC. However, some human studies have shown that sex differences in the structure of the lateral PFC, such as cortical thickness, emerge at the onset of puberty (Nelson and Guyer, 2011, Raznahan et al., 2010), suggesting that puberty may have a unique influence in this area of the brain. Furthermore, VLPFC is related to stopping motor responses and response uncertainty (Levy and Wagner, 2011) as well as flexibly supporting specific inhibitory control demands depending on the task (Ryman et al., 2019). Thus, VLPFC connectivity to motor and performance monitoring (dACC) regions may support the ability to plan and execute a timely response. Accordingly, the VLPFC may be the region of the prefrontal cortex allowing for the performance optimization that we are associating with pubertal development. Activity in the cingulate region identified here, which is also known as the “mid-cingulate zone” or the “rostral cingulate motor zone” in prior literature, has previously been associated with variation in response speed (Hahn et al., 2007) and time to plan a cognitive response (Domic-Siede et al., 2021), providing a link to its association with antisaccade response latency in the current study. This area of the brain may also underlie important areas of general cognitive function such as between-network integration (Margulies and Uddin, 2019, Tang et al., 2019) and goal-directed behavior (Touroutoglou et al., 2020, Touroutoglou et al., 2019). Our findings, therefore, may support a potential role of VLPFC-cingulate circuitry in this puberty-related optimization of cognitive performance as contributing to pubertally-dependent changes in cognitive response speed.
Some limitations of this study should be noted. First, the age range during which puberty occurs varies dramatically across individuals based on many characteristics. Because this sample was not originally recruited with a focus on full pubertal development, resulting in relatively fewer young children, the onset of puberty may not be well-characterized in this sample, with more timepoints representing the latter half of pubertal development. Thus, we may not have been able to detect age-by-puberty interactions specific to early puberty, which are possible since the onset of puberty (transition from Stage 1 to Stage 2) represents a significant biological change. In addition, while this study took several steps to try to separate the effects of age and puberty, the fact remains that these variables still represent strongly related processes, and therefore it is still highly likely that these age and puberty effects are capturing some overlapping change.
Sex differences are also a significant aspect of discerning variability in cognitive development, particularly with respect to puberty. While we did not have the statistical power to test for sex differences directly in this sample, sex was included as a variable in our models. This revealed a main effect of sex on response latency when combined with puberty, but no significant effects of sex on fMRI task activation or PPI connectivity. The presence of a sex effect in the behavioral analyses but not in the fMRI analysis highlights the need to explore these sex differences in future studies. Additionally, due to the lack of statistical power that is a known weakness of PPI analysis, we were unable to test age and puberty in the same models when examining voxelwise whole-brain effects because of the high correlation between these two variables. This low statistical power characteristic of PPI analyses, as well as the dearth of prior research in the area of puberty and inhibitory control, also compelled us to use somewhat lenient thresholds in our exploratory voxelwise PPI analyses to provide a comprehensive examination of potential brain regions that may play a role in puberty’s influence on this development. Future studies with much larger samples, optimized for equal representation of pubertal stages, will be needed to replicate these findings and identify the inhibitory control-related regions most affected by pubertal development.
Another limitation that is common across many puberty-related studies is the fact that existing pubertal assessments reflect a number of different factors, rather than a singular measure of pubertal stage. Self-report measures, such as those used in this study, likely reflect pubertal development, but may also be affected by the individual’s self-image, their peer group, and many other factors that impact how adolescents report the status of their own development. The other commonly used method of defining pubertal status is clinician-rated Tanner stage, but this involves a physical exam that can be uncomfortable for study participants, especially adolescents. Further, puberty itself encompasses multiple neurobiological processes across both the adrenal and gonadal axes (Blakemore et al., 2010, Ladouceur et al., 2019, Marceau et al., 2015, Saxbe et al., 2015), and isolating the underlying mechanisms may require biological assays, for example by measuring hormone levels, which were not collected in this sample. Future studies should investigate whether these puberty-specific findings are associated with variation in one or multiple pubertal hormones, such as estradiol or testosterone.
5. Conclusions
These findings have important implications for our understanding of puberty and its critical importance in adolescent brain and cognitive development. By elucidating the role of pubertal development in the brain, we may gain new insights into the reasons that many psychiatric disorders, and the sex differences in their prevalence, emerge in adolescence. Furthermore, this research may provide novel targets for psychiatric intervention during the adolescence years, allowing for better preventative mental health care and greater ability to treat adolescents earlier and more effectively for enhanced long-term outcomes.
Funding
This research was supported by the National Institutes of Health: R01MH067924 (BL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
OR designed and performed data analysis, wrote manuscript. BL and FC designed study and data collection protocol. FC and WF assisted in code development and data analysis design. All authors contributed to the manuscript.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101162.
Appendix A. Supplementary material
Supplementary material
.
Data availability
Data will be made available on request.
References
- Akaike H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974;19:716–723. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Almey A., Milner T.A., Brake W.G. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse M.E.A., Byrne M.L., Flournoy J.C., McNeilly E.A., Guazzelli Williamson V., Barrett A.-M.Y., Chavez S.J., Shirtcliff E.A., Allen N.B., Pfeifer J.H. Multimethod assessment of pubertal timing and associations with internalizing psychopathology in early adolescent girls. J. Psychopathol. Clin. Sci. 2022;131:14–25. doi: 10.1037/abn0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A., Robbins T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol. 2013;108:44–79. doi: 10.1016/j.pneurobio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Bates D., Mächler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014;67:1–48. [Google Scholar]
- Bimonte H.A., Denenberg V.H. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/S0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Burnett S., Dahl R.E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 2010;31:926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin J.R., Piekarski D.J., Thomas A.W., Wilbrecht L. Adolescent pruning and stabilization of dendritic spines on cortical layer 5 pyramidal neurons do not depend on gonadal hormones. Dev. Cogn. Neurosci. 2018;30:100–107. doi: 10.1016/j.dcn.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond L., Clements J., Bertalli N., Evans-Whipp T., McMorris B.J., Patton G.C., Toumbourou J.W., Catalano R.F. A comparison of self-reported puberty using the pubertal development scale and the sexual maturation scale in a school-based epidemiologic survey. J. Adolesc. 2006;29:709–720. doi: 10.1016/j.adolescence.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Castagna P.J., Crowley M.J. Relationship between puberty and inhibitory control: computational modeling of the drift-diffusion process. Dev. Neuropsychol. 2021;46:360–380. doi: 10.1080/87565641.2021.1952206. [DOI] [PubMed] [Google Scholar]
- Castellanos-Ryan N., Struve M., Whelan R., Banaschewski T., Barker G.J., Bokde A.L.W., Bromberg U., Büchel C., Flor H., Fauth-Bühler M., Frouin V., Gallinat J., Gowland P., Heinz A., Lawrence C., Martinot J.-L., Nees F., Paus T., Pausova Z., Rietschel M., Robbins T.W., Smolka M.N., Schumann G., Garavan H., Conrod P.J. Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am. J. Psychiatry. 2014;171:1310–1319. doi: 10.1176/appi.ajp.2014.13111499. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Potenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am. J. Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Saad Z.S., Adleman N.E., Leibenluft E., Cox R.W. Detecting the subtle shape differences in hemodynamic responses at the group level. Front. Neurosci. 2015:9. doi: 10.3389/fnins.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Zijdenbos A.P., Baaré W.F.C., Evans A.C. In: Information Processing in Medical Imaging, Lecture Notes in Computer Science. Kuba A., Šáamal M., Todd-Pokropek A., editors. Springer; Berlin Heidelberg: 1999. ANIMAL+INSECT: improved cortical structure segmentation; pp. 210–223. [Google Scholar]
- Colzato L.S., Hommel B. Effects of estrogen on higher-order cognitive functions in unstressed human females may depend on individual variation in dopamine baseline levels. Front. Neurosci. 2014:8. doi: 10.3389/fnins.2014.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato L.S., Hertsig G., van den Wildenberg W.P.M., Hommel B. Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience. 2010;167:709–715. doi: 10.1016/j.neuroscience.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster F.N. The rise and fall of the inhibitory mechanism: toward a unified theory of cognitive development and aging. Dev. Rev. 1992;12:45–75. doi: 10.1016/0273-2297(92)90003-K. [DOI] [Google Scholar]
- Domic-Siede M., Irani M., Valdés J., Perrone-Bertolotti M., Ossandón T. Theta activity from frontopolar cortex, mid-cingulate cortex and anterior cingulate cortex shows different roles in cognitive planning performance. NeuroImage. 2021;226 doi: 10.1016/j.neuroimage.2020.117557. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D., MacMaster F.P., Peterson B.S., Niesink R., Andersen S., Braams B.R. Experience during adolescence shapes brain development: from synapses and networks to normal and pathological behavior. Neurotoxicol. Teratol. 2019;76 doi: 10.1016/j.ntt.2019.106834. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Shirtcliff E.A., Boyce W.T., Deardorff J., Essex M.J. Quality of early family relationships and the timing and tempo of puberty: effects depend on biological sensitivity to context. Dev. Psychopathol. 2011;23:85–99. doi: 10.1017/S0954579410000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V., Evans A., McKinstry R., Almli C., Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. doi: 10.1016/S1053-8119(09)70884-5. [DOI] [Google Scholar]
- Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L.A., Moffitt T.E., McGee R. Neuropsychological correlates of psychopathology in an unselected cohort of young adolescents. J. Abnorm. Psychol. 1989;98:307–313. doi: 10.1037/0021-843X.98.3.307. [DOI] [PubMed] [Google Scholar]
- Gibbs R.B. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm. Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A.-L., Mills K.L., Clasen L.S., Giedd J.N., Viner R.M., Blakemore S.-J. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K., Burns C.D., Madison C., Clark D., Halchenko Y.O., Waskom M.L., Ghosh S.S. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front. Neuroinform. 2011:5. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B., Ross T.J., Stein E.A. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cereb. Cortex. 2007;17:1664–1671. doi: 10.1093/cercor/bhl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting M.M., Kim R., Uban K.A., Kan E., Binley A., Sowell E.R. Longitudinal changes in pubertal maturation and white matter microstructure. Psychoneuroendocrinology. 2017;81:70–79. doi: 10.1016/j.psyneuen.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M.M., Wide J.K., Galea L.A.M. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav. Neurosci. 2002;116:928–934. doi: 10.1037/0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Hwang K., Velanova K., Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J. Neurosci. 2010;30:15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K., Ghuman A.S., Manoach D.S., Jones S.R., Luna B. Frontal preparatory neural oscillations associated with cognitive control: a developmental study comparing young adults and adolescents. NeuroImage. 2016;136:139–148. doi: 10.1016/j.neuroimage.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. The inhibition of automatic saccades in early infancy. Dev. Psychobiol. 1995;28:281–291. doi: 10.1002/dev.420280504. [DOI] [PubMed] [Google Scholar]
- Ladouceur C.D. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front. Integr. Neurosci. 2012:6. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur C.D., Kerestes R., Schlund M.W., Shirtcliff E.A., Lee Y., Dahl R.E. Neural systems underlying reward cue processing in early adolescence: the role of puberty and pubertal hormones. Psychoneuroendocrinology. 2019;102:281–291. doi: 10.1016/j.psyneuen.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen B., Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube C., van den Bos W., Fandakova Y. The relationship between pubertal hormones and brain plasticity: Implications for cognitive training in adolescence. Dev. Cogn. Neurosci. 2020:42. doi: 10.1016/j.dcn.2020.100753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B.J., Wagner A.D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann. N. Y. Acad. Sci. 2011;1224:40–62. doi: 10.1111/j.1749-6632.2011.05958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Adv. Child Dev. Behav. 2009;37:233–278. doi: 10.1016/S0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Marek S., Larsen B., Tervo-Clemmens B., Chahal R. An integrative model of the maturation of cognitive control. Annu. Rev. Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K., Ruttle P.L., Shirtcliff E.A., Essex M.J., Susman E.J. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Dev. Psychobiol. 2015;57:742–768. doi: 10.1002/dev.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies, D.S., Uddin, L.Q., 2019. Chapter 7 – Network convergence zones in the anterior midcingulate cortex. In: Vogt, B.A. (Ed.), Handbook of Clinical Neurology, Cingulate Cortex. Elsevier, pp. 103–111. 〈 10.1016/B978-0-444-64196-0.00007-8〉. [DOI] [PubMed]
- Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980:271. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Guyer A.E. The development of the ventral prefrontal cortex and social flexibility. Dev. Cogn. Neurosci. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick A.M., Forster G.L., Tejani-Butt S.M., Watt M.J. Adolescent social defeat alters markers of adult dopaminergic function. Brain Res. Bull. 2011;86:123–128. doi: 10.1016/j.brainresbull.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A. Ojha A.C. Parr W. Foran F.J. Calabro B. Luna. Puberty contributes to adolescent development of fronto-striatal functional connectivity supporting inhibitory control 2022 doi: 10.1101/2022.05.02.490303. [DOI] [PMC free article] [PubMed]
- Ordaz S.J., Foran W., Velanova K., Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J. Neurosci. 2013;33:18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz S.J., Fritz B.L., Forbes E.E., Luna B. The influence of pubertal maturation on antisaccade performance. Dev. Sci. 2018;21 doi: 10.1111/desc.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchanathan K., Frankenhuis W.E. The evolution of sensitive periods in a model of incremental development. Proc. R. Soc. B Biol. Sci. 2016;283:20152439. doi: 10.1098/rspb.2015.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A.X., Bullmore E.T. A wavelet-based estimator of the degrees of freedom in denoised fMRI time series for probabilistic testing of functional connectivity and brain graphs. NeuroImage. 2016;142:14–26. doi: 10.1016/j.neuroimage.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen D.J., Hallquist M.N., Geier C.F., Luna B. Effects of incentives, age, and behavior on brain activation during inhibitory control: a longitudinal fMRI study. Dev. Cogn. Neurosci. Proc. Inaug. Flux Congr. Towards Integr. Dev. Cogn. Neurosci. 2015;11:105–115. doi: 10.1016/j.dcn.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence. Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z., Judaš M., Šimić G., Rašin M.R., Uylings H.B.M., Rakic P., Kostović I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A.C., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Raznahan A., Lee Y., Stidd R., Long R., Greenstein D., Clasen L., Addington A., Gogtay N., Rapoport J.L., Giedd J.N. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc. Natl. Acad. Sci. 2010;107:16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman S.G., El Shaikh A.A., Shaff N.A., Hanlon F.M., Dodd A.B., Wertz C.J., Ling J.M., Barch D.M., Stromberg S.F., Lin D.S., Abrams S., Mayer A.R. Proactive and reactive cognitive control rely on flexible use of the ventrolateral prefrontal cortex. Hum. Brain Mapp. 2019;40:955–966. doi: 10.1002/hbm.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom N.J., Kim J.H., Wasserman M.A. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Saxbe D.E., Negriff S., Susman E.J., Trickett P.K. Attenuated hypothalamic–pituitary–adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Dev. Psychopathol. 2015;27:819–828. doi: 10.1017/S0954579414000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E.A., Dahl R.E., Pollak S.D. Pubertal development: correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gómez A.B., Rojas D., Juárez I., Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/S0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Tang W., Jbabdi S., Zhu Z., Cottaar M., Grisot G., Lehman J.F., Yendiki A., Haber S.N. A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. eLife. 2019;8 doi: 10.7554/eLife.43761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toren P., Sadeh M., Wolmer L., Eldar S., Koren S., Weizman R., Laor N. Neurocognitive correlates of anxiety disorders in children:: a preliminary report. J. Anxiety Disord. 2000;14:239–247. doi: 10.1016/S0887-6185(99)00036-5. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A., Andreano J., Dickerson B.C., Barrett L.F. The tenacious brain: How the anterior mid-cingulate contributes to achieving goals. Cortex. 2020;123:12–29. doi: 10.1016/j.cortex.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou, A., Andreano, J.M., Adebayo, M., Lyons, S., Barrett, L.F., 2019. Chapter one – motivation in the service of allostasis: the role of anterior mid-cingulate cortex. In: Elliot, A.J. (Ed.), Advances in Motivation Science. Elsevier, pp. 1–25. 〈 10.1016/bs.adms.2018.09.002〉. [DOI] [PMC free article] [PubMed]
- van Duijvenvoorde A.C.K., Westhoff B., de Vos F., Wierenga L.M., Crone E.A. A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: testing age- and puberty-related changes. Hum. Brain Mapp. 2019;40:3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb. Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Op de Macks Z., Shirtcliff E.A., Pfeifer J.H. Puberty and the human brain: insights into adolescent development. Neurosci. Biobehav. Rev. 2018;92:417–436. doi: 10.1016/j.neubiorev.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N., Youssef G.J., Allen N.B., Anderson V., Efron D., Hazell P., Mundy L., Nicholson J.M., Patton G., Seal M.L., Simmons J.G., Whittle S., Silk T. A longitudinal analysis of puberty‐related cortical development. NeuroImage. 2021;228 doi: 10.1016/j.neuroimage.2020.117684. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L., Holtmaat A., Wright N., Fox K., Svoboda K. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J. Neurosci. 2010;30:4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Wright L.D., Hébert K.E., Perrot-Sinal T.S. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A.R., Minkowski A. Regional Development of the Brain in Early Life. Blackwell Scientific; Oxford: 1967. The myelogenetic cycles of regional maturation of the brain; pp. 3–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.