Abstract
STUDY QUESTION
Does inoculation with inactivated vaccines against coronavirus disease 2019 (Covid-19) before frozen-thawed embryo transfer (FET) affect live birth and neonatal outcomes?
SUMMARY ANSWER
Inactivated Covid-19 vaccines did not undermine live birth and neonatal outcomes of women planning for FET.
WHAT IS KNOWN ALREADY
Accumulating reports are now available indicating the safe use of mRNA vaccines against Covid-19 in pregnant and lactating women, and a few reports indicate that they are not associated with adverse effects on ovarian stimulation or early pregnancy outcomes following IVF. Evidence about the safety of inactivated Covid-19 vaccines is very limited.
STUDY DESIGN, SIZE, DURATION
This is a retrospective cohort analysis from Reproductive Medical Center of a tertiary teaching hospital. Clinical records and vaccination record of 2574 couples with embryos transferred between 1 March 2021 and 30 September 2021 were screened for eligibility of this study.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Clinical and vaccination data of infertile couples planning for FET were screened for eligibility of the study. The reproductive and neonatal outcomes of FET women inoculated with inactivated Covid-19 vaccines or not were compared. The primary outcomes were live birth rate per embryo transfer cycle and newborns’ birth height and weight. Secondary outcomes included rates of ongoing pregnancy, clinical pregnancy, biochemical pregnancy and spontaneous miscarriage. Multivariate logistical regression and propensity score matching (PSM) analyses were performed to minimize the influence of confounding factors. Subgroup analyses, including single dose versus double dose of the vaccines and the time intervals between the first vaccination and embryo transfer, were also performed.
MAIN RESULTS AND THE ROLE OF CHANCE
Vaccinated women have comparable live birth rates (43.6% versus 45.0% before PSM, P = 0.590; and 42.9% versus 43.9% after PSM, P = 0.688), ongoing pregnancy rates (48.2% versus 48.1% before PSM, P = 0.980; and 52.2% versus 52.7% after PSM, P = 0.875) and clinical pregnancy rate (55.0% versus 54.8% before PSM, P = 0.928; and 54.7% versus 54.2% after PSM, P = 0.868) when compared with unvaccinated counterparts. The newborns’ birth length (50.0 ± 1.6 versus 49.0 ± 2.9 cm before PSM, P = 0.116; and 49.9 ± 1.7 versus 49.3 ± 2.6 cm after PSM, P = 0.141) and birth weight (3111.2 ± 349.9 versus 3030.3 ± 588.5 g before PSM, P = 0.544; and 3053.8 ± 372.5 versus 3039.2 ± 496.8 g after PSM, P = 0.347) were all similar between the two groups. Neither single dose nor double dose of vaccines, as well as different intervals between vaccination and embryo transfer showed any significant impacts on reproductive and neonatal outcomes.
LIMITATIONS, REASONS FOR CAUTION
The main findings might be limited by retrospective design. Besides, inoculations of triple dose of Covid-19 vaccines were not available by the time of data collection, thus the results cannot reflect the safe use of triple dose of inactivated Covid-19 vaccines. Finally, history of Covid-19 infection was based on patients’ self-report rather than objective laboratory tests.
WIDER IMPLICATIONS OF THE FINDINGS
Eligible individuals of inactivated vaccines against Covid-19 should not postpone vaccination plan because of their embryo transfer schedule, or vice versa.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Medical Key Discipline of Guangzhou (2021–2023). All authors had nothing to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: Covid-19, inactivated vaccine, frozen-thawed embryo transfer, live birth, neonatal outcomes
Introduction
The global pandemic of coronavirus disease 2019 (Covid-19) has added tremendous burdens to the world’s public health and economy. Fighting the pandemic is largely dependent on Covid-19 vaccines coverage. The global coverage of Covid-19 vaccines is 46.5% by 8 December 2021 (CNN, 2021). Meanwhile, the fully vaccinated population in China has reached 77.3% (CNN, 2021). According to our retrospective data, the vaccination rate of male partners planning for frozen-thawed embryo transfer (FET) in assisted reproductive therapy (ART) field was 70.4% (Supplementary Fig. S1), which is comparable to the national coverage in China. However, only 23.9% of women were vaccinated prior to FET (Supplementary Fig. S1). Similarly, low rates of vaccination and acceptance of vaccines were also observed among pregnant women all around the world (Battarbee et al., 2021; Razzaghi et al., 2021; Blakeway et al., 2022; Shamshirsaz et al., 2022). The main reason is the lack of proper information about the safety concerns of vaccines on fertility and pregnancy (de Figueiredo et al., 2020).
Multiple strategies are now available for design and production of Covid-19 vaccines (Fathizadeh et al., 2021; Ita, 2021). (i) Inactivated virus vaccines are one of the traditional methods of preparing vaccines (Soleimanpour and Yaghoubi, 2021). The vaccines are involved with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2 virus), which is inactivated or killed by chemical or physical methods with no risk of virus reversion. (ii) Attenuated-virus vaccines were produced using viruses with decreased pathogenesis, and thus provide powerful immune response. However, one major concern of live attenuated-virus vaccine is that the virus might regain toxicity after vaccination (Okamura and Ebina, 2021). (iii) Viral vectors vaccines use adenovirus to insert the Covid-19 virus gene into human body. Viral vector vaccines are considered to be safe and can induce potent immune response, but the construction of adenovirus vector is demanding and challenging (Luo et al., 2019). (iv) mRNA vaccines have received much attention because of their low risk of virus infection, high potency of immune response, safe administration and low cost of production (Pardi et al., 2018).
Inactivated Covid-19 vaccine are now account for almost half of all doses delivered globally, and had been tremendously crucial in fighting the pandemic (Mallapaty, 2021a,b). Accumulating publications are now available promoting the usage of mRNA vaccines in pregnant and lactating women (Bertrand et al., 2021; Dagan et al., 2021; Jamieson and Rasmussen, 2022), but only a few reports indicate that mRNA vaccines are not related to adverse effects on ovarian reserve, ovarian stimulation or early pregnancy outcomes following IVF (Orvieto et al., 2021; Aharon et al., 2022; Avraham et al., 2022; Mohr-Sasson et al., 2022). Less evidence is available about the safe use of inactivated vaccines, especially very few reports indicate that inactivated vaccines do not affect IVF outcomes (Wu et al., 2022).
Many women with embryos frozen before the pandemic were now also hesitant to receive either FET or vaccine inoculation. Given the unknown effect of vaccines on their oocytes and embryos, some couples prefer to have their oocytes collected and embryos prepared before Covid-19 vaccination. However, the question remains that whether vaccination before FET would lead to any adverse impact on reproductive outcomes of the following FET cycle. Besides, the appropriate time interval between vaccination and conception was also unknown. Therefore, how to make a proper recommendation for scheduled FET after Covid-19 vaccination remains a big challenge for patients and physicians due to lack of precise information.
Here, we performed this retrospective data analysis aiming to determine the impact of inactivated Covid-19 vaccines on reproductive and neonatal outcomes of women attempting FET. Updated information of the vaccination’s impact on pregnancy and newborns’ outcomes will be crucial for physicians to make proper recommendations, and for patients to make conception schedule.
Materials and methods
Study design and inclusion of populations
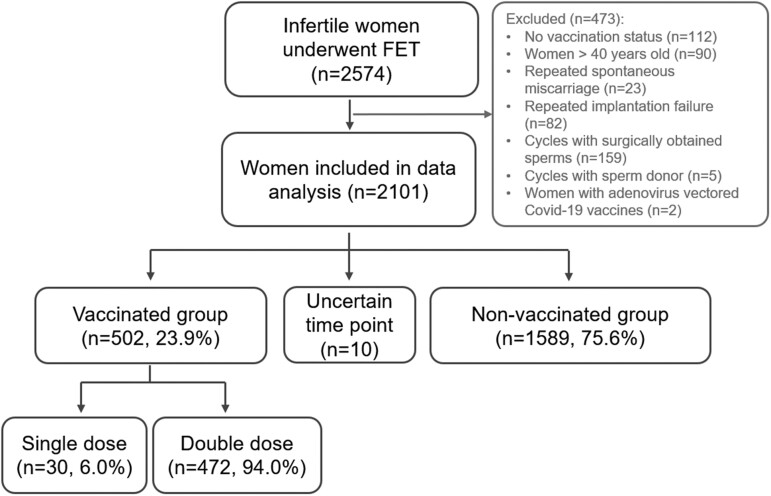
In this retrospective cohort study, clinical records of couples who had frozen embryo transfer between 1 March and 30 September 2021 at the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, were screened for eligibility for this study. March 2021 was chosen as the first month for screening because this is the first month of a massive vaccination campaign initiated in Guangzhou, China. The detailed inclusion and exclusion process of patients are illustrated in Fig. 1. The inclusion criteria of participants were (i) women with confirmed vaccination status from public health surveillance system record of their smartphone app (see Supplementary Fig. S2 for an example of vaccination status record), (ii) the first or second cycle of frozen-thawed embryos transferred, (iii) women in vaccinated group had embryos frozen prior to the exposure to Covid-19 vaccines and (iv) women aged 20–40 years old. Women fulfilled the following conditions were excluded: (i) women with three or more cycles of controlled ovarian stimulation (COS), (ii) women with repeated spontaneous miscarriage, (iii) women with repeated implantation failure, (iv) cycles with surgically obtained sperms, (v) cycles with sperm donor and (vi) infertile couples with severe systemic disease which might reduce conception chance.
Figure 1.
Flow chart of patients’ inclusion. FET, frozen-thawed embryo transfer; ET, embryo transfer. Vaccination status was updated on 1 October 2021.
ART-related data were extracted from the electronic database of reproductive center. Detailed vaccination status, including the date and manufacturer of the vaccines, was recorded in the mobile phone build-in app which was developed by local public health surveillance system. Women were queried about their vaccination status during the hospital visit by research nurses. Vaccination details of their spouses were also cross-checked with their personal records. The study protocol was approved by the hospital ethics committee (approval number is 2021-116). All the personal information were kept confidential.
None of the participants were ever infected with Covid-19 virus based on their self-reports. Almost all women were vaccinated with inactivated Covid-19 vaccines (Sinopharm from Beijing Bio-Institute Ltd., Beijing, China; and CoronaVac from Sinovac Biotech Ltd., Beijing, China) (Doroftei et al., 2021), except for only two women received adenovirus vectored vaccines (Ad5-nCoV from CanSino Biologics Inc., Tianjin, China) who were excluded from the current data analysis. The entire course of inactivated Covid-19 vaccines included two jabs, with an interval of at least 28 days. People with double doses of inactivated vaccines were defined as the fully vaccinated. A booster injection or a triple dose of inactivated Covid-19 vaccines was recommended for over 18 years old fully vaccinated population since the end of October 2021 by the provincial government (GD-government, 2021). The interval of last injection and booster injection should be at least 6 months. Since the data analysis of the present study was performed before the vaccination campaign of booster vaccines, booster vaccine status was not included in the present study.
Endometrial preparation protocols and frozen-thawed embryo transfer
Embryos were frozen with vitrification following COS and fertilization with either IVF or ICSI. The vitrification and thawing process were performed with protocols provided by Cryotop vitrification device and media (Cryotop®, Kitazato BioPharma, Japan). Regimes for endometrial preparation in the FET cycles included natural cycle, hormonal replacement cycle (HRT), GnRH agonist (GnRHa) + HRT cycle and stimulation cycle. In natural cycles, natural ovulation was monitored with ultrasonography. Luteal phase support was initiated since the day of ovulation with dydrogesterone (20 mg/day, Abbott, The Netherlands). In HRT cycles, estradiol valerate (Progynova, 6–8 mg/day, Bayer, Germany) was started since Days 2–3 of menstruation. Luteal phase support with dydrogesterone (20 mg/day) and vaginal progesterone gel (Crinone, 90 mg/day, Merck, Germany) were added if the endometrial thickness reached 7 mm or above. In GnRHa + HRT cycles, GnRHa (Triptorelin, 3.75 mg, Ipsen, France) was administrated on Day 2 or Day 3 of menstrual cycle. Estradiol valerate was provided 28 days later for endometrial proliferation. In stimulation cycle, mild ovarian stimulation was performed with intramuscular human menopausal gonadotropin (HMG, Lizhu Pharmaceuticals, Zhuhai, China). Follicle development and endometrial growth were monitored with transvaginal sonography. Luteal phase support with dydrogesterone (20 mg/day) and progesterone capsule (Utrogestan, 0.2 g/day, Besins, France) and were added if ovulation were confirmed. In all above-mentioned regimes of endometrial preparation, frozen embryos were thawed and transferred either on Day 3 or Day 5 after luteal phase support. The morphologies of embryos were re-evaluated 2–4 h after warming. One or two embryos were transferred to endometrium. Biochemical pregnancy test with serum HCG levels was scheduled 14 days after embryo transfer. Clinical pregnancy would be confirmed with the detection of intrauterine gestational sac(s) by transvaginal ultrasound 4 weeks after embryo transfer. If clinical pregnancy was confirmed, continuous luteal phase will last till gestational 10 weeks. Ultrasonography will be performed at around gestational 12 weeks to confirm ongoing pregnancy, which was defined as the detection of intrauterine viable pregnancy.
Definitions of study outcomes
The primary outcomes of this study were live birth rate per embryo transfer cycle and neonatal outcomes. Live birth was defined as delivery of live newborn(s) at least over gestational 28 weeks. Delivery of twins or triplets was defined as one live birth. Newborns’ birth height and weight were followed-up and recorded by experienced nurses. The secondary outcomes included rates of ongoing pregnancy, clinical pregnancy, biochemical pregnancy, spontaneous miscarriage and ectopic pregnancy. Ongoing pregnancy was defined as intrauterine pregnancy with live fetus over gestational 12 weeks. Clinical pregnancy was confirmed with observation of intrauterine gestational sac on ultrasonography at around gestational 6 weeks. Biochemical pregnancy was determined as detection of serum level of HCG more than 10 mIU/ml 14 days after embryo transfer. Biochemical pregnancy loss was determined as elevated HCG levels but no detectable gestational sac was observed with transvaginal sonography 4 weeks following embryo transfer. Spontaneous miscarriages were those pregnancy losses with detectable intrauterine gestational sacs within gestational 12 weeks. Ectopic pregnancy was identified as embryos implant at any other sites except for intrauterine cavity. Rates of live birth, ongoing pregnancy, clinical pregnancy and biochemical pregnancy were calculated based on the proportion of women with above-mentioned outcomes out of women with embryo transfer. Biochemical pregnancy loss rate, spontaneous miscarriage rate and ectopic pregnancy rate were calculated based on the proportion of women with the outcomes out of women with biochemical pregnancy.
Statistical analysis
All statistical description and analysis were conducted with SPSS (version 22.0, IBM Inc., USA). Normally distributed quantitative parameters were expressed as mean ± SD, and compared using Students’ t test or one-way ANOVA when appropriate. Quantitative parameters which were not normally distributed were expressed as median (25th and 75th quartiles), and compared using Mann–Whitney U test. Comparisons of frequencies and proportions were made using Chi-squared test. Fisher’s exact test would be applied if expected count was <5 or the total sample size was <40. A P value <0.05 was considered to have statistical significance.
Multivariable logistic regressions were performed to determine the independent impact of Covid-19 vaccination status on reproductive outcomes, and data were presented as adjusted odds ratios (aORs) and 95% CI. Propensity score matching (PSM) was conducted to match the basic clinical characteristics of vaccinated and non-vaccinated groups, which might potentially differ between the two groups. The study groups were matched 1:4 based on the vaccination rate of women planning for FET. The PSM was conducted with a caliper width of 0.2 of the SD of the logit of the propensity score. The SD for independent variables before and after PSM was calculated. SD values after PSM <10% were considered to be balanced (Wu et al., 2021). After PSM, the effect of vaccination status was calculated using generalized estimation equations, and demonstrated as aOR and 95% CI. The possible confounders including female age, infertility duration, number of COS cycles, protocols of COS, endometrial preparation protocol, number of embryo(s) transferred and number of top-quality embryo(s) transferred were included in the multivariable logistic regression and PSM.
Results
Vaccination status of infertile couples seeking for FET
Clinical data of 2574 FET cycles were included. After exclusion of 473 cycles (see Fig. 1 for detailed reasons of exclusion), 502 women (23.9%) were confirmed to be vaccinated prior to FET, and were labeled as vaccinated group. Ten women were vaccinated but with uncertain time point, and hence were not included for data analysis. The remaining 1589 women were unvaccinated and labeled as unvaccinated group (Fig. 1). There were 1479 (70.4%) male partners of women underwent FET cycles were fully vaccinated, although details of their vaccination time points were not available (Supplementary Data, Supplementary Fig. S1).
Comparisons of basic clinical characteristics and fertility treatment-related characteristics between vaccinated and unvaccinated groups
The baseline characteristics were comparable considering of their age, anti-Mullerian hormone (AMH), antral follicle counting (AFC), body mass index (BMI), type of infertility and causes of infertility between vaccinated and unvaccinated group (Table I). Infertility duration in vaccinated women was notably longer than unvaccinated group (P < 0.001).
Table I.
Comparisons of basic clinical characteristics and fertility treatment-related features between vaccinated and unvaccinated groups.
| Vaccinated group (n = 502) | Unvaccinated group (n = 1589) | t/Z/X2 | P | |
|---|---|---|---|---|
| Age (years) | 32.43 ± 3.97 | 32.70 ± 4.40 | −0.550 | 0.582 |
| Infertility duration (years) | 5 (2, 8) | 4 (0.5, 8) | −4.084 | <0.001 |
| AMH (ng/ml) | 4.93 (0.16, 9.70) | 4.65 (0.43, 8.87) | −1.146 | 0.145 |
| Total AFC | 22 (8, 36) | 22 (11, 33) | −0.094 | 0.925 |
| BMI (kg/m2) | 21.74 (17.55, 25.93) | 21.45 (17.26, 25.64) | −0.220 | 0.826 |
| Type of infertility % (n) | 2.523 | 0.112 | ||
| Primary | 42.0% (211) | 45.9% (729) | ||
| Secondary | 58.0% (291) | 53.7% (853) | ||
| Causes of infertility % (n) | 7.273 | 0.296 | ||
| Male | 10.4% (52) | 11.4% (181) | ||
| Tubal factors | 43.6% (219) | 37.7% (599) | ||
| Ovulatory disorder | 7.57% (38) | 8.37% (133) | ||
| Endometriosis | 2.8% (14) | 2.5% (39) | ||
| Unexplained infertility | 10.4% (52) | 10.3% (164) | ||
| Mixed factors | 20.5% (103) | 24.9% (395) | ||
| PGT | 4.8% (24) | 4.8% (76) | ||
| Indications of PGT | 5.172 | 0.075 | ||
| PGT-A | 25.0% (6) | 27.6% (21) | ||
| PGT-M | 54.2% (13) | 30.3% (23) | ||
| PGT-SR | 20.8% (5) | 42.1% (32) | ||
| COS cycle number | 6.193 | 0.013 | ||
| First cycle | 86.5% (434) | 90.4% (1436) | ||
| Second cycle | 13.5% (68) | 9.6% (153) | ||
| COS protocols % (n) | 8.528 | 0.014 | ||
| Antagonist | 66.9% (336) | 70.7% (1123) | ||
| Agonist | 29.9% (150) | 24.2% (385) | ||
| Others | 3.2% (16) | 5.1% (81) | ||
| Endometrial preparation protocol | 9.135 | 0.028 | ||
| Natural cycle | 23.9% (120) | 18.8% (299) | ||
| HRT cycle | 70.1% (352) | 72.3% (1149) | ||
| GnRHa + HRT cycle | 5.4% (27) | 7.9% (125) | ||
| Stimulation cycle | 0.6% (3) | 1.0% (16) | ||
| Endometrial thickness (mm) | 8.6 (6.4, 10.8) | 8.7 (6.7, 10.7) | −1.135 | 0.257 |
| Endometrial type | 3.106 | 0.212 | ||
| Type A | 42.0% (211) | 45.9% (729) | ||
| Type B | 57.6% (289) | 53.5% (850) | ||
| Type C and others | 0.4% (2) | 0.2% (3) | ||
| Fertilization type | 1.591 | 0.451 | ||
| IVF | 80.5% (404) | 77.9% (1238) | ||
| ICSI | 15.7% (79) | 18.1% (288) | ||
| IVF + ICSI | 3.8% (19) | 4.0% (63) | ||
| Number of embryos for transfer | 5.252 | 0.022 | ||
| N = 1 | 64.1% (322) | 69.6% (1106) | ||
| N = 2 | 35.9% (180) | 30.4% (483) | ||
| Embryo stage | 0.010 | 0.920 | ||
| Cleavage | 20.1% (101) | 20.3% (323) | ||
| Blastocyst | 79.9% (401) | 79.7% (1266) | ||
| Day 3 embryo# | 20.97% (143/682) | 22.88% (474/2072) | 1.077 | 0.584 |
| Day 5 embryo | 56.01% (382/682) | 54.73% (1134/2072) | ||
| Others | 23.02% (157/682) | 22.39% (464/2072) | ||
| Number of top-quality embryos for transfer | 18.085 | <0.001 | ||
| N = 0 | 24.5% (123) | 17.4% (276) | ||
| N = 1 | 59.4% (298) | 69.4% (1102) | ||
| N = 2 | 16.1% (81) | 13.3% (211) |
AMH, anti-Mullerian hormone; AFC, antral follicle counting; COS, controlled ovarian stimulation; PGT, preimplantation genetic test; PGT-A, PGT for aneuploidies; PGT-M, PGT for monogenic; PGT-SR, PGT for chromosome structural rearrangements; NA, not available/applicable; HRT, hormonal replacement therapy.
Proportions of Day 3, Day 5 embryos and others were calculated based on total number of embryos transferred. Values are mean ± SD; median (Quartile1, Quartile3) or percent (n).
Fertility treatment-related features were also compared and presented in Table I. In vaccinated group, more women had repeated cycles of COS (13.5% versus 9.6%, P = 0.013), GnRHa protocol for COS (29.9% versus 24.2%, P = 0.014) and natural cycle for endometrial preparation (23.9% versus 18.8%, P = 0.028) compared with unvaccinated women. Endometrial thickness, endometrial type and fertilization types were comparable between the two groups. More women in vaccinated group had double embryo transfer (35.9% versus 30.4%, P = 0.022), but less of them were transferred with at least one top-quality embryo (75.5% versus 82.7%, P < 0.001).
Comparisons of reproductive and neonatal outcomes between groups before and after PSM
Since several baseline characteristics were notably differed between vaccinated and unvaccinated women, further balance of those features using multivariable logistic regression and PSM were performed. Basic clinical characteristics of 492 women in vaccinated group and 1263 unvaccinated women were further matched by PSM. Data in Supplementary Table SI demonstrate that the baseline characteristics of vaccinated and unvaccinated women were well balanced after PSM, with all SD values of basic clinical characteristics <10%. As demonstrated in Table II, vaccinated women have comparable live birth rates (43.6% versus 45.0% before PSM, P = 0.590; and 42.9% versus 43.9% after PSM, P = 0.688), ongoing pregnancy rates (48.2% versus 48.1% before PSM, P = 0.980; and 52.2% versus 52.7% after PSM, P = 0.875) and clinical pregnancy rate (55.0% versus 54.8% before PSM, P = 0.928; and 54.7% versus 54.2% after PSM, P = 0.868) when compared with unvaccinated counterparts. Other outcomes, including biochemical pregnancy rate, biochemical pregnancy loss rate, early miscarriage rate and ectopic pregnancy rate were all comparable between vaccinated and unvaccinated women regardless of analyses before or after PSM. The newborns’ birth length (50.0 ± 1.6 versus 49.0 ± 2.9 cm before PSM, P = 0.116; and 49.9 ± 1.7 versus 49.3 ± 2.6 cm after PSM, P = 0.141) and birth weight (3111.2 ± 349.9 versus 3030.3 ± 588.5 g before PSM, P = 0.544; and 3053.8 ± 372.5 versus 3039.2 ± 496.8 g after PSM, P = 0.347) were all similar between the two groups.
Table II.
Comparisons of reproductive outcomes before and after propensity score matching (PSM).
| Before PSM |
After PSM |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated n = 502 | Unvaccinated n = 1589 | P | aOR (95% CI) | P | Vaccinated n = 492 | Unvaccinated n = 1263 | P | aOR (95% CI) | P | |
| Live birth | 43.6% (219/502) | 45.0% (715/1589) | 0.590 | 1.045 (0.844–1.293) | 0.689 | 42.9% (211/492) | 43.9% (555/1263) | 0.688 | 0.984 (0.792–1.223) | 0.887 |
| Ongoing pregnancy | 48.2% (242/502) | 48.1% (765/1589) | 0.980 | 1.111 (0.893–1.383) | 0.343 | 52.2% (257/492) | 52.7% (665/1263) | 0.875 | 0.983 (0.798–1.211) | 0.875 |
| Clinical pregnancy | 55.0% (276/502) | 54.8% (870/1589) | 0.928 | 1.135 (0.910–1.414) | 0.262 | 54.7% (269/492) | 54.2% (685/1263) | 0.868 | 1.018 (0.826–1.255) | 0.868 |
| Biochemical pregnancy | 61.2% (307/502) | 59.0% (937/1589) | 0.384 | 1.268 (1.012–1.589) | 0.039 | 61.0% (300/492) | 58.3% (736/1263) | 0.301 | 1.119 (0.904–1.384) | 0.301 |
| Biochemical pregnancy loss | 10.1% (31/307) | 7.2% (67/937) | 0.096 | 1.537 (0.944–2.502) | 0.084 | 10.0% (30/300) | 6.7% (49/736) | 0.066 | 1.558 (0.968–2.507) | 0.068 |
| Early miscarriage | 11.1% (34/307) | 11.2% (105/937) | 0.927 | 1.003 (0.643–1.563) | 0.99 | 11.3% (34/300) | 11.4% (84/736) | 0.971 | 0.992 (0.650–1.515) | 0.971 |
| Ectopic pregnancy | 0.3% (1/307) | 0.2% (2/937) | 0.573# | NA | 0.3% (1/300) | 0.3% (2/736) | 1.000# | NA | ||
| Newborns’ birth height (cm) | 50.0 ± 1.6 | 49. 0 ± 2.9 | 0.116 | NA | 49.9 ± 1.7 | 49.3 ± 2.6 | 0.141 | NA | ||
| Newborns’ birth weight (g) | 3111.2 ± 349.9 | 3030.3 ± 588.5 | 0.544 | NA | 3053.8 ± 372.5 | 3039.2 ± 496.8 | 0.347 | NA | ||
Fisher exact test. aOR, adjusted odds ratio; NA, not available/applicable.
Subgroup analysis of vaccinated women
Subgroup analysis of the impact of vaccination status on reproductive and neonatal outcomes was performed. As presented in Table III, the number of doses of inactivated vaccines prior to FET showed no obvious impacts on reproductive outcomes (single dose 53.5% versus double dose 43.0% for live birth rate, P = 0.269). The median time interval between first vaccination and FET was 117.5 days (minimum to maximum days were 7–311 days). Time intervals between the first vaccination and FET were further divided into three subgroups, <3 months (n = 126), 3–6 months (n = 338) and >6 month (n = 38). No significant differences of reproductive outcomes were observed among the three subgroups (38.1% versus 45.0% versus 50.0% for live birth rate, P = 0.295; 42.1% versus 49.7% versus 55.3% for ongoing pregnancy rate, P = 0.227; 50.0% versus 55.6% versus 65.8% for clinical pregnancy rate, P = 0.211; and 15.9% versus 10.6% versus 16.0% for miscarriage rate, P = 0.371). Multivariable logistic regression analyses were performed to eliminate the impact of possible confounders. As demonstrated in Table III, either the dose of vaccination or the interval between the first vaccination and FET showed any significant influence on any reproductive outcomes. The newborns’ birth length and birth weight were all comparable among the subgroups (see Table III for details).
Table III.
Subgroup analysis of reproductive outcomes within vaccinated group.
| Doses of vaccination % (n) |
Intervals between the first vaccination and FET % (n) |
||||||
|---|---|---|---|---|---|---|---|
| Single dose n = 30 | Double dose n = 472 | P value | <3 months n = 126 | 3–6 months n = 338 | >6 months n = 38 | P value | |
| Live birth | |||||||
| % (n) | 53.3% (16) | 43.0% (203) | 0.269 | 38.1% (48) | 45.0% (152) | 50.0% (19) | 0.295 |
| aOR | 1.000 | 0.646 | 1.000 | 1.420 | 2.006 | ||
| 95% CI | 1.000 | 0.298–1.405 | 1.000 | 0.908–2.222 | 0.919–4.379 | ||
| P value | 0.271 | 0.125 | 0.081 | ||||
| Ongoing pregnancy | |||||||
| % (n) | 53.3% (16) | 47.9% (226) | 0.562 | 42.1% (53) | 49.7% (168) | 55.3% (21) | 0.227 |
| aOR | 1.000 | 0.893 | 1.000 | 1.409 | 1.962 | ||
| 95% CI | 1.000 | 0.413–1.934 | 1.000 | 0.914–2.172 | 0.913–4.216 | ||
| P value | 0.775 | 0.120 | 0.084 | ||||
| Clinical pregnancy | |||||||
| % (n) | 53.3% (16) | 55.1% (260) | 0.852 | 50.0% (63) | 55.6% (188) | 65.8% (25) | 0.211 |
| aOR | 1.000 | 1.169 | 1.000 | 1.191 | 2.115 | ||
| 95% CI | 1.000 | 0.537–2.545 | 1.000 | 0.769–1.846 | 0.952–4.699 | ||
| P value | 0.695 | 0.433 | 0.066 | ||||
| Biochemical pregnancy | |||||||
| % (n) | 63.3% (19) | 61.0% (288) | 0.801 | 56.3% (71) | 62.1% (210) | 68.4% (26) | 0.332 |
| aOR | 1.000 | 0.935 | 1.000 | 1.239 | 1.805 | ||
| 95% CI | 1.000 | 0.421–2.077 | 1.000 | 0.796–1.926 | 0.805–4.044 | ||
| P value | 0.870 | 0.342 | 0.151 | ||||
| Biochemical pregnancy loss | |||||||
| % (n) | 15.8% (3) | 9.7% (28) | 0.422# | 11.3% (8) | 10.5% (22) | 3.8% (1) | 0.649# |
| aOR | 1.000 | 0.563 | 1.000 | 1.130 | 0.393 | ||
| 95% CI | 1.000 | 0.150–2.114 | 1.000 | 0.433–2.951 | 0.044–3.483 | ||
| P value | 0.395 | 0.803 | 0.401 | ||||
| Miscarriage | |||||||
| % (n) | 0% (0) | 11.8% (34) | 0.245# | 15.9% (10) | 10.6% (20) | 16.0% (4) | 0.371# |
| aOR | 1.000 | NA | 1.000 | 0.617 | 0.982 | ||
| 95% CI | 1.000 | NA | 1.000 | 0.272–1.399 | 0.278–3.465 | ||
| P value | 0.998 | 0.248 | 0.977 | ||||
| Newborns’ birth height (cm) | 49.8 ± 1.1 | 50.0 ± 1.5 | 0.133 | 50.1 ± 1.7 | 50.3 ± 0.6 | 49.8 ± 1.3 | 0.876 |
| Newborns’ birth weight (g) | 3067.2 ± 356.2 | 3122.3 ± 348.7 | 0.254 | 3075.6 ± 377.8 | 3226.7 ± 328.8 | 3287.5 ± 243.6 | 0.491 |
Fisher exact test. FET, frozen-thawed embryo transfer; aOR, adjusted odds ratio; NA, not available/applicable.
Discussion
The present study, to our knowledge, is one of the first studies focusing on the impact of inactivated Covid-19 vaccination on live birth and neonatal outcomes of women with FET. Our data demonstrate that vaccination prior to FET did not undermine live birth rate and neonatal outcomes, nor other assisted reproductive outcomes. Results of this study could help to improve the social confidence and acceptance of inactivated Covid-19 vaccines. Updated knowledge of inactivated Covid-19 vaccines can also help health professionals for consultation of potential benefit and risk of vaccines to make an informed decision.
Inactivated vaccine against Covid-19 has become the most widely offered vaccine around the world (Croda and Ranzani, 2022). There are abundant reports suggesting that vaccination against Covid-19 did not cause harm in preconception women, or women during gestation or breastfeeding (Nana and Nelson-Piercy, 2021; Blakeway et al., 2022; Jacobs and Van Voorhis, 2022). Recently, Aizer et al. (2022) also reported the mRNA vaccination against coronavirus or virus infection showed no significant impact on women’s performance in FET cycles. However, almost all of this evidence focused on mRNA vaccines, and only rare reports provide safety data of inactivated vaccines in the assisted reproduction field. Unlike live attenuated vaccines, immunization with inactivated vaccines showed no evidence of notable adverse maternal or fetal harm and is generally considered to be safe for pregnant and preconceptional women (Arora and Lakshmi, 2021; Dad et al., 2021). Several other kinds of inactivated vaccines, for instance, tetanus, influenza, polio, rabies, Hepatitis B and Hepatitis A, would be administrated for pregnancy women if high risk of exposure is presented (Arora and Lakshmi, 2021; Dad et al., 2021). Our results provide some of the first evidence confirming the safety of inactivated Covid-19 vaccines in preconceptional women planning for FET.
Even though SARS-CoV-2 has not been isolated from human endometrium, uterine endometrium might still be susceptible to SARS-CoV-2 infection, especially during the implantation window (Henarejos-Castillo et al., 2020; Chandi and Jain, 2021). Virus-infectivity-related genes, ACE2 and TMPRSS4, were detected to be expressed in human endometrium, through which, SARS-CoV-2 virus can enter and infect cells and cause tissue damage. The expression abundance of virus-infectivity-related genes increased from proliferative phase to secretory phase, indicating a possible infectious risk of SARS-CoV-2 during embryo implantation period (Henarejos-Castillo et al., 2020). Given this infectious risk of SARS-CoV-2, protection from Covid-19 vaccine might be necessary for those planning for embryo transfer. However, whether inactivated Covid-19 vaccines might have any impact on human endometrial receptivity, has not been determined yet. Our real-world data of reproductive outcomes in FET cycles, which also largely relied on endometrial receptivity, provides evidence indicating that human endometrial receptivity might not be affected by inactivated Covid-19 vaccines.
An optimal time interval between Covid-19 vaccines and conception were not investigated previously, and no proper recommendation can be made. Here, in our study, almost two-thirds women (67.3% 338/502) had FET within 3–6 months after Covid-19 vaccination. Only 12 (2.4%) women had FET within 1 month after vaccination. One-month interval, hence, cannot be analyzed separately. Although slight increase trends of pregnancy rates were observed with prolonged interval from vaccination and embryo transfer, no obvious detrimental effects on their assisted reproductive outcomes were observed. The clinical pregnancy rates in all three subgroups with various time intervals were all above 50%, which is acceptable compared to the benchmark value (35.5%) suggested by Maribor consensus (Vlaisavljevic et al., 2021). Moreover, the aim to reach clinical pregnancy or live birth was affected by multiple factors besides of vaccination status (Vlaisavljevic et al., 2021). And those influencing factors included demographical, clinical, laboratory features and even psychological status. Furthermore, to eliminate the possible impact from calendar time of FET, we also re-evaluate selected cases with FET performed between August and October. Because most cases were vaccinated since June 2021 after the local outbreak of coronavirus pandemic, majority of them in this study planned their FET between August and October after vaccination. No obvious differences were detected in pregnancy outcomes (data not shown) in those selected cases, and hence the time of FET might have little impact on pregnancy outcomes. We believe that data from the current study did not identify any notable impact on pregnancy outcomes with various intervals between vaccination and embryo transfer. Given the safety of a shortened time interval between vaccination and conception within 3 months, deferral of FET and conception plan for more than 3 months due to Covid-19 vaccination is not necessary. Nevertheless, the optimal time interval between vaccination and conception would be further confirmed with the ongoing multi-center prospective cohort study (registration number: ChiCTR2200055622).
This study included a large sample size comparing the live birth and neonatal outcomes of vaccinated and unvaccinated women with FET. Furthermore, by integrating accurate information from public health surveillance databases and fertility treatment database, reliability and authenticity of our retrospective results can be reassured. However, several drawbacks of the study should be taken with discreet. First, our main findings might be limited by its retrospective design. To avoid the potential selection biases and interference of potential confounders, both multi-variable regression analysis and PSM were performed to identify the independent impact of inactivated Covid-19 vaccination status. Besides, inoculations of triple dose of Covid-19 vaccines were not available by the time of data collection, thus the results cannot reflect the safe use of triple dose of inactivated Covid-19 vaccines. Finally, we should acknowledge that the history of Covid-19 infection was based on patients’ self-report rather than objective laboratory tests.
Conclusion
A low vaccination coverage (23.9%) was observed among women planning for FET. Inactivated Covid-19 vaccines did not undermine live birth rate and other assisted reproductive outcomes, of women who attempt FET. Newborns’ birth weight and height showed no alterations after vaccination. Eligible individuals of Covid-19 vaccines should not postpone vaccination plan because of embryo transfer schedule, or vice versa.
Supplementary Material
Contributor Information
Mingzhu Cao, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Yixuan Wu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Yanshan Lin, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Zijin Xu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Zhu Liang, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Qing Huang, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Sichen Li, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Hanyan Liu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Chunyan An, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Yiqun Luo, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Haiying Liu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Jianqiao Liu, Department of Obstetrics and Gynecology, Center for Reproductive Medicine, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China; Key Laboratory for Reproductive Medicine of Guangdong Province, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ roles
M.C., Y.W. and Y.L. contributed in study design, data acquisition, data analysis and data interpretation. Z.X., Z.L., Q.H., S.L., H.L., C.A. and Y.L. contributed in data acquisition and data interpretation. H.L. and J.L. contributed in study design, data interpretation and draft revision. All authors have approved the final version of the manuscript.
Funding
This study was supported by the Medical Key Discipline of Guangzhou (2021–2023).
Conflict of interest
All authors had nothing to disclose.
References
- Aharon D, Lederman M, Ghofranian A, Hernandez-Nieto C, Canon C, Hanley W, Gounko D, Lee JA, Stein D, Buyuk E. et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol 2022;139:490–497. [DOI] [PubMed] [Google Scholar]
- Aizer A, Noach-Hirsh M, Dratviman-Storobinsky O, Nahum R, Machtinger R, Yung Y, Haas J, Orvieto R.. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril 2022;117:974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora M, Lakshmi R.. Vaccines—safety in pregnancy. Best Pract Res Clin Obstet Gynaecol 2021;76:23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, Gat I, Gidoni Y, Hochberg A, Baum M. et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril 2022;117:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battarbee AN, Stockwell MS, Varner M, Newes-Adeyi G, Daugherty M, Gyamfi-Bannerman C, Tita AT, Vorwaller K, Vargas C, Subramaniam A. et al. Attitudes toward COVID-19 illness and COVID-19 vaccination among pregnant women: a cross-sectional multicenter study during August-December 2020. Am J Perinatol 2021;39:75–83. [DOI] [PubMed] [Google Scholar]
- Bertrand K, Honerkamp-Smith G, Chambers CD.. Maternal and child outcomes reported by breastfeeding women following messenger RNA COVID-19 vaccination. Breastfeed Med 2021;16:697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, Magee LA, O’Brien P, Rezvani A, von Dadelszen P. et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol 2022;226:236.e1–236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandi A, Jain N.. State of assisted reproduction technology in the coronavirus disease 2019 era and consequences on human reproductive system. Biol Reprod 2021;105:808–821. [DOI] [PubMed] [Google Scholar]
- CNN. Tracking Covid-19 Vaccinations Worldwide. 2021. https://edition.cnn.com/interactive/2021/health/global-covid-vaccinations/ (8 December 2021, date last accessed).
- Croda J, Ranzani OT.. Booster doses for inactivated COVID-19 vaccines: if, when, and for whom. Lancet Infect Dis 2022;22:430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dad N, Buhmaid S, Mulik V.. Vaccination in pregnancy—the when, what and how? Eur J Obstet Gynecol Reprod Biol 2021;265:1–6. [DOI] [PubMed] [Google Scholar]
- Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, Hernán MA, Lipsitch M, Hernandez-Diaz S, Reis BY. et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021;27:1693–1695. [DOI] [PubMed] [Google Scholar]
- de Figueiredo A, Simas C, Karafillakis E, Paterson P, Larson HJ.. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study. Lancet 2020;396:898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroftei B, Ciobica A, Ilie OD, Maftei R, Ilea C.. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics (Basel) 2021;11:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathizadeh H, Afshar S, Masoudi MR, Gholizadeh P, Asgharzadeh M, Ganbarov K, Köse Ş, Yousefi M, Kafil HS.. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: a review. Int J Biol Macromol 2021;188:740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GD-government. A Booster Vaccination is Recommended for People Over 18 Years Old and Fully Vaccinated for Free. 2021. https://www.gd.gov.cn/gdywdt/bmdt/content/post_3595275.html (10 December 2021, date last accessed).
- Henarejos-Castillo I, Sebastian-Leon P, Devesa-Peiro A, Pellicer A, Diaz-Gimeno P.. SARS-CoV-2 infection risk assessment in the endometrium: viral infection-related gene expression across the menstrual cycle. Fertil Steril 2020;114:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ita K. Coronavirus disease (COVID-19): current status and prospects for drug and vaccine development. Arch Med Res 2021;52:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs E, Van Voorhis BJ.. COVID-19 vaccination in obstetrics and gynecology: addressing concerns while paving a way forward. Obstet Gynecol 2022;139:479–480. [DOI] [PubMed] [Google Scholar]
- Jamieson DJ, Rasmussen SA.. An update on COVID-19 and pregnancy. Am J Obstet Gynecol 2022;226:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Zhang P, Ma X, Wang Q, Lu J, Liu B, Zhao W, Allain JP, Li C, Li T.. A rapid strategy for constructing novel simian adenovirus vectors with high viral titer and expressing highly antigenic proteins applicable for vaccine development. Virus Res 2019;268:1–10. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. China's COVID vaccines have been crucial - now immunity is waning. Nature 2021a;598:398–399. [DOI] [PubMed] [Google Scholar]
- Mallapaty S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature 2021b;594:161–162. [DOI] [PubMed] [Google Scholar]
- Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Doitch Amdurski H, Dadon T, Blumenfeld S, Derazne E, Hemi R, Orvieto R. et al. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod 2022;37:534–541. [DOI] [PubMed] [Google Scholar]
- Nana M, Nelson-Piercy C.. COVID-19 in pregnancy. Clin Med 2021;21:e446–e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura S, Ebina H.. Could live attenuated vaccines better control COVID-19? Vaccine 2021;39:5719–5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas J, Nahum R, Aizer A.. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reprod Biol Endocrinol 2021;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardi N, Hogan MJ, Porter FW, Weissman D.. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaghi H, Meghani M, Pingali C, Crane B, Naleway A, Weintraub E, Kenigsberg TA, Lamias MJ, Irving SA, Kauffman TL. et al. COVID-19 vaccination coverage among pregnant women during pregnancy—Eight Integrated Health Care Organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep 2021;70:895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamshirsaz AA, Hessami K, Morain S, Afshar Y, Nassr AA, Arian SE, Asl NM, Aagaard K.. Intention to receive COVID-19 vaccine during pregnancy: a systematic review and meta-analysis. Am J Perinatol 2022;39:492–500. [DOI] [PubMed] [Google Scholar]
- Soleimanpour S, Yaghoubi A.. COVID-19 vaccine: where are we now and where should we go? Expert Rev Vaccines 2021;20:23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaisavljevic V, Apter S, Capalbo A, D'Angelo A, Gianaroli L, Griesinger G, Kolibianakis EM, Lainas G, Mardesic T, Motrenko T. et al. ; ESHRE Clinic PI Working Group. The Maribor consensus: report of an expert meeting on the development of performance indicators for clinical practice in ART. Hum Reprod Open 2021;2021:hoab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cao M, Lin Y, Xu Z, Liang Z, Huang Q, Li S, Li L, Meng Y, An C. et al. Inactivated COVID-19 vaccination does not affect in vitro fertilization outcomes in women. Hum Reprod 2022;37:2054–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Ying Y, Cao M, Liu J, Liu H.. Trophectoderm biopsy of blastocysts for a preimplantation genetic test does not affect serum beta-hCG levels in early pregnancy: a study using propensity score matching. J Ovarian Res 2021;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.