Abstract
Cellular senescence, once thought an artifact of in vitro culture or passive outcome of aging, has emerged as fundamental to tissue development and function. The senescence mechanism importantly halts cell cycle progression to protect against tumor formation, while transiently present senescent cells produce a complex secretome (or SASP) of inflammatory mediators, proteases, and growth factors that guide developmental remodeling and tissue regeneration. Transiently present senescence is important for skin repair, where it accelerates extracellular matrix formation, limits fibrosis, promotes reepithelialization, and modulates inflammation. Unfortunately, advanced age and diabetes drive pathological accumulation of senescent cells in chronic wounds, which is perpetuated by a proinflammatory SASP, advanced glycation end-products, and oxidative damage. Although the biology of wound senescence remains incompletely understood, drugs that selectively target senescent cells are showing promise in clinical trials for diverse pathological conditions. It may not be long before senescence-targeted therapies will be available for the management, or perhaps even prevention, of chronic wounds.
Hayflick and Moorhead (1961) first identified cellular senescence when they observed cultured primary human fibroblasts had a finite life span, undergoing irreversible cell cycle arrest following a limited number of population doublings. What was originally dismissed as a cell culture artifact is now widely acknowledged as a biological program that globally regulates cell fate. In the years following Hayflick's discovery of replicative senescence, it emerged that cellular senescence could also be triggered by the activation of oncogenes (Serrano et al. 1997), highlighting the importance of senescence as a robust antitumor mechanism. More recently, it has transpired that senescent cells are far from passive bystanders, instead displaying diverse phenotypes that contribute to tissue maintenance and dysfunction. In this review, we discuss the diverse roles of cellular senescence in tissue repair and pathology, highlighting exciting opportunities to develop senescence-targeted therapies to treat or prevent chronic wounds.
CELLULAR SENESCENCE PATHWAYS
Replicative and oncogenic stimuli are well-documented drivers of senescence (Demaria et al. 2015), but senescence can be induced by various other intrinsic and extrinsic stressors including DNA breaks (d'Adda di Fagagna 2008), oxidative and genotoxic stress (Nair et al. 2015; Erusalimsky 2020), epigenetic damage (Sidler et al. 2017), inflammation (Freund et al. 2010), and mitochondrial dysfunction (Chapman et al. 2019). Following insult, transient cell cycle arrest is induced via the p53/p21WAF1/CIP1 axis. If the damage stimulus persists, the p16INK4A/pRB tumor suppressor pathway can be engaged, causing irreversible senescence (He and Sharpless 2017). At the nexus of cell cycle regulation, p53 responds to telomere attrition (d'Adda di Fagagna et al. 2003; Herbig et al. 2004) and broad damage-induced signals (Ou and Schumacher 2018). Cell fate following DNA damage and p53 activation is context-dependent with subsequently activated signaling pathways directing a cell toward quiescence, programmed cell death (apoptosis), or senescence. A plethora of extrinsic factors sway this response, including the cell type and stress severity, while at the molecular level elaborate post-translational modifications affect p53 activity and function (Childs et al. 2014; Bourgeois and Madl 2018).
In most cases, p53 activation occurs in a DNA damage response (DDR)-dependent manner, whereby DDR sensors (e.g., ATM) phosphorylate p53 and its ubiquitin ligase, MDM2, leading to p53 stabilization (Hu et al. 2012). p53, in turn, transactivates p21, which governs the switch between proliferation, quiescence, and senescence. p21 acts by modulating the expression of many p53 targets (Mijit et al. 2020) and can broadly prevent cyclin-dependent kinase (CDK)-mediated inactivation of pRb (Bertoli et al. 2013). Importantly, p21 can induce transient growth arrest, because inactivation of the p53-p21 pathway in the absence of p16 reverses senescence (Beauséjour et al. 2003). Thus, engagement of the pRB/p16 pathway is often required for irreversible senescence. p16 is one of three tumor suppressors encoded by the INK4A/ARF locus, whose main role is preventing phosphorylation of pRb via CDK4 and CDK6 (Takahashi et al. 2006). pRb binds to E2F family transcription factors, repressing the activation of E2F target genes required for replication (Giacinti and Giordano 2006). Hence, failure to phosphorylate pRb prevents cell cycle progression. In addition to stimulating pRb-mediated inhibition of cell cycle progression, p53 promotes senescence by directly targeting E2F7, the only E2F family member up-regulated during senescence (Aksoy et al. 2012), and a number of p53-responsive miRNAs (Xu et al. 2019). E2F7 and pRB then reinforce repression of E2F target genes by promoting heterochromatization of E2F-responsive elements via recruitment of histone deacetylases and histone methyltransferases (Martínez-Zamudio et al. 2017).
While all forms of growth arrest are characterized by the presence of hypophosphorylated pRb family members (He and Sharpless 2017), there remain considerable differences between cells undergoing senescence versus those that become quiescent or terminally differentiated. Unlike cell quiescence, where cells enter cell cycle arrest in G0 and can reenter at any time in response to mitogenic signals (Terzi et al. 2016), cellular senescence occurs at G1, G1/S, and even G2 phases of the cell cycle (Blagosklonny 2011). Terminally differentiated cells develop specialized identities in response to developmental programming, whereas senescence is a fate shared by many cell types, often as a result of a sustained DDR (Chandler and Peters 2013). There are instances where the demarcations are less clear, because senescence is known to play a role in developmental processes (Muñoz-Espín et al. 2013; Storer et al. 2013), and terminally differentiated cells can undergo senescence (Jurk et al. 2012; Moreno-Blas et al. 2019), suggesting active inhibition of cell cycle is not always required for senescence.
In nonpathological states, senescent cells are typically removed from the body via apoptosis and immune clearance mechanisms. Following senescence induction, senescent cells undergo immunogenic conversion, producing a secretome of factors collectively known as the senescence-associated secretory phenotype (SASP) (Burton and Faragher 2015). The SASP can include a range of inflammatory chemokines that attract and activate various subsets of immune cells depending on their chemokine receptor repertoires (Acosta et al. 2013). This response thus leads to elimination of senescent cells by virtue of the immune system. Indeed, various immune cell types, including natural killer (NK) cells, T cells, neutrophils, and macrophages, are known to be involved in immune-mediated clearance of senescent cells (summarized in Sagiv and Krizhanovsky 2013).
CHARACTERIZING CELLULAR SENESCENCE—DETECTION
Reliable biomarkers for senescence detection are essential given its major role in aging and disease. However, detection of senescent cells remains challenging in the real-world setting for a number of reasons: (1) Senescence is context-dependent and cell-type specific (Kirschner et al. 2020); (2) Senescence is highly dynamic with different markers associated with early and late stages (Mijit et al. 2020); and (3) Several senescence markers are shared with other growth-arrested states (Itahana et al. 2007). It is important to note that not all senescence markers outlined in the following paragraphs will be a feature of every senescent cell. Therefore, it is essential that more than one senescence marker is present for a cell to be identified as senescent.
Senescent cells possess a range of morphological, functional, and molecular characteristics that can be influenced by intrinsic and extrinsic cues (Fig. 1). Typically, senescent cells take on a flattened, elongated appearance with enlarged vacuoles and nuclei (Wang and Dreesen 2018; Neurohr et al. 2019). They exhibit heterochromatin at E2F promoters, termed senescence-associated heterochromatin foci (SAHF), which potentiate senescence by preventing E2F target gene transcription (Narita et al. 2003). SAHF are relatively easy to visualize as they stain notably with DAPI (Kosar et al. 2011) but are not useful for senescence detection in murine cells, which are unable to produce robust SAHF (Aird and Zhang 2013). Histone loss and cytoplasmic chromatin fragments (positive for γH2AX and H3K9me3) have likewise been observed in vitro following replicative and oncogene-induced senescence (Ivanov et al. 2013). This observation suggests a compromised nuclear envelope, and is supported by loss of lamin B1, a major structural nuclear envelope protein, following senescence in vitro and in vivo (Shah et al. 2013; Wang et al. 2017; Saito et al. 2019). Given that persistent DNA damage is a primary cause of senescence, it is not surprising that senescent cells exhibit markers of DNA damage, such as γH2AX and ATM kinase, with reduced expression of DNA repair genes (Collin et al. 2018). However, DDR markers have been suggested to be of limited use for in vivo senescence detection, where the majority of cells with a DDR are responding to reparable damage (Herranz and Gil 2018).
Figure 1.
Characteristics of senescent cells. Senescent cells undergo cell cycle arrest and feature DNA alterations such as senescence-associated heterochromatin foci (SAHF), DNA-SCARS, and markers of DNA damage. A disrupted nuclear envelope is accompanied by reduction in the structural nuclear envelope protein, lamin B1, and the release of chromatin into the cytoplasm. Senescent cells show mitochondrial dysfunction, with increased production of reactive oxygen species (ROS) and up-regulation of prosurvival (antiapoptotic) pathways. Morphologically, senescent cells appear flattened and elongated with enlargement of lysosomes, enabling detection by senescence-associated β-galactosidase (SA-βGAL) and lipofuscin. In addition, senescent cells feature a senescence-associated secretory phenotype (SASP) containing proteases, cytokines, matrix metalloproteinases (MMPs), and extracellular vesicles (ECVs).
Senescence-associated β-galactosidase (SA-βGal) is the archetypical senescence biomarker, due to its expression across a broad range of senescent cell types and aged tissues (Dimri et al. 1995; Debacq-Chainiaux et al. 2009). The lysosomal hydrolase detected by SA-βGal, B-D-galactosidase, is ordinarily detected in nonsenescent cells at pH 4. However, senescence causes expansion of the lysosomal compartment, allowing detection of B-D-galactosidase at higher pH (Kuilman et al. 2010). Despite its wide use as a senescence biomarker, there remains controversy around the specificity of SA-βGal, with suggested nonspecific staining of skin appendages in vivo and quiescent cells in vitro (Krishna et al. 1999; Lee et al. 2006). Prolonged incubation can also lead to nonspecific staining, while the staining itself requires fresh tissue. Sudan Black B, which stains age-associated lipofuscin, has been recommended as an alternative to SA-βGal that can be used on archived tissues (Georgakopoulou et al. 2013). Nevertheless, SA-βGal staining remains the most extensively used method for detecting senescent cells, with commercial SA-βGal staining kits widely available. Note, in a recent study, Chia et al. (2021) failed to detect SA-βGal in young or aged human skin or acute wound tissue, despite observing up-regulation of other senescence markers following injury.
Arguably the most specific marker of senescence in vivo is p16, accumulating with age in a variety of tissues (Jeyapalan et al. 2007; Hall et al. 2017; Hudgins et al. 2018). However, not all tissues show age-dependent accumulation of p16 (Idda et al. 2020), senescence can occur in a p16-independent manner (Prieur et al. 2011), and some cancer cells express p16 (Romagosa et al. 2011). In addition, p16 has been suggested as a characteristic of “normal” immunological phenotypes, such as macrophage polarization (Hall et al. 2017) and T-cell exhaustion (Sharpless and Sherr 2015). Other methods to detect senescence include the absence of proliferation (Biran et al. 2017), the presence of a SASP (Coppé et al. 2008), loss of lamin B1 (Freund et al. 2012), SAHF (Aird and Zhang 2013), and DNA damage markers (Wang et al. 2009; Hooten and Evans 2017). Given the variability in senescence phenotype/markers (Wang et al. 2009; Idda et al. 2020), combinatorial approaches to validate senescence in tissues are preferred, such as using SA-βGAL with proliferation markers (Itahana et al. 2013; Biran et al. 2017) or in conjunction with p16 and p21 staining (Ritschka et al. 2017).
CHARACTERIZING CELLULAR SENESCENCE—OUTCOMES
It is now widely accepted that senescent cells actively contribute to progressive tissue dysfunction. One major feature of senescent cells, important in the context of the tissue microenvironment, is their complex secretome, termed a SASP (Coppé et al. 2008). The SASP, like senescence, is a dynamic process regulated by factors such as Notch1 (Hoare et al. 2016) and established in a temporal- and situation-dependent manner (Basisty et al. 2020). The SASP can include proinflammatory mediators, proteases, extracellular matrix (ECM) components, and growth factors (Coppé et al. 2008; Freund et al. 2010; Elzi et al. 2012). Lipids and exosomal cargo are also important SASP components (Basisty et al. 2020; Wallis et al. 2020; Narzt et al. 2021). Interestingly, damage-associated molecular patterns, such as HMGB1 and specific Toll-like receptors, are required for SASP induction (Davalos et al. 2013; Hari et al. 2019).
SASP factors not only reinforce cell cycle arrest in an autocrine manner (e.g., Acosta et al. 2008), but exacerbate inflammation, accelerate tissue breakdown, and promote paracrine induction of senescence (Acosta et al. 2013; Davalos et al. 2013; Severino et al. 2013). Indeed, the proinflammatory SASP feature of senescent cells is often a DDR, controlled at the transcriptional level by NF-κB (Rodier et al. 2009), C/EBP (Shao et al. 2016), mTOR (Herranz et al. 2015), p38MAPK (Freund et al. 2011), and Gata4 (Kang et al. 2015). Notably, the SASP can also be beneficial in particular situations, reinforcing senescence (Acosta et al. 2008), promoting senescent cell clearance (Eggert et al. 2016), preventing tumors (Lujambio et al. 2013), and aiding tissue repair (Demaria et al. 2014). While the SASP has clear implications for tissue homeostasis and pathology, understanding the context-dependent diversity of the SASP remains a key challenge.
SENESCENCE FROM EMBRYOGENESIS TO TISSUE AGING
It is well established that senescence is a dynamic stress response, evolved to prevent incipient neoplastic transformation (Campisi and d'Adda di Fagagna 2007). Tens of thousands of DNA alterations occur in an individual cell per day (Jackson and Bartek 2009). Therefore, along with other proofreading mechanisms, senescence is crucial to avert unrestrained proliferation of mutated cells. In young organisms, this process is highly efficient, with resulting senescent cells effectively cleared by the immune system. However, cellular senescence is often considered a double-edged sword because, as we age, this process becomes perturbed, resulting in disease (Kowald et al. 2020).
Intriguingly, senescence has emerged as far more than an anticancer mechanism, or outcome of advanced cellular age. Indeed, it has now been shown to play diverse roles in the development and maintenance of tissues. Seminal publications documented senescent cell accumulation in the signaling hubs of murine and human embryos at restricted time windows (Muñoz-Espín et al. 2013; Storer et al. 2013). Detailed evaluation revealed the developmental importance of p21-dependent senescence, whereby senescent cells directed macrophage-mediated clearance and embryonic remodeling (Muñoz-Espín et al. 2013; Storer et al. 2013). Senescence and macrophage-mediated clearance has since been shown to be important for development of the inner ear in mice and chickens (Gibaja et al. 2019), and in the patterning of kidney, cement gland, and brain of amphibians (Davaapil et al. 2017; Villiard et al. 2017). Senescence may be vital even earlier in development, as extravillous trophoblasts, required for placenta formation, lose their replicative potential and develop a SASP following invasion into the uterine lining (Velicky et al. 2018). Senescent cells are subsequently cleared from the endometrium by uterine NK cells (Brighton et al. 2017). These studies open questions around the origins of senescence, and whether its links to development (and tissue repair) precede its antitumorigenic role. However, the degree of conservation of senescence programming and its functional requirements during development remain largely unknown.
In contrast to aging and pathology, where senescence is a stochastic damage response, senescence observed during development is instead a highly organized transient process with complimentary apoptotic and immune clearance mechanisms. At face value, it appears that transiently present and pathological senescence are dichotomous. A key factor governing the switch between beneficial and detrimental states appears to be effective immune-mediated clearance (Rhinn et al. 2019). During transiently present senescence observed during development (Storer et al. 2013), regeneration (Yun et al. 2015), and wound healing (Demaria et al. 2014), senescent cells are removed by macrophages, neutrophils, T lymphocytes, and NK cells (Xue et al. 2007; Song et al. 2020). Immune cells are able to locate senescent cells by the factors they secrete (Iannello et al. 2013; Sagiv and Krizhanovsky 2013), controlled at the epigenetic level by BRD4 (Tasdemir et al. 2016). Senescent cells also express stimulatory ligands that bind the NKG2D receptor and activate killing by T cells (Sagiv et al. 2016). In mouse tissues, developmental senescence appears to be solely modulated via p21, independent of p16 (Muñoz-Espín et al. 2013; Storer et al. 2013). However, similar to DDR-linked senescence, developmental senescence and SASP requires TGF-β/SMAD signaling (Muñoz-Espín et al. 2013; Tominaga and Suzuki 2019).
The processes underpinning senescence accumulation in aged tissues are also not fully understood. Initial induction is likely mediated by replicative exhaustion, shortening of telomeres, and activation of senescence pathways (Reaper et al. 2004). Subsequently, advanced age is associated with long-term exposure to intrinsic and extrinsic damage signals. At the molecular level, aging perturbs the developmental machinery responsible for repressing senescence such that the INK/ARF locus loses repressive marks, hence increasing p16 sensitivity to induction (Martin et al. 2014). This combination of increased susceptibility and continuous damage signals heighten senescence onset, which is then reinforced in an intracrine manner by the production of a proinflammatory SASP (Acosta et al. 2008; Martien et al. 2013; Hsieh et al. 2017). Additionally, the SASP can potentiate senescence to the neighboring microenvironment in a paracrine manner by activating a number of receptor pathways, including CCR2 (Acosta et al. 2013), TGFBR1 (Acosta et al. 2013; Bird et al. 2018; Ferreira-Gonzalez et al. 2018), and CXCR2 (Wilkinson et al. 2019a). The age-related SASP differs from developmental SASP, containing secreted factors known to drive widespread inflammation and tissue destruction. Ironically, the age-related SASP is also rich in potent mitogenic drivers, including proteases, growth factors, and cytokines, which can enhance tumorigenesis (Coppé et al. 2008; Yoshimoto et al. 2013; Eggert et al. 2016). Consequently, a mechanism selected for its beneficial anti-effects in the young can become maladaptive in later life. The diverse roles of senescence are summarized in Figure 2.
Figure 2.
Diverse roles for senescence throughout life. Transiently present (short-term) senescence is required during development, tissue regeneration, and wound repair. Here, senescent cells produce a beneficial senescence-associated secretory phenotype (SASP) that guides developmental patterning and tissue restoration following injury. Effective clearance of senescent cells during these processes prevents chronicity. By contrast, chronological aging leads to accumulation of cellular stress, which drives senescence. Chronic senescence is exacerbated by defective clearance mechanisms and unrestrained inflammation, leading to widespread tissue damage and increased risk of pathology.
Another reason senescent cells accumulate during aging is impairment in clearance mechanisms, such as redistribution of NK cell subtypes (Solana et al. 2014; Sagiv et al. 2016; Ovadya et al. 2018). It is widely acknowledged that aging causes dysfunction to both the innate and adaptive immune systems, termed immunosenescence (Song et al. 2020). Some senescent cells evade NK- and CD8+ T-cell-mediated clearance by expressing high levels of HLA-E, which bind the inhibitory receptor NK2GA (Pereira et al. 2019). Others shed MICA and MICB to avoid detection by NKG2D (Muñoz et al. 2019). Moreover, age decreases expression of NKG2A in NK cells, thus reducing clearance mechanisms (Lutz et al. 2005). The SASP may also aid senescent cell evasion from immune clearance in certain contexts (Ruhland et al. 2016; Pereira et al. 2019).
The detrimental role of senescence during aging is well established, where elevated numbers of senescent cells are associated with reduced tissue functionality in mice (Molofsky et al. 2006; Ovadya et al. 2018; Xu et al. 2018; Palmer et al. 2019; Cai et al. 2020) and humans (Justice et al. 2018; Gustafson et al. 2019), while the proinflammatory SASP contributes to many pathologies (Xu et al. 2015; Oubaha et al. 2016). Direct evidence comes from studies where transplantation of senescent cells to young mice induces disease states, such as osteoarthritis (Xu et al. 2017) and lower physical activity (Xu et al. 2018), likely via enhanced paracrine induction of senescence (da Silva et al. 2019). By contrast, selective removal of senescent cells is known to alleviate many age-related pathologies and extend life span in experimental models (Baker et al. 2011, 2016; Hashimoto et al. 2016; Roos et al. 2016; Ogrodnik et al. 2017; Xu et al. 2018; Yousefzadeh et al. 2018).
SENESCENCE IN WOUND REPAIR AND REGENERATION
When a tissue repairs or regenerates, it reuses processes associated with development and morphogenesis. This is true for senescence, where the SASP can enable reprogramming of cells to a stem cell–like fate following tissue injury (Mosteiro et al. 2016; Chiche et al. 2017) or during regeneration (Yun et al. 2015; Ritschka et al. 2017). The parallels between development and tissue regeneration are clear. Senescence is tightly regulated to aid limb regeneration in the salamander, with effective clearance mediated by macrophages, even following multiple rounds of amputation and regeneration (Yun et al. 2015). Senescence, mediated via Ccn1, is also required for regeneration of the embryonic murine heart (Feng et al. 2019), while ablation of senescent cells in zebrafish abrogates pectoral fin regeneration (Da Silva-Álvarez et al. 2020).
The sophisticated host response to injury can be described as four overlapping phases: hemostasis, inflammation, proliferation, and remodeling (Gurtner et al. 2008; Eming et al. 2014). Each of these stages requires a temporal and dynamic interplay between various signaling cascades and cell types, where the role of senescence remains less well understood (Wilkinson and Hardman 2020a). The processes that occur during wound healing involve many mitogenic factors that enable partial epithelial-to-mesenchymal transition in keratinocytes to aid wound closure, and rapid proliferation of fibroblasts to restore the dermal matrix. Additionally, the complex population of immune cells provide an environment enriched for secreted factors that promote plasticity (Shaw and Martin 2016). Given the close links to cancer, developmental remodeling, and regeneration, it is perhaps unsurprising that research is now uncovering vital roles for transient presence of senescence in tissue repair.
There is now strong evidence that induction of transiently present senescence is able to prevent excessive fibrosis following injury in multiple murine tissues (Krizhanovsky et al. 2008; Jun and Lau 2010; Meyer et al. 2016). Pivotal studies revealed that transiently present senescence occurs during murine skin wound repair, and that Ccn1 and Ccn2 are important drivers of this response (Jun and Lau 2010, 2017). Intriguingly, Ccn1 has also been reported as a pattern-recognition receptor vital to prevent wound infection (Jun and Lau 2020). A demonstration that transiently present senescence is beneficial to healing was provided by Demaria et al. (2014), where specific ablation of p16- and p21-expressing cells significantly delayed cutaneous wound closure and reduced ECM deposition. Here, injury-induced senescent cells produced a PDGF-AA-rich SASP, crucial for stimulating myofibroblast differentiation and enabling effective healing. More recently, Hiebert et al. (2018) showed that Nrf2-triggered induction of senescence in fibroblasts accelerated both reepithelialization and ECM deposition. Transiently present senescence is not confined to skin wounds, with reported observations during corneal (Wang et al. 2019) and lung (Kobayashi et al. 2020) injury. In acute lung injury, p21 activation limits apoptosis and ameliorates tissue damage (Blázquez-Prieto et al. 2021).
The above investigations provide new insight into the importance of transiently present senescence during tissue repair. However, many questions remain unanswered. At what point do cells become susceptible to acute injury-induced senescence? Which cell types and why? How are these cells effectively cleared to prevent the switch to a chronic state? It is likely that injury causes the release of a myriad of factors stimulating senescence induction, such as reactive oxygen species (ROS) (Jun and Lau 2010; Passos et al. 2010) with others still to be identified. Effective clearance likely occurs by virtue of the diverse inflammatory profile of wounds (Wilkinson and Hardman 2020a), yet uncertainties remain around specificity and regulation. Finally, a major limitation of existing studies is that they are almost exclusively limited to in vivo models of wound repair, and we currently have a limited understanding of how these observations will translate to human healing. In a recent proof-of-concept study, Chia et al. (2021) demonstrated that p21 and p53, but not p16, were induced in acute wound repair in young subjects, while neither p21, p53, nor p16 were induced in older subjects.
SENESCENCE IN CHRONIC WOUND HEALING
Unlike internal organs, senescence in the skin is induced by a combination of intrinsic chronological aging and external factors such as ultraviolet radiation exposure (Rittié and Fisher 2015). Indeed, the skin is characterized by a dense matrix of structural proteins, with degradation and remodeling of this ECM leading to loss of physiological and biomechanical integrity (Wilkinson and Hardman 2021a). Ultraviolet radiation is widely reported to induce skin senescence by increasing ROS levels (Herrling et al. 2006; Jenkins et al. 2011; Wang et al. 2017), with aging epidermis and dermis both characterized by increased p16- and p21-positive cells (Ressler et al. 2006; Waaijer et al. 2012; Idda et al. 2020). Aged skin additionally exhibits loss of lamin b1 (Dreesen et al. 2013), shortened telomeres (particularly in the epidermis; Sugimoto et al. 2006), increased mutations in mitochondrial DNA (Berneburg et al. 1997), and an elevated SASP, including MMPs (Quan et al. 2009) and PAI-1 (Goldstein et al. 1994; Baker et al. 2008). Factors contributing to the age-related accumulation of SASP include higher levels of histone variant H2A.J in the epidermis, which promotes inflammation (Contrepois et al. 2017), and higher numbers of immunosuppressive cell types, reducing senescent cell clearance (Ruhland et al. 2016).
An important characteristic of skin is the high turnover of epidermal keratinocytes, essential to maintain the skin barrier (and repair epidermal damage). This high renewal capacity is aided by stem cell niches in the epidermal basal layer, hair follicles, and sebaceous glands (Pincelli and Marconi 2010; Donati and Watt 2015). Sebaceous gland function declines with age (Zouboulis et al. 2008), causing decreased production of enzymes required to synthesize long-chain fatty acids and cholesterol (Seyfarth et al. 2011). Age-associated loss of lipids and reduced keratinocyte renewal lead to an impaired skin barrier. Aged keratinocytes show altered cell cycle kinetics and lower proliferation rates (Giangreco et al. 2008; Charruyer et al. 2009), coinciding with accumulation of senescence (Zou et al. 2021). Intriguingly, hair follicle stem cell abundance is not altered with chronological age in mice (Giangreco et al. 2008), yet aged hair follicle stem cells possess lower chromatin accessibility, which is linked to decreased renewal capacity (Koester et al. 2021). The ability to regenerate tissues and repair injuries declines with age throughout the body (e.g., in the muscle) (Jang et al. 2011; Sousa-Victor et al. 2014), while an age-related increase in p16 is associated with reduced stem cell capacity in the brain, kidney, hemopoietic system, and other tissues (Janzen et al. 2006; Krishnamurthy et al. 2006; Molofsky et al. 2006).
Age-associated decline in skin barrier increases susceptibility to injury and infection. Thus, it is unsurprising that age is a major risk factor for the development of chronic, nonhealing wounds, with high morbidity and mortality in patients (Guest et al. 2015; Han and Ceilley 2017). A second key risk factor for chronic wound development is diabetes. Diabetes is also closely linked to senescence as hyperglycemia accelerates the formation of advanced glycation end products, triggering oxidative damage and driving unrestrained inflammation (Stegenga et al. 2008; Fang et al. 2016; Moura et al. 2019; Wilkinson and Hardman 2021b). It has been known for more than 20 years that fibroblasts isolated from chronic wounds are predisposed to senescence (Mendez et al. 1998; Vande Berg et al. 1998; Ågren et al. 1999; Stanley and Osler 2001). However, there has been little attempt to determine the molecular and cellular drivers of pathological wound senescence, nor to functionally demonstrate a link to poor healing outcome. We recently reported that diabetic macrophages are susceptible to senescence, showing that they delay healing in a non-aged murine model of diabetic wound repair (Wilkinson et al. 2019a). Interestingly, this process was modulated by CXCR2, an important senescence mediator (Acosta et al. 2008).
A hallmark of chronic wounds is prolonged and excessive local inflammation, where bacterial colonization and impaired cell behaviors combine to drive extensive immune cell recruitment and retention (Wilkinson and Hardman 2020b). This provides an optimum environment for rapid senescence induction. For example, neutrophils produce high levels of ROS, which cause paracrine induction of senescence in neighboring fibroblasts by telomere shortening (Lagnado et al. 2021). Chronic wounds also display elevated cytokines and chemokines, key SASP components that skew macrophages toward a proinflammatory state (Lujambio et al. 2013). A range of local factors, including pathogenic bacterial products (Muller et al. 2009; Elsayed et al. 2021) and tissue iron (Sindrilaru et al. 2011; Wilkinson et al. 2019b), likely reinforce chronic wound senescence by contributing to unresolved inflammation and perturbed immune cell function. A chronic wound milieu, which induces cellular perturbations associated with chronicity, such as epidermal hyperproliferation (Stojadinovic et al. 2008) and reduced angiogenesis (Lauer et al. 2000), has been shown to directly induce senescence in neonatal fibroblasts (Mendez et al. 1999).
What remains somewhat perplexing is that senescence and the SASP naturally induce pluripotency and regenerative capacity, yet during tissue aging they contribute to inflammation and pathology (summarized in Fig. 3). It could be that this disparity reflects differences in the level and persistence of the response. This has been demonstrated recently, where short-term senescence induction in keratinocytes induced pluripotency and regeneration in skin grafts, while prolonged exposure to the SASP reduced pluripotency and increased numbers of p16+ senescent cells (Ritschka et al. 2017). Conversely, the SASP itself could change over time in a context-dependent manner. For example, Hoare et al. (2016) showed that fibroblast SASP is characterized by a Notch-high early phase, and Notch-low late phase. The early phase was TGF-β-dependent and associated with tissue regeneration and immunosuppression, while the late phase was NF-κB dependent and linked to inflammation.
Figure 3.
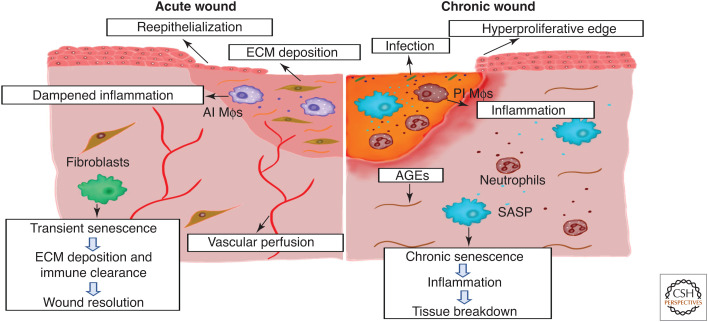
Senescence in acute versus chronic wound healing. In acute wounds, transiently present senescent cells appear during late-stage healing, producing a senescence-associated secretory phenotype (SASP) that aids extracellular matrix (ECM) deposition but prevents tissue fibrosis. Senescent cells are then cleared by the immune system, allowing full tissue resolution. During aging and diabetes, advanced glycation end products (AGEs) and sterile inflammation promote the accumulation of senescent cells. Following injury, these resident senescent cells contribute to a proinflammatory environment that perpetuates senescence, causes tissue breakdown, and prevents healing. Senescence (and inflammation) can also be exacerbated by chronic wound infection. (AI Mφs) Anti-inflammatory macrophages, (PI Mφs) proinflammatory macrophages.
From a clinical perspective, extensive chronic wound recalcitrance highlights an urgent need to improve intervention strategies. While we are far from fully understanding the contribution of senescence to chronic wounds, studies into the role of senescence in other pathologies could provide a timely opportunity to repurpose new and existing therapies. Indeed, the outcomes of senescent cell ablation using genetic models (Baker et al. 2011, 2016) have now been confirmed using drugs that selectively target cellular senescence, referred to as senolytics (Kirkland and Tchkonia 2020). Senolytics can act upon senescence machinery, for example, by targeting the prosurvival pathways (BCL-2 and others) that provide senescent cells with apoptosis resistance (Zhu et al. 2015; Chang et al. 2016; Hohmann et al. 2019). Elimination of senescent cells using senolytics in these models alleviated many age-related diseases and restored tissue function. Moreover, a BCL-2 family inhibitor reduced epidermal senescence and promoted hair follicle stem cell proliferation in mice with epidermal overexpression of p14Arf (Yosef et al. 2016). Another potential strategy is to target the SASP by blocking NF-κB nuclear translocation (Moiseeva et al. 2013), inhibiting the JAK/STAT pathway (Xu et al. 2015; Farr et al. 2017), or suppressing BRD4 (Tasdemir et al. 2016).
To date, the majority of studies using senolytics to target chronic senescence have been preclinical. Therapeutic intervention for human disease is complex, particularly as most elderly patients suffer multimorbidity, presenting with two or more conditions (Guisado-Clavero et al. 2018). As chronic wounds primarily affect the elderly and/or diabetic, compatibility with other treatments must be considered to prevent contraindication or reduced efficacy. Despite these limitations, many senolytics are FDA-approved cancer drugs or natural products, making the clinical pathway for wound repurposing highly attractive. Indeed, a handful of recent clinical trials are starting to suggest the benefit of senolytics in other chronic indications, such as patients with diabetic kidney disease (Hickson et al. 2019) or idiopathic pulmonary fibrosis (Justice et al. 2019), while others are underway (summarized in Robbins et al. 2021).
CONCLUDING REMARKS
Understanding of cellular senescence has progressed rapidly since the concept was first proposed in the 1960s. A process once thought to be an artifact of cell culture is now known to be essential for tumor suppression, developmental reprogramming, regeneration, and wound repair. Whereas the presence of transient senescence is beneficial for tissue maintenance, excessive senescence, as a result of age-related accumulation and defective immune clearance, contributes to many disease states. Although we are still a long way from unraveling the role of senescence in poor wound healing, preliminary in vivo studies have revealed the therapeutic potential of targeting senescence to promote wound repair. When coupled with emerging efficacy data from senolytic clinical trials in other chronic indications, it is clear that a senolytic-based strategy for chronic wounds treatment could be a reality in the not so distant future.
Footnotes
Editors: Xing Dai, Sabine Werner, Cheng-Ming Chuong, and Maksim Plikus
Additional Perspectives on Wound Healing: From Bench to Bedside available at www.cshperspectives.org
COMPETING INTEREST STATEMENT
The authors have no direct funding to declare.
REFERENCES
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018. 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, et al. 2013. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15: 978–990. 10.1038/ncb2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. 1999. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Invest Dermatol 112: 463–469. 10.1046/j.1523-1747.1999.00549.x [DOI] [PubMed] [Google Scholar]
- Aird KM, Zhang R. 2013. Detection of senescence-associated heterochromatin foci (SAHF). Methods Mol Biol 965: 185–196. 10.1007/978-1-62703-239-1_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy O, Chicas A, Zeng T, Zhao Z, McCurrach M, Wang X, Lowe SW. 2012. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes Dev 26: 1546–1557. 10.1101/gad.196238.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Perez-Terzic C, Jin F, Pitel KS, Niederländer NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ et al. 2008. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol 10: 825–836. 10.1038/ncb1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, Van De Sluis B, Kirkland JL, Van Deursen JM. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, et al. 2016. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530: 184–189. 10.1038/nature16932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, et al. 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18: e3000599. 10.1371/journal.pbio.3000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. 2003. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22: 4212–4222. 10.1093/emboj/cdg417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneburg M, Gattermann N, Stege H, Grewe M, Vogelsang K, Ruzicka T, Krutmann J. 1997. Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochem Photobiol 66: 271–275. 10.1111/j.1751-1097.1997.tb08654.x [DOI] [PubMed] [Google Scholar]
- Bertoli C, Klier S, McGowan C, Wittenberg C, de Bruin RA. 2013. Chk1 inhibits E2F6 repressor function in response to replication stress to maintain cell-cycle transcription. Curr Biol 23: 1629–1637. 10.1016/j.cub.2013.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. 2017. Quantitative identification of senescent cells in aging and disease. Aging Cell 16: 661–671. 10.1111/acel.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TG, Müller M, Boulter L, Vincent DF, Ridgway RA, Lopez-Guadamillas E, Lu WY, Jamieson T, Govaere O, Campbell AD, et al. 2018. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci Transl Med 10: eaan1230. 10.1126/scitranslmed.aan1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. 2011. Cell cycle arrest is not senescence. Aging (Albany NY) 3: 94–101. 10.18632/aging.100281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez-Prieto J, Huidobro C, López-Alonso I, Amado-Rodriguez L, Martín-Vicente P, López-Martínez C, Crespo I, Pantoja C, Fernandez-Marcos PJ, Serrano M, et al. 2021. Activation of p21 limits acute lung injury and induces early senescence after acid aspiration and mechanical ventilation. Transl Res 233: 104–116. 10.1016/j.trsl.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois B, Madl T. 2018. Regulation of cellular senescence via the FOXO 4-p53 axis. FEBS Lett 592: 2083–2097. 10.1002/1873-3468.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L, et al. 2017. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 6: e31274. 10.7554/eLife.31274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DGA, Faragher RGA. 2015. Cellular senescence: from growth arrest to immunogenic conversion. Age (Omaha) 37: 1–19. 10.1007/s11357-015-9764-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Zhou H, Zhu Y, Sun Q, Ji Y, Xue A, Wang Y, Chen W, Yu X, Wang L, et al. 2020. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res 30: 574–589. 10.1038/s41422-020-0314-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d'Adda di Fagagna FD. 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8: 729–740. 10.1038/nrm2233 [DOI] [PubMed] [Google Scholar]
- Chandler H, Peters G. 2013. Stressing the cell cycle in senescence and aging. Curr Opin Cell Biol 25: 765–771. 10.1016/j.ceb.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, et al. 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83. 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Fielder E, Passos JF. 2019. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593: 1566–1579. 10.1002/1873-3468.13498 [DOI] [PubMed] [Google Scholar]
- Charruyer A, Barland CO, Yue L, Wessendorf HB, Lu Y, Lawrence HJ, Mancianti ML, Ghadially R. 2009. Transit-amplifying cell frequency and cell cycle kinetics are altered in aged epidermis. J Invest Dermatol 129: 2574–2583. 10.1038/jid.2009.127 [DOI] [PubMed] [Google Scholar]
- Chia CW, Sherman-Baust CA, Larson SA, Pandey R, Withers R, Karikkineth AC, Zukley LM, Campisi J, Egan JM, Sen R, et al. 2021. Age-associated expression of p21and p53 during human wound healing. Aging Cell 20: e13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiche A, Le Roux I, von Joest M, Sakai H, Aguín SB, Cazin C, Salam R, Fiette L, Alegria O, Flamant P, et al. 2017. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20: 407–414.e4. 10.1016/j.stem.2016.11.020 [DOI] [PubMed] [Google Scholar]
- Childs BG, Baker DJ, Kirkland JL, Campisi J, Van Deursen JM. 2014. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15: 1139–1153. 10.15252/embr.201439245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Huna A, Warnier M, Flaman JM, Bernard D. 2018. Transcriptional repression of DNA repair genes is a hallmark and a cause of cellular senescence. Cell Death Dis 9: 1–4. 10.1038/s41419-018-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contrepois K, Coudereau C, Benayoun BA, Schuler N, Roux PF, Bischof O, Courbeyrette R, Carvalho C, Thuret JY, Ma Z, et al. 2017. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat Commun 8: 1–8. 10.1038/ncomms14995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun YU, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: e301. 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Adda di Fagagna F. 2008. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer 8: 512–522. 10.1038/nrc2440 [DOI] [PubMed] [Google Scholar]
- d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. 2003. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198. 10.1038/nature02118 [DOI] [PubMed] [Google Scholar]
- da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, et al. 2019. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18: e12848. 10.1111/acel.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva-Álvarez S, Guerra-Varela J, Sobrido-Cameán D, Quelle A, Barreiro-Iglesias A, Sánchez L, Collado M. 2020. Cell senescence contributes to tissue regeneration in zebrafish. Aging Cell 19: e13052. 10.1111/acel.13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaapil H, Brockes JP, Yun MH. 2017. Conserved and novel functions of programmed cellular senescence during vertebrate development. Development 144: 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J. 2013. p53-dependent release of alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201: 613–629. 10.1083/jcb.201206006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O. 2009. Protocols to detect senescence-associated β-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protoc 4: 1798–1806. 10.1038/nprot.2009.191 [DOI] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé ME, et al. 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Desprez PY, Campisi J, Velarde MC. 2015. Cell autonomous and non-autonomous effects of senescent cells in the skin. J Invest Dermatol 135: 1722–1726. 10.1038/jid.2015.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens MA, Rubelj I, Pereira-Smith O. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci 92: 9363–9367. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati G, Watt FM. 2015. Stem cell heterogeneity and plasticity in epithelia. Cell Stem Cell 16: 465–476. 10.1016/j.stem.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Dreesen O, Chojnowski A, Ong PF, Zhao TY, Common JE, Lunny D, Lane EB, Lee SJ, Vardy LA, Stewart CL, et al. 2013. Lamin B1 fluctuations have differential effects on cellular proliferation and senescence. J Cell Biol 200: 605–617. 10.1083/jcb.201206121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Wolter K, Ji J, Ma C, Yevsa T, Klotz S, Medina-Echeverz J, Longerich T, Forgues M, Reisinger F, et al. 2016. Distinct functions of senescence-associated immune responses in liver tumor surveillance and tumor progression. Cancer Cell 30: 533–547. 10.1016/j.ccell.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsayed R, Elashiry M, Liu Y, El-Awady A, Hamrick M, Cutler CW. 2021. Porphyromonas gingivalis provokes exosome secretion and paracrine immune senescence in bystander dendritic cells. Front Cell Infect Microbiol 11: 669989. 10.3389/fcimb.2021.669989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi DJ, Lai Y, Song M, Hakala K, Weintraub ST, Shiio Y. 2012. Plasminogen activator inhibitor 1-insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc Natl Acad Sci 109: 12052–12057. 10.1073/pnas.1120437109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. 2014. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6: 265sr6. 10.1126/scitranslmed.3009337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erusalimsky JD. 2020. Oxidative stress, telomeres and cellular senescence: what non-drug interventions might break the link? Free Radic Biol Med 150: 87–95. 10.1016/j.freeradbiomed.2020.02.008 [DOI] [PubMed] [Google Scholar]
- Fang M, Wang J, Li S, Guo Y. 2016. Advanced glycation end-products accelerate the cardiac aging process through the receptor for advanced glycation end-products/transforming growth factor-β-smad signaling pathway in cardiac fibroblasts. Geriatr Gerontol Int 16: 522–527. 10.1111/ggi.12499 [DOI] [PubMed] [Google Scholar]
- Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, et al. 2017. Targeting cellular senescence prevents age-related bone loss in mice. Nature Med 23: 1072–1079. 10.1038/nm.4385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Meng J, Kou S, Jiang Z, Huang X, Lu Z, Zhao H, Lau LF, Zhou B, Zhang H. 2019. CCN1-induced cellular senescence promotes heart regeneration. Circulation 139: 2495–2498. 10.1161/CIRCULATIONAHA.119.039530 [DOI] [PubMed] [Google Scholar]
- Ferreira-Gonzalez S, Lu WY, Raven A, Dwyer B, Man TY, O'Duibhir E, Lewis PJ, Campana L, Kendall TJ, Bird TG, et al. 2018. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Commun 9: 1–5. 10.1038/s41467-018-03299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16: 238–246. 10.1016/j.molmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Patil CK, Campisi J. 2011. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J 30: 1536–1548. 10.1038/emboj.2011.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Laberge RM, Demaria M, Campisi J. 2012. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 23: 2066–2075. 10.1091/mbc.e11-10-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez MP, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG. 2013. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5: 37–50. 10.18632/aging.100527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacinti C, Giordano A. 2006. RB and cell cycle progression. Oncogene 25: 5220–5227. 10.1038/sj.onc.1209615 [DOI] [PubMed] [Google Scholar]
- Giangreco A, Qin M, Pintar JE, Watt FM. 2008. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell 7: 250–259. 10.1111/j.1474-9726.2008.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibaja A, Aburto MR, Pulido S, Collado M, Hurle JM, Varela-Nieto I, Magariños M. 2019. TGFβ2-induced senescence during early inner ear development. Sci Rep 9: 5912. 10.1038/s41598-019-42040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Moerman EJ, Fujii S, Sobel BE. 1994. Overexpression of plasminogen activator inhibitor type-1 in senescent fibroblasts from normal subjects and those with Werner syndrome. J Cell Physiol 161: 571–579. 10.1002/jcp.1041610321 [DOI] [PubMed] [Google Scholar]
- Guest JF, Ayoub N, McIlwraith T, Uchegbu I, Gerrish A, Weidlich D, Vowden K, Vowden P. 2015. Health economic burden that wounds impose on the national health service in the UK. BMJ Open 5: e009283. 10.1136/bmjopen-2015-009283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado-Clavero M, Roso-Llorach A, López-Jimenez T, Pons-Vigués M, Foguet-Boreu Q, Muñoz MA, Violán C. 2018. Multimorbidity patterns in the elderly: a prospective cohort study with cluster analysis. BMC Geriatr 18: 16. 10.1186/s12877-018-0705-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. 2008. Wound repair and regeneration. Nature 453: 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- Gustafson B, Nerstedt A, Smith U. 2019. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun 10: 2757. 10.1038/s41467-019-10688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BM, Balan V, Gleiberman AS, Strom E, Krasnov P, Virtuoso LP, Rydkina E, Vujcic S, Balan K, Gitlin II, Leonova KI. 2017. p16Ink4a and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 9: 1867. 10.18632/aging.101268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Ceilley R. 2017. Chronic wound healing: a review of current management and treatments. Adv Ther 34: 599–610. 10.1007/s12325-017-0478-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari P, Millar FR, Tarrats N, Birch J, Quintanilla A, Rink CJ, Fernández-Duran I, Muir M, Finch AJ, Brunton VG, et al. 2019. The innate immune sensor toll-like receptor 2 controls the senescence-associated secretory phenotype. Sci Adv 5: eaaw0254. 10.1126/sciadv.aaw0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, Sugimoto K, Sato T, Maruyama M, Sugimoto M. 2016. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight 1: e87732. 10.1172/jci.insight.87732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. 1961. The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]
- He S, Sharpless NE. 2017. Senescence in health and disease. Cell 169: 1000–1011. 10.1016/j.cell.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol Cell 14: 501–513. 10.1016/S1097-2765(04)00256-4 [DOI] [PubMed] [Google Scholar]
- Herranz N, Gil J. 2018. Mechanisms and functions of cellular senescence. J Clin Invest 128: 1238–1246. 10.1172/JCI95148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz N, Gallage S, Mellone M, Wuestefeld T, Klotz S, Hanley CJ, Raguz S, Acosta JC, Innes AJ, Banito A, et al. 2015. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol 17: 1205–1217. 10.1038/ncb3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrling T, Jung K, Fuchs J. 2006. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc 63: 840–845. 10.1016/j.saa.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Hickson LJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, et al. 2019. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine 47: 446–456. 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert P, Wietecha MS, Cangkrama M, Haertel E, Mavrogonatou E, Stumpe M, Steenbock H, Grossi S, Beer HD, Angel P, et al. 2018. Nrf2-mediated fibroblast reprogramming drives cellular senescence by targeting the matrisome. Dev Cell 46: 145–161.e10. 10.1016/j.devcel.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, et al. 2016. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18: 979–992. 10.1038/ncb3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr, Hogaboam CM. 2019. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol 60: 28–40. 10.1165/rcmb.2017-0289OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten NN, Evans MK. 2017. Techniques to induce and quantify cellular senescence. J Vis Exp 123: e55533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HH, Chen YC, Jhan JR, Lin JJ. 2017. The serine protease inhibitor serpinB2 binds and stabilizes p21 in senescent cells. J Cell Sci 130: 3272–3281. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Levine AJ. 2012. The regulation of multiple p53 stress responses is mediated through MDM2. Genes Cancer 3: 199–208. 10.1177/1947601912454734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins AD, Tazearslan C, Tare A, Zhu Y, Huffman D, Suh Y. 2018. Age-and tissue-specific expression of senescence biomarkers in mice. Front Genet 9: 59. 10.3389/fgene.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Lowe SW, Raulet DH. 2013. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med 210: 2057–2069. 10.1084/jem.20130783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M. 2020. Survey of senescent cell markers with age in human tissues. Aging (Albany NY) 12: 4052–4066. 10.18632/aging.102903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itahana K, Campisi J, Dimri GP. 2007. Methods to detect biomarkers of cellular senescence: the senescence-associated β-galactosidase assay. Methods Mol Biol 371: 21–31. 10.1007/978-1-59745-361-5_3 [DOI] [PubMed] [Google Scholar]
- Itahana K, Itahana Y, Dimri GP. 2013. Colorimetric detection of senescence-associated β galactosidase. Methods Mol Biol 965: 143–156. 10.1007/978-1-62703-239-1_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Pawlikowski J, Manoharan I, van Tuyn J, Nelson DM, Rai TS, Shah PP, Hewitt G, Korolchuk VI, Passos JF, et al. 2013. Lysosome-mediated processing of chromatin in senescence. J Cell Biol 202: 129–143. 10.1083/jcb.201212110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. 2009. The DNA-damage response in human biology and disease. Nature 461: 1071–1078. 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Sinha M, Cerletti M, Dall'Osso C, Wagers AJ. 2011. Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb Symp Quant Biol 76: 101–111. 10.1101/sqb.2011.76.010652 [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443: 421–426. 10.1038/nature05159 [DOI] [PubMed] [Google Scholar]
- Jenkins NC, Liu T, Cassidy P, Leachman SA, Boucher KM, Goodson AG, Samadashwily G, Grossman D. 2011. The p16INK4A tumor suppressor regulates cellular oxidative stress. Oncogene 30: 265–274. 10.1038/onc.2010.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. 2007. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev 128: 36–44. 10.1016/j.mad.2006.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. 2010. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685. 10.1038/ncb2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. 2017. CCN2 induces cellular senescence in fibroblasts. J Cell Commun Signal 11: 15–23. 10.1007/s12079-016-0359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. 2020. CCN1 is an opsonin for bacterial clearance and a direct activator of Toll-like receptor signaling. Nat Commun 11: 1–5. 10.1038/s41467-020-15075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Jill Saffrey M, Cameron K, et al. 2012. Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11: 996–1004. 10.1111/j.1474-9726.2012.00870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ. 2018. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci 73: 939–945. 10.1093/gerona/glx134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, et al. 2019. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine 40: 554–563. 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L, Lu T, Yankner BA, Campisi J, Elledge SJ. 2015. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 349: aaa5612. 10.1126/science.aaa5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T. 2020. Senolytic drugs: from discovery to translation. J Intern Med 288: 518–536. 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner K, Rattanavirotkul N, Quince MF, Chandra T. 2020. Functional heterogeneity in senescence. Biochem Soc Trans 48: 765–773. 10.1042/BST20190109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Tata A, Konkimalla A, Katsura H, Lee RF, Ou J, Banovich NE, Kropski JA, Tata PR. 2020. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol 22: 934–946. 10.1038/s41556-020-0542-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester J, Miroshnikova YA, Ghatak S, Chacón-Martínez CA, Morgner J, Li X, Atanassov I, Altmüller J, Birk DE, Koch M, et al. 2021. Niche stiffening compromises hair follicle stem cell potential during ageing by reducing bivalent promoter accessibility. Nat Cell Biol 23: 771–781. 10.1038/s41556-021-00705-x [DOI] [PubMed] [Google Scholar]
- Kosar M, Bartkova J, Hubackova S, Hodny Z, Lukas J, Bartek J. 2011. Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type-and insult-dependent manner and follow expression of p16ink4a. Cell Cycle 10: 457–468. 10.4161/cc.10.3.14707 [DOI] [PubMed] [Google Scholar]
- Kowald A, Passos JF, Kirkwood TB. 2020. On the evolution of cellular senescence. Aging Cell 19: e13270. 10.1111/acel.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna DR, Sperker B, Fritz P, Klotz U. 1999. Does pH 6 β-galactosidase activity indicate cell senescence? Mech Ageing Dev 109: 113–123. 10.1016/S0047-6374(99)00031-7 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. 2006. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443: 453–457. 10.1038/nature05092 [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. 2008. Senescence of activated stellate cells limits liver fibrosis. Cell 134: 657–667. 10.1016/j.cell.2008.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. 2010. The essence of senescence. Genes Dev 24: 2463–2479. 10.1101/gad.1971610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagnado A, Leslie J, Ruchaud-Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, Collins AL, Vizioli MG, Habiballa L, Saretzki G, et al. 2021. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J 40: e106048. 10.15252/embj.2020106048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer G, Sollberg S, Cole M, Krieg T, Eming SA, Flamme I, Stürzebecher J, Mann K. 2000. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Invest Dermatol 115: 12–18. 10.1046/j.1523-1747.2000.00036.x [DOI] [PubMed] [Google Scholar]
- Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. 2006. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 5: 187–195. 10.1111/j.1474-9726.2006.00199.x [DOI] [PubMed] [Google Scholar]
- Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. 2013. Non-cell-autonomous tumor suppression by p53. Cell 153: 449–460. 10.1016/j.cell.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CT, Moore MB, Bradley S, Shelton BJ, Lutgendorf SK. 2005. Reciprocal age related change in natural killer cell receptors for MHC class I. Mech Ageing Dev 126: 722–731. 10.1016/j.mad.2005.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martien S, Pluquet O, Vercamer C, Malaquin N, Martin N, Gosselin K, Pourtier A, Abbadie C. 2013. Cellular senescence involves an intracrine prostaglandin E2 pathway in human fibroblasts. Biochim Biophys Acta Mol Cell Biol Lipids 1831: 1217–1227. 10.1016/j.bbalip.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Martin N, Beach D, Gil J. 2014. Ageing as developmental decay: insights from p16INK4a. Trends Mol Med 20: 667–674. 10.1016/j.molmed.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Martínez-Zamudio RI, Robinson L, Roux PF, Bischof O. 2017. Snapshot: cellular senescence pathways. Cell 170: 816–816.e1. 10.1016/j.cell.2017.07.049 [DOI] [PubMed] [Google Scholar]
- Mendez MV, Stanley A, Park HY, Shon K, Phillips T, Menzoian JO. 1998. Fibroblasts cultured from venous ulcers display cellular characteristics of senescence. J Vasc Surg 28: 876–883. 10.1016/S0741-5214(98)70064-3 [DOI] [PubMed] [Google Scholar]
- Mendez MV, Raffetto JD, Phillips T, Menzoian JO, Park HY. 1999. The proliferative capacity of neonatal skin fibroblasts is reduced after exposure to venous ulcer wound fluid: a potential mechanism for senescence in venous ulcers. J Vasc Surg 30: 734–743. 10.1016/S0741-5214(99)70113-8 [DOI] [PubMed] [Google Scholar]
- Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. 2016. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol 67: 2018–2028. 10.1016/j.jacc.2016.02.047 [DOI] [PubMed] [Google Scholar]
- Mijit M, Caracciolo V, Melillo A, Amicarelli F, Giordano A. 2020. Role of p53 in the regulation of cellular senescence. Biomolecules 10: 420. 10.3390/biom10030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva O, Deschênes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN, Ferbeyre G. 2013. Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-κB activation. Aging Cell 12: 489–498. 10.1111/acel.12075 [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443: 448–452. 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Blas D, Gorostieta-Salas E, Pommer-Alba A, Muciño-Hernández G, Gerónimo-Olvera C, Maciel-Barón LA, Konigsberg M, Massieu L, Castro-Obregón S. 2019. Cortical neurons develop a senescence-like phenotype promoted by dysfunctional autophagy. Aging (Albany NY) 11: 6175–6198. 10.18632/aging.102181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, Fernandez-Marcos PJ, Muñoz-Martin M, Blanco-Aparicio C, Pastor J, et al. 2016. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354: aaf4445. 10.1126/science.aaf4445 [DOI] [PubMed] [Google Scholar]
- Moura J, Madureira P, Leal EC, Fonseca AC, Carvalho E. 2019. Immune aging in diabetes and its implications in wound healing. Clin Immunol 200: 43–54. 10.1016/j.clim.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Li Z, Maitz PK. 2009. Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns 35: 500–508. 10.1016/j.burns.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Muñoz DP, Yannone SM, Daemen A, Sun Y, Vakar-Lopez F, Kawahara M, Freund AM, Rodier F, Wu JD, Desprez PY, et al. 2019. Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight 4: e124716. 10.1172/jci.insight.124716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espín D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118. 10.1016/j.cell.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Nair RR, Bagheri M, Saini DK. 2015. Temporally distinct roles of ATM and ROS in genotoxic-stress-dependent induction and maintenance of cellular senescence. J Cell Sci 128: 342–353. 10.1242/jcs.159517 [DOI] [PubMed] [Google Scholar]
- Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. 2003. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716. 10.1016/S0092-8674(03)00401-X [DOI] [PubMed] [Google Scholar]
- Narzt MS, Pils V, Kremslehner C, Nagelreiter IM, Schosserer M, Bessonova E, Bayer A, Reifschneider R, Terlecki-Zaniewicz L, Waidhofer-Söllner P, et al. 2021. Epilipidomics of senescent dermal fibroblasts identify lysophosphatidylcholines as pleiotropic senescence-associated secretory phenotype (SASP) factors. J Invest Dermatol 141: 993–1006.e15. 10.1016/j.jid.2020.11.020 [DOI] [PubMed] [Google Scholar]
- Neurohr GE, Terry RL, Lengefeld J, Bonney M, Brittingham GP, Moretto F, Miettinen TP, Vaites LP, Soares LM, Paulo JA, et al. 2019. Excessive cell growth causes cytoplasm dilution and contributes to senescence. Cell 176: 1083–1097.e18. 10.1016/j.cell.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, et al. 2017. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8: 15691. 10.1038/ncomms15691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HL, Schumacher B. 2018. DNA damage responses and p53 in the aging process. Blood 131: 488–495. 10.1182/blood-2017-07-746396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oubaha M, Miloudi K, Dejda A, Guber V, Mawambo G, Germain MA, Bourdel G, Popovic N, Rezende FA, Kaufman RJ, et al. 2016. Senescence-associated secretory phenotype contributes to pathological angiogenesis in retinopathy. Sci Transl Med 8: 362ra144. 10.1126/scitranslmed.aaf9440 [DOI] [PubMed] [Google Scholar]
- Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, et al. 2018. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun 9: 5435. 10.1038/s41467-018-07825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, et al. 2019. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18: e12950. 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, Miwa S, Olijslagers S, Hallinan J, Wipat A, et al. 2010. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol Syst Biol 6: 347. 10.1038/msb.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, et al. 2019. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun 10: 2387. 10.1038/s41467-019-10335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincelli C, Marconi A. 2010. Keratinocyte stem cells: friends and foes. J Cell Physiol 225: 310–315. 10.1002/jcp.22275 [DOI] [PubMed] [Google Scholar]
- Prieur A, Besnard E, Babled A, Lemaitre JM. 2011. P53 and p16 INK4a independent induction of senescence by chromatin-dependent alteration of S-phase progression. Nat Commun 2: 473. 10.1038/ncomms1473 [DOI] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. 2009. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc 14: 20–24. 10.1038/jidsymp.2009.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaper PM, Fagagna FD, Jackson SP. 2004. Activation of the DNA damage response by telomere attrition: a passage to cellular senescence. Cell Cycle 3: 541–544. 10.4161/cc.3.5.835 [DOI] [PubMed] [Google Scholar]
- Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Dürr P, Wlaschek M. 2006. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell 5: 379–389. 10.1111/j.1474-9726.2006.00231.x [DOI] [PubMed] [Google Scholar]
- Rhinn M, Ritschka B, Keyes WM. 2019. Cellular senescence in development, regeneration and disease. Development 146: dev151837. 10.1242/dev.151837 [DOI] [PubMed] [Google Scholar]
- Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM. 2017. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev 31: 172–183. 10.1101/gad.290635.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittié L, Fisher GJ. 2015. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med 5: a015370. 10.1101/cshperspect.a015370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, Jurk D, Khosla S, Kirkland JL, LeBrasseur NK, Miller JD, Passos JF, Pignolo RJ, Tchkonia T, Niedernhofer LJ. 2021. Senolytic drugs: reducing senescent cell viability to extend health span. Annu Rev Pharmacol Toxicol 61: 779–803. 10.1146/annurev-pharmtox-050120-105018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. 2009. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11: 973–979. 10.1038/ncb1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagosa C, Simonetti S, López-Vicente L, Mazo A, Lleonart ME, Castellvi J, Ramon y Cajal S. 2011. p16Ink4a overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 30: 2087–2097. 10.1038/onc.2010.614 [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, et al. 2016. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15: 973–977. 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, Belt BA, Alspach E, Leahy K, Luo J, et al. 2016. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun 7: 1–8. 10.1038/ncomms11762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv A, Krizhanovsky V. 2013. Immunosurveillance of senescent cells: the bright side of the senescence program. Biogerontology 14: 617–628. 10.1007/s10522-013-9473-0 [DOI] [PubMed] [Google Scholar]
- Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, Golani O, Polic B, Krizhanovsky V. 2016. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 8: 328–344. 10.18632/aging.100897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Araya J, Ito S, Tsubouchi K, Minagawa S, Hara H, Ito A, Nakano T, Hosaka Y, Ichikawa A, et al. 2019. Involvement of lamin B1 reduction in accelerated cellular senescence during chronic obstructive pulmonary disease pathogenesis. J Immunol 202: 1428–1440. 10.4049/jimmunol.1801293 [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88: 593–602. 10.1016/S0092-8674(00)81902-9 [DOI] [PubMed] [Google Scholar]
- Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. 2013. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 4: e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth F, Schliemann S, Antonov D, Elsner P. 2011. Dry skin, barrier function, and irritant contact dermatitis in the elderly. Clin Dermatol 29: 31–36. 10.1016/j.clindermatol.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T, et al. 2013. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 27: 1787–1799. 10.1101/gad.223834.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao AW, Sun H, Geng Y, Peng Q, Wang P, Chen J, Xiong T, Cao R, Tang J. 2016. Bclaf1 is an important NF-κB signaling transducer and C/EBPβ regulator in DNA damage-induced senescence. Cell Death Differ 23: 865–875. 10.1038/cdd.2015.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ. 2015. Forging a signature of in vivo senescence. Nat Rev Cancer 15: 397–408. 10.1038/nrc3960 [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. 2016. Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol 42: 29–37. 10.1016/j.ceb.2016.04.001 [DOI] [PubMed] [Google Scholar]
- Sidler C, Kovalchuk O, Kovalchuk I. 2017. Epigenetic regulation of cellular senescence and aging. Front Genet 8: 138. 10.3389/fgene.2017.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, et al. 2011. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997. 10.1172/JCI44490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solana R, Campos C, Pera A, Tarazona R. 2014. Shaping of NK cell subsets by aging. Curr Opin Immunol 29: 56–61. 10.1016/j.coi.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Song P, An J, Zou MH. 2020. Immune clearance of senescent cells to combat ageing and chronic diseases. Cells 9: 671. 10.3390/cells9030671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, et al. 2014. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506: 316–321. 10.1038/nature13013 [DOI] [PubMed] [Google Scholar]
- Stanley A, Osler T. 2001. Senescence and the healing rates of venous ulcers. J Vasc Surg 33: 1206–1211. 10.1067/mva.2001.115379 [DOI] [PubMed] [Google Scholar]
- Stegenga ME, Crabben SV, Dessing MC, Pater JM, Van Den Pangaart PS, De Vos AF, Tanck MW, Roos D, Sauerwein HP, Van Der Poll T. 2008. Effect of acute hyperglycaemia and/or hyperinsulinaemia on proinflammatory gene expression, cytokine production and neutrophil function in humans. Diabet Med 25: 157–164. 10.1111/j.1464-5491.2007.02348.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojadinovic O, Pastar I, Vukelic S, Mahoney MG, Brennan D, Krzyzanowska A, Golinko M, Brem H, Tomic-Canic M. 2008. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med 12: 2675–2690. 10.1111/j.1582-4934.2008.00321.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al. 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130. 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- Sugimoto M, Yamashita R, Ueda M. 2006. Telomere length of the skin in association with chronological aging and photoaging. J Dermatol Sci 43: 43–47. 10.1016/j.jdermsci.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Takahashi A, Ohtani N, Yamakoshi K, Iida SI, Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, Hara E. 2006. Mitogenic signalling and the p16 INK4a–Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 8: 1291–1297. 10.1038/ncb1491 [DOI] [PubMed] [Google Scholar]
- Tasdemir N, Banito A, Roe JS, Alonso-Curbelo D, Camiolo M, Tschaharganeh DF, Huang CH, Aksoy O, Bolden JE, Chen CC, et al. 2016. BRD4 connects enhancer remodeling to senescence immune surveillance. Cancer Discover 6: 612–629. 10.1158/2159-8290.CD-16-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzi MY, Izmirli M, Gogebakan B. 2016. The cell fate: senescence or quiescence. Mol Biol Rep 43: 1213–1220. 10.1007/s11033-016-4065-0 [DOI] [PubMed] [Google Scholar]
- Tominaga K, Suzuki HI. 2019. TGF-β signaling in cellular senescence and aging-related pathology. Int J Mol Sci 20: 5002. 10.3390/ijms20205002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vande Berg JS, Rudolph R, Hollan C, Haywood-Reid PL. 1998. Fibroblast senescence in pressure ulcers. Wound Repair Regen 6: 38–49. 10.1046/j.1524-475X.1998.60107.x [DOI] [PubMed] [Google Scholar]
- Velicky P, Meinhardt G, Plessl K, Vondra S, Weiss T, Haslinger P, Lendl T, Aumayr K, Mairhofer M, Zhu X, et al. 2018. Genome amplification and cellular senescence are hallmarks of human placenta development. PLoS Genet 14: e1007698. 10.1371/journal.pgen.1007698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villiard É, Denis JF, Hashemi FS, Igelmann S, Ferbeyre G, Roy S. 2017. Senescence gives insights into the morphogenetic evolution of anamniotes. Biol Open 6: 891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, Sedivy JM, Westendorp RG, Gunn DA, Maier AB. 2012. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 11: 722–725. 10.1111/j.1474-9726.2012.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R, Mizen H, Bishop CL. 2020. The bright and dark side of extracellular vesicles in the senescence-associated secretory phenotype. Mech Ageing Dev 189: 111263. 10.1016/j.mad.2020.111263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AS, Dreesen O. 2018. Biomarkers of cellular senescence and skin aging. Front Genet 9: 247. 10.3389/fgene.2018.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, Von Zglinicki T. 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8: 311–323. 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
- Wang AS, Ong PF, Chojnowski A, Clavel C, Dreesen O. 2017. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci Rep 7: 15678. 10.1038/s41598-017-15901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qu M, Li J, Danielson P, Yang L, Zhou Q. 2019. Induction of fibroblast senescence during mouse corneal wound healing. Invest Ophthalmol Vis Sci 60: 3669–3679. 10.1167/iovs.19-26983 [DOI] [PubMed] [Google Scholar]
- Wilkinson HN, Hardman MJ. 2020a. Wound healing: cellular mechanisms and pathological outcomes. Open Biol 10: 200223. 10.1098/rsob.200223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson HN, Hardman MJ. 2020b. Senescence in wound repair: emerging strategies to target chronic healing wounds. Front Cell Dev Biol 8: 773. 10.3389/fcell.2020.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson HN, Hardman MJ. 2021a. A role for estrogen in skin ageing and dermal biomechanics. Mech Ageing Dev 197: 111513. 10.1016/j.mad.2021.111513 [DOI] [PubMed] [Google Scholar]
- Wilkinson HN, Hardman MJ. 2021b. Wound senescence: a functional link between diabetes and ageing? Exp Dermatol 30: 68–73. 10.1111/exd.14082 [DOI] [PubMed] [Google Scholar]
- Wilkinson HN, Clowes C, Banyard KL, Matteuci P, Mace KA, Hardman MJ. 2019a. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J Invest Dermatol 139: 1171–1181.e6. 10.1016/j.jid.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Wilkinson HN, Roberts ER, Stafford AR, Banyard KL, Matteucci P, Mace KA, Hardman MJ. 2019b. Tissue iron promotes wound repair via m2 macrophage polarization and the chemokine (cc motif) ligands 17 and 22. Am J Pathol 189: 2196–2208. 10.1016/j.ajpath.2019.07.015 [DOI] [PubMed] [Google Scholar]
- Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, et al. 2015. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci 112: E6301–E6310. 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, et al. 2017. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci 72: 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, et al. 2018. Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256. 10.1038/s41591-018-0092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Wu W, Huang H, Huang R, Xie L, Su A, Liu S, Zheng R, Yuan Y, Zheng HL, et al. 2019. The p53/miRNAs/Ccna2 pathway serves as a novel regulator of cellular senescence: complement of the canonical p53/p21 pathway. Aging Cell 18: e12918. 10.1111/acel.12918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. 2007. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660. 10.1038/nature05529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, Vadai E, Dassa L, Shahar E, Condiotti R, et al. 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun 7: 1–1. 10.1038/ncomms11190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et al. 2013. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499: 97–101. 10.1038/nature12347 [DOI] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhu YI, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, et al. 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36: 18–28. 10.1016/j.ebiom.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun MH, Davaapil H, Brockes JP. 2015. Recurrent turnover of senescent cells during regeneration of a complex structure. eLife 4: e05505. 10.7554/eLife.05505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu YI, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, et al. 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14: 644–658. 10.1111/acel.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Long X, Zhao Q, Zheng Y, Song M, Ma S, Jing Y, Wang SI, He Y, Esteban CR, et al. 2021. A single-cell transcriptomic atlas of human skin aging. Dev Cell 56: 383–397.e8. 10.1016/j.devcel.2020.11.002 [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Baron JM, Böhm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. 2008. Frontiers in sebaceous gland biology and pathology. Exp Dermatol 17: 542–551. 10.1111/j.1600-0625.2008.00725.x [DOI] [PubMed] [Google Scholar]