Abstract
The discoveries of EGFR mutations and ALK rearrangements as actionable oncogenic drivers in non-small-cell lung cancer (NSCLC) has propelled a biomarker-directed treatment paradigm for patients with advanced-stage disease. Numerous EGFR and ALK tyrosine kinase inhibitors (TKIs) with demonstrated efficacy in patients with EGFR-mutant and ALK-rearranged NSCLCs have been developed, culminating in the availability of the highly effective third-generation TKIs osimertinib and lorlatinib, respectively. Despite their marked efficacy, resistance to these agents remains an unsolved fundamental challenge. Both ‘on-target’ mechanisms (largely mediated by acquired resistance mutations in the kinase domains of EGFR or ALK) and ‘off-target’ mechanisms of resistance (mediated by non-target kinase alterations such as bypass signalling activation or phenotypic transformation) have been identified in patients with disease progression on osimertinib or lorlatinib. A growing understanding of the biology and spectrum of these mechanisms of resistance has already begun to inform the development of more effective therapeutic strategies. In this Review, we discuss the development of third-generation EGFR and ALK inhibitors, predominant mechanisms of resistance, and approaches to tackle resistance in the clinic, ranging from novel fourth-generation TKIs to combination regimens and other investigational therapies.
Introduction
The evaluation and treatment of patients with non-small-cell lung cancer (NSCLC) has evolved dramatically over the past two decades with the discovery of multiple oncogenic driver alterations and the development of matched molecularly targeted therapies1-3. The experience with somatic activating mutations in EGFR, detected in approximately 15% of lung adenocarcinomas4-7, provided the first demonstration that a subset of NSCLCs harbour ‘actionable’ driver oncogenes that confer exquisite sensitivity to targeted therapy using tyrosine kinase inhibitors (TKIs). Chromosomal rearrangements involving ALK were subsequently identified as the oncogenic driver in a further 3–7% of patients with NSCLC8,9. In the intervening years since the discoveries of EGFR and ALK-driven NSCLCs, increasingly effective targeted small-molecule inhibitors have been developed, culminating in the FDA approvals of highly potent and central nervous system (CNS)-penetrant, third-generation EGFR and ALK TKIs. In parallel, lessons learned from EGFR-mutant and ALK-rearranged NSCLC have informed the paradigm of targeted therapy more generally, providing a framework for understanding the burgeoning number of molecularly defined subsets of NSCLC. In this Review, we provide an overview of the development of EGFR and ALK targeted therapies with a focus on third-generation agents, including the predominant mechanisms of resistance, strategies for overcoming resistance and future research directions.
EGFR mutations in NSCLC
EGFR is a member of the ErbB family of receptor tyrosine kinases (RTKs). Binding of ligands to the extracellular domain induces a conformational change that leads to receptor dimerization, tyrosine phosphorylation and activation of downstream signalling pathways including RAS–MAPK, PI3K–AKT and JAK–STAT, resulting in cell growth and survival10. Although EGFR is expressed in a range of nonmalignant tissues, including those of the skin and gastrointestinal tract11, it is overexpressed across multiple epithelial cancers 5-fold or greater 12, including NSCLC13,14, and thus has long been considered a therapeutic target. Yet, the initial development of effective EGFR-targeted therapies was not straightforward.
Early generation EGFR TKIs
The first-generation reversible EGFR inhibitors, gefitinib and erlotinib, were initially tested in unselected patients with advanced-stage NSCLC in the early 2000s and demonstrated only modest activity (objective response rates (ORRs) of around 10–20% and median progression-free survival (PFS) durations of around 3 months)15-18, although increased response rates were observed among never-smokers and patients of Asian ethnicity. Subsequent translational studies of exceptional responders led to the discovery of somatic oncogenic alterations in the EGFR kinase domain that were enriched in both of these populations5-7, thus explaining the previously noted clinical correlates of response. The most common types of activating EGFR mutations were found to be a family of deletions in a small region of exon 19 or the L858R point mutation, both of which confer a 100-fold higher sensitivity to EGFR TKIs compared to the wild-type receptor5,19. Prospective trials evaluating gefitinib or erlotinib specifically in patients with EGFR-mutant advanced-stage NSCLC soon followed, and the efficacy observed in this population was, at the time, unprecedented (typical ORRs of ~75% and median PFS durations of ~7-12 months). The rapid, deep tumour responses seen in these early studies offered one of the first glimpses into the potential of targeted therapy for NSCLC4,20-27. Thereafter, randomized clinical trials from the early 2010s demonstrated clearly superior ORRs (typically around 75% versus <40%) and median PFS durations (typically 8–10 months versus 4–8 months) with first-generation EGFR TKIs compared to chemotherapy28-34, establishing EGFR TKIs as the preferred first-line therapy for patients with EGFR-mutant NSCLC (FIG. 1).
Figure 1. Timeline of genomic discoveries and drug development in EGFR-mutant and ALK-rearranged NSCLCs.
Key events, in a. ∣ EGFR-mutant non-small-cell lung cancers (NSCLC) and b. ∣ ALK-rearranged NSCLC, including the discovery of the oncogenic drivers and gene alterations (orange), FDA approvals of tyrosine-kinase inhibitors for unselected patients (grey), approvals of therapies for biomarker-selected populations in the advanced-stage disease setting (blue), approvals for biomarker-selected populations in the adjuvant setting (green), and important ongoing trials (purple).
Despite excellent initial responses, acquired resistance to first-generation EGFR inhibitors developed, usually within a year. The most abundant mechanism of resistance, seen in ~60% of patients, was the T790M mutation located at the gatekeeper residue of EGFR35,36, which enhances the affinity of the kinase for ATP and thus limits opportunities for erlotinib and gefitinib to bind37. Subsequent efforts to overcome EGFRT790M-driven resistance led to the development of second-generation and third-generation EGFR TKIs. The second-generation, irreversible pan-ErbB inhibitors afatinib and dacomitinib were predicted to have activity against EGFRT790M based on data from preclinical models. However, this early promise was not borne out in clinical trials38,39, potentially owing to inhibition of wild-type EGFR, resulting in dose-limiting dermatological and gastrointestinal toxicities that might have abrogated the ability to achieve the drug concentrations required to overcome EGFRT790M. Both TKIs were ultimately approved by the FDA as first-line therapies (owing to improvements in median overall survival (OS) of about 8 months compared with first-generation EGFR TKIs)40,41, although the role of these agents is now unclear given the successful development of osimertinib.
Osimertinib
Osimertinib is a third-generation, irreversible EGFR TKI with greater potency and selectivity against both the canonical EGFR activating mutations and the T790M resistance mutation42. Despite being a substrate of several drug efflux transporters, such as permeability glycoprotein (Pgp) and breast cancer resistance protein (BCRP), the reduced affinity of osimertinib for these transporters enables greater retention within the cerebrospinal fluid after crossing the blood-brain barrier, resulting in greater CNS activity relative to earlier-generation EGFR TKIs43,44. When compared with standard chemotherapy in patients with T790M-driven acquired resistance to first-generation EGFR TKIs, osimertinib demonstrated a superior ORR (71% versus 31%) and PFS (10.1 months versus 4.4 months), including in patients with CNS metastases (median PFS 8.5 months versus 4.2 months)45. Osimertinib was granted accelerated FDA approval in 2015 for patients with EGFRT790M -positive NSCLC following disease progression on a prior EGFR TKI and subsequently received regular approval in 2017.
Subsequently, osimertinib was compared with gefitinib or erlotinib as first-line therapy for patients with advanced-stage EGFR-mutant NSCLC in the pivotal FLAURA trial, demonstrating improved median PFS (18.9 months versus 10.2 months)46, a lower incidence of CNS progression (in 6% versus 15% of patients)46 and prolonged OS (median 38.6 months versus 31.8 months)47. Osimertinib is now established as the standard-of-care first-line therapy for patients with advanced-stage EGFR-mutant NSCLC (FIG. 1). Other third-generation EGFR TKIs have been developed, with nazartinib (NCT02335944), lazertinib (NCT04248829, NCT04077463, NCT04487080) and aumolertinib (NCT04923906) in ongoing clinical trials, while the development of rociletinib, mavelertinib, avitinib and naquotinib has been halted48.
ALK rearrangements in NSCLC
ALK rearrangements were first identified in patients with NSCLC in 20078, more than a decade after the initial discovery of the NPM1–ALK rearrangement in patients with anaplastic large cell lymphoma49. Since the initial report of EML4–ALK rearrangements in patients with NSCLC8, >90 ALK fusion partners have been identified50 across 3–7% of all NSCLCs9,51,52. Mechanistically, ALK rearrangements result in constitutive activation of the ALK kinase and the associated downstream cellular signalling pathways, such as RAS–MAPK, PI3K–AKT and JAK–STAT, leading to dysregulated cellular proliferation and survival53,54. Indeed, cancers harbouring ALK rearrangements are dependent on ALK signalling for their survival55.
First-generation and second-generation ALK TKIs
In the clinic, ALK rearrangements are associated with adenocarcinomas, a younger age than average at diagnosis and a minimal smoking history9. Crizotinib, the first targeted therapy evaluated in patients with ALK-rearranged NSCLC, is a broad-spectrum TKI initially developed as a MET inhibitor but discovered to also be a potent ALK and ROS1 inhibitor in kinase screens56. This agent had substantial efficacy in patients with advanced-stage ALK-rearranged NSCLC in phase I/II trials, with ORRs of ~60%57,58. This led to FDA approval for this indication in 2011, followed by full approval in 2013, illustrating that biomarker-directed treatment approaches can be extended beyond EGFR-mutant NSCLC and supporting the major paradigm shift toward personalized management of advanced-stage NSCLC57. Subsequent randomized phase III trials demonstrated crizotinib to be superior to chemotherapy for patients with ALK-rearranged NSCLC59,60.
As with early generation EGFR TKIs, acquired resistance to crizotinib was common, typically emerging within a year of starting treatment (median PFS 7.7–10.9 months)59,60. More-potent second-generation ALK TKIs were developed, including ceritinib, alectinib and brigatinib, with the initial goal of overcoming resistance to crizotinib. These second-generation ALK TKIs were active in patients with crizotinib-pretreated disease and had improved CNS efficacy owing to improved CNS penetration 61-66. Mirroring the experience with osimertinib in EGFR-mutant NSCLC, the second-generation ALK TKIs then supplanted crizotinib as the standard first-line therapy for patients with advanced-stage ALK-rearranged NSCLC, with superior efficacy demonstrated in phase III trials comparing alectinib, brigatinib or ensartinib with crizotinib67-69 (12-month PFS typically ~65% versus ~45%, median PFS by blinded independent review ranging 24-26 months) (FIG 1).
Lorlatinib
Lorlatinib is a third-generation, macrocyclic, highly potent and selective ALK/ROS1 TKI developed to address acquired resistance to earlier-generation ALK TKIs, with potency against a broad spectrum of ALK kinase domain resistance mutations70-73. Furthermore, given the known high incidence of CNS metastases in patients with ALK-rearranged NSCLC (lifetime prevalence of ~60% - 73%74,75, lorlatinib was intentionally designed to accumulate within the CNS by minimizing efflux in Pgp-overexpressing cell lines70-72 and was confirmed to achieve marked CNS penetration in patients with a mean ratio of cerebrospinal fluid (CSF):plasma (unbound) of 0.75 among four patients in the phase I study72. In a global phase II study, lorlatinib demonstrated efficacy in patients who had received at least one prior ALK TKI, with an ORR of 47% and an intracranial response rate of 63%73. Specifically among patients who had received at least one prior second-generation ALK TKI, the ORR and median PFS were 40% and 6.9 months, respectively76. Of note, lorlatinib has shown higher efficacy among patients with tumours harbouring mutations associated with resistance to second-generation ALK TKIs at baseline versus those without76, suggesting that the former remain dependent on ALK signalling. On the basis of these data, lorlatinib received accelerated FDA approval in 2018 for patients with advanced-stage ALK-rearranged NSCLC with disease progression on first-line alectinib or ceritinib, or on crizotinib followed by at least one other ALK TKI.
More recently, lorlatinib was compared with crizotinib as a first-line therapy in the phase III CROWN trial. Lorlatinib was clearly superior to crizotinib with a 72% reduction in the risk of disease progression or death and a significantly longer time to CNS progression (96% versus 60% of patients were alive without CNS progression at 12 months; HR 0.07, 95% CI, 0.03 – 0.17)77, resulting in the regular approval of lorlatinib in March 2021 for patients with advanced-stage ALK-rearranged NSCLC, including those with previously untreated disease (FIG. 1).
Acquired resistance
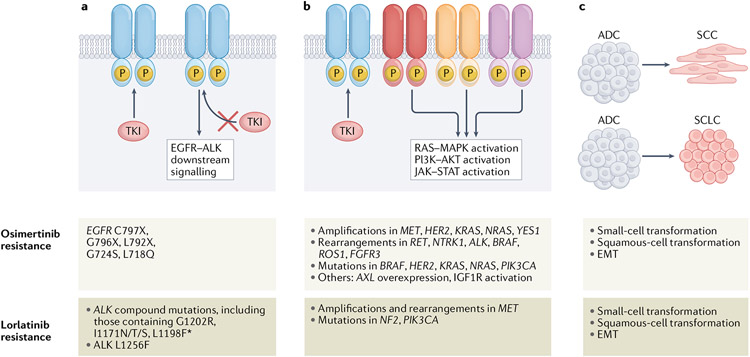
Despite the efficacy of the highly potent, third-generation EGFR and ALK TKIs, acquired resistance to these agents is inevitable and remains a critical unsolved challenge. Further investigations with the aim of elucidating the underlying mechanisms of resistance to these agents are therefore essential. Broadly speaking, acquired resistance can be categorized as either ‘on-target’ (or target-dependent; involving alterations to the target kinase, such that persistent RTK activation and signalling occurs even when the TKI is present) or ‘off-target’ (or target-independent; involving the activation of one or more bypass signalling pathways following upregulation of signalling proteins downstream of the target, or phenotypic transformation). In the following sections, we focus on mechanisms of acquired resistance to osimertinib and lorlatinib (FIG. 2).
Figure 2. Mechanisms of acquired resistance to osimertinib and lorlatinib in EGFR-mutant and ALK-rearranged NSCLCs, respectively.
Mechanisms of resistance to these agents can be divided into several main categories, including: a ∣ alterations that prevent inhibition of the target receptor tyrosine kinase by the tyrosine kinase inhibitor (TKI); b ∣ Activation of bypass and/or downstream signalling pathways that promote cell survival and proliferation despite adequate TKI binding; and c ∣ Changes in tumour cell lineage, such as transformation from an adenocarcinoma to a squamous cell carcinoma or small-cell lung cancer phenotype and epithelial–mesenchymal transition (EMT). Specific selected examples of these mechanisms are also provided.
On-target resistance
An array of kinase-domain mutations can occur in EGFR and ALK, causing steric hindrance with the binding of a TKI or inducing a conformational change, resulting in drug resistance. Whether these alterations reflect pre-existing resistance mutations present in a few subclones at diagnosis, or the occurrence of one or more de novo alterations during treatment, is beyond the scope of this Review.
On-target resistance to osimertinib.
Osimertinib binds EGFR by forming a covalent bond with the cysteine 797 residue located in the ATP-binding cleft42. The C797 residue is therefore a vulnerable site for the development of resistance to osimertinib, and indeed, EGFRC797S mutations have been identified in 10–26% of patients with disease progression on second-line osimertinib78-83. Other rarer EGFR mutations causing resistance to osimertinib have also been identified at lower incidences than that of C797S, including but not limited to: G796X, which interferes with osimertinib binding84-87; the solvent-front mutation L792X 84,85,88,89, which affects the ‘hinge’ region of the kinase domain, and L718X84,89,90 located in the p-loop, both of which causes steric hindrance; and G724S in the p-loop, which induces a conformational change in the glycine-rich loop that is incompatible with drug binding91-93. Nonetheless, the collective incidence of EGFR mutations that confer resistance to osimertinib84 is substantially lower than that of the T790M mutation identified in 50–60% of patients with resistance to earlier-generation EGFR TKIs35,36. EGFR amplifications have also been observed after treatment with third-generation EGFR TKIs94,95; however, because EGFR amplifications can also co-occur with an activating EGFR mutation in treatment-naïve tumours, detailed comparisons of EGFR copy number between pretreatment and post-treatment samples will be required to define the role of this alteration in resistance to osimertinib96.
In the first-line setting, on-target resistance to osimertinib seems to be even less common than in later-line settings97,98, with EGFRC797X identified in only 7% of patients in the preliminary analysis of resistance mechanisms from the FLAURA trial98. As expected, EGFRT790M was not detected in biopsy samples from patients with osimertinib-resistant tumours. These findings highlight the role of prior treatment exposures in determining the types of acquired TKI resistance that emerge.
On-target resistance to lorlatinib.
ALK kinase-domain mutations confer resistance to second-generation ALK TKIs in approximately 50–60% of patients, and each second-generation TKI has been associated with a unique distribution of ALK resistance mutations99. For example, the most common ALK resistance mutations following alectinib treatment are G1202R (in 25–30% of patients) and I1171X (in 10–15%)99. At disease progression on later-line lorlatinib, the overall frequency of on-target resistance mutations is lower compared to that with second-generation ALK TKIs, at only 25–30%100,101, which likely reflects the high potency of lorlatinib against wild-type ALK as well as a broad spectrum of single ALK resistance mutations including against G1202R and I1171X99. Indeed, the majority of on-target ALK mutations conferring resistance to lorlatinib are compound ALK mutations (such as C1156Y/L1198F, G1202R/L1196M, I1171N/D1203N, and others)100-107. These compound ALK mutations are diverse in range and involve two or more mutations in the kinase domain, which cumulatively reduce the potency of lorlatinib (requiring concentrations of lorlatinib that are not clinically achievable) or steric interference with drug binding101,102. Whole-exome sequencing and analysis of the clonality of serial biopsy samples obtained from patients with acquired resistance indicate that ALK resistance mutations can accumulate in a stepwise manner following sequential treatment with ALK inhibitors, culminating in highly refractory compound mutations that are challenging to target therapeutically and, in many patients, recalcitrant to the majority of currently available ALK TKIs100,101.
Lorlatinib was only approved by the FDA for first-line use in 2021 and only 28% of patients in the CROWN study had disease progression at the interim analysis77; therefore, insights into mechanisms of resistance to lorlatinib in this setting are currently lacking. However, data from preclinical studies suggest that the incidence of on-target resistance to first-line lorlatinib will be substantially lower than the 25–30% observed with later-line use. In a study using N-ethyl-N-nitrosourea (ENU) mutagenesis to generate Ba/F3 cells expressing non-mutant EML4–ALK (modelling treatment-naive ALK-rearranged tumour cells) no single ALK mutation emerged that conferred high level resistance to lorlatinib following prolonged exposure. However, in Ba/F3 cells expressing EML4–ALK harbouring a single ALK kinase domain mutation (modelling ALK TKI-pretreated tumours) exposure to lorlatinib resulted in the emergence of a range of compound ALK mutations conferring resistance100. This finding supports the suggestion that the range of single ALK mutations that can emerge de novo and confer resistance to first-line lorlatinib will be narrow. In another study, a novel single ALK mutation (L1256F) was found to confer resistance to lorlatinib in a cell line model, with predictions suggesting that this mutation causes steric clash with the fluorobenzene group of lorlatinib108; however, this mutation has not yet been reported in patients. The findings by Yoda et al.100 also suggest that first-line lorlatinib might suppress or delay the development of on-target resistance, because compound ALK mutations are less likely to develop in tumours harbouring wild-type ALK compared with variant forms harbouring single resistance mutations.
Of note, the underlying ALK fusion variants might have implications for the development of on-target resistance to ALK inhibitors109. In one study, ALK resistance mutations and, in particular, ALKG1202R (the most frequent ALK mutation arising after treatment with second-generation ALK TKIs), were more common in tumours harbouring EML4–ALK variant 3 (exon 6a/b of EML4 fused with exon 20 of ALK) compared to variant 1 (exon 13 of EML4 fused with exon 20 of ALK)110. Whether ALK fusion variants will influence the distribution of mechanisms of acquired resistance to lorlatinib remains undetermined.
Off-target resistance
The prevalence of off-target resistance is anticipated to increase as third-generation inhibitors become more widely used in the first-line96,100. Historically, mechanisms of off-target resistance have been more challenging to study as these can involve nongenomic or non-cell-autonomous mechanisms that might not be discernable using tumour sequencing. Indeed, although substantial progress has been made in understanding off-target resistance to EGFR/ALK TKIs, the mechanisms of resistance remain unknown in a substantial proportion of patients (up to 40–50% after first-line osimertinib)97,98, underscoring the need for further investigations in this area.
Parallel bypass pathways
A key feature of resistance owing to activation of bypass signalling pathways is the requirement for dual inhibition of both the driver oncogene and the bypass pathway in order to successfully overcome resistance. MET amplification was the first mechanism of resistance involving bypass signalling to be identified in EGFR-mutant NSCLC111, observed in 7–15% of patients following first-line osimertinib96,98 and 9.8–30% following later-line osimertinib83,112,113. Importantly, MET amplification is clinically actionable using a combination approach that incorporates a MET-targeted inhibitor114-120. For example, the TATTON trial evaluated the combination of osimertinib plus the MET TKI savolitinib, which was tolerable and had encouraging efficacy with an ORR of 30% among patients with EGFR-mutant NSCLC with disease progression and concomitant MET amplification on a third-generation EGFR TKI. MET amplifications have also been identified as an actionable mechanism of resistance across other molecular subsets of NSCLC, including ALK-rearranged NSCLC. In an analysis of 207 tumour biopsy samples, MET amplification was detected in 15% of samples from patients relapsing on next-generation ALK inhibitors (including in 22% with disease relapse on lorlatinib)121. Experiences with combined ALK and MET inhibition have thus far been limited, although this approach is reported to be feasible121.
Additional mechanisms of bypass-mediated resistance have been described, including acquisition of gene rearrangements that are established drivers of NSCLC (TABLES 1 and 2). A representative example is provided by the identification of RET rearrangements at disease progression on osimertinib83,96,112,113,122-124 or brigatinib125. Resistance mediated by acquired RET rearrangements can be overcome using a combination of EGFR and RET inhibitors (such as selpercatinib or pralsetinib)83,126,127. Several other mechanisms of resistance involving activation of bypass signalling pathways have been reported in patients with EGFR-mutant or ALK-rearranged NSCLCs (TABLES 1 and 2)35,36,83,96,98,111-113,121-125,128-165.
Table 1.
Bypass resistance pathways in EGFR-mutant NSCLC
| Bypass mechanism | Prior EGFR TKIa | Prevalence (%) | Refs |
|---|---|---|---|
| MET amplifications | Osimertinib | 7–15% in first line | 98,96 |
| 9.8–30% in second or later lines | 83,112,113,124,128 | ||
| First-generation TKIs | 5–22%, line of therapy mixed/unspecified | 36, 35, 36, 111 | |
| RET rearrangements | Osimertinib | 3.7% in first line | 96 |
| 1–2.4% in second or later lines | 83,112,113,122-124 | ||
| Not specified, case reports | 164 b | ||
| Erlotinib | Unknown, data limited to case reports | 129 c | |
| NTRK rearrangements | Osimertinib | 1–2.4% in second line | 83, 112 |
| Earlier-generation TKIs | Unknown, data limited to case reports | 133,164 b | |
| ALK rearrangements | Osimertinib | 3% in first line | 96 |
| Earlier-generation TKIs | Unknown, data limited to case reports | 129 c, 164 b | |
| BRAF rearrangements | Osimertinib | 3.7% in first line | 96 |
| Erlotinib or osimertinib | 2.4% in later lines/mixed cohorts | 113,134, 129 c, 165 | |
| BRAF mutations | Osimertinib | 4–5% in second or later lines | 112,113,135 |
| Nazartinib | Unknown, data limited to case reports | 136 | |
| ROS1 rearrangements | Osimertinib | Unknown, data limited to case reports | 164 b,137,138 |
| FGFR3 rearrangements | Osimertinib | 1–2.4%, primarily in later lines | 112,113,124,140,164 b |
| Earlier-generation TKIs | Unknown, data limited to case reports | 129 c, 139, 159 | |
| HER2 amplifications | Osimertinib | 2% in first line | 98 |
| 5% in second line, data from later lines limited to case reports | 112, 131 | ||
| First-generation TKIs | 12% in first-line | 130 | |
| HER2 mutations | Osimertinib | Unknown, data limited to case reports | 132 |
| KRAS mutations | Osimertinib | 2–7% in first line | 98,96 |
| 1–8.3%, primarily in second line or later | 112,113,124,161-163 | ||
| KRAS gains | Osimertinib | 2.4%, primarily in later lines | 124 |
| NRAS mutations | Osimertinib | Preclinical data only | 158 |
| NRAS gains | Third-generation TKIs | Preclinical data only | 158,160 |
| PIK3CA mutations | Osimertinib | 4% in first line | 98 |
| 2.4–16.7% in second line or later | 112,113,124,163 | ||
| YES1 amplifications | Earlier-generaton TKIs | 2.9%, line of therapy mixed/unspecified | 141 |
| AXL overexpression | Osimertinib | Preclinical only | 142,143 |
| IGF1R activation | Third-generation TKI | Preclinical only | 144,145 |
This table includes selected studies, focusing primarily on those demonstrated as conferring resistance to third-generation EGFR TKIs, and is not meant to represent the entirety of clinical and preclinical work on bypass mechanisms in EGFR-mutant NSCLC.
The EGFR TKI received immediately before biopsy sampling is reported here.
This study included samples from 93 patients with concomitant EGFR alterations and receptor tyrosine kinase (RTK) fusions, 16 of which demonstrated acquired RTK fusions at resistance, including: 6 with RET rearrangements (1 with co-occurring KRAS amplification, 1 with MET amplification and 1 with EGFR amplification), 5 with ALK rearrangements (2 with co-occurring EGFR amplifications and 1 with KRAS G12D), 4 with NTRK1 rearrangements (2 with co-occurring MET amplifications, 1 with HER2 amplification and ROS1 rearrangement, and 1 with EGFR amplification), 1 with ROS1 rearrangement (with co-occurring HER2 amplification and NTRK1 rearrangement), and 1 with FGFR3 rearrangement.
This study included 12 paired pre/post-EGFR TKI samples with acquired RTK fusions identified following treatment: 2 with RET rearrangements, 4 with ALK rearrangements, 4 with FGFR3 rearrangements and 1 with BRAF rearrangement. NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor.
Table 2.
Bypass resistance pathways in ALK-rearranged NSCLC
| Bypass mechanism | Prior ALK TKIa | Prevalence | Refs |
|---|---|---|---|
| MET amplifications | Second-generation TKIs | 12% in first or later lines | 121 |
| Lorlatinib | 22% in later lines | 121 | |
| MET rearrangements | Alectinib or lorlatinib | 3% in later lines | 121 |
| MET exon 14 mutations | Alectinib | Unknown, data limited to case reports | 146 |
| RET rearrangements | Brigatinib | Unknown, data limited to case reports | 125 |
| EGFR activation | Crizotinib | 44% in first line | 151 |
| EGFR mutations | Crizotinib | 9–14% in first line | 152, 153 |
| HER2 amplifications | Crizotinib, alectinib | Unknown, data limited to case reports | 148, 149 |
| KIT amplifications/activation | Crizotinib | 15% in first line | 151 |
| IGF1R activation | Crizotinib | 80% in first line | 154 |
| SHP2 signalling | Ceritinib | Preclinical data only | 157 |
| NF2 mutations | Lorlatinib | 20% in later lines | 107 |
| YES1 amplifications | Crizotinib, ceritinib | 11.8% in later lines | 141 |
| KRAS mutations | Crizotinib | 18% in first line | 153 |
| BRAFV600E mutations | Alectinib | Unknown, data limited to case reports | 147 |
| MAP2K1 mutations | Ceritinib | Unknown, data limited to case reports | 150 |
| DUSP6 loss | Crizotinib | 83% | 166 |
| PIK3CA mutations | Lorlatinib or ceritinib | Unknown, data limited to case reports | 100,150 |
| AXL overexpression | Earlier-generation TKIs | Preclinical data only | 155,156 |
This table includes selected studies and is not meant to represent the entirety of clinical and preclinical work on bypass mechanisms in ALK-rearranged NSCLC.
The ALK TKI received immediately before biopsy sampling is reported here. NSCLC, non-small-cell lung cancer; TKI, tyrosine kinase inhibitor
Downstream signalling pathways
Downstream effector pathways of EGFR and/or ALK can become reactivated and mediate resistance, although the path towards targeting these mechanisms of resistance clinically has been less clear. As a key example, the RAS–MAPK pathway is a pivotal downstream effector of both EGFR and ALK that can be reactivated by multiple mechanisms affecting each node of the pathway (such as acquired BRAF fusions96,104,112,113,129,134-136,147, KRAS mutations,96,104,112,113,125, NRAS mutations158, MAP2K1 mutations150, DUSP6 loss166, or gain of wild-type NRAS or KRAS158,166). Data from preclinical models demonstrate the ability of upregulated ERK signalling to confer resistance to third-generation EGFR inhibitors and that combined MEK/ERK inhibition can restore sensitivity to EGFR TKIs167.
Histological transformation
Repeat biopsy samples obtained at the time of disease progression on EGFR or ALK TKIs are crucial not only for enabling genomic analyses but also for identifying histological transformation. Transformation to small-cell lung cancer (SCLC) accounts for 3–14% of patients with resistance to early generation EGFR inhibitors35,36,168, and is likely even more common after first-line osimertinib169-171. The exact mechanisms leading to this dramatic shift in cellular appearance and biology remain unclear. The founder EGFR mutation is known to persist in the transformed tumour specimens, and yet, the dependence on EGFR no longer exists, rendering EGFR TKIs ineffective172. Nonetheless, the presence of baseline RB1 and TP53 inactivation confers a strikingly elevated risk (43-fold) of transformation to SCLC173. A retrospective review of data from 67 patients with EGFR-mutant SCLC revealed generally poorer outcomes than those with EGFR-mutant NSCLC, including transient responses to chemotherapy and unfavourable OS (mOS following transformation of 10.9 months)174. Transformation from adenocarcinoma to squamous-cell carcinoma can also occur and has been observed in 5 of 62 patients progressing on first-line osimertinib in one study; this phenotype is also associated with short post-transformation survival durations96. Both small-cell and squamous-cell transformation have also been identified following ALK TKIs101,175-180. In one study evaluating samples from 168 patients with ALK-rearranged NSCLCs with resistance to next-generation ALK TKIs, small-cell transformation was identified in 1.2% of patients, which is lower than for EGFR-mutant NSCLC181. Whether the underlying tumour cell plasticity and the prevalence of histological transformation differs across the various molecular subsets of NSCLC currently remains undetermined. Nevertheless, repeat tissue biopsy sampling at the time of progression on next-generation TKIs should be strongly considered (if feasible), given the possibility of histological transformation that might warrant the use of histology-appropriate therapies. This recommendation also reflects the inability to detect such transformations using current liquid biopsy platforms182.
Epithelial-to-mesenchymal transition (EMT) has also been detected both in patients with EGFR-mutant36 and ALK-rearranged cancers99 and is thought to facilitate invasiveness183. EMT is a conserved developmental process in which epithelial cells assume a migratory and invasive mesenchymal phenotype with the loss of cell–cell junctions and polarity183. This process has been implicated in both primary and acquired184 resistance to anticancer therapies, and EMT transcriptional regulators have been identified as possible therapeutic targets in patients with lung adenocarcinomas184. Over the past few years, EMT has been implicated in resistance to osimertinib185 and lorlatinib107. In the case of EMT-mediated resistance to osimertinib, the ATR–CHK1–aurora B signalling cascade has been found to be activated in preclinical studies, with the combined inhibition of EGFR and aurora B kinases overcoming this resistance186.
Primary Resistance
Across clinical trials of EGFR and ALK TKIs involving patients with EGFR-mutant and ALK-rearranged lung cancers, respectively, primary disease progression (the lack of an initial response to therapy or disease control) on therapy has been observed in as many as 4–10% of newly diagnosed patients, indicative of primary, rather than acquired, TKI resistance46,77,187,188. The biology of primary resistance remains an area of active investigation. Mechanisms implicated in primary resistance to TKIs include the presence of a pre-existing concomitant non-TKI-sensitizing alterations (such as EGFR exon 20 insertions, which do not confer sensitivity to earlier-generation EGFR TKIs)189,190, co-occurring mutations in other pathways (such as NF-κB)191, or germline deletions of the gene encoding BCL-2-like protein 11 (BIM)192-194. Conceptually, any pre-existing on-target or off-target alterations that are refractory to a specific TKI could contribute to primary resistance to that inhibitor. Next-generation sequencing offers the advantage of delineating co-occurring alterations in the target genes at baseline, which can help elucidate tumour biology.
Strategies to manage resistance
The clinical pace and pattern of disease progression varies widely following treatment with targeted therapies, including osimertinib and lorlatinib. The behaviour of the progressive cancer, together with the understanding of the mechanisms of resistance outlined above, can inform decision-making regarding the optimal management strategy. TKI-resistant tumours might be biologically complex and harbour polyclonal resistance (with resistance mediated by multiple simultaneous mechanisms) with further diversification of underlying intratumour heterogeneity (as reviewed elsewhere)195,196, posing additional challenges in overcoming resistance.
Addressing oligoprogression
In the scenario of indolent and/or asymptomatic disease progression, a patient might nonetheless continue to receive therapy if they are deemed to be deriving clinical benefit182. For example, in patients with oligoprogression, defined as small metastases located at one or a limited number of sites in a patient with disseminated disease that is otherwise well controlled197, the incorporation of locally ablative therapy can be a valid treatment strategy198,199. In particular, data from several small-cohort studies demonstrate that stereotactic body radiotherapy can provide meaningful local control of progressive disease located at several sites, with favourable PFS outcomes, in patients with targetable oncogene-driven NSCLCs198,200.
Addressing intracranial progression
Oligoprogressive CNS disease that occurs despite receiving CNS-active, next-generation EGFR or ALK TKIs might be addressed using stereotactic radiosurgery or surgical resection. By contrast, multifocal CNS progression typically requires an alternative management strategy, such as the addition of pemetrexed (which has some CNS efficacy in patients with metastatic adenocarcinomas)201, whole-brain radiotherapy, a switch to a more CNS-penetrant TKI if available, or — for select TKIs — dose escalation in an attempt to increase the CNS drug concentration. The potential for escalation of osimertinib from 80 mg to 160 mg daily dosing in rescuing CNS progression in patients with EGFR-mutant NSCLC has been evaluated in a small subgroup analysis of a phase II trial. In this study, 54% of patients with isolated intracranial disease progression on standard-dose osimertinib had intracranial responses to the escalated dose of osimertinib, although many of these responses were not durable (median intracranial PFS 3.8–7.0 months)202, consistent with findings from a separate multicentre retrospective study demonstrating only modest benefit after escalation of the osimertinib dose following CNS progression203. In another phase II study in which patients with EGFRT790M-positive NSCLC (following disease progression on earlier-generation EGFR inhibitors) received 160 mg osimertinib, the intracranial ORR among patients with brain metastases and prior exposure to T790M-targeting agents was 50%204. Conversely, escalation of lorlatinib beyond 100 mg daily is unlikely to rescue CNS progression in patients with ALK-rearranged NSCLC given the already marked CNS penetration of this agent72. By contrast, successful re-induction of CNS disease control has been reported with dose-escalation of second-generation ALK TKIs, such as alectinib and brigatinib205,206. Ultimately, the robust CNS efficacy of later-generation TKIs has meant that any subsequent CNS progression on these therapies is exceptionally challenging to manage with the limited treatment options available, thus highlighting the need for novel strategies.
Addressing systemic progression
For patients with diffuse, systemic disease progression, changes in the approach to systemic therapy are warranted. Here, options can be broadly categorized as an alternative TKI (aimed at overcoming on-target resistance) versus strategies beyond TKI monotherapy (aimed at overcoming off-target resistance).
Novel later-generation TKIs.
A number of fourth-generation EGFR and ALK TKIs are currently in development with the goal of overcoming on-target resistance to third-generation TKIs. EAI045 is an allosteric EGFR inhibitor that binds at a site displaced by the C-helix in the inactive kinase conformation. EAI045 alone has been shown to inhibit L858R–T790M-mutant forms of EGFR in vitro but not in vivo; however, this agent was successful in inhibiting L858R–T790M and L858R–T790M–C797S-driven lung cancers in mouse models when combined with cetuximab207,208. This apparent lack of activity as a monotherapy and the toxicities associated with inhibition of wild-type EGFR by cetuximab might ultimately limit clinical applicability of this combination. JBJ-04-125-02 is another allosteric EGFR inhibitor currently in development that has been found to inhibit the L858R–T790M–C797S forms of EGFR both in vitro and in vivo. JBJ-04-125-02 might induce more-potent responses when combined with osimertinib209. Most recently, BLU-945 and BLU-701 have emerged as potent, CNS-penetrant and wild-type EGFR-sparing fourth-generation EGFR TKIs. In preclinical studies, BLU-945 has been shown to inhibit the triple-mutant EGFR subtype (harbouring a sensitizing EGFR L858R mutation or exon 19 deletion, T790M and C797S), while BLU-701 is active against the double-mutant (with sensitizing mutations and C797S)210,211. BLU-701 might be a particularly important agent given the lack of emergence of T790M observed with first-line use of osimertinib. BLU-945 (NCT04862780) and BLU-701 (NCT05153408) are both currently being tested in early phase clinical trials.
Novel ALK TKIs are being developed in an attempt to overcome some of the known compound ALK mutations associated with resistance to lorlatinib. TPX-0131 is a next-generation ALK TKI with a compact macrocyclic structure designed to fit completely within the ATP-binding pocket and inhibit ALK even in the presence of ALK resistance mutations. TPX-0131 has demonstrated enhanced potency against wild-type ALK and various single as well as compound ALK mutations compared with the currently approved ALK TKIs in xenograft models212. This compound is currently in phase I testing (NCT04849273). NVL-655 is a selective, CNS-penetrant next-generation ALK TKI with activity against lorlatinib-resistant G1202R–L1196M, G1202R–L1198F and G1202R–G1269A compound ALK mutations that is anticipated to enter phase I testing in 2022213. Of note, a single novel ALK TKI might not be able to overcome the entire spectrum of compound ALK mutations conferring resistance to lorlatinib. Indeed, an analysis involving ALK-rearranged cell lines and patient-derived xenograft models demonstrates that certain lorlatinib analogues are able to more potently inhibit G1202R-based than I1171N/S/T-based compound ALK mutations, and vice versa101. Similarly, TPX-0131 is a potent inhibitor of ALK G1202R as well as G1202R-based compound ALK mutations but not those containing I1171212. These findings highlight the challenges associated with effective target inhibition once tumours have acquired compound mutations following disease progression on sequential TKIs.
Cycling or repurposing of TKIs.
Outside of the development of novel TKIs, a potential role for ‘recycling’ of older-generation TKIs or ‘repurposing’ of broad-spectrum kinase inhibitors in overcoming on-target resistance to third-generation EGFR or ALK TKIs has been investigated in certain scenarios. For example, the combination of brigatinib, an ALK TKI that also has potency against ROS1, FLT3 and EGFR in preclinical studies214, with cetuximab has been found to inhibit triple-mutant EGFR and overcome resistance to osimertinib in both preclinical studies and case reports215-218. Certain lorlatinib-resistance mutations can re-sensitize ALK-rearranged NSCLCs to earlier-generation ALK TKIs. For example, a patient who had disease relapse on crizotinib owing to the emergence of an ALK C1156Y mutation subsequently had disease progression on lorlatinib owing to an ALK C1156Y–L1198F compound mutation102. This compound mutation unexpectedly restored sensitivity to crizotinib in cell-line models, enabling the patient to be successfully re-treated with crizotinib. Other reports describe additional compound ALK mutations that might confer sensitivity to earlier-generation ALK TKIs106,151, and the aforementioned lorlatinib-resistant ALK L1256F mutation has been shown to be sensitive to alectinib in preclinical studies108.
Combination therapies
Combination therapies are an alternative treatment strategy that could potentially address, or delay, the onset of resistance to third-generation inhibitors. We highlight that all combination strategies discussed here remain investigational. One approach involves the administration of both an early generation and third-generation TKI targeting the same RTK in order to overcome or delay the emergence of on-target resistance. In EGFR-mutant NSCLC, the combination of osimertinib with a first-generation EGFR TKI could overcome the resistance associated with the C797S mutation in certain allelic contexts. Niederst and colleagues demonstrated that when EGFR T790M and C797S occur in trans (that is, in different EGFR alleles), a combination of a first-generation EGFR TKI (targeting C797S) and a third-generation TKI (targeting T790M) could restore EGFR inhibition219. Subsequent case reports indicate that such dual EGFR TKI therapy offers at least a transient clinical benefit220,221, with some potential for durable responses222. The phase II multi-arm ORCHARD trial (NCT03944772) is now evaluating the efficacy of osimertinib plus gefitinib in patients with known EGFR C797X mutations after first-line osimertinib. In parallel, other clinical trials are assessing combinations comprising a third-generation plus an earlier-generation EGFR TKI in patients with treatment-naive EGFR-mutant NSCLC, with the goal of delaying the emergence of on-target resistance (TABLE 3). In ALK-rearranged NSCLC, a proof-of-concept clinical trial at two centres in Australia (ACTRN12619000844145) is evaluating an alternating schedule of crizotinib and lorlatinib with the goal of delaying the development of resistance.
A second combination approach involves the use of an EGFR or ALK TKI together with another targeted agent to address off-target resistance; evidence supporting the rationale for such strategies is currently accumulating. The previously mentioned ORCHARD trial (NCT03944772) includes arms assessing the safety and efficacy of osimertinib combined with various non-EGFR-targeted therapies in biomarker-selected patient subpopulations. Several other trials testing osimertinib-based combinations in patients with EGFR-mutant NSCLC are currently either planned or ongoing (Supplementary Table 1). Similarly in patients with ALK-rearranged NSCLC, combinations of lorlatinib with other targeted therapies such as MET inhibitors (targeting MET-driven resistance), MEK inhibitors (targeting RAS–MAPK pathway reactivation), or SHP2 inhibitors (targeting resistance driven by RTK signalling) are all being explored (TABLE 3).
A third combination approach involves the addition of chemotherapy to an EGFR or ALK TKI. After disease relapse on prior third-generation EGFR or ALK TKIs, the goal of chemotherapy is to target TKI-resistant tumour cells, whereas the goal of continuing to administer a CNS-penetrant TKI is often to confer CNS-specific protection or efficacy. Experiences in patients with EGFR-mutant NSCLC suggest that adding chemotherapy to osimertinib following disease progression on osimertinib monotherapy does not lead to intolerable toxicities and might extend PFS223,224. The randomized phase III COMPEL study is designed to determine whether osimertinib should be continued alongside platinum–pemetrexed chemotherapy following extracranial disease progression on first-line osimertinib (NCT04765059). In ALK-rearranged NSCLC, prospective data on this question are lacking, although data from one small retrospective study suggest that continuing an ALK TKI together with chemotherapy following progression on a second-generation ALK TKI extends PFS225. A separate question is whether the combination of an EGFR or ALK TKI with chemotherapy as initial therapy — prior to clinical relapse on a TKI — offers a PFS and/or OS advantage compared to sequential use of TKIs followed by chemotherapy. Theoretically, maximal use of cytoreductive therapy upfront could either minimize or eradicate any TKI-tolerant persister cell subclones, thus delaying the emergence of resistant clones and clinical relapse226. In patients with EGFR-mutant NSCLC, first-line therapy with gefitinib plus chemotherapy has been demonstrated to significantly prolong PFS (by 8–10 months) and OS (by around 12 months) compared with gefitinib alone in two independent randomized trials227,228. The ongoing phase III FLAURA2 trial (NCT04035486) is evaluating the efficacy of upfront osimertinib plus chemotherapy versus osimertinib alone in patients with previously untreated EGFR-mutant non-squamous NSCLC.
Lastly, combinations of EGFR or ALK TKIs with anti-angiogenic agents are being explored, most notably in the first-line setting, with mixed results to date. Anti-angiogenic agents such as bevacizumab (an anti-VEGFA monoclonal antibody) can modify the tumour vasculature, which can affect both drug delivery and efficacy and also suppress tumour growth. Data from preclinical studies demonstrate synergistic effects of anti-angiogenic agents with both EGFR and ALK TKIs229. Earlier phase II and III studies in which patients with treatment-naïve EGFR-mutant NSCLCs received erlotinib plus ramucirumab or bevacizumab demonstrated improved PFS, but not OS, from the addition of the anti-angiogenic agent230-232. However, a randomized study comparing the efficacy of osimertinib plus bevacizumab versus osimertinib alone as later-line therapy failed to demonstrate any significant improvement in PFS or OS233,234. These results are supported by those from a small cohort in Japan that also did not demonstrate any significant improvement in PFS with osimertinib plus bevacizumab in this setting235. A phase III trial evaluating osimertinib plus bevacizumab versus osimertinib alone as first-line therapy for patients with advanced-stage EGFR-mutant NSCLC is currently under way (NCT04181060). Similarly, the potential benefit from adding an anti-angiogenic agent to an ALK TKI in patients with ALK-rearranged NSCLC remains to be determined. In phase I/II trials, the combination of alectinib and bevacizumab has been demonstrated to be feasible and tolerable, although the size of both study cohorts was small (11 and 12 patients, respectively)236,237.
Consideration of immunotherapy
The incorporation of immunotherapy into the treatment of patients with NSCLC harbouring EGFR mutations or ALK rearrangements has remained controversial, despite the widespread adoption of immune-checkpoint inhibitors (ICIs) for patients with NSCLC who lack a targetable driver alteration. Data from previous studies indicate that the efficacy of anti-PD-1/PD-L1 antibodies in patients with EGFR-mutant or ALK-rearranged NSCLC is limited238-242, and the pivotal phase III trials of first-line ICI monotherapy or ICI–chemotherapy combinations in patients with advanced-stage NSCLC excluded patients with tumours of these genotypes. Potential contributors to the modest efficacy of ICIs in these NSCLC subsets include the disproportional representation of never or minimal smokers with lower tumour mutational burdens and lower levels of tumour-infiltrating lymphocytes compared to those with EGFR/ALK-wild-type NSCLC243. Moreover, PD-L1 expression can be upregulated in tumours harbouring EGFR mutations or ALK rearrangements through constitutive oncogenic signalling (also known as innate immune resistance), resulting in the predictive correlation between level of PD-L1 expression and benefit from ICIs being lost, thus negating the reliability of PD-L1 level as a biomarker in this population238,243. Overall, the available data do not support the general use of ICI monotherapy among patients with EGFR-mutant or ALK-rearranged NSCLCs after progression on targeted therapies. The role of ICIs in combination with chemotherapy and/or anti-angiogenic therapy requires further clarification. In the initial subgroup analysis of data from IMpower150, atezolizumab in combination with carboplatin, paclitaxel and bevacizumab (ABCP) demonstrated a higher ORR (71% versus 42%), duration of response (11.1 versus 4.7 months) and median OS (not estimable versus 17.5 months) than those observed without atezolizumab (BCP) in patients with EGFR-mutant NSCLC with disease progression on at least one previous TKI244,245. However, the updated final exploratory OS analyses with longer follow-up revealed no significant difference in OS with ABCP versus BCP in all patients with EGFR-mutant NSCLC (HR 0.60, 95% CI 0.31–1.14) and in those who had received a previous TKI (HR 0.74, 95% CI 0.38–1.46)246. The global ORIENT-31 trial evaluated a quadruplet regimen comprising the anti-PD-1 antibody sintilimab plus bevacizumab biosimilar and chemotherapy (pemetrexed plus cisplatin) in patients with EGFR-mutant NSCLC and found that median PFS was significantly prolonged for patients who received all four therapies compared with chemotherapy alone247. Whether an ICI plus chemotherapy without an anti-angiogenic agent has meaningful benefit in this patient population also remains unknown248. The phase III KEYNOTE-789 trial (NCT03515837) is assessing the efficacy of chemotherapy with or without pembrolizumab in patients with EGFR-mutant NSCLC with disease progression on osimertinib and the phase III ATLAS trial is evaluating the efficacy of atezolizumab and bevacizumab in combination with chemotherapy in patients with EGFR-mutant or ALK-rearranged NSCLC (NCT03991403). Together, these trials should help inform the evolving conversation on the most effective therapies following disease progression on third-generation TKIs.
A growing number of studies have demonstrated an increased risk of severe toxicities with concomitant or sequential use of ICIs and specific EGFR or ALK TKIs249-258. For example, ICI–EGFR TKI combinations have been associated with substantial risks of grade ≥3 pneumonitis and hepatitis259,260. In the TATTON trial, specifically, combination of osimertinib and durvalumab was deemed not feasible due to an increased rate of interstitial lung disease (38% with combination versus 2.9% with osimertinib or 2.0% with durvalumab)259. Sequential rather than concomitant treatment with an ICI followed by TKI also can confer greater risk of adverse events260. For example, in 41 patients treated with immunotherapy followed by osimertinib, 15% developed severe immune-related adverse events, including grade 3 pneumonitis, grade 3 colitis, and grade 4 hepatitis260.
Hence, concomitant use of ICIs and EGFR or ALK TKIs is currently not advised outside of well-designed clinical trials owing to the considerable risks this might pose to the patient. Furthermore, premature exposure of patients to ICIs following an initial diagnosis should be avoided while genotyping data are pending, in order to minimize the risk of incurring toxicities upon subsequent switch to a TKI243.
Novel therapies and future directions
Antibody–drug conjugates (ADCs) and bispecific antibodies are novel classes of therapies that are currently under development and might be effective in patients with TKI-resistant cancers. These agents are of particular interest owing to the potential to overcome a diverse range of mechanisms of resistance. Amivantamab is a bispecific antibody targeting EGFR and MET that received accelerated approval for patients with NSCLCs harbouring EGFR exon 20 insertion mutations in May 2021 based on the results of the CHRYSALIS trial 261. The combination of amivantamab and lazertinib, a third-generation EGFR TKI, has demonstrated preliminary efficacy among 45 chemotherapy-naive patients with NSCLCs harbouring EGFR exon 19 deletions or L858R mutations that had progressed on osimertinib, with an ORR of 36% and a median PFS of 4.9 months262. Of note, although the ORR was higher (47%) among patients with tumours positive for EGFR-based biomarkers (i.e., EGFR mutations and amplification) or MET-based biomarkers (i.e., MET amplification or MET exon 14 skipping mutation), responses were observed even in their absence (ORR 29%), supporting a broader applicability of this approach263. As another example, patritumab deruxtecan is a HER3-directed ADC with a topoisomerase I inhibitor payload that has activity in patients with disease progression on at least one prior EGFR TKI (86% of whom received osimertinib)264, with efficacy observed across an array of mechanisms of resistance. An analysis of 48 paired pre-treatment and post-treatment tumour samples indicates that HER3 expression is often upregulated in EGFR-mutant tumours with acquired resistance to EGFR TKIs265 and that EGFR inhibition can potentiate the anticancer activity of patritumab deruxtecan in patient-derived xenograft models266, thus supporting the rationale for exploring the combination of patritumab deruxtecan and an EGFR TKI such as osimertinib. The activity of patritumab deruxtecan in patients with previously treated EGFR-mutant NSCLCs is currently being investigated further, both as a monotherapy (NCT04619004) and in combination with osimertinib (NCT04676477). The potential role of alternative ADCs targeting TROP2267 (NCT04152499) and MET268 (NCT03859752) is also being evaluated in patients with lung cancers, potentially including NSCLCs with EGFR mutations or ALK rearrangements. Of note, ALK fusions (which lack the transmembrane domain of native ALK) are not membrane bound; therefore, ADCs directly targeting ALK are not feasible for patients with ALK-rearranged NSCLCs.
Other novel therapeutic strategies, such as cancer vaccines, might hold some promise for patients with EGFR-driven or ALK-driven NSCLCs. For example, data from several studies demonstrate that ALK is antigenic in patients with ALK-positive anaplastic large-cell lymphomas269-272. Furthermore a DNA-based ALK vaccine has been shown to induce potent immune responses in mouse models of ALK-driven lymphoma and NSCLC273,274, providing the basis for ongoing efforts to develop anti-ALK vaccines as a novel treatment approach. Personalized neoantigen vaccines, either as monotherapy (NCT04397926) or together with EGFR TKIs (NCT04487093), are currently being tested in early phase studies, and numerous additional trials of personalized cancer vaccines, targeting patient-specific predicted tumour neoantigens are enrolling unselected patients with advanced-stage NSCLC, regardless of tumour genotype. Whether such vaccines are a viable therapeutic strategy for patients with EGFR-mutant or ALK-rearranged NSCLCs remains to be established.
Conclusions
The discovery of activating EGFR mutations and ALK rearrangements as actionable driver oncogenes launched a paradigm shift in the management of NSCLC. Subsequent advances with the development of increasingly potent and CNS-active EGFR and ALK TKIs, and — in tandem — the growing understanding of the mechanisms of resistance to these agents, have come to serve as a roadmap for the discovery of targeted therapies for other genomic subsets of NSCLC. Despite these advances, advanced-stage EGFR-mutant and ALK-rearranged NSCLCs remain incurable. Resistance remains an unsolved challenge. New therapeutic strategies are clearly still urgently needed to extend the lives of patients living with these cancers. Ongoing collaborative laboratory, transitional and clinical research efforts will enable the rational development of novel therapies based on a deeper and broader mechanistic understanding of the biology of TKI-resistant cancers.
Supplementary Material
Key points.
Non-small-cell lung cancers (NSCLCs) harbouring oncogenic EGFR mutations or ALK rearrangements can be effectively treated with EGFR and ALK tyrosine kinase inhibitors (TKIs), respectively.
The third-generation EGFR TKI osimertinib and the ALK TKI lorlatinib are currently the most advanced and effective clinically approved agents in their respective NSCLC subsets; both agents are highly effective including against CNS metastases.
Resistance to third-generation EGFR or ALK TKIs can be mediated by acquired EGFR or ALK kinase-domain mutations, respectively; fourth-generation TKIs designed to overcome this on-target resistance are currently in development.
Off-target resistance to third-generation EGFR and ALK TKIs is more prevalent than on-target resistance and is mediated by various mechanisms, including the activation of bypass signalling or phenotypic transformation.
Novel approaches designed to overcome resistance beyond fourth-generation TKIs, including combination therapies, antibody–drug conjugates, bispecific antibodies and immune-directed approaches, are at various stages of clinical investigation.
Acknowledgements
We would like to acknowledge the myriad research groups that have contributed to advances in EGFR-mutant and ALK-rearranged lung cancers, including those whose important work was not able to be featured in this Review owing to space constraints. We thank the patients, their families and clinical trial teams whose generosity and dedication have enabled drug development and advances in the mechanistic understanding of drug resistance.
Footnotes
Competing interests:
AJC has no competing interests to declare.
LVS has received consulting fees from AstraZeneca, Genentech, Pfizer, Takeda, and Janssen, and has received institutional research support from Boehringer-Ingelheim, Novartis, AstraZeneca, and Delfi.
JJL has served as a compensated consultant for Genentech, C4 Therapeutics, Blueprint Medicines, Nuvalent, Bayer, Elevation Oncology, Novartis, Mirati Therapeutics, and Turning Point Therapeutics; received honorarium and travel support from Pfizer; received institutional research funds from Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, Bayer, Elevation Oncology, Roche, Linnaeus, Nuvalent, and Novartis; received CME funding from OncLive, MedStar Health, and Northwell Health.
REFERENCES
- 1.Barlesi F et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). The Lancet 387, 1415–1426, doi: 10.1016/s0140-6736(16)00004-0 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Kris MG et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311, 1998–2006, doi: 10.1001/jama.2014.3741 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thai AA, Solomon BJ, Sequist LV, Gainor JF & Heist RS Lung cancer. The Lancet 398, 535–554, doi: 10.1016/s0140-6736(21)00312-3 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Rosell R et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 361, 958–967, doi: 10.1056/NEJMoa0904554 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Lynch TJ et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350, 2129–2139, doi: 10.1056/NEJMoa040938 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Paez JG et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500, doi: 10.1126/science.1099314 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Pao W et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101, 13306–13311, doi: 10.1073/pnas.0405220101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soda M et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448, 561–566, doi: 10.1038/nature05945 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 27, 4247–4253, doi: 10.1200/JCO.2009.22.6993 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee P & Wang Z Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 9, doi: 10.3390/cancers9050052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000146648-EGFR/tissue (Accessed 2021).
- 12.Mendelsohn J Targeting the Epidermal Growth Factor Receptor for Cancer Therapy. Journal of Clinical Oncology 20, doi: 10.1200/JCO.2002.07.121 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Rikova K et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203, doi: 10.1016/j.cell.2007.11.025 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Ding L et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075, doi: 10.1038/nature07423 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa K et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib ('Iressa', ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 14, 922–930, doi: 10.1093/annonc/mdg250 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Fukuoka M et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 21, 2237–2246, doi: 10.1200/JCO.2003.10.038 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Kris MG et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290, 2149–2158, doi: 10.1001/jama.290.16.2149 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353, 123–132, doi: 10.1056/NEJMoa050753 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Sordella R, Bell DW, Haber DA & Settleman J Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305, 1163–1167, doi: 10.1126/science.1101637 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Asahina H et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. Br J Cancer 95, 998–1004, doi: 10.1038/sj.bjc.6603393 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue A et al. Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations. J Clin Oncol 24, 3340–3346, doi: 10.1200/JCO.2005.05.4692 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Sutani A et al. Gefitinib for non-small-cell lung cancer patients with epidermal growth factor receptor gene mutations screened by peptide nucleic acid-locked nucleic acid PCR clamp. Br J Cancer 95, 1483–1489, doi: 10.1038/sj.bjc.6603466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sunaga N et al. Phase II prospective study of the efficacy of gefitinib for the treatment of stage III/IV non-small cell lung cancer with EGFR mutations, irrespective of previous chemotherapy. Lung Cancer 56, 383–389, doi: 10.1016/j.lungcan.2007.01.025 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Yoshida K et al. Prospective Validation for Prediction of Gefitinib Sensitivity by Epidermal Growth Factor Receptor Gene Mutation in Patients with Non-Small Cell Lung Cancer. Journal of Thoracic Oncology 2, 22–28, doi: 10.1016/s1556-0864(15)30013-7 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Sequist LV et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 26, 2442–2449, doi: 10.1200/JCO.2007.14.8494 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Tamura K et al. Multicentre prospective phase II trial of gefitinib for advanced non-small cell lung cancer with epidermal growth factor receptor mutations: results of the West Japan Thoracic Oncology Group trial (WJTOG0403). Br J Cancer 98, 907–914, doi: 10.1038/sj.bjc.6604249 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugio K et al. Prospective phase II study of gefitinib in non-small cell lung cancer with epidermal growth factor receptor gene mutations. Lung Cancer 64, 314–318, doi: 10.1016/j.lungcan.2008.09.010 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Mok TS et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361, 947–957, doi: 10.1056/NEJMoa0810699 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Han JY et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 30, 1122–1128, doi: 10.1200/JCO.2011.36.8456 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Mitsudomi T et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. The Lancet Oncology 11, 121–128, doi: 10.1016/s1470-2045(09)70364-x (2010). [DOI] [PubMed] [Google Scholar]
- 31.Maemondo M et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362, 2380–2388, doi: 10.1056/NEJMoa0909530 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Zhou C et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. The Lancet Oncology 12, 735–742, doi: 10.1016/s1470-2045(11)70184-x (2011). [DOI] [PubMed] [Google Scholar]
- 33.Rosell R et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. The Lancet Oncology 13, 239–246, doi: 10.1016/s1470-2045(11)70393-x (2012). [DOI] [PubMed] [Google Scholar]
- 34.Wu YL et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 26, 1883–1889, doi: 10.1093/annonc/mdv270 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Yu HA et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 19, 2240–2247, doi: 10.1158/1078-0432.CCR-12-2246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sequist LV et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 3, 75ra26, doi: 10.1126/scitranslmed.3002003 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun CH et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 105, 2070–2075, doi: 10.1073/pnas.0709662105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller VA et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. The Lancet Oncology 13, 528–538, doi: 10.1016/s1470-2045(12)70087-6 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Ellis PM et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. The Lancet Oncology 15, 1379–1388, doi: 10.1016/s1470-2045(14)70472-3 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Mok TS et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib With Gefitinib in Patients With Advanced Non-Small-Cell Lung Cancer and EGFR-Activating Mutations. J Clin Oncol 36, 2244–2250, doi: 10.1200/JCO.2018.78.7994 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Sequist LV et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31, 3327–3334, doi: 10.1200/JCO.2012.44.2806 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Janne PA et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372, 1689–1699, doi: 10.1056/NEJMoa1411817 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Colclough N et al. Preclinical Comparison of the Blood-brain barrier Permeability of Osimertinib with Other EGFR TKIs. Clin Cancer Res 27, 189–201, doi: 10.1158/1078-0432.CCR-19-1871 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Ballard P et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 22, 5130–5140, doi: 10.1158/1078-0432.CCR-16-0399 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Mok TS et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 376, 629–640, doi: 10.1056/NEJMoa1612674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soria JC et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378, 113–125, doi: 10.1056/NEJMoa1713137 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Ramalingam SS et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382, 41–50, doi: 10.1056/NEJMoa1913662 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Andrews Wright NM & Goss GD Third-generation epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Transl Lung Cancer Res 8, S247–S264, doi: 10.21037/tlcr.2019.06.01 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris SW et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263, 1281–1284, doi: 10.1126/science.8122112 (1994). [DOI] [PubMed] [Google Scholar]
- 50.Ou SI, Zhu VW & Nagasaka M Catalog of 5' Fusion Partners in ALK-positive NSCLC Circa 2020. JTO Clin Res Rep 1, 100015, doi: 10.1016/j.jtocrr.2020.100015 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi K et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 15, 3143–3149, doi: 10.1158/1078-0432.CCR-08-3248 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Togashi Y et al. KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only. PLoS One 7, e31323, doi: 10.1371/journal.pone.0031323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw AT & Engelman JA ALK in lung cancer: past, present, and future. J Clin Oncol 31, 1105–1111, doi: 10.1200/JCO.2012.44.5353 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiarle R, Voena C, Ambrogio C, Piva R & Inghirami G The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 8, 11–23, doi: 10.1038/nrc2291 (2008). [DOI] [PubMed] [Google Scholar]
- 55.McDermott U et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 68, 3389–3395, doi: 10.1158/0008-5472.CAN-07-6186 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Zou HY et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67, 4408–4417, doi: 10.1158/0008-5472.CAN-06-4443 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Kwak EL et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363, 1693–1703, doi: 10.1056/NEJMoa1006448 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camidge DR et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. The Lancet Oncology 13, 1011–1019, doi: 10.1016/s1470-2045(12)70344-3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw AT et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368, 2385–2394, doi: 10.1056/NEJMoa1214886 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Solomon BJ et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371, 2167–2177, doi: 10.1056/NEJMoa1408440 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Shaw AT et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 370, 1189–1197, doi: 10.1056/NEJMoa1311107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D-W et al. Activity and safety of ceritinib in patients with ALK -rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. The Lancet Oncology 17, 452–463, doi: 10.1016/s1470-2045(15)00614-2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ou SH et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 34, 661–668, doi: 10.1200/JCO.2015.63.9443 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Shaw AT et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. The Lancet Oncology 17, 234–242, doi: 10.1016/s1470-2045(15)00488-x (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gettinger SN et al. Activity and safety of brigatinib in ALK -rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. The Lancet Oncology 17, 1683–1696, doi: 10.1016/s1470-2045(16)30392-8 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Kim DW et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 35, 2490–2498, doi: 10.1200/JCO.2016.71.5904 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Peters S et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 377, 829–838, doi: 10.1056/NEJMoa1704795 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Camidge DR et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 379, 2027–2039, doi: 10.1056/NEJMoa1810171 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Horn L et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol, doi: 10.1001/jamaoncol.2021.3523 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou HY et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 28, 70–81, doi: 10.1016/j.ccell.2015.05.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson TW et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 57, 4720–4744, doi: 10.1021/jm500261q (2014). [DOI] [PubMed] [Google Scholar]
- 72.Shaw AT et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. The Lancet Oncology 18, 1590–1599, doi: 10.1016/s1470-2045(17)30680-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Solomon BJ et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. The Lancet Oncology 19, 1654–1667, doi: 10.1016/s1470-2045(18)30649-1 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Gainor JF et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017, doi: 10.1200/PO.17.00063 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drilon A et al. Frequency of Brain Metastases and Multikinase Inhibitor Outcomes in Patients With RET-Rearranged Lung Cancers. J Thorac Oncol 13, 1595–1601, doi: 10.1016/j.jtho.2018.07.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaw AT et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 37, 1370–1379, doi: 10.1200/JCO.18.02236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaw AT et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 383, 2018–2029, doi: 10.1056/NEJMoa2027187 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Thress KS et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21, 560–562, doi: 10.1038/nm.3854 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ercan D et al. EGFR Mutations and Resistance to Irreversible Pyrimidine-Based EGFR Inhibitors. Clin Cancer Res 21, 3913–3923, doi: 10.1158/1078-0432.CCR-14-2789 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yu HA et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol 1, 982–984, doi: 10.1001/jamaoncol.2015.1066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chabon JJ et al. Corrigendum: Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 7, 13513, doi: 10.1038/ncomms13513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leonetti A et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 121, 725–737, doi: 10.1038/s41416-019-0573-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Piotrowska Z et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 8, 1529–1539, doi: 10.1158/2159-8290.CD-18-1022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Z et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 24, 3097–3107, doi: 10.1158/1078-0432.CCR-17-2310 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Ou SI et al. Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung Cancer 108, 228–231, doi: 10.1016/j.lungcan.2017.04.003 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Klempner SJ, Mehta P, Schrock AB, Ali SM & Ou SI Cis-oriented solvent-front EGFR G796S mutation in tissue and ctDNA in a patient progressing on osimertinib: a case report and review of the literature. Lung Cancer (Auckl) 8, 241–247, doi: 10.2147/LCTT.S147129 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng D et al. EGFR G796D mutation mediates resistance to osimertinib. Oncotarget 8, 49671–49679, doi: 10.18632/oncotarget.17913 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen K et al. Novel Mutations on EGFR Leu792 Potentially Correlate to Acquired Resistance to Osimertinib in Advanced NSCLC. J Thorac Oncol 12, e65–e68, doi: 10.1016/j.jtho.2016.12.024 (2017). [DOI] [PubMed] [Google Scholar]
- 89.Ou Q et al. Investigating novel resistance mechanisms to third generation EGFR TKI osimertinib in non-small cell lung cancer patients using next generation sequencing. Journal of Clinical Oncology 35, 2572–2572, doi: 10.1200/JCO.2017.35.15_suppl.2572 (2017). [DOI] [Google Scholar]
- 90.Bersanelli M et al. L718Q Mutation as New Mechanism of Acquired Resistance to AZD9291 in EGFR-Mutated NSCLC. J Thorac Oncol 11, e121–123, doi: 10.1016/j.jtho.2016.05.019 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Fassunke J et al. Overcoming EGFR(G724S)-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun 9, 4655, doi: 10.1038/s41467-018-07078-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, He B, Zhou D, Li M & Hu C Newly emergent acquired EGFR exon 18 G724S mutation after resistance of a T790M specific EGFR inhibitor osimertinib in non-small-cell lung cancer: a case report. Onco Targets Ther 12, 51–56, doi: 10.2147/OTT.S188612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oztan A et al. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer 111, 84–87, doi: 10.1016/j.lungcan.2017.07.002 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Nukaga S et al. Amplification of EGFR Wild-Type Alleles in Non-Small Cell Lung Cancer Cells Confers Acquired Resistance to Mutation-Selective EGFR Tyrosine Kinase Inhibitors. Cancer Res 77, 2078–2089, doi: 10.1158/0008-5472.CAN-16-2359 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Knebel FH et al. Sequential liquid biopsies reveal dynamic alterations of EGFR driver mutations and indicate EGFR amplification as a new mechanism of resistance to osimertinib in NSCLC. Lung Cancer 108, 238–241, doi: 10.1016/j.lungcan.2017.04.004 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Schoenfeld AJ et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 26, 2654–2663, doi: 10.1158/1078-0432.CCR-19-3563 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schoenfeld AJ et al. Tissue-based molecular and histological landscape of acquired resistance to osimertinib given initially or at relapse in patients with EGFR-mutant lung cancers. Journal of Clinical Oncology 37, 9028–9028, doi: 10.1200/JCO.2019.37.15_suppl.9028 (2019). [DOI] [Google Scholar]
- 98.Ramalingam SS et al. Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Annals of Oncology 29, doi: 10.1093/annonc/mdy424.063 (2018). [DOI] [Google Scholar]
- 99.Gainor JF et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 6, 1118–1133, doi: 10.1158/2159-8290.CD-16-0596 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoda S et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 8, 714–729, doi: 10.1158/2159-8290.CD-17-1256 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiba-Ishii A et al. Structural and functional analysis of lorlatinib analogs reveals roadmap for targeting diverse compound resistance mutations in ALK-positive lung cancer. bioRxiv, doi: 10.1101/2021.07.16.452681 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaw AT et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 374, 54–61, doi: 10.1056/NEJMoa1508887 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dagogo-Jack I et al. Treatment with Next-Generation ALK Inhibitors Fuels Plasma ALK Mutation Diversity. Clin Cancer Res 25, 6662–6670, doi: 10.1158/1078-0432.CCR-19-1436 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pailler E et al. Acquired Resistance Mutations to ALK Inhibitors Identified by Single Circulating Tumor Cell Sequencing in ALK-Rearranged Non-Small-Cell Lung Cancer. Clin Cancer Res 25, 6671–6682, doi: 10.1158/1078-0432.CCR-19-1176 (2019). [DOI] [PubMed] [Google Scholar]
- 105.Zhu VW et al. A Novel Sequentially Evolved EML4-ALK Variant 3 G1202R/S1206Y Double Mutation In Cis Confers Resistance to Lorlatinib: A Brief Report and Literature Review. JTO Clin Res Rep 2, 100116, doi: 10.1016/j.jtocrr.2020.100116 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takahashi K et al. Overcoming resistance by ALK compound mutation (I1171S + G1269A) after sequential treatment of multiple ALK inhibitors in non-small cell lung cancer. Thorac Cancer 11, 581–587, doi: 10.1111/1759-7714.13299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Recondo G et al. Diverse Resistance Mechanisms to the Third-Generation ALK Inhibitor Lorlatinib in ALK-Rearranged Lung Cancer. Clin Cancer Res 26, 242–255, doi: 10.1158/1078-0432.CCR-19-1104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okada K et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug repurposing to overcome the resistance. EBioMedicine 41, 105–119, doi: 10.1016/j.ebiom.2019.01.019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Song Z et al. Deep RNA Sequencing Revealed Fusion Junctional Heterogeneity May Predict Crizotinib Treatment Efficacy in ALK-Rearranged NSCLC. J Thorac Oncol 17, 264–276, doi: 10.1016/j.jtho.2021.09.016 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Lin JJ et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J Clin Oncol 36, 1199–1206, doi: 10.1200/JCO.2017.76.2294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engelman JA et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043, doi: 10.1126/science.1141478 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Papadimitrakopoulou VA et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Annals of Oncology 29, doi: 10.1093/annonc/mdy424.064 (2018). [DOI] [Google Scholar]
- 113.Oxnard GR et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 4, 1527–1534, doi: 10.1001/jamaoncol.2018.2969 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ou SI, Agarwal N & Ali SM High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 98, 59–61, doi: 10.1016/j.lungcan.2016.05.015 (2016). [DOI] [PubMed] [Google Scholar]
- 115.York ER, Varella-Garcia M, Bang TJ, Aisner DL & Camidge DR Tolerable and Effective Combination of Full-Dose Crizotinib and Osimertinib Targeting MET Amplification Sequentially Emerging after T790M Positivity in EGFR-Mutant Non-Small Cell Lung Cancer. J Thorac Oncol 12, e85–e88, doi: 10.1016/j.jtho.2017.02.020 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Deng L, Kiedrowski LA, Ravera E, Cheng H & Halmos B Response to Dual Crizotinib and Osimertinib Treatment in a Lung Cancer Patient with MET Amplification Detected by Liquid Biopsy Who Acquired Secondary Resistance to EGFR Tyrosine Kinase Inhibition. J Thorac Oncol 13, e169–e172, doi: 10.1016/j.jtho.2018.04.007 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Zhu VW, Schrock AB, Ali SM & Ou SI Differential response to a combination of full-dose osimertinib and crizotinib in a patient with EGFR-mutant non-small cell lung cancer and emergent MET amplification. Lung Cancer (Auckl) 10, 21–26, doi: 10.2147/LCTT.S190403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kang J et al. Osimertinib and Cabozantinib Combinatorial Therapy in an EGFR-Mutant Lung Adenocarcinoma Patient with Multiple MET Secondary-Site Mutations after Resistance to Crizotinib. J Thorac Oncol 13, e49–e53, doi: 10.1016/j.jtho.2017.10.028 (2018). [DOI] [PubMed] [Google Scholar]
- 119.Oxnard GR et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol 31, 507–516, doi: 10.1016/j.annonc.2020.01.013 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Sequist LV et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. The Lancet Oncology 21, 373–386, doi: 10.1016/s1470-2045(19)30785-5 (2020). [DOI] [PubMed] [Google Scholar]
- 121.Dagogo-Jack I et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res 26, 2535–2545, doi: 10.1158/1078-0432.CCR-19-3906 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iams W & Chae Y P3.02-034 Acquired Resistance to Osimertinib by CCDC6-RET Fusion in a Patient with EGFR T790M Mutant Metastatic Lung Adenocarcinoma. Journal of Thoracic Oncology 12, S2249–S2250, doi: 10.1016/j.jtho.2017.09.1564 (2017). [DOI] [Google Scholar]
- 123.Offin M et al. Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. JCO Precis Oncol 2, doi: 10.1200/PO.18.00126 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le X et al. Landscape of EGFR-Dependent and -Independent Resistance Mechanisms to Osimertinib and Continuation Therapy Beyond Progression in EGFR-Mutant NSCLC. Clin Cancer Res 24, 6195–6203, doi: 10.1158/1078-0432.CCR-18-1542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McCoach CE et al. Resistance Mechanisms to Targeted Therapies in ROS1(+) and ALK(+) Non-small Cell Lung Cancer. Clin Cancer Res 24, 3334–3347, doi: 10.1158/1078-0432.CCR-17-2452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rotow J et al. FP14.07 Combination Osimertinib plus Selpercatinib for EGFR-mutant Non-Small Cell Lung Cancer (NSCLC) with Acquired RET fusions. Journal of Thoracic Oncology 16, doi: 10.1016/j.jtho.2021.01.150 (2021). [DOI] [Google Scholar]
- 127.Kim L, Chae YK, Jung CM, Lee AD & Yu E Addition of selpercatinib to overcome osimertinib resistance in non-small cell lung cancer (NSCLC) with acquired RET fusion detected in ctDNA at very low allele frequency. Journal of Clinical Oncology 39, 3046–3046, doi: 10.1200/JCO.2021.39.15_suppl.3046 (2021). [DOI] [Google Scholar]
- 128.Piotrowska Z et al. MET amplification (amp) as a resistance mechanism to osimertinib. Journal of Clinical Oncology 35, 9020–9020, doi: 10.1200/JCO.2017.35.15_suppl.9020 (2017). [DOI] [Google Scholar]
- 129.Schrock AB et al. Receptor Tyrosine Kinase Fusions and BRAF Kinase Fusions are Rare but Actionable Resistance Mechanisms to EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 13, 1312–1323, doi: 10.1016/j.jtho.2018.05.027 (2018). [DOI] [PubMed] [Google Scholar]
- 130.Takezawa K et al. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2, 922–933, doi: 10.1158/2159-8290.CD-12-0108 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Planchard D et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 26, 2073–2078, doi: 10.1093/annonc/mdv319 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Hsu CC et al. Exon 16-Skipping HER2 as a Novel Mechanism of Osimertinib Resistance in EGFR L858R/T790M-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 15, 50–61, doi: 10.1016/j.jtho.2019.09.006 (2020). [DOI] [PubMed] [Google Scholar]
- 133.Xia H et al. Evidence of NTRK1 Fusion as Resistance Mechanism to EGFR TKI in EGFR+ NSCLC: Results From a Large-Scale Survey of NTRK1 Fusions in Chinese Patients With Lung Cancer. Clin Lung Cancer 21, 247–254, doi: 10.1016/j.cllc.2019.09.004 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Vojnic M et al. Acquired BRAF Rearrangements Induce Secondary Resistance to EGFR therapy in EGFR-Mutated Lung Cancers. J Thorac Oncol 14, 802–815, doi: 10.1016/j.jtho.2018.12.038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ho CC et al. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Osimertinib. J Thorac Oncol 12, 567–572, doi: 10.1016/j.jtho.2016.11.2231 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Piotrowska Z et al. Heterogeneity and Coexistence of T790M and T790 Wild-Type Resistant Subclones Drive Mixed Response to Third-Generation Epidermal Growth Factor Receptor Inhibitors in Lung Cancer. JCO Precis Oncol 2018, doi: 10.1200/PO.17.00263 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu C, Li D, Duan W & Tao M TPD52L1-ROS1 Rearrangement as a New Acquired Resistance Mechanism to Osimertinib That Responds to Crizotinib in Combination With Osimertinib in Lung Adenocarcinoma. JTO Clin Res Rep 1, 100034, doi: 10.1016/j.jtocrr.2020.100034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeng L, Yang N & Zhang Y GOPC-ROS1 Rearrangement as an Acquired Resistance Mechanism to Osimertinib and Responding to Crizotinib Combined Treatments in Lung Adenocarcinoma. J Thorac Oncol 13, e114–e116, doi: 10.1016/j.jtho.2018.02.005 (2018). [DOI] [PubMed] [Google Scholar]
- 139.Allen JM et al. Genomic Profiling of Circulating Tumor DNA in Relapsed EGFR-mutated Lung Adenocarcinoma Reveals an Acquired FGFR3-TACC3 Fusion. Clin Lung Cancer 18, e219–e222, doi: 10.1016/j.cllc.2016.12.006 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Haura EB, Hicks JK & Boyle TA Erdafitinib Overcomes FGFR3-TACC3-Mediated Resistance to Osimertinib. J Thorac Oncol 15, e154–e156, doi: 10.1016/j.jtho.2019.12.132 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Fan PD et al. YES1 amplification is a mechanism of acquired resistance to EGFR inhibitors identified by transposon mutagenesis and clinical genomics. Proc Natl Acad Sci U S A 115, E6030–E6038, doi: 10.1073/pnas.1717782115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taniguchi H et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun 10, 259, doi: 10.1038/s41467-018-08074-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scaltriti M, Elkabets M & Baselga J Molecular Pathways: AXL, a Membrane Receptor Mediator of Resistance to Therapy. Clin Cancer Res 22, 1313–1317, doi: 10.1158/1078-0432.CCR-15-1458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park JH et al. Activation of the IGF1R pathway potentially mediates acquired resistance to mutant-selective 3rd-generation EGF receptor tyrosine kinase inhibitors in advanced non-small cell lung cancer. Oncotarget 7, 22005–22015, doi: 10.18632/oncotarget.8013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cortot AB et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res 73, 834–843, doi: 10.1158/0008-5472.CAN-12-2066 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Daniel C, Callens C, Melaabi S, Bieche I & Girard N Acquired Exon 14 MET Mutation Associated With Resistance to Alectinib in a Patient With ALK-Rearranged NSCLC. JTO Clin Res Rep 1, 100082, doi: 10.1016/j.jtocrr.2020.100082 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sui A et al. BRAF V600E mutation as a novel mechanism of acquired resistance to ALK inhibition in ALK-rearranged lung adenocarcinoma: A case report. Medicine (Baltimore) 100, e24917, doi: 10.1097/MD.0000000000024917 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gu FF et al. Lung adenocarcinoma harboring concomitant SPTBN1-ALK fusion, c-Met overexpression, and HER-2 amplification with inherent resistance to crizotinib, chemotherapy, and radiotherapy. J Hematol Oncol 9, 66, doi: 10.1186/s13045-016-0296-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Minari R et al. Emergence of a HER2-amplified clone during disease progression in an ALK-rearranged NSCLC patient treated with ALK-inhibitors: a case report. Transl Lung Cancer Res 9, 787–792, doi: 10.21037/tlcr.2020.04.03 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crystal AS et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346, 1480–1486, doi: 10.1126/science.1254721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Katayama R et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 4, 120ra117, doi: 10.1126/scitranslmed.3003316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim S et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol 8, 415–422, doi: 10.1097/JTO.0b013e318283dcc0 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Doebele RC et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 18, 1472–1482, doi: 10.1158/1078-0432.CCR-11-2906 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lovly CM et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med 20, 1027–1034, doi: 10.1038/nm.3667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wilson FH et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 27, 397–408, doi: 10.1016/j.ccell.2015.02.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nakamichi S et al. Overcoming drug-tolerant cancer cell subpopulations showing AXL activation and epithelial-mesenchymal transition is critical in conquering ALK-positive lung cancer. Oncotarget 9, 27242–27255, doi: 10.18632/oncotarget.25531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dardaei L et al. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med 24, 512–517, doi: 10.1038/nm.4497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eberlein CA et al. Acquired Resistance to the Mutant-Selective EGFR Inhibitor AZD9291 Is Associated with Increased Dependence on RAS Signaling in Preclinical Models. Cancer Res 75, 2489–2500, doi: 10.1158/0008-5472.CAN-14-3167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ou SI et al. Emergence of FGFR3-TACC3 fusions as a potential by-pass resistance mechanism to EGFR tyrosine kinase inhibitors in EGFR mutated NSCLC patients. Lung Cancer 111, 61–64, doi: 10.1016/j.lungcan.2017.07.006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ninomiya K et al. MET or NRAS amplification is an acquired resistance mechanism to the third-generation EGFR inhibitor naquotinib. Sci Rep 8, 1955, doi: 10.1038/s41598-018-20326-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ortiz-Cuaran S et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin Cancer Res 22, 4837–4847, doi: 10.1158/1078-0432.CCR-15-1915 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Mu Y et al. Acquired resistance to osimertinib in patients with non-small-cell lung cancer: mechanisms and clinical outcomes. J Cancer Res Clin Oncol 146, 2427–2433, doi: 10.1007/s00432-020-03239-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hong MH et al. Molecular landscape of osimertinib resistance revealed by targeted panel sequencing and patient-derived cancer models in non-small cell lung cancer patients. Annals of Oncology 29, doi: 10.1093/annonc/mdy292.051 (2018). [DOI] [Google Scholar]
- 164.Xu H et al. Characterization of acquired receptor tyrosine-kinase fusions as mechanisms of resistance to EGFR tyrosine-kinase inhibitors. Cancer Manag Res 11, 6343–6351, doi: 10.2147/CMAR.S197337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dagogo-Jack I et al. Response to the Combination of Osimertinib and Trametinib in a Patient With EGFR-Mutant NSCLC Harboring an Acquired BRAF Fusion. J Thorac Oncol 14, e226–e228, doi: 10.1016/j.jtho.2019.05.046 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Hrustanovic G et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med 21, 1038–1047, doi: 10.1038/nm.3930 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ercan D et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov 2, 934–947, doi: 10.1158/2159-8290.CD-12-0103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Oser MG, Niederst MJ, Sequist LV & Engelman JA Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. The Lancet Oncology 16, e165–e172, doi: 10.1016/s1470-2045(14)71180-5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ham JS et al. Two Cases of Small Cell Lung Cancer Transformation from EGFR Mutant Adenocarcinoma During AZD9291 Treatment. J Thorac Oncol 11, e1–4, doi: 10.1016/j.jtho.2015.09.013 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Li L et al. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: A case report. Oncotarget 8, 18609–18614, doi: 10.18632/oncotarget.14506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Taniguchi Y, Horiuchi H, Morikawa T & Usui K Small-Cell Carcinoma Transformation of Pulmonary Adenocarcinoma after Osimertinib Treatment: A Case Report. Case Rep Oncol 11, 323–329, doi: 10.1159/000489603 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Niederst MJ et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 6, 6377, doi: 10.1038/ncomms7377 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lee JK et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 35, 3065–3074, doi: 10.1200/JCO.2016.71.9096 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Marcoux N et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 37, 278–285, doi: 10.1200/JCO.18.01585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Takegawa N et al. Transformation of ALK rearrangement-positive adenocarcinoma to small-cell lung cancer in association with acquired resistance to alectinib. Ann Oncol 27, 953–955, doi: 10.1093/annonc/mdw032 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Miyamoto S et al. Transformation to small-cell lung cancer as a mechanism of acquired resistance to crizotinib and alectinib. Jpn J Clin Oncol 46, 170–173, doi: 10.1093/jjco/hyv173 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Fujita S, Masago K, Katakami N & Yatabe Y Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol 11, e67–72, doi: 10.1016/j.jtho.2015.12.105 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Park S, Han J & Sun JM Histologic transformation of ALK-rearranged adenocarcinoma to squamous cell carcinoma after treatment with ALK inhibitor. Lung Cancer 127, 66–68, doi: 10.1016/j.lungcan.2018.11.027 (2019). [DOI] [PubMed] [Google Scholar]
- 179.Kaiho T, Nakajima T, Iwasawa S, Yonemori Y & Yoshino I ALK Rearrangement Adenocarcinoma with Histological Transformation to Squamous Cell Carcinoma Resistant to Alectinib and Ceritinib. Onco Targets Ther 13, 1557–1560, doi: 10.2147/OTT.S236706 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ueda S et al. Transformation from adenocarcinoma to squamous cell lung carcinoma with MET amplification after lorlatinib resistance: A case report. Thorac Cancer 12, 715–719, doi: 10.1111/1759-7714.13829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin JJ et al. Small cell transformation of ROS1 fusion-positive lung cancer resistant to ROS1 inhibition. NPJ Precis Oncol 4, 21, doi: 10.1038/s41698-020-0127-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Piper-Vallillo AJ, Sequist LV & Piotrowska Z Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. J Clin Oncol, JCO1903123, doi: 10.1200/JCO.19.03123 (2020). [DOI] [PubMed] [Google Scholar]
- 183.Singh A & Settleman J EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751, doi: 10.1038/onc.2010.215 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Voulgari A & Pintzas A Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796, 75–90, doi: 10.1016/j.bbcan.2009.03.002 (2009). [DOI] [PubMed] [Google Scholar]
- 185.Weng CH et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 38, 455–468, doi: 10.1038/s41388-018-0454-2 (2019). [DOI] [PubMed] [Google Scholar]
- 186.Tanaka K et al. Targeting Aurora B kinase prevents and overcomes resistance to EGFR inhibitors in lung cancer by enhancing BIM- and PUMA-mediated apoptosis. Cancer Cell 39, 1245–1261 e1246, doi: 10.1016/j.ccell.2021.07.006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lin JJ & Shaw AT Resisting Resistance: Targeted Therapies in Lung Cancer. Trends Cancer 2, 350–364, doi: 10.1016/j.trecan.2016.05.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu C et al. Potential resistance mechanisms using next generation sequencing from Chinese EGFR T790M+ non-small cell lung cancer patients with primary resistance to osimertinib: A multicenter study. Annals of Oncology 30, doi: 10.1093/annonc/mdz063.012 (2019). [DOI] [Google Scholar]
- 189.Oxnard GR et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol 8, 179–184, doi: 10.1097/JTO.0b013e3182779d18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arcila ME et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther 12, 220–229, doi: 10.1158/1535-7163.MCT-12-0620 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bivona TG et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature 471, 523–526, doi: 10.1038/nature09870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ng KP et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 18, 521–528, doi: 10.1038/nm.2713 (2012). [DOI] [PubMed] [Google Scholar]
- 193.Karachaliou N et al. BIM and mTOR expression levels predict outcome to erlotinib in EGFR-mutant non-small-cell lung cancer. Sci Rep 5, 17499, doi: 10.1038/srep17499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang L et al. Clinical features of Bim deletion polymorphism and its relation with crizotinib primary resistance in Chinese patients with ALK/ROS1 fusion-positive non-small cell lung cancer. Cancer 123, 2927–2935, doi: 10.1002/cncr.30677 (2017). [DOI] [PubMed] [Google Scholar]
- 195.Dagogo-Jack I & Shaw AT Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15, 81–94, doi: 10.1038/nrclinonc.2017.166 (2018). [DOI] [PubMed] [Google Scholar]
- 196.Marusyk A, Janiszewska M & Polyak K Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 37, 471–484, doi: 10.1016/j.ccell.2020.03.007 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rheinheimer S et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers (Basel) 12, doi: 10.3390/cancers12041046 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Campo M et al. Integration of Stereotactic Body Radiation Therapy With Tyrosine Kinase Inhibitors in Stage IV Oncogene-Driven Lung Cancer. Oncologist 21, 964–973, doi: 10.1634/theoncologist.2015-0508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Weickhardt AJ et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 7, 1807–1814, doi: 10.1097/JTO.0b013e3182745948 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Franceschini D et al. The use of radiation therapy for oligoprogressive/oligopersistent oncogene-driven non small cell lung cancer: State of the art. Crit Rev Oncol Hematol 148, 102894, doi: 10.1016/j.critrevonc.2020.102894 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Bearz A et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer 68, 264–268, doi: 10.1016/j.lungcan.2009.06.018 (2010). [DOI] [PubMed] [Google Scholar]
- 202.Goldstein IM et al. Dose escalation of osimertinib for intracranial progression in EGFR mutated non-small-cell lung cancer with brain metastases. Neurooncol Adv 2, vdaa125, doi: 10.1093/noajnl/vdaa125 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Piper-Vallillo A et al. High-dose osimertinib for CNS progression in EGFR+ non-small cell lung cancer (NSCLC): A multi-institutional experience. Journal of Clinical Oncology 38, 9586–9586, doi: 10.1200/JCO.2020.38.15_suppl.9586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Park S et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 31, 1397–1404, doi: 10.1016/j.annonc.2020.06.017 (2020). [DOI] [PubMed] [Google Scholar]
- 205.Gainor JF et al. Alectinib Dose Escalation Reinduces Central Nervous System Responses in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer Relapsing on Standard Dose Alectinib. J Thorac Oncol 11, 256–260, doi: 10.1016/j.jtho.2015.10.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Urbanska EM, Santoni-Rugiu E, Melchior LC, Carlsen JF & Sorensen JB Intracranial Response of ALK(+) Non-Small-cell Lung Cancer to Second-line Dose-escalated Brigatinib After Alectinib Discontinuation Due to Drug-induced Hepatitis and Relapse After Whole Brain Radiotherapy Followed by Stereotactic Radiosurgery. Clin Lung Cancer 22, e528–e532, doi: 10.1016/j.cllc.2020.04.012 (2021). [DOI] [PubMed] [Google Scholar]
- 207.Jia Y et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 534, 129–132, doi: 10.1038/nature17960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang S, Song Y & Liu D EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett 385, 51–54, doi: 10.1016/j.canlet.2016.11.008 (2017). [DOI] [PubMed] [Google Scholar]
- 209.To C et al. Single and Dual Targeting of Mutant EGFR with an Allosteric Inhibitor. Cancer Discov 9, 926–943, doi: 10.1158/2159-8290.CD-18-0903 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lim SM et al. BLU-945, a fourth generation, potent and highly selective epidermal growth factor receptor tyrosine kinase inhibitor with intracranial activity, demonstrates robust in vivo anti-tumor activity in models of osimertinib-resistant non-small cell lung cancer. Presented at AACR 2021 Abstract 1467 (2021). [Google Scholar]
- 211.Conti C et al. BLU-701 is a highly potent, brain-penetrant and WT-sparing next-generation EGFR TKI for the treatment of sensitizing (ex19del, L858R) and C797S resistance mutations in metastatic NSCLC. Presented at AACR 2021 Abstract 1262 (2021). [Google Scholar]
- 212.Murray BW et al. TPX-0131, a Potent CNS-Penetrant, Next-Generation Inhibitor of Wild-Type ALK and ALK-Resistant Mutations. Mol Cancer Ther, doi: 10.1158/1535-7163.MCT-21-0221 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tangpeerachaikul A, Deshpande A, Kohl NE, Horan JC & Pelish HE NVL-655 Exhibits Antitumor Activity in Lorlatinib-Resistant Subcutaneous and Intracranial Models of ALK-Rearranged NSCLC (P244). AACR-NCI-EORTC Molecular Targets Conference (2021). [Google Scholar]
- 214.Zhang S et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 22, 5527–5538, doi: 10.1158/1078-0432.CCR-16-0569 (2016). [DOI] [PubMed] [Google Scholar]
- 215.Uchibori K et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 8, 14768, doi: 10.1038/ncomms14768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Noda-Narita S & Kanda S Overcoming resistance to third-generation epidermal growth factor receptor tyrosine kinase inhibitor in non-small cell lung cancer. Translational Cancer Research 6, S1187–S1190, doi: 10.21037/tcr.2017.09.04 (2017). [DOI] [Google Scholar]
- 217.Wang X et al. Lung Adenocarcinoma Harboring EGFR 19del/C797S/T790M Triple Mutations Responds to Brigatinib and Anti-EGFR Antibody Combination Therapy. J Thorac Oncol 14, e85–e88, doi: 10.1016/j.jtho.2019.01.015 (2019). [DOI] [PubMed] [Google Scholar]
- 218.Zhao J et al. Effective treatment of pulmonary adenocarcinoma harboring triple EGFR mutations of L858R, T790M, and cis-C797S by osimertinib, bevacizumab, and brigatinib combination therapy: a case report. Onco Targets Ther 11, 5545–5550, doi: 10.2147/OTT.S170358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Niederst MJ et al. The Allelic Context of the C797S Mutation Acquired upon Treatment with Third-Generation EGFR Inhibitors Impacts Sensitivity to Subsequent Treatment Strategies. Clin Cancer Res 21, 3924–3933, doi: 10.1158/1078-0432.CCR-15-0560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wang Z et al. Lung Adenocarcinoma Harboring EGFR T790M and In Trans C797S Responds to Combination Therapy of First- and Third-Generation EGFR TKIs and Shifts Allelic Configuration at Resistance. J Thorac Oncol 12, 1723–1727, doi: 10.1016/j.jtho.2017.06.017 (2017). [DOI] [PubMed] [Google Scholar]
- 221.Arulananda S et al. Combination Osimertinib and Gefitinib in C797S and T790M EGFR-Mutated Non-Small Cell Lung Cancer. J Thorac Oncol 12, 1728–1732, doi: 10.1016/j.jtho.2017.08.006 (2017). [DOI] [PubMed] [Google Scholar]
- 222.Zhou Z et al. Durable Clinical Response of Lung Adenocarcinoma Harboring EGFR 19Del/T790M/in trans-C797S to Combination Therapy of First- and Third-Generation EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 14, e157–e159, doi: 10.1016/j.jtho.2019.04.020 (2019). [DOI] [PubMed] [Google Scholar]
- 223.Patil T et al. Effect of continuing osimertinib with chemotherapy in the post-progression setting on progression-free survival among patients with metastatic epidermal growth factor receptor (EGFR) positive non-small cell lung cancer. Journal of Clinical Oncology 39, 9124–9124, doi: 10.1200/JCO.2021.39.15_suppl.9124 (2021). [DOI] [Google Scholar]
- 224.White MN et al. Combining Osimertinib With Chemotherapy in EGFR-Mutant NSCLC at Progression. Clin Lung Cancer 22, 201–209, doi: 10.1016/j.cllc.2021.01.010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lin JJ et al. Efficacy of Platinum/Pemetrexed Combination Chemotherapy in ALK-Positive NSCLC Refractory to Second-Generation ALK Inhibitors. J Thorac Oncol 15, 258–265, doi: 10.1016/j.jtho.2019.10.014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cabanos HF & Hata AN Emerging Insights into Targeted Therapy-Tolerant Persister Cells in Cancer. Cancers (Basel) 13, doi: 10.3390/cancers13112666 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hosomi Y et al. Gefitinib Alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer With Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J Clin Oncol 38, 115–123, doi: 10.1200/JCO.19.01488 (2020). [DOI] [PubMed] [Google Scholar]
- 228.Noronha V et al. Gefitinib Versus Gefitinib Plus Pemetrexed and Carboplatin Chemotherapy in EGFR-Mutated Lung Cancer. J Clin Oncol 38, 124–136, doi: 10.1200/JCO.19.01154 (2020). [DOI] [PubMed] [Google Scholar]
- 229.Watanabe H et al. Significant combination benefit of anti-VEGFR antibody and oncogene-targeted agents in EGFR or ALK mutant NSCLC cells. Cancer Res 79 (2019). [Google Scholar]
- 230.Nakagawa K et al. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res, doi: 10.1158/1078-0432.CCR-21-0273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kawashima Y et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. The Lancet Respiratory Medicine 10, 72–82, doi: 10.1016/s2213-2600(21)00166-1 (2022). [DOI] [PubMed] [Google Scholar]
- 232.Zhou Q et al. CTONG 1509: Phase III study of bevacizumab with or without erlotinib in untreated Chinese patients with advanced EGFR-mutated NSCLC. Annals of Oncology 30, doi: 10.1093/annonc/mdz260.002 (2019). [DOI] [Google Scholar]
- 233.Soo R et al. VP3-2021: A randomized phase II study of second-line osimertinib (Osi) and bevacizumab (Bev) versus Osi in advanced non-small-cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) and T790M mutations (mt): Results from the ETOP BOOSTER trial. Annals of Oncology 32, 942–944, doi: 10.1016/j.annonc.2021.04.010 (2021). [DOI] [Google Scholar]
- 234.Akamatsu H et al. Efficacy of Osimertinib Plus Bevacizumab vs Osimertinib in Patients With EGFR T790M-Mutated Non-Small Cell Lung Cancer Previously Treated With Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: West Japan Oncology Group 8715L Phase 2 Randomized Clinical Trial. JAMA Oncol 7, 386–394, doi: 10.1001/jamaoncol.2020.6758 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kenmotsu H et al. Primary results of a randomized phase II study of osimertinib plus bevacizumab versus osimertinib monotherapy for untreated patients with non-squamous non-small cell lung cancer harboring EGFR mutations: WJOG9717L study. Presented at ESMO 2021 Abstract LBA44 (2021). [Google Scholar]
- 236.Lin JJ et al. Safety and activity of alectinib plus bevacizumab in patients with advanced ALK-rearranged non-small-cell lung cancer: a phase I/II study. ESMO Open 7, 100342, doi: 10.1016/j.esmoop.2021.100342 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Watanabe S et al. MA21.05 Phase II Trial of the Combination of Alectinib with Bevacizumab in ALK-Positive Nonsquamous Non-Small Cell Lung Cancer. Journal of Thoracic Oncology 14, doi: 10.1016/j.jtho.2019.08.676 (2019). [DOI] [Google Scholar]
- 238.Gainor JF et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 22, 4585–4593, doi: 10.1158/1078-0432.CCR-15-3101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Garassino MC et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. The Lancet Oncology 19, 521–536, doi: 10.1016/s1470-2045(18)30144-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bylicki O et al. Efficacy and safety of programmed cell-death-protein-1 and its ligand inhibitors in pretreated patients with epidermal growth-factor receptor-mutated or anaplastic lymphoma kinase-translocated lung adenocarcinoma. Medicine (Baltimore) 99, e18726, doi: 10.1097/MD.0000000000018726 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Huang Q et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology 7, e1396403, doi: 10.1080/2162402X.2017.1396403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lee CK et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 12, 403–407, doi: 10.1016/j.jtho.2016.10.007 (2017). [DOI] [PubMed] [Google Scholar]
- 243.Calles A, Riess JW & Brahmer JR Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am Soc Clin Oncol Educ Book 40, 372–384, doi: 10.1200/EDBK_280795 (2020). [DOI] [PubMed] [Google Scholar]
- 244.Socinski MA et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 378, 2288–2301, doi: 10.1056/NEJMoa1716948 (2018). [DOI] [PubMed] [Google Scholar]
- 245.Reck M et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. The Lancet Respiratory Medicine 7, 387–401, doi: 10.1016/s2213-2600(19)30084-0 (2019). [DOI] [PubMed] [Google Scholar]
- 246.Nogami N et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J Thorac Oncol 17, 309–323, doi: 10.1016/j.jtho.2021.09.014 (2022). [DOI] [PubMed] [Google Scholar]
- 247.Lu S et al. VP9-2021: ORIENT-31: Phase III study of sintilimab with or without IBI305 plus chemotherapy in patients with EGFR mutated nonsquamous NSCLC who progressed after EGFR-TKI therapy. Annals of Oncology 33, 112–113, doi: 10.1016/j.annonc.2021.10.007 (2022). [DOI] [Google Scholar]
- 248.Muthusamy B & Pennell N Chemoimmunotherapy for EGFR-Mutant NSCLC: Still No Clear Answer. J Thorac Oncol 17, 179–181, doi: 10.1016/j.jtho.2021.11.012 (2022). [DOI] [PubMed] [Google Scholar]
- 249.Rudin C et al. MA15.02 Long-Term Safety and Clinical Activity Results from a Phase Ib Study of Erlotinib Plus Atezolizumab in Advanced NSCLC. Journal of Thoracic Oncology 13, doi: 10.1016/j.jtho.2018.08.440 (2018). [DOI] [Google Scholar]
- 250.Gettinger S et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol 13, 1363–1372, doi: 10.1016/j.jtho.2018.05.015 (2018). [DOI] [PubMed] [Google Scholar]
- 251.Creelan BC et al. A Phase 1 study of gefitinib combined with durvalumab in EGFR TKI-naive patients with EGFR mutation-positive locally advanced/metastatic non-small-cell lung cancer. Br J Cancer 124, 383–390, doi: 10.1038/s41416-020-01099-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yang JC et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol 14, 553–559, doi: 10.1016/j.jtho.2018.11.028 (2019). [DOI] [PubMed] [Google Scholar]
- 253.Spigel DR et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370). J Thorac Oncol 13, 682–688, doi: 10.1016/j.jtho.2018.02.022 (2018). [DOI] [PubMed] [Google Scholar]
- 254.Felip E et al. Ceritinib plus Nivolumab in Patients with Advanced ALK-Rearranged Non-Small Cell Lung Cancer: Results of an Open-Label, Multicenter, Phase 1B Study. J Thorac Oncol 15, 392–403, doi: 10.1016/j.jtho.2019.10.006 (2020). [DOI] [PubMed] [Google Scholar]
- 255.Kim D-W et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). Journal of Clinical Oncology 36, 9009–9009, doi: 10.1200/JCO.2018.36.15_suppl.9009 (2018). [DOI] [Google Scholar]
- 256.Shaw AT et al. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. Journal of Clinical Oncology 36, 9008–9008, doi: 10.1200/JCO.2018.36.15_suppl.9008 (2018). [DOI] [Google Scholar]
- 257.Patel SP et al. Phase Ib Study of Crizotinib plus Pembrolizumab in Patients with Previously Untreated Advanced Non-Small Cell Lung Cancer with ALK Translocation. Oncologist 25, 562–e1012, doi: 10.1634/theoncologist.2020-0034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ahn MJ et al. Brief Report: Osimertinib Plus Durvalumab in Patients with EGFR-Mutated, Advanced Non-Small Cell Lung Cancer: A Phase 1b, Open-Label, Multicenter Trial. J Thorac Oncol, doi: 10.1016/j.jtho.2022.01.012 (2022). [DOI] [PubMed] [Google Scholar]
- 259.Ahn MJ et al. 136O: Osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: Results from the TATTON phase Ib trial. Journal of Thoracic Oncology 11, doi: 10.1016/s1556-0864(16)30246-5 (2016). [DOI] [Google Scholar]
- 260.Schoenfeld AJ et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 30, 839–844, doi: 10.1093/annonc/mdz077 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Park K et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol 39, 3391–3402, doi: 10.1200/JCO.21.00662 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cho BC et al. 1258O - Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. (2020). [Google Scholar]
- 263.Bauml J et al. Amivantamab in combination with lazertinib for the treatment of osimertinib-relapsed, chemotherapy-naïve EGFR mutant (EGFRm) non-small cell lung cancer (NSCLC) and potential biomarkers for response. Journal of Clinical Oncology 39, 9006–9006, doi: 10.1200/JCO.2021.39.15_suppl.9006 (2021). [DOI] [Google Scholar]
- 264.Janne PA et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov 12, 74–89, doi: 10.1158/2159-8290.CD-21-0715 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yonesaka K et al. HER3 Augmentation via Blockade of EGFR/AKT Signaling Enhances Anticancer Activity of HER3-Targeting Patritumab Deruxtecan in EGFR-Mutated Non-Small Cell Lung Cancer. Clin Cancer Res 28, 390–403, doi: 10.1158/1078-0432.CCR-21-3359 (2022). [DOI] [PubMed] [Google Scholar]
- 266.Haikala HM et al. EGFR Inhibition Enhances the Cellular Uptake and Antitumor-Activity of the HER3 Antibody-Drug Conjugate HER3-DXd. Cancer Res 82, 130–141, doi: 10.1158/0008-5472.CAN-21-2426 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Zaman S, Jadid H, Denson AC & Gray JE Targeting Trop-2 in solid tumors: future prospects. Onco Targets Ther 12, 1781–1790, doi: 10.2147/OTT.S162447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gymnopoulos M et al. TR1801-ADC: a highly potent cMet antibody-drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol Oncol 14, 54–68, doi: 10.1002/1878-0261.12600 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Pulford K et al. Immune response to the ALK oncogenic tyrosine kinase in patients with anaplastic large-cell lymphoma. Blood 96, 1605–1607, doi: 10.1182/blood.V96.4.1605 (2000). [DOI] [PubMed] [Google Scholar]
- 270.Passoni L et al. ALK as a novel lymphoma-associated tumor antigen: identification of 2 HLA-A2.1-restricted CD8+ T-cell epitopes. Blood 99, 2100–2106, doi: 10.1182/blood.v99.6.2100 (2002). [DOI] [PubMed] [Google Scholar]
- 271.Ait-Tahar K et al. B and CTL responses to the ALK protein in patients with ALK-positive ALCL. Int J Cancer 118, 688–695, doi: 10.1002/ijc.21410 (2006). [DOI] [PubMed] [Google Scholar]
- 272.Ait-Tahar K, Barnardo MC & Pulford K CD4 T-helper responses to the anaplastic lymphoma kinase (ALK) protein in patients with ALK-positive anaplastic large-cell lymphoma. Cancer Res 67, 1898–1901, doi: 10.1158/0008-5472.CAN-06-4427 (2007). [DOI] [PubMed] [Google Scholar]
- 273.Chiarle R et al. The anaplastic lymphoma kinase is an effective oncoantigen for lymphoma vaccination. Nat Med 14, 676–680, doi: 10.1038/nm1769 (2008). [DOI] [PubMed] [Google Scholar]
- 274.Voena C et al. Efficacy of a Cancer Vaccine against ALK-Rearranged Lung Tumors. Cancer Immunol Res 3, 1333–1343, doi: 10.1158/2326-6066.CIR-15-0089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.