Abstract
Background
Non‐infectious intermediate, posterior, and panuveitis (NIIPPU) represent a heterogenous collection of autoimmune and inflammatory disorders isolated to or concentrated in the posterior structures of the eye. Because NIIPPU is typically a chronic condition, people with NIIPPU frequently require treatment with steroid‐sparing immunosuppressive therapy. Methotrexate, mycophenolate, cyclosporine, azathioprine, and tacrolimus are non‐biologic, disease‐modifying antirheumatic drugs (DMARDs) which have been used to treat people with NIIPPU.
Objectives
To compare the effectiveness and safety of selected DMARDs (methotrexate, mycophenolate mofetil, tacrolimus, cyclosporine, and azathioprine) in the treatment of NIIPPU in adults.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register), MEDLINE, Embase, the Latin American and Caribbean Health Sciences database, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform, most recently on 16 April 2021.
Selection criteria
We included randomized controlled trials (RCTs) comparing selected DMARDs (methotrexate, mycophenolate, tacrolimus, cyclosporine, and azathioprine) with placebo, standard of care (topical steroids, with or without oral steroids), or with each other.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 11 RCTs with a total of 601 participants in this review.
DMARDs versus control
Two studies compared an experimental DMARD (cyclosporine A or enteric‐coated mycophenolate [EC‐MPS]) plus oral steroid with steroid monotherapy. We did not pool these results into a meta‐analysis because the dose of cyclosporine used was much higher than that used in current clinical practice. The evidence is very uncertain about whether EC‐MPS plus low‐dose oral steroid results in a higher proportion of participants achieving control of inflammation over steroid monotherapy (risk ratio [RR] 2.81, 95% confidence interval [CI] 1.10 to 7.17; 1 study, 41 participants; very low‐certainty evidence). The change in best‐corrected visual acuity (BCVA) was reported separately for right and left eyes. The evidence for improvement (lower logarithm of the minimum angle of resolution (logMAR) indicates better vision) between the groups is very uncertain (mean difference [MD] ‐0.03 and ‐0.10, 95% CI ‐0.96 to 0.90 and ‐0.27 to 0.07 for right and left, respectively; 1 study, 82 eyes; very low‐certainty evidence). No data were available for the following outcomes: proportion of participants achieving a 2‐line improvement in visual acuity, with confirmed macular edema, or achieving steroid‐sparing control. The evidence for the proportion of participants requiring cessation of medication in the DMARD versus control group is very uncertain (RR 2.61, 95% CI 0.11 to 60.51; 1 study, 41 participants; very low‐certainty evidence).
Methotrexate versus mycophenolate
We were able to combine two studies into a meta‐analysis comparing methotrexate versus mycophenolate mofetil. Methotrexate probably results in a slight increase in the proportion of participants achieving control of inflammation, including steroid‐sparing control, compared to mycophenolate at six months (RR 1.23, 95% CI 1.01 to 1.50; 2 studies, 261 participants; moderate‐certainty evidence). Change in BCVA was reported per eye and the treatments likely result in little to no difference in change in vision (MD 0.01 logMAR higher [worse] for methotrexate versus mycophenolate; 2 studies, 490 eyes; moderate‐certainty evidence). No data were available for the proportion of participants achieving a 2‐line improvement in visual acuity. The evidence is very uncertain regarding the proportion of participants with confirmed macular edema between methotrexate versus mycophenolate (RR 0.49, 95% CI 0.19 to 1.30; 2 studies, 35 eyes; very low‐certainty). Methotrexate versus mycophenolate may result in little to no difference in the proportion of participants requiring cessation of medication (RR 0.99, 95% CI 0.43 to 2.27; 2 studies, 296 participants; low‐certainty evidence).
Steroids with or without azathioprine versus cyclosporine A
Four studies compared steroids with or without azathioprine (oral steroids, intravenous [IV] steroids, or azathioprine) to cyclosporine A. We excluded two studies from the meta‐analysis because the participants were treated with 8 mg to 15 mg/kg/day of cyclosporine A, a significantly higher dose than is utilized today because of concerns for nephrotoxicity.
The remaining two studies were conducted in all Vogt‐Koyanagi‐Harada disease (VKH) populations and compared cyclosporine A to azathioprine or IV pulse‐dose steroids. The evidence is very uncertain for whether the steroids with or without azathioprine or cyclosporine A influenced the proportion of participants achieving control of inflammation (RR 0.84, 95% CI 0.70 to 1.02; 2 studies, 112 participants; very low‐certainty evidence), achieving steroid‐sparing control (RR 0.64, 95% CI 0.33 to 1.25; 1 study, 21 participants; very low‐certainty evidence), or requiring cessation of medication (RR 0.85, 95% 0.21 to 3.45; 2 studies, 91 participants; very low‐certainty evidence). The evidence is uncertain for improvement in BCVA (MD 0.04 logMAR lower [better] with the steroids with or without azathioprine versus cyclosporine A; 2 studies, 91 eyes; very low‐certainty evidence). There were no data available (with current cyclosporine A dosing) for the proportion of participants achieving a 2‐line improvement in visual acuity or with confirmed macular edema.
Studies not included in synthesis
We were unable to include three studies in any of the comparisons (in addition to the aforementioned studies excluded based on historic doses of cyclosporine A). One was a dose‐response study comparing cyclosporine A to cyclosporine G, a formulation which was never licensed and is not clinically available. We excluded another study from meta‐analysis because it compared cyclosporine A and tacrolimus, considered to be of the same class (calcineurin inhibitors). We were unable to combine the third study, which examined tacrolimus monotherapy versus tacrolimus plus oral steroid, with any group.
Authors' conclusions
There is a paucity of data regarding which DMARD is most effective or safe in NIIPPU. Studies in general were small, heterogenous in terms of their design and outcome measures, and often did not compare different classes of DMARD with each other. Methotrexate is probably slightly more efficacious than mycophenolate in achieving control of inflammation, including steroid‐sparing control (moderate‐certainty evidence), although there was insufficient evidence to prefer one medication over the other in the VKH subgroup (very low‐certainty evidence). Methotrexate may result in little to no difference in safety outcomes compared to mycophenolate.
Plain language summary
Treatment of inflammatory conditions of the eye with medications
What was the aim of this review?
In this systematic review, we studied the available evidence on the effectiveness and safety of several medications used to treat a specific subset of inflammatory conditions of the back part of the eye.
Key message
We found that the medication methotrexate may be slightly more effective than mycophenolate. Otherwise, there was no strong evidence to suggest one medication is more effective or safe than the others.
What did we study in this review?
Non‐infectious intermediate, posterior, and panuveitis (NIIPPU) are a collection of several diseases which cause inflammation restricted to or including the back part of the eye. NIIPPU is not caused by an infection. NIIPPU is usually treated first with oral steroids, which tamp down the overactive immune system. However, because steroids have many other side effects when used over the long term, people with NIIPPU often require other immunosuppressive medications to control their disease. Several medications have been developed, but it is not clear which is most effective or safest for NIIPPU. This systematic review attempted to answer how safe and effective the medications methotrexate, mycophenolate, azathioprine, tacrolimus, and cyclosporine are for NIIPPU.
What were the main results of this review?
The main result of this review is that the medication methotrexate may be slightly more effective than mycophenolate for the treatment of NIIPPU in terms of how many people had their disease controlled and were able to be weaned from (taken off) steroid medications. The two medications are likely similar in terms of their safety, but this evidence is not strong. There were not many other instances in which we could combine evidence from different studies. There is a general lack of data in this area, and we cannot draw any other conclusions regarding the superiority of one medication over the others in terms of safety or efficacy.
What are the limitations of this review?
This review does not include biologic therapies, which are increasingly becoming the class of medications preferred by most doctors for treating inflammatory rheumatologic conditions such as NIIPPU. This review is also limited by the lack of large, randomized controlled trials (RCTs) in the field, wide differences in the available studies' design, and absence of studies comparing multiple medications head‐to‐head.
How up to date is the review?
This study is up to date as of 16 April 2021.
Summary of findings
Summary of findings 1. Non‐biologic disease‐modifying antirheumatic drugs (DMARDs) versus steroid for NIIPPU.
| Non‐biologic disease‐modifying antirheumatic drugs (DMARDs) versus steroid for NIIPPU | ||||||
|
Patient or population: NIIPPU Setting: ophthalmology clinics (Netherlands, Germany) Intervention: DMARDs (CsA, EC‐MPS plus steroid) Comparison: control (placebo plus steroid or steroid alone) | ||||||
| Outcomes | Anticipated absolute effect (95% CI)* | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with control | Assumed risk with DMARDs | |||||
|
Proportion of participants achieving control of inflammation (higher number of events is better) Follow‐up: 0 to 12 months |
21 events per 100 participants |
59 events per 100 participants (2 to 100) |
RR 2.81 (95% CI 1.10 to 7.17) | 41 (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
One study comparing CsA plus steroid with placebo plus steroid also reported this outcome but used CsA doses no longer in practice (De Vries 1990); thus, we did not include it in meta‐analysis. |
|
Change in BCVA (lower logMAR indicate better vision) Follow‐up: 0 to 6 months |
Right eyes MD ‐0.03 logMAR (95% CI ‐0.96 logMAR to 0.90 logMAR); Left eyes MD ‐0.10 logMAR (95% CI ‐0.27 logMAR to 0.07 logMAR) | ‐ | 82 eyes (1 RCT) |
⊕⊝⊝⊝ Very lowa,b |
||
|
Proportion of participants achieving a 2‐line improvement in visual acuity (Snellen chart) (higher scores indicate better vision) |
No data were reported for this outcome | ‐ | ‐ | ‐ | ‐ | |
|
Proportion of participants with confirmed macular edema (lower number of events is better) Follow‐up: 0 to 6 months |
No data were reported for this outcome | ‐ | ‐ | ‐ | ‐ | |
|
Proportion of participants achieving steroid‐sparing control (higher number of events is better) Follow‐up: 0 to 12 months |
No data were reported for this outcome | ‐ | ‐ | ‐ | ‐ | |
|
Proportion of participants experiencing complications or requiring cessation of medication (lower number of events is better) Follow‐up: 0 to 12 months |
See comment | ‐ | 41 (1 RCT) |
⊕⊝⊝⊝ Very lowb,c |
One event reported in DMARDs group (RR 2.61, 95% CI 0.11 to 60.51). Another study reported this outcome but used CsA doses no longer in practice (De Vries 1990). | |
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; CsA: cyclosporin A; DMARD: disease‐modifying antirheumatic drug;EC‐MPS: enteric‐coated mycophenolate sodium; logMAR: logarithm of the minimum angle of resolution;MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation | ||||||
| The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence: High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
aDowngraded (‐2) for risk of bias (unclear risk of bias for the randomization process, deviations from intended interventions, and high risk of bias overall) bDowngraded (‐1) for imprecision (small sample size) cDowngraded (‐2) for risk of bias (unclear risk of bias for the randomization process and missing outcome data, and high risk of bias for measurement of outcome, selection of the reported result, and high risk of bias overall)
Summary of findings 2. Methotrexate versus mycophenolate for NIIPPU.
| Mycophenolate versus methotrexate for NIIPPU | ||||||
|
Patient or population: NIIPPU Setting: ophthalmology clinics (India, United States, Australia, India, Mexico, Saudi Arabia) Intervention: methotrexate Comparison: mycophenolate | ||||||
| Outcomes | Anticipated absolute effect (95% CI)* | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with mycophenolate | Assumed risk with methotrexate | |||||
|
Proportion of participants achieving control of inflammation§ (higher number of events is better) Follow‐up: 0 to 6 months |
55 events per 100 participants | 67 events per 100 participants (55 to 88) | RR 1.23 (1.01 to 1.50) | 261 (2 RCTs) | ⊕⊕⊕⊝ Moderatea |
|
|
Change in BCVA (lower logMAR indicate better vision) Follow‐up: 0 to 6 months |
The mean BCVA score across the control group ranged from ‐0.12 to ‐0.19 logMAR | The mean logMAR was 0.01 higher (worse) on average (0.04 lower to 0.05 higher logMAR) | 490 eyes (2 RCTs)ɸ | ⊕⊕⊕⊝ Moderatea |
||
|
Proportion of participants achieving a 2‐line improvement in visual acuity (Snellen chart) (higher scores indicate better vision) Follow‐up: 0 to 6 months |
No data were reported for this outcome |
‐ | ‐ | ‐ | ‐ | |
|
Proportion of participants with confirmed macular edema (lower number of events is better) Follow‐up: 0 to 6 months |
46 events per 100 participant eyes | 23 events per 100 participant eyes (9 to 60) | RR 0.49 (0.19 to 1.30) | 35 eyes (1 RCT)† | ⊕⊝⊝⊝ Very lowb,c |
|
|
Proportion of participants achieving steroid‐sparing control§ (higher number of events is better) Follow‐up: 0 to 6 months |
55 events per 100 participants | 67 events per 100 participants (55 to 88) | RR 1.23 (1.01 to 1.50) | 261 (2 RCTs) | ⊕⊕⊕⊝ Moderatea |
|
|
Proportion of participants experiencing complications or requiring cessation of medication (lower number of events is better) Follow‐up: 0 to 6 months |
7 events per 100 participants | 7 events per 100 participants (3 to 17) | RR 0.99 (0.43 to 2.27) | 296 (2 RCTs) | ⊕⊕⊝⊝ Lowa,d |
|
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; logMAR: logarithm of the minimum angle of resolution;MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation; VKH: Vogt‐Koyanagi‐Harada | ||||||
| The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence: High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
§Study primary outcome of steroid sparing control of inflammation defined as ≤ 0.5 + anterior chamber cells, ≤ 0.5 + vitreous cells, ≤ 0.5 + vitreous haze, and no active retinal or choroidal lesions; ≤ 2 drops of prednisolone acetate 1% a day in both studies and daily prednisolone ≤ 10 mg in one study and ≤ 7.5 mg/day in the other study ɸVisual acuity of uveitic eyes only †Subgroup of VKH participants' eyes with macular edema at baseline aDowngraded (‐1) for imprecision (small sample size) bDowngraded (‐1) for indirectness (single study subgroup of VKH in India) cDowngraded (‐2) for serious imprecision (small sample size) dDowngraded (‐1) for risk of bias (both studies have some concern for risk of bias in outcome measurement)
Summary of findings 3. Steroids with or without azathioprine versus cyclosporine A for NIIPPU.
| Steroids with or without azathioprine versus cyclosporine A for NIIPPU | ||||||
|
Patient or population: NIIPPU Setting: ophthalmology clinics (Germany, Japan, Chile, USA) Intervention: Steroids with or without azathioprine (oral steroids, IV steroids, or azathioprine) Comparison: cyclosporine A (CsA) | ||||||
| Outcomes | Anticipated absolute effect (95% CI)* | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk with cyclosporine A | Assumed risk with steroids ± azathioprine | |||||
|
Proportion of participants achieving control of inflammation (higher number of events is better) Follow‐up: 0 to 12 months |
87 events per 100 participants | 73 events per 100 participants (61 to 88) | RR 0.84 (0.70 to 1.02) | 112 (2 RCTs)a | ⊕⊝⊝⊝ Very lowa,b,c |
Two other studies reported this outcome but used CsA doses no longer in practice (Nussenblatt 1991; Wiederholt 1986). |
|
Change in BCVA (lower logMAR indicate better vision) Follow‐up: 0 to 12 months |
The mean BCVA score across the CsA group ranged from ‐0.32 to ‐0.21 logMAR | The mean logMAR was 0.04 lower (better) on average (‐0.14 to 0.07 logMAR) | ‐ | 91 eyes (2 RCTs)a | ⊕⊝⊝⊝ Very lowa,b,c |
|
|
Proportion of participants achieving a 2‐line improvement in visual acuity (Snellen chart) (higher scores indicate better vision) Follow‐up: 0 to 6 months |
See comment | ‐ | ‐ | ‐ | ‐ | One study reported this outcome but used CsA doses no longer in practice (Wiederholt 1986). |
|
Proportion of participants with confirmed macular edema (lower number of events is better) Follow‐up: 0 to 6 months |
See comment | ‐ | ‐ | ‐ | ‐ | One study reported this outcome but used CsA doses no longer in practice (Nussenblatt 1991). |
|
Proportion of participants achieving steroid‐sparing control (higher number of events is better) Follow‐up: 0 to 12 months |
78 events per 100 participants | 50 events per 100 participants (26 to 97) | RR 0.64 (0.33 to 1.25) | 21 (1 RCT)a | ⊕⊝⊝⊝ Very lowa,b,c |
|
|
Proportion of participants experiencing complications or requiring cessation of medication (lower number of events is better) Follow‐up: 0 to 12 months |
7 events per 100 participants | 6 events per 100 participants (1 to 24) | RR 0.85 (0.21 to 3.45) | 91 participants (2 RCTs)§ | ⊕⊝⊝⊝ Very lowa,b,c |
One other study reported this outcome but used CsA doses no longer in practice (Nussenblatt 1991). |
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; CsA: cyclosporin A; IV: intravenouslogMAR: logarithm of the minimum angle of resolution;MD: mean difference; RCT: randomized controlled trial; RR: risk ratio; SD: standard deviation; VKH: Vogt‐Koyanagi‐Harada | ||||||
| The Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group grades of evidence: High certainty: further research is very unlikely to change our confidence in the estimate of effect Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low certainty: we are very uncertain about the estimate | ||||||
aDowngraded (‐2) for risk of bias (one or more studies with unclear or high risk of bias overall, randomization process, deviations from intended interventions) bDowngraded (‐1) for imprecision (small sample size) cDowngraded (‐1) for indirectness (VKH not representative of all NIIPPU)
§Only VKH participants
Background
Description of the condition
Uveitis (inflammation of the middle layer of the eye) is one of the main causes of visual morbidity, specifically in the working‐age population (Durrani 2004). The incidence of uveitis in high‐income countries is estimated to be between 17 and 52 per 100,000 people per year, and the prevalence is estimated to be between 38 and 714 cases per 100,000 people (Tsirouki 2018). Uveitis involves inflammation of the vascularized uveal tissues of the eye — the iris, ciliary body, and choroid — along with neighboring tissues, including the retina, vitreous, and optic nerve. As its name suggests, non‐infectious intermediate, posterior, and panuveitis (NIIPPU) represent a collection of anatomically‐defined, heterogeneous inflammatory conditions, rather than a single clinical entity. NIIPPU includes inflammation secondary to isolated, well‐described uveitic conditions; inflammation associated with systemic autoimmune conditions such as Behçet’s disease, the spondyloarthropathies, and sarcoidosis; and idiopathic disease. The heterogeneity of these conditions and uncertainty about their pathophysiologic causes present a challenge for both treatment and research. Although these diseases are classified by phenotype and anatomic location, it is not clear that they are truly separate entities. Even well‐defined clinical entities, such as sarcoidosis, are probably heterogeneous responses to a variety of unknown processes, rather than a single disease.
According to the Standardization of Uveitis Nomenclature (SUN) Working Group, intermediate uveitis is defined by primary inflammation of the vitreous, including inflammation of the vitreous also involving the peripheral retina (Jabs 2005). Globally, intermediate uveitis is estimated to comprise approximately 15% of all uveitides (Tsirouki 2018). Pars planitis, posterior cyclitis, and hyalitis are all described variants of intermediate uveitis, according to this definition. Non‐infectious intermediate uveitis is associated with a variety of systemic conditions, including sarcoidosis, multiple sclerosis, and inflammatory bowel disease, although the vast majority of intermediate uveitis is idiopathic, at least in high‐income countries (Tsirouki 2018). People with intermediate uveitis are typically aged 15 to 40 and may present with floaters and decreased or blurry vision. Inflammatory cells, typically T cells, localized in the anterior vitreous are a characteristic finding, along with vitreous condensations and focal collections of inflammatory cells ('snowballs'), fibrovascular peripheral plaques ('snowbanks'), and peripheral periphlebitis.
Like intermediate uveitis, the majority of non‐infectious posterior uveitis, which primarily affects the retina and choroid, is idiopathic: that is, it is not found in conjunction with a systemic disease. Posterior uveitis can primarily affect the retina (retinitis), the choroid (choroiditis), some combination of the two (chorioretinitis or retinochoroiditis), and may variably involve inflammation of the optic nerve as well (neuroretinitis). Common non‐infectious associations with posterior uveitis include sarcoidosis, birdshot chorioretinopathy, and Behçet's disease. Panuveitis, in which people develop inflammation of the anterior chamber, vitreous, and retina or choroid, is also frequently seen in Behçet’s disease, sarcoidosis, and Vogt‐Koyanagi‐Harada (VKH) disease. As many of these conditions are associated with specific human leukocyte antigen haplotypes, it is not surprising that their incidence varies dramatically between countries: for instance, VKH panuveitis is rare in Europe and common in Asia (Tsirouki 2018). A wide spectrum of clinical signs may be seen in the posterior pole in posterior and panuveitis, including cystoid macular edema, choroidal and retinal exudates, retinal hemorrhages, periphlebitis, disc edema, and choroidal neovascularization. People with posterior and panuveitis may present with decreased vision, eye pain or redness, floaters, flashes, and photophobia.
To date, our understanding of the pathophysiology of NIIPPU remains incomplete. It is likely that both autoinflammatory and autoimmune processes play a role. Some of these diseases are thought to be driven by autoinflammation, caused by unwarranted activation of effectors of the innate immune system, namely myeloid cells, with or without the influence of the cytokine interleukin‐1 (IL‐1) (Lee 2014). Other subtypes of NIIPPU, including VKH syndrome, seem to be autoimmune in nature, arising from a dysregulated cellular or humoral response to autoantigens found in ocular tissues themselves (Sugita 2006). It is likely that infectious agents, in certain situations, act as the spark that ignites a susceptible host into a dysregulated immune response, further complicating the question of who develops uveitis, and why. A variety of cytokines and immune mediators has been identified in the eye during intermediate and posterior uveitis, including IL‐1, IL‐2, IL‐6, tumor necrosis factor (TNF‐α), interferon gamma (IFN‐γ), as well as CD4+ and CD8+ T cells, natural killer (NK) cells, B cells, and myeloid lineage cells (Boyd 2001). Elevated levels of IL‐17A have been identified in the serum of people with uveitis and sarcoidosis (Jawad 2013), and animal models of uveitis supported a role for this cytokine in disease (Chi 2011; Zhang 2009). However, secukinumab, a human monoclonal anti‐IL‐17 antibody, failed in three randomized controlled trials (RCTs) in people with NIIPPU (Dick 2013), underscoring the tenuous connection that observational and animal data have to this complex human disease.
In general, people with NIIPPU suffer vision‐limiting sequelae, including cataracts, glaucoma, and retinopathy, that often necessitate surgical management. Some of these problems are caused by recurrent episodes of inflammation, but many are secondary to long‐term steroid use, the traditional mainstay of treatment. Uveitic eyes are often more surgically complex and have been shown to have higher rates of postoperative complications than non‐uveitic eyes (Chu 2017). Steroids are powerful, broad‐spectrum immunosuppressants — in some ways, an ideal choice for a group of diseases with a poorly understood pathophysiology and likely disparate etiologies. Because steroids effectively suppress both the innate and adaptive immune system, are reasonably well‐tolerated (at least in the short term), and are inexpensive and widely available, they will likely always be first‐line therapy for people with uveitis. However, given the chronicity of many forms of uveitis, it is necessary to find other treatment modalities that spare people the side effects of steroids. Monoclonal antibody treatments, including anti‐TNF and anti‐IL‐6 agents, have been studied in several RCTs. A non‐Cochrane meta‐analysis assessed three RCTs of anti‐TNF agents in people with NIIPPU, finding high‐quality evidence that adalimumab reduces the risk of decreased best‐corrected visual acuity (Leal 2019). However, non‐biologic, immune‐modifying therapies, such as methotrexate, mycophenolate, cyclosporine, tacrolimus, and azathioprine, are often used as first‐line, steroid‐sparing agents. In many resource‐limited countries, these may be the only medications available as an alternative to chronic steroids, as biologics as a class remain expensive.
Description of the intervention
This review focused on the following non‐biologic, immune‐modifying therapies: methotrexate, mycophenolate, cyclosporine, tacrolimus, and azathioprine. We selected these non‐biologic, disease‐modifying antirheumatic drugs (DMARDs) for review because they are the agents most utilized in clinical practice due to their cost and perceived efficacy. None of these medications are approved by the US Food and Drug Administration for usage in NIIPPU.
Methotrexate, a folate antimetabolite, has a long history of use in cancer treatment. At high doses, it inhibits multiple enzymes involved in DNA synthesis, specifically those involved in critical steps in the production of purines and pyrimidines. However, at lower doses, methotrexate is efficacious for a number of inflammatory diseases, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease. This may be due to suppression of lymphoproliferation by the drug, although alternate immunomodulatory functions are also likely, given that co‐administration of folate to people on methotrexate often ameliorates side effects without decreasing the drug’s efficacy (Ortiz 1998), a finding further supported by animal models (Graffner‐Nordberg 2003). The most common side effects of low‐dose methotrexate include gastrointestinal upset, stomatitis, headache, and fatigue. Hepatotoxicity and, more rarely, severe myelosuppression, may also occur.
Mycophenolate mofetil is another antimetabolite, inhibiting synthesis of the purine guanosine, required for the production of DNA and RNA. Lymphocytes are unable to generate guanosine‐derived nucleotides from alternate pathways, requiring de novo synthesis of these molecules in order to proliferate. Therefore, mycophenolate mofetil is a potent and selective inhibitor of B cells, CD4+ and CD8+ lymphocytes, and dendritic cells (Allison 2002). The most common adverse effect of mycophenolate mofetil is persistent diarrhea; the most serious is cytopenia, for which people must undergo regular monitoring of blood counts.
Azathioprine, a prodrug converted to its active form by glutathione in red blood cells, also inhibits purine synthesis. Inhibition of DNA and RNA synthesis in B and T cells reduces the number of circulating lymphocytes, the production of immunoglobulins, and the secretion of IL‐2 (Bacon 1987). Common adverse effects of azathioprine include anorexia, nausea, and vomiting. A small increase in the risk of malignancy, particularly lymphoma, has been reported in people with inflammatory bowel disease treated with azathioprine (Kandiel 2005).
Tacrolimus and cyclosporine are fungally‐derived calcineurin receptor inhibitors. By forming complexes with other receptors, these molecules block calcineurin signaling, preventing T cell activation, and limiting their proliferation and production of IL‐2, IL‐6, IFN‐γ, and TNF‐α, among others. Both of these agents were previously thought to lack any clinically significant myelosuppressive activity (Ishida 1995), although there is evidence that tacrolimus also inhibits B cells, dendritic cells, and macrophages (van Dieren 2006). The most serious adverse effect of calcineurin inhibitors is nephrotoxicity: both reversible, acute kidney injury and irreversible chronic, progressive renal damage may occur.
How the intervention might work
Given the importance of both cellular and humoral immunity in the pathogenesis of NIIPPU, non‐biologic, steroid‐sparing therapies are thought to suppress the lymphocytes, myeloid cells, and cytokines responsible for the aberrant immune response central to these diseases.
Why it is important to do this review
Although there have been several RCTs studying non‐biologic, steroid‐sparing therapies in NIIPPU, heterogeneity in outcomes, study design, and participants has made it difficult to draw comparisons between publications. A rigorous systematic review of these agents may provide insights into which medication is most efficacious or best tolerated, providing a useful resource for both clinicians and people with uveitis. Gaps in the data which are identified through this review could be equally informative, providing guidance on experimental design or outcomes that should be taken into consideration in future studies. By selecting agents which are widely accessible and inexpensive, this review aims to be relevant to ophthalmologists globally.
Objectives
To compare the effectiveness and safety of selected DMARDs (methotrexate, mycophenolate mofetil, tacrolimus, cyclosporine, and azathioprine) in the treatment of NIIPPU in adults.
Methods
Criteria for considering studies for this review
Types of studies
This review included only RCTs.
Types of participants
We included trials in which the investigators enrolled individuals with NIIPPU, as defined by the SUN criteria (Jabs 2005). We planned on including trials with adult participants only (age 18 and over) (Edwards Mayhew 2021). We changed this to include trials with a mix of adults, adolescents, and children but excluded trials where all participants were under 18 years old (Differences between protocol and review). We excluded trials in which: (i) participants had uveitis of infectious origin; (ii) participants were on combination therapy with multiple steroid‐sparing agents, or (iii) participants were either currently on biologic therapy or had been within the past six months.
Types of interventions
The original pharmacologic interventions included the following DMARDs, alone or in combination with topical or oral steroid therapy:
methotrexate, a folate antimetabolite;
mycophenolate mofetil, an inhibitor of purine synthesis;
azathioprine, a purine antimetabolite;
cyclosporine, a fungally‐derived calcineurin inhibitor; and
tacrolimus, a macrolide inhibitor of T cells.
We compared each steroid‐sparing therapy described above with placebo or with standard of care (e.g. topical steroids, with or without systemic steroids), or with each other. We decided comparisons of the same DMARD (tacrolimus versus tacrolimus) or overlapping mechanism of action (tacrolimus versus cyclosporine) were not of interest for this review (Differences between protocol and review).
Types of outcome measures
Critical outcomes
Proportion of participants achieving control of inflammation, defined as a two‐step reduction in vitreous haze grade/score or decrease to grade 0 (Jabs 2005; Nussenblatt 1985); or clinically comparable study definition
Change in best corrected visual acuity (BCVA), measured as a continuous outcome on a logMAR (logarithm of the minimum angle of resolution) chart (or equivalent)
Proportion of participants achieving a 2‐line improvement in visual acuity (Snellen chart)
Proportion of participants with macular edema, confirmed by optical coherence tomography (OCT) (macular thickness, at the center point ≥ 240 μm) or by fluorescein angiogram (macular leakage ≥ 0.44 disc areas) or by slit‐lamp biomicroscopy through a dilated pupil
Key time points for these outcomes include follow‐up at six and 12 months.
Important outcomes
Mean time to relapse
Reduction in cumulative hazard of disease relapse
Proportion of participants with change in anterior chamber flare and cells, as defined by the SUN Working Group (Jabs 2005)
Mean change in central macular thickness (CMT), measured in microns on OCT imaging
Change (resolution, yes/no) in other activity domains, including vitreous cells; vitreous 'snow‐balls'; chorioretinal inflammatory lesions; and retinovascular inflammation
Proportion of participants to achieve steroid‐sparing control
Proportion of participants to achieve reduction in oral steroid dose (to < 10 mg/day)
Cost‐effectiveness, e.g. the incremental cost‐effectiveness ratio (ICER)
Mean change in vision‐related quality of life, measured using the Visual Function Questionnaire 25 (VFQ‐25), or other validated questionnaire (Mangione 2001)
Mean change in general health‐related quality of life (HRQoL), measured using the EuroQoL five dimensions questionnaire (EQ‐5D), or other validated questionnaire
-
Adverse events:
Proportion of participants experiencing any adverse effects, including ocular and systemic complications
Proportion of participants experiencing complications or requiring cessation of medication, such as bone marrow suppression (absolute neutrophil count [ANC] < 1500 cells/μL), hepatotoxicity (elevation in liver enzyme alanine transaminase [ALT] > 45 IU/L in men and ALT > 35 IU/L in women), as well as severe allergic reaction
Proportion of participants experiencing ocular complications, including elevated eye pressure (≥ 21 mmHg), lens opacity, hypotony, choroidal neovascular membrane
We used the same time points as above for the critical outcomes (six and 12 months).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision (CEV) Information Specialist searched the following electronic databases for RCTs on 16 April 2021. There were no restrictions by language or year of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (Issue 4) (Appendix 1)
MEDLINE Ovid (1946 to 16 April 2021) (Appendix 2)
Embase.com (1947 to 16 April 2021) (Appendix 3)
PubMed (1946 to 16 April 2021) (Appendix 4)
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to 16 April 2021) (Appendix 5)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (Appendix 6)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp) (Appendix 7)
Searching other resources
We handsearched the references of included studies, review articles, and guidelines for additional trials. We did not search conference abstracts as many eyes and vision conference abstracts are included in Embase, which was searched as part of our electronic search.
Data collection and analysis
Selection of studies
The Information Specialist removed duplicate references and imported the search results into the web‐based review management software Covidence. Review authors (REM, PMcC, LL, ASC) worked in pairs to independently screen titles and abstracts using Covidence. Based on the eligibility criteria, we classified each record as 'relevant', 'possibly relevant', and 'not relevant' for further full‐text review. We then retrieved the full‐text articles for records considered 'relevant' or 'possibly relevant'. Review authors (REM, PMcC, LL) worked in pairs to independently screen the full‐text articles for eligibility and classified articles as 'to be included' or 'to be excluded'. When there were questions regarding the eligibility of the studies, we contacted the authors of the studies to obtain additional information. If the authors did not respond within two weeks, we used information available from publications and trial registries to determine eligibility. For non‐English studies, we used GoogleTranslate. We recorded reasons for exclusion of full‐text reports in the Characteristics of excluded studies table. We also classified eligible studies that had not yet been completed as 'ongoing'. In contrast, we classified eligible studies whose results were unavailable as 'awaiting classification'. In case of any disagreement or discrepancies regarding the classification of the studies, we adjudicated via discussion with the senior authors (AP or TL). We did not exclude studies on the basis of outcomes reported or study status.
Data extraction and management
We used Covidence for data extraction. Review authors (REM, PMcC, LL) independently extracted data from the selected full‐text articles, including the following information: study setting, countries where participant recruitment took place, study design, sample size, study duration (planned and actual), participants, interventions, comparators, outcomes, sources of funding, and potential conflicts of interests (Appendix 8). Where data were only available in graphical displays, two review authors independently extracted the data using GetData Graph Digitizer 2.24. One review author (LL) exported data from Covidence into RevMan Web (Review Manager Web 2020) and a second review author (PMcC) verified all data entries to ensure that data were consistent and free of errors. Any discrepancies in data extraction were resolved through discussion.
Assessment of risk of bias in included studies
Review authors (REM, PMcC, LL) worked in pairs to independently apply the Risk of Bias tool 2 (RoB 2) to assess the risk of bias for the effect of assignment to interventions according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022a). In case of authors' disagreement, we resolved the conflicts via discussion with the senior authors (AP or TL). We assessed the risk of bias for the 'proportion of participants achieving control of inflammation' efficacy outcome and the 'proportion of participants experiencing complications or requiring cessation of medication' safety outcome. We used RoB2 Excel tool and 2019 guidance to implement RoB 2 (available on the riskofbiasinfo.org website).We considered the following domains of bias:
bias arising from the randomization process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome; and
bias in selection of the reported result.
We assessed each domain as guided by domain‐specific signaling questions. For an overall 'risk of bias' judgement, we considered each study to have:
'low risk of bias' if it was at low risk of bias for all domains for this result;
'some concerns' if the trial raised some concerns in at least one domain for this result, but we did not consider it to be at high risk of bias for any domain; and
'high risk of bias' if we judged the trial to be at high risk of bias in at least one domain, or to have some concerns for multiple domains in a way that substantially lowers confidence in the result.
Measures of treatment effect
We conducted data analysis using guidance from Chapter 9 and Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022; McKenzie 2022). We calculated the mean difference (MD) with 95% confidence intervals (CIs) for continuous outcomes. We calculated the standardized mean difference (SMD) for quality of life scores. We used risk ratios (RR) with 95% CIs for dichotomous outcomes. There were no time‐to‐event data reported, so we did not calculate hazard ratios.
Unit of analysis issues
For the purpose of this review, the unit of analysis was the study participant. When a study randomized both eyes of participants (to the same or different interventions), we extracted the results that accounted for the correlation between eyes. If the included studies failed to consider the correlation between two eyes, we planned to exclude those studies in the sensitivity analysis. We followed the guidance in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions regarding including variants on randomized trials (Higgins 2022b).
Dealing with missing data
While dealing with missing data, we followed recommendations in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). If there were missing data (e.g. missing full‐text report from a trial, missing information regarding study methods, missing summary data for an outcome, or individual participants missing from the summary data), we contacted the authors of the primary trials to request the information of interest. As prespecified in the protocol, we planned to assess the impact of missing data by excluding studies at high risk of bias of missing outcome data in a sensitivity analysis. However, there were only trials at 'low' or 'some concern' for this domain.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity among studies by evaluating the potential differences in participants, interventions, and outcomes, as well as differences in study design, outcome measurement tools, and risk of bias. We evaluated the statistical heterogeneity by assessing if CIs for the results of individual studies showed poor overlap. We also considered the I2 statistic, which described the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance). As described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022), we consider the following ranges while interpreting the I2 statistics:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We evaluated selective outcome reporting for each study by comparing the outcomes specified in the protocol or clinical trial registry with those in study reports. Where trial protocols or trial registry records were unavailable or inaccessible, we compared outcomes specified in the Methods section with outcomes reported in the Results section of the study reports. Because we did not include more than 10 trials in a meta‐analysis, we did not use funnel plots to assess small‐study effects.
Data synthesis
For data synthesis and analysis, we followed the guidelines in Chapter 9 and Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022; McKenzie 2022). We analyzed data using a random‐effects model whenever feasible; we used fixed‐effects models when fewer than three studies contributed data in a given meta‐analysis. If the direction of treatment effects was inconsistent across studies or there was evidence of considerable statistical heterogeneity (I2 > 75%), we did not combine results in a meta‐analysis and presented a narrative summary of results instead. The primary analysis included all eligible studies and did not restrict them based on risk of bias.
Subgroup analysis and investigation of heterogeneity
Given the limited number of trials included, we did not perform subgroup analyses by study design or by uveitis subtype. We only performed subgroup analysis by uveitis etiology when there was sufficient information reported by trials that enrolled mainly participants with Vogt‐Koyanagi‐Harada (VKH) disease versus trials that enrolled participants with non‐VKH uveitis (Differences between protocol and review).
Sensitivity analysis
There were too few studies included in analyses to perform meaningful sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables to present the main findings of the review, including key information concerning the certainty of evidence, the magnitude of effect of the interventions examined, and the summary of available data on the main outcomes, following guidelines in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2022).
We included the following outcomes at six and 12 months' follow‐up in the summary of findings tables:
control of inflammation;
change in BCVA;
proportion of participants achieving a 2‐line improvement in visual acuity (Snellen chart);
proportion of participants with confirmed macular edema;
proportion of participants achieving steroid‐sparing control; and
proportion of participants experiencing complications or requiring cessation of medication.
Authors (REM, PMcC, LL) worked in pairs and each independently performed the GRADE assessment to evaluate the certainty of the review findings by grading the certainty of evidence as 'high', 'moderate', 'low', or 'very low' (GRADEpro GDT), based on (i) high risk of bias among included studies, (ii) indirectness of evidence, (iii) unexplained heterogeneity or inconsistency of results, (iv) imprecision of results, and (v) high probability of publication bias. When there was any disagreement or discrepancy between the two review authors, a third review author (AP or TL) adjudicated the grading.
Results
Description of studies
Results of the search
We identified 5070 records through electronic database searches and two additional records through supplemental search methods. After the removal of duplicates, 4299 records remained. Fifty of these records underwent full‐text screening. We excluded six studies (11 records) and listed three studies (8 records) as 'awaiting classification.' We included 11 studies (31 records) in this review (Figure 1). Because some studies included a mixture of children and adults, we made a post hoc decision to include studies with children and adults, but no studies with only children (Differences between protocol and review).
1.
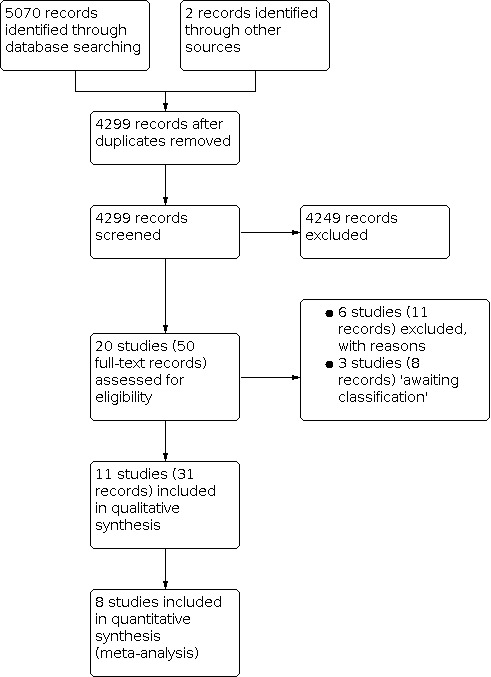
Study flow diagram
Included studies
For a detailed description of the included studies, please see the Characteristics of included studies table.
We included 11 studies with a total of 601 participants. The majority of the included studies were small: all but one had fewer than 100 participants, and seven of the 11 had fewer than 50 participants (Cuchacovich 2010; Deuter 2018; De Vries 1990; Lee 2012; Murphy 2005; Nussenblatt 1993; Wiederholt 1986). The First‐line Antimetabolites as Steroid‐sparing Treatment (FAST) uveitis trials enrolled 46% (274 of 601) of the total participants included (Rathinam 2014; Rathinam 2019). Trials conducted after 2005 incorporated the Standardization of Uveitis Nomenclature (SUN) working group definition of NIIPPU; studies conducted prior to this generally had similar eligibility criteria (De Vries 1990; Nussenblatt 1991; Nussenblatt 1993; Wiederholt 1986). Most studies included only adults; four studies also included children and teenagers (Cuchacovich 2010; Nussenblatt 1991; Rathinam 2014; Rathinam 2019).
There was a broad global distribution of studies, with representation from the United Kingdom, USA, Germany, the Netherlands, Chile, Japan, India, Australia, Saudi Arabia, and Mexico. Given the wide range of clinical entities making up NIIPPU, many of which have strong geographic or ethnic associations, or both, it is not surprising that the specific uveitis diagnoses varied greatly between studies. For example, studies conducted in the UK, the Netherlands, and Germany (Deuter 2018; De Vries 1990; Lee 2012; Murphy 2005), composed of largely white populations, contained no VKH patients, while studies with large Asian or South American populations had high rates of VKH, or were entirely composed of VKH patients (Cuchacovich 2010; Ono 2021; Rathinam 2014; Rathinam 2019).
There was also significant heterogeneity between studies in the clinical interventions and definitions of clinical outcomes (see below).
Types of interventions
The included studies evaluated a variety of treatments, dosages, and regimens.
Non‐biologic disease‐modifying antirheumatic drugs (DMARDs) versus steroids
Two studies compared a DMARD plus steroid to steroid monotherapy. De Vries 1990 compared CsA plus oral steroid with steroid monotherapy plus placebo whereas Deuter 2018 compared enteric‐coated mycophenolate sodium (1440 mg/day) in combination with a steroid to steroid monotherapy.
Mycophenolate versus methotrexate
Two studies compared mycophenolate to methotrexate. The FAST pilot study and main study both compared a higher dose of mycophenolate (2000 mg/day) and a slightly different formulation (oral mycophenolate mofetil) with methotrexate (Rathinam 2014; Rathinam 2019).
Steroids with or without azathioprine versus cyclosporine A
Four studies compared other steroids with or without azathioprine (oral steroid, IV steroid, or azathioprine) to cyclosporine A. Nussenblatt 1991 and Wiederholt 1986 used oral steroids while Cuchacovich 2010 compared oral steroid and azathioprine to oral steroid and cyclosporine A at a dosage of 3 to 5 mg/kg/day. Ono 2021 compared a 3‐day pulse‐dose of IV methylprednisolone to a 3 mg/kg/day dose of cyclosporine A, both in combination with a gradually tapered dose of oral steroid.
Three studies included comparisons outside the scope of this review (see Differences between protocol and review). Two studies used tacrolimus: Murphy 2005 compared tacrolimus plus oral steroid with cyclosporine A, but was not included in the synthesis as these two medications were considered to be of the same class (both are calcineurin inhibitors); Lee 2012 compared tacrolimus monotherapy with tacrolimus in combination with oral steroids, with the same target tacrolimus trough level as used in Murphy 2005 (8 to 12 ng/L). Nussenblatt 1993 was designed as a dose‐escalation trial with four different doses of cyclosporine A to cyclosporine G. Because the formulation of cyclosporine G is not clinically available, we have omitted this study as well.
Dosing of cyclosporine A
The dosages of cyclosporine A ranged from 2.5 mg to 15 mg/kg/day. Three studies published from 1986 to 1991 used dosages of 8 mg to 15 mg/kg/day (De Vries 1990; Nussenblatt 1991; Wiederholt 1986). Around that time, dose‐response and tolerability studies revealed that chronic use of cyclosporine at high doses was associated with the development of interstitial fibrosis, glomerular atrophy, decreased glomerular filtration rate, and increased risk of hypertension (Palestine 1986; Nussenblatt 1993). Two studies published more recently used modern dosing of 3 mg to 5 mg/kg/day of cyclosporine A (Cuchacovich 2010; Ono 2021). We excluded the studies using the higher, outdated dosing of cyclosporine from our primary analyses as these data are not informative to current clinical practice. However, we have included a description of their results.
Steroid regimens
Steroid regimens varied considerably across included studies. All studies either explicitly allowed the use of topical steroids or did not comment on the use of topical drops (Cuchacovich 2010; Deuter 2018; Murphy 2005; Nussenblatt 1993); no studies explicitly prohibited the use of topical steroid drops. Regimens for oral steroids and steroid tapers ranged widely among the studies. Four studies started at relatively low doses of oral steroids (10 mg to 15 mg/day or 0.3 mg/kg/day to a maximum of 20 mg) with subsequent tapers (De Vries 1990; Lee 2012; Nussenblatt 1993; Rathinam 2014). Six studies started at a high dose of oral steroid (60 mg to 100 mg/day) for a variable time period, usually one to four weeks, although this was not always explicitly stated, followed by a taper (Cuchacovich 2010; Deuter 2018; Nussenblatt 1991; Ono 2021; Rathinam 2019; Wiederholt 1986). Participants in the Murphy 2005 study were allowed to be taking anywhere from 14 mg to 51 mg/day of oral steroids at the start of the trial. Participants in two studies received an IV pulse‐dose of steroids at the start of the trial (1.0 g to 3.0 g over one to three days in Lee 2012, and 1.5 g to 3.0 g over three days in Ono 2021). Steroid tapers were generally aimed to achieve a dose of 5 mg to 10 mg/day, but the time period over which the taper occurred varied from two to six months, and the exact tapering regimen was not always explicitly stated by the investigators. The steroid regimens between arms within a study were similar except for the comparisons with one steroid group (Lee 2012, Nussenblatt 1991; Wiederholt 1986.)
The overall length and time points for data collection varied between studies, as detailed below.
Types of outcomes
There was a wide variety in the study design and reported outcomes of each study, with variation in how authors defined outcomes such as control of inflammation or treatment success. This made comparisons across studies challenging.
Active to improvement versus inactive to relapse
Most studies tested an experimental therapy in participants with active disease, and reported measures such as time to control of inflammation; that is, treatment success. Some of these studies would report a time to relapse after control was achieved, often with a loading dose of oral steroids while the experimental medication was being initiated (Cuchacovich 2010; Murphy 2005; Deuter 2018; Ono 2021). However, another study design, utilized by Lee 2012 and Deuter 2018, instead examined participants with inactive disease and tested how long the experimental therapeutic regimen could prevent a relapse; that is, treatment failure. For example, Lee 2012 required inactive disease for four weeks with target tacrolimus trough levels on a maximum of 10 mg/day of steroid, and then randomized participants to tacrolimus monotherapy (after a quick steroid taper), or to continue dual therapy. Those with active inflammation on this regimen were excluded from the study.
Definitions of control of inflammation or time to relapse/hazard of relapse, or both
All studies used a composite measure of multiple clinical exam findings to define "control of inflammation," many in combination with a visual acuity outcome. These definitions were heterogenous. To define inflammatory activity, Wiederholt 1986 used a combination of anterior chamber cell and vitreous opacities grading but did not explicitly define what constituted control of inflammation with these parameters. De Vries 1990 utilized a modified Hogan‐Thygeson‐Kimura scale with a variety of domains in both the anterior and posterior segment (congestion, anterior chamber cell/flare, nerve edema, cystoid macular edema [CME], 'snowballs'), and treatment failure was, in part, defined by worsening of this score. A composite weighted visual morbidity scale was also used by Cuchacovich 2010 and incorporated anterior chamber Tyndall and flare measured from grade 0 to 4 and vitreous haze graded from 0 to 4; an improvement in inflammation was then defined as a two‐step decrease in the level of inflammation or a decrease to 0. Murphy 2005, Lee 2012, and Cuchacovich 2010 utilized a binocular indirect ophthalmoscopy (BIO) score, along with a visual acuity measure, as their definitions of control of inflammation, treatment failure, or treatment success, respectively. Nussenblatt 1991 and Nussenblatt 1993 utilized a two‐step improvement in vitreous haze as a component, along with a visual acuity measurement, in their definition of treatment success, with the latter reporting at 16 weeks and the former at three months. Deuter 2018 used vitreous haze, anterior chamber cells, CME, retinal vasculitis, and a visual acuity outcome in their criteria for relapse. Ono 2021, a study in all VKH patients, also used a two‐step increase (or increase from 3+ to 4+) of vitreous haze or anterior chamber cells to define "worsening," while "recurrence" was defined as reappearance of a serous retinal detachment on optical coherence tomography (OCT) that had previously resolved, or a return of systemic symptoms of VKH disease.
Rathinam 2014 and Rathinam 2019 used similar definitions of control of inflammation, utilizing the 0 to 4 grading scale for anterior chamber cells and vitreous haze, and requiring less than or equal to 0.5 + for each, as part of this outcome. The 2014 study also used vitreous cells, again at a level less than or equal to 0.5 +, as a requirement for control of inflammation, and both required participants to have no active retinal or choroidal lesions (Rathinam 2014). Other features required for control of inflammation in these studies were a limitation on amount of oral and topical steroid and lack of failure due to safety or intolerability concerns (see below). These outcomes were primarily determined at six months of follow‐up, although the 2019 study followed participants for an additional six months after this (Rathinam 2019).
Time to relapse, hazard of relapse, or relapse rate were not reported by several studies (Nussenblatt 1991; Nussenblatt 1993; Rathinam 2014; Rathinam 2019). Wiederholt 1986 did not go into detail about parameters for relapse, but did report the number of relapses and timing of these relapses over 12 months. Murphy 2005 determined relapse as a decrease in visual acuity of two lines or an increase in the BIO score of at least one point after achieving a clinical response, and reported a rate of relapse among responders and average time to first relapse over one year of follow‐up. Cuchacovich 2010 reported a rate of relapse and average time to relapse over one year of follow‐up after one year of steroid treatment; Lee 2012 reported rates of relapse for nine months of follow‐up in a group with established remission at the start of the trial. Time to relapse was the primary outcome for the Deuter 2018 study, and the criteria for relapse involved a visual acuity measure, a two‐step change in vitreous haze or anterior chamber cells using a 0 to 4 grading system, new‐onset or worsening CME on OCT, or new‐onset or worsening of retinal vasculitis on fundus fluorescein angiography in the first six months of treatment. Ono 2021 reported the risk of relapse (recurrence or worsening) over 12 months of observation.
Change in BCVA
All studies reported some visual acuity outcome. A variety of vision tests were used, including Snellen tables (Cuchacovich 2010; Wiederholt 1986), Landolt C optotypes (De Vries 1990), the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart (Deuter 2018; Lee 2012; Murphy 2005; Nussenblatt 1991; Nussenblatt 1993), tumbling E chart (Rathinam 2014), or a combination of these (Rathinam 2019). Ono 2021 did not report which eye chart was used for visual acuity assessment.
Most studies included a change in visual acuity as a part of their definition of treatment success or failure. De Vries 1990 considered a decrease in visual acuity of two rank numbers or more (presumably in either eye, although this is not explicitly mentioned) compared with the best visual acuity measured at six months, as part of their definition of treatment failure. Nussenblatt 1991 also included visual acuity outcomes as primary endpoints: improvement of three lines of BCVA at three months in at least one eye was a part of their definition of treatment success, whereas worsening by two lines or more after maximal therapy of one week was a criterion for treatment failure. Therapy with cyclosporine A or G was considered successful in Nussenblatt 1993 if there was a 2‐line improvement in baseline visual acuity by 16 weeks; similarly, Murphy 2005 considered cyclosporine or tacrolimus treatment a success if there was a 2‐line improvement by 12 weeks in at least one eye. The primary outcome in Cuchacovich 2010 was change in best‐corrected visual acuity at 54 weeks of follow‐up. Lee 2012, studying participants with inactive disease for time to treatment failure, defined relapse in part as a 2‐line decrease in visual acuity at any point after the start of the trial, or by a subjective decrease in vision accompanied by a change in the BIO score, in either eye. Deuter 2018 also used a decrease in visual acuity as a criterion for relapse, requiring a three‐line or greater decrease in BCVA from baseline.
Rathinam 2014 and Rathinam 2019 both reported a mean change in BCVA at six months, but this was a secondary outcome, and change in visual acuity was not included in their definition of control of inflammation. Change in mean BCVA at each study visit was also a reported secondary outcome in Ono 2021 over 12 months of follow‐up. The change in BCVA was reported at three and 12 months by Wiederholt 1986, but it is not clear whether one or both eyes were reported in cases of bilateral disease.
Macular edema
Two studies discussed the incidence or resolution of macular edema without providing specifics about how this outcome was measured or how presence/worsening was defined (De Vries 1990; Nussenblatt 1991). Nussenblatt 1993 determined CME by biomicroscopic examination and reported the proportion with resolution at 16 weeks. Lee 2012 allowed an increase in central macular thickness to qualify a subjective change in visual acuity as a treatment failure, and required there to be no evidence of CME (by clinical examination, fundus FA, or OCT) at the start of the trial. Rathinam 2014 reported the proportion of participants with resolution of macular edema (measured by OCT) at six months, while Rathinam 2019 reported the mean change in central macular thickness on OCT at six months compared to baseline. Deuter 2018 included new‐onset or worsening macular edema, measured by OCT, as a criterion for relapse.
Proportion achieving steroid‐sparing control
Several studies did not allow oral steroids to be used in combination with the experimental treatment (Lee 2012; Nussenblatt 1991; Nussenblatt 1993; Rathinam 2014; Rathinam 2019; Wiederholt 1986). Thus, all treatment successes in this group had, by definition, achieved steroid‐sparing control. Similarly, participants whose disease remained active at the start of the Lee 2012 study on 10 mg or less per day of steroid (plus tacrolimus) were excluded.
All participants in the Cuchacovich 2010 study initially received oral steroid monotherapy with a long steroid taper to 5 mg to 10 mg/day over 12 months, and the proportion who developed chronic inflammation despite this therapy was reported. The proportion with chronic inflammation randomized to azathioprine or tacrolimus who could then taper oral steroids to 10 mg or less per day without clinical relapse was also reported, along with the average steroid dose at remission, mean time to reach remission with a steroid dose of 10 mg or less per day, and cumulative steroid dose.
The FAST trial and preceding pilot study both utilized steroid‐sparing control as a key feature for treatment success (Rathinam 2014; Rathinam 2019), requiring participants to be taking 10 mg or less per day of oral steroid in the 2014 study and 7.5 mg or less per day in the 2019 study (and two drops or less per day of topical 1% prednisolone drops or equivalent in both). Both studies reported the mean time to reach steroid‐sparing control.
Other studies discussed steroid‐sparing control but did not explicitly report a proportion or likelihood of steroid‐sparing control. For example, De Vries 1990 only reported the number of participants still tapering from oral steroids at the time of their treatment failure between the cyclosporine‐tapered group and the control group. Nussenblatt 1993 reported the number of participants who received more than 20 mg/kg/day of steroids but did not go into detail about the likelihood of steroid‐sparing control. Murphy 2005 reported the median steroid dose at baseline and months one, three, and six. Deuter 2018 required both treatment and control groups to attempt to taper to a maintenance oral steroid dose of 5 mg/day over the course of three months. The proportion of participants achieving steroid‐sparing control was not reported explicitly but can be inferred from the number of participants with disease‐free survival after the three‐month taper. The average steroid dose at the time of first relapse was also reported. In the Ono 2021 study, a complete taper from oral steroid was attempted in both groups, with only a 20% or one‐week adjustment in steroid dose/duration allowed. Systemic therapy terminated at approximately 26 weeks in this study, and follow‐up was conducted for 12 months. Therefore, any participant experiencing treatment success after the conclusion of the systemic therapy would, by definition, also have steroid‐sparing success.
Change in other activity domains (anterior chamber cells, 'snowballs', etc.)
Wiederholt 1986 reported the change in vitreous opacities over the course of 12 months (in three‐month increments) using a 0 to 4 scale. As mentioned above, De Vries 1990 collected a variety of activity domains in the anterior and posterior segment as a part of a composite "inflammatory activity score," and reported these findings as a part of their definition of treatment failure (an increase in inflammatory activity score of 4 points or more at any point over the one‐year study).
Many studies' definitions of control of inflammation used grading of anterior chamber cells (Cuchacovich 2010; Deuter 2018; De Vries 1990; Rathinam 2014; Rathinam 2019; Wiederholt 1986). For example, a maximum amount of anterior chamber cells (grade ≤ 0.5) was a part of the definition of control of inflammation in Rathinam 2014 and Rathinam 2019 at the six‐month time point, while many other studies used a two‐step change in anterior chamber cell to define a treatment success or failure. Nussenblatt 1991 reported anterior chamber cell and flare in addition to vitreous cell and haze in monthly increments for months 0 to three but did not include this in their definition of treatment success/failure.
Cuchacovich 2010, a study with only VKH patients, also discussed the rate of serous retinal detachment. Ono 2021 also examined a VKH‐only population, and incorporated recurrence of a serous retinal detachment into their definition of relapse. They also reported other VKH‐specific activity domains, such as mean sub‐foveal choroidal thickness by enhanced‐depth imaging OCT (EDI‐OCT), incidence of hypofluorescent dark dots evaluated by indocyanine green angiography (ICGA), and 'sunset glow' fundus, in addition to more general uveitic activity domains, such as anterior chamber cell and flare, at all study time points through 12 months of follow‐up.
Most studies did not report other activity domains, such as 'snowballs'.
Quality of life
Murphy 2005 reported both vision‐related and health‐related quality of life data through self‐administered questionnaires at one, three, six, and 12 months of follow‐up using the Vision Core Module‐1 (VCM‐1) and UK standard version of the 36‐item Short‐Form Heath Survey (SF‐36), respectively. Rathinam 2014 and Rathinam 2019 reported vision‐related quality of life with the National Eye Institute Visual Function Questionnaire (NEI‐VFQ) or the Indian Visual Function Questionnaire (IND‐VFQ), a Tamil version validated for use in southern India. Health‐related quality of life data were collected with version 2 of the SF‐36 (SF‐36v2). These studies collected quality of life metrics at baseline and six months, and for the 2019 study, at 12 months. The rest of the included studies did not report quality of life measures.
Cost
No studies reported cost‐effectiveness outcomes.
Adverse events/safety reporting
Collection and reporting of adverse events also varied widely from study to study, with some reporting incidences (i.e. number) of events, some reporting the proportion or percentage of participants experiencing events (De Vries 1990; Nussenblatt 1991), or some combination of both. Many of the smaller studies simply listed systemic and ocular adverse events which had occurred without commenting on the proportion of participants experiencing them or specific laboratory values (Wiederholt 1986). Included studies frequently did not report when adverse events had occurred in the course of the study. Given the wide range of study durations, this made temporal comparisons about adverse events challenging.
Nussenblatt 1993 reported detailed parameters for renal toxicity, including glomerular filtration rate and effective renal plasma flow, liver toxicity, hypertension, and blood counts. Murphy 2005 reported median serum creatinine levels at baseline, month 1, and month 3, along with median total serum cholesterol levels and median mean arterial pressures at the same time points. Lee 2012 did not report values for serum creatinine levels but did use a rise in serum creatinine as part of their definition of medication intolerance.
Murphy 2005 utilized a self‐reported adverse‐events questionnaire with a 0 to 5 scale in which participants ranked how much adverse reactions affected quality of life. However, only proportions of participants experiencing each of these were reported in their publication rather than the self‐reported severity. Cuchacovich 2010 utilized predefined hematologic parameters (leukocyte count, platelet count, and hemoglobin) as indications for withdrawal of azathioprine or tacrolimus immunosuppression, and also reported the proportion of participants experiencing a variety of ocular and systemic complications. Lee 2012 reported rates of blood test abnormalities as incidence rates per patient‐year of treatment, but other adverse events, such as headache and tremor, as a proportion of participants experiencing the event at any time post‐randomization. Deuter 2018 also reported adverse events in patient‐years, and grouped them by serious adverse events, all adverse events, laboratory abnormalities, events requiring discontinuation of the medication, and listed a few specific adverse events, such as infections, headache, and gastrointestinal problems.
Rathinam 2014 and Rathinam 2019 formally incorporated "no safety or intolerability concerns" in their definition of treatment success. These studies collected adverse events in three categories: ocular, systemic, and laboratory (changes in alanine transaminase [ALT], aspartate transaminase [AST], hemoglobin, leukocyte count, creatinine), and reported them as proportions of participants experiencing each over the six months of follow‐up.
Ono 2021 reported a survival curve of cataract formation over the course of 12 or more months of follow‐up. "Serious" adverse events (hyponatremia, severe headache) were reported as a proportion. "Non‐serious adverse events", including systemic events (fatigue, tremor) and laboratory abnormalities (hyperglycemia, elevation in liver enzymes), were also grouped together into an overall proportion of participants experiencing any of these over the course of the study.
All studies did report if serious adverse events or intolerability required cessation of medication. Dose reductions were allowed in some studies (Deuter 2018; De Vries 1990; Murphy 2005; Nussenblatt 1991; Nussenblatt 1993; Ono 2021; Rathinam 2014; Rathinam 2019).
Subgroup analyses
Most studies were too small to analyze subgroups with any statistical power. Rathinam 2014 and Rathinam 2019 analyzed treatment success by anatomic subgroup, breaking participants into anterior and intermediate/intermediate only versus posterior/panuveitis groups. Both trials also carried out separate subgroup analyses for participants with VKH.
Excluded studies
We excluded six studies (11 records) at the full‐text screening stage. The primary reasons for exclusion were: ineligible study design (one study); ineligible comparator (two studies); ineligible intervention or co‐intervention (two studies); ineligible population (one study). Please see the Characteristics of excluded studies for further details.
Risk of bias in included studies
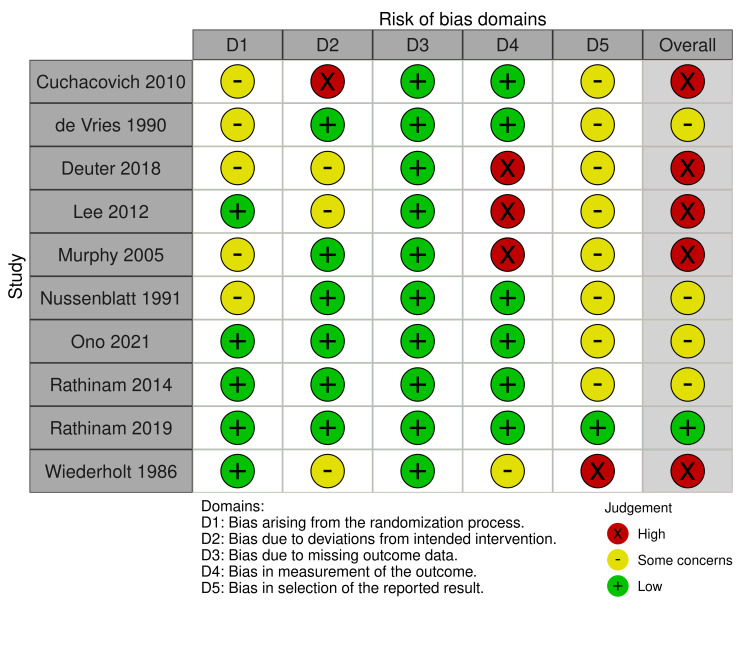
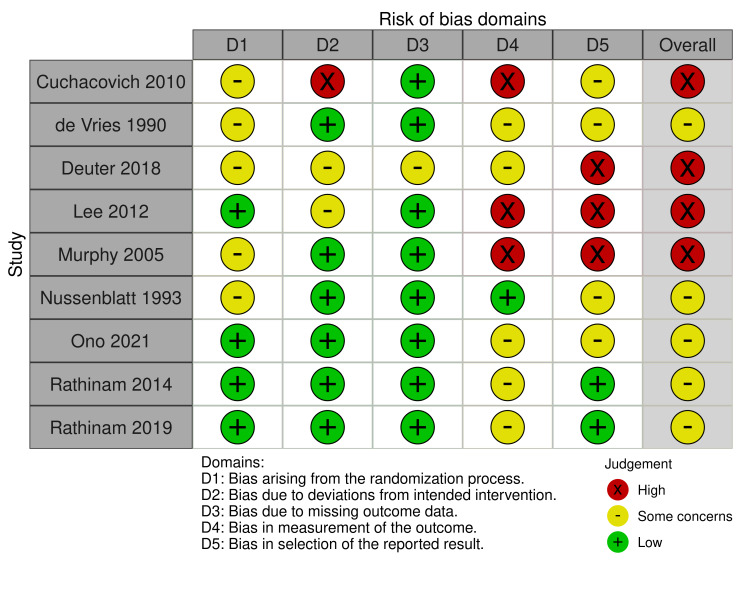
We evaluated the risk of bias using the Risk of Bias 2 (RoB2) tool for two outcomes, "proportion of participants achieving control of inflammation" (Figure 2) and "proportion of participants experiencing complications or requiring cessation of medication" (Figure 3). One study did not report the efficacy outcome (Nussenblatt 1993), and two studies did not report the safety outcome to allow for the risk of bias assessment (Nussenblatt 1991; Wiederholt 1986). Therefore, we included 10 and nine studies, respectively, in the risk of bias assessments of these outcomes.
2.
Risk of bias summary: proportion of participants achieving control of inflammation
3.
Risk of bias summary: proportion of participants experiencing complications or requiring cessation of medication
Regarding the efficacy outcome, we judged only Rathinam 2019 to be at low risk of bias overall. We judged five of the 10 studies (50%) to have a high risk of bias (Cuchacovich 2010; Deuter 2018; Lee 2012; Murphy 2005; Wiederholt 1986). We judged the remaining RCTs to have "some concerns" in the overall risk of bias (Nussenblatt 1991; Ono 2021; Rathinam 2014). We considered no study to be at low risk of bias for the safety outcome. We judged five studies to have "some concerns" overall (De Vries 1990; Nussenblatt 1993; Ono 2021; Rathinam 2014; Rathinam 2019). We determined the overall risk of bias in the safety outcome to be high in four (44%) of the nine studies (Cuchacovich 2010; Deuter 2018; Lee 2012; Murphy 2005).
Bias arising from the randomization process
Three studies did not describe the randomization process whatsoever (Cuchacovich 2010; Nussenblatt 1991; Nussenblatt 1993). Allocation sequence concealment was also frequently not mentioned in the included studies, and was missing from three studies (Deuter 2018; De Vries 1990; Murphy 2005). We judged these studies to have some concerns for bias arising from the randomization process (five out of 10 studies for efficacy; five out of nine studies for safety). Baseline characteristics were generally felt to be similar between groups for the evaluated studies. In the case of the smaller studies, we judged large differences in baseline characteristics to be likely due to chance alone. We judged five studies have a low risk of bias arising from the randomization process (Lee 2012; Ono 2021; Rathinam 2014; Rathinam 2019; Wiederholt 1986).
Bias due to deviations from the intended intervention
We judged most studies (six out of 10 for efficacy; six out of nine for safety) to be at low risk of bias for deviations from the intended intervention (De Vries 1990; Murphy 2005; Nussenblatt 1991; Nussenblatt 1993; Ono 2021; Rathinam 2014; Rathinam 2019). We had some concerns about risk of bias in this domain for Deuter 2018, Lee 2012, and Wiederholt 1986. The participants and investigators were aware of assignment in Lee 2012 and treatment tolerance was a factor prior to randomization, which could lead to deviations from intended interventions, even though they used an intention‐to‐treat analysis. Although Deuter 2018 reported utilizing an intention‐to‐treat analysis, the authors required participants to have received one dose of medication and undergone one follow‐up visit to be analyzed in the group to which they were randomized. Wiederholt 1986 did not provide a detailed protocol and thus did not state whether non‐experimental interventions (such as vitrectomy) should be considered deviations from the intended intervention. We judged Cuchacovich 2010 to be at high risk of bias for this domain because of unbalanced additions of other immunomodulating therapies to the azathioprine group (five participants total: three received additional cyclosporine, the comparator medication, and two were switched to other immunomodulating therapies).
Bias due to missing outcome data
Given the small size of most of the studies included in this review, it is not surprising that we had few concerns with bias arising from missing outcome data. We judged all studies to be at low risk of bias for this domain for both safety and efficacy, except for Deuter 2018, which we judged to be at some concern for the safety outcome in this domain. This was due to missing data for three (6.8%) out of 44 participants. Participants experiencing severe side effects may have stopped following up, and the missingness in the outcome could have influenced the true rate of serious side effects, although we felt this was unlikely.
Bias in measurement of the outcome
We judged Cuchacovich 2010, De Vries 1990, Nussenblatt 1991, Ono 2021, Rathinam 2014, and Rathinam 2019 to be at low risk of bias in measurement of the efficacy outcome (six out of 10 studies). We determined Deuter 2018, Lee 2012, and Murphy 2005 to be at high risk of bias in the measurement of control of inflammation. Outcome assessors were not blinded in these studies, and they used somewhat subjective measures (e.g. vitreous haze grading and BIO score) as the main determinant of control of inflammation, which could have been influenced by knowledge of the participants' treatment assignment. We judged Wiederholt 1986 to be at some concern for risk of bias due to a lack of blinding of outcome assessors: although the scales were standardized, lack of blinding could lead to some bias in outcome measurement.
We determined there was a low risk of bias in measurement of the safety outcome in Nussenblatt 1993, which provided clear laboratory thresholds necessitating cessation of medication, collected detailed adverse effects data, and was ostensibly a safety and tolerability study for cyclosporine A versus G. We had some concerns for bias in most other studies (five of nine studies) for the safety outcome (Deuter 2018; De Vries 1990; Ono 2021; Rathinam 2014; Rathinam 2019), often because of lack of masking to treatment assignment for participants/outcome assessors, or the use of subjective outcomes (fatigue, dizziness, etc.) without clear descriptions for how these self‐reported outcomes might trigger cessation. We judged Cuchacovich 2010, Lee 2012, and Murphy 2005 to be at high risk of bias for this domain because participants and investigators were not masked, some adverse events were self‐reported, and criteria for requiring cessation of medication were not described. Therefore, the measurement of this outcome could have been influenced by the observers' or participants' biases, or both.
Bias in selection of the reported result
No studies provided a predetermined statistical analysis plan (SAP), except for Rathinam 2019, which we judged to be at an overall low risk of bias for selection of the reported result for both the efficacy and safety measures.
Regarding efficacy data, most studies did not appear to utilize multiple eligible analyses of the data, although it is not possible to determine if final analyses matched prespecified outcome measures without a statistical analysis plan. We judged these studies (seven out of 10) to have some concerns of bias in this domain (Cuchacovich 2010; Deuter 2018; De Vries 1990; Murphy 2005; Nussenblatt 1991; Ono 2021; Rathinam 2014). Because there was no detailed analysis plan and minimal information on the possibility of multiple measurements and analyses, we judged Lee 2012 to be at some concern for risk of bias for this outcome domain. We judged Wiederholt 1986 to be at high risk of bias in selection of the reported result for control of inflammation because definitions of relapse and treatment success were not laid out, and multiple time points at which a participant could be declared to have relapsed seem possible. As mentioned previously, we judged Rathinam 2019 to be at low risk of bias for this domain and outcome.
We determined Deuter 2018, Lee 2012, and Murphy 2005 to be at high risk of bias for the safety outcome in this domain. These studies reported multiple adverse effects without a description of prespecified adverse effects which would result in cessation of medication; therefore, need for discontinuation of the drug may have depended on multiple eligible outcome measurements (i.e. different definitions of severe renal toxicity). In addition to Rathinam 2019, we judged Rathinam 2014 to be at low risk of bias for the safety outcome in terms of selection of the reported result. We had some concerns in this domain for Cuchacovich 2010, De Vries 1990, Nussenblatt 1993, and Ono 2021, generally because of a lack of information provided about whether the adverse effects triggering cessation of medication were prespecified. Overall, we judged four of nine studies to have some concerns for bias for the safety outcome in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1: DMARDs plus steroid versus steroid monotherapy
Two studies with a total of 68 participants compared oral steroids with or without an experimental DMARD (Deuter 2018; De Vries 1990). The De Vries 1990 study compared cyclosporine with placebo, both in combination with oral steroid (0.3 mg/kg/day), while Deuter 2018 compared enteric‐coated mycophenolate sodium (EC‐MPS) plus low‐dose oral steroid (5 mg/day) versus steroid monotherapy. We did not combine these studies in a meta‐analysis because the dose of cyclosporine A used in De Vries 1990 (10 mg/kg/day) is higher than that used in current clinical practice. See Table 1.
Critical outcomes
Control of inflammation
Deuter 2018 was designed as a time‐to‐relapse study measuring time to first relapse; therefore, in this context, control of inflammation meant a lack of relapse. Relapses were defined as a decrease in BCVA of three or more lines compared to baseline, a two‐step increase in vitreous haze or anterior chamber cells compared with the lowest grade from baseline (or an increase from 3+ to 4+), a new onset or worsening of pre‐existing CME, or a new onset or worsening of retinal vasculitis proven by fluorescein angiography. The evidence may suggest that participants treated with EC‐MPS and low‐dose oral steroid have a higher chance of gaining control of inflammation at six months compared to low‐dose steroid alone (risk ratio [RR] of 2.81, where > 1 favors EC‐MPS plus steroid, 95% CI 1.10 to 7.17; Figure 4), but is uncertain.
4.

Forest plot of comparison 1: DMARDs versus control, outcome: 1.1 Control of inflammation
We judged the overall certainty of the evidence from this single study with 41 events to be of very low certainty, downgrading two levels because of the overall high risk of bias and one level for imprecision.
De Vries 1990 measured control of inflammatory activity, defined using a modified Hogan‐Thygeson‐Kimura scale which scored congestion, keratic precipitates, anterior chamber cells and flare, vitreous opacity, macular edema, optic disc edema, vasculitis, infiltrates, 'snowballs' and 'snowbanks', exudates, and hemorrhages. The evidence is very uncertain about the effect of cyclosporine plus oral steroid versus placebo plus oral steroid on the control of inflammation (RR 0.93, where > 1 favors cyclosporine plus steroid, 95% CI 0.06 to 13.37; Analysis 1.2). The high dose of cyclosporine used in this study (10 mg/kg/day) is not consistent with current clinical practice and therefore the results of this study provide only indirect evidence on the effectiveness of cyclosporine A plus low dose steroid versus steroid monotherapy. This single study represented 27 total events for this outcome.
1.2. Analysis.

Comparison 1: DMARDs plus steroid versus steroid monotherapy, Outcome 2: Control of inflammation: high‐dose cyclosporine A
Because De Vries 1990 did not use modern cyclosporine dosing, we did not apply the GRADE assessment.
Change in BCVA
Change in BCVA was reported by Deuter 2018 separately for the right and left eyes. EC‐MPS with oral low‐dose steroid may result in little to no change in BCVA over control, but the evidence is very uncertain (MD ‐0.03 for right eyes, MD ‐0.10 for left eyes, where < 0 favors combination therapy; Analysis 1.3). Both analyses have CIs which cross the null threshold (95% CI ‐0.96 to 0.90 for right eyes, ‐0.27 to 0.07 for left eyes). There were 22 eyes, right and left, in the EC‐MPS plus steroid group, and 19, right and left, in the control group.
1.3. Analysis.

Comparison 1: DMARDs plus steroid versus steroid monotherapy, Outcome 3: Change in BCVA
We judged the results from this single small study to be of very low certainty, downgrading two levels because of risk of bias and one level for imprecision.
Proportion of participants achieving a 2‐line improvement in visual acuity
No included studies reported this outcome.
Proportion of participants with macular edema
No included studies reported this outcome.
Important outcomes
Mean time to relapse
The median time to relapse was reported by Deuter 2018 as a primary outcome; however, the mean times were not reported, and we could not extract data on means in a useable format. The authors also reported the probability of relapse‐free survival at 15 months and the percentage of participants that experienced a first relapse. For all of these measures, EC‐MPS plus oral steroid may increase the median time to first relapse than control (MD > 15 months versus [vs] 2.8 months; P = 0.07), have a higher probability of relapse‐free survival at 15 months (52.9% vs 19.7%; P = 0.01), and reduce the proportion of participants experiencing a first relapse (40.9% vs 78.9%; P = 0.03). However, the evidence is very uncertain: the authors do not report confidence intervals or the effect measure. These results represent only 41 participants total.
We judged the data for this outcome to be of very low certainty, downgrading two levels given concerns for risk of bias and one level for imprecision.
Reduction in cumulative hazard of disease relapse
No included studies reported this outcome.
Proportion of participants with change in anterior chamber flare and cells
No included studies reported this outcome.
Mean change in CMT
Change in CMT was reported by Deuter 2018 separately for the right and left eyes. The low‐dose oral steroid may result in a larger mean decrease in CMT than combination therapy with EC‐MPS (MD 17.00 for right eyes, MD 1.00 for left eyes, where < 0 favors combination therapy; Analysis 1.4). However, the data are very uncertain. Both analyses have CIs which cross the null threshold (95% CI ‐2.18 to 36.18 for right eyes, ‐1.41 to 3.41 for left eyes).
1.4. Analysis.

Comparison 1: DMARDs plus steroid versus steroid monotherapy, Outcome 4: Change in CMT
The data for this outcome were derived from a single study with 41 events. We judged the evidence to be of very low certainty, downgrading one level because of imprecision and two levels because of risk of bias.
Change in other activity domains
Other activity domains (including anterior chamber cell, vitreous cell/opacities, vascular leakage, etc.) were included in the definition of control of inflammation or inflammatory activity in both Deuter 2018 and De Vries 1990 (see above). No included studies reported this outcome directly.
Proportion of participants to achieve steroid‐sparing control or to achieve reduction in oral steroid dose
No included studies reported this outcome directly. Deuter 2018 required participants to be taking a low‐dose of oral steroid (5 mg/day) without relapse and therefore all participants without a relapse can be considered to have achieved steroid‐sparing control. De Vries 1990 used a higher dose of oral steroid (0.3 mg/kg/day) which does not meet our definition of steroid‐sparing control (dose ≤ 10 mg/day).
Cost‐effectiveness
No included studies measured this outcome.
Mean change in vision‐related quality of life
No included studies reported this outcome.
Mean change in general health‐related quality of life
No included studies reported this outcome.
Adverse effects
Proportion of participants experiencing any adverse effects
No included studies reported this outcome.
Proportion of participants experiencing complications or requiring cessation of medication
Both studies reported the proportion of participants requiring cessation of medication. However, the risk ratio was not estimable for De Vries 1990, with no events reported for cyclosporine A (0 of 14 participants) or placebo (0 of 13 participants). As reported by the Deuter 2018 study, the evidence is uncertain whether combination therapy with EC‐MPS plus oral steroid reduces the risk of patient cessation compared to oral steroid monotherapy (RR 2.61, where < 1 favors combination therapy, 95% CI 0.11 to 60.51; Analysis 1.5).
1.5. Analysis.

Comparison 1: DMARDs plus steroid versus steroid monotherapy, Outcome 5: Proportion of participants experiencing complications or requiring cessation of medication
We judged the data to be of very low overall certainty, downgrading two levels because of concerns for risk of bias and one level for imprecision.
Proportion of participants experiencing ocular complications
No included studies reported this outcome.
Comparison 2: methotrexate versus mycophenolate
Two studies with a total of 261 participants compared methotrexate versus mycophenolate mofetil and were analyzed together in a meta‐analysis (Rathinam 2014; Rathinam 2019). See Table 2.
Critical outcomes
Control of inflammation
At six months of follow‐up, the two studies reported that 88 of 131 participants taking methotrexate and 71 of 130 participants taking mycophenolate mofetil had achieved treatment success (261 participants total). Both studies defined control of ocular inflammation as less than or equal to 0.5 + anterior chamber cells, less than or equal to 0.5 + vitreous haze, and no active retinal/choroidal lesions in both eyes, and required that participants be on a low dose of oral steroid (≤ 10 mg/day in the 2014 study, ≤ 7.5 mg/day in the 2019 study) and two drops or fewer per day of topical 1% prednisolone acetate. There is evidence that methotrexate slightly increases the likelihood of control of inflammation compared to mycophenolate (RR 1.23, where > 1 favors methotrexate, 95% CI 1.01 to 1.50; Figure 5). Heterogeneity was low (I² = 0%, Chi² = 0.84, degrees of freedom [df] = 1, P = 0.36).
5.

Forest plot of comparison 2: Methotrexate versus mycophenolate, outcome 2.1: Control of inflammation
We graded the overall certainty of the evidence as moderate, downgrading by one level for imprecision because of the small number of events.
We also analyzed these outcomes for the VKH subgroup using data from Rathinam 2014. Outcomes for the VKH subgroup were not reported in Rathinam 2019 in a way in which we could combine the data. For control of inflammation, mycophenolate probably results in little to no difference in outcome compared to methotrexate, but the evidence is very uncertain (RR 1.39, where > 1 favors methotrexate, 95% CI 0.81 to 2.36; Analysis 2.2). A similar, statistically uncertain finding for this outcome was reported in Rathinam 2019 for all participants (RR 1.17, 95% CI 0.93 to 1.50; Figure 5).
2.2. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 2: Control of inflammation: subset (VKH patients only)
We graded the overall certainty of the evidence as very low, downgrading two levels for imprecision and one level for indirectness of the evidence, as the data were derived from a small VKH subgroup from a single study.
Change in BCVA
Methotrexate likely results in little to no difference in BCVA over six months compared to mycophenolate for the 490 eyes included. For this outcome, a negative MD means better vision. Rathinam 2014 found a ‐0.26 change (standard deviation [SD] 0.33) in BCVA for participants treated with methotrexate versus a ‐0.19 change (SD 0.36) in participants treated with mycophenolate. Rathinam 2019 reported MDs of ‐0.10 (SD 0.24) and ‐0.12 (SD 0.23) for participants treated with methotrexate and mycophenolate, respectively. Overall, the change in BCVA in 490 uveitic eyes was 0.01 (95% CI ‐0.04 to 0.05; P = 0.18, I2 = 44%; Analysis 2.3), with a negative MD favoring methotrexate.
2.3. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 3: Change in BCVA
We graded the overall certainty of the evidence as moderate, downgrading one level due to the imprecision of the results.
Proportion of participants achieving a 2‐line improvement in visual acuity
No included studies reported this outcome.
Proportion of participants with macular edema
Rathinam 2014 reported the proportion of participants with unresolved macular edema in a subgroup of participants with macular edema at the start of the study (35 eyes). Macular edema was measured by masked OCT operators with thresholds derived from normative data for central subfield thickness for each OCT device (> 252 micrometers for Stratus and > 308 micrometers for Cirrus). Five of 22 participants treated with methotrexate and six of 13 participants treated with mycophenolate still had residual macular edema at six months of follow‐up. The evidence is very uncertain regarding residual macular edema in participants treated with methotrexate compared with those treated with mycophenolate (RR 0.49, where < 1 favors methotrexate, 95% CI 0.19 to 1.30; Analysis 2.4).
2.4. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 4: Proportion of participants with confirmed (residual) macular edema
We judged the evidence to be of very low certainty, downgrading two levels for imprecision and one level for indirectness of the evidence, as the data for this outcome were derived from a small VKH subgroup from a single study.
Important outcomes
Mean time to relapse
No included studies reported this outcome.
Reduction in cumulative hazard of disease relapse
No included studies reported this outcome.
Proportion of participants with change in anterior chamber flare and cells
No included studies reported this outcome.
Mean change in CMT
Rathinam 2019 reported a mean change in central macular thickness recorded on OCT over six months of follow‐up in 97 eyes, excluding participants who had a serous retinal detachment in the setting of VKH. Mean change in CMT was ‐26 (SD 69.6) for participants receiving methotrexate (55 eyes) and ‐14 (SD 61.7) for those receiving mycophenolate (42 eyes). The evidence suggests that mycophenolate may result in little to no difference in mean change in central macular thickness compared to methotrexate (MD ‐12.00, where < 0 favors methotrexate, 95% CI ‐38.20 to 14.20; Analysis 2.5).
2.5. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 5: Change in central macular thickness
We judged the data to be of overall low certainty, downgrading one level due to imprecision.
Change in other activity domains
No included studies reported this outcome.
Proportion of participants to achieve steroid‐sparing control or to achieve reduction in oral steroid dose
As part of their definition of control of inflammation, both Rathinam 2014 and Rathinam 2019 included a requirement for steroid‐sparing control, albeit with different steroid cutoffs. Therefore, the RR of control of inflammation for this outcome is the same as for Analysis 2.1, suggesting that participants treated with methotrexate have a slightly higher chance of achieving steroid‐sparing control compared to those treated with mycophenolate (RR 1.23, where > 1 favors methotrexate, 95% CI 1.01 to 1.50; I2 = 0%; Analysis 2.6).
2.1. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 1: Control of inflammation
2.6. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 6: Proportion of participants achieving steroid reduction
We graded the overall certainty of the evidence as moderate, downgrading by one level due to imprecision of the results.
Cost‐effectiveness
No included studies measured this outcome.
Mean change in vision‐related quality of life
Both Rathinam 2014 and Rathinam 2019 reported outcomes for the Indian Visual Function Questionnaire (IND VFQ‐25), and Rathinam 2019 also reported the National Eye Institute Visual Function Questionnaire (NEI‐VFQ), with measurements compared between baseline and primary study endpoint (either at six months of follow‐up if treatment was successful, or at treatment failure if occurring before six months). Scores were compared between treatment groups (methotrexate versus mycophenolate) using multivariate models that controlled for baseline score, age, sex, and site (included as a random effect), and were reported as β coefficients. Together, 186 participants were included, and combined, the evidence suggests mycophenolate does not increase improvement in vision‐related quality of life over methotrexate (MD 2.27, where MD < 0 favors mycophenolate, 95% CI ‐2.25 to 6.79; P = 0.26, I2 = 20%; Analysis 2.7). The NEI‐VFQ data from Rathinam 2019 also suggest that mycophenolate does not improve vision‐related quality of life scores over methotrexate (MD 2.20, where MD < 0 favors mycophenolate, 95% CI ‐1.39 to 5.79; Analysis 2.7).
2.7. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 7: Change in vision‐related quality of life scores
We considered the data to be of moderate overall certainty, downgrading one level due to imprecision.
Mean change in general health‐related quality of life
Health‐related quality of life scores were reported by both Rathinam 2014 and Rathinam 2019 utilizing the Medical Outcomes Study 36‐Item Short‐Form Survey (SF‐36v2), with the same comparison as used in the VFQ measurements (baseline compared to follow‐up at six months if treatment successful, or at treatment failure if occurring prior to six months). Scores were compared between treatment groups (methotrexate versus mycophenolate) using multivariate models that controlled for baseline score, age, sex, and site (included as a random effect). Both studies reported physical and mental health components of the SF‐36 for 260 participants total. Mycophenolate likely does not result in an improvement in self‐reported physical (MD 1.18, where MD > 0 favors methotrexate, 95% CI ‐0.48 to 2.85; P = 0.25, I2 = 23%; Analysis 2.8) or mental (MD 0.04, 95% CI ‐2.24 to 2.31; I2 = 0%; Analysis 2.8) quality of life scores over methotrexate. Both components of the SF‐36v2 had wide CIs crossing the null threshold.
2.8. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 8: Change in general health‐related quality of life scores
We considered the data to be of moderate overall certainty, downgrading one level due to imprecision.
Adverse effects
Proportion of participants experiencing any adverse effects
In the two studies, 140 participants out of 148 on methotrexate, and 139 participants out of 148 on mycophenolate experienced any adverse effect over the course of six months of follow‐up, including non‐serious and serious ocular, systemic, and laboratory events. The evidence suggests that treatment with mycophenolate results in little to no difference in the proportion of participants experiencing any adverse effects compared to methotrexate (RR 1.02, 95% CI 0.99 to 1.05; I2 = 0; Analysis 2.9).
2.9. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 9: Proportion of participants experiencing any adverse event
We rated the overall certainty of evidence as low, downgrading one level because of imprecision in the data and one level due to indirectness of the evidence due to differing adverse event definitions and thresholds for reporting.
Proportion of participants experiencing complications or requiring cessation of medication
Rathinam 2014 and Rathinam 2019 reported the number of participants experiencing complications requiring cessation of medication. Cessation of medication because of safety or intolerability occurred in 10 of 137 participants on methotrexate and 10 of 137 participants on mycophenolate, or about 7% of participants in both groups. Therefore, treatment with mycophenolate likely results in little to no difference in the proportion of participants requiring cessation of medication over methotrexate (RR 0.99, where RR < 1 favors methotrexate, 95% CI 0.43 to 2.27; P = 0.31, I2 = 2%; Figure 6). We had some concerns for risk of bias for this safety outcome due to bias in the measurement of the outcome. We downgraded this evidence by one level, to moderate certainty, due to imprecision.
6.

Forest plot of comparison 2: Methotrexate versus mycophenolate, outcome: 2.10 Proportion of participants experiencing complications or requiring cessation of medication
Mycophenolate likely does not influence the rate of cessation in the VKH subgroup from Rathinam 2014 compared to methotrexate, although the data are uncertain (RR 1.19, 95% CI 0.12 to 12.05; Analysis 2.11). We downgraded the evidence to very low certainty, downgrading one level for indirectness and two levels for imprecision as it was from a subgroup from a single study with a small size.
2.11. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 11: Proportion of participants experiencing complications or requiring cessation of medication: subset (VKH patients only)
Both Rathinam 2014 and Rathinam 2019 reported participants experiencing elevated liver enzymes (AST or ALT) or abnormal hemoglobin. Elevations in AST or ALT, or both, greater than two times the upper limit of normal were observed in 18 of 148 participants on methotrexate, versus 10 of 148 participants on mycophenolate, or 12% versus 7% of participants. Mycophenolate likely results in little to no difference in liver test abnormalities compared to methotrexate (RR 1.81, where < 1 favors methotrexate, 95% CI 0.86 to 3.78; Analysis 2.12). Rathinam 2019 further separated liver enzyme elevations into serious and non‐serious categories, with non‐serious defined as elevations greater than two to five times the upper limit of normal lasting less than 28 days, and serious defined as greater than two to five times the upper limit of normal for at least 28 days or greater than five times the upper limit of normal. Three participants had serious elevations in AST or ALT, or both, on methotrexate and two participants on mycophenolate. The study reported by Rathinam 2019 did not demonstrate a difference between treatments (RR 1.53, 95% CI 0.26 to 8.96; 216 participants; Analysis 2.12). We judged the evidence to be of moderate overall certainty, downgrading one level due to imprecision of results.
2.12. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 12: Proportion of participants experiencing systemic complications
Two of 148 participants on methotrexate developed a non‐serious abnormal hemoglobin level (defined as > 6.5 to < 9 g/dL lasting less than 28 days in the Rathinam 2019 study, and simply listed as "abnormal" in the Rathinam 2014 study), compared to five of 148 participants on mycophenolate. No participants in Rathinam 2019 developed a serious change in hemoglobin level (< 9 g/dL for at least 28 days or < 6.5 g/dL). Mycophenolate likely results in little to no difference in the risk of abnormal hemoglobin levels compared to methotrexate (RR 0.45 for this outcome, where < 1 favors fewer events with methotrexate, 95% CI 0.10 to 1.97; Analysis 2.12). Heterogeneity was low (I2 = 0). We judged the evidence to be of moderate overall certainty, downgrading one level because of imprecision in the results.
Rathinam 2019 also reported the number of participants that developed low leukocyte counts (non‐serious defined as > 1000 and < 2500/μL lasting less than 28 days, serious defined as < 2500/μL for at least 28 days or < 1000/μL) and elevated creatinine (non‐serious defined as ≥ 1.5 to < 2 mg/dL lasting less than 28 days, serious defined as ≥ 1.5 to < 2 mg/dL for at least 28 days or ≥ 2 mg/dL). No participants in the study had low leukocyte or elevated creatinine levels qualifying as serious events. Three of 107 participants on methotrexate versus one of 109 participants on mycophenolate developed a non‐serious low leukocyte level. Mycophenolate likely results in little to no difference in the risk of leukopenia compared to methotrexate (RR 3.06, where < 1 favors methotrexate, 95% CI 0.32 to 28.92; Analysis 2.12). One participant on methotrexate out of 107 developed a non‐serious elevated creatinine versus 0 of 107 on mycophenolate. Mycophenolate probably results in no difference in the risk of elevated creatinine compared to methotrexate (RR 3.06, 95% CI 0.13 to 74.18; Analysis 2.12). We judged the evidence to be of moderate overall certainty, downgrading one level because of imprecision.
Proportion of participants experiencing ocular complications
Both Rathinam 2014 and Rathinam 2019 reported the number of participants experiencing ocular adverse events. We analyzed data for glaucoma/increased intraocular pressure (IOP), hypotony, and cataract formation.
Eighteen (12%) of 148 participants in the methotrexate group developed ocular hypertension (IOP > 24 mmHg) or suspected or confirmed glaucoma, compared to 20 (13.5%) of 148 participants in the mycophenolate group. Mycophenolate likely results in little to no difference in the risk of ocular hypertension/glaucoma compared to methotrexate (RR 0.90, where < 1 favors methotrexate, 95% CI 0.50 to 1.64; Analysis 2.13). Heterogeneity was low (I2 = 0%). We judged the data to be of moderate overall certainty, downgrading one level for imprecision.
2.13. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 13: Proportion of participants experiencing ocular complications
No significant differences were observed in cataract formation between the groups. Five (3%) of 148 participants on methotrexate developed a visually significant cataract or cataract in which surgery was indicated versus 10 (7%) of 148 participants on mycophenolate. Mycophenolate probably does not increase rate of cataract formation compared to mycophenolate (RR 0.51, where < 1 favors methotrexate, 95% CI 0.18 to 1.44; Analysis 2.13). Heterogeneity was low (I2 = 0%). We judged the data to be of moderate overall certainty, downgrading one level given imprecision in the data.
Similarly, the incidence of hypotony (IOP < 5mmHg) was not found to differ significantly between the treatment groups: three participants in the methotrexate group out of 148 developed hypotony, versus 0 in the mycophenolate group. Mycophenolate likely does not increase the risk of hypotony compared to methotrexate (RR 4.08, where < 1 favors methotrexate, 95% CI 0.47 to 35.72; I2 = 0%; Analysis 2.13). We judged the data to be of moderate overall certainty, downgrading one level for imprecision.
We also analyzed the VKH subgroup from Rathinam 2014. Mycophenolate probably results in little to no difference in the risk for glaucoma/increased IOP (RR 6.68, where < 1 favors methotrexate, 95% CI 0.39 to 113.36; Analysis 2.14), cataract formation (RR 5.46, 95% CI 0.31 to 95.31; Analysis 2.14), or hypotony (RR 3.50, 95% CI 0.17 to 70.14; Analysis 2.14) compared to methotrexate, although the evidence is very uncertain. We judged the data to be of very low certainty, downgrading two levels for imprecision and one level for indirectness of the evidence, as they arose from a small VKH subgroup from a single study.
2.14. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 14: Proportion of participants experiencing ocular complications: subset (VKH patients only)
Comparison 3: steroids with or without azathioprine versus cyclosporine A
Four studies with a total of 145 participants compared steroids with or without azathioprine to cyclosporine A (Cuchacovich 2010; Nussenblatt 1991; Ono 2021; Wiederholt 1986). Wiederholt 1986 and Nussenblatt 1991 compared cyclosporine A with oral steroid monotherapy. The other studies examined cyclosporine in combination with oral steroid: Cuchacovich 2010 compared cyclosporine A plus oral steroid with azathioprine plus oral steroid; Ono 2021 studied an oral steroid taper in combination with cyclosporine with three days of pulse‐dose IV steroids plus a steroid taper.
Two of the studies included in this analysis were based on all‐VKH populations (Cuchacovich 2010; Ono 2021), and the VKH subgroup analyses are included where relevant. For further information, see Table 3.
Critical outcomes
Control of inflammation
We included two studies in the analysis of control of inflammation at 12 months (Cuchacovich 2010; Ono 2021). Together, these two trials comprised 112 participants, all of whom had VKH. Control of inflammation was defined heterogeneously between the studies. Cuchacovich 2010 used a weighted visual morbidity scale based on anterior chamber cells and flare and vitreous haze, while Ono 2021 used a definition for worsening based on anterior chamber cells or vitreous haze, or recurrence based on the reappearance of serous retinal detachment or other systemic signs of VKH disease. The evidence may suggest the steroids with or without azathioprine result in little to no difference in control of inflammation over cyclosporine, but is very uncertain (RR 0.84, where < 1 favors cyclosporine A, 95% CI 0.70 to 1.02; I2 = 0%; Figure 7).
7.

Forest plot of comparison 3: steroids ± azathioprine versus cyclosporin A, outcome: 3.1 Control of inflammation
We judged both studies included in this outcome to be at some concern or high risk of bias, including bias due to deviations from intended interventions and in selection of reported result. Overall, we judged these data to be of very low overall certainty, downgrading two levels because of concerns for bias and one level for imprecision in the data.
We excluded from the evidence synthesis two studies using high‐dose cyclosporine A which reported control of inflammation (Nussenblatt 1991; Wiederholt 1986). Both were conducted in non‐VKH patients, with a total of 64 participants. Wiederholt 1986 used a grading scheme involving anterior chamber cells and vitreous opacities; Nussenblatt 1991 used a vitreal haze grading scheme to define the control of inflammation. Regarding the superiority of steroid monotherapy over high‐dose cyclosporine A, the RR was 1.21 (95% CI 0.72 to 2.01; P = 0.14, I2 = 53%; Analysis 3.2).
3.2. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 2: Control of inflammation: high‐dose cyclosporine A
Change in BCVA
Two trials, comprising 91 eyes, reported data on the change in BCVA (Cuchacovich 2010; Ono 2021). For this outcome, a more negative mean change indicates improved vision. The mean change and SD in BCVA at 12 months, extracted from a graphical reading in Ono 2021, were nearly identical for the IV steroid plus oral steroid taper group versus the cyclosporine plus oral steroid taper group (‐0.34 ± 0.25 versus ‐0.32 ± 0.24). Similarly, Cuchacovich 2010 reported a mean change of ‐0.32 ± 0.24 for the azathioprine plus oral steroid group compared to ‐0.21 ± 0.31 for the cyclosporine plus oral steroid group. The evidence is very uncertain whether the steroids with or without azathioprine improve vision over cyclosporine (RR ‐0.04, where < 0 favors comparators, 95% CI ‐0.14 to 0.07; I² = 0%; Analysis 3.3).
3.3. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 3: Change in BCVA
We judged the evidence to be of very low overall certainty, downgrading two levels because of concerns for bias and one level for imprecision.
Proportion of participants achieving a 2‐line improvement in visual acuity
This outcome was not reported by any study which used modern cyclosporine dosing. Two studies using high doses of cyclosporine A reported the proportion of participants achieving improvement in visual acuity at three months (Nussenblatt 1991; Wiederholt 1986), with a higher score indicating a larger proportion with vision improvement. Improvement was defined as 2‐3 Snellen optotypes by Wiederholt 1986 or as 15 or more ETDRS letters by Nussenblatt 1991. Overall, 13 out of 32 participants treated with cyclosporine and 12 out of 32 participants treated with steroid monotherapy had improvement in visual acuity. The evidence is very uncertain whether steroid monotherapy produces a higher proportion of participants achieving improvement in visual acuity compared to cyclosporine (RR 0.92, where > 1 favors steroid monotherapy, 95% CI 0.50 to 1.69; I² = 0%; Analysis 3.4).
3.4. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 4: Proportion of participants achieving improvement in visual acuity
Because these studies do not use modern cyclosporine dosing, we did not apply the GRADE assessment.
Proportion of participants with macular edema
No study using modern cyclosporine dosing reported this outcome. Nussenblatt 1991 reported the proportion of participants with macular edema, finding that macular edema persisted in eight of 15 participants treated with cyclosporine A and in six of 16 participants treated with oral steroid monotherapy at three months of follow‐up. The evidence is very uncertain whether oral steroid treatment leads to a lower proportion of participants with macular edema compared to cyclosporine A (RR 0.70, 95% CI 0.32 to 1.55; Analysis 3.5).
3.5. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 5: Proportion of participants with macular edema
Because these studies do not use modern cyclosporine dosing, we did not apply the GRADE assessment.
Important outcomes
Mean time to relapse
No included studies reported this outcome.
Reduction in cumulative hazard of disease relapse
No included studies reported this outcome.
Proportion of participants with change in anterior chamber flare and cells
No included studies reported this outcome.
Mean change in CMT
No included studies reported this outcome.
Change in other activity domains
No included studies reported this outcome.
Proportion of participants to achieve steroid‐sparing control or to achieve reduction in oral steroid dose
Cuchacovich 2010 reported the proportion of participants who achieved steroid‐sparing control (< 10 mg/day) at 12 months of follow‐up. The authors found that more VKH patients on cyclosporine (seven of nine) achieved this low maintenance steroid dose than those on azathioprine (six of 12). The evidence is very uncertain if there is a difference in the proportion of participants achieving steroid‐sparing control with azathioprine or cyclosporine A (RR 0.64, where < 1 favors cyclosporine, 95% CI 0.33 to 1.25; Analysis 3.6).
3.6. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 6: Proportion of participants achieving steroid reduction
We considered the data to be of very low overall certainty, downgrading one level given the imprecision of the data and two levels due to risk of bias.
Cost‐effectiveness
No included studies measured this outcome.
Mean change in vision‐related quality of life
No included studies reported this outcome.
Mean change in general health‐related quality of life
No included studies reported this outcome.
Adverse effects
Proportion of participants experiencing any adverse effects
Cuchacovich 2010 reported the number of participants experiencing any adverse event over the course of 12 months of follow‐up. Two‐thirds of participants on cyclosporine (six of nine) and azathioprine (eight of 12) experienced any adverse event. The risk ratio of 1.00 is situated at the null value (95% CI 0.54 to 1.84) and therefore there may be no difference between the treatments; however, the evidence is very uncertain (Analysis 3.7).
3.7. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 7: Proportion of participants with any adverse events
We judged the evidence to be of very low overall certainty, downgrading one level for imprecision of the data and two levels for risk of bias.
Proportion of participants experiencing complications or requiring cessation of medication
Using data from Ono 2021 and Cuchacovich 2010, three of 43 participants with VKH treated with cyclosporine required cessation of the medication, versus three of 48 participants treated with the comparators (IV steroid plus oral steroid taper or azathioprine). There may be no difference between the comparators and cyclosporine A regarding the risk of participants requiring cessation of medication, but the data are very uncertain (RR 0.87, where > 1 favors cyclosporine; P = 0.08, I2 = 68%; Figure 8).
8.

Forest plot of comparison 3: steroids ± azathioprine versus cyclosporin A, outcome: 3.8 Proportion of participants experiencing complications or requiring cessation of medication
We judged both studies included for this outcome to be at some concerns for or high risk of bias. Therefore, we graded the overall certainty of the data as very low, downgrading one level for imprecision and two levels for risk of bias.
Alongside the above three serious adverse events, a variety of non‐serious systemic issues, such as fatigue, tremor, moon‐face, and hyperglycemia, were reported as a percentage in Ono 2021, with nine (26.5%) of 34 participants treated with IV steroid versus five (13.9%) of 36 participants receiving cyclosporine. Cuchacovich 2010 reported the number of participants experiencing a variety of systemic adverse events, including respiratory infections, urinary infections, and hypertension. Two (16.67%) of 12 participants on azathioprine versus 0 of nine participants on cyclosporine had elevated liver enzymes, although the cutoffs used for this designation were not described. One of 12 participants on azathioprine versus 0 of nine on cyclosporine had leukopenia (with laboratory cutoff not explicitly described), and no participants in either group had "renal damage," again without a definition of this provided. The evidence is very uncertain whether the comparators reduce the risk of complications (RR 0.61, where >1 favors cyclosporine, 95% CI 0.35 to 1.06; P = 0.07, I² = 70%; Analysis 3.9).
3.9. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 9: Proportion of participants experiencing systemic complications
We judged the overall certainty of the data as very low, downgrading one level for imprecision and two levels for risk of bias.
Proportion of participants experiencing ocular complications
Cuchacovich 2010 and Ono 2021 reported specific ocular complications. In their year‐long study of people with VKH, Cuchacovich 2010 reported that 10 of 24 participants on azathioprine versus nine of 18 participants on cyclosporine developed increased IOP. There may be little to no difference between azathioprine and cyclosporine A in terms of risk of ocular complications, but the data are statistically very uncertain (RR 0.83, 95% CI 0.43 to 1.61; Analysis 3.10).
3.10. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 10: Proportion of participants experiencing ocular complications
Cuchacovich 2010 and Ono 2021 also reported the rate of cataract formation. Fewer participants on cyclosporine developed cataract (16 out of 80) versus the steroids with or without azathioprine (24 out of 88). Statistical heterogeneity was moderate (P = 0.09, I2 = 64%) and estimates from both trials were largely overlapping, therefore we decided to combine reported adverse event rates of the two trials. The evidence is very uncertain about the effect of the steroids with or without azathioprine versus cyclosporine A on the risk of cataract formation (RR 1.59, 95% CI 0.90 to 2.81; Analysis 3.10). We judged the data as being of very low overall certainty, downgrading one level for imprecision and two levels for risk of bias.
Nussenblatt 1991, a high‐dose cyclosporine study, also reported the risk of cataract development. The evidence is very uncertain regarding the effect of the therapies on cataract development (RR 0.25, 95% CI 0.03 to 2.10; Analysis 3.11). We did not perform a GRADE assessment on this high‐dose trial.
3.11. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 11: Proportion of participants experiencing ocular complications: high‐dose cyclosporine A
Descriptions of studies with comparisons not included in meta‐analysis
Nussenblatt 1993 tested cyclosporine A and G at four escalating doses in 32 participants. They reported the number of participants with resolution of macular edema at 12 weeks for each of the four doses tested. The proportion of participants with remaining macular edema at this time point was similar between the groups (two out of 14 for cyclosporine G, three out of 14 for cyclosporine A). The evidence is very uncertain whether cyclosporine G causes a lower rate of residual macular edema than cyclosporine A (RR 0.67, where < 1 favors cyclosporine G, 95% CI 0.13 to 3.40). Nussenblatt 1993 also reported improvement in visual acuity, which was similar between the two groups at four months of follow‐up. Detailed analyses were carried out regarding changes in renal parameters, liver function, and blood pressure. Although statistically significant differences were not noted between cyclosporine A and G, increased renal toxicity and rate of hypertension development were noted in participants on higher doses of either formulation of cyclosporine, suggesting a dose‐response. Four participants, all taking cyclosporine G, had hepatic abnormalities (abnormal AST, ALT, or bilirubin).
Murphy 2005 analyzed cyclosporine A versus tacrolimus in 37 participants with NIIPPU, none of whom had VKH. They defined control of inflammation as improvement in logMAR visual acuity or binocular indirect ophthalmoscopy (BIO) score, and found that 13 of 19 participants taking tacrolimus versus 12 of 18 taking cyclosporine had control of inflammation at three months. Four participants taking cyclosporine versus six taking tacrolimus had a relapse during the 12‐month follow‐up period. The authors did not find a difference in the ability to taper oral steroids between the two groups. Two participants taking cyclosporine and one taking tacrolimus required cessation of medication because of intolerability or safety concerns. Quality of life measures were also similar between the two groups.
Lee 2012 compared tacrolimus monotherapy versus tacrolimus plus oral steroid in 35 participants (most of whom were white and none of whom had VKH), who were successfully tapered from an oral steroid dose of 10 mg daily. The main outcome of this study was an improvement in visual acuity; cessation of medication because of safety/intolerability was also a primary outcome. There were no significant differences between the groups in the change in visual acuity (change in logMAR vision) or in the number of participants able to maintain remission at nine months. There were more treatment failures in the combination group than in the tacrolimus monotherapy group.
Discussion
Rather than representing a single clinical entity, non‐infectious intermediate, posterior, and panuveitis (NIIPPU) is an anatomic classification of a variety of disparate diseases not associated with infectious organisms that produce inflammation primarily in the vitreous, retina, choroid, and, in the case of panuveitis, the entire eye. Although many clinical syndromes within NIIPPU are well‐defined based on clinical features, genetic markers, and other diagnostic modalities, the pathophysiologic underpinnings of these inflammatory conditions remain poorly understood. Treatment for these potentially blinding conditions typically begins with systemic or topical corticosteroids, or both, but given the chronicity of most NIIPPU, steroid‐sparing agents are frequently required. Non‐biologic, disease‐modifying antirheumatic drugs (DMARDs) are often used in clinical practice for this purpose. In this review, we attempted to survey and synthesize the available data regarding the effectiveness and safety of DMARDs in the management of NIIPPU, specifically assessing the data on cyclosporine, azathioprine, tacrolimus, mycophenolate, and methotrexate in this heterogeneous group of conditions.
Summary of main results
We included 11 RCTs in this analysis.
We evaluated two individual studies comparing an experimental DMARD to oral steroid but did not combine them in a meta‐analysis: Deuter 2018 found that mycophenolate plus low‐dose oral steroid was associated with significantly better control of inflammation (improved time to relapse) over oral steroid monotherapy (low‐certainty evidence), whereas De Vries 1990 found no evidence of a difference between high‐dose cyclosporine plus oral steroid and steroid monotherapy.
Methotrexate was found to be slightly more likely to result in control of inflammation, including steroid‐sparing control, over mycophenolate (moderate‐certainty evidence), although there was no benefit for one medication over the other in the Vogt‐Koyanagi‐Harada (VKH) subgroup (Rathinam 2014; Rathinam 2019). Mycophenolate or methotrexate are likely similar in other clinical outcomes, such as cystoid macular edema (CME) or best‐corrected visual acuity (BCVA), quality of life measures, and systemic or ocular adverse events.
Randomized controlled trials testing cyclosporine fell into two categories: studies published between 1986 and 1993, which used a higher dose of cyclosporine (8 mg to 15 mg/kg/day) than is currently used in clinical practice; and studies published between 2010 and 2021, which used modern dosing (3 mg to 5 mg/kg/day). We did not include the high‐dose cyclosporine studies in any meta‐analysis as we felt these did not inform current clinical practice.
Seven studies examined cyclosporine A versus another DMARD or oral steroid. We combined two of these in a meta‐analysis comparing individual DMARDs plus oral steroids to modern dosing cyclosporine A plus oral steroids. Cyclosporine A plus oral steroid was not found to be superior to azathioprine plus oral steroid (Cuchacovich 2010) or IV pulse of steroid plus steroid taper (Ono 2021) in any efficacy or safety outcome (low‐ or very low‐certainty evidence). High‐dose cyclosporine A plus oral steroid was not found to be superior to steroid monotherapy (De Vries 1990; Nussenblatt 1991; Wiederholt 1986). We excluded one study from the meta‐analysis because it compared tacrolimus to cyclosporine A, two medications of the same class (Murphy 2005), and another because it compared cyclosporine A to cyclosporine G, which is not clinically available (Nussenblatt 1993).
Finally, we were not able to add Lee 2012 to any of the comparison groups. This study tested tacrolimus monotherapy versus tacrolimus plus oral steroid. There were no significant differences in the change in visual acuity or in the number of participants able to maintain remission at nine months between the groups, but more treatment failures occurred throughout the whole course of study in the combination group than in the tacrolimus monotherapy group.
Overall completeness and applicability of evidence
We included 11 RCTs with a total of 601 participants. Seven of the 11 trials were small, with fewer than 50 participants. The studies included only adult participants, except for Cuchacovich 2010, which included one child aged five years, and the two FAST trials, Rathinam 2014 and Rathinam 2019, which included participants 16 years of age and older. The prevalence of various subtypes of NIIPPU, such as VKH disease, varies widely depending on geographic region, and our review included studies from the USA, Western Europe, Mexico, Chile, Australia, Japan, Saudi Arabia, and India. Notably, no studies from the African subcontinent or which contained majority Black/Afro‐Caribbean participants were included. Two studies had exclusively VKH populations (Cuchacovich 2010; Ono 2021), two had sizable VKH populations (Rathinam 2014; Rathinam 2019), and four studies had either no participants with VKH (Deuter 2018; De Vries 1990; Murphy 2005; Lee 2012), or a small proportion (Nussenblatt 1991). Two studies did not report the proportion of participants with VKH (Nussenblatt 1993; Wiederholt 1986). Subgroup analyses with the VKH patients did not differ from the main analyses, in that frequently the evidence was very uncertain regarding one treatment's superiority over another in terms of safety or efficacy. The high proportion of VKH patients in some included studies makes their results less generalizable to certain parts of the world, with lower rates of VKH, and vice‐versa.
In the search, we found few trials which compared various DMARDs head‐to‐head, except for the FAST trials (Rathinam 2014; Rathinam 2019), which compared mycophenolate to methotrexate, and the Cuchacovich 2010 study, which compared azathioprine to cyclosporine in combination with a low‐dose oral steroid. The other studies compared a DMARD with or without oral steroid to steroid monotherapy (Deuter 2018; De Vries 1990; Nussenblatt 1991; Ono 2021; Wiederholt 1986), a DMARD to the same DMARD plus oral steroid (Lee 2012), or two DMARDs with same mechanism of action (Murphy 2005; Nussenblatt 1993). Although oral steroids are efficacious and should be viewed as the initial standard of care, there is a clear clinical need for steroid‐sparing medications. Because of the lack of direct comparisons, data are sparse to answer the most clinically relevant question: which DMARD is most effective or safest when compared to other, dissimilar DMARDs? This review provides little practical evidence to the clinician wondering which medication to start as the best first choice, or which medication to choose when an initial DMARD fails. Further studies in which medications are tested head‐to‐head are needed to answer this important clinical question.
There was variation in terms of comparator treatments, especially in steroid dosing and tapers, between studies. The length of steroid tapers varied from several weeks to six months, while the amount the dose was decreased and the frequency at which these decreases occurred also differed between studies. There were few instances in which an experimental and control pair were represented by multiple studies; most were single studies without comparable replicates. Studies that compared cyclosporine A also utilized different doses of the medication, with earlier studies (De Vries 1990; Nussenblatt 1991; Wiederholt 1986) treating participants with a higher dose (8 mg to 15 mg/kg/day) than what is typically used today in clinical practice (2 mg to 5 mg/kg/day; Cuchacovich 2010; Murphy 2005), owing to known nephrotoxicity at higher doses (Palestine 1986). One study specifically addressed cyclosporine A and G toxicity in a dose‐escalation trial (Nussenblatt 1993), which found that higher doses (7.5 mg or 10 mg/kg/day) of both formulations were associated with higher rates of moderately severe renal toxicity and the need for dose reductions.
There was a high degree of heterogeneity in how studies defined control of inflammation, relapse, and clinical activity. Because of this, we were unable to make any useful comparisons based on a single, prespecified definition of control of inflammation, and instead based our analyses on how the individual study had defined "control of inflammation." Frequently, several components of this definition were challenging to quantify, such as the number of retinal hemorrhages, retinal vasculitis, or "activity" of retinal or choroidal lesions. Even a two‐step change in the grade of vitreous cell/flare is somewhat subjective between observers. Other activity domains (such as anterior chamber cells, vitreous opacities like 'snowballs', rate of serous retinal detachment, etc.) were frequently included as a part of the study's definition of control of inflammation. This lack of standardization of primary outcome makes it challenging to compare results across studies or group results together in meta‐analyses, a particularly unfortunate situation in a relatively rare condition like NIIPPU, in which studies are frequently small. All studies reported a visual acuity outcome, although a number of different methods were employed, including the ETDRS chart, Snellen tables, Landolt C optotypes, and tumbling Es. Some metric for macular edema was reported by seven studies, but there was a variety in how this was measured (biomicroscopy, optical coherence tomography, or fluorescein angiography), whether macular edema was reported as a change from baseline, as a proportion of participants with resolution, or as a criterion for relapse. The proportion of participants to achieve steroid‐sparing control was not reported by all studies but was generally defined as those on less than or equal to 5 mg to 10 mg/day of oral steroid. Quality of life and vision‐related quality of life metrics were only reported by a few studies using the SF‐36 scale and VFQ or VCM‐1 scales, respectively (Murphy 2005; Rathinam 2014; Rathinam 2019). No studies reported data on cost‐effectiveness.
Adverse events were not reported in a standardized way across studies: some studies reported the incidence of adverse events over the study period, some reported the proportion or percentage of participants experiencing various adverse events, and some simply listed events which had occurred during the study without clarifying how common the adverse event was. Laboratory cut‐offs varied from study to study, and many adverse events were self‐reported without detailed protocols regarding how self‐reporting was collected and standardized. Because all studies reported the number of participants requiring cessation of medication because of safety or intolerability concerns, and because this number was discrete (participants either had to stop or did not), we utilized this as our main safety metric when considering the risk of bias. We also felt that, on a practical level, this metric may be the most important to clinicians counselling their patients on the risk of adverse events with a new DMARD.
In general, follow‐up times for studies ranged widely from three months to over a year. Two studies began their follow‐up period once the participant was quiet on the experimental regimen (Deuter 2018; Lee 2012), and then measured for time to relapse, while the others were designed to measure how long it took participants with active disease to achieve control of inflammation on an experimental medication.
Certainty of the evidence
Despite changes from the original protocol to expand the number of qualifying trials by including trials that investigated both pediatric and adult participants, many factors led to the downgrading of the certainty of the evidence to moderate, low or very low. These factors included heterogeneity in study population characteristics, heterogeneity in the underlying causes of NIIPPU, various pharmacologic agents of different formulations and dosages, some of which are no longer in clinical use, heterogeneity in study designs, and small study sizes. Also, we assessed the risk of bias as high in at least one‐third of the studies, with the remaining studies demonstrating at least some concerns in the risk of bias assessment for at least one of the assessed outcomes.
Potential biases in the review process
An Information Specialist assisted with the comprehensive electronic database search of multiple databases, including trial registries. We applied standard Cochrane methods to conduct this review and avoid potential biases associated with the literature search, appraisal, data extraction, analysis, and interpretation. We also performed an additional handsearch of the reference lists of included studies and made frequent contacts with many trial investigators and study authors to obtain additional details regarding study design, analytic methods, and outcome measures where these were lacking or unclear.
Agreements and disagreements with other studies or reviews
Karam 2022 performed a systematic review and meta‐analysis and compared methotrexate to mycophenolate mofetil for the treatment of all types of non‐infectious uveitis. Similar to our findings, methotrexate had better treatment success compared to mycophenolate for posterior and panuveitis. Interestingly, mycophenolate had a faster median resolution of disease compared to methotrexate, but this included anterior uveitis in the analysis.
Pato 2011 and Gómez‐Gómez 2020 performed systematic reviews, while Knickelbein 2017 performed only a limited PubMed search. None of these reviews performed a meta‐analysis. Gómez‐Gómez 2020 included RCTs and studied non‐anterior, non‐infectious uveitis while Pato 2011 included RCTs, uncontrolled masked trials, prospective, and retrospective studies and examined posterior uveitis, anterior uveitis associated with juvenile idiopathic arthritis, and macular edema. Finally, Knickelbein 2017 included RCTs and retrospective studies and evaluated treatment effects for all forms of non‐infectious uveitis. Findings from these reviews were similar to ours.
The 2018 guidance report from the American Academy of Ophthalmology concluded that mycophenolate mofetil, tacrolimus, cyclosporine, azathioprine, and methotrexate are effective treatments for NIIPPU, but these recommendations were based on findings from studies including RCTs, prospective and retrospective studies, case series, and peer‐reviewed articles, and a meta‐analysis was not performed (Dick 2018).
Authors' conclusions
Implications for practice.
Methotrexate may be more effective than mycophenolate in control of inflammation, including achievement of steroid‐sparing control, in non‐infectious intermediate, posterior, and panuveitis (NIIPPU). Otherwise, there is insufficient evidence to consider one non‐biologic, disease‐modifying antirheumatic drug (DMARD) more effective or safe than the others, or more effective or safe compared to systemic steroid therapy, in the treatment of NIIPPU.
DMARDs are generally well‐tolerated and few patients require cessation of medication while on these therapies.
Implications for research.
Significant heterogeneity exists in the NIIPPU literature in virtually every aspect of randomized controlled trials (RCTs): study design (active to control design versus remission to relapse design), study population (dependent on geographic location, with significant alterations in the proportion of people with Vogt‐Koyanagi‐Harada [VKH] disease included), length of follow‐up, outcome definition (control of inflammation, relapse), reporting of adverse events, and so on. Because studies of this relatively rare condition tend to be small, it would be useful to combine results into meta‐analyses.
Standardization of outcome definitions would make synthesizing all evidence more feasible and valid. A core outcome set published in 2021 identified 16 outcomes of importance for studies of NIIPPU covering four domains: visual function, health‐related quality of life, treatment side effects, and disease control (Tallouzi 2021). This provides an important framework for future study design, but the authors acknowledge that many of the outcomes identified remain based on somewhat subjective clinician estimates. The optimal timing and method of measurement of these outcomes are also not codified. Furthermore, the issue of which outcomes to measure when comparing clinical activity between different conditions within NIIPPU (i.e. serous retinal detachments in VKH, retinal vasculitis in vasculitis subtypes) remains to be dealt with.
Because diseases within NIIPPU have a wide variety of clinical presentations, with different features (vitreous cell, serous retinal detachment, enlargement of lesion size) indicating disease activity, it would be useful going forward to develop standardized, widely‐adopted definitions of clinical activity and inflammation control for various NIIPPU subtypes.
There are significant gaps in the literature: there are few RCTs testing the same comparators or testing different DMARDs head‐to‐head. Clinical practice, therefore, is currently being based on small, single studies. Larger, multicenter studies testing DMARDs head‐to‐head are needed.
History
Protocol first published: Issue 3, 2021
Risk of bias
Risk of bias for analysis 1.1 Control of inflammation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Deuter 2018 | Some concerns | Randomisation was performed centrally via a web‐based randomisation system for each site by permuted blocks. No information provided about the allocation sequence concealment. Baseline characterisitics differences could be compatable with chance. | Some concerns | The study is "open label" with no blinding described. There is no information about deviations in treatment due to trial context. Analysis by intention‐to‐treat (ITT) last observation carried forward (LOCF). Participants appear to be analyzed by assigned group. | Low risk of bias | Data are available for most randomized participants. | High risk of bias | Description not given about determination of change in baseline vitreous haze score. Unblinded outcome assessment could have differed between groups. | Some concerns | The primary endpoint has multiple assessments (BCVA, vitreous haze, CME by OCT, etc.) with standard scales for each. No information provided about a pre specified analysis plan. | High risk of bias | Deuter 2018 was judged to have a potentially high risk of bias based on concerns regarding bias in the measurement of the outcome (open lable, lack of second unblinded outcome assessor), along with some concerns about bias arising from the randomization process, deviations from intended interventions, and selection of the reported result. |
Risk of bias for analysis 1.2 Control of inflammation: high‐dose cyclosporine A.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| De Vries 1990 | Some concerns | No information on method of allocation concealment. There are few differences between groups and could be due to chance. | Low risk of bias | Participants and treating investigator were blinded to treatment assignment. The ITT analysis with exclusions for ocular side effects and protocol noncompliance is probably appropriate. Impact is balanced between treatment groups (three participants from each group). | Low risk of bias | Efficacy data are not available for 6/27 participants but is similar between groups (3/13 vs 3/14). There are differences between text and figures on how drop‐outs may be handled, but any missing data is unlikely to depend on the true value. | Low risk of bias | The blinded outcome assessor used appropriate predefined inflammatory activity score. | Some concerns | There is no information provided about statistical analysis plans. It is unlikely that the result could be selected from multiple measurements or analyses. | Some concerns | Some concerns regarding risk of bias were identified for the efficacy outcome in De Vries 1990. The study was largely assessed to have low risk of bias in most domains; however, there were some concerns regarding the randomization process as there was no description given of allocation concealment, and in selection of the reported result, as information about a statistical analysis plan was not provided. |
Risk of bias for analysis 1.5 Proportion of participants experiencing complications or requiring cessation of medication.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Deuter 2018 | Some concerns | Randomisation was performed centrally via a web‐based randomisation system for each site by permuted blocks. No information provided about the allocation sequence concealment. Baseline characterisitics differences could be compatable with chance. | Some concerns | The study "open label" with no blinding described. There is no information about deviations in treatment due to trial context. Analysis described as ITT. Discontinuation due to adverse events included all participants. Participants appear to be analyzed by assigned group. | Some concerns | Data is missing for 3/44 (6.8%) participants. Patients who were experiencing severe side effects may been lost to follow up. If there are missing data from later assessments for these patients, the missingness in the outcome could influence the true rate of serious side effects. It is unlikely this occured. | Some concerns | Patients and obervers were not blinded and this could have influenced their perception of adverse events. In addition, follow up visits were variable which could have also influenced adverse event reporting/detection. Adverse event that caused discontinuation was a laboratory test. | High risk of bias | No information is provided about a statistical analysis plan. No information provided about pre‐determined cutoffs or scales used for adverse events. Adverse events reported in per patient‐years; this may have been one of several eligible analyses. | High risk of bias | Deuter 2018 was judged to be at possibly high risk of bias in the safety outcome because of concerns in selection of the reported result, as no information was provided about pre‐determined cutoffs or scales used for adverse events. There were some concerns about risk of bias in the other domains for this outcome. |
Risk of bias for analysis 2.1 Control of inflammation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Rathinam 2014 | Low risk of bias | Participants were randomized by program generated permuted, block randomization by principle statistician. Baseline characteristics were similar; statistically significantly differences could be from chance based on the number of characteristics. | Low risk of bias | Participants and study coordinators were not masked. No clear evidence of deviations from the intended intervention that arose because of the study protocol. All complete case analysis and ITT analysis with regression‐based multiple imputation. | Low risk of bias | There were 6/35 and 7/32 participants excluded for not receiving the intervention, loss to follow‐up, or dropout. A complete case analysis and additional regression‐based multiple imputation ITT analysis were performed. | Low risk of bias | Study ophthalmologists were blinded and measured AC cells according the SUN guidelines, vitreous cells were graded according to a scale from the Multicenter Uveitis Steroid Treatment trial, vitreous haze assessed by the NEI scoring system, which are somewhat subjective but scaled. Activity of retinal/choroidal lesions judged by the treating ophthalmologist and is more subjective. Observers were blinded and while inter‐observer variability may have occurred, it is unlikely that intra‐observer measurements differed based on intervention groups. | Some concerns | No information provided about a pre‐specified analysis plan. The time points of 5 and 6 month visits are not clearly clinically relevant. No evidence of multiple eligible outcome measurements although time points of 5 and 6 months are not clearly clinically relevant. No evidence of multiple eligible analyses of data. | Some concerns | Rathinam 2014 was judged to have some concerns in risk of bias in efficacy outcome becuase of concerns regarding bias in selection of the reported result. No pre‐specified analysis plan was reported. The other 4 domains were judged to be at low risk of bias in this study. |
| Rathinam 2019 | Low risk of bias | Block randomization with varying block sizes only known to the biostatistician. Individuals with access to randomization list and study coordinators enrolling participants are separate. Baseline demographics and clinical characteristics were similar between the two groups. | Low risk of bias | All participants and study coordinators were unmasked to treatment assignment after enrolment; study drugs are different in appearance and must be administered on different weekly schedules. Deviations from protocol interventions were detailed and may not be due to trial context. All patients with a 6 month visit or who were declared as having early treatment failure were analyzed according to their randomization group. | Low risk of bias | There were 22 participants (10%) were lost to follow‐up; 194 patients (96 in the methotrexate group and 98 in the mycophenolate group) had complete information for the primary outcome. | Low risk of bias | Pre‐defined grading scale for grading vitreous haze, etc., with reporting of inter‐observer variation for several time points. Unlikely to have variability between intervention groups as same site assessor; between sites may differ. Outcome assessors were masked to treatment group. | Low risk of bias | Final analysis did match pre‐specified, published statistical analysis plan. Multiple measurements and types included in treatment success definition. The outcome domain is a known adaptation of established SUN criteria. Pre specified analyses are reported as outlined and post hoc are stated. | Low risk of bias | A low risk of bias was determined for Rathinam 2019 in the efficacy outcome measure. All domains for this outcome were considered at low risk of bias, with no concerns identified for the randomization process, outcome measurement, or reporting of the result. |
Risk of bias for analysis 2.10 Proportion of participants experiencing complications or requiring cessation of medication.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Rathinam 2014 | Low risk of bias | Participants were randomized by program generated permuted, block randomization by principle statistician. Baseline characteristics were similar; statistically significantly differences could be from chance based on the number of characteristics. | Low risk of bias | Patients and study coordinators were not masked. No clear evidence of deviations from the intended intervention that arose because of the study protocol. An additional intention‐to‐treat analysis was conducted using regression based multiple‐imputation, all 80 patients were included. | Low risk of bias | There were 6/35 and 7/32 participants excluded for not receiving the intervention, loss to follow‐up, or dropout. | Some concerns | Serious and non serious adverse events were ascertained at each visit by the coordinator and ophthalmologist. Information about methodology of collecting these outcomes is not provided but is likely appropriate for laboratory values; more concerns arise from subjective, patient‐reported measures like headache/fatigue. | Low risk of bias | No information is provided about a pre‐specified analysis plan, although frequency of adverse events is listed as a "prespecified secondary outcome". Total adverse events over the entire study period are reported and do not seem to have been influenced by multiple eligible outcome measurements within this outcome domain. There is no evidence of multiple eligible analysis of these data. | Some concerns | Most domains for the safety outcome in the Rathinam 2014 study were judged to be at low risk of bias. However, overall the study is judged to be at some risk of bias because of concerns in the measurement of the safety outcome; information about methodology for collection of subjective, patient‐reported issues with tolerability/safety is not provided. |
| Rathinam 2019 | Low risk of bias | Block randomization with varying block sizes only known to the biostatistician. Individuals with access to randomization list and study coordinators enrolling participants are separate. Baseline demographics and clinical characteristics were similar between the two groups. | Low risk of bias | All participants and study coordinators were not masked to treatment assignment after enrolment; study drugs are different in appearance and must be administered on different weekly schedules. Deviations from protocol interventions were detailed and may not be due to trial context. All patients with a 6 month visit or who were declared as having early treatment failure were analyzed according to their randomization group. | Low risk of bias | AEs reported in trial registry include all participants in both arms (MTX 107/107 vs MM 109/109). | Some concerns | Defined laboratory abnormalities are not inappropriate. Participant reported measures have varying subjectivity (e.g., mood or dizziness). Participants were not masked to treatment assignment. It is not clear if the safety outcome assessors were blinded in the same manner as the efficacy outcome assessments. There is the potential for diagnostic detection bias for the non‐study visits outlined for this outcome assessor. The AEs seem equal between groups. | Low risk of bias | Final analysis did match pre‐specified, published statistical analysis plan. There are multiple possible eligible analyses but those beyond the pre‐specified are reported as post hoc. | Some concerns | The safety outcome in Rathinam 2019 was judged to be at low risk of bias for 4 out of 5 domains; however, there were some concerns in measurement of the outcome domain which rendered an overall judgement of some concerns of bias. There was judged to be potential for diagnostic detection bias for adverse events, especially subjective adverse events such as dizziness, as patients were not masked to treatment assignment, and it is not clear if safety outcome assessors were blinded in the same way as efficacy outcome assessors. |
Risk of bias for analysis 3.1 Control of inflammation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.1.1 IV pulse methylprednisolone for 3 days | ||||||||||||
| Ono 2021 | Low risk of bias | Randomization for all sites performed at single data center and "numbered container method" noted for concealment. There do not appear to be substantial differences between groups at baseline. | Low risk of bias | Treating clinicians and participants were not masked to treatment. No detailed protocol of acceptable non intervention treatments was provided, however the reported topical steroid use and changes to intervention protocol are reported. Analysis is described as "missing values were treated as missing" for this outcome. | Low risk of bias | Almost all participants (70 / 72) had outcome data reported. | Low risk of bias | Validated outcome measurements used for primary outcome and unlikely to differ between groups. Efficacy outcome measurements performed by masked assessors. | Some concerns | No detailed information about prespecified analysis plan. There are multiple possible measures included in the composite outcome. There are multiple possible analyses for each measure to contribute to the final effect measure. | Some concerns | Ono 2021 was judged to be at some risk of bias for the efficacy measure. Four out of 5 domains were considered at low risk of bias. There were some concerns in the selection of the reported result, as there was not information included about a prespecified analysis plan and multiple possible measures were included in the composite outcome, introducing the possibility of multiple analyses for each measure contributing to the final effect measure. |
| Subgroup 3.1.2 Azathioprine 2 to 3 mg/kg/day | ||||||||||||
| Cuchacovich 2010 | Some concerns | Participants treatment groups randomized with a table of random, computer‐generated numbers. Allocation concealment is not detailed. The authors note that age may be a source of bias. The small study size means differences in baseline characteristics could be due to chance alone. | High risk of bias | Participants and intervention providers were not masked. In the AZA group, CsA was added to AZA for 3 participants and 2 participants were switched to other IMTs (cyclophosphamide, mycophenolate) after reactivation. In the CsA arm, 2 participants had AZA added after reactivation. These deviations following reactivation are likely to influence the outcome especially since they are not balanced between groups. Participants are analyzed in the group they were assigned to at randomization. | Low risk of bias | All participant data appear available. | Low risk of bias | Inflammatory score, anterior chamber Tyndall and flare, and vitreous haze were measured using published scales. Outcomes were assessed at specific times by an independent ophthalmologist, not passively collected. Measurements unlikely to differ between groups. | Some concerns | No information provided about a pre‐specified statistical analysis plan. The outcome "improvement in inflammation" is a composite of multiple assessments with differing scales and subjectivity. Though are multiple possible combinations for this analysis. There is no evidence the results was selected from multiple measurements or analyses. | High risk of bias | The efficacy outcome in Cuchacovich 2010 was judged to have high risk of bias because of concerns with deviations from intended interventions, as patients were allowed to receive additional IMTs, including the experimental medication from the opposite group. There were also some concerns with the randomization process and the selection of the reported result. |
Risk of bias for analysis 3.2 Control of inflammation: high‐dose cyclosporine A.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Nussenblatt 1991 | Some concerns | No information provided about how patients were randomized with allocation concealed. Baseline demographics are not provided; diagnosis of randomized participants is fairly similar between groups. | Low risk of bias | The prednisolone was prepared into a liquid solution that had the taste and consistency of the cyclosporine liquid preparation. This made it impossible for the patients to konw which drug they were taking on the basis of physical characteristics of the two preparations.
Patients were observed at monthly intervals by two masked observers. No information provided on whether an intent‐to‐treat analysis was done, but all patients randomized to each group appear to have been analyzed. |
Low risk of bias | 28 per group, however only 15 with vitreal haze in Cyclosporine A group and 13 with vitreal haze in the prednisolone group as denominators | Low risk of bias | Vitreal haze was measured by two mased observers who had to agree. Observers were masked and had to agree. Assessors were blinded. |
Some concerns | No information provided about a statistical analysis plan. No evidence of multiple eligible outcome measurements used. No evidence of muliple eligible anlayses of the data. |
Some concerns | [same as 3.1] |
| Wiederholt 1986 | Low risk of bias | No detailed information is provided about randomization procedure. The allocation sequence was concealed using sealed envelopes that determine randomized treatment group. Baseline differences with so few participants may be due to chance alone. | Some concerns | Modes of therapy delivery differed (liquid versus tablet form). The study is not described as masked or blinded. A detailed protocol is not provided. There are interventions, such as vitrectomy, that may have been deviations or acceptable (e.g. participant 3 had vitrectomy at 3 months). All participants are included in analyses by their assigned group with detailed information. |
Low risk of bias | Data were available for all participants. | Some concerns | All visual acuity measurements were carried out under standardized conditions by a ward doctor who was not involved in the study, and the inflammatory activity of uveitis was determined using a previously introduced scheme by two investigators involved in the study. Outcome assessment for inflammation required two assessors and was scheduled at specific intervals. Outcome assessors for inflammatory activity were not blinded. Somewhat subjective scaling scoring could have been influenced by the knowledge of treatment, but this is not likely as both observers would have had to be influenced similarly. It is not clear that the outcome was influenced. |
High risk of bias | No information provided about a statistical analysis plan. Definitions of relapse and success not laid out, multiple time points at which a patient could be declared a relapse seem possible. Results of some participants are reported over 12 months and others go to 18 months and a 3 month vs 12 month comparision provided. |
High risk of bias | [same as 3.1] |
Risk of bias for analysis 3.8 Proportion of participants experiencing complications or requiring cessation of medication.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 3.8.1 IV pulse methylprednisolone for 3 days | ||||||||||||
| Ono 2021 | Low risk of bias | Randomization for all sites performed at single data center and "numbered container method" noted for concealment. There do not appear to be substantial differences between groups at baseline. | Low risk of bias | Treating clinicians and participants were not masked to treatment. No detailed protocol of acceptable non intervention treatments was provided, however the reported topical steroid use and changes to intervention protocol are reported. Analysis is described as "missing values were treated as missing" for this outcome. | Low risk of bias | Almost all participants (70/72) had outcome data reported. | Some concerns | Combination of clinical and participants reported AEs. Possible diagnostic detection bias if passive collection of AE data. Ophthalmologists monitoring safety was not masked. Stopping rules for serious adverse events not described; if the decision to stop treatment because of serious AEs is decided by the unmasked treating ophthalmologist, the outcome is determined by the intervention provider. | Some concerns | No SAP or protocol for comparison to results in report. The results is in line with the Methods section. Lab safety threshold for d/c are reported on fixed scales combined with reported AEs that could be selected. Protocol for assessors' stopping is not described. Comparing to the Methods section, it does not appear that the results was selected. No SAP for comparison. The analyses outlined for labs and reported AEs do not appear to have been selected. | Some concerns | Ono 2021 was judged to have some concerns of bias in the safety outcome. Measurement of the outcome and selection of the reported result were both felt to have some risk of bias. Stopping rules for serious AEs were not described, the safety assessor was not blinded, and no statistical analysis plan provided were some of the reasons for this determination. The other domains were judged to be of low risk of bias. |
| Subgroup 3.8.2 Azathioprine 2 to 3 mg/kg/day | ||||||||||||
| Cuchacovich 2010 | Some concerns | Participants treatment groups randomized with a table of random, computer‐generated numbers. Allocation concealment is not detailed. The authors note that age may be a source of bias. The small study size means differences in baseline characteristics could be due to chance alone. | High risk of bias | Participants and intervention providers were not masked. In the AZA group, CsA was added to AZA for 3 participants and 2 participants were switched to other IMTs (cyclophosphamide, mycophenolate) after reactivation. In the CsA arm, 2 participants had AZA added after reactivation. These deviations following reactivation are likely to influence the outcome especially since they are not balanced between groups. Participants are analyzed in the group they were assigned to at randomization. | Low risk of bias | All participant data appear available. | High risk of bias | Passive collection of participant reported adverse events could result in diagnostic detection bias. Patients and investigators were not blinded, some adverse events were self‐reported, and criteria for requiring cessation of a medication are not described; therefore the measurement of this outcome could have been influenced by the observers' and/or participants' biases. The outcome reflects decisions made by the intervention provider (treatment termination). | Some concerns | No pre‐specified statistical analysis plan available for comparison. There is no information about the multiple systematic AEs assessed for the outcome results; discrepency between tables and text regarding neurologic sequelae indicates other AEs may have been assessed. No evidence of results selected from multiple eligible analyses. | High risk of bias | Cuchacovich 2010 was judged to be at high risk of bias for the safety outcome measure, with some concerns arising from the randomization process and selection of the reported result, and a high risk of bias determined for the measurement of outcome and deviations from intended intervention domains. This arose because of lack of blinding, self‐reported safety outcomes, without pre‐specified criteria for requiring cessation of the medication, as well as discrepancies between data reported in text and tables which suggested other AEs may have been assessed. |
Acknowledgements
We thank Lori Rosman, Information Specialist for Cochrane Eyes and Vision (CEV), who created and executed the electronic search strategies.
We also thank Su‐Hsun Liu, Project Director of CEV@US, for methodology support; Anupa Shah, Managing Editor for CEV, for support and guidance in the preparation of this review.
We would like to acknowledge the contributions of Naira Khachatryan (University of Colorado), an author on the review protocol.
We would also like to thank the following peer reviewers for their comments: Dr. Renee Bovelle (Howard University Hospital) and Dr. Vishal Jhanji (University of Pittsburgh) for the review protocol; Renee Bovelle (Howard University) and Mohammad Rafieetary (Charles Retina) for the review manuscript. We thank Faith Armitage for proofreading the review.
This review was managed by CEV@US and was signed off for publication by Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Uveitis] explode all trees #2 uveiti* #3 MeSH descriptor: [Panuveitis] explode all trees #4 Panuveitis #5 MeSH descriptor: [Ophthalmia, Sympathetic] explode all trees #6 (Ophthalm* near/2 Sympathetic) #7 MeSH descriptor: [Pars Planitis] explode all trees #8 Pars Planitis or cyclitis or hyalitis #9 MeSH descriptor: [Panophthalmitis] explode all trees #10 Panophthalmiti* #11 MeSH descriptor: [Uveomeningoencephalitic Syndrome] explode all trees #12 (Uveomeningoencephaliti* or Vogt Koyanagi Harada or VKH or fuch or Harada disease or harada syndrome or vogt koyanagi disease) #13 MeSH descriptor: [Behcet Syndrome] explode all trees #14 (behcet* or triple symptom complex) #15 MeSH descriptor: [Iridocyclitis] explode all trees #16 (Iridocycliti* or Heterochromic Cycliti* or anterior scleritis) #17 MeSH descriptor: [Iritis] explode all trees #18 Iriti* #19 Choroiditis #20 (choroiditi* or retinochoroiditi* or chorioretinitis) #21 (Blau* syndrome or familial juvenile systemic granulomatosis or Jabs disease) #22 (Reiter* disease or reiter* syndrome or conjunctivo urethro synovial or urethrooculosynovial syndrome or uroarthritis) #23 (uveoretinitis or uveo retinitis) #24 vitritis* #25 MeSH descriptor: [Retinitis] explode all trees #26 retinitis or neuroretinitis #27 {OR #1‐#26} #28 MeSH descriptor: [Methotrexate] explode all trees #29 Methotrexate or "a methopterine" or abitrexate or amethopterin or amethopterine or ametopterine or antifolan or biotrexate or canceren or "cl 14377" or cl14377 or emtexate or emthexat or emthexate or emtrexate or enthexate or farmitrexat or farmitrexate or farmotrex or folex or ifamet or imeth or "intradose MTX" or jylamvo or lantarel or ledertrexate or maxtrex or metex or methoblastin or methohexate or methotrate or methotrexat or methotrexato or methoxtrexate or methrotrexate or methylaminopterin or methylaminopterine or meticil or metoject or metothrexate or metotrexat or metotrexate or metotrexin or metrex or mexate or "mpi 5004" or mpi5004 or MTX or neotrexate or nordimet or novatrex or "nsc 740" or nsc740 or otrexup or rasuvo or reditrex or reumatrex or rheumatrex or texate or texorate or trexall or xaken or xatmep or zexate or "15475‐56‐6" or "59‐05‐2" or "7413‐34‐5" #30 MeSH descriptor: [Mycophenolic Acid] explode all trees #31 "mycophenolate mofetil" or "mycophenolic acid" or "cell cept" or cellcept or cellmune or cellsept or munoloc or myclausen or "mycophenolate sodium" or myfenax or myfortic or "rs 61443" or rs61443 or "116680‐01‐4" or "128794‐94‐5" #32 MeSH descriptor: [Azathioprine] explode all trees #33 Azathioprine or arathioprin or arathioprine or "aza‐q" or azafalk or azahexal or azamedac or azamun or azamune or azanin or azapin or azapress or azaprine or azarex or azasan or azathiodura or azathiopine or azathioprim or azathioprin or azathiopurine or azathropsin or azatioprina or azatox or azatrilem or azopi or azoran or azothioprin or azothioprine or "bw 57 322" or "bw 57322" or "bw57‐322" or bw57322 or colinsan or immuran or immurel or immuthera or imunen or imuprin or imuran or imurane or imurek or imurel or imuren or methylnitroimidazolylmercaptopurine or "nsc 39084" or nsc39084 or thioazeprine or thioprine or transimune or zytrim or "446‐86‐6" #34 MeSH descriptor: [Cyclosporins] explode all trees #35 Cyclosporine or "adi 628" or adi628 or cequa or "cgc 1072" or cgc1072 or ciclomulsion or cicloral or ciclosporin or ciclosporine or cipol or consupren or CsANeoral or cyclasol or cyclokat or cyclosporin or "cyclosporine a" or "CyA NOF" or "de 076" or de076 or deximune or equoral or gengraf or ikervis or iminoral or implanta or imusporin or "lx 201" or lx201 or "mc2 03" or mc203 or "mtd 202" or mtd202 or neoral or "neuro‐stat" or neurostat or "nm 0133" or "nm 133" or nm0133 or nm133 or "nova 22007" or nova22007 or "ol 27400" or ol27400 or "ol 27 400" or "olo 400" or olo500 or "opph 088" or opph088 or opsisporin or "otx 101" or otx101 or "p 3072" or p3072 or padciclo or papilock or pulminiq or restasis or restaysis or sanciclo or sandimmun or sandimmune or sandimun or sandimune or "sang 35" or sang35 or sangcya or "sp 14019" or sp14019 or "sti 0529" or sti0529 or "t 1580" or t1580 or vekacia or verkazia or "59865‐13‐3" or "63798‐73‐2" or "79217‐60‐0" #36 MeSH descriptor: [Tacrolimus] explode all trees #37 Tacrolimus or advagraf or astagraf or envarsus or "fk 506" or fk506 or "fr 900506" or fr900506 or fujimycin or hecoria or modigraf or "mustopic oint" or prograf or prograft or protopic or protopy or tacforius or tsukubaenolide or "104987‐11‐3" #38 {OR #28‐#37} #39 #27 AND #38
Appendix 2. MEDLINE (Ovid) search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp Uveitis/ 13. uveiti*.tw. 14. exp Panuveitis/ 15. (Panuveitis or cyclitis or hyalitis).tw. 16. exp Ophthalmia, Sympathetic/ 17. (Ophthalm* adj2 Sympathetic).tw. 18. exp Pars Planitis/ 19. Pars Planitis.tw. 20. exp Panophthalmitis/ 21. Panophthalmiti*.tw. 22. exp Uveomeningoencephalitic Syndrome/ 23. (Uveomeningoencephaliti* or Vogt Koyanagi Harada or VKH or fuch or Harada disease or harada syndrome or vogt koyanagi disease).tw. 24. exp Behcet Syndrome/ 25. (behcet* or triple symptom complex).tw. 26. exp Iridocyclitis/ 27. (Iridocycliti* or Heterochromic Cycliti* or anterior scleritis).tw. 28. exp Iritis/ 29. Iriti*.tw. 30. exp Choroiditis/ 31. (choroiditi* or retinochoroiditi* or chorioretinitis).tw. 32. (Blau* syndrome or familial juvenile systemic granulomatosis or Jabs disease).tw. 33. (Reiter* disease or reiter* syndrome or conjunctivo urethro synovial or urethrooculosynovial syndrome or uroarthritis).tw. 34. (uveoretinitis or uveo retinitis).tw. 35. vitritis*.tw. 36. exp Retinitis/ 37. (retinitis or neuroretinitis).tw. 38. or/12‐37 39. exp Methotrexate/ 40. (Methotrexate or "a methopterine" or abitrexate or amethopterin or amethopterine or ametopterine or antifolan or biotrexate or canceren or "cl 14377" or cl14377 or emtexate or emthexat or emthexate or emtrexate or enthexate or farmitrexat or farmitrexate or farmotrex or folex or ifamet or imeth or "intradose MTX" or jylamvo or lantarel or ledertrexate or maxtrex or metex or methoblastin or methohexate or methotrate or methotrexat or methotrexato or methoxtrexate or methrotrexate or methylaminopterin or methylaminopterine or meticil or metoject or metothrexate or metotrexat or metotrexate or metotrexin or metrex or mexate or "mpi 5004" or mpi5004 or MTX or neotrexate or nordimet or novatrex or "nsc 740" or nsc740 or otrexup or rasuvo or reditrex or reumatrex or rheumatrex or texate or texorate or trexall or xaken or xatmep or zexate or "15475‐56‐6" or "59‐05‐2" or "7413‐34‐5").tw,rn. 41. exp Mycophenolic Acid/ 42. ("mycophenolate mofetil" or "mycophenolic acid" or "cell cept" or cellcept or cellmune or cellsept or munoloc or myclausen or "mycophenolate sodium" or myfenax or myfortic or "rs 61443" or rs61443 or "116680‐01‐4" or "128794‐94‐5").tw,rn. 43. exp Azathioprine/ 44. (Azathioprine or arathioprin or arathioprine or "aza‐q" or azafalk or azahexal or azamedac or azamun or azamune or azanin or azapin or azapress or azaprine or azarex or azasan or azathiodura or azathiopine or azathioprim or azathioprin or azathiopurine or azathropsin or azatioprina or azatox or azatrilem or azopi or azoran or azothioprin or azothioprine or "bw 57 322" or "bw 57322" or "bw57‐322" or bw57322 or colinsan or immuran or immurel or immuthera or imunen or imuprin or imuran or imurane or imurek or imurel or imuren or methylnitroimidazolylmercaptopurine or "nsc 39084" or nsc39084 or thioazeprine or thioprine or transimune or zytrim or "446‐86‐6").tw,rn. 45. exp Cyclosporine/ 46. (Cyclosporine or "adi 628" or adi628 or cequa or "cgc 1072" or cgc1072 or ciclomulsion or cicloral or ciclosporin or ciclosporine or cipol or consupren or CsANeoral or cyclasol or cyclokat or cyclosporin or "cyclosporine a" or "CyA NOF" or "de 076" or de076 or deximune or equoral or gengraf or ikervis or iminoral or implanta or imusporin or "lx 201" or lx201 or "mc2 03" or mc203 or "mtd 202" or mtd202 or neoral or "neuro‐stat" or neurostat or "nm 0133" or "nm 133" or nm0133 or nm133 or "nova 22007" or nova22007 or "ol 27400" or ol27400 or "ol 27 400" or "olo 400" or olo500 or "opph 088" or opph088 or opsisporin or "otx 101" or otx101 or "p 3072" or p3072 or padciclo or papilock or pulminiq or restasis or restaysis or sanciclo or sandimmun or sandimmune or sandimun or sandimune or "sang 35" or sang35 or sangcya or "sp 14019" or sp14019 or "sti 0529" or sti0529 or "t 1580" or t1580 or vekacia or verkazia or "59865‐13‐3" or "63798‐73‐2" or "79217‐60‐0").tw,rn. 47. exp Tacrolimus/ 48. (Tacrolimus or advagraf or astagraf or envarsus or "fk 506" or fk506 or "fr 900506" or fr900506 or fujimycin or hecoria or modigraf or "mustopic oint" or prograf or prograft or protopic or protopy or tacforius or tsukubaenolide or "104987‐11‐3").tw,rn. 49. or/39‐48 50. 11 and 38 and 49
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'uveitis'/exp #34 uveiti*:ab,ti,kw #35 'autoimmune uveitis'/exp #36 'behcet disease'/exp #37 behcet*:ab,ti,kw OR 'triple symptom complex':ab,ti,kw #38 'blau syndrome'/exp #39 ((blau* NEXT/1 syndrome):ab,ti,kw) OR 'familial juvenile systemic granulomatosis':ab,ti,kw OR 'jabs disease':ab,ti,kw #40 'choroiditis'/exp #41 choroiditi*:ab,ti,kw OR chorioiditi*:ab,ti,kw #42 'chorioretinitis'/exp #43 retinochoroiditi*:ab,ti,kw OR chorioretiniti*:ab,ti,kw #44 'vogt koyanagi syndrome'/exp #45 uveomeningoencephaliti*:ab,ti,kw OR 'vogt koyanagi harada':ab,ti,kw OR vkh:ab,ti,kw OR fuch:ab,ti,kw OR 'harada disease':ab,ti,kw OR 'harada syndrome':ab,ti,kw OR 'vogt koyanagi disease':ab,ti,kw #46 'intermediate uveitis'/exp #47 'pars planitis':ab,ti,kw OR cyclitis:ab,ti,kw OR hyalitis:ab,ti,kw #48 'iridocyclitis'/exp #49 iridocycliti*:ab,ti,kw OR ((heterochromic NEXT/1 cycliti*):ab,ti,kw) OR 'anterior scleritis':ab,ti,kw #50 'iritis'/exp #51 iriti*:ab,ti,kw #52 'kirisawa uveitis'/exp #53 'reiter syndrome'/exp #54 ((reiter* NEXT/1 disease):ab,ti,kw) OR ((reiter* NEXT/1 syndrome):ab,ti,kw) OR 'conjunctivo urethro synovial':ab,ti,kw OR 'urethrooculosynovial syndrome':ab,ti,kw OR uroarthritis:ab,ti,kw #55 'sympathetic ophthalmia'/exp #56 (ophthalm* NEXT/2 sympathetic):ab,ti,kw #57 'uveoretinitis'/exp #58 uveoretinitis:ab,ti,kw OR 'uveo retinitis':ab,ti,kw #59 'vitritis'/exp #60 vitritis*:ab,ti,kw #61 panuveitis:ab,ti,kw #62 panophthalmiti*:ab,ti,kw #63 'retinitis'/exp #64 retinitis:ab,ti,kw OR neuroretinitis:ab,ti,kw #65 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 #66 'methotrexate'/exp #67 methotrexate:ab,ti,kw,tn,rn OR 'a methopterine':ab,ti,kw,tn,rn OR abitrexate:ab,ti,kw,tn,rn OR amethopterin:ab,ti,kw,tn,rn OR amethopterine:ab,ti,kw,tn,rn OR ametopterine:ab,ti,kw,tn,rn OR antifolan:ab,ti,kw,tn,rn OR biotrexate:ab,ti,kw,tn,rn OR canceren:ab,ti,kw,tn,rn OR 'cl 14377':ab,ti,kw,tn,rn OR cl14377:ab,ti,kw,tn,rn OR emtexate:ab,ti,kw,tn,rn OR emthexat:ab,ti,kw,tn,rn OR emthexate:ab,ti,kw,tn,rn OR emtrexate:ab,ti,kw,tn,rn OR enthexate:ab,ti,kw,tn,rn OR farmitrexat:ab,ti,kw,tn,rn OR farmitrexate:ab,ti,kw,tn,rn OR farmotrex:ab,ti,kw,tn,rn OR folex:ab,ti,kw,tn,rn OR ifamet:ab,ti,kw,tn,rn OR imeth:ab,ti,kw,tn,rn OR 'intradose mtx':ab,ti,kw,tn,rn OR jylamvo:ab,ti,kw,tn,rn OR lantarel:ab,ti,kw,tn,rn OR ledertrexate:ab,ti,kw,tn,rn OR maxtrex:ab,ti,kw,tn,rn OR metex:ab,ti,kw,tn,rn OR methoblastin:ab,ti,kw,tn,rn OR methohexate:ab,ti,kw,tn,rn OR methotrate:ab,ti,kw,tn,rn OR methotrexat:ab,ti,kw,tn,rn OR methotrexato:ab,ti,kw,tn,rn OR methoxtrexate:ab,ti,kw,tn,rn OR methrotrexate:ab,ti,kw,tn,rn OR methylaminopterin:ab,ti,kw,tn,rn OR methylaminopterine:ab,ti,kw,tn,rn OR meticil:ab,ti,kw,tn,rn OR metoject:ab,ti,kw,tn,rn OR metothrexate:ab,ti,kw,tn,rn OR metotrexat:ab,ti,kw,tn,rn OR metotrexate:ab,ti,kw,tn,rn OR metotrexin:ab,ti,kw,tn,rn OR metrex:ab,ti,kw,tn,rn OR mexate:ab,ti,kw,tn,rn OR 'mpi 5004':ab,ti,kw,tn,rn OR mpi5004:ab,ti,kw,tn,rn OR mtx:ab,ti,kw,tn,rn OR neotrexate:ab,ti,kw,tn,rn OR nordimet:ab,ti,kw,tn,rn OR novatrex:ab,ti,kw,tn,rn OR 'nsc 740':ab,ti,kw,tn,rn OR nsc740:ab,ti,kw,tn,rn OR otrexup:ab,ti,kw,tn,rn OR rasuvo:ab,ti,kw,tn,rn OR reditrex:ab,ti,kw,tn,rn OR reumatrex:ab,ti,kw,tn,rn OR rheumatrex:ab,ti,kw,tn,rn OR texate:ab,ti,kw,tn,rn OR texorate:ab,ti,kw,tn,rn OR trexall:ab,ti,kw,tn,rn OR xaken:ab,ti,kw,tn,rn OR xatmep:ab,ti,kw,tn,rn OR zexate:ab,ti,kw,tn,rn OR '15475‐56‐6':ab,ti,kw,tn,rn OR '59‐05‐2':ab,ti,kw,tn,rn OR '7413‐34‐5':ab,ti,kw,tn,rn #68 'mycophenolic acid'/exp #69 'mycophenolate mofetil':ab,ti,kw,tn,rn OR 'mycophenolic acid':ab,ti,kw,tn,rn OR 'cell cept':ab,ti,kw,tn,rn OR cellcept:ab,ti,kw,tn,rn OR cellmune:ab,ti,kw,tn,rn OR cellsept:ab,ti,kw,tn,rn OR munoloc:ab,ti,kw,tn,rn OR myclausen:ab,ti,kw,tn,rn OR 'mycophenolate sodium':ab,ti,kw,tn,rn OR myfenax:ab,ti,kw,tn,rn OR myfortic:ab,ti,kw,tn,rn OR 'rs 61443':ab,ti,kw,tn,rn OR rs61443:ab,ti,kw,tn,rn OR '116680‐01‐4':ab,ti,kw,tn,rn OR '128794‐94‐5':ab,ti,kw,tn,rn #70 'azathioprine'/exp #71 azathioprine:ab,ti,kw,tn,rn OR arathioprin:ab,ti,kw,tn,rn OR arathioprine:ab,ti,kw,tn,rn OR 'aza‐q':ab,ti,kw,tn,rn OR azafalk:ab,ti,kw,tn,rn OR azahexal:ab,ti,kw,tn,rn OR azamedac:ab,ti,kw,tn,rn OR azamun:ab,ti,kw,tn,rn OR azamune:ab,ti,kw,tn,rn OR azanin:ab,ti,kw,tn,rn OR azapin:ab,ti,kw,tn,rn OR azapress:ab,ti,kw,tn,rn OR azaprine:ab,ti,kw,tn,rn OR azarex:ab,ti,kw,tn,rn OR azasan:ab,ti,kw,tn,rn OR azathiodura:ab,ti,kw,tn,rn OR azathiopine:ab,ti,kw,tn,rn OR azathioprim:ab,ti,kw,tn,rn OR azathioprin:ab,ti,kw,tn,rn OR azathiopurine:ab,ti,kw,tn,rn OR azathropsin:ab,ti,kw,tn,rn OR azatioprina:ab,ti,kw,tn,rn OR azatox:ab,ti,kw,tn,rn OR azatrilem:ab,ti,kw,tn,rn OR azopi:ab,ti,kw,tn,rn OR azoran:ab,ti,kw,tn,rn OR azothioprin:ab,ti,kw,tn,rn OR azothioprine:ab,ti,kw,tn,rn OR 'bw 57 322':ab,ti,kw,tn,rn OR 'bw 57322':ab,ti,kw,tn,rn OR 'bw57‐322':ab,ti,kw,tn,rn OR bw57322:ab,ti,kw,tn,rn OR colinsan:ab,ti,kw,tn,rn OR immuran:ab,ti,kw,tn,rn OR immurel:ab,ti,kw,tn,rn OR immuthera:ab,ti,kw,tn,rn OR imunen:ab,ti,kw,tn,rn OR imuprin:ab,ti,kw,tn,rn OR imuran:ab,ti,kw,tn,rn OR imurane:ab,ti,kw,tn,rn OR imurek:ab,ti,kw,tn,rn OR imurel:ab,ti,kw,tn,rn OR imuren:ab,ti,kw,tn,rn OR methylnitroimidazolylmercaptopurine:ab,ti,kw,tn,rn OR 'nsc 39084':ab,ti,kw,tn,rn OR nsc39084:ab,ti,kw,tn,rn OR thioazeprine:ab,ti,kw,tn,rn OR thioprine:ab,ti,kw,tn,rn OR transimune:ab,ti,kw,tn,rn OR zytrim:ab,ti,kw,tn,rn OR '446‐86‐6':ab,ti,kw,tn,rn #72 'cyclosporine'/exp #73 cyclosporine:ab,ti,kw,tn,rn OR 'adi 628':ab,ti,kw,tn,rn OR adi628:ab,ti,kw,tn,rn OR cequa:ab,ti,kw,tn,rn OR 'cgc 1072':ab,ti,kw,tn,rn OR cgc1072:ab,ti,kw,tn,rn OR ciclomulsion:ab,ti,kw,tn,rn OR cicloral:ab,ti,kw,tn,rn OR ciclosporin:ab,ti,kw,tn,rn OR ciclosporine:ab,ti,kw,tn,rn OR cipol:ab,ti,kw,tn,rn OR consupren:ab,ti,kw,tn,rn OR csaneoral:ab,ti,kw,tn,rn OR cyclasol:ab,ti,kw,tn,rn OR cyclokat:ab,ti,kw,tn,rn OR cyclosporin:ab,ti,kw,tn,rn OR 'cyclosporine a':ab,ti,kw,tn,rn OR 'cya nof':ab,ti,kw,tn,rn OR 'de 076':ab,ti,kw,tn,rn OR de076:ab,ti,kw,tn,rn OR deximune:ab,ti,kw,tn,rn OR equoral:ab,ti,kw,tn,rn OR gengraf:ab,ti,kw,tn,rn OR ikervis:ab,ti,kw,tn,rn OR iminoral:ab,ti,kw,tn,rn OR implanta:ab,ti,kw,tn,rn OR imusporin:ab,ti,kw,tn,rn OR 'lx 201':ab,ti,kw,tn,rn OR lx201:ab,ti,kw,tn,rn OR 'mc2 03':ab,ti,kw,tn,rn OR mc203:ab,ti,kw,tn,rn OR 'mtd 202':ab,ti,kw,tn,rn OR mtd202:ab,ti,kw,tn,rn OR neoral:ab,ti,kw,tn,rn OR 'neuro‐stat':ab,ti,kw,tn,rn OR neurostat:ab,ti,kw,tn,rn OR 'nm 0133':ab,ti,kw,tn,rn OR 'nm 133':ab,ti,kw,tn,rn OR nm0133:ab,ti,kw,tn,rn OR nm133:ab,ti,kw,tn,rn OR 'nova 22007':ab,ti,kw,tn,rn OR nova22007:ab,ti,kw,tn,rn OR 'ol 27400':ab,ti,kw,tn,rn OR ol27400:ab,ti,kw,tn,rn OR 'ol 27 400':ab,ti,kw,tn,rn OR 'olo 400':ab,ti,kw,tn,rn OR olo500:ab,ti,kw,tn,rn OR 'opph 088':ab,ti,kw,tn,rn OR opph088:ab,ti,kw,tn,rn OR opsisporin:ab,ti,kw,tn,rn OR 'otx 101':ab,ti,kw,tn,rn OR otx101:ab,ti,kw,tn,rn OR 'p 3072':ab,ti,kw,tn,rn OR p3072:ab,ti,kw,tn,rn OR padciclo:ab,ti,kw,tn,rn OR papilock:ab,ti,kw,tn,rn OR pulminiq:ab,ti,kw,tn,rn OR restasis:ab,ti,kw,tn,rn OR restaysis:ab,ti,kw,tn,rn OR sanciclo:ab,ti,kw,tn,rn OR sandimmun:ab,ti,kw,tn,rn OR sandimmune:ab,ti,kw,tn,rn OR sandimun:ab,ti,kw,tn,rn OR sandimune:ab,ti,kw,tn,rn OR 'sang 35':ab,ti,kw,tn,rn OR sang35:ab,ti,kw,tn,rn OR sangcya:ab,ti,kw,tn,rn OR 'sp 14019':ab,ti,kw,tn,rn OR sp14019:ab,ti,kw,tn,rn OR 'sti 0529':ab,ti,kw,tn,rn OR sti0529:ab,ti,kw,tn,rn OR 't 1580':ab,ti,kw,tn,rn OR t1580:ab,ti,kw,tn,rn OR vekacia:ab,ti,kw,tn,rn OR verkazia:ab,ti,kw,tn,rn OR '59865‐13‐3':ab,ti,kw,tn,rn OR '63798‐73‐2':ab,ti,kw,tn,rn OR '79217‐60‐0':ab,ti,kw,tn,rn #74 'tacrolimus'/exp #75 tacrolimus:ab,ti,kw,tn,rn OR advagraf:ab,ti,kw,tn,rn OR astagraf:ab,ti,kw,tn,rn OR envarsus:ab,ti,kw,tn,rn OR 'fk 506':ab,ti,kw,tn,rn OR fk506:ab,ti,kw,tn,rn OR 'fr 900506':ab,ti,kw,tn,rn OR fr900506:ab,ti,kw,tn,rn OR fujimycin:ab,ti,kw,tn,rn OR hecoria:ab,ti,kw,tn,rn OR modigraf:ab,ti,kw,tn,rn OR 'mustopic oint':ab,ti,kw,tn,rn OR prograf:ab,ti,kw,tn,rn OR prograft:ab,ti,kw,tn,rn OR protopic:ab,ti,kw,tn,rn OR protopy:ab,ti,kw,tn,rn OR tacforius:ab,ti,kw,tn,rn OR tsukubaenolide:ab,ti,kw,tn,rn OR '104987‐11‐3':ab,ti,kw,tn,rn #76 #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 #77 #32 AND #65 AND #76
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 uveiti*[tw] #3 behcet*[tw] OR “triple symptom complex”[tw] #4 “blau syndrome*”[tw] OR “familial juvenile systemic granulomatosis”[tw] OR “jabs disease”[tw] #5 choroiditi*[tw] OR chorioiditi*[tw] #6 retinochoroiditi*[tw] OR chorioretiniti*[tw] #7 uveomeningoencephaliti*[tw] OR “vogt koyanagi harada”[tw] OR vkh[tw] OR fuch[tw] OR “harada disease”[tw] OR “harada syndrome”[tw] OR “vogt koyanagi disease”[tw] #8 “pars planitis”[tw] OR cyclitis[tw] OR hyalitis[tw] #9 iridocycliti*[tw] OR “heterochromic cycliti*”[tw] OR “anterior scleritis”[tw] #10 iriti*[tw] #11 “reiter disease*”[tw] OR “reiter syndrome*”[tw] OR “conjunctivo urethro synovial”[tw] OR “urethrooculosynovial syndrome*”[tw] OR uroarthritis[tw] #12 (ophthalm*[tw] AND sympathetic*[tw]) #13 uveoretinitis[tw] OR “uveo retinitis”[tw] #14 vitritis*[tw] #15 panuveitis[tw] #16 panophthalmiti*[tw] #17 retinitis[tw] OR neuroretinitis[tw] #18 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #19 methotrexate[tw] OR “a methopterine”[tw] OR abitrexate[tw] OR amethopterin[tw] OR amethopterine[tw] OR ametopterine[tw] OR antifolan[tw] OR biotrexate[tw] OR canceren[tw] OR “cl 14377”[tw] OR cl14377[tw] OR emtexate[tw] OR emthexat[tw] OR emthexate[tw] OR emtrexate[tw] OR enthexate[tw] OR farmitrexat[tw] OR farmitrexate[tw] OR farmotrex[tw] OR folex[tw] OR ifamet[tw] OR imeth[tw] OR “intradose mtx”[tw] OR jylamvo[tw] OR lantarel[tw] OR ledertrexate[tw] OR maxtrex[tw] OR metex[tw] OR methoblastin[tw] OR methohexate[tw] OR methotrate[tw] OR methotrexat[tw] OR methotrexato[tw] OR methoxtrexate[tw] OR methrotrexate[tw] OR methylaminopterin[tw] OR methylaminopterine[tw] OR meticil[tw] OR metoject[tw] OR metothrexate[tw] OR metotrexat[tw] OR metotrexate[tw] OR metotrexin[tw] OR metrex[tw] OR mexate[tw] OR “mpi 5004”[tw] OR mpi5004[tw] OR mtx[tw] OR neotrexate[tw] OR nordimet[tw] OR novatrex[tw] OR “nsc 740”[tw] OR nsc740[tw] OR otrexup[tw] OR rasuvo[tw] OR reditrex[tw] OR reumatrex[tw] OR rheumatrex[tw] OR texate[tw] OR texorate[tw] OR trexall[tw] OR xaken[tw] OR xatmep[tw] OR zexate[tw] OR “15475‐56‐6”[tw] OR “59‐05‐2”[tw] OR “7413‐34‐5”[tw] #20 “mycophenolate mofetil”[tw] OR “mycophenolic acid”[tw] OR “cell cept”[tw] OR cellcept[tw] OR cellmune[tw] OR cellsept[tw] OR munoloc[tw] OR myclausen[tw] OR “mycophenolate sodium”[tw] OR myfenax[tw] OR myfortic[tw] OR “rs 61443”[tw] OR rs61443[tw] OR “116680‐01‐4”[tw] OR “128794‐94‐5”[tw] #21 azathioprine[tw] OR arathioprin[tw] OR arathioprine[tw] OR “aza‐q”[tw] OR azafalk[tw] OR azahexal[tw] OR azamedac[tw] OR azamun[tw] OR azamune[tw] OR azanin[tw] OR azapin[tw] OR azapress[tw] OR azaprine[tw] OR azarex[tw] OR azasan[tw] OR azathiodura[tw] OR azathiopine[tw] OR azathioprim[tw] OR azathioprin[tw] OR azathiopurine[tw] OR azathropsin[tw] OR azatioprina[tw] OR azatox[tw] OR azatrilem[tw] OR azopi[tw] OR azoran[tw] OR azothioprin[tw] OR azothioprine[tw] OR “bw 57 322”[tw] OR “bw 57322”[tw] OR “bw57‐322”[tw] OR bw57322[tw] OR colinsan[tw] OR immuran[tw] OR immurel[tw] OR immuthera[tw] OR imunen[tw] OR imuprin[tw] OR imuran[tw] OR imurane[tw] OR imurek[tw] OR imurel[tw] OR imuren[tw] OR methylnitroimidazolylmercaptopurine[tw] OR “nsc 39084”[tw] OR nsc39084[tw] OR thioazeprine[tw] OR thioprine[tw] OR transimune[tw] OR zytrim[tw] OR “446‐86‐6”[tw] #22 cyclosporine[tw] OR “adi 628”[tw] OR adi628[tw] OR cequa[tw] OR “cgc 1072”[tw] OR cgc1072[tw] OR ciclomulsion[tw] OR cicloral[tw] OR ciclosporin[tw] OR ciclosporine[tw] OR cipol[tw] OR consupren[tw] OR csaneoral[tw] OR cyclasol[tw] OR cyclokat[tw] OR cyclosporin[tw] OR “cyclosporine a”[tw] OR “cya nof”[tw] OR “de 076”[tw] OR de076[tw] OR deximune[tw] OR equoral[tw] OR gengraf[tw] OR ikervis[tw] OR iminoral[tw] OR implanta[tw] OR imusporin[tw] OR “lx 201”[tw] OR lx201[tw] OR “mc2 03”[tw] OR mc203[tw] OR “mtd 202”[tw] OR mtd202[tw] OR neoral[tw] OR “neuro‐stat”[tw] OR neurostat[tw] OR “nm 0133”[tw] OR “nm 133”[tw] OR nm0133[tw] OR nm133[tw] OR “nova 22007”[tw] OR nova22007[tw] OR “ol 27400”[tw] OR ol27400[tw] OR “ol 27 400”[tw] OR “olo 400”[tw] OR olo500[tw] OR “opph 088”[tw] OR opph088[tw] OR opsisporin[tw] OR “otx 101”[tw] OR otx101[tw] OR “p 3072”[tw] OR p3072[tw] OR padciclo[tw] OR papilock[tw] OR pulminiq[tw] OR restasis[tw] OR restaysis[tw] OR sanciclo[tw] OR sandimmun[tw] OR sandimmune[tw] OR sandimun[tw] OR sandimune[tw] OR “sang 35”[tw] OR sang35[tw] OR sangcya[tw] OR “sp 14019”[tw] OR sp14019[tw] OR “sti 0529”[tw] OR sti0529[tw] OR “t 1580”[tw] OR t1580[tw] OR vekacia[tw] OR verkazia[tw] OR “59865‐13‐3”[tw] OR “63798‐73‐2”[tw] OR “79217‐60‐0”[tw] #23 tacrolimus[tw] OR advagraf[tw] OR astagraf[tw] OR envarsus[tw] OR “fk 506”[tw] OR fk506[tw] OR “fr 900506”[tw] OR fr900506[tw] OR fujimycin[tw] OR hecoria[tw] OR modigraf[tw] OR “mustopic oint”[tw] OR prograf[tw] OR prograft[tw] OR protopic[tw] OR protopy[tw] OR tacforius[tw] OR tsukubaenolide[tw] OR “104987‐11‐3”[tw] #24 #19 OR #20 OR #21 OR #22 OR #23 #25 #1 AND #18 AND #24 #26 medline[sb] #27 #25 NOT #26
Appendix 5. LILACS (Latin American and Caribbean Health Sciences Literature Database) search strategy
(Uveitis or Uveítis or Uveíte or MH:C11.941.879$ or Panuveitis or Panuveítis or Panuveíte or "Ophthalmia Sympathetic" or "Oftalmía Simpática" or "Oftalmia Simpática" or "Pars Planitis" or "Pars Planite" or "Panophthalmitis" or "Panoftalmitis" or "Panoftalmite" or cyclitis or hyalitis or MH:C01.252.354.900.675$ or MH:C01.539.375.354.900.675$ or MH:C01.539.375.450.900.675$ or MH:C01.703.343.900.675$ or MH:C11.294.354.900.675$ or MH:C11.294.450.900.675$ or "Uveomeningoencephalitic Syndrome" or "Síndrome Uveomeningoencefálico" or "Síndrome Uveomeningoencefálica" or MH:C10.114.843$ or MH:C10.228.228.553.900$ or MH:C20.111.258.925$ or Uveomeningoencephalitis or "Vogt Koyanagi Harada" or "Harada disease" or "harada syndrome" or "vogt koyanagi disease" or "Behcet syndrome" or "Síndrome de Behçet" or MH:C07.465.075$ or MH:C14.907.940.100$ or MH:C17.800.862.150$ or "triple symptom complex" or Iridocyclitis or Iridociclitis or Iridociclite or MH:C11.941.375.360$ or "Heterochromic Cyclitis" or MH:C11.941.160.478$ or chorioretinitis or Retinitis or Retinite or MH:C11.768.773$) AND (Methotrexate or Metotrexato or MH: D03.633.100.733.631.192.500$ or "Mycophenolic Acid" or "Ácido Micofenólico" or MH:D02.241.081.193.678$ or MH:D10.251.618$ or "a methopterine" or abitrexate or amethopterin or amethopterine or ametopterine or antifolan or biotrexate or canceren or "cl 14377" or cl14377 or emtexate or emthexat or emthexate or emtrexate or enthexate or farmitrexat or farmitrexate or farmotrex or folex or ifamet or imeth or "intradose MTX" or jylamvo or lantarel or ledertrexate or maxtrex or metex or methoblastin or methohexate or methotrate or methotrexat or methotrexato or methoxtrexate or methrotrexate or methylaminopterin or methylaminopterine or meticil or metoject or metothrexate or metotrexat or metotrexate or metotrexin or metrex or mexate or "mpi 5004" or mpi5004 or MTX or neotrexate or nordimet or novatrex or "nsc 740" or nsc740 or otrexup or rasuvo or reditrex or reumatrex or rheumatrex or texate or texorate or trexall or xaken or xatmep or zexate or "15475‐56‐6" or "59‐05‐2" or "7413‐34‐5" or "mycophenolate mofetil" or "mycophenolic acid" or "cell cept" or cellcept or cellmune or cellsept or munoloc or myclausen or "mycophenolate sodium" or myfenax or myfortic or "rs 61443" or rs61443 or "116680‐01‐4" or "128794‐94‐5" or Azathioprine or Azatioprina or MH:D02.886.759.111$ or MH:D03.633.100.759.570.090$ or MH:D13.570.900.111$ or arathioprin or arathioprine or "aza‐q" or azafalk or azahexal or azamedac or azamun or azamune or azanin or azapin or azapress or azaprine or azarex or azasan or azathiodura or azathiopine or azathioprim or azathioprin or azathiopurine or azathropsin or azatioprina or azatox or azatrilem or azopi or azoran or azothioprin or azothioprine or "bw 57 322" or "bw 57322" or "bw57‐322" or bw57322 or colinsan or immuran or immurel or immuthera or imunen or imuprin or imuran or imurane or imurek or imurel or imuren or methylnitroimidazolylmercaptopurine or "nsc 39084" or nsc39084 or thioazeprine or thioprine or transimune or zytrim or "446‐86‐6" or Cyclosporine or Ciclosporina or MH:D04.345.566.235.300$ or MH:D12.644.641.235.300$ or "adi 628" or adi628 or cequa or "cgc 1072" or cgc1072 or ciclomulsion or cicloral or ciclosporin or ciclosporine or cipol or consupren or CsANeoral or cyclasol or cyclokat or cyclosporin or "cyclosporine a" or "CyA NOF" or "de 076" or de076 or deximune or equoral or gengraf or ikervis or iminoral or implanta or imusporin or "lx 201" or lx201 or "mc2 03" or mc203 or "mtd 202" or mtd202 or neoral or "neuro‐stat" or neurostat or "nm 0133" or "nm 133" or nm0133 or nm133 or "nova 22007" or nova22007 or "ol 27400" or ol27400 or "ol 27 400" or "olo 400" or olo500 or "opph 088" or opph088 or opsisporin or "otx 101" or otx101 or "p 3072" or p3072 or padciclo or papilock or pulminiq or restasis or restaysis or sanciclo or sandimmun or sandimmune or sandimun or sandimune or "sang 35" or sang35 or sangcya or "sp 14019" or sp14019 or "sti 0529" or sti0529 or "t 1580" or t1580 or vekacia or verkazia or "59865‐13‐3" or "63798‐73‐2" or "79217‐60‐0" or Tacrolimus or Tacrolimo or MH:D02.540.505.810$ or advagraf or astagraf or envarsus or "fk 506" or fk506 or "fr 900506" or fr900506 or fujimycin or hecoria or modigraf or "mustopic oint" or prograf or prograft or protopic or protopy or tacforius or tsukubaenolide or "104987‐11‐3")
Appendix 6. ClinicalTrials.gov search strategy
(uveitis OR iritis OR iridocyclitis OR cyclitis OR “pars planitis” OR hyalitis OR choroiditis OR chorioretinitis OR retinochoroiditis OR retinitis OR neuroretinitis OR panuveitis) AND (methotrexate OR mycophenolate OR cyclosporine OR tacrolimus OR azathioprine)
Appendix 7. WHO ICTRP search strategy
uveitis AND methotrexate OR uveitis AND mycophenolate OR uveitis AND cyclosporine OR uveitis AND tacrolimus OR uveitis AND azathioprine OR iritis AND methotrexate OR iritis AND mycophenolate OR iritis AND cyclosporine OR iritis AND tacrolimus OR iritis AND azathioprine OR iridocyclitis AND methotrexate OR iridocyclitis AND mycophenolate OR iridocyclitis AND cyclosporine OR iridocyclitis AND tacrolimus OR iridocyclitis AND azathioprine OR cyclitis AND methotrexate OR cyclitis AND mycophenolate OR cyclitis AND cyclosporine OR cyclitis AND tacrolimus OR cyclitis AND azathioprine OR “pars planitis” AND methotrexate OR “pars planitis” AND mycophenolate OR “pars planitis” AND cyclosporine OR “pars planitis” AND tacrolimus OR “pars planitis” AND azathioprine OR hyalitis AND methotrexate OR hyalitis AND mycophenolate OR hyalitis AND cyclosporine OR hyalitis AND tacrolimus OR hyalitis AND azathioprine
choroiditis AND methotrexate OR choroiditis AND mycophenolate OR choroiditis AND cyclosporine OR choroiditis AND tacrolimus OR choroiditis AND azathioprine OR chorioretinitis AND methotrexate OR chorioretinitis AND mycophenolate OR chorioretinitis AND cyclosporine OR chorioretinitis AND tacrolimus OR chorioretinitis AND azathioprine OR retinochoroiditis AND methotrexate OR retinochoroiditis AND mycophenolate OR retinochoroiditis AND cyclosporine OR retinochoroiditis AND tacrolimus OR retinochoroiditis AND azathioprine OR retinitis AND methotrexate OR retinitis AND mycophenolate OR retinitis AND cyclosporine OR retinitis AND tacrolimus OR retinitis AND azathioprine OR neuroretinitis AND methotrexate OR neuroretinitis AND mycophenolate OR neuroretinitis AND cyclosporine OR neuroretinitis AND tacrolimus OR neuroretinitis AND azathioprine OR panuveitis AND methotrexate OR panuveitis AND mycophenolate OR panuveitis AND cyclosporine OR panuveitis AND tacrolimus OR panuveitis AND azathioprine
Appendix 8. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design | · Parallel group RCTi.e. people randomized to treatment · Within‐person RCTi.e. eyes randomized to treatment · Cluster‐RCTi.e. communities randomized to treatment · Cross‐over RCT · Other, specify |
Exclusions after randomization Losses to follow‐up Number randomized/analyzed How were missing data handled? e.g. available case analysis, imputation methods Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomization/unit of analysis |
· One eye included in study, specify how eye selected · Two eyes included in study, both eyes received same treatment, briefly specify how analyzed (best/worst/average/both and adjusted for within person correlation/both and not adjusted for within person correlation) and specify if mixture one eye and two eye · Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done. |
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
· Number of people randomized to this group · Drug (or intervention) name · Dose · Frequency · Route of administration |
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
Data and analyses
Comparison 1. DMARDs plus steroid versus steroid monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Control of inflammation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Control of inflammation: high‐dose cyclosporine A | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Change in BCVA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Right eyes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.2 Left eyes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Change in CMT | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Right eyes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4.2 Left eyes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.5 Proportion of participants experiencing complications or requiring cessation of medication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.

Comparison 1: DMARDs plus steroid versus steroid monotherapy, Outcome 1: Control of inflammation
Comparison 2. Methotrexate versus mycophenolate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Control of inflammation | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.01, 1.50] |
| 2.2 Control of inflammation: subset (VKH patients only) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2.1 VKH patients | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Change in BCVA | 2 | 490 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.04, 0.05] |
| 2.4 Proportion of participants with confirmed (residual) macular edema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5 Change in central macular thickness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5.1 Change in central macular thickness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.6 Proportion of participants achieving steroid reduction | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.01, 1.50] |
| 2.6.1 Steroid reduction to ≤ 7.5 mg/day oral prednisone | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.93, 1.46] |
| 2.6.2 Steroid reduction to ≤ 10 mg/day oral prednisone | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.95, 2.25] |
| 2.7 Change in vision‐related quality of life scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.7.1 IND‐VFQ | 2 | Mean Difference (IV, Fixed, 95% CI) | 2.27 [‐2.25, 6.79] | |
| 2.7.2 NEI‐VFQ | 1 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐1.39, 5.79] | |
| 2.8 Change in general health‐related quality of life scores | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.8.1 SF‐36, Physical component summary | 2 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐0.48, 2.85] | |
| 2.8.2 SF‐36, Mental component summary | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐2.24, 2.31] | |
| 2.9 Proportion of participants experiencing any adverse event | 2 | 296 | Risk Ratio (IV, Fixed, 95% CI) | 1.02 [0.99, 1.05] |
| 2.10 Proportion of participants experiencing complications or requiring cessation of medication | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.43, 2.27] |
| 2.11 Proportion of participants experiencing complications or requiring cessation of medication: subset (VKH patients only) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.12 Proportion of participants experiencing systemic complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.12.1 Elevated AST or ALT (nonserious) | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.86, 3.78] |
| 2.12.2 Elevated AST or ALT (serious) | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.26, 8.96] |
| 2.12.3 Abnormal Hb | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 1.97] |
| 2.12.4 Low leukocyte count | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.32, 28.92] |
| 2.12.5 Elevated creatinine | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.13, 74.18] |
| 2.13 Proportion of participants experiencing ocular complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.13.1 Increased IOP | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.50, 1.64] |
| 2.13.2 Cataract | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.44] |
| 2.13.3 Hypotony | 2 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [0.47, 35.72] |
| 2.14 Proportion of participants experiencing ocular complications: subset (VKH patients only) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.14.1 Increased IOP ‐ VKH | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.68 [0.39, 113.36] |
| 2.14.2 Cataract ‐ VKH | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.46 [0.31, 95.31] |
| 2.14.3 Hypotony ‐ VKH | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.17, 70.14] |
2.10. Analysis.

Comparison 2: Methotrexate versus mycophenolate, Outcome 10: Proportion of participants experiencing complications or requiring cessation of medication
Comparison 3. Steroids with or without azathioprine versus cyclosporine A.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Control of inflammation | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.02] |
| 3.1.1 IV pulse methylprednisolone for 3 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.05] |
| 3.1.2 Azathioprine 2 to 3 mg/kg/day | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 3.2 Control of inflammation: high‐dose cyclosporine A | 2 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.72, 2.01] |
| 3.3 Change in BCVA | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| 3.3.1 IV pulse methylprednisolone for 3 days | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 3.3.2 Azathioprine 2 to 3 mg/kg/day | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.35, 0.13] |
| 3.4 Proportion of participants achieving improvement in visual acuity | 2 | 64 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.50, 1.69] |
| 3.4.1 ETDRS chart | 1 | 56 | Risk Ratio (IV, Fixed, 95% CI) | 0.92 [0.49, 1.72] |
| 3.4.2 Snellen table | 1 | 8 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.09, 11.03] |
| 3.5 Proportion of participants with macular edema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Proportion of participants achieving steroid reduction | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7 Proportion of participants with any adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.8 Proportion of participants experiencing complications or requiring cessation of medication | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.21, 3.45] |
| 3.8.1 IV pulse methylprednisolone for 3 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.52] |
| 3.8.2 Azathioprine 2 to 3 mg/kg/day | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.38 [0.31, 92.73] |
| 3.9 Proportion of participants experiencing systemic complications | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.06] |
| 3.9.1 IV pulse methylprednisolone for 3 days | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.15, 1.00] |
| 3.9.2 Azathioprine 2 to 3 mg/kg/day | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.84] |
| 3.10 Proportion of participants experiencing ocular complications | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.10.1 Increased IOP | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.61] |
| 3.10.2 Cataract | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.90, 2.81] |
| 3.11 Proportion of participants experiencing ocular complications: high‐dose cyclosporine A | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11.1 Cataract | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 1: Control of inflammation
3.8. Analysis.

Comparison 3: Steroids with or without azathioprine versus cyclosporine A, Outcome 8: Proportion of participants experiencing complications or requiring cessation of medication
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cuchacovich 2010.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): 44 total, 23 not randomized were treated with extended prednisone alone. Total randomized 21 participants; 12 participants in AZA + prednisone group and 9 in CsA + prednisone group Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): not reported (NR) Number analyzed (total and per group): 21 total; 12 steroid + AZA group; 9 steroid + CsA group Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): eye (worst eye for change in visual acuity, inflammatory score); individual (for adverse outcomes, reduction in steroid dose, mean time to reach control of inflammation) Length of follow‐up (planned; actual): 52 weeks; 54 weeks How were missing data handled? (e.g. available case analysis, imputation methods): NR Reported power calculation (Y/N), if yes, sample size and power: N |
|
| Participants |
Baseline characteristics Intervention: AZA + prednisone (n = 12)
Comparison: CsA + prednisone (n = 9)
Overall (N = 21):
Inclusion criteria: diagnosed VKH (criteria recommended by the American Uveitis Society in 1978 and later 2001 VKH Workshop Revised Criteria) and at least three of the following: bilateral chronic iridocyclitis, posterior uveitis, neurologic signs, and cutaneous findings. Exclusion criteria: immunodeficiency; blood disorder; abnormal renal or liver function; history of eye surgery or trauma. Pretreatment differences: no statistically significant baseline differences per authors. The CsA group is younger, includes pediatrics, and has only female participants. Other notes: some children included in study (age of cyclosporine group includes participants 5 to 52 years old) |
|
| Interventions |
Intervention: azathioprine in combination with oral steroid therapy
Comparator intervention: cyclosporine in combination with oral steroid therapy
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y, systemic and ocular adverse events Measurement time points (specify intervals at which outcomes were assessed): "Clinical variables were assessed at baseline and at 4 weeks interval for at least 1 year" Other issues with outcome assessment (e.g. quality control for outcomes, if any): an analysis of covariance (ANCOVA) using baseline visual acuity and ocular inflammation as covariates for clinical outcomes: "visual acuity, ocular inflammation, and corticosteroid daily dose at remission and at 6 and 12 months" |
|
| Notes | Sponsorship source: NR Country: Chile Setting: Los Andes Ophthalmologic Foundation ‐ "tertiary national center for diagnosis and management of ocular inflammatory diseases" Comments: "The study was approved by the Ethical Committee of each study site, and all patients gave their informed consent." Author's name: Miguel Cuchacovich, MD Institution: Clinical Hospital University of Chile Conflicts of interest (verbatim report): "The authors report no conflicts of interest." Publication language: English Study period: 1998 to 2005 Trial registration: NR | |
Deuter 2018.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): 44 total, 23 in enteric‐coated mycophenolate sodium + low‐dose corticosteroid (prednisolone) group ("treatment group"), 21 in control group (prednisolone monotherapy) ("control group") Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): exclusions: 3 excluded after randomization due to insufficient data collection (1 treatment group, 2 control group), 5 left the study due to other reasons (withdrew consent or lost to follow‐up), 13 were discontinued by reaching an endpoint; lost to follow‐up: 1 treatment group, 2 control group; withdrawal of informed consent: 2 treatment group, 0 control group; 18 (43.9%) did not complete study to follow‐up month 15 Number analyzed (total and per group): total: 41, treatment group: 22; control group: 19 Unit of analysis (individual or eye): individual for relapse; eye (change from baseline and stratified by laterality) for best corrected visual acuity (BCVA), central foveal thickness (CFT), number of quadrants with vessel leakage, vitreous haze score, and anterior chamber (AC) cell score Length of follow‐up (planned; actual): planned 15 months; actual 15 months How were missing data handled? (e.g. available case analysis, imputation methods): "Intent‐to‐treat population and safety population were defined as all subjects who received at least one dose of prednisolone or EC‐MPS and did have at least one post‐baseline assessment or at least one post‐therapy safety assessment, respectively." Missing values after the first relapse were continued using the last observation carried forward (LOCF) method. [Cross‐over from control to treatment after the first relapse.] Reported power calculation (Y/N), if yes, sample size and power: Y; for the sample size determination, we assumed the rate of relapse‐free participants after 15 months as 0.40 for the control group and 0.65 for the treatment group. A two‐sided log‐rank test with an overall sample size of 144 participants (72 per group) and a dropout rate of 15% achieves 80% power at a 0.05 significance level to detect this difference. |
|
| Participants |
Baseline characteristics Intervention: mycophenolate mofetil in combination with topical or oral steroid therapy (n = 22)
Comparison: standard of care (e.g. topical steroids, with or without systemic steroids) (n = 19)
Overall (N = 41):
Inclusion criteria: age 18 and older, non‐infectious uveitis (unilateral or bilateral) due to systemic disease (sarcoidosis, multiple sclerosis); uveitis (grade ≥ 2+ vitreous haze, ≥ 2+ anterior chamber cells, presence of CME in OCT, retinal vessel leakage in fluorescein angiogram); requires systemic treatment based on investigator's judgment; not planning to undergo elective ocular surgery. Exclusion criteria: infectious uveitis, primary diagnosis of anterior or posterior uveitis, history of infections (herpes zoster, varicella, HIV), uncontrolled glaucoma, malignancy; previously treated with systemic immunosuppressants (biologics or study drugs), periocular steroids within six weeks; laboratory abnormalities; pregnant or lactating, history of substance abuse, and psychiatric disorders. Pretreatment differences: control group was predominately female; the treatment group was more evenly split. Criteria for discontinuation from study: ocular disease did not show an improvement under prednisolone after 1 month; a relapse occurred in any eye of a participant who had been primarily randomized to the treatment group; a relapse occurred more than 6 months after day 0 in any eye of a participant who had been primarily randomized to the control group; a further relapse occurred in any eye of a participant after a change from the control group to the cross‐over group. |
|
| Interventions |
Intervention: mycophenolate mofetil in combination with topical or oral steroid therapy
Comparator intervention: standard of care
|
|
| Outcomes |
Primary outcome:
Secondary outcomes
Adverse outcomes (Y/N), if yes, please describe: Y, recorded in incidence per patient year: serious adverse event; adverse event (all); infections; headache; alopecia; laboratory abnormalities; gastrointestinal problems; discontinuation of enteric‐coated mycophenolate due to adverse events Measurement time points (specify intervals at which outcomes were assessed):
Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes | Sponsorship source: "This work was supported by a research grant from Novartis Pharma, Nuernberg. Novartis Pharma also supplied the investigational drug (Myfortic) free of charge. Novartis Pharma had no role in the design or conduct of this research." Country: Germany Setting: multicentre; phase III, open‐label, prospective, controlled, randomized national trial Comments: allowed for cross‐over from the control group who developed the first relapse within 6 months after randomization Author's name: Dr Christoph ME Deuter Institution: Centre for Ophthalmology, University of Tuebingen Conflicts of interest (verbatim report): "Competing interests None declared." Publication language: English Study period: March 2010 to October 2015 Trial registration: ClinicalTrials.gov Identifier: NCT01092533; Protocol MYCUV‐IIT02; EUDRA CT number: 2009‐009998‐10 | |
De Vries 1990.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): total: 27, cyclosporin: 14, placebo: 13 Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): total: 8 (2 withdrawn, 6 dropouts). Cyclosporin: 4 (1 withdrawn, 3 dropouts). Placebo: 4 (1 withdrawn, 3 dropouts) Number analyzed (total and per group): total: 27, cyclosporin: 14, placebo: 13 Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual Length of follow‐up (planned; actual): one year; premature termination was allowed (treatment failure or dropout) How were missing data handled? (e.g. available case analysis, imputation methods): missing individuals were censored in survival analysis and thus were excluded from the Kaplan‐Meier curve. Reported power calculation (Y/N), if yes, sample size and power: N |
|
| Participants |
Baseline characteristics Intervention: cyclosporine in combination with oral steroid therapy (n = 14)
Comparator: placebo in combination with oral steroid therapy (n = 13)
Overall:
Inclusion criteria: active idiopathic posterior uveitis, panuveitis or intermediate uveitis; insufficient response to systemic corticosteroids; best‐corrected visual acuity of 0.5 or less in their best eye (does not apply to Behçet's disease or sympathetic ophthalmia). Exclusion criteria: under 18 years of age, infectious uveitis, irreversible retinal damage, lens or cornea opacities preventing assessment, intraocular surgery, uncontrolled infection, history of malignancy, contraindications to study drugs, abnormal kidney or liver function, hypertension, pregnancy, malabsorption syndrome, substance abuse. Pretreatment differences: "None of the differences between the groups was statistically significant at the 5% level." Only one participant had visual acuity of more than 0.5 at entry, diagnosed with Behçet’s disease. They were in the cyclosporin group. Other notes: two participants had previously received cytostatic agents. Groups not reported. *Proportion of 'diagnosis' and 'uveitis location' are reported combined as "Diagnostic subgroups" in study. |
|
| Interventions |
Intervention: cyclosporine in combination with oral steroid therapy:
Comparator: placebo in combination with oral steroid therapy:
|
|
| Outcomes |
Primary outcome
Secondary outcomes
Adverse outcomes (Y/N), if yes, please describe: Y
Measurement time points (specify intervals at which outcomes were assessed): baseline, weeks 1, 2, and 4, and at 1‐month intervals thereafter for up to one year Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
|
| Notes | Sponsorship source: NR Country: the Netherlands Setting: four ophthalmology departments Comments: none Author's name: J de Vries, MD Institution: Eye Hospital Department of Ophthalmology, Erasmus University Rotterdam Conflicts of interest (verbatim report): NR Publication language: English Study period: NR Trial registration: NR | |
Lee 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled non‐inferiority trial Study grouping: parallel group Numbers randomized (total and per group): total randomized (successfully tapered to prednisone 10 mg/day): 35; tacrolimus monotherapy: 16; tacrolimus and prednisolone dual therapy: 19 Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): enrolled (not randomized): 58, withdrawn pre‐randomization: 23 total; treatment inefficacy (n = 14); hand tremor (n = 2); hypertension (n = 1); headache and facial swelling (n = 1); diarrhea (n = 1); unable to comply with visit schedule (n = 2); revised diagnosis (n = 2). Total lost to follow‐up: 0/35, tacrolimus monotherapy lost to follow‐up: 0/19; tacrolimus and prednisolone lost to follow‐up: 0/16 Number analyzed (total and per group): total: 35, tacrolimus monotherapy: 16, tacrolimus and prednisolone: 19 Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): logarithm of the minimum angle of resolution (LogMAR) visual acuity (VA) unit of analysis: "All analyses were carried out separately for study eyes and fellow eyes. In cases of bilateral disease, the study eye was designated as the eye with the worst BIO score on enrollment. If both eyes had the same binocular indirect ophthalmoscopy (BIO) score, the eye with the worst VA was designated. If both these attributes were the same, the right eye was designated." Individual was unit of analysis for other outcomes. Length of follow‐up (planned; actual): 9 months How were missing data handled? (e.g. available case analysis, imputation methods): no missing data. LogMAR VA per protocol analysis uses last observation carried forward (LOCF) Reported power calculation (Y/N), if yes, sample size and power: Y, 80% power with inferiority threshold of 0.1 (mean change in monotherapy logMAR VA ‐ mean change in dual therapy log MAR VA < 0.1), to obtain 80% with a 1‐sided alpha equal to 0.05, 14 participants were needed per study arm to establish non‐inferiority of tacrolimus monotherapy versus dual therapy. |
|
| Participants |
Baseline characteristics Intervention: tacrolimus monotherapy +/‐ in combination topical steroid therapy (n = 16)
Comparator intervention: tacrolimus dual therapy in combination with oral steroid therapy +/‐ topical steroid therapy (n = 19)
Overall (N = 35):
Inclusion criteria: sight‐threatening non‐infectious posterior segment intraocular inflammation; 18 years of age or older; plus 1 or more of the following: disease reactivation after prednisone taper to < 10 mg daily; recurrent corticosteroid rescue for relapsing disease; immediately sight‐threatening features warranting systemic corticosteroid treatment combined with a second‐line immunosuppressive agent. Exclusion criteria: contraindication to study drugs; use of cyclosporin within three months; abnormal labwork; diabetes mellitus; pregnant or breastfeeding; history of infections (tuberculosis, HIV); history of substance abuse. Other notes: concomitant medications based on a history of peptic ulcers and bone density scan |
|
| Interventions | All trial recruits started tacrolimus either before, or at the time of, enrollment in conjunction with 10 mg or more prednisone daily. Participants whose disease was inactive for 4 weeks while taking 10 mg prednisone daily in the presence of target tacrolimus levels (trough serum level of 8 ng to 12 ng/mL) were allocated randomly to: Intervention: tacrolimus and prednisone tapered rapidly and discontinued over 2 weeks, or Comparator: tacrolimus and oral prednisone (10 mg/day for 3 months then tapering to a minimum of 7.5 mg/day). | |
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y,
Measurement time points (specify intervals at which outcomes were assessed): after randomization, participants allocated to receive tacrolimus monotherapy were reviewed the day before they stopped prednisone to ensure that there had been no disease reactivation upon reducing their prednisone dose to 5 mg daily, and again 2 weeks later to check that clinically defined remission was maintained after prednisone cessation. Participants then were seen 4 weeks later, with subsequent reviews at 4‐ to 8‐week intervals until study completion 9 months after randomization. Participants allocated to receive dual tacrolimus and prednisone therapy were reviewed at 4‐ to 8‐week intervals throughout their participation in the study. Participants in either treatment group could request early review at any time. Other issues with outcome assessment (e.g. quality control for outcomes, if any): NR |
|
| Notes | Sponsorship source: "Supported by Astellas Pharma Ltd, Staines, United Kingdom (R.W.J.L., A.D.D.); and the National Eye Research Center, Bristol, UK (grant no.: RJ4750 [R.W.J.L., and A.D.D.])." Country: UK Setting: Bristol Eye Hospital, Bristol, UK, and Aberdeen Royal Infirmary, Aberdeen, UK Author's name: Richard W.J. Lee Institution: Department of Clinical Sciences, University of Bristol, Bristol, United Kingdom Conflicts of interest: "The author(s) have made the following disclosure(s): Richard W. J. Lee ‐ Financial support ‐ Astellas Pharma Ltd. Andrew D. Dick ‐ Financial support ‐ Astellas Pharma Ltd." Publication language: English Study period: enrollment started May 2004 and ended December 2008 Trial registration: ISRCTN46576063 | |
Murphy 2005.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): total: 37, tacrolimus: 19, cyclosporine: 18 Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): none (in terms of study main outcomes) Number analyzed (total and per group): total: 37, tacrolimus: 19, cyclosporine: 18 Unit of analysis (individual or eye. Please report what was used for the analysis if both eyes were included; e.g. the worse eye): individual level Length of follow‐up (planned; actual): 3 months; 3 months for study main outcomes; 12 months, 12 months for QoL How were missing data handled? (e.g. available case analysis, imputation methods): NR Reported power calculation (Y/N), if yes, sample size and power: Y, "To detect a difference in efficacy between cyclosporine and tacrolimus, we estimated that 72 patients would need to be recruited to each treatment group. This estimate was performed using a χ2 test with a 2‐sided significance level of.05 and 80% power." |
|
| Participants |
Baseline characteristics Intervention: cyclosporine in combination with oral steroid therapy (n = 18)
Comparator intervention: tacrolimus in combination with oral steroid therapy (n = 19)
Overall:
Inclusion criteria: chronic, noninfectious, sight‐threatening posterior segment intraocular inflammation, defined as: high doses of prednisolone (> 10 mg/d); recurrent high‐dose steroid rescue for recurrent relapsing disease (> 2 relapses per year despite maintenance prednisolone of > 10 mg/d); severe sight‐threatening disease that warranted immediate institution of high‐dose prednisolone and a second‐line agent. Exclusion criteria: pregnant; diabetes mellitus; renal disease; current infection; recent live vaccination. Pretreatment differences
|
|
| Interventions |
Intervention: cyclosporine in combination with oral steroid therapy
Comparator intervention: tacrolimus in combination with oral steroid therapy
|
|
| Outcomes |
Primary outcomes
Secondary outcomes
Adverse outcomes (Y/N), if yes, please describe: Y,
Measurement time points (specify intervals at which outcomes were assessed):
Other issues with outcome assessment (e.g. quality control for outcomes, if any): NR |
|
| Notes | Sponsorship source: "Funding/Support: Dr Murphy was funded by Fujisawa UK (London, England) and The National Eye Research Centre (Bristol, England), and Dr Greiner was funded by The Grampian University Hospital Trust for Research and Development (Aberdeen, Scotland)." Country: United Kingdom Setting: 2 regional referral centers Comments: ethics: "All patients who were invited to enroll in the study during this period agreed to participate. The study was approved by the ethics committee of each center, and informed consent was obtained from all patients." Author's name: Conor C. Murphy Institution: University of Bristol, Bristol Eye Hospital Conflicts of interest: "Financial Disclosure: None." Publication language: English Study period: May 2001 to April 2003 Trial registration: NR | |
Nussenblatt 1991.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: cross‐over Numbers randomized (total and per group): total: 56; cyclosporine group: 28; prednisolone group: 28 Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): not reported Number analyzed (total and per group): total: 56, cyclosporine group: 28, prednisolone group: 28 Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual and eye (bilateral disease) Length of follow‐up (planned; actual): planned: 3 months (parallel); 6 months; 9 months; 12 months (cross‐over) How were missing data handled? (e.g. available‐case analysis, imputation methods): available‐case analysis Reported power calculation (Y/N), if yes, sample size and power: N |
|
| Participants |
Baseline characteristics Intervention: cyclosporine A (n = 28)
Comparator Intervention: prednisolone (n = 28)
Overall (N = 56):
Inclusion criteria: 10 years of age or older, and were capable of understanding the goals of the project; active bilateral disease with a visual acuity of 20/40 or worse in both eyes because of intraocular inflammation (active inflammation was defined as a recent [within four months] decrease of two or more lines of visual acuity or active retinal lesions or vitreal haze); and no underlying chronic infectious disorder, major organ dysfunction, or history of neoplasia. Exclusion criteria: anticipated ocular surgery within the first six months of the study; treatment for ocular disease with systemic corticosteroids or cytotoxic agents for at least one month before entering the study; diabetes; hypertension; pregnancy Other notes: None |
|
| Interventions |
Intervention: cyclosporine A
Intervention: prednisolone
|
|
| Outcomes |
Primary outcome Treatment success at three months:
Secondary outcomes
Adverse outcomes (Y/N), if yes, please describe: Y, systemic side effects Measurement time points (specify intervals at which outcomes were assessed): monthly intervals for first 3‐month period, then every 3 months for 1 year Other issues with outcome assessment (e.g. quality control for outcomes, if any): outcome data at month 3 considered and compared because, after this period, the random allocation of treatments was breached and was based on clinical responses. |
|
| Notes | Sponsorship source: NR Country: USA Setting: Clinical Center at the National Institutes of Health Comments: there is cross‐over design to this study after 3‐month follow‐up. Author's name: Robert B. Nussenblatt Institution: Laboratory of Immunology, National Eye Institute, National Institutes of Health Conflicts of interest: NR Publication language: English Study period: NR Trial registration: NR | |
Nussenblatt 1993.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group (4‐arm) Numbers randomized (total and per group): 32; 16 to 4 doses each of cyclosporine A (2.5 mg/kg body weight, 5.0 mg/kg body weight, 7.5 mg/kg body weight, or 10.0 mg/kg body weight) 16 to 4 doses each of cyclosporine G (2.5 mg/kg body weight, 5.0 mg/kg body weight, 7.5 mg/kg body weight, or 10.0 mg/kg body weight) Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): one participant from each therapeutic group (CsA and CsG) stopped because of side effects Number analyzed (total and per group): 32 total, 16 in cyclosporine A group, 16 in cyclosporine G group Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual Length of follow‐up (planned; actual): 4 months of masked follow‐up, with weekly follow‐ups for the first month, then at least monthly How were missing data handled? (e.g. available case analysis, imputation methods): NR Reported power calculation (Y/N), if yes, sample size and power: N |
|
| Participants |
Baseline characteristics Intervention: cyclosporine A in combination with oral steroid therapy (n = 16)
Comparator intervention: cyclosporine G in combination with oral steroid therapy (n = 16)
Overall (N = 32):
Inclusion criteria: 18 years or older; active bilateral intraocular inflammation not deemed to be infectious in origin; and visual acuity of less than 20/40 in both eyes. Exclusion criteria: chronic disease (cardiovascular, hypertension, HIV, hepatitis); pregnant; glomerular filtration rate < 90 mL/min; expectation of intraocular surgery within the first four months of the study. Group differences: NR Other notes: None *uveitis location and diagnosis are reported together in the study |
|
| Interventions |
Intervention: cyclosporine A in combination with oral steroid therapy
Intervention: cyclosporine G in combination with oral steroid therapy
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y,
Measurement time points (specify intervals at which outcomes were assessed):
Other issues with outcome assessment (e.g. quality control for outcomes, if any): None |
|
| Notes | Sponsorship source: NR Country: USA Setting: Clinical Center of the National Institutes of Health Comments: None Author's name: Robert B. Nussenblatt Institution: Laboratory of Immunology, National Eye Institute Conflicts of interest: NR Publication language: English Study period: NR Trial registration: NR | |
Ono 2021.
| Study characteristics | ||
| Methods |
Study design: randomized controlled non‐inferiority trial Study grouping: parallel group Numbers randomized (total and per group): total 72: 36 in cyclosporine and oral prednisolone (combination) group and 36 in corticosteroid pulse therapy (corticosteroid) group Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): 2 losses to follow‐up in the combination group Number analyzed (total and per group): 70 analyzed: 34 in the combination group and 36 in pulse group Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual and eye, no description on eye selection Length of follow‐up (planned; actual): 12 months; 12 months. How were missing data handled? (e.g. available case analysis, imputation methods): missing values were treated as missing Reported power calculation (Y/N), if yes, sample size and power: Y, 45 participants per group, power, 80% alpha, 0.05 (one‐sided) |
|
| Participants |
Baseline characteristics Intervention: cyclosporin A combination with prednisolone (n = 34)
Comparator intervention: corticosteroid pulse therapy (n = 36)
Overall (N = 70):
Inclusion criteria: at least 20 years of age, people who presented with both ocular and systemic inflammation, VKH disease within 2 months after the onset. Exclusion criteria: abnormal complete blood cell counts; abnormal hepatic or renal functions; systemic infectious diseases such as active tuberculosis, hepatitis B, hepatitis C, or syphilis; current treatment with conventional systemic immunosuppressive agents such as cyclosporine, azathioprine, methotrexate, and others, or prior administration of periocular or intravitreal injections of corticosteroids within the past 90 days. Pretreatment differences: None Other notes: None |
|
| Interventions |
Intervention: cyclosporin A combined with prednisolone
Comparator: corticosteroid pulse therapy
In case of need, prednisolone dose and treatment duration were allowed a ± 20% and ± 1 week adjustment from the standard protocol, respectively. Topical corticosteroid eye drops could be used between 2 and 6 times daily depending on the intensity of inflammatory activity in the anterior chamber during the study period. |
|
| Outcomes |
Primary outcome: Incidence of a composite of recurrence (serous retinal detachment by OCT; recurrence of systemic VKH symptoms) or worsening (two‐step increase in AC cells and vitreous haze, or an increase from grade 3+ to 4+ according to SUN criteria) Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y,
Measurement time points (specify intervals at which outcomes were assessed): outcomes assessed at baseline before initiation of therapy, outpatient week 2, and 1, 3, 6, 9, and 12 months. Other issues with outcome assessment (e.g. quality control for outcomes, if any): NR |
|
| Notes | Sponsorship source: NR Country: Japan Setting: 11 tertiary eye centers Comments: None Author's name: Takashi Ono Institution: Miyata Eye Hospital Conflicts of interest (verbatim report): "T. Ono, Lecture fee (Bayer, Santen, AbbVie); H. Goto, None; T. Sakai, None; F. Nitta, None; N. Mizuki, None; H. Takase, Lecture fee (Santen, Novartis, AbbVie, Senju, Eisai, Otsuka); Y. Kaneko, None; J. Hori, None; S. Nakano, Grant (Novartis); N. Nao‐I, None; N. Ohguro, None; K. Miyata, Grant, Lecture fee (AMO, Alcon, Senju, Santen), Grant (Kissei, Chugai, Bayer, M's science, Novartis), Lecture fee (Otsuka, Kowa, HOYA, J‐TEC, JFC Sales Plan); M. Tomita, None; M. Mochizuki, None." Publication language: English Study period: July 2014 to March 2018 Trial registration: UMIN000014387 | |
Rathinam 2014.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): total: 80, methotrexate group: 41, mycophenolate mofetil group: 39 Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): total: 13 participants. Methotrexate group: 6 excluded (did not receive intervention, lost to follow‐up, or dropout); mycophenolate mofetil group: 7 excluded (did not receive intervention, lost to follow‐up, or dropout) Number analyzed (total and per group): Total 67 contributed to the primary outcome; 35 in methotrexate group, 32 in mycophenolate mofetil group Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual and eye Length of follow‐up (planned; actual): 6 months How were missing data handled? (e.g. available‐case analysis, imputation methods): available‐case analysis, confirmed by imputation method Reported power calculation (Y/N), if yes, sample size and power: Y, "a sample of 40 patients per arm was estimated to provide 80% power to detect a difference of approximately 30% in treatment success, assuming a dropout rate of 10% and a 2‐tailed α of 0.05" |
|
| Participants |
Baseline characteristics Intervention: methotrexate in combination with topical or oral steroid therapy (n = 41)
Intervention comparator: mycophenolate mofetil in combination with topical or oral steroid therapy (n = 39)
Overall (N = 80):
Inclusion criteria: 16 years of age or older; noninfectious intermediate, posterior or panuveitis which is active in the 60 days prior to enrollment, according to SUN criteria, in at least 1 eye; more than or equal to 15 mg oral prednisolone dose at enrollment; a history of a corticosteroid taper failure (i.e. inability to taper to prednisolone 10 mg or less) or an obvious chronic disease necessitating corticosteroid‐sparing immunosuppressive treatment (i.e. Behçet's disease, multifocal choroiditis and panuveitis, serpiginous choroidopathy or birdshot retinochoroidopathy) as per Standardization of Uveitis Nomenclature (SUN) Working Group guidelines. Participants with VKH and bullous retinal detachment were also considered for this category. Exclusion criteria: infectious uveitis; active infection (tuberculosis, hepatitis, syphilis); laboratory abnormalities (anemia); pregnant or breast‐feeding; chronic hypotony (intraocular pressure < 5 mm Hg for > 3 months); prior use of any immunosuppressive drug for the treatment of uveitis in the past 6 months or prior failed treatment with methotrexate or mycophenolate mofetil; periocular or intravitreal corticosteroid injection in the past 3 months or fluocinolone acetonide implant surgery in either eye in < 3 years; intraocular surgery in < 30 days, or any ocular surgery scheduled during the 6‐month study period; visual acuity of hand motions or worse in better eye. Pretreatment differences:
Other notes: "Other than corticosteroids, all patients were immunosuppressant naïve, except for 2 who had briefly received methotrexate >1 year before enrollment and had stopped for financial reasons." |
|
| Interventions |
Intervention: methotrexate in combination with topical or oral steroid therapy
Comparator: mycophenolate mofetil in combination with topical or oral steroid therapy
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y, "Serious and nonserious ocular and systemic adverse events were ascertained at each visit by the coordinator and ophthalmologist. Blood samples were drawn at each visit to monitor laboratory values (complete blood count, aspartate aminotransferase, alanine aminotransferase, and creatinine)" Measurement time points (specify intervals at which outcomes were assessed): participants completed study visits at baseline, 2 weeks, and every month for up to 6 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): "All personnel responsible for outcome measurements (i.e., ophthalmologists, visual acuity examiners, and optical coherence tomography operators) were masked. Patients and study coordinators were unmasked." |
|
| Notes | Sponsorship source: funding for this trial was provided by That Man May See Foundation at University of California, San Francisco (UCSF), and the South Asia Research Fund. "Dr. Acharya is currently supported by a National Eye Institute grant (no. U10 EY021125‐01). The UCSF Department of Ophthalmology is supported by the National Eye Institute (NIH‐NEI EY002162 ‐ Core grant for Vision Research) and by the Research to Prevent Blindness Unrestricted grant. The sponsor or funding organization had no role in the design or conduct of this research." Country: India Setting: multicenter, block‐randomized, observer‐masked comparative effectiveness trial at 2 Aravind Eye Hospital located in Madurai and Coimbatore, South India Comments: None Author's name: Nisha R. Acharya, MD, MS Institution: F.I. Proctor Foundation Conflicts of interest: NR Publication language: English Study period: October 2011 to June 2012 Trial registration: ClinicalTrials.gov: NCT01232920 | |
Rathinam 2019.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): 216 total, 107 methotrexate (206 uveitic eyes), 109 mycophenolate (201 uveitic eyes) Unit of randomization (individual or eye): individuals Exclusions and losses to follow‐up (total and per group): 6 months total losses to follow‐up ‐ 22 individuals (19 lost to follow‐up; 3 missed 6‐month visit) 6 months methotrexate losses to follow‐up ‐ 11 individuals (9 lost to follow‐up; 2 missed 6‐month visit) 6 months mycophenolate mofetil losses to follow‐up ‐ 11 individuals (10 lost to follow‐up; 1 missed 6‐month visit) Number analyzed (total and per group): 6 months total ‐ 194 individuals. 6 months methotrexate ‐ 96 individuals. 6 months mycophenolate mofetil ‐ 98 individuals Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual ‐ primary outcome; eye ‐ change in visual acuity and central subfield macular thickness Length of follow‐up (planned; actual): planned 6‐month and 12‐month follow‐up How were missing data handled? (e.g. available case analysis, imputation methods): all participants with a 6‐month visit or who were declared as having early treatment failure were analyzed according to their randomization group. Participants missing a primary outcome visit were not included in the primary analysis. As a sensitivity analysis, multiple imputation was used to infer missing primary outcome data. Reported power calculation (Y/N), if yes, sample size and power: Y, enrollment of 216 participants was estimated to provide 80% power to detect a clinically meaningful absolute difference of approximately 20% in treatment success between the methotrexate group (40%) and the mycophenolate mofetil group (60%), assuming 10% loss to follow‐up and a 2‐tailed α of 0.05. This risk difference in favor of mycophenolate mofetil was supported by retrospective studies. |
|
| Participants |
Baseline characteristics Intervention: methotrexate in combination with topical or oral steroid therapy (n = 107)
Comparator: mycophenolate mofetil in combination with topical or oral steroid therapy (n = 109)
Overall (N = 216):
Inclusion criteria: 16 years of age or older; historical non‐infectious intermediate, anterior and intermediate, posterior or panuveitis in at least one eye; active inflammation within the last 180 days in at least one eye) according to SUN criteria, at enrollment, and after prior high‐dose regional or systemic corticosteroid treatment, or a known chronic condition necessitating corticosteroid‐sparing immunosuppressive treatment; willingness to start corticosteroid treatment at 1 mg/kg or 60 mg a day of prednisone, whichever is less; willingness to limit alcohol consumption. Exclusion criteria: any infectious cause of uveitis; prior immunosuppressive therapy other than corticosteroids in the past 12 months; prior intolerability or safety issues with methotrexate or mycophenolate mofetil or treatment failure with these drugs; prior biologic therapy at any time; media opacity (such as cataract and/or corneal scar) and/or extensive posterior synechiae such that examination of the posterior segment is not possible in both eyes; chronic hypotony (IOP < 5 mmHg for > 3 months) in both eyes; periocular or intravitreal corticosteroid injection in the past 4 weeks or fluocinolone acetonide implant in either eye in < 3 years; intraocular surgery in < 30 days, or planning on getting surgery within the next 6 months; BCVA of hand motions or worse in better eye; history of cancer, systemic autoimmune disease, or active systemic infection; laboratory abnormalities such as anemia. Pretreatment differences: slightly more females in methotrexate group than mycophenolate group than mycophenolate group (70.1% vs 55.0%) |
|
| Interventions |
Intervention: methotrexate in combination with topical or oral steroid therapy
Comparator: mycophenolate mofetil in combination with topical steroid therapy
|
|
| Outcomes |
Primary outcome: Proportion with treatment success ‐ defined by the following:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y, adverse events:
Measurement time points (specify intervals at which outcomes were assessed): primary outcome ‐ 6 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): randomization broken after 6 months, so only the results reported at 6 months were pertinent |
|
| Notes | Sponsorship source: this study was supported by NEI cooperative agreement U10 EY021125 (to Dr Acharya, primary investigator). The University of California San Francisco pharmacy provided study drugs for all sites. The Department of Ophthalmology at UCSF is supported by an unrestricted grant from the Research to Prevent Blindness Foundation, a core grant (EY06190) from the NEI, and That Man May See Foundation. The National Institutes of Health (NIH) did not have any direct role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; and decision to submit the manuscript for publication. The data and safety monitoring committee, appointed by the NIH, reviewed and approved submitting this manuscript for publication. Countries: USA; Australia; India; Mexico; Saudi Arabia Setting: 9 referral eye care centers Comments: RCT for the first 6 months, followed by additional 6 months of observation under preferred treatment Author's name: Nisha R. Acharya, MD, MS Institution: F.I. Proctor Foundation, University of California, San Francisco Conflicts of interest: "Dr Rathinam reports receipt of grants from Aravind Eye Hospital during the conduct of the study. Dr Lim reports receipt of grants from the National Eye Institute (NEI) during the conduct of the study; personal fees fromAllergan, Novartis, and Novotech; and grants and personal fees from AbbVie and Bayer outside the submitted work. Dr Al‐Dhibi reports receipt of personal fees from AbbVie, Allergan, and Novartis(consultancies) outside the submitted work. Dr Porco reports receipt of grants from NEI during the conduct of the study. Dr Acharya reports receipt of grants from NEI during the conduct of the study andpersonal fees from Santen and AbbVie (consultancies for advisory board participation) outside the submitted work. Dr Suhler reports receipt of personal fees from AbbVie, Clearside, EyeGate, EyePoint, Gilead, Santen, and Eyevensys and grants from AbbVie, Aldeyra, Clearside, EyeGate, EyePoint, Genentech, and Gilead. Drs Gonzales, Doan, and Keenan report receipt of grant funding from the NEI. No other disclosures were reported." Publication language: English Study period: enrollment 22 August 2013 to 16 August 2017; follow‐up ended 20 August 2018 Trial registration: ClinicalTrials.gov Identifier: NCT01829295 | |
Wiederholt 1986.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Study grouping: parallel group Numbers randomized (total and per group): 8 total, 4 to cyclosporine A, and 4 to systemic prednisone Unit of randomization (individual or eye): individual Exclusions and losses to follow‐up (total and per group): none Number analyzed (total and per group): total: 8, cyclosporine A: 4, prednisolone: 4 Unit of analysis (individual or eye): please report what was used for the analysis if both eyes were included (e.g. the worse eye): individual Length of follow‐up (planned; actual): 12 months How were missing data handled? (e.g. available case analysis, imputation methods): NR Reported power calculation (Y/N), if yes, sample size and power: N |
|
| Participants |
Baseline characteristics Intervention: cyclosporine in combination with topical steroid therapy (n = 4)
Comparator: standard of care (e.g. topical steroids, with or without systemic steroids) (n = 4)
Overall
Inclusion criteria: unilateral or bilateral active, non‐infectious posterior uveitis; visual acuity of 0.5 or worse. Exclusion criteria: contraindications to high‐dose oral corticosteroid therapy (diabetes mellitus); contraindications to immunosuppression (infectious diseases, malignancies); infectious uveitis; creatinine > 130 pmol/L; impaired liver function; simultaneous therapy with nephrotoxic antibiotics; substance abuse. Pretreatment differences: the average age of the cyclosporin group was 48.75 years (range: 33 to 73) vs prednisone 32.75 years (range: 18 to 47). Other notes: None |
|
| Interventions |
Intervention: cyclosporine in combination with topical steroid therapy
Comparator: standard of care (e.g. topical steroids, with or without systemic steroids)
|
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Adverse outcomes (Y/N), if yes, please describe: Y,
Measurement time points (specify intervals at which outcomes were assessed): baseline, 1 and 2 weeks, 1 month, 2 months, 3 months, 6 months, 9 months, 12 months (not reported, based on common visits shown in figures) Other issues with outcome assessment (e.g. quality control for outcomes, if any): NR |
|
| Notes | Sponsorship source: NR (Cyclosporin supplied by Sandoz AG, Basel) Country: Germany Setting: university hospital inpatient and outpatient Comments: None Author's name: Wiederholt, M Institution: Freie Universität Berlin Conflicts of interest: NR Publication language: German (English translation via Google translation) Study period: February to December 1984 Trial registration: NR | |
AE: adverse event ALT: alanine aminotransferase AST: aspartate aminotransferase AZA: azathioprine BCVA: best‐corrected visual acuity BIO: binocular indirect ophthalmoscopy CBC: complete blood count CsA: cyclosporine A CsG: cyclosporine G CME: cystoid macular edema DMARD: (non‐biologic) disease‐modifying antirheumatic drug EC‐MPS: enteric‐coated mycophenolate sodium ETDRS: Early Treatment Diabetic Retinopathy Study FA: fluorescein angiography IV: intravenous IQR: interquartile range LOCF: last observation carried forward LogMAR: logarithm of the minimum angle of resolution NEI: National Eye Institute NIIPPU: non‐infectious intermediate, posterior, and panuveitis NR: not reported OCT: optical coherence tomography QoL: quality of life SD: standard deviation SF‐36: 36‐item short form health survey SUN: Standardization of Uveitis Nomenclature VA: visual acuity VCM1: vision core module 1 VKH: Vogt‐Koyanagi‐Harada
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BenEzra 1988 | Ineligible comparator and population: chlorambucil and Behcet's disease |
| Chavis 1992 | Ineligible study design: not a randomized controlled trial |
| Masuda 1989 | Ineligible comparison: colchicine is an anti‐inflammatory agent |
| Rosenbaum 2008 | Ineligible intervention: voclosporin is an analog of cyclosporine A |
| Shalaby 2017 | Ineligible co‐intervention: diltiazem with cyclosporin A |
| Yazici 1990 | Ineligible participant population: includes Behçet's disease with and without ocular disease |
Characteristics of studies awaiting classification [ordered by study ID]
EUCTR2006‐004709‐24‐NL.
| Methods | Parallel, single‐blinded, randomized controlled trial |
| Participants |
Inclusion criteria: Eligible patients with uveitis not responding to steroids due to systemic disease (ocular sarcoidosis; intermediate uveitis; Behçet’s syndrome; idiopathic retinal vasculitis; Birdshot; VKH disease; sympathetic ophthalmia; idiopathic panuveitis); no systemic immunomodulatory agents (other than steroids); significant flare requiring intensification of therapy (prednisone); visual acuity of 0.1 or better in at least one eye; adequate birth control measures; normal screening laboratory; normal chest x‐ray; 18 years of age or older. Exclusion criteria: Inability to visualize the fundus; ocular surgery within three months; pregnant, nursing; investigational drugs < 1 month or < 5 x T½; systemic immunosuppressive therapy, other than steroids; creatinine clearance of < 20 mL/min; hypersensitivity to study medications; clinically significant infection (HIV, tuberculosis, Lues infection) organ transplant; malignancy, lymphoproliferative disease; recent live vaccinations. |
| Interventions |
Intervention
Active control
|
| Outcomes |
Primary outcome
Secondary outcomes
Time points: Weeks 0, 2, 4, 8, 12, 16, 28, 40, 52 |
| Notes | Requests for information: investigator confirmed there was no publication or meeting abstracts for this trial. Unable to obtain unpublished data. |
JPRN‐jRCTs011180031.
| Methods | Open‐label, parallel‐group, active control, randomized trial |
| Participants |
Inclusion criteria: early onset of Vogt‐Koyanagi‐Harada (VKH) disease (defined as within 4 weeks from onset, anterior chamber cells less than or equal to 2+, typical serous retinal detachment, without 'sunset glow' fundus); presence of "cerebrospinal fluid pleocytosis or meningeal irritating sign except for headache"; able to receive treatment within 4 weeks from onset; age between 20 to 70 years old. Exclusion criteria: renal dysfunction; receiving immunosuppressant or corticosteroid therapy; state of immunosuppression; pregnant, unwilling to practice contraception during the study, or lactating female; malignant tumor; intraocular surgery (except for cataract surgery) or glaucoma surgery in the past or future. |
| Interventions |
Intervention: prednisolone only
Comparator: prednisolone and cyclosporine
|
| Outcomes | Primary outcome: proportion of participants showing recurrences of uveitis with VKH disease |
| Notes | Requests for information: attempts to contact investigators for information were unsuccessful. Investigators for the Ono 2021 study stated that these publications were separate from their trial. |
UVEXATE 2017.
| Methods | Parallel‐assignment, double‐blind, randomized, placebo‐controlled study |
| Participants |
Inclusion criteria: over 18 years old; oral contraception; posterior uveitis or panuveitis associated with macular edema; people with histologically proven sarcoidosis or presumed sarcoidosis. Exclusion criteria: other causes of uveitis; extra ophthalmologic manifestations of sarcoidosis justifying corticosteroids; people previously treated with immunosuppressive agents or corticosteroids of more than 10 mg daily over 15 days; people with life‐threatening conditions; chronic hepatopathy or renal failure; uncontrolled diabetes mellitus |
| Interventions |
Intervention: methotrexate plus prednisone
Comparator: placebo plus prednisone
All participants receive three pulses of methylprednisolone (5 mg/kg) followed by four weeks of prednisone (1 mg/kg daily). After randomization, participants receive either prednisone (1 mg/kg daily) plus methotrexate (0.3 mg/kg weekly) or prednisone (1 mg/kg daily) plus placebo. Corticosteroids will be progressively tapered when macular edema completely resolve. Unilateral macular edema relapses will be treated with a single local injection triamcinolone acetonide. Increasing dose of prednisone will be administered in case of bilateral macular edema relapses. |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes |
Requests for information: attempts to contact investigators for information were unsuccessful. Partial results from editorial publication.
Total: N = 7, halted for low enrollment
Intervention: methotrexate plus prednisone (n = 2)
Comparator: placebo plus prednisone (n = 5)
Partial results: "lower efficiency in the methotrexate group (mean cumulated prednisone dose 6812 mg vs. 5565)" "No rescue triamcinolone injection had to be performed in the placebo group, while 1 patient out of 2 received it in the methotrexate group." |
BCVA: best‐corrected visual acuity IV: intravenous(ly) OCT: optical coherence tomography VKH: Vogt‐Koyanagi‐Harada disease
Differences between protocol and review
Population
We decided to include studies with a mix of ages but to exclude studies solely in pediatric populations (under 18 years old). We made this decision having seen that Rathinam 2014 and Rathinam 2019 included participants 16 and older. One study included a single participant aged 5 years old (Cuchacovich 2010).
Protocol population: age 18 and over
Review population: includes multiple studies with participants under 18 years old
Subgroups
We decided to add a subgroup analysis of Vogt‐Koyanagi‐Harada (VKH) versus non‐VKH participants. In the included trials, some enrolled only VKH participants, some contained a mix of NIIPPU etiologies, and some reported subgroup analysis outcomes by VKH subgroup participants.
Protocol subgroups: studies treating people with active disease versus preventing relapse in people with inactive inflammation; intermediate uveitis versus posterior uveitis or panuveitis; with cystoid macular edema versus without
Review subgroup: VKH versus non‐VKH
Comparisons
There were a number of older trials included in the review, some used cyclosporin A (CsA) dosing that we now know to cause nephrotoxicity (Palestine 1986). We did not feel that including these in the main analyses was informative to current practice. We omitted the comparison of tacrolimus to tacrolimus and tacrolimus to CsA as the mechanism of action was overlapping. These comparisons were not of interest to this review.
Protocol: compare each steroid‐sparing pharmacologic therapy with each other;
Review: omit comparisons of tacrolimus versus CsA or tacrolimus.
Protocol: no dosage threshold specified for comparisons;
Review: omit older studies using CsA doses.
Outcomes
Limiting outcomes to only those studies meeting the criteria of reduction in oral steroid dose to less than 7.5 mg/day would omit trial results that did show an important reduction in steroid use. We have noted where there was less than 7.5 mg/day versus less than 10 mg/day.
Protocol: proportion of participants to achieve reduction in oral steroid dose (to < 7.5 mg/day)
Review: proportion of participants to achieve reduction in oral steroid dose (to < 10 mg/day)
The included studies did not report the exact timeline specified in the protocol. Steroid‐sparing control of inflammation is an important outcome of interest, so we decided to remove the specified three months to make the best use of the available evidence.
Protocol: proportion of participants to achieve steroid‐sparing control at three months
Review: proportion of participants to achieve steroid‐sparing control
We changed the wording of the safety outcome to reflect the possibility that participants may stop treatment for reasons that differ from clinical indicators of intolerance.
Protocol: proportion of participants experiencing systemic complications requiring cessation of medication
Review: proportion of participants experiencing complications or requiring cessation of medication
Some studies predated the criteria outlined by Jabs 2005 but we felt these were clinically comparable.
Protocol: proportion of participants achieving control of inflammation, defined as a two‐step reduction in vitreous haze grade/score or decrease to grade 0 (Jabs 2005; Nussenblatt 1985)
Review: proportion of participants achieving control of inflammation, defined as a two‐step reduction in vitreous haze grade/score or decrease to grade 0 (Jabs 2005; Nussenblatt 1985); or clinically comparable study definition
Summary of findings
During the review process, we felt that the overall adverse events did not reflect the more clinically important complications leading to the cessation of medication.
Protocol summary of findings (SoF) table: proportion of participants experiencing adverse effects
Review SoF table: proportion of participants experiencing complications or requiring cessation of medication
We decided that the outcome of highest interest regarding steroid‐sparing therapy was the broadest definition.
Protocol: proportion of participants needing less than 7.5 mg/day glucocorticoids for over three months
Review: proportion of participants achieving steroid‐sparing control
Contributions of authors
All protocol authors (REM, NK, TL, AP) contributed to the conception and design of study. REM drafted the review proposal form (RPF). NK, AP, TL reviewed the RPF and provided detailed feedback. NK and TL finalized the RPF.
The protocol was written by REM (Background) and NK (Methods). TL and AP commented on it critically for intellectual content. All authors reviewed the protocol in detail and provided the final approval of the protocol to be published.
All review authors (REM, TL, PMcC, LL, ASC, AP) contributed to the conception and design of the study, participated in study selection, data extraction and/or analysis, drafted portions of the review, commented on drafts critically regarding intellectual content, and approved the final version for publication.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Eye Institute, National Institutes of Health, USA
Cochrane Eyes and Vision Group US Project, supported by grant UG1EY020522 (PI: Tianjing Li, MD, MHS, PhD)
-
Queen’s University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland
-
Public Health Agency, UK
This review was supported by the HSC Research and Development (R&D) Division of the Public Health Agency which funds the Cochrane Eyes and Vision editorial base at Queen's University Belfast.
Declarations of interest
REM: none known.
TL: serves as the Principal Investigator for Cochrane Eyes and Vision US Satellite, which is support by grant UG1 EY020522 from the National Eye Institute, National Institutes of Health, USA.
PMcC: work undertaken as part of a postdoctoral research fellowship which is supported by Cochrane Eyes and Vision US Satellite (Grant UG1 EY020522), from the National Eye Institute, National Institutes of Health, USA.
LL: reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution.
ASC: none.
AP: serves as a Co‐investigator for Cochrane Eyes and Vision US Satellite, which is support by grant UG1 EY020522 from the National Eye Institute, National Institutes of Health, USA.
New
References
References to studies included in this review
Cuchacovich 2010 {published data only}
- Cuchacovich M, Solanes F, Díaz G, Cermenati T, Avila S, Verdaguer J, et al. Comparison of the clinical efficacy of two different immunosuppressive regimens in patients with chronic vogt-koyanagi-harada disease. Ocular Immunology and Inflammation 2010;18(3):200‐7. [CENTRAL: CN-00781196] [DOI: 10.3109/09273941003587541] [DOI] [PubMed] [Google Scholar]
Deuter 2018 {published data only}
- Deuter C, Engelmann K, Heiligenhaus A, Lanzl I, Mackensen F, Ness T, et al. Enteric-coated mycophenolate sodium in the treatment of non-infectious intermediate uveitis: results of a prospective, controlled, randomised, open-label, early terminated multicentre trial. British Journal of Ophthalmology 2018;102(5):647‐53. [CENTRAL: CN-00781196] [DOI: 10.1136/bjophthalmol-2017-310156] [DOI] [PubMed] [Google Scholar]
- EUCTR2009-009998-10-DE. Myfortic (enteric-coated mycophenolate sodium) for the treatment of non-infectous intermediate uveitis – a prospective, controlled randomized multicenter trial. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2009-009998-10 (first received 2 December 2009).
- NCT01092533. Myfortic for the treatment of non-infectious intermediate uveitis (MYCUV-IIT02) [Myfortic (enteric-coated mycophenolate sodium) for the treatment of non-infectious intermediate uveitis - a prospective, controlled randomized multicenter trial]. clinicaltrials.gov/ct2/show/study/NCT01092533 (first received 25 March 2010).
De Vries 1990 {published data only}
- De Vries J, Baarsma G, Zaal M, Boen-Tan T, Rothova A, Buitenhuis H, et al. Cyclosporin in the treatment of severe chronic idiopathic uveitis. British Journal of Ophthalmology 1990;74(6):344‐9. [CENTRAL: CN-00069296] [DOI: 10.1136/bjo.74.6.344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- ISRCTN46576063. Tacrolimus monotherapy for uveitis [Dual steroid and tacrolimus therapy versus steroid withdrawal and tacrolimus therapy in the treatment of posterior segment intraocular inflammation (uveitis)]. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN46576063 (first received 16 April 2008). [DOI: ]
- Lee R, Greenwood R, Taylor H, Amer R, Biester S, Heissigerova J, et al. A randomized trial of tacrolimus versus tacrolimus and prednisone for the maintenance of disease remission in noninfectious uveitis. Ophthalmology 2012;119(6):1223‐30. [DOI: 10.1016/j.ophtha.2011.12.030] [DOI] [PubMed] [Google Scholar]
- Lee RW, Greenwood R, Amer R, Biester S, Plskova J, Forrester JV, et al. Tacrolimus monotherapy versus tacrolimus and prednisone for the maintenance of disease remission in non-infectious posterior segment intraocular inflammation. Investigative Ophthalmology and Visual Science 2009;50(13):2024. [Google Scholar]
Murphy 2005 {published data only}
- Murphy C, Greiner K, Plskova J, Duncan L, Frost N, Forrester J, et al. Cyclosporine vs tacrolimus therapy for posterior and intermediate uveitis. Archives of Ophthalmology 2005;123(5):634‐41. [CENTRAL: CN-00511918] [DOI: 10.1001/archopht.123.5.634] [DOI] [PubMed] [Google Scholar]
Nussenblatt 1991 {published data only}
- Nussenblatt RB, Palestine A, Chan CC, Fujikawa LS, Stevens G, Green SB. A randomised, double-masked trial of sandimmune vs. prednisolone for endogenous uveitis. American Academy of Ophthalmology 1990;112:105. [CENTRAL: CN-00485269] [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB, Palestine AG, Chan CC, Stevens G, Mellow SD, Green SB. Randomized, double-masked study of cyclosporine compared to prednisolone in the treatment of endogenous uveitis. American Journal of Ophthalmology 1991;112(2):138‐46. [CENTRAL: CN-00077346] [DOI: 10.1016/s0002-9394(14)76692-9] [DOI] [PubMed] [Google Scholar]
Nussenblatt 1993 {published data only}
- Nussenblatt RB, De Smet MD, Rubin B, Freidlin V, Whitcup SM, Davis J, et al. A masked, randomized, dose-response study between cyclosporine A and G in the treatment of sight-threatening uveitis of noninfectious origin. American Journal of Ophthalmology 1993;115(5):583‐91. [CENTRAL: CN-00093004] [DOI: 10.1016/s0002-9394(14)71454-0] [DOI] [PubMed] [Google Scholar]
Ono 2021 {published data only}
- JPRN-UMIN000014387. A prospective multicenter comparative clinical trial between corticosteroid pulse therapy vs. combination therapy of oral corticosteroid and cyclosporine on Vogt-Koyanagi-Harada disease. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000014387 (first received 27 June 2014). [CENTRAL: CN-01827271]
- Ono T, Goto H, Sakai T, Nitta F, Mizuki N, Takase H, et al. Comparison of combination therapy of prednisolone and cyclosporine with corticosteroid pulse therapy in Vogt–Koyanagi–Harada disease. Japanese Journal of Ophthalmology 2021 Oct 24 [Epub ahead of print]. [DOI: 10.1007/s10384-021-00878-w] [DOI] [PubMed]
- Ono T, Mochizuki M, Goto H, Sakai T, Nitta F, Mizuki N, et al. A prospective multi-center randomized clinical study comparing steroid-pulse therapy and a combined therapy with oral prednisolone and cyclosporine for new-onset acute Vogt-Koyanagi-Harada disease. Investigative Ophthalmology and Visual Science 2020;61(7):3169. [CENTRAL: CN-02179109] [EMBASE: 632697033] [Google Scholar]
Rathinam 2014 {published data only}
- NCT01232920. First-line antimetabolites as steroid-sparing treatment uveitis pilot trial. clinicaltrials.gov/ct2/show/NCT01232920 (first received 2 November 2010). [CENTRAL: CN-02032098]
- Niemeyer KM, Gonzales JA, Rathinam SR, Babu M, Thundikandy R, Kanakath A, et al. Quality-of-life outcomes from a randomized clinical trial comparing antimetabolites for intermediate, posterior, and panuveitis. American Journal of Ophthalmology 2017;179:10‐17. [CENTRAL: CN-01374518] [DOI: 10.1016/j.ajo.2017.04.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer KM, Gonzales JA, Rathinam SR, Babu M, Thundikandy R, Kanakath A, et al. Quality of life outcomes from a randomized controlled trial comparing methotrexate to mycophenolate mofetil for noninfectious uveitis. Investigative Ophthalmology and Visual Science 2017;58(8):2174. [CENTRAL: CN-01616554] [EMBASE: 621489608] [Google Scholar]
- Rathinam SR, Babu M, Thundikandy R, Kanakath A, Nardone N, Esterberg E, et al. A randomized clinical trial comparing methotrexate and mycophenolate mofetil for noninfectious uveitis. Ophthalmology 2014;121(10):1863‐70. [CENTRAL: CN-01053754] [DOI: 10.1016/j.ophtha.2014.04.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E, Rathinam SR, Babu M, Browne E, Weinrib R, Porco T, et al. Uveitic macular edema outcomes: results of a randomized controlled trial. Clinical and Translational Science 2014;7(3):236. [CENTRAL: CN-01049997] [DOI: 10.1111/cts.12171] [EMBASE: 71734851] [DOI] [Google Scholar]
- Shen E, Rathinam SR, Babu M, Kanakath A, Thundikandy R, Lee S, et al. Outcomes of vogt-koyanagi-harada disease from a randomized clinical trial of antimetabolite therapies. Investigative Ophthalmology and Visual Science 2016;57(12):1899. [CENTRAL: CN-01377043] [EMBASE: 616083527] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E, Rathinam SR, Babu M, Kanakath A, Thundikandy R, Lee SM, et al. Outcomes of vogt-koyanagi-harada disease: a subanalysis from a randomized clinical trial of antimetabolite therapies. American Journal of Ophthalmology 2016;168:279‐86. [CENTRAL: CN-01177618] [DOI: 10.1016/j.ajo.2016.06.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathinam 2019 {published data only}
- CTRI 2017/09/009699. Immunosuppresants comparison for UVEITIS [First-line Antimetabolites as Steroid-sparing Treatment (FAST) Uveitis Trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=13782 (first received 9 November 2017).
- Jabs DA. Antimetabolite therapy for uveitis: methotrexate or mycophenolate? JAMA Ophthalmology 2019;137(12):1449‐51. [CENTRAL: CN-02136445] [DOI: 10.1001/jamaophthalmol.2019.3964] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Jabs DA. Stratification clarification for methods for randomized clinical trials - reply. JAMA Ophthalmology 2020;138(7):801. [DOI: 10.1001/jamaophthalmol.2020.1864] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NK, Chattopadhyay A, Rathinam SR, Gonzales JA, Thundikandy R, Kanakath A, et al. Health- and vision-related quality of life in a randomized controlled trial comparing methotrexate and mycophenolate mofetil for uveitis. Ophthalmology 2021;128(9):1337-45. [CENTRAL: CN-02268128] [DOI: 10.1016/j.ophtha.2021.02.024] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CL, Kelly NK, Sundararajan M, Rathinam SR, Gonzales JA, Thundikandy R, et al. Comparison of CD4 counts with mycophenolate mofetil versus methotrexate from the first-line antimetabolites as steroid-sparing treatment (FAST) uveitis trial. Ocular Immunology and Inflammation 2020;30(1):1-5. [CENTRAL: CN-02161428] [DOI: 10.1080/09273948.2020.1774906] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01829295. First-line Antimetabolites as Steroid-sparing Treatment (FAST) Uveitis Trial. clinicaltrials.gov/ct2/show/NCT01829295 (first received 11 April 2013). [CENTRAL: CN-01541548]
- Porco TC, Kim E, Acharya NR. Stratification clarification for methods for randomized clinical trials. JAMA Ophthalmology 2020;138(7):800-1. [DOI: 10.1001/jamaophthalmol.2020.1551] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Rathinam SR, Gonzales JA, Thundikandy R, Kanakath A, Murugan SB, Vedhanayaki R, et al. Effect of corticosteroid-sparing treatment with mycophenolate mofetil vs methotrexate on inflammation in patients with uveitis: a randomized clinical trial. JAMA 2019;322(10):936‐45. [CENTRAL: CN-01990886] [DOI: 10.1001/jama.2019.12618] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiederholt 1986 {published data only}
- Wiederholt M, Kämpe C, Hansen LL, Hövener G. Treatment of posterior uveitis with cyclosporin or prednisolone--one-year report of a randomized prospective study [Behandlung der uveitis posterior mit cyclosporin oder prednisolon--jahresbericht über eine randomisierte prospektive studie]. Fortschritte der Ophthalmologie 1986;83(3):348‐52. [CENTRAL: CN-00044224] [EMBASE: 1986247564] [PMID: ] [PubMed] [Google Scholar]
References to studies excluded from this review
BenEzra 1988 {published data only}
- BenEzra D, Cohen E, Chajek T, Friedman G, Pizanti S, Courten C, et al. Evaluation of conventional therapy versus cyclosporine A in Behçet's syndrome. Transplantation Proceedings 1988;20(3 Suppl 4):136‐43. [PMID: ] [PubMed] [Google Scholar]
Chavis 1992 {published data only}
- Chavis PS, Antonios SR, Tabbara KF. Cyclosporine effects on optic nerve and retinal vasculitis in Behçet's disease. Documenta Ophthalmologica [Advances in Ophthalmology] 1992;80(2):133-42. [DOI: 10.1007/BF00161239] [DOI] [PubMed] [Google Scholar]
Masuda 1989 {published data only}
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Ciclosporin treatment for Behcet's disease - double-masked group comparative study. Rinsho Hyoka [Clinical Evaluation] 1986;14:437‐61. [CENTRAL: CN-00320614] [Google Scholar]
- Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet's disease. Lancet 1989;1(8647):1093‐6. [DOI: 10.1016/s0140-6736(89)92381-7] [DOI] [PubMed] [Google Scholar]
Rosenbaum 2008 {published data only}
- EUCTR2006-006543-31. A double-masked, placebo-controlled, parallel group, multi-centre, dose-ranging study with an optional extension to assess the efficacy and safety of LX211 as therapy in subjects with active sight threatening, non-infectious intermediate-, anterior and intermediate-, posterior-, or pan-uveitis. who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006-006543-31-GB (first received 09 March 2007).
- EUCTR2006-006544-66. A double-masked, placebo-controlled, multi-centre, parallel group, dose-ranging study to assess the efficacy and safety of LX211 as therapy in subjects with clinically quiescent sight threatening, non-infectious intermediate-, anterior and intermediate-, posterior-, or pan-uveitis. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-006544-66-FR (first received 06 March 2007).
- NCT00404612. A double-masked, placebo-controlled, parallel group, multi-centre, dose-ranging study with an optional extension to assess the efficacy and safety of LX211 as therapy in subjects with active sight threatening, non-infectious intermediate-, anterior and intermediate-, posterior-, or pan-uveitis. clinicaltrials.gov/ct2/show/NCT00404612 (first received 29 November 2006).
- NCT00404742. A study of LX211 in clinically quiescent non-infectious intermediate, anterior and intermediate, posterior or pan-uveitis (LUMINATE). clinicaltrials.gov/ct2/show/NCT00404742 (first received 29 November 2006).
- Rosenbaum JT, LUMINATE Uveitis Program Study Group. Design of phase 2/3 clinical trials of a novel calcineurin inhibitor, Lx 211, for the treatment of non-infection uveitis. Investigative Ophthalmology and Visual Science 2008;49(13):ARVO-E abstract 3883. [Google Scholar]
Shalaby 2017 {published data only}
- Shalaby U. Diltiazem co treatment with cyclosporine for induction of disease remission in sight-threatening non-infectious intraocular inflammation. Japanese Journal of Ophthalmology 2017;61(2):169-78. [DOI: 10.1007/s10384-016-0490-9.] [DOI] [PubMed] [Google Scholar]
Yazici 1990 {published data only}
- Yazici H, Pazarli H, Barnes CG, Tüzün Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behçet's syndrome. New England Journal of Medicine 1990;322(5):281‐5. [DOI: 10.1056/NEJM199002013220501] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
EUCTR2006‐004709‐24‐NL {published data only (unpublished sought but not used)}
- EUCTR2006-004709-24-NL. Mycophenolate sodium (Myfortic®) in the treatment of uveitis: a pilot study. trialsearch.who.int/Trial2.aspx?TrialID=EUCTR2006-004709-24-NL (first received 1 September 2006).
- NTR1126. Myfortic in uveitis [Mycophenolate sodium (Myfortic®) in the treatment of uveitis: a pilot study]. trialsearch.who.int/?TrialID=NTR1126 (first received 2 November 2007).
JPRN‐jRCTs011180031 {published data only}
- JPRN-jRCTs011180031. The combination therapy of prednisolone and cyclosporine for initial onset of Vogt-Koyanagi-Harada disease [Prednisolone and cyclosporine combination therapy for early onset of Vogt-Koyanagi-Harada disease - PSL/CYA therapy for early onset of VKH disease]. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs011180031 (first received 25 March 2019).
- UMIN000013373. The combination therapy of prednisolone and cyclosporine for initial onset of Vogt-Koyanagi-Harada disease. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000013373 (first received 1 April 2014).
- UMIN000014041. The combination therapy of prednisolone and cyclosporine for initial onset of Vogt-Koyanagi-Harada disease. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000014041 (first received 22 May 2014).
- UMIN000017093. The combination therapy of prednisolone and cyclosporine for initial onset of Vogt-Koyanagi-Harada disease. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000017093 (first received 10 April 2015).
UVEXATE 2017 {published data only}
- NCT00918554. Efficacy study of methotrexate to treat sarcoid-associated uveitis [Corticosteroid sparing effect of methotrexate in patients with sarcoid-associated uveitis: a double blind, randomized, placebo controlled-study - UVEXATE]. ClinicalTrials.gov/show/NCT00918554 (first received 11 June 2009). [CENTRAL: CN-01524320]
- Rodrigues F, Vicaut E, Abad S. Abortion of the UVEXATE study: are we unable to evaluate cheap and well-tolerated treatments? Clinical and Experimental Rheumatology 2017;35(1):175. [CENTRAL: CN-01338179] [EMBASE: 614510198] [PMID: ] [PubMed] [Google Scholar]
Additional references
Allison 2002
- Allison AC. Mechanisms of action of mycophenolate mofetil in preventing chronic rejection. Transplantation Proceedings 2002;34(7):2863-6. [DOI: 10.1016/s0041-1345(02)03538-8] [DOI] [PubMed] [Google Scholar]
Bacon 1987
- Bacon PA, Salmon M. Modes of action of second-line agents. Scandinavian Journal of Rheumatology 1987;16(Suppl 64):17-24. [DOI: 10.3109/03009748709096717] [DOI] [PubMed] [Google Scholar]
Boyd 2001
- Boyd SR, Young S, Lightman S. Immunopathology of the noninfectious posterior and intermediate uveitides. Survey of Ophthalmology 2001;46(3):209-33. [DOI: 10.1016/s0039-6257(01)00275-2] [DOI] [PubMed] [Google Scholar]
Chi 2011
- Chi W, Zhou S, Yang P, Chen L. CD4⁺ T cells from behcet patients produce high levels of IL-17. Eye Science 2011;26(2):65-9. [DOI: 10.3969/j.issn.1000-4432.2011.02.013] [DOI] [PubMed] [Google Scholar]
Chu 2017
- Chu CJ, Dick AD, Johnston RL, Yang YC, Denniston AK, UK Pseudophakic Macular Edema Study Group. Cataract surgery in uveitis: a multicentre database study. British Journal of Ophthalmology 2017;101(8):1132. [DOI: 10.1136/bjophthalmol-2016-309047] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 13 March 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Dick 2013
- Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology 2013;120(4):777-87. [DOI: 10.1016/j.ophtha.2012.09.040] [DOI] [PubMed] [Google Scholar]
Dick 2018
- Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R Jr, Brézin AP, Fundamentals of Care for Uveitis International Consensus Group. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS) Initiative. Ophthalmology 2018;125(5):757-73. [DOI: 10.1016/j.ophtha.2017.11.017] [DOI] [PubMed] [Google Scholar]
Durrani 2004
- Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. British Journal of Ophthalmology 2004;88(9):1159-62. [DOI: 10.1136/bjo.2003.037226] [DOI] [PMC free article] [PubMed] [Google Scholar]
GetData Graph Digitizer 2.24 [Computer program]
- GetData Graph Digitizer 2.24. S. Fedorov, accessed 17 August 2020. Available at getdata-graph-digitizer.com.
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Gómez‐Gómez 2020
- Gómez-Gómez A, Loza E, Rosario MP, Espinosa G, Morales JM, Spanish Society of Ocular Inflammation (SEIOC). Efficacy and safety of immunomodulatory drugs in patients with non-infectious intermediate and posterior uveitis, panuveitis and macular edema: a systematic literature review. Seminars in Arthritis and Rheumatism 2020;50(6):1299-306. [DOI: 10.1016/j.semarthrit.2020.08.010] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), version accessed 3 March 2022. Available at gradepro.org.
Graffner‐Nordberg 2003
- Graffner-Nordberg M, Fyfe M, Brattsand R, Mellgård B, Hallberg A. Design and synthesis of dihydrofolate reductase inhibitors encompassing a bridging ester group. Evaluation in a mouse colitis model. Journal of Medicinal Chemistry 2003;46(16):3455-62. [DOI: 10.1021/jm021062y] [DOI] [PubMed] [Google Scholar]
Higgins 2022a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from handbook.cochrane.org.
Higgins 2022b
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Ishida 1995
- Ishida Y, Matsuda H, Kida K. Effect of cyclosporin A on human bone marrow granulocyte-macrophage progenitors with anti-cancer agents. Acta Paediatrica Japonica : Overseas Edition 1995;37(5):610-3. [DOI: 10.1111/j.1442-200x.1995.tb03386.x] [DOI] [PubMed] [Google Scholar]
Jabs 2005
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American Journal of Ophthalmology 2005;140(3):509-16. [DOI: 10.1016/j.ajo.2005.03.057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jawad 2013
- Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocular Immunology and Inflammation 2013;21(6):434-9. [DOI: 10.3109/09273948.2013.815786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandiel 2005
- Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54(8):1121-5. [DOI: 10.1136/gut.2004.049460] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karam 2022
- Karam M, Alsaif A, Al-Naseem A, Hayre A, Al Jabbouri A, Aldubaikhi A, et al. Mycophenolate versus methotrexate in non-infectious ocular inflammatory disease: a systematic review and meta-analysis. Ocular Immunology and Inflammation 2022 Feb 24 [Epub ahead of print]. [DOI: 10.1080/09273948.2022.2034166] [DOI] [PubMed]
Knickelbein 2017
- Knickelbein JE, Kim M, Argon E, Nussenblatt RB, Sen NH. Comparative efficacy of steroid-sparing therapies for non-infectious uveitis. Expert Review of Ophthalmology 2017;12(4):313-9. [DOI: 10.1080/17469899.2017.1319762] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leal 2019
- Leal I, Rodrigues FB, Sousa DC, Duarte GS, Romão VC, Marques-Neves C, et al. Anti-TNF drugs for chronic uveitis in adults - a systematic review and meta-analysis of randomized controlled trials. Frontiers in Medicine 2019;6:104. [DOI: 10.3389/fmed.2019.00104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014
- Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, et al. Autoimmune and autoinflammatory mechanisms in uveitis. Seminars in Immunopathology 2014;36(5):581-94. [DOI: 10.1007/s00281-014-0433-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mangione 2001
- Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Archives of Ophthalmology (Chicago, Ill. : 1960) 2001;119(7):1050-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
McKenzie 2022
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Nussenblatt 1985
- Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985;92(4):467-71. [DOI: 10.1016/s0161-6420(85)34001-0] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ortiz 1998
- Ortiz Z, Shea B, Suarez-Almazor ME, Moher D, Wells GA, Tugwell P. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A meta-analysis of randomized controlled trials. Journal of Rheumatology 1998;25(1):36-43. [PMID: ] [PubMed] [Google Scholar]
Palestine 1986
- Palestine AG, Austin HA 3rd, Balow JE, Antonovych TT, Sabnis SG, Preuss HG, et al. Renal histopathologic alterations in patients treated with cyclosporine for uveitis. New England Journal of Medicine 1986;314(20):1293-8. [DOI: 10.1056/NEJM198605153142005] [DOI] [PubMed] [Google Scholar]
Pato 2011
- Pato E, Muñoz-Fernández S, Francisco F, Abad MA, Maese J, Uveitis Working Group from Spanish Society of Rheumatology. Systematic review on the effectiveness of immunosuppressants and biological therapies in the treatment of autoimmune posterior uveitis. Seminars in Arthritis and Rheumatism 2011;40(4):314-23. [DOI: 10.1016/j.semarthrit.2010.05.008] [DOI] [PubMed] [Google Scholar]
Review Manager Web 2020 [Computer program]
- Review Manager Web (RevMan Web). Version 1.22.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Schünemann 2022
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Sugita 2006
- Sugita S, Takase H, Taguchi C, Imai Y, Kamoi K, Kawaguchi T, et al. Ocular infiltrating CD4+ T cells from patients with Vogt-Koyanagi-Harada disease recognize human melanocyte antigens. Investigative Ophthalmology and Visual Science 2006;47(6):2547-54. [DOI: 10.1167/iovs.05-1547] [DOI] [PubMed] [Google Scholar]
Tallouzi 2021
- Tallouzi MO, Mathers JM, Moore DJ, Bucknall N, Calvert MJ, COSUMO Working Group. Development of a Core Outcome Set for clinical trials in non-infectious uveitis of the posterior segment. Ophthalmology 2021;128(8):1209-21. [DOI: 10.1016/j.ophtha.2021.01.022] [DOI] [PubMed] [Google Scholar]
Tsirouki 2018
- Tsirouki T, Dastiridou A, Symeonidis C, Tounakaki O, Brazitikou I, Kalogeropoulos C, et al. A focus on the epidemiology of uveitis. Ocular Immunology and Inflammation 2018;26(1):2-16. [DOI: 10.1080/09273948.2016.1196713] [DOI] [PubMed] [Google Scholar]
van Dieren 2006
- Van Dieren JM, Kuipers EJ, Samsom JN, Nieuwenhuis EE, Van der Woude CJ. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: their mechanisms of action and role in the treatment of IBD. Inflammatory Bowel Diseases 2006;12(4):311-27. [DOI: 10.1097/01.MIB.0000209787.19952.53] [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang R, Qian J, Guo J, Yuan YF, Xue K. Suppression of experimental autoimmune uveoretinitis by Anti-IL-17 antibody. Current Eye Research 2009;34(4):297-303. [DOI: 10.1080/02713680902741696] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Edwards Mayhew 2021
- Edwards Mayhew R, Khachatryan N, Li T, Palestine A. Non‐biologic, steroid‐sparing therapies for non‐infectious intermediate, posterior, and panuveitis in adults. Cochrane Database of Systematic Reviews 2021, Issue 3. Art. No: CD014831. [DOI: 10.1002/14651858.CD014831] [DOI] [PMC free article] [PubMed] [Google Scholar]