Abstract
Background
Polycystic ovary syndrome (PCOS) is a condition of the female reproductive system and it remains imperative to identify target genes responsible for its pathogenesis and develop therapeutic drugs capable of effectively treating it.
Methods
We performed primary screening, staging, functional analysis as well as screening of target genes and therapeutic drugs based on single cell sequencing data of 34 oocytes from the GEO database.
Results
Oxidative phosphorylation played a pivotal role in the development of oocytes, insulin resistance and ovulation disorders. At the cellular level, GV and MI phases were particularly critical for the biology of pregnancy. We screened PGR, SIRT1 and ADAMTS1 as hub differentially expressed genes (DEGs) and found relevant drugs using the Drug-Gene Interaction Database. In clinical study, oral contraceptives and insulin sensitisers were found to be effective in the treatment of PCOS.
Conclusion
PGR, SIRT1 and ADAMTS1 were found to be down-regulated in oocytes, ovulation and female pregnancy. These 3 genes are likely biomarkers important in the treatment of PCOS. Insulin sensitiser in combination with oral contraceptive administration were found to significantly improve PCOS.
Key messages
Our study used a new bioinformatics approach to find target genes for the treatment of PCOS.
Our study sought to identify target genes that affect human oocyte quality by analysing single-cell sequencing data from oocytes.
We testified to our data by analysing a subset of clinical data.
Keywords: Differentially expressed genes, Insulin resistance, Polycystic ovary syndrome, Biomarkers, Gene therapy
1. Introduction
Polycystic ovary syndrome (PCOS) is a female endocrine disorder affecting 5–10% of reproductive age patients [1]. The Rotterdam diagnostic criteria is generally utilized in the diagnosis of this condition [2] and ovulatory dysfunction, hyperandrogenism and polycystic ovarian morphology (PCOM) are typical manifestations [3]. Insulin resistance (IR) is an especially prominent characteristic of PCOS [4] and about 75% of patients suffer impaired insulin sensitivity [5]. Infertility in the setting of PCOS accounts for a large percentage of anovulatory infertility [6]. Despite significant research efforts the underlying mechanisms of abnormal follicular development and anovulation remains unclear [7]. However, evidence indicates that environmental, developmental, genetic and epigenetic factors are all important in the pathogenesis of PCOS [8,9]. Furthermore, hyperandrogenism is understood to play a major role in metabolic disorders [3,10]. As elevated serum testosterone and luteinizing hormone (LH) are typical biochemical features of PCOS [11], oligoovulation, androgen excess and IR are accepted treatment targets [12]. Therapeutic strategies include metformin administration and weight management [13,14], both of which can reduce IR but can’t reverse IR [15].
Single-cell RNA sequencing (scRNA-seq) [16] measures gene expression at the cellular level and yields higher resolution data as compared to bulk RNA-sequencing in addition to improving the understanding of cellular activity, disease progression and treatment response [17,18]. Here, we used scRNA-seq (GEO, https://www.earthobservations.org/index.php; accession number PRJNA600740) to study 34 oocytes obtained from both healthy individuals and PCOS patients to identify important processes in the pathogenesis of PCOS and screened drugs for treatment potential. Alterations in endocrinology and glucose metabolism were evaluated for the purposes of clinical validation of treatment.
2. Methods
2.1. Data acquisition
The data of 20 oocytes from PCOS patients and of 14 from healthy individuals were acquired from the Intergovernmental Group on Earth Observations (GEO, https://www.earthobservations.org/index.php; accession number PRJNA600740). Data were processed using RStudio software and R v. 4.0.4, platform x86_64-w64-mingw32/x64 (64-bit).
2.2. Data filtering, dimensional reduction, and pseudotime analysis
As only 34 oocytes were studied, data were not filtered according to gene and cell quantity or mitochondrial DNA percentage. Gene quantity and mitochondrial DNA percentage were noted, however, and the top 10 genes with highly variable expression among cells were identified. Available dimensions above the dotted line are detailed using a JackStraw plot. The DimHeatmap function based on individual principal components was applied, and cells and genes were sorted based on principal component scores. Nonlinear dimensionality reduction using t-SNE clustering was also performed. The FindAllMarkers function was applied to identify markers significantly expressed among clusters, and t-SNE plots of the top 4 markers in the cluster were constructed and total mRNAs of the two groups were shown. Pseudotime analysis differentiated the relationship among subpopulations and revealed functional alterations during the differentiation process.
2.3. Cellphonedb, GO and signalling pathway analyses of oocytes
CellPhoneDB is a publicly available repository of curated receptors, ligands and their interactions [19]. EdgeR packages were used to identify differentially expressed genes (DEGs), P value <0.05 was considered as the differential expression thresholds, and a value of logFC >1.5 was considered as signifying up regulation while logFC <−1.5 considered as signifying down regulation; P value >0.05 were considered as signifying stability. PCA plots, Volcano plots and Heatmaps were constructed and DEGs with P values of <0.00001 & abs (DEG$logFC) ≥3 were labelled. Up regulated DEGs were marked red, down regulated DEGs were marked blue while stable DEGs were marked. Ggplot2, clusterProfiler and org.Hs.eg.db packages were utilised to constructed bar graphs of gene ontology (GO) functions [20,21], and KEGG pathways of studied oocytes.
2.4. Copykat, GSEA GO and KEGG pathway analyses of aneuploid oocytes
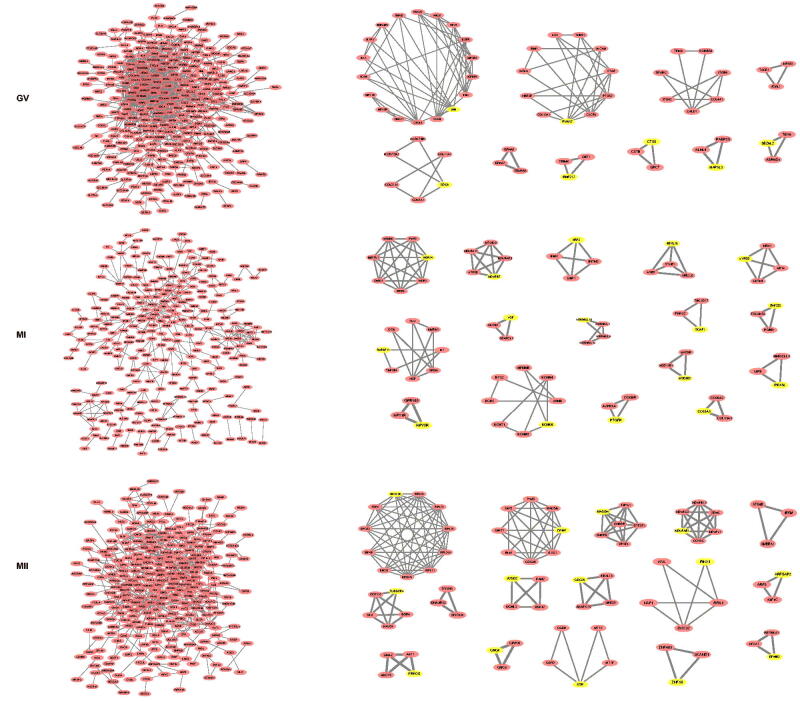
Cells exhibiting aneuploidy alterations were analysed using CopyKAT analysis and t-SNE plots were constructed. Screening conditions for DEG evaluation as well as volcano plots construction were as detailed above. Gene Set Enrichment Analysis (GSEA) was applied for GO and KEGG pathway study of aneuploid oocytes and Biological processes (BP) cnetplots and gseaplot2 analyses of GV, MI, and MII phases was used. Protein-Protein Interaction (PPI) networks [22,23] of aneuploid oocytes were constructed using STRING (v. 11.0), with a combined score of >0.4 (medium confidence); DEGs were selected with |logFC| of >3 using Cytoscape software (v. 3.7.1). MCODE (v. 1.6.1) was used to identify modules of greatest significance; criteria for selection were as follows: degree cut-off = 2, node score cut-off = 0.2, max depth = 100 and k-score = 2.
2.5. Meaningful biological process, target DEG and relevant drug screening analyses
Circular cnetplots and gseaplot2 of significant BPs in all 3 phrases were constructed by using RStudio. Venn diagrams of DEGs were constructed using Bioinformatics & Evolutionary Genomics (http://bioinformatics.psb.ugent.be/webtools/Venn/) and target DEGs were identified. The Drug-Gene Interaction Database (DGIdb, http://www.dgidb.org), a website that consolidates disparate data sources detailing drug-gene interactions [24], was utilised to identify drug-gene interactions.
2.6. Validation of clinical drug therapy in patients with PCOS
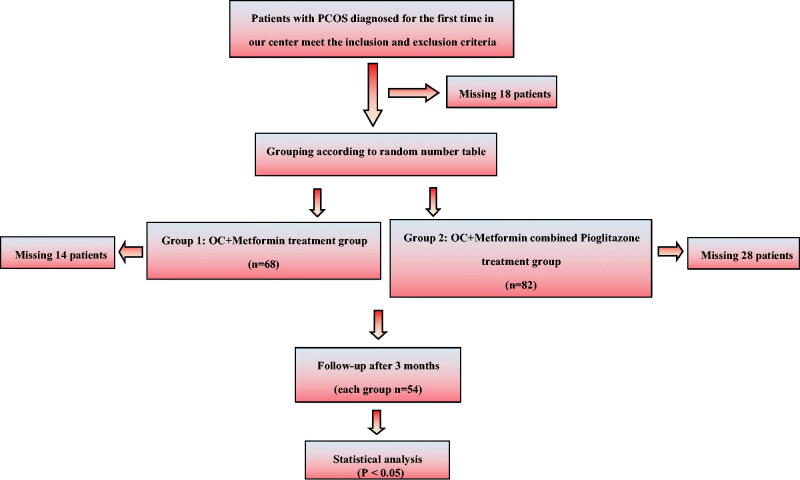
A total of 168 patients were selected according to inclusion and exclusion criteria (We declared that our research was guided by the principles of the Declaration of Helsinki, and all treatments received verbal consent from patients, this clinical study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (ethical approval number for human research: 0241-01) ), after missing 18 patients in the course of the study, there remained 150 patients. During the follow-up group treatment, OC + Metformin group missed 14 patients, OC + Metformin combined Pioglitazone missed 28 patients, so 108 were eventually tested on their 2nd-5th days of menstruation. Endocrine and metabolic data from January 2017 to December 2017 were compiled (Figure 1). Patients in group 1 were administered oral contraceptives (OC; Diane-35) starting on day 5 of menstruation along with metformin 500 mg bid/d for 3 consecutive menstrual cycles. Patients in group 2 were administered Diane-35 starting on day 5 of menstruation along with metformin 500 mg bid/d and pioglitazone 15 mg bid/d for 3 consecutive menstrual cycles. After 3 months, endocrine and glucose metabolism data were statistically analysed using SPSS v.26.0; p < 0.05 was considered statistically significant.
Figure 1.
Clinical research flow chart.
3. Results
3.1. Data filtering, dimensional reduction and pseudotime analysis
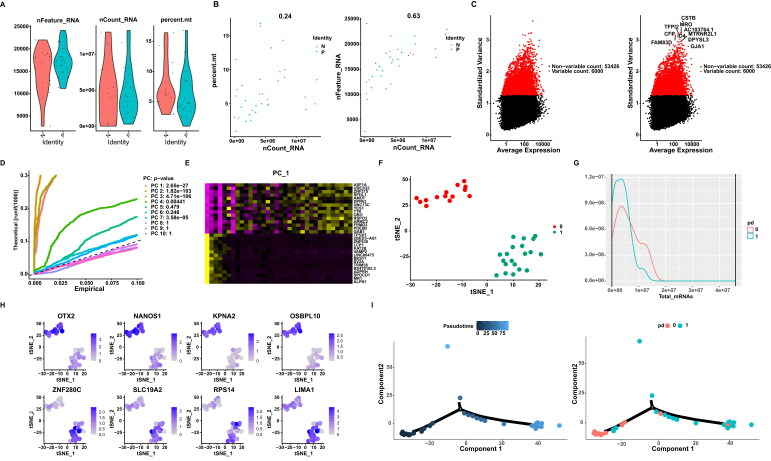
Single cell sequencing data were filtered and discriminated for characteristics. Violin diagram construction revealed gene and cell quantities and mitochondrial gene percentages (Figure 2(A)). Gene quantities and mitochondrial gene percentages in relation to cell quantity were shown in Figure 2(B). The 10 most highly variable genes were shown in Figure 2(C). JackStraw plotting was used to detail available data dimensions of the data as shown in Figure 2(D). A heatmap of PC1 was shown in Figure 2(E), it represents the new variables obtained by transforming the variables in the original data. Nonlinear dimensionality reduction clustering via t-SNE was shown in Figure 2(F) and mRNAs of the two groups Figure 2(G), the top 4 DEGs in each cluster were shown in Figure 2(H). Pseudotime analysis plots and total mRNA content of the 2 clusters were shown in Figure 2(I).
Figure 2.
A. VlnPlot. B. FeatureScatter Plot. C. The top 10 VariableFeature Plot. D. JackStraw Plot. E. PC_1 heatmap. F. TSNE Plots. G Total mRNAs of the two groups. H. FeaturePlot of top 4 markers. I. Proposed timing analysis plots.
3.2. Cellphonedb, GO and signalling pathway analyses of oocytes
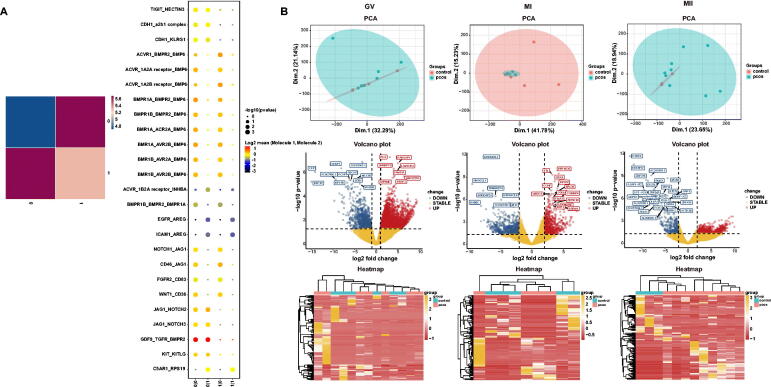
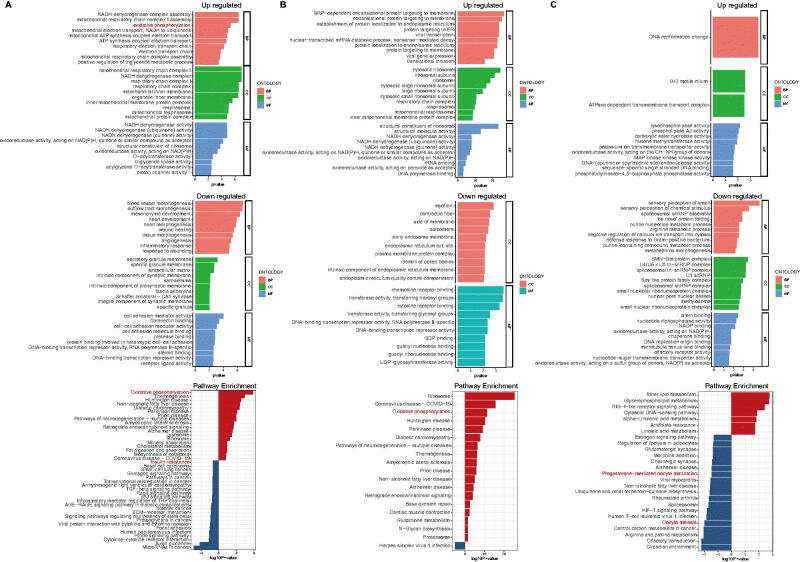
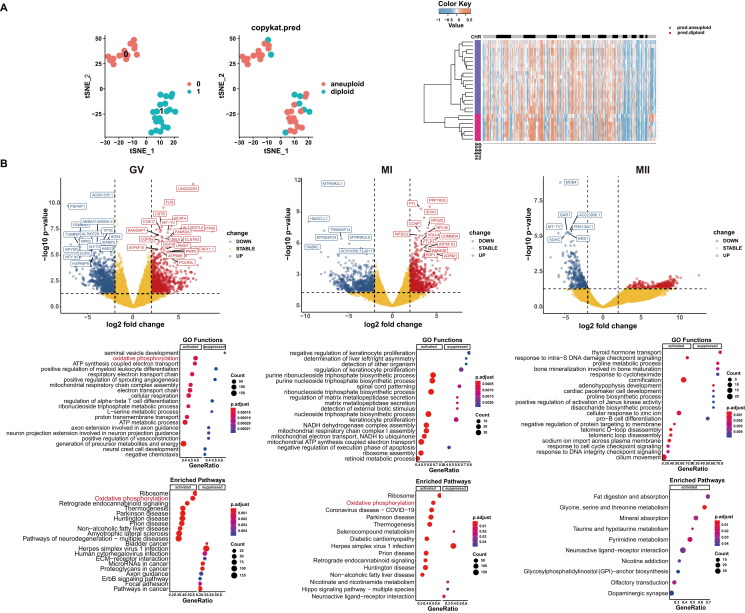
CellphoneDB analysis of ligand/receptor expression using scRNA-seq data revealed inter-oocyte interactions and were shown in Figure 3(A). PCA, volcano and heatmap plots (Figure 3(B)), up and down of GO functions, and signalling pathways of GV, MI and MII oocyte phase (Figure 4). In the three phases, oxidative phosphorylation was found to be important both in GO and oocyte pathway during the development of oocyte. In phase GV, IR and glucagon signalling were down regulated and in MII phase, progesterone-mediated oocyte maturation and oocyte meiosis were down regulated.
Figure 3.
A. Heatmap of logCount and CellphoneDB. B. PCA plots, volcano plots, heatmaps of GV, MI and MII phases.
Figure 4.
A. GO functions and signalling pathways in GV phase. B. GO functions and signalling pathways in MI phase. C. GO functions and signalling pathways in MII phases.
3.3. Copykat, GSEA GO and KEGG pathway analyses of aneuploid oocytes
Data detailing the t-SNE of clusters 0 and 1, the number 0 represented normal oocytes and the number 1 represented oocytes with PCOS, aneuploid and diploid oocytes, while heatmaps of pred.aneuploid and pred.diploid data were shown in Figure 5(A). In Figure 5(B), Volcano plots of GV, MI and MII phases were shown. Oxidative phosphorylation was determined the most important factor in all the three phases of oogenesis. Biological processes related to ovulation disorders, female pregnancy, and meiosis were shown greater detail in the three phases. The PPI networks of DEGs from all phases of oocytes were constructed (Figure 6), three phases of the MCODE modules were constructed, and seed DEGs were highlighted yellow.
Figure 5.
A. t-SNE plots and CopyKAT plot of aneuploid oocytes. B. Volcano plots, GSEA GO and KEGG enriched bubble plots of GV, MI and MII phases in aneuploid cells.
Figure 6.
PPI networks of GV, MI and MII in aneuploid cell.
3.4. Meaningful BP, target DEGs, and relevant drug analyses
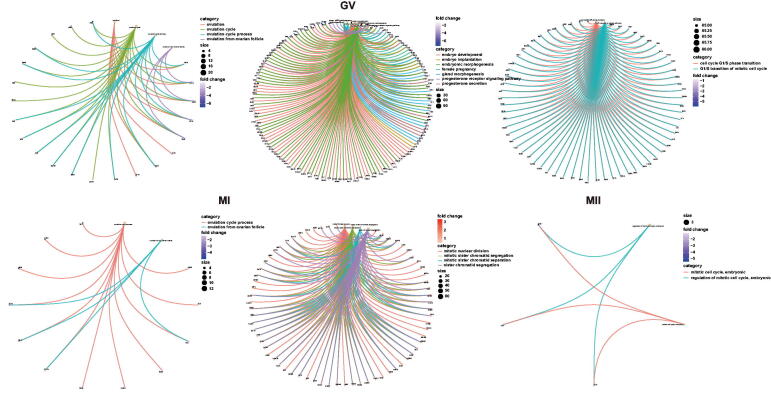
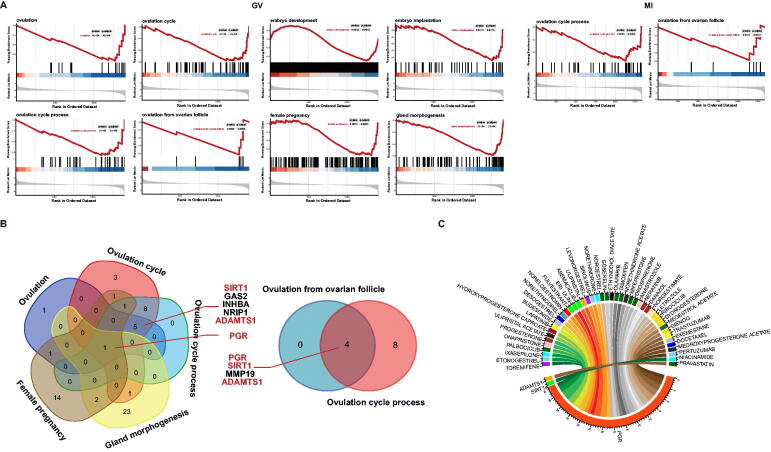
Cnetplots of significant BP from GV to MII phases were shown in Figure 7, and GSEA plots from GV to MI phases in aneuploid oocytes were shown in Figure 8(A). In the GV phase, BPs associated with ovulation were found to be significantly down regulated, including ovulation, ovulation cycle, ovulation cycle process and ovulation from ovarian follicle. Those BPs associated with pregnancy were found to be down regulated, including those associated with embryo development and embryo implantation, female pregnancy, gland morphogenesis and progesterone secretion. Meiosis-associated BPs were found to be down regulated, including those associated with meiotic G1/S and G1/S transitions. Throughout the MI phase, processes associated with the ovulation cycle were found to be down regulated, although BPs related to meiosis were up regulated, including meiotic nuclear division and mitotic sister chromatid segregation. In the MII phase, In MII phase, mitotic cell cycle, and regulation of mitotic cell cycle were also down regulated, but absence of BP associated with ovulation in MII phase. Because the main BPs associated with obstacles to ovulation were noted in GV and MI phases, Venn diagrams were utilised to identify common DEGs involved in BPs relevant to ovulation and pregnancy (Figure 8(B)). Notably, PGR (Progesterone Receptor), SIRT1 (Sirtuin 1) and ADAMTS1 (A disintegrin and metalloproteinase with thrombospondin motifs 1) were found to be involved in ovulation; PGR also was noted to participate in pregnancy and gland morphogenesis (Table 1). Finally, PGR, SIRT1 and ADAMTS1 were input into DGIdb to identify potential drugs; PGR was found to be potentially targeted by 42 drugs, and SIRT1 and ADAMTS1 each by 1 drugs (Figure 8(C), Table 2).
Figure 7.
Cnetplots of significant biological process from GV, MI, and MII phases in aneuploid cells.
Figure 8.
A. GSEA plots from GV, MI phases in aneuploid cells. B. Venn diagrams of significant biological processes in GV and MI phases. C. Drug-Gene Interaction Chord Diagram of PGR, SIRT1, and ADAMTS1.
Table 1.
DEGs of significant biological processes in GV and MI phases.
| Phase | Biological Processes | Gene symbol |
|---|---|---|
| GV | ovulation | GAS2, SIRT1, NRIP1, PGR, ADAMTS1, TNFAIP6, INHBA, PTGS2 |
| ovulation cycle | PDGFRA, ADNP, MAP2K6, PLEKHA1, GAS2, AMH, BMPR1B, CASP3, SIRT1, NRIP1, PGR, ADAMTS1, OXTR, AXL, INHBA, PAM, PTX3, HAS2, EGFR, NPY5R | |
| ovulation cycle process | PDGFRA, MAP2K6, PLEKHA1, GAS2, AMH, BMPR1B, CASP3, SIRT1, NRIP1, PGR, ADAMTS1, INHBA, PAM, PTX3, NPY5R | |
| female pregnancy | ACSL4, AR, STC1, CRHBP, PGR, OXTR, PTHLH, VEGFA, ITGA5, IGFBP7, PRDM1, SLC38A2, TRO, PAPPA, PAM, ITGA2, IGFBP5, GJA1, STC2, PTGS2 | |
| gland morphogenesis | FGF7, PROX1, NTN4, GLI3, LAMA1, FGFR2, SFRP1, NFIB, AR, TWSG1, EPHA2, AREG, PGR, MSN, WNT5A, CAV1, NOTCH2, SEMA3A, SOX9, TNFAIP3 | |
| MI | ovulation cycle process | SLIT3, CASP2, MMP19, MAP2K6, ZNF830, PAM, SIRT1, ADAMTS1, PGR, NPY5R, EREG, LHCGR |
| ovulation from ovarian follicle | MMP19, SIRT1, ADAMTS1, PGR |
Table 2.
Drugs targeting PGR, SIRT1 and ADAMTS1 from DGIdb.
| Gene | Drug | Interaction Type & Directionality | PMIDs | Score |
|---|---|---|---|---|
| PGR | ULIPRISTAL | modulator | 23437846 | 5.7 |
| LEVONORGESTREL | agonist, modulator, binder | 17484506 17531625 17077229 16157482 11752352 16084894 | 4.18 | |
| DYDROGESTERONE | agonist | 6827166 436795 11752352 16045524 8486204 15128769 | 4.18 | |
| DESOGESTREL | agonist | 6645495 3139361 11041225 11752352 7750284 10601100 | 2.85 | |
| DROSPIRENONE | agonist | 7625729 8922878 16291771 17000933 16157482 15493951 11024226 | 2.85 | |
| NORELGESTROMIN | agonist | 17107221 1324557 19962254 7750291 8384965 | 2.66 | |
| ETONOGESTREL | agonist | 15063480 11041225 11752352 10601100 | 2.28 | |
| NORGESTIMATE | agonist | 17107221 1324557 11752352 7750291 8384965 | 2.05 | |
| NORETHINDRONE | agonist | 15063480 15261304 10494488 15189034 7711211 11752352 16084894 | 1.52 | |
| ETHYNODIOL DIACETATE | agonist | 166800 858280 11752352 | 1.52 | |
| ONAPRISTONE | antagonist | None found | 1.14 | |
| ULIPRISTAL ACETATE | modulator | None found | 1.14 | |
| DIENOGEST | agonist | 18061638 | 1.14 | |
| NORGESTREL | agonist, binder | 11521119 | 0.76 | |
| NORETHYNODREL | agonist | None found | 0.57 | |
| NORETHINDRONE ACETATE | agonist | None found | 0.57 | |
| MIFEPRISTONE | agonist, antagonist | 10699595 10682471 10374120 10806733 11783365 11752352 | 0.57 | |
| MEDROXYPROGESTERONE ACETATE | agonist | 12846422 | 0.41 | |
| DANAZOL | agonist | 17636649 2404115 8000225 18061638 | 0.36 | |
| TOREMIFENE | n/a | 2147123 | 0.33 | |
| ERIBULIN | n/a | None found | 0.33 | |
| GOSERELIN | n/a | None found | 0.33 | |
| HYDROXYPROGESTERONE CAPROATE | agonist | None found | 0.28 | |
| MEGESTROL ACETATE | agonist | None found | 0.28 | |
| PERTUZUMAB | n/a | None found | 0.28 | |
| ANASTROZOLE | n/a | None found | 0.24 | |
| LETROZOLE | n/a | None found | 0.23 | |
| PROGESTERONE | agonist | 17109827 9506743 21315613 17138644 17013809 17015480 17169175 30013421 | 0.22 | |
| NERATINIB | n/a | None found | 0.21 | |
| FULVESTRANT | n/a | None found | 0.16 | |
| EXEMESTANE | n/a | None found | 0.16 | |
| RIBOCICLIB | n/a | None found | 0.14 | |
| IXABEPILONE | n/a | None found | 0.11 | |
| BUDESONIDE | agonist | None found | 0.09 | |
| TAMOXIFEN | n/a | 3793272 | 0.09 | |
| TRASTUZUMAB | n/a | None found | 0.09 | |
| LAPATINIB | n/a | None found | 0.08 | |
| ABEMACICLIB | n/a | None found | 0.08 | |
| OLAPARIB | n/a | None found | 0.08 | |
| SIROLIMUS | n/a | 1284044 | 0.04 | |
| DOCETAXEL | n/a | None found | 0.03 | |
| PALBOCICLIB | n/a | None found | 0.02 | |
| SIRT1 | NIACINAMIDE | n/a | 25884115 | 1.64 |
| ADAMTS1 | PRAVASTATIN | n/a | 18174457 | 3.65 |
3.5. Efficacy of insulin sensitisers in the treatment of PCOS
In the comparison of the underlying conditions, both groups significantly differed in terms of weight and BMI reduction. Androgen and serum LH levels were found to be significantly lower in both groups. The differences among group 1 subject glucose indices were not statistically significant, while those in group 2 were found to have significantly decreased HOMA-IR, glycosylated haemoglobin level and CRP, suggesting that a combination of insulin sensitisers produced superior results in terms of improving glucose metabolism (Table 3).
Table 3.
Comparison of patient groups before and after treatment in terms of general condition, endocrine parameters and glucolipid metabolism.
| Terms | group 1 (n = 54) |
T or Z | P* | group 2 (n = 54) |
T or Z | P* | △group1 | △group1 | T or Z | P* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-treatment | Post-treatment | Pre-treatment | Post-treatment | ||||||||||
| Basic conditions | Age | 26 | 25 | ||||||||||
| Height (cm) | 161 | 162 | |||||||||||
| Weight (kg) | 64.2 | 61.7 | −5.396 | <0.001 | 63.9 | 61.2 | −4.007 | <0.001 | −3.15 ± 3.11 | −2.133 ± 3.64 | 1.572 | 0.119 | |
| BMI (kg/m2) | 25.35 | 24.55 | −4.591 | <0.001 | 24.65 | 23.65 | −4.158 | <0.001 | −1.25 | −0.85 | −0.732 | 0.464 | |
| Waist-hip ratio | 0.88 | 0.88 | −2.377 | 0.017 | 0.88 | 0.87 | −0.867 | 0.386 | −0.01 | −0.01 | −0.903 | 0.367 | |
| Visceral fat area | 115.23 ± 35.17 | 109.09 ± 30.97 | 2.771 | 0.008 | 109.44 ± 30.48 | 105.78 ± 28.72 | 1.832 | 0.073 | −4.70 | −3.15 | −1.241 | 0.215 | |
| Body fat percentage | 25.89 ± 5.44 | 34.75 ± 5.89 | 0.147 | 0.147 | 35.00 ± 5.38 | 34.63 ± 4.90 | 0.618 | 0.540 | −0.85 | −0.10 | −1.530 | 0.126 | |
| Endocrine changes | FSH | 5.89 | 5.97 | −1.124 | 0.261 | 5.71 | 6.02 | −1.881 | 0.060 | 0.32 | 0.27 | −0.461 | 0.645 |
| LH | 9.78 | 7.47 | −2.722 | 0.006 | 12.99 | 7.55 | −3.414 | <0.001 | −1.98 | −4.79 | −1.220 | 0.223 | |
| LH/FSH | 1.82 | 1.16 | −4.077 | <0.001 | 2.08 | 1.18 | −4.275 | <0.001 | −0.65 | −0.78 | −1.238 | 0.216 | |
| T | 1.50 ± 0.59 | 1.27 ± 0.53 | 2.944 | 0.005 | 1.58 ± 0.58 | 1.56 | −0.194 | 0.847 | −0.27 | −0.13 | −2.089 | 0.037 | |
| DHEA | 32.14 | 23.21 | −2.614 | 0.009 | 29.73 | 25.17 | −2.174 | 0.030 | 487 ± 13.26 | −5.79 ± 17.17 | −0.279 | 0.781 | |
| DHT | 9.74 | 6.83 | −4.244 | <0.001 | 9.18 | 7.22 | −2.587 | 0.010 | −2.29 ± 3.47 | -l.34 ± 3.43 | 1.432 | 0.155 | |
| FT | 9.92 | 5.28 | −4.490 | <0.001 | 7.93 | 5.74 | −5.106 | <0.001 | −3.14 | −2.79 | −0.012 | 0.990 | |
| El | 267.4 | 193.65 | −4.736 | <0.001 | 235.85 | 174.85 | −2.915 | 0.004 | •89.62 | −53.45) | −2.363 | 0.018 | |
| E2 | 136.25 | 104.0 | −2.860 | <0.001 | 128.05 | 132.35 | −1.402 | 0.161 | −44.11 | −22.03 | −0.826 | 0.409 | |
| SHBG | 36.02 | 147.70 | •5.739 | <0.001 | 42.77 | 200 | −5.704 | <0.001 | 91.24 | 128.61 | −2.529 | 0.011 | |
| FAI | 4.04 | 0.82 | −5.954 | <0.001 | 3.31 | 1.01 | −5.928 | <0.001 | −3.44 | −2.52 | −0.568 | 0.570 | |
| Glucose metabolism | CRP | 1.21 | 1.51 | −0.198 | 0.843 | 0.83 | 0.55 | −2.700 | 0.007 | 0.09 | −0.29 | −1.865 | 0.062 |
| FPG | 5.40 | 5.05 | −1.519 | 0.129 | 5.11 ± 0.57 | 5.00 ± 0.65 | −2.470 | 0.014 | −0.25 | −0.30 | −0.188 | 0.198 | |
| FINS | 13.99 | 14.42 | −0.495 | 0.621 | 14.31 | 11.31 | −1.890 | 0.059 | −0.54 | −1.81 | −0.919 | 0.358 | |
| IUCA | 276.62 | 325.70 | −1.985 | 0.047 | 265.05 | 269.84 | −0.564 | 0.573 | 33.06 | −4.02 | −1.674 | 0.094 | |
| HOMA-IR | 3.74 | 3.21 | −0.254 | 0.799 | 3.20 | 2.48 | −2.174 | 0.030 | −0.34 | −0.53 | −1.115 | 0.265 | |
| HbAlc | 5.15 | 5.10 | −1.072 | 0.284 | 5.1610.28 | 5.1610.28 | −2.540 | 0.011 | −0.05 | −0.10 | −0.754 | 0.451 | |
| 0.5G1U | 8.90 | 8.95 | −0.164 | 0.870 | 9.18 ± 1.43 | 8.70 ± 1.23 | 2.191 | 0.033 | 0.45 | −0.40 | −1.288 | 0.198 | |
| IhGlu | 9.35 | 9.35 | −0.818 | 0.413 | 8.97 ± 2.67 | 8.90 ± 2.09 | 0.180 | 0.858 | −0.20 | −0.05 | −0.688 | 0.491 | |
| 2hGlu | 7.20 | 7.30 | −0.108 | 0.914 | 6.65 | 7.60 | −1.637 | 0.102 | 0.00 | 0.30 | −1.202 | 0.230 | |
| 3hGlu | 4.80 | 4.90 | .0.173 | 0.863 | 4.66 ± 1.07 | 4.6811.45 | −0.061 | 0.952 | 0.10 | 0.15 | −0.212 | 0.832 | |
| 0.5hIns | 109.45 | 116.97 | −1.795 | 0.073 | 102.63 | 96.59 | −0.555 | 0.579 | 15.55 ± 66. 19 | −8.94 ± 65.79 | −1.929 | 0.056 | |
| Ihlns | 117.19 | 149.47 | −2.553 | 0.011 | 114.02 | 125.60 | −0.047 | 0.962 | 19.10 | −10.08 | −1.816 | 0.069 | |
| 2hlns | 116.10 | 128.55 | −1.649 | 0.099 | 99.14 | 97.75 | −0.504 | 0.614 | 16.01 ± 66. 35 | −8.94 ± 65.79 | −1.658 | 0.100 | |
| 3hlns | 44.63 | 35.49 | −0.779 | 0.436 | 31.18 | 26.22 | −2.001 | 0.045 | −7.94 | −3.75 | −0.802 | 0.423 | |
| HOMA•B | 185.57 | 190.14 | −0.883 | 0.377 | 143.77 | 138.16 | −0.022 | 0.983 | 15.80 | 4.98 | −0.752 | 0.452 | |
4. Discussion
Here, GO and KEGG functions of 34 oocytes at GV, MI and MII stages, as well as of aneuploid oocytes, revealed that the pathway responsible for IR mainly functioned in the GV phase, while processes associated with ovulation disorders were mainly down regulated in GV and MI phases. Analysis of DEGs involved in the ovulation process during GV and MI phases revealed PGR, SIRT1 and ADAMTS1 to be hub DEGs, and all to down regulated in the setting of PCOS. Diseases associated with PGR mainly include progesterone resistance and myoma formation, with the responsible cellular process being oocyte meiosis. PGR plays a central part in reproductive events associated with the establishment and maintenance of pregnancy, and encodes a member of the steroid receptor superfamily, its expression was previously found to be up regulated in the PCOS endometrium [25–27], while in our study, it was found to be down regulated in oocytes. SIRT1 was associated with ageing, cellular senescence and the p53 pathway, the level of serum SIRT1 was to be higher in PCOS patients as compared to controls [28], but the expression of SIRT1 was significantly lower in ovarian tissues as compared to controls in the setting of PCOS, and it was consistent with our findings. Exenatide has been reported to be of therapeutic value for PCOS by its up regulation of SITR1 expression [29,30]. ADAMTS1 was reported relevant to premature ovarian failure and menopause and to be significantly increased in granulosa cells in PCOS [31]. ADAMTS1 expression was found to exhibit a positive correlation with oocyte fertilisation rate [32] and more mature oocytes, transplantable embryos and better-quality embryos [33]; however, we found it to be significantly down regulated in our analysis. via the screening of drugs relevant to these 3 DEGs, we found that most therapeutic drugs targeted PGR and the majority of these were progesterone drugs, exerting both progestogenic and anti-androgenic effects. In clinical treatment, the most common strategy targeting IR includes the use of insulin sensitiser drugs, particularly metformin, which produces similar effects to lifestyle interventions such as a decreased body weight, but is superior in terms of decreasing androgen concentrations, and it was consistent with our clinical study. Furthermore, the combination of metformin and OC administration likely prevents any deterioration in metabolic function.
Oligoovulation and anovulation are the primary aetiologies for female infertility in PCOS, resulting in menstrual dysfunction and endometrial hyperplasia, thereby increasing the risk of endometrial cancer [34]. Administration of OC has been traditionally used for endometrial protection and attenuation of hyperandrogenism [35] and the use of a levonorgestrel-releasing intrauterine device, these were essentially consistent with screening drugs in Table 2. Metformin was also reported to assist with the prevention of ovarian hyperstimulation syndrome in the setting of in vitro fertilisation [36] and improved ovulation and pregnancy rates [8]. Here, we validated Diane-35 tablets which contained cyproterone acetate (2 mg) and ethinylestradiol (0.035 mg). Cyproterone acetate, previously screened for associating with foetal growth restriction, exerts both progestogenic and anti-androgenic effects. Metformin and pioglitazone are insulin sensitisers that improve IR. Diane-35 combined with metformin was found to significantly increase SIRT1 expression in rat PCOS ovarian tissues [30], and combination therapy resulted in decreased body weights, levels of luteinizing hormone and testosterone and IR[30]. Here, the combination of Diane-35 with metformin was found to significantly reduce weight and BMI in both groups as compared to pre-treatment. No statistical difference among group 1, using one insulin sensitiser, and group 2, using two insulin sensitisers, was noted. In addition, serum androgen and luteinizing hormone levels in both groups decreased significantly compared to pre-treatment, with no significant difference noted among groups. This phenomenon likely manifested due to cyproterone acetate reducing androgen levels and inhibiting hypothalamic GnRHa, thereby leading to a decrease in serum luteinizing hormone levels. Ultimately, a combination of insulin sensitisers is likely more effective in improving glucose metabolism.
5. Conclusion
The pathogenesis of PCOS and the development of ovulation disorders in this condition remains unclear. Metformin, however, is known to improve IR and ovulation. In the clinical setting, clomiphene citrate and letrozole are considered to be the first-line agents for ovulation induction [36]. Metformin reduces the risk of ovarian hyperstimulation syndrome, which together with ovarian laparoscopic surgery are used as second-line treatment [36]. Although the PGR progesterone agonist drugs have been studied in greater detail, methods of SIRT1 and ADAMTS1 modulation require further investigation. Besides it is difficult to collect oocyte from PCOS and control patients to do further validation, which is a limitation of our research, so it need to be validated by further subsequent studies.
Acknowledgment
The authors thanks to Yanfang Lu for his help in the data analysis process.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (ethical approval number for human research: 0241-01) and based on the World Medical Association Declaration of Helsinki.
Author contributions
We certify that Prof. Gao Ying and Yanhui Li have participated sufficiently in the intellectual content, and Zhenzhen Lu was major involved in the work of conception, design of this research, analysis and interpretation of the data, as well as the writing and revising of the manuscript, and Chunyan Chen was mainly involved in the design and analysis process of clinical data. And all other authors were involved in the process of the design, the analysis of bioinformatics data, the integration of the figures and the revisions. All authors agreed to be accountable for all aspects of the work and on the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All datasets involved in the article are available in the GEO database (GEO, https://www.earthobservations.org/index.php; accession number PRJNA600740).
References
- 1.Bannigida DM, Nayak BS, Vijayaraghavan R.. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem. 2020;126(2):183–186. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam EA-S. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Garrido MA, Tena-Sempere M.. Metabolic dysfunction in polycystic ovary syndrome: Pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghetti P, Tosi F.. Insulin resistance and PCOS: chicken or egg? J Endocrinol Invest. 2021;44(2):233–244. [DOI] [PubMed] [Google Scholar]
- 5.Tosi F, Bonora E, Moghetti P.. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. 2017;32(12):2515–2521. [DOI] [PubMed] [Google Scholar]
- 6.Gorry A, White DM, Franks S.. Infertility in polycystic ovary syndrome: focus on low-dose gonadotropin treatment. Endocrine. 2006;30(1):27–33. [DOI] [PubMed] [Google Scholar]
- 7.Zhou R, Li S, Liu J, et al. Up-regulated FHL2 inhibits ovulation through interacting with androgen receptor and ERK1/2 in polycystic ovary syndrome. EBioMedicine. 2020;52:102635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. [DOI] [PubMed] [Google Scholar]
- 9.Dapas M, Lin FTJ, Nadkarni GN, et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17(6):e1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. The Lancet. 2007;370(9588):685–697. [DOI] [PubMed] [Google Scholar]
- 11.Laven JSE. Follicle stimulating hormone receptor (FSHR) polymorphisms and polycystic ovary syndrome (PCOS). Front Endocrinol (Lausanne). 2019;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway G, Dewailly D, Diamanti-Kandarakis E, ESE PCOS Special Interest Group, et al. The polycystic ovary syndrome: a position statement from the european society of endocrinology. Eur J Endocrinol. 2014;171(4):P1–29. [DOI] [PubMed] [Google Scholar]
- 13.Teede HJ, Misso ML, Costello MF, International PCOS Network, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stepto NK, Patten RK, Tassone EC, et al. Exercise recommendations for women with polycystic ovary syndrome: is the evidence enough? Sports Med. 2019;49(8):1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepto N, Hiam D, Gibson-Helm M, et al. Exercise and insulin resistance in PCOS: muscle insulin signalling and fibrosis. Endocr Connect. 2020;9:346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grun D, van Oudenaarden A.. Design and analysis of single-cell sequencing experiments. Cell. 2015;163(4):799–810. [DOI] [PubMed] [Google Scholar]
- 17.Gao S. Data analysis in Single-Cell transcriptome sequencing. Methods Mol Biol. 2018;1754:311–326. [DOI] [PubMed] [Google Scholar]
- 18.Ziegenhain C, Vieth B, Parekh S, et al. Comparative analysis of Single-Cell RNA sequencing methods. Mol Cell. 2017;65(4):631–643 e4. [DOI] [PubMed] [Google Scholar]
- 19.Efremova M, Vento-Tormo M, Teichmann SA, et al. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc. 2020;15(4):1484–1506. [DOI] [PubMed] [Google Scholar]
- 20.Thomas PD, Hill DP, Mi H, et al. Gene ontology causal activity modeling (GO-CAM) moves beyond GO annotations to structured descriptions of biological functions and systems. Nat Genet. 2019;51(10):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Gene Ontology, C. The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabbani G, Baig MH, Ahmad K, et al. Protein-protein interactions and their role in various diseases and their prediction techniques. Curr Protein Pept Sci. 2018;19(10):948–957. [DOI] [PubMed] [Google Scholar]
- 23.Athanasios A, Charalampos V, Vasileios T, et al. Protein-Protein interaction (PPI) network: Recent advances in drug discovery. Curr Drug Metab. 2017;18(1):5–10. [DOI] [PubMed] [Google Scholar]
- 24.Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong F, Xiao J, Bai Y, et al. Metformin inhibits estradiol and progesterone-induced decidualization of endometrial stromal cells by regulating expression of progesterone receptor, cytokines and matrix metalloproteinases. Biomed Pharmacother. 2019;109:1578–1585. [DOI] [PubMed] [Google Scholar]
- 26.Hu M, Zhang YH, Feng JX, et al. Uterine progesterone signaling is a target for metformin therapy in PCOS-like rats. J Endocrinol. 2018;237(2):123–137. [DOI] [PubMed] [Google Scholar]
- 27.Hu M, Li J, Zhang Y, et al. Endometrial progesterone receptor isoforms in women with polycystic ovary syndrome. Am J Transl Res. 2018;10(8):2696–2705. [PMC free article] [PubMed] [Google Scholar]
- 28.Kiyak Caglayan E, Engin-Ustun Y, Gocmen AY, et al. Serum sirtuin 1 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2015;35(6):608–611. [DOI] [PubMed] [Google Scholar]
- 29.Tao X, Zhang X, Ge SQ, et al. Expression of SIRT1 in the ovaries of rats with polycystic ovary syndrome before and after therapeutic intervention with exenatide. Int J Clin Exp Pathol. 2015;8(7):8276–8283. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Tu H, Yao J, et al. Combined use of diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod Biol Endocrinol. 2020;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G, Yao G, Xu Z, et al. Expression level of ADAMTS1 in granulosa cells of PCOS patients is related to granulosa cell function, oocyte quality, and embryo development. Front Cell Dev Biol. 2021;9:647522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil K, Hinduja I, Mukherjee S.. Alteration in angiogenic potential of granulosa-lutein cells and follicular fluid contributes to luteal defects in polycystic ovary syndrome. Hum Reprod. 2021;36(4):1052–1064. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Jin J, Tong X, et al. ADAMTS1 and HSPG2 mRNA levels in cumulus cells are related to human oocyte quality and controlled ovarian hyperstimulation outcomes. J Assist Reprod Genet. 2020;37(3):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azziz R, Carmina E, Dewailly D, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society, et al. The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488., [DOI] [PubMed] [Google Scholar]
- 35.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 36.Balen AH, Morley LC, Misso M, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets involved in the article are available in the GEO database (GEO, https://www.earthobservations.org/index.php; accession number PRJNA600740).