Abstract
Purpose:
CT900 is a novel small molecule thymidylate synthase inhibitor that binds to α-folate receptor (α-FR) and thus is selectively taken up by α-FR–overexpressing tumors.
Patients and Methods:
A 3+3 dose escalation design was used. During dose escalation, CT900 doses of 1–6 mg/m2 weekly and 2–12 mg/m2 every 2 weeks (q2Wk) intravenously were evaluated. Patients with high-grade serous ovarian cancer were enrolled in the expansion cohorts.
Results:
109 patients were enrolled: 42 patients in the dose escalation and 67 patients in the expansion cohorts. At the dose/schedule of 12 mg/m2/q2Wk (with and without dexamethasone, n = 40), the most common treatment-related adverse events were fatigue, nausea, diarrhea, cough, anemia, and pneumonitis, which were predominantly grade 1 and grade 2. Levels of CT900 more than 600 nmol/L needed for growth inhibition in preclinical models were achieved for >65 hours at a dose of 12 mg/m2. In the expansion cohorts, the overall response rate (ORR), was 14/64 (21.9%). Thirty-eight response-evaluable patients in the expansion cohorts receiving 12 mg/m2/q2Wk had tumor evaluable for quantification of α-FR. Patients with high or medium expression had an objective response rate of 9/25 (36%) compared with 1/13 (7.7%) in patients with negative/very low or low expression of α-FR.
Conclusions:
The dose of 12 mg/m2/q2Wk was declared the recommended phase II dose/schedule. At this dose/schedule, CT900 exhibited an acceptable side effect profile with clinical benefit in patients with high/medium α-FR expression and warrants further investigation.
Translational Relevance.
α-Folate receptor (α-FR) is overexpressed in a variety of solid tumors including high-grade serous ovarian cancer, triple-negative breast cancer, and non–small cell lung cancer. CT900 is a small-molecule thymidylate synthase (TS) inhibitor that binds to and is taken up by α-FR. This is the first clinical trial to show reproducible single-agent responses caused by a small molecule in α-FR–overexpressing cancers. The toxicity profile did not show classical TS inhibitor toxicity such as myelosuppression suggesting differential α-FR–mediated uptake in tumor tissue. This trial is proof of concept for developing α-FR–targeting small molecules that are selectively taken up in α-FR–overexpressing tumors for patient benefit and are an alternate approach to antibody–drug conjugates that already target α-FR.
Introduction
The α-folate receptor (α-FR) is highly overexpressed during malignant transformation (1) and is known to be expressed on the surface of tumor cells in ovarian cancer, triple-negative breast cancer, endometrial cancer, mesothelioma, and lung cancer (2–11).
Up to 90% of ovarian cancers are reported to constitutively express α-FR, whereas it is scarcely expressed in nonmalignant tissues (6), making it an important cancer drug target (12). Multiple therapeutic approaches, including antibodies, folate drug conjugates, and antibody–drug conjugates, have been used and, despite promising results from early phase trials, there are currently no approved α-FR–targeted anticancer drugs (12).
CT900 (also known as ONX-0801 and BCG945) is a novel, cyclopentaquinazoline-based, α-FR–targeted thymidylate synthase inhibitor that specifically enters cancer cells following binding to α-FR and does not utilize the reduced folate carrier (RFC; refs. 13, 14). CT900, however, does not have a classic small-molecule or antibody-cleavable linker–payload structure of several other α-FR–targeted therapies, such as vintafolide (15) or mirvetuximab soravtansine (16).
A multicenter, open-label, dose-escalation, phase I clinical study in adult patients with histologically or cytologically proven solid tumors was conducted with the primary aim to identify a recommended dose and schedule for phase II evaluation by establishing the MTD and a safety profile. Secondary aims included defining a pharmacokinetic profile and tertiary aims included establishing antitumor activity, evaluating pharmacodynamic activity, and evaluating correlations of clinical response to α-FR in archival tumor tissue.
Patients and Methods
Study conduct
This multicenter, open-label, dose-escalation, phase I clinical study (EudraCT number: 2013-000569-34; ClinicalTrials.gov identifier: NCT02360345; ref. 17) of CT900 was cosponsored by The Institute of Cancer Research (ICR) and The Royal Marsden NHS Foundation Trust (RM). The study was conducted in accordance with Good Clinical Practice (GCP) legislation and the ethical principles enunciated in the Declaration of Helsinki (October 2013). Approvals from the Medicines and Healthcare products Regulations Agency (MHRA) and the relevant Research Ethics Committee (REC) were obtained before the start of the study. All patients provided fully informed written consent.
The study comprised two stages: the dose escalation phase, in which the MTD was determined, and the expansion phase, in which evaluable patients were treated at the MTD and schedule to further support the design of subsequent studies of CT900.
Inclusion criteria
Main inclusion criteria for the dose escalation included patients with advanced cancers who had received standard-of-care treatment, age at least 18 years with measurable or evaluable disease, life expectancy of at least 12 weeks, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. The patients were required to have adequate organ bone marrow, renal, and liver function routine for phase I oncology trials. In addition, patients were required to have a forced vital capacity (FVC) of >70% and a diffusion capacity for carbon monoxide corrected for hemoglobin (DLCOc) of >60%. The expansion cohorts were limited to patients with high-grade serous ovarian cancer.
Treatment schedules
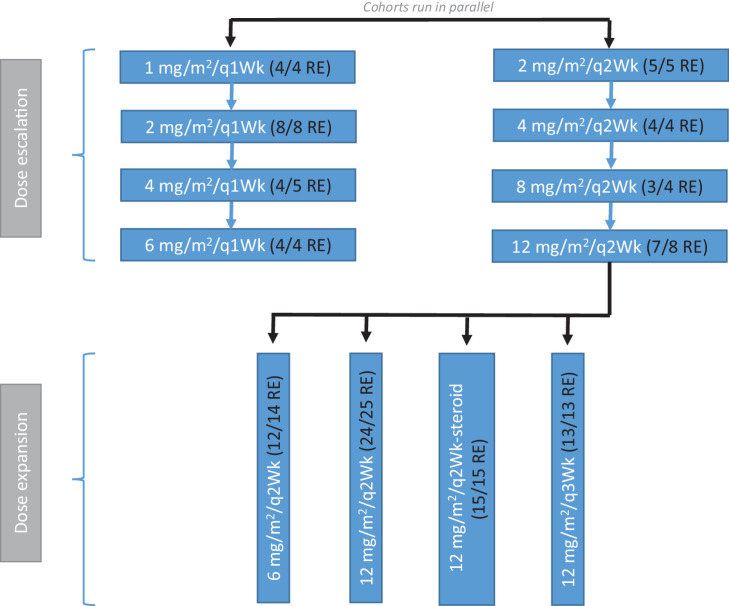
For the dose escalation portion of the study, patients were dosed either weekly (qWk) or once every 2 weeks (q2Wk). A period of 4 weeks was considered one cycle (four doses in the qWk schedule and two doses in the q2Wk schedule). The first two cycles were considered to be the dose-limiting toxicity (DLT) window. Following the establishment of the recommended phase II dose and an amendment, two further schedules were studied: once every 2 weeks with dexamethasone prophylaxis (q2Wk-steroid) or once every 3 weeks (q3Wk; Fig. 1).
Figure 1.
CONSORT diagram. RE, response evaluable patients/cohort.
Patients could initially receive up to six cycles of treatment. Patients in the q3Wk cohort could have up to eight cycles if they completed six cycles without pulmonary toxicity or disease progression. The limit of the total number of cycles based on data from a previous unpublished trial of this drug Eudra CT 2009-012933-31 where pulmonary toxicity was shown to occur at a median cumulative dose of 162 mg/m2. Pharmacokinetic modeling suggested relevant concentrations approximately 10 mg/m2, thus at 12 mg/m2 of CT900 administered once every two weeks (24 mg/m2/month or cycle), 6 months of treatment would reach a cumulative dose of 144 mg/m2 which would be lower than 162 mg/m2.
Patients who received less than 75% of the planned doses of CT900 in the first two treatment cycles for reasons other than toxicity in either phase were replaced. Patients had to receive two cycles of CT900 to be evaluable for escalation decisions in the dose escalation phase.
Study design
The study used a 3+3 dose escalation design during dose escalation. Each cohort consisted of 3 patients, and this was expanded to 6 evaluable patients if a DLT was observed. The qWk and q2Wk cohorts in the expansion were recruited to in parallel. The recommended phase II dose was based on a minimum of 6 patients. The expansion cohort was started as a q2Wk schedule and following that three further expansion cohorts, that is, 6 mg/m2, 12 mg/m2/q2Wk-steroid, and 12 mg/m2/q3Wk were recruited to in parallel (Fig. 1).
Criteria for evaluation
Safety and tolerability
Safety and tolerability of CT900 were assessed by monitoring the nature and frequency of adverse events (AE) using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI CTCAE v4.0) and laboratory abnormalities (biochemistry, hematology, urinalysis), pulmonary function tests (PFT), vital signs, electrocardiogram (ECG), and physical examination. The dose-limiting period window was two cycles (8 weeks for the weekly and once every 2 weekly schedules and 6 weeks for the once every 3 week schedules).
Pharmacokinetics
The plasma concentration/time data were analyzed using noncompartmental methods. The PK parameters determined for CT900 included area under the plasma concentration versus time curve (AUC) from zero to the last value above the limit of quantification (AUClast), from zero extrapolated to infinite time (AUC∞), and from 0 to 24 hours (AUC0–24); maximum observed plasma concentration (Cmax); time to Cmax (Tmax); terminal elimination half-life (t1/2); terminal rate constant (λz); clearance (CL); and steady-state volume of distribution (Vss).
Pharmacodynamics
Patients who provided a separate, optional consent underwent an 18F-FLT PET/CT (3′-Deoxy-3′-[18F]-fluorothymidine (FLT) PET/CT) scan at either dose escalation or dose expansion. Each patient received an 18F-FLT PET/CT scan at baseline and a second scan 16–26 hours after the first dose of CT900. Maximum standardized uptake value (SUVmax) was quantified.
Efficacy
To enroll, patients were required to have measurable disease according to the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1). For patients with ovarian cancer, CA125 levels were assessed according to the Gynecologic Cancer InterGroup (GCIG) criteria. Clinical and radiologic assessments were used to assess response to CT900. Disease response in patients with ovarian cancer was determined by CA125 levels according to GCIG criteria. All patients who met the eligibility criteria received at least two cycles of study medication and had a baseline assessment of disease evaluable for response by RECIST v1.1.
Predictive biomarkers
α-FR expression in tumor biopsy samples was primarily evaluated using the Ventana FOLR1 (FOLR1-2.1) CDx assay (Roche Diagnostics), which comprises IHC-compatible murine mAb (clone FOLR1-2.1) with high specificity for human α-FR. Samples were scored by the percentage of cells with greater than or equal to 2+ membranous staining intensity (PS2+ Scoring). Patients were then classified as follows based on PS2+ scoring percentage: 0%–24%, negative/very low; 25%–49%, low; 50%–74%, medium; and ≥75%, high (18–21). Sample images of the Ventana assay are shown in the Supplementary Data (Supplementary Fig. S1).
α-FR expression in tumor biopsy samples was also assessed using an in-house IHC assay with a commercially available monoclonal antibody (BN3.2) developed by ICR to detect the protein. Patients were scored on the basis of the level of staining observed at the cell membrane and the proportion of cells that were stained positive.
Statistical analysis
All analyses in this study were descriptive in nature. Descriptive summaries of continuous variables included the mean, median, SD, range, and, where appropriate, 95% confidence interval (CI) and/or interquartile range (IQR). Descriptive summaries of categorical variables included number of patients with an observation (n) and proportion (%).
Role of the funding source
This is an academically sponsored study. Funding was provided by the following pharmaceutical companies: Onyx Pharmaceuticals, BTG International, and Carrick Therapeutics. In addition to the academic sponsors, Carrick Therapeutics was involved in the analysis, interpretation, writing of the report, and decision to submit the article for publication.
Data availability
Qualified scientific and medical researchers can request patient level anonymized clinical data for research purposes. These will be reviewed by sponsor prior to granting access and decisions depend on purpose/scientific merit of the proposal and availability of the data. Requests are to be sent to the corresponding author.
Results
Demographics
A total of 109 patients were enrolled in the study (42 patients in the dose escalation and 67 patients in the expansion cohorts) between 2013 and 2019. The male-to-female ratio of 11:98 reflected the expansions conducted in patients with ovarian cancer. The median age of patients was 62 (range 55–67) years, and the median number of previous lines of treatment was 5 (range 1–13; Table 1).
Table 1.
Demographics in escalation and expansion.
| Escalation | Expansion | |
|---|---|---|
| Total | Total | |
| N = 42 | N = 67 | |
| N (%) | N (%) | |
| Age (years) | ||
| Median (IQR) | 61.0 (55.0–63.8) | 62.0 (57.5–68.0) |
| Sex | ||
| Female | 31 (73.8) | 67 (100.0) |
| Male | 11 (26.2) | 0 (0) |
| ECOG performance status | ||
| 0 | 15 (35.7) | 14 (20.9) |
| 1 | 27 (64.3) | 53 (79.1) |
| Unknown | 0 (0) | 0 (0) |
| Primary diagnosis | ||
| Breast | 1 (2.4) | 0 (0) |
| Colonic/Rectal | 7 (16.7) | 0 (0) |
| Oesophageal | 1 (2.4) | 0 (0) |
| Gastric | 1 (2.4) | 0 (0) |
| Head and Neck | 1 (2.4) | 0 (0) |
| Ocular | 2 (4.8) | 0 (0) |
| Ovarian/Cervical/Endometrial | 25 (59.5) | 63 (94.0) |
| Fallopian tube/Omentum/Peritoneum | 0 (0) | 4 (6.0) |
| Pancreatic | 3 (7.1) | 0 (0) |
| Renal | 1 (2.4) | 0 (0) |
| Prior lines of treatment | ||
| Median (range) | 4 (1–12) | 5 (1–13) |
Dose escalation
Two schedules were explored in parallel: qWk and q2Wk (Fig. 1). During the dose escalation cohort, there was only one protocol-defined DLT within the first 8 weeks in the qWk schedule: cellulitis at 2 mg/m2, which occurred in a patient with ovarian cancer who had extensive pre-existing edema of the abdominal wall. The DLT necessitated an expansion to 6 patients in the cohort. One patient treated at 4 mg/m2/qWk developed a grade 3 pneumonitis after completing six cycles of treatment. Thus, although 6 mg/m2/qWk and 12 mg/m2/q2Wk were considered tolerable by protocol-defined criteria (i.e., no DLTs in the first 8 weeks of treatment), the decision was made to explore 12 mg/m2/q2Wk in the expansion.
Following treatment of 25 patients at 12 mg/m2/q2Wk, three further cohorts were explored to evaluate tolerability and efficacy. These were 6 mg/m2/q2Wk, 12 mg/m2/q2Wk-steroid prophylaxis, and 12 mg/m2/q3Wk.
Tolerability
qWk schedule
The qWk schedule explored dose levels of 1, 2, 4, and 6 mg/m2 and a total of 21 patients were treated. There were three drug-related NCI CTCAE ≥grade 3 events: anemia (6 mg/m2 in Cycle 3), cellulitis (2 mg/m2 in Cycle 1), and pneumonitis (4 mg/m2 after completing Cycle 6; Supplementary Table S1). The grade 3 cellulitis that occurred was a DLT; therefore, the 2 mg/m2/qWk cohort was expanded to 6 patients.
q2Wk schedule
The q2Wk schedule explored dose levels of 2, 4, 8, and 12 mg/m2 (n = 21). This schedule was better tolerated than the qWk schedule, with only one drug-related grade 3 event of anemia recorded (8 mg/m2 in Cycle 1; Supplementary Table S2).
Expansion cohorts
Two expansion cohorts explored doses other than 12 mg/m2/q2Wk. These were 6 mg/m2/q2Wk (n = 14) and 12 mg/m2/q3Wk (n = 13). Drug-related ≥grade 3 toxicities included anemia (two patients at 6 mg/m2/q2Wk), alanine aminotransferase increase (one patient at 6 mg/m2/q2Wk), and hyponatremia (two patients at 12 mg/m2/q3Wk) (Supplementary Table S3).
Two expansion cohorts explored 12 mg/m2/q2Wk: one without prophylactic steroids (n = 25) and the other with prophylactic steroids (n = 15). Drug-related NCI CTCAE ≥grade 3 events were anemia (two patients in the 12 mg/m2/q2Wk-steroid cohort), diarrhea (one patient each in the 12 mg/m2/q2Wk and 12 mg/m2/q2Wk-steroid cohorts), influenza-like illness (one patient at 12 mg/m2/q2Wk), and atrial fibrillation (one patient at 12 mg/m2/q2Wk). The most common side effects (all grades) reported with 12 mg/m2/q2Wk (without or with steroid prophylaxis) were fatigue (53%), nausea (40%), diarrhea (23%), cough (23%), anemia (20%), and pneumonitis (20%; Table 2). All these side effects were grade 1–2 apart from two cases each of grade 3 diarrhea and grade 3 anemia. The use of steroid prophylaxis had minimal impact on the tolerability profile.
Table 2.
Treatment-related toxicities reported in the 12 mg/m2/q2Wk-steroid expansion cohort by the dose level assigned and worst recorded NCI CTCAE grade (only seen in 10% or more in cohorts specified below shown).
| 12 mg/m2/q2Wk | 12 mg/m2/q2Wk-steroid | ||||||
|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G1 | G2 | G3 | Total | |
| Preferred terms | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Fatigue | 12 (48) | 1 (4) | 0 (0) | 6 (40) | 2 (13.3) | 0 (0) | 21 (53) |
| Nausea | 8 (32) | 4 (16) | 0 (0) | 4 (26.7) | 0 (0) | 0 (0) | 16 (40) |
| Diarrhea | 4 (16) | 1 (4) | 1 (4) | 2 (13.3) | 0 (0) | 1 (6.7) | 9 (23) |
| Cough | 8 (32) | 0 (0) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 9 (23) |
| Anemia | 2 (8) | 2 (8) | 0 (0) | 1 (6.7) | 1 (6.7) | 2 (13.3) | 8 (20) |
| Pneumonitis | 0 (0) | 4 (16) | 0 (0) | 1 (6.7) | 3 (20) | 0 (0) | 8 (20) |
| Pyrexia | 3 (12) | 0 (0) | 0 (0) | 4 (26.7) | 0 (0) | 0 (0) | 7 (18) |
| Aspartate aminotransferase increased | 3 (12) | 1 (4) | 0 (0) | 3 (20) | 0 (0) | 0 (0) | 7 (18) |
| Vomiting | 4 (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 4 (10) |
| Chills | 2 (8) | 1 (4) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 4 (10) |
| Decreased appetite | 3 (12) | 0 (0) | 0 (0) | 1 (6.7) | 0 (0) | 0 (0) | 4 (10) |
Note: Grade 3 toxicities in bold text; there were no grade 4 toxicities.
Ten cases of pneumonitis were reported in the expansion cohorts (n = 67) and were considered adverse effects of special interest. One patient in the 4 mg/m2/qWk dose escalation cohort had grade 3 pneumonitis, observed radiologically, after completing six cycles of treatment. There were no signs of pneumonitis on CT scans after Cycle 2 and Cycle 4 in this patient. There were no cases of fatal pneumonitis during the trial. All other cases were grade 1–2 and were diagnosed radiologically.
Overall, there was no clinically significant difference in tolerability between the 12 mg/m2/q2Wk and 12 mg/m2/q2Wk-steroid cohorts (Table 2).
Pharmacokinetics
The pharmacokinetic parameters are shown in Table 2. At the 12 mg/m2 dose level the geometric mean of the Cmax was 5,101 ng/mL [percentage coefficient of variation of the geometric mean (CV% Geo Mean), 12.2] and the geometric mean of the AUClast was 85,858 h.ng/mL (CV% Geo Mean, 22; Table 3). The terminal half-life was 28.7 hours. The plasma concentration exceeded the target concentration of 600 nmol/L for more than 65 hours which is predicted to be efficacious based on preclinical studies (ref. 13; Supplementary Fig. S2).
Table 3.
Summary of CT900 plasma pharmacokinetic parameters from dose escalation phase, Cycle 1 Day 1.
| Cohort (mg/m2) | Descriptive statistic | T max (h) | C max (ng/mL) | HL Lambda_z (h) | AUC0-24 (h.ng/mL) | AUClast (h.ng/mL) | Cl (mL/h) | Vss (mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | Mean | 1.0 | 402 | 6.7 | 1,654 | 1,654 | 1,219 | 7,594 |
| CV% | 1.7 | 7.2 | 47.6 | 39.7 | 39.7 | 47.7 | 19.7 | |
| Geometric Mean | 1.0 | 402 | 6.1 | 1,565 | 1,565 | 1,116 | 7,501 | |
| CV% Geo Mean | 1.8 | 7.2 | 62.2 | 43.4 | 43 | 57.6 | 19.0 | |
| Median | 1.0 | 401 | 7.5 | 1,618 | 1,618 | 1,250 | 6,745 | |
| Range | 1.0–1.0 | 374–432 | 3.2–9.4 | 1,017–2,329 | 1,017–2,329 | 623–1,784 | 6,712–9,324 | |
| N | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |
| 2 | Mean | 0.9 | 746 | 12.6 | 4,892 | 6,857 | 674 | 8,519 |
| CV% | 29.6 | 14.4 | 42.1 | 42.9 | 63.0 | 75.1 | 34.2 | |
| Geometric Mean | 0.9 | 740 | 11.8 | 4,541 | 5,855 | 552 | 8,193 | |
| CV% Geo Mean | 37.1 | 14.9 | 41.9 | 48.2 | 75 | 82.6 | 32.1 | |
| Median | 1.0 | 759 | 11.3 | 4,733 | 6,046 | 523 | 7,407 | |
| Range | 0.5–1.1 | 613–855 | 7.5–20.0 | 2,516–7,588 | 2,516–12,818 | 244–1,405 | 6,534–12,730 | |
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |
| 6 | Mean | 1.0 | 2,030 | 19.9 | 15,104 | 25,574 | 460 | 12,024 |
| N | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 12 | Mean | 1.0 | 5,132 | 28.7 | 47,191 | 87,560 | 216 | 7,621 |
| CV% | 1.4 | 12.4 | 24.6 | 13.9 | 23.5 | 29.6 | 11.1 | |
| Geometric Mean | 1.0 | 5,101 | 28.1 | 46,862 | 85,858 | 207 | 7,586 | |
| CV% Geo Mean | 1.4 | 12.2 | 22.4 | 13.0 | 22 | 33.0 | 10.6 | |
| Median | 1.0 | 4,830 | 26.5 | 45,428 | 83,497 | 221 | 7,333 | |
| Range | 1.0–1.0 | 4,460–6,010 | 23.0–41.0 | 42,328–58,611 | 68,732–122,559 | 126–303 | 6,986–9,065 | |
| N | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
Abbreviations: CV%, percentage coefficient of variation; Tmax, time to maximum observed plasma concentration; Cmax, maximum observed plasma concentration; HL λz, half-life terminal rate constant; AUC0-24, area under the plasma concentration versus time curve from 0 to 24 hours; AUClast, area under the plasma concentration versus time curve from 0 to the last value above the limit of quantification; Cl, clearance; Vss, steady state volume of distribution.
Pharmacodynamics
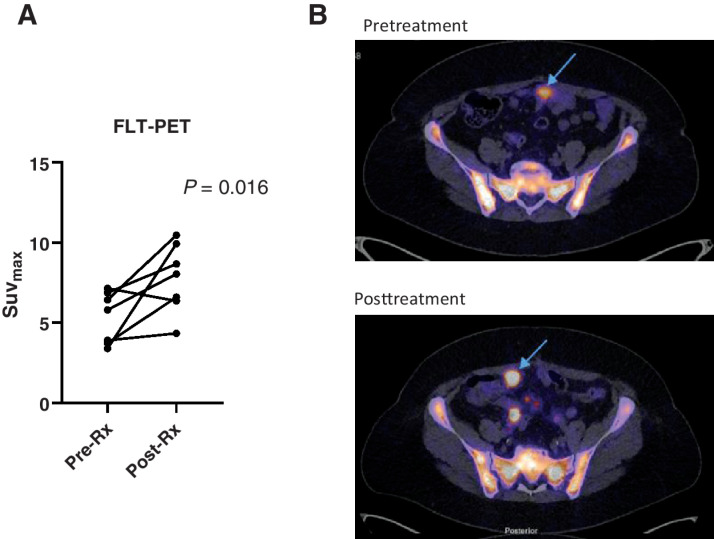
Seven patients treated with CT900 at 12 mg/m2/q2Wk underwent FLT PET/CT studies pretreatment and 16–24 hours posttreatment to study changes in SUVmax in 18F-FLT PET/CT scans. There was an increase in SUVmax in tumor posttreatment in 6 of 7 patients (P = 0.016; Fig. 2A). An example of an increase in 18F-FLT PT signal in ovarian cancer metastasis is shown in Fig. 2B. One further patient treated at 6 mg/m2/q2Wk underwent PET scans at the same time and had a pre- and posttreatment SUVmax of 6.14 and 9.61, respectively.
Figure 2.
Pharmacodynamic studies of CT900. A, Results in 7 patients showing difference in SUVmax in pre- and posttreatment scans. B, Representative images of a patient's 18F-FLT PET/CT scan showing increase in uptake of FLT in tumor in posttreatment.
Efficacy
Twelve patients with high-grade serous ovarian cancer entered the dose escalation cohort in whom 4 of 11 (36.4%) partial responses were observed.
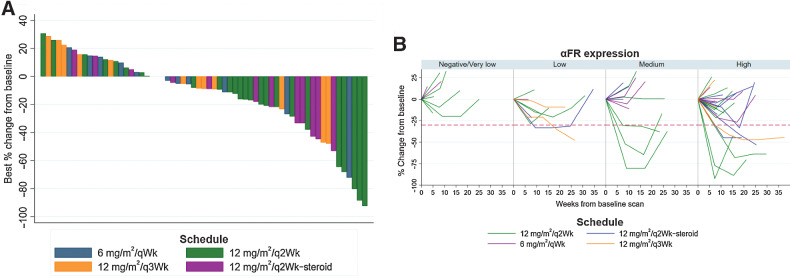
Sixty-four patients with high-grade serous ovarian cancer were treated on the expansion cohorts and were evaluable for response. The overall response rate (ORR) determined by CT or MRI, regardless of α-FR status, was 14/64 [21.9%; 95% CI, 12.5–34; Fig. 3A].
Figure 3.
Efficacy of CT900/Idetrexed in expansion cohorts of patients with ovarian cancer. A, Waterfall plot of patients with high-grade serous ovarian cancer treated on the expansion cohort. Percentage change from baseline in tumor size in the expansion cohort (n = 64*). *All patients with at least one complete follow-up scan, n = 63 shown on waterfall plot. B, Depth and duration of response of patients receiving 12 mg/m2/q2Wk based on α-FR expression.
Thirty-eight response-evaluable patients in the expansion cohorts treated at 12 mg/m2/q2Wk had tumor evaluable for the Ventana FOLR2.1 assay. For patients who received treatment in the 12 mg/m2/qWk cohorts, the RECIST v1.1 ORR was 9/25 (36%; 95% CI, 18–57.5) in patients with medium or high α-FR expression compared with 1/13 (7.7%; 95% CI, 0.2–36) in patients with negative/very low or low expression of α-FR (Fig. 3B; Supplementary Table S4).
In the medium and high α-FR–expressing patients treated at 12 mg/m2/q2Wk, 5/25 (20%; 95% CI, 6.8–40.7) patients received treatment for ≥16 weeks and 2/25 (8%; 95% CI 1–26) received treatment for ≥24 weeks (Supplementary Table S5).
Of interest, 3 patients had previously received an α-FR–targeted antibody–drug conjugate prior to study entry and 1 of these 3 patients had a partial response.
Recommended phase II dose
Grade 3 pneumonitis was observed radiologically in one patient on the qWk schedule at a dose of 4 mg/m2 after six cycles, which is outside the DLT window. Treating additional patients on weekly schedules would likely have increased the incidence of this event, thus weekly schedules were not considered for evaluation in expansions.
Within the q2Wk dosing schedules, 12 mg/m2/q2Wk was the highest dose reached. At this dose, there is a plasma concentration >600 nmol/L for >65 hours. An increase in the 18F-FLT PET signal has been shown to correspond to thymidylate synthase activity in preclinical models (22) and it was possible to show pharmacodynamic activity of CT900 within tumors of patients treated at 12 mg/m2/q2Wk using this approach. Not accounting for α-FR expression status, the RECIST v1.1 response rates in the medium and high α-FR–expressing patients were lower in the 6 mg/m2/q2Wk [0/9 (0%)] and 12 mg/m2/q3Wk [1/5 (20%)] cohorts than in the 12 mg/m2/q2Wk [9/25 (36%)] cohorts. Furthermore, there was no significant improvement in toxicity or reduction in radiologic incidence of pneumonitis with the addition of prophylactic dexamethasone. Taking all these factors into consideration, the dose of 12 mg/m2/q2Wk was considered the recommended phase II dose.
Discussion
This is the first publication about clinical results of CT900 and has multiple novel aspects. It is the first α-FR–targeted drug that has thymidylate synthase inhibitor properties that is internalized by the α-FR but is not linked to an antibody or folic acid moiety by a linker (13) to be evaluated in a clinical trial (13, 15). This is in contrast to antibody–drug conjugates that target α-FR and have maytansine payloads (16), or small molecules that target α-FR and have vinca payloads (15). This is also the first report of a small molecule binding to α-FR that has shown single-agent responses in α-FR–overexpressing cancers. This provides proof of concept for further drug development opportunities focusing on α-FR as a target.
CT900 exhibited an acceptable side effect profile. The majority of AEs experienced during the study were reported as unrelated and mild or moderate in severity and were generally typical for patients with an advanced malignancy. A drug-related, predominantly low-grade pneumonitis was observed in 10/109 (9%) patients on CT900, manifesting as radiologic changes rather than significant symptomatic impairment. The addition of steroid prophylaxis did not alter this clinical picture. The cases of pneumonitis were reported prior to the first reported case of COVID-19 infections in the United Kingdom where the trial was run and were not associated with fever and thus are unlikely to be associated with COVID-19 infections. With appropriate pulmonary monitoring, long-term administration of CT900 appears feasible. The α-FR is known to be expressed on the luminal surface of alveolar cells. Pneumonitis was observed in 2.9% and 9% of patients in trials of another α-FR–targeted agent, mirvetuximab soravtansine, used as a single agent (20) and when combined with bevacizumab, respectively (23).
After a single intravenous infusion, CT900 PK showed low variability in Cmax. Total plasma concentration after infusion of 12 mg/m2 CT900 was maintained above >600 nmol/L for at least 65 hours, a concentration when corrected for plasma binding that has shown efficacy in vitro in α-FR–expressing cells (13). Preclinical studies with BCG945/CT900 have shown that thymidylate synthase (TS) inhibition causes increase in thymidine uptake which can be measured by the increase in 18F-FLT PET signals in tumor tissue (22). A subset of patients underwent pre- and posttreatment 18F-FLT PET scans and showed an increase in 18F-FLT PET signals, posttreatment in tumor tissue, consistent with TS inhibition in tumor tissue.
This study showed the significant clinical benefit of identifying patients with solid tumors which expressed α-FR. In line with the mechanism of action of CT900, patients with a high or medium α-FR expression determined by an IHC assay showed an objective response rate of 36%.
This phase I study has thus used toxicity–pharmacokinetic–pharmacodynamic–biomarker of sensitivity, all important pillars of the pharmacologic audit trial to optimize and recommend a phase II dose and schedule CT900 (24).
CT900 therefore has the potential to help address the significant unmet medical need and poor outcomes for patients with platinum-resistant high-grade serous ovarian cancer where current control treatment arms in randomized trials have a response rate of 4%–12% and progression-free survival of 3.5–4.4 months (20, 25). Of note, apart from mirvetuximab soravtansine with an ORR of 22%–34%, there have been few single-agent objective responses seen with any α-FR–targeting drugs (12, 20, 26). The authors acknowledge the progression-free intervals in patients with CT900 was modest and ways of maximizing this should be explored. Preclinical studies have found synergistic combinations of TS inhibitors and PARP inhibitors (27) and we have shown synergistic growth inhibition caused by the combination of CT900 and PARP inhibition in an α-FR–overexpressing preclinical model (Supplementary Fig. S3). This is a possible path for further development. Further clinical trials of CT900 as a single agent or in combination in α-FR–overexpressing tumors such as high-grade serous ovarian cancer are warranted.
Supplementary Material
Acknowledgments
This is an academically sponsored study, jointly sponsored by The Institute of Cancer Research (ICR) and The Royal Marsden NHS Foundation Trust (RM). Funding was provided by the following pharmaceutical companies: Onyx Pharmaceuticals, BTG International, and Carrick Therapeutics. The National Institute for Health Research Biomedical Research Centre (NIHR BRC) at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research funded 18F-FLT PET/CT scans. Medical writing assistance was provided by Dr. Brigitte Scott, MarYas Editorial Services. The clinical trial was supported by the NIHR BRC to The Institute of Cancer Research (ICR) and The Royal Marsden Hospital NHS Foundation Trust and a Cancer Research UK Centre grant to The ICR. In Manchester this study was supported by the NIHR Manchester Clinical Research Facility. This research was supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014). All recruiting sites acknowledge funding to the Experimental Cancer Medicine Centre initiatives. U. Banerji acknowledges the NIHR grant funding (NIHR-RP-2016-07-028).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Highlights of This Issue, p. 4591
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
S. Banerjee reports other support from Carrick Therapeutics during the conduct of the study; S. Banerjee also reports grants and personal fees from AstraZeneca and GlaxoSmithKline, as well as personal fees from Amgen, Immunogen, Merck Sharpe Dohme, Mersana, Oncxerna, Shattuck Labs, and Clovis outside the submitted work. A. Biondo reports employment with Astex Pharmaceuticals. F. Raynaud reports grants from The Institute of Cancer Research during the conduct of the study. J.C. Porter reports grants from Breathing Matters, GSK, LifeArc, and NIHR, as well as personal fees from Carrick Therapeutics outside the submitted work. A. Turner reports grants from Onyx Pharmaceuticals, Carrick Therapeutics, and BTG during the conduct of the study. S. McIntosh is an employee and shareholder of Carrick Therapeutics. E. Ainscow reports personal fees and other support from Carrick Therapeutics, as well as other support from AstraZeneca PLC during the conduct of the study; E. Ainscow also reports personal fees from Alethiomics Ltd outside the submitted work. In addition, E. Ainscow has a patent for WO2019057825A1 pending. A. Minchom reports personal fees from Janssen Pharmaceuticals, GSK Pharmaceuticals, Merck Pharmaceuticals, Takeda Pharmaceuticals, Genmab Pharmaceuticals, Chugai Pharmaceuticals, Novartis Oncology, and Faron Pharmaceuticals, as well as other support from Amgen Pharmaceuticals outside the submitted work. J. de Bono reports grants and personal fees from Amgen, Astellas, Bayer, Bioxcel Therapeutics, Boehringer Ingelheim, Cellcentric, Daiichi, Eisai, Genentech/Roche, Genmab, GSK, Harpoon, ImCheck Therapeutics, Janssen, Merck Serono, Merck Sharp & Dohme, Menarini/Silicon Biosystems, Orion, Pfizer, Qiagen, Sanofi Aventis, Sierra Oncology, Taiho, Terumo, and Vertex Pharmaceuticals outside the submitted work. In addition, J. de Bono has a patent for DNA repair defects PARP (ICR owned patent; does not receive royalties) issued, licensed, and with royalties paid, as well as a patent for Abiraterone acetate (ICR owned patent; does not receive royalties) issued, licensed, and with royalties paid. The Institute of Cancer Research has a commercial interest in CT900. R. Jones reports personal fees from AstraZeneca outside the submitted work. E. Hall reports grants from Merck Sharpe & Dohme, Accuray Inc., Varian Medical Systems Inc., AstraZeneca, Janssen-Cilag, Aventis Pharma Limited (Sanofi), and Roche Products Ltd, as well as grants and non-financial support from Bayer outside the submitted work. N. Cook reports grants from Cancer Research UK during the conduct of the study, as well as other support from Roche, Taiho, AstraZeneca, Orion, UCB, Starpharma, RedX, Avacta, Bayer, Eisai, Tarveda, Boehringer, and Stemline outside the submitted work. U. Banerji reports other support from The Institute of Cancer Research during the conduct of the study; U. Banerji also reports other support from Pegasy, Boehringer Ingelheim, and Idea Pharma (Janssen), as well as grants from Verastem, Carrick Therapeutics, and Chugai outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
S. Banerjee: Writing–original draft, writing–review and editing, data interpretation, patient recruitment. V. Michalarea: Formal analysis, supervision, writing–review and editing. J.E. Ang: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, writing–review and editing. A. Ingles Garces: Investigation, writing–original draft, writing–review and editing, patient recruitment, data acquisition. A. Biondo: Writing–review and editing, patient recruitment, data acquisition. I.-G. Funingana: Writing–review and editing, patient recruitment, data acquisition. M. Little: Data curation, investigation, writing–review and editing, data curation. R. Ruddle: Formal analysis, writing–review and editing, data analysis. F. Raynaud: Writing–review and editing, data analysis. R. Riisnaes: Formal analysis, methodology, writing–review and editing, data collection, data analysis. B. Gurel: Validation, investigation, visualization. S. Chua: Formal analysis, writing–review and editing, data acquisition, data analysis. N. Tunariu: Formal analysis, writing–original draft, writing–review and editing, data acquisition, data analysis. J.C. Porter: Writing–review and editing, review of clinical data. T. Prout: Data curation, formal analysis, data collection, data cleaning and data analysis. M. Parmar: Data curation, formal analysis, figures, data collection, data analysis, data interpretation. A. Zachariou: Data curation, project administration, writing–review and editing. A. Turner: Funding acquisition, project administration. B. Jenkins: Formal analysis, writing–review and editing, data analysis. S. McIntosh: Writing–review and editing. E. Ainscow: Writing–review and editing. A. Minchom: Writing–review and editing, consenting, treatment of patients. J. Lopez: Data curation, formal analysis, supervision, writing–original draft, writing–review and editing, data collection and interpretation of data. J. de Bono: Conceptualization, data curation, supervision, validation, visualization, writing–original draft, writing–review and editing, resources. R. Jones: Formal analysis, writing–review and editing, review of clinical data, analysis of data. E. Hall: Formal analysis, writing–review and editing, data analysis. N. Cook: Formal analysis, writing–original draft, writing–review and editing, recruitment of patients, data analysis and interpretation. B. Basu: Formal analysis, supervision, writing–original draft, writing–review and editing, data interpretation. U. Banerji: Conceptualization, formal analysis, writing–original draft, writing–review and editing, consent, treatment of patients, analysis of clinical data and biomarkers.
References
- 1. Siu MK, Kong DS, Chan HY, Wong ES, Ip PP, Jiang L, et al. Paradoxical impact of two folate receptors, FRalpha and RFC, in ovarian cancer: effect on cell proliferation, invasion and clinical outcome. PLoS One 2012;7:e47201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338:284–93. [DOI] [PubMed] [Google Scholar]
- 3. O'Shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget 2012;3:414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boogerd LS, Boonstra MC, Beck AJ, Charehbili A, Hoogstins CE, Prevoo HA, et al. Concordance of folate receptor-alpha expression between biopsy, primary tumor and metastasis in breast cancer and lung cancer patients. Oncotarget 2016;7:17442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kobel M, Madore J, Ramus SJ, Clarke BA, Pharoah PD, Deen S, et al. Evidence for a time-dependent association between FOLR1 expression and survival from ovarian carcinoma: implications for clinical testing. An ovarian tumour tissue analysis consortium study. Br J Cancer 2014;111:2297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Markert S, Lassmann S, Gabriel B, Klar M, Werner M, Gitsch G, et al. Alpha-folate receptor expression in epithelial ovarian carcinoma and non-neoplastic ovarian tissue. Anticancer Res 2008;28:3567–72. [PubMed] [Google Scholar]
- 7. Nunez MI, Behrens C, Woods DM, Lin H, Suraokar M, Kadara H, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol 2012;7:833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nutt JE, Razak AR, O'Toole K, Black F, Quinn AE, Calvert AH, et al. The role of folate receptor alpha (FRalpha) in the response of malignant pleural mesothelioma to pemetrexed-containing chemotherapy. Br J Cancer 2010;102:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Shannessy DJ, Somers EB, Maltzman J, Smale R, Fu YS. Folate receptor alpha (FRA) expression in breast cancer: identification of a new molecular subtype and association with triple negative disease. Springerplus 2012;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senol S, Ceyran AB, Aydin A, Zemheri E, Ozkanli S, Kosemetin D, et al. Folate receptor alpha expression and significance in endometrioid endometrium carcinoma and endometrial hyperplasia. Int J Clin Exp Pathol 2015;8:5633–41. [PMC free article] [PubMed] [Google Scholar]
- 11. Cheung A, Opzoomer J, Ilieva KM, Gazinska P, Hoffmann RM, Mirza H, et al. Anti-folate receptor alpha-directed antibody therapies restrict the growth of triple-negative breast cancer. Clin Cancer Res 2018;24:5098–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor alpha in oncology. Nat Rev Clin Oncol 2020;17:349–59. [DOI] [PubMed] [Google Scholar]
- 13. Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Res 2005;65:11721–8. [DOI] [PubMed] [Google Scholar]
- 14. Tochowicz A, Dalziel S, Eidam O, O'Connell JD, 3rd, Griner S, Finer-Moore JS, et al. Development and binding mode assessment of N-[4-[2-propyn-1-yl[(6S)-4,6,7,8-tetrahydro-2-(hydroxymethyl)-4-oxo-3H-cyclopenta [g]quinazolin-6-yl]amino]benzoyl]-l-gamma-glutamyl-D-glutamic acid (BGC 945), a novel thymidylate synthase inhibitor that targets tumor cells. J Med Chem 2013;56:5446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vlahov IR, Santhapuram HK, Kleindl PJ, Howard SJ, Stanford KM, Leamon CP. Design and regioselective synthesis of a new generation of targeted chemotherapeutics. Part 1: EC145, a folic acid conjugate of desacetylvinblastine monohydrazide. Bioorg Med Chem Lett 2006;16:5093–6. [DOI] [PubMed] [Google Scholar]
- 16. Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D, et al. IMGN853, a folate receptor-alpha (FRalpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRalpha-expressing tumors. Mol Cancer Ther 2015;14:1605–13. [DOI] [PubMed] [Google Scholar]
- 17. The Institute of Cancer Research, The Royal Marsden NHS Foundation Trust. A phase I trial of ONX-0801 (a novel α-folate receptor-mediated thymidylate synthase inhibitor) exploring once weekly and alternate week dosing regimens in patients with solid tumors. Available from: https://clinicaltrials.gov/ct2/show/NCT02360345?term=NCT02360345.
- 18. Martin LP, Konner JA, Moore KN. Characterization of folate receptor alpha (FRα) expression in archival tumor and biopsy samples in a phase I study of mirvetuximab soravtansine, a FRα-targeting antibody drug conjugate (ADC), in relapsed epithelial ovarian cancer patients. In SGO Annual Meeting 2017. Available from: https://www.immunogen.com/wp-content/uploads/2018/05/IMGN-SGO2017Ph1biopsyab61.pdf. [DOI] [PMC free article] [PubMed]
- 19. Martin LP, Konner JA, Moore KN, Seward SM, Matulonis UA, Perez RP, et al. Characterization of folate receptor alpha (FRalpha) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: A phase I expansion study of the FRalpha-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol Oncol 2017;147:402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore KN, Oza AM, Colombo N, Oaknin A, Scambia G, Lorusso D, et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I. Ann Oncol 2021;32:757–65. [DOI] [PubMed] [Google Scholar]
- 21. Zhao J, Labelle A, Ab O, Acosta K, Yates A, Zhou Y, et al. Development and application of an immunohistochemistry-based clinical assay for evaluating folate receptor alpha (FRα) expression in the clinical setting. In AACR Annual Meeting 2015. [Accessed 20 July 2021]. Available from: https://www.immunogen.com/wp-content/uploads/2018/05/FRalpha_assay_AACR_2015_3400A.pdf.
- 22. Pillai RG, Forster M, Perumal M, Mitchell F, Leyton J, Aibgirhio FI, et al. Imaging pharmacodynamics of the alpha-folate receptor-targeted thymidylate synthase inhibitor BGC 945. Cancer Res 2008;68:3827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Malley DM, Matulonis UA, Birrer MJ, Castro CM, Gilbert L, Vergote I, et al. Phase Ib study of mirvetuximab soravtansine, a folate receptor alpha (FRalpha)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol 2020;157:379–85. [DOI] [PubMed] [Google Scholar]
- 24. Banerji U, Workman P. Critical parameters in targeted drug development: the pharmacological audit trail. Semin Oncol 2016;43:436–45. [DOI] [PubMed] [Google Scholar]
- 25. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 2021;22:1034–46. [DOI] [PubMed] [Google Scholar]
- 26. Immunogen. ImmunoGen announces positive top-line results from pivotal SORAYA trial of mirvetuximab soravtansine in ovarian cancer. Immunogen. Available from: https://investor.immunogen.com/news-releases/news-release-details/immunogen-announces-positive-top-line-results-pivotal-soraya.
- 27. Huehls AM, Wagner JM, Huntoon CJ, Karnitz LM. Identification of DNA repair pathways that affect the survival of ovarian cancer cells treated with a poly(ADP-ribose) polymerase inhibitor in a novel drug combination. Mol Pharmacol 2012;82:767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified scientific and medical researchers can request patient level anonymized clinical data for research purposes. These will be reviewed by sponsor prior to granting access and decisions depend on purpose/scientific merit of the proposal and availability of the data. Requests are to be sent to the corresponding author.