Abstract
Objective
To determine whether the use of glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, and sodium-glucose co-transporter-2 (SGLT-2) inhibitors, separately, is associated with a decreased risk of exacerbations of chronic obstructive pulmonary disease among patients with chronic obstructive pulmonary disease and type 2 diabetes.
Design
Population based cohort study using an active comparator, new user design.
Setting
The United Kingdom Clinical Practice Research Datalink linked with the Hospital Episode Statistics Admitted Patient Care and Office for National Statistics databases.
Participants
Three active comparator, new user cohorts of patients starting the study drugs (GLP-1 receptor agonists, DPP-4 inhibitors, or SGLT-2 inhibitors) or sulfonylureas with a history of chronic obstructive pulmonary disease. The first cohort included 1252 patients starting GLP-1 receptor agonists and 14 259 starting sulfonylureas, the second cohort included 8731 patients starting DPP-4 inhibitors and 18 204 starting sulfonylureas, and the third cohort included 2956 patients starting SGLT-2 inhibitors and 10 841 starting sulfonylureas.
Main outcome measures
Cox proportional hazards models with propensity score fine stratification weighting were fitted to estimate hazard ratios and 95% confidence intervals of severe exacerbation of chronic obstructive pulmonary disease (defined as hospital admission for chronic obstructive pulmonary disease), separately for GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors. Whether these drugs were associated with a decreased risk of moderate exacerbation (defined as a co-prescription of an oral corticosteroid and an antibiotic along with an outpatient diagnosis of acute chronic obstructive pulmonary disease exacerbation on the same day) was also assessed.
Results
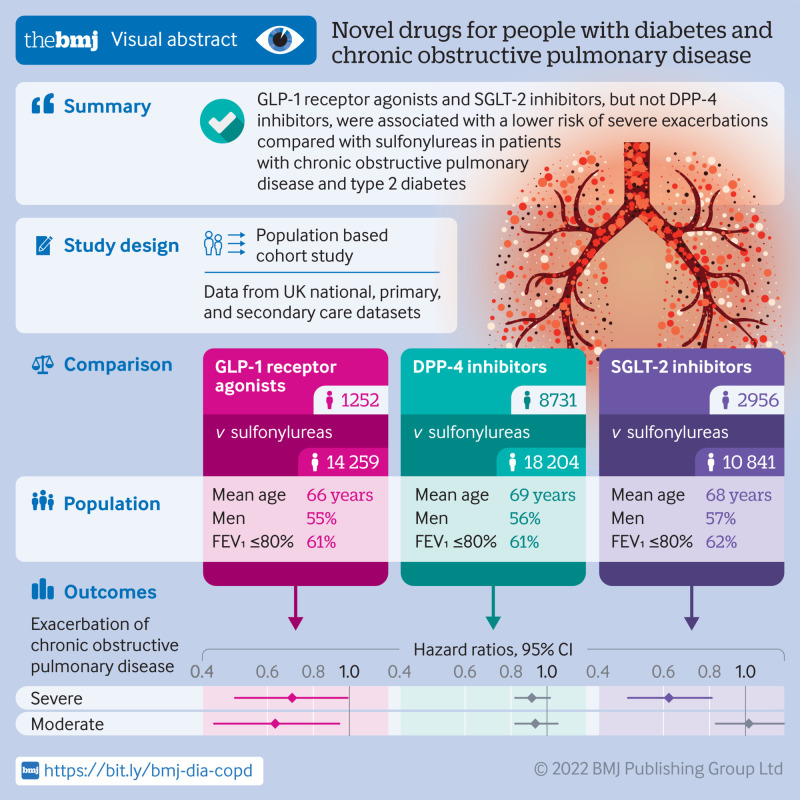
Compared with sulfonylureas, GLP-1 receptor agonists were associated with a 30% decreased risk of severe exacerbation (3.5 v 5.0 events per 100 person years; hazard ratio 0.70, 95% confidence interval 0.49 to 0.99) and moderate exacerbation (0.63, 0.43 to 0.94). DPP-4 inhibitors were associated with a modestly decreased incidence of severe exacerbation (4.6 v. 5.1 events per 100 person years; hazard ratio 0.91, 0.82 to 1.02) and moderate exacerbation (0.93, 0.82 to 1.07), with confidence intervals including the null value. Finally, SGLT-2 inhibitors were associated with a 38% decreased risk of severe exacerbation (2.4 v 3.9 events per 100 person years; hazard ratio 0.62, 0.48 to 0.81) but not moderate exacerbation (1.02, 0.83 to 1.27).
Conclusions
In this population based study, GLP-1 receptor agonists and SGLT-2 inhibitors were associated with a reduced risk of severe exacerbations compared with sulfonylureas in patients with chronic obstructive pulmonary disease and type 2 diabetes. DPP-4 inhibitors were not clearly associated with a decreased risk of chronic obstructive pulmonary disease exacerbations.
Introduction
Glucagon-like peptidase 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose co-transporter-2 (SGLT-2) inhibitors are commonly prescribed novel antihyperglycaemic drugs.1 2 In addition to their favourable cardiovascular effects,2 emerging evidence suggests that these drugs may also have beneficial effects on lung function, such as among patients with chronic obstructive pulmonary disease.
In laboratory studies in murine models of obstructive lung disease, direct stimulation of the GLP-1 receptors by GLP-1 receptor agonists reduced airway hyperresponsiveness and inflammation leading to improved survival.3 4 5 Furthermore, GLP-1 receptor agonists have been shown to improve forced vital capacity among patients with compromised lung function in randomised controlled trials.6 7 DPP-4 inhibitors may reduce bronchial hyperresponsiveness by increasing endogenous GLP-1 concentration and directly blocking DPP-4, a molecule up-regulated in the lungs of patients with exacerbations of chronic obstructive pulmonary disease.8 Finally, although SGLT-2 receptors are not expressed in the lungs, these drugs may exert beneficial effects by reducing carbon dioxide retention by inducing glucosuria and by reducing the risk of pneumonia, a major precipitator of chronic obstructive pulmonary disease exacerbations.9 10 11 12 To date, observational studies investigating the effects of these novel antihyperglycaemic drugs on respiratory disease exacerbations have been limited.13 14 In one study, DPP-4 inhibitors were not associated with a decreased risk of severe exacerbation of chronic obstructive pulmonary disease.13 In another study, GLP-1 receptor agonists were associated with a 48% decreased risk of severe exacerbation among patients with type 2 diabetes and chronic lower respiratory diseases (asthma or chronic obstructive pulmonary disease).14 However, these studies did not consider other clinically important outcomes, such as moderate exacerbations, and were unable to adjust for potentially important confounders, such as body mass index, smoking, and measures of lung function. To our knowledge, no real world study has been conducted to assess whether SGLT-2 inhibitors are associated with a decreased risk of chronic obstructive pulmonary disease exacerbations.
Given that patients with type 2 diabetes are at a high risk of chronic obstructive pulmonary disease related morbidity and mortality,15 16 we did a large population based cohort study to determine whether GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors, separately, are associated with a decreased risk of chronic obstructive pulmonary disease exacerbation among patients with chronic obstructive pulmonary disease and type 2 diabetes, compared with sulfonylureas.
Methods
Data sources
We did this population based cohort study by linking the GOLD (Gp OnLine Data) and Aurum databases of the Clinical Practice Research Datalink (CPRD) with the Hospital Episode Statistics Admitted Patient Care (HES APC) and Office for National Statistics databases. The CPRD is a large primary care database from the UK that includes approximately 60 million patients from nearly 2000 general practices and is representative of the general population of the UK with respect to age, sex, and ethnicity.17 The Read code and SNOMED-CT classification systems are used to record medical diagnoses and procedures, and a coded drug dictionary based on the British National Formulary is used to record prescription details.17 Several studies have confirmed the validity of the data recorded in the CPRD.18 19 20
The HES APC database contains hospital admission records of patients who have received care from English National Health Services hospitals.21 Diagnoses in the HES APC are recorded using the ICD-10 (international classification of diseases, 10th revision) classification.21 The Office for National Statistics is a vital statistics database that we used to accurately identify patients who died during follow-up. The linkage of the CPRD with other databases has been well validated.17
Study population
We constructed three separate active comparator, new user cohorts that compared patients starting GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors with those starting sulfonylureas. The first cohort consisted of patients starting GLP-1 receptor agonists (dulaglutide, exenatide, liraglutide (except the 3 mg/0.5mL formulation indicated for weight loss), lixisenatide, semaglutide) and those starting sulfonylureas (glibenclamide, gliclazide, glipizide, glimepiride, tolbutamide) and extended between 1 January 2007 (the year the first GLP-1 receptor agonists entered the UK market) and 31 December 2019. The second cohort consisted of patients starting DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin, vildagliptin) and those starting sulfonylureas between 1 January 2007 (the year the first DPP-4 inhibitors entered the UK market) and 31 December 2019. The third cohort consisted of patients starting SGLT-2 inhibitors (canagliflozin, dapagliflozin, empagliflozin) and those starting sulfonylureas from 1 January 2013 (the year the first SGLT-2 inhibitor entered the UK market) to 30 December 2019. Overall, 6679 sulfonylurea users were present in all three cohorts. However, we constructed each cohort independently of the others, so inclusion in one cohort was not conditional on inclusion in another cohort.
We defined cohort entry in each cohort by the date of the first prescription of either the drug of interest (GLP-1 receptor agonists, DPP-4 inhibitors, or SGLT-2 inhibitors) or the comparator (sulfonylureas) during the study period, whichever came first. This procedure avoided classifying exposure in a hierarchical fashion (that is, identifying the drug of interest first and then the comparator), which would have introduced immortal time bias.22 In all cohorts, we excluded patients under 40 years of age and those concomitantly treated with the study drugs at cohort entry. Furthermore, we required all patients to have at least one year of medical history in the CPRD before cohort entry and at least one diagnostic code for chronic obstructive pulmonary disease at any time before cohort entry. Chronic obstructive pulmonary disease codes in the CPRD have been previously validated, achieving a positive predictive value of 86.5% when the assessment of respiratory physicians is used as the gold standard.23 To identify patients starting a drug, we excluded those previously treated with the drugs of interest or sulfonylureas in the year before cohort entry. In the GLP-1 receptor agonist and DPP-4 inhibitor cohorts, we additionally excluded patients who used the other incretin based drug class in the year before cohort entry, given their shared mechanism of action24 (that is, DPP-4 inhibitor users in the GLP-1 receptor agonist versus sulfonylurea cohort and GLP-1 receptor agonist users in the DPP-4 inhibitor versus sulfonylurea cohort). Finally, we excluded patients diagnosed as having end stage renal disease at any time before cohort entry, as several GLP-1 receptor agonists, SGLT-2 inhibitors, and sulfonylureas are contraindicated in these patients.
We chose sulfonylureas as the active comparator as they are used at a similar disease stage as incretin based drugs and SGLT-2 inhibitors,2 continue to be widely prescribed worldwide,1 25 26 27 and have not been previously associated with an increased incidence of exacerbation of chronic obstructive pulmonary disease.13 By contrast, metformin and insulin are drugs usually started in early or advanced diabetes, respectively,2 which can introduce confounding by indication. Thiazolidinediones are second to third line drugs, but they have been associated with several adverse events and are now used infrequently.1 19
Exposure definition
We followed all patients by using an on-treatment exposure definition, in which patients were followed while they were continuously exposed to the study drugs. We considered patients to be continuously exposed if the duration of one prescription overlapped the date of the next prescription. In the event of non-overlapping prescriptions, we used a 60 day grace period to bridge consecutive prescriptions. We defined treatment discontinuation by the absence of a new prescription by the end of the 60 day grace period. Thus, we followed patients from cohort entry until a chronic obstructive pulmonary disease exacerbation (detailed below), treatment discontinuation, switching to or adding on one of the study drugs, death from any cause, end of registration with the CPRD, or the end of the study period (31 March 2020), whichever occurred first.
Primary and secondary outcomes
The primary outcome was time to the first episode of severe chronic obstructive pulmonary disease exacerbation during the follow-up period. We defined this as a hospital admission (identified using HES APC) with a diagnosis of chronic obstructive pulmonary disease in the primary position (ICD-10 codes J41-J44).28 This definition has been previously validated with a positive predictive value of 86% and a specificity of 99%.28 We also considered three secondary outcomes: time to the first moderate chronic obstructive pulmonary disease exacerbation during the follow-up period (using a validated outcome definition based on a co-prescription of an oral corticosteroid and a respiratory antibiotic (penicillins, cephalosporins, aminoglycosides, macrolides, quinolones, tetracyclines, vancomycin, cotrimoxazole, clindamycin) along with an outpatient diagnosis of acute chronic obstructive pulmonary disease exacerbation on the same day)29; count of moderate exacerbations; and count of severe exacerbations during the follow-up period. Events within 30 days of each other counted as the continuation of the same exacerbation episode for the count based outcomes.
Potential confounders
We considered a wide range of potential confounders, all measured before or at cohort entry. Our rationale for selecting potential confounders focused on variables previously associated with the outcome, which may also be associated with the exposures of interest, on the basis of the available literature and subject matter expertise. The variables included age (modelled using cubic splines with five interior knots), sex, body mass index, smoking status, and alcohol related disorders. We also included variables as proxies for severity of diabetes, including haemoglobin A1c, duration of diabetes before cohort entry (defined by the date of the first of a haemoglobin A1c≥6.5%, a diagnosis of type 2 diabetes, or prescription for any antihyperglycaemic drug, modelled using cubic splines with five interior knots), microvascular complications (nephropathy, neuropathy, retinopathy), macrovascular complications (myocardial infarction, stroke, peripheral arteriopathy), and different antihyperglycaemic drugs prescribed in the year before and including cohort entry. The models also included proxies for severity of chronic obstructive pulmonary disease, such as duration of disease (time between the initial diagnosis and cohort entry), respiratory drugs prescribed in the year before cohort entry (including long acting and short acting β agonist drugs, long acting and short acting anti-muscarinic drugs, inhaled and oral corticosteroids, leukotriene antagonists, methylxanthines, respiratory antibiotics), and respiratory events in the year before cohort entry (hospital admission for chronic obstructive pulmonary disease (any position), pneumonia, or influenza). The models included relevant spirometry parameters, such as per cent predicted forced expiratory volume in one second (FEV1),30 and FEV1 to forced vital capacity ratio,31 and dyspnoea (modified Medical Research Council scale assessing the impact on physical function),32 along with blood eosinophil count (<2%, 2-4%, >4%) at cohort entry. We also considered other chronic respiratory disorders that could influence chronic obstructive pulmonary disease exacerbations (asthma, interstitial lung diseases, cystic fibrosis, bronchiectasis, pulmonary embolism, pulmonary hypertension) and lung cancer at any time before cohort entry. As markers of general health, we adjusted for common comorbidities (cancer other than non-melanoma skin cancer, heart failure, hypertension, arrhythmia, dyslipidaemia, non-alcoholic fatty liver disease, hypothyroidism, gastro-oesophageal reflux disease, obesity, sleep apnoea, osteoarthritis, depression) and drugs prescribed in the year before cohort entry (angiotensin converting enzyme inhibitors, angiotensin receptor blockers, β blockers, calcium channel blockers, thiazides, other diuretics, antiarrhythmic agents, antiplatelet agents, statins, proton pump inhibitors, non-steroidal anti-inflammatory drugs, and opioids). As markers of health seeking behaviour, we considered the uptake of cancer screening (faecal occult blood testing or colonoscopy, mammography, prostate specific antigen testing) and influenza and pneumococcal vaccinations, all measured in the year before cohort entry. Finally, we considered season (winter (December-February), spring (March-May), summer (June-August), and fall/autumn (September-November)), as chronic obstructive pulmonary disease exacerbations might be influenced by seasonality, and calendar year (entered as a categorical variable) at cohort entry to adjust for potential variations in trends in the treatment of type 2 diabetes and chronic obstructive pulmonary disease during the study period. Variables with missing values (body mass index, smoking, haemoglobin A1c, FEV1, FEV1 to forced vital capacity ratio, and eosinophil count) were modelled with an unknown category.
Statistical analysis
We used propensity score fine stratification to adjust for confounding.33 In each new user cohort, we estimated the predicted probability of receiving the study drugs of interest (GLP-1 receptor agonist, DPP-4 inhibitor, or SGLT-2 inhibitor) versus a sulfonylurea by using multivariable logistic regression models conditional on the covariates listed above. After trimming patients in the non-overlapping regions of the propensity score distributions, we created 50 strata based on the propensity score distribution of the patients who received the study drugs. Within each stratum, patients in the GLP-1 receptor agonist, DPP-4 inhibitor, and SGLT-2 inhibitor groups received a weight of one, whereas patients in the sulfonylurea group were reweighted in proportion to the number exposed in the corresponding stratum. This method aims to balance the covariate distribution within each stratum, and the estimand generated by this approach is the average treatment effect among treated patients.
We used descriptive statistics to summarise the baseline characteristics of the exposure groups before and after propensity score weighting. We calculated absolute standardised differences to assess covariate balance, with differences less than 0.10 indicating good balance between the exposure groups.34 We calculated incidence rates of chronic obstructive pulmonary disease exacerbation with 95% confidence intervals based on the Poisson distribution for each exposure group. Additionally, we plotted weighted Kaplan-Meier curves for each exposure group to show the cumulative incidence of chronic obstructive pulmonary disease exacerbation over the follow-up period. We fitted weighted Cox proportional hazards models to estimate hazard ratios with 95% confidence intervals, using robust variance estimators, of incident severe and moderate chronic obstructive pulmonary disease exacerbation, comparing GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors, separately, with sulfonylureas. We also calculated the number needed to treat to prevent one severe chronic obstructive pulmonary disease exacerbation event after one and five years of use by using the Kaplan-Meier method.35 Finally, we used weighted Poisson regression to estimate rate ratios with 95% confidence intervals for count of severe and moderate exacerbations chronic obstructive pulmonary disease, with log of follow-up time as the offset.
Secondary analyses
We did four secondary analyses in each of the three cohorts described above. Firstly, we assessed whether a duration-response relation (duration of use <1 year, 1-2 years, >2 years) existed between the use of the study drugs (GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT-2 inhibitors, separately) and the incidence of severe chronic obstructive pulmonary disease exacerbation. Secondly, we assessed whether the association varied with individual drugs within each study drug class. We restricted this analysis to those drugs with sufficient person time to yield stable estimates. Thirdly, we assessed potential effect measure modification by age (<75 and ≥75 years), sex, history of asthma, severity of dyspnoea, and predicted FEV1 (<30%, 30–50%, 51-80%, >80%). We assessed effect measure modification on a multiplicative scale by including interaction terms between the exposure variable and these variables in the models. Finally, given that our cohort extended over a 14 year period, we examined whether a potential period effect on the findings was present. For this analysis, we modelled the hazard ratio according to year of cohort entry year as a continuous variable by using restricted cubic splines (five knots at the 5th, 27.5th, 50th, 72.5th, and 95th centiles).
Sensitivity analyses
We did four sensitivity analyses to assess the robustness of the findings for the primary outcome. Firstly, we varied the grace period between consecutive prescriptions to 30 and 90 days. Secondly, we used time varying inverse probability of censoring weighting to account for potential informative censoring due to discontinuation or switching between the exposure groups, administrative censoring, and competing risk by death.36 37 38 This involved taking the product of the weights calculated from the conditional probabilities of treatment discontinuation or switching, administrative censoring, and death by using the covariates listed above. Thirdly, we used multiple imputation to examine the effect of missing data on our results. We fitted multiple regression models to impute variables with missing information and then combined the datasets by using Rubin’s rules.39 40 41 Finally, we repeated the analyses using herpes zoster as a negative control outcome to assess the impact of residual confounding. This outcome has been associated with type 2 diabetes42 but not with the drugs of interest. This analysis was based on the same cohorts as the ones used in the primary analysis but also excluded patients found to have herpes zoster at any time before cohort entry. We used SAS version 9.4 for all analyses.
Patient and public involvement
Our study was a secondary data analysis and did not include patients as study participants. No patients were involved in setting the research question or the outcome measures, and nor were they involved in the design and implementation of the study. This is because no specific funding had been allocated for this purpose. Moreover, CPRD data are not publicly available, and the analysis plan required specialised training.
Results
GLP-1 receptor agonists versus sulfonylureas
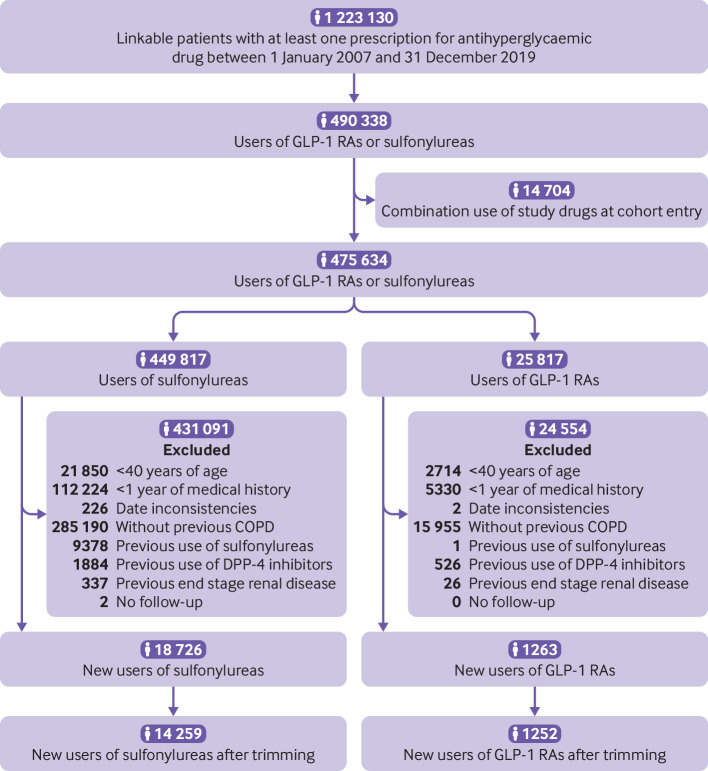
Overall, 1252 patients starting GLP-1 receptor agonists and 14 259 starting sulfonylureas were included in the first cohort (fig 1). This cohort was followed for a median of 1.0 (interquartile range 0.4-2.3) years. The most common reason for the end of follow-up was drug discontinuation or switching (supplementary table A). A total of 1325 incident events of severe COPD exacerbations occurred during 25 979 person years of follow-up, yielding a crude incidence rate of 5.1 (95% confidence interval 4.8 to 5.4) per 100 person years. Before propensity score weighting, patients starting GLP-1 receptor agonists were younger, were more likely to be obese, had elevated haemoglobin A1c levels, had a longer duration of diabetes, and had a higher prevalence of microvascular complications of diabetes compared with those starting sulfonylureas (table 1). The exposure groups were well balanced across all covariates after propensity score weighting.
Fig 1.
Study flow diagram of patients starting sulfonylureas or glucagon-like peptide-1 receptor agonists (GPL-1 RAs) in UK Clinical Practice Research Datalink between 2007 and 2019. COPD=chronic obstructive pulmonary disease
Table 1.
Baseline characteristics of glucagon-like peptide 1 (GLP-1) receptor agonist and sulfonylurea exposure groups before and after propensity score weighting. Values are numbers (percentages) unless stated otherwise
| Characteristics | Before weighting* | After weighting* | |||||
|---|---|---|---|---|---|---|---|
| GLP-1 receptor agonists (n=1252) | Sulfonylureas (n=14 259) | ASD | GLP-1 receptor agonists (n=1252) | Sulfonylureas (n=14 259) | ASD | ||
| Mean (SD) age, years | 61.4 (8.9) | 66.7 (9.9) | 0.56 | 61.4 (8.9) | 61.1 (9.2) | 0.04 | |
| Male sex | 626 (50.0) | 7887 (55.3) | 0.11 | 626 (50.0) | 7129 (50.0) | 0.00 | |
| Year of cohort entry: | |||||||
| 2007-10 | 312 (24.9) | 5040 (35.3) | 0.23 | 312 (24.9) | 3493 (24.5) | 0.01 | |
| 2011-14 | 359 (28.7) | 4891 (34.3) | 0.12 | 359 (28.7) | 3668 (25.7) | 0.07 | |
| 2015-19 | 581 (46.4) | 4328 (30.4) | 0.33 | 581 (46.4) | 7098 (49.8) | 0.07 | |
| Body mass index: | |||||||
| <30 | 75 (6.0) | 4426 (31.0) | 0.68 | 75 (6.0) | 948 (6.6) | 0.03 | |
| ≥30.0 | 1147 (91.6) | 9594 (67.3) | 0.63 | 1147 (91.6) | 12 960 (90.9) | 0.03 | |
| Unknown | 30 (2.4) | 239 (1.7) | 0.05 | 30 (2.4) | 352 (2.5) | 0.00 | |
| Smoking status: | |||||||
| Ever | 1181 (94.3) | 13 446 (94.3) | 0.00 | 1181 (94.3) | 13 412 (94.1) | 0.01 | |
| Never | 71 (5.7) | 813 (5.7) | 0.00 | 71 (5.7) | 847 (5.9) | 0.01 | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Haemoglobin A1c: | |||||||
| ≤7.0% | 185 (14.8) | 2244 (15.7) | 0.03 | 185 (14.8) | 2460 (17.3) | 0.07 | |
| 7.1-8.0% | 267 (21.3) | 3727 (26.1) | 0.11 | 267 (21.3) | 2558 (17.9) | 0.09 | |
| >8.0% | 780 (62.3) | 7571 (53.1) | 0.19 | 780 (62.3) | 8952 (62.8) | 0.01 | |
| Unknown | 20 (1.6) | 717 (5.0) | 0.19 | 20 (1.6) | 289 (2.0) | 0.03 | |
| Alcohol related disorders | 124 (9.9) | 1664 (11.7) | 0.06 | 124 (9.9) | 1537 (10.8) | 0.03 | |
| Mean (SD) duration of diabetes, years | 10.4 (7.6) | 5.5 (5.2) | 0.75 | 10.4 (7.6) | 10.2 (7.5) | 0.04 | |
| Type of antihyperglycaemic drugs: | |||||||
| Metformin | 1040 (83.1) | 11 705 (82.1) | 0.03 | 1040 (83.1) | 11 994 (84.1) | 0.03 | |
| Thiazolidinedione | 133 (10.6) | 1084 (7.6) | 0.11 | 133 (10.6) | 1661 (11.6) | 0.03 | |
| Meglitinides | 10 (0.8) | 78 (0.5) | 0.03 | 10 (0.8) | 134 (0.9) | 0.02 | |
| α-glucosidase inhibitors | 11 (0.9) | 22 (0.2) | 0.10 | 11 (0.9) | 66 (0.5) | 0.05 | |
| Sodium-glucose co-transporter-2 inhibitors | 116 (9.3) | 131 (0.9) | 0.39 | 116 (9.3) | 1506 (10.6) | 0.04 | |
| Insulin | 671 (53.6) | 806 (5.7) | 1.23 | 671 (53.6) | 7474 (52.4) | 0.02 | |
| Peripheral vascular disease | 244 (19.5) | 2077 (14.6) | 0.13 | 244 (19.5) | 2625 (18.4) | 0.03 | |
| Stroke | 101 (8.1) | 1239 (8.7) | 0.02 | 101 (8.1) | 1125 (7.9) | 0.01 | |
| Myocardial infarction | 210 (16.8) | 2114 (14.8) | 0.05 | 210 (16.8) | 2411 (16.9) | 0.00 | |
| Renal disease | 304 (24.3) | 3563 (25.0) | 0.02 | 304 (24.3) | 3259 (22.9) | 0.03 | |
| Retinopathy | 518 (41.4) | 3200 (22.4) | 0.41 | 518 (41.4) | 5493 (38.5) | 0.06 | |
| Neuropathy | 456 (36.4) | 3119 (21.9) | 0.32 | 456 (36.4) | 5082 (35.6) | 0.02 | |
| Mean (SD) duration of COPD, years | 7.0 (6.4) | 7.6 (7.2) | 0.09 | 7.0 (6.4) | 7.1 (6.3) | 0.02 | |
| Long acting β-agonist | 682 (54.5) | 7461 (52.3) | 0.04 | 682 (54.5) | 7644 (53.6) | 0.02 | |
| Short acting β-agonist | 898 (71.7) | 9993 (70.1) | 0.04 | 898 (71.7) | 10 517 (73.8) | 0.05 | |
| Long acting anti-muscarinic | 457 (36.5) | 4931 (34.6) | 0.04 | 457 (36.5) | 5123 (35.9) | 0.01 | |
| Short acting anti-muscarinic | 111 (8.9) | 1723 (12.1) | 0.11 | 111 (8.9) | 1560 (10.9) | 0.07 | |
| Inhaled corticosteroids, | 765 (61.1) | 8390 (58.8) | 0.05 | 765 (61.1) | 8915 (62.5) | 0.03 | |
| Oral corticosteroids, | 458 (36.6) | 5354 (37.5) | 0.02 | 458 (36.6) | 4969 (34.8) | 0.04 | |
| Leukotriene antagonists, | 112 (8.9) | 648 (4.5) | 0.18 | 112 (8.9) | 1048 (7.4) | 0.06 | |
| Methylxanthines, | 72 (5.8) | 728 (5.1) | 0.03 | 72 (5.8) | 910 (6.4) | 0.03 | |
| Respiratory antibiotics, | 913 (72.9) | 10 013 (70.2) | 0.06 | 913 (72.9) | 9835 (69.0) | 0.09 | |
| Hospital admission for COPD | 323 (25.8) | 3955 (27.7) | 0.04 | 323 (25.8) | 3722 (26.1) | 0.01 | |
| Pneumonia | 48 (3.8) | 850 (6.0) | 0.10 | 48 (3.8) | 660 (4.6) | 0.04 | |
| Influenza | 8 (0.6) | 107 (0.8) | 0.01 | 8 (0.6) | 109 (0.8) | 0.01 | |
| Asthma | 833 (66.5) | 8517 (59.7) | 0.14 | 833 (66.5) | 9633 (67.6) | 0.02 | |
| Interstitial lung disease | 32 (2.6) | 427 (3.0) | 0.03 | 32 (2.6) | 290 (2.0) | 0.04 | |
| Bronchiectasis | 46 (3.7) | 722 (5.1) | 0.07 | 46 (3.7) | 371 (2.6) | 0.06 | |
| Pulmonary embolism | 274 (21.9) | 2544 (17.8) | 0.10 | 274 (21.9) | 2950 (20.7) | 0.03 | |
| Pulmonary hypertension | 24 (1.9) | 246 (1.7) | 0.01 | 24 (1.9) | 212 (1.5) | 0.03 | |
| Lung cancer | 5 (0.4) | 227 (1.6) | 0.12 | 5 (0.4) | 53 (0.4) | 0.01 | |
| FEV1 (% predicted): | |||||||
| <30 | 32 (2.6) | 503 (3.5) | 0.06 | 32 (2.6) | 399 (2.8) | 0.02 | |
| 30-80 | 698 (55.8) | 8179 (57.4) | 0.03 | 698 (55.8) | 7768 (54.5) | 0.03 | |
| >80 | 156 (12.5) | 1678 (11.8) | 0.02 | 156 (12.5) | 1780 (12.5) | 0.00 | |
| Unknown | 366 (29.2) | 3899 (27.3) | 0.04 | 366 (29.2) | 4312 (30.2) | 0.02 | |
| FEV1-FVC ratio: | |||||||
| <35 | 5 (0.4) | 126 (0.9) | 0.06 | 5 (0.4) | 47 (0.3) | 0.01 | |
| 35-59.9 | 110 (8.8) | 2193 (15.4) | 0.20 | 110 (8.8) | 1202 (8.4) | 0.01 | |
| ≥60 | 608 (48.6) | 5943 (41.7) | 0.14 | 608 (48.6) | 6875 (48.2) | 0.01 | |
| Unknown | 529 (42.3) | 5997 (42.1) | 0.00 | 529 (42.3) | 6136 (43.0) | 0.02 | |
| Severity of dyspnoea: | |||||||
| None/mild | 259 (20.7) | 3496 (24.5) | 0.09 | 259 (20.7) | 2793 (19.6) | 0.03 | |
| Moderate/severe | 661 (52.8) | 7144 (50.1) | 0.05 | 661 (52.8) | 7417 (52.0) | 0.02 | |
| Unknown | 332 (26.5) | 3619 (25.4) | 0.03 | 332 (26.5) | 4049 (28.4) | 0.04 | |
| Blood eosinophil count: | |||||||
| <2% | 380 (30.4) | 4825 (33.8) | 0.07 | 380 (30.4) | 4707 (33.0) | 0.06 | |
| 2-4% | 561 (44.8) | 5873 (41.2) | 0.07 | 561 (44.8) | 6471 (45.4) | 0.01 | |
| >4% | 265 (21.2) | 2942 (20.6) | 0.01 | 265 (21.2) | 2486 (17.4) | 0.09 | |
| Unknown | 46 (3.7) | 619 (4.3) | 0.03 | 46 (3.7) | 595 (4.2) | 0.03 | |
| Cancer† | 115 (9.2) | 1940 (13.6) | 0.14 | 115 (9.2) | 1120 (7.9) | 0.05 | |
| Heart failure | 242 (19.3) | 2684 (18.8) | 0.01 | 242 (19.3) | 3034 (21.3) | 0.05 | |
| Hypertension | 1065 (85.1) | 11 170 (78.3) | 0.17 | 1065 (85.1) | 12 199 (85.6) | 0.01 | |
| Arrhythmia | 256 (20.4) | 3333 (23.4) | 0.07 | 256 (20.4) | 2742 (19.2) | 0.03 | |
| Dyslipidaemia | 690 (55.1) | 6373 (44.7) | 0.21 | 690 (55.1) | 8033 (56.3) | 0.02 | |
| Non-alcoholic fatty liver disease | 74 (5.9) | 403 (2.8) | 0.15 | 74 (5.9) | 883 (6.2) | 0.01 | |
| Hypothyroidism | 210 (16.8) | 1924 (13.5) | 0.09 | 210 (16.8) | 2571 (18.0) | 0.03 | |
| Gastro-oesophageal reflux disease | 407 (32.5) | 3701 (26.0) | 0.14 | 407 (32.5) | 4561 (32.0) | 0.01 | |
| Sleep apnoea | 339 (27.1) | 1248 (8.8) | 0.49 | 339 (27.1) | 4420 (31.0) | 0.09 | |
| Osteoarthritis | 592 (47.3) | 6180 (43.3) | 0.08 | 592 (47.3) | 6744 (47.3) | 0.00 | |
| Depression | 737 (58.9) | 6488 (45.5) | 0.27 | 737 (58.9) | 8493 (59.6) | 0.01 | |
| Angiotensin converting enzyme inhibitors | 658 (52.6) | 6758 (47.4) | 0.10 | 658 (52.6) | 7314 (51.3) | 0.03 | |
| Angiotensin receptor blockers | 295 (23.6) | 2684 (18.8) | 0.12 | 295 (23.6) | 3055 (21.4) | 0.05 | |
| β blockers | 315 (25.2) | 3447 (24.2) | 0.02 | 315 (25.2) | 3760 (26.4) | 0.03 | |
| Calcium channel blockers | 476 (38.0) | 4996 (35.0) | 0.06 | 476 (38.0) | 5554 (39.0) | 0.02 | |
| Diuretics | 558 (44.6) | 5522 (38.7) | 0.12 | 558 (44.6) | 6206 (43.5) | 0.02 | |
| Antiarrhythmic agents | 74 (5.9) | 783 (5.5) | 0.02 | 74 (5.9) | 771 (5.4) | 0.02 | |
| Antiplatelet agents | 151 (12.1) | 1565 (11.0) | 0.03 | 151 (12.1) | 1912 (13.4) | 0.04 | |
| Statins | 1062 (84.8) | 11 052 (77.5) | 0.19 | 1062 (84.8) | 12 015 (84.3) | 0.02 | |
| Proton pump inhibitors | 728 (58.1) | 7422 (52.1) | 0.12 | 728 (58.1) | 8475 (59.4) | 0.03 | |
| Non-steroidal anti-inflammatory drugs | 767 (61.3) | 7932 (55.6) | 0.11 | 767 (61.3) | 8725 (61.2) | 0.00 | |
| Opioids | 762 (60.9) | 7299 (51.2) | 0.20 | 762 (60.9) | 8543 (59.9) | 0.02 | |
| Faecal occult blood testing or colonoscopy | 200 (16.0) | 1945 (13.6) | 0.07 | 200 (16.0) | 2214 (15.5) | 0.01 | |
| Mammography | 120 (9.6) | 1033 (7.2) | 0.08 | 120 (9.6) | 1292 (9.1) | 0.02 | |
| Prostate specific antigen testing | 89 (7.1) | 1196 (8.4) | 0.05 | 89 (7.1) | 1096 (7.7) | 0.02 | |
| Influenza vaccination | 409 (32.7) | 5900 (41.4) | 0.18 | 409 (32.7) | 4733 (33.2) | 0.01 | |
| Pneumococcal vaccination | 52 (4.2) | 706 (5.0) | 0.04 | 52 (4.2) | 546 (3.8) | 0.02 | |
| Season: | |||||||
| Spring | 281 (22.4) | 3702 (26.0) | 0.08 | 281 (22.4) | 3291 (23.1) | 0.02 | |
| Summer | 334 (26.7) | 3515 (24.7) | 0.05 | 334 (26.7) | 3789 (26.6) | 0.00 | |
| Autumn/fall | 358 (28.6) | 3602 (25.3) | 0.08 | 358 (28.6) | 3845 (27.0) | 0.04 | |
| Winter | 279 (22.3) | 3440 (24.1) | 0.04 | 279 (22.3) | 3334 (23.4) | 0.03 | |
ASD=absolute standardised difference; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; SD=standard deviation.
Weighted using propensity score fine stratification within study populations with overlapping propensity scores.
Excludes lung cancer and non-melanoma skin cancer.
Table 2 shows the results of the GLP-1 receptor agonist versus sulfonylurea analyses. Overall, GLP-1 receptor agonists were associated with a 30% lower risk of a severe exacerbation of chronic obstructive pulmonary disease compared with sulfonylureas (3.5 v 5.0 events per 100 person years; hazard ratio 0.70, 95% confidence interval 0.49 to 0.99). The cumulative incidence curves diverged after around eight months of use (fig 2), and the number needed to treat corresponded to 79 and 11 patients to prevent one severe chronic obstructive pulmonary disease exacerbation event after one and five years of use, respectively. GLP-1 receptor agonists were also associated with a decreased risk of a moderate exacerbation of chronic obstructive pulmonary disease (hazard ratio 0.63, 0.43 to 0.94). Similarly, GLP-1 receptor agonists were associated with a lower rate of severe exacerbation (4.7 v 7.9 events per 100 person years; rate ratio 0.59, 95% confidence interval 0.37 to 0.94) and moderate exacerbation (4.1 v 7.8 events per 100 person years; 0.52, 0.34 to 0.80), compared with sulfonylureas.
Table 2.
Relative risks for severe and moderate exacerbation of chronic obstructive pulmonary disease comparing glucagon-like peptide 1 (GLP-1) receptor agonists with sulfonylureas
| Patients | Events | Person years | Incidence rate (95% CI)* † | Crude HR or RR‡ | Weighted HR or RR‡ (95% CI)† | |
|---|---|---|---|---|---|---|
| Time to first exacerbation | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 14 259 | 1261 | 24 126 | 5.0 (4.7 to 5.3) | 1.00 | 1.00 (reference) |
| GLP-1 receptor agonists | 1252 | 64 | 1853 | 3.5 (2.7 to 4.4) | 0.64 | 0.70 (0.49 to 0.99) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 14 259 | 909 | 22 718 | 5.4 (5.0 to 5.7) | 1.00 | 1.00 (reference) |
| GLP-1 receptor agonists | 1252 | 58 | 1765 | 3.3 (2.5 to 4.3) | 0.79 | 0.63 (0.43 to 0.94) |
| No of exacerbations | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 14 259 | 2053 | 25 765 | 7.9 (7.5 to 8.3) | 1.00 | 1.00 (reference) |
| GLP-1 receptor agonists | 1252 | 89 | 1913 | 4.7 (3.8 to 5.7) | 0.58 | 0.59 (0.37 to 0.94) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 14 259 | 1482 | 24 126 | 7.8 (7.4 to 8.2) | 1.00 | 1.00 (reference) |
| GLP-1 receptor agonists | 1252 | 75 | 1853 | 4.1 (3.2 to 5.1) | 0.66 | 0.52 (0.34 to 0.80) |
CI=confidence interval; HR=hazard ratio; RR=rate ratio.
Per 100 person years.
Weighted using propensity score fine stratification.
HR for time to first exacerbation; RR for number of exacerbations.
Fig 2.
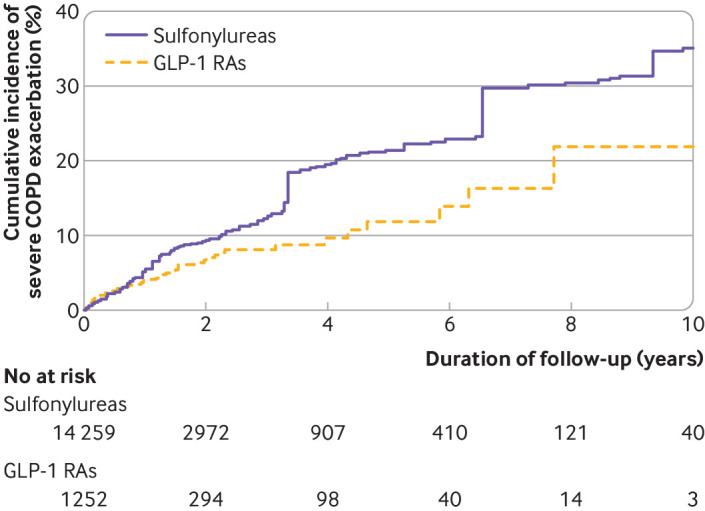
Weighted cumulative incidence curves of severe chronic obstructive pulmonary disease (COPD) exacerbations in glucagon-like peptide-1 receptor agonist (GLP-1 RA) versus sulfonylurea cohort
The results of the secondary analyses are shown in supplementary figure A. In the duration-response analysis, use of GLP-1 receptor agonists for more than two years were associated with a lower risk of severe chronic obstructive pulmonary disease exacerbation (hazard ratio 0.37, 0.16 to 0.82). With respect to drug specific effects, liraglutide, the most prescribed GLP-1 receptor agonist in our cohort, generated a hazard ratio below the null but with a wide confidence interval (hazard ratio 0.81, 0.51 to 1.27). We observed no effect measure modification on a multiplicative scale for age, sex, severity of dyspnoea, or predicted FEV1. However, patients with a history of asthma had a 37% lower risk of severe chronic obstructive pulmonary disease exacerbation with GLP-1 receptor agonists, whereas this association was not present in patients without a history of asthma. The hazard ratio was below the null for nearly all the study period, although with wide confidence intervals owing to the few events in each calendar year (supplementary figure B). Overall, the findings generated by the sensitivity analyses are consistent with the primary analysis, with all confidence intervals overlapping with those from the primary analysis (supplementary figure C).
DPP-4 inhibitors versus sulfonylureas
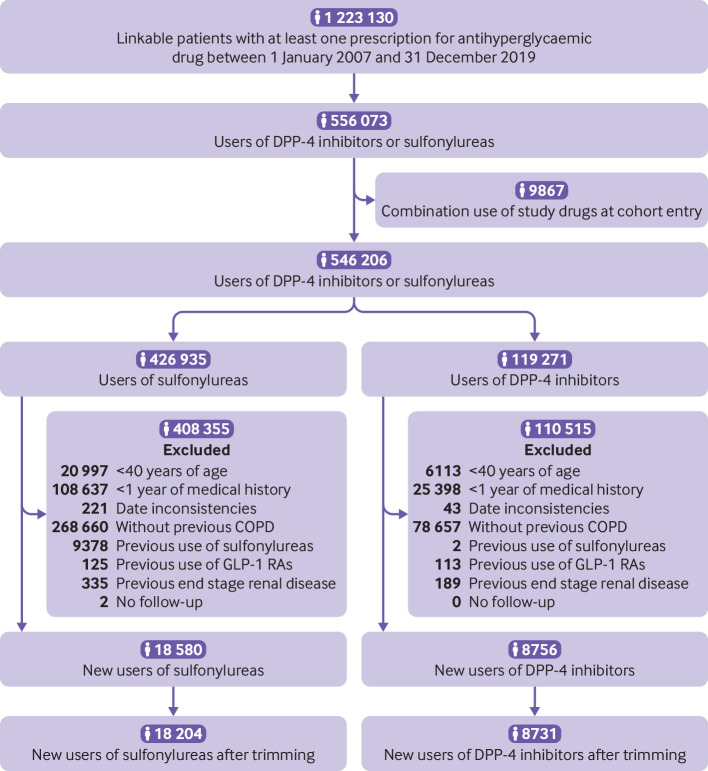
The second cohort included 8731 patients starting DPP-4 inhibitors and 18 204 starting sulfonylureas (fig 3). This cohort was followed for a median of 1.0 (0.4-2.2) years. As with the GLP-1 receptor agonist versus sulfonylurea cohort, the most common reason for the end of follow-up was drug discontinuation or switching (supplementary table B). A total of 2438 incident events of severe chronic obstructive pulmonary disease exacerbations occurred during 43 756 person years of follow-up, resulting in a crude incidence rate of 5.6 (5.4 to 5.8) per 100 person years. Before propensity score weighting, patients starting DPP-4 inhibitors were more likely to be obese, were more likely to have had diabetes for a longer period, and had a higher prevalence of microvascular complications compared with those starting sulfonylureas (table 3). The exposure groups were well balanced after propensity score weighting.
Fig 3.
Study flow diagram of patients starting sulfonylureas or dipeptidyl peptidase-4 (DPP-4) inhibitors in UK Clinical Practice Research Datalink between 2007 and 2019. COPD=chronic obstructive pulmonary disease
Table 3.
Baseline characteristics of dipeptidyl peptidase 4 (DPP-4) inhibitor and sulfonylurea exposure groups before and after propensity score weighting. Values are numbers (percentages) unless stated otherwise
| Characteristics | Before weighting* | After weighting* | |||||
|---|---|---|---|---|---|---|---|
| DPP-4 inhibitors (n=8731) | Sulfonylureas (n=18 204) | ASD | DPP-4 inhibitors (n=8731) | Sulfonylureas (n=18 204) | ASD | ||
| Mean (SD) age, years | 69.3 (10.7) | 69.4 (10.6) | 0.01 | 69.3 (10.7) | 68.8 (10.7) | 0.05 | |
| Male sex | 4836 (55.4) | 10 363 (56.9) | 0.03 | 4836 (55.4) | 10 044 (55.2) | 0.00 | |
| Year of cohort entry: | |||||||
| 2007-10 | 767 (8.8) | 7108 (39.0) | 0.76 | 767 (8.8) | 1582 (8.7) | 0.00 | |
| 2011-14 | 2183 (25.0) | 6270 (34.4) | 0.21 | 2183 (25.0) | 4518 (24.8) | 0.00 | |
| 2015-19 | 5781 (66.2) | 4826 (26.5) | 0.87 | 5781 (66.2) | 12 104 (66.5) | 0.01 | |
| Body mass index: | |||||||
| <30 | 3068 (35.1) | 8193 (45.0) | 0.20 | 3068 (35.1) | 6249 (34.3) | 0.02 | |
| ≥30.0 | 5576 (63.9) | 9759 (53.6) | 0.21 | 5576 (63.9) | 11 783 (64.7) | 0.02 | |
| Unknown | 87 (1.0) | 252 (1.4) | 0.04 | 87 (1.0) | 171 (0.9) | 0.01 | |
| Smoking status: | |||||||
| Ever | 8212 (94.1) | 17 059 (93.7) | 0.01 | 8212 (94.1) | 17 159 (94.3) | 0.01 | |
| Never | 515 (5.9) | 1135 (6.2) | 0.01 | 515 (5.9) | 1041 (5.7) | 0.01 | |
| Unknown | 4 (0.0) | 10 (0.1) | 0.00 | 4 (0.0) | 4 (0.0) | 0.01 | |
| Haemoglobin A1c: | |||||||
| ≤7.0% | 1318 (15.1) | 3012 (16.5) | 0.04 | 1318 (15.1) | 2736 (15.0) | 0.00 | |
| 7.1-8.0% | 2920 (33.4) | 4847 (26.6) | 0.15 | 2920 (33.4) | 5916 (32.5) | 0.02 | |
| >8.0% | 4429 (50.7) | 9345 (51.3) | 0.01 | 4429 (50.7) | 9397 (51.6) | 0.02 | |
| Unknown | 64 (0.7) | 1000 (5.5) | 0.28 | 64 (0.7) | 154 (0.8) | 0.01 | |
| Alcohol related disorders | 966 (11.1) | 1970 (10.8) | 0.01 | 966 (11.1) | 2024 (11.1) | 0.00 | |
| Mean (SD) duration of diabetes, years | 7.7 (6.8) | 5.3 (5.3) | 0.39 | 7.7 (6.8) | 7.4 (6.5) | 0.04 | |
| Type of antihyperglycaemic drugs: | |||||||
| Metformin | 7400 (84.8) | 14 332 (78.7) | 0.16 | 7400 (84.8) | 15 775 (86.7) | 0.05 | |
| Thiazolidinedione | 545 (6.2) | 1165 (6.4) | 0.01 | 545 (6.2) | 1210 (6.6) | 0.02 | |
| Meglitinides | 54 (0.6) | 87 (0.5) | 0.02 | 54 (0.6) | 136 (0.7) | 0.02 | |
| α-glucosidase inhibitors | 15 (0.2) | 25 (0.1) | 0.01 | 15 (0.2) | 33 (0.2) | 0.00 | |
| Sodium-glucose co-transporter-2 inhibitors | 243 (2.8) | 104 (0.6) | 0.17 | 243 (2.8) | 494 (2.7) | 0.00 | |
| Insulin | 1016 (11.6) | 800 (4.4) | 0.27 | 1016 (11.6) | 2000 (11.0) | 0.02 | |
| Peripheral vascular disease | 1484 (17.0) | 2762 (15.2) | 0.05 | 1484 (17.0) | 3008 (16.5) | 0.01 | |
| Stroke | 892 (10.2) | 1895 (10.4) | 0.01 | 892 (10.2) | 1856 (10.2) | 0.00 | |
| Myocardial infarction | 1349 (15.5) | 2907 (16.0) | 0.01 | 1349 (15.5) | 2737 (15.0) | 0.01 | |
| Renal disease | 2765 (31.7) | 5244 (28.8) | 0.06 | 2765 (31.7) | 5496 (30.2) | 0.03 | |
| Retinopathy | 2605 (29.8) | 4009 (22.0) | 0.18 | 2605 (29.8) | 5279 (29.0) | 0.02 | |
| Neuropathy | 2237 (25.6) | 3849 (21.1) | 0.11 | 2237 (25.6) | 4588 (25.2) | 0.01 | |
| Mean (SD) duration of COPD, years | 8.3 (7.6) | 8.0 (7.6) | 0.05 | 8.3 (7.6) | 8.2 (7.6) | 0.01 | |
| Long acting β-agonist | 4682 (53.6) | 9480 (52.1) | 0.03 | 4682 (53.6) | 9734 (53.5) | 0.00 | |
| Short acting β-agonist | 6009 (68.8) | 12 724 (69.9) | 0.02 | 6009 (68.8) | 12 587 (69.1) | 0.01 | |
| Long acting anti-muscarinic | 3454 (39.6) | 6210 (34.1) | 0.11 | 3454 (39.6) | 7160 (39.3) | 0.00 | |
| Short acting anti-muscarinic | 537 (6.2) | 2506 (13.8) | 0.26 | 537 (6.2) | 1129 (6.2) | 0.00 | |
| Inhaled corticosteroids, | 4979 (57.0) | 10 793 (59.3) | 0.05 | 4979 (57.0) | 10 385 (57.0) | 0.00 | |
| Oral corticosteroids, | 3166 (36.3) | 7112 (39.1) | 0.06 | 3166 (36.3) | 6541 (35.9) | 0.01 | |
| Leukotriene antagonists, | 406 (4.7) | 724 (4.0) | 0.03 | 406 (4.7) | 829 (4.6) | 0.00 | |
| Methylxanthines, | 343 (3.9) | 987 (5.4) | 0.07 | 343 (3.9) | 722 (4.0) | 0.00 | |
| Respiratory antibiotics, | 5848 (67.0) | 12 948 (71.1) | 0.09 | 5848 (67.0) | 12 173 (66.9) | 0.00 | |
| Hospital admission for COPD | 2346 (26.9) | 5531 (30.4) | 0.08 | 2346 (26.9) | 4826 (26.5) | 0.01 | |
| Pneumonia | 586 (6.7) | 1390 (7.6) | 0.04 | 586 (6.7) | 1251 (6.9) | 0.01 | |
| Influenza | 57 (0.7) | 161 (0.9) | 0.03 | 57 (0.7) | 114 (0.6) | 0.00 | |
| Asthma | 5123 (58.7) | 10 819 (59.4) | 0.02 | 5123 (58.7) | 10 712 (58.8) | 0.00 | |
| Interstitial lung disease | 259 (3.0) | 661 (3.6) | 0.04 | 259 (3.0) | 553 (3.0) | 0.00 | |
| Bronchiectasis | 504 (5.8) | 1016 (5.6) | 0.01 | 504 (5.8) | 1082 (5.9) | 0.01 | |
| Pulmonary embolism | 1880 (21.5) | 3435 (18.9) | 0.07 | 1880 (21.5) | 3825 (21.0) | 0.01 | |
| Pulmonary hypertension | 214 (2.5) | 356 (2.0) | 0.03 | 214 (2.5) | 401 (2.2) | 0.02 | |
| Lung cancer | 113 (1.3) | 487 (2.7) | 0.10 | 113 (1.3) | 235 (1.3) | 0.00 | |
| FEV1 (% predicted): | |||||||
| <30 | 257 (2.9) | 715 (3.9) | 0.05 | 257 (2.9) | 543 (3.0) | 0.00 | |
| 30-80 | 5060 (58.0) | 10 478 (57.6) | 0.01 | 5060 (58.0) | 10 631 (58.4) | 0.01 | |
| >80 | 1233 (14.1) | 2032 (11.2) | 0.09 | 1233 (14.1) | 2601 (14.3) | 0.00 | |
| Unknown | 2181 (25.0) | 4979 (27.4) | 0.05 | 2181 (25.0) | 4429 (24.3) | 0.02 | |
| FEV1-FVC ratio: | |||||||
| <35 | 64 (0.7) | 235 (1.3) | 0.06 | 64 (0.7) | 133 (0.7) | 0.00 | |
| 35-59.9 | 1406 (16.1) | 3101 (17.0) | 0.03 | 1406 (16.1) | 2906 (16.0) | 0.00 | |
| ≥60 | 4122 (47.2) | 7116 (39.1) | 0.16 | 4122 (47.2) | 8718 (47.9) | 0.01 | |
| Unknown | 3139 (36.0) | 7752 (42.6) | 0.14 | 3139 (36.0) | 6448 (35.4) | 0.01 | |
| Severity of dyspnoea: | |||||||
| None/mild | 2617 (30.0) | 4369 (24.0) | 0.13 | 2617 (30.0) | 5566 (30.6) | 0.01 | |
| Moderate/severe | 4486 (51.4) | 9323 (51.2) | 0.00 | 4486 (51.4) | 9297 (51.1) | 0.01 | |
| Unknown | 1628 (18.6) | 4512 (24.8) | 0.15 | 1628 (18.6) | 3342 (18.4) | 0.01 | |
| Blood eosinophil count: | |||||||
| <2% | 2860 (32.8) | 6476 (35.6) | 0.06 | 2860 (32.8) | 5919 (32.5) | 0.01 | |
| 2-4% | 3695 (42.3) | 7200 (39.6) | 0.06 | 3695 (42.3) | 7839 (43.1) | 0.01 | |
| >4% | 2016 (23.1) | 3772 (20.7) | 0.06 | 2016 (23.1) | 4113 (22.6) | 0.01 | |
| Unknown | 160 (1.8) | 756 (4.2) | 0.14 | 160 (1.8) | 333 (1.8) | 0.00 | |
| Cancer† | 1330 (15.2) | 2844 (15.6) | 0.01 | 1330 (15.2) | 2664 (14.6) | 0.02 | |
| Heart failure | 1780 (20.4) | 3819 (21.0) | 0.01 | 1780 (20.4) | 3555 (19.5) | 0.02 | |
| Hypertension | 7272 (83.3) | 14 172 (77.9) | 0.14 | 7272 (83.3) | 15 073 (82.8) | 0.01 | |
| Arrhythmia | 2432 (27.9) | 4951 (27.2) | 0.01 | 2432 (27.9) | 4853 (26.7) | 0.03 | |
| Dyslipidaemia | 4474 (51.2) | 8003 (44.0) | 0.15 | 4474 (51.2) | 9284 (51.0) | 0.00 | |
| Non-alcoholic fatty liver disease | 334 (3.8) | 421 (2.3) | 0.09 | 334 (3.8) | 714 (3.9) | 0.00 | |
| Hypothyroidism | 1375 (15.7) | 2409 (13.2) | 0.07 | 1375 (15.7) | 2790 (15.3) | 0.01 | |
| Gastro-oesophageal reflux disease | 2567 (29.4) | 4596 (25.2) | 0.09 | 2567 (29.4) | 5346 (29.4) | 0.00 | |
| Sleep apnoea | 935 (10.7) | 1264 (6.9) | 0.13 | 935 (10.7) | 2019 (11.1) | 0.01 | |
| Osteoarthritis | 4155 (47.6) | 7914 (43.5) | 0.08 | 4155 (47.6) | 8523 (46.8) | 0.02 | |
| Depression | 3998 (45.8) | 7756 (42.6) | 0.06 | 3998 (45.8) | 8558 (47.0) | 0.02 | |
| Angiotensin converting enzyme inhibitors | 4091 (46.9) | 8487 (46.6) | 0.00 | 4091 (46.9) | 8424 (46.3) | 0.01 | |
| Angiotensin receptor blockers | 1844 (21.1) | 3306 (18.2) | 0.07 | 1844 (21.1) | 3826 (21.0) | 0.00 | |
| β blockers | 2582 (29.6) | 4439 (24.4) | 0.12 | 2582 (29.6) | 5301 (29.1) | 0.01 | |
| Calcium channel blockers | 3295 (37.7) | 6410 (35.2) | 0.05 | 3295 (37.7) | 6769 (37.2) | 0.01 | |
| Diuretics | 3372 (38.6) | 7392 (40.6) | 0.04 | 3372 (38.6) | 6859 (37.7) | 0.02 | |
| Antiarrhythmic agents | 457 (5.2) | 1033 (5.7) | 0.02 | 457 (5.2) | 969 (5.3) | 0.00 | |
| Antiplatelet agents | 1118 (12.8) | 2151 (11.8) | 0.03 | 1118 (12.8) | 2295 (12.6) | 0.01 | |
| Statins | 7125 (81.6) | 13 829 (76.0) | 0.14 | 7125 (81.6) | 14 846 (81.6) | 0.00 | |
| Proton pump inhibitors | 4860 (55.7) | 9674 (53.1) | 0.05 | 4860 (55.7) | 10 100 (55.5) | 0.00 | |
| Non-steroidal anti-inflammatory drugs | 4502 (51.6) | 10 145 (55.7) | 0.08 | 4502 (51.6) | 9369 (51.5) | 0.00 | |
| Opioids | 4143 (47.5) | 9087 (49.9) | 0.05 | 4143 (47.5) | 8623 (47.4) | 0.00 | |
| Faecal occult blood testing or colonoscopy | 1604 (18.4) | 2208 (12.1) | 0.17 | 1604 (18.4) | 3364 (18.5) | 0.00 | |
| Mammography | 610 (7.0) | 1100 (6.0) | 0.04 | 610 (7.0) | 1312 (7.2) | 0.01 | |
| Prostate specific antigen testing | 851 (9.7) | 1706 (9.4) | 0.01 | 851 (9.7) | 1757 (9.7) | 0.00 | |
| Influenza vaccination | 1524 (17.5) | 8166 (44.9) | 0.62 | 1524 (17.5) | 3169 (17.4) | 0.00 | |
| Pneumococcal vaccination | 353 (4.0) | 845 (4.6) | 0.03 | 353 (4.0) | 742 (4.1) | 0.00 | |
| Season: | |||||||
| Spring | 2335 (26.7) | 4864 (26.7) | 0.00 | 2335 (26.7) | 4850 (26.6) | 0.00 | |
| Summer | 2166 (24.8) | 4437 (24.4) | 0.01 | 2166 (24.8) | 4544 (25.0) | 0.00 | |
| Autumn/fall | 2164 (24.8) | 4411 (24.2) | 0.01 | 2164 (24.8) | 4484 (24.6) | 0.00 | |
| Winter | 2066 (23.7) | 4492 (24.7) | 0.02 | 2066 (23.7) | 4326 (23.8) | 0.00 | |
ASD=absolute standardised difference; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; SD=standard deviation.
Weighted using propensity score fine stratification within study populations with overlapping propensity scores.
Excludes lung cancer and non-melanoma skin cancer.
Table 4 shows the results of the primary analysis. DPP-4 inhibitors were associated with a small decrease in the incidence of severe exacerbation of chronic obstructive pulmonary disease, compared with sulfonylureas, although the confidence interval included the null value (4.6 v 5.1 events per 100 person years; hazard ratio 0.91, 0.82 to 1.02). The cumulative incidence curves diverged after around six months of use but reconverged at around five years of follow-up (fig 4). DPP-4 inhibitors were not associated with a decreased risk of moderate exacerbation of chronic obstructive pulmonary disease (4.2 v 4.5 events per 100 person years; hazard ratio 0.93, 0.82 to 1.07). Furthermore, DPP-4 inhibitors were not associated with a lower rate of severe (6.5 v 7.2 events per 100 person years; rate ratio 0.90, 0.80 to 1.03) or moderate chronic obstructive pulmonary disease exacerbations (5.8 v 6.4 events per 100 person years; 0.91, 0.77 to 1.07).
Table 4.
Relative risks for severe and moderate exacerbation of chronic obstructive pulmonary disease comparing dipeptidyl peptidase 4 (DPP-4) inhibitors with sulfonylureas
| Patients | Events | Person years | Incidence rate (95% CI) * † | Crude HR or RR‡ | Weighted HR or RR‡ (95% CI)† | |
|---|---|---|---|---|---|---|
| Time to first exacerbation | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 18 204 | 1827 | 30 537 | 5.1 (4.9 to 5.4) | 1.00 | 1.00 (reference) |
| DPP-4 inhibitors | 8731 | 611 | 13 219 | 4.6 (4.3 to 5.0) | 0.74 | 0.91 (0.82 to 1.02) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 18 204 | 1136 | 28 809 | 4.5 (4.3 to 4.8) | 1.00 | 1.00 (reference) |
| DPP-4 inhibitors | 8731 | 528 | 12 604 | 4.2 (3.8 to 4.6) | 1.03 | 0.93 (0.82 to 1.07) |
| No of exacerbations | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 18 204 | 2974 | 32 737 | 7.2 (6.9 to 7.5) | 1.00 | 1.00 (reference) |
| DPP-4 inhibitors | 8731 | 904 | 13 944 | 6.5 (6.1 to 6.9) | 0.71 | 0.90 (0.80 to 1.03) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 18 204 | 1829 | 30 537 | 6.4 (6.1 to 6.7) | 1.00 | 1.00 (reference) |
| DPP-4 inhibitors | 8731 | 764 | 13 219 | 5.8 (5.4 to 6.2) | 0.97 | 0.91 (0.77 to 1.07) |
CI=confidence interval; HR=hazard ratio; RR=rate ratio.
Per 100 person years.
Weighted using propensity score fine stratification.
HR for time to first exacerbation; RR for number of exacerbations.
Fig 4.
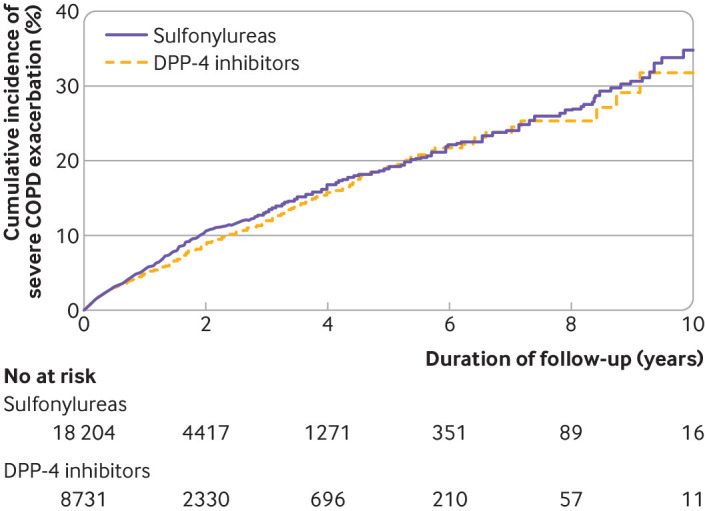
Weighted cumulative incidence curves of severe chronic obstructive pulmonary disease (COPD) exacerbations in dipeptidyl peptidase-4 (DPP-4) inhibitor versus sulfonylurea cohort
The results of the secondary analyses are shown in supplementary figure D. The duration-response analysis showed that the incidence of severe exacerbation of chronic obstructive pulmonary disease decreased by 23% between one and two years of use (hazard ratio 0.77, 0.60 to 0.99), but this benefit did not persist beyond two years of use. Although alogliptin generated the lowest hazard ratio among individual drugs (0.64, 0.46 to 0.89), its confidence interval overlapped with others in the class. We observed potential effect measure modification on a multiplicative scale with history of asthma, but not for age, sex, severity of dyspnoea, or predicted FEV1. The hazard ratios associated with DPP-4 inhibitors remained close to the null value throughout the study period (supplementary figure E). Finally, the sensitivity analyses generated consistent results (supplementary figure F).
SGLT-2 inhibitors versus sulfonylureas
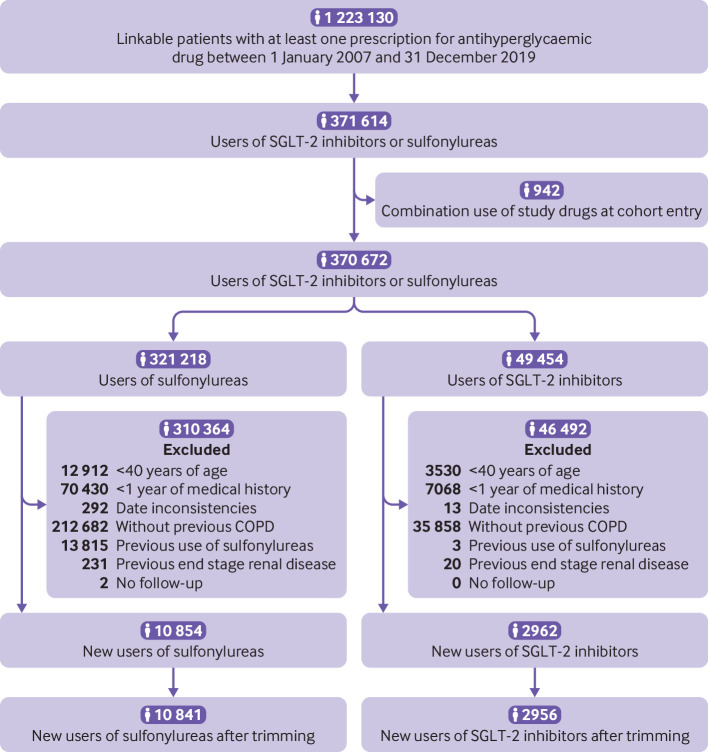
The third cohort included 2956 patients starting SGLT-2 inhibitors and 10 841 starting sulfonylureas (fig 5). This cohort was followed for a median of 0.9 (0.3-2.0) years, and the most common reason for end of follow-up was drug discontinuation or switching (supplementary table C). During 19 542 person years of follow-up, 1098 patients had a severe exacerbation of chronic obstructive pulmonary disease (crude incidence rate 5.6 (5.3 to 6.0) per 100 person years). Before weighting, SGLT-2 inhibitor users were more likely to be younger, to be obese, and to have retinopathy but less likely to have renal diseases than sulfonylurea users. The groups were well balanced after weighting (table 5).
Fig 5.
Study flow diagram of patients starting sulfonylureas or sodium-glucose co-transporter-2 (SGLT-2) inhibitors in UK Clinical Practice Research Datalink between 2013 and 2019. COPD=chronic obstructive pulmonary disease
Table 5.
Baseline characteristics of sodium-glucose co-transporter-2 (SGLT-2) inhibitor and sulfonylurea exposure groups before and after propensity score weighting. Values are numbers (percentages) unless stated otherwise
| Characteristics | Before weighting* | After weighting* | |||||
|---|---|---|---|---|---|---|---|
| SGLT-2 inhibitors (n=2956) | Sulfonylureas (n=10 841) | ASD | SGLT-2 inhibitors (n=2956) | Sulfonylureas (n=10 841) | ASD | ||
| Mean (SD) age, years | 62.9 (9.0) | 69.3 (10.6) | 0.65 | 62.9 (9.0) | 62.7 (9.1) | 0.02 | |
| Male sex | 1729 (58.5) | 6106 (56.3) | 0.04 | 1729 (58.5) | 6290 (58.0) | 0.01 | |
| Year of cohort entry: | |||||||
| 2013-14 | 285 (9.6) | 4035 (37.2) | 0.69 | 285 (9.6) | 889 (8.2) | 0.05 | |
| 2015-19 | 2671 (90.4) | 6806 (62.8) | 0.69 | 2671 (90.4) | 9952 (91.8) | 0.05 | |
| Body mass index: | |||||||
| <30 | 614 (20.8) | 4716 (43.5) | 0.50 | 614 (20.8) | 2357 (21.7) | 0.02 | |
| ≥30.0 | 2314 (78.3) | 5981 (55.2) | 0.51 | 2314 (78.3) | 8396 (77.4) | 0.02 | |
| Unknown | 28 (0.9) | 144 (1.3) | 0.04 | 28 (0.9) | 88 (0.8) | 0.01 | |
| Smoking status: | |||||||
| Ever | 2793 (94.5) | 10 227 (94.3) | 0.01 | 2793 (94.5) | 10 272 (94.8) | 0.01 | |
| Never | 163 (5.5) | 614 (5.7) | 0.01 | 163 (5.5) | 569 (5.2) | 0.01 | |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Haemoglobin A1c: | |||||||
| ≤7.0% | 204 (6.9) | 1561 (14.4) | 0.24 | 204 (6.9) | 819 (7.6) | 0.03 | |
| 7.1-8.0% | 749 (25.3) | 2522 (23.3) | 0.05 | 749 (25.3) | 2653 (24.5) | 0.02 | |
| >8% | 1995 (67.5) | 6437 (59.4) | 0.17 | 1995 (67.5) | 7333 (67.6) | 0.00 | |
| Unknown | 8 (0.3) | 321 (3.0) | 0.21 | 8 (0.3) | 35 (0.3) | 0.01 | |
| Alcohol related disorders | 410 (13.9) | 1479 (13.6) | 0.01 | 410 (13.9) | 1439 (13.3) | 0.02 | |
| Mean (SD) duration of diabetes, years | 9.1 (6.9) | 6.7 (6.0) | 0.37 | 9.1 (6.9) | 8.5 (6.4) | 0.09 | |
| Type of antihyperglycaemic drugs: | |||||||
| Metformin | 2648 (89.6) | 8204 (75.7) | 0.37 | 2648 (89.6) | 9808 (90.5) | 0.03 | |
| Thiazolidinedione | 127 (4.3) | 286 (2.6) | 0.09 | 127 (4.3) | 544 (5.0) | 0.03 | |
| Meglitinides | 16 (0.5) | 42 (0.4) | 0.02 | 16 (0.5) | 43 (0.4) | 0.02 | |
| α-glucosidase inhibitors | 5 (0.2) | 9 (0.1) | 0.02 | 5 (0.2) | 19 (0.2) | 0.00 | |
| Glucagon-like peptide 1 receptor agonists | 337 (11.4) | 239 (2.2) | 0.37 | 337 (11.4) | 1197 (11.0) | 0.01 | |
| Dipeptidyl peptidase 4 inhibitors | 972 (32.9) | 2020 (18.6) | 0.33 | 972 (32.9) | 3964 (36.6) | 0.08 | |
| Insulin | 744 (25.2) | 487 (4.5) | 0.61 | 744 (25.2) | 2484 (22.9) | 0.05 | |
| Peripheral vascular disease | 450 (15.2) | 1727 (15.9) | 0.02 | 450 (15.2) | 1497 (13.8) | 0.04 | |
| Stroke | 200 (6.8) | 1148 (10.6) | 0.14 | 200 (6.8) | 924 (8.5) | 0.07 | |
| Myocardial infarction | 408 (13.8) | 1731 (16.0) | 0.06 | 408 (13.8) | 1387 (12.8) | 0.03 | |
| Renal disease | 378 (12.8) | 3081 (28.4) | 0.39 | 378 (12.8) | 1354 (12.5) | 0.01 | |
| Retinopathy | 969 (32.8) | 2993 (27.6) | 0.11 | 969 (32.8) | 3141 (29.0) | 0.08 | |
| Neuropathy | 775 (26.2) | 2476 (22.8) | 0.08 | 775 (26.2) | 2626 (24.2) | 0.05 | |
| Mean (SD) duration of COPD, years | 7.8 (7.2) | 8.3 (7.7) | 0.06 | 7.8 (7.2) | 7.9 (7.1) | 0.00 | |
| Long acting β-agonist | 1588 (53.7) | 5971 (55.1) | 0.03 | 1588 (53.7) | 5877 (54.2) | 0.01 | |
| Short acting β-agonist | 2012 (68.1) | 7644 (70.5) | 0.05 | 2012 (68.1) | 7311 (67.4) | 0.01 | |
| Long acting anti-muscarinic | 1160 (39.2) | 4396 (40.5) | 0.03 | 1160 (39.2) | 4272 (39.4) | 0.00 | |
| Short acting anti-muscarinic | 106 (3.6) | 749 (6.9) | 0.15 | 106 (3.6) | 353 (3.3) | 0.02 | |
| Inhaled corticosteroids, | 1660 (56.2) | 6313 (58.2) | 0.04 | 1660 (56.2) | 6073 (56.0) | 0.00 | |
| Oral corticosteroids, | 1033 (34.9) | 4617 (42.6) | 0.16 | 1033 (34.9) | 3776 (34.8) | 0.00 | |
| Leukotriene antagonists, | 205 (6.9) | 515 (4.8) | 0.09 | 205 (6.9) | 744 (6.9) | 0.00 | |
| Methylxanthines, | 108 (3.7) | 506 (4.7) | 0.05 | 108 (3.7) | 391 (3.6) | 0.00 | |
| Respiratory antibiotics, | 1900 (64.3) | 7666 (70.7) | 0.14 | 1900 (64.3) | 6854 (63.2) | 0.02 | |
| Hospital admission for COPD | 620 (21.0) | 3699 (34.1) | 0.30 | 620 (21.0) | 2321 (21.4) | 0.01 | |
| Pneumonia | 87 (2.9) | 1041 (9.6) | 0.28 | 87 (2.9) | 325 (3.0) | 0.00 | |
| Influenza | 12 (0.4) | 88 (0.8) | 0.05 | 12 (0.4) | 38 (0.4) | 0.01 | |
| Asthma | 1776 (60.1) | 6271 (57.8) | 0.05 | 1776 (60.1) | 6577 (60.7) | 0.01 | |
| Interstitial lung disease | 72 (2.4) | 481 (4.4) | 0.11 | 72 (2.4) | 253 (2.3) | 0.01 | |
| Bronchiectasis | 134 (4.5) | 748 (6.9) | 0.10 | 134 (4.5) | 438 (4.0) | 0.02 | |
| Pulmonary embolism | 545 (18.4) | 2451 (22.6) | 0.10 | 545 (18.4) | 2044 (18.9) | 0.01 | |
| Pulmonary hypertension | 30 (1.0) | 296 (2.7) | 0.13 | 30 (1.0) | 96 (0.9) | 0.01 | |
| Lung cancer | 30 (1.0) | 323 (3.0) | 0.14 | 30 (1.0) | 112 (1.0) | 0.00 | |
| FEV1 (% predicted): | |||||||
| <30 | 53 (1.8) | 382 (3.5) | 0.11 | 53 (1.8) | 196 (1.8) | 0.00 | |
| 30-80 | 1711 (57.9) | 6356 (58.6) | 0.02 | 1711 (57.9) | 6211 (57.3) | 0.01 | |
| >80 | 435 (14.7) | 1512 (13.9) | 0.02 | 435 (14.7) | 1616 (14.9) | 0.01 | |
| Unknown | 757 (25.6) | 2591 (23.9) | 0.04 | 757 (25.6) | 2818 (26.0) | 0.01 | |
| FEV1-FVC ratio: | |||||||
| <35 | 20 (0.7) | 158 (1.5) | 0.08 | 20 (0.7) | 81 (0.8) | 0.01 | |
| 35-59.9 | 374 (12.7) | 1805 (16.6) | 0.11 | 374 (12.7) | 1385 (12.8) | 0.00 | |
| ≥60 | 1563 (52.9) | 4990 (46.0) | 0.14 | 1563 (52.9) | 5661 (52.2) | 0.01 | |
| Unknown | 999 (33.8) | 3888 (35.9) | 0.04 | 999 (33.8) | 3713 (34.3) | 0.01 | |
| Severity of dyspnoea: | |||||||
| None/mild | 1005 (34.0) | 3204 (29.6) | 0.10 | 1005 (34.0) | 3660 (33.8) | 0.01 | |
| Moderate/severe | 1367 (46.2) | 5689 (52.5) | 0.12 | 1367 (46.2) | 4980 (45.9) | 0.01 | |
| Unknown | 584 (19.8) | 1948 (18.0) | 0.05 | 584 (19.8) | 2201 (20.3) | 0.01 | |
| Blood eosinophil count: | |||||||
| <2% | 964 (32.6) | 4050 (37.4) | 0.10 | 964 (32.6) | 3430 (31.6) | 0.02 | |
| 2-4% | 1317 (44.6) | 4344 (40.1) | 0.09 | 1317 (44.6) | 4961 (45.8) | 0.02 | |
| >4% | 639 (21.6) | 2249 (20.7) | 0.02 | 639 (21.6) | 2296 (21.2) | 0.01 | |
| Unknown | 36 (1.2) | 198 (1.8) | 0.05 | 36 (1.2) | 153 (1.4) | 0.02 | |
| Cancer† | 303 (10.3) | 1914 (17.7) | 0.21 | 303 (10.3) | 1037 (9.6) | 0.02 | |
| Heart failure | 358 (12.1) | 2295 (21.2) | 0.25 | 358 (12.1) | 1235 (11.4) | 0.02 | |
| Hypertension | 2392 (80.9) | 8757 (80.8) | 0.00 | 2392 (80.9) | 8612 (79.4) | 0.04 | |
| Arrhythmia | 547 (18.5) | 3113 (28.7) | 0.24 | 547 (18.5) | 2017 (18.6) | 0.00 | |
| Dyslipidaemia | 1509 (51.0) | 5309 (49.0) | 0.04 | 1509 (51.0) | 5612 (51.8) | 0.01 | |
| Non-alcoholic fatty liver disease | 214 (7.2) | 392 (3.6) | 0.16 | 214 (7.2) | 759 (7.0) | 0.01 | |
| Hypothyroidism | 448 (15.2) | 1669 (15.4) | 0.01 | 448 (15.2) | 1543 (14.2) | 0.03 | |
| Gastro-oesophageal reflux disease | 924 (31.3) | 3220 (29.7) | 0.03 | 924 (31.3) | 3378 (31.2) | 0.00 | |
| Sleep apnoea | 565 (19.1) | 1058 (9.8) | 0.27 | 565 (19.1) | 2093 (19.3) | 0.00 | |
| Osteoarthritis | 1300 (44.0) | 5039 (46.5) | 0.05 | 1300 (44.0) | 4660 (43.0) | 0.02 | |
| Depression | 1651 (55.9) | 5191 (47.9) | 0.16 | 1651 (55.9) | 6034 (55.7) | 0.00 | |
| Angiotensin converting enzyme inhibitors | 1425 (48.2) | 4771 (44.0) | 0.08 | 1425 (48.2) | 5268 (48.6) | 0.01 | |
| Angiotensin receptor blockers | 579 (19.6) | 1918 (17.7) | 0.05 | 579 (19.6) | 2008 (18.5) | 0.03 | |
| β blockers | 781 (26.4) | 3212 (29.6) | 0.07 | 781 (26.4) | 2704 (24.9) | 0.03 | |
| Calcium channel blockers | 990 (33.5) | 3728 (34.4) | 0.02 | 990 (33.5) | 3488 (32.2) | 0.03 | |
| Diuretics | 804 (27.2) | 4100 (37.8) | 0.23 | 804 (27.2) | 2908 (26.8) | 0.01 | |
| Antiarrhythmic agents | 156 (5.3) | 560 (5.2) | 0.01 | 156 (5.3) | 668 (6.2) | 0.04 | |
| Antiplatelet agents | 299 (10.1) | 1383 (12.8) | 0.08 | 299 (10.1) | 1113 (10.3) | 0.00 | |
| Statins | 2442 (82.6) | 8258 (76.2) | 0.16 | 2442 (82.6) | 9011 (83.1) | 0.01 | |
| Proton pump inhibitors | 1669 (56.5) | 6479 (59.8) | 0.07 | 1669 (56.5) | 6056 (55.9) | 0.01 | |
| Non-steroidal anti-inflammatory drugs | 1460 (49.4) | 5373 (49.6) | 0.00 | 1460 (49.4) | 5145 (47.5) | 0.04 | |
| Opioids | 1474 (49.9) | 5330 (49.2) | 0.01 | 1474 (49.9) | 5227 (48.2) | 0.03 | |
| Faecal occult blood testing or colonoscopy | 745 (25.2) | 2148 (19.8) | 0.13 | 745 (25.2) | 2693 (24.8) | 0.01 | |
| Mammography | 247 (8.4) | 695 (6.4) | 0.07 | 247 (8.4) | 882 (8.1) | 0.01 | |
| Prostate specific antigen testing | 248 (8.4) | 1098 (10.1) | 0.06 | 248 (8.4) | 916 (8.5) | 0.00 | |
| Influenza vaccination | 57 (1.9) | 454 (4.2) | 0.13 | 57 (1.9) | 222 (2.0) | 0.01 | |
| Pneumococcal vaccination | 135 (4.6) | 420 (3.9) | 0.03 | 135 (4.6) | 458 (4.2) | 0.02 | |
| Season: | |||||||
| Spring | 759 (25.7) | 3029 (27.9) | 0.05 | 759 (25.7) | 2724 (25.1) | 0.01 | |
| Summer | 729 (24.7) | 2608 (24.1) | 0.01 | 729 (24.7) | 2618 (24.1) | 0.01 | |
| Autumn/fall | 760 (25.7) | 2463 (22.7) | 0.07 | 760 (25.7) | 2736 (25.2) | 0.01 | |
| Winter | 708 (24.0) | 2741 (25.3) | 0.03 | 708 (24.0) | 2763 (25.5) | 0.04 | |
ASD=absolute standardised difference; COPD=chronic obstructive pulmonary disease; FEV1=forced expiratory volume in the first second; FVC=forced vital capacity; SD=standard deviation.
Weighted using propensity score fine stratification within study populations with overlapping propensity scores.
Excludes lung cancer and non-melanoma skin cancer.
Table 6 shows the relative risks for the primary analyses. SGLT-2 inhibitors were associated with a decreased incidence of severe exacerbations of chronic obstructive pulmonary disease (2.4 v 3.9 events per 100 person years; hazard ratio 0.62, 0.48 to 0.81), with the cumulative incidence curves diverging around five months of follow-up, and the numbers needed to treat were 78 and 31 at one and five years, respectively (fig 6). Similarly, SGLT-2 inhibitors were associated with a decreased count of severe exacerbations (3.2 v 5.4 events per 100 person years; rate ratio 0.59, 0.43 to 0.80). However, we saw no association with moderate exacerbations, whether considering the incidence (4.5 v 4.3 events per 100 person years; hazard ratio 1.02, 0.83 to 1.27) or count (5.9 v 5.9 events per 100 person years; rate ratio 1.00, 0.79 to 1.26).
Table 6.
Relative risks for severe and moderate exacerbation of chronic obstructive pulmonary disease comparing sodium-glucose co-transporter-2 (SGLT-2) inhibitors with sulfonylureas
| Patients | Events | Person years | Incidence rate (95% CI)* † | Crude HR or RR‡ | Weighted HR or RR‡ (95% CI)† | |
|---|---|---|---|---|---|---|
| Time to first exacerbation | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 10 841 | 1006 | 15 740 | 3.9 (3.6 to 4.2) | 1.00 | 1.00 (reference) |
| SGLT-2 inhibitors | 2956 | 92 | 3803 | 2.4 (2.0 to 3.0) | 0.36 | 0.62 (0.48 to 0.81) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 10 841 | 704 | 14 860 | 4.3 (4.0 to 4.7) | 1.00 | 1.00 (reference) |
| SGLT-2 inhibitors | 2956 | 162 | 3629 | 4.5 (3.8 to 5.2) | 0.91 | 1.02 (0.83 to 1.27) |
| No of exacerbations | ||||||
| Severe exacerbations: | ||||||
| Sulfonylureas | 10 841 | 1586 | 16 816 | 5.4 (5.1 to 5.8) | 1.00 | 1.00 (reference) |
| SGLT-2 inhibitors | 2956 | 125 | 3911 | 3.2 (2.7 to 3.8) | 0.34 | 0.59 (0.43 to 0.80) |
| Moderate exacerbations: | ||||||
| Sulfonylureas | 10 841 | 1021 | 15 740 | 5.9 (5.5 to 6.3) | 1.00 | 1.00 (reference) |
| SGLT-2 inhibitors | 2956 | 224 | 3803 | 5.9 (5.2 to 6.7) | 0.91 | 1.00 (0.79 to 1.26) |
CI=confidence interval; HR=hazard ratio; RR=rate ratio.
Per 100 person years.
Weighted using propensity score fine stratification.
HR for time to first exacerbation; RR for number of exacerbations.
Fig 6.
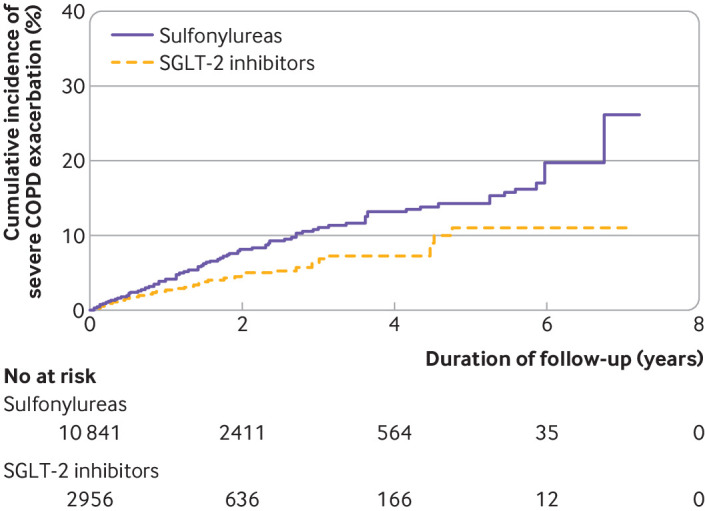
Weighted cumulative incidence curves of severe chronic obstructive pulmonary disease (COPD) exacerbations in sodium-glucose co-transporter-2 inhibitor (SGLT-2) versus sulfonylurea cohort
Supplementary figure G shows the results of secondary analyses. We observed a 32% lower incidence of chronic obstructive pulmonary disease exacerbation during the first year of follow-up (hazard ratio 0.68, 0.49 to 0.94). All three available SGLT-2 inhibitors were associated with a reduced risk of severe exacerbations. We saw no effect measure modification on a multiplicative scale by age, sex, dyspnoea severity, predicted FEV1, or history of asthma. The hazard ratio remained below the null value during most of the study period (supplementary figure H). Overall, the results of the sensitivity analyses were consistent with those of the primary analyses (supplementary figure I).
Discussion
The results of this population based cohort study indicate that, compared with sulfonylureas, GLP-1 receptor agonists were associated with a reduced risk of severe and moderate exacerbations of chronic obstructive pulmonary disease among patients with type 2 diabetes and chronic obstructive pulmonary disease. By contrast, DPP-4 inhibitors were not consistently associated with an overall lower risk of these outcomes, and SGLT-2 inhibitors were associated with a reduced risk of severe, but not moderate, exacerbations. Overall, these findings remained robust in several sensitivity analyses.
Comparison with previous studies
Our findings indicate that GLP-1 receptor agonists are associated with a 30% lower risk of severe exacerbation and a 37% lower risk of moderate exacerbation, compared with sulfonylureas. These results align with those of a recent study conducted using the US IBM MarketScan Commercial Claims Database.14 In that study, GLP-1 receptor agonists were associated with a decreased risk of severe respiratory disease exacerbations in patients with concomitant type 2 diabetes and chronic lower respiratory diseases (hazard ratio 0.52, 0.32 to 0.85). However, the active comparator in that study was DPP-4 inhibitors, drugs that may not have a neutral effect on exacerbations. Furthermore, the cohort included patients with either chronic obstructive pulmonary disease or asthma, two different disease entities. Finally, the study truncated the follow-up to one year, whereas our study followed patients for up to 14 years.
With respect to DPP-4 inhibitors, we observed a numerically lower risk of chronic obstructive pulmonary disease exacerbations, with confidence intervals around all effect estimates including the null value. A previous Taiwanese nested case-control study conducted among patients with both type 2 diabetes and chronic obstructive pulmonary disease found that DPP-4 inhibitors were not associated with a decreased risk of exacerbation (odds ratio 1.13, 0.92 to 1.40).13 However, that study had some limitations, including use of a comparator group consisting of various antihyperglycaemic drugs and not matching on the duration of follow-up, which can introduce confounding and potential time window bias.43 To date, the effectiveness of SGLT-2 inhibitors in preventing exacerbations of chronic obstructive pulmonary disease has not been investigated in observational studies.
By contrast to previous work, our study was specifically designed to assess the effects of novel antihyperglycaemic drugs among patients with type 2 diabetes and chronic obstructive pulmonary disease. In addition, we defined exacerbations by using codes for chronic obstructive pulmonary disease, avoiding potential outcome misclassification with regard to exacerbations due to chronic obstructive pulmonary disease versus asthma. Importantly, we considered additional outcomes, such as moderate exacerbation, an important endpoint in randomised controlled trials in chronic obstructive pulmonary disease. Finally, we avoided using DPP-4 inhibitors as the active comparator, given their possible association with the outcomes. These drugs were associated with a modest effect on the outcomes in our study, with some drugs such as alogliptin having potentially stronger effects on exacerbations. Overall, our findings add to the therapeutic options physicians could explore for patients with both type 2 diabetes and chronic obstructive pulmonary disease. Whether these drugs can be used in patients with chronic obstructive pulmonary disease without type 2 diabetes will need to be investigated in future randomised controlled trials.
Biological mechanisms
Several potential mechanisms may explain the decreased risk of chronic obstructive pulmonary disease exacerbation observed with GLP-1 receptor agonists. Firstly, GLP-1 receptor agonists have been shown to reduce local inflammation and airway hyperresponsiveness in murine models,4 5 44 in isolated human airways,3 and among patients with type 2 diabetes and chronic obstructive pulmonary disease.45 In short term randomised controlled trials, GLP-1 receptor agonists have been shown to improve surrogate measures of lung function, such as forced vital capacity.6 7 Secondly, chronic obstructive pulmonary disease is increasingly conceptualised as the respiratory manifestation of a chronic systemic inflammation, which results from a constellation of underlying risk factors, including smoking, obesity, and hypertension. GLP-1 receptor agonists are known to have systemic anti-inflammatory effects.46 Thirdly, abdominal obesity increases the risk of chronic obstructive pulmonary disease exacerbations by physically reducing the lung capacity by pushing the diaphragm against the thorax.47 GLP-1 receptor agonists may reduce chronic obstructive pulmonary disease exacerbations as these drugs have been associated with significant weight loss.2 48 Additional laboratory and observational studies are needed to explore further the mechanisms by which GLP-1 receptor agonists may exert their beneficial effects on chronic obstructive pulmonary disease exacerbations. Any small effect of DPP-4 inhibitors may be attributed to the raised concentration of endogenous GLP-1 that may produce a local and systemic anti-inflammatory effect,49 albeit at much lower levels than with the GLP-1 receptor agonists, which are more potent than endogenous GLP-1.50 The glucosuric effect SGLT-2 inhibitors decreases serum concentrations of glucose available for tissue metabolism, resulting in decreased endogenous carbon dioxide production; this may be beneficial for patients with chronic obstructive pulmonary disease who have difficulty expelling carbon dioxide from the body.9 Some evidence also suggests that SGLT-2 inhibitors may have beneficial effects on chronic obstructive pulmonary disease outcomes. In a meta-analysis of nine randomised controlled trials, SGLT-2 inhibitors were associated with a trend towards a reduced risk of new onset of chronic obstructive pulmonary disease (risk ratio 0.79, 0.61 to 1.02).51 Furthermore, large observational studies and a meta-analysis of cardiovascular outcome trials have associated SGLT-2 inhibitors with a decreased risk of pneumonia,10 11 52 a condition that often leads to chronic obstructive pulmonary disease exacerbations. The mechanisms behind heterogeneous findings of SGLT-2 inhibitors preventing severe but not moderate chronic obstructive pulmonary disease exacerbations are unclear and should be explored further.
An interesting finding of this study was that GLP-1 receptor agonists and DPP-4 inhibitors were associated with a lower risk of severe chronic obstructive pulmonary disease exacerbations among patients who also had asthma but not among those without a history of asthma. Laboratory studies finding benefits of an up-regulated GLP-1 pathway in airway diseases showed a reduction in airway hyperresponsiveness by local anti-inflammatory effects.3 Airway hyperresponsiveness is a feature that is present in both asthma and chronic obstructive pulmonary disease patients.53 54 Thus, among patients with chronic obstructive pulmonary disease and asthma, hyperresponsiveness is more prevalent, which may potentiate the effects of incretin based drugs in this subpopulation. This highlights a potential role of GLP-1 receptor agonists and DPP-4 inhibitors in the asthma-chronic obstructive pulmonary disease overlap syndrome, a major phenotype among older patients with chronic obstructive pulmonary disease.55 On the other hand, such effect measure modification on a multiplicative scale with history of asthma was not seen in the case of SGLT-2 inhibitors, for which we found a reduced risk in patients with or without a history of asthma.
Strengths and limitations of study
Our study has several strengths. Firstly, the use of the CPRD and its linked databases allowed us to control for potentially important confounders, such as body mass index, spirometry parameters, and laboratory measures, which are typically absent in claims databases. Secondly, we used an active comparator, new user design, an approach that limits biases due to the inclusion of prevalent users. Thirdly, we granulated our outcome into severe and moderate exacerbations of chronic obstructive pulmonary disease, thus enabling a more nuanced identification of the role of the novel antihyperglycaemic drugs in this therapeutic area compared with previous studies.
Our study also has some limitations. Firstly, exposure misclassification is possible, given that the CPRD is a general practice database and does not record prescriptions written by specialists. However, this is unlikely to be an important source of misclassification because general practitioners are responsible for the management of type 2 diabetes in the UK.56 Furthermore, the CPRD does not record patients’ adherence to the treatment regimens, which can be another source of exposure misclassification. However, using an on-treatment exposure definition in which patients were followed while continuously exposed to the study drugs likely reduced the impact of this bias. Secondly, some outcome misclassification may have been present, although the definition of severe chronic obstructive pulmonary disease exacerbation requiring hospital admission has been validated and has a high specificity.28 In the case of moderate exacerbation, we used a validated algorithm that required patients to have a diagnostic code for chronic obstructive pulmonary disease exacerbation along with co-prescription of a corticosteroid and antibiotic on the same day. Although this restrictive definition likely affected the sensitivity of the outcome, we prioritised specificity as this should have no impact on relative effect measures in the context of non-differential misclassification. Thirdly, residual confounding is possible given the observational nature of the study. However, using an active comparator typically prescribed at the same disease stage as the novel antihyperglycaemic drugs, and propensity score models that considered 72 variables that are potential and proxy confounders, likely reduced the possibility of major unmeasured confounding. Fourthly, although sulfonylureas were not associated with an increased risk of chronic obstructive pulmonary disease exacerbation in a previous study,13 these drugs may not be entirely neutral owing to their weight inducing effects. Sulfonylureas have been shown to inhibit the NLRP3 inflammasome and interleukin-1β release, thereby having potential anti-inflammatory effects.57 58 59 Although this is possible, the choice of this comparator needs to be considered in relation to the other antihyperglycaemic drugs that are less likely to have neutral effects on chronic obstructive pulmonary disease exacerbations. Furthermore, sulfonylureas continue to be widely used in the treatment of diabetes in the UK,1 25 the US,26 and worldwide,27 attesting to the clinical meaningfulness of our comparative effectiveness findings. Our study indicates that the prescribing of sulfonylureas outnumbered the prescribing of GLP-1 receptor agonists in an 11 to one ratio, DPP-4 inhibitors in a two to one ratio, and SGLT-2 inhibitors in a four to one ratio. Fifthly, our study had a median follow-up of one year, although the potential follow-up extended for up to 14 years. The cumulative incidence curves in the GLP-1 receptor agonist versus sulfonylurea cohort diverged after eight months of use. The timing of this effect fits with the hypothesised mechanisms of GLP-1 receptor agonists on exacerbations of chronic obstructive pulmonary disease, which include local anti-inflammatory, smooth muscle relaxation, and structural modifications.44 On the other hand, the cumulative incidence curves in the SGLT-2 inhibitor versus sulfonylurea cohort diverged earlier. Lastly, some secondary analyses, such as those stratifying on individual drugs, generated fewer exposed events and wide confidence intervals. Thus, these results should be interpreted with caution.
Conclusions
In summary, in this large population based study, the use of GLP-1 receptor agonists and SGLT-2 inhibitors was associated with a lower risk of severe exacerbations among patients with type 2 diabetes and chronic obstructive pulmonary disease compared with sulfonylureas, whereas a risk reduction associated with DPP-4 inhibitor use, if any, was small. Further research, including confirmatory randomised controlled trials, will be needed to investigate the potential of GLP-1 receptor agonists and SGLT-2 inhibitors as a therapeutic option in patients with type 2 diabetes and chronic obstructive pulmonary disease.
What is already known on this topic
Glucagon-like 1 peptide (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose co-transporter 2 (SGLT-2) inhibitors are novel antihyperglycaemic drugs
Laboratory and clinical studies suggest that these drugs might have a therapeutic role in chronic obstructive pulmonary disease (COPD)
To date, real world studies investigating this potentially beneficial effect have been limited
What this study adds
GLP-1 receptor agonists were associated with a lower risk of severe and moderate COPD exacerbations among patients with type 2 diabetes and COPD, compared with sulfonylureas
DPP-4 inhibitors generated very modest risk reductions in COPD exacerbations, but these were associated with wide confidence intervals that included the null value
SGLT-2 inhibitors were associated with a decreased risk of severe COPD exacerbations but not of moderate exacerbations
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: All authors were involved in conceiving and designing the study. LA acquired the data. RP, HY, and LA did the statistical analyses. All authors analysed and interpreted the data. RP and SL wrote the manuscript, and all authors critically revised it. All authors approved the final version of the manuscript and agree to be accountable for the accuracy of the work. LA supervised the study and is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by a foundation scheme grant from the Canadian Institutes of Health Research (FDN-143328). The sponsors had no influence on the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. RP is the recipient of a doctoral award from the Fonds de Recherche du Québec – Santé. OHYY holds a Chercheur-Boursier Clinicien Junior 1 award from the Fonds de Recherche du Québec - Santé. SS is the recipient of the Distinguished James McGill Award from McGill University. LA holds a distinguished research scholar award from the Fonds de Recherche du Québec - Santé and is the recipient of a William Dawson Scholar Award from McGill University.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: this study was funded by the Canadian Institutes of Health Research; LA has received consulting fees from Janssen and Pfizer unrelated to this project; SS has attended scientific advisory committee meetings for AstraZeneca, Atara, Bristol-Myers-Squibb, Merck, Novartis, Panalgo, Pfizer, and Seqirus and has received speaking fees from Boehringer-Ingelheim and Novartis; no other relationships or activities that could appear to have influenced the submitted work.
The guarantor (LA) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will disseminate the results through our general media communications plan, by organising a press release through the Public Relations Department of the Lady Davis Institute. Additionally, we will directly communicate our results to diabetes associations and regulatory agencies.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study protocol was approved by the Research Data Governance of the CPRD (protocol 21_000702) and by the Research Ethics Board of the Jewish General Hospital, Montreal, Canada. The study was a secondary data analysis and did not include patients as study participants. Hence the Research Data Governance of the Clinical Practice Research Datalink and the Research Ethics Board deemed it exempt by from the need to obtain patient consent.
Data availability statement
This study is based on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Because electronic health records are classified as “sensitive data” by the UK Data Protection Act, information governance restrictions (to protect patient confidentiality) prevent data sharing via public deposition. Data are available with approval through the individual constituent entities controlling access to the data. Specifically, the primary care data can be requested via application to the Clinical Practice Research Datalink (https://www.cprd.com).
References
- 1. Curtis HJ, Dennis JM, Shields BM, et al. Time trends and geographical variation in prescribing of drugs for diabetes in England from 1998 to 2017. Diabetes Obes Metab 2018;20:2159-68. 10.1111/dom.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Diabetes Association . 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2021 . Diabetes Care 2021;44(Suppl 1):S111-24. 10.2337/dc21-S009 [DOI] [PubMed] [Google Scholar]
- 3. Rogliani P, Calzetta L, Capuani B, et al. Glucagon-Like Peptide 1 Receptor: A Novel Pharmacological Target for Treating Human Bronchial Hyperresponsiveness. Am J Respir Cell Mol Biol 2016;55:804-14. 10.1165/rcmb.2015-0311OC [DOI] [PubMed] [Google Scholar]
- 4. Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology 2013;154:4503-11. 10.1210/en.2013-1666 [DOI] [PubMed] [Google Scholar]
- 5. Zhu T, Wu XL, Zhang W, Xiao M. Glucagon Like Peptide-1 (GLP-1) Modulates OVA-Induced Airway Inflammation and Mucus Secretion Involving a Protein Kinase A (PKA)-Dependent Nuclear Factor-κB (NF-κB) Signaling Pathway in Mice. Int J Mol Sci 2015;16:20195-211. 10.3390/ijms160920195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Altintas Dogan AD, Hilberg O, Hess S, Jensen TT, Bladbjerg EM, Juhl CB. Respiratory Effects of Treatment with a Glucagon-Like Peptide-1 Receptor Agonist in Patients Suffering from Obesity and Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis 2022;17:405-14. 10.2147/COPD.S350133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López-Cano C, Ciudin A, Sánchez E, et al. Liraglutide Improves Forced Vital Capacity in Individuals With Type 2 Diabetes: Data From the Randomized Crossover LIRALUNG Study. Diabetes 2022;71:315-20. 10.2337/db21-0688 [DOI] [PubMed] [Google Scholar]
- 8. Kotnala S, Kim Y, Rajput C, et al. Contribution of dipeptidyl peptidase 4 to non-typeable Haemophilus influenzae-induced lung inflammation in COPD. Clin Sci (Lond) 2021;135:2067-83. 10.1042/CS20210099 [DOI] [PubMed] [Google Scholar]
- 9. Brikman S, Dori G. Sodium glucose cotransporter2 inhibitor-possible treatment for patients with diabetes, pulmonary disease and CO2 retention. Med Hypotheses 2020;139:109631. 10.1016/j.mehy.2020.109631 [DOI] [PubMed] [Google Scholar]
- 10. Au PCM, Tan KCB, Cheung BMY, Wong ICK, Wong Y, Cheung CL. Association Between SGLT2 Inhibitors vs DPP-4 Inhibitors and Risk of Pneumonia Among Patients With Type 2 Diabetes. J Clin Endocrinol Metab 2022;107:e1719-26. 10.1210/clinem/dgab818 [DOI] [PubMed] [Google Scholar]
- 11. Brunetti VC, Reynier P, Azoulay L, et al. SGLT-2 inhibitors and the risk of hospitalization for community-acquired pneumonia: A population-based cohort study. Pharmacoepidemiol Drug Saf 2021;30:740-8. 10.1002/pds.5192 [DOI] [PubMed] [Google Scholar]
- 12. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786-96. 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang MT, Lai JH, Huang YL, et al. Use of antidiabetic medications and risk of chronic obstructive pulmonary disease exacerbation requiring hospitalization: a disease risk score-matched nested case-control study. Respir Res 2020;21:319. 10.1186/s12931-020-01547-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albogami Y, Cusi K, Daniels MJ, Wei YJ, Winterstein AG. Glucagon-Like Peptide 1 Receptor Agonists and Chronic Lower Respiratory Disease Exacerbations Among Patients With Type 2 Diabetes. Diabetes Care 2021;44:1344-52. 10.2337/dc20-1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castañ-Abad MT, Montserrat-Capdevila J, Godoy P, et al. Diabetes as a risk factor for severe exacerbation and death in patients with COPD: a prospective cohort study. Eur J Public Health 2020;30:822-7. 10.1093/eurpub/ckz219 [DOI] [PubMed] [Google Scholar]
- 16. Ho TW, Huang CT, Ruan SY, Tsai YJ, Lai F, Yu CJ. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS One 2017;12:e0175794. 10.1371/journal.pone.0175794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4-14. 10.1111/j.1365-2125.2009.03537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jick SS, Kaye JA, Vasilakis-Scaramozza C, et al. Validity of the general practice research database. Pharmacotherapy 2003;23:686-9. 10.1592/phco.23.5.686.32205 [DOI] [PubMed] [Google Scholar]
- 20. Lawrenson R, Williams T, Farmer R. Clinical information for research; the use of general practice databases. J Public Health Med 1999;21:299-304. 10.1093/pubmed/21.3.299 [DOI] [PubMed] [Google Scholar]
- 21.Medicines & Healthcare products Regulatory Agency. CRPD linked data. https://cprd.com/linked-data.
- 22. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 23. Quint JK, Müllerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open 2014;4:e005540. 10.1136/bmjopen-2014-005540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009;5:262-9. 10.1038/nrendo.2009.48 [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson S, Douglas I, Stirnadel-Farrant H, et al. Changing use of antidiabetic drugs in the UK: trends in prescribing 2000-2017. BMJ Open 2018;8:e022768. 10.1136/bmjopen-2018-022768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fang M, Wang D, Coresh J, Selvin E. Trends in Diabetes Treatment and Control in U.S. Adults, 1999-2018. N Engl J Med 2021;384:2219-28. 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mohan V, Saboo B, Khader J, et al. Position of Sulfonylureas in the Current ERA: Review of National and International Guidelines. Clin Med Insights Endocrinol Diabetes 2022;15:11795514221074663. 10.1177/11795514221074663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein BD, Bautista A, Schumock GT, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest 2012;141:87-93. 10.1378/chest.11-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of the Recording of Acute Exacerbations of COPD in UK Primary Care Electronic Healthcare Records. PLoS One 2016;11:e0151357. 10.1371/journal.pone.0151357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quanjer PH, Stanojevic S, Cole TJ, et al. ERS Global Lung Function Initiative . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324-43. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rothnie KJ, Chandan JS, Goss HG, Müllerová H, Quint JK. Validity and interpretation of spirometric recordings to diagnose COPD in UK primary care. Int J Chron Obstruct Pulmon Dis 2017;12:1663-8. 10.2147/COPD.S133891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rebordosa C, Plana E, Aguado J, et al. GOLD assessment of COPD severity in the Clinical Practice Research Datalink (CPRD). Pharmacoepidemiol Drug Saf 2019;28:126-33. 10.1002/pds.4448 [DOI] [PubMed] [Google Scholar]
- 33. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. 10.1136/bmj.l5657 [DOI] [PubMed] [Google Scholar]
- 34. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suissa D, Brassard P, Smiechowski B, Suissa S. Number needed to treat is incorrect without proper time-related considerations. J Clin Epidemiol 2012;65:42-6. 10.1016/j.jclinepi.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 36. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550-60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 37. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561-70. 10.1097/00001648-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 38. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656-64. 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40-9. 10.1002/mpr.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982-98. 10.1002/sim.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rubin D. Multiple imputation for nonresponse in surveys. John Wiley & Sons, 2004. [Google Scholar]
- 42. Lai SW, Liu CS, Kuo YH, Lin CL, Hwang BF, Liao KF. The incidence of herpes zoster in patients with diabetes mellitus: A meta-analysis of cohort studies. Medicine (Baltimore) 2021;100:e25292. 10.1097/MD.0000000000025292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suissa S, Dell’Aniello S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol Drug Saf 2020;29:1101-10. 10.1002/pds.5083 [DOI] [PubMed] [Google Scholar]
- 44. Wu AY, Peebles RS. The GLP-1 receptor in airway inflammation in asthma: a promising novel target? Expert Rev Clin Immunol 2021;17:1053-7. 10.1080/1744666X.2021.1971973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rogliani P, Matera MG, Calzetta L, et al. Long-term observational study on the impact of GLP-1R agonists on lung function in diabetic patients. Respir Med 2019;154:86-92. 10.1016/j.rmed.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 46. Lee YS, Jun HS. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediators Inflamm 2016;2016:3094642. 10.1155/2016/3094642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goto T, Hirayama A, Faridi MK, Camargo CA, Jr, Hasegawa K. Obesity and Severity of Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc 2018;15:184-91. 10.1513/AnnalsATS.201706-485OC [DOI] [PubMed] [Google Scholar]
- 48. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. 10.1136/bmj.m4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomovic K, Lazarevic J, Kocic G, Deljanin-Ilic M, Anderluh M, Smelcerovic A. Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med Res Rev 2019;39:404-22. 10.1002/med.21513 [DOI] [PubMed] [Google Scholar]
- 50. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72-130. 10.1016/j.molmet.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qiu M, Ding LL, Zhan ZL, Liu SY. Use of SGLT2 inhibitors and occurrence of noninfectious respiratory disorders: a meta-analysis of large randomized trials of SGLT2 inhibitors. Endocrine 2021;73:31-6. 10.1007/s12020-021-02644-x [DOI] [PubMed] [Google Scholar]
- 52. Barkas F, Anastasiou G, Milionis H, Liberopoulos E. Sodium-glucose cotransporter inhibitors may reduce the risk of pneumonia: an updated meta-analysis of cardiovascular outcome trials. Diabetol Int 2021;13:325-9. 10.1007/s13340-021-00515-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scichilone N, Battaglia S, La Sala A, Bellia V. Clinical implications of airway hyperresponsiveness in COPD. Int J Chron Obstruct Pulmon Dis 2006;1:49-60. 10.2147/copd.2006.1.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postma DS, Kerstjens HA. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:S187-92. 10.1164/ajrccm.158.supplement_2.13tac170 [DOI] [PubMed] [Google Scholar]
- 55. Freiler JF. The Asthma-COPD Overlap Syndrome. Fed Pract 2015;32(Suppl 10):19S-23S. [PMC free article] [PubMed] [Google Scholar]
- 56. Sharma M, Nazareth I, Petersen I. Trends in incidence, prevalence and prescribing in type 2 diabetes mellitus between 2000 and 2013 in primary care: a retrospective cohort study. BMJ Open 2016;6:e010210. 10.1136/bmjopen-2015-010210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lowes DJ, Hevener KE, Peters BM. Second-Generation Antidiabetic Sulfonylureas Inhibit Candida albicans and Candidalysin-Mediated Activation of the NLRP3 Inflammasome. Antimicrob Agents Chemother 2020;64:e01777-19. 10.1128/AAC.01777-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol 2009;187:61-70. 10.1083/jcb.200903124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21:248-55. 10.1038/nm.3806 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
This study is based on data from the Clinical Practice Research Datalink obtained under licence from the UK Medicines and Healthcare products Regulatory Agency. The data are provided by patients and collected by the UK National Health Service as part of their care and support. The interpretation and conclusions contained in this study are those of the authors alone. Because electronic health records are classified as “sensitive data” by the UK Data Protection Act, information governance restrictions (to protect patient confidentiality) prevent data sharing via public deposition. Data are available with approval through the individual constituent entities controlling access to the data. Specifically, the primary care data can be requested via application to the Clinical Practice Research Datalink (https://www.cprd.com).