Abstract
Four undescribed sesquiterpenoids, crannenols A–D (1–4), have been isolated from CHCl2 and MeOH extracts of the deep-sea bamboo coral Acanella arbuscula. The corals were collected from a submarine canyon on the edge of Ireland’s Porcupine Bank via a remotely operated vehicle. The structure elucidation of these (Z,E)-α-farnesene derivatives was achieved using a combination of 1D and 2D NMR, electron impact (1, 2), and electrospray ionization (3, 4) mass spectrometry.
Natural products (NPs) remain a vital point of inspiration for the development of modern medicines with roughly 50% of newly approved drugs in the last 40 years deriving their roots from secondary metabolites extracted from nature.1 Among more than 200 000 known NPs, only ∼30 000 derive from the marine environment and are primarily from organisms living in shallow, temperate, and tropical waters, despite roughly 93% of the oceans existing at depths greater than 1000 m, highlighting the need for investigation of biota from the deep.2,3 Technological advances including remotely operated vehicles (ROVs) and manned submersibles have afforded researchers new opportunities for targeted collections of such organisms at previously inaccessible depths.
Deep-sea octocorals of the order Alcyonacea are known producers of diverse terpenoids that often possess notable bioactivity; examples include the cytotoxic diterpene alcyonolide and the illuldalene sesquiterpenoids alcyopterosins.4−6 The current study was carried out to investigate the chemical diversity of the Irish deep-sea bamboo coral Acanella arbuscula, a species from which no prior chemical investigation has been reported.
Results and Discussion
Dichloromethane and methanol
extracts were subjected to a panel
of bioassays where preliminary data indicated inhibitory activity
against the bacteria Clostridium difficile and Mycobacterium tuberculosis, as well as respiratory syncytial
virus. Repeat rounds of fractionation altering between normal and
reversed phase chromatography yielded four new sesquiterpenoid (Z,E)-α-farnesene derivatives, crannenols A–D
(1–4). Deriving their name from the
Irish word “crann” meaning tree due to the branching
resemblance of A. arbuscula to that of a small tree,
the isolation and subsequent structure determination of compounds 1–4 are described herein.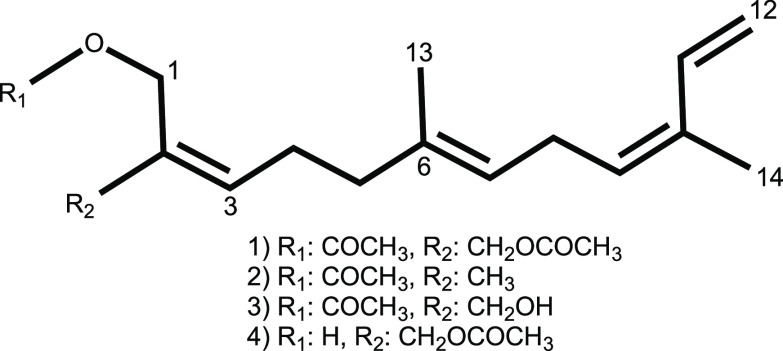
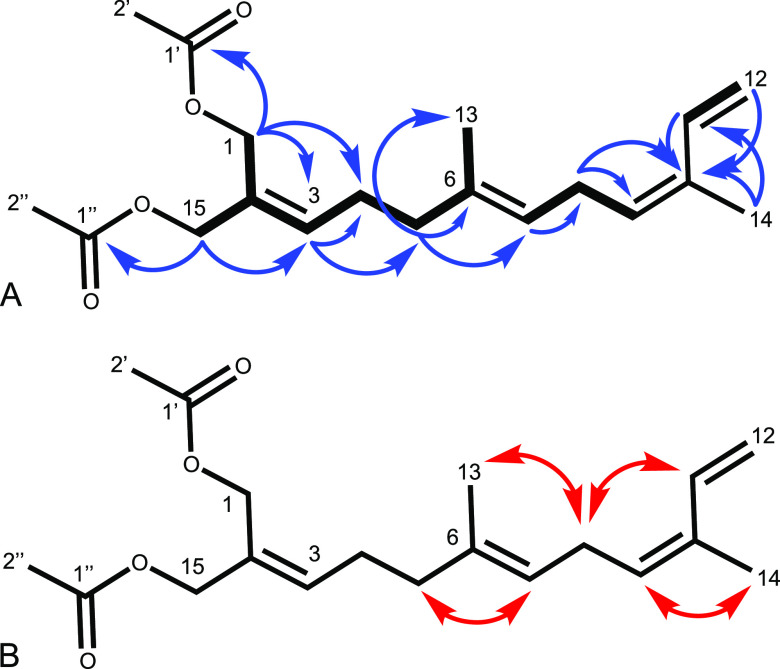
Crannenol A (1) was isolated as a clear oil. A molecular formula of C19H28O4 was established by HREIMS corroborated by signals in the 1H and 13C NMR spectra (Table 1). Key 1H NMR signals (Figure 1a) included two acetoxy-bearing methylene singlets H2-1 and H2-15 (δH 4.65 and 4.56, respectively), which demonstrated long-range (allylic and W) coupling in the COSY spectrum to each other as well as through C-2 (δC 129.1) to H-3 (δH 5.75) (Figure 1a). H2-1 and H2-15 both showed HMBC correlations to their respective acetate carbonyls C-1′ (δC 170.9) and C-1″ (δC 170.7), as well as to C-3 (δC 136.1), with H2-1 extending a four-bond correlation to C-4 (δC 26.1). COSY correlations of H-3 linked to both quartet and triplet methylenes in H2-4 (δH 2.26) and H2-5 (δH 2.06), respectively, which were confirmed by HMBC correlations of H-3 to C-4 and C-5 (δC 38.9). H2-5 displayed a further COSY correlation to a singlet methyl, H3-13 (δH 1.64), as well as the corresponding HMBC correlation to C-13 (δC16.0), and additional correlations to the vinyl olefin C-6 (δC 134.4) and methine C-7 (δC 123.4), establishing a trisubstituted alkene. H-7 (δH 5.13) was shown to correlate in the COSY spectrum to the triplet methylene H2-8 (δH 2.87) and triplet methine H-9 (δH 5.34), corroborated with an HMBC correlation from H-7 to C-8 (δC 26.3). H2-8 showed a correlation in the COSY spectrum only to H-9, yet displayed HMBC correlations to both C-9 (δC129.3) and C-10 (δC 132.1), indicative of C-10 as a quaternary olefin. C-10 correlated in the HMBC spectrum with the singlet methyl H3-14 (δH 1.82) as well as the methine H-11 (δH 6.80), displaying a doublet-of-doublets splitting pattern, and two doublets accounting for the terminal olefinic protons of H2-12 (δH 5.11 and 5.21). The proximity of H-11 and H-12 was further confirmed by a COSY correlation between the two. The double-bond configurations of crannenol A were determined using 2D NOESY NMR correlations (Figure 1b), which demonstrated proximity of protons H2-5/H-7 and H2-8/H3-13, suggesting the E configuration for the C-6/C-7 olefin. The C-9/C-10 olefin was assigned the Z configuration based on the observations of correlations between H2-8/H-11 and H-9/H3-14.
Table 1. NMR Data for Crannenol A (1)a.
| pos | δC,b type | δHc (J in Hz) | HMBCc | COSYc | NOESYc |
|---|---|---|---|---|---|
| 1 | 59.8, CH2 | 4.65, s | 3, 4, 1′ | 3, 15 | |
| 2 | 129.1, C | ||||
| 3 | 136.1, CH | 5.75, t (7.4) | 4, 5 | 4, 5 | |
| 4 | 26.1, CH2 | 2.26, q (7.6) | 13 | ||
| 5 | 38.9, CH2 | 2.06, m | 6, 7, 13 | 13 | 7 |
| 6 | 134.4, C | ||||
| 7 | 123.4, CH | 5.13, t | 8 | 8, 9 | |
| 8 | 26.3, CH2 | 2.87, t (7.3) | 9, 10 | 9 | 13, 11 |
| 9 | 129.3, CH | 5.34, t (7.5) | 14 | ||
| 10 | 132.1, C | ||||
| 11 | 133.5, CH | 6.80, dd (17.3, 10.8) | 10 | 12 | |
| 12 | 113.6, CH2 | 5.11, d (10.7) | 10 | ||
| 5.21, d (17.2) | 10 | ||||
| 13 | 16.0, CH3 | 1.64, s | |||
| 14 | 19.7, CH3 | 1.82, s | 10, 11 | ||
| 15 | 66.7, CH2 | 4.56, s | 3, 1″ | ||
| 1′ | 170.9, C | ||||
| 1″ | 170.7, C | ||||
| 2′ | 20.9, CH3 | 2.07, s | |||
| 2″ | 21.0, CH3 | 2.07, s |
CDCl3, ppm, multiplicity determined by HSQC.
150 MHz.
600 MHz.
Figure 1.
Key HMBC (blue →) and COSY (thick ―) correlations (A) and key NOESY (red ↔) correlations (B) establishing the configuration of crannenol A (1).
Crannenol B (2) was isolated as a clear oil with spectroscopic data similar to that of crannenol A (1). A molecular formula of C17H26O2 for compound 2 was established from HREIMS corroborated by 1H and 13C NMR spectra (Table 2). Crannenol B departed from the motif of 1 by displaying only a single acetate methyl group in the 1H NMR spectrum. A new singlet methyl present in 2, H3-15 (δH 1.75), was confirmed through COSY correlations of H3-15 to both the triplet methine H-3 (δH 5.38) and singlet methylene H2-1 (δH 4.58), as well as HMBC correlations of H3-15 to olefinic carbons C-2 (δC 129.8) and C-3 (δC 130.5), and the acetoxy-bearing methylene C-1 (δC 63.2). The remainder of the carbon skeleton of 2 was determined to mirror that of 1 on the basis of 1H and 13C NMR data. The configuration of the C-2/C-3 olefin in compound 2 was found to be Z on the basis of 2D NOESY correlations between H2-1/H2-4 (δH 2.17) and H-3/H3-15. This assignment was further confirmed through 1D NOE experiments in which H2-1 and H3-15 were separately irradiated and found to display proximity through space to H2-4 and H-3, respectively. This proposed structure for 2 differs from that of iso-α-sinensyl acetate isolated from the terrestrial Lomatium mohavense ssp. longilobum by Beauchamp et al. (2010) only in the appearance of the C-2/C-3 olefin in the Z configuration.7
Table 2. NMR Data for Crannenols B, C, and D (2, 3, and 4)a.
| crannenol
B (2) |
crannenol
C (3) |
crannenol
D (4) |
||||
|---|---|---|---|---|---|---|
| pos | δC,b type | δHc (J in Hz) | δC,b type | δHc (J in Hz) | δC,b type | δHc (J in Hz) |
| 1 | 63.2, CH2 | 4.58, s | 60.2, CH2 | 4.71, s | 58.5, CH2 | 4.18, s |
| 2 | 129.8, C | 133.7, C | 133.7, C | |||
| 3 | 130.5, CH | 5.38, t | 133.3, CH | 5.68, t,(7.5) | 133.9, CH | 5.64, t (7.5) |
| 4 | 26.3, CH2 | 2.17, q (7.99) | 26.0, CH2 | 2.25, q (7.5) | 26.0, CH2 | 2.24, q (7.5) |
| 5 | 39.5, CH2 | 2.02, t (7.81) | 39.1, CH2 | 2.06, t | 39.1, CH2 | 2.06, t (7.5) |
| 6 | 134.9, C | 134.5, C | 134.6, C | |||
| 7 | 122.9, CH | 5.12, m | 123.3, CH | 5.13, t | 123.4, CH | 5.13, t |
| 8 | 26.2, CH2 | 2.87, t (7.27) | 26.3, CH2 | 2.87, t (7.3) | 26.3, CH2 | 2.87, t (7.3) |
| 9 | 129.5, CH | 5.35, t | 129.4, CH | 5.35, t (7.6) | 129.3, CH | 5.34, t (7.5) |
| 10 | 132.0, C | 132.2, C | 132.2, C | |||
| 11 | 133.6, CH | 6.81, q (17.4, 10.2) | 133.6, CH | 6.80, dd (17.4, 10.8) | 133.5, CH | 6.80, dd (18.2, 10.9) |
| 12 | 113.5, CH2 | 5.11, d (10.9) | 113.7, CH2 | 5.11, d (10.9) | 113.7, CH2 | 5.11, d (10.9) |
| 5.21, d (17.1) | 5.22, d (16.7) | 5.22, d (17.1) | ||||
| 13 | 16.0, CH3 | 1.64, s | 16.0, CH3 | 1.65, s | 16.1, CH3 | 1.64, s |
| 14 | 19.7, CH3 | 1.83, s | 19.7, CH2 | 1.82, s | 19.7, CH2 | 1.82, s |
| 15 | 21.4, CH3 | 1.75, s | 65.9, CH2 | 4.10, s | 67.3, CH2 | 4.64, s |
| 1′ | 171.2,d C | 171.4, C | 171.4, C | |||
| 2′ | 21.0, CH3 | 2.08, s | 21.0, CH3 | 2.08, s | 21.0, CH3 | 2.09, s |
CDCl3, ppm, multiplicity determined by HSQC.
150 MHz.
600 MHz.
Chemical shift confirmed by HMBC.
Crannenols C (3) and D (4) were isolated as clear oils with spectroscopic data similar to that of crannenols A (1) and B (2). A molecular formula of C17H26O3 for both compounds 3 and 4 was established by HRESIMS corroborated by 1H and 13C NMR spectra (Table 2). Both 3 and 4 differed from 2 by the presence of a new singlet methylene, H2-15 and H2-1, respectively (δH 4.10 and 4.18, respectively), signal in the 1H NMR spectra. The presence of an alcohol at C-15 and C-1 in 3 and 4 was suggested by the chemical shift of these carbons (δC 65.9 and 58.5, respectively). The assignment of this portion of 3 was determined through long-range COSY correlations between the singlet hydroxy-bearing methylene H2-15 and singlet acetoxy-bearing methylene H2-1 (δH 4.71) and to the triplet methine H-3 (δH 5.68), as well as HMBC correlations from H2-15 to C-1 (δC 60.2), C-2 (δC133.7), and C-3 (δC 133.3). The assignment of this portion of 4 was determined through COSY correlations between the equivalent H2-1 to the triplet methine H-3 (δH 5.64) and the quartet methylene H2-4 (δH 2.24). Additionally, HMBC correlations from H2-1 to C-15 (δC 67.3), C-2 (δC 133.7), and C-3 (δC 133.9) confirmed this portion of the structure. The remainder of the carbon skeletons of 3 and 4 were found to mirror those of compounds 1 and 2 on the basis of 1H and 13C NMR data. The configuration of the C-2/C-3 olefin in 3 was found to be Z on the basis of 2D NOESY correlations between H2-1/H2-4 and H-3/H2-15. The configuration of the C-2/C-3 olefin in 4 was found to be E on the basis of 2D NOESY correlations of H2-15/H-3 and H2-4/H2-1.
Due to the abundance of crannenol A (1) isolated, this metabolite was used as a probe for the evaluation of biological activity of the series. Despite the observed activity of the organic extracts, analyses of purified compound 1 revealed no discernible antibiotic activity, based on screening seven strains of Candida spp., the ESKAPE pathogens, Mycobacterium tuberculosis, and human respiratory syncytial virus.
Despite the lack of retention of biological activity from extract to metabolite, this study reports the isolation and elucidation of a series of four undescribed compounds, crannenols A–D (1–4), from a genus of deep-sea soft coral for which no chemical investigations have been reported.
Experimental Section
General Experimental Procedures
Solvents were obtained from Fisher Scientific Co. and were HPLC grade (>99% purity) unless otherwise stated. UV absorptions were measured with a Shimadzu LC-20AT HPLC system equipped with a Shimadzu SPD-M20A diode array detector in CH3OH. IR spectra were recorded with an Agilent Cary 630 FTIR. NMR spectra were acquired in CDCl3 with residual solvent referenced as the internal standard (δH 7.27; δC 77.0) for 1H and 13C NMR spectra, respectively. The NMR spectra were recorded on a Varian 600 MHz broadband instrument operating at 600 MHz for 1H and 150 MHz for 13C. GC/MS analysis was performed on an Agilent 7890A GC using a Zebron ZB-5HT Inferno (30 m × 0.25 mm, 0.25 μm film thickness) column coupled to an Agilent 7200 accurate-mass QToF with electron impact ionization. LC/MS analysis was performed on an Agilent 1260 Infinity LC using an analytical C18 (150 × 3.0 mm, 2.6 μm) column coupled to an Agilent 6540 UHD accurate-mass QToF with electrospray ionization. MPLC fractionation and analysis were performed on a Teledyne-Isco CombiFlash Rf system equipped with built-in UV detection at 254 and 280 nm. HPLC fractionation and analysis were performed on a Shimadzu LC-20AR system equipped with a Shimadzu SPD-20A UV/vis detector using preparative silica or semipreparative C18 ((250 × 21.2 mm, 5 μm) or (250 × 10.0 mm, 5 μm)) conditions.
Biological Materials
Thirty-one specimens of Acanella arbuscula (Cnidaria, Alcyonacea, Calcaxonia, Keratoisididae) were collected from the Whittard Canyon, an extensive submarine canyon system southwest of Ireland on the northeast Atlantic margin, between May 30 and June 10, 2016, using the ROV Holland I deployed from the Irish national research vessel RV Celtic Explorer. Specimens were collected from depths of 984–2011 m during a series of eight ROV dives that ranged in latitude from 48° 25′ 42″ N to 48° 40′ 23″ N and in longitude from 9° 52′ 58″ W to 10° 40′ 50″ W. Specimens were stored in bioboxes on the ROV and immediately identified, logged, labeled, and frozen at −80 °C when the ROV was recovered to the vessel. Specimens were freeze-dried on return to land and then stored until analysis at −20 °C. Specimens were identified as A. arbuscula based on a distinctive skeleton of alternating proteinaceous nodes with calcium carbonate internodes and the densely branched, bush-like structure of the colonies.
According to the latest taxonomic revision, A. arbuscula displays widely divergent morphotypes within this general morphology but is the only species of Acanella present in the northeast Atlantic.8
Extraction and Isolation
Crannenols A, C, and D (1, 3, 4) were isolated from 720.5 g of 31 combined freeze-dried A. arbuscula specimens extracted via Soxhlet extraction in CHCl2 and dried in vacuo, resulting in 5.9 g of organic extract. The extract was fractionated using MPLC utilizing a gradient from 100% hexanes to 100% EtOAc with a normal phase silica column over 25 min, resulting in eight fractions following the recombination of fractions with similar UV profiles. Fraction D/E (320 mg) was shown by 1H NMR spectroscopy to contain a chemical shift pattern consistent with terpene-like secondary metabolites and was thus subjected to normal phase HPLC utilizing a gradient from 100% hexanes to 60% EtOAc over 25 min on a preparative silica column, affording crannenol A (1, 35.6 mg). In MPLC fraction F a doublet of doublets at a chemical shift of 6.80 ppm was observed, mirroring that seen in compound 1 as the β-vinylic protons on C-11. Further HPLC separation of this fraction was conducted on a preparative silica column with a gradient from 78% hexanes to 53% EtOAc over 17 min to yield two terpene-containing fractions, 7 and 8. Each of these fractions was individually subjected to reversed phase HPLC on a semipreparative C18 column with a gradient from 75% to 100% MeOH over 17 min to afford crannenols C (3, 2.2 mg) and D (4, 1.8 mg), respectively.
Crannenol B (2) was isolated from subsequent Soxhlet extraction in MeOH of the same 31 combined freeze-dried A. arbuscula specimens following CHCl2 extraction and dried in vacuo, resulting in 35.6 g of organic extract. The extract was fractionated using MPLC utilizing a solvent system of 5% MeOH with a reversed phase C18 column for 10 min to elute the majority of highly polar compounds followed by an immediate increase to 100% MeOH over 0.1 min that was held for 15 min to elute less polar secondary metabolites, resulting in two fractions. 1H NMR analysis of the nonpolar fraction 2 confirmed the presence of a similar analog to that of crannenols A, C, and D (1, 3, 4) and was thus subjected to normal phase HPLC utilizing a gradient from 100% hexanes to 70% EtOAc over 14 min and shown to contain both crannenol A (1) in the resulting fraction 2 and a separate analog in fraction 1. Fraction 1 was further subjected to reversed phase HPLC on an analytical C18 column with a gradient from 50% to 100% MeOH over 20 min, affording crannenol B (2, 0.1 mg).
Crannenol A (1):
clear oil; UV (MeOH) λmax 236 nm; IR ν (thin film) 2943, 1744, 1446, 1379, 1223, 1029, 970, 910, 612 cm–1; 1H and 13C NMR data, Table 1; 70 eV HREIMS m/z 320.1984 [M]+ (calcd for C19H28O4, 320.1982)
Crannenol B (2):
clear oil; UV (MeOH) λmax 236 nm; IR ν (thin film) 2935, 1744, 1454, 1379, 1245, 1037, 992 cm–1; 1H and 13C NMR data, Table 2; 70 eV HREIMS m/z 262.1922 [M]+ (calcd for C17H26O2, 262.1927).
Crannenol C (3):
clear oil; UV (MeOH) λmax 235 nm; IR ν (thin film) 3441, 2943, 1744, 1454, 1379, 1245, 1037 cm–1; 1H and 13C NMR data, Table 2; HRESIMS m/z 279.1955 [M + H]+ (calcd for C17H26O3, 279.1955).
Crannenol D (4):
clear oil; UV (MeOH) λmax 235 nm; IR ν (thin film) 3449, 2935, 1744, 1446, 1387, 1238, 1029 cm–1; 1H and 13C NMR data, Table 2; HRESIMS m/z 279.1953 [M + H]+ (calcd for C17H26O3, 279.1955).
Acknowledgments
The authors wish to thank the crew and scientists of research expedition CE16006 aboard RV Celtic Explorer. We also thank the USF Chemical Purification, Analysis, and Screening core facility and its director, Dr. L. Calcul, as well as the USF NMR core facility. This work was supported by Science Foundation Ireland (SFI) and the Marine Institute under the Investigators Programme Grant No. SFI/15/1A/3100, cofunded under the European Regional Development Fund 2014-2020, to A.L.A. and the U.S. National Institutes of Health grant R56 AI154922 to B.J.B. Expedition CE16006 was funded under the Irish National Shiptime Programme.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jnatprod.2c00602.
NMR spectra of crannenols A–D (1–4); HREIMS of 1 and 2; HRESIMS of 3 and 4; UV λmax of 1–4; IR spectra of 1–4 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Newman D. J.; Cragg G. M. J. Nat. Prod 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Skropeta D. Nat. Prod. Rep 2008, 25, 1131–1166. 10.1039/b808743a. [DOI] [PubMed] [Google Scholar]
- Pilkington L. I. Molecules 2019, 24, 1–24. 10.3390/molecules24213942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M.; Yasuzawa T.; Kobayashi Y.; Kyogoku Y.; Kitagawa I. Tetrahedron Lett. 1981, 22, 4445–4448. 10.1016/S0040-4039(01)82979-8. [DOI] [Google Scholar]
- Roy P. K; Maarisit W.; Roy M. C; Taira J.; Ueda K. Mar. Drugs 2012, 10, 2741–2748. 10.3390/md10122741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo J. A; Brasco M. F; Spagnuolo C.; Seldes A. M. J. Org. Chem. 2000, 65, 4482–4486. 10.1021/jo991740x. [DOI] [PubMed] [Google Scholar]
- Beauchamp P. S; Dev V.; Kittisanthanon K.; Ly B. Flavour Fragr. J. 2010, 25, 427–430. 10.1002/ffj.1990. [DOI] [Google Scholar]
- Saucier E. H; Sajjadi A.; France S. C. Zootaxa 2017, 4323 (3), 359–390. 10.11646/zootaxa.4323.3.2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.