Abstract
Objective:
Respiratory illnesses, including COVID-19, have resulted in millions of deaths globally. Guidance on mask-wearing in community settings has been inconsistent. This review examined the effectiveness of mask-wearing on respiratory virus transmission in community settings.
Methods:
A search was conducted for English language reports of randomized controlled trials of mask-wearing in the community and effect on laboratory-confirmed respiratory infections or influenza-like-illness. Investigators abstracted study characteristics and assessed bias. Meta-analysis was conducted to calculate pooled risk estimates.
Results:
Eleven studies were included. In 7 studies that evaluated influenza-like-illness symptoms as an outcome (3,029 participants), this study found mask-wearing associated with a decreased risk of influenza-like-illness (overall risk ratio [RR] 0.83, 95% confidence interval [CI] 0.71 to 0.96). Studies examining laboratory-confirmed respiratory infections as an outcome (10,531 participants) showed no statistically significant association between mask-wearing and infections (RR 1.04, 95% CI 0.60 to 1.80). However, masking combined with enhanced hand hygiene was associated with a decreased risk for both influenza-like-illness symptoms (RR 0.88, 95% CI 0.51 to 1.51) and laboratory-confirmed respiratory infection (RR 0.79, 95% CI 0.52 to 1.18).
Conclusions:
Masking in community settings decreases transmission of influenza-like-illness. Mask-wearing combined with enhanced hand hygiene reduces transmission of influenza-likeillness and laboratory-confirmed respiratory infection.
Keywords: masking, community, transmission, respiratory, infection
INTRODUCTION
On March 11, 2020, the World Health Organization (WHO) classified “coronavirus disease 2019” (COVID-19) as a pandemic, as the levels of spread and severity of the disease increased rapidly worldwide.1 Non-pharmaceutical interventions are necessary to decrease the spread of the virus in community settings. These interventions include face mask-wearing, physical distancing, and hand hygiene.
Face mask use in healthcare facilities is widely accepted, with a number of studies now comparing the efficacy of various types of medical masks for the prevention of infection transmission.2–6 However, even amid the pandemic, with many states and municipalities mandating face mask use in enclosed community settings such as offices and schools, there has been no definitive answer on the efficacy of mask-wearing in community settings. In fact, opinions and policies vary greatly worldwide. Governments of various countries and the Centers for Disease Control and Prevention and WHO have disagreed on masking guidelines and changed views throughout the course of the pandemic.7–15
Under the threat of a relentless pandemic that could result in many more deaths across the globe, it is imperative that infection prevention and control interventions are based on current evidence. Therefore, this review aimed to generate data to support evidence-based public health policy development regarding mask-wearing in community settings. As there is little data examining the effectiveness of mask-wearing in the community for COVID-19, this rapid review examined the transmission of respiratory infections in general. It includes randomized controlled trials and summarizes the efficacy of mask-wearing on the transmission of respiratory infections in the community setting.
METHODS
Data Sources and Searches
This review followed the methods outlined in the McMaster Rapid Review Guidebook.16 The original search was conducted via PubMed from May 20–26, 2020. The search was then expanded in the following 2 weeks (end date, June 10, 2020) to include Google Scholar, Scopus, Health Evidence, and medRxiv (search terms in Appendix Table 1). The search was limited to English language articles. In addition, all relevant systematic reviews arising from the search were reviewed for additional primary literature matching the search criteria.2,17–29
Study Selection
Article titles were parsed for relevance in terms of study topic and setting. Relevant articles included studies focused on the efficacy of mask-wearing to reduce the spread of respiratory infections in community settings. Respiratory infections included in the analysis were any infection spread by aerosol or droplet, including but not limited to influenza A or B, rhinoviruses, coronaviruses, picornaviruses, enteroviruses, adenoviruses, respiratory syncytial virus human metapneumovirus and parainfluenza viruses.
Full texts of the articles deemed relevant were reviewed and reduced by study type. This rapid review focused solely on randomized controlled trials reporting quantitative data. Excluded were studies relating to respiratory virus transmission in healthcare settings and non-randomized controlled trials. Additional inclusion criteria included English language articles conducted with participants of any age in a community setting that measured influenza-like-illness (ILI) symptoms or laboratory-confirmed respiratory viruses as a study outcome. A comprehensive list of inclusion and exclusion criteria can be found in Appendix Table 2. The selected articles that met all inclusion criteria were confirmed by a second investigator and further examined for duplication; duplicate studies were removed.
Data Extraction and Quality Assessment
Two investigators extracted study data: title, author, year of study, year of publication, study design, methods, participants, number of participants, primary outcome, measurement of outcome, other outcomes, type of mask, comparator, results of control, results of interventions, whether the paper reported a significant difference according to their statistical standards, and papers’ self-identified strengths and limitations. After extraction by both investigators, data were compared to ensure accuracy and completeness. To establish consensus on the data extracted between the 2 investigators, any discrepancies were reviewed by the senior investigator.
Critical appraisal of each study was performed using the Cochrane Risk of Bias Tool30 via Covidence software (Melbourne, Australia). This assessment evaluated risk of bias in the following areas: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias. The risk of bias for each aspect of the study was rated as high, low, or unclear by 2 investigators, and the individual assessment results were compared; any discrepancies were discussed until consensus was met. Following consensus evaluation, the reviewers gave each article an overall risk-of-bias rating. If an article had a high risk-of-bias rating in ≥2 categories, it was considered to have an overall high risk of bias. If an article had a high riskof-bias rating in 1 category and ≤2 unclear ratings, it was considered to have an overall medium risk of bias. Finally, if an article had no high risk-of-bias ratings and ≤2 unclear ratings, it was considered to have an overall low risk of bias (Appendix Tables 3 and 4).
Data Synthesis and Analysis
This analysis used the “META” package in STATA to construct forest plots for all the studies, examining masking data alone, enhanced hand hygiene alone and a combination of masking and enhanced hand hygiene, to compare against a control.31 Only quantitative data on rates of ILI and laboratory-confirmed respiratory infection from included publications were analyzed in forest plots. A fixed effects model was first used to calculate the individual and pooled risk ratios (RR). The level of heterogeneity was then calculated using the I2 statistic.32 Forest plots reporting substantial to considerable heterogeneity based on the following I2 index (low heterogeneity [I2 =0 – 40%], moderate heterogeneity [I2 =30 – 50%], substantial heterogeneity [I2 =50 – 90], and considerable heterogeneity [I2 = 75 – 100%], were re-calculated using the Dersimonian and Laird random effects model.33 Publication bias was assessed using contour-enhanced funnel plots and the Egger’s test. Two sided tests were used with all p-values ≤0.05 considered statistically significant. All analyses were conducted using STATA 16 (StataCorp LLC, College Station, TX).
RESULTS
Literature search
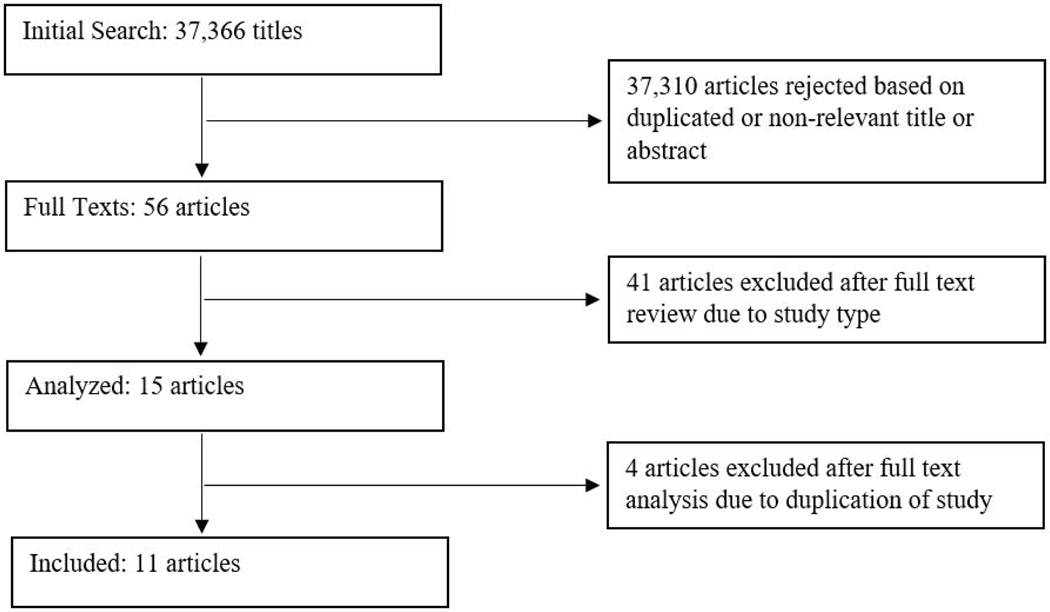
The database search yielded 37,366 titles. After removing duplicate articles and reviewing titles and abstracts, 56 full texts met initial inclusion criteria. Narrowing to randomized controlled trials left 11 eligible articles that were included in this rapid review (Figure 1).
Figure 1:
Literature search and selection.
Eleven studies were identified as randomized controlled trials of face mask use in the community, taking place between 2006 and 2015 (Table 1). In total, there were 9,140 participants across all intervention groups, and 6,918 participants across all control groups. Studies were conducted across the United States, Australia, China, Thailand, Germany, and France. Two studies were conducted in university residence halls, 2 during Hajj pilgrimages, and 7 in households. Nine studies collected data on self-reported ILI symptoms, and 10 studies used laboratory tests to confirm respiratory infection. Seven studies reported multiple interventions, including enhanced hand hygiene, education and masking or a combination. All studies used surgical masks for the masking intervention.
Table 1:
Randomized controlled trials measuring the effects of mask-wearing on the spread of respiratory viruses in community settings.
| Author | Setting | Arms | Primary Outcomes | Type of Mask | Control Group Adherence | Intervention Group Adherence | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| Aiello A et al., 2010 39 | US universities |
− Control (n=552) − Mask (n=378) − Mask and Hand Hygiene (n=367) All groups were provided education on proper hand hygiene. |
Self-reported ILI symptoms and laboratory-confirmed influenza | Tecnol procedure mask - Kimberly Clark | Not reported | Not reported | Low |
| Aiello A et al., 2012 38 | US universities |
− Control (n=370)
− Mask (n=392) − Mask and Hand Hygiene (n=349) |
Self-reported ILI symptoms and laboratory-confirmed influenza | Tecnol procedure mask - Kimberly Clark | Used hand sanitizer 1.51 times per day |
− Mask and Hand Hygiene: wore face mask on average 5.08 hours per day, used hand sanitizer 4.49 times per day − Mask: wore face mask on average 5.04 hours per day, used hand sanitizer 1.29 times per day |
Low |
| Alfelali M et al., 2019 35 | Hajj pilgrimage with pilgrims from Australia, Saudi Arabia, and Qatar |
− Control (n=3823)
− Mask (n=3864) |
Laboratory-confirmed vRTIs and CRIs | 3M standard tie-on surgical mask | 14% reported wearing face mask daily, 35% reported wearing face mask intermittently | − Mask: 25% reported wearing face mask daily, 48% reported wearing face mask intermittently | Medium |
| Barasheed O et al., 2014 34 | Hajj pilgrimage with Australian pilgrims |
− No Supervised Mask Group (n=53)
− Supervised Mask Group (n=36) |
Self-reported ILI symptoms and laboratory-confirmed respiratory infections | 3M standard tie-on surgical mask | 12% wore face masks | − Supervised Mask Group: 76% wore face mask | High |
| Canini L et al., 2010 48 | Households with an infection in France |
− Control (n=158)
− Mask (n=148) |
Self-reported ILI symptoms | AEROKYNH - LCH medical products for ages 10 and up; Face Mask KC47127 - Kimberly Clark for ages 5–10 | Not reported | − Mask: Used 2.5±1.3 masks per day, wore mask for 3.7±2.7 hours per day | Low |
| Cowling B et al., 2009 40 | Households with an infection in China |
− Control (n=279) − Hand Hygiene (n=257) − Hand Hygiene plus Mask (n=258) All groups received education about the importance of healthy diet and lifestyle. |
Self-reported ILI symptoms and laboratory-confirmed influenza | Surgical mask: Tecnol - The Lite One - Kimberly Clark | 15% of index patients wore masks often, 7% of contacts wore masks often |
− Hand Hygiene: 85.7g of soap used, index patients used 2.7g of hand sanitizer, contacts used 1.4g of hand sanitizer, 31% of index patients wore masks often, 5% of contacts wore masks often − Mask and Hand Hygiene: 78.9g of soap used, index patients used 1.6g of hand sanitizer, contacts used 1.4g of hand sanitizer, 49% of index patients wore masks often, 26% of contacts wore masks often |
Low |
| Larson E et al., 2010 36 | Households with an infection in the US |
− Control (n=904) − Hand Sanitizer (n=946) − Hand Sanitizer and Face Mask (n=938) All groups received materials regarding the prevention and treatment of URIs and influenza |
Self-reported ILI symptoms and URI and laboratory-confirmed influenza | Procedure Face Masks for adults and children - Kimberly Clark | 44.2% reported using hand sanitizer occasionally |
− Hand Hygiene: used a mean 12.1 ounces of hand sanitizer per month − Mask and Hand Hygiene: used a mean 11.6 ounces of hand sanitizer per month, 50% of households with ILI reported using mask within 48 hours of episode onset |
Medium |
| MacIntyre C et al., 2009 50 | Households with an infection in Australia |
− Control (n=100) − Surgical Masks (n=94) P2 Masks (n=92) − Mask groups were always advised to wear their face masks when in the same room as the infected child. |
Self-reported ILI symptoms and laboratory-confirmed respiratory infections | 3M surgical masks and 3M flat-fold P2 masks | Not reported |
− Surgical Masks: 38% stated that they were wearing mask most or all the time on day 1, 31% on day 5 − P2 Masks: 46% stated that they were wearing mask most or all of the time on day 1, 25% on day 5 Overall 21% of participants in mask groups reported wearing mask often or always |
Low |
| MacIntyre C et al., 2016 49 | Households with an infection in China |
− Control (n=295)
− Mask (n=302) |
CRI, ILI symptoms, and laboratory-confirmed respiratory infections | 3M 1817 surgical mask | Used mask for 1.4 hours per day | − Mask: used mask for 4.4 hours per day | Low |
| Simmerman J et al., 2011 41 | Households with an infection in Thailand |
− Control (n=302)
− Hand Hygiene (n=292) − Hand Hygiene plus Mask (n=291) |
Self-reported ILI symptoms and laboratory-confirmed influenza | Standard paper surgical face masks (MedCon Company) | 3.9 hand washes per day |
− HH: 4.7 hand washes per day − Mask and HH: 4.9 hand washes per day, averaged 12 masks per person per week, averaged 211 minutes of mask-wearing per day |
Low |
| Suess T et al., 2012 42 | Households with an infection in Germany |
− Control (n=82)
− Mask and Hand Hygiene (n=67) − Mask Only (n=69) |
Clinical ILI and laboratory-confirmed influenza | Child’s Face Mask - Kimberly Clark for children under 14, Aérokyn Masques - LCH Medical Products for adults | Not reported |
− Mask: 12.9 face masks per person, 1.8 masks per day in 2010/2011 − Mask and HH: 12.6 masks per person, 1.7 masks per day in 2010/2011, 85.2mL of hand sanitizer in 2009/2010, 42.7mL of hand sanitizer in 2010/2011 |
Low |
HH = hand hygiene; ILI = influenza-like-illness; vRTI = viral respiratory tract infection; CRI = clinical respiratory infection; URI = upper-respiratory infection. Risk of bias based on Cochrane Risk of Bias tool30
The overall risk of bias for the studies was generally low-to-medium. One study was identified, Barasheed et al.34, that rated high for potential risk of bias based on the criteria, due to small sample size and demographic differences between intervention groups. Alfelali et al.35 and Larson et al.36 both received an overall medium rank for risk of bias due to differences between intervention groups (Appendix Table 4).
Bias testing
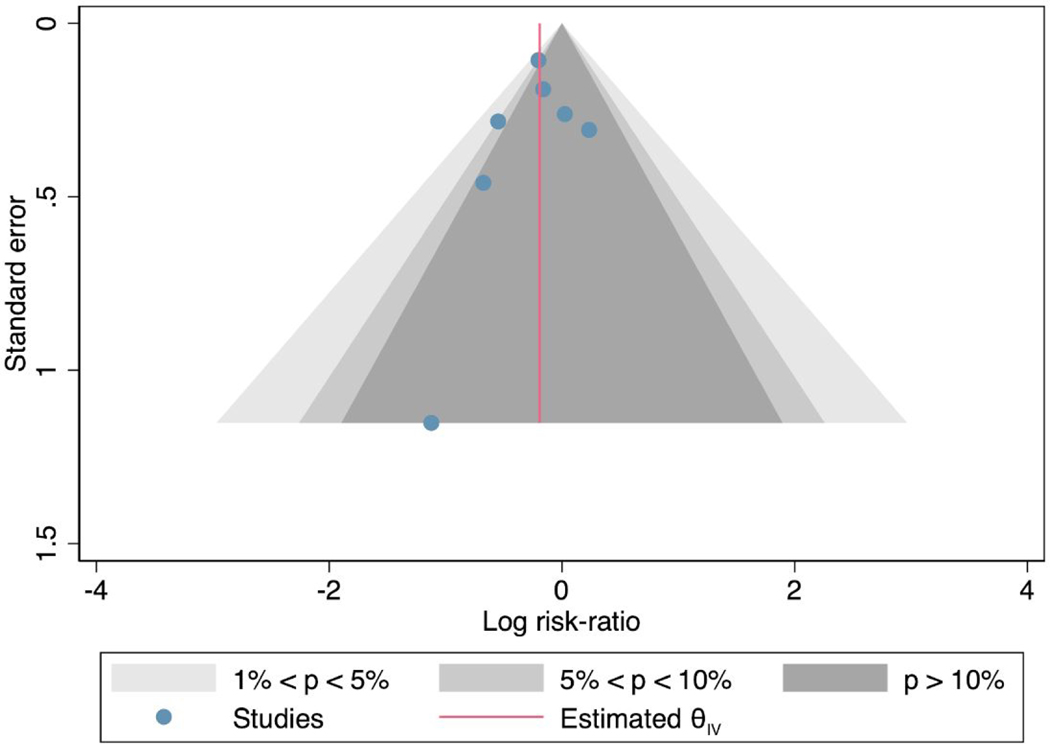
On the contour enhanced funnel plot (Appendix Figure 1), the measure of the study effect size (log risk) against the variance (standard error) was relatively symmetrical showing no signs of publication bias. The Egger’s regression test of asymmetry was not statistically significant (p=0.54), also indicative that publication bias is absent.
Meta-analysis
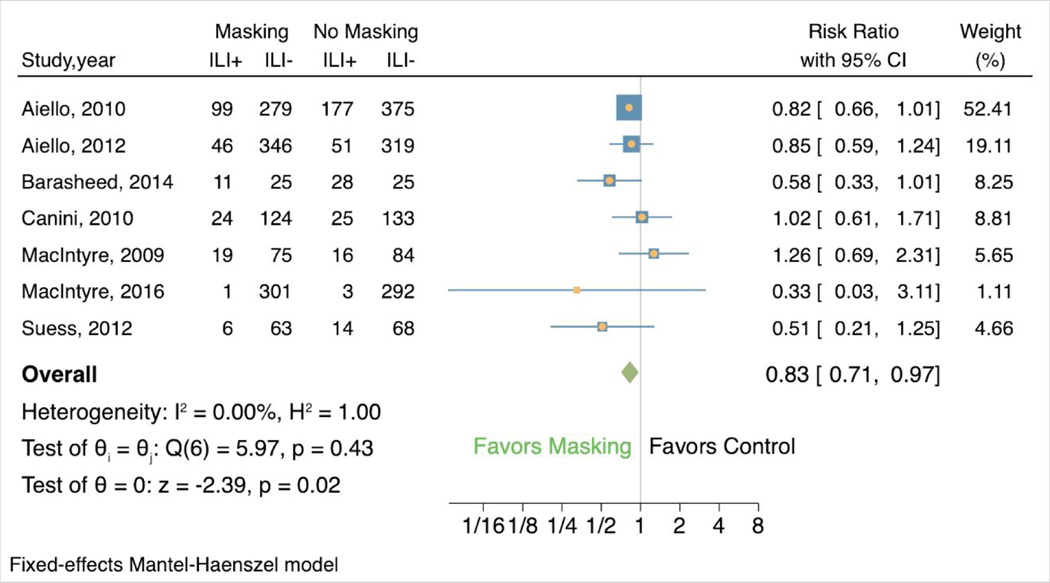
Among the 7 studies evaluating mask-wearing alone that included ILI symptoms as an outcome, 14.5% (206/1419) of participants in the mask groups vs. 19.5% (314/1610) of those in the control groups showed ILI symptoms, and masking was associated with decreased risk of ILI symptoms (RR 0.83, 95% confidence interval [CI] 0.71to 0.96) (Figure 2). There was no heterogeneity (I2 0.00%, H2 1.00). Two of the 7 studies found results that favored the control group (weight 9.15, RR 1.02, 95% CI 0.61 to 1.71; weight 6.64, RR 1.26, 95% CI 0.69 to 2.31). In the other 5 studies, comprising 84.21% of the weight, masking was associated with reduced risk of ILI symptoms.
Figure 2:
Forest plot of estimations of the association between face mask use and influenza-like-illness symptoms.
ILI+ = showed influenza-like-illness symptoms; ILI- = did not show influenza-like-illness symptoms
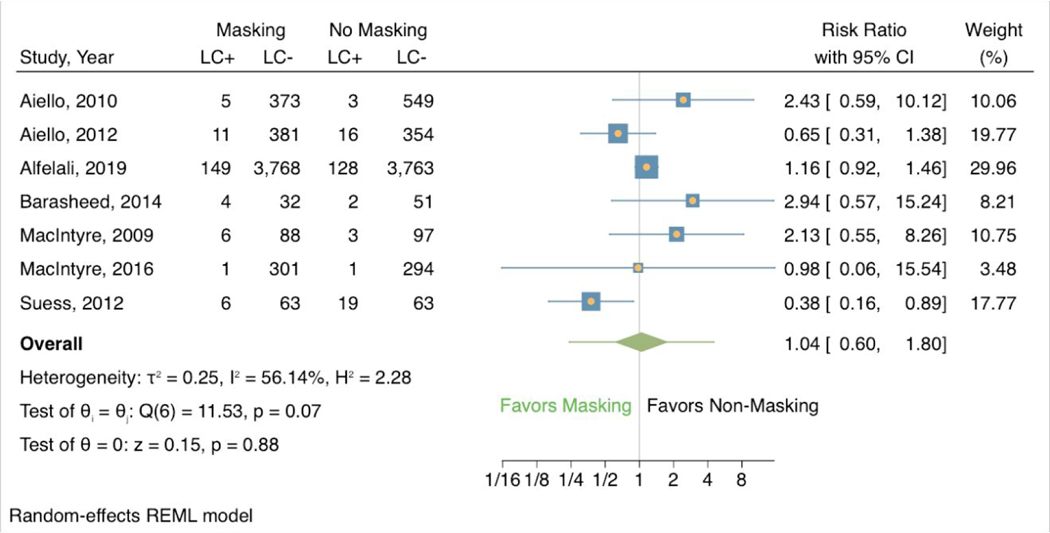
Across the 7 studies evaluating mask-wearing alone that included laboratory-confirmed respiratory infection as an outcome, 3.51% (182/5188) of participants in mask groups vs. 3.22% (172/5343) of those in control groups had a laboratory-confirmed respiratory infection. There was no statistically significant association between masking and risk of infection (RR 1.04, 95% CI 0.60 to 1.80) (Figure 3). In contrast to ILI symptoms, these studies showed a noteworthy effect of heterogeneity (I2 56.14%, H2 2.28). Three of the 7 studies found masking associated with reduced risk of illness. The other 4 studies found masking was associated with increased risk of illness, comprising 58.98% of the total weight.
Figure 3:
Forest plot of estimations of the association between face mask use and laboratory-confirmed respiratory infection.
LC+ = laboratory-confirmed respiratory infection; LC- = no laboratory-confirmed respiratory infection
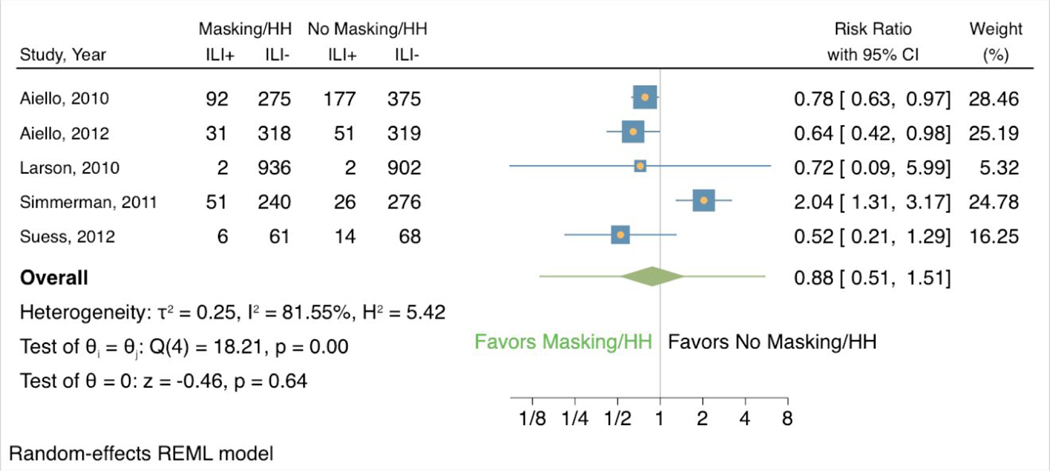
In the 5 studies that also examined the effects of hand hygiene, masking combined with elevated hand hygiene was associated with a lower risk of ILI symptoms (RR 0.88, 95% CI 0.51 to 1.51) (Appendix Figure 2). These 5 studies showed a larger effect of heterogeneity (I2 81.55%, H2 5.42). Only 1 of the studies showed results that favored the control group (weight 24.78, RR 2.04, 95% CI 1.31 to 3.17), while the other 4 studies associated masking and elevated hand hygiene with a reduced risk of ILI symptoms.
Lastly, in the 6 studies where it was examined, masking combined with improved hand hygiene was associated with a decreased risk of laboratory-confirmed respiratory infection (Appendix Figure 3). This was the strongest association among all the comparisons (RR 0.79, 95% CI 0.52 to 1.18). These 6 studies showed modest heterogeneity (I2 41.87%, H2 1.72). Only 1 of the studies showed results that favored the control group (weight 35.93, RR 1.18, 95% CI 0.86 to 1.62), and another study found no difference in infections between the groups (weight 4.67, RR 1.00, 95% CI 0.17 to 5.97). The other 4 studies, making up 59.40% of the total weight, favored masking and elevated hand hygiene as associated with a reduced risk of laboratory-confirmed respiratory infection.
DISCUSSION
This rapid review summarizes the evidence regarding the efficacy of face masks for reducing respiratory illness in the community setting – a critical question given the ongoing COVID-19 pandemic. The evidence derived from randomized controlled trials shows that masking in a community setting reduces self-reported ILI symptoms by 17%. However, it found limited evidence to support the same association between masking and transmission of laboratory-confirmed respiratory infections. On the other hand, masking combined with enhanced hand hygiene was associated with significantly reduced transmission both in terms of ILI (12% reduction) and laboratory-confirmed respiratory infection (21% reduction).
Surprisingly, the results of the analysis of the effect of face masks alone for reducing laboratory confirmed-respiratory were inconsistent with those for the analysis of reduction of ILI symptoms. One possible explanation for this could be bias and variation in self-reporting. ILI symptoms were measured by participant reports of qualifying conditions, such as cough, sore throat, chills, fever, headaches, body aches, etc., though exact definitions differed. Some studies used the CDC definition of ILI, which is a “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza,”37 while other studies simply required any listed symptoms to be noted by participants. Not only was reporting inconsistent across studies and participants, but certain symptoms could also have been due to allergies or other conditions beyond viral infection, ultimately skewing the ILI measurements. Multiple included papers addressed self-reporting issues in their limitations, stating specific problems with subjects underreporting symptoms36, reporting what they thought was expected20,38,39, and reporting ILI symptoms that were likely not due to influenza infection.39 Additionally, it is important to note that because only a subset of all upper respiratory infection-causing viruses were included in the analyses, ILI symptoms may also have resulted from viruses that were not included in laboratory analyses. For example, 3 of the 7 studies reporting laboratory confirmed infection rates in the face mask alone category tested only for influenza.
Furthermore, discrepancies also exist between the results of the analysis of face masks alone versus face masks in combination with enhanced hand hygiene, which may be due to viral transmissibility. In 5 out of the 6 studies included in the analysis of the effects of face masks with advanced hand hygiene38–42, influenza A and B were the only respiratory viruses tested for. According to a 2021 review by Leung evaluating the transmissibility of respiratory viruses43, infectious influenza viral particles have been recovered on surfaces suggesting possible roles for direct and indirect surface transmission of the influenza virus. Thus, hand hygiene may play a larger role in reducing transmission of influenza viruses – as was indicated in the studies analyzed here – than in other respiratory viruses that do not appear to use direct and/or indirect contact as a major mode of transmission, such as SARS-CoV-244. Future studies are needed to evaluate the roles of mask wearing versus hand hygiene in a wider range of respiratory viruses.
Overall, the findings were similar to other systematic reviews that have examined the benefit of masking in both healthcare and community settings2,17,18 with some important differences. Bin-Reza et al.18 found a limited evidence base to support the use of masks in healthcare or community settings but concluded that mask use is best undertaken as a package of personal protective measures, including good hand hygiene. The community randomized controlled trials included in the 2015 review by MacIntyre et al.2 suggested that face masks provide protection against respiratory infection in various community settings, though this protection is subject to adherence. Chu et al.17 also reported a lack of robust data but suggested that face masks were protective for people in the community who had been exposed to infection. Another rapid review relevant to COVID-19 found that data on the effectiveness of masking in community settings for preventing infections associated with coronaviruses were limited but suggest possible reduced risk for SARS1 transmission associated with masking45. Adding to this evidence, a cohort study conducted in Beijing, China during the pandemic produced results showing that face mask use by a COVID-19 positive patient prior to symptom development was 79% effective in reducing transmission in the household, while face mask use after the development of symptoms was not significantly effective46. In contrast to the prior systematic reviews, this review was limited to randomized controlled trials specific to community settings, providing us with the highest quality evidence from which to draw conclusions specific to the community setting.
A limitation of this analysis is that many studies reported adherence issues with mask-wearing or difficulty measuring and reporting adherence. Self-report was used as a measurement tool in 8 of 11 studies, and there was great variability in how adherence was measured amongst the studies, ranging from daily self-reported data to measurement of the amount of intervention material remaining at the conclusion of the study. As a result, it was difficult to set a uniform scale for adherence in order to assess its impact on measured outcomes. Lack of consistency related to adherence may influence individual study results, and thereby the results of this analysis. With many studies reporting low mask-wearing adherence among study participants, the inability to consider these data may have resulted in an underestimation of the effects of the interventions in this analysis and also may explain why this review did not find a reduction with masking and laboratory-confirmed respiratory infection. However, the prevalence of adherence issues among the studies may provide practical insight for public health officials when considering maskwearing mandates within a community setting. Looking at the greatest public health successes over the past few decades (e.g., tobacco control, seatbelt use, motor vehicle safety, childhood lead poisoning, fluoridation, immunization), few can be attributed to changing personal or individual behavior; instead, these successes are directly attributed to laws or mandates.47
When discussing limitations, it is also important to consider the additional limitations of the individual studies included in this rapid review. Foremost, there were different definitions of ILI within included studies, which made it difficult to make comparisons. In addition to varying definitions of ILI and the adherence issues discussed previously, several studies had small sample sizes and therefore were underpowered, limiting detection of small differences between intervention arms.34,36,39,42,48,49 Self-reported data, as discussed above, also present a potential source of bias. Moreover, in 2 studies, there was potential for infection in situations where masking was not required for the masking group – outside of the residence halls in Aiello et al.38 and at dinner time in MacIntyre et al.49 In the Cowling et al.40 study, there was potential bias due to the inclusion of symptomatic patients which may have resulted in higher viral shedding, unavoidable delay between symptom onset and application of intervention, and households with existing immunity. Lastly, in multiple studies there were widespread community hygiene efforts in place that may have influenced control group practices.35,36,41
CONCLUSIONS
In conclusion, this analysis found that masking in the community setting may be an effective way to slow the spread of respiratory illnesses. Future research should examine the impact of universal masking by the public on SARS-CoV-2 transmission. It should also address how best to maintain and measure adherence in a community or household setting as well as how to eliminate bias from reporting of ILI symptoms.
To reduce transmission of COVID-19, masking in community settings in combination with a sustained emphasis on adherence with hand hygiene best practices is recommended. The benefits of masking may be lost if hand hygiene is de-emphasized in community settings.
Acknowledgments:
MB was supported by a National Library of Medicine (NLM) training grant to the Computation and Informatics in Biology and Medicine Training Program (5T15LM007359). Research reported in this publication was also supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health (DP2AI144244). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sources of Support:
MB was supported by a National Library of Medicine (NLM) training grant to the Computation and Informatics in Biology and Medicine Training Program (5T15LM007359). Research reported in this publication was also supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health (DP2AI144244). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- COVID-19
Coronavirus Disease 2019
- CI
Confidence interval
- CRI
Clinical respiratory infection
- HH
Hand hygiene
- ILI
Influenza-like illness
- RR
Risk ratio
- URI
Upper-respiratory infection
- vRTI
Viral respiratory tract infection
- WHO
World Health Organization
APPENDIX
Appendix Figure 1:
Contour enhance funnel plot measuring study effect size (log risk) against variance (standard error).
Appendix Figure 2:
Forest plot of estimations of the association between face mask use combined with elevated hand hygiene and influenza-like illness symptoms.
ILI+ = showed influenza-like-illness symptoms; ILI- = did not show influenza-like-illness symptoms; HH = hand hygiene
Appendix Figure 3:
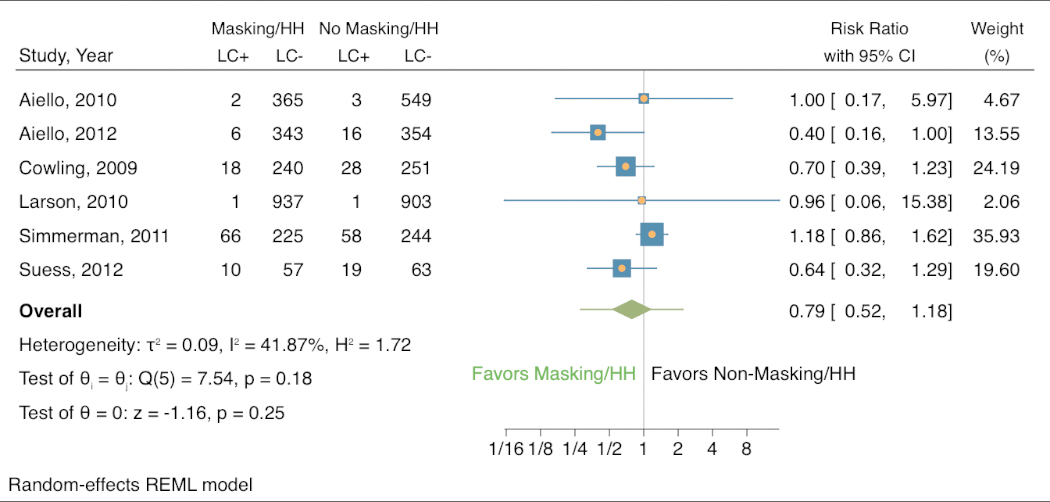
Forest plot of estimations of the association between face mask use combined with elevated hand hygiene and laboratory-confirmed respiratory infection.
LC+ = laboratory-confirmed respiratory infection; LC- = no laboratory-confirmed respiratory infection; HH = hand hygiene
Appendix Table 1:
Search strategies in various databases.
| Database | Search Terms |
|---|---|
| PubMed | “public mask AND infection”, “mask AND infections”, “facemask AND MERS”, “facemask AND community”, “face mask AND community”, “community AND facemask AND infection”, “community AND face mask AND infection”, “((community settings) AND (infection prevention)) AND ((face masks OR face mask OR facemask OR mask))” |
| Google Scholar | “((community settings) AND (infection prevention)) AND ((face masks OR face mask OR facemask OR mask))” |
| Scopus | “((community settings) AND (infection prevention)) AND ((face masks OR face mask OR facemask OR mask))”, “community AND mask AND infection(s)” |
| Health Evidence | “face mask AND community”, “facemask AND community”, “mask AND community” |
| medRxiv | “face mask AND infection”, “facemask AND infection”, “((community settings) AND (infection prevention)) AND ((face masks OR face mask OR facemask OR mask))” |
Appendix Table 2:
Summary of criteria for article inclusion.
| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| Type of Study | Cluster randomized trial | Type of Study | Cohort, cross sectional, case study, experimental, metaanalysis, systematic review, rapid review |
| Participants | Humans | Participants | Animals/non-humans |
| Setting | Community or public | Setting | Healthcare or laboratory |
| Language | English | Language | Non-English |
| Abstract | Available | Abstract | Not available |
| Outcome | ILI symptoms or laboratory-confirmed respiratory virus | Outcome | No measure of ILI symptoms or laboratory-confirmed respiratory virus |
Appendix Table 3:
Description of biases addressed by the Cochrane Risk of Bias tool.
| Bias Domain | Issues Addressed |
|---|---|
| Sequence generation | Was the allocation random? Would it result in comparable groups? |
| Allocation concealment | Was the allocation sequence properly concealed? Could allocations have been foreseen? |
| Blinding of participants and personnel | Were study participants blinded from the study intervention that they received? |
| Blinding of outcome assessors | Were outcome assessors blinded from the study intervention participants received? |
| Incomplete outcome data | Was the outcome data complete? Were there any exclusions? Were they properly reported? |
| Selective outcome reporting | Was the possibility of selective outcome reporting addressed? |
| Other sources of bias | Were limitations addressed? Was there a large enough sample size for generalizations? Were there confounding variables contributing to the results? Were intervention groups demographically comparable? |
| Overall risk of bias | High means ≥ 2 high ratings; Medium means 1 high rating and ≤ 2 unclear ratings; Low means no high ratings and ≤ 2 unclear ratings |
Appendix Table 4:
Individual category and overall risk of bias scores for each study.
| Author | Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessors | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Aiello A et al., 2010 (36) | Low | Low | Low | Unclear | Low | Unclear | Low | Low |
| Aiello A et al., 2012 (37) | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Alfelali M et al., 2019 (38) | Low | Low | Low | Low | Low | Unclear | High | Medium |
| Barasheed O et al., 2014 (39) | Unclear | Unclear | Low | High | Low | Low | High | High |
| Canini L et al., 2010 (40) | Low | Low | Low | Low | Low | Low | High | Low |
| Cowling B et al., 2009 (41) | Low | Low | Low | Unclear | Low | Low | Low | Low |
| Larson E et al., 2010 (42) | High | Unclear | Low | Unclear | Low | Low | Low | Medium |
| MacIntyre C et al., 2009 (43) | Unclear | Unclear | Low | Low | Low | Low | Low | Low |
| MacIntyre C et al., 2016 (44) | Low | Low | Low | Low | Unclear | Low | Low | Low |
| Simmerman J et al., 2011 (45) | Low | Low | Unclear | Unclear | Low | Low | Low | Low |
| Suess T et al., 2012 (46) | Low | Low | Low | Unclear | Low | Low | Low | Low |
High = study showed clear red flags when addressing the bias issues in Appendix Table 3; Unclear = study did not address the bias issues in Appendix Table 3; Low = study properly addressed bias issues in Appendix Table 3
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Archived: WHO Timeline - COVID-19. World Health Organization. Updated April 27, 2020. Accessed May 29, 2020. https://www.who.int/news-room/detail/27-04-2020-whotimeline---covid-19
- 2.MacIntyre CR, Chughtai AA. Facemasks for the prevention of infection in healthcare and community settings. Bmj. Apr 9 2015;350:h694. doi: 10.1136/bmj.h694 [DOI] [PubMed] [Google Scholar]
- 3.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. Jama. Nov 4 2009;302(17):1865–71. doi: 10.1001/jama.2009.1466 [DOI] [PubMed] [Google Scholar]
- 4.MacIntyre CR, Wang Q, Cauchemez S, et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respir Viruses. May 2011;5(3):170–9. doi: 10.1111/j.1750-2659.2011.00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacIntyre CR, Wang Q, Seale H, et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. May 1 2013;187(9):960–6. doi: 10.1164/rccm.201207-1164OC [DOI] [PubMed] [Google Scholar]
- 6.MacIntyre CR, Wang Q, Rahman B, et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med. May 2014;62:1–7. doi: 10.1016/j.ypmed.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan H. Top health officials have changed their minds about face mask guidance -- but for good reason. CNN Health. Updated July 20. Accessed July 20, 2020. https://www.cnn.com/2020/07/19/health/face-masks-us-guidance/index.html
- 8.The government requires the wearing of protective equipment and reserved time for pensioners to do their food shopping. Government of the Czech Republic. Updated March 18. Accessed May 29, 2020. https://www.vlada.cz/en/media-centrum/aktualne/the-government-hasdecided-to-require-the-wearing-of-protective-equipment-and-reserved-time-for-senior-citizensto-do-their-food-shopping-180465/
- 9.South Korea unveils new coronavirus rules, including bars registering all patrons. CBS News. Updated May 25. Accessed July 16, 2020. https://www.cbsnews.com/news/coronavirussouth-korea-unveils-new-covid-rules-travel-face-masks-high-risk-businesses/
- 10.Coronavirus (COVID-19) - what you need to know. Public Health England. Updated July 10. Accessed July 13, 2020. https://publichealthmatters.blog.gov.uk/2020/01/23/wuhan-novelcoronavirus-what-you-need-to-know/
- 11.Dutch measures against coronavirus: basic rules for everyone. Government of the Netherlands. Accessed July 16, 2020. https://www.government.nl/topics/coronavirus-covid-19/tackling-new-coronavirus-in-the-netherlands/basic-rules-for-everyone
- 12.Considerations for Wearing Cloth Face Coverings. US Centers for Disease Control and Prevention. Updated June 28. Accessed May 29, 2020. https://www.cdc.gov/coronavirus/2019ncov/prevent-getting-sick/cloth-face-coverguidance.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprevent-getting-sick%2Fcloth-face-cover.html
- 13.World Health O. Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak: interim guidance, 29 January 2020. 2020. 2020. https://apps.who.int/iris/handle/10665/330987
- 14.World Health O. Advice on the use of masks in the context of COVID-19: interim guidance, 6 April 2020. 2020. 2020. https://apps.who.int/iris/handle/10665/331693
- 15.Advice on the use of masks in the context of COVID-19. 2020:16. June 5, 2020.
- 16.Dobbins M. Rapid Review Guidebook. The National Collaborating Centre for Methods and Tools; 2017:25. [Google Scholar]
- 17.Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. Jun 27 2020;395(10242):1973–1987. doi: 10.1016/s0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bin-Reza F, Lopez Chavarrias V, Nicoll A, Chamberland ME. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respir Viruses. Jul 2012;6(4):257–67. doi: 10.1111/j.1750-2659.2011.00307.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aledort JE, Lurie N, Wasserman J, Bozzette SA. Non-pharmaceutical public health interventions for pandemic influenza: an evaluation of the evidence base. BMC Public Health. Aug 15 2007;7:208. doi: 10.1186/1471-2458-7-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello AE, Coulborn RM, Aragon TJ, et al. Research findings from nonpharmaceutical intervention studies for pandemic influenza and current gaps in the research. Am J Infect Control. May 2010;38(4):251–8. doi: 10.1016/j.ajic.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Barasheed O, Alfelali M, Mushta S, et al. Uptake and effectiveness of facemask against respiratory infections at mass gatherings: a systematic review. Int J Infect Dis. Jun 2016;47:105–11. doi: 10.1016/j.ijid.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong VW, Cowling BJ, Aiello AE. Hand hygiene and risk of influenza virus infections in the community: a systematic review and meta-analysis. Epidemiol Infect. May 2014;142(5):922–32. doi: 10.1017/s095026881400003x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim SW, Moey KS, Tan NC. The use of facemasks to prevent respiratory infection: a literature review in the context of the Health Belief Model. Singapore Med J. Mar 2014;55(3):160–7. doi: 10.11622/smedj.2014037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders-Hastings P, Crispo JAG, Sikora L, Krewski D. Effectiveness of personal protective measures in reducing pandemic influenza transmission: A systematic review and meta-analysis. Epidemics. Sep 2017;20:1–20. doi: 10.1016/j.epidem.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 25.Benkouiten S, Brouqui P, Gautret P. Non-pharmaceutical interventions for the prevention of respiratory tract infections during Hajj pilgrimage. Travel Med Infect Dis. Sep-Oct 2014;12(5):429–42. doi: 10.1016/j.tmaid.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainard JS, Jones N, Lake I, Hooper L, Hunter P. Facemasks and similar barriers to prevent respiratory illness such as COVID-19: A rapid systematic review. medRxiv. 2020:2020.04.01.20049528. doi: 10.1101/2020.04.01.20049528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. Bmj. Apr 9 2020;369:m1435. doi: 10.1136/bmj.m1435 [DOI] [PubMed] [Google Scholar]
- 28.Cowling BJ, Zhou Y, Ip DK, Leung GM, Aiello AE. Face masks to prevent transmission of influenza virus: a systematic review. Epidemiol Infect. Apr 2010;138(4):449–56. doi: 10.1017/s0950268809991658 [DOI] [PubMed] [Google Scholar]
- 29.Brienen NC, Timen A, Wallinga J, van Steenbergen JE, Teunis PF. The effect of mask use on the spread of influenza during a pandemic. Risk Anal. Aug 2010;30(8):1210–8. doi: 10.1111/j.1539-6924.2010.01428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. Aug 28 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 31.Anzures-Cabrera J, Higgins JP. Graphical displays for meta-analysis: An overview with suggestions for practice. Res Synth Methods. Jan 2010;1(1):66–80. doi: 10.1002/jrsm.6 [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. Jun 15 2002;21(11):1539–58. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. Sep 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 34.Barasheed O, Almasri N, Badahdah AM, et al. Pilot Randomised Controlled Trial to Test Effectiveness of Facemasks in Preventing Influenza-like Illness Transmission among Australian Hajj Pilgrims in 2011. Infect Disord Drug Targets. 2014;14(2):110–6. doi: 10.2174/1871526514666141021112855 [DOI] [PubMed] [Google Scholar]
- 35.Alfelali M, Haworth EA, Barasheed O, et al. Facemask versus no facemask in preventing viral respiratory infections during hajj: a cluster randomised open label trial. 2019; [Google Scholar]
- 36.Larson EL, Ferng YH, Wong-McLoughlin J, Wang S, Haber M, Morse SS. Impact of non-pharmaceutical interventions on URIs and influenza in crowded, urban households. Public Health Rep. Mar-Apr 2010;125(2):178–91. doi: 10.1177/003335491012500206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Influenza Surveillance System US: Purpose and Methods. Centers for Disease Control and Prevention. https://www.cdc.gov/flu/weekly/overview.htm
- 38.Aiello AE, Perez V, Coulborn RM, Davis BM, Uddin M, Monto AS. Facemasks, hand hygiene, and influenza among young adults: a randomized intervention trial. PLoS One. 2012;7(1):e29744. doi: 10.1371/journal.pone.0029744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aiello AE, Murray GF, Perez V, et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis. Feb 15 2010;201(4):491–8. doi: 10.1086/650396 [DOI] [PubMed] [Google Scholar]
- 40.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. Oct 6 2009;151(7):437–46. doi: 10.7326/0003-4819-151-7-200910060-00142 [DOI] [PubMed] [Google Scholar]
- 41.Simmerman JM, Suntarattiwong P, Levy J, et al. Findings from a household randomized controlled trial of hand washing and face masks to reduce influenza transmission in Bangkok, Thailand. Influenza Other Respir Viruses. Jul 2011;5(4):256–67. doi: 10.1111/j.17502659.2011.00205.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suess T, Remschmidt C, Schink SB, et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009–2011. BMC Infect Dis. Jan 26 2012;12:26. doi: 10.1186/1471-2334-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung NHL. Transmissibility and transmission of respiratory viruses. Nature Reviews Microbiology. 2021/08/01 2021;19(8):528–545. doi: 10.1038/s41579-021-00535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mondelli MU, Colaneri M, Seminari EM, Baldanti F, Bruno R. Low risk of SARS-CoV2 transmission by fomites in real-life conditions. The Lancet Infectious diseases. 2021;21(5):e112–e112. doi: 10.1016/S1473-3099(20)30678-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou R, Dana T, Jungbauer R, Weeks C, McDonagh MS. Masks for Prevention of Respiratory Virus Infections, Including SARS-CoV-2, in Health Care and Community Settings: A Living Rapid Review. Ann Intern Med. Jun 24 2020;doi: 10.7326/m20-3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Global Health. 2020;5(5):e002794. doi: 10.1136/bmjgh-2020-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ten Great Public Health Achievements --- United States, 2001−−2010. Vol. 60. 2011:619623. Morbidity and Mortality Weekly Report. May 20. [PubMed] [Google Scholar]
- 48.Canini L, Andréoletti L, Ferrari P, et al. Surgical mask to prevent influenza transmission in households: a cluster randomized trial. PLoS One. Nov 17 2010;5(11):e13998. doi: 10.1371/journal.pone.0013998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacIntyre CR, Zhang Y, Chughtai AA, et al. Cluster randomised controlled trial to examine medical mask use as source control for people with respiratory illness. BMJ Open. Dec 30 2016;6(12):e012330. doi: 10.1136/bmjopen-2016-012330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacIntyre CR, Cauchemez S, Dwyer DE, et al. Face mask use and control of respiratory virus transmission in households. Emerg Infect Dis. Feb 2009;15(2):233–41. doi: 10.3201/eid1502.081167 [DOI] [PMC free article] [PubMed] [Google Scholar]