Abstract
Introduction
The prevalence of asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap (ACO) in patients with COVID-19 varies, as well as their risks of mortality. The present study aimed to assess the prevalence of asthma, COPD, and ACO as comorbidities, and to determine their risks of mortality in patients with COVID-19 using a systematic review and meta-analysis.
Methods
We systematically reviewed clinical studies that reported the comorbidities of asthma, COPD, and ACO in patients with COVID-19. We searched various databases including PubMed (from inception to 27 September 2021) for eligible studies written in English. A meta-analysis was performed using the random-effect model for measuring the prevalence of asthma, COPD, and ACO as comorbidities, and the mortality risk of asthma, COPD, and ACO in patients with COVID-19 was estimated. A stratified analysis was conducted according to country.
Results
One hundred one studies were eligible, and 1,229,434 patients with COVID-19 were identified. Among them, the estimated prevalence of asthma, COPD, and ACO using a meta-analysis was 10.04% (95% confidence interval [CI], 8.79–11.30), 8.18% (95% CI, 7.01–9.35), and 3.70% (95% CI, 2.40–5.00), respectively. The odds ratio for mortality of pre-existing asthma in COVID-19 patients was 0.89 (95% CI, 0.55–1.4; p = 0.630), while that in pre-existing COPD in COVID-19 patients was 3.79 (95% CI, 2.74–5.24; p<0.001). France showed the highest prevalence of asthma followed by the UK, while that of COPD was highest in the Netherlands followed by India.
Conclusion
Pre-existing asthma and COPD are associated with the incidence of COVID-19. Having COPD significantly increases the risk of mortality in patients with COVID-19. These differences appear to be influenced by the difference of locations of disease pathophysiology and by the daily diagnosis and treatment policy of each country.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in Wuhan, China [1], and it is the causative agent of the coronavirus disease 2019 (COVID-19) pandemic [2]. As many as 50% of patients have reported having at least one comorbidity with COVID-19 [3]. Among them, the highest prevalent comorbidity was hypertension (21.1%), followed by diabetes (9.7%), cardiovascular disease (8.4%), and respiratory system disease (1.5%) [3]. However, the prevalence of asthma, as a comorbidity of patients with COVID-19, has been reported to vary from 1.10% [4] to 36.3% [5]. Additionally, the prevalence of chronic obstructive pulmonary disease (COPD) in COVID-19 ranges from 0.70% [6] to 70.60% [7] and that of asthma-COPD overlap (ACO) ranges from 0.40% [8] to 29.40% [7]. Previous reports have indicated that the global prevalence of asthma in adults is estimated to be 4.3% [9], that of COPD is estimated to be 12.16% [10], and that of ACO ranges from 0.9% to 11.1% [11]. While some studies have reported that asthma, COPD, and ACO are related to an increase in the mortality rate of COVID-19 [12, 13], some studies have reported that they may not be risk factors or may not increase the mortality of COVID-19 [14–17]. However, studies on detailed examinations of the prevalence and risk of mortality of asthma, COPD, and ACO in patients with COVID-19 are still lacking.
Therefore, this study aimed to systematically review and integrate the data from studies with various results on the prevalence of asthma, COPD, and ACO in patients with COVID-19. We also aimed to determine the mortality risks of asthma, COPD, and ACO in patients with COVID-19.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement and the statement by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group [18–20].
Search strategy
Two investigators (Y.U. and T.M.) independently searched for eligible studies in PubMed, the Cochrane Library, and MedRxiv from inception to 27 September 2021. We used the following key words: “asthma” OR “asthmatic” OR “COPD” OR “Chronic Obstructive Lung” OR “Chronic Obstructive Pulmonary Disease” OR “chronic bronchitis” OR “pulmonary emphysema” OR “pulmonary disease” OR “Chronic Obstructive” OR “Chronic Obstructive Airway Disease” OR “COAD” OR “Chronic Obstructive Lung Disease” OR “Chronic Airflow Obstruction” OR “Obstructive Lung Disease” OR “Obstructive pulmonary Disease” OR “Lung Disease” OR “ACO” OR “asthma-COPD overlap” OR “Asthma-chronic obstructive pulmonary disease overlap syndrome” OR “Asthma and chronic obstructive pulmonary disease overlap syndrome” OR “asthma-COPD overlap syndrome” OR “asthma-COPD” OR “ACOS” OR “mixed asthma-COPD phenotype” OR “Asthma combined with COPD” OR “coexistence of asthma and COPD” OR “coexistence of asthma and COPD” OR “COPD with asthmatic features” OR “overlap of asthma-COPD” AND “COVID-19” OR “novel coronavirus” OR “new coronavirus” OR “emerging coronavirus” OR “2019-nCoV” OR “SARS-CoV-2” OR “COVID” OR “coronavirus” OR “nCov” OR “coronavirus disease 2019” OR “coronavirus 2019”. We also reviewed the reference lists of eligible studies using Google Scholar and performed a manual search to ensure that all appropriate studies were included.
Eligibility criteria and outcome measures
Studies fulfilling the following selection criteria were included in the meta-analysis: (1) randomized, clinical trials, observational studies, and case series involving >20 patients written in English; and (2) patients with positive laboratory-confirmed SARS-CoV-2 infection who had asthma, COPD, or ACO as comorbidities. The exclusion criteria were as follows: (1) systematic reviews, (2) reviews, (3) animal experimental reports, (4) ≤20 patients in case series, (5) insufficient or incomplete data, (6) unpublished articles, and (7) pediatrics reports.
Data extraction
Two reviewers (Y.U. and T.M.) extracted the data independently. Articles that were retrieved in the search were stored in a citation manager. After removing redundant articles, titles, and abstracts, full-text articles were then investigated. We extracted the following data: study design, observational period, study site, and inclusion/exclusion criteria of each study. Outcome variables were extracted into predesigned data collection forms. We verified the accuracy of the data by comparing the collection of each investigator, and any discrepancies were resolved through discussion.
Level of evidence
The level of evidence was determined using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework, which classifies the level of evidence for each outcome on the basis of the risk of bias, imprecision, inconsistency, indirectness, and publication bias [21]. The authors classified the evidence level for each eligible study in accordance with the revised grading system for recommendation in the evidence-based guideline [22] (S1 Table).
Data analysis
In the meta-analysis, we estimated the odds ratios (ORs) or the proportions of patients for primary outcome variables with 95% confidence intervals (CIs) using the random-effects model (generic inverse variance method). To assess the proportions of the outcome variables in patients with COVID-19, the standard error was calculated using the Agresti-Coull method [23]. Heterogeneity among the original studies was evaluated using the I2 statistic [24]. Publication bias was examined using a funnel plot. For all analyses, significant levels were two-tailed, and p<0.01 was considered significant. All statistical tests were performed using Review Manager (RevMan) ver. 5.4.1 (Cochrane Collaboration, Copenhagen, Denmark) [25].
Ethics approval and consent to participate
The institutional review board and patient consent were not required because of the review nature of this study.
Results
Study selection and characteristics
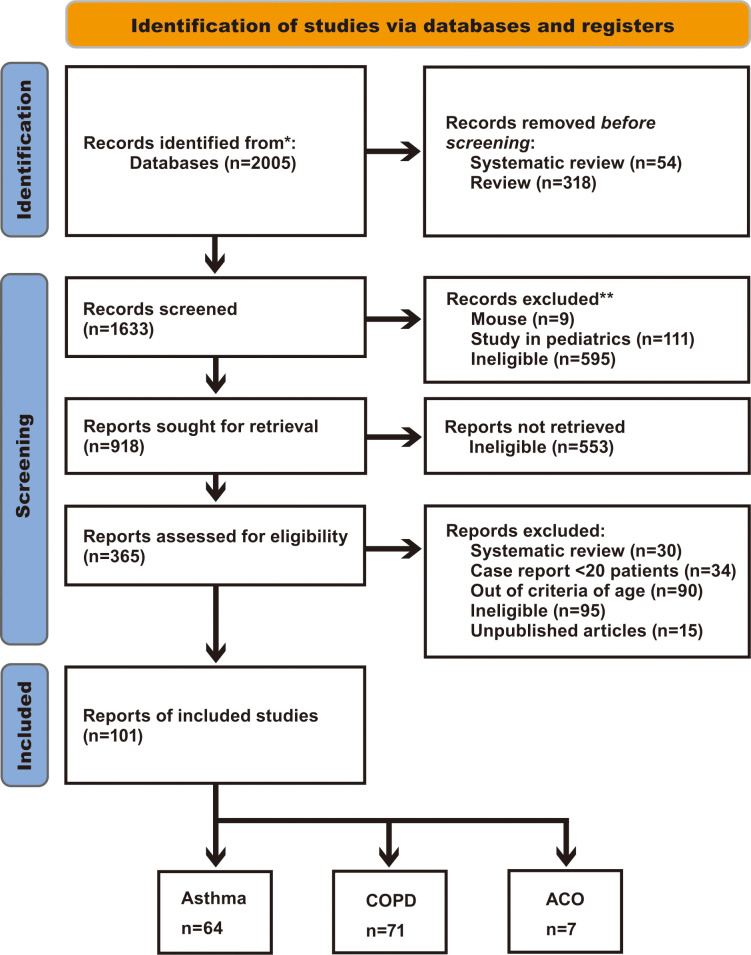
Of the 2005 references screened, 101 studies reported the outcome variables (Fig 1).
Fig 1. PRISMA flow diagram.
N indicates the number of articles.
We analyzed 64 studies on asthma, 71 on COPD, and 7 on ACO. Thirty-three studies were duplicated for asthma and COPD, two for asthma and ACO, and four for asthma, COPD, and ACO. Table 1 shows the characteristics of the included studies.
Table 1. The characteristics of the included studies.
| Study, year | Country | Observational period | Study Design | No. of participants | Sex-Male, n (%) | Age, Median (IQR) or mean±SD, years | Severity of COVID-19, n (%) | Standard of the evidence level |
|---|---|---|---|---|---|---|---|---|
| Zhang JJ, 2020 [26] | China | Jan.16-Feb.3, 2020 | - | 140 | 71 (50.7) | 57 (range, 25–87) | Nonsevere 82 Severe 58 |
2- |
| Wang L, 2020 [27] | China | Jan.1-Feb.6, 2020 | Retrospective single-center study | 339 | 166 | 69 (65–76) | Moderate100(29.5) severe159(46.9) critical80(23.6) | 2- |
| Bhatraju PK, 2020 [28] | USA | Feb.24-Mar.9, 2020 | - | 24 | 15 (63) | 64±18 | 2- | |
| Turan O, 2021 [29] | Turkey | Mar-Aug, 2020 | Multicenter, retrospective cohort study | 1069 | 634 | >18 | 2- | |
| Barrasa H, 2020 [30] | Spain | Mar.4-Mar.31, 2020 | - | 48 | 27 (56) | 63 (12)≥18 | 2- | |
| Mahdavinia M, 2020 [31] | USA | Mar.12-Apr.3 2020 | - | 935 | 417 | ≥18 | 2- | |
| Li P, 2020 [32] | China | Jan.31-Feb.20, 2020 | - | 204 | 100 | 68 (64–75) Range, 60–95 | Mild 64.7%, severe 33.3%, critical 2% | 2- |
| Lian J, 2020 [33] | China | Jan.17-Feb.12, 2020 | retrospective | 136 | 58 | 68.28±7.314 | Mild 102, severe 22, critical 12 | 2- |
| Iaccarino G, 2020 [34] | Italy | Mar.9-Apr.9, 2020 | Cross-sectional, multicenter, observational study | 1591 | 64% | 66.5±0.4 range18-101 | 2- | |
| Toussie D, 2020 [35] | USA | Mar.10-Mar.26, 2020 | Retrospective study | 338 | 210 (62) | 39 (31–45) Between 21 and 50 | Low chest radiograph severity score 0–1:202 2–6:136 |
2- |
| Argenziano M, 2020 [36] | USA | Mar.1-Apr.5, 2020 | Retrospective case series | 1000 | 596 | Emergency department55 (40–69) In hospital 64 (51–77) ICU, 62 (52–72) |
2- | |
| Cummings MJ, 2020 [37] | USA | Mar.2-Apr.1, 2020 | Prospective cohort study | 257 critical | 171 (67) | 62 (51–72) | 2- | |
| Zhu Z, 2020 [38] | USA | Mar.16 2020- | Population-based prospective cohort study | 641 | 288 (45) | 56±8 Aged 40–69 | 2- | |
| Grandbastien M, 2020 [39] | France | Mar.4-Apr.6, 2020 | Monocentric, retrospective, cohort study | 106 | 66 (62.3) | 63. 5(54.2–72.0) | 2- | |
| Yao Y, 2020 [40] | China | -Mar.10, 2020 | Retrospective, multicenter, cohort study | 171 | 92 (53.8) | 50.5±15.2 | Severe 71(41.5), critical 29(17.0) | 2- |
| Lieberman-Cribbin W, 2020 [41] | USA | Feb.29-Apr.24, 2020 | - | 6245 | 49% | 57 | 2- | |
| Wang J, 2020 [42] | China | Jan.24-Feb.23, 2020 | Retrospective study | 307 | 156 (50.8) | 57.65±15.754 | Mild/moderate 259(84.6) Severe/critical 48(15.6) |
2- |
| Aggarwal A, 2020 [43] | India | Apr.10-Apr.30, 2020 | Retrospective, single-center case series | 32 | 19 (59.4) | 54. 5 (46.25–60) | Severe 24 Non-severe 8 |
2- |
| Caminati M, 2020 [44] | Italy | Mar.1-Apr.30, 2020 | - | Brescia20 Verona6 |
Brescia8 Verona3 |
Brescia41-77 (mean 61.5) Verona55-79 (69.3) | 2- | |
| Bello-Chavolla OY, 2020 [4] | Mexico | -June.3, 2020 | Population-based statistics | 20804 | 12257 (58.9) | ≥ 60 | 2- | |
| Bravi F, 2020 [45] | Italy | -Apr.24, 2020 | Case-control, retrospective study | 1603 | 47.3% | 58.0 (20.9) All adults | Mild957 Severe454 Very severe/lethal192 |
2- |
| Song J, 2021 [46] | China | Feb.1-Mar.6 2020 | Retrospective observational study | 961 | 500 (52.0) | 63 (49–70) | Nonsevere719(74.8) Severe242(25.2) | 2- |
| Wang L, 2020 [47] | USA | Mar .3-June.8, 2020 | - | 1827 | 595 | 54 (37–66) | Hospitalized 565, Non-hospitalized 1262 | 2- |
| Canevelli M, 2020 [48] | Italy | Feb.21-Apr.29, 2020 | - | 2687 | 1807 | Natives 78.3±10.8 Migrants 71.1±13.1 |
2- | |
| De Vito A, 2020 [49] | Italy | Mar.8-Apr.8, 2020 | Retrospective, monocentric study | 87 | 56 (64.4) | 72 (62.5–83.5) | 2- | |
| Atkins JL, 2020 [50] | UK | Mar.16-Apr.26, 2020 | - | 507 | 311 | 74.3 (4.5) Aged 65 and older | 2- | |
| Zhao Z, 2020 [51] | USA | Mar.9-Apr.20, 2020 | Retrospective study | 641 | Died 53 (64.6), ICU admission136 (69.7) General admission222 (55.8) | Died 77 (66–85) ICU admission 60 (50–70) General admission 58 (46–71) | 2- | |
| Pérez-Sastré MA, 2020 [52] | Mexico | Feb.28-June.21, 2020 | - | 159017 | (52.2) | ≥20 | 2- | |
| Guner R, 2020 [53] | Turkey | Mar.10-Apr.10, 2020 | - | 222 | 132 (59.5) | 50.6±16.5 (18–93) | Mild172 Critical50 |
2- |
| Somani SS, 2020 [54] | USA | Feb.27-Apr.12 | Retrospective cohort study | 2864 | 1663 | ≥18 | 2- | |
| Yang JM, 2020 [55] | Korea | Jan.1-May.15, 2020 | Propensity-score-matched nationwide cohort | 7430 | 2970 (40.5) | 49.0±19.9 | 2- | |
| Campioli CC, 2020 [56] | USA | Feb.1-May.15, 2020 | Retrospective study | 251 | 103 (41.0) | 53 (27) adult | 2- | |
| He Y, 2020 [57] | China | Jan.20-Apr.1 2020 | - | 336 | 201 (59.8) | 65 (50–77) | severe | 2- |
| Mushtaq J, 2021 [58] | Italy | Feb.25-Apr.9, 2020 | Retrospective single-center study | 697 | 465 (66.7) | 62 (52–75) | 2- | |
| Goel N, 2020 [59] | India | May.8-July.3, 2020 | Retrospective observational study | 35 | 20 (57.1) | 46±17 | Symptomatic 29(82.9%), asymptomatic 6(17.1%) | 2- |
| Brendish NJ, 2020 [60] | UK | Mar.20-Apr.29, 2020 | Prospective, interventional, non-randomised study | 352 | 202 (57.4) | 68 (50–80) | 2- | |
| Ioannou GN, 2020 [61] | USA | Feb.28-May.14, 2020 | Longitudinal cohort study | 10131 | 9221 (91.0) | 63.6 (16.2) ≥18 | 2- | |
| Xiong Q, 2021 [62] | China | -Mar.1, 2020 | Longitudinal study | 538 | 245 (45.5) | 52.0 (41.0–62.0) From 20 to 80 | General 331(61.5), severe 180(33.5), critical 27(5) | 2- |
| Seaton RA, 2020 [63] | UK | Apr.20-30, 2020 | - | 531 | 274 (51.6) | 72(61–82) Range25-104 | 2- | |
| Abrams MP, 2020 [64] | USA | Mar.1-Apr.3, 2020 | cohort | 133 | 74 (55.6) | 81.0 (70.5–88.0) | Arrhythmic death11 Nonarrhythmic death122 |
2- |
| Akpinar G, 2021 [65] | Turkey | Mar.1-May.31, 2020 | Retrospective cross-sectional design | 88 | 46 | 48.0±17.3 | 2- | |
| Schiavone M, 2021 [66] | Italy | Feb.23-Apr.1, 2020 | Retrospective study | 844 | 521 (61.7) | 63.4±16.1 | 2- | |
| Calmes D, 2020 [67] | Belgium | Mar.18-Apr.17 2020 | - | 596 | 294 | ≥35 | 2- | |
| Rial MJ, 2021 [68] | Spain | Mar-June, 2020 | Multicenter retrospective cohort | 35 | 14 | ≥20 | 2- | |
| Robinson LB, 2020 [69] | USA | Mar.8-Apr.27, 202 | Matched cohort study | 403 | 191 | ≥18 | 2- | |
| Cates J, 2020 [70] | USA | Mar.1-Mar.31, 2020 | - | 3948 | 3710 (94.0) | 70 (61–77) | 2- | |
| Şanlı DET, 2020 [71] | Turkey | Mar.11-Apr.11, 2020 | Local institutional reveiw | 102 | 73 (72) | 48.62±14.42 Ranging 19–94 |
2- | |
| Hussein MH, 2020(USA) [72] | USA | Mar.15-June.9, 2020 | Multi-center retrospective study | 502 | 238 | Mean age 60.7 ≥18 | qSOFA score, CURB65 score | 2- |
| Liao SY, 2021(USA) [5] | USA | Mar.11-June.23, 2020 | Prospective observational study | 113 | 53 (47) | 50±16 | 2- | |
| Tabarsi P, 2021 [73] | Iran | ? | Randomized controlled trial | 84 | 65 | IVIg, 54.29±12.85 Control group, 52.47±14.49 Between 18 and 65 |
All severe patients | 1- |
| Lee SC, 2020 [74] | Korea | Jan.20-May.27 2020 | Retrospective cohort study | 7272 | 2927 | ≥20 | Non-severe, severe | 2- |
| Jiang Y, 2020 [75] | China | Jan.30-Mar.8, 2020 | Retrospective observational study | 281 | 143 | ≥60 | 2- | |
| Xiao J, 2020 [76] | China | Dec.25, 2019-Feb.16, 2020 | Retrospective single-center study | 243 | 105 (43.2) | 47.0 (range20-89) | Moderate203, severe/critical 40 | 2- |
| Signes-Costa J, 2021 [77] | Spain | Mar.23-May.5, 2020 | Retrospective, multicenter, cohort study | 5847 | 3432 | 65.1±16.6 | 2- | |
| Bello-Chavolla OY, 2020 [78] | Mexico | Mar.16-Aug.17, 2020 | - | 3007 | Non-severe1227 (50.5) Severe403 (70.1) |
Non-severe 44 (33–55) Severe 56 (47–66) | Non-severe2432 Severe 575 |
2- |
| Lokken EM, 2020 [79] | USA | Jan.21-Apr.17, 2020 | Retrospective study | 46 | 0 Pregnant women |
29 (26–34) | 2- | |
| Gómez Antúnez M, 2021 [80] | Spain | Mar 2020 | Retrospective cohort study | 10420 | 5893 (56.7) | 69 (55–79) | 2- | |
| Ferastraoaru D, 2021 [8] | USA | Mar.14-Apr.27, 2020 | Retrospective study | 4558 | (31.8) | Asthma 60.5±17.07 | 2- | |
| Monterrubio-Flores E, 2021 [81] | Mexico | Feb.28-July.31, 2020 | - | 406966 | 216908 (53.2) | ≥20 | 2- | |
| Mortaz E, 2021 [82] | Iran | Apr.10-May.9, 2020 | retrospective observational study | 29 | 17 | 54.45±2.536 (range, 32–79) | 2- | |
| Değerli E, 2021 [83] | Turkey | Mar.23-Oct.23, 2020 | Retrospective study | 45 | 23 (51) | 60.3±15.65 | 2- | |
| Laake JH, 2020 [84] | Norway | Mar.10-June.19, 2020 | National cohort | 217 | 162 | 63 (54.2–72.2) | 2- | |
| Lee SC, 2021 [85] | Korea | Jan.20-May.27, 2020 | Retrospective cohort study | 4610 | 1710 | ≥40 | 2- | |
| Jungo S, 2021 [86] | France | Apr.1-Apr.29, 2020 | - | 79 | 37 (46.8) | 44 (36–53) Range, 21–86 |
COVID-19-related phenotypes 68(86.1) | 2- |
| Cao L, 2021 [87] | USA | Mar-Sep, 2020 | Prospectively collected cohort | 343 | 192 | >18 | 2- | |
| Fong WCG, 2021 [88] | UK | Mar.1-May.31, 2020 | retrospective | 6638(with, w/o covid) | 3079 (46.4) | 65 (42–79) | 2- | |
| Jongbloed M, 2021 [89] | Netherland | Feb.28-Apr.1, 2020 | Retrospective cohort study | 303 | 195 (64) | 72±12 | 2- | |
| Artero A, 2021 [90] | Spain | Mar.1-May.28, 2020 | Multicenter retrospective cohort study | 10238 | 5924 (57.9) | 66.6±16.2 | 2- | |
| Ho KS, 2021 [91] | USA | Mar.7-June.7, 2020 | Retrospective multicenter cohort study | 10523 | 5707 | 58.35±18.81 | 2- | |
| Yoshida Y, 2021 [92] | USA | Feb.27-July.15, 2020 | Retrospective case series | 776 | 365 (47.3) | 60.5 (16.1) >18 | 2- | |
| Nanda S, 2021 [93] | USA | Jan.1-May.23, 2020 | retrospective | 1169 | 575 (49.2) | 43.9 (17.6) [range18.0–99.0] | 2- | |
| De Vito A, 2021 [94] | Italy | Apr.9-May.31, 2020 | Observational retrospective cohort study | 264 | 99 (37.5) | 81.93±10.11 | Symptomatic 132 Asymptomatic 132 |
2- |
| Rodriguez C, 2021 [95] | France | Mar.9-30, 2020 | - | 104 | 59 | Outpatient 50 (range, 19–87) Hospitalized 61 (31–82) ICU, 68 (33–90) | 2- | |
| Garibaldi BT, 2021 [96] | USA | Mar.4-Aug.29, 2020 | Retrospective comparative effectiveness research | 2299 | 1193 adults | All remdesivir, 60 (46–69) All control, 60 (44–74) | 2- | |
| Giovannetti G, 2021 [97] | Italy | May.18-July.25, 2020 | Prospective observational study | 38 | 27 (71.1) | 60.6 (10.4) Between 18 and 75 |
Mild11(28.9) Moderate11(28.9) Severe3(7.9) |
2- |
| Khan MS, 2021 [98] | USA | Jan.1-June.15, 2020 | Retrospective, observational cohort study | 470 | 224 (47.7) | ≥18 | 2- | |
| Tsai S, 2021 [99] | USA | Feb.24-Nov.25, 2020 | Retrospective cohort | 8308 | 0 All women | 50.69±12.80 Adult | 2- | |
| Lobelo F, 2021 [100] | USA | Mar.3-Oct.29, 2020 | Retrospective cohort | 5721 | 2416 (42.2) | 44.8 (15.7) ≥18 | 2- | |
| Chatterjee A, 2021 [101] | Netherland | Mar.1-July.1, 2020 | Retrospective study | 2337 | Non-mortality1078 (60.9) Mortality393 (69.2) |
Non-mortality, 65 (55–75) Mortality, 77 (70–83) |
Non-mortality1769 Mortality568 |
2- |
| Yordanov Y, 2021 [102] | France | Mar.9-Aug.11, 2020 | Prospective cohort | 7320 | 2301 (31.5) | 43.0±13.9 | 2- | |
| Riou M, 2021 [103] | France | June-Dec, 2020 | descriptive | 81 | 59 (73) | 61 (51–68) | Mild-to-moderate 21, severe 15, critical 45 | 2- |
| Wei W, 2021 [104] | USA | June.1-Dec.9, 2021 | Retrospective study | 206741 | 85228 | 46.7 (17.8) ≥18 | - | 2- |
| Hou X, 2021 [105] | China | Jan.28-Feb.25, 2020 | Single-center retrospective cohort study | 113 | 61 (54) | 55.1±14.2 | Severe113 | 2- |
| Valverde-Monge M, 2021 [106] | Spain | Jan.31-Apr.17, 2020 | Retrospective analysis | 2539 | 1275 | NCRD 61.1±19.3, CRD 71.4±14.8 | - | 2- |
| Cosio BG, 2021 [107] | Spain | Mar.15-Apr.30, 2020 | Case-control study | 52 | 48 (92.3) | 72.96±10.75 | - | 2- |
| Adir Y, 2021 [108] | Israel | Mar.1-Dec.7, 2020 | Case-control study | 8242 | 4343 | 43.3±20.4 | Moderate, severe | 2- |
| Sen P, 2021 [7] | USA | Mar.8-Sap.16, 2020 | - | 1288 | 499 (38.8) | 63.7 (15.2)≥35 | - | 2- |
| Chandel A, 2021 [6] | USA | Mar.1-June.9, 2020 | Multicenter retrospective observational study | 272 | 180 | 57±13 | - | 2- |
| Chaudhary S, 2021 [109] | USA | Mar.15-May.10, 2020 | Single-center retrospective observational study | 128 | 71 | 68 (61–75.5) All adult | - | 2- |
| Williamson EJ, 2020 [110] | UK | Feb.1-May.6, 2020 | Cohort study | 10926 | 6126 (0.07) | ≥18 | - | 2- |
| Abayomi A, 2021 [111] | Nigeria | Feb.27-Jul.6, 2020 | Retrospective cohort study | 2075 | 1379 | 40 (32–50) Range18-98 | Mild/asymptomatic 1179, moderate 743, severe 107, critical 42 | 2- |
| Liu YH, 2021 [112] | China | Feb.10-Apr.10, 2020 | Cross-sectional study | 1539 | 738 (47.95) | 69 (66–75) | Severe238 Non-severe1301 |
2- |
| Munblit D, 2021 [113] | Russia | Dec.2-Jan.14, 2020 | Longitudinal cohort study | 1358 | 675 | 57 (47–67) | Mild841(61.9) Moderate479(35.3) Severe38(2.8) |
2- |
| Sandoaval M, 2021 [114] | USA | Mar.1-Dec.7, 2020 | Retrospective registry-based chart reveiw | 1853 | 704(38.0) | 24 (21–27) From 18 to 29 | - | 2- |
| Lokken EM, 2021 [115] | USA | Mar.1-June.30, 2020 | Multicenter retrospective cohort study | 240 | 0 (pregnant woman) | 28 (24–34) | Mild218(90.8) Severe18(7.5) Critical4(1.7) |
2- |
| Cataño-Correa JC, 2021 [116] | Colombia | Mar-Aug, 2020 | - | 399 | 235(58.9) | >18 | - | 2- |
| Meza D, 2021 [117] | USA | Feb.2021 | - | 387008 | COPD 3949, no COPD 126324 | COPD 70.5, no COPD 57.9 Aged over 35 | - | 2- |
| Sun Y, 2021 [118] | China | Feb.2-Mar.25, 2020 | Retrospective study | 268 | 139(51.9) | 57.75 (67–73) Range, 20–88 | Severe 96 Non-severe 172 |
2- |
| Fernández-Martínez NF, 2021 [119] | Spain | Mar.1-Apr.15, 2020 | Observational longitudinal study | 968 | 530(55) | 67 (55–77) | - | 2- |
| Cosma S, 2021 [120] | Italy | Sep.20-Jan.9, 2020 | Case-control study | 21 | 0 | ≥18 | - | 2- |
| Chudasama YV, 2021 [121] | UK | Mar.16-July.26, 2020 | - | 1706 | 981(57.5) | 68 (range48-85) | severe | 2- |
IQR, Interquartile range; SD, Standard deviation; NCRD, non-chronic respiratory disease; CRD, chronic respiratory disease
In the 101 included studies, we identified 1,229,434 patients with COVID-19, and 32,301, 10,827, and 818 had asthma, COPD, and ACO, respectively, as the comorbidities. Among the studies, there were 34 reports from USA, 14 from China, 10 from Italy, 8 from Spain, 6 from the UK, 5 from Turkey, 4 from Mexico, 3 from Korea, 2 from the Netherlands, 2 from Iran, and 1 each from Israel, Nigeria, Russia, Norway, and Columbia. The study designs were 52 retrospective studies, 7 prospective studies, 1 population-based statistics, 2 matched cohort studies, 4 longitudinal cohort studies, 2 local institutional reviews, 1 randomized, controlled trial, 2 nation cohort studies, 1 descriptive study, 3 case–control studies, 2 cross-sectional studies, and 24 with an unknown design. The total number of male patients was 616,380 and that of female patients was 737,188. Among the studies, the severity of patients with COVID varied from asymptomatic to a critical condition.
Frequency of asthma, COPD, and ACO in patients with COVID-19
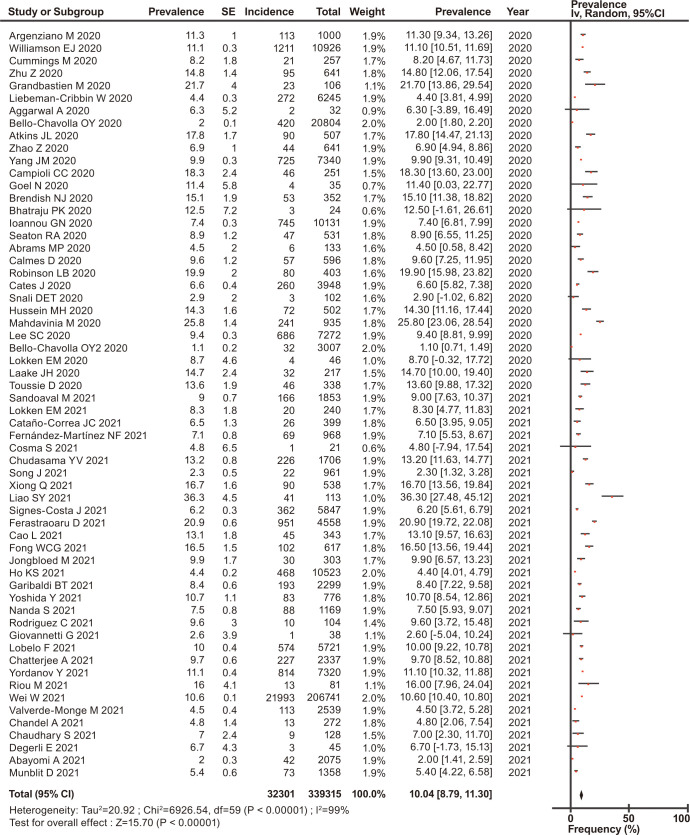
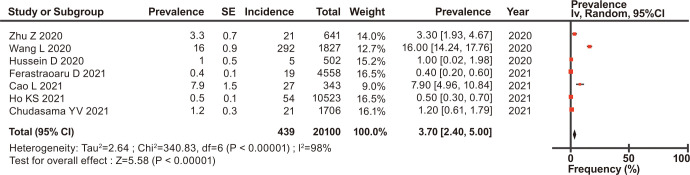
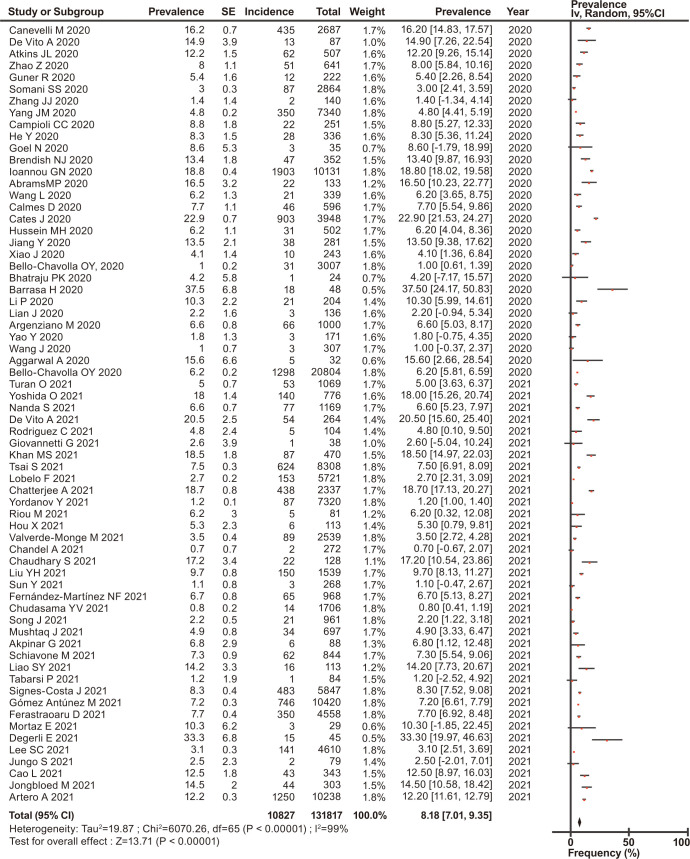
The overall prevalence of asthma, COPD, and ACO was estimated, and their forest plots are shown in Figs 2–4, respectively.
Fig 2. Forest plots of the prevalence of asthma in patients with COVID-19.
Fig 4. Forest plots of the prevalence of ACO in patients with COVID-19.
Among the eligible patients with COVID-19, the prevalence of asthma, COPD, and ACO was 10.04% (95% CI, 8.79–11.30) for asthma (Fig 2), 8.18% (95% CI, 7.01–9.35) for COPD (Fig 3), and 3.70% (95% CI, 2.40–5.00) for ACO (Fig 4). In the stratified analysis, the frequencies of asthma in different countries are shown in Table 2, and their forest plots are shown in S1 Fig.
Fig 3. Forest plots of the prevalence of COPD in patients with COVID-19.
Table 2. Estimated frequencies of asthma, COPD, and ACO in patients with COVID-19 according to countries.
| Asthma | COPD | ACO | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) | No. of studies | No. of patients | Estimated frequency (95% CI) |
| USA | 28 | 26692 | 11.14 (9.55–12.73) | 19 | 4600 | 10.48 (7.56–13.40) | 6 | 418 | 4.24 (2.74–5.73) |
| Mexico | 2 | 452 | 1.57 (0.69–2.45) | 2 | 1329 | 3.60 (-1.50–8.70) | - | - | - |
| UK | 6 | 1729 | 13.45 (11.23–15.66) | 3 | 123 | 8.69 (-0.83–18.22) | 1 | 21 | 1.20 (0.61–1.79) |
| Italy | 3 | 28 | 0.10 (0.09-.011) | 6 | 599 | 11.09 (5.64–16.54) | - | - | - |
| Spain | 3 | 544 | 5.83 (4.44–7.23) | 6 | 2651 | 8.84 (5.77–11.91) | - | - | - |
| France | 4 | 860 | 13.50 (9.08–17.92) | 4 | 99 | 2.60 (0.33–4.88) | - | - | - |
| Netherland | 2 | 257 | 9.72 (8.61–10.83) | 2 | 482 | 17.00 (12.96–21.04) | - | - | - |
| Turkey | 2 | 6 | 3.58 (0.02–7.13) | 4 | 86 | 8.23 (3.47–12.98) | - | - | - |
| Iran | - | - | - | 2 | 4 | 3.84 (-4.25–11.93) | - | - | - |
| India | 2 | 6 | 8.57 (0.98–16.16) | 2 | 8 | 11.34 (3.24–19.44) | - | - | - |
| China | 2 | 112 | 9.42 (-4.69–23.53) | 13 | 309 | 4.93 (2.89–6.96) | - | - | - |
| Korea | 2 | 1411 | 9.65 (9.16–10.14) | 2 | 491 | 3.96 (2.30–5.63) | - | - | - |
With regard to the frequency of asthma, France showed a rate of 13.50% (95% CI, 9.08–17.92), which was the highest, followed by 13.45% in the UK (95% CI, 11.23–15.66). The frequency of COPD in patients with COVID-19 was the highest in the Netherlands at 17.00% (95% CI, 12.96–21.04), followed by India at 11.34% (95% CI, 3.24–19.44). The frequency of ACO on the USA and the UK was 4.24% (95% CI, 2.74–5.73) and 1.20% (95% CI, 0.61–1.79), respectively. The forest plots of these data are shown in supplementary figures (S1 Fig).
Prevalence of death in patients with COVID-19 and asthma or COPD
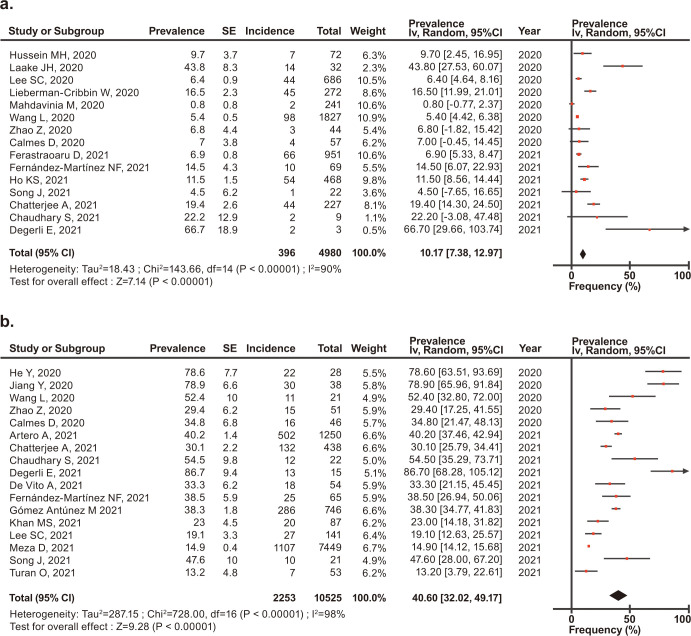
Forest plots of the prevalence of death in patients with COVID-19 and asthma or COPD are shown in Fig 5A and 5B.
Fig 5.
Forest plots of the prevalence of death a) in patients with asthma and COVID-19 and b) in patients with COPD and COVID-19.
Among 4,980 patients with asthma and COVID-19, the prevalence of death was 10.17% (95% CI, 7.38–12.97) (Fig 5A). Among 10,525 patients with COPD and COVID-19, the prevalence of death was 40.60% (95% CI, 32.02–49.17) (Fig 5B).
Risk of mortality due to COVID-19 in patients with asthma or COPD
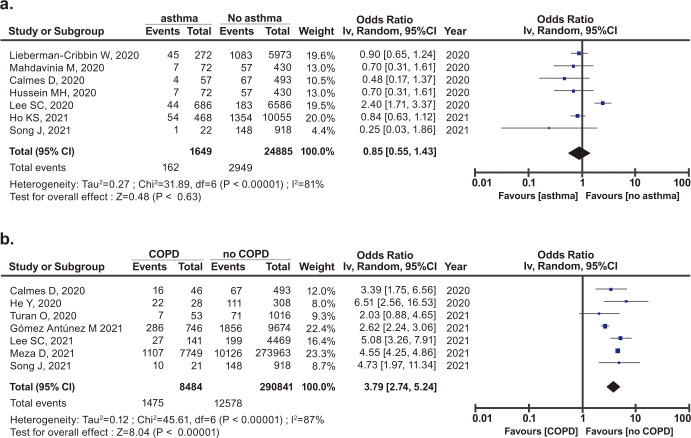
The risk to mortality due to COVID-19 in patients with asthma or COPD was estimated and it is shown in forest plots in Fig 6.
Fig 6.
Forest plots of the risk of mortality in patients with COVID-19 and a) asthma or b) COPD.
The risk of mortality in pre-existing asthma in COVID-19 patients was not significant (OR, 0.89; 95% CI, 0.55–1.43; p = 0.630) (Fig 6A). However, the risk of mortality in pre-existing COPD in COVID-19 patients was significant (OR, 3.79; 95% CI, 2.74–5.24; p<0.001) (Fig 6B).
Discussion
The present systematic review and meta-analysis on 101 studies showed that pre-existing asthma and COPD affected the incidence of COVID-19, and asthma had a greater effect than COPD. However, pre-existing asthma did not have a significant effect on mortality in patients with COVID-19, while patients with COPD had a 3.8-fold increased risk of mortality among COVID-19 cases. Among patients with COVID-19, the highest prevalence of asthma was observed in France followed by the UK, while the highest prevalence of COPD was observed in the Netherlands followed by India. The various prevalence of these disease in each county indicates the importance of daily clinical control of asthma, COPD, and ACO for preventing and reducing the severity of COVID-19.
The COVID-19 pandemic has disproportionately affected people with chronic diseases, such as asthma and COPD, which are the most common respiratory diseases. Generally, viral infection to the respiratory tract is thought to be one of the triggers for the exacerbation of pre-existing diseases [110]. Respiratory viral infection that is initiated in the upper respiratory tract and innate immunity are critical for the initial control of infection at this site [122]. If the innate immune response is inadequate, the infection can spread to the lower respiratory tract, causing pneumonia [123]. Before the COVID-19 pandemic, the reported global prevalence of adult asthma and COPD was 3.5% [9] and 12.16% [10], respectively. However, in patients with COVID-19 in the present study, which assessed studies published after COVID-19 emerged, the prevalence of pre-existing asthma was 10.04% and that of COPD was 8.18%. The prevalence of asthma after COVID-19 emerged was higher than that before the pandemic. These results indicated that asthma affected the incidence of COVID-19. The increased susceptibility of viral infection in the bronchial airway might be caused by pathophysiological impairment in both of these diseases. Especially in asthma, the main involved sites of the bronchial airway are the upper and lower bronchi [124]. In case of COVID-19, more than 80% of patients have mild illness [123], and the locations where mild COVID-19 is involved are similar to those in patients with asthma. Consequently, the number of patients with asthma may have increased as the number and proportion of mild COVID-19 cases increased. This possibility may also explain why the prevalence of pre-existing asthma was higher than that of COPD. In fact, the Omicron variant was associated with a large number of mild COVID-19 cases [125]. Additionally, a previous meta-analysis, which used only data before the Omicron variant emerged, reported that the prevalence of asthma was similar to that before the COVID-19 pandemic [126]. This result is different from that in the present study, which assessed COVID-19 cases that included infected patients with the Omicron variant. However, a study including hospitalized COVID-19 cases with a history of asthma indicated that none of these patients presented with asthma exacerbation [127]. Owing to the nature of the meta-analysis, we could not evaluate asthma exacerbation after admission among the patients in this study.
The present study showed that pre-existing COPD in patients had a 3.8-fold higher risk of mortality than in those who did not have COPD. The risk of mortality for pre-existing COPD was stronger than that for pre-existing asthma. Unlike asthma, of which the main involved sites are the upper and lower bronchi, the main impaired lesion of COPD extends from the peripheral small airway to alveolar tissues with architectural damage, which can cause the severe illness. These locations of lesions are compatible with those in COVID-19 when the disease severity is moderate to severe. Indeed, patients with COPD have a high risk of mortality in other respiratory infectious diseases, such as influenza [128] and community-acquired pneumonia [129]. A previous study showed that the long-term use of inhaled corticosteroids for controlling asthma is likely to have a beneficial modulatory effect on COVID-19 [130]. This finding suggests that this efficacy is achieved by reducing epithelial damage and improving the T-cell response. Several studies reported a large number of patients who were receiving either inhaled steroids or systemic steroids at the time of COVID-19 diagnosis [55, 127, 131, 132]. However, the effect of inhaled corticosteroids at the early stage of COVID-19 is controversial [133]. The benefit of systemic corticosteroids for patients with asthma may outweigh the risk of severe outcomes in patients with COVID-19 [134]. Systemic corticosteroids are effective for treating bronchial wall inflammation and bronchial spasm. As the result, uncontrolled asthma is associated with increased intensive care unit admission and intensive respiratory support [135], whereas well-controlled asthma does not have an increased risk of COVID-19-related death [136]. The present study showed that the prevalence of pre-existing asthma in COVID-19 cases varied according to the countries. This finding may be partly due to the fact that each country has different treatment policies and guidelines, as well as available medical resources. In addition, owing to the nature of the meta-analysis in which we did not use individual patient data, we were unable to examine the impact on COVID-19 diagnosis according to age, sex, and stage at which therapy was started. These differences may also influence the severity of COVID-19 in different countries. These factors may be also related to the heterogeneity in the results of the meta-analysis in the present study. A large-sample study showed that the contribution of inhaled corticosteroids for patients with COPD to COVID-19-related death was lower than that for patients with asthma [137]. Additionally, the association with mortality was confounded by the presence of other risk factors for severe COVID-19, such as an older age, cardiovascular disease, hypertension, and diabetes mellitus [123], which are common in people with COPD.
ACO has clinical characteristics derived from asthma and COPD. The risk of mortality from ACO in patients with COVID-19 might be significant and as high as that for COPD. However, in the present study, the prevalence of pre-existing ACO was lower than that of asthma and COPD. Our results regarding ACO cannot be properly assessed because of the number of eligible studies, and the countries that reported pre-existing ACO were only from the USA, UK, and China among the eligible studies. These issues might be due to the short history of the concept of ACO and a lack of global recognition. However, even with the small number of eligible studies, the prevalence of ACO was highest in studies from the USA. Additionally, a study in the USA before the COVID-19 pandemic reported that the prevalence of ACO was 1.05% (0.74%–1.37%) [138], while that in the present study was 4.24%. One of the reasons for this discrepancy between studies may be related to the high smoking rate (14%) in the USA [139–141]. This discrepancy suggests the necessity of considering other cofounding factors for assessing the risk of ACO, such as the rate of smokers and obesity.
Our study has some limitations, including mainly those inherent to the nature of systematic reviews and meta-analyses using observational studies and case series. The eligible studies were limited to articles written in English. The treatment guidelines and available medical resources for COVID-19, and the examined comorbidities may be different according to the different countries, and these could have affected the risk of infection and mortality of COVID-19. The eligible studies were selected from published papers during 1 year and 9 months from the beginning of the COVID-19 pandemic. We were not able to evaluate the change in the risk of COVID-19 caused by the change in SARS-CoV-2 variants and the vaccination availability during this observational period.
Despite these limitations, the present systematic review and meta-analysis of 101 studies suggests the importance of daily clinical management for patients with asthma, COPD, or ACO. Additionally, this study suggests that attention should be paid to the prevention of COVID-19 infection and disease progression, as well as to patients with other high-risk diseases of COVID-19.
Conclusion
The present systematic review and meta-analysis using 101 studies shows that pre-existing asthma and COPD are associated with the incidence of COVID-19. Asthma has a stronger influence on the incidence of COVID-19 than COPD. The presence of COPD as a comorbidity in patients with COVID-19 has a 3.8 times higher risk of mortality, while asthma has no significant effect on COVID-19 related death. These differences appear to be affected by the difference in locations of disease pathophysiology, and by the daily diagnosis and treatment policy of each country.
Supporting information
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Ellen Knapp, for editing a draft of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI, Promotion of Joint International Research B, #20KK0218), and a grant from Japan Science and Technology (JST), JST-Mirai Program (#20345310). The funders had no role in the design, methods, participant recruitment, data collection, analysis, or preparation of the paper. There was no additional external funding received for this study.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020. Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 January 2022. Available at https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—12-january-2022 (Access January 30, 2022) [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. Mar 28;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bello-Chavolla OY, González-Díaz A, Antonio-Villa NE, Fermín-Martínez CA, Márquez-Salinas A, Vargas-Vázquez A, et al. Unequal Impact of Structural Health Determinants and Comorbidity on COVID-19 Severity and Lethality in Older Mexican Adults: Considerations Beyond Chronological Aging. J Gerontol A Biol Sci Med Sci. 2021. Feb 25;76(3):e52–e59. doi: 10.1093/gerona/glaa163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao SY, Petrache I, Fingerlin TE, Maier LA. Association of inhaled and systemic corticosteroid use with Coronavirus Disease 2019 (COVID-19) test positivity in patients with chronic pulmonary diseases. Respir Med. 2021. Jan;176:106275. doi: 10.1016/j.rmed.2020.106275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandel A, Patolia S, Brown AW, Collins AC, Sahjwani D, Khangoora V, et al. High-Flow Nasal Cannula Therapy in COVID-19: Using the ROX Index to Predict Success. Respir Care. 2021. Jun;66(6):909–919. doi: 10.4187/respcare.08631 [DOI] [PubMed] [Google Scholar]
- 7.Sen P, Majumdar U, Zein J, Hatipoğlu U, Attaway AH. Inhaled corticosteroids do not adversely impact outcomes in COVID-19 positive patients with COPD: An analysis of Cleveland Clinic’s COVID-19 registry. PloS One. 2021. Jun 3;16(6):e0252576. doi: 10.1371/journal.pone.0252576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, et al. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J Allergy Clin Immunol Pract. 2021. Mar;9(3):1152–1162.e3. doi: 10.1016/j.jaip.2020.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattiuzzi C, Lippi G. Worldwide asthma epidemiology: insights from the Global Health Data Exchange database. Int Forum Allergy Rhinol. 2020;10(1):75–80. doi: 10.1002/alr.22464 [DOI] [PubMed] [Google Scholar]
- 10.Varmaghani M, Dehghani M, Heidari E, Sharifi F, Moghaddam SS, Farzadfar F. Global prevalence of chronic obstructive pulmonary disease: systematic review and meta-analysis. East Mediterr Health J. 2019. Mar 19;25(1):47–57. doi: 10.26719/emhj.18.014 [DOI] [PubMed] [Google Scholar]
- 11.Leung C, Sin DD. Asthma-COPD Overlap: What Are the Important Questions? Chest. 2021. Oct 6:S0012-3692(21)04078–2. doi: 10.1016/j.chest.2021.09.036 [DOI] [PubMed] [Google Scholar]
- 12.Rothe T, Spagnolo P, Bridevaux PO, Clarenbach C, Eich-Wanger C, Meyer F, et al. Diagnosis and Management of Asthma–The Swiss Guidelines. Respiration. 2018;95(5):364–380. doi: 10.1159/000486797 [DOI] [PubMed] [Google Scholar]
- 13.Vogelmeier CF, Román-Rodríguez M, Singh D, Han MK, Rodríguez-Roisin R, Ferguson GT. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir Med. 2020. May;166:105938. doi: 10.1016/j.rmed.2020.105938 [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa S, Ichinose M. Definition and diagnosis of asthma-COPD overlap (ACO). Allergol Int. 2018. Apr;67(2):172–178. doi: 10.1016/j.alit.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021. Feb;76(2):428–455. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, et al. Does Asthma Increase the Mortality of Patients with COVID-19?: A Systematic Review and Meta-Analysis. Int Arch Allergy Immunol. 2021;182(1):76–82. doi: 10.1159/000510953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan WJ, Liang WH, Shi Y, Gan LX, Wang HB, He JX, et al. Chronic Respiratory Diseases and the Outcomes of COVID-19: A Nationwide Retrospective Cohort Study of 39,420 Cases. J Allergy Clin Immunol Pract. 2021. Jul;9(7):2645–2655.e14. doi: 10.1016/j.jaip.2021.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 20.Lee SW., Koo MJ. PRISMA 2020 statement and guidelines for systematic review and meta-analysis articles, and their underlying mathematics: Life Cycle Committee Recommendations. Life Cycle. 2022;2:e9. doi: 10.54724/lc.2022.e9 [DOI] [Google Scholar]
- 21.Balshem H, Helfand M, Schünemann HJ, Andrew Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 22.Harbour R, Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001. Aug 11;323(7308):334–6. doi: 10.1136/bmj.323.7308.334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–26. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Review Manager (RevMan) [Computer program] Version 5.4.1 Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014 [Google Scholar]
- 26.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020. Jul;75(7):1730–1741. doi: 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020. Jun;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N Engl J Med. 2020. May 21;382(21):2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turan O, Arpınar Yigitbas B, Turan PA, Mirici A. Clinical characteristics and outcomes of hospitalized COVID-19 patients with COPD. Expert Rev Respir Med. 2021. Aug;15(8):1069–1076. doi: 10.1080/17476348.2021.1923484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. ; Alava COVID-19 Study Investigators. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020. Oct;39(5):553–561. doi: 10.1016/j.accpm.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahdavinia M, Foster KJ, Jauregui E, Moore D, Adnan D, Andy-Nweye AB, et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. 2020. Jul-Aug;8(7):2388–2391. doi: 10.1016/j.jaip.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Chen L, Liu Z, Pan J, Zhou D, Wang H, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis. 2020. Aug;97:245–250. doi: 10.1016/j.ijid.2020.05.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, et al. Analysis of Epidemiological and Clinical Features in Older Patients With Coronavirus Disease 2019 (COVID-19) Outside Wuhan. Clin Infect Dis. 2020. Jul 28;71(15):740–747. doi: 10.1093/cid/ciaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iaccarino G, Grassi G, Borghi C, Ferri C, Salvetti M, Volpe M; SARS-RAS Investigators. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 2020. Aug;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 35.Toussie D, Voutsinas N, Finkelstein M, Cedillo MA, Manna S, Maron SZ, et al. Clinical and Chest Radiography Features Determine Patient Outcomes in Young and Middle-aged Adults with COVID-19. Radiology. 2020. Oct;297(1):E197–E206. doi: 10.1148/radiol.2020201754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020. May 29;369:m1996. doi: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020. Jun 6;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA Jr, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. 2020. Aug;146(2):327–329.e4. doi: 10.1016/j.jaci.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandbastien M, Piotin A, Godet J, Abessolo-Amougou I, Ederlé C, Enache I, et al. SARS-CoV-2 Pneumonia in Hospitalized Asthmatic Patients Did Not Induce Severe Exacerbation. J Allergy Clin Immunol Pract. 2020. Sep;8(8):2600–2607. doi: 10.1016/j.jaip.2020.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao Y, Chen W, Wu X, Shen L, Shen L, Fu Y, et al. Clinical characteristics of COVID-19 patients in three consecutive generations of spread in Zhejiang, China. Clin Microbiol Infect. 2020. Oct;26(10):1380–1385. doi: 10.1016/j.cmi.2020.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lieberman-Cribbin W, Rapp J, Alpert N, Tuminello S, Taioli E. The Impact of Asthma on Mortality in Patients With COVID-19. Chest. 2020. Dec;158(6):2290–2291. doi: 10.1016/j.chest.2020.05.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Zhu X, Xu Z, Yang G, Mao G, Jia Y, et al. Clinical and CT findings of COVID-19: differences among three age groups. BMC Infect Dis. 2020. Jun 22;20(1):434. doi: 10.1186/s12879-020-05154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and Epidemiological Features of SARS-CoV-2 Patients in SARI Ward of a Tertiary Care Centre in New Delhi. J Assoc Physicians India. 2020. Jul;68(7):19–26. [PubMed] [Google Scholar]
- 44.Caminati M, Lombardi C, Micheletto C, Roca E, Bigni B, Furci F, et al. Asthmatic patients in COVID-19 outbreak: Few cases despite many cases. J Allergy Clin Immunol. 2020. Sep;146(3):541–542. doi: 10.1016/j.jaci.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Togni A, et al. Predictors of severe or lethal COVID-19, including Angiotensin Converting Enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS One. 2020. Jun 24;15(6):e0235248. doi: 10.1371/journal.pone.0235248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song J, Zeng M, Wang H, Qin C, Hou HY, Sun ZY, et al. Distinct effects of asthma and COPD comorbidity on disease expression and outcome in patients with COVID-19. Allergy. 2021. Feb;76(2):483–496. doi: 10.1111/all.14517 [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020. Oct;146(4):808–812. doi: 10.1016/j.jaci.2020.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canevelli M, Palmieri L, Raparelli V, Punzo O, Donfrancesco C, Lo Noce C, et al. ; Italian National Institute of Health COVID-19 Mortality Group. COVID-19 mortality among migrants living in Italy. Ann Ist Super Sanita. 2020. Jul-Sep;56(3):373–377. doi: 10.4415/ANN_20_03_16 [DOI] [PubMed] [Google Scholar]
- 49.De Vito A, Geremia N, Fiore V, Princic E, Babudieri S, Madeddu G. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci. 2020. Jul;24(14):7861–7868. doi: 10.26355/eurrev_202007_22291 [DOI] [PubMed] [Google Scholar]
- 50.Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Preexisting Comorbidities Predicting COVID-19 and Mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020. Oct 15;75(11):2224–2230. doi: 10.1093/gerona/glaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020. Jul 30;15(7):e0236618. doi: 10.1371/journal.pone.0236618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pérez-Sastré MA, Valdés J, Ortiz-Hernández L. Clinical characteristics and severity of COVID-19 among Mexican adults. Gac Med Mex. 2020;156(5):373–381. English. doi: 10.24875/GMM.M20000424 [DOI] [PubMed] [Google Scholar]
- 53.Güner R, Hasanoğlu İ, Kayaaslan B, Aypak A, Kaya Kalem A, Eser F, et al. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic’s first month in Turkey. Turk J Med Sci. 2020. Dec 17;50(8):1801–1809. doi: 10.3906/sag-2006-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Somani SS, Richter F, Fuster V, De Freitas JK, Naik N, Sigel K, et al. Characterization of Patients Who Return to Hospital Following Discharge from Hospitalization for COVID-19. J Gen Intern Med. 2020. Oct;35(10):2838–2844. doi: 10.1007/s11606-020-06120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: A nationwide cohort study. J Allergy Clin Immunol. 2020. Oct;146(4):790–798. doi: 10.1016/j.jaci.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corsini Campioli C, Cano Cevallos E, Assi M, Patel R, Binnicker MJ, O’Horo JC. Clinical predictors and timing of cessation of viral RNA shedding in patients with COVID-19. J Clin Virol. 2020. Sep;130:104577. doi: 10.1016/j.jcv.2020.104577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He Y, Xie M, Zhao J, Liu X. Clinical Characteristics and Outcomes of Patients with Severe COVID-19 and Chronic Obstructive Pulmonary Disease (COPD). Med Sci Monit. 2020. Sep 4;26:e927212. doi: 10.12659/MSM.927212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mushtaq J, Pennella R, Lavalle S, Colarieti A, Steidler S, Martinenghi CMA, et al. Initial chest radiographs and artificial intelligence (AI) predict clinical outcomes in COVID-19 patients: analysis of 697 Italian patients. Eur Radiol. 2021. Mar;31(3):1770–1779. doi: 10.1007/s00330-020-07269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goel N, Spalgais S, Mrigpuri P, Khanna M, Menon B, Kumar R. Characteristics of COVID-19 at a non-COVID tertiary pulmonary care centre in Delhi, India. Monaldi Arch Chest Dis. 2020. Nov 9;90(4). doi: 10.4081/monaldi.2020.1568 [DOI] [PubMed] [Google Scholar]
- 60.Brendish NJ, Poole S, Naidu VV, Mansbridge CT, Norton N, Borca F, et al. Clinical characteristics, symptoms and outcomes of 1054 adults presenting to hospital with suspected COVID-19: A comparison of patients with and without SARS-CoV-2 infection. J Infect. 2020. Dec;81(6):937–943. doi: 10.1016/j.jinf.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ioannou GN, Locke E, Green P, Berry K, O’Hare AM, Shah JA, et al. Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10131 US Veterans With SARS-CoV-2 Infection. JAMA Netw Open. 2020. Sep 1;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021. Jan;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seaton RA, Gibbons CL, Cooper L, Malcolm W, McKinney R, Dundas S, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020. Dec;81(6):952–960. doi: 10.1016/j.jinf.2020.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrams MP, Wan EY, Waase MP, Morrow JP, Dizon JM, Yarmohammadi H, et al., Clinical and cardiac characteristics of COVID-19 mortalities in a diverse New York City Cohort. J Cardiovasc Electrophysiol. 2020;31(12):3086–3096. doi: 10.1111/jce.14772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akpinar G, Demir MC, Sultanoglu H, Sonmez FT, Karaman K, Keskin BH, et al. The Demographic Analysis of the Probable COVID-19 Cases in Terms of RT-PCR Results and Age. Clin. Lab. 2021. Sep 67:1058–1064 doi: 10.7754/Clin.Lab.2020.200844 [DOI] [PubMed] [Google Scholar]
- 66.Schiavone M, Gasperetti A, Mancone M, Curnis A, Mascioli G, Mitacchione G, et al. Oral anticoagulation and clinical outcomes in COVID-19: An Italian multicenter experience. Int J Cardiol. 2021. Jan 15;323:276–280. doi: 10.1016/j.ijcard.2020.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calmes D, Graff S, Maes N, Frix AN, Thys M, Bonhomme O, et al. Asthma and COPD Are Not Risk Factors for ICU Stay and Death in Case of SARS-CoV2 Infection. J Allergy Clin Immunol Pract. 2021. Jan;9(1):160–169. doi: 10.1016/j.jaip.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rial MJ, Valverde M, Del Pozo V, González-Barcala FJ, Martínez-Rivera C, Muñoz X, et al. Clinical characteristics in 545 patients with severe asthma on biological treatment during the COVID-19 outbreak. J Allergy Clin Immunol Pract. 2021. Jan;9(1):487–489.e1. doi: 10.1016/j.jaip.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson LB, Fu X, Bassett IV, Triant VA, Foulkes AS, Zhang Y, et al. COVID-19 severity in hospitalized patients with asthma: A matched cohort study. J Allergy Clin Immunol Pract. 2021. Jan;9(1):497–500. doi: 10.1016/j.jaip.2020.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cates J, Lucero-Obusan C, Dahl RM, Schirmer P, Garg S, Oda G, et al. Risk for In-Hospital Complications Associated with COVID-19 and Influenza—Veterans Health Administration, United States, October 1, 2018-May 31, 2020. MMWR Morb Mortal Wkly Rep. 2020. Oct 23;69(42):1528–1534. doi: 10.15585/mmwr.mm6942e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tekcan Şanlı DE, Yıldırım D. A new imaging sign in COVID-19 pneumonia: vascular changes and their correlation with clinical severity of the disease. Diagn Interv Radiol. 2021. Mar;27(2):172–180. doi: 10.5152/dir.2020.20346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hussein MH, Toraih EA, Attia AS, Burley N, Zhang AD, Roos J, et al. Asthma in COVID-19 patients: An extra chain fitting around the neck? Respir Med. 2020. Dec;175:106205. doi: 10.1016/j.rmed.2020.106205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tabarsi P, Barati S, Jamaati H, Haseli S, Marjani M, Moniri A, et al. Evaluating the effects of Intravenous Immunoglobulin (IVIg) on the management of severe COVID-19 cases: A randomized controlled trial. Int Immunopharmacol. 2021. Jan;90:107205. doi: 10.1016/j.intimp.2020.107205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SC, Son KJ, Han CH, Jung JY, Park SC. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep. 2020. Dec 11;10(1):21805. doi: 10.1038/s41598-020-77791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang Y, Abudurexiti S, An MM, Cao D, Wei J, Gong P. Risk factors associated with 28-day all-cause mortality in older severe COVID-19 patients in Wuhan, China: a retrospective observational study. Sci Rep. 2020. Dec 22;10(1):22369. doi: 10.1038/s41598-020-79508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao J, Li X, Xie Y, Huang Z, Ding Y, Zhao S, et al. Maximum chest CT score is associated with progression to severe illness in patients with COVID-19: a retrospective study from Wuhan, China. BMC Infect Dis. 2020. Dec 11;20(1):953. doi: 10.1186/s12879-020-05683-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Signes-Costa J, Núñez-Gil IJ, Soriano JB, Arroyo-Espliguero R, Eid CM, Romero R, et al. ; HOPE COVID-19 investigators. Prevalence and 30-Day Mortality in Hospitalized Patients With Covid-19 and Prior Lung Diseases. Arch Bronconeumol. 2021. Apr;57:13–20. doi: 10.1016/j.arbres.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bello-Chavolla OY, Antonio-Villa NE, Ortiz-Brizuela E, Vargas-Vázquez A, González-Lara MF, de Leon AP, et al. Validation and repurposing of the MSL-COVID-19 score for prediction of severe COVID-19 using simple clinical predictors in a triage setting: The Nutri-CoV score. PLoS One. 2020. Dec 16;15(12):e0244051. doi: 10.1371/journal.pone.0244051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lokken EM, Walker CL, Delaney S, Kachikis A, Kretzer NM, Erickson A, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020. Dec;223(6):911.e1–911.e14. doi: 10.1016/j.ajog.2020.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gómez Antúnez M, Muiño Míguez A, Bendala Estrada AD, Maestro de la Calle G, Monge Monge D, Boixeda R, et al. ; SEMI-COVID-19 Network. Clinical Characteristics and Prognosis of COPD Patients Hospitalized with SARS-CoV-2. Int J Chron Obstruct Pulmon Dis. 2021. Jan 5;15:3433–3445. doi: 10.2147/COPD.S276692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monterrubio-Flores E, Ramírez-Villalobos MD, Espinosa-Montero J, Hernandez B, Barquera S, Villalobos-Daniel VE, et al. Characterizing a two-pronged epidemic in Mexico of non-communicable diseases and SARS-Cov-2: factors associated with increased case-fatality rates. Int J Epidemiol. 2021. May 17;50(2):430–445. doi: 10.1093/ije/dyab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mortaz E, Bassir A, Dalil Roofchayee N, Dezfuli NK, Jamaati H, Tabarsi P, et al. Serum cytokine levels of COVID-19 patients after 7 days of treatment with Favipiravir or Kaletra. Int Immunopharmacol. 2021. Apr;93:107407. doi: 10.1016/j.intimp.2021.107407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Değerli E, Derin S, Oruç K, Şengül Samancı N, Bedir Ş, Çelik E, et al. The demographic characteristics, prognosis, and relationship with cancer subtypes of hospitalized COVID-19 patients with malignancy: A single-center experience. J Med Virol. 2021. Oct;93(10):5839–5845. doi: 10.1002/jmv.27123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laake JH, Buanes EA, Småstuen MC, Kvåle R, Olsen BF, Rustøen T, et al. Characteristics, management and survival of ICU patients with coronavirus disease-19 in Norway, March-June 2020. A prospective observational study. Acta Anaesthesiol Scand. 2021. May;65(5):618–628. doi: 10.1111/aas.13785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee SC, Son KJ, Han CH, Park SC, Jung JY. Impact of COPD on COVID-19 prognosis: A nationwide population-based study in South Korea. Sci Rep. 2021. Feb 12;11(1):3735. doi: 10.1038/s41598-021-83226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jungo S, Moreau N, Mazevet ME, Ejeil AL, Biosse Duplan M, Salmon B, et al. Prevalence and risk indicators of first-wave COVID-19 among oral health-care workers: A French epidemiological survey. PLoS One. 2021. Feb 11;16(2):e0246586. doi: 10.1371/journal.pone.0246586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao L, Lee S, Krings JG, Rauseo AM, Reynolds D, Presti R, et al. Asthma in patients with suspected and diagnosed coronavirus disease 2019. Ann Allergy Asthma Immunol. 2021. May;126(5):535–541.e2. doi: 10.1016/j.anai.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fong WCG, Borca F, Phan H, Moyses HE, Dennison P, Kurukulaaratchy RJ, et al. Asthma did not increase in-hospital COVID-19-related mortality in a tertiary UK hospital. Clin Exp Allergy. 2021. Jul;51(7):939–941. doi: 10.1111/cea.13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jongbloed M, Leijte WT, Linssen CFM, van den Hoogen BG, van Gorp ECM, de Kruif MD. Clinical impact of human metapneumovirus infections before and during the COVID-19 pandemic. Infect Dis (Lond). 2021. Jul;53(7):488–497. doi: 10.1080/23744235.2021.1887510 [DOI] [PubMed] [Google Scholar]
- 90.Artero A, Madrazo M, Fernández-Garcés M, Muiño Miguez A, González García A, Crestelo Vieitez A, et al. ; SEMI-COVID-19 Network. Severity Scores in COVID-19 Pneumonia: a Multicenter, Retrospective, Cohort Study. J Gen Intern Med. 2021. May;36(5):1338–1345. doi: 10.1007/s11606-021-06626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ho KS, Howell D, Rogers L, Narasimhan B, Verma H, Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021. Jul;127(1):42–48. doi: 10.1016/j.anai.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshida Y, Gillet SA, Brown MI, Zu Y, Wilson SM, Ahmed SJ, et al. Clinical characteristics and outcomes in women and men hospitalized for coronavirus disease 2019 in New Orleans. Biol Sex Differ. 2021. Feb 5;12(1):20. doi: 10.1186/s13293-021-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nanda S, Toussaint L, Vincent A, Fischer KM, Hurt R, Schroeder DR, et al. A Midwest COVID-19 Cohort for the Evaluation of Multimorbidity and Adverse Outcomes from COVID-19. J Prim Care Community Health. 2021. Jan-Dec;12:21501327211010991. doi: 10.1177/21501327211010991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS One. 2021. Mar 16;16(3):e0248009. doi: 10.1371/journal.pone.0248009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez C, de Prost N, Fourati S, Lamoureux C, Gricourt G, N’debi M, et al. Viral genomic, metagenomic and human transcriptomic characterization and prediction of the clinical forms of COVID-19. PLoS Pathog. 2021. Mar 29;17(3):e1009416. doi: 10.1371/journal.ppat.1009416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garibaldi BT, Wang K, Robinson ML, Zeger SL, Bandeen-Roche K, Wang MC, et al. Comparison of Time to Clinical Improvement With vs Without Remdesivir Treatment in Hospitalized Patients With COVID-19. JAMA Netw Open. 2021. Mar 1;4(3):e213071. doi: 10.1001/jamanetworkopen.2021.3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giovannetti G, De Michele L, De Ceglie M, Pierucci P, Mirabile A, Vita M, et al. Lung ultrasonography for long-term follow-up of COVID-19 survivors compared to chest CT scan. Respir Med. 2021. May;181:106384. doi: 10.1016/j.rmed.2021.106384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khan MS, Dogra R, Miriyala LKV, Salman FNU, Ishtiaq R, Patti DK, et al. Clinical characteristics and outcomes of patients with Corona Virus Disease 2019 (COVID-19) at Mercy Health Hospitals, Toledo, Ohio. PLoS One. 2021. Apr 22;16(4):e0250400. doi: 10.1371/journal.pone.0250400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsai S, Nguyen H, Ebrahimi R, Barbosa MR, Ramanan B, Heitjan DF, et al. COVID-19 associated mortality and cardiovascular disease outcomes among US women veterans. Sci Rep. 2021. Apr 19;11(1):8497. doi: 10.1038/s41598-021-88111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lobelo F, Bienvenida A, Leung S, Mbanya A, Leslie E, Koplan K, et al. Clinical, behavioural and social factors associated with racial disparities in COVID-19 patients from an integrated healthcare system in Georgia: a retrospective cohort study. BMJ Open. 2021. May 19;11(5):e044052. doi: 10.1136/bmjopen-2020-044052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chatterjee A, Wu G, Primakov S, Oberije C, Woodruff H, Kubben P, et al. Can predicting COVID-19 mortality in a European cohort using only demographic and comorbidity data surpass age-based prediction: An externally validated study. PLoS One. 2021. Apr 15;16(4):e0249920. doi: 10.1371/journal.pone.0249920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yordanov Y, Dinh A, Bleibtreu A, Mensch A, Lescure FX, Debuc E, et al. ; AP-HP/Universities/Inserm COVID-19 research collaboration. Clinical characteristics and factors associated with hospital admission or death in 43 103 adult outpatients with coronavirus disease 2019 managed with the Covidom telesurveillance solution: a prospective cohort study. Clin Microbiol Infect. 2021. Aug;27(8):1158–1166. doi: 10.1016/j.cmi.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riou M, MarcoT C, Oulehri W, Enache I, Pistea C, Chatron E, et al. Respiratory follow-up after hospitalization for COVID-19: Who and when? Eur J Clin Invest. 2021. Aug;51(8):e13603. doi: 10.1111/eci.13603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei W, Sivapalasingam S, Mellis S, Geba GP, Jalbert JJ. A Retrospective Study of COVID-19-Related Urgent Medical Visits and Hospitalizations After Outpatient COVID-19 Diagnosis in the US. Adv Ther. 2021. Jun;38(6):3185–3202. doi: 10.1007/s12325-021-01742-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hou X, Tian L, Zhou L, Jia X, Kong L, Xue Y, et al. Intravenous immunoglobulin-based adjuvant therapy for severe COVID-19: a single-center retrospective cohort study. Virol J. 2021. May 21;18(1):101. doi: 10.1186/s12985-021-01575-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valverde-Monge M, Cañas JA, Barroso B, Betancor D, Ortega-Martin L, Gómez-López A, et al. Eosinophils and Chronic Respiratory Diseases in Hospitalized COVID-19 Patients. Front Immunol. 2021. Jun 2;12:668074. doi: 10.3389/fimmu.2021.668074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cosio BG, Shafiek H, Toledo-Pons N, Iglesias A, Barcelo M, Represas-Represas C, et al. Characterization of COPD Admissions During the First COVID-19 Outbreak. Int J Chron Obstruct Pulmon Dis. 2021. Jun 3;16:1549–1554. doi: 10.2147/COPD.S312493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adir Y, Humbert M, Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J Allergy Clin Immunol. 2021. Aug;148(2):361–367.e13. doi: 10.1016/j.jaci.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chaudhary S, Benzaquen S, Woo JG, Rubinstein J, Matta A, Albano J, et al. Clinical Characteristics, Respiratory Mechanics, and Outcomes in Critically Ill Individuals With COVID-19 Infection in an Underserved Urban Population. Respir Care. 2021. Jun;66(6):897–908. doi: 10.4187/respcare.08319 [DOI] [PubMed] [Google Scholar]
- 110.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020. Aug;584(7821):430–436. doi: 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abayomi A, Osibogun A, Kanma-Okafor O, Idris J, Bowale A, Wright O, et al. Morbidity and mortality outcomes of COVID-19 patients with and without hypertension in Lagos, Nigeria: a retrospective cohort study. Glob Health Res Policy. 2021. Jul 29;6(1):26. doi: 10.1186/s41256-021-00210-6 Erratum in: Glob Health Res Policy. 2021 Aug 13;6(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu YH, Wang YR, Wang QH, Chen Y, Chen X, Li Y, et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener. 2021. Jul 19;16(1):48. doi: 10.1186/s13024-021-00469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Munblit D, Bobkova P, Spiridonova E, Shikhaleva A, Gamirova A, Blyuss O, et al. Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19. Clin Exp Allergy. 2021. Sep;51(9):1107–1120. doi: 10.1111/cea.13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sandoval M, Nguyen DT, Vahidy FS, Graviss EA. Risk factors for severity of COVID-19 in hospital patients age 18–29 years. PLoS One. 2021. Jul 30;16(7):e0255544. doi: 10.1371/journal.pone.0255544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. ; Washington State COVID-19 in Pregnancy Collaborative. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021. Jul;225(1):77.e1–77.e14. doi: 10.1016/j.ajog.2020.12.1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cataño-Correa JC, Cardona-Arias JA, Porras Mancilla JP, García MT. Bacterial superinfection in adults with COVID-19 hospitalized in two clinics in Medellín-Colombia, 2020. PLoS One. 2021. Jul 13;16(7):e0254671. doi: 10.1371/journal.pone.0254671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meza D, Khuder B, Bailey JI, Rosenberg SR, Kalhan R, Reyfman PA. Mortality from COVID-19 in Patients with COPD: A US Study in the N3C Data Enclave. Int J Chron Obstruct Pulmon Dis. 2021. Aug 13;16:2323–2326. doi: 10.2147/COPD.S318000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun Y, Jiang N, Li Z, Li X, Yang B, Si D, et al. A Retrospective Study of 268 Patients with SARS-CoV-2 Infection to Evaluate the Association Between Blood Glucose and Severity of COVID-19 Pneumonia and Patient Mortality. Med Sci Monit. 2021. Aug 7;27:e932156. doi: 10.12659/MSM.932156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fernández-Martínez NF, Ortiz-González-Serna R, Serrano-Ortiz Á, Rivera-Izquierdo M, Ruiz-Montero R, Pérez-Contreras M, et al. Sex Differences and Predictors of In-Hospital Mortality among Patients with COVID-19: Results from the ANCOHVID Multicentre Study. Int J Environ Res Public Health. 2021. Aug 26;18(17):9018. doi: 10.3390/ijerph18179018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cosma S, Carosso AR, Cusato J, Borella F, Carosso M, Gervasoni F, et al. Preterm birth is not associated with asymptomatic/mild SARS-CoV-2 infection per se: Pre-pregnancy state is what matters. PLoS One. 2021. Aug 5;16(8):e0254875. doi: 10.1371/journal.pone.0254875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chudasama YV, Zaccardi F, Gillies CL, Razieh C, Yates T, Kloecker DE, et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: an observational study in the U.K. BMC Infect Dis. 2021. Sep 4;21(1):908. doi: 10.1186/s12879-021-06600-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Girkin JLN, Maltby S, Bartlett NW. Toll-like receptor-agonist-based therapies for respiratory viral diseases: thinking outside the cell. Eur Respir Rev. 2022. May 4;31(164):210274. doi: 10.1183/16000617.0274-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020. Apr 7;323(13):1239–1242. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 124.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011. Dec;128(6):1165–74. doi: 10.1016/j.jaci.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abdullah F, Myers J, Basu D, Tintinger G, Ueckermann V, Mathebula M, et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int J Infect Dis. 2022. Mar;116:38–42. doi: 10.1016/j.ijid.2021.12.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han X, Xu J, Hou H, Yang H, Wang Y. Impact of asthma on COVID-19 mortality in the United States: Evidence based on a meta-analysis. Int Immunopharmacol. 2022. Jan;102:108390. doi: 10.1016/j.intimp.2021.108390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beurnier A, Jutant EM, Jevnikar M, Boucly A, Pichon J, Preda M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J. 2020. Nov 5;56(5):2001875. doi: 10.1183/13993003.01875-2020 ; PMCID: PMC7397950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulpuru S, Li L, Ye L, Hatchette T, Andrew MK, Ambrose A, et al. ; Serious Outcomes Surveillance (SOS) Network of the Canadian Immunization Research Network (CIRN). Effectiveness of Influenza Vaccination on Hospitalizations and Risk Factors for Severe Outcomes in Hospitalized Patients With COPD. Chest. 2019. Jan;155(1):69–78. doi: 10.1016/j.chest.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 129.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006. Aug;28(2):346–51. doi: 10.1183/09031936.06.00131905 [DOI] [PubMed] [Google Scholar]
- 130.Baker JR, Mahdi M, Nicolau DV Jr, Ramakrishnan S, Barnes PJ, Simpson JL, et al. Early Th2 inflammation in the upper respiratory mucosa as a predictor of severe COVID-19 and modulation by early treatment with inhaled corticosteroids: a mechanistic analysis. Lancet Respir Med. 2022;10(6):545–556. doi: 10.1016/S2213-2600(22)00002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chhiba KD, Patel GB, Vu THT, Chen MM, Guo A, Kudlaty E, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol. 2020. Aug;146(2):307–314.e4. doi: 10.1016/j.jaci.2020.06.010 Epub 2020 Jun 15. ; PMCID: PMC7295471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lombardi C, Roca E, Bigni B, Cottini M, Passalacqua G. Clinical course and outcomes of patients with asthma hospitalized for severe acute respiratory syndrome coronavirus 2 pneumonia: A single-center, retrospective study. Ann Allergy Asthma Immunol. 2020. Dec;125(6):707–709. doi: 10.1016/j.anai.2020.07.029 Epub 2020 Aug 1. ; PMCID: PMC7395222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Klimek L, Akdis CA, Jutel M, Zuberbier T, Bousquet J. Inhaled corticosteroids in early COVID-19-A tale of many facets. Allergy. 2021;76(11):3540–3542. doi: 10.1111/all.15041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hasan SS, Capstick T, Zaidi STR, Kow CS, Merchant HA. Use of corticosteroids in asthma and COPD patients with or without COVID-19. Respir Med. 2020. Aug-Sep;170:106045. doi: 10.1016/j.rmed.2020.106045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang BZ, Chen Z, Sidell MA, Eckel SP, Martinez MP, et al. Asthma Disease Status, COPD, and COVID-19 Severity in a Large Multiethnic Population. J Allergy Clin Immunol Pract. 2021. Oct;9(10):3621–3628.e2. doi: 10.1016/j.jaip.2021.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zaki N, Alashwal H, Ibrahim S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review. Diabetes Metab Syndr. 2020. Sep-Oct;14(5):1133–1142. doi: 10.1016/j.dsx.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, et al.; OpenSAFELY Collaborative. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. 2020;8(11):1106–1120. doi: 10.1016/S2213-2600(20)30415-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mendy A, Forno E, Niyonsenga T, Carnahan R, Gasana J. Prevalence and features of asthma-COPD overlap in the United States 2007–2012. Clin Respir J. 2018. Aug;12(8):2369–2377. doi: 10.1111/crj.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.WHO global report on trends in prevalence of tobacco smoking 2015 9789241564922_eng.pdf;sequence = 1 (who.int) (Accessed February 2, 2022)
- 140.Chandrupatla SG, Tavares M, Natto ZS. Tobacco Use and Effects of Professional Advice on Smoking Cessation among Youth in India. Asian Pac J Cancer Prev. 2017. Jul 27;18(7):1861–1867. doi: 10.22034/APJCP.2017.18.7.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Office for National Statistics. Smoking prevalence in the UK and the impact of data collection changes: 2020. Available at https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/bulletins/smokingprevalenceintheukandtheimpactofdatacollectionchanges/2020#:~:text=In%20Quarter%201%202020%2C%2013.5,(around%204.9%20million%20people)(Accessed February 4, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.