Abstract
Background:
The harmful vascular effects of smoking are well established, but the effects of chronic use of electronic cigarettes (e-cigarettes) on endothelial function are less understood. We hypothesized that e-cigarette use causes changes in blood milieu that impair endothelial function.
Methods:
Endothelial function was measured in chronic e-cigarette users, chronic cigarette smokers, and nonusers. We measured effects of participants’ sera, or e-cigarette aerosol condensate, on nitric oxide (NO) and H2O2 release and cell permeability in cultured endothelial cells (ECs).
Results:
E-cigarette users and smokers had lower FMD than nonusers. Sera from e-cigarette users and smokers reduced VEGF-induced NO secretion by ECs relative to nonuser sera, without significant reduction in endothelial NO synthase mRNA or protein levels. E-cigarette user sera caused increased endothelial release of H2O2, and more permeability than nonuser sera. E-cigarette users and smokers exhibited changes in circulating biomarkers of inflammation, thrombosis, and cell adhesion relative to nonusers, but with distinct profiles. E-cigarette user sera had higher concentrations of the RAGE ligands S100A8 and HMGB1 than smoker and nonuser sera, and RAGE inhibition reduced permeability induced by e-cigarette user sera but did not affect NO production.
Conclusion:
Chronic vaping and smoking both impair FMD and cause changes in the blood that inhibit endothelial NO release. Vaping, but not smoking, causes changes in the blood that increase microvascular endothelial permeability and may have a vaping-specific effect on intracellular oxidative state. Our results suggest a role for RAGE in e-cigarette-induced changes in endothelial function.
Introduction
Smoking is a leading cause of mortality and morbidity in the US and in the world, accounting for over 140,000 cardiovascular deaths each year in the US. Public awareness of the deleterious effects of tobacco smoke on health has led many people to turn to electronic cigarettes (e-cigarettes) in the hope that they offer a less harmful alternative to smoking. However, the relative safety or danger of e-cigarette use (vaping) is a matter of fierce scientific and regulatory debate.1 A growing body of literature indicates that e-cigarette use has adverse cardiovascular effects,2–8 but the mechanism of e-cigarette-induced cardiovascular toxicity is unclear.
Cigarette smoking and e-cigarette vaping both are known to cause endothelial dysfunction. There is a growing awareness and understanding of the effects of cigarette smoke and its constituents on endothelial cells and how they cause atherogenesis.9 Smoking induces vascular dysfunction by reducing the bioavailability of nitric oxide (NO), leading to endothelial dysfunction.10 The mechanism underlying this impairment is incompletely understood. Evidence points to interference with endothelial production of NO via endothelial NO synthase (eNOS)11, 12 by one or more of the thousands of chemicals in the particulate or gas phase of smoke. Barua et al.11, 12 demonstrated that smoking causes changes in the blood that inhibit endothelial generation of NO and impair arterial flow-mediated dilation (FMD) as originally reported by Celermajer et al.13, 14 Barua et al. showed that when human umbilical vein and coronary artery endothelial cells (HUVECs and HCAECs) were incubated in serum from smokers vs. non-smokers, basal and substance P-stimulated NO production was lower with smokers’ serum. Smokers not only exhibited reduced FMD, but their serum could blunt NO generation and eNOS activity in the endothelial cells.
Similarly, the acute use of e-cigarettes adversely affects endothelial function and markers of oxidative stress in humans and rodents.4, 7, 15, 16 Lee et al.6 demonstrated toxicity and functional impairment in human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs) from acute exposure to flavored e-liquids. Moreover, smoking and vaping can increase platelet activation in young, otherwise healthy individuals.17 Together, these studies indicate that at least some of the adverse cardiovascular effects caused by smoking are also caused by e-cigarette use.
Therefore, to answer the important question of whether chronic e-cigarette use carries vascular risks similar to those of cigarette smoke, we conducted a clinical observational study to investigate the association of chronic e-cigarette use and vascular impairment on the physiological and cellular levels. Because endothelial dysfunction is an early predictor of cardiovascular diseases, we measured brachial artery FMD.18 We also performed cell culture experiments to determine the effects of chronic e-cigarette use on the NO pathway and endothelial permeability. Biomarkers of inflammation, cell adhesion, thrombosis, and tissue fibrosis were analyzed in sera of participants. To further investigate the causal factors of endothelial dysfunction, we generated condensates of e-liquids with various combinations of nicotine and flavors to determine whether any inhibitory activities within e-cigarette user serum were already present in the aerosols as opposed to having been formed de novo in the body after vaping.
Methods
All data have been made publicly available at the Dryad repository and can be accessed initially at https://datadryad.org/stash/share/90rbwOW8DllgXvmDP0PKqLLcQPy9V1ABkrjPSV9c05A and then permanently at https://doi.org/10.7272/Q6GB229G.
Population:
Participants were recruited at three institutions: University of California, San Francisco (UCSF); Boston University Medical School; and the University of Louisville. Informed Consent was provided to all study participants and all study protocols were approved by the UCSF, Boston Medical Center, and University of Louisville institutional review boards. Participants in each group were matched by age and sex to the greatest extent possible. A total of 120 healthy volunteers were recruited, with 80 recruited at UCSF Zuckerberg San Francisco General Hospital and 40 at American Heart Association Tobacco Regulation and Addiction Centers (A-TRAC) at Boston University and University of Louisville. FMD data were not available for the participants from the A-TRAC collaborators and some of the UCSF participants; serum samples were provided from participants at all three locations. Therefore, while the total number of the participants in our study was 120, the total number for FMD calculation was 51. Furthermore, because we had different volumes of serum from each participant, some of the serum experiments involved more participants than others based on sample availability; details are provided separately for each experiment. All the user/smoker study participants used one or the other product; there were no dual users and no marijuana smokers. Nicotine and cotinine levels were measured in all participants.19 Participants at UCSF or the A-TRAC centers were told not to vape, smoke, or drink coffee for 12 or 6 hours, respectively.
Inclusion Criteria:
The study included participants of both sexes, free of CVD, age 21–50 years. Inclusion criteria for e-cigarette users included current use of e-cigarettes >5 times/week for >3 months, and for cigarette smokers included current smoking of >5 cigarettes/day with ≥1 pack year. The e-cigarette users had been mainly using earlier generation e-cigarette devices rather than 4th generation types (e.g., JUUL). See Supplementary materials for complete inclusion criteria.
Exclusion criteria:
We excluded participants with diabetes, hypertension, past history of coronary artery disease and hyperlipidemia, liver disease, chronic respiratory disease, chronic kidney disease, current use of illicit drugs (by history or urine test), history of marijuana smoking, exposure to SHS, active psychiatric disease, pregnancy, or breast feeding. See Supplementary materials for complete exclusion criteria.
FMD measurement:
Vasomotor endothelial function was assessed after 15 minutes of supine rest in a 21°C room, using a standard clinical ultrasound-based method to measure FMD18. High-resolution ultrasound of the right brachial artery was performed one cm distal to the antecubital fossa with a 10 MHz linear array probe coupled to a GE Vivid 7 Imaging System and Sonosite M-turbo (FMD studies were performed only at UCSF). To assess FMD, after recording baseline B-mode ultrasound images of the brachial artery and spectral Doppler images of flow velocity, a forearm cuff was inflated to 250 mmHg for five minutes to induce transient ischemia. Immediately after deflation, Doppler images were obtained to measure reactive hyperemia.20 Digital images for FMD were analyzed by a blinded investigator with dedicated software (Information Integrity Inc.; Iowa City, Iowa) and Doppler velocity signal with NIH ImageJ. To minimize the variation in FMD during the ovarian cycle, menstruating women were tested during the first 5 days of their menstrual period.21
Blood serum collection and analysis:
Study participants abstained from vaping, smoking, and drinking coffee for 12 hours before the study visit. Blood was collected by venipuncture at fasting state from the antecubital area. Serum was prepared and samples were aliquoted and immediately stored at −80°C for subsequent assays. Serum was sent to the appropriate core lab for the measurement of nicotine and cotinine level.
Generation of e-cigarette aerosol and its condensates:
A Gram Universal Vaping Machine (Gram Research, Oakland, CA) was used to generate aerosol from an Aspire® Nautilus tank-style e-cigarette (Aspire, Shenzhen, China) and a USONICIG Zip ultrasonic vaping device (USONICIG, London, UK), with a 15-second draw of 60–80 mL puff volume every 30 seconds. The vaping devices were connected to a one-way polypropylene plastic valve connected to a 140 mL syringe with an exhale line with another one-way valve (Supp. Figure S1). With a continuous run of aerosol generation, the aerosol naturally condensed and collected in the one-way valve at room temperature. To prevent cross-contamination of different aerosols, separate syringes, one-way valves, and tubing were used for each e-liquid. Condensates of each condition were aliquoted and stored at −80°C for further analysis.
HUVEC culture:
Commercially available pooled donor HUVECs (Lonza, Basel, Switzerland) were cultured in 24-well, flat-bottom tissue culture plates with EGM-2 medium (Lonza) at 37°C in 5% CO2 and used at passages 3–6. When appropriate, cell viability was assessed with 0.4% trypan blue (Thermo Fisher Scientific, Waltham, MA).
Measurement of unstimulated and stimulated NO production:
Supp. Figure S2 summarizes our sequential cell culture conditions for studies of NO production and gene/protein expression. 24-well culture plates were coated with 0.1 mg/mL human fibronectin (Sigma-Aldrich, St. Louis, MO) by incubation at 37°C for 30 minutes. HUVECs were cultured at 20,000 cells/well and grown to confluency. A 1:1 mixture of serum from each individual participant and EGM-2 medium was added to different wells (2–3 wells/participant depending on the experiment) and incubated for 12 hours. The supernatants were harvested for measurement of NO. The cells were washed twice with Dulbecco’s PBS, and fresh EGM-2 (not containing serum samples) was added to each well followed by stimulation with 50 ng/mL recombinant human VEGF (Sigma-Aldrich) for 30 minutes, after which supernatants were harvested for measurement of stimulated NO levels. The amount of NO released by the cells before and after VEGF stimulation was measured by the chemiluminescence method using an NO analyzer (model 280i, GE/Zysense, Weddington, NC) and normalized to cell count per well. Similar experiments were performed by incubating cells in varying dilutions of e-cigarette aerosol condensates depending on the experiment.
Measurement of relative expression of NOS3 gene:
80,000 HUVECs were cultured in 24-well culture plates. Total RNA was isolated with RNeasy Mini Kit (Qiagen, Hilden, Germany) and reverse transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA). qPCR was conducted using TaqMan™ Gene Expression Master Mix with NOS3 TaqMan™ FAM-MGB Probes in a ViiA7 Real-Time PCR system (Applied Biosystems). NOS3 expression was normalized to GAPDH expression as an endogenous control.
Measurement of eNOS protein levels:
40,000 HUVECs were cultured in 24-well plates and incubated with participants’ sera for 12 hours as described above for NO measurement. The cells were lysed using Sample Diluent 1 (R&D Systems, Minneapolis, MN) and the lysates were analyzed using Human eNOS DuoSet ELISA kits (DY950-05, R&D Systems). eNOS protein levels were normalized to the number of cells plated per well.
Measurement of H2O2 in cell culture supernatant, lysed cells, and serum:
40,000 HUVECs were cultured in 24-well plates, incubated with participants’ sera for 12 hours, and lysed as described above. The concentration of H2O2 was measured in the cell culture supernatant, cell lysate, and serum using an Amplex™ Red Hydrogen Peroxide/Peroxidase Assay Kit (Thermo Fisher Scientific, Waltham, MA).
Assessment of endothelial cell permeability:
Permeability of human microvascular endothelial cells (HMVEC-Ls; PromoCell, Heidelberg, Germany) was inferred from trans-endothelial resistance (TEER) to the flow of electrical current. TEER was measured repeatedly over the timeframe of the experiment using electric cell-substrate impedance sensing (ECIS® Z-Theta system, Applied Biophysics, Troy, NY).22 A decrease in TEER reflects an increase in permeability. Briefly, 50,000 HMVEC-Ls were plated in 96-well arrays (96W20idf PET plate; Applied Biophysics) and were cultured (37°C, 5% CO2) to confluence based on reaching stable plateau in resistance measured at 4000Hz as we have previously described.23 Confluent HMVEC-L monolayers were treated with 40% (by volume) of sera of participants in EGM™-MV medium (Lonza), or with varying concentrations of aerosol condensates and 40% (by volume) of commercial human serum (Sigma-Aldrich) in EGM-MV (see Results). TEER was measured repeatedly throughout the experiment. To account for the differences in baseline resistances of each well, the TEER data was normalized to the resistance of the well immediately before the addition of the samples.
The experiments were then repeated in the presence of inhibitors as follows. For the RAGE inhibitor FPS-ZM1 (Millipore Sigma, Burlington, MA), 25 mg was dissolved in 250 μl DMSO for stock solution. For the TLR4 inhibitor TAK-242 (Cayman Chemical, Ann Arbor, MI), 5 mg was dissolved in 500 ul of DMSO for stock solution.24, 25 Cells were pretreated with inhibitors at 1 μg/mL for 1 hour, followed by replacement of media with 40% (by volume) of participants’ sera containing 1 μg/mL of each inhibitor. 200 pg/mL of recombinant human calprotectin (S100A8/S100A9) (R&D Systems) was used as a positive control. Endothelial permeability was measured as described above. We also repeated the experiment without participants’ serum samples in the presence of inhibitors and additional RAGE ligands (360 pg/mL S100A8 and 500 pg/ml HMGB1).
Measurement of circulating biomarkers:
Circulating biomarkers in participants’ sera were measured by Human Magnetic Luminex Assay (R&D Systems). Each sample was measured as recommended by the manufacturer. See supplementary materials for details of biomarkers. Calprotectin was measured using Human Calprotectin L1/S100-A8/A9 Complex ELISA Kit (Invitrogen, Waltham, MA).
Statistics:
Power calculations based on data from a previous similar study26 indicate that for an alpha of 0.05 and power of 0.8, 30 participants are sufficient to detect a significant difference in FMD between groups of 1.7 percentage points. Cardiovascular measures (e.g. FMD) were summarized with mean±SD. Histogram and Shapiro-Wilk test were used to check the normality assumption on the distributions of cardiovascular measures. An overall test among all the groups was conducted with the analysis of variance (ANOVA) for approximately normally distributed measures and Kruskal-Wallis for non-normally distributed measures. If there was a significant overall difference among all the groups, specific two-group comparisons were performed with independent t test for approximately normally distributed measures and Wilcoxon rank sum for non-normally distributed measures. Multiple variable analysis was conducted as a sensitivity analysis to examine whether or not the results may change after controlling for age, sex, and baseline measures if not balanced by group. Holm’s step-down procedure was used to compute adjusted p values for multiple comparisons. We used α=0.05 as the significant threshold. Stata 13.1 software was used for analyses.
Results
Characteristics of participants in each group are shown in Table 1. There were no significant differences observed between groups in mean body mass index, systolic and diastolic blood pressure, and heart rate. Nicotine and cotinine levels were measured in urine from all participants to confirm tobacco product use or lack thereof.
Table 1.
Characteristics of research participants in each group. Mean±SD or frequency (percentage).
| Variables | E-cigarette users (n=42) | Cigarette smokers (n=28) | Nonusers (n=50) | P value |
|---|---|---|---|---|
| Age | 29±4.6 | 34±8 | 28±4 | Overall: 0.0005 e-cigarette vs cigarette: 0.01 e-cigarette vs nonusers: 1.0 cigarette vs nonusers: 0.004 |
| Female | 10 (23.8%) | 17 (60.7%) | 20 (40.0%) | |
| Other | 2.6 | 3.7 | 6 | |
| Mean body mass index, kg/m2 | 27.7 ±4.4 | 28±6.2 | 31±12 | Overall: 0.4 e-cigarette vs cigarette: 0.6 e-cigarette vs nonusers: 1.0 cigarette vs nonusers: 0.6 |
| Systolic blood pressure, mm Hg | 113.1±12.5 | 110.±11.2 | 115±13 | Overall: 0.4 e-cigarette vs cigarette: 0.5 e-cigarette vs nonusers: 1.0 cigarette vs nonusers: 0.8 |
| Diastolic blood pressure, mm Hg | 68.6±8.3 | 69.8±8.2 | 69±6.8 | Overall: 0.6 e-cigarette vs cigarette: 0.9 e-cigarette vs nonusers: 0.7 cigarette vs nonusers: 1.0 |
| Heart rate, bpm | 61.1±9 | 63.2±8.5 | 66±12 | Overall: 0.04 e-cigarette vs cigarette: 0.07 e-cigarette vs nonusers: 0.1 cigarette vs nonusers: 1.0 |
| Duration of product use, yrs | 1.7±0.7 | 10.2±10.4 | N/A | e-cigarette vs cigarette: 0.001 |
| Cigarettes smoked per day, n | N/A | 15.5 | N/A | N/A |
| Packs per day, n | N/A | 0.77 | N/A | N/A |
| Pack years | N/A | 7.9 | N/A | N/A |
| Mean urine nicotine, ng/ml | 1135.1±1314.8 | 1659±2408.9 | 7±7.71 | Overall: 0.0001 e-cigarette vs cigarette: 0.7 e-cigarette vs nonusers: 0.0001 cigarette vs nonusers: 0.001 |
| Mean urine cotinine, ng/ml | 923±965 | 1735±1367 | 2±0.84 | Overall: 0.0001 e-cigarette vs cigarette: 0.07 e-cigarette vs nonusers: 0.0001 cigarette vs nonusers: 0.0001 |
Chronic e-cigarette and cigarette use impaired FMD:
To determine endothelial function on the physiological level, we measured brachial artery FMD in our combined cohort. FMD was reduced in both e-cigarette users and cigarette smokers relative to the nonusers (5.3±2.3% and 6.5±2.8% vs. 10.7±5.2%, respectively; Figure 1). FMD did not differ significantly between males and females (p>0.1; Supp. Figure S3A). Baseline brachial artery diameter did not differ significantly between the groups (p>0.1; Supp. Figure S3B).
Figure 1. FMD of brachial artery.
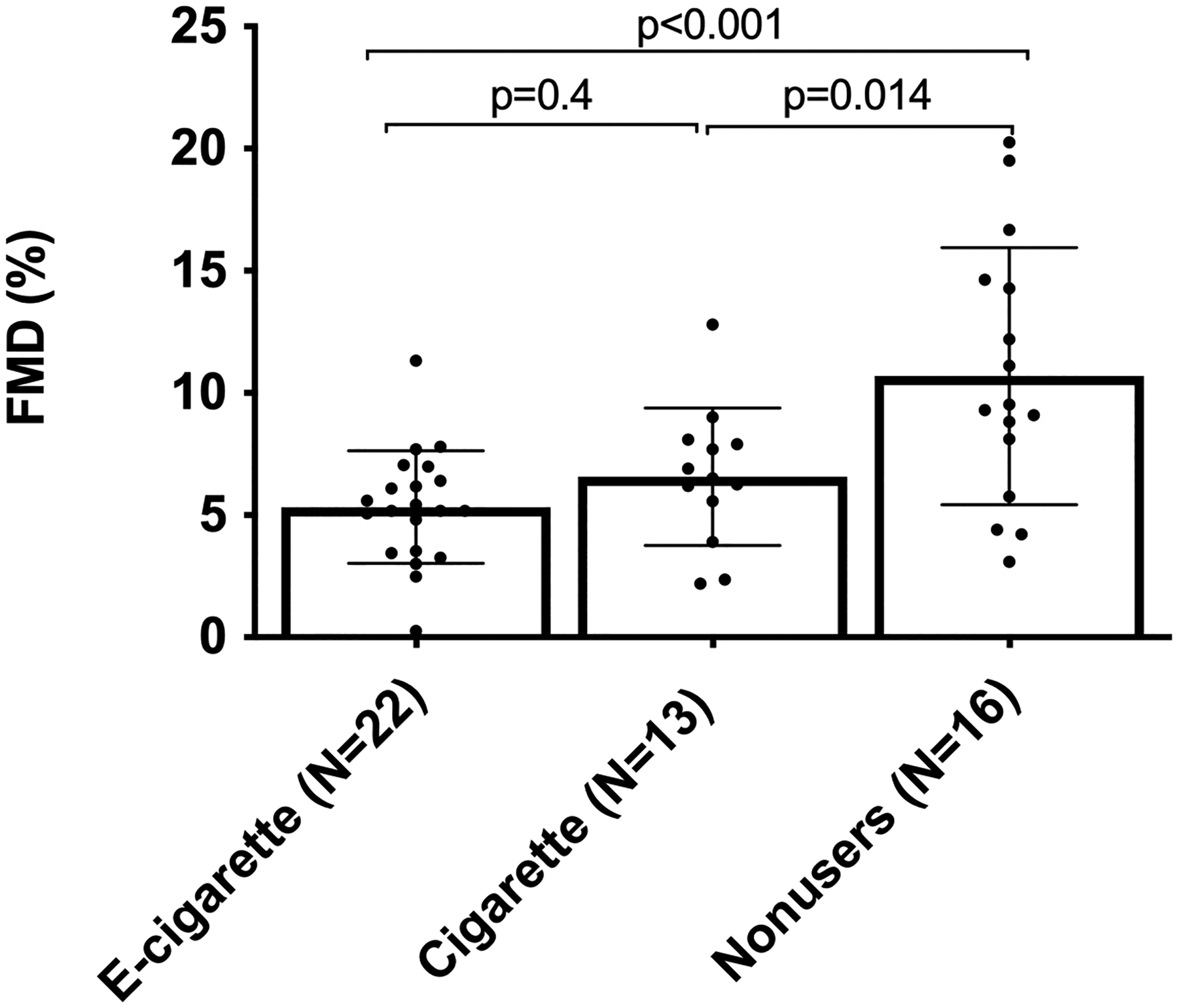
Reduced brachial artery FMD in both e-cigarette users and cigarette smokers relative to nonusers. Group means were compared by one-way ANOVA with Holm-Šidák post-hoc adjustment. Bars=SD.
HUVECs incubated with serum from e-cigarette users or smokers released less NO:
Barua et al.11, 12 demonstrated that HUVECs and HCAECs that had been incubated in serum from smokers released less NO in basal and stimulated states than those incubated in nonusers’ serum. We therefore measured NO release from HUVECs incubated individually in sera from each participant. We observed that serum from e-cigarette users and smokers caused comparable reductions in NO release by unstimulated and stimulated HUVECs (for unstimulated: 0.65±0.24 nM and 0.69±0.29 nM vs. 0.95±0.37 nM, respectively; for stimulated: 0.21±0.08 nM and 0.25±0.12 nM vs. 0.41±0.04 nM; Figure 2A). The results shown in Figures 1 and 2a indicate that clinically observed vascular dysfunction in smokers and e-cigarette users is paralleled by the inhibitory effects of serum on endothelial cell NO production, although correlation was not observed on the per-participant level by Pearson analysis (r=0.2; not shown). We determined cell viability after treatment with serum samples with trypan blue as being >95%.
Figure 2. Effects of user sera (every participant) on NO production and eNOS gene expression.
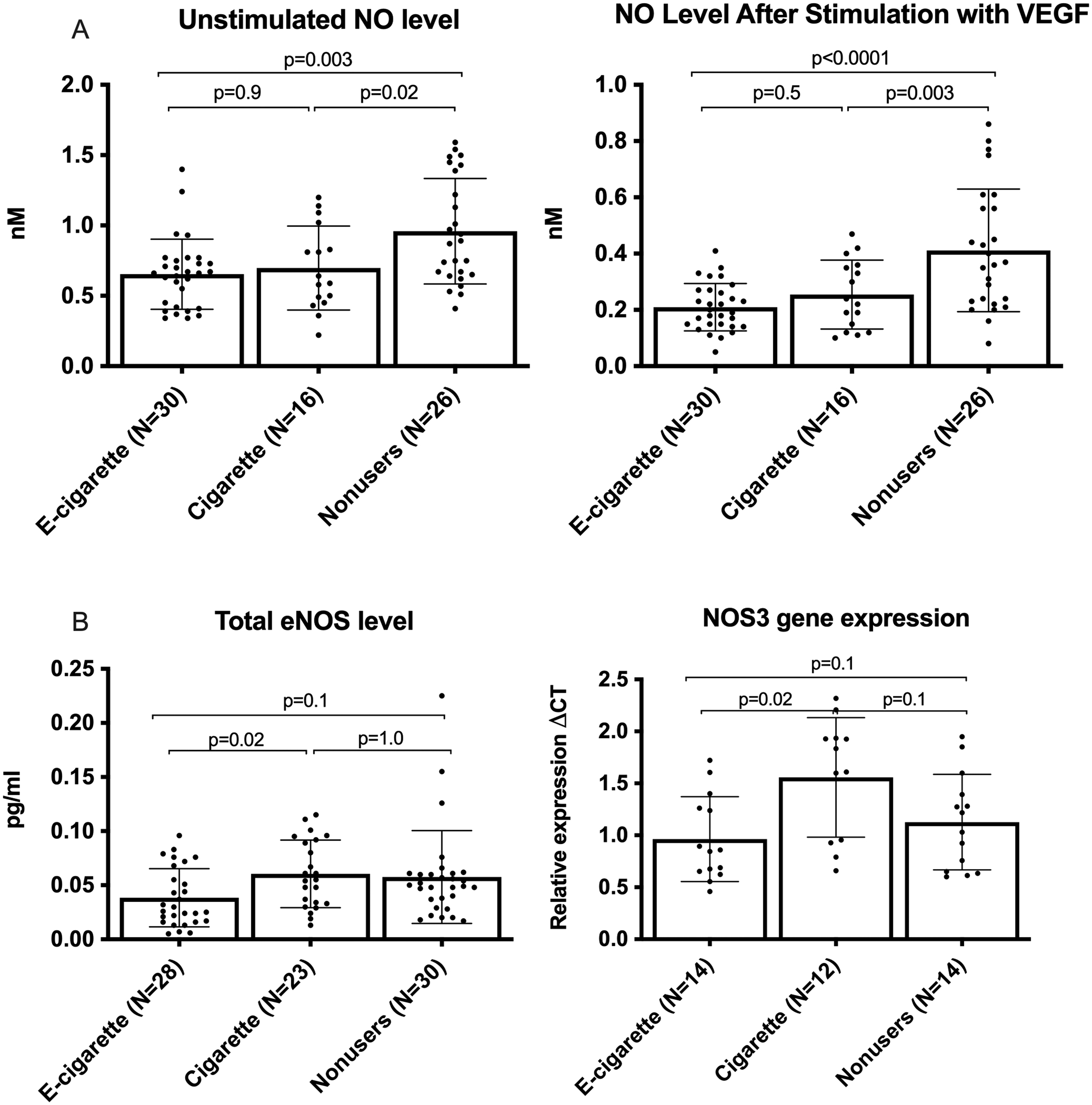
(A) NO level released from cultured HUVECs before and after stimulation with VEGF. Stimulated values are lower than unstimulated values due to a 30-minute stimulated collection period vs. 12 hours for basal conditions. (B) eNOS protein and gene expression in HUVECs treated with individual serum samples. eNOS protein and gene expression were significantly lower in the e-cigarette group relative to smokers but not nonusers. There were 3 replicates per participant for NO and NOS3 measurements, and 2 replicates per participant for eNOS protein measurements. Group means were compared by one-way ANOVA with Holm-Šidák post-hoc adjustment. Bars=SD.
Sensitivity multiple variable analysis:
Because we observed differences in FMD and NO production in the smoker group, for which age and sex slightly varied from the other groups in ways that may affect those outcomes, analysis of covariance was used to compare FMD and NO production after stimulation among tobacco use groups. After controlling for age, sex, and baseline cardiovascular measures, there was consistently significant difference in FMD (p=0.0014) and NO production (p=0.0093) after stimulation among smokers, e-cigarette users, and nonusers. Specifically, smokers and e-cigarette users had significantly lower FMD than nonusers, respectively (adjusted p=0.0496 for smokers vs. nonusers, 0.0020 for ecig vs. nonusers) and significantly lower NO production after stimulation than nonusers (adjusted p=0.0496 for smokers vs. nonusers, 0.0093 for ecig vs. nonusers). That is, sensitivity analysis with multiple variables controlled showed a similar pattern in cardiovascular measures. Neither FMD nor post-stimulation NO production were significantly affected by age or sex (FMD: p=0.5137 for age and 0.3511 for sex; NO production: p=0.5778 for age and 0.0728 for sex).
No clear difference in levels of eNOS protein and NOS3 mRNA in HUVECs incubated with sera from e-cigarette users or cigarette smokers versus sera from nonusers:
Because sera from e-cigarette users or from smokers reduced NO release relative to nonuser sera, we asked if this reduction reflected changes in expression of eNOS on the protein or gene level. The cells treated with sera from e-cigarette users contained significantly less eNOS protein and NOS3 mRNA than those treated with sera from cigarette smokers; however, neither user group differed significantly from the nonuser group despite a trend toward lower eNOS (Figure 2B).
Physiological dose of e-cigarette aerosol condensates did not directly inhibit VEGF-stimulated endothelial NO production in HUVECs:
The rationale for treating endothelial cells with individual participants’ serum samples was to bathe the cells in a medium reflecting the participants’ own blood milieu, including not just substances in smoke or aerosol that reach the blood, but also factors made by the body in response to their chronic inhalational exposures. To explore which of those alternatives might be responsible for the functional changes we had observed, we next treated HUVECs with condensates of e-cigarette aerosol diluted in growth medium to see if any components of the aerosols could directly mimic the ability of serum to decrease NO release. Condensates of aerosols were used instead of the e-liquids themselves to ensure that chemical reaction products generated during the heating process (e.g., acrolein, formaldehyde, etc.27) were included in the mixture. In each set, the e-liquids consisted of 67% propylene glycol (PG) and 33% vegetable glycerin (VG) for Aspire, and 40% PG / 60% VG for the USONICIG Zip (concentrations optimized for each device); with the following variations: for both devices, with and without nicotine (12 mg/ml free-base form); and for Aspire, with or without each of four common flavorants (vanillin, cinnamaldehyde, menthol, or ethyl maltol),28 all at 2 mg/mL.29 The USONICIG device uses vibration to aerosolize rather than a heating coil (negative control for heating). Culture medium alone was a negative control for aerosol condensates.
0.3% v/v dilution of the condensates (41.25 mM for PG+VG), the condition used by Lee et al.6, significantly decreased VEGF-stimulated NO release relative to the culture medium control, regardless of flavors, nicotine, or heating coil, whereas no significant differences between condensates and culture medium were detected under unstimulated conditions (Supp. Figure S4). There was no difference in cell viability when confirmed by CCK-8 in all the conditions except menthol+nicotine condensate, which showed decreased, but not significant, cell viability (Supp. Figure S5).
However, since 41.25 mM of aerosol condensate was not necessarily representative of the circulating levels in vapers, we repeated the study with a physiologically relevant concentration. We determined a reasonable physiologic level of PG/VG in the circulation to be ~0.1 mM (see Discussion). At this concentration, no significant change (p>0.3) was observed in unstimulated or stimulated states compared to culture medium control (Figure 3). The lack of effect on NO release at physiologically realistic levels of aerosol condensate suggests that the circulating chemicals from e-cigarette devices may not play a direct role in the impairment of endothelial NO production.
Figure 3. NO release from cells treated with e-liquid aerosol condensates at estimated physiological circulating level (0.1 mM).
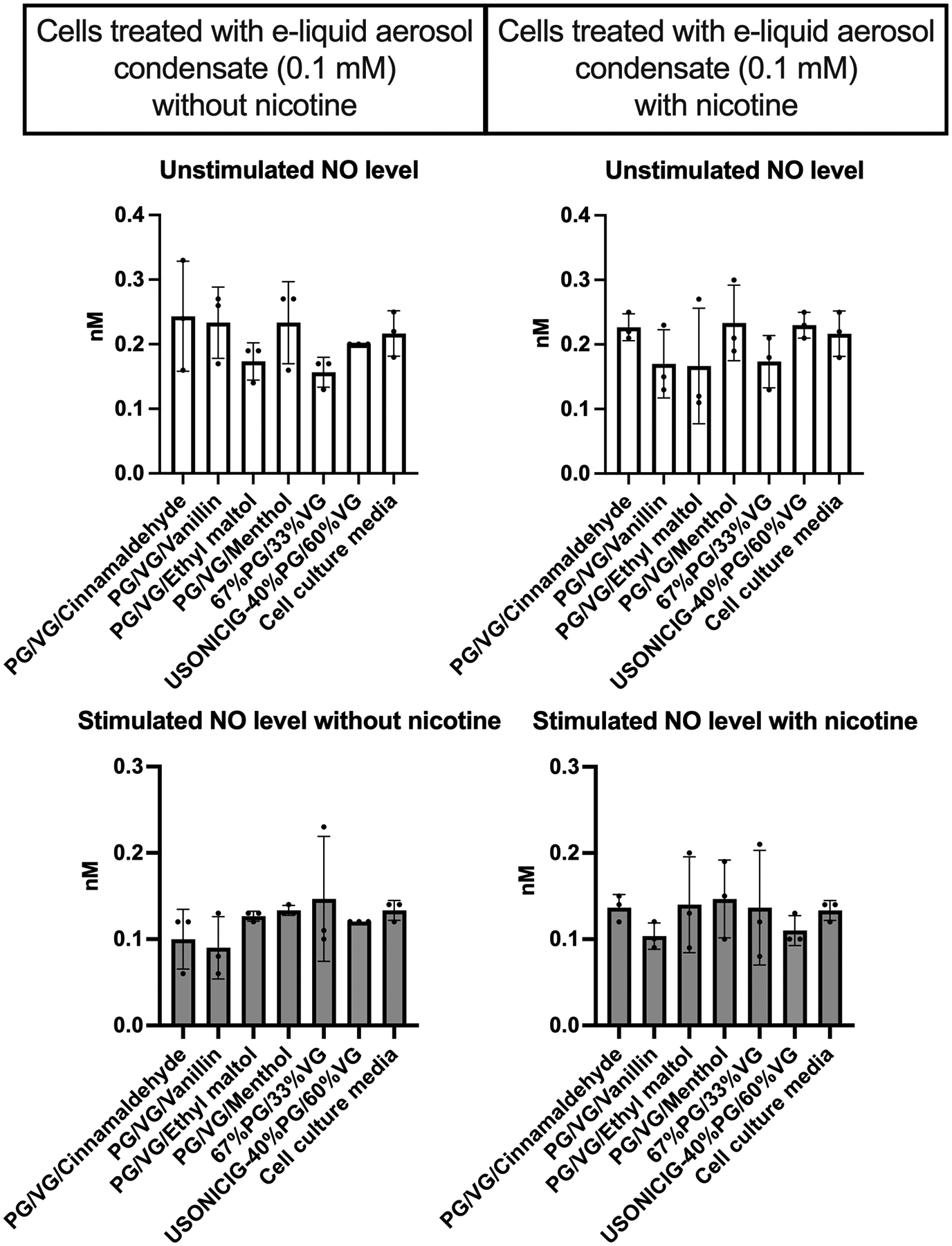
Aerosol condensates did not significantly change NO production under basal or stimulated conditions (p<.05 required for significance; all p values were >0.3). Group means were compared by Kruskal-Wallis test with Dunn’s post-hoc adjustment. Each condition was run in triplicate. Bars=SD.
Cell culture supernatant from HUVECs exposed to serum from e-cig users contained significantly higher levels of H2O2 than nonusers:
As shown in Figure 4, H2O2 level was significantly higher in supernatant of HUVECs exposed to serum from ecig users. No differences were observed in H2O2 level in the serum samples themselves, or in lysed cells, suggesting that serum from e-cigarette users caused reactive oxygen species (ROS) production in naïve HUVECs detectable as increased H2O2 secretion. Limited availability of serum from the cigarette smokers prevented us from including that group in this experiment; therefore, we do not know if this effect is specific to e-cig users.
Figure 4. Significant increase in H2O2 level in cell culture supernatant exposed to e-cigarette users sera.
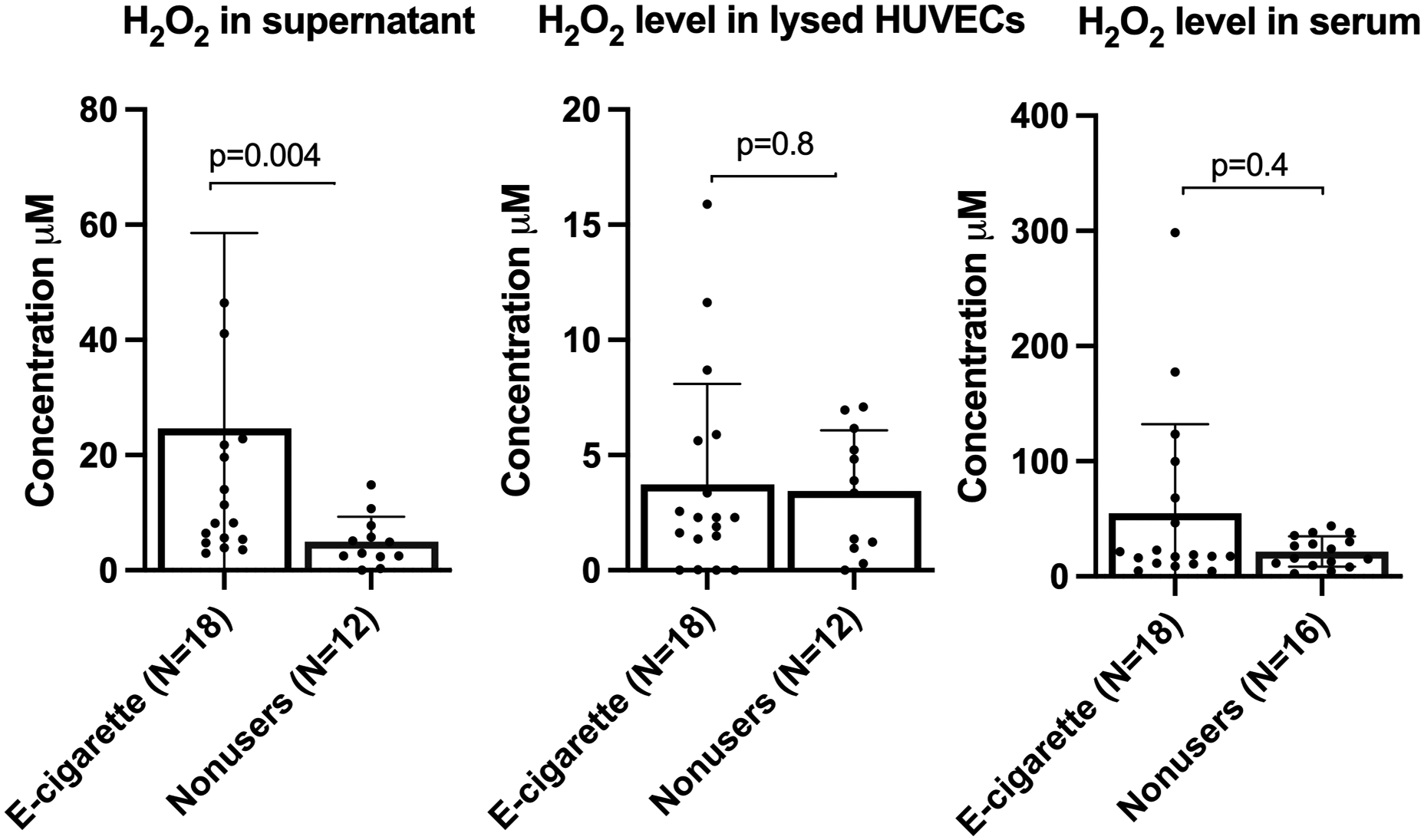
No significant changes in H2O2 level was observed in participants serum or lysed cells of all groups. Group means were compared by Mann-Whitney test. Bars=SD.
Microvascular endothelial cells exhibit greater permeability after incubation with sera from e-cigarette users than from smokers or nonusers:
We explored the effects of sera from the user groups vs. nonusers on endothelial cell permeability by measuring TEER (normalized resistance) of HMVEC-Ls, which are a physiologically relevant cell type. As compared with the culture medium control, treatment with all sera led to decreased TEER, which reflects increased permeability. Notably, serum samples from the e-cigarette users induced a larger increase in permeability than sera from the smokers or nonusers after 17 hours of incubation (Figure 5; lower numbers correspond to greater permeability; p<0.05).
Figure 5. Increased microvascular endothelial permeability induced by e-cigarette user sera (every participant).
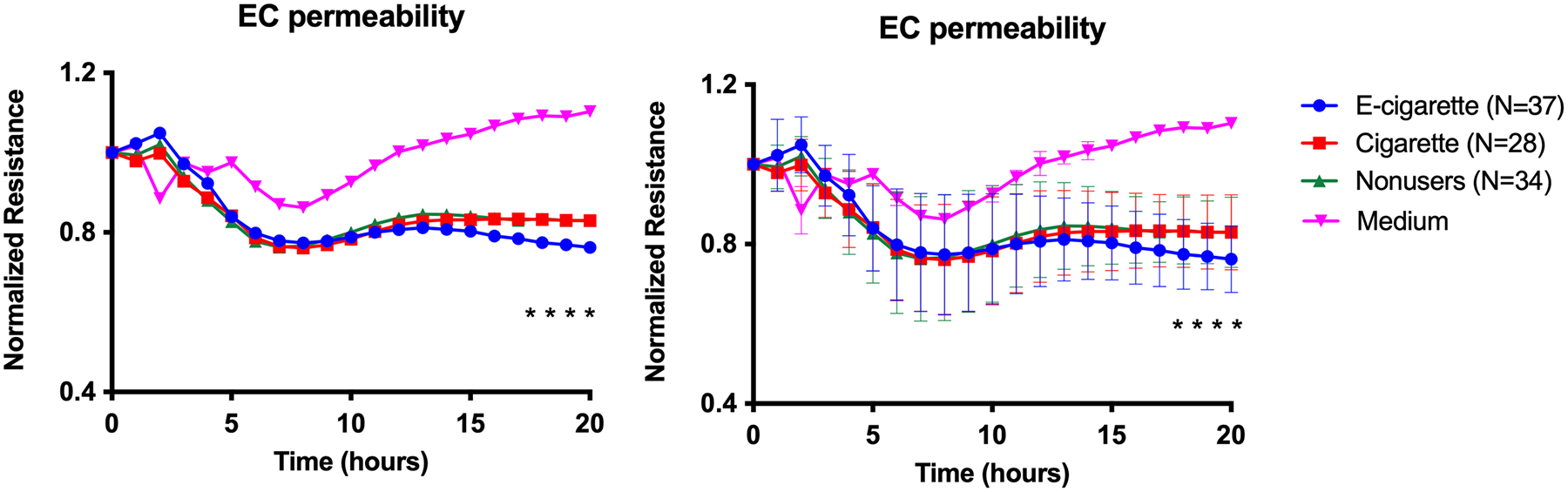
Graph shows resistance across a monolayer of HMVEC-Ls; lower resistance values correspond to greater permeability. *p<0.05 between e-cigarette user group compared to cigarette smoker and nonuser groups starting at 17 hours of incubation. There were 3 replicates per participant. Group means at each timepoint were compared by repeated measures ANOVA with Holm-Šidák post-hoc adjustment. Bars=SD; the graphs are equivalent but error bars have been omitted from one of them to enhance readability.
Physiological levels of e-cigarette aerosol condensates did not directly alter HMVEC-L permeability:
To determine whether the increased permeability caused by sera from e-cigarette users in Figure 4 is a direct effect of aerosol chemicals that reach the circulation, or an indirect response mediated by the pulmonary epithelium, we repeated the HMVEC-L permeability experiment with same e-liquid aerosol condensates used in the earlier HUVEC NO release experiment shown in Figure 3. Treatment with 0.1 mM aerosol condensate with or without serum in the medium (Figure 6 and Supp. Figure S6) did not alter cell permeability. In fact, incubation of HMVEC-Ls with 41.25 mM e-cigarette aerosol condensates from most e-liquids, with and without nicotine, decreased cell permeability (Supp. Figure S7), in contrast to the increased permeability caused by incubation with e-cigarette user serum) (Figure 5). The exception was menthol + nicotine, which increased permeability while also reducing cell viability. There was no significant decrease in cell viability when confirmed by CCK-8 in all the conditions except menthol + nicotine condensate (Supp. Figure S5).
Figure 6. Endothelial cell permeability was not changed in cells treated with e-liquid aerosol condensate (0.1 mM).
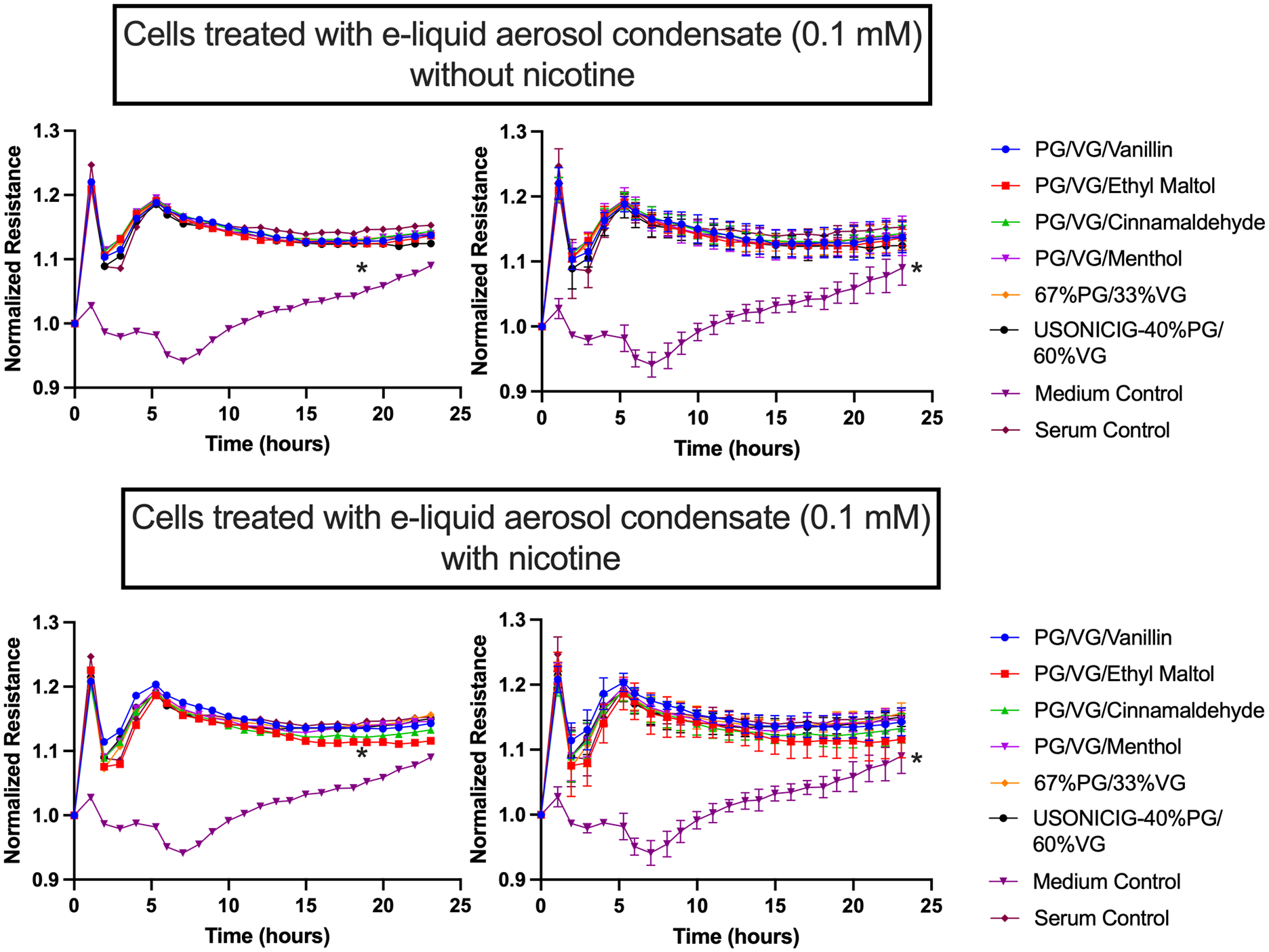
Graphs show resistance across a monolayer of HMVEC-Ls; lower resistance values correspond to greater permeability. There were no significant differences in all comparisons except for Medium Control versus all other conditions (*p<0.05). Each condition was run in triplicate. Group means at each timepoint were compared by repeated measures ANOVA with Holm-Šidák post-hoc adjustment. Bars=SD; the left hand and right hand graphs are equivalent but error bars have been omitted from the left hand graphs to enhance readability.
Chronic e-cigarette users have increased circulating levels of endothelial cell adhesion and inflammatory biomarkers:
To assess potential changes in the inflammatory, thrombotic, or fibrotic states of the research participants, we analyzed a panel of biomarkers in all the serum samples (Figure 7). S100A8, HMGB1, IFN-β, soluble ICAM-1, vWF, and myeloperoxidase (MPO) were unchanged in smokers but were substantially higher in e-cigarette users than in the other groups. Conversely, some biomarkers that were unchanged in e-cigarette users were elevated in cigarette smokers, such as IL-1β (trend as p=0.06), RAGE, and soluble PECAM-1. There were no significant differences in serum levels of the following biomarkers: GM-CSF, IL-6, IL-8, IL-10, CRP, S100A12, tissue factor, serpin E, CCL2, THPO, D-Dimer, soluble VCAM-1, TGF-β, Angiopoietin-1/−2, VEGF, S100A9, and TNF-α (Supp. Figure S8–S9). We observed a significant increase in the level of RAGE ligands S100A8 and HMGB1 in serum from e-cigarette users compared to the other groups.
Figure 7. Circulating biomarkers.
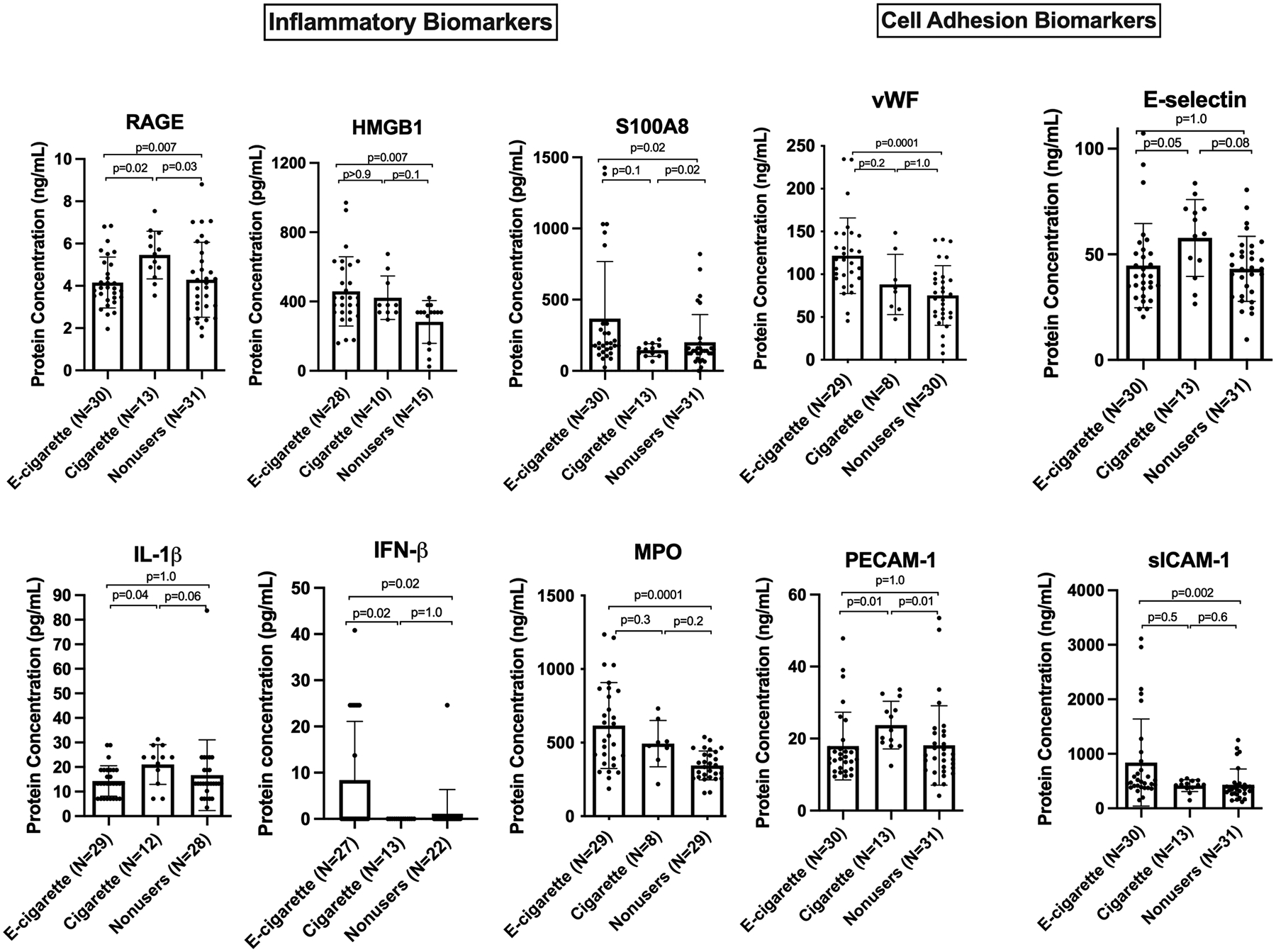
Chronic e-cigarette use led to changes in inflammatory and cell adhesion biomarkers relevant to endothelial dysfunction. Group means were compared by Kruskal-Wallis test with Dunn’s post-hoc adjustment. Bars=SD. (The mean level of IFN-β in cigarette smokers was below detection with one outlier with value >500 removed, and in nonusers, except for one person, it was below detection as well).
Measurement of endothelial cell permeability and NO level with RAGE and TLR4 inhibitors:
While e-cigarette user’s sera increased permeability of HMVEC-Ls (Figure 5), direct exposure to e-cigarette aerosol condensates at physiologically relevant concentrations did not increase HMVEC-L permeability (Figure 6). Therefore, we hypothesized that the permeability increasing effects of serum from e-cigarette users might be mediated by circulating factors and not specific components of the original aerosol. We focused on the RAGE pathway because its activation modulates cell permeability, NO production, and eNOS activity;30, 31 and because we observed that the RAGE ligands S100A8 and HMGB1 are elevated in e-cigarette user sera (Figure 7). We assessed the effects of a RAGE inhibitor on the permeability enhancing effects of serum from e-cigarette users on HMVEC-L permeability. We also tested the effects of a TLR4 inhibitor because many ligands of RAGE also interact with TLR4.32 Figure 8A shows that RAGE inhibition partially prevented the increased permeability caused by e-cigarette user serum starting at 16 hours. In contrast, we observed that TLR4 inhibition had no effect on e-cigarette user serum-induced HMVEC-L permeability. The simultaneous inhibition of TLR4 and RAGE led to a trend towards reduced permeability, but significance was not reached for reasons that are unclear. Notably, although RAGE inhibition limited the increased permeability of HMVEC-Ls caused by sera from e-cigarette users, RAGE inhibition had no effect on permeability with sera from cigarette smokers or nonusers (Figure 8B,C). Moreover, we observed that the administration of calprotectin (S100A8/S100A9) increased endothelial permeability, which was reversed by the inhibition of RAGE (Figure 8D). However, there was no significant difference in the level of S100A9 and calprotectin between sera of e-cigarette users, cigarette smokers, and nonusers (Supp. Figure S8, S10). Together, these results indicate a crucial role for the RAGE pathway in the increased endothelial permeability resulting from e-cigarette use but not from cigarette smoking.
Figure 8. Inhibition of RAGE reduces the otherwise increased permeability from exposure to sera from e-cigarette users, but not from cigarette smokers or nonusers.
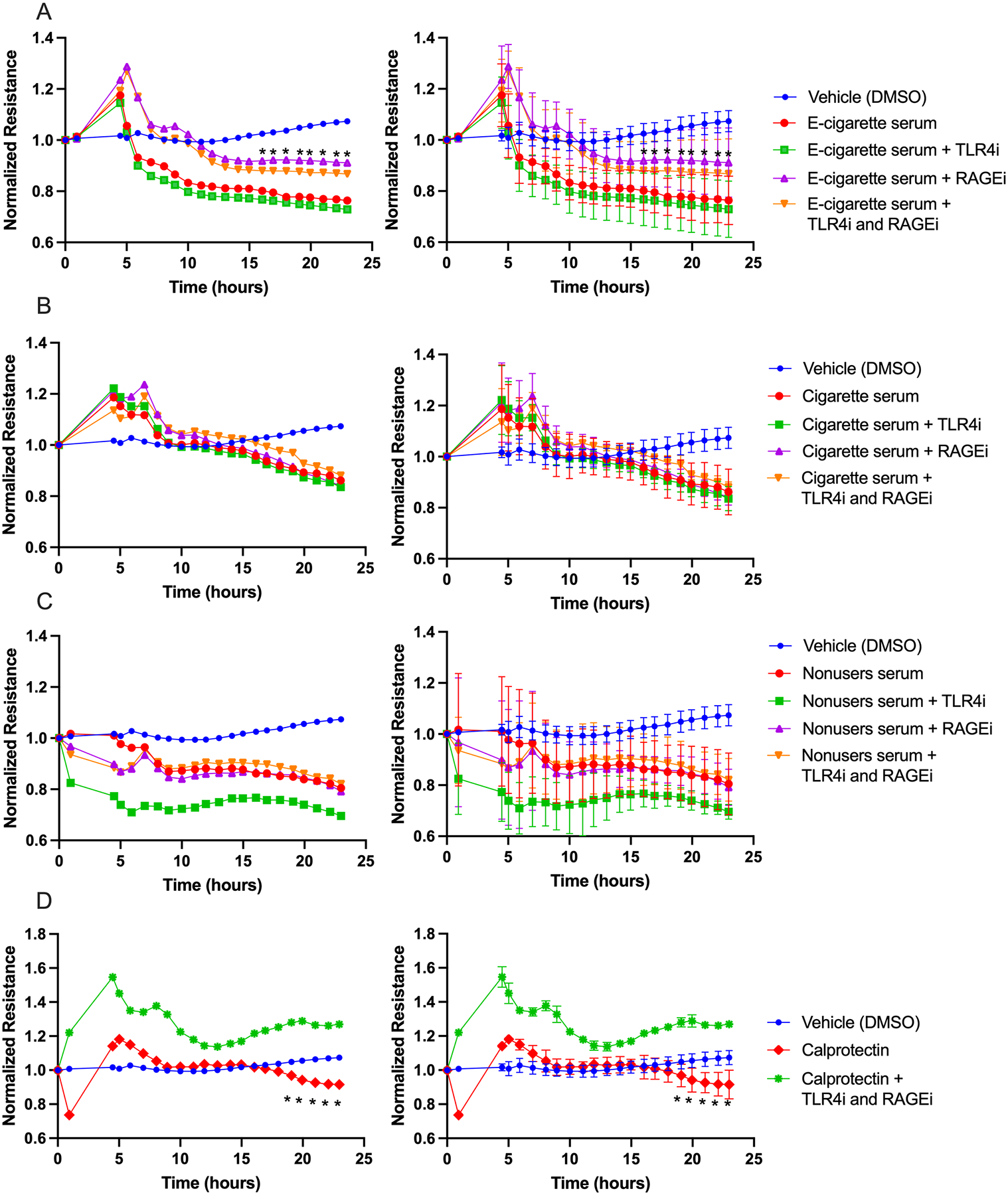
Graphs show resistance across a monolayer of HMVEC-Ls; lower resistance values correspond to greater permeability. (A) Exposure to e-cigarette user serum with and without inhibitors (*p<0.05 between e-cigarette serum and e-cigarette serum + RAGEi). (B) Similar exposure to cigarette smoker serum (no significant differences). (C) Similar exposure to nonuser serum (no significant differences). (D) With no serum, calprotectin led to increased cell permeability, which was reversed by adding both inhibitors (*p<0.05 between cells treated with only calprotectin and those treated with calprotectin and both inhibitors). For A-C, there were 10 participants per condition and 1 well per participant. For D, there were 3 replicates per condition. Group means at each timepoint were compared by repeated measures ANOVA with Holm-Šidák post-hoc adjustment. RAGEi = RAGE inhibitor, TLR4i = TLR4 inhibitor. Bars=SD; the left hand and right hand graphs are equivalent but error bars have been omitted from the left hand graphs to enhance readability.
After observing the above results and confirming the role of the RAGE pathway in endothelial cell permeability, it made sense to explore whether the RAGE pathway also has a role in e-cigarette-induced change in endothelial cell NO production. We repeated the NO release experiment as in Figure 2A, incubating HUVECs in several remaining e-cigarette user serum samples with addition of treating HUVECs with RAGE inhibitor or TLR4 inhibitor or both or DMSO for 1 hour as described earlier. Neither inhibitor significantly altered NO release (Supp. Figure S11).
To determine how individual RAGE ligands influence cell permeability, we treated cultured HMVEC-Ls with S100A8 or HMGB1 without study participant serum. The cells treated with each RAGE ligand had significantly greater cell permeability compared to the cells treated with medium alone (Figure 9A). In a different experiment, cells were treated with either RAGE ligand at the average concentrations at which they had been detected in e-cig user serum (see Figure 7), followed by RAGEi, TLR4i, or both inhibitors. In wells treated with S100A8 followed by the inhibitors, those treated with both inhibitors showed a significant decrease in permeability compared to other wells (Figure 9B). Cells treated with HMGB1 followed by both inhibitors or only RAGEi showed significantly less permeability than cells treated with media or TLR4i. (Figure 9C). Treatment of cells with inhibitors without RAGE ligands led to no significant changes in cell permeability compared to culture medium alone (Figure 9D). These results suggest that the RAGE ligands HMGB1 and S100A8 have substantial roles in vaping-induced cell permeability at the physiological level.
Figure 9. Ligands of RAGE (S100A8 and HMGB1) increase cell permeability. RAGEi and TLR4i reduced cell permeability caused by the RAGE ligands.
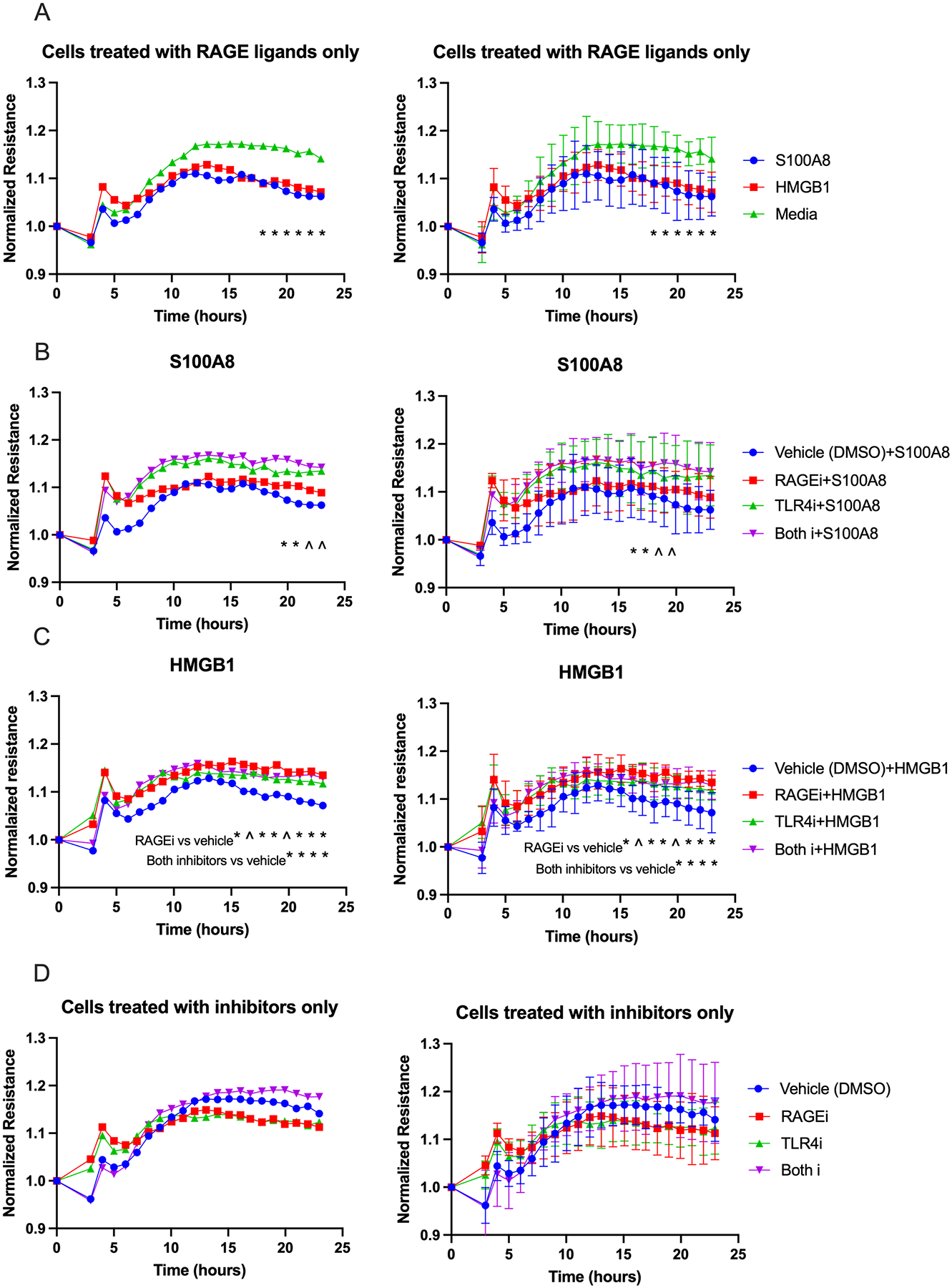
Graphs show resistance across a monolayer of HMVEC-Ls; lower resistance values correspond to greater permeability. (A) Cells treated with each RAGE ligand alone had significantly greater permeability compared to the control starting at 17 hours of exposure (*p<.0.05 for media vs. S100A8 or HMBG1). (B) Cells treated with S100A8 followed by both inhibitors showed significant decrease in permeability starting at 19 hours of exposure compared to cells treated with each inhibitor or vehicle (*significant at p<.05 for both inhibitors vs. all other conditions; ^0.05<p<0.06 is also shown for reference). (C) Cells treated with HMGB1 followed by RAGEi showed a significant reduction in permeability compared to vehicle starting at 15 hours of exposure, and starting at 19 hours of exposure when treated with both inhibitors (see notes on graph; *significant at p<.05; ^0.05<p<0.06 is also shown for reference). (D) Inhibitors alone did not change cell permeability. Group means at each timepoint were compared by repeated measures ANOVA with Holm-Šidák post-hoc adjustment. RAGEi = RAGE inhibitor, TLR4i = TLR4 inhibitor. Bars=SD; the left hand and right hand graphs are equivalent but error bars have been omitted from the left hand graphs to enhance readability.
Discussion
Our findings offer important insight about a timely and much debated issue of public health and regulatory policy: whether incomplete switching from smoking to e-cigarette use (resulting in dual product use) leads to reduced harm. This is a common assumption by the public, and this assumption is present in the FDA Marketing Granted Orders for RJ Reynolds’ Vuse, Logic, and NJOY e-cigarette and heated tobacco products.33, 34 We found that chronic e-cigarette use and smoking resulted in comparable impairment of vascular endothelial function and in altered serum that blunted NO production from endothelial cells. However, the distinct patterns of circulating CVD risk biomarkers from chronic users of each product indicate that despite the similar physiological effects, e-cigarette use and smoking trigger fundamentally different molecular responses. These differences indicate that at least for vascular health, dual product use may not result in reduced harm, and may actually be worse. This important concept is bolstered by the finding that chronic e-cigarette use, but not smoking, resulted in serum that increased microvascular permeability, an example of adverse endothelial consequences specific to vaping that may further augment the vascular harm of dual use vs. exclusive smoking.
Celermajer et al.14 showed that chronic smokers have impaired brachial artery FMD relative to non-smokers, a finding that has been reproduced in most, but not all, subsequent studies.2, 3, 11, 12 While several studies in humans and animals indicate that acute exposure to e-cigarette aerosol with or without nicotine can lead to reduced FMD and increased vascular stiffness,2, 4, 15, 16 our results indicate that chronic vaping reduces FMD as much as chronic smoking. Brachial artery FMD correlates closely with coronary endothelial function and predicts long-term risk of CVD events,35 and an absolute reduction in FMD of 2% (i.e., 2 percentage points, FMD units) has been associated with a 15% increase in CVD risk.36 In this study, FMD was significantly lower in both e-cigarette users and cigarette smokers relative to nonusers by more than 5%, suggesting that the effect is clinically significant. Conclusions from studies of the association of chronic e-cig use and cardiovascular disease in humans have been mixed,37–48 our study is important in showing a clear increase in early indicators of cardiovascular risk in otherwise healthy vapers.
To focus our study on the differences in how individual participants’ sera influenced endothelial cells in culture, we did not take endothelial cells from study participants, but instead used commercially available HUVECs and HMVEC-Ls. This provided a common pool of cells from multiple donors and was consistent with the original conditions of Barua et al. in their study of smokers’ serum.11
Because HUVECs are not from a typical adult conduit vessel, their use as an endothelial cell model is not always appropriate. For some applications, the use of cells derived from a major adult vessel like HCAECs is a more relevant model. However, Barua et al. researched the differences between HUVECs and HCAECs in their original seminal studies of how smokers’ serum impairs endothelial NO release in culture, and they reported similar changes in NO secretion and eNOS level in both cell types,11, 12 validating the use of HUVECs for such studies, especially in a large cohort study like this in which the large number of assays made use of HCAECs economically less feasible. It has also been reported that the two cell types respond similarly to mediators of inflammation and activation, although responsiveness of HCAECs tended to be higher.49 Moreover, the umbilical vein, which serves as a conduit feeder vessel to the fetus, undergoes endothelium-dependent vasodilatory vascular reactivity similar to that of adult arteries,50 further validating the use of HUVECs as a relevant in vitro model of reactive endothelium.
It is notable that the decrease in cultured endothelial cell NO release caused by incubation in smoker serum (relative to nonuser serum) also resulted from incubation in e-cigarette user serum, and that the decrease in NO release reflected a similar decrease in FMD of the serum donors. Barua et al.11, 12 reported that the reduction in NO release involved an increase in oxidative stress. We did not detect a clear difference in eNOS protein and NOS3 mRNA levels in the cells treated with e-cigarette user serum relative to nonuser serum, indicating post-translational regulation presumably at the level of eNOS activation, eNOS uncoupling, or NO destruction or scavenging. Due to limited serum sample volumes, we were unable to study their effects on phosphorylated (activated) eNOS protein level in incubated cells. Therefore, despite a difference in means between the e-cigarette and nonuser groups that was comparable to the difference in NO production, the specific effect on eNOS remains inconclusive.
The use of individual serum samples from vapers ensured that the cultured endothelial cells were exposed both to aerosol components and to circulating factors produced in response to inhalational exposure. Our experiment to determine if aerosol components themselves can lower NO production, although not directly physiologically relevant, is informative regarding theoretical ability of any aerosol component to directly mimic this effect of serum incubation. It been shown that some common e-liquid flavorants can impair the functional properties of endothelial cells regardless of the presence or absence of PG/VG.2, 51 Rather than exposing the cells directly to flavors, as is a common approach,2, 6 we made condensates of aerosols from e-liquids containing or lacking individual flavors, nicotine, and heating coil reaction products (via use of the ultrasonic device) and exposed HUVECs to the condensates in culture. Notably, unheated PG/VG (USONICIG) alone was able to mimic the effect of the serum incubation.
The exact circulating levels of e-cigarette aerosol components after vaping sessions are unknown. However, the fasting serum level of glycerol is reported to be 0.003 mM – 0.01 mM.52, 53 The baseline level of PG in former smokers is undetectable,54 but a single session of vaping without nicotine resulted in an average serum PG concentration of 0.2 mM and 0.15 mM after 30 minutes and 150 minutes of the exposure respectively.54 While the participant samples in our study were collected before JUUL was popular, the known properties of JUUL pods enabled the following rough theoretical yield calculation for PG and VG inhaled from one session of JUUL use:55, 56 maximum of 0.043 mM of PG and 0.086 mM of VG would appear in the serum assuming 100% of aerosol absorption through the lungs (see supplement).
Cigarette smoking leads to increased expression of several types of endothelial cell activation and adhesion proteins,57 and certain inflammatory proteins are elevated in immune cells of tobacco smokers but not e-cig users.58 Interestingly, in this study, we observed that chronic e-cigarette use also alters the expression profile of relevant proteins, but with relatively little overlap with those increased by smoking. This is consistent with the growing awareness that vaping and smoking involve overlapping potential health risks such that dual use may be more risky than either smoking or vaping alone.59
Notably, we observed a significant increase in levels of circulating ICAM-1 and S100A8 proteins in serum from e-cigarette users. ICAM-1 facilitates inflammation by promoting leukocyte recruitment, and the extravasation cascade through injured endothelium and increased ICAM-1 level increases endothelial leakiness.60 Moreover, soluble ICAM-1 is associated with lung injury and increased vascular permeability in rats. S100A861 is a ligand that induces RAGE activity and plays a role in trans-endothelial migration and cell permeability.31 HMGB1 is another RAGE ligand and a cytokine that has an important role in mediating inflammatory responses.62 Although we found no significant increase in calprotectin and S100A9 levels, serum levels of S100A8 and HMGB1 were significantly increased in e-cigarette users and it is known that S100A8 alone can increase HUVEC permeability.63 We confirmed the relationship between e-cigarette use and the level of RAGE or its ligands HMGB1 and S100A8 in sera by our findings that endothelial permeability relies on activation of the RAGE pathway and can be partially prevented by inhibition of RAGE, whereas RAGE ligands alone significantly increased permeability, an effect that was reversed by RAGEi. These findings suggest a potential link between vaping and disruptions in the control of what can cross from the airways into the lung capillary bed.
We observed substantially higher MPO and vWF levels in sera of e-cigarette users. vWF is produced mainly by endothelial cells and platelets, and increased vWF level is strongly linked to atherosclerosis, blood hypercoagulability, and thrombosis.64 The increased platelet aggregation from acute e-cigarette exposure in mice further supports the link between vWF and e-cigarette use.15, 65 MPO causes endothelial dysfunction by decreasing NO availability and increasing oxidative stress,65 and plays a role in atherosclerosis and reperfusion/ischemia injuries. Our findings of elevated MPO level may explain the impaired NO production in HUVECS exposed to sera from e-cigarette users.
H2O2 is a ROS molecule and another important mediator of endothelial cell dysfunction. H2O2 impacts endothelial function in cultured cells via the Fas signaling pathway,66 and cellular oxidative stress in immune cells is elevated not only but also in non-users after a single vaping session.67 In this study we found that HUVECs that were exposed to serum from e-cigarette users produced significantly more H2O2 than cells exposed to serum from nonusers, with no significant differences in the serum samples themselves. These findings suggest that the serum from e-cigarette users causes an increase in endothelial oxidative stress, leading to endothelial dysfunction and an increased risk of future cardiovascular events.
Our study has many strengths and some limitations. A limitation is that the FMD results were aggregated from similar cohorts in two different UCSF sites in San Francisco; and were measured and interpreted by different FMD experts, potentially increasing variability. However, we used the same standardized FMD procedure and analysis software, minimizing the potential added variability. The blood samples from study participants are subject to the same variability. Biomarkers of cardiovascular health and functional properties of the endothelium may be influenced by geographic variabilities in the climate, air pollution level, socioeconomic status, etc.; but this variability also results in a more generalizable sample of the population. Most of the e-cigarette users were using earlier generation products than those that are currently the most popular.
Conclusions
Chronic e-cigarette use impaired FMD comparably to chronic smoking, and led to changes in blood serum that decreased NO production from cultured endothelial cells, without conclusive changes in eNOS gene and protein expression. It is likely that the serum also decreases NO release in the users’ own vascular endothelium. However, this effect was not caused by direct exposure of the cells to components of e-cigarette aerosol at presumptive physiological levels in e-cigarette users. Serum of e-cigarette users and smokers contained elevated levels of endothelial cell adhesion, pro-inflammatory, and thrombotic mediators; but some of these circulating mediators showed distinct patterns of elevation specifically in smoker sera (including RAGE), whereas others were elevated in e-cig user sera (including the RAGE ligands S100A8 and HMGB1). E-cigarette user serum, but not smoker serum, increased microvascular endothelial cell permeability in a process involving RAGE and its ligands. Serum from e-cigarette users also led to elevated ROS production in endothelial cells that was not caused by serum from nonusers. Our results support the emerging consensus that e-cigarette use is not without cardiovascular risk, causing adverse effects that may overlap with those of smoking but with divergent underlying mechanisms. These findings suggest a potential explanation for how dual use of cigarettes and e-cigarettes could be more harmful than smoking or vaping alone.
Supplementary Material
Highlights.
Not only did both chronic e-cigarette users (vapers) and chronic smokers have more impaired endothelial function than nonusers (manifested by lower brachial artery FMD), both vapers’ serum and smokers’ serum significantly reduced NO secretion by endothelial cells relative to nonusers’ serum.
Condensed e-cigarette aerosol did not directly reduce NO secretion, indicating that the serum’s inhibitory activity was produced in response to the inhalation rather than directly from the aerosol.
Vapers’ serum, but not smokers’ serum, caused more permeability than nonusers’ serum in microvascular endothelial cells, contrasting the effects of these two products.
Vapers and smokers exhibited changes in circulating levels of biomarkers of inflammation, cell adhesion, and thrombosis relative to nonusers, but the profiles of changes in these two cohorts differed considerably. Notably, vapers’ serum had higher concentrations of the RAGE ligand S100A8 than smoker serum and nonuser serum, and inhibition of RAGE reduced permeability changes induced by vapers’ serum but not on smokers’ serum, and had no effect on endothelial NO production.
Serum from chronic vapers does not contain elevated H2O2, but incubation of cultured endothelial cells with the chronic vaper serum increases H2O2 in the culture supernatant.
Acknowledgements
We thank Drs. Wenhui Gong and Jeffrey Fineman for the use of their NO analyzer. The graphic abstract for this paper was created with BioRender.com.
Funding
This research was supported by grants U54HL147127, P50HL120163, and R01HL120062 from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH/NHLBI) and the US Food and Drug Administration Center for Tobacco Products (FDA CTP); and grant P50CA180890 from the National Cancer Institute at the NIH and FDA CTP. Dr. Mohammadi was supported by a postdoctoral fellowship from the American Heart Association (19POST34380462), and by a generous gift from the Elfenworks Foundation in memory of Deb O’Keefe. The UCSF Flow Core facility (RRID:SCR_018206) is supported in part by the DRC Center Grant NIH P30DK063720. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Abbreviations:
- CCL-2
monocyte chemoattractant protein-1, MCP-1
- CVD
cardiovascular disease
- e-cigarette
electronic cigarette
- EGM-2
Endothelial Cell Growth Media
- EGM-MV
Microvascular Endothelial Cell Growth Media
- e-liquid
e-cigarette liquid
- eNOS
endothelial nitric oxide synthase
- FMD
flow-mediated dilation
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- H2O2
hydrogen peroxide
- HCAECs
human coronary artery endothelial cells
- HMGB1
high mobility group box 1
- HMVEC-Ls
human lung microvascular endothelial cells
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intracellular adhesion molecule-1
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-8
interleukin-8
- IL-10
interleukin-10
- IFN-β
Interferon- β
- iPSC-ECs
induced pluripotent stem cell-derived endothelial cells
- MPO
myeloperoxidase
- NO
nitric oxide
- NOS3
nitric oxide synthase 3
- PECAM-1
platelet endothelial cell adhesion molecule-1, CD31
- PG
propylene glycol
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxygen species
- SHS
secondhand smoke
- TEER
trans-endothelial electrical resistance
- THPO
thrombopoietin
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- VEGF
vascular endothelial growth factor
- VG
vegetable glycerin
- vWF
von Willebrand factor
Footnotes
Conflict of interest: None.
References
- 1.Fairchild AL, Bayer R and Lee JS. The E-Cigarette Debate: What Counts as Evidence? Am J Public Health. 2019;109:1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A and Hamburg NM. Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetterman JL, Keith RJ, Palmisano JN, McGlasson KL, Weisbrod RM, Majid S, Bastin R, Stathos MM, Stokes AC, Robertson RM, Bhatnagar A and Hamburg NM. Alterations in Vascular Function Associated With the Use of Combustible and Electronic Cigarettes. J Am Heart Assoc. 2020;9:e014570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao P, Liu J and Springer ML. JUUL and Combusted Cigarettes Comparably Impair Endothelial Function. Tob Regul Sci. 2020;6:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan ND, Grimmer JA, Tanwar V, Schwieterman N, Mohler PJ and Wold LE. Cardiovascular risk of electronic cigarettes: a review of preclinical and clinical studies. Cardiovasc Res. 2020;116:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee WH, Ong SG, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, Springer ML, Schick SF, Bhatnagar A and Wu JC. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J Am Coll Cardiol. 2019;73:2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, Mandler WK, Erdreich BH, Ducatman BS, Bryner RW, Dasgupta P and Chantler PD. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol. 2017;124:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL and Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conklin DJ, Schick S, Blaha MJ, Carll A, DeFilippis A, Ganz P, Hall ME, Hamburg N, O’Toole T, Reynolds L, Srivastava S and Bhatnagar A. Cardiovascular injury induced by tobacco products: assessment of risk factors and biomarkers of harm. A Tobacco Centers of Regulatory Science compilation. Am J Physiol Heart Circ Physiol. 2019;316:H801–H827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messner B and Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:509–15. [DOI] [PubMed] [Google Scholar]
- 11.Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG and Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104:1905–10. [DOI] [PubMed] [Google Scholar]
- 12.Barua RS, Ambrose JA, Srivastava S, DeVoe MC and Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: an in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–7. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A and Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–4. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J and Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–55. [DOI] [PubMed] [Google Scholar]
- 15.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, Valenti V, Biondi-Zoccai G and Frati G. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest. 2016;150:606–12. [DOI] [PubMed] [Google Scholar]
- 16.Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S and Wehrli FW. Acute Effects of Electronic Cigarette Aerosol Inhalation on Vascular Function Detected at Quantitative MRI. Radiology. 2019;293:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nocella C, Biondi-Zoccai G, Sciarretta S, Peruzzi M, Pagano F, Loffredo L, Pignatelli P, Bullen C, Frati G and Carnevale R. Impact of Tobacco Versus Electronic Cigarette Smoking on Platelet Function. Am J Cardiol. 2018;122:1477–1481. [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK and Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. [DOI] [PubMed] [Google Scholar]
- 19.Marsot A and Simon N. Nicotine and Cotinine Levels With Electronic Cigarette: A Review. Int J Toxicol. 2015;35:179–185. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S and Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–9. [DOI] [PubMed] [Google Scholar]
- 21.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K and Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–95. [DOI] [PubMed] [Google Scholar]
- 22.Giaever I and Keese CR. Micromotion of mammalian cells measured electrically. Proc Natl Acad Sci. 1991;88:7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffre J, Lloyd E, Wong E, Chung-Yeh C, Nguyen N, Xu F, Legrand M and Hellman J. Catecholaminergic Vasopressors Reduce Toll-Like Receptor Agonist-Induced Microvascular Endothelial Cell Permeability But Not Cytokine Production. Crit Care Med. 2021;49:e315–e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JG, Williams JC, Davis BK, Jacobson K, Doerschuk CM, Ting JPY and Mackman N. Monocytic microparticles activate endothelial cells in an IL-1β–dependent manner. Blood. 2011;118:2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi L, Zhang Y, Xu Y, Zheng X, Zhang X, Wang Z, Xue M and Jin X. HMGB1/RAGE pro-inflammatory axis promotes vascular endothelial cell apoptosis in limb ischemia/reperfusion injury. Biomed Pharmacother. 2019;116:109005. [DOI] [PubMed] [Google Scholar]
- 26.Podzolkov VI, Tarzimanova AI and Mokhammadi LN. [The altered endothelial function in patients with arterial hypertension and different forms of atrial fibrillation]. Klin Med (Mosk). 2014;92:42–6. [PubMed] [Google Scholar]
- 27.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ and Fu X-A. Aldehyde Detection in Electronic Cigarette Aerosols. ACS Omega. 2017;2:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erythropel HC, Jabba SV, DeWinter TM, Mendizabal M, Anastas PT, Jordt SE and Zimmerman JB. Formation of flavorant–propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob Res. 2019;21:1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tierney PA, Karpinski CD, Brown JE, Luo W and Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldin A, Beckman JA, Schmidt AM and Creager MA. Advanced Glycation End Products. Circulation. 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- 31.Averill MM, Kerkhoff C and Bornfeldt KE. S100A8 and S100A9 in Cardiovascular Biology and Disease. Arterioscler Thromb Vasc Biol. 2012;32:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olejarz W, Łacheta D, Głuszko A, Migacz E, Kukwa W, Szczepański MJ, Tomaszewski P and Nowicka G. RAGE and TLRs as Key Targets for Antiatherosclerotic Therapy. Biomed Res Int. 2018;2018:7675286-7675286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Food and Drug Administration. Technical Product Lead (TPL) Review of PMTAs PM0000551,PM0000553, PM0000560 (RJ Reynolds Vapor Co). 2021.
- 34.Food and Drug Administration. Technical Product Lead (TPL) Review of PMTAs PM0000529-PM0000541 (Logic Technology). 2022.
- 35.Charakida M, Masi S, Loukogeorgakis SP and Deanfield JE. The role of flow-mediated dilatation in the evaluation and development of antiatherosclerotic drugs. Curr Opin Lipidol. 2009;20:460–6. [DOI] [PubMed] [Google Scholar]
- 36.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR and Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzahrani T, Pena I, Temesgen N and Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med. 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Shahawy O, Shah T, Obisesan OH, Durr M, Stokes AC, Uddin I, Pinjani R, Benjamin EJ, Mirbolouk M, Osei AD, Loney T, Sherman SE and Blaha MJ. Association of E-Cigarettes With Erectile Dysfunction: The Population Assessment of Tobacco and Health Study. American Journal of Preventive Medicine. 2022;62:26–38. [DOI] [PubMed] [Google Scholar]
- 39.Berlowitz JB, Xie W, Harlow AF, Hamburg NM, Blaha MJ, Bhatnagar A, Benjamin EJ and Stokes AC. E-Cigarette Use and Risk of Cardiovascular Disease: A Longitudinal Analysis of the PATH Study (2013–2019). Circulation. 2022;145:1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osei AD, Mirbolouk M, Orimoloye OA, Dzaye O, Uddin SMI, Benjamin EJ, Hall ME, DeFilippis AP, Stokes A, Bhatnagar A, Nasir K and Blaha MJ. Association Between E-Cigarette Use and Cardiovascular Disease Among Never and Current Combustible-Cigarette Smokers. Am J Med. 2019;132:949–954.e2. [DOI] [PubMed] [Google Scholar]
- 41.Bricknell RAT, Ducaud C, Figueroa A, Schwarzman LS, Rodriguez P, Castro G, Zevallos JC and Barengo NC. An association between electronic nicotine delivery systems use and a history of stroke using the 2016 behavioral risk factor surveillance system. Medicine (Baltimore). 2021;100:e27180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farsalinos KE, Polosa R, Cibella F and Niaura R. Is e-cigarette use associated with coronary heart disease and myocardial infarction? Insights from the 2016 and 2017 National Health Interview Surveys. Ther Adv Chronic Dis. 2019;10:2040622319877741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gathright EC, Wu WC and Scott-Sheldon LAJ. Electronic cigarette use among heart failure patients: Findings from the Population Assessment of Tobacco and Health study (Wave 1: 2013–2014). Heart Lung. 2020;49:229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller CR, Shi H, Li D and Goniewicz ML. Cross-Sectional Associations of Smoking and E-cigarette Use with Self-Reported Diagnosed Hypertension: Findings from Wave 3 of the Population Assessment of Tobacco and Health Study. Toxics. 2021;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parekh T, Pemmasani S and Desai R. Risk of Stroke With E-Cigarette and Combustible Cigarette Use in Young Adults. Am J Prev Med. 2020;58:446–452. [DOI] [PubMed] [Google Scholar]
- 46.Patel U, Patel N, Khurana M, Parulekar A, Patel A, Ortiz JF, Patel R, Urhoghide E, Mistry A, Bhriguvanshi A, Abdulqader M, Mehta N, Arumaithurai K and Shah S. Effect Comparison of E-Cigarette and Traditional Smoking and Association with Stroke-A Cross-Sectional Study of NHANES. Neurol Int. 2022;14:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Mahoney MC, Rivard C, Kimmel HL, Hammad HT, Sharma E, Halenar MJ, Sargent J, Cummings KM, Niaura R, Goniewicz ML, Bansal-Travers M, Hatsukami D, Gaalema D, Fong G, Gravely S, Christensen CH, Haskins R, Silveira ML, Blanco C, Compton W, Stanton CA and Hyland A. Cardiovascular Outcomes among Combustible-Tobacco and Electronic Nicotine Delivery System (ENDS) Users in Waves 1 through 5 of the Population Assessment of Tobacco and Health (PATH) Study, 2013–2019. Int J Environ Res Public Health. 2022;19:4137. doi: 10.3390/ijerph19074137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Lakhani K, Ruedisueli I, Gornbein J and Middlekauff HR. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am J Physiol Heart Circ Physiol. 2020;319:H547–H556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lakota K, Mrak-Poljsak K, Rozman B and Sodin-Semrl S. Increased responsiveness of human coronary artery endothelial cells in inflammation and coagulation. Mediators Inflamm. 2009;2009:146872-146872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leiva A, Fuenzalida B, Salsoso R, Barros E, Toledo F, Gutiérrez J, Pardo F and Sobrevia L. Tetrahydrobiopterin Role in human umbilical vein endothelial dysfunction in maternal supraphysiological hypercholesterolemia. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016;1862:536–544. [DOI] [PubMed] [Google Scholar]
- 51.Wölkart G, Kollau A, Stessel H, Russwurm M, Koesling D, Schrammel A, Schmidt K and Mayer B. Effects of flavoring compounds used in electronic cigarette refill liquids on endothelial and vascular function. PLoS One. 2019;14:e0222152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson JL, Harmon ME and Robergs RA. Identifying Plasma Glycerol Concentration Associated with Urinary Glycerol Excretion in Trained Humans. J Anal Toxicol. 2011;35:617–623. [DOI] [PubMed] [Google Scholar]
- 53.McCurdy DK, Schneider B and Scheie HG. Oral Glycerol: The Mechanism of Intraocular Hypotension. Am J Ophthalmol. 1966;61:1244–1249. [DOI] [PubMed] [Google Scholar]
- 54.Chaumont M, van de Borne P, Bernard A, Van Muylem A, Deprez G, Ullmo J, Starczewska E, Briki R, de Hemptinne Q, Zaher W and Debbas N. Fourth generation e-cigarette vaping induces transient lung inflammation and gas exchange disturbances: results from two randomized clinical trials. Am J Physiol Lung Cell Mol Physiol. 2019;316:L705–L719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A and Przulj D. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e-cigarette products. Addiction. 2020;115:1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YO, Nonnemaker JM, Bradfield B, Hensel EC and Robinson RJ. Examining Daily Electronic Cigarette Puff Topography Among Established and Nonestablished Cigarette Smokers in their Natural Environment. Nicotine Tob Res. 2018;20:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams MR, Jessup W and Celermajer DS. Cigarette Smoking Is Associated With Increased Human Monocyte Adhesion to Endothelial Cells: Reversibility With Oral l-Arginine but Not Vitamin C. J Am Coll Cardiol. 1997;29:491–497. [DOI] [PubMed] [Google Scholar]
- 58.Kelesidis T, Zhang Y, Tran E, Sosa G and Middlekauff HR. Expression of Key Inflammatory Proteins Is Increased in Immune Cells From Tobacco Cigarette Smokers But Not Electronic Cigarette Vapers: Implications for Atherosclerosis. J Am Heart Assoc. 2021;10:e019324-e019324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang JB, Olgin JE, Nah G, Vittinghoff E, Cataldo JK, Pletcher MJ and Marcus GM. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13:e0198681-e0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark PR, Manes TD, Pober JS and Kluger MS. Increased ICAM-1 Expression Causes Endothelial Cell Leakiness, Cytoskeletal Reorganization and Junctional Alterations. J Invest Dermatol. 2007;127:762–774. [DOI] [PubMed] [Google Scholar]
- 61.Schmal H, Czermak BJ, Lentsch AB, Bless NM, Beck-Schimmer B, Friedl HP and Ward PA. Soluble ICAM-1 Activates Lung Macrophages and Enhances Lung Injury. J Immunol. 1998;161:3685. [PubMed] [Google Scholar]
- 62.Chen R, Kang R and Tang D. The mechanism of HMGB1 secretion and release. Experimental & Molecular Medicine. 2022;54:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Luo H, Chen X, Jiang Y and Huang Q. Functional Characterization of S100A8 and S100A9 in Altering Monolayer Permeability of Human Umbilical Endothelial Cells. PLoS One. 2014;9:e90472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dmitrieva NI and Burg MB. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci. 2014;111:6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartman CL and Ford DA. MPO (Myeloperoxidase) Caused Endothelial Dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1676–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suhara T, Fukuo K, Sugimoto T, Morimoto S, Nakahashi T, Hata S, Shimizu M and Ogihara T. Hydrogen Peroxide Induces Up-Regulation of Fas in Human Endothelial Cells. J Immunol. 1998;160:4042. [PubMed] [Google Scholar]
- 67.Kelesidis T, Tran E, Nguyen R, Zhang Y, Sosa G and Middlekauff HR. Association of 1 Vaping Session With Cellular Oxidative Stress in Otherwise Healthy Young People With No History of Smoking or Vaping: A Randomized Clinical Crossover Trial. JAMA Pediatrics. 2021;175:1174–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


