Abstract
One of the principal uncertainties when estimating population risk of late effects from epidemiological data is that few radiation-exposed cohorts have been followed up to extinction. Therefore, the relative risk model has often been used to estimate radiation-associated risk and to extrapolate risk to the end of life. Epidemiological studies provide evidence that children are generally at higher risk of cancer induction than adults for a given radiation dose. However, the strength of evidence varies by cancer site and questions remain about site-specific age at exposure patterns. For solid cancers, there is a large body of evidence that excess relative risk (ERR) diminishes with increasing age at exposure. This pattern of risk is observed in the Life Span Study (LSS) as well as in other radiation-exposed populations for overall solid cancer incidence and mortality and for most site-specific solid cancers. However, there are some disparities by endpoint in the degree of variation of ERR with exposure age, with some sites (e.g., colon, lung) in the LSS incidence data showing no variation, or even increasing ERR with increasing age at exposure. The pattern of variation of excess absolute risk (EAR) with age at exposure is often similar, with EAR for solid cancers or solid cancer mortality decreasing with increasing age at exposure in the LSS. We shall review the human data from the Japanese LSS cohort, and a variety of other epidemiological data sets, including a review of types of medical diagnostic exposures, also some radiobiological animal data, all bearing on the issue of variations of radiation late-effects risk with age at exposure and with attained age. The paper includes a summary of several oral presentations given in a Symposium on “Age effects on radiation response” as part of the 67th Annual Meeting of the Radiation Research Society, held virtually on 3–6 October 2021.
Keywords: Ionizing radiation, age at exposure, Japanese atomic bomb survivors, in vivo radiobiological data, attained age
Introduction
Few groups exposed to radiation have been followed across their lifespan, so there remain substantial uncertainties about estimating radiation cancer risk. In particular, nearly sixty years after the bombings of Hiroshima and Nagasaki, over 40% of the survivors remained alive (Ozasa et al. 2012). Therefore, the relative risk model has often been used to estimate radiation-associated risk and to extrapolate risk to extinction.
Epidemiological studies provide a growing body of evidence that children are, in general, at higher risk of cancer compared to adults from a given radiation dose. However, the strength of evidence varies by cancer site and questions remain about site-specific age at exposure patterns. For solid cancers, many data suggest that excess relative risk (ERR) diminishes at large exposure ages (Little 1993; Little 2003; United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008). This pattern of risk is observed in the Life Span Study (LSS) as well as in other radiation-exposed populations (e.g., radiotherapy patients) (Little 1993, 2003) for overall solid cancer incidence and mortality (Preston et al. 2007; Ozasa et al. 2012) and for most site-specific solid cancers (particularly for mortality). However, there are some disparities by endpoint in the degree of variation of ERR with exposure age, with some sites (e.g., colon, lung) in the LSS incidence data showing no variation, or even ERR that increases at large exposure ages (Preston et al. 2007). This matter has been considered further by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2013) and addressed by a number of studies of the LSS described in more detail below. Excess absolute risk (EAR) often varies similarly with exposure age, with decreasing EAR for solid cancers incidence or mortality at large exposure ages in the LSS (Preston et al. 2007; Ozasa et al. 2012).
For leukemia, ERRs display a more complex pattern of variation with exposure age, with ERR increasing with age at exposure for some subtypes (e.g., acute myeloid leukemia) (Hsu et al. 2013).
The pioneering study of Stewart et al. (1956), Stewart et al. (1958), Bithell and Stewart (1975), the so-called Oxford Survey of Childhood Cancers (OSCC), implied excess risk of many varieties of childhood cancer following in utero X-ray exposure of about 0.01–0.03 Gy. There are many other data of childhood exposure from various other sources also suggesting excess risk associated with childhood exposure at about this level of dose (Wakeford and Bithell 2021; Little et al. 2022a, 2022b).
In this paper we review the human data from the Japanese LSS cohort, and other groups, including a review of types of medical diagnostic exposures, also some radiobiological animal data, all bearing on the issue of variations of radiation risk with exposure age and with age attained. The current paper includes a summary of several oral presentations given in a Symposium on “Age effects on radiation response” as part of the 67th Annual Meeting of the Radiation Research Society, held virtually 3–6 October 2021.
Studies of the Japanese atomic bomb survivors exposed postnatally
The LSS of Japanese atomic bomb survivors is notable for its size, population exposed at all ages to a wide range of well-characterized doses, and long-term follow-up. The most up-to-date report on solid cancer incidence in the LSS for the 1958–2009 period (80,205 subjects with individual dose estimates and 25,239 subjects not in Hiroshima or Nagasaki at the time of the bombings) extended the previous report by 11 years, adding 5,918 cases for a total of 22,538 (i.e., 36% increase) (Grant et al. 2017). Importantly, 72% of new cases occurred among survivors aged <20 years at exposure allowing a more accurate evaluation of radiation risks with childhood exposure.
As the cohort was exposed to atomic bomb radiation simultaneously, the age at exposure and year of birth are negatively correlated. For individuals born between 1860 and 1945, age-specific cancer rates have been increasing in successive birth cohorts for many sites (although an opposite trend has occurred for stomach and cervical cancers), similar to trends in the general Japanese population. Consequently, careful modeling of effects of year of birth on rates of cancer in the LSS was conducted while evaluating site-specific patterns of radiation risk with age at exposure. Another issue related to the interpretation of temporal patterns of radiation risk in the LSS is the weakening correlation between exposure age and age attained over time that allows for discrimination of the two effects more clearly as follow-up is extended.
Similar to the previous LSS cancer incidence report (Preston et al. 2007), both the all solid cancers ERR and EAR varied significantly and independently with attained age (modelled as a power trend) and exposure age (modelled as a log-linear trend). For a given exposure age, solid cancer ERR was lower for larger ages attained while the EAR were larger with increasing age, slightly less rapidly than the baseline rates of cancer. Continuing increase in EAR above the baseline rates of cancer suggests the persistent nature of radiation effects. By contrast to the effects of attained age, the modifications of all solid cancer ERR and EAR by exposure age were similar, reducing for larger exposure ages by 22% and 30% per decade, respectively (Grant et al. 2017). Estimates of ERR effect modification by age at exposure varied substantially across individual cancer sites (from −66% for salivary gland to 24% for cancer of the proximal and distal colon (Sugiyama et al. 2020)) although, for many sites, there was considerable uncertainty in these estimates (i.e., wide confidence intervals). As in the previous analysis of Preston et al. (2007), significant age at exposure trends in ERR were found for thyroid cancer and non-melanoma skin cancer (NMSC) whereas a previously suggested trend for salivary gland cancer reached significance in the current data. Also, as before (Preston et al. 2007), estimates for an age at exposure effect on ERR were positive but not significant for colon, esophageal, lung, and bladder cancers.
Comparisons of ERR and EAR patterns with exposure age and age attained for three sites – thyroid, lung, and female breast cancers, illustrate the varied nature of these age-related effect modifiers; however, the importance of both age and sex in these cancers and the changing age of menarche with improved diet (leading to younger age of menarche), also smoking (active and passive) make these complex sites to study. Specifically, a significant independent effect of exposure age but not age attained was observed for thyroid cancer ERR and EAR whereas a significant independent effect of attained age but not exposure age was observed for lung cancer ERR and EAR (Cahoon et al. 2017). Further, for female breast cancer, comparison of temporal patterns of radiation risks in the current LSS data (Brenner et al. 2018) with those in previous reports (Tokunaga et al. 1987; Preston et al. 2007) highlighted how our understanding of modifying effects on ERR and EAR of age attained and exposure age could change over time. In early LSS data (Tokunaga et al. 1987), the breast cancer ERR was significantly lower for large exposure age and age attained considered separately. As the follow-up period increased and the correlation in the LSS between age attained and exposure age diminished, the evidence for a log-linear trend in the breast cancer ERR with exposure age lessened and was largely explained by the effect of attained age (Preston et al. 2007). By contrast, the evidence for a simple exposure age trend in EAR persisted. In recent breast cancer data, new ERR and EAR patterns emerged, best described by a non-monotonic spline in exposure age with a changepoint at age at menarche (Brenner et al. 2018). Under this model, the ERR increased as exposure age approached menarche and slowly decreased as exposure age increased after menarche. A largely similar ERR pattern was found for uterine corpus cancer (Utada et al. 2018). For both sites, the highest radiation risks were estimated for exposures around the time of puberty.
The presence of many new cancers in the LSS for the 1958–2009 period, particularly among those exposed in childhood, allowed us to estimate radiation risks and temporal patterns more precisely. For all solid cancers combined, both ERR and EAR varied significantly and independently with exposure age and age attained. The two measures of risk provide complementary information on temporal patterns of the risks of radiation exposure. Exposure age and age attained modify radiation risks (both ERR and EAR) in various ways by cancer site and, for some sites, our ability to characterize these effects has evolved over time. The strongest evidence for higher ERR with childhood exposure in LSS has been found for thyroid, NMSC, and salivary gland cancers.
Biological and genetic effects of medical fetal radiation exposure
Pregnant patient, and fetal exposure, to ionizing radiation may be necessary to address urgent maternal health conditions, or may at other times be unintentional, such as when the woman does not yet know she is pregnant; both diagnostic and therapeutic radiographic procedures may be involved. In 1982, Mossman and Hill (Mossman and Hill 1982) found that approximately one percent of women were exposed to abdominal or pelvic radiation imaging, most often in the first trimester before being aware of pregnancy. When it does occur, radiation exposure of the pregnant or possibly pregnant patient is one of the most contentious issues that radiologists, radiologic technologists and clinicians face in communicating with anxious families and patients (International Commission on Radiological Protection (ICRP) 2000; National Council on Radiation Protection and Measurements (NCRP) 2013). Planned diagnostic imaging exposures of pregnant patients address the clinical conditions for which pregnant women are at risk (e.g., trauma, pulmonary thromboembolism, stroke, arteriovenous malformations, urinary stone, appendicitis). One in one-thousand pregnant women have a new cancer diagnosis — with the most common cancer being breast cancer — and these women may need staging imaging as well as radiotherapy. Women are also accidentally exposed from procedures using ionizing radiation — most commonly after trauma - including radiotherapy, interventional procedures, diagnostic procedures and nuclear medicine imaging.
It is therefore important to consider non-ionizing radiation imaging or treatment techniques (e.g., ultrasound and magnetic resonance imaging (MRI)) where feasible and limit the intentional use of ionizing radiation as far as is reasonably possible. The needs of the mother must be balanced with those of the embryo/fetus; the imaging exam should be optimized so that it answers the clinical question and the dose is minimized to the developing embryo/fetus. These risks to the fetus from radiation are principally cancer at low dose (<100 mGy) and teratogenic risks (growth retardation, microcephaly, neuropathology, congenital malformations) at moderate (>100 mGy but <1 Gy) or high doses (>1 Gy). Based on a number of epidemiological and cytological marker studies, there are no known human germline mutation risks from radiation. However, there are known germline changes in animal research. A new International Commission on Radiological Protection (ICRP) task group will be reviewing the literature (International Commission on Radiological Protection (ICRP) 2022).
Ionizing radiation and fetal effects
Fetal teratogenic effects (malformations) are dependent on the timing of the exposures. Much of the evidence is based on animal studies. The epidemiological data are very limited, relying on the in utero cohort from Japanese bomb survivors (e.g., Sugiyama et al. (2021)), and some data from Chernobyl (Hatch et al. 2017). If the exposure occurs within 14 days post-conception, there is a risk of pregnancy loss at a threshold dose of 150 mGy or above. This may occur before the woman knows she is pregnant and it is called the ‘all or none phenomenon’. The most radiosensitive fetal period is the late organogenesis time period (8–15 weeks post-conception) radiation exposure in which may result in malformations, particularly for neuropathology, with a threshold dose of at least 100 mGy. During this most radiosensitive fetal period, the research from the Japanese in utero cohort found a linear increase in intellectual disability of 40% per Gy and a 25 point intelligence quotient (IQ) drop per Gy (Otake and Schull 1984). After 27 weeks post-conception, the risk is very low for fetal malformations but there is risk for growth retardation.
Fetal cancer risk from radiation exposures at 14 days post-conception to birth is not considered dependent on fetal age, although evidence exists from animal studies of increased risk at later pregnancy stage. Most of the information from epidemiologic data comes from the Japanese in utero cohort (2463 individuals) and the OSCC, a large case-control follow-up study of people that were exposed in utero to pelvimetry radiographs (Stewart et al. 1956; Bithell and Stewart 1975), but there is also information from several groups receiving clinical diagnostic and environmental exposures (Wakeford and Bithell 2021; Little et al. 2022a, 2022b). Recent follow-up in the Japanese cohort demonstrated that females in late adulthood continue to have excess mortality risk for solid cancer, although males do not (Sugiyama et al. 2021). The comprehensive review by Wakeford and Bithell (Wakeford and Bithell 2021) of the in utero medical exposures concludes that radiation increased the risk of leukemia and most common childhood cancers. In the OSCC, they estimated the unadjusted excess relative risk of fatal cancer associated with medical diagnostic radiation to be about 1.4–1.5 (up to age 15 years). Similar relative risks were observed in a systematic review and meta-analysis of all published studies (Little et al. 2022b). The estimated pelvimetry doses (on average, 10 mGy) from many decades ago are similar to modern, and optimized, single pass CT scans of the abdomen. Therefore, there may be opportunities to understand fetal risks from epidemiological studies of pregnant women undergoing medical imaging.
Frequency and dose estimates of medical imaging
In many regions, there has been a strong shift in awareness and availability of ultrasound and MRI, alternatives to ionizing radiation imaging, especially for radiosensitive populations like pregnant women and children. Yet, the concern in the medical radiation protection communities is the continued trend upward in use of ionizing radiation imaging, in particular, CT and fluorscopically-guided interventional procedures. Brambilla and colleagues documented recurrent imaging leading to doses >100mSv in 1% of patients in many developed nations (Brambilla et al. 2020). These doses may occur within one day, one year, or five years; they also note that 20% of these patients are childbearing-aged girls and women for which special radiation protection education and guidance is needed. Kwan et al. (2019) studied a twenty-year trend in use of imaging in a large cohort of 2.2 million pregnant women and showed a 3.7 fold increase in CT use in the USA and 2.0 fold increase in CT use in Canada; in fact, 0.8% of pregnant women received a CT in the USA and 0.4% in Canada.
Angel et al. (2008) estimated that the fetal dose from a typical abdomino-pelvic CT ranged from 16 mGy to 31 mGy with a mean value of 24 mGy. These doses should be lower with modern scanners and optimized protocols. As a general conservative rule, the fetal dose estimate would be 15% of the skin entrance dose.
Age effects in the Wake Forest experimental animal data
The Wake Forest Primate Late Effects Cohort is a unique National Institute of Allergic and Infective Diseases (NIAID) funded population of rhesus macaques previously exposed to single-dose ionizing radiation and followed long-term for assessment of late effects. Major diseases reported in this cohort to date comprise cardiovascular diseases such as diastolic dysfunction and myocardial fibrosis (DeBo et al. 2016; Michalson et al. 2020); type 2 diabetes mellitus due to peripheral insulin resistance (Kavanagh et al. 2015; Bacarella et al. 2020) and with diminished microvascular density (Fanning et al. 2017); cerebrovascular disease (Andrews, Metheny-Barlow et al. 2017; Andrews et al. 2020); and immune impairment (Hale et al. 2019; Macintyre et al. 2021). The work reported in this section seeks to combine observed patterns of multi-organ morbidity in this unique cohort.
For the analysis, reported attention was focused on 260 rhesus macaques exposed to single dose total body ionizing radiation (TBI), including clinical, imaging, and pathology outcomes across all major organ systems, and 51 non-irradiated controls. TBI animals were exposed to 1.1–8.5 Gy TBI, with a mean age at exposure of 4.8 years (range 2–15 y) and mean follow-up of 4.6 years (range 0–14 y). Forty percent of irradiated animals received a single mitigating agent such as a hematopoietic growth factor, cytokine, toll-like receptor (TLR) agonist, antibiotic, or another agent. Clinical conditions were diagnosed based on standardized diagnostic criteria, summarized in Table 1. Emergent disease patterns were analyzed with respect to radiation dose, age at irradiation, current age, mitigator use, and sex. Morbidities were each considered independent categorical variables for this analysis. Mitigator use was coded as any mitigator use (all mitigators combined into one category). Dependent continuous variables were radiation dose, age at irradiation, and current age. Proportions of animals affected with a given morbidity (yes/no) and radiation status (yes/no) were assessed by a chi-squared test applied to 2 × 2 tables. Proportions of irradiated animals, by mitigator status (yes/no), were assessed by a chi-squared test applied to 2 × 2 tables. Effects of current age (irrespective of irradiation status); radiation dose; and age at irradiation (if irradiated) were analyzed by morbidity status (yes/no) for each disease condition, using a nonparametric (Wilcoxon) test.
Table 1.
Diagnostic criteria for major disease processes in the Wake Forest cohort examined.
| Organ/Disease | Criteria for Diagnosis |
|---|---|
| Diabetes | Hemoglobin A1c (HbA1c) >6.5% |
| 3 fasting blood glucose measurements > 100 mg/dL | |
| Any non-fasted blood glucose > 200 mg/dL | |
| Kidney | BUN > 30 mg/dl or Cr > 1.1 mg/dl |
| Loss of renal volume >50% | |
| Lung | Pulmonary consolidation on CT scan or emphysema >25%; SPO2 < 80%; Respiratory rate >80 bpm |
| GI | Any lesion on endoscopy |
| Diarrhea (severity code >2 for >5 days) | |
| Skin | Persistent dermatitis, alopecia or loss of pigmentation |
| Heart/CV | Murmur detected on auscultation or echocardiography; MAP >120 |
| Adult stroke volume <5 ml & CO <0.5 L/min at >7y | |
| Brain | Y/N MRI lesions visible on SWI |
| Neurologic abnormality on clinical exam | |
| Cataracts | Any lens opacity on annual slit-lamp exam |
| Behavior | Any behavioral abnormality requiring individual management plan |
| Cancer | Any neoplastic disease (imaging or biopsy) |
| Testicular atrophy | Testis volume <10 ml each after 7 years of age |
| Bone | Adult bone mineral content (BMC) < 274 g or density (BMD) < 0.373 g/cc |
| Obese Underweight | Obesity: Waist Circumference > 45 cm or DEXA Body Fat > 30% |
| Underweight: Waist Circumference < 25 cm or DEXA Adult Body Fat < 12.3% |
BMC: bone mineral content; BMD: bone mineral density; bpm: beats per minute; BUN: blood urea nitrogen; CO: cardiac output; Cr: serum creatinine; CT: computerized tomography; CV: cardiovascular; DEXA: dual energy X-ray absorptiometry; MAP: maximum arterial pressure; mg: milligram; ml: milliliter; MRI: magnetic resonance imaging; SPO2: percent blood oxygen saturation; SWI: susceptibility-weighted imaging
Relationships between disease prevalence and findings are shown in Table 2. Both body weight and percentage body fat declined with radiation dose and increased with age. Irradiated animals were osteopenic. Major patterns of radiation-associated disease in TBI animals included cardiac disease, diabetes mellitus, osteoarthritis, cancers, renal disease, gastrointestinal disease/diarrhea, gonadal atrophy, posterior pole cataracts, and dermatitis/alopecia. Age at irradiation was significantly lower (p < 0.05) in animals with subsequent low body weight, testicular atrophy, brain lesions, osteopenia, and dermatitis/alopecia. Time since irradiation was longer in animals with at least one type of morbidity (mean number of morbidities 4.8, range 1–12), relative to animals with no morbidities (p < 0.0001). Age of animals with any morbidity was greater than unaffected animals (p < 0.0001), except for those with gastrointestinal tract (GI) disease or testicular atrophy. Mean radiation dose was higher for animals with diabetes, cataracts, testicular atrophy, neoplasia, and brain lesions (p < 0.05), and lower in those with cutaneous disease, periodontal disease, and obesity (p < 0.05).
Table 2.
Differences in disease prevalence for Irradiation and Mitigator use in Wake Forest animal cohort; or difference between affected and unaffected animals for Current Age, Age or radiation Dose.
| Disease Condition | Proportion of Irradiated Animals Affected | Proportion of Non-Irradiated Animals Affected | Difference in Proportion Affected if Irradiated | Difference in Current Age in Affected Animals | Difference in Age at Irradiation in Irradiated Animals with this Morbidity | Difference in Radiation Dose (Gy) in Irradiated Animals with this Morbidity | Difference in Proportion of Irradiated Animals Affected Given Any Mitigator |
|---|---|---|---|---|---|---|---|
| Diabetes | 9% | 4% | NSD | +4 yrs | NSD | +1 Gy | NSD |
| (22/239) | (2/51) | <0.001b | 0.0197b | ||||
| Renal Disease | 21.2% | 15.7% | NSD | +4 yrs | NSD | +0.8 Gy | 20% |
| (41/239) | (8/51) | <0.0001b | 0.0534b | 0.0023a | |||
| Osteoarthritis | 23.0% | 31.4% | NSD | +5 yrs | NSD | NSD | NSD |
| (55/239) | (16/51) | <0.0001b | |||||
| GI Disease | 13.6% | 7.8% | NSD | NSD | NSD | NSD | NSD |
| (25/239) | (4/51) | ||||||
| Dermatitis/Alopecia | 23.0% | 39.2% | +16% | +3 yrs | +0.7 yrs | −0.8 Gy | −21% |
| (55/239) | (20/51) | 0.0204a | <0.0001b | 0.0347b | 0.0393b | 0.0032a | |
| Cardiac Disease | 41.8% | 35.3% | NSD | +3 yrs | NSD | NSD | −22% |
| (76/239) | (18/51) | <0.0001b | 0.0075a | ||||
| Hypertension | 17.6% | 3.9% | +14% | +1 yr | NSD | NSD | −20% |
| 42/239 | 2/51 | 0.0052a | 0.0145b | 0.0014a | |||
| Brain (any lesion) | 8.4% | 0% | +8% | +2 yrs | NSD | +2.2 Gy | −18% |
| (20/239) | (0/51) | 0.0046a | 0.0119b | <0.0001b | <0.0001a | ||
| Cataracts | 26.4% | 17.6% | NSD | +3 yrs | NSD | +1 Gy | −21% |
| (63/239) | (9/51) | <0.0001b | 0.0024b | 0.0058a | |||
| Cancer | 17.9% | 2% | +12% | +2 yrs | NSD | +1.8 Gy | NSD |
| (33/239) | (1/51) | 0.0050a | 0.0035b | <0.0001b | |||
| Testicular atrophy | 33.9% | 2% | +32% | NSD | −0.9 yrs | +2 Gy | −33% |
| (81/239) | (1/51) | <0.0001a | 0.0002b | <0.0001b | <0.0001a | ||
| Osteopenia | 14.1% | 9.8% | NSD | +4 yrs | NSD | NSD | NSD |
| (26/239) | (7/51) | <0.0001b | |||||
| Obesity | 12.1% | 43.1% | −31% | +6 yrs | +0.9 yrs | −1.6 Gy | −8% |
| (29/239) | (22/51) | <0.0001a | <0.0001b | 0.0431b | 0.0024b | 0.0466a | |
| Underweight | 20.1% | 15.7% | NSD | +1 yr | −0.6 yrs | NSD | NSD |
| (33/239) | (8/51) | 0.0218b | 0.0168b | ||||
| Periodontal Disease | 55% | 92.1% | −37% | +5 yrs | NSD | −1.4 Gy | −50% |
| (132/239) | (47/51) | <0.0001a | <0.0001b | <0.0001b | <0.0001a |
Chi-squared analysis, or
Wilcoxon test. p-value (2-sided) for departure from null.
NSD: no significant difference.
yr: year.
Use of any mitigator vs none was associated with lower prevalence of periodontal disease, cataracts, skin disease, cardiac disease, renal disease, overweight body condition, and presence of brain lesions (p < 0.05); however, animals receiving mitigators were significantly younger (p < 0.0001). Sex differences could not be determined due to insufficient numbers of females in the cohort.
Influence of genetic background and age at irradiation on the induction of cancer and non-cancer diseases in mice
Individual risk variations for induction of cancer and non-cancer diseases following irradiation, are important concerns for present system and regulation. Tissue reactions (formerly termed deterministic effects) and stochastic effects after radiation exposure show wide individual variation with genetic and other factors, including age at irradiation, which substantially contributes to individual radiation response. Animal studies are critical for the mechanistic understanding of the biological factors modifying risk of radiation-induced health effects, including age at irradiation, dose, dose-rate, organ/tissue specificity and genetic factors.
Sonic hedgehog (Shh) pathway signaling is fundamental for development of many organs in mice and humans, such as cerebellum, hippocampus and eye lens. As a consequence, Patched1 heterozygous mice (Ptch1+/−), characterized by constitutive Shh pathway activation, are predisposed to cerebellar tumors such as medulloblastoma (MB), defects in the hippocampus and lens opacity (Antonelli et al. 2015; De Stefano et al. 2015; Antonelli et al. 2018), and show a marked radiation hypersensitivity to the induction of cancer and non-cancer effects (Pazzaglia et al. 2009). Ptch1+/− mice of different age have been X-irradiated to identify critical susceptible windows for radiation carcinogenesis, revealing a high sensitivity to MB in neonatal Ptch1+/− mice (Pazzaglia et al. 2009). Furthermore, a strong decrease in sensitivity to radiation-induced lens opacity was also reported with increasing of mouse age at irradiation.
A given genetic mutation may produce different phenotypic consequences in genetically distinct individuals. The individual risk is in fact modulated by genetic background effects that can strongly modify the phenotype of a genetic mutation, as well as its way of interacting with environmental agents such as ionizing radiations. Under the Euratom LDLensRad project (Ainsbury et al. 2022), in order to assess the genetic background-related variations in sensitivity to the induction of cancer and non-cancer pathologies, neonatal Ptch1+/− mice maintained on CD1 and C57BL/6 background, have been irradiated at postnatal day 2 with 0.5 Gy, 1 Gy and 2 Gy of γ-rays (60Co). As this mouse developmental phase may be peculiarly susceptible to radiation effects, being critical for development of the eye lens, cerebellum, and dentate gyrus, we monitored the irradiated wild-type and mutant mice for induction of lens opacity, MB and hippocampal neurogenesis defects (Antonelli et al. 2021).
There are reports suggesting a strong genetic background dependence for both cancer and non-cancer radiation-induced pathologies (Antonelli et al. 2018, 2021; De Stefano et al. 2022). However, no concordance in the strain-dependent radiosensitivity was reported for the 3 pathologies in exam. We identified an inverse genetic background-related relationship between susceptibility to radiation-induced MB and lens opacity and that to neurogenesis defects, with elevated incidences of MB and cataract and no consequence of irradiation on neurogenesis in Ptch1+/−/CD1 mutant mice (De Stefano et al. 2022) (see Figure 1). On the other hand, Ptch1+/−/C57BL/6 mutants resistant to cataract and MB induction following irradiation, were instead susceptible to the induction of neurogenesis deficit (De Stefano et al. 2022). Increased radiosensitivity to apoptosis was observed in the cerebellum of Ptch1+/−/C57BL/6 mice compared to Ptch1+/−/CD1 mice, suggesting that the opposite strain-related radiosensitivity to cancer and non-cancer pathologies may be dependent on differences in processing radiation-induced DNA damage. (De Stefano et al. 2022) (see Figure 1).
Figure 1.
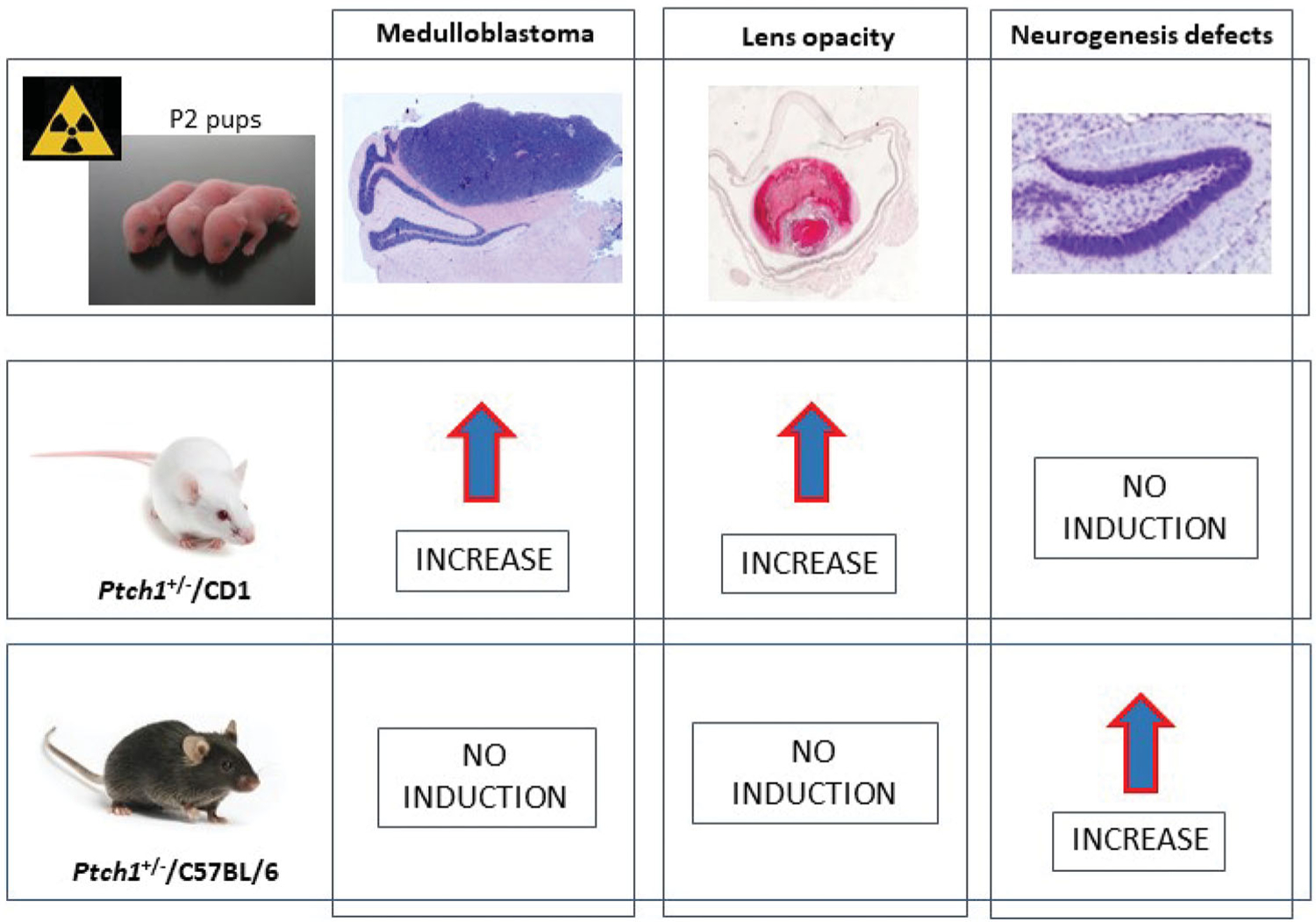
Summary of genetic background effects on radiation risk for cancer and non-cancer pathologies. P2: postnatal day 2.
Discussion and conclusions
There is abundant data from the Japanese atomic bomb survivors and numerous other groups exposed to radiation of the modifying effects of exposure age and age attained on the absolute and relative radiogenic excess cancer risks (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008).
With more than 50 years of follow-up, the LSS continues to add new insights into knowledge of radiogenic cancer risks. At the end of 2009, nearly 73% of the LSS survivors exposed at age under 20 years remained alive. As this population ages, the site-specific ERR and EAR modifications by exposure age will likely be further refined. Extended follow-up of the LSS will be important to fully characterize temporal patterns of radiation risks and estimate the lifetime risk of cancer.
The OSCC study (Stewart et al. 1956, 1958; Bithell and Stewart 1975) suggested that most childhood cancer subtypes were associated with obstetric exposure to doses of no more than 0.03 Gy. A number of other antenatal case-control studies have also suggested cancer risks expressed in childhood associated with medical diagnostic exposure in utero (Wakeford and Bithell 2021; Little et al. 2022a, 2022b). There are few studies other than those of natural background radiation suggesting excess cancer risks at about this level of dose (United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008). Case-control studies are commonly employed in this setting, which can be subject to a number of biases, in particular due to selection, participation and recall; however, with appropriate care being taken with the design, large case-control studies of medically diagnostically exposed groups have been employed without appreciable bias (MacMahon 1962). Although, the interpretation of these studies remains some-what controversial, Doll and Wakeford (Doll and Wakeford 1997) concluded that there are strong grounds for a causal interpretation of the association. Although there have been some significant exceptions (International Commission on Radiological Protection (ICRP) 2003; National Council on Radiation Protection and Measurements (NCRP) 2013) there has been a degree of consensus among recent reviews (Wakeford and Little 2003; Armstrong et al. 2012; Wakeford and Bithell 2021) that this association may represent a causal relationship. More generally, several recent studies have suggested that there are significant excess risks below 100 mGy for leukemia and thyroid cancer (Lubin et al. 2017; Little et al. 2018) after exposure in childhood. The low and moderate dose literature relating in general to all exposure ages has been recently reviewed by various groups including the National Council on Radiation Protection and Measurements (NCRP) (National Council on Radiation Protection and Measurements (NCRP) 2018; Shore et al. 2018; Shore et al. 2019) and by the National Cancer Institute (NCI) (Berrington de Gonzalez et al. 2020; Daniels et al. 2020; Gilbert et al. 2020; Hauptmann et al. 2020; Linet et al. 2020, Schubauer-Berigan et al. 2020). Little et al. (2022a) reviewed and summarized a large body (60 studies in all) of data relating to exposure in early life (including in utero), which they concluded provide support for the existence of excess cancer risk for radiation doses of about 0.02 Gy.
The findings from epidemiological studies are paralleled in experimental animal radiobiological data. In particular, the analysis of the Wake Forest primate data indicates that patterns of delayed morbidity vary by age, age at exposure, dose, and time post-exposure, influencing the interpretation of mitigator effects. Radiation exposure is associated with a higher proportion of animals with dermatitis, hypertension, brain lesions, cancers, and testicular atrophy; a lower prevalence of obesity and periodontal disease is seen in irradiated animals. Age has a profound parallel adverse effect on nearly all delayed radiation health effects, outweighing the effects of radiation exposure and mitigator status; therefore, a “time to disease” model may be most appropriate for future analysis. Age at irradiation had little effect on chronic disease in the dose ranges and age ranges in the rhesus monkeys in the cohort, with the exception of an association of younger-age exposures with greater testicular injury and lower attained adult body weight. Animals receiving mitigators were significantly younger, and age bias and survivor bias are undoubtedly present in the higher-dose animals. Because of these confounders within the population, it is not yet clear whether there are long-term benefits of specific mitigators; additional analysis will be possible as mitigator-treated animals age.
The results of Antonelli et al. (2018), Antonelli et al. (2021) and De Stefano et al. (2022) demonstrate dependence of cancer and non-cancer radiation risk on age at irradiation, as well as on the genetic background, that significantly modulates induction of MB, neurogenesis and lens opacity. In addition, their data demonstrate that individual differences, specifically Ptch1+/− mutation genetic background, might modify the relationship between dose and disease. Further understanding interactions of genetic background with various diseases associated with the same mutation might be important to gain a deeper understanding of radiation-induced detrimental health effects. Furthermore, developing mechanistic risk models accounting for individual risk factors could yield important insights into individual sensitivity and thereby inform the need for revising the system of radiological protection.
There is growing use of ionizing radiation in medicine that exposes the pregnant patient and the fetus (sometimes accidentally, sometimes to save the mother’s life). This situation suggests an opportunity to develop national and global registries of those exposed in utero to study long term outcomes.
Acknowledgements
The authors are grateful for the detailed and helpful comments of the two referees.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Biographies
Mark P. Little, DPhil, joined the National Cancer Institute, Radiation Epidemiology Branch as a Senior Scientist in 2010, and was promoted to a Senior Investigator in 2012. Previously (2000–2010), he worked in Imperial College London, and before that (1992–2000) at UK National Radiological Protection Board (now part of UK Health Security Agency). He is a member of Council of NCRP, and has served as consultant to UNSCEAR, to IAEA, to ICRP (in particular on ICRP Task Group 91 and ICRP Task Group 119), to the UK COMARE, and to NCRP committees SC 1–21 (NCRP Commentary No. 24) and 1–26 (NCRP Report No. 186). He has particular statistical interests in machine learning algorithms and dose measurement error models. He has over 320 publications in the peer-reviewed literature.
Alina Brenner, MD, PhD, is a Senior Scientist in the Department of Epidemiology at the Radiation Effects Research Foundation (RERF), Hiroshima since 2017, and for the period 2005–2017 was a Staff Scientist in the Radiation Epidemiology Branch, National Cancer Institute. The overall goal of her research is to improve our understanding and quantification of cancer risk following exposure to ionizing radiation. Current research interests include a comparison of radiation risk patterns (by sex, age, and time) across different cancer sites and tissues in the LSS cohort of atomic bomb survivors and how these might be related to lifestyle factors and underlying biology. A special emphasis is placed on etiology of radiation-related breast, endometrial, central nervous system and thyroid cancers and includes collaboration with pathologists and geneticists at RERF. An additional area of interest concerns uncertainty in low dose risk estimates as related to different cancer outcomes: mortality and incidence.
Eric J. Grant, PhD, is trained in epidemiology with a background in engineering, his research is primarily focused on cancer after ionizing radiation exposure. Dr. Grant has a 20-year career working for RERF and previously served as the Assistant Chief of the Department of Epidemiology at RERF. Dr. Grant was the Scientific Co-Chair of the 2018 Conference on Radiation and Health. He is a member of the US National Council on Radiation Protection and Measurements as well as a contributing author on an UNSCEAR expert group on cancer after radiation exposure, among other societal memberships.
Hiromi Sugiyama, PhD, is an epidemiologist, she assumed her position at RERF in 2004. Dr. Sugiyama has studied the effects of radiation on risks of mortality and cancer incidence among atomic bomb survivors. She has also led a study of mortality in individuals exposed to the atomic bombings in utero, with interpretations that include not only radiation effects but also social factors. She is responsible for the management of the population-based cancer registry in Hiroshima Prefecture and provides academic support. She has conducted descriptive epidemiological studies based on population-based cancer registry data in Japan.
Dale L. Preston, PhD, is a biostatistician who worked on atomic-bomb survivor studies at RERF for 23 years. He developed statistical modeling software that is widely used for dose-response modeling in radiation epidemiology and other areas, and took a lead role in reports of radiation-associated cancer and noncancer risk in the survivor cohorts, and oversaw the implementation of two new dosimetry systems. Since returning to the United States in 2004, Dr. Preston has continued to work on the analyses of cancer risks in various other exposed groups, including the Mayak Nuclear Workers, Techa River residents and U.S. radiologic technologists. He has served as a consultant to UNSCEAR and various Biological Effects of Ionizing Radiation committees, as a member of ICRP Committee 1, and as an associate editor of Radiation Research. In 2017 he was awarded the Failla Memorial Lecture Award by the Radiation Research Society in recognition of his contributions to radiation research.
Ritsu Sakata, PhD, is trained in epidemiology and biostatistics, she serves as the Assistant Chief of the Department of Epidemiology. Dr. Sakata studies radiation effects among atomic-bomb survivors by incorporating an LSS mail survey data on lifestyle factors as well as other information that was accumulated by the ABCC-RERF during the long-term study period but has not been used in risk estimation previously. She oversees data collection and LSS follow-up activities and collaborates with the internal and external investigators who use the RERF data.
John Cologne, PhD, ELS, is a Senior Scientist in the Statistics Department at RERF, Hiroshima where, for over 30 years, he has been analyzing radiation effects on health outcomes in the major RERF study cohorts of atomic-bomb survivors, in collaboration with RERF epidemiologists, clinical researchers, and laboratory scientists. Much of his applied work has been related to quantifying radiation-associated cancer risk in the LSS, assessing radiation-related risk of liver disease associated with viral hepatitis in the Adult Health Study, and evaluating laboratory markers as potential biodosimeters. He has particular expertise in the design and analysis of epidemiologic studies based on sampling from a cohort. He has published methodological work on the choice of primary time scale for Cox regression, omitted-variable bias with general risk models, and the effect of mis-specified background rate models on inference about the shape of the dose response. Current research interests include application of structural equations to causal modeling with latent factors and mediation, genomic analyses using pathways and gene sets rather than individual single nucleotide polymorphism loci, risk regression based on joint analysis of multiple disease endpoints, and joint modeling of longitudinal and event-time outcomes using novel summaries of longitudinal trajectories as risk factors.
Raquel Velazquez-Kronen, PhD, is a research epidemiologist who joined the Field Research Branch of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention in 2018. Dr. Velazquez-Kronen currently studies chronic disease risks associated with occupational cosmic radiation exposure among aircrew. Previously, she studied risks of cancer and cataract associated with protracted low-dose occupational radiation exposure in U.S. radiologic technologists with the Radiation Epidemiology Branch, National Cancer Institute.
Mai Utada, PhD, is an epidemiologist who joined RERF in 2014. She studies age at exposure effects on radiation risk and carcinogenesis. She led assessment of risks for uterine and ovarian cancers among the LSS women. Currently, Dr. Utada investigates the impact of prostate specific antigen testing in the Adult Health Study on radiation risk estimates for prostate cancer. Dr. Utada collaborates extensively with researchers from different disciplines and institutions.
Kiyohiko Mabuchi, MD, DrPH, joined the Radiation Epidemiology Branch at NCI in 2000 where he has been a Senior Scientist since 2008 and served as a head for the Chernobyl Research Unit. Before joining the NCI, he had been head of the Department of Epidemiology at RERF, leading follow-up studies in the LSS cohort and in the F1 mortality study. He was a member of ICRP Committee 1 between 1993 and 2001, consultant to the 2006 UNSCEAR Report on cardiovascular disease, the ICRP Tissue Reaction Task Group and the NCRP, Scientific Committee on Biological Effectiveness of Photons. His research interests include the radiation risk of solid and hematological cancers and their temporal patterns in atomic bomb survivors and Chernobyl populations.
Kotaro Ozasa, MD, PhD, is an epidemiologist for community-based cohort studies and others targeting cancer and lifestyle-related health conditions and was also involved in public health activities. He has been the Chief of the Department of Epidemiology, RERF since 2008, and has been involved in the cohort studies of health effects due to atomic bomb radiation exposure among the survivors, in utero-exposed people, and survivor’s children.
John D. Olson, MS is a Research Associate with 16 years of experience in preclinical research. Most of his work has been in preclinical imaging, quantitative image analysis, experimental design, and data analysis. He is now the Resource Manager for the NIAID Wake Forest Primate Late Effects Program (U01 AI150578).
Gregory O. Dugan, DVM, is a veterinarian with over 10 years’ experience in the clinical management and study of late effects of radiation in nonhuman primates, in support of the Wake Forest Radiation Late Effects Program.
Simonetta Pazzaglia, PhD, is a senior scientist group leader at the “Division of Health Protection Technologies” of ENEA (Italian National Agency for the New Technologies, Energy and Sustainable Economic Development). She has considerable experience in experimental oncology and molecular biology, with particular emphasis on in vivo experiments using genetically modified mice. She also has a considerable experience in studying molecular events associated with radiation-induced cancer, with notable concentration on brain cancers. She is also interested in radiation-associated normal tissue effects and induction of non-cancer diseases, with a main focus on neurocognitive effects and lens opacity at low radiation doses.
J. Mark Cline, DVM, PhD, is an experienced researcher with over 25 years of continuous NIH funding. He is a board-certified veterinary pathologist with many years’ experience in the discovery and development of animal models of cancer and radiation effects. Much of his past work focused on primate studies of hormonal and dietary effects on pathophysiology of breast and reproductive cancer risk, with comparative work in rodents, and translational studies in human subjects. He is now the Principal Investigator for the NIAID Wake Forest Primate Late Effects Program (U01 AI150578), funded from 2007–2027, which includes assessments of multiple organ systems in male and female primates, and serves a network of over 50 investigators across the US. Finding to date from this program include hematopoietic, immune metabolic, cardiovascular, pulmonary, neurovascular, and skeletal abnormalities, and gonadal injury in both males and females.
Kimberly Applegate, MD, is the chair of Committee 3 of the ICRP, focusing on radiation protection in medicine. Dr Applegate is a retired professor of radiology and pediatrics from the University of Kentucky in Lexington. Dr. Applegate’s policy and research work, including 200 publications, has contributed to an improved understanding of the structure, process, and outcomes of the practice of imaging, especially in pediatric groups, toward its improvement and standardization. She has worked collaboratively around the world in a number of medical specialties to improve imaging practice. From its start in 2007 to the present, she has worked on the Steering Committee for the Image Gently Campaign to improve children’s care worldwide. Kimberly has received a number of awards that include the 2019 AAPM’s Honorary Membership and the American Association for Women in Radiology’s Marie Sklowdoska Curie Award for her unique roles in leadership and outstanding contributions to the advancement of women in the Radiology professions.
Footnotes
Disclosure statement
The authors declare no conflicts of interest.
References
- Ainsbury EA, Dalke C, Mancuso M, Kadhim M, Quinlan RA, Azizova T, Dauer LT, Dynlacht JR, Tanner R, Hamada N. 2022. Introduction to the Special LDLensRad Focus Issue. Radiat Res 197(1):1–6. [DOI] [PubMed] [Google Scholar]
- Andrews RN, Bloomer EG, Olson JD, Hanbury DB, Dugan GO, Whitlow CT, Cline JM. 2020. Non-human primates receiving high-dose total-body irradiation are at risk of developing cerebrovascular injury years postirradiation. Radiat Res 194(3):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, Hampson RE, Deadwyler SA, Cline JM. 2017. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res 187(5):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel E, Wellnitz CV, Goodsitt MM, Yaghmai N, DeMarco JJ, Cagnon CH, Sayre JW, Cody DD, Stevens DM, Primak AN, et al. 2008. Radiation dose to the fetus for pregnant patients undergoing multi-detector ct imaging: Monte Carlo simulations estimating fetal dose for a range of gestational age and patient size. Radiology 249(1): 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli F, Campa A, Esposito G, Giardullo P, Belli M, Dini V, Meschini S, Simone G, Sorrentino E, Gerardi S, et al. 2015. Induction and repair of DNA DSB as revealed by H2AX phosphorylation foci in human fibroblasts exposed to low- and high-LET radiation: relationship with early and delayed reproductive cell death. Radiat Res 183(4):417–431. [DOI] [PubMed] [Google Scholar]
- Antonelli F, Casciati A, Belles M, Serra N, Linares-Vidal MV, Marino C, Mancuso M, Pazzaglia S. 2021. Long-term effects of ionizing radiation on the hippocampus: linking effects of the sonic hedgehog pathway activation with radiation response. IJMS 22(22):12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli F, Casciati A, Tanori M, Tanno B, Linares-Vidal MV, Serra N, Bellés M, Pannicelli A, Saran A, Pazzaglia S. 2018. Alterations in morphology and adult neurogenesis in the dentate gyrus of Patched1 heterozygous mice. Front Mol Neurosci 11:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B, Brenner DJ, Baverstock K, Cardis E, Green A, Guilmette RA, Hall J, Hill MA, Hoel D, Krewski D, et al. 2012. Radiation. In: A review of human carcinogens Vol. 100D. Lyon: International Agency for Research on Cancer. p. 1–341. [Google Scholar]
- Bacarella N, Ruggiero A, Davis AT, Uberseder B, Davis MA, Bracy DP, Wasserman DH, Cline JM, Sherrill C, Kavanagh K. 2020. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys 106(4):878–886. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Daniels RD, Cardis E, Cullings HM, Gilbert E, Hauptmann M, Kendall G, Laurier D, Linet MS, Little MP, et al. 2020. Epidemiological studies of low-dose ionizing radiation and cancer: rationale and framework for the monograph and overview of eligible studies. J Natl Cancer Inst Monogr 2020(56):97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell JF, Stewart AM. 1975. Pre-natal irradiation and childhood malignancy: a review of British data from the Oxford Survey. Br J Cancer 31(3):271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla M, Vassileva J, Kuchcinska A, Rehani MM. 2020. Multinational data on cumulative radiation exposure of patients from recurrent radiological procedures: call for action. Eur Radiol 30(5):2493–2501. [DOI] [PubMed] [Google Scholar]
- Brenner AV, Preston DL, Sakata R, Sugiyama H, de Gonzalez AB, French B, Utada M, Cahoon EK, Sadakane A, Ozasa K, et al. 2018. Incidence of breast cancer in the Life Span Study of atomic bomb survivors: 1958–2009. Radiat Res 190(4):433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon EK, Preston DL, Pierce DA, Grant E, Brenner AV, Mabuchi K, Utada M, Ozasa K. 2017. Lung, laryngeal and other respiratory cancer incidence among Japanese atomic bomb survivors: an updated analysis from 1958 through 2009. Radiat Res 187(5):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Kendall GM, Thierry-Chef I, Linet MS, Cullings HM. 2020. Strengths and weaknesses of dosimetry used in studies of low-dose radiation exposure and cancer. J Natl Cancer Inst Monogr 2020(56):114–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano I, Leonardi S, Casciati A, Pasquali E, Giardullo P, Antonelli F, Novelli F, Babini G, Tanori M, Tanno B, LDLensRad Consortium, et al. 2022. Contribution of genetic background to the radiation risk for cancer and non-cancer diseases in Ptch1+/− Mice. Radiat Res 197(1):43–56. [DOI] [PubMed] [Google Scholar]
- De Stefano I, Tanno B, Giardullo P, Leonardi S, Pasquali E, Antonelli F, Tanori M, Casciati A, Pazzaglia S, Saran A, et al. 2015. The patched 1 tumor-suppressor gene protects the mouse lens from spontaneous and radiation-induced cataract. Am J Pathol 185(1): 85–95. [DOI] [PubMed] [Google Scholar]
- DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, Kavanagh K, Cline JM, Register TC. 2016. Late effects of total-body gamma irradiation on cardiac structure and function in male rhesus macaques. Radiat Res 186(1):55–64. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Wakeford R. 1997. Risk of childhood cancer from fetal irradiation. Br J Radiol 70:130–139. [DOI] [PubMed] [Google Scholar]
- Fanning KM, Pfisterer B, Davis AT, Presley TD, Williams IM, Wasserman DH, Cline JM, Kavanagh K. 2017. Changes in microvascular density differentiate metabolic health outcomes in monkeys with prior radiation exposure and subsequent skeletal muscle ECM remodeling. Am J Physiol Regul Integr Comp Physiol 313(3): R290–R297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ES, Little MP, Preston DL, Stram DO. 2020. Issues in interpreting epidemiologic studies of populations exposed to low-dose, high-energy photon radiation. J Natl Cancer Inst Monogr 2020(56): 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EJ, Brenner A, Sugiyama H, Sakata R, Sadakane A, Utada M, Cahoon EK, Milder CM, Soda M, Cullings HM, et al. 2017. Solid cancer incidence among the life span study of atomic bomb survivors: 1958–2009. Radiat Res 187(5):513–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale LP, Rajam G, Carlone GM, Jiang C, Owzar K, Dugan G, Caudell D, Chao N, Cline JM, Register TC, et al. 2019. Late effects of total body irradiation on hematopoietic recovery and immune function in rhesus macaques. PLoS One 14(2):e0210663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M, Little MP, Brenner AV, Cahoon EK, Tereshchenko V, Chaikovska L, Pasteur I, Likhtarov I, Bouville A, Shpak V, et al. 2017. Neonatal outcomes following exposure in utero to fallout from Chernobyl. Eur J Epidemiol 32(12):1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann M, Daniels RD, Cardis E, Cullings HM, Kendall G, Laurier D, Linet MS, Little MP, Lubin JH, Preston DL, et al. 2020. Epidemiological studies of low-dose ionizing radiation and cancer: summary bias assessment and meta-analysis. J Natl Cancer Inst Monogr 2020(56):188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, et al. 2013. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res 179(3):361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP) 2000. Pregnancy and medical radiation. Publication 84. Annals ICRP 30(1):i–viii. + 1–43. [DOI] [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP) 2003. Biological effects after prenatal irradiation (embryo and fetus). ICRP publication 90. Annals ICRP 33(1–2):1–206. [PubMed] [Google Scholar]
- International Commission on Radiological Protection (ICRP) 2022. Task Group 121. Effects of ionising radiation exposure in offspring and next generations https://www.icrp.org/icrp_group.asp?id=189
- Kavanagh K, Dendinger MD, Davis AT, Register TC, DeBo R, Dugan G, Cline JM. 2015. Type 2 diabetes is a delayed late effect of whole-body irradiation in nonhuman primates. Radiat Res 183(4):398–406. 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan ML, Miglioretti DL, Marlow EC, Aiello Bowles EJ, Weinmann S, Cheng SY, Deosaransingh KA, Chavan P, Moy LM, Bolch WE, T. Radiation-Induced Cancers Study, et al. 2019. Trends in medical imaging during pregnancy in the United States and Ontario, Canada, 1996 to 2016. JAMA Netw Open 2(7):e197249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linet MS, Schubauer-Berigan MK, Berrington de Gonzalez A. 2020. Outcome assessment in epidemiological studies of low-dose radiation exposure and cancer risks: sources, level of ascertainment, and misclassification. J Natl Cancer Inst Monogr 2020(56):154–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP. 1993. Risks of radiation-induced cancer at high doses and dose rates. J Radiol Prot 13(1):3–25. [Google Scholar]
- Little MP. 2003. Risks associated with ionizing radiation. Br Med Bull 68:259–275. [DOI] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Borrego D, French B, Zablotska LB, Adams MJ, Allodji R, de Vathaire F, Lee C, Brenner AV, et al. 2018. Leukaemia and myeloid malignancy among people exposed to low doses (<100 mSv) of ionising radiation during childhood: a pooled analysis of nine historical cohort studies. Lancet Haematol 5(8): e346–e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Bouffler SD, Abalo K, Hauptmann M, Hamada N, Kendall GM. 2022a. Review of the risk of cancer following low and moderate doses of sparsely ionising radiation received in early life in groups with individually estimated doses. Environ Int 159: 106983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MP, Wakeford R, Bouffler SD, Abalo K, Hauptmann M, Hamada N, Kendall MG. 2022b. Cancer risks among studies of medical diagnostic radiation exposure in early life without quantitative estimates of dose. Sci Tot Environ 832:154723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Adams MJ, Shore R, Holmberg E, Schneider AB, Hawkins MM, Robison LL, Inskip PD, Lundell M, Johansson R, et al. 2017. Thyroid cancer following childhood low-dose radiation exposure: a pooled analysis of nine cohorts. J Clin Endocrinol Metab 102(7): 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre AN, French MJ, Sanders BR, Riebe KJ, Shterev ID, Wiehe K, Hora B, Evangelous T, Dugan G, Bourland JD, et al. 2021. Long-term recovery of the adaptive immune system in rhesus macaques after total body irradiation. Adv Radiat Oncol 6(5):100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon B 1962. Prenatal x-ray exposure and childhood cancer. J. Natl. Cancer Inst 28:1173–1191. [PubMed] [Google Scholar]
- Michalson KT, Dugan GO, Caudell DL, Cline JM, Kitzman DW, Register TC. 2020. Diastolic dysfunction accompanies alterations in myocardial structure, cellular composition and macrophage polarization in survivors of ionizing radiation exposure. Biorxiv doi: 10.1101/22020.2002.2018.953190. [DOI] [Google Scholar]
- Mossman KL, Hill LT. 1982. Radiation risks in pregnancy. Obstet Gynecol 60(2):237–242. [PubMed] [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). 2013. Report No. 174. Preconception and prenatal radiation exposure: health effects and protective guidance. 7910 Woodmont Avenue, Suite 400/Bethesda, MD 20814–3095, USA, National Council on Radiation Protection and Measurements (NCRP): 1–418. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP) 2018. Implications of recent epidemiologic studies for the linear-nonthreshold model and radiation protection. NCRP Commentary no 27. Bethesda, MD, USA, National Council on Radiation Protection and Measurements (NCRP). SC 1–27: i–ix. + 1–199. [Google Scholar]
- Otake M, Schull WJ. 1984. In utero exposure to A-bomb radiation and mental retardation; a reassessment. Br J Radiol 57(677):409–414. [DOI] [PubMed] [Google Scholar]
- Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. 2012. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res 177(3):229–243. [DOI] [PubMed] [Google Scholar]
- Pazzaglia S, Pasquali E, Tanori M, Mancuso M, Leonardi S, V M d, Rebessi S, Saran A. 2009. Physical, heritable and age-related factors as modifiers of radiation cancer risk in patched heterozygous mice. Int J Radiat Oncol Biol Phys 73(4):1203–1210. [DOI] [PubMed] [Google Scholar]
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. 2007. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168(1):1–64. [DOI] [PubMed] [Google Scholar]
- Schubauer-Berigan MK, Berrington de Gonzalez A, Cardis E, Laurier D, Lubin JH, Hauptmann M, Richardson DB. 2020. Evaluation of Confounding and Selection Bias in Epidemiological Studies of Populations Exposed to Low-Dose, High-Energy Photon Radiation. J Natl Cancer Inst Monogr 2020(56):133–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, Mettler FA, Preston RJ, Till JE, Wakeford R, et al. 2018. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot 38(3): 1217–1233. [DOI] [PubMed] [Google Scholar]
- Shore RE, Beck HL, Boice JDJ, Caffrey EA, Davis S, Grogan HA, Mettler FAJ, Preston RJ, Till JE, Wakeford R, et al. 2019. Recent Epidemiologic Studies and the Linear No-Threshold Model For Radiation Protection-Considerations Regarding NCRP Commentary 27. Health Phys 116(2):235–246. [DOI] [PubMed] [Google Scholar]
- Stewart A, Webb J, Hewitt D. 1958. A survey of childhood malignancies. Br Med J 1(5086):1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Webb J, Giles D, Hewitt D. 1956. Malignant disease in childhood and diagnostic irradiation in utero. Lancet 268(6940):447. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Misumi M, Brenner A, Grant EJ, Sakata R, Sadakane A, Utada M, Preston DL, Mabuchi K, Ozasa K. 2020. Radiation risk of incident colorectal cancer by anatomical site among atomic bomb survivors: 1958–2009. Int J Cancer 146(3):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama H, Misumi M, Sakata R, Brenner AV, Utada M, Ozasa K. 2021. Mortality among individuals exposed to atomic bomb radiation in utero: 1950–2012. Eur J Epidemiol 36(4):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M, Land CE, Yamamoto T, Asano M, Tokuoka S, Ezaki H, Nishimori I. 1987. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1980. Radiat Res 112(2):243–272. [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2008. UNSCEAR 2006 Report. Annex A. Epidemiological Studies of Radiation and Cancer New York, United Nations. E.08.IX.6: 13–322. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2013. Volume II. Scientific Annex B: Effects of radiation exposure of children New York, United Nations. E.14.IX.2: 1–269. [Google Scholar]
- Utada M, Brenner AV, Preston DL, Cologne JB, Sakata R, Sugiyama H, Sadakane A, Grant EJ, Cahoon EK, Ozasa K, et al. 2018. Radiation risks of uterine cancer in atomic bomb survivors: 1958–2009. JNCI Cancer Spectr 2(4):pky081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeford R, Bithell JF. 2021. A review of the types of childhood cancer associated with a medical X-ray examination of the pregnant mother. Int J Radiat Biol 97(5):571–592. [DOI] [PubMed] [Google Scholar]
- Wakeford R, Little MP. 2003. Risk coefficients for childhood cancer after intrauterine irradiation: a review. Int J Radiat Biol 79(5): 293–309. [DOI] [PubMed] [Google Scholar]


