Summary
Diet has a profound impact on the microbial community in the gastrointestinal tract, the intestinal microbiota, to the benefit or detriment of human health. To understand the influence of diet on the intestinal microbiota, research has focused on individual macronutrients. Some macronutrients (e.g. fiber) have been studied in great detail and have been found to strongly influence the intestinal microbiota. The relationship between dietary protein, a vital macronutrient, and the intestinal microbiota has gone largely unexplored. Emerging evidence suggests that dietary protein strongly impacts intestinal microbiota composition and function and that protein-microbiota interactions can have critical impacts on host health. In this review, we focus on recent studies investigating the impact of dietary protein quantity and source on the intestinal microbiota and resulting host health consequences. We highlight major open questions critical to understanding health outcomes mediated by interactions between dietary protein and the microbiota.
Subject areas: Microbiome, Nutrition, Diet
Graphical abstract
Microbiome; Nutrition; Diet.
Introduction
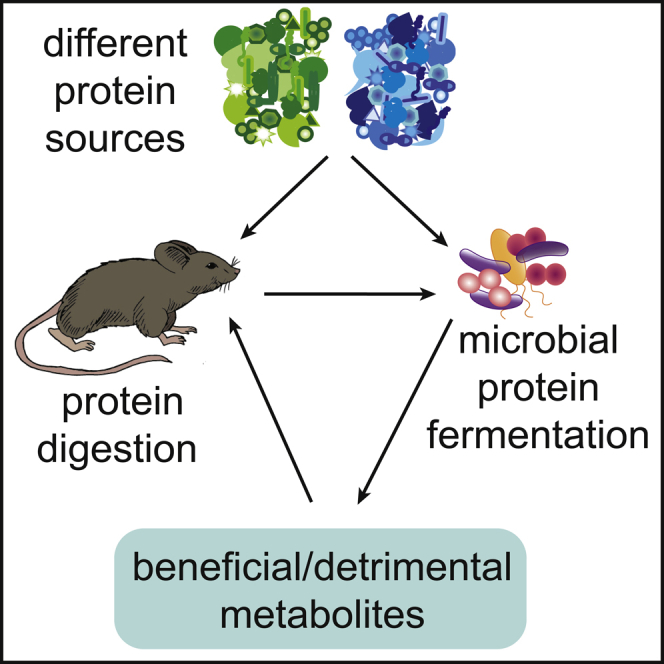
The community of microorganisms living in the digestive tract, the intestinal microbiota, greatly impacts human health. Diet is known to affect this vital microbial community and interactions between dietary components and the microbiota have been implicated in a variety of human diseases (Bäckhed et al., 2005; David et al., 2014; Patnode et al., 2019; Turnbaugh et al., 2009). To unravel the complex relationship between host, diet, and the intestinal microbiota, research has focused on the impact of specific dietary components including individual macro- and micronutrients. Some macronutrients, such as fiber, have been extensively researched in regard to their impact on the microbiota and the resulting consequences to host health (Desai et al., 2016; Patnode et al., 2019). Other macronutrients, particularly protein, remain vastly understudied (Reese and Carmody, 2019). There is accumulating evidence that suggests protein is an important driver of microbiota composition and function and that microbiota-protein interactions may have major impacts on long-term host health (Budhathoki et al., 2019; Faith et al., 2011; Holmes et al., 2017; Huang et al., 2020; Reese et al., 2018; Song et al., 2019).
Over 50% of the United States population is estimated to surpass the daily protein recommendation and higher than recommended daily protein consumption has been observed in other countries as well (Jantchou et al., 2010, USDA, Center for Nutrition Policy, 2015). The amount of protein in a given meal influences how much protein reaches the colon, where it can drive microbial processes such as protein fermentation. Studies have shown an increase in protein derivatives (e.g. branched-chain amino acids and nitrogen) in the colon and fecal material following an increase in the quantity of protein consumed (Beaumont et al., 2017; Evenepoel et al., 1999; Reese et al., 2018; Russell et al., 2011; Silvester and Cummings, 1995; Young et al., 2000). The source of dietary protein may also influence colonic protein fermentation due to differences in digestibility of different protein sources (Joye, 2019; Marinangeli and House, 2017; Mathai et al., 2017). This has important implications to host health, as increased microbial protein fermentation in the colon has been associated with intestinal diseases such as inflammatory bowel disease and colorectal cancer, as well as metabolic diseases (Beaumont et al., 2017; Choi et al., 2021; Jantchou et al., 2010; Jowett et al., 2004; Newgard et al., 2009; Russell et al., 2011). However, it is unclear if the negative health effects of microbial protein fermentation are a general phenomenon or if they are limited to high protein intake or specific dietary protein sources.
In this review, we provide an overview of recent research on how the quantity and source of dietary protein impacts microbiota composition and function, with a focus on human and murine studies. To put these studies into context, we first discuss factors that influence how much dietary protein is available to the intestinal microbiota. Additionally, we identify major open questions about protein-microbiota-host interactions and potential pathways on how they can be addressed. While protein fermentation is relevant to microbiota-protein interactions, it is not at the center of this review and we would like to point the reader to excellent reviews by Oliphant and Allen-Vercoe (2019) and Portune et al. for further reading on protein fermentation (Portune et al., 2016).
Protein availability in the colon
To understand interactions between dietary protein and the microbiota, one must first consider the process of protein digestion. Protein digestion and absorption influences how much protein reaches the colon, where most of the microbiota resides. Dietary protein is hydrolyzed to small peptides and amino acids by host proteases before it can be absorbed in the small intestine (primarily in the duodenum and jejunum). The majority of dietary protein (>90%) is absorbed in the small intestine but a fraction escapes digestion and eventually reaches the colon. Protein that reaches the colon is largely unavailable to the host, as peptides and amino acids are minimally absorbed in the mammalian large intestine, except in the case of neonates (Portune et al., 2016). This undigested dietary protein therefore serves as a substrate for microbial metabolism. Undigested dietary protein that reaches the large intestine is hydrolyzed to peptides and amino acids, which may be used by the microbiota as a source of carbon, nitrogen, and energy through a diversity of metabolic pathways (Moreno-Pérez et al., 2018; Oliphant and Allen-Vercoe, 2019).
There are a number of factors that influence how much dietary protein goes undigested by the host and reaches the intestinal microbiota. One factor is the quantity of dietary protein consumed in a given meal by the host (See: impact of protein quantity on microbiota composition). Another factor is dietary protein digestibility. Dietary protein digestibility is influenced by dietary protein source which dictates a protein’s amino acid composition, protein accessibility, processing, and anti-nutritional factor content (Joye, 2019). Dietary protein source generally refers to the species from which the protein was derived, but can also be more specific (e.g. whey versus casein versus beef, all from cows).
The dietary protein source determines the amino acid composition of a protein. The amino acid composition of a protein influences protein hydrolysis due to amino acid specificities of proteases. In addition, amino acid composition influences protein chain flexibility (Joye, 2019). A less flexible protein chain is less accessible for protease cleavage (Joye, 2019). Dietary protein accessibility can furthermore be influenced by other dietary components consumed with the protein or the matrix in which the protein resides (Acton et al., 1982; Barbé et al., 2014). For example, if a protein is surrounded by fiber, the fiber will need to be metabolized before bacterial proteases can access the protein. Dietary protein processing, which can range from simple heating to more in-depth processes of protein purification, has the potential to both increase and decrease the digestibility of dietary protein (Carmody and Wrangham, 2009). Anti-nutritional factors (e.g. protease inhibitors, tannins, and phytates) can be found both in animal- and plant-based dietary proteins but are more commonly cited as negatively influencing the digestibility of plant-based dietary protein sources (Carmody and Wrangham, 2009; Joye, 2019).
Other sources of protein for microbes in the colon that are not diet derived but need to be potentially considered in studies of interactions between the microbiota and proteinaceous substrates, are host protein and microbial protein. Host and microbial protein are sources of non-dietary protein that may contribute to the protein pool available in the colon. Protein from the host is potentially available from the secretion of digestive enzymes, tissue sloughing throughout the digestive tract, and the mucosal layer. 18 g/day of host protein is estimated to contribute to the protein pool in the colon in humans (Moughan and Rutherfurd, 2012). Another source of protein available to the microbiota is microbial protein in the form of secreted proteins or those released through cell lysis in the upper regions of the digestive tract. An important differentiation here is that the diet itself can also contribute microbial protein to the protein pool in the colon, as the processing of numerous foods, such as cheese, involves microbes (David et al., 2014). We only highlight this potential confounder of dietary protein-microbiota studies and will not further discuss non-dietary proteins in this review.
Dietary protein impact on microbiota composition and function
Impact of protein quantity on microbiota composition
High-protein diets are frequently suggested for weight loss, elderly individuals, and athletes, yet our understanding of how an increase in undigested protein in the colon impacts the composition and function of the microbiota is still limited (Beaumont et al., 2017; Moreno-Pérez et al., 2018; Ni Lochlainn et al., 2018). When a high-protein meal is consumed, a corresponding increase in protein derivatives (nitrogen, urea, and protein fermentation products) has been observed in the lower intestinal tract and fecal material of humans and other mammals, suggesting there is a limit for how much protein can be absorbed by the small intestine at one time (Beaumont et al., 2017; Evenepoel et al., 1999; Reese et al., 2018; Russell et al., 2011; Silvester and Cummings, 1995; Young et al., 2000). Initial studies have shown dietary protein quantity to be influential to the microbiota particularly through providing nitrogen, a limiting nutrient for the intestinal microbiota (Faith et al., 2011; Holmes et al., 2017; Reese et al., 2018). Considering the prevalence of high-protein diets, it is essential to understand how an increase in undigested dietary protein in the colon influences the composition and function of the microbiota and the accompanying host health consequences.
Controlled diet studies in humans have presented contradictory results regarding compositional changes of the microbiota in response to an increase in dietary protein consumption. These contradictions may be due to 1) interference from other dietary components, 2) differences in the protein supplement sources included in the diets, or 3) high interindividual variation between participants. When high-protein diets are designed in diet-microbiota studies, carbohydrates or fat are often substituted with extra protein, resulting in high-protein diets that are simultaneously low-carbohydrate/fat diets (Cotillard et al., 2013; David et al., 2014; Russell et al., 2011). Intervention studies have largely shown that microbiota members with the capacity to metabolize a substrate quickly increase in abundance following consumption of a diet high in the respective substrate. The contribution of protein quantity to this transient effect of microbiota composition can be difficult to assess when the quantities of other dietary components are also altered. For example, microbiota compositional changes were evident when participants consumed animal-based diets with 30% protein or plant-based diets with 10% protein, but it was not possible to disentangle the influence of other dietary components from the results, such as limited fiber content in the animal-based diet (David et al., 2014). Nonprotein dietary components can influence the availability of protein and offer additional substrates to the microbiota, as discussed above (See: protein availability in the colon).
Protein supplements offer a way to increase dietary protein without altering other dietary components, yet the few human studies that have used protein supplements have come to contradictory conclusions. When overweight individuals consumed casein or soy protein supplements, no significant changes of microbiota composition were observed as compared to controls (Beaumont et al., 2017). However, microbiota compositional changes were observed when athletes consumed protein supplements consisting of whey isolate and beef hydrolysate (Moreno-Pérez et al., 2018). After a ten-week supplementation period, 11 taxa were found to be significantly different between the control and protein-supplemented groups (Moreno-Pérez et al., 2018). Five of the taxa, all belonging to the Bacteroidetes phylum, were more abundant in the protein-supplemented group, while six taxa, belonging to the Proteobacteria or Firmicutes phyla, were more abundant in the control group (Moreno-Pérez et al., 2018). It is worth noting that the protein sources of the supplements differed between these contradictory studies. Beaumont et al. suggested that high interindividual variation may have masked compositional changes due to dietary intervention (Beaumont et al., 2017). High interindividual variation of intestinal microbiota is well documented and often renders studies in human populations difficult to interpret (Eckburg et al., 2005; Laukens et al., 2016; Salonen et al., 2014; Walker et al., 2011). One way to tackle issues with high variation is to use mouse models in which microbial communities can be more homogeneous due to a controlled environment or the introduction of defined microbial communities. Below, we describe murine studies which allow for greater control of diet and environment while offering microbiota models functionally similar to the human microbiota (Doré et al., 2015).
In gnotobiotic mice with a defined microbiota consisting of ten microbial species, dietary protein was shown to be more influential on microbial community biomass and the individual abundance of all ten community members compared to fat (corn oil), polysaccharide (cornstarch), or simple sugar (sucrose) (Faith et al., 2011). Dietary protein was found to limit the biomass of the community, but changes in species abundances were not uniform (Faith et al., 2011). Three of the ten community members, Eubacterium rectale, Desulfovibrio piger, and Marvinbryantia formatexigens, decreased in absolute abundance with an increase in dietary protein, while all others increased (Faith et al., 2011). Faith et al. speculated that the decrease in the three community members may reflect resource competition between community members. The overall microbial biomass response to protein amount can be attributed to the fact that nitrogen is a limiting nutrient in the large intestine, which has been demonstrated by additional studies in conventional mice (Holmes et al., 2017; Reese et al., 2018). When mice were fed diets ranging from 6% to 40% protein, microbial load increased in fecal samples with a corresponding increase in protein content (Reese et al., 2018).
Dietary protein amount has also been shown to mediate changes in relative abundance of specific taxonomic groups, as well as impact overall taxonomic diversity of the microbiota (Holmes et al., 2017; Kim et al., 2016). Increased dietary protein has been shown to lead to a significant decrease in diversity at both the family and genus level in studies examining murine microbiota response to high-protein diets (Holmes et al., 2017; Kim et al., 2016). Holmes et al. modeled microbiota composition after feeding mice 25 different diets with varying quantities of protein (5%–60%) (Holmes et al., 2017). Their approach divided taxa into guilds that represented responses to resource availability (rather than phylogeny) for their model. An increase in dietary protein was associated with significant increases in Clostridium, unnamed Clostridiales and Allobaculum and significant decreases in the genera Eubacterium, Akkermansia, Mucispirillum, Ruminococcus, Johnsonella, Alistipes, Butyrivibrio, and Blautia (Holmes et al., 2017). Another study found that the abundance of Bacteroidaceae increased with an increase in dietary protein (Reese et al., 2018). However, the modeling done by Holmes et al. found Bacteroidaceae to decrease with an increase in protein, demonstrating a lack of consensus between studies (Holmes et al., 2017). Bacteroidales was found to consume higher quantities of dietary nitrogen than other taxa when mice were fed diets in which the protein source was isotopically labeled, supporting the idea that Bacteroidales are stimulated by higher availability of nitrogen from dietary protein (Reese et al., 2018). Further support for the idea that some groups within the Bacteroidales order are stimulated by high dietary protein input comes from a recent study using isotopically heavy water to track microbial activity in bioreactors inoculated with a human fecal microbiota (Kleiner et al., 2021; Starke et al., 2020). The authors found that several Bacteroides species showed higher activity in bioreactors fed with a medium simulating a high-protein diet as compared to a high-fiber diet medium.
In summary, murine studies have provided evidence that overall microbial biomass increases but diversity decreases with an increase in dietary protein (Faith et al., 2011; Holmes et al., 2017; Kim et al., 2016; Reese et al., 2018). Decreased diversity has been linked with negative host health consequences based on associations with numerous diseases and clinical disease indicators (Manor et al., 2020; Turnbaugh et al., 2009). While compositionally different microbiotas have been shown to be similar in terms of function, due to functional redundancy (Lozupone et al., 2012), additional studies are needed to clarify compositional changes of individual taxa in response to protein quantity, as well as health impacts due to high protein-induced diversity decreases. Multiple Clostridia have been shown to increase in abundance with an increase in dietary protein but there are also instances in which Clostridia decrease in abundance with increased dietary protein (Faith et al., 2011; Holmes et al., 2017). Although there is no consensus (Holmes et al., 2017), several studies have associated an increase in abundance of Bacteroidia with an increase in dietary protein (Faith et al., 2011; Kleiner et al., 2021; Reese et al., 2018).
Impact of protein quantity on microbiota function
Studies in humans and animal models have connected an increase in dietary protein consumption with increased microbial protein fermentation in the colon (Beaumont et al., 2017; David et al., 2014; Mayneris-Perxachs et al., 2016). Protein in the colon is hydrolyzed by microbial proteases to peptides and amino acids. The amino acids can then be assimilated or fermented by the microbiota to produce a variety of end products, including short-chain fatty acids (SCFAs), ammonia, phenols, hydrogen sulfide, amines, and indoles (Oliphant and Allen-Vercoe, 2019). Murine studies that include diets with varying protein content have provided evidence that the functional response of the microbiota to an increase in dietary protein involves increased protein hydrolysis and amino acid fermentation (Faith et al., 2011; Mayneris-Perxachs et al., 2016). Untargeted mass spectrometry-based metabolomics has revealed significant differences in the plasma concentrations of many amino acid-derived metabolites between conventional and germ-free mice (Wikoff et al., 2009). Wikoff et al. found lower concentrations of tryptophan and N-acetyltryptophan in conventional mice, compared to germ-free mice. The authors speculated that this difference is likely due to the metabolism of dietary tryptophan by microbiota members in the conventional mice that express tryptophanase (Wikoff et al., 2009).
SCFAs are microbial metabolites produced from the fermentation of all amino acids (Table 2 in Oliphant and Allen-Vercoe, 2019). Acetate, butyrate, and propionate are among the most abundant SCFAs produced from microbial fermentation of many different amino acids (Smith and Macfarlane, 1997). Beneficial host health effects are frequently attributed to SCFAs (Nogal et al., 2021). However, studies investigating the health effects of SCFAs have largely examined these metabolites in the context of carbohydrates rather than protein, as carbohydrates are a major source of SCFAs.
Human studies examining the impact of a high-protein diet on microbiota function have reported inconsistent findings regarding changes to fecal butyrate following protein supplementation. Beaumont et al. associated an increase in dietary protein consumption with significantly lower levels of fecal butyrate (Beaumont et al., 2017). However, Moreno-Pérez et al. found no significant difference in fecal butyrate, or any other SCFAs measured, between individuals supplemented with protein and controls (Moreno-Pérez et al., 2018). Beaumont et al. found no significant differences in fecal propionate or acetate concentrations between individuals consuming protein supplements and control groups (Beaumont et al., 2017).
A specific group of SCFAs, namely branched short-chain fatty acids (BCFAs), is exclusively produced by microbial fermentation of the branched-chain amino acids leucine, isoleucine, and valine, and are thus often used as a marker of protein fermentation (Diether and Willing, 2019). Increased concentrations of the protein fermentation marking BCFAs isovalerate and isobutyrate were measured following protein supplementation of obese individuals (Beaumont et al., 2017). In contrast, no change in concentrations of BCFAs was observed following protein supplementation in athletes (Moreno-Pérez et al., 2018). Increased concentrations of BCFAs from microbial fermentation of protein have been shown to negatively impact liver insulin resistance in mice fed a high-fat high-sugar diet (Choi et al., 2021).
Additional protein fermentation end products that have been investigated and are associated with detrimental effects to host health, particularly in large quantities, include ammonia, phenol, and imidazole propionate (Gilbert et al., 2018; Portune et al., 2016; Russell et al., 2011). Ammonia can be produced through the deamination of numerous amino acids and phenol is a product of microbial fermentation of tyrosine (Portune et al., 2016; Smith and Macfarlane, 1997). Evidence suggests that both ammonia and phenol can reduce epithelial barrier function in vitro (Hughes et al., 2008). Imidazole propionate, a microbial metabolite of histidine, has been shown to induce glucose intolerance in mice and higher plasma concentrations have been found in individuals with type 2 diabetes (Koh et al., 2018; Molinaro et al., 2020). Increased plasma concentrations of this metabolite have also been shown to correlate with lower levels of microbial diversity (Molinaro et al., 2020). However, increased histidine consumption, based on food frequency questionnaires, was not found to correlate with increased levels of imidazole propionate.
There are a number of understudied microbial protein fermentation products for which preliminary evidence suggests benefits to the host at low concentrations but potentially detrimental host health consequences at higher concentrations. One example of this is indole, a fermentation product of tryptophan. Indole is not only a quorum-sensing molecule but has also been shown to be beneficial to maintaining the epithelial barrier of the host (Bansal et al., 2010; Shimada et al., 2013). Additionally, indole concentrations were found to be lower in mice upon induction of colitis (Alexeev et al., 2018). However, upon host absorption of indole, it is metabolized to indoxyl-sulfate in the liver. Indoxyl-sulfate has been associated with neuropsychiatric disorders and accumulates in patients with chronic kidney disease (Brydges et al., 2021). Another example of a protein fermentation metabolite for which host health consequences are dependent on concentration is hydrogen sulfide, which is produced by microbial fermentation of cysteine and methionine (Buret et al., 2022).
When considering protein quantity, it is also worth taking into account the influence of protein-deficient diets relevant for the study of malnourishment. Little research has been done in this area. One of the few exceptions is a study in mice with a conventional microbiota, in which a diet deficient in protein (2%) resulted in lower concentrations of protein fermentation metabolites in urine, including BCFAs, compared to mice consuming standard (20%) protein diets (Mayneris-Perxachs et al., 2016).
Impact of protein source on microbiota composition
The majority of dietary protein sources can be divided into two overarching categories, plant-derived protein and animal-derived protein. Plant-based protein is generally considered to be less digestible compared to animal-based protein. The current approach for assessing protein quality is the Digestible Indispensable Amino Acid Score (DIAAS). The DIAAS of plant-based proteins is typically lower than animal-based proteins (Marinangeli and House, 2017; Mathai et al., 2017). Based on the lower digestibility of plant protein, one would expect that if equal quantities of animal and plant protein were consumed, a greater proportion of plant protein would reach the colon and correspondingly result in greater protein fermentation by the microbiota. Microbial fermentation of protein in the colon has been implicated in a number of diseases including inflammatory bowel disease, colorectal cancer, as well as metabolic diseases (Beaumont et al., 2017; Choi et al., 2021; Jantchou et al., 2010; Jowett et al., 2004; Newgard et al., 2009; Russell et al., 2011). Therefore, one would expect a diet high in plant protein would be associated with negative host health outcomes due to increased protein fermentation in the colon. However, multiple large cohort studies contradict this expectation, as increased plant protein intake has been associated with beneficial host health outcomes (Budhathoki et al., 2019; Huang et al., 2020; Jantchou et al., 2010; Song et al., 2019). Thus, a major conundrum exists regarding our understanding of the relationship between dietary protein source, the microbiota, and resulting host health outcomes. One may consider fiber to play a role in this conundrum, as increased plant protein consumption likely corresponds to increased fiber consumption from the accompanying plant material. However, adjustments for fiber were made in the most recent large cohort studies (Budhathoki et al., 2019; Huang et al., 2020; Song et al., 2019). It is also worth noting that there is evidence that microbiota interactions with other dietary components of animal-based protein sources, such as the microbial production of trimethylamine from choline in red meat, contribute to negative host health consequences (Koeth et al., 2013).
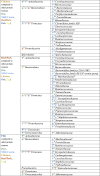
Controlled human studies that exclusively investigate the influence of different dietary protein sources on microbiota composition are extremely limited, thus the current knowledge in the field is primarily based on animal studies. Studies in rodents have found several dietary protein sources to differentially impact microbiota composition including soy, milk/casein, chicken, beef, pork, fish, and egg (An et al., 2014; Bai et al., 2016, 2018; Butteiger et al., 2016; Sivixay et al., 2021; Xia et al., 2020; Zhu et al., 2015, 2017) (Table 1). Soy is the most common plant-based protein source examined in these dietary protein-microbiota rodent studies. The microbiota of rodents on a soy-based protein diet has been found to be more diverse in comparison to multiple animal-based protein diets, including milk and egg protein (An et al., 2014; Butteiger et al., 2016; Sivixay et al., 2021; Xia et al., 2020). However, these diversity differences are not consistent across all studies (Zhu et al., 2015, 2017). While some studies have found differences in microbiota diversity when comparing casein (the main protein in milk) and soy-fed rodents, when these two protein sources are compared with other protein sources, microbiota composition is more similar for casein and soy-fed rodents compared to other dietary protein sources (An et al., 2014; Bai et al., 2016; Sivixay et al., 2021; Xia et al., 2020; Zhu et al., 2015, 2017). When rats were fed six different protein sources (casein, soy, pork, beef, chicken, and fish), the phyla composition of the rats fed casein and soy was more similar to each other than to the other protein sources (Zhu et al., 2015). Zhu et al. reported a higher relative abundance of Ruminococcacea OTUs in soy-fed rats that positively correlated with glucose, galactose, and ribose (Zhu et al., 2017). The authors speculated that the relative abundance increases of these microbiota members indicate that soy protein may favor bacteria with the ability to degrade glycans. Fiber-like effects have also been suggested for egg white proteins but additional studies are needed to clarify and elucidate this mechanism (Xia et al., 2020).
Table 1.
Impact of dietary protein source on microbiota composition at the phylum and family levels
 |
 |
 |
Casein-based diets are used as a control group in some studies, as casein is the protein component of the defined AIN-93 laboratory rodent diet recommended by the American Institute of Nutrition (Bai et al., 2016; Reeves et al., 1993; Zhu et al., 2015, 2017). When Choi et al. compared casein-fed mice to mice fed a protein mix (composed of 10 different protein sources), they found changes to the microbiota regardless of fat and carbohydrate content of the diets (Choi et al., 2021). Microbial diversity was higher for mice that consumed the protein mix both when it was consumed in the context of a low-fat low-sucrose diet or high-fat high-sucrose diet. Additionally, there were particular abundance changes of individual community members, such as a decrease in Akkermansia muciniphila, when mice consumed the protein mix diets (Choi et al., 2021).
Microbiota compositional changes have also been associated with other animal-based protein sources including chicken, beef, pork, fish, and egg (An et al., 2014; Bai et al., 2018; Sivixay et al., 2021; Xia et al., 2020; Zhu et al., 2015, 2017). We have summarized significant differences in relative abundance at the phylum and family levels from studies performed in rodent models in Table 1. Changes in microbiota composition have the potential to influence the colonic metabolite pool and in turn host health. However, as described above (See: impact of protein quantity on microbiota function), there is much to be investigated regarding the impact of microbial protein fermentation products to host health. Furthermore, the concentration of BCFAs and other protein fermentation metabolites will depend not only on the microbiota species composition but also on the quantity of protein consumed, the source of the protein, and the digestibility (See: protein availability in the colon).
Microbiota compositional changes have also been reported in gnotobiotic mice following consumption of whole foods providing some of the protein sources described above. In mice colonized with bacterial isolates from Bangladeshi children, diets with milk powder, tilapia, and eggs correlated with significant abundance changes of multiple community members (Gehrig et al., 2019). Milk powder was positively correlated with the abundance of Bifidobacterium longum and negatively correlated with the abundance of eight different community members. The relative abundance of six clostridia (Ruminococcus torques, Dorea longicatena, Blautia obeum (previously Ruminococcus obeum), Faecalibacterium prausnitzii, Dorea formicigenerans, and Blautia luti), a Gammaproteobacteria (Escherichia fergusonii), and a Bacilli (Streptococcus pasteurianus) was negatively correlated with milk powder. Tilapia was positively correlated with an increase in the relative abundance of D. longicatena, B.obeum, B. luti, E.fergusonii, and S. pasteurianus and negatively correlated with B. longum. Egg was positively correlated with the relative abundance of B. longum and negatively correlated with D. formicigenerans, Streptococcus constellatus, B. luti, and S. pasteurianus.
The differences in digestibility between plant and animal proteins may provide some explanation as to why plant proteins impact the microbiota composition differently than animal proteins. However, it has been shown that even within the category of animal proteins, microbiota composition can be differentially impacted, exemplified above by the differences between meat, casein, fish, and egg protein diets (An et al., 2014; Bai et al., 2018; Sivixay et al., 2021; Xia et al., 2020; Zhu et al., 2015, 2017). Furthermore, microbiota composition responds differently to protein from the same organism (e.g. casein and beef), illustrating the uniqueness of each protein source (Zhu et al., 2015). These findings demonstrate the urgent need for additional studies investigating the impact of purified protein and protein from whole foods on the composition of the intestinal microbiota.
Impact of protein source on microbiota function
The few studies investigating the relationship between dietary protein source and the intestinal microbiota suggest protein source influences microbiota function. Microbiota functional changes characterized so far are largely based on differences in metabolites produced by protein hydrolysis and amino acid fermentation (Beaumont et al., 2017; Zhu et al., 2017).
Differences in fecal pH and branched-chain amino acid concentrations have been found in overweight humans following consumption of different protein sources (Beaumont et al., 2017). Individuals supplemented with casein-based protein were found to have a significantly higher fecal pH (0.5 higher at pH 6.9) compared to the soy protein and control groups (Beaumont et al., 2017). The increase in pH was negatively correlated with the concentration of butyrate in the casein group. Additionally, greater concentrations of branched-chain amino acids were measured in the casein group compared to the soy and control groups, suggesting increased protein degradation by the microbiota (Beaumont et al., 2017). Similarly, another study found casein-fed rats to have significantly higher concentrations of branched-chain amino acids in cecal material compared to rats on beef, chicken, or soy diets (Zhu et al., 2017).
Studies in rodents have associated changes in microbiota function with different dietary protein sources, including soy, casein, fish, beef, chicken, and egg (An et al., 2014; Bai et al., 2016, 2018; Choi et al., 2021; Sivixay et al., 2021; Zhu et al., 2017). As previously discussed (See: impact of protein quantity on Microbiota Function), the SCFAs butyrate, propionate, and acetate are major products of microbial fermentation of amino acids. Butyric acid concentrations were found to be significantly higher in soy-fed rats compared to rats on casein or fish diets (An et al., 2014). However, more recent studies have not found significant differences in butyric acid concentrations between mice on soy, casein, fish, meat, or egg-based protein diets (Bai et al., 2016, 2018; Sivixay et al., 2021). Bai et al. reported a significantly lower concentration of propionic acid in soy-fed rats compared to meat-fed rats (Bai et al., 2018). When mice were fed diets with either casein or a protein mix (composed of 10 different protein sources), differences in microbiota function were revealed in the context of low-fat low-sucrose and high-fat high-sucrose diets (Choi et al., 2021). Butyric, propionic, and acetic acids in fecal content were higher for the mice consuming the protein mix compared to mice consuming casein in the context of a low-fat low-sucrose diet (Choi et al., 2021). BCFAs were higher in the fecal content of mice consuming the protein mix compared to casein in the context of a high-fat high-sucrose diet (Choi et al., 2021). Furthermore, mice consuming the protein mix had more severe health consequences from the high-sucrose high-fat diet compared to purified casein (Choi et al., 2021). Outside of SCFAs, indole, hydrogen sulfide, and phenol levels were reported as significantly higher in rats fed fish or soy compared to casein (An et al., 2014).
Fecal and colonic metabolite profiles have been found to vary with dietary protein source in both humans and animal models (Beaumont et al., 2017; Zhu et al., 2017). When overweight individuals consumed soy or casein protein supplements, fecal metabolite profiles of each supplement group differed according to protein source (Beaumont et al., 2017). For example, acetoin, a bacterial metabolite produced from the fermentation of soy, was only detected in the soy-supplemented group (Beaumont et al., 2017). In participants consuming the soy supplement, the relative concentrations of the microbial fermentation metabolites valerate, phenylacetate, and tyramine, all of which can be produced from amino acids, were higher in fecal samples compared to casein-supplemented and control groups (Beaumont et al., 2017). Colonic metabolite profiles of rats on a chicken-based diet have been shown to be distinct from metabolite profiles of rats fed casein, beef, and soy diets (Zhu et al., 2017). A significantly higher concentration of lactate in the chicken-fed rats distinguished the diet group in this study (Zhu et al., 2017). Zhu et al. also found similarity in the colonic metabolite profiles of rats fed soy and beef diets.
Although current evidence suggests dietary protein source to be influential to microbiota function, it is clear that additional studies are needed to clarify conflicting results and investigate functional changes in greater depth. We have outlined pressing questions and potential strategies for investigation below.
Major open questions
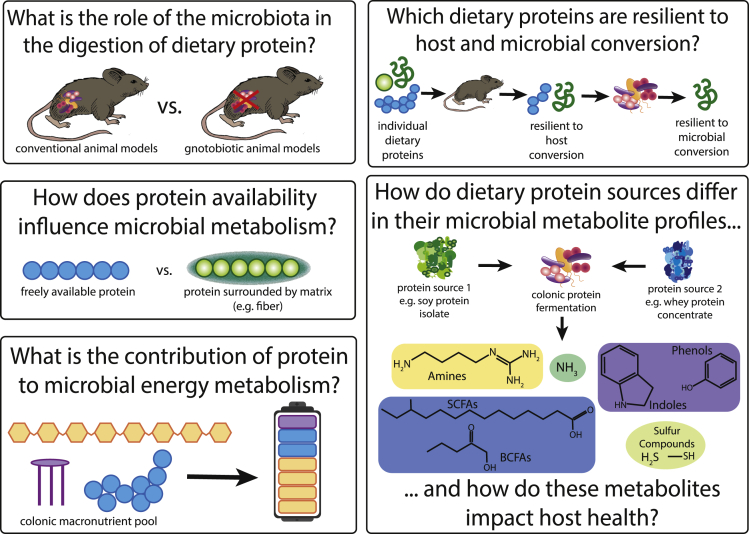
As we illustrate in this review, our understanding of interactions between dietary protein, the microbiota, and host health is limited and there are many open questions (Figure 1). One key point that hopefully has become obvious in this review is that not only protein quantity has strong effects on microbiota composition and function but also that protein source has similar or even stronger effects. This highlights that, similar to type of fiber, which has been shown to have strong effects on microbiota function, type of protein consumed matters. While an understanding of the mechanism behind responses to protein quantity has been emerging with protein alleviating nitrogen limitation for microbes in the intestinal tract, the mechanisms behind effects of protein source are poorly understood. Potential mechanisms that have been suggested or can be speculated on include: (1) variability in host digestion of protein sources in the upper intestinal tract driven by protein accessibility and antinutritional factors leads to differences in the quality and amount of protein that reaches the colon; (2) different amino acid composition of dietary proteins favors different microbes; (3) non-proteinaceous substances that are associated with dietary protein impact microbiota composition and function; and (4) post-translational modifications of proteins, such as glycosylations, interact with the microbiota. Investigation of these potential mechanisms is urgently needed to develop our understanding of effects of types of protein sources on the microbiota and subsequent impacts on host health.
Figure 1.
Pressing questions in the field of dietary protein-microbiota-host interactions
All of the questions we have outlined in Figure 1 require carefully controlled studies that extend beyond the current correlation-based data in order to assess microbiota functional responses. Fortunately, there are multiple emerging methods to potentially address these questions. Two important questions to answer in the realm of protein-microbiota interactions include, which individual dietary proteins are resilient to host and microbial conversion? and how does protein availability influence microbial metabolism? Determining which dietary proteins are resilient to host digestion and absorption will inform which proteins and how much reach the microbiota. For example, of the hundreds of individual soy proteins that make up a soy protein isolate, which individual soy proteins are most likely to be absorbed by the host? Which individual soy proteins are likely to be resilient to host digestion, and reach the colon? Of the individual proteins that reach the colon, are they accessible to microbial proteases (or are they trapped in fiber, etc.)?
To answer these questions relating to protein digestibility, identification and quantification of individual dietary proteins throughout the intestinal tract is required. Identification and quantification of individual proteins throughout the intestinal tract will provide insight on which proteins are largely resilient to host digestion (proteins that reach the end of the small intestine) and resilient to microbial fermentation (proteins that reach the fecal material). Measurement of individual protein abundances throughout the intestinal tract will also elucidate the role of the microbiota in the digestion of dietary protein.
Germ-free animal models can be used to assess the role of not only the microbial community in the colon but also the microbes in the upper intestinal tract. One reason it is important to understand the role of the upper intestinal tract microbiota in protein digestion is that the current method for assessing host protein digestibility overlooks the contribution of upper intestinal tract microbes. Protein digestibility is currently measured from ileal effluent. Ileal effluent allows for assessment of protein digestibility before protein is fermented by the colonic microbiota. However, there are still a large number of bacteria in the upper intestinal tract (103–104 bacteria/mL in the duodenum and jejunum, 108 bacteria/mL in the ileum) that may play a role in protein digestion (Sender et al., 2016). Comparing germ-free and conventional (or conventionalized/gnotobiotic) model animals will allow for differentiation between host and microbiota-driven dietary protein digestion. Understanding the role of the entire intestinal microbiota in the digestion of dietary protein is vital to assess the impact of microbial protein fermentation to the host.
The next set of questions relates to the functional interactions of the microbiota and dietary protein; more specifically, how does dietary protein in the colon impact microbial activity and metabolism? As described above, nitrogen has been shown to be a limiting nutrient in the colon for the microbiota and increased nitrogen availability from higher amounts of dietary protein has been shown to stimulate specific microbial species in the colon (Faith et al., 2011; Holmes et al., 2017; Reese et al., 2018). We expect that stimulation of specific microbial species leads to changes in the colonic metabolite pool based on changes in the substrates consumed by the microbiota. Colonic metabolite changes may reflect not only increased protein fermentation but also changes to the consumption of other substrates. Which species are responsible for changing substrate and end product concentrations is difficult to assess by correlating taxonomic abundance changes with metabolite changes alone. To understand the role of microbial species in metabolite conversions and assimilation, studies are needed that directly measure assimilation of carbon and nitrogen by microbial species, as well as stimulation of specific species without increased assimilation. These direct evidence-based studies will allow us to answer, what is the contribution of protein to microbial energy metabolism and biomass assimilation?
There is a wide array of microbial metabolites that can be produced by the fermentation of amino acids by the microbiota. Different protein sources have different amino acid compositions (Joye, 2019; Marinangeli and House, 2017; Mathai et al., 2017). The varying amino acid compositions of different protein sources mean that microbial metabolite profiles should vary depending upon the source and type of protein. Many end products of protein fermentation may be detrimental to the host. However, the amounts and types of microbial metabolites produced from different protein sources are currently understudied. For example, hydrogen sulfide is known to be a product of microbial fermentation of cysteine (Oliphant and Allen-Vercoe, 2019; Portune et al., 2016). Does consumption of a dietary protein source high in cysteine result in increased concentrations of hydrogen sulfide in the colon?
In addition, it would be valuable to differentiate the source of metabolites that are produced from different substrates. For example, SCFAs primarily originate from carbohydrates but are also a product of amino acid fermentation. SCFAs represent protein fermentation products that may be beneficial to host health but the exact quantity of SCFAs contributed from protein is currently unknown. Quantifying the contribution of SCFAs from dietary protein will elucidate the functional impact of protein fermentation to host health through the differentiation of protein fermentation products that may be beneficial versus detrimental to host health.
Future directions
There are a number of emerging approaches to address pressing questions in the field of dietary protein-microbiota interactions. A combination of meta-omics techniques will be particularly useful in unraveling the relationship between dietary protein and the intestinal microbiota. Notably, metaproteomic analysis of intestinal samples allows for the identification and quantification of thousands of proteins from the diet, host, and microbiota in one measurement and thus this approach is key to understanding dietary protein-microbiota interactions (Kleiner et al., 2017; McNulty et al., 2013; Mottawea et al., 2016; Patnode et al., 2019; Salvato et al., 2021). In addition to basic metaproteomic approaches that allow for identifying and quantifying proteins in microbiome samples, there are advanced metaproteomic methods to directly link dietary components to their microbial metabolizers (Kleiner et al., 2018, 2021; Smyth et al., 2020). Metaproteomics can be combined with additional approaches to further address the questions we have outlined above. For example, Patnode et al. used metaproteomics, genetic screening, and artificial food particles (magnetic beads bound with dietary fibers) to assess fiber degradation by specific microbiota species (Patnode et al., 2019). This technique could be applied to studying dietary protein by using similar magnetic beads bound with different dietary proteins. We point readers to a recent mini-review by Salvato et al., for an introduction to metaproteomics (Salvato et al., 2021).
Well-controlled studies that focus exclusively on dietary protein source and quantity will aid in furthering our understanding of this dietary component that constitutes a vital portion of the human diet. When designing protein-microbiota studies, it is important to consider interference of nonprotein dietary components and to disambiguate host effects from microbiota effects, which can be achieved with the use of fully defined diets and gnotobiotic animal models. Such studies will elucidate the currently understudied relationship of dietary protein and the intestinal microbiota and contribute to the ultimate goal of modulating the microbiota to benefit host health.
Acknowledgments
We are grateful to Dr. Heather Maughan and Dr. Matthew Foley for feedback on the manuscript. We thank all members of the Kleiner Lab for insightful comments on the figures, in particular Angie Mordant for her contribution. This work was supported by the USDA National Institute of Food and Agriculture Hatch project 1014212, by the National Institutes of Health P30 DK034987, the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R35GM138362, and the Foundation for Food and Agriculture Research Grant ID: 593607.
Author contributions
A.B. and M.K. both wrote the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Alexandria Bartlett, Email: aebartl2@ncsu.edu.
Manuel Kleiner, Email: manuel_kleiner@ncsu.edu.
References
- Acton J.C., Breyer L., Satterlee L.D. Effect of dietary fiber constituents on the in vitro digestibility of casein. J. Food Sci. 1982;47:556–560. doi: 10.1111/j.1365-2621.1982.tb10122.x. [DOI] [Google Scholar]
- Alexeev E.E., Lanis J.M., Kao D.J., Campbell E.L., Kelly C.J., Battista K.D., Gerich M.E., Jenkins B.R., Walk S.T., Kominsky D.J., Colgan S.P. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 2018;188:1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C., Kuda T., Yazaki T., Takahashi H., Kimura B. Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl. Microbiol. Biotechnol. 2014;98:2779–2787. doi: 10.1007/s00253-013-5271-5. [DOI] [PubMed] [Google Scholar]
- Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Bai G., Ni K., Tsuruta T., Nishino N. Dietary casein and soy protein isolate modulate the effects of raffinose and fructooligosaccharides on the composition and fermentation of gut microbiota in rats. J. Food Sci. 2016;81:H2093–H2098. doi: 10.1111/1750-3841.13391. [DOI] [PubMed] [Google Scholar]
- Bai G., Tsuruta T., Nishino N. Dietary soy, meat, and fish proteins modulate the effects of prebiotic raffinose on composition and fermentation of gut microbiota in rats. Int. J. Food Sci. Nutr. 2018;69:480–487. doi: 10.1080/09637486.2017.1382454. [DOI] [PubMed] [Google Scholar]
- Bansal T., Alaniz R.C., Wood T.K., Jayaraman A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA. 2010;107:228–233. doi: 10.1073/pnas.0906112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbé F., Le Feunteun S., Rémond D., Ménard O., Jardin J., Henry G., Laroche B., Dupont D. Tracking the in vivo release of bioactive peptides in the gut during digestion: mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrix fed mini-pigs. Food Res. Int. 2014;63:147–156. doi: 10.1016/j.foodres.2014.02.015. [DOI] [Google Scholar]
- Beaumont M., Portune K.J., Steuer N., Lan A., Cerrudo V., Audebert M., Dumont F., Mancano G., Khodorova N., Andriamihaja M., et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 2017;106:1005–1019. doi: 10.3945/ajcn.117.158816. [DOI] [PubMed] [Google Scholar]
- Brydges C.R., Fiehn O., Mayberg H.S., Schreiber H., Dehkordi S.M., Bhattacharyya S., Cha J., Choi K.S., Craighead W.E., Krishnan R.R., et al. Mood Disorders Precision Medicine Consortium Indoxyl sulfate, a gut microbiome-derived uremic toxin, is associated with psychic anxiety and its functional magnetic resonance imaging-based neurologic signature. Sci. Rep. 2021;11:21011. doi: 10.1038/s41598-021-99845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhathoki S., Sawada N., Iwasaki M., Yamaji T., Goto A., Kotemori A., Ishihara J., Takachi R., Charvat H., Mizoue T., et al. Japan Public Health Center–based Prospective Study Group. For the Japan public health center–based prospective study group Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern. Med. 2019;179:1509–1518. doi: 10.1001/jamainternmed.2019.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buret A.G., Allain T., Motta J.-P., Wallace J.L. Effects of hydrogen sulfide on the microbiome: from toxicity to therapy. Antioxid. Redox Signal. 2022;36:211–219. doi: 10.1089/ars.2021.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butteiger D.N., Hibberd A.A., McGraw N.J., Napawan N., Hall-Porter J.M., Krul E.S. Soy protein compared with milk protein in a western diet increases gut microbial diversity and reduces serum lipids in golden Syrian hamsters. J. Nutr. 2016;146:697–705. doi: 10.3945/jn.115.224196. [DOI] [PubMed] [Google Scholar]
- Carmody R.N., Wrangham R.W. The energetic significance of cooking. J. Hum. Evol. 2009;57:379–391. doi: 10.1016/j.jhevol.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Choi B.S.-Y., Daniel N., Houde V.P., Ouellette A., Marcotte B., Varin T.V., Vors C., Feutry P., Ilkayeva O., Ståhlman M., et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat. Commun. 2021;12:3377. doi: 10.1038/s41467-021-23782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotillard A., Kennedy S.P., Kong L.C., Prifti E., Pons N., Le Chatelier E., Almeida M., Quinquis B., Levenez F., Galleron N., Gougis S., Rizkalla S., Batto J.M., Renault P., ANR MicroObes consortium. Doré J., Zucker J.D., Clément K., Ehrlich S.D., Leclerc M., Juste C., De Wouters T., Lepage P., Fouqueray C., Basdevant A., Henegar C., Godard C., Fondacci M., Rohia A., Hajduch F., Weissenbach J., Pelletier E., Le Paslier D., Gauchi J.P., Gibrat J.F., Loux V., Carré W., Maguin E., Van De Guchte M., Jamet A., Boumezbeur F., Layec S. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M.S., Seekatz A.M., Koropatkin N.M., Kamada N., Hickey C.A., Wolter M., Pudlo N.A., Kitamoto S., Terrapon N., Muller A., et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353.e21. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diether N.E., Willing B.P. Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms. 2019;7:19. doi: 10.3390/microorganisms7010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Feng Q., Liang S., Sonne S.B., Xia Z., Qiu X., Li X., Long H., Zhang J., Zhang D., et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015;33:1103–1108. doi: 10.1038/nbt.3353. [DOI] [PubMed] [Google Scholar]
- Eckburg P.B., Bik E.M., Bernstein C.N., Purdom E., Dethlefsen L., Sargent M., Gill S.R., Nelson K.E., Relman D.A. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenepoel P., Claus D., Geypens B., Hiele M., Geboes K., Rutgeerts P., Ghoos Y. Amount and fate of egg protein escaping assimilation in the small intestine of humans. Am. J. Physiol. 1999;277:G935–G943. doi: 10.1152/ajpgi.1999.277.5.G935. [DOI] [PubMed] [Google Scholar]
- Faith J.J., Mcnulty N.P., Rey F.E., Gordon J.I. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025.Predicting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrig J.L., Venkatesh S., Chang H.-W., Hibberd M.C., Kung V.L., Cheng J., Chen R.Y., Subramanian S., Cowardin C.A., Meier M.F., et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science. 2019;365:eaau4732. doi: 10.1126/science.aau4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M.S., Ijssennagger N., Kies A.K., van Mil S.W.C. Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G159–G170. doi: 10.1152/ajpgi.00319.2017. [DOI] [PubMed] [Google Scholar]
- Holmes A.J., Chew Y.V., Colakoglu F., Cliff J.B., Klaassens E., Read M.N., Solon-Biet S.M., McMahon A.C., Cogger V.C., Ruohonen K., et al. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metabol. 2017;25:140–151. doi: 10.1016/j.cmet.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Huang J., Liao L.M., Weinstein S.J., Sinha R., Graubard B.I., Albanes D. Association between plant and animal protein intake and overall and cause-specific mortality. JAMA Intern. Med. 2020;180:1173–1184. doi: 10.1001/jamainternmed.2020.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R., Kurth M.J., McGilligan V., McGlynn H., Rowland I. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr. Cancer. 2008;60:259–266. doi: 10.1080/01635580701649644. [DOI] [PubMed] [Google Scholar]
- Jantchou P., Morois S., Clavel-Chapelon F., Boutron-Ruault M.C., Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: the E3N prospective study. Am. J. Gastroenterol. 2010;105:2195–2201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- Jowett S.L., Seal C.J., Pearce M.S., Phillips E., Gregory W., Barton J.R., Welfare M.R. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joye I. Protein digestibility of cereal products. Foods. 2019;8:199. doi: 10.3390/foods8060199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Kim D.B., Park J.Y. Changes of mouse gut microbiota diversity and composition by modulating dietary protein and carbohydrate contents: a pilot study. Prev. Nutr. Food Sci. 2016;21:57–61. doi: 10.3746/pnf.2016.21.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M., Dong X., Hinzke T., Wippler J., Thorson E., Mayer B., Strous M. Metaproteomics method to determine carbon sources and assimilation pathways of species in microbial communities. Proc. Natl. Acad. Sci. USA. 2018;115:E5576–E5584. doi: 10.1073/pnas.1722325115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner M., Kouris A., Jensen M., Liu Y., McCalder J., Strous M. Ultra-sensitive Protein-SIP to quantify activity and substrate uptake in microbiomes with stable isotopes. bioRxiv. 2021 doi: 10.1101/2021.03.29.437612. Preprint at. [DOI] [Google Scholar]
- Kleiner M., Thorson E., Sharp C.E., Dong X., Liu D., Li C., Strous M. Assessing species biomass contributions in microbial communities via metaproteomics. Nat. Commun. 2017;8:1558–1614. doi: 10.1038/s41467-017-01544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L., et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh A., Molinaro A., Ståhlman M., Khan M.T., Schmidt C., Mannerås-Holm L., Wu H., Carreras A., Jeong H., Olofsson L.E., et al. Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell. 2018;175:947–961.e17. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]
- Laukens D., Brinkman B.M., Raes J., De Vos M., Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol. Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor O., Dai C.L., Kornilov S.A., Smith B., Price N.D., Lovejoy J.C., Gibbons S.M., Magis A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020;11:5206. doi: 10.1038/s41467-020-18871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinangeli C.P.F., House J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017;75:658–667. doi: 10.1093/nutrit/nux025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai J.K., Liu Y., Stein H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS) Br. J. Nutr. 2017;117:490–499. doi: 10.1017/S0007114517000125. [DOI] [PubMed] [Google Scholar]
- Mayneris-Perxachs J., Bolick D.T., Leng J., Medlock G.L., Kolling G.L., Papin J.A., Swann J.R., Guerrant R.L. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr. 2016;104:1253–1262. doi: 10.3945/ajcn.116.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty N.P., Wu M., Erickson A.R., Pan C., Erickson B.K., Martens E.C., Pudlo N.A., Muegge B.D., Henrissat B., Hettich R.L., Gordon J.I. Effects of diet on resource utilization by a model human gut microbiota containing Bacteroides cellulosilyticus WH2, a symbiont with an extensive glycobiome. PLoS Biol. 2013;11:e1001637. doi: 10.1371/journal.pbio.1001637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaro A., Bel Lassen P., Henricsson M., Wu H., Adriouch S., Belda E., Chakaroun R., Nielsen T., Bergh P.-O., Rouault C., André S., Marquet F., Andreelli F., Salem J.-E., Assmann K., Bastard J.-P., Forslund S., Le Chatelier E., Falony G., Pons N., Prifti E., Quinquis B., Roume H., Vieira-Silva S., Hansen T.H., Pedersen H.K., Lewinter C., Sønderskov N.B., MetaCardis Consortium. Køber L., Vestergaard H., Hansen T., Zucker J.D., Galan P., Dumas M.E., Raes J., Oppert J.M., Letunic I., Nielsen J., Bork P., Ehrlich S.D., Stumvoll M., Pedersen O., Aron-Wisnewsky J., Clément K., Bäckhed F. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020;11:5881. doi: 10.1038/s41467-020-19589-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez D., Bressa C., Bailén M., Hamed-Bousdar S., Naclerio F., Carmona M., Pérez M., González-Soltero R., Montalvo-Lominchar M.G., Carabaña C., Larrosa M. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients. 2018;10:337. doi: 10.3390/nu10030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W., Chiang C.-K., Mühlbauer M., Starr A.E., Butcher J., Abujamel T., Deeke S.A., Brandel A., Zhou H., Shokralla S., et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat. Commun. 2016;7:13419. doi: 10.1038/ncomms13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moughan P.J., Rutherfurd S.M. Gut luminal endogenous protein: implications for the determination of ileal amino acid digestibility in humans. Br. J. Nutr. 2012;108:S258–S263. doi: 10.1017/S0007114512002474. [DOI] [PubMed] [Google Scholar]
- Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., Haqq A.M., Shah S.H., Arlotto M., Slentz C.A., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Lochlainn M., Bowyer R.C.E., Steves C.J. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10:929. doi: 10.3390/nu10070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogal A., Valdes A.M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microb. 2021;13:1–24. doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnode M.L., Beller Z.W., Han N.D., Cheng J., Peters S.L., Terrapon N., Henrissat B., Le Gall S., Saulnier L., Hayashi D.K., et al. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179:59–73.e13. doi: 10.1016/j.cell.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portune K.J., Beaumont M., Davila A.-M., Tomé D., Blachier F., Sanz Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci. Technol. 2016;57:213–232. doi: 10.1016/j.tifs.2016.08.011. [DOI] [Google Scholar]
- Reese A.T., Carmody R.N. Thinking outside the cereal box: noncarbohydrate routes for dietary manipulation of the gut microbiota. Appl. Environ. Microbiol. 2019;85:e022466-18. doi: 10.1128/AEM.02246-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese A.T., Pereira F.C., Schintlmeister A., Berry D., Wagner M., Hale L.P., Wu A., Jiang S., Durand H.K., Zhou X., et al. Microbial nitrogen limitation in the mammalian large intestine. Nat. Microbiol. 2018;3:1441–1450. doi: 10.1038/s41564-018-0267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.G., Nielsen F.H., Fahey G.C. AIN-93 purified diets for laboratory rodents: final report of the American Institute of nutrition ad hoc writing committee on the reformulation of the AIN-76a rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Russell W.R., Gratz S.W., Duncan S.H., Holtrop G., Ince J., Scobbie L., Duncan G., Johnstone A.M., Lobley G.E., Wallace R.J., et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 2011;93:1062–1072. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- Salonen A., Lahti L., Salojärvi J., Holtrop G., Korpela K., Duncan S.H., Date P., Farquharson F., Johnstone A.M., Lobley G.E., et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvato F., Hettich R.L., Kleiner M. Five key aspects of metaproteomics as a tool to understand functional interactions in host-associated microbiomes. PLoS Pathog. 2021;17:e1009245. doi: 10.1371/journal.ppat.1009245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y., Kinoshita M., Harada K., Mizutani M., Masahata K., Kayama H., Takeda K. Commensal bacteria-dependent indole production enhances epithelial barrier function in the colon. PLoS One. 2013;8:e80604. doi: 10.1371/journal.pone.0080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvester K.R., Cummings J.H. Does digestibility of meat protein help explain large bowel cancer risk? Nutr. Cancer. 1995;24:279–288. doi: 10.1080/01635589509514417. [DOI] [PubMed] [Google Scholar]
- Sivixay S., Bai G., Tsuruta T., Nishino N. Cecum microbiota in rats fed soy, milk, meat, fish, and egg proteins with prebiotic oligosaccharides. AIMS Microbiol. 2021;7:1–12. doi: 10.3934/microbiol.2021001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E.A., Macfarlane G.T. Dissimilatory amino Acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- Smyth P., Zhang X., Ning Z., Mayne J., Moore J.I., Walker K., Lavallée-Adam M., Figeys D. Studying the temporal dynamics of the gut microbiota using metabolic stable isotope labeling and metaproteomics. Anal. Chem. 2020;92:15711–15718. doi: 10.1021/acs.analchem.0c02070. [DOI] [PubMed] [Google Scholar]
- Song M., Fung T.T., Hu F.B., Willett W.C., Longo V.D., Chan A.T., Giovannucci E.L. 02114. 2019. pp. 1453–1463. (Association of Animal and Plant Protein Intake with All-Cause and Cause-specific Mortality). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke R., Oliphant K., Jehmlich N., Schäpe S.S., Sachsenberg T., Kohlbacher O., Allen-Vercoe E., von Bergen M. Tracing incorporation of heavy water into proteins for species-specific metabolic activity in complex communities. J. Proteomics. 2020;222:103791. doi: 10.1016/j.jprot.2020.103791. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA, Center for Nutrition Policy . Government Printing Office; 2015. Dietary Guidelines for Americans 2015-2020. [Google Scholar]
- Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A., et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Fukunaga M., Kuda T., Goto M., Chiaraluce G., Hoshiba H., Takahashi H., Kimura B. Detection and isolation of protein susceptible indigenous bacteria affected by dietary milk-casein, albumen and soy-protein in the caecum of ICR mice. Int. J. Biol. Macromol. 2020;144:813–820. doi: 10.1016/j.ijbiomac.2019.09.159. [DOI] [PubMed] [Google Scholar]
- Young V.R., El-Khoury A.E., Raguso C.A., Forslund A.H., Hambraeus L. Rates of urea production and hydrolysis and leucine oxidation change linearly over widely varying protein intakes in healthy adults. J. Nutr. 2000;130:761–766. doi: 10.1093/jn/130.4.761. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Lin X., Zhao F., Shi X., Li H., Li Y., Zhu W., Xu X., Li C., Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015;5:15220–15314. doi: 10.1038/srep15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Shi X., Lin X., Ye K., Xu X., Li C., Zhou G. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front. Microbiol. 2017;8:1395. doi: 10.3389/fmicb.2017.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]