Abstract
Medicinal applications of turmeric-derived curcumin have been known to mankind for long ages. Its potential in managing “cystic fibrosis” has also been evaluated. This autosomal recessive genetic disease is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) which involves an impaired secretion of chloride ions and leads to hypersecretion of thick and sticky mucus and serious complications including airway obstruction, chronic lung infection, and inflammatory reactions. This narrative review aims to highlight the available evidence for the efficacy of curcumin nanoformulations in its potential treatment of cystic fibrosis. Recent research has shown that curcumin acts on the localized mutant CFTR ion channel at the plasma membrane. Preclinical studies have also shown that curcumin nanoformulations have promising effects in the treatment of cystic fibrosis. In this context, the purpose of this narrative review is to highlight the general bioactivity of curcumin, the types of formulations and related studies, thus opening new therapeutic perspectives for CF.
Keywords: Cystic fibrosis, Curcumin nanoparticles, Bioavailability, Molecular mechanisms, Signalling pathways
Introduction
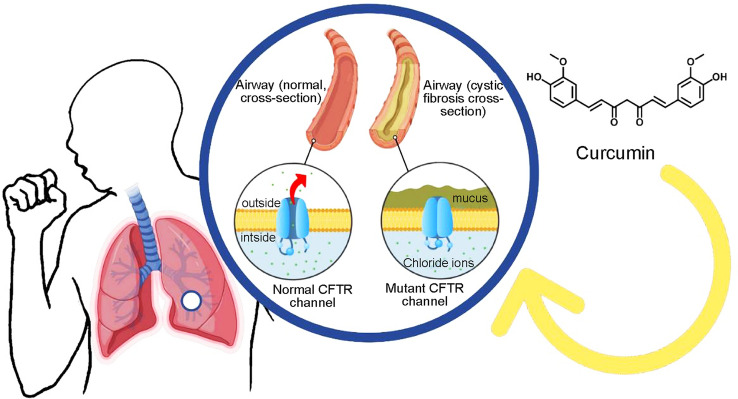
Cystic fibrosis (CF) is an autosomal recessive genetic disease, affecting today more than 70,000 people globally, whereas approximately 1000 new cases are diagnosed each year. More than 75% of the patients are diagnosed at the age of 2, while over half of the patients are age 18 or older (Cystic Fibrosis Foundation, 2020) and the median survival age is 53 years (Stephenson et al. 2017). The disease is caused by the mutations in the gene located on chromosome 7 in the region of 7q31.2 that encodes the CF transmembrane conductance regulator (CFTR) protein (Trandafir et al. 2019; Lukacs and Verkman 2012). To date, more than 2000 mutations associated with CF have been identified. Amongst all the mutations, the most prevalent one affecting more than 70% of the patients is caused by deletion of a phenylalanine residue at position 508 (ΔF508) of the CFTR protein (Villamizar et al. 2019).
CFTR protein predominantly functions as a chloride channel on the apical membrane of epithelial cells, and it is responsible for the regulation of the secretion of chloride ions and re-absorption of sodium ions. As a result of mutations on CFTR protein, the secretion of chloride ions is impaired and sodium ions are hyper-absorbed across epithelia, leading to hypersecretion of thick and sticky mucus (Saint-Criq and Gray 2017; Cristallini et al. 2019). The over-production of the mucus secreted in the lungs, along with its altered appearance and composition, results in serious complications including airway obstruction, chronic lung infections with inflammatory reactions germs difficult to treat and resistant to antibiotics such as methicillin-resistant Staphylococcus aureus, Aspergillus or Pseudomonas aeruginosa (Taheri et al. 2021).
Although most severe symptoms related to CF occur in the lungs, patients also often suffer from several other diseases formed within other epithelial-lined organs such as small intestine bacterial overgrowth, pancreatic exocrine insufficiency, cirrhosis of the liver and progressive hepatic dysfunction, and infertility (Kelly and Buxbaum 2015; Velino et al. 2019). Currently, there is no cure for CF; however, there have been significant advances being made in available treatments in recent years.
The use of natural compounds, in particular, curcumin has been proposed as a complementary and effective strategy in the treatment of this disease (Yavarpour-Bali et al. 2019).
Curcumin is the most active and major compound of turmeric discovered by Vogel and Pelletier 205 years ago (Vogel and Pelletier 1815). Many years later, it has been obtained as a pure compound in 1842 (Vogel 1842) and its chemical structure was described by Milobedeska et al. (1910). The synthesis of curcumin was described in 1913 by Lampe and Milobedeska (1913). Curcumin is the main curcuminoid of turmeric rhizomes and presents between 1.5 to 3% of turmeric, which is responsible for the extreme yellow colour of turmeric (Guo et al. 2020).
The medicinal use of this plant has been documented in Ayurveda, i.e., the Indian system of medicine, and traditional Chinese medicine, for at least 2500 years (Kocaadam and Sanlier 2017). From a global traditional perspective, turmeric was used as an important ingredient of dietary spices about 4,000 years ago in India (Li et al. 2020). While it might have reached China before 700 BCE, East Africa before 800 BCE, West Africa before 1200 BCE, and Jamaica in the eighteenth century (Prasad et al. 2014). In traditional medicine, turmeric is used for the treatment of rheumatoid arthritis, chronic anterior uveitis, conjunctivitis, skin cancer, smallpox, chickenpox, wound healing, urinary tract infections, liver ailments, strengthening the overall energy of the body, dispelling worms, regulating menstruation, dissolving gall stones, and for several digestive disorders, amongst other conditions (Quispe et al. 2022; Akaberi et al. 2021).
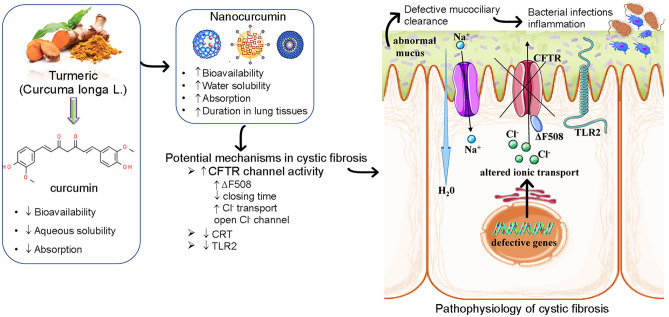
Many studies reported that curcumin, constituting 2–5% of turmeric (Kalaycioglu et al. 2017), possesses a wide range of important pharmacological activities including antioxidants, antimicrobial, anti-inflammatory and anti-cancer effects (Alexa et al. 2020). Because curcumin has a low biodisponibility and absorption, various nanoformulations including liposomes, polymeric nanoparticles, solid lipid nanoparticles, micelles, nanogels, nanosuspensions, nanoemulsions, complexes, and dendrimer/dimer are studied for the delivery of an active form of curcumin (Sun et al. 2012; Pinzaru et al. 2018). It has been reported that curcumin nanoparticles possess special efficacies both in vivo (Cartiera et al. 2010) and in vitro (Goncalves et al. 2017; Lababidi et al. 2019) for the treatment of CF.
In the light of these aspects, this updated review aims to highlight the available evidence on the effectiveness of curcumin nanomedicine in the treatment of CF. In this context, the general bioactivity of curcumin, formulation types and related studies were discussed along with the current challenges and future perspectives.
Purpose and review methodology
A search was performed in the literature to highlight published data on the potential effects of pharmacological effects of nano-curcumin in CF. The databases accessed were: PubMed, ScienceDirect, and Google Scholar using the following MeSH terms: "cystic fibrosis", "curcumin/pharmacokinetics", "curcumin/therapeutic use", "nanoparticles", "polyethylene glycols', "bioavailability", "diffusion", "hydrophobic and hydrophilic interactions", "nanomedicine", drug carriers/administration", "molecular targeted therapy", "humans". Data regarding the uses of nano-curcumin as a complementary therapy for the treatment of CF were extracted from papers published in English that included in vitro/in vivo pharmacological studies highlighting the molecular mechanisms of action. Exclusion criteria: papers without preclinical studies (in vivo) or cell lines (in vitro), or control group; articles which may include homoeopathic preparations; duplicates, and articles are not written in English.
Curcumin as potential therapeutic agent for CF
CF is a progressive and ultimately fatal inherited disorder caused by a mutation in the gene encoding CFTR (Zeitlin, 2004), in this regard, Egan et al. (2004) evaluated the curative effects of curcumin on CF defects. They developed a weak and safe sarco/endoplasmic reticulum calcium ATPases inhibitor in a CF mouse model and the curcumin corrected the electrolyte abnormalities and prohibited intestinal blockage.
Song et al. (2004) tried to re-investigate the preclinical data of Egan et al. (2004) through in vitro and in vivo studies and their results indicated that curcumin was not able to produce functional correction of ΔF508-CFTR processing in both studies (Fig. 1).
Fig. 1.
Curcumin CF correction is initiated by CFTR mutation
The effect of curcumin on cultured baby hamster kidney (BHK) cells transfected with ΔF508-CFTR and nasal epithelial cell lines was evaluated by Dragomir et al. (2004). The compound curcumin showed a significant positive effect (small increase) on ΔF508-CFTR BHK cells or CFBE cells mediated chloride transport in airway epithelial cells (Dragomir et al. 2004).
The stimulating effect of curcumin on CFTR channel functioning was reported by Berger et al. (2005). They observed and found that curcumin reduces the channel closing time and increased CFTR channel activity. In other words, curcumin displayed dose-dependent, reversible and better stimulating effects on channel activity compared to another well-known compound (Berger et al. 2005).
Sodium/potassium pumps are prominent examples of P2 type superfamily. These pumps are involved in specialized tissue functions including trans-epithelial Na+ transport, muscle and nerve excitability, and secretory and signal transduction processes (Li et al. 2021).
The modulatory effects of curcumin on Na, K-ATPase activity and kinetic properties were evaluated by Mahmmoud (2005). Curcumin showed dose-dependent inhibitory Na, K-ATPase activity (K0.5 ῀14.6 µM) and partially blocks the K+ occlusion site. The intermediate filament cytoskeleton of all epithelial cells is made up of keratins (type I and II) and they are encoded by 54 evolutionarily conserved genes (28 types I, 26 types II) (Jacob et al. 2018).
Keratin 18 (K18) is a type 1 keratin and is directly associated with ΔF508-CFTR trafficking. Lipecka et al. (2006) reported the effects of curcumin on ΔF508-CFTR localization and the keratin 18 (K18) network. They performed a functional assay for the CFTR chloride channel in CFPAC-1 cells and treated them with/without curcumin. The curcumin displayed an increase in a cAMP-dependent chloride efflux in treated ΔF508-CFTR expressing cells. The K18 network was analyzed using immunohistochemistry and immunoblotting assays. Curcumin treatment induced a considerable alteration/remodelling in the K18 network and significantly increased the K18 Ser52 phosphorylation. It is already established that curcumin has been reported to correct CF brought about by ΔF508 mutation of the CFTR (Egan et al. 2004; Zeitlin, 2004) but its actual action or mechanism is still unclear (Fig. 1). Harada et al. (2007) described curcumin and its role in the down-regulation of endoplasmic reticulum chaperone calreticulin (CRT). The Chinese hamster ovary cells treated with curcumin displayed suppressing CRT expression and increased wild type CFTR without affecting ΔF508 CFTR expression. CRT negatively regulates the CFTR cell surface expression and its activity. Wang and co-workers reported about the curcumin and its role as an important chelator of Fe3+. The compound inhibits channel dimerization and opening despite phosphorylation of the R domain (Wang, 2015). Curcumin also potentially activates ion channels mutated with G551D and W1282X mutations that cause impairment of ATP-modulated channel gating (Wang et al. 2007). It opens the Cl− ion channel independent of ATP binding. However, the influence of curcumin depends on the prior phosphorylation of the CFTR ion channel R-domain (Wang et al. 2007) (Fig. 2).
Fig. 2.
Summarized scheme with potential molecular mechanisms of curcumin nanomedicine formulations in CF. Abbreviations and symbols: ↑increased, ↓decreased, CF transmembrane conductance regulator (CFTR), Na+ sodium ion, Cl− chloride ion, endoplasmic reticulum chaperone calreticulin (CRT), toll-like receptor-2 (TLR2)
Cell migration is an essential process in skin wound healing and photodynamic therapy (PDT) enhances wound healing by photo-activating at a specific wavelength of light (Matei et al. 2021). In this context, Chiu et al. (2019) reported the CFTR and its involvement in indocyanine green (ICG)–mediated PDT regulated cell migration in skin wound healing. ICG–PDT conditioned medium activates CFTR and regulates other molecules in the focal adhesion (focal adhesion kinase and paxillin). Curcumin treatment enhanced cell migration (dose-dependent manner) in a similar manner as 5 J/cm2 ICG–PDT conditioned medium which is related to CFTR activation (p < 0.05). Chaudhary et al. (2019) reported curcumin and its role as an inhibitor of TLR2 expression in CF bronchial cell lines (CFBE410 cells). A strong suppression was displayed by curcumin treatment (40 µM) against TLR2 gene and protein expression in CFBE410 cells. Curcumin treatment also decreased the expression of transcription factors specificity protein 1, which is responsible for the increased basal TLR2 expression in the CF cell line.
Curcumin nanomedicines nanoformulation types and related studies
In vitro and in vivo studies
Numerous types of nanoparticles have been designed and evaluated for their efficacy in drug delivery in several diseases (Docea et al. 2020). Cell line-based and animal model-based studies have highlighted curcumin's effectiveness in treating CF. In the case of CF, the major barrier in delivering nanoparticles is the thick, sticky mucus membrane, characteristic of CF. This mucus layer brings hindrance to cilia movement and prevents the elimination of microbes that leads to infection. Mucus is mostly comprised of low-viscosity fluid, made up of mucin fibres (70–80%) and macromolecules. These mucin fibres create mesh architecture by cross-linking through hydrophobic interactions and disulfide bonds. The presence of macromolecules including DNA and actin gives rise to electrostatic hindrance and hydrophobicity, thus, giving rise to a need to develop a delivery system that has less adhesive ability with mucus layers (Rubin, 2007; Bengtson et al. 2021; Sala et al. 2021). Small structures like nanoparticles hold great potential in crossing highly steric environments and enhancing the rate of drug delivery. Nanoparticles coated with inert mucus material might facilitate enhancing curcumin delivery to the target site. Polyethene glycol (PEG) has been identified as mucus inert material and has been analyzed for its efficiency in delivering numerous drugs to the lungs in CF. A gold standard therapy, recombinant human deoxyribonuclease I (rhDNase), for CF has recently been investigated for improving its delivery mechanism and half-life through PEGylation. The rhDNase1 PEGylation enhanced drug stability and hence, availability further ameliorated the need for the daily administration of the drug (Guichard et al. 2021, 2017, 2018). Investigation in model animals further indicated that drug delivery once a week was enough to manifest its therapeutic influence (Guichard et al. 2021). Further, PEGylated rhDNase is reported to make thin viscous mucus (Guichard et al. 2018). Drug PEGylation did not have any cytotoxicity or adverse reaction in studied animals (Guichard et al. 2021).
PEG had been coated on mesoporous silica nanoparticles and gadolinium molybdate nanoparticles in previous studies and curcumin was loaded in these particles. PEG-coated curcumin nanoparticles have been analyzed for their efficiency in different cancer cell lines and were demonstrated to have improved bioavailability, loading capacity, and efficiency (Ma'mani et al. 2014; Kuang et al. 2020; Ayubi et al. 2019; Lin et al. 2018).
PEG co-polymerized curcumin, created through chemo-enzymatic methodology, in a study was reported to enhance curcumin bioavailability and water solubility in human bronchial epithelial cells (Pandey et al. 2011). Curcumin was attached to the hydroxyl group of PEG through EDC/NHS (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide) activation reactions (Lin et al. 2018). Furthermore, dry powdered inhalers (DPI) of curcumin were also developed through methyl ether-PEG (5 kDa). In vitro analysis depicted sustained delivery of PEGylated curcumin-DPI in the pulmonary system (Muralidharan et al. 2014; El-Sherbiny and Smyth, 2012).
Nano-spray-dried proliposomes were also employed for delivering curcumin to the lungs. Hydroxypropyl β-cyclodextrin was used as a carrier for proliposomes production. Spray-dried proliposomes loaded with curcumin were reported to have superior properties of aerosolization that capacitated the drug to access deep regions of the lungs. Comparative to curcumin powder, proliposomal curcumin was reported to have high absorption and increased mean residence duration in lung tissues (Adel et al. 2021).
The bioavailability of curcumin was attempted to enhance by encapsulating it in PLGA nanoparticles. These nanoparticles have a diameter of 77 ± 16 nm and a 7.6% (w/w) drug loading capacity. The study demonstrated that PLGA-encapsulated curcumin (PLGA-cur) was more readily bioavailable in comparison to non-encapsulated curcumin (Cartiera et al. 2010; Roointan et al. 2016). Further, the IC50 value of PLGA-cur was also determined to be lower than non-encapsulated curcumin (Roointan et al. 2016).
PLGA-cur effectiveness was validated in mice models harbouring ΔF508 mutation. PLGA-cur oral administration enhanced the effectiveness of curcumin effect (Cartiera et al. 2010). In another study, microfluid technology was employed to fabricate Pluronic (muco-penetrating stabilizer)-coated PLGA nanoparticles to penetrate the mucus layer in CF lungs. It is reported that pluronic-PLGA nanoparticles (size 40–160 nm) have a high capacity to encapsulate curcumin. Further, nanoparticles under 100 nm size have faster and more efficient penetration capability in pulmonary mucus (Lababidi et al. 2019).
Curcumin is known to negatively modulate the expression of pro-inflammatory toll-like receptor 2 (TLR2) that contributes to exerting its anti-inflammatory influence (Chaudhary et al. 2019). Thus, nano-curcumin can be considered as being a potential nutritional strategy for treating CF (Talebi et al. 2021).
Chronic inflammation, massive infiltration of immune cells, tissue damage, and chronic infections are the characteristics of CF (Tsoukalas et al. 2019). Lababidi et al. (2020) developed a micro-particle formulation using spray drying combining multiple drugs. Three antibiotics including tobramycin, ciprofloxacin or azithromycin, N-acetylcysteine (NAC), and curcumin were used in a synergistic mode. The antibacterial activity of three drugs and multidrug formulations were tested against Pseudomonas aeruginosa and the combination of azithromycin and ciprofloxacin with NAC and curcumin did not show better antibacterial activity. Whereas NAC and the addition of curcumin-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles displayed significant inhibitory activity against tumour necrosis factor (TNF)-α, interleukin (IL)-8, and IL-1β release. On the other hand, Cartiera et al. (2010) reported the oral administration of PLGA nanoparticles encapsulating curcumin enhanced the effects of curcumin therapy in CF mice models.
In a recent investigation, curcumin was loaded on amphiphilic nanomicelles particles and activated through blue laser light. This synergistic approach was reported to be effective in inhibiting resistant P. aeruginosa species in eukaryotic HaCaT cells (Rupel et al. 2021). Antimicrobial PDT-coupled with curcumin nanomicelles approach seems to show promising outcomes against P. aeruginosa, however, independent research on analyzing these therapeutic strategy efficacies on P. aeruginosa in CF still needs to be done.
Nanotechnology is also being utilized to make curcumin bioavailable to remediate symptoms and morbidity associated with CF. P. aeruginosa is an opportunistic, Gram-negative bacteria that readily infects CF lungs and is responsible for chronic infection. The mutation rate in the bacteria is high that has been reported to induce antibiotic resistance, rendering treatment strategies barely effective (Oliver et al. 2000; Talwalkar and Murray, 2016; Jennings et al. 2021).
Curcumin nanoparticle formulations have been evaluated for their potential to target this opportunistic microbe in CF patients. The study indicated that a concentration of 25 µg/mL of nano-curcumin affirmatively inhibited the biofilm formation of P. aeruginosa strain ATCC 10,145. Further, nano-curcumin, prepared through Planetary Ball Mill technology, has more resistance to enzyme-induced hydrolysis, along with improved tissue solubility (Sharifian et al. 2020). Spray-dried technology was used to co-administer curcumin-loaded PLGA nanoparticles and NAC to evaluate their co-effect in inhibiting infection in the pulmonary system. Co-treatment of PLGA-curcumin and NAC elicited an anti-inflammatory response that highlighted its potential application as therapeutic for pulmonary infections (Lababidi et al. 2020).
Clinical studies
The effect of curcumin supplementation on children and their quality of life with CF was investigated by Rafeey et al. (2020). They performed a controlled, randomized clinical study in 40 patients with CF (n = 20 in the intervention group, n = 20 control group, 5–18 years). Intervention group was treated with curcumin nanoparticles for up to 6 months (dose: 80 mg, three times). Height, weight, and quality of life were measured using the Pediatric Quality of Life Inventory (PedsQL) 4.0 (CITE) before and after treatment. The curcumin-treated group showed a significant increase in the percentage of weight change (7.48 ± 4.68 kg) compared to the control group (4.15 ± 4.68) at p = 0.03. Curcumin-treated group also displayed an improvement in terms of percentage change in physical functioning (19.28 ± 31.65) and school functioning score (40.96 ± 42.93) compared to the control group (15.24 ± 47.14 at p = 0.08 and 23.90 ± 14.82 at p = 0.06) (Rafeey et al. 2020).
A double-blind clinical trial conducted in Iran assessed the clinical significance and influence of nano-curcumin (Exir Nano Sina Drug Company, Iran) on the inflammatory markers of CF patients below 18. The study delineated that nano-curcumin has shorter liver metabolism and is more readily absorbed in the gastrointestinal tract. Furthermore, its use for 3 months elicited the anti-inflammatory response required to ameliorate pulmonary inflammation in CF (Talebi et al. 2021).
Limitations and prospects
Curcumin is a yellow-coloured hydrophobic polyphenol derived from the rhizomes of turmeric (Curcuma longa L.), a plant species belonging to the Zingiberaceae family. For a long time, curcumin has drawn the attention of researchers around the world in multidisciplinary fields related to therapeutic applications including antioxidant, anti-inflammatory, anti-arthritic, antimicrobial, cardio-protective, antithrombotic, hepato-protective, hypoglycemic, wound healing, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, rheumatoid arthritis, diabetes, gastro-related disorders, pulmonary diseases, atherosclerosis, and different types of tumours such as colorectal cancer, lung cancer, pancreatic cancer, breast cancer, multiple myeloma, melanoma, and sarcoma. Curcumin molecule is considered “generally recognized as safe” (GRAS) by the United States Food and Drug Administration (FDA) as a food additive at levels up to 20 mg per serving (Nelson et al. 2017). Curcumin is capable of inhibiting the progression of CF through two different mechanisms of action. One mechanism suggests that it can control the signalling pathways initiated by several cytokines and chemokines that directly cause fibrosis, and second, it can act through the induction of apoptosis in stellate cells of affected organs (Flora et al. 2013).
As in the case of several other hydrophobic therapeutic small drug molecules, curcumin also has limitations in its effective clinical use to treat diseases. These limitations include:
-
(i)
Low hydrophilicity and intrinsic dissolution rate(s),
-
(ii)
Low physicochemical instability,
-
(iii)
Poor pharmacokinetics and bioavailability,
-
(iv)
Low bioactive absorption,
-
(v)
Rapid metabolization,
-
(vi)
Low penetration and targeting efficacy (Yallapu et al. 2015).
In addition to these limitations, CF presents an additional challenge in the form of a thick mucus layer blocking access to diseased cells. To overcome these difficulties and thus to provide the desired biological effects, several nanotechnology-based delivery approaches have been applied (Ong et al. 2019; Salehi et al. 2020). These nanotechnology-based approaches have many attractive properties including:
-
(i)
Improved encapsulation or solubilization of therapeutic drugs for protective and targeted delivery,
-
(ii)
High surface to volume ratio allowing modifications to surface functional groups to achieve extensive stabilization and internalization,
-
(iii)
Biocompatibility, superior pharmacokinetics, and minimal clearance from the body,
-
(iv)
Controlled, stimuli-responsive, remote actuation, and on-demand drug release properties (Yallapu et al. 2013).
Its clinical implementation has been limited due to its poor aqueous solubility and lower bioavailability that consequently, cause hindrance in its uptake by cells and lead to rapid clearance. Limitations in therapeutic applications of curcumin can be overcome by designing an effective delivery system that could improve its systematic bioavailability, pharmacokinetics, and bioactivity. Applications of nanotechnology have been extended to encapsulate and load curcumin in several types of nanoformulations.
Overall conclusions
The potential of curcumin in treating CF has been delineated through numerous scientific validations. Limitations that restrict its clinical implementations can be addressed by employing advancements in nanotechnology. Studies have been performed at cell-line and animal model levels to evaluate the efficacy of several curcumin nanoformulations in ameliorating the deleterious effect of ΔF508 mutation and pathogenicity linked with CF.
Outcomes of this research have validated the potential of nano-curcumin as a therapeutic drug at the clinical level. Curcumin is well-tolerated and reported to have insignificant toxicity even at higher doses. However, further investigations are necessary to enhance the efficacy of nano-curcumin. Moreover, co-treatment of nano-curcumin with antibiotics also holds great potential that should be probed in CF cell lines and model animal. Spray-dried curcumin nanoparticles have so far shown great capacity to reach deep portions of the lungs with enhanced half-life and null cytotoxicity, highlighting the essentiality of more investigations in the area to further improve the strategy for clinical purposes (Table 1).
Table 1.
Nanoformulations’ list of curcumin that can be potentially applied for the treatment of CF
| Nanoformulations | Experimental model/clinical study | Results | References |
|---|---|---|---|
| PEG-curcumin | Human bronchial epithelial cells in vitro |
↑Aqueous solubility ↑Bioavailability |
(Pandey et al. 2011) |
| DPI-curcumin | RAW 264.7 cells/ in vitro | High loading and sustained release of drug | (El-Sherbiny and Smyth 2012) |
| PLGA-curcumin | Mice/in vivo | Effective in curing ΔF508 mutation | (Cartiera et al. 2010) |
| Nano-curcumin (Exir Nano Sina Drug Company, Iran) | Humans/clinical study |
No cytotoxicity ↓ liver metabolism ↑Gastrointestinal absorption |
(Talebi et al. 2021) |
DPI dry powdered inhalers, PEG polyethylene glycol, PLGA poly (lactic-co-glycolic acid)
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data deposition information
Yes.
Declarations
Conflict of Interest
No conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cristina Quispe, Email: elquispe@unap.cl.
Khushbukhat Khan, Email: khan2011@hotmail.com.
Zeeshan Javed, Email: zeeshan_javed456@yahoo.com.
Prabhakar Semwal, Email: semwal.prabhakar@gmail.com.
Sakshi Painuli, Email: sakshipainulii@gmail.com.
Senem Kamiloglu, Email: skamiloglu@uludag.edu.tr.
Miquel Martorell, Email: mmartorell@udec.cl.
Daniela Calina, Email: calinadaniela@gmail.com.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
References
- Adel IM, Elmeligy MF, Abdelrahim MEA, Maged A, Abdelkhalek AA, Abdelmoteleb AMM, Elkasabgy NA. Design and characterization of spray-dried proliposomes for the pulmonary delivery of curcumin. Int J Nanomed. 2021;16:2667–2687. doi: 10.2147/IJN.S306831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaberi M, Sahebkar A, Emami SA. Turmeric and curcumin: from traditional to modern medicine. Adv Exp Med Biol. 2021;1291:15–39. doi: 10.1007/978-3-030-56153-6_2. [DOI] [PubMed] [Google Scholar]
- Alexa ID, Ilie AC, Prada G, Herghelegiu AM, Luca A, Rotaru TS, Dondas A, Rusu-zota G, Alexa-stratulat T, Bohotin CR. A comprehensive behavioural assessment of curcumin's effect on inflammatory and non-inflammatory pain in mice. Farmacia. 2020;68:829–834. doi: 10.31925/farmacia.2020.5.8. [DOI] [Google Scholar]
- Ayubi M, Karimi M, Abdpour S, Rostamizadeh K, Parsa M, Zamani M, Saedi A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: synthesis, toxicity and biocompatibility study. Mater Sci Eng: C. 2019;104:109810. doi: 10.1016/j.msec.2019.109810. [DOI] [PubMed] [Google Scholar]
- Bengtson CD, Kim MD, Anabtawi A, He J, Dennis JS, Miller S, Yoshida M, Baumlin N, Salathe M. Hyperglycaemia in cystic fibrosis adversely affects BK channel function critical for mucus clearance. Eur Respir J. 2021;57(1):2000509. doi: 10.1183/13993003.00509-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl- channel activity. J Biol Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- Cartiera MS, Ferreira EC, Caputo C, Egan ME, Caplan MJ, Saltzman WM. Partial correction of cystic fibrosis defects with PLGA nanoparticles encapsulating curcumin. Mol Pharm. 2010;7:86–93. doi: 10.1021/mp900138a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Ueno-shuto K, Ono T, Ohira Y, Watanabe K, Nasu A, Fujikawa H, Nakashima R, Takahashi N, Suico MA, Kai H, Shuto T. Curcumin down-regulates toll-like receptor-2 gene expression and function in human cystic fibrosis bronchial epithelial cells. Biol Pharm Bull. 2019;42:489–495. doi: 10.1248/bpb.b18-00928. [DOI] [PubMed] [Google Scholar]
- Chiu WT, Tran TV, Pan SC, Huang HK, Chen YC, Wong TW. Cystic fibrosis transmembrane conductance regulator: a possible new target for photodynamic therapy enhances wound healing. Adv Wound Care (new Rochelle) 2019;8:476–486. doi: 10.1089/wound.2018.0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristallini C, Barbani N, Ventrelli L, Summa C, Filippi S, Capeloa T, Vitale E, Albera C, Messore B, Giachino C. Biodegradable microparticles designed to efficiently reach and act on cystic fibrosis mucus barrier. Mater Sci Eng C Mater Biol Appl. 2019;95:19–28. doi: 10.1016/j.msec.2018.10.064. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Foundation (2020) About Cystic Fibrosis. Retrieved from: https://www.cff.org
- Docea AO, Calina D, Buga AM, Zlatian O, Paoliello MMB, Mogosanu GD, Streba CT, Popescu EL, Stoica AE, Birca AC, Vasile BS, Grumezescu AM, Mogoanta L. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. Int J Mol Sci. 2020;21:17. doi: 10.3390/ijms21041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragomir A, Bjorstad J, Hjelte L, Roomans GM. Curcumin does not stimulate cAMP-mediated chloride transport in cystic fibrosis airway epithelial cells. Biochem Biophys Res Commun. 2004;322:447–451. doi: 10.1016/j.bbrc.2004.07.146. [DOI] [PubMed] [Google Scholar]
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny IM, Smyth HD. Controlled release pulmonary administration of curcumin using swellable biocompatible microparticles. Mol Pharm. 2012;9:269–280. doi: 10.1021/mp200351y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Gupta D, Tiwari A. Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30:331–368. doi: 10.1615/CritRevTherDrugCarrierSyst.2013007236. [DOI] [PubMed] [Google Scholar]
- Goncalves C, Gomez JP, Meme W, Rasolonjatovo B, Gosset D, Nedellec S, Hulin P, Huin C, Le Gall T, Montier T, Lehn P, Pichon C, Guegan P, Cheradame H, Midoux P. Curcumin/poly(2-methyl-2-oxazoline-b-tetrahydrofuran-b-2-methyl-2-oxazoline) formulation: An improved penetration and biological effect of curcumin in F508del-CFTR cell lines. Eur J Pharm Biopharm. 2017;117:168–181. doi: 10.1016/j.ejpb.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Guichard MJ, Patil HP, Koussoroplis SJ, Wattiez R, Leal T, Vanbever R. Production and characterization of a PEGylated derivative of recombinant human deoxyribonuclease I for cystic fibrosis therapy. Int J Pharm. 2017;524:159–167. doi: 10.1016/j.ijpharm.2017.03.057. [DOI] [PubMed] [Google Scholar]
- Guichard MJ, Kinoo D, Aubriot AS, Bauwens N, Gougue J, Vermeulen F, Lebecque P, Leal T, Vanbever R. Impact of PEGylation on the mucolytic activity of recombinant human deoxyribonuclease I in cystic fibrosis sputum. Clin Sci (lond) 2018;132:1439–1452. doi: 10.1042/CS20180315. [DOI] [PubMed] [Google Scholar]
- Guichard MJ, Wilms T, Mahri S, Patil HP, Hoton D, Ucakar B, Vanvarenberg K, Cheou P, Beka M, Marbaix E. PEGylation of recombinant human deoxyribonuclease I provides a long-acting version of the mucolytic for patients with cystic fibrosis. Adv Ther. 2021;4:2000146. doi: 10.1002/adtp.202000146. [DOI] [Google Scholar]
- Guo YH, An BZ, Lang ZF, Zhou FB, Zhang XH, Wang HL. Effects of curcumin on inhibiting the proliferation of pulmonary artery smooth muscle cells and relieving pulmonary arterial hypertension. Farmacia. 2020;68:307–312. doi: 10.31925/farmacia.2020.2.16. [DOI] [Google Scholar]
- Harada K, Okiyoneda T, Hashimoto Y, Oyokawa K, Nakamura K, Suico MA, Shuto T, Kai H. Curcumin enhances cystic fibrosis transmembrane regulator expression by down-regulating calreticulin. Biochem Biophys Res Commun. 2007;353:351–356. doi: 10.1016/j.bbrc.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Jacob JT, Coulombe PA, Kwan R, Omary MB. Types I and II keratin intermediate filaments. Cold Spring Harb Perspect Biol. 2018;10(4):a018275. doi: 10.1101/cshperspect.a018275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings LK, Dreifus JE, Reichhardt C, Storek KM, Secor PR, Wozniak DJ, Hisert KB, Parsek MR. Pseudomonas aeruginosa aggregates in cystic fibrosis sputum produce exopolysaccharides that likely impede current therapies. Cell Rep. 2021;34:108782. doi: 10.1016/j.celrep.2021.108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaycioglu Z, Torlak E, Akin-Evingur G, Ozen I, Erim FB. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int J Biol Macromol. 2017;101:882–888. doi: 10.1016/j.ijbiomac.2017.03.174. [DOI] [PubMed] [Google Scholar]
- Kelly T, Buxbaum J. Gastrointestinal manifestations of cystic fibrosis. Dig Dis Sci. 2015;60:1903–1913. doi: 10.1007/s10620-015-3546-7. [DOI] [PubMed] [Google Scholar]
- Kocaadam B, Sanlier N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 2017;57:2889–2895. doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- Kuang G, Zhang Q, He S, Liu Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020;10:24624–24630. doi: 10.1039/D0RA04778C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lababidi N, Sigal V, Koenneke A, Schwarzkopf K, Manz A, Schneider M. Microfluidics as tool to prepare size-tunable PLGA nanoparticles with high curcumin encapsulation for efficient mucus penetration. Beilstein J Nanotechnol. 2019;10:2280–2293. doi: 10.3762/bjnano.10.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lababidi N, Montefusco-Pereira CV, De Souza C-W, Lehr CM, Schneider M. Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur J Pharm Biopharm. 2020;157:200–210. doi: 10.1016/j.ejpb.2020.10.010. [DOI] [PubMed] [Google Scholar]
- Lampe V, Milobedeska J. Studien über curcumin. Eur J Pharm Biopharm. 1913;46:2235–2240. [Google Scholar]
- Li H, Sureda A, Devkota HP, Pittala V, Barreca D, Silva AS, Tewari D, Xu S, Nabavi SM. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343. doi: 10.1016/j.biotechadv.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Li F, Egea PF, Vecchio AJ, Asial I, Gupta M, Paulino J, Bajaj R, Dickinson MS, Ferguson-Miller S, Monk BC, Stroud RM. Highlighting membrane protein structure and function: a celebration of the protein data bank. J Biol Chem. 2021;296:100557. doi: 10.1016/j.jbc.2021.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cai Q, Tang Y, Xu Y, Wang Q, Li T, Xu H, Wang S, Fan K, Liu Z, Jin Y, Lin D. PEGylated lipid bilayer coated mesoporous silica nanoparticles for co-delivery of paclitaxel and curcumin: Design, characterization and its cytotoxic effect. Int J Pharm. 2018;536:272–282. doi: 10.1016/j.ijpharm.2017.10.043. [DOI] [PubMed] [Google Scholar]
- Lipecka J, Norez C, Bensalem N, Baudouin-Legros M, Planelles G, Becq F, Edelman A, Davezac N. Rescue of DeltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J Pharmacol Exp Ther. 2006;317:500–505. doi: 10.1124/jpet.105.097667. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Verkman AS. CFTR: folding, misfolding and correcting the DeltaF508 conformational defect. Trends Mol Med. 2012;18:81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmmoud YA. Curcumin modulation of Na, K-ATPase: phosphoenzyme accumulation, decreased K+ occlusion, and inhibition of hydrolytic activity. Br J Pharmacol. 2005;145:236–245. doi: 10.1038/sj.bjp.0706185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma'mani L, Nikzad S, Kheiri-Manjili H, Al-Musawi S, Saeedi M, Askarlou S, Foroumadi A, Shafiee A. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: practical strategy for the breast cancer therapy. Eur J Med Chem. 2014;83:646–654. doi: 10.1016/j.ejmech.2014.06.069. [DOI] [PubMed] [Google Scholar]
- Matei AM, Caruntu C, Tampa M, Georgescu SR, Matei C, Constantin MM, Constantin TV, Calina D, Ciubotaru DA, Badarau IA, Scheau C, Caruntu A. Applications of nanosized-lipid-based drug delivery systems in wound care. Appl Sci. 2021;11:4915. doi: 10.3390/app11114915. [DOI] [Google Scholar]
- Milobedeska J, Kostanecki V, Lampe V. Structure of curcumin. Eur J Pharm Biopharm. 1910;43:2163–2170. [Google Scholar]
- Muralidharan P, Mallory E, Malapit M, Hayes D, Mansour HM. Inhalable PEGylated phospholipid nanocarriers and PEGylated therapeutics for respiratory delivery as aerosolized colloidal dispersions and dry powder inhalers. Pharmaceutics. 2014;6:333–353. doi: 10.3390/pharmaceutics6020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin: miniperspective. J Med Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- Ong V, Mei V, Cao L, Lee K, Chung EJ. Nanomedicine for cystic fibrosis. SLAS Technol. 2019;24:169–180. doi: 10.1177/2472630318824334. [DOI] [PubMed] [Google Scholar]
- Pandey MK, Kumar S, Thimmulappa RK, Parmar VS, Biswal S, Watterson AC. Design, synthesis and evaluation of novel PEGylated curcumin analogs as potent Nrf2 activators in human bronchial epithelial cells. Eur J Pharm Sci. 2011;43:16–24. doi: 10.1016/j.ejps.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Pinzaru I, Coricovac D, Dehelean C, Moaca EA, Mioc M, Baderca F, Sizemore I, Brittle S, Marti D, Calina CD, Tsatsakis AM, Soica C. Stable PEG-coated silver nanoparticles - A comprehensive toxicological profile. Food Chem Toxicol. 2018;111:546–556. doi: 10.1016/j.fct.2017.11.051. [DOI] [PubMed] [Google Scholar]
- Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Quispe C, Herrera-bravo J, Javed Z, Khan K, Raza S, Gulsunoglu-Konuskan Z, Daştan SD, Sytar O, Martorell M, Sharifi-Rad J, Calina D. Therapeutic applications of curcumin in diabetes: a review and perspective. Biomed Res Int. 2022;2022:1375892. doi: 10.1155/2022/1375892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafeey M, Nikniaz Z, Farshiradvar F, Sameni Z, Faramarzi E. Effects of curcumin supplementation on quality of life of cystic fibrosis patients. Int J Pediatr. 2020;8:11169–11176. [Google Scholar]
- Roointan A, Sharifi-Rad M, Badrzadeh F, Sharifi-Rad J. A comparison between PLGA-PEG and NIPAAm-MAA nanocarriers in curcumin delivery for hTERT silencing in lung cancer cell line. Cell Mol Biol. 2016;62:51–56. [PubMed] [Google Scholar]
- Rubin BK. Mucus structure and properties in cystic fibrosis. Paediatr Respir Rev. 2007;8:4–7. doi: 10.1016/j.prrv.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Rupel K, Zupin L, Brich S, Mardirossian M, Ottaviani G, Gobbo M, Di Lenarda R, Pricl S, Crovella S, Zacchigna S, Biasotto M. Antimicrobial activity of amphiphilic nanomicelles loaded with curcumin against Pseudomonas aeruginosa alone and activated by blue laser light. J Biophotonics. 2021;14:e202000350. doi: 10.1002/jbio.202000350. [DOI] [PubMed] [Google Scholar]
- Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74:93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala V, Cnudde SJ, Murabito A, Massarotti A, Hirsch E, Ghigo A. Therapeutic peptides for the treatment of cystic fibrosis: Challenges and perspectives. Eur J Med Chem. 2021;213:113191. doi: 10.1016/j.ejmech.2021.113191. [DOI] [PubMed] [Google Scholar]
- Salehi B, Calina D, Docea AO, Koirala N, Aryal S, Lombardo D, Pasqua L, Taheri Y, Castillo CMS, Martorell M, Martins N, Iriti M, Suleria HAR, Sharifi-Rad J. Curcumin's nanomedicine formulations for therapeutic application in neurological diseases. J Clin Med. 2020;9:35. doi: 10.3390/jcm9020430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian P, Yaslianifard S, Fallah P, Aynesazi S, Bakhtiyari M, Mohammadzadeh M. Investigating the effect of nano-curcumin on the expression of biofilm regulatory genes of pseudomonas aeruginosa. Infect Drug Resist. 2020;13:2477–2484. doi: 10.2147/IDR.S263387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Sonawane ND, Salinas D, Qian L, Pedemonte N, Galietta LJ, Verkman AS. Evidence against the rescue of defective DeltaF508-CFTR cellular processing by curcumin in cell culture and mouse models. J Biol Chem. 2004;279:40629–40633. doi: 10.1074/jbc.M407308200. [DOI] [PubMed] [Google Scholar]
- Stephenson AL, Stanojevic S, Sykes J, Burgel PR. The changing epidemiology and demography of cystic fibrosis. Presse Med. 2017;46:e87–e95. doi: 10.1016/j.lpm.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Sun M, Su X, Ding B, He X, Liu X, Yu A, Lou H, Zhai G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine. 2012;7:1085–1100. doi: 10.2217/nnm.12.80. [DOI] [PubMed] [Google Scholar]
- Taheri Y, Jokovic N, Vitorovic J, Grundmann O, Maroyi A, Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Front Pharmacol. 2021;11:18. doi: 10.3389/fphar.2020.578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi S, Safarian M, Jaafari MR, Sayedi SJ, Abbasi Z, Ranjbar G, Kianifar HR. The effects of nano-curcumin as a nutritional strategy on clinical and inflammatory factors in children with cystic fibrosis: the study protocol for a randomized controlled trial. Trials. 2021;22:1–11. doi: 10.1186/s13063-021-05224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwalkar JS, Murray TS. The approach to pseudomonas aeruginosa in cystic fibrosis. Clin Chest Med. 2016;37:69–81. doi: 10.1016/j.ccm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Trandafir LM, Leon MM, Frasinariu O, Baciu G, Dodi G, Cojocaru E. Current practices and potential nanotechnology perspectives for pain related to cystic fibrosis. J Clin Med. 2019;8:1023. doi: 10.3390/jcm8071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukalas D, Sarandi E, Thanasoula M, Docea AO, Tsilimidos G, Calina D, Tsatsakis A. Metabolic fingerprint of chronic obstructive lung diseases: a new diagnostic perspective. Metabolites. 2019;9:18. doi: 10.3390/metabo9120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velino C, Carella F, Adamiano A, Sanguinetti M, Vitali A, Catalucci D, Bugli F, Iafisco M. Nanomedicine approaches for the pulmonary treatment of cystic fibrosis. Front Bioeng Biotechnol. 2019;7:406. doi: 10.3389/fbioe.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamizar O, Waters SA, Scott T, Saayman S, Grepo N, Urak R, Davis A, Jaffe A, Morris KV. Targeted activation of cystic fibrosis transmembrane conductance regulator. Mol Ther. 2019;27:1737–1748. doi: 10.1016/j.ymthe.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel E. Die Bibliothek Der Benediktinerabtei Sponheim. Serapeum. 1842;3:312–328. [Google Scholar]
- Vogel HA, Pelletier J (1815) Curcumin–biological and medicinal properties. J Pharma 2:50
- Wang G. Interplay between inhibitory ferric and stimulatory curcumin regulates phosphorylation-dependent human cystic fibrosis transmembrane conductance regulator and DeltaF508 activity. Biochemistry. 2015;54:1558–1566. doi: 10.1021/bi501318h. [DOI] [PubMed] [Google Scholar]
- Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanomedicine: a road to cancer therapeutics. Curr Pharm Des. 2013;19:1994–2010. doi: 10.2174/138161213805289219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yallapu MM, Nagesh PK, Jaggi M, Chauhan SC. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17:1341–1356. doi: 10.1208/s12248-015-9811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int J Nanomedicine. 2019;14:4449–4460. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin P. Can curcumin cure cystic fibrosis? N Engl J Med. 2004;351:606–608. doi: 10.1056/NEJMcibr041584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.