Abstract
Background and aims
Risk factor cutoffs are derived from associations with clinical cardiovascular disease (CVD), but how these risk factors associate with preserved cardiovascular health into old age is not well studied. We investigated midlife determinants of healthy versus nonhealthy cardiovascular aging in the Atherosclerosis Risk in Communities (ARIC) study.
Methods
ARIC participants were categorized by cardiovascular status in older age (mean age 75.8±5.3 years, range 66–90): healthy, subclinical disease (assessed by biomarkers and left ventricular function), clinical CVD (coronary heart disease, stroke, or heart failure), or prior death. We examined associations of midlife (mean age 52.1±5.1 years) systolic and diastolic blood pressure (SBP, DBP), low-density lipoprotein cholesterol (LDL-C), triglycerides, hemoglobin A1c (HbA1c), and body mass index (BMI) with cardiovascular status in older age using multinomial logistic regression analyses.
Results
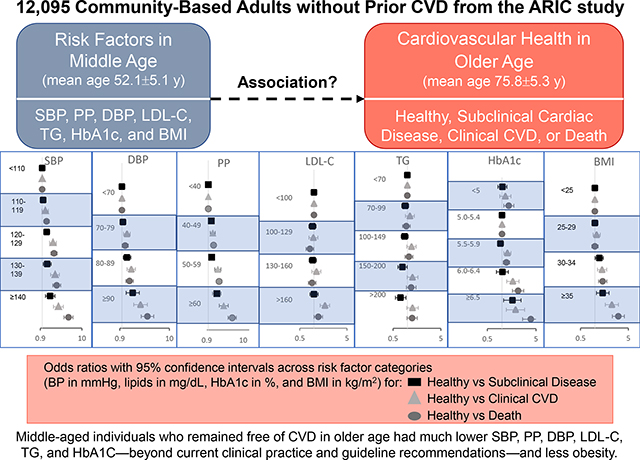
Compared with healthy status, odds for subclinical disease (odds ratio [OR] 1.30, 95% confidence interval [CI] 1.09–1.55) and clinical CVD (OR 1.87, 95% CI 1.53–2.29) at older age increased starting with midlife SBP 120–129 mmHg, whereas odds for death increased starting with SBP 110–119 mmHg (OR 1.29, 95% CI 1.10–1.52); findings were similar for DBP. Odds for subclinical disease increased for HbA1c ≥6.5% and BMI starting at 30–<35 kg/m2; odds for clinical CVD or death increased starting at HbA1c 5.5–5.9%, LDL-C >160 mg/dL, and BMI 30–<35 kg/m2.
Conclusions
More-stringent levels of modifiable risk factors in midlife beyond current clinical practice and guidelines were associated with preserved cardiovascular health in older age.
Keywords: Cardiovascular risk factors, healthy aging, cardiovascular disease prevention
Graphical Abstract
1. Introduction
Individuals older than 70 are the fasting growing age group in most Western countries, and cardiovascular disease (CVD) imparts significant morbidity and mortality in older adults [1]. CVD risk increases with age, likely a result of age-related pathophysiologic changes of the cardiovascular system and the cumulative effects of risk factors over time. Healthy cardiovascular aging may be thought of as aging without clinical or subclinical manifestation of CVD, noting that subclinical myocardial injury assessed by elevated cardiac biomarkers predicts increased short-term risk for clinical CVD [2,3].
Determinants of cardiovascular health include a combination of genetic and environmental factors. Well-established risk factors for clinical CVD and subclinical myocardial injury, including blood pressure, blood sugar, blood cholesterol, and body mass index (BMI) [4–7], begin affecting the cardiovascular system early in life, with cumulative exposure to suboptimal ranges resulting in increased risk over time [8]. Risk factor cutoffs defined by guidelines are derived from associations with increased risk for adverse events, but the optimal ranges of these risk factors that are associated with preserved cardiovascular health into older age are not well studied. A better understanding of these associations has significant implications for both individual and population health. Therefore, the principal aim of our study was to assess the effect of modifiable risk factors at midlife on cardiovascular health in older age.
2. Patients and methods
2.1. Study population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective population-based study of CVD incidence in adults who were middle-aged (aged 45–64 years) when recruited from 4 U.S. communities in 1987–1989 (visit 1) [9]. The study protocol complies with the Declaration of Helsinki and was approved by the institutional review boards of all participating centers; all participants provided written informed consent. In the present study, ARIC visit 1 served as the baseline visit, and cardiovascular health status was assessed at visit 5 (2011–2013). Of the 15,792 participants at visit 1, we excluded individuals with race other than White or Black as well as non-White individuals at the Minneapolis or Washington field centers because of small numbers. Of participants who attended visit 5, 6336 had complete data on high-sensitivity troponin T (hs-TnT), N-terminal pro–B-type natriuretic peptide (NT-proBNP), left ventricular ejection fraction (LVEF), and clinical CVD status and were included. Pre–visit 5 death (n=5759) was also included as a cardiovascular health status category, resulting in a total of 12,095 individuals included for the primary analysis (Supplementary Figure 1). Participants without confirmed death but who did not attend visit 5, and participants who attended visit 5 but were missing data on hs-TnT, NT-proBNP, LVEF, or clinical CVD status, were categorized as “missing” (n=3594) and were addressed by imputation in a sensitivity analysis.
2.2. Modifiable clinical risk factors
The main exposure variables of interest were midlife (visit 1 except as noted) modifiable clinical risk factors modeled as predefined categories: systolic blood pressure (SBP: <110, 110–119, 120–129, 130–139, ≥140 mmHg), diastolic blood pressure (DBP: <70, 70–79, 80–89, >90 mmHg), pulse pressure (PP: <40, 40–49, 50–59, ≥60 mmHg), low-density lipoprotein cholesterol (LDL-C: <100, 100–129, 130–160, >160 mg/dL), triglycerides (TG: <70, 70–99, 100–149, 150–200, >200 mg/dL), hemoglobin A1c (HbA1c [visit 2; not measured at visit 1]: <5%, 5.0–5.4%, 5.5–5.9%, 6.0–6.5%, ≥6.5%), and BMI (<25, 25–<30, 30–<35, ≥35 kg/m2). Methods for measuring modifiable risk factors are described in Supplementary Methods. In secondary analyses, we also assessed modifiable risk factors across ARIC visits 1 through 5 as described below. To evaluate risk factor exposure over time, we calculated time-weighted means of each risk factor measurement across visits.
2.3. Outcome variable: cardiovascular health status in older age
Cardiovascular health status at visit 5 was categorized into 4 groups: healthy, subclinical cardiac disease, clinical CVD, and pre–visit 5 death. Clinical CVD was defined as incident coronary heart disease, stroke, or heart failure event between visits 1 and 5. Coronary heart disease and stroke were defined as adjudicated myocardial infarction, silent myocardial infarction (diagnosed by ECG changes), coronary revascularization, or stroke between visits 1 and 5. A heart failure event was determined by diagnosis code (ICD-9 code 428) prior to 2005 or physician-adjudicated heart failure event after 2005 [10].
Subclinical cardiac disease was defined as hs-TnT ≥14 ng/L, NT-proBNP ≥125 pg/mL, or LVEF <50% at visit 5 [3,11,12]. Echocardiography was performed at visit 5 as described previously [13]. hs-TnT and NT-proBNP measurements in ARIC have been described [11,14]. Left ventricular volumes were calculated by the modified Simpson’s method using the apical 4- and 2-chamber views, and LVEF was derived from volumes [13].
Healthy cardiovascular status at visit 5 was defined as the absence of known clinical CVD or subclinical disease (both hs-TnT and NT-proBNP levels below their respective cutpoints and LVEF ≥50%).
2.4. Other covariates of interest
Other covariates of interest included baseline age, sex, race, antihypertensive medication use, lipid-lowering therapy, diabetes status, and smoking status.
2.5. Statistical analysis
To evaluate whether ideal ranges of the clinical measures of interest (SBP, DBP, LDL-C, TG, HbA1c, BMI) at midlife are associated with cardiovascular health in older age, we created categories across the range of measurements for each clinical measure. We then performed multinomial logistic regression analyses estimating odds ratio (ORs) and 95% confidence intervals (CIs) for cardiovascular health status categories at visit 5 across categories of each risk factor measured at midlife. The “optimal” category for each clinical factor was assigned as the reference group. For example, SBP was categorized as defined above and SBP <110 mmHg was assigned as the reference. OR may be interpreted as odds of having a healthy cardiovascular status versus a nonhealthy one at older age for a given risk factor measure at midlife (as compared to the optimal range). To account for dropout and missingness, we employed multiple imputation by chained equations (MICE) [15]. In sensitivity analysis, we repeated the multinomial regressions assessing the association between midlife risk factors and cardiovascular health status in older age while accounting for missingness using MICE.
Among participants at ARIC visit 5, we further compared differences between cardiovascular health status categories in older age (healthy vs subclinical cardiac disease vs clinical CVD) for SBP, DBP, PP, LDL-C, TG, and BMI across visits 1 through 5. HbA1c data were available only at visits 2 and 5 and therefore not included in this analysis. Relationship of changes in clinical measures over time and cardiovascular health status categories was graphically modeled using mixed-effect linear regression models with cardiovascular health categories as the independent variable and each clinical measure (modeled continuously) as time-updated dependent variables. Associations were reported as coefficients of cardiovascular health status and interactions between cardiovascular health status and time. The interaction term can be interpreted as how the association of clinical risk factors over time is related to cardiovascular health status. To understand the relationship between exposure of risk factor over time and cardiovascular health in older age, we performed multinomial logistic regression of the time-weighted mean of each risk factor from visit 1 through visit 5 with cardiovascular risk categories. OR was expressed as odds per unit increase in time-weighted mean for each risk factor.
3. Results
Of the included participants in the main analysis (n=12,095), 52.4% (n=6336) were alive and attended ARIC visit 5 (mean age 75.8±5.3 years; range 66–90); 47.6% (n=5759) had died prior to visit 5. Of the individuals who attended visit 5, 29.6% (n=1875) were categorized as free of subclinical cardiac disease or clinical CVD (“healthy”: mean age 73.4±4.4 years; 64.6% women), 43.4% (n=2749) were categorized as having subclinical cardiac disease (mean age 76.6±5.2 years; 60.8% women), and 27.0% (n=1712) were categorized as having clinical CVD (mean age 77.1±5.4 years; 48.0% women). The median follow-up between visits 1 and 5 for this analysis was 23.7 years. Baseline characteristics at visit 1 across categories of cardiovascular health status are shown in Table 1. The characteristics of the study population at visit 5 are shown in Supplementary Table 1.
Table 1.
Baseline (visit 1) characteristics of cardiovascular health status categories at visit 5.
| Healthy (N=1875, 11.95%) |
Subclinical disease (N=2749, 17.52%) |
Clinical CVD (N=1712, 10.91%) |
Pre-visit 5 death (N=5759, 36.71%) |
p value | ||
|---|---|---|---|---|---|---|
| Age, years | 49.9±4.29 | 52.8±5.09 | 53.3±5.34 | 56.8±5.47 | <0.001 | |
| Black, % | 25.01 | 18.26 | 26.52 | 31.53 | <0.001 | |
| Female, % | 64.59 | 60.82 | 48.01 | 46.05 | <0.001 | |
| Smoking status, % | Never | 52.19 | 50.87 | 41.96 | 30.88 | <0.001 |
| Former | 31.09 | 32.59 | 37.17 | 31.95 | ||
| Current | 16.72 | 16.53 | 20.86 | 37.17 | ||
| Current drinking, % | 62.58 | 61.41 | 56.63 | 51.68 | <0.001 | |
| BMI, kg/m2 | 26.5±4.47 | 26.8±4.68 | 28.3±5.32 | 28.3±5.79 | <0.001 | |
| Fasting glucose, mg/dL | 98.5±16.91 | 100.9±22.37 | 106.3±31.15 | 119.8±56.28 | <0.001 | |
| Hypertension, % | 16.94 | 22.21 | 37.93 | 47.71 | <0.001 | |
| Diabetes, % | 2.79 | 4.93 | 10.20 | 21.00 | <0.001 | |
| Total cholesterol, mg/dL | 205.7±39.06 | 210.8±39.00 | 218.1±42.27 | 217.8±43.79 | <0.001 | |
| LDL cholesterol, mg/dL | 128.8±36.81 | 132.8±36.52 | 142.6±40.53 | 140.5±40.28 | <0.001 | |
| HDL cholesterol, mg/dL | 54.4±16.64 | 54.9±17.23 | 48.9±16.21 | 49.0±17.04 | <0.001 | |
| Triglycerides, mg/dL | 94 (71, 136) | 98 (72, 140) | 116 (83, 163) | 121 (86, 174) | <0.001 | |
| Lipid-lowering medication use, % | 1.56 | 1.65 | 3.65 | 3.63 | <0.001 | |
| HbA1c, % | 5.3 (5.1, 5.6) | 5.4 (5.1, 5.6) | 5.5 (5.3, 5.9) | 5.7 (5.3, 6.2) | <0.001 | |
| eGFR, mL/min/1.73m2 | 107.2±13.93 | 102.3±13.20 | 103.1±14.80 | 99.9±18.16 | <0.001 | |
| SBP, mmHg | 113.2±14.81 | 116.3±15.70 | 121.3±16.73 | 127.0±21.19 | <0.001 | |
| DBP, mmHg | 72.0±9.97 | 72.2±10.08 | 75.1±11.05 | 74.8±12.48 | <0.001 | |
| Pulse pressure, mmHg | 41.2±9.56 | 44.0±10.98 | 46.2±11.83 | 52.1±15.94 | <0.001 | |
| BP-lowering medication use, % | 14.56 | 19.18 | 35.75 | 41.23 | <0.001 | |
Data presented as mean±SD, median (25th, 75th percentiles), or percentage. P-values for linear trend were calculated by using trend test across ordered groups. Healthy cardiovascular status was defined as visit 5 hs-TnT <14 ng/L, NT-proBNP <125 pg/mL, and LVEF ≥50%, and without prevalent coronary heart disease, stroke, or heart failure. Pre-visit 5 death included those who did not participate in visit 5 whose dates of death were before the end of visit 5 (August 31, 2013). Missing included individuals who did not attend visit 5 (not including pre-visit 5 death) or those missing data on NT-proBNP, hs-TnT, LVEF, or known clinical CVD status at visit 5.
BMI = body mass index; BP = blood pressure; CVD = cardiovascular disease; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; hs-TnT = high-sensitivity troponin T; LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; SBP = systolic blood pressure
3.1. Midlife risk factors and association of cardiovascular health status at visit 5
3.1.1. Blood pressure
Using SBP <110 mmHg as the reference category, we observed significantly higher odds for subclinical cardiac disease and clinical CVD compared with healthy status at visit 5 starting with midlife SBP 120–129 mmHg, with a stepwise increase in odds with increasing SBP categories. Significantly increased odds for pre–visit 5 death compared with healthy status was observed starting with midlife SBP 110–119 mmHg; a stepwise increase in odds was again observed with increasing SBP categories (Table 2).
Table 2.
Multinomial logistic regression analysis evaluating the association between categories of clinical risk factors measured in midlife (visit 1a) with cardiovascular health status in older age (visit 5). Association expressed as odds ratio (95% confidence interval).
| Healthy vs Subclinical Disease | Healthy vs Clinical CVD | Healthy vs Death | ||
|---|---|---|---|---|
|
| ||||
| SBP, mmHg | <110 | -Ref- | -Ref- | -Ref- |
| 110–119 | 1.06 (0.91–1.24) | 1.20 (0.99–1.44) | 1.29 (1.10–1.52) | |
| 120–129 | 1.30 (1.09–1.55) | 1.87 (1.53–2.29) | 1.99 (1.66–2.39) | |
| 130–139 | 1.34 (1.06–1.70) | 2.11 (1.63–2.72) | 2.32 (1.84–2.93) | |
| ≥140 | 1.59 (1.21–2.09) | 2.56 (1.92–3.41) | 4.60 (3.55–5.96) | |
|
| ||||
| DBP, mmHg | <70 | -Ref- | -Ref- | -Ref- |
| 70–79 | 1.05 (0.91–1.21) | 1.28 (1.08–1.50) | 1.17 (1.01–1.36) | |
| 80–89 | 1.28 (1.07–1.54) | 1.57 (1.28–1.93) | 1.45 (1.21–1.75) | |
| >90 | 1.69 (1.23–2.33) | 2.47 (1.77–3.45) | 3.46 (2.55–4.68) | |
|
| ||||
| PP, mmHg | <40 | -Ref- | -Ref- | -Ref- |
| 40–49 | 1.22 (1.06–1.40) | 1.35 (1.15–1.59) | 1.42 (1.23–1.64) | |
| 50–59 | 1.25 (1.03–1.50) | 1.88 (1.53–2.31) | 2.05 (1.70–2.48) | |
| ≥60 | 1.76 (1.32–2.33) | 2.78 (2.07–3.74) | 4.88 (3.73–6.40) | |
|
| ||||
| LDL-C, mg/dL | <100 | -Ref- | -Ref- | -Ref- |
| 100–129 | 0.93 (0.78–1.11) | 0.97 (0.78–1.21) | 0.82 (0.68–0.99) | |
| 130–160 | 0.94 (0.79–1.13) | 1.13 (0.90–1.40) | 0.91 (0.75–1.10) | |
| >160 | 1.00 (0.82–1.23) | 1.71 (1.36–2.15) | 1.24 (1.01–1.52) | |
|
| ||||
| TG, mg/dL | <70 | -Ref- | -Ref- | -Ref- |
| 70–99 | 0.90 (0.76–1.06) | 1.13 (0.91–1.40) | 0.95 (0.79–1.15) | |
| 100–149 | 0.85 (0.71–1.01) | 1.29 (1.04–1.59) | 1.08 (0.89–1.30) | |
| 150–200 | 0.79 (0.63–0.99) | 1.28 (0.99–1.66) | 1.20 (0.95–1.51) | |
| >200 | 0.72 (0.56–0.93) | 1.28 (0.97–1.69) | 1.26 (0.98–1.61) | |
|
| ||||
| HbA1c, % | <5 | 1.02 (0.82–1.26) | 1.17 (0.90–1.53) | 1.38 (1.08–1.75) |
| 5.0–5.4 | -Ref- | -Ref- | -Ref- | |
| 5.5–5.9 | 0.93 (0.81–1.08) | 1.29 (1.09–1.53) | 1.23 (1.06–1.44) | |
| 6.0–6.4 | 1.02 (0.77–1.35) | 1.67 (1.25–2.24) | 2.15 (1.64–2.80) | |
| ≥6.5 | 1.64 (1.08–2.47) | 1.90 (1.23–2.91) | 3.91 (2.64–5.79) | |
|
| ||||
| BMI, kg/m 2 | <25 | -Ref- | -Ref- | -Ref- |
| 25–<30 | 1.00 (0.87–1.16) | 1.09 (0.92–1.30) | 1.00 (0.86–1.17) | |
| 30–<35 | 1.24 (1.01–1.51) | 1.41 (1.13–1.77) | 1.46 (1.19–1.78) | |
| ≥35 | 1.46 (1.08–1.98) | 2.26 (1.65–3.10) | 3.10 (2.32–4.15) | |
Adjustment for age, sex, race, SBP, antihypertensive medication use, total cholesterol, HDL cholesterol, diabetes status, and current smoking status. (Note: SBP was omitted in SBP, PP, and DBP models; total cholesterol and HDL cholesterol were omitted in LDL-C and TG models).
HbA1c measured at visit 2; data not available for visit 1.
BMI = body mass index; CVD = cardiovascular disease; DBP = diastolic blood pressure; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein; LDL-C = low-density lipoprotein cholesterol; PP = pulse pressure; SBP = systolic blood pressure; TG = triglycerides
For DBP, with <70 mmHg as the reference category, odds were significantly higher for subclinical cardiac disease compared with healthy status at visit 5 starting with midlife DBP 80–89 mmHg and increased stepwise across increasing DBP categories. Odds for clinical CVD and death compared with healthy status were significantly increased starting at DBP 70–79 mmHg (Table 2).
Increasing PP was significantly associated with nonhealthy cardiovascular status as compared with healthy status. Using PP <40 mmHg as the reference, midlife PP of 40–49 mmHg was associated with higher odds for subclinical disease and clinical CVD at visit 5 and pre–visit 5 death. Midlife PP ≥60 mmHg was associated with the highest odds for subclinical disease, clinical CVD, and death (Table 2).
3.1.2. Lipids
Using LDL-C <100 mg/dL as the reference category, odds for clinical CVD at visit 5 and death prior to visit 5 were significantly increased starting at midlife LDL-C >160 mg/dL. We did not observe any significant difference in odds across categories of LDL-C in analyses comparing subclinical disease with healthy status (Table 2).
For TG, using <70 mg/dL as the reference category, individuals with midlife TG 150–200 mg/dL or >200 mg/dL paradoxically had lower odds for subclinical disease than healthy status at visit 5. Odds for clinical CVD were significantly higher than for healthy status in individuals with TG 100–149 mg/dL, but not higher TG categories. We did not find any additional association across TG categories in our analyses (Table 2).
3.1.3. HbA1c
Across increasing HbA1c categories, using 5.0–5.4% as the reference category [16], odds for subclinical disease did not significantly increase until the highest HbA1c category of ≥6.5% (Table 2). Compared with healthy status, odds for clinical CVD at visit 5 and death prior to visit 5 increased starting with HbA1c of 5.5–5.9% and increased stepwise across higher HbA1c categories. We further noted an increased odds for death with HbA1c <5%.
3.1.4. BMI
With a midlife BMI of <25 kg/m2 as the reference group, odds for subclinical disease, clinical CVD, and pre–visit 5 death were higher than for healthy status beginning with BMI 30–<35 kg/m2. We did not observe any significant associations for BMI 25–<30 kg/m2 (Table 2).
3.1.5. Sensitivity analysis
Visit 5 participants included in the study were younger and generally healthier than living ARIC participants not attending visit 5 or missing data (Supplementary Table 2). Multinomial regression analysis with MICE to account for dropout and missingness of data did not yield any significant changes in the associations between risk factors in midlife and cardiovascular health status in older age (Supplementary Table 3).
3.2. Cardiovascular status in older age and risk factors over time
Among participants who attended visit 5, clinical measures (SBP, DBP, PP, LDL-C, TG, BMI) across visits were significantly different in those with healthy cardiovascular status versus those with clinical CVD (all p<0.001), and SBP, DBP, and BMI across visits were significantly different in those with healthy cardiovascular status versus those with subclinical disease (all p≤0.001). However, no significant difference in LDL-C or TG across visits was noted between the healthy and subclinical disease groups (p=0.135 and p=0.303, respectively) (Supplementary Tables 4–8). Graphical representation of the linear mixed-effect models minimally adjusted for age, sex, and race is shown in Figure 1.
Figure 1.
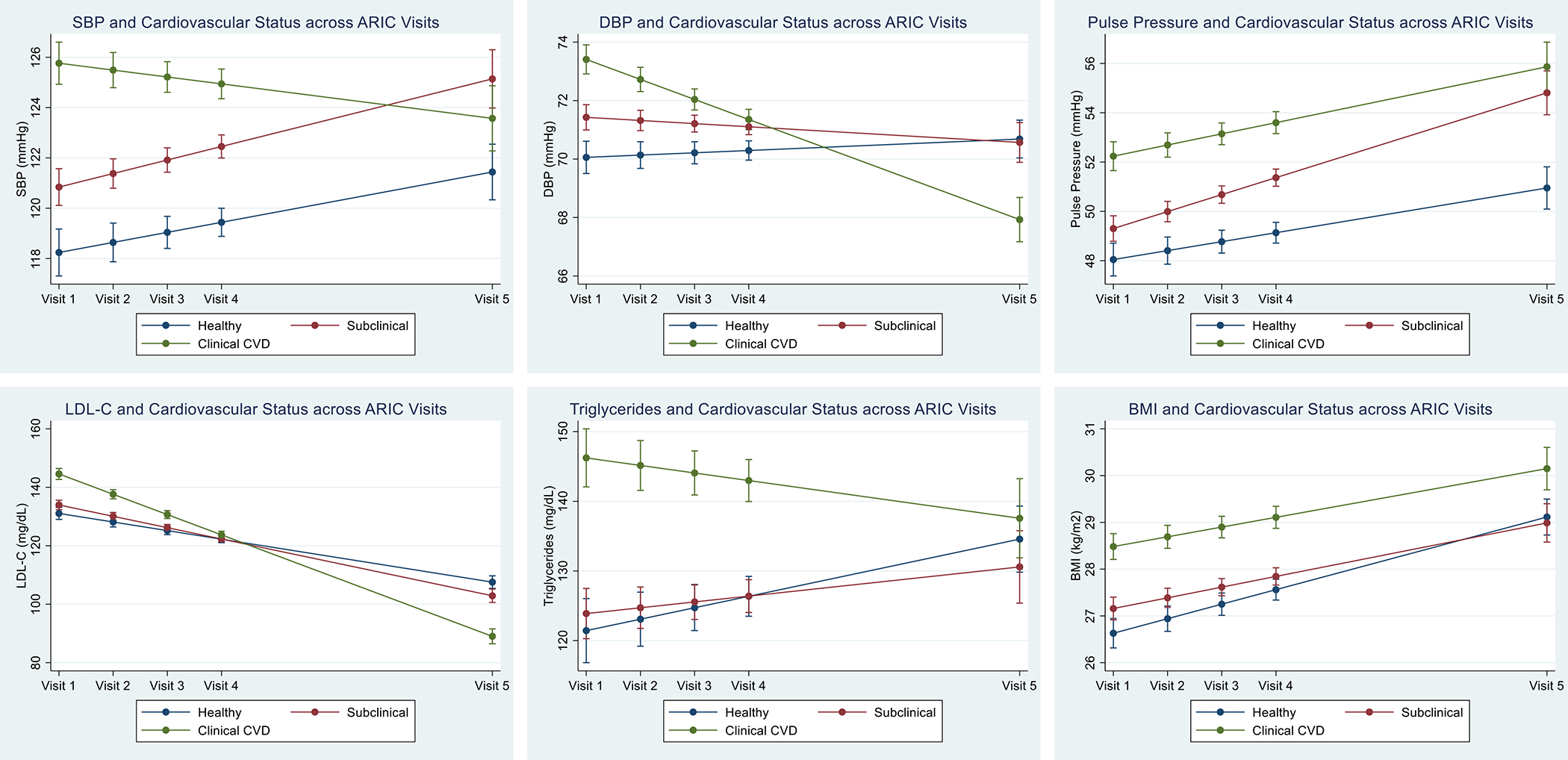
Results of linear mixed effect models for 25-year change in cardiac risk factors by cardiovascular health categories at visit 5, adjusted by age, sex, and race.
Cardiac risk factor estimates and 95% confidence intervals are shown for ARIC visits 1–5.
With respect to exposure over time, the time-weighted means for SBP were numerically higher for the subclinical disease (124.5±12.7 mmHg) and clinical CVD (127.0±13.2 mmHg) groups compared with the healthy group (120.6±12.4 mmHg). Increased time-weighted means for SBP were significantly associated with subclinical disease and clinical CVD even after adjusting for antihypertensive medication use across all visits. Increasing time-weighted means for DBP were not associated with subclinical disease but inversely associated with clinical CVD. Time-weighted means for PP were significantly and positively associated with subclinical disease and clinical CVD (Table 3).
Table 3.
Association of time-weighted average of risk factor measurements from visit 1 to visit 5 and cardiovascular health status at visit 5. Association expressed as odds ratio (95% confidence interval).
| Healthy (N=1691) | Subclinical Disease (N=2516) | Clinical CVD (N=1498) | |
|---|---|---|---|
| SBP | |||
| Time-weighted average (mmHg) | 120.6±12.37 | 124.5±12.70 | 127.0±13.19 |
| Model 1 | Ref | 1.021 (1.016–1.027) | 1.033 (1.026–1.039) |
| Model 2a | Ref | 1.014 (1.008–1.020) | 1.008 (1.0003–1.015) |
| DBP | |||
| Time-weighted average (mmHg) | 70.3±7.52 | 70.0±7.41 | 70.2±7.85 |
| Model 1 | Ref | 1.012 (1.003–1.021) | 1.006 (0.995–1.016) |
| Model 2a | Ref | 1.001 (0.991–1.011) | 0.977 (0.966–0.989) |
| PP | |||
| Time-weighted average (mmHg) | 50.2±9.19 | 54.6±10.31 | 56.7±11.11 |
| Model 1 | Ref | 1.031 (1.023–1.038) | 1.052 (1.044–1.061) |
| Model 2a | Ref | 1.024 (1.016–1.032) | 1.027 (1.017–1.036) |
| LDL-C | |||
| Time-weighted average (mg/dL) | 120.8±24.68 | 119.8±24.40 | 119.0±25.69 |
| Model 1 | Ref | 0.997 (0.995–0.9998) | 0.996 (0.993–0.999) |
| Model 2b | Ref | 0.997 (0.994–0.9999) | 0.994 (0.991–0.998) |
| TG | |||
| Time-weighted average (mg/dL) | 128.4±58.32 | 129.2±61.69 | 141.7±65.01 |
| Model 1 | Ref | 0.9996 (0.998–1.0008) | 1.003 (1.002–1.005) |
| Model 2b | Ref | 0.999 (0.998–1.000) | 1.000 (0.999–1.002) |
| BMI | |||
| Time-weighted average (kg/m2) | 27.9±4.63 | 27.9±4.90 | 29.1±5.31 |
| Model 1 | Ref | 1.018 (1.004–1.032) | 1.069 (1.053–1.085) |
| Model 2c | Ref | 1.023 (1.008–1.039) | 1.043 (1.025–1.061) |
Model 1 adjusted for visit 5 age, sex, and race.
Model 2 adjusted for model 1 plus visit 5 total cholesterol, HDL cholesterol, diabetes status, smoking status, number of hypertensive medications at visits 1, 2, 3, 4, and 5.
Model 2 adjusted for model 1 plus visit 5 SBP, diabetes status, smoking status, number of lipid-lowering medications at visits 1, 2, 3, 4, and 5.
Model 2 adjusted for model 1 plus visit 5 SBP, total cholesterol, HDL cholesterol, diabetes status, and smoking status.
BMI = body mass index; CVD = cardiovascular disease; DBP = diastolic blood pressure; HDL = high-density lipoprotein; LDL-C = low-density lipoprotein cholesterol; PP = pulse pressure; SBP = systolic blood pressure; TG = triglycerides.
We noted a negative association between time-weighted means for LDL-C across visits and subclinical disease or clinical CVD after adjusting for covariates. No significant association between time-weighted means for TG and cardiovascular health status was observed. Finally, increasing time-weighted means for BMI were significantly associated with subclinical disease and clinical CVD (Table 3).
4. Discussion
While numerous studies have shown that increased blood pressure, lipid parameters, and other risk measures are associated with increased risk for clinical as well as subclinical cardiac disease, assessment of optimal ranges of these risk factors for maintaining disease-free cardiovascular health into older age is limited. In this large cohort of 15,792 community-based adults who were middle-aged at baseline, at a median follow-up of 23.7 years more than a third (n=5759) were deceased and only 30% of the 6336 who attended visit 5 had “healthy” cardiovascular status as defined by no history of clinical CVD, no elevation of cardiac biomarkers, and LVEF ≥50% on echocardiogram, whereas 27% had clinical CVD and 43% had subclinical CVD. Incremental increases in midlife SBP, DBP, HbA1c, and BMI beyond ideal ranges (per guidelines) were associated with increased odds for subclinical and clinical CVD and death compared with preserved cardiovascular health at older age. However, midlife LDL-C and TG levels within the ranges evaluated in this study were generally not as predictive of healthy versus nonhealthy cardiovascular status.
Since the publication of the Systolic Blood Pressure Intervention Trial (SPRINT) [17], some but not all medical organizations have adopted lower blood pressure thresholds for the definition of hypertension, resulting in a discordance among clinical practice guidelines for the diagnosis and treatment of hypertension. For instance, current guidelines from the American College of Cardiology and American Heart Association define normal blood pressure as <120/80 mmHg, elevated blood pressure as 120–129/<80 mmHg, and hypertension as ≥130/80 mmHg, with a treatment target of <130/80 mmHg for most patients, including older adults (≥65 years) [18]. In contrast, guidelines from the American College of Physicians and the American Academy of Family Physicians recommend initiating treatment at higher blood pressure levels (SBP ≥150 mmHg) in older adults (≥60 years) [19].
Even modest elevation of blood pressure exerts stress on the cardiovascular system, leading to a myriad of pathophysiological changes including myocardial hypertrophy, endothelial dysfunction, vascular resistance, and promotion of atheroma formation, which over time result in myocardial damage and eventually the manifestation of clinical heart failure and atherosclerotic CVD [20]. In our study, we observed an increased likelihood for subclinical cardiac disease, clinical CVD, and death as compared with disease-free cardiovascular health in older age even at midlife blood pressure ranges of 110–119/70–79 mmHg. These observational data over a period of almost 25 years are also consistent with genetic data that show the lifelong impact of lower blood pressure levels on CVD and support the concept that the duration of exposure to elevated SBP is related to adverse effects on CVD [21].
We observed incrementally increased odds for CVD and death with increasing DBP categories in midlife without evidence of a J-curve, which is consistent with recent Mendelian randomization studies showing lack of causality between lower DBP and CVD [22]. Lower DBP later in life, however, is likely a marker for poorer cardiovascular health. Our linear mixed-effect models showed a marked decrease in DBP over time in the CVD subgroup that was greater than the reduction in SBP. Moreover, whereas DBP in midlife was positively associated with nonhealthy cardiovascular status in later life, time-weighted mean DBP, incorporating measurements across visits as participants aged, was inversely associated with CVD. The association between lower DBP and CVD in older age likely reflects a combination of more-intensive blood pressure therapy in a higher-risk group and more-rapid progression of vascular stiffness with aging in patients who have clinical or subclinical CVD. During our study, antihypertensive medication use increased from 19% to 73% of participants with subclinical disease and from 36% to 93% of participants with clinical CVD at visit 5, when the respective groups used on average 1.34 and 2.09 medications compared with 0.98 in the healthy group (see Supplementary Table 1). The larger decrease in DBP than in SBP in these groups can be explained by the difference in PP between the healthy group and those with subclinical or clinical CVD, which widened over time; time-weighted mean PP remained positively associated with disease, suggestive of more advanced progression of vascular stiffness in the groups with clinical or subclinical CVD [23]. The utility of PP as a marker for cardiovascular risk may warrant further study in older individuals, for whom more attention should be given to PP along with SBP.
Similar to blood pressure elevation, hyperglycemia is associated with increased risk for CVD outcomes as well as accelerated atherosclerosis and subclinical myocardial damage [5,24,25]. HbA1c at a range of 5.5% to <6.0% has been associated with increased risk for coronary heart disease, incident heart failure, and all-cause death, with incrementally increasing risk at higher HbA1c ranges [5,16,26]. Moreover, elevated HbA1c even within the prediabetes range of 5.7% to 6.4% was associated with development of subclinical myocardial damage [25]. Our results are in line with these previous findings. Odds for clinical CVD or pre–visit 5 death compared with healthy cardiovascular status increased starting at HbA1c levels of 5.5–5.9%. We did not observe increased odds for subclinical disease relative to healthy cardiovascular status at prediabetes HbA1c levels, but HbA1c ≥6.5% was associated with significantly higher odds for subclinical cardiac disease. Whereas our study used biomarkers of myocardial injury to determine subclinical disease, studies that used cardiovascular imaging have noted increased odds for subclinical atherosclerosis starting at HbA1c measurements within the prediabetes range [27].
We further showed that BMI ≥30 kg/m2 at midlife was associated with increased odds for subclinical cardiac disease, clinical CVD, and death compared with healthy cardiovascular status, with further increased odds for nonhealthy status with BMI ≥35 kg/m2. Obesity exerts multiple adverse effects that can increase CVD risk, including metabolic effects on blood pressure, lipids, insulin resistance, and glucose intolerance [28]. Elevated BMI has also been shown to increase risk for subclinical myocardial damage as assessed by hs-TnT [8,29].
Although elevated LDL-C >160 mg/dL was associated with higher odds for clinical CVD versus healthy cardiovascular status in our study, milder LDL-C elevations at midlife did not distinguish among cardiovascular health status categories in older age. Elevated LDL-C is a well-established causal risk factor for CVD. However, CVD risk in patients with hypercholesterolemia increases with the presence of other risk factors, as modeled by various risk estimate calculators such as the pooled cohort risk equations [30]. Importantly, the risk for CVD is strongly dependent on length of exposure to hypercholesterolemia [31]. Our data suggest that LDL-C trended downwards in all groups over time, with the greatest reductions in participants with clinical CVD at visit 5, whose LDL-C decreased from a mean of 143 mg/dL at visit 1 to 93 mg/dL at visit 5, which may be one reason for the lack of association. Statins were approved in 1987 [32], around the time of ARIC visit 1 (when <5% of the study population were on lipid-lowering therapy), and increased statin use (lipid-lowering medication use at visit 5 in 49% of the healthy group, 51% of the group with subclinical disease, and 72% of the group with clinical CVD) likely contributed to the decrease in LDL-C across visits. Thus, though LDL-C and TG are associated with increased risk for CVD, especially atherosclerotic CVD, they are highly modifiable risk factors with commonly used statin therapy, as shown by the changes over time in this study, and quantification at a single time point may not be as strong a predictor of cardiovascular status in older age as parameters such as blood pressure, diabetes, and obesity, which also may not have been treated as effectively.
Unfortunately, recent data on patterns of risk factor control have revealed a disturbing trend. These studies have noted a decline in the age-adjusted estimated proportion of the population with controlled blood pressure, a decline in glycemic control among individuals with diabetes, and a significant increase in prevalence of obesity [33–35]. In addition, racial and ethnic minorities appear to be disproportionately affected, underscoring the disparity in CVD preventive care in these groups [36]. For instance, in our study, Black adults had much higher odds of death than White adults. The worsening trend in risk factor modification highlights the need for augmented preventive efforts to lessen the future morbidity and mortality associated with the manifestation of clinical CVD, including greater focus on early and intensive treatment of blood pressure and HbA1c as well as developing better strategies for treating obesity. While lifestyle approaches to controlling risk factors at all times is important, delayed initiation of treatment may be too little too late and result in diminishing returns. Individuals older than age 75 are the fastest growing segment of the population in the United States and many European countries. Because randomized clinical trials of antihypertensive treatment with 2 decades of follow-up are impractical, genetic and observational data are important to provide additional information on optimal blood pressure to promote healthy cardiovascular aging. These data also highlight that our current approach to the treatment of hypertension, which usually begins late in middle age with therapy that is not intensive, has not been very successful in slowing or reversing the progression of vascular stiffness in large arteries, as shown by the progressive increase in PP over time.
Our study has several limitations. First, the definition of optimal cardiovascular health in older age has not been established. Our model of cardiovascular health was based on the absence of major clinical CVD and appreciable subclinical myocardial injury. We acknowledge that this construct might be made more stringent, and our model does not include arrhythmias, valvulopathies, peripheral vascular disease, and other potential criteria. However, our approach was to balance the selectiveness of the criteria with the practical consideration of defining a large enough sample size for analysis. The significant associations noted in this study, even with a less-stringent definition, underscore the importance of certain midlife factors for cardiovascular health at older age. Second, we acknowledge that the biomarkers used in our study are associated with subclinical myocardial injury and structural heart disease and less directly associated with subclinical atherosclerosis. While we included clinical CVD events in our analysis, we did not have data on measures of subclinical atherosclerosis such as coronary artery calcium score at visit 5. Further studies are warranted that include measures of subclinical atherosclerosis to model overall subclinical disease burden in older adults. Another important consideration is the long time-lapse between visits 1 and 5 (1987–1989 to 2011–2013) and considerable attrition of participants during this period, which we attempted to account for with imputation analysis. Finally, the trends of risk factor measurements over time are strongly influenced by medication use. Although we sought to address this limitation by adjusting for the number of medications used at each visit in our time-weighted mean analyses, we did not have sufficient data on medication dosing to account fully for medication effect on risk factor measurements over time.
In conclusion, we found that lower SBP, PP, HbA1c, and BMI measurements in middle age were significantly associated with lower odds for subclinical disease and clinical CVD in later life, which suggests additional value in more-stringent control of these risk factors than recommended in current guidelines. Our findings show the importance of addressing modifiable clinical risk factors early in life to preserve cardiovascular health into older age, which has significant implications at both the individual and population health levels.
Supplementary Material
Highlights.
Only 30% of ARIC participants maintained healthy CV status at mean age 75
Midlife traditional risk factors are associated with CV health in older age
Associations of some traditional risk measures with CV health change with aging
Pulse pressure may be a better marker of CV health with aging than SBP or DBP
Early more-aggressive risk factor modification may promote healthy CV aging
Acknowledgments:
The authors thank the staff and participants of the ARIC study for their important contributions.
Financial support:
This work was supported by the National Institutes of Health [grant numbers R01-HL134320 to E.S. and C.M.B., K24-HL152440 to E.S., R01-DK089174 to E.S.], Department of Veterans Affairs [Merit grant to V.N., grant/research support to S.V.]; and World Heart Federation [grant/research support to S.V.]. The Atherosclerosis Risk in Communities study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services [contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I].
Footnotes
Declaration of interests
☒The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Conflict of interest: Dr. Virani has received honorarium from the American College of Cardiology in his role as the Associate Editor for Innovations, acc.org. Dr. Taffet has received grant/research support (to his institution) from Kiromics, and has been a consultant for Animatus Biosciences, Baylor Global, and Novartis. Dr. de Lemos reports grant support from Roche Diagnostics and Abbott Diagnostics, and consulting fees from Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc., Beckman Coulter, Siemen’s Health Care Diagnostics, Astra Zeneca, Novo Nordisc, Eli Lilly, Regeneron, and Amgen. Dr. Ballantyne has received grant/research support (to his institution) from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostic, and has been a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma Inc, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, and Sanofi-Synthelabo. The other authors declare that they have no potential conflict of interest.
Credit Author Statement
Xiaoming Jia: Conceptualization, methodology, writing—original draft, writing—review and editing
Caroline Sun: Formal analysis
Vijay Nambi: Writing—review and editing
Salim S. Virani: Writing—review and editing
George Taffet: Writing—review and editing
Eric Boerwinkle: Writing—review and editing
Jan Bressler: Writing—review and editing
Chiadi Ndumele: Writing—review and editing
B. Gwen Windham: Writing—review and editing
James A. de Lemos: Writing—review and editing
Kunihiro Matsushita: Writing—review and editing
John William McEvoy: Writing—review and editing
Ron C. Hoogeveen: Writing—review and editing
Elizabeth Selvin: Writing—review and editing
Christie M. Ballantyne: Conceptualization, methodology, writing—original draft, Writing—review and editing, supervision, funding acquisition
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Stern S, Behar S, Gottlieb S, Cardiology patient pages. Aging and diseases of the heart, Circulation, 2003;108:e99–101. [DOI] [PubMed] [Google Scholar]
- [2].Saeed A, Nambi V, Sun W, Virani SS, Taffet GE, Deswal A, et al. , Short-term global cardiovascular disease risk prediction in older adults, J Am Coll Cardiol, 2018;71:2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. , Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure, J Card Fail, 2021. [DOI] [PubMed] [Google Scholar]
- [4].Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. , Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people, Lancet, 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. , Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults, N Engl J Med, 2010;362:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stamler J, Wentworth D, Neaton JD, Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT), JAMA, 1986;256:2823–2828. [PubMed] [Google Scholar]
- [7].Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. , Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity, JAMA Cardiol, 2018;3:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ndumele CE, Cobb L, Lazo M, Bello NA, Shah A, Nambi V, et al. , Weight History and Subclinical Myocardial Damage, Clin Chem, 2018;64:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Investigators ARIC, The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives, Am J Epidemiol, 1989;129:687–702. [PubMed] [Google Scholar]
- [10].Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. , Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria, Circ Heart Fail, 2012;5:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, et al. , Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study, Circulation, 2011;123:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. , 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure, Eur Heart J, 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- [13].Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, et al. , Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study, Circ Cardiovasc Imaging, 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, et al. , N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population, Eur Heart J, 2007;28:1374–1381. [DOI] [PubMed] [Google Scholar]
- [15].White IR, Royston P, Wood AM, Multiple imputation using chained equations: Issues and guidance for practice, Stat Med, 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- [16].Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. , The association of hemoglobin A1C with incident heart failure among people without diabetes: the Atherosclerosis Risk in Communities study, Diabetes, 2010;59:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control, N Engl J Med, 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. , 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation, 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA, et al. , Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians, Ann Intern Med, 2017;166:430–437. [DOI] [PubMed] [Google Scholar]
- [20].Drazner MH, The progression of hypertensive heart disease, Circulation, 2011;123:327–334. [DOI] [PubMed] [Google Scholar]
- [21].Ference BA, Bhatt DL, Catapano AL, Packard CJ, Graham I, Kaptoge S, et al. , Association of Genetic Variants Related to Combined Exposure to Lower Low-Density Lipoproteins and Lower Systolic Blood Pressure With Lifetime Risk of Cardiovascular Disease, JAMA, 2019;322:1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Arvanitis M, Qi G, Bhatt DL, Post WS, Chatterjee N, Battle A, et al. , Linear and nonlinear Mendelian randomization analyses of the association between diastolic blood pressure and cardiovascular events: the J-curve revisited, Circulation, 2021;143:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gaffney B, Jacobsen AP, Pallippattu AW, Leahy N, McEvoy JW, The diastolic blood pressure J-curve in hypertension management: links and risk for cardiovascular disease, Integr. Blood Press. Control, 2021;14:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meigs JB, Larson MG, D’Agostino RB, Levy D, Clouse ME, Nathan DM, et al. , Coronary artery calcification in type 2 diabetes and insulin resistance: the framingham offspring study, Diabetes Care, 2002;25:1313–1319. [DOI] [PubMed] [Google Scholar]
- [25].Selvin E, Lazo M, Chen Y, Shen L, Rubin J, McEvoy JW, et al. , Diabetes mellitus, prediabetes, and incidence of subclinical myocardial damage, Circulation, 2014;130:1374–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB, Hemoglobin a1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women, J Am Heart Assoc, 2013;2:e000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rossello X, Raposeiras-Roubin S, Oliva B, Sanchez-Cabo F, Garcia-Ruiz JM, Caimari F, et al. , Glycated Hemoglobin and Subclinical Atherosclerosis in People Without Diabetes, J Am Coll Cardiol, 2021;77:2777–2791. [DOI] [PubMed] [Google Scholar]
- [28].Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. , Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity, Circulation, 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- [29].Ndumele CE, Coresh J, Lazo M, Hoogeveen RC, Blumenthal RS, Folsom AR, et al. , Obesity, subclinical myocardial injury, and incident heart failure, JACC Heart Fail, 2014;2:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. , 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation, 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Duncan MS, Vasan RS, Xanthakis V, Trajectories of Blood Lipid Concentrations Over the Adult Life Course and Risk of Cardiovascular Disease and All-Cause Mortality: Observations From the Framingham Study Over 35 Years, J Am Heart Assoc, 2019;8:e011433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tobert JA, Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors, Nat Rev Drug Discov, 2003;2:517–526. [DOI] [PubMed] [Google Scholar]
- [33].Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, et al. , Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018, JAMA, 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fang M, Wang D, Coresh J, Selvin E, Trends in Diabetes Treatment and Control in U.S. Adults, 1999–2018, N Engl J Med, 2021;384:2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, et al. , Trends in Obesity Prevalence by Race and Hispanic Origin-1999–2000 to 2017–2018, JAMA, 2020;324:1208–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rodgers GP, Gibbons GH, Obesity and Hypertension in the Time of COVID-19, JAMA, 2020;324:1163–1165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


