Abstract
Background
Hypertrophic cardiomyopathy (HCM) is defined as left ventricular end-diastolic maximal wall thickness (WTMax) ≥15.0 mm, without accounting for ethnicity, sex, and body size. It is well-established that Asians have smaller hearts than do Caucasians.
Objectives
This study aims to examine the implications of this single absolute WTMax threshold on the diagnosis of HCM in Asians.
Methods
The study consisted of 360 healthy volunteers (male: n = 174; age: 50 ± 12 years) and 114 genetically characterized patients with HCM (male: n = 83; age: 52 ± 13 years; genotype-positive, n = 39). All participants underwent cardiovascular magnetic resonance. WTMax was measured semiautomatically at end-diastole according to the standard 16 myocardial segments.
Results
Healthy male volunteers had increased WTMax compared with that of female volunteers (8.4 ± 1.2 mm vs 6.6 ± 1.1 mm, respectively; P < 0.001). Conversely, WTMax was similar between male and female patients with HCM (15.2 ± 3.4 mm vs 14.7 ± 3.0 mm, respectively; P = 0.484) and between those with and without a pathogenic gene variant (P = 0.828). Using the recommended diagnostic threshold of 15.0 mm, 56 patients with HCM had WTMax <15.0 mm and no healthy volunteers had WTMax >15.0 mm (specificity of 100% and sensitivity of 51%). Lowering WTMax thresholds to 10.0 mm in female patients and 12.0 mm in male patients did not affect specificity (100%) but significantly improved sensitivity (84%). Despite lower left ventricular mass, female patients with HCM demonstrated more features of adverse cardiac remodeling than did male patients: increased myocardial fibrosis, higher asymmetric ratio, and disproportionately worse myocardial strain.
Conclusions
The study highlights cautious application of guideline-recommended WTMax to diagnose HCM in Asians. Lowering WTMax to account for ethnicity and sex improves diagnostic sensitivity without compromising specificity.
Key Words: asymmetrical hypertrophy, cardiovascular magnetic resonance, hypertrophic cardiomyopathy, sex-specific diagnostic thresholds
Abbreviations and Acronyms: AUC, area under the curve; CMR, cardiovascular magnetic resonance; HCM, hypertrophic cardiomyopathy; LV, left ventricle/left ventricular; LVH, left ventricular hypertrophy; NPV, negative predictive value; PPV, positive predictive value; WTMax, maximal wall thickness
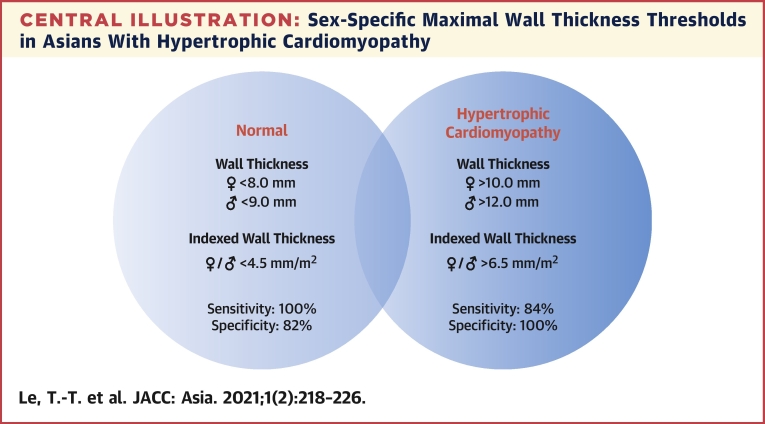
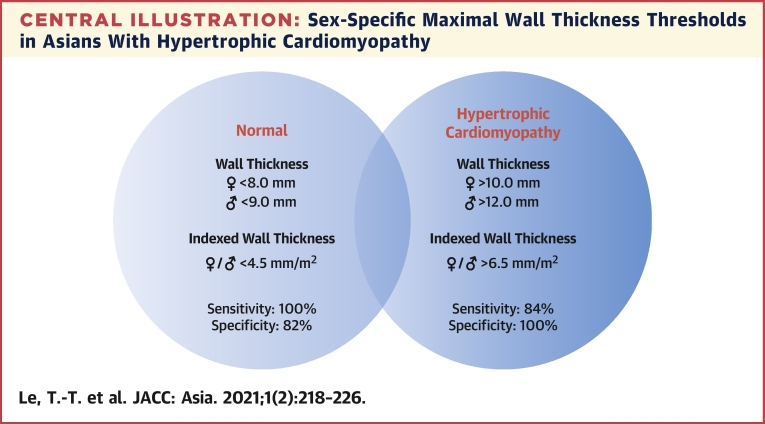
Central Illustration
Hypertrophic cardiomyopathy (HCM) is an important cause of arrhythmias, heart failure, and strokes (1). Accurate measurement of wall thickness is crucial in establishing diagnosis and assessing prognosis (2, 3, 4). The current diagnosis of HCM in adults is defined as unexplained left ventricular (LV) end-diastolic maximal wall thickness (WTMax) ≥15.0 mm (3,4). Of note, the same threshold is used regardless of the ethnicity, sex, and body surface area of the patient (5).
It is well-established that Asians have smaller LV mass compared with that of Caucasians even after accounting for their smaller body sizes. Regardless of ethnicities, male persons have increased LV mass over that of female persons (6). The implications of using a single threshold of 15.0 mm to diagnose HCM in Asians have not been examined.
In this study, we aim to examine the distribution of WTMax in a large population of healthy Asians and explore WTMax thresholds in a well-characterized HCM cohort. We hypothesize that Asians with HCM require lower WTMax thresholds than the published recommendations because of their smaller heart sizes compared with those of Caucasians.
Methods
Study populations
Healthy adults (>18 years of age) were prospectively recruited in the National Heart Centre Singapore Biobank to examine health and cardiovascular risk in the general population. Those without cardiovascular risk factors were included in this analysis.
Patients with a clinical diagnosis of HCM based on contemporary guidelines (3,4) were prospectively recruited from the Cardiomyopathy Clinic at the National Heart Centre Singapore between June 1, 2014, and June 30, 2019. These patients were referred for diagnosis, risk stratification, and management of HCM. Because the study aimed to examine WTMax in Asians with HCM, using the recommended threshold of ≥15.0 mm will introduce a selection bias. Instead, this study defined HCM as nondilated left ventricular hypertrophy (LVH) on cardiovascular magnetic resonance (CMR) according to indexed LV mass and volumes established in Asians (7) that was not explained by another cardiac or systemic disease (such as hypertension and other cardiomyopathies). This was according to the clinical definition reported in the 2011 and 2020 American College of Cardiology Foundation/American Heart Association Guidelines for the Diagnosis and Management of Hypertrophic Cardiomyopathy (3,8). Furthermore, the study included only probands, and family members identified by screening and pedigree studies were excluded.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Singhealth Centralised Institutional Review Board. Written informed consent was obtained from all individuals.
CMR protocol and image analysis
Cardiovascular phenotyping was performed in all participants (Siemens MAGNETOM Aera 1.5-T). Balanced steady-state free precession cines were acquired in the standard long-axis (2-, 3- and 4-chamber) and short-axis views, extending from the base to the apex (8-mm thick and 2-mm gap; 30 phases per cardiac cycle). In patients with HCM, replacement myocardial fibrosis was assessed using late gadolinium enhanced imaging (Gadovist, Bayer Pharma AG) based on the inversion-recovery fast gradient echo sequence. Myocardial T1 mapping (Modified Look-Locker Inversion-recovery sequence) was used to assess for diffuse myocardial fibrosis. Extracellular volume fraction was estimated from the native and 15-minute postcontrast T1 maps (T1 mapping module: CVI42 [Circle Cardiovascular Imaging]) that included regions of nonischemic fibrosis.
LV measures of geometry and function were analyzed using CVI42 at our NHRIS CMR Core Laboratory, according to standardized protocols as published previously (7,9,10). Specifically, WTMax was measured at end-diastole according to the standard 16 myocardial segments (Figure 1). Asymmetric LV wall thickness ratio was defined as the ratio of the thickest myocardial segment compared with the opposing segment (9). All patients and healthy volunteers with abnormal CMR findings that would confound wall thickness assessment were excluded: other cardiomyopathies and regional/global myocardial thinning caused by myocardial infarction, significant valvular regurgitation, and burnt-out HCM.
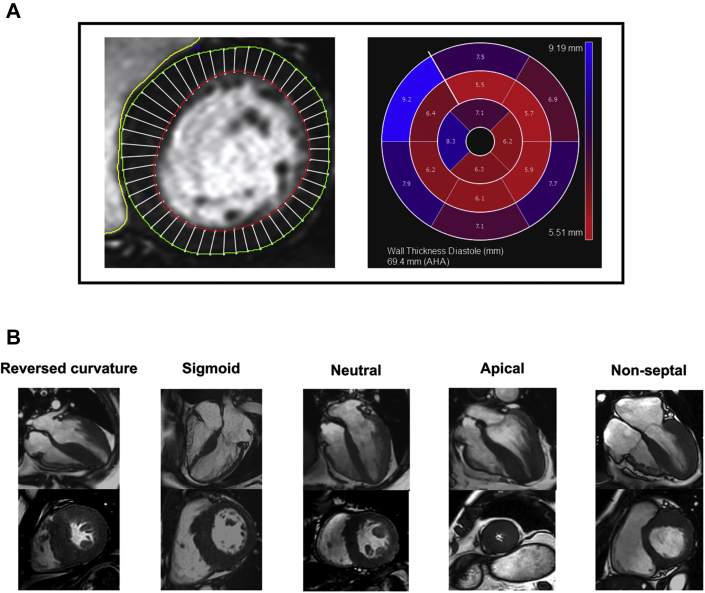
Figure 1.
Assessment of Myocardial Wall Thickness and Morphology
Myocardial wall thickness was measured using a semiautomated approach (CVI42). (A) Wall thickness was estimated using 50 chords per myocardial slice in the short-axis view, according to the 16-segment model. (B) The distribution of hypertrophy in hypertrophic cardiomyopathy was classified based on the following: reversed curvature, neutral, sigmoid septum; apical and nonseptal hypertrophy. AHA = American Heart Association.
The distribution of hypertrophy in HCM was assessed qualitatively on the long-axis views based on the following definitions (Figure 1) (11):
-
•
Reversed curvature septum: septum convexes into LV cavity and a crescentic LV cavity
-
•
Neutral septum: straight septum; neither concaves nor convexes into LV cavity
-
•
Sigmoid septum: prominent septal bulge and septum concaves into LV cavity
-
•
Apical hypertrophy: hypertrophy of apical with or without mid-ventricular segments and an “ace of spade” LV cavity
-
•
Nonseptal hypertrophy: hypertrophy in segments other than the septum
Targeted sequencing of HCM genes and defining pathogenic variants
In all patients with HCM, targeted genome sequencing was performed using TruSight Cardio sequencing kit (Illumina) as previously described (12). Libraries were individually indexed, purified, and enriched for genes related to inherited cardiac conditions including HCM. Pooled libraries were sequenced using Illumina MiSeq (v2 kit) or NextSeq 500 (Mid Output v2 kit) benchtop sequencers using paired-end, 150 base pair reads. Raw sequencing data were demultiplexed, trimmed, and mapped to University of California, Santa Cruz GRCh37/hg19 reference genome before variant calling using Genome Analysis Toolkit version 3.5 HaplotypeCaller and UnifiedGenotyper.
Variants of 15 genes (ACTC1, CSRP3, FHL1, GLA, LAMP2, MYBPC3, MYH7, MYL2, MYL3, PLN, PRKAG2, TNNC1, TNNI3, TNNT2, TPM1) that are robustly associated with either HCM or its known genocopies were annotated using CardioClassifier (13,14). The pathogenicity of the variants for each patient was further curated by an expert cardiologist in genetics and cardiomyopathies (S.A.C.), who was blinded to the imaging and other clinical data.
Statistical analysis
Distribution of continuous variables was assessed using the Shapiro-Wilk test. Data were presented as mean ± SD or median (interquartile range), as appropriate. Groups of continuous data were compared using either the parametric Student’s t-test and 1-way analysis of variance or nonparametric Mann-Whitney U test and Kruskal-Wallis test. Categorical variables were expressed as absolute values and percentage and compared using the Pearson chi-square test. The ability to discriminate between patients with HCM and healthy volunteers and the optimal sensitive/specific WTMax thresholds were derived from the area under the curve (AUC).
All statistical analyses were performed using SPSS (version 24, IBM Corp) and GraphPad (version 8, GraphPad Software Inc). A 2-sided P value <0.05 was considered statistically significant.
Results
Myocardial wall thickness in healthy volunteers and patients with HCM
The study consisted of 360 healthy volunteers (male: n = 174; age: 50 ± 12 years) (Table 1). Male, compared with female, volunteers have increased WTMax (8.4 ± 1.2 vs 6.6 ± 1.1; P < 0.001). A positive correlation was demonstrated between WTMax and body surface area in both male (r = 0.38; P < 0.001) and female (r = 0.30; P < 0.001) volunteers. After accounting for the differences in body sizes, the difference in wall thickness between healthy male and female volunteers was reduced but remained statistically significant (indexed WTMax: 4.6 ± 0.6 mm/m2 vs 4.2 ± 0.7 mm/m2, respectively; P < 0.001). Age was associated with WTMax in the healthy volunteers, but the effect was small. With every decade increase in age, WTMax increased 0.11 mm in male volunteers (linear regression: WTMax = 0.011 × age + 7.612) and 0.34 mm in female volunteers (WTMax = 0.034 × age + 5.189).
Table 1.
Clinical and CMR Characteristics in Healthy Volunteers
| All Healthy Volunteers (n = 360) | Male Volunteers (n = 174) | Female Volunteers (n = 186) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 50 ± 12 | 51 ± 12 | 49 ± 12 | 0.120 |
| Race | 0.143 | |||
| Chinese | 329 (91) | 159 (91) | 170 (91) | |
| Malay | 15 (4) | 10 (6) | 5 (3) | |
| Indian | 16 (4) | 5 (3) | 11 (6) | |
| Height, m | 1.64 ± 0.09 | 1.71 ± 0.06 | 1.58 ± 0.06 | <0.001 |
| Weight, kg | 64 ± 13 | 72 ± 11 | 57 ± 11 | <0.001 |
| Body surface area, m2 | 1.70 ± 0.20 | 1.83 ± 0.16 | 1.57 ± 0.14 | <0.001 |
| Systolic blood pressure, mm Hg | 131 ± 17 | 137 ± 15 | 125 ± 17 | <0.001 |
| Diastolic blood pressure, mm Hg | 80 ± 12 | 85 ± 11 | 75 ± 11 | <0.001 |
| CMR characteristics | ||||
| Indexed LV EDV, mL/m2 | 73 ± 11 | 76 ± 11 | 70 ± 11 | <0.001 |
| Indexed LV ESV, mL/m2 | 30 ± 7 | 32 ± 6 | 27 ± 6 | <0.001 |
| Indexed LV SV, mL/m2 | 43 ± 7 | 44 ± 7 | 43 ± 6 | 0.080 |
| Indexed LV mass, g/m2 | 43 ± 8 | 49 ± 7 | 38 ± 5 | <0.001 |
| LV ejection fraction, % | 59 ± 5 | 58 ± 5 | 61 ± 5 | <0.001 |
| Indexed RV EDV, mL/m2 | 80 ± 15 | 87 ± 14 | 74 ± 13 | <0.001 |
| Indexed RV ESV, mL/m2 | 37 ± 10 | 42 ± 10 | 31± 8 | <0.001 |
| Indexed RV SV, mL/m2 | 44 ± 7 | 44 ± 7 | 43 ± 7 | 0.062 |
| RV ejection fraction, % | 55 ± 7 | 52 ± 5 | 58 ± 6 | <0.001 |
| Global longitudinal strain, % | −19.0 ± 2.9 | −17.7 ± 2.5 | −20.4 ± 2.6 | <0.001 |
| Global radial strain, % | 44.2 ± 11.8 | 39.5 ± 9.6 | 48.9 ± 11.9 | <0.001 |
| Global circumferential strain, % | −21.1 ± 2.9 | −19.7 ± 2.5 | −22.4 ± 2.5 | <0.001 |
| LV mass/EDV | 0.66 ± 0.11 | 0.65 ± 0.10 | 0.55 ± 0.10 | <0.001 |
| WTMax, mm | 7.5 ± 1.5 | 8.4 ± 1.2 | 6.6 ± 1.1 | <0.001 |
| Indexed WTMax, mm/m2 | 4.4 ± 0.7 | 4.6 ± 0.6 | 4.2 ± 0.7 | <0.001 |
| Asymmetric ratio | 1.27 ± 0.18 | 1.26 ± 0.17 | 1.27 ± 0.19 | 0.586 |
Values are mean ± SD or n (%).
CMR = cardiovascular magnetic resonance; EDV = end-diastolic volume; ESV = end-systolic volume; LV = left ventricular; RV = right ventricular; SV = stroke volume; WTMax= maximal wall thickness.
A total of 114 patients with HCM (male: n = 83; age: 52 ± 13 years) were analyzed (Table 2). Patients with HCM and healthy volunteers had similar systolic blood pressures (134 ± 20 mm Hg vs 131 ± 17 mm Hg, respectively; P = 0.108). Pathogenic HCM gene variants were identified in 39 of 114 patients with HCM. WTMax was similar between those with and without a pathogenic gene variant (WTMax: 15.1 ± 3.4 mm vs 15.0 ± 3.2 mm, respectively; P = 0.828) (Supplemental Figure 1). Reverse curvature (n = 57; 50%) and apical hypertrophy (n = 27; 24%) were the most common patterns in patients with HCM. The remaining patients had neutral (n = 17; 15%), sigmoid (n = 7; 6%) septum or hypertrophy at a nonseptal location (n = 6; 5%).
Table 2.
Clinical and Baseline Characteristics of Patients with HCM
| All HCM Patients (n = 114) | Male HCM Patients (n = 83) | Female HCM Patients (n = 31) | P Value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, y | 52 ± 13 | 52 ± 14 | 53 ± 12 | 0.570 |
| Race | 0.749 | |||
| Chinese | 100 (88) | 72 (87) | 28 (90) | |
| Malay | 6 (5) | 4 (5) | 2 (6) | |
| Indian | 8 (7) | 7 (8) | 1 (3) | |
| Height, m | 1.68 ± 0.08 | 1.71 ± 0.06 | 1.59 ± 0.06 | <0.001 |
| Weight, kg | 71 ± 14 | 75 ± 12 | 60 ± 13 | <0.001 |
| Body surface area, m2 | 1.80 ± 0.19 | 1.87 ± 0.15 | 1.61 ± 0.17 | <0.001 |
| Systolic blood pressure, mm Hg | 134 ± 20 | 136 ± 18 | 128 ± 24 | 0.044 |
| Diastolic blood pressure, mm Hg | 77 ± 12 | 79 ± 12 | 73 ± 11 | 0.018 |
| CMR characteristics | ||||
| Indexed LV EDV, mL/m2 | 79 ± 12 | 80 ± 13 | 78 ± 10 | 0.312 |
| Indexed LV ESV, mL/m2 | 33 ± 9 | 34 ± 10 | 32 ± 9 | 0.419 |
| Indexed LV SV, mL/m2 | 46 ± 9 | 46 ± 9 | 45 ± 8 | 0.587 |
| Indexed LV mass, g/m2 | 81 ± 26 | 85 ± 26 | 70 ± 21 | 0.006 |
| LV ejection fraction, % | 58 ± 9 | 58 ± 9 | 59 ± 9 | 0.701 |
| Indexed RV EDV, mL/m2 | 72 ± 16 | 74 ± 16 | 66 ± 15 | 0.016 |
| Indexed RV ESV, mL/m2 | 27 ± 12 | 29 ± 12 | 23 ± 10 | 0.010 |
| Indexed RV SV, mL/m2 | 45 ± 9 | 45 ± 9 | 43 ± 8 | 0.310 |
| RV ejection fraction, % | 63 ± 10 | 62 ± 10 | 67 ± 10 | 0.025 |
| Global longitudinal strain, % | −11.8 ± 3.1 | −11.6 ± 3.3 | −12.2 ± 2.5 | 0.386 |
| Global radial strain, % | 29.1 ± 9.4 | 27.9 ± 8.9 | 32.4 ± 9.7 | 0.025 |
| Global circumferential strain, % | −16.9 ± 3.6 | −16.5 ± 3.7 | −18.0 ± 3.4 | 0.062 |
| LV mass/EDV | 1.03 ± 0.29 | 1.07 ± 0.30 | 0.91 ± 0.25 | 0.009 |
| WTMax, mm | 15.0 ± 3.3 | 15.2 ± 3.4 | 14.7 ± 3.0 | 0.484 |
| Indexed WTMax, mm/m2 | 8.5 ± 2.0 | 8.2 ± 2.0 | 9.2 ± 1.8 | 0.018 |
| Asymmetric ratio | 1.97 ± 0.80 | 1.78 ± 0.61 | 2.47 ± 1.02 | <0.001 |
| Nonischemic fibrosis | 87 (76) | 61 (73) | 26 (84) | 0.600 |
| Native T1, ms | 1,058 ± 41 | 1,050 ± 40 | 1,080 ± 37 | 0.007 |
| Extracellular volume fraction, % | 30.4 ± 4.4 | 29.5 ± 4.0 | 32.9 ± 4.7 | 0.005 |
Values are mean ± SD or n (%).
HCM = hypertrophic cardiomyopathy; other abbreviations as in Table 1.
Implications of WTMax thresholds in Asians with HCM
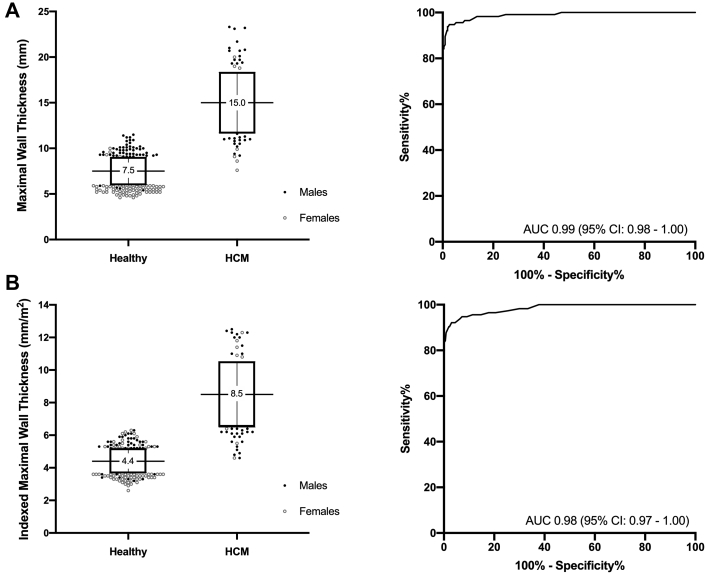
Female and male patients with HCM had similar WTMax (14.7 ± 3.0 mm vs 15.2 ± 3.4 mm, respectively; P = 0.484) (Table 2). WTMax demonstrated excellent ability to discriminate between healthy volunteers and patients with HCM (AUC: 0.99; 95% CI: 0.98-1.00; P < 0.001) (Figure 2), with similar findings observed in either sex.
Figure 2.
WTMax in Patients With HCM
Compared to healthy volunteers, patients with hypertrophic cardiomyopathy (HCM) had increased maximal wall thickness (WTMax) either in absolute values (A) or indexed to body surface area (B). (A, B) WTMax demonstrated excellent ability to discriminate HCM from healthy volunteers. The recommended threshold of 15.0 mm in contemporary guidelines had excellent specificity of 100% but low sensitivity of 51% in diagnosing HCM in Asians. Lowering the absolute WTMax to 12.0 mm in male and 10.0 mm in female populations will improve sensitivity (84%) without changing specificity (100%). To account for differences in body sizes, an indexed WTMax of 6.5 mm/m2 and 4.5 mm/m2 are associated with 100% specificity and sensitivity, respectively (same threshold in either sex). Box-plot shows mean ± SD. AUC = area under the curve.
Using the recommended diagnostic threshold of 15.0 mm in contemporary guidelines, a total of 56 patients (female: n = 16) had wall thickness <15.0 mm. No healthy volunteers had WTMax >15.0 mm. These findings accounted for a low sensitivity of 51% and negative predictive value (NPV) of 87% but excellent specificity and positive predictive value (PPV) of 100%. Based on the AUC values, the most sensitive and specific sex-specific WTMax values were determined. A WTMax of 10.0 mm in female volunteers and 12.0 mm in male volunteers were associated with a specificity and PPV of 100% and a sensitivity and NPV of 84% and 95%, respectively. Lowering WTMax to 8.0 mm in female volunteers and 9.0 mm in male volunteers was associated with a sensitivity and NPV of 100% and at an expected lower specificity and PPV of 82% and 64%, respectively.
When corrected for their smaller body sizes, female patients with HCM had increased indexed WTMax compared with that of male patients (9.2 ± 1.8 mm/m2 vs 8.2 ± 2.0 mm/m2, respectively; P = 0.018). Like absolute WTMax, indexed WTMax demonstrated excellent discrimination between healthy volunteers and patients with HCM (AUC: 0.98; 95% CI: 0.97-1.00; P < 0.001) (Figure 2). Incidentally, the most sensitive and specific indexed WTMax values derived from the AUC curves were the same for either sex. An indexed WTMax of 6.5 mm/m2 was associated with a specificity and PPV of 100% (sensitivity and NPV of 84% and 95%, respectively), and an indexed WTMax of 4.5 mm/m2 was associated with a sensitivity and NPV of 100% (specificity and PPV of 62% and 46%, respectively). Similar thresholds were observed when patients with apical hypertrophy were excluded (indexed WTMax values of 6.4 mm/m2 and 4.5 mm/m2 were associated with 100% specificity and sensitivity, respectively) and when analysis was stratified by genotypic status.
Despite lower LV mass and concentricity, female patients with HCM had increased measures of diffuse myocardial fibrosis (native T1 and extracellular volume fraction) and similar proportions of nonischemic replacement myocardial fibrosis compared with male patients with HCM. Similarly, asymmetric ratio in WTMax segments was significantly higher in female compared with male patients (2.47 ± 1.02 vs 1.78 ± 0.61, respectively; P < 0.001). Multidirectional strain was significantly higher in healthy female compared with male volunteers (P < 0.001 for all) but was similar in female and male patients with HCM, suggesting a relative worse strain in female compared with male patients with HCM (Table 2).
Discussion
We have examined WTMax in Asian volunteers and patients with HCM. Sex-specific WTMax of 12.0 mm in male subjects and 10.0 mm in female subjects (or indexed WTMax of 6.5 mm/m2 in either sex) discriminate between healthy volunteers and Asians with HCM with 100% specificity. Lower WTMax of 9.0 mm in male subjects and 8.0 mm in female subjects (or indexed WTMax of 4.5 mm/m2 in either sex) is associated with 100% sensitivity (Central Illustration). Despite smaller LV mass, female patients with HCM have increased indexed WTMax, elevated CMR markers of diffuse myocardial fibrosis, increased asymmetric ratio (a potential adverse prognostic marker), and disproportionately worse LV strain compared to male patients with HCM.
Central Illustration.
Sex-Specific Maximal Wall Thickness Thresholds in Asians With Hypertrophic Cardiomyopathy
Sex-specific (or indexed for both sexes) maximal wall thickness thresholds established in the study demonstrate high diagnostic sensitivity and specificity in Asians with hypertrophic cardiomyopathy.
The study has shown that absolute WTMax in Asians is about 2.0 mm less than that reported in Caucasians (male: 8.4 ± 1.2 mm vs 10.6 ± 1.9 mm, respectively; female: 6.6 ± 1.1 mm vs 8.6 ± 1.6 mm, respectively) (15). Based on this, the 15.0-mm diagnostic threshold is >5 SDs above the WTMax in Asians compared with ∼2 to 4 SDs in Caucasians. Therefore, it is perhaps not surprising that the current recommended wall thickness thresholds that were established in Caucasians can be applied in Asians with very high specificity. However, this threshold will “miss” about a half of Asians with HCM as demonstrated in this study. Our study has important clinical implications. Asians and female patients are at risk of delayed (or under-) diagnosis because their smaller hearts may not satisfy the WTMax criteria until more advanced stages of disease. This may partly explain why female patients present with more adverse cardiac remodeling (also demonstrated in our study) and experience worse outcomes than male patients do, and Asians with HCM have higher rates of sudden cardiac deaths than Western populations do (>2% vs 1%-2%) (16, 17, 18).
In this study, we have presented data using both absolute sex-specific WTMax and indexed WTMax thresholds. Indexed WTMax threshold, although less frequently described, accounts for the important differences in body surface area. Of note, the value of 6.5 mm/m2 in our study is very similar to thresholds demonstrated in a recent study conducted in the Netherlands: 6.5 mm/m2 for male and 6.7 mm/m2 for female participants (19). Whether a single indexed WTMax can be applied to different ethnicities, sexes, and body sizes requires further investigations. Increasing evidence suggesting the recommendation of a single absolute WTMax of 15.0 mm to diagnose HCM should be re-examined (5,16). Because patients with WTMax lower than thresholds using either sex-specific or indexed WTMax have less severe disease (Supplemental Tables 1 and 2), it is reasonable to consider serial CMR to monitor disease progression in a patient referred for suspected HCM and WTMax in the “gray zone” between 4.5 and 6.5 mm/m2 (males: 9.0-12.0 mm; females: 8.0-10.0 mm). In these patients with WTMax in the gray zone, the pattern of hypertrophy, clinical history, and/or genetic testing may also be helpful.
The heterogeneous phenotypic expression of HCM is contributed in part by differences in sex, ethnicity, and body size. Female persons have smaller heart sizes and LV mass than male persons do. In the general population, female persons develop more concentric remodeling and demonstrate elevated measures of myocardial fibrosis than do male persons [20]. Of note, the regions of increased concentricity in female subjects correspond to regions of increased wall stress [20]. Despite a lower LV mass, female patients with HCM demonstrate adverse features of cardiac remodeling compared to male patients with HCM: increased asymmetric ratio (a potential adverse prognostic marker [9]), elevated CMR measures of diffuse myocardial fibrosis, and relative worse strain measures. How the smaller heart sizes predispose female patients with HCM to an increased cardiovascular risk warrants further investigation.
Study strengths and limitations
The study was conducted using the same imaging and analysis protocols for all patients. Furthermore, all patients with HCM underwent genome sequencing. In this first Asian study to examine implications of diagnostic thresholds, an irrefutable diagnosis of HCM was essential. We had excluded other causes of LVH in the HCM cohort that could confound study validity. As hypertension is a common cause of LVH in individuals in the older age group, this partly explains the relatively small sample size of younger patients with HCM in the study. We have limited our study to patients with HCM and nondilated LVH on CMR. These thresholds will need to be validated in other Asian cohorts with HCM, including those with segmental myocardial thickening and normal LV mass (presumably with milder disease and lower WTMax). Female persons were underrepresented in the study, and the thresholds will need to be validated in larger Asian cohorts.
Conclusions
Current recommended WTMax threshold of 15.0 mm is highly specific for the diagnosis of HCM in Asians. Lower thresholds to account for ethnicity, sex, and body size improve sensitivity without affecting specificity and should be considered to improve diagnostic accuracy in Asians.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: It is well-established Asians have smaller hearts than do Caucasians, even after accounting for the smaller body surface areas. This study demonstrates the potential challenge of using a single absolute WTMax value in diagnosing Asians with HCM.
TRANSLATIONAL OUTLOOK: The thresholds examined in the study should be validated in larger and more diverse Asian cohorts of patients with HCM.
Funding Support and Author Disclosures
This study was funded by the National Medical Research Council, Singapore. Dr Cook has consulted for Illumina. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank the radiographers at the Department of Cardiovascular Magnetic Resonance Imaging, National Heart Center Singapore for their help in the study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Maron B.J., Maron M.S. Hypertrophic cardiomyopathy. Lancet. 2013;381(9862):242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 2.Spirito P., Bellone P., Harris K.M., Bernabo P., Bruzzi P., Maron B.J. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342(24):1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 3.Gersh B.J., Maron B.J., Bonow R.O., et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011 doi: 10.1016/j.jacc.2011.10.825. 58(25):2703-2738. [DOI] [PubMed] [Google Scholar]
- 4.Authors/Task Force members. Elliott P., Anastasaki A., et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35(39):2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 5.Sen-Chowdhry S., Jacoby D., Moon J.C., McKenna W.J. Update on hypertrophic cardiomyopathy and a guide to the guidelines. Nat Rev Cardiol. 2016;13(11):651–675. doi: 10.1038/nrcardio.2016.140. [DOI] [PubMed] [Google Scholar]
- 6.Raisi-Estabragh Z., Kenawy A., Aung N., et al. Variation in left ventricular cardiac magnetic resonance normal reference ranges: systemic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2021;22(5):494–504. doi: 10.1093/ehjci/jeaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le T.T., Tan R.S., De Deyn M., et al. Cardiovascular magnetic resonance reference ranges for the heart and aorta in Chinese at 3T. J Cardiovasc Magn Reson. 2016;18:21. doi: 10.1186/s12968-016-0236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76(25):e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Cai J., Bryant J.A., Le T.T., et al. Fractal analysis of left ventricular trabeculations is associated with impaired myocardial deformation in healthy Chinese. J Cardiovasc Magn Reson. 2017;19(1):102. doi: 10.1186/s12968-017-0413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiecinski J., Chin C., Everett R.J., et al. Adverse prognosis associated with asymmetric myocardial thickening in aortic stenosis. Eur Heart J Cardiovasc Imaging. 2018;19(3):3479356. doi: 10.1093/ehjci/jex052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Williams L., Rakowski H. In: Naidu S., editor. Hypertrophic Cardiomyopathy; Springer: 2015. Natural history of untreated hypertrophic cardiomyopathy; pp. 9–22. [Google Scholar]
- 12.Pua C.J., Bhalshankar J., Miao K., et al. Development of a comprehensive sequencing assay for inherited cardiac condition genes. J Cardiovasc Transl Res. 2016;9(1):3–11. doi: 10.1007/s12265-016-9673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pua C.J., Tham N. Genetic studies of hypertrophic cardiomyopathy in Singaporeans identify variants in TNNI3 and TNNT2 that are common in Chinese patients. Circ Genom Precis Med. 2020;13:424–434. doi: 10.1161/CIRCGEN.119.002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whiffin N., Walsh R., Govind R., et al. CardioClassifier: disease- and gene-specific computational decision support for clinical genome interpretation. Genet Med. 2018;20:1246–1254. doi: 10.1038/gim.2017.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawel N., Turkbey E.B., Carr J.J., et al. Normal left ventricular myocardial thickness for middle-aged and older subjects with steady-state free precession cardiac magnetic resonance: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2012;5(4):500–508. doi: 10.1161/CIRCIMAGING.112.973560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Driel B., Nijenkamp L., Huurman R., Michels M., van der Velden J. Sex differences in hypertrophic cardiomyopathy: new insights. Curr Opin Cardiol. 2019;34(3):254–259. doi: 10.1097/HCO.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 17.Choi Y.J., Kim H.K., Lee S.C., et al. Validation of the hypertrophic cardiomyopathy risk-sudden cardiac death calculator in Asians. Heart. 2019;105(24):1892–1897. doi: 10.1136/heartjnl-2019-315160. [DOI] [PubMed] [Google Scholar]
- 18.Elliott P.M., Gimeno J.R., Thaman R., et al. Historical trends in reported survival rates in patients with hypertrophic cardiomyopathy. Heart. 2006;92(6):785–791. doi: 10.1136/hrt.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huurman R., Schinkel A.F.L., van der Velde N., et al. Effects of body surface area and gender on wall thickness thresholds in hypertrophic cardiomyopathy. Neth Heart J. 2020;28(1):37–43. doi: 10.1007/s12471-019-01349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phua A.I.H., Le T.T., Tara S.W., et al. Paradoxical higher myocardial wall stress and increased cardiac remodeling despite lower mass in females. J Am Heart Assoc. 2020;9(4) doi: 10.1161/JAHA.119.014781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.