Abstract
Objective
To investigate the effects of discontinuing antihypertensive drugs on the characteristics of patients with frailty syndrome.
Methods
This prospective pilot study was conducted between March 2016 and July 2019. Among patients who visited the frailty clinic within this period, outpatients who received antihypertensive drugs at their first visit and were followed-up for about 1 year were enrolled. Participants who discontinued or continued antihypertensive drugs during 1 year of follow-up were classified into a discontinuation group or continuation group, respectively. Each domain in the Kihon checklist (KCL), fall risk score, short physical performance battery (SPPB) score, and skeletal muscle index (SMI) were assessed at the first visit and 1-year follow-up assessment, and were compared between the two groups.
Results
Among 498 patients who attended the frailty clinic, 78 were enrolled (discontinuation group, n = 19; continuation group, n = 59). At the first visit, SMI scores were significantly higher in the discontinuation versus continuation group. At the 1-year assessment, physical strength in the KCL for the discontinuation group and various SPPB scores for both groups were significantly improved, and the fall risk score was improved in the continuation group.
Conclusion
Discontinuation of antihypertensive drugs may positively affect physical performance.
Keywords: Hypertension, frailty syndrome, discontinuation of antihypertensive drugs, Kihon checklist, physical performance, fall risk
Introduction
Frailty, one of the greatest challenges faced by our aging population,1 is defined as a state of high vulnerability associated with adverse health outcomes, such as falls, hospitalization, physical disability, and mortality.2
Hypertension may be a risk factor for frailty because detrimental outcomes induced by chronically high blood pressure target the organs associated with cerebrovascular, cardiac, and kidney diseases, and bring about mental health repercussions, including anxiety, stress, depression, and impaired cognitive function.3,4
Evidence from observational studies suggests that low blood pressure and multiple antihypertensive prescriptions may harm older patients through polypharmacy and multimorbidity.5 Low blood pressure is also shown to be associated with high morbidity and mortality rates in frail older patients receiving blood pressure-lowering drugs, but not in those with spontaneously low blood pressure unrelated to drugs.4
Deprescribing is a term used with varying degrees of precision, and for which there is no accepted definition. Page et al.6 analyzed the concept of deprescribing and defined it as a patient-centered process for withdrawing medication, aimed at improving health outcomes through the discontinuation of one or more medications that are either potentially harmful or no longer required.
In a previous study, no significant differences in frailty indices, assessed at 12 weeks, were observed between older patients who were deprescribed antihypertensive drugs and those receiving multiple antihypertensive drugs to control systolic blood pressure and treat frailty.5 However, the characteristics of patients in whom frailty syndrome may be effectively improved after discontinuing antihypertensive drugs remain unclear.
The Kihon checklist (KCL), a reliable tool for predicting frailty in older adults,7 is a self-administered questionnaire developed by the Japanese Ministry of Health, Labor, and Welfare to identify frail older individuals requiring new certification for long-term care insurance.8 The KCL includes 25 yes/no questions covering several domains, comprising instrumental activities of daily living (IADL), physical strength, nutrition, oral function, isolation, memory, and mood.
At the National Center for Geriatrics and Gerontology in Obu, Japan, a multidisciplinary therapeutic approach that involves physicians, nutritionist, physical therapists, pharmacists, and medical social workers, and includes nutritional management, physical therapy, and clinical medication review using some evaluation items, such as the KCL, is employed when treating outpatients who visit the frailty clinic. Using data obtained from this therapeutic approach, including each KCL domain plus other detailed evaluation items, the aim of the current pilot study was to investigate the effects of discontinuing antihypertensive drugs on frailty syndrome in outpatients.
Patients and methods
Study population
This prospectively designed retrospective chart-review study was conducted between March 2016 and July 2019. The inclusion criteria were as follows: consecutive patients who attended the frailty clinic at the National Center for Geriatrics and Gerontology, Obu, Japan, who were receiving antihypertensive drug treatment at their first visit and who attended a follow-up visit. Patients who discontinued antihypertensive drug treatment within 1 year after the first visit were allocated to the discontinuation group, while those who continued antihypertensive drug treatment up to 1 year after the first visit were allocated to the continuation group. Patients who were not receiving antihypertensive drug treatment at their first visit, those with missing data, and patients who were lost to follow-up after the first visit and up to 1 year thereafter, were excluded from the study. Discontinuation or continuation of antihypertensive drugs were identified using the Essence of Japanese Society of Hypertension Guidelines for the Management of Hypertension.9
This study was approved by the ethics review board of the National Center for the Geriatrics and Gerontology, Obu, Japan (approval No: 881–13; 2 February 2022). All participants provided written and/or verbal informed consent for routine medical care and for study registration. All patient details were deidentified for this study, and study was reported in accordance with the STROBE guidelines.10
Antihypertensive drugs
Antihypertensive drugs were classified as ‘antihypertensive drugs’, ‘vasodilators’, and ‘thiazide derivatives and preparations’ in accordance with the drugs and related commodities class in the Standard Commodity Classification system of Japan.11
Kihon checklist scores
The KCL scores were assessed as the first visit and at approximately 1 year following the first visit.7,8 The KCL comprises 25 questions corresponding to different KCL domains as follows: IADL, questions 1–5; physical activity, questions 6–10; nutrition, questions 11–12; oral function, questions 13–15; isolation, questions 16–17; memory, questions 18–20; and mood, questions 21–25. The total score is calculated (range, 0–25), with higher scores equating to increased frailty.
Data collection and outcome measures
At the first visit, data regarding age, sex, medical history, prescribed antihypertensive drugs, and use of vitamin D analogs were obtained, and systolic blood pressure, diastolic blood pressure, mean arterial pressure, pulse pressure, and serum 25(OH) vitamin D and albumin levels were measured. In addition to KCL scores, total fall risk score;12 short physical performance battery (SPPB) balance score (range, 0–4), SPPB gait speed score (range, 0–4), SPPB chair stand score (range, 0–4), and total SPPB score (range 0–12);13 body weight, and skeletal muscle index (SMI) were obtained at the first and follow-up visit for each participant. Serum vitamin D measurements were outsourced to Hoken Kagaku, Inc, Nagoya Laboratory (Nagoya, Japan). Blood samples drawn at the National Center for Geriatrics and Gerontology were allowed to clot, then centrifuged at 2000 × g for 5 min. An approximately 2 ml serum sample was collected and stored at –18°C or below until measurement by electrochemiluminescence immunoassay using an Access 25 (OH) Vitamin D Total Kit (Beckman Coulter, Brea, CA, USA) within 1 day of arrival at Hoken Kagaku. SMI was calculated by dividing limb muscle mass (kg) by height squared (m2). After measuring SMI by dual-energy X-ray absorptiometry and bioelectrical impedance analysis, the lower value was selected.14 Changes in the above parameters, between the first and follow-up visit, were evaluated. Higher SPPB and SMI values equate to better physical performance.
Statistical analyses
Data are presented as mean ± SD, n prevalence or median (range) for each group. Between-group differences at the first visit were analyzed with Student’s t-test (continuous variables) or Fisher’s exact test (categorical variables). Wilcoxon rank-sum test (Mann–Whitney U-test) was performed to determine between-group differences in KCL scores at the first and follow-up visit. Within-group differences in KCL scores between the first and follow-up visit were assessed using Wilcoxon signed–rank test. Within-group differences in physical performance data between the first and follow-up visit were measured with paired sample t-test. The collected data were analyzed using BellCurve for Excel software, version 2.15 (Social Survey Research Information Co., Tokyo, Japan), and statistical significance was set at P < 0.05.
Results
Patient enrollment
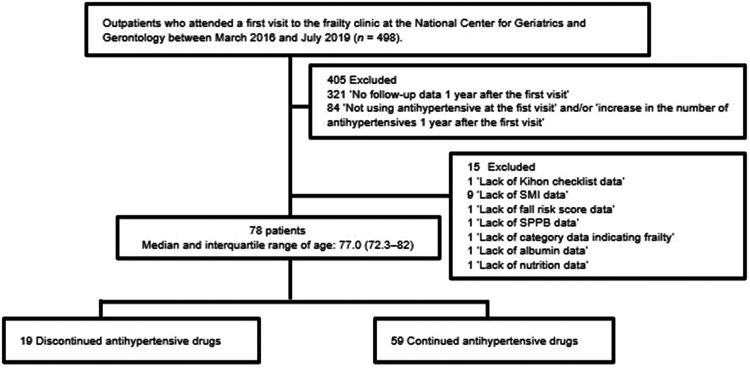
Of 498 outpatients who visited the frailty clinic at the National Center for Geriatrics and Gerontology between March 2016 and July 2019, a total of 78 patients aged 59–96 years were included in this study. The discontinuation (n = 19) and continuation groups (n = 59) completed follow-up within 1 year after their first visit, and had a median (interquartile range) age of 77.0 (72.3–82.0) years (Figure 1).
Figure 1.
Flow diagram of study participant enrollment. SMI, skeletal muscle index; SPPB, short physical performance battery.
Participant characteristics at the first visit
At the first visit, when all participants were taking antihypertensive medication, there were no statistically significant between-group differences in age, sex, body weight, antihypertensive drugs, medical histories, use of vitamin D analogs, blood pressure, pulse pressure, serum 25 (OH) vitamin D levels or albumin levels (Table 1). However, the discontinuation group showed significantly higher SMI (P < 0.01) than the continuation group (Table 1). The prescribed antihypertensive drugs at the first visit, and medical histories of the participants, are summarized in Table 1. Conditions recorded and included in the total morbidity count are listed in supplemental eTable 1 and eTable 2.
Table 1.
Characteristics of participants at the first visit in 78 patients who attended the frailty clinic at the National Center for Geriatrics and Gerontology, Obu, Japan.
| Characteristic | Discontinuation (n = 19) | Continuation (n = 59) | Statistical significance |
|---|---|---|---|
| Age, years | 76.1 ± 6.6 | 76.7 ± 7.1 | P = 0.72* |
| Sex | |||
| Male | 8 | 16 | P = 0.26$ |
| Female | 11 | 43 | |
| Body weight, kg | 62.2 ± 14.1 | 56.7 ± 12.4 | P = 0.11* |
| SMI, kg/m2 | 7.2 ± 1.7 | 6.2 ± 1.0 | P < 0.01* |
| Antihypertensive drugs | |||
| ARB/ACEI | 13 | 36 | P = 0.60$ |
| Calcium channel blocker | 18 | 49 | P = 0.28$ |
| αβ-blocker | 2 | 5 | P > 0.99$ |
| βblocker | 1 | 1 | P = 0.43$ |
| Thiazide and related diuretics | 3 | 3 | P = 0.15$ |
| Others | 6 | 7 | P = 0.07$ |
| Vitamin D analogs | |||
| Use | 4 | 7 | P = 0.45$ |
| Non-use | 15 | 52 | |
| aMedical history | |||
| Cancer | 1 | 12 | P = 0.17$ |
| bCardiac disease | 8 | 21 | P = 0.79$ |
| Cerebral aneurysm or infarction | 1 | 2 | P = 0.57$ |
| Diabetes | 6 | 11 | P = 0.34$ |
| Dyslipidemia | 11 | 32 | P > 0.99$ |
| Osteoarthritis | 9 | 24 | P = 0.79$ |
| Osteoporosis | 5 | 16 | P > 0.99$ |
| Blood pressure, mmHg | |||
| Systolic blood pressure | 133.3 ± 22.0 | 136.6 ± 20.0 | P = 0.54* |
| Diastolic blood pressure | 70.7 ± 11.5 | 72.1 ± 12.0 | P = 0.67* |
| Mean arterial pressure | 91.6 ± 1.37 | 93.6 ± 12.9 | P = 0.57* |
| Pulse pressure, mmHg | 62.5 ± 16.7 | 64.5 ± 16.8 | P = 0.66* |
| 25(OH) vitamin D level, ng/mL | 18.7 ± 5.0 | 18.1 ± 4.7 | P = 0.62* |
| ALB level, g/dL | 4.2 ± 0.29 | 4.2 ± 0.27 | P = 0.50* |
Data presented as mean ± SD or n prevalence.
ARB, angiotensin II receptor blocker; ACEI, angiotensin converting enzyme inhibitor; SMI, skeletal muscle index.
aThe seven most common morbidities observed in the study population are listed. Comorbidities are listed in Supplemental eTable 1 and eTable 2.
bCardiac disease is defined as the presence of angina, aortic regurgitation, aortic stenosis, arrhythmia, arteriosclerosis, aortic regurgitation, atrial fibrillation, premature ventricular contraction, or heart failure.
*Student’s t-test or $Fisher's exact test (versus continuation group).
Influence of antihypertensive drug discontinuation on KCL domains
Total KCL scores (Discontinuation; P < 0.05, Continuation; P < 0.01) and KCL isolation domain scores (Both group; P < 0.05) were significantly decreased in both groups at the 1-year follow-up visit (Table 2). KCL physical strength domain scores were significantly lower in the discontinuation group, but not the continuation group, at the 1-year follow-up visit (P < 0.05; Table 2). A statistically significant decrease in KCL mood domain scores in KCL were also observed for the continuation group, but not the discontinuation group (P < 0.05; Table 2). No changes in the KCL IADL, nutrition, or oral function domains were observed in either group (Table 2). Furthermore, there were no statistically significant differences between the two groups for any of the KCL scores, either at the first visit or the 1-year follow-up visit (Table 2).
Table 2.
Influence of discontinuation or continuation of antihypertensive drugs on KCL score in 78 patients who attended the frailty clinic at the National Center for Geriatrics and Gerontology, Obu, Japan.
| KCL domain | Discontinuation (n = 19) | Continuation (n = 59) | Statistical significance |
|---|---|---|---|
| IADL | |||
| First visit | 1 (0–4) | 1 (0–4) | P = 0.40‡ |
| 1-year follow-up visit | 1 (0–3) | 1 (0–5) | P = 0.69‡ |
| Statistical significance | P = 0.64† | P = 0.30† | |
| Physical strength | |||
| First visit | 3 (1–5) | 3 (0–5) | P = 0.27‡ |
| 1-year follow-up visit | 2 (0–4) | 3 (0–5) | P = 0.51‡ |
| Statistical significance | P < 0.05† | P = 0.20† | |
| Nutrition | |||
| First visit | 0 (0–2) | 0 (0–1) | P = 0.53‡ |
| 1-year follow-up visit | 0 (0–2) | 0 (0–1) | P = 0.61‡ |
| Statistical significance | P = 0.22† | P = 0.08† | |
| Oral function | |||
| First visit | 1 (0–3) | 1 (0–3) | P = 0.64‡ |
| 1-year follow-up visit | 1 (0–3) | 1 (0–3) | P = 0.98‡ |
| Statistical significance | P = 0.75† | P = 0.67† | |
| Isolation | |||
| First visit | 1 (0–2) | 0 (0–2) | P = 0.49‡ |
| 1-year follow-up visit | 0 (0–1) | 0 (0–2) | P = 0.89‡ |
| Statistical significance | P < 0.05† | P < 0.05† | |
| Memory | |||
| First visit | 0 (0–3) | 0 (0–2) | P = 0.72‡ |
| 1-year follow-up visit | 0 (0–2) | 0 (0–2) | P = 0.54‡ |
| Statistical significance | P = 0.18† | P = 0.20† | |
| Mood | |||
| First visit | 2 (0–4) | 2 (0–3) | P = 0.78‡ |
| 1-year follow-up visit | 0 (0–5) | 1 (0–2) | P = 0.17‡ |
| Statistical significance | P = 0.26† | P < 0.01† | |
| Total score | |||
| First visit | 8 (1–20) | 7 (1–19) | P = 0.74‡ |
| 1-year follow-up visit | 6 (1–14) | 5 (0–17) | P = 0.64‡ |
| Statistical significance | P < 0.05† | P < 0.01† |
Data presented as median (range).
IADL, instrumental activities of daily living.
†Wilcoxon signed–rank test; ‡Wilcoxon rank-sum test.
Influence of antihypertensive drug discontinuation on physical performance
There were no changes in the SMI for either group (Table 3). However, the continuation group showed a statistically significant decrease in total fall risk score (P < 0.05), with no such change observed in the discontinuation group (Table 3). The discontinuation group showed a statistically significant increase in SPPB balance score (P < 0.05), gait speed score (P < 0.05), and total score (P < 0.05; Table 3). However, there were no changes in the SPPB chair stand score for the discontinuation group, or total score for the continuation group (Table 3). SPPB gait speed score (P < 0.01) and SPPB chair stand score (P < 0.05) significantly increased, whereas SPPB balance score significantly decreased (P < 0.05), at the 1-year follow-up visit in the continuation group (Table 3).
Table 3.
The influence of discontinuation or continuation of antihypertensive drugs on physical performance in 78 patients who attended the frailty clinic at the National Center for Geriatrics and Gerontology, Obu, Japan.
| Physical performance measure | Discontinuation(n = 19) | Statistical significance | Continuation(n = 59) | Statistical significance |
|---|---|---|---|---|
| Total fall risk score | ||||
| First visit | 9.8 ± 3.8 | P = 0.27# | 10.2 ± 3.2 | P < 0.05# |
| 1-year follow-up visit | 8.7 ± 3.9 | 9.3 ± 3.6 | ||
| SPPB balance score | ||||
| First visit | 2.9 ± 1.4 | P < 0.05# | 3.6 ± 0.7 | P < 0.05# |
| 1-year follow-up visit | 3.6 ± 0.8 | 3.3 ± 1.0 | ||
| SPPB gait speed score | ||||
| First visit | 2.9 ± 1.1 | P < 0.05# | 3.2 ± 0.9 | P < 0.01# |
| 1-year follow-up visit | 3.4 ± 0.6 | 3.5 ± 0.8 | ||
| SPPB chair stand score | ||||
| First visit | 3.0 ± 1.4 | P = 0.27# | 3.1 ± 1.2 | P < 0.05# |
| 1-year follow-up visit | 3.3 ± 1.2 | 3.4 ± 1.0 | ||
| Total SPPB score | ||||
| First visit | 8.9 ± 3.1 | P < 0.05# | 9.9 ± 2.1 | P = 0.20# |
| 1-year follow-up visit | 10.4 ± 1.9 | 10.2 ± 2.1 | ||
| SMI (kg/m2) | ||||
| First visit | 7.2 ± 1.7 | P = 0.24# | 6.2 ± 1.0 | P = 0.15# |
| 1-year follow-up visit | 6.9 ± 1.1 | 6.3 ± 1.1 |
Data presented as mean ± SD.
SPPB, short physical performance battery; SMI, skeletal muscle index.
#Paired sample t-test.
Discussion
In the present study, patients who discontinued antihypertensive medication showed a significant decrease in the KCL physical strength domain. Therefore, the study focused on physical performance and further investigations were conducted using detailed evaluation items. Of note, at the first frailty clinic visit, during which all patients were receiving antihypertensive medication, the SMI was significantly higher in the discontinuation group compared with the continuation group, and no changes were observed in either group at the 1-year follow-up visit. Serum 25(OH) vitamin D levels play an essential role in normal muscle function,15 and vitamin D analogs have been reported to maintain SMI in postmenopausal women.16 The SMI values and serum 25(OH) vitamin D levels were not significantly different between patients who continued or discontinued antihypertensive medication; thus, the persistently high SMI corresponding to the discontinuation group appeared to be independent of 25(OH) vitamin D. Exercise contributes to the prevention of hypertension in normotensive participants and control of blood pressure in hypertensive patients.17 The constant SMI for both groups in the present study may be associated with exercise, as these study participants received multidisciplinary treatment during the study period that included physical therapy. The SPPB total score was significantly improved in the discontinuation group, but not in the continuation group, suggesting that the persistently high SMI and/or the discontinuation of antihypertensive drugs may have exerted a positive effect on physical performance. Further studies involving larger sample sizes are needed to validate this result, as the sample size in the present pilot study was relatively small.
The SPPB balance score was significantly decreased in the present continuation group. Orthostatic hypotension has been observed in 50–65% of older patients taking antihypertensive medications;18 thus, the present observation might have resulted from the antihypertensive drugs. However, the study participants were not evaluated for orthostatic hypotension. Thus, further studies on blood pressure before and after SPPB measurements are needed to confirm whether taking antihypertensive drugs will decrease the SPPB balance score.
Sarcopenia, a degenerative loss of skeletal muscle mass and strength is associated with aging and accelerates frailty syndrome.19 One of the limitations of the present study was the age group of the participants (aged ≥59 years). Thus, the study did not focus on sarcopenia, which was evaluated in a previous study in participants aged ≥65 years in Japan.19 Another limitation of the present pilot study was that the sample size was relatively small. Therefore, further studies with a larger sample size, including participants aged ≥65 years are needed to validate the results.
It was difficult to propose an index of blood pressure that may be used to determine the effect of discontinuing or continuing antihypertensive drugs on frailty syndrome, as the current blood pressure values were not obtained via resting blood pressure measurements. Thus, further investigations involving the measurement of resting blood pressure are necessary.
The present study focused on the effect of discontinuing antihypertensive drugs on frailty syndrome, which to date, remains unclear. As it was difficult to determine whether the antihypertensive drugs were deliberately deprescribed or discontinued for other reasons, such as non-compliance, further studies are needed to clarify whether deliberately deprescribing antihypertensive drugs has a positive effect on physical performance.
Conclusion
Discontinuation of antihypertensive drugs may have a positive effect on physical performance.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221130716 for Effects of discontinuation of antihypertensive drugs on frailty syndrome in outpatients: a 1-year prospectively designed retrospective chart-review pilot study by Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Yuji Hayakawa, Atsuya Shimizu and Yasumoto Matsui in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605221130716 for Effects of discontinuation of antihypertensive drugs on frailty syndrome in outpatients: a 1-year prospectively designed retrospective chart-review pilot study by Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Yuji Hayakawa, Atsuya Shimizu and Yasumoto Matsui in Journal of International Medical Research
Acknowledgements
We would like to thank Editage (https://www.editage.com/) for editing and reviewing this manuscript for English language.
Author contributions: Conceptualization: Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Atsuya Shimizu.
Data curation: Sho Hasegawa, Fumihiro Mizokami, Yasumoto Matsui.
Formal analysis: Sho Hasegawa, Fumihiro Mizokami.
Methodology: Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Atsuya Shimizu.
Writing (original draft): Sho Hasegawa, Fumihiro Mizokami.
Writing (review and editing): Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Yuji Hayakawa, Atsuya Shimizu.
The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by The Research Funding for Longevity Sciences (20-42) from the National Center for Geriatrics and Gerontology (NCGG), Japan, and by JSPS Grant-in-Aid for Scientific Research 22K15352.
Data accessibility
The data that support the findings of this study are available from the corresponding author, Sho Hasegawa, upon reasonable request.
ORCID iD
Sho Hasegawa https://orcid.org/0000-0003-1236-1623
Supplemental material
Supplemental material for this article is available online.
References
- 1.Feng Z, Lugtenberg M, Franse C, et al. Risk factors and protective factors associated with incident or increase of frailty among community-dwelling older adults: a systematic review of longitudinal studies. PLoS One 2017; 12: e0178383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesari M, Leeuwenburgh C, Lauretani F, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr 2006; 83: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endomba FT, Mazou TN, Bigna JJ. Epidemiology of depressive disorders in people living with hypertension in Africa: a systematic review and meta-analysis. BMJ Open 2020; 10: e037975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res 2019; 124: 1045–1060. [DOI] [PubMed] [Google Scholar]
- 5.Sheppard JP, Burt J, Lown M, et al. Effect of antihypertensive medication reduction versus usual Care on short-term blood pressure control in patients with hypertension aged 80 years and older: The OPTIMISE randomized clinical trial. JAMA 2020; 323: 2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page A, Clifford R, Potter K, et al. A concept analysis of deprescribing medications in older people. Journal of Pharmacy Practice and Research 2018; 48: 132–148. [Google Scholar]
- 7.Sewo Sampaio PY, Sampaio RA, Yamada M, et al. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr Gerontol Int 2016; 16: 893–902. [DOI] [PubMed] [Google Scholar]
- 8.Honzawa A, Nishitani-Yokoyama M, Shimada K, et al. Relationship between Kihon Checklist score and anxiety levels in elderly patients undergoing early phase II cardiac rehabilitation. Cardiol Res 2020; 11: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Japanese Society of Hypertension. Guidelines for the management of hypertension 2019, https://www.jpnsh.jp/data/jsh2019/JSH2019_hp.pdf (2019, accessed 1 October 2022. [In Japanese]).
- 10.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 11.Japan Standard Commodity Classification: Division 87 – Drugs and related commodities, https://www.soumu.go.jp/main_content/000294493.pdf (2019, accessed 1 October 2022. [In Japanese]).
- 12.Kikuchi R, Kozaki K, Iwata A, et al. Evaluation of risk of falls in patients at a memory impairment outpatient clinic. Geriatr Gerontol Int 2009; 9: 298–303. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 14.Oba H, Matsui Y, Arai H, et al. Evaluation of muscle quality and quantity for the assessment of sarcopenia using mid-thigh computed tomography: a cohort study. BMC Geriatr 2021; 21: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunton JE, Girgis CM. Vitamin D and muscle. Bone Rep 2018; 8: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda K, Imatani J, Moritani S, et al. Effects of eldecalcitol alone or a bone resorption inhibitor with eldecalcitol on bone mineral density, muscle mass, and exercise capacity for postmenopausal women with distal radius fractures. J Orthop Sci 2022; 27: 139–145. [DOI] [PubMed] [Google Scholar]
- 17.Fagard RH. Exercise therapy in hypertensive cardiovascular disease. Prog Cardiovasc Dis 2011; 53: 404–411. [DOI] [PubMed] [Google Scholar]
- 18.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther 2005; 30: 173–178. [DOI] [PubMed] [Google Scholar]
- 19.Yuki A, Ando F, Otsuka R, et al. Epidemiology of sarcopenia in elderly Japanese. J Phys Fitness Sports Med 2015; 4: 111–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221130716 for Effects of discontinuation of antihypertensive drugs on frailty syndrome in outpatients: a 1-year prospectively designed retrospective chart-review pilot study by Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Yuji Hayakawa, Atsuya Shimizu and Yasumoto Matsui in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605221130716 for Effects of discontinuation of antihypertensive drugs on frailty syndrome in outpatients: a 1-year prospectively designed retrospective chart-review pilot study by Sho Hasegawa, Fumihiro Mizokami, Hiroki Mase, Yuji Hayakawa, Atsuya Shimizu and Yasumoto Matsui in Journal of International Medical Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Sho Hasegawa, upon reasonable request.