Abstract
Interstitial lung abnormality (ILA) is defined as an interstitial change detected incidentally on CT images. It is seen in 4%–9% of smokers and 2%–7% of nonsmokers. ILA has a tendency to progress with time and is associated with respiratory symptoms, decreased exercise capability, reduced pulmonary function, and increased mortality. ILAs can be classified into three subcategories: nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic. In cases of ILA, clinically significant interstitial lung disease should be identified and requires clinically driven management by a pulmonologist. Risk factors for the progression of ILA include clinical elements (ie, inhalation exposures, medication use, radiation therapy, thoracic surgery, physiologic findings, and gas exchange findings) and radiologic elements (ie, basal and peripheral predominance and fibrotic findings). It is recommended that individuals with one or more clinical or radiologic risk factors for progression of ILA be actively monitored with pulmonary function testing and CT. To avoid overcalling ILA at CT, radiologists must recognize the imaging pitfalls, including centrilobular nodularity, dependent abnormality, suboptimal inspiration, osteophyte-related lesions, apical cap and pleuroparenchymal fibroelastosis–like lesions, aspiration, and infection. There is a close association between ILA and lung cancer, and many studies have reported an increased incidence of lung cancer, worse prognoses, and/or increased pulmonary complications in relation to cancer treatment in patients with ILA. ILA is considered to be an important comorbidity in patients with lung cancer. Accordingly, all radiologists involved with body CT must have sound knowledge of ILAs owing to the high prevalence and potential clinical significance of these anomalies. An overview of ILAs, including a literature review of the associations between ILAs and lung cancer, is presented.
©RSNA, 2022
Created with BioRender.com
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the general concept, findings, and management of ILAs.
■ Describe the evidence of ILA in patients with lung cancer.
■ Explain the clinical importance of ILAs in the management of patients with lung cancer.
Introduction
Interstitial lung abnormality (ILA) is a recently emerged concept regarding incidental CT findings of subtle interstitial change (1–3). Many studies (4–12) have shown that ILAs commonly progress and are associated with a worse prognosis. Idiopathic interstitial pneumonia, especially idiopathic pulmonary fibrosis (IPF), is a major concern at thoracic imaging. Although IPF has been widely studied, its origin and pathogenesis are not fully understood. Therefore, the relationship between ILA and the subsequent development of IPF has been discussed in numerous studies (3,6,13,14).
Tobacco smoking is a known risk factor for ILA and lung cancer (2,15). The association between ILA and lung cancer has been investigated in many studies (16–19). For patients with lung cancer, complications during treatment are an important issue, and it is widely accepted that clinically significant interstitial lung disease (ILD) is a risk factor for complications and that patients with ILD have limited options for treatment of lung cancer (20). However, at present, the concept and importance of ILA in patients with lung cancer are not well known among clinicians.
This article has two main purposes. The first purpose is to provide an overview of ILA, including its definition and associated imaging pitfalls, epidemiologic factors, clinical significance, subcategories, and management. The second purpose is to present a literature review of the association between ILA and lung cancer in terms of the incidence of lung cancer, the prognosis for patients with ILAs, and the associated complications that can occur in patients with ILA during the treatment for lung cancer.
General Overview of ILA
Definition
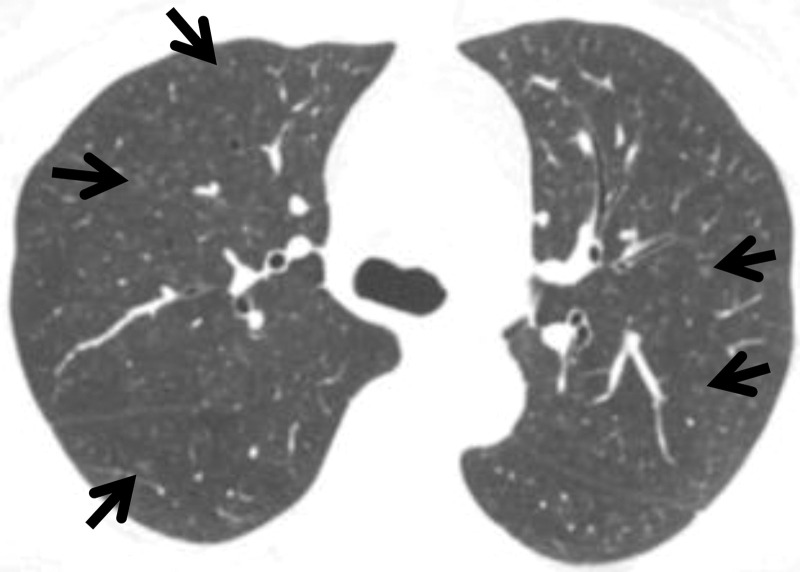
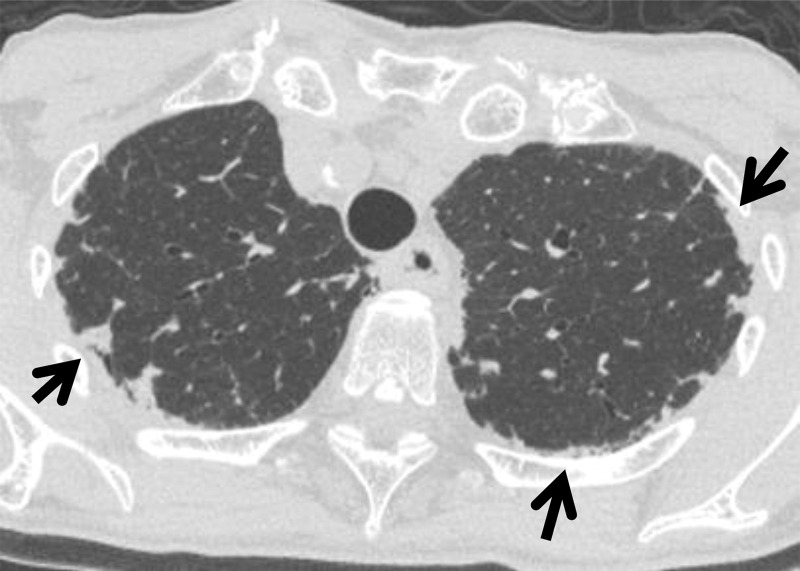
Interstitial lung abnormality is defined as the incidental finding of nondependent abnormalities that affect more than 5% of the cross-sectional area of at least one of three lung zones on CT images (2). Although ILAs previously were regarded as insignificant, they should now be included in the CT report because of their potential clinical significance. ILAs include ground-glass abnormality, reticulation, architectural distortion, traction bronchiectasis, honeycombing, and nonemphysematous cysts (Fig 1). As shown in Figure 2, even relatively subtle findings can affect more than 5% of a lung zone. The threshold of 5% is arbitrary and is used as a rough reference standard. Although the findings of ILA can be in the dependent area, they are not associated with dependent positioning. An ILA can be detected in the lung area covered on abdominal or coronary CT images, as well as the lung area covered on chest CT images. It is assumed that an ILA will not resolve within a short period.
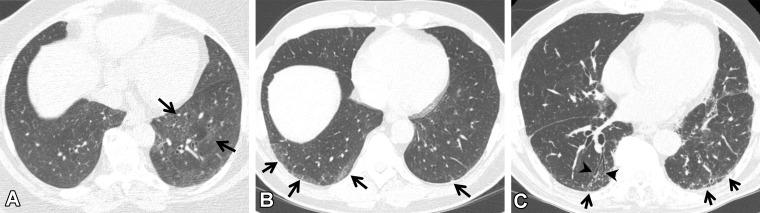
Figure 1.
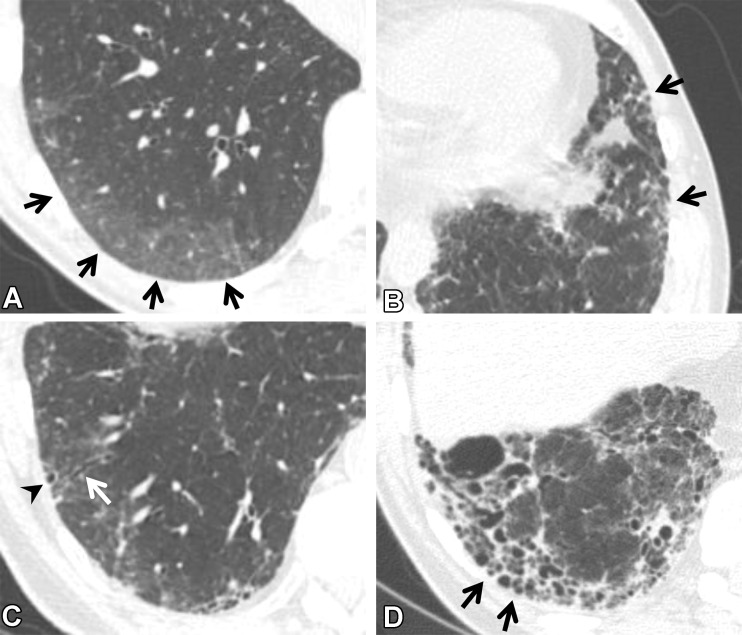
CT findings that constitute ILAs. Axial images show ground-glass abnormality (arrows in A), reticulation (arrows in B), traction bronchiectasis (arrow in C), nonemphysematous cyst (arrowhead in C), and honeycombing (arrows in D).
Figure 2.
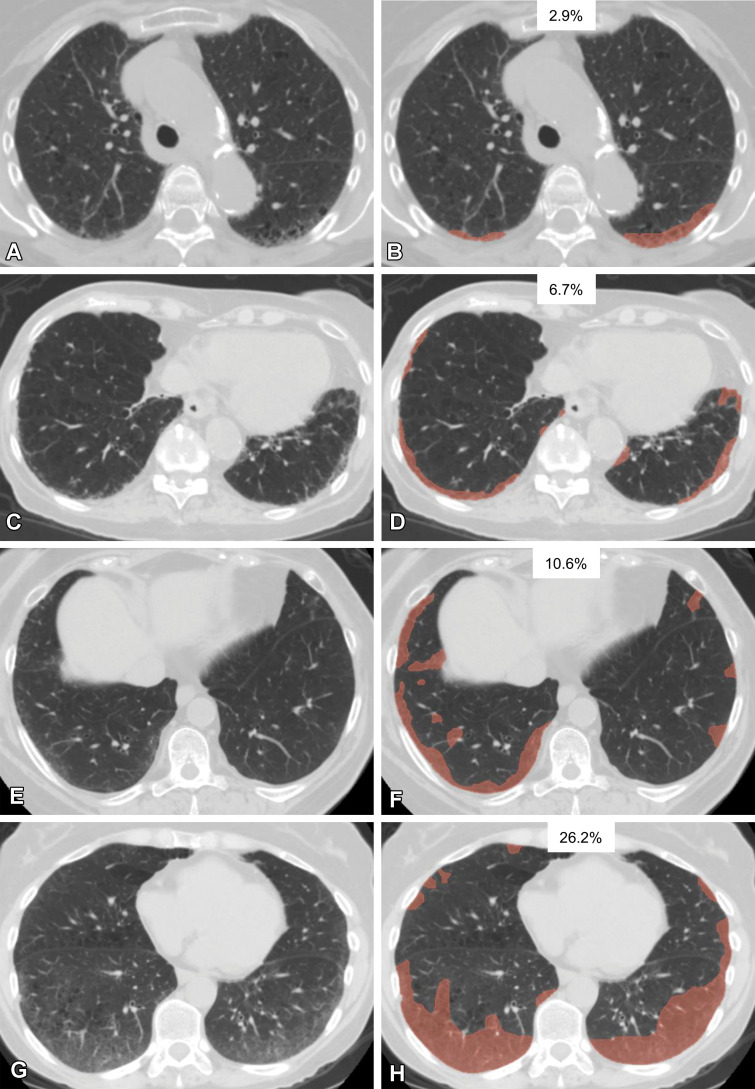
Various extents of lung abnormality in four patients. Original axial CT images (A, C, E, G) and the same CT images with a segmented overlay of ILA (B, D, F, and H) are shown. (A, B) Scans in a 76-year-old woman show subtle subpleural ground-glass abnormality that accounts for 2.9% of the lung area, indicating insignificant abnormality. (C, D) Scans in an 84-year-old woman show ground-glass and reticular abnormalities that account for 6.7% of the lung area, indicating significant abnormality. (E, F) Scans in a 73-year-old woman show faint ground-glass abnormality that accounts for 10.6% of the lung area, indicating significant abnormality. (G, H) Scans in a 60-year-old woman show widespread ground-glass abnormality that affects 26.2% of the lung area, indicating significant abnormality. The segmentations shown were performed manually by using 3D Slicer software (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA).
ILA does not imply the absence of symptoms or a functional decline. Rather, these abnormalities are defined purely according to CT findings, while an ILD is ultimately defined at a multidisciplinary conference that includes assessment of symptoms, pulmonary function test results, and CT and histopathologic findings. Among the patients deemed to have ILA, those who have at least one of three criteria—respiratory symptoms or physical examination findings possibly attributable to ILD, decrements in pulmonary function or gas exchange possibly attributable to ILD, and extensive disease at CT—are considered to have potentially clinically significant ILD and should be evaluated by clinicians and at a multidisciplinary conference and managed separately from the remainder of patients with ILA.
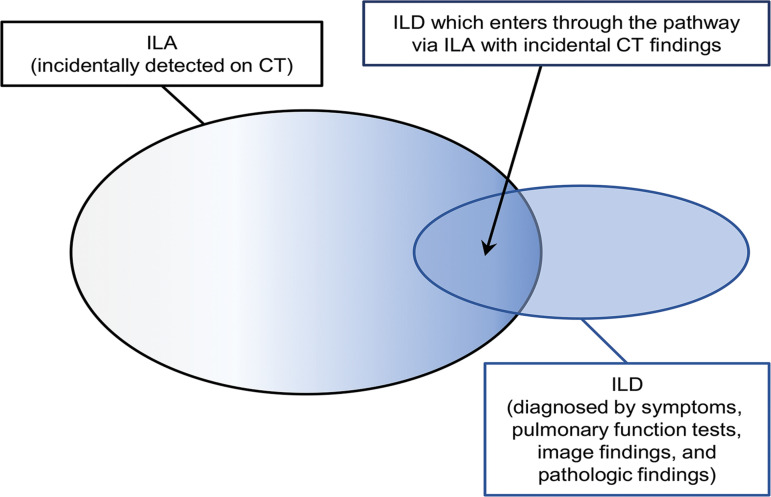
Extensive disease seen at CT refers to the findings of ILA that are seen in three or more of six zones of the bilateral lungs (above the bottom of the aortic arch, between the aortic arch and top of the inferior pulmonary vein, and below the inferior pulmonary vein). A portion of patients with ILD are diagnosed by way of this pathway (having ILA with incidental CT findings), whereas many other patients are diagnosed directly at clinics because of their respiratory symptoms and abnormal pulmonary function test results that indicate ILD. Practically speaking, ILA and ILD are on the spectrum of fibrotic lung diseases with overlapping boundaries (Fig 3) (21).
Figure 3.
Diagram shows the relationship between ILA and ILD.
Abnormalities detected during screening for ILD in high-risk groups such as patients with connective tissue disease (CTD) are not included in the current definition of ILA because they are not incidental. However, early detection of interstitial lung findings and understanding of the evolving process of CTD-ILD are important in the management of CTD and the intervention in affected patients. The concept of CTD-related ILA is proposed by some researchers (22).
Imaging Pitfalls
Several entities are considered to be potential imaging pitfalls of ILA (Table 1). Knowledge of these pitfalls is mandatory for distinguishing clinically significant abnormalities from insignificant findings and identifying cases that require further clinical workup. In principle, these findings and disease entities are not considered to be ILAs, but they are sometimes difficult to distinguish from ILAs and may be considered equivocal. Comparisons with previous images, additional prone scanning, and/or follow-up may be necessary to diagnose equivocal cases.
Table 1:
Pitfalls of ILA Depicted at Imaging
Centrilobular Nodularity.—Although initial descriptions of ILA included centrilobular nodularity (Fig 4), this is not included in the current definition (2). Centrilobular nodularity is a typical manifestation of smoking-related respiratory bronchiolitis, and a previous study (23) showed that it is usually nonprogressive. The differential diagnosis for centrilobular nodules includes bronchiolitis due to infection or aspiration, hypersensitivity pneumonitis, pneumoconiosis (especially welder’s lung), diffuse alveolar hemorrhage, and lipoid pneumonia.
Figure 4.
Centrilobular nodularity in a 50-year-old woman who currently smoked and had a history of 35 pack-years without respiratory symptoms. Axial CT image shows small nodules (arrows) with centrilobular distribution in the bilateral lungs.
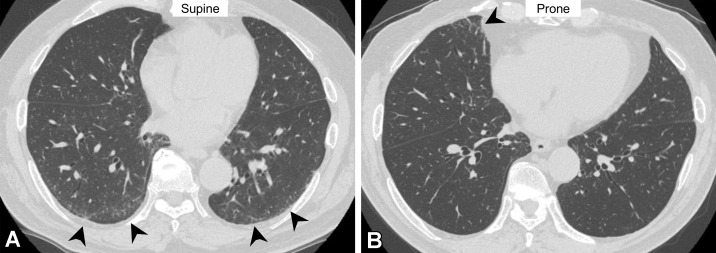
Dependent Abnormality and Suboptimal Inspiration.—Ground-glass abnormalities sometimes occur in the dependent lung area, especially when inspiration is suboptimal (24). The increased attenuation is caused mainly by transient lung atelectasis, most commonly at the lung bases. The lack of deep inspiration is suggested by tortuosity of the vessels, anterior bulging of the posterior membranous portion of the intrathoracic trachea, and decreased lung volume compared with the lung volume at previous scanning (Fig 5) (24,25). The findings of fibrosis, reticulation, and ground-glass abnormality, all beyond the dependent area, may suggest ILA. However, it is often difficult to differentiate true ILA from transient lung atelectasis on supine scans alone, and an additional prone scan may be required to confirm true ILA (Fig 6).
Figure 5.
Suboptimal inspiration in an 87-year-old woman who underwent CT for evaluation of a lung nodule. (A, B) Axial CT images show ground-glass abnormalities (arrowheads) in subpleural and central areas of the lung zone. Anterior bulging of the posterior membranous portion of the trachea (arrow in A) and tortuosity of the vessels (circle) suggest suboptimal inspiration. (C, D) Follow-up axial CT images show that the ground-glass abnormality has disappeared, and the normal round shape of the trachea is seen (arrow in C). Tortuosity of the vessels (circle) is no longer seen, and the lung volume, as compared with that in A and B, has improved.
Figure 6.
Dependent abnormality in a 72-year-old man with rheumatoid arthritis. (A) Axial CT image obtained with the patient supine shows ground-glass abnormality (arrowheads) in the subpleural lung area. (B) On the axial CT image obtained with the patient prone, the ground-glass abnormality has disappeared in the subpleural area but is seen in the middle lobe of the right lung (arrowhead). These findings are considered to indicate transient lung atelectasis.
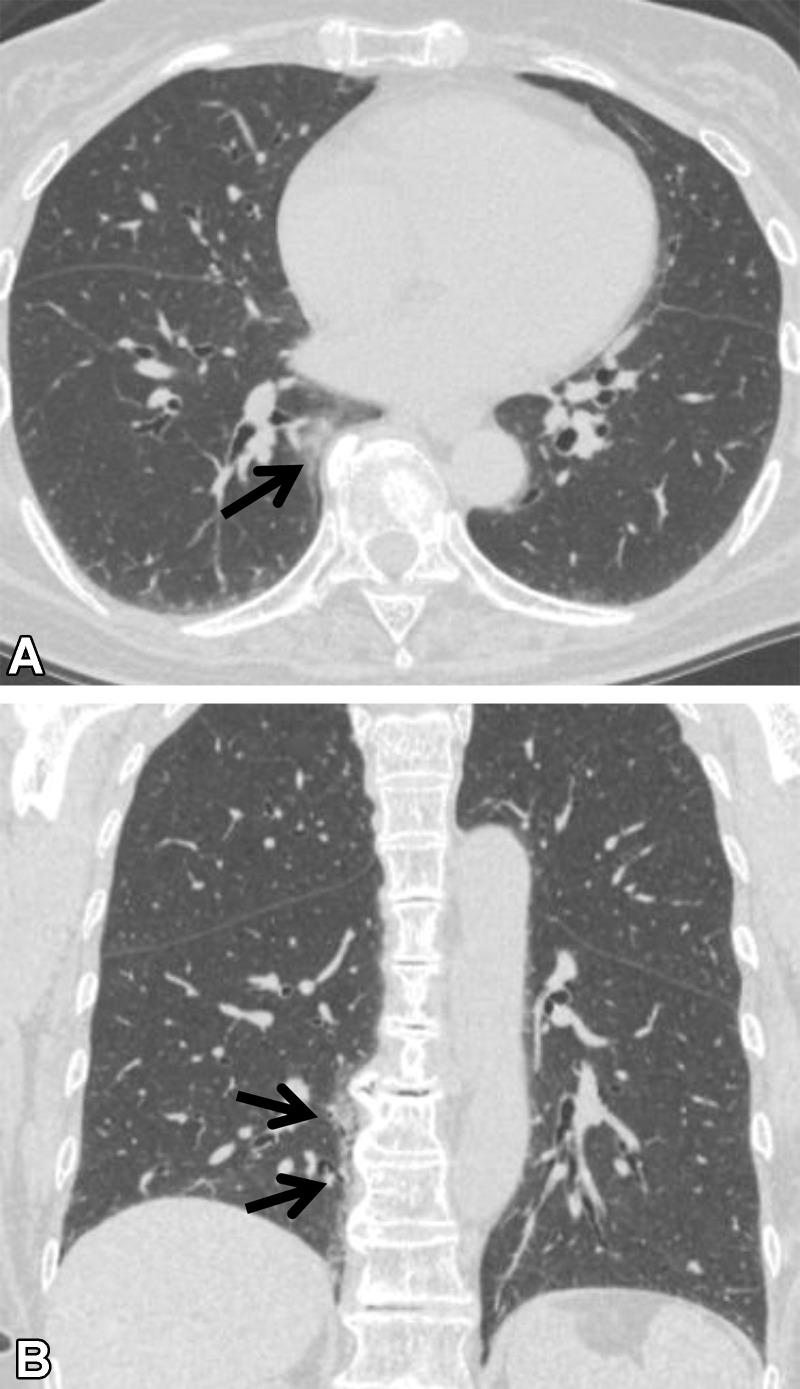
Osteophyte-related Lesions.—Focal pulmonary reticulation and ground-glass abnormality adjacent to thoracic spinal osteophytes are commonly seen at thoracic CT, especially in older individuals (Fig 7) (26). These opacities are typically seen in the medial right lower lung lobe, as thoracic vertebral osteophytes tend to be more prominent on the right side (27). These lesions are believed to be caused by mechanical irritation from the osteophytes and represent compressive atelectasis or fibrosis (26). They have close contact with osteophytes and are usually aligned craniocaudally. Such limited abnormalities rarely progress and are not considered to be a preclinical form of more extensive lung disease. Therefore, localized osteophyte-related opacities are excluded from the definition of ILA.
Figure 7.
Osteophyte-related lesion in a 72-year-old woman with no respiratory symptoms. (A) Axial CT image shows ground-glass abnormality adjacent to an osteophyte (arrow). (B) Coronal CT image shows the craniocaudal alignment (arrows) of the abnormality.
Apical Cap and Pleuroparenchymal Fibroelastosis–like Lesions.—Apical cap refers to a caplike lesion at the apex of the lung. The prevalence of apical cap increases with age, and this lesion is believed to be caused by chronic ischemia that results in hyaline pleural plaque formation or fibrosis pulling down extrapleural fat (28). Apical cap is usually seen as homogeneous soft-tissue attenuation in the lung apex unilaterally or bilaterally.
Pleuroparenchymal fibroelastosis (PPFE) is a relatively rare condition characterized by pleural and subpleural parenchymal fibrosis and elastosis, mainly in the upper lobes. PPFE is suggested by pleural thickening or multiple subpleural foci of airspace consolidation, with associated subpleural fibrosis located predominantly in the bilateral upper lobes (29,30). Volume loss in the upper lobes is often seen on CT images.
It is reported that PPFE-like lesions (Fig 8) are identified at CT in 30.9% of patients (31). In the early stage, it is difficult to distinguish PPFE from apical cap radiologically and pathologically (32). Apical cap and PPFE-like lesions are often detected incidentally but are not included in the definition of ILA because they are distinct radiologic entities.
Figure 8.
PPFE-like lesion in a 73-year-old woman. Axial CT image shows subpleural soft-tissue attenuation with irregular borders (arrows) in the upper lungs. There was no interval change in this abnormality at follow-up.
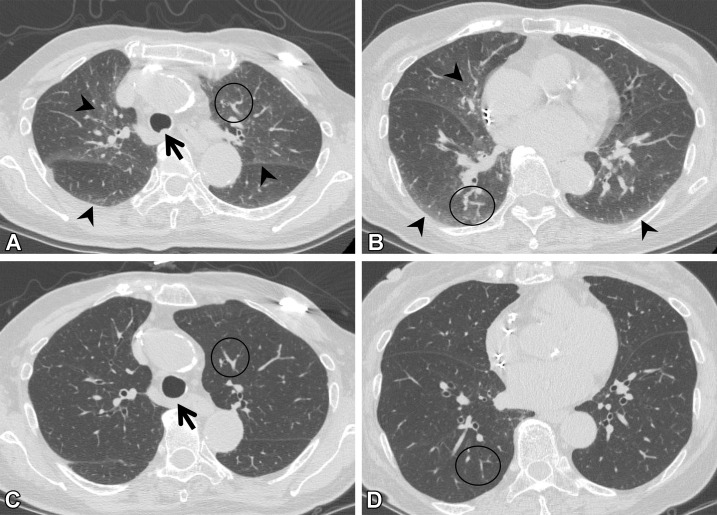
Aspiration.—Chronic and recurrent aspiration results in incidental pulmonary findings at CT (Fig 9). Risk factors for aspiration in adults include structural abnormalities of the pharynx and esophagus, neuromuscular disorders, deglutition abnormalities, alcoholism, loss of consciousness, and general anesthesia (33). Aspiration lesions show nodularity and the "tree-in-bud" sign with a lobar or segmental distribution on CT images (34). Bronchiectasis can be seen if the patient has a chronic course of disease. Central plugging of the airways is sometimes seen. Posterior segments of the upper lung lobes and superior segments of the lower lobes are predominantly affected when patients aspirate while in the supine position (34). In contrast, in patients who are standing during imaging, the right middle lobe, lingular segment, and bibasilar segments are involved (34). These typical findings of aspiration are excluded from ILA.
Figure 9.
Aspiration in a 64-year-old man with corticobasal degeneration. (A) Axial CT image shows central airway plugging (arrows) and centrilobular nodularity (arrowheads) bilaterally in the lower lung lobes. (B) Axial CT image at the level of the lung base shows ground-glass abnormality (arrowheads) in the subpleural area and peripheral centrilobular nodules (arrow).
Gastroesophageal reflux is a known risk factor for pulmonary fibrosis (35), but its association with ILA is unknown. George et al (36) reported that hiatus hernia was associated with the usual interstitial pneumonia (UIP) pattern but not with ILA. Further investigations are necessary to clarify whether recurrent aspiration correlates with ILA.
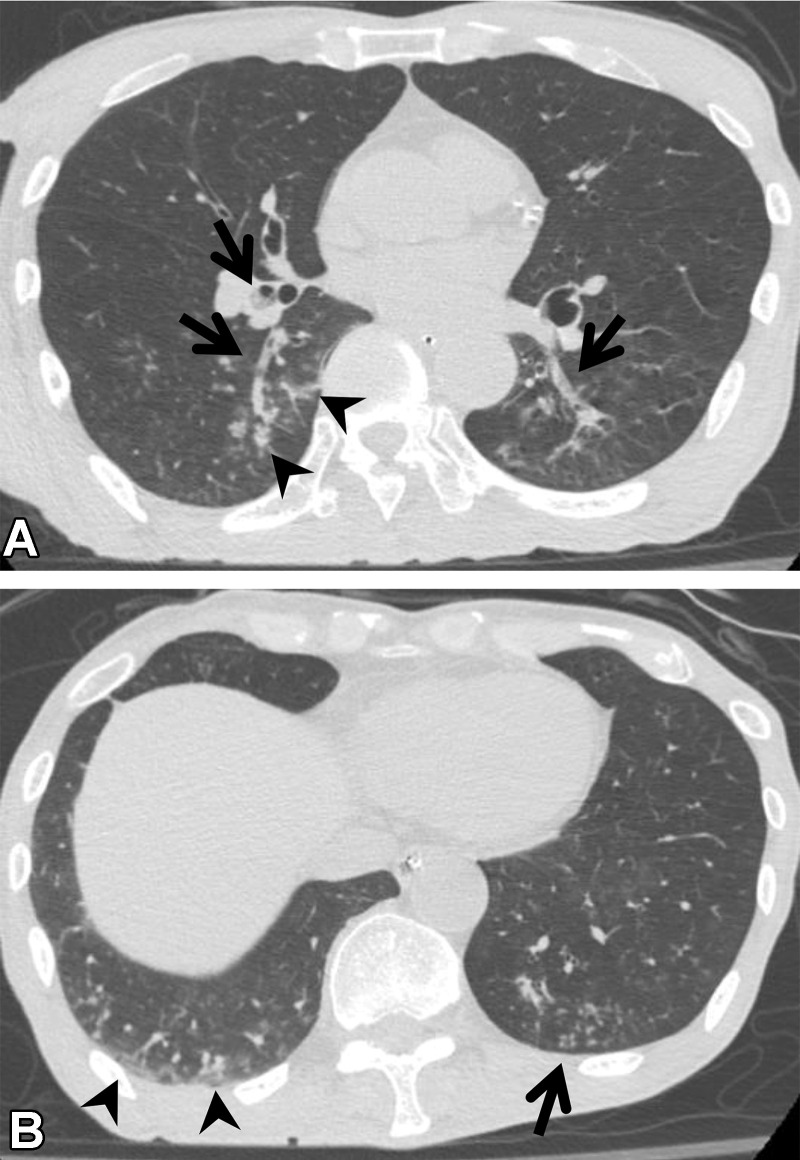
Infection and Postinflammatory Change.—Pulmonary infectious diseases can show resolving opacity or persistent scarring at imaging. The findings usually are focal or multifocal and often are linear. It is sometimes difficult to distinguish infectious change and postinflammatory change from ILA. In particular, the changes seen with bronchiectasis are challenging to differentiate from those seen with traction bronchiectasis in ILA. Chronic airway inflammation such as nontuberculous mycobacteria shows bronchiectasis, nodules, consolidation, and scarring and/or volume loss (Fig 10) (37). Centrilobular nodules, bronchial wall thickening, and mucous plugging are usually accompanied by infectious chronic airway inflammation (38). In patients with findings such as these, which suggest infection, bronchiectasis is considered to be excluded from ILA.
Figure 10.
Mycobacterium intracellulare infection (non-ILA) in a 72-year-old woman. (A, B) Axial CT images show linear opacity and ground-glass abnormality (arrowheads) in the subpleural area of the upper lung lobes. (C) Axial CT image at a lower level shows bronchiectasis and reticulation (straight arrows) in the middle lobe and lingula and centrilobular nodularity (curved arrows) in the left lower lobe. These findings are typical of nontuberculous mycobacterial pulmonary disease, suggesting that the abnormalities at the upper level are related to infection or postinfection change. The findings in this patient were not considered to indicate ILA.
Recently, Han et al (39) reported that fibrotic ILA was seen at 1-year follow-up CT after severe COVID-19–related pneumonia. Strictly speaking, the term interstitial lung abnormality may be used to refer to the incidental CT findings (2,3). However, the framework of ILA appears to be suited for the analysis of CT scans after COVID-19–related pneumonia. We believe that ILA-like findings after SARS-CoV-2 infection seen on follow-up CT scans may be best described as post–COVID-19 lung abnormalities.
Epidemiologic Factors
Among older (>60 years of age) individuals, the prevalence of ILA has been reported to be 4%–9% in persons who smoke and 2%–7% in those who do not smoke (3–10). Risk factors for ILA include increasing age, inhalational exposures (eg, tobacco, vapors, gases, dust, fumes, and traffic-related air pollution), and genetic factors (3,5,7,8,10,40,41). A common promoter polymorphism (rs35705950) in MUC5B, the gene that encodes mucin 5B, is associated with the presence of ILA and ILA progression (7,9). The MUC5B gene is associated with IPF, suggesting that ILA may involve the common process of fibrosis with IPF. Male sex is an equivocal risk factor, as an association between male sex and ILA prevalence has been reported in some (10,42) but not all studies.
Clinical Significance
The clinical significance of ILA has been investigated in many studies. ILA often shows progression on images, with reported progression rates of 20% in 2 years to 73% in 5 years (8,9,23,43). In addition, increased respiratory symptoms, reduced exercise capability, decreased total lung capacity, impaired gas exchange, and increased mortality are seen in individuals with ILA (5,7,10,11,43).
ILA Subcategories
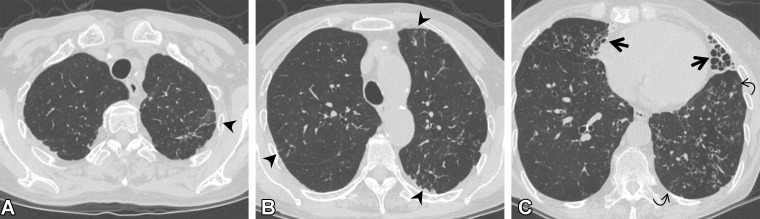
In the 2020 position paper by the Fleischner Society (2), ILA subcategories (nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic ILA) are proposed according to the distribution of ILA and the presence of fibrosis (Fig 11) (2). Fibrosis refers to architectural distortion with traction bronchiectasis and/or honeycombing. This proposed subcategorization is based on a study in which the imaging patterns of ILA and the associated prognoses were investigated (23). Subpleural ILA was correlated with greater likelihood of progression. Compared with the findings in individuals without ILA, the fibrosis in individuals with ILA correlated with increased mortality. The differential diagnosis and imaging pitfalls of nonsubpleural ILA include aspiration and infection, those of subpleural nonfibrotic ILA include dependent abnormality, and those of subpleural fibrotic ILA include apical cap and PPFE-like lesions and osteophyte-related lesions.
Figure 11.
Subcategories of ILA seen on axial CT images. (A) Nonsubpleural ILA: faint ground-glass abnormality (arrows) in the central and subpleural areas of the lungs but not predominant in the subpleural area. (B) Subpleural nonfibrotic ILA: ground-glass abnormality (arrows) without fibrosis such as bronchiectasis and architectural distortion. (C) Subpleural fibrotic ILA: ground-glass abnormality and linear opacities (arrows), with traction bronchiectasis (arrowheads).
Recommended CT Protocol
Chest CT with a thin section thickness and a high-spatial-frequency algorithm is recommended for the detailed evaluation of ILAs. An additional prone CT scan is helpful for differentiating dependent lung atelectasis from ILA. Expiratory CT is not necessary for the evaluation of ILA. Ultra-low-dose CT with use of iterative reconstruction is not recommended because the interstitial findings might be obscured.
Management
The following plan for the management of ILA was proposed by the Fleischner Society (2): After the identification of individuals with clinically significant ILD, those with ILA are divided into high- and low-risk groups. The individuals with one or more risk factors (Table 2) are categorized as having high risk of ILA progression, and all others are assigned to the low-risk group. Individuals in both risk groups are advised to reduce their risk factors such as cigarette smoking. The high-risk group is advised to undergo active monitoring, including pulmonary function testing in 3–12 months and CT in 12–24 months. The low-risk group is advised to undergo reassessment when they demonstrate symptoms or other evidence of ILD progression. Radiologists should identify ILA, compare the findings with those on previously obtained CT scans, classify ILA into subcategories, and recommend clinical evaluation of ILDs to physicians. The concept of ILA is relatively new and evolving. Thus, ILA terminology, classifications, and management may be updated in the future.
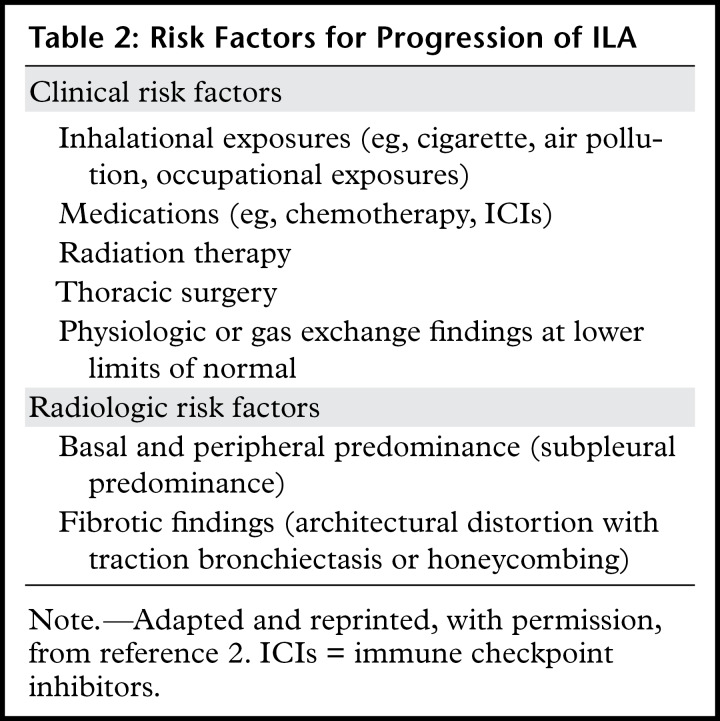
Table 2:
Risk Factors for Progression of ILA
Association between ILA and Lung Cancer
ILA and lung cancer commonly coexist, particularly in persons who smoke. There are three main issues regarding the association between ILA and lung cancer: risk of lung cancer, prognosis of patients with lung cancer, and complications during lung cancer treatment.
Risk of Lung Cancer
The increased incidence of lung cancer among patients with IPF is well known (44). Similarly, ILA has been reported as a risk factor for lung cancer. The relationship between ILA and lung cancer has been investigated in lung cancer screening cohorts (16–18). Among 25 041 participants in the National Lung Cancer Trial, the prevalence of lung cancer was 2.4% (121 of 5053 persons) in the ILA group and 1.5% (304 of 19 988) in the non-ILA group (17). Analysis adjusted according to the presence of radiographic emphysema indicated a higher lung cancer incidence in the ILA group (incidence ratio, 1.33 [95% CI: 1.07, 1.65]).
Risk of lung cancer was also evaluated in the AGES (Age, Gene/Environment Susceptibility)-Reykjavik study (16), which involved a population-based cohort. The participants with fibrosis in the study were at greater risk of having lung cancer than the participants who did not have fibrosis, although both groups were at risk (with fibrosis: hazard ratio [HR], 3.95 [95% CI: 2.07, 7.57] [P < .001]; without fibrosis: HR, 2.26 [95% CI: 1.29, 3.96] [P = .004]). In terms of having a cancer diagnosis other than lung cancer, there was no significant difference between the participants with and those without ILA.
Lung Cancer Prognosis
The lung cancer mortality associated with ILA has been assessed in several studies. Earlier cited cohort studies (16,17) showed an association between ILA and increased mortality (National Lung Screening Trial: HR, 1.82 [95% CI: 1.37, 2.42]; AGES-Reykjavik study: HR, 1.47 [95% CI: 1.12, 1.94]) (P = .005). In addition, an association between ILA and higher mortality has been reported in patients with early-stage lung cancer and those with advanced-stage lung cancer (with stage I: HR, 2.88 [P = .005]; with stage IV: HR, 2.09 [P = .004]) (45,46). Iwasawa et al (47) reported that the existence of ILA was a predictor of poorer disease-free survival in patients with stage I or II lung cancer (HR, 3.3 [95% CI: 1.8, 6.2]; P =.001), suggesting that patients who have lung cancer with ILA have an increased risk of early death owing to lung cancer. Furthermore, Cho et al (48) reported an association between ILA and larger tumor size (P = .04 at linear regression analysis) and advanced cancer staging in patients with early-stage non–small cell lung cancer (stage I or II: odds ratio, 1.81 [95% CI: 1.10, 2.96]; P = .02).
Complications Related to Lung Cancer Treatment
The treatment options for lung cancer include surgery, radiation therapy, cytotoxic chemotherapy, molecularly targeted drugs, and immune checkpoint inhibitors (ICIs). Complications are not uncommon with lung cancer treatment, especially in patients with ILD. In addition, an increased prevalence of treatment-related complications in patients with ILA has been reported. Further investigation is necessary, because the criteria for ILA might not be unified among these studies; however, ILA is considered an important comorbidity in individuals who have lung cancer. Clinicians should carefully discuss with patients the benefits of cancer therapy and the increased risk of complications, although there is no clear evidence or guidelines regarding how to manage patients who have ILA and lung cancer.
Postoperative Complications.—Postoperative pulmonary complications after lung cancer surgery are common and include pneumonia and acute respiratory distress syndrome (Fig 12). These complications are the major causes of perioperative morbidity and mortality and occur in 14%–40% of patients after lung resection (49,50). Poor performance status, underlying comorbidities, and impaired lung function due to old age are considered to be poor prognostic factors for postoperative pulmonary complications (51). In a study in which patients older than 70 years who had lung cancer with preserved lung function were evaluated (51), ILA was reported as an independent risk factor (odds ratio, 1.91; P = .003) for postoperative pulmonary complications in 488 patients with stage I or II non–small cell lung cancer. When the analysis was limited to patients with severe postoperative pulmonary complications (ie, pneumonia, acute respiratory distress syndrome, and respiratory failure), the presence of ILA remained a significant risk factor (odds ratio, 1.09; P = .002). These results suggest that ILA could be an important determinant that increases the incidence of postoperative pulmonary complications in elderly patients.
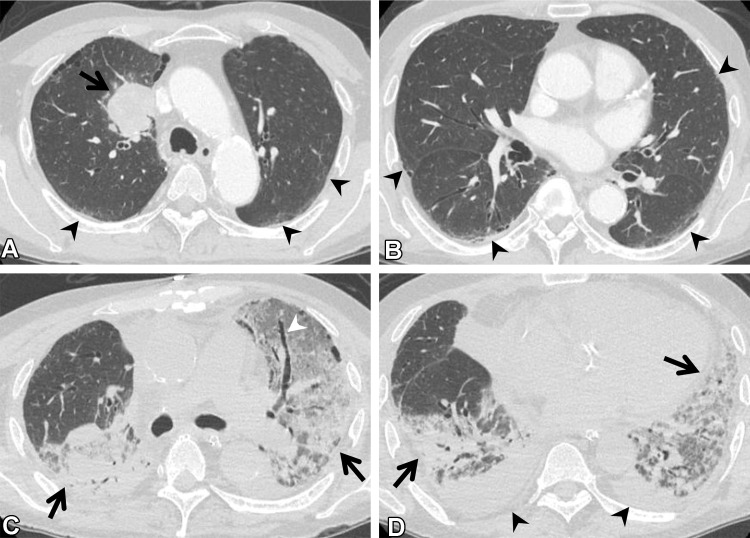
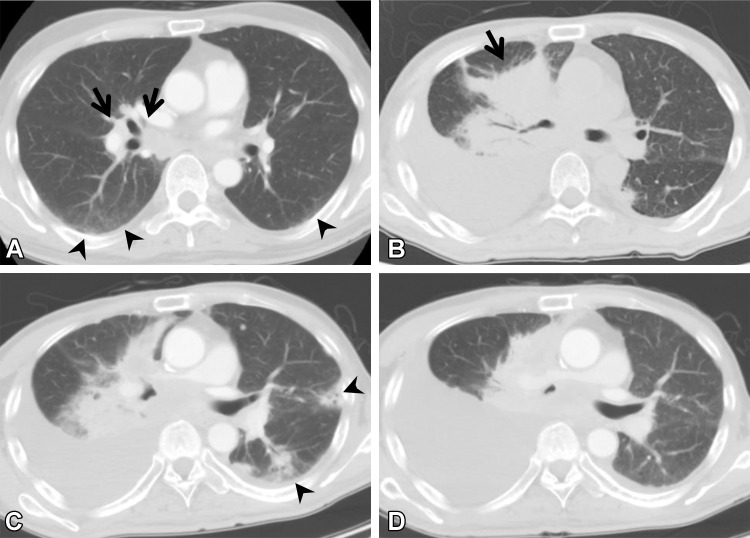
Figure 12.
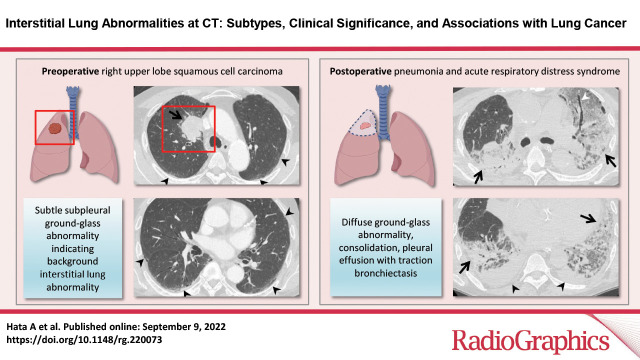
Postoperative complications in a 66-year-old man with squamous cell cancer. (A, B) Axial preoperative CT images show a mass (arrow in A) in the right upper lobe of the lung. Subtle ground-glass abnormality (arrowheads), indicating ILA, is seen in the subpleural area. (C, D) Axial postoperative CT images show diffuse ground-glass abnormality and consolidation (arrows) with traction bronchiectasis (arrowhead in C). Pleural effusion (arrowheads in D) also is seen. The patient was clinically diagnosed to have pneumonia and acute respiratory distress syndrome.
Radiation Pneumonitis.—Radiation pneumonitis (RP) is a major complication during radiation therapy in patients with lung cancer (Fig 13). An organizing pneumonia pattern is the most common imaging pattern of symptomatic RP in patients with lung cancer (52). RP is generally graded according to common terminology criteria for adverse events, as follows: Grade 1 RP is asymptomatic and based on clinical or diagnostic observations only; intervention is not indicated. Grade 2 RP is symptomatic, with affected patients having limited ability to perform instrumental activities of daily living; medical intervention is indicated. Grade 3 RP is associated with severe symptoms that limit self-care activities of daily living; supplemental oxygen is indicated. Grade 4 RP involves life-threatening respiratory compromise, with urgent intervention (eg, tracheotomy or intubation) indicated. Grade 5 RP leads to death (53). It is well known that clinically significant ILD is associated with RP (54). The correlation between ILA and RP has been investigated in several studies (55–58) but remains unclear.
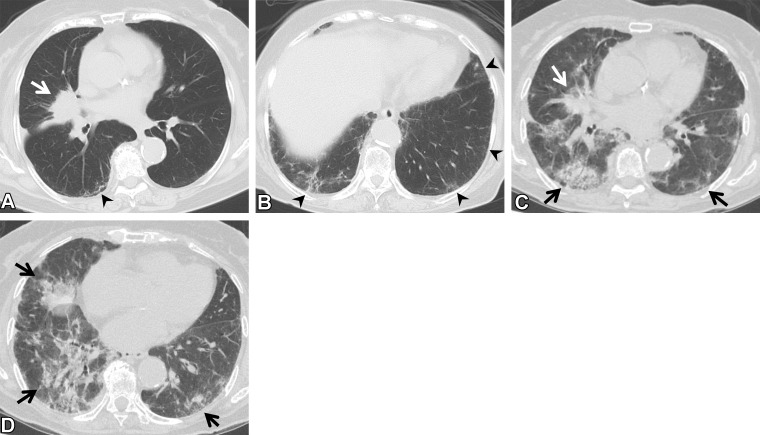
Figure 13.
RP in a 64-year-old man with squamous cell cancer. (A, B) Axial pretreatment CT images show a nodule (arrow in A) in the right upper lobe and ground-glass abnormality (arrowheads) in the subpleural area, suggesting ILA. (C) Axial CT image after radiation therapy shows ground-glass abnormality and consolidation (arrows) in the right lung, beyond the irradiated field. The patient required steroid therapy for RP.
Li et al (55) evaluated 95 patients with small cell lung cancer who were treated with greater than or equal to 50 Gy of thoracic radiation therapy. The cumulative prevalence of grade 2 or higher RP at 1 year was 50.0% (95% CI: 18.1%, 75.3%) for the group with ILA and 23.3% (95% CI: 13.1%, 31.9%) for the group without ILA (P = .017) (55). On the other hand, Higo et al (56) reported that ILA was not a risk factor for RP (odds ratio, 1.86 [95% CI: 0.48, 7.20]; P = .37) at multivariate analysis of data on 77 patients who underwent chemoradiotherapy with 30–60 Gy for locally advanced lung cancer.
Study populations and radiation techniques vary among studies. Kashihara et al (58) noted that the definition of significant RP at evaluation of the association between ILA and RP was itself controversial. In the common terminology criteria for adverse events, an RP grade of 2 or higher is generally considered to be significant. However, this broad definition includes entities that range from mild disorders requiring cough medicine to moderate diseases requiring steroids. Moreover, the extent of ILA was not fully considered. These results might also have been affected by the regional extent or fibrosis severity of ILA. Further investigations are necessary to clarify whether ILA is associated with the incidence of RP.
ICI–related Pneumonitis.—Anti–programmed cell death protein 1 (PD-1) antibodies are ICIs that are used for the treatment of various advanced cancers (59,60). ICIs are associated with immune-related adverse events, and using them sometimes results in lung toxicities referred to as ICI-related pneumonitis (Fig 14) (61–63). The prevalence of ICI-related pneumonitis was reported to be 4.1% in patients with lung cancer treated in the PD-1 inhibitor trials (64). A significant correlation between anti–PD-1–related pneumonitis risk and preexisting pulmonary fibrosis has been reported (65). A distinction needs to be made between the baseline preexisting ILD and drug-related pneumonitis. In addition, it should be noted that acute exacerbations of the preexisting ILD during lung cancer treatment also are different from drug-related pneumonitis.
Figure 14.
ICI-related pneumonitis in a 62-year-old man with squamous cell cancer. The patient was treated with nivolumab as the sixth line of therapy. (A) Axial pretreatment CT image shows subtle ground-glass abnormality (arrowheads) in the subpleural area, indicating ILA. Right hilar lymphadenopathy (arrows) also is apparent. (B) Axial CT image 10 months later shows a mass (arrow) in the right lung and pleural effusion, suggesting tumor exacerbation. (C) Axial CT image 20 days after the patient started nivolumab therapy shows a newly emergent patchy ground-glass abnormality and consolidation (arrowheads), representing ICI-related pneumonitis with an organizing pneumonia pattern; nivolumab was discontinued. (D) Axial CT image 3 months after the discontinuation of nivolumab shows that the patchy opacities have disappeared.
In terms of ILA, Nakanishi et al (66) examined 83 patients with non–small cell lung cancer who were treated with anti–PD-1 antibodies. They reported that the prevalence of ICI-related pneumonitis was significantly higher in patients with preexisting ILA than in those without it (six of 14 [42.9%] patients with ILA vs seven of 69 [10.1%] patients without ILA; P = .007) and that ground-glass attenuation in ILA was a significant risk factor for ICI-related pneumonitis (odds ratio, 44.0; P < .001). In their cohort, nine patients with ICI-related pneumonitis were treated with steroid therapy, but three of these patients died. The same group (67) also reported an association between ILA and ICI-related pneumonitis in patients with nonlung cancers.
Other Treatment-related Complications.—Cytotoxic chemotherapy and targeted drug therapy are major treatment options for patients with lung cancer, but it is well known that both of these therapies can cause exacerbation of ILD or drug-related pneumonitis (Fig 15). In the 2020 position paper published by the Fleischner Society (2), it is recommended that great care be taken when using these drugs in patients with ILA. Thus, patients with ILA have a worse prognosis than those who do not have it. In the Fleischner Society position paper (2), it is recommended that active monitoring for symptoms, physiologic alterations, and imaging-depicted progression of drug-related pneumonitis be considered.
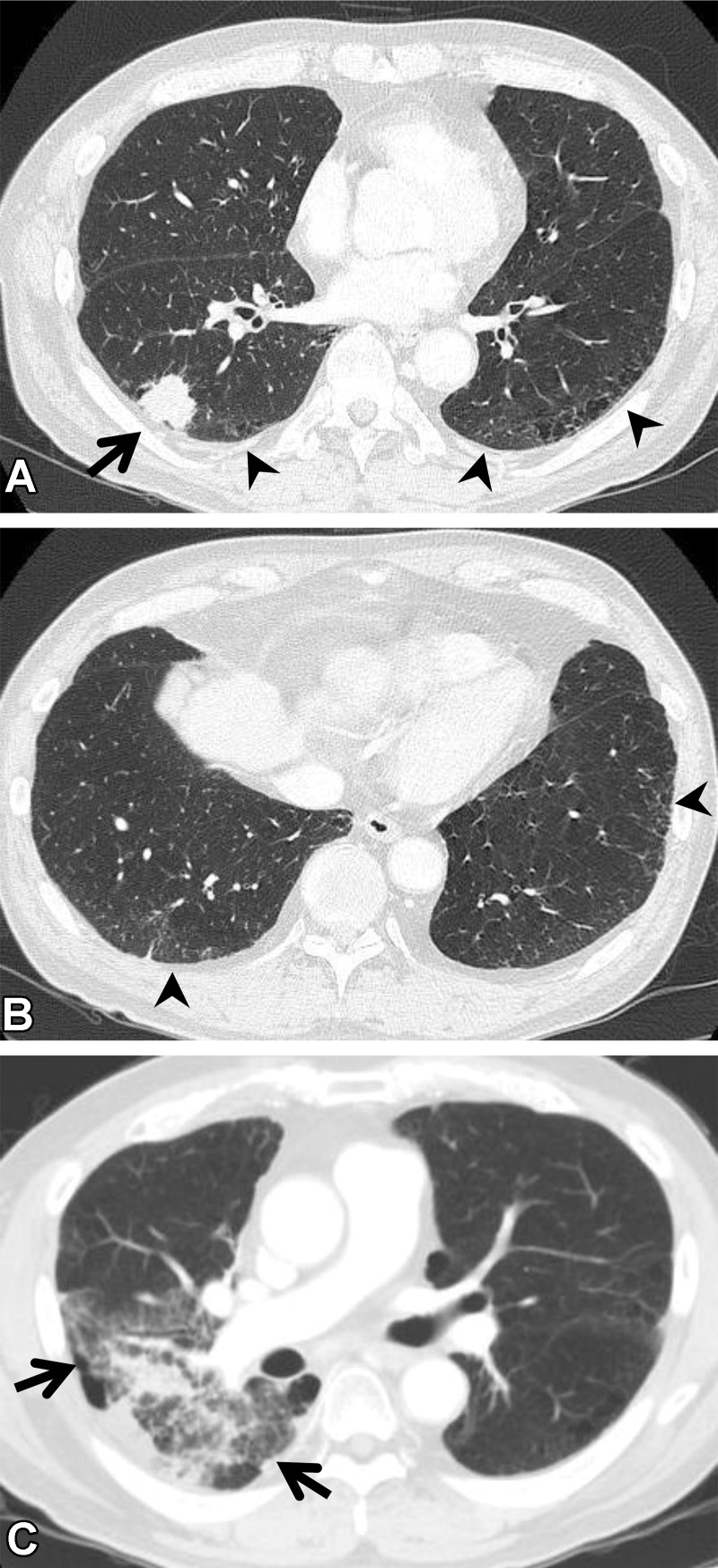
Figure 15.
Complications of targeted drug therapy in an 85-year-old woman with adenocarcinoma who was treated with osimertinib. (A, B) Axial pretreatment CT images show a mass (arrow in A) in the right middle lobe. The ground-glass abnormality and reticulation (arrowheads) seen in the subpleural area suggest ILA. The patient’s Krebs von den Lungen 6 and surfactant protein D levels were normal. (C, D) Axial CT images at 5 months of treatment show the mass (white arrow in C), and diffuse ground-glass abnormality and consolidation (black arrows), which indicate pneumonitis. The patient was short of breath and was treated with steroids.
Speculated Causes of ILA
The reason for the associations between higher incidence of lung cancer, increased mortality, and higher risk of lung injury in patients with ILA is not fully understood. ILA is not uniform and includes several histologic patterns, such as UIP, smoking-related interstitial fibrosis, and nonspecific interstitial pneumonia (68). A study of the radiologic–histopathologic correlation of ILA (69) revealed that the large percentage of patients with subpleural fibrotic ILA have histologic evidence of typical or probable UIP at biopsy. In that study (69), 69% (25 of 36) of subpleural fibrotic ILAs had histologically typical or probable UIP patterns, whereas 11% (one of nine) of subpleural nonfibrotic ILAs had these patterns.
In IPF, chronic damage of the alveolar epithelium is driven by pathogenic events common to cancer, including epigenetic and genetic disorders, abnormal response to regulatory signals, and activation of specific signaling pathways (70). Although ILA includes a variety of histopathologic lung injuries, subpleural fibrotic ILA may have a cause in common with IPF.
It has been reported that ILA is associated with decreased telomere length (71). Telomeres consist of long repetitive nucleotide sequences, and their function is to cap the ends of chromosomes and protect chromosomes from loss of genetic information (72). Progressive telomere shortening leads to insufficient telomeric caps and chromosomal instability, which can increase the rate of mutation of oncogenes and tumor suppressor genes. It has been reported that telomere shortening is associated with higher cancer risk during aging and chronic illness and with familial and sporadic IPF (70,73,74). Thus, telomere shortening may be a cause of the association between ILA and lung cancer. Further etiologic research is necessary to better understand and prevent ILA progression and tumorigenesis.
Conclusion
ILA is an incidental CT finding with potentially clinical significance. ILA is classified into three subcategories: nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic. Potentially clinically significant ILD should be identified in individuals with ILA. Risk factors for the progression of ILA include clinical elements (inhalational exposures, medication use, radiation therapy, thoracic surgery, physiologic findings, and gas exchange findings) and radiologic elements (basal and peripheral predominance and fibrotic findings). Subpleural fibrotic ILA is associated with a high incidence of histologic UIP. In addition, ILA is closely linked to lung cancer incidence, poor prognoses, and complications related to lung cancer treatment. Radiologists have the important roles of identifying ILA and recommending further workup. Recognition of this concept and knowledge of the imaging pitfalls of ILA are mandatory for radiologists.
Recipient of a Certificate of Merit award for an education exhibit at the 2021 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors M.N., G.M.H., and H.H. have provided disclosures (see end of article); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
M.Y. supported by Grants-in-Aid for Scientific Research (KAKENHI) (JP21K07672, JP21H03840) and Japan Agency for Medical Research and Development (AMED) (JP20gk0110051). M.N. supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) (R01CA240592). M.N., G.M.H., and H.H. supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) (RO1HL111024). M.N., D.C.C., and H.H. supported by NIH/NCI (U01CA209414). M.N. and H.H. supported by NIH (R01CA203636). T. Hida supported by Japan Society for the Promotion of Science (KAKENHI) (JP20K16827). G.M.H. and H.H. supported by NIH/NHLBI (R01HL130974) and NIH (R01HL135142).
Disclosures of conflicts of interest.—: M.N. Institutional research grants from Canon Medical Systems, Daiichi Sankyo, and AstraZeneca; consulting fees from Daiichi Sankyo and AstraZeneca. G.M.H. Consulting fees from Boehringer-Ingelheim, Chugai Pharmaceuticals, and Gerson Lehrman Group; participation on Data Safety Monitoring Board or Advisory Board of Biogen. H.H. Institutional research grants from Canon Medical Systems and Konica-Minolta; personal consulting fees from Mitsubishi Chemical and Canon Medical Systems.
Abbreviations:
- CTD
- connective tissue disease
- HR
- hazard ratio
- ICI
- immune checkpoint inhibitor
- ILA
- interstitial lung abnormality
- ILD
- interstitial lung disease
- IPF
- idiopathic pulmonary fibrosis
- PPFE
- pleuroparenchymal fibroelastosis
- RP
- radiation pneumonitis
- UIP
- usual interstitial pneumonia
References
- 1. Hatabu H , Hunninghake GM , Lynch DA . Interstitial lung abnormality: recognition and perspectives . Radiology 2019. ; 291 ( 1 ): 1 – 3 . [DOI] [PubMed] [Google Scholar]
- 2. Hatabu H , Hunninghake GM , Richeldi L , et al . Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society . Lancet Respir Med 2020. ; 8 ( 7 ): 726 – 737 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hata A , Schiebler ML , Lynch DA , Hatabu H . Interstitial Lung Abnormalities: State of the Art . Radiology 2021. ; 301 ( 1 ): 19 – 34 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Washko GR , Lynch DA , Matsuoka S , et al . Identification of early interstitial lung disease in smokers from the COPDGene Study . Acad Radiol 2010. ; 17 ( 1 ): 48 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Washko GR , Hunninghake GM , Fernandez IE , et al . Lung volumes and emphysema in smokers with interstitial lung abnormalities . N Engl J Med 2011. ; 364 ( 10 ): 897 – 906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seibold MA , Wise AL , Speer MC , et al . A common MUC5B promoter polymorphism and pulmonary fibrosis . N Engl J Med 2011. ; 364 ( 16 ): 1503 – 1512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hunninghake GM , Hatabu H , Okajima Y , et al . MUC5B promoter polymorphism and interstitial lung abnormalities . N Engl J Med 2013. ; 368 ( 23 ): 2192 – 2200 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin GY , Lynch D , Chawla A , et al . Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate . Radiology 2013. ; 268 ( 2 ): 563 – 571 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Araki T , Putman RK , Hatabu H , et al . Development and progression of interstitial lung abnormalities in the Framingham Heart Study . Am J Respir Crit Care Med 2016. ; 194 ( 12 ): 1514 – 1522 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Putman RK , Hatabu H , Araki T , et al . Association between interstitial lung abnormalities and all-cause mortality . JAMA 2016. ; 315 ( 7 ): 672 – 681 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doyle TJ , Washko GR , Fernandez IE , et al . Interstitial lung abnormalities and reduced exercise capacity . Am J Respir Crit Care Med 2012. ; 185 ( 7 ): 756 – 762 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lederer DJ , Enright PL , Kawut SM , et al . Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study . Am J Respir Crit Care Med 2009. ; 180 ( 5 ): 407 – 414 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobbs BD , Putman RK , Araki T , et al . Overlap of genetic risk between interstitial lung abnormalities and idiopathic pulmonary fibrosis . Am J Respir Crit Care Med 2019. ; 200 ( 11 ): 1402 – 1413 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells AU , Kokosi MA . Subclinical Interstitial Lung Abnormalities: Toward the Early Detection of Idiopathic Pulmonary Fibrosis? Am J Respir Crit Care Med 2016. ; 194 ( 12 ): 1445 – 1446 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loeb LA , Ernster VL , Warner KE , Abbotts J , Laszlo J . Smoking and lung cancer: an overview . Cancer Res 1984. ; 44 ( 12 Pt 1 ): 5940 – 5958 . [PubMed] [Google Scholar]
- 16. Axelsson GT , Putman RK , Aspelund T , et al . The associations of interstitial lung abnormalities with cancer diagnoses and mortality . Eur Respir J 2020. ; 56 ( 6 ): 1902154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whittaker Brown SA , Padilla M , Mhango G , et al . Interstitial Lung Abnormalities and Lung Cancer Risk in the National Lung Screening Trial . Chest 2019. ; 156 ( 6 ): 1195 – 1203 . [DOI] [PubMed] [Google Scholar]
- 18. Hoyer N , Thomsen LH , Wille MMW , et al . Increased respiratory morbidity in individuals with interstitial lung abnormalities . BMC Pulm Med 2020. ; 20 ( 1 ): 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wille MMW , Thomsen LH , Petersen J , et al . Visual assessment of early emphysema and interstitial abnormalities on CT is useful in lung cancer risk analysis . Eur Radiol 2016. ; 26 ( 2 ): 487 – 494 . [DOI] [PubMed] [Google Scholar]
- 20. Naccache JM , Gibiot Q , Monnet I , et al . Lung cancer and interstitial lung disease: a literature review . J Thorac Dis 2018. ; 10 ( 6 ): 3829 – 3844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hino T , Lee KS , Han J , Hata A , Ishigami K , Hatabu H . Spectrum of Pulmonary Fibrosis from Interstitial Lung Abnormality to Usual Interstitial Pneumonia: Importance of Identification and Quantification of Traction Bronchiectasis in Patient Management . Korean J Radiol 2021. ; 22 ( 5 ): 811 – 828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoo H , Hino T , Hwang J , et al . Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnormality (ILA): Evolving concept of CT findings, pathology and management . Eur J Radiol Open 2022. ; 9 : 100419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Putman RK , Gudmundsson G , Axelsson GT , et al . Imaging patterns are associated with interstitial lung abnormality progression and mortality . Am J Respir Crit Care Med 2019. ; 200 ( 2 ): 175 – 183 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Pasquier C , Hajri R , Lazor R , et al . Pitfalls in diagnosis of infiltrative lung disease by CT . BJR Open 2019. ; 1 ( 1 ): 20190036 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gaeta M , Minutoli F , Girbino G , et al . Expiratory CT scan in patients with normal inspiratory CT scan: a finding of obliterative bronchiolitis and other causes of bronchiolar obstruction . Multidiscip Respir Med 2013. ; 8 ( 1 ): 44 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hansell DM . Thin-section CT of the lungs: the Hinterland of normal . Radiology 2010. ; 256 ( 3 ): 695 – 711 . [DOI] [PubMed] [Google Scholar]
- 27. Otake S , Takahashi M , Ishigaki T . Focal pulmonary interstitial opacities adjacent to thoracic spine osteophytes . AJR Am J Roentgenol 2002. ; 179 ( 4 ): 893 – 896 . [DOI] [PubMed] [Google Scholar]
- 28. Hansell DM , Bankier AA , MacMahon H , McLoud TC , Müller NL , Remy J . Fleischner Society: glossary of terms for thoracic imaging . Radiology 2008. ; 246 ( 3 ): 697 – 722 . [DOI] [PubMed] [Google Scholar]
- 29. Chua F , Desai SR , Nicholson AG , et al . Pleuroparenchymal Fibroelastosis. A Review of Clinical, Radiological, and Pathological Characteristics . Ann Am Thorac Soc 2019. ; 16 ( 11 ): 1351 – 1359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe K , Ishii H , Kiyomi F , et al . Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: a proposal . Respir Investig 2019. ; 57 ( 4 ): 312 – 320 . [DOI] [PubMed] [Google Scholar]
- 31. Sumikawa H , Johkoh T , Iwasawa T , Nakanishi K , Tomiyama N . Pleuroparenchymal fibroelastosis-like lesions on chest computed tomography in routine clinical practice . Jpn J Radiol 2019. ; 37 ( 3 ): 230 – 236 . [DOI] [PubMed] [Google Scholar]
- 32. Lagstein A . Pulmonary Apical Cap: What’s Old Is New Again . Arch Pathol Lab Med 2015. ; 139 ( 10 ): 1258 – 1262 . [DOI] [PubMed] [Google Scholar]
- 33. Franquet T , Giménez A , Rosón N , Torrubia S , Sabaté JM , Pérez C . Aspiration diseases: findings, pitfalls, and differential diagnosis . RadioGraphics 2000. ; 20 ( 3 ): 673 – 685 . [DOI] [PubMed] [Google Scholar]
- 34. Kim M , Lee KY , Lee KW , Bae KT . MDCT evaluation of foreign bodies and liquid aspiration pneumonia in adults . AJR Am J Roentgenol 2008. ; 190 ( 4 ): 907 – 915 . [DOI] [PubMed] [Google Scholar]
- 35. Raghu G , Freudenberger TD , Yang S , et al . High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis . Eur Respir J 2006. ; 27 ( 1 ): 136 – 142 . [DOI] [PubMed] [Google Scholar]
- 36. George PM , Hida T , Putman RK , et al . Hiatus hernia and interstitial lung abnormalities . Eur Respir J 2020. ; 56 ( 5 ): 2001679 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moore EH . Atypical mycobacterial infection in the lung: CT appearance . Radiology 1993. ; 187 ( 3 ): 777 – 782 . [DOI] [PubMed] [Google Scholar]
- 38. Milliron B , Henry TS , Veeraraghavan S , Little BP . Bronchiectasis: mechanisms and imag13. ing clues of associated common and uncommon diseases . RadioGraphics 2015. ; 35 ( 4 ): 1011 – 1030 . [DOI] [PubMed] [Google Scholar]
- 39. Han X , Fan Y , Alwalid O , et al . Fibrotic Interstitial Lung Abnormalities at 1-year Follow-up CT after Severe COVID-19 . Radiology 2021. ; 301 ( 3 ): E438 – E440 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sack CS , Doney BC , Podolanczuk AJ , et al . Occupational Exposures and Subclinical Interstitial Lung Disease. The MESA (Multi-Ethnic Study of Atherosclerosis) Air and Lung Studies . Am J Respir Crit Care Med 2017. ; 196 ( 8 ): 1031 – 1039 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoyer N , Wille MMW , Thomsen LH , et al . Interstitial lung abnormalities are associated with increased mortality in smokers . Respir Med 2018. ; 136 : 77 – 82 . [DOI] [PubMed] [Google Scholar]
- 42. Pompe E , de Jong PA , Lynch DA , et al . Computed tomographic findings in subjects who died from respiratory disease in the National Lung Screening Trial . Eur Respir J 2017. ; 49 ( 4 ): 1601814 . [DOI] [PubMed] [Google Scholar]
- 43. Tsushima K , Sone S , Yoshikawa S , Yokoyama T , Suzuki T , Kubo K . The radiological patterns of interstitial change at an early phase: over a 4-year follow-up . Respir Med 2010. ; 104 ( 11 ): 1712 – 1721 . [DOI] [PubMed] [Google Scholar]
- 44. Park J , Kim DS , Shim TS , et al . Lung cancer in patients with idiopathic pulmonary fibrosis . Eur Respir J 2001. ; 17 ( 6 ): 1216 – 1219 . [DOI] [PubMed] [Google Scholar]
- 45. Hida T , Hata A , Lu J , et al . Interstitial lung abnormalities in patients with stage I non-small cell lung cancer are associated with shorter overall survival: the Boston lung cancer study . Cancer Imaging 2021. ; 21 ( 1 ): 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Araki T , Dahlberg SE , Hida T , et al . Interstitial lung abnormality in stage IV non-small cell lung cancer: a validation study for the association with poor clinical outcome . Eur J Radiol Open 2019. ; 6 : 128 – 131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Iwasawa T , Okudela K , Takemura T , et al . Computer-aided Quantification of Pulmonary Fibrosis in Patients with Lung Cancer: Relationship to Disease-free Survival . Radiology 2019. ; 292 ( 2 ): 489 – 498 . [DOI] [PubMed] [Google Scholar]
- 48. Cho SW , Jeong WG , Lee JE , et al . Clinical implication of interstitial lung abnormality in elderly patients with early-stage non-small cell lung cancer . Thorac Cancer 2022. ; 13 ( 7 ): 977 – 985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stéphan F , Boucheseiche S , Hollande J , et al . Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors . Chest 2000. ; 118 ( 5 ): 1263 – 1270 . [DOI] [PubMed] [Google Scholar]
- 50. Agostini P , Cieslik H , Rathinam S , et al . Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax 2010. ; 65 ( 9 ): 815 – 818 . [DOI] [PubMed] [Google Scholar]
- 51. Im Y , Park HY , Shin S , et al . Prevalence of and risk factors for pulmonary complications after curative resection in otherwise healthy elderly patients with early stage lung cancer . Respir Res 2019. ; 20 ( 1 ): 136 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomas R , Chen YH , Hatabu H , Mak RH , Nishino M . Radiographic patterns of symptomatic radiation pneumonitis in lung cancer patients: imaging predictors for clinical severity and outcome . Lung Cancer 2020. ; 145 : 132 – 139 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. National Institutes of Health . Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 . https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed June 7, 2022 .
- 54. Ueki N , Matsuo Y , Togashi Y , et al . Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer . J Thorac Oncol 2015. ; 10 ( 1 ): 116 – 125 . [DOI] [PubMed] [Google Scholar]
- 55. Li F , Zhou Z , Wu A , et al . Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy . Radiat Oncol 2018. ; 13 ( 1 ): 82 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Higo H , Kubo T , Makimoto S , et al . Chemoradiotherapy for locally advanced lung cancer patients with interstitial lung abnormalities . Jpn J Clin Oncol 2019. ; 49 ( 5 ): 458 – 464 . [DOI] [PubMed] [Google Scholar]
- 57. Yamaguchi S , Ohguri T , Ide S , et al . Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis . Lung Cancer 2013. ; 82 ( 2 ): 260 – 265 . [DOI] [PubMed] [Google Scholar]
- 58. Kashihara T , Nakayama Y , Ito K , et al . Usefulness of Simple Original Interstitial Lung Abnormality Scores for Predicting Radiation Pneumonitis Requiring Steroidal Treatment After Definitive Radiation Therapy for Patients With Locally Advanced Non-Small Cell Lung Cancer . Adv Radiat Oncol 2020. ; 6 ( 1 ): 100606 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nishino M , Hatabu H , Hodi FS . Imaging of Cancer Immunotherapy: Current Approaches and Future Directions . Radiology 2019. ; 290 ( 1 ): 9 – 22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ribas A , Wolchok JD . Cancer immunotherapy using checkpoint blockade . Science 2018. ; 359 ( 6382 ): 1350 – 1355 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishino M , Sholl LM , Hodi FS , Hatabu H , Ramaiya NH . Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy . N Engl J Med 2015. ; 373 ( 3 ): 288 – 290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nishino M , Ramaiya NH , Awad MM , et al . PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course . Clin Cancer Res 2016. ; 22 ( 24 ): 6051 – 6060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johkoh T , Lee KS , Nishino M , et al . Chest CT Diagnosis and Clinical Management of Drug-related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper from the Fleischner Society . Radiology 2021. ; 298 ( 3 ): 550 – 566 . [DOI] [PubMed] [Google Scholar]
- 64. Nishino M , Giobbie-Hurder A , Hatabu H , Ramaiya NH , Hodi FS . Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis . JAMA Oncol 2016. ; 2 ( 12 ): 1607 – 1616 . [DOI] [PubMed] [Google Scholar]
- 65. Yamaguchi T , Shimizu J , Hasegawa T , et al . Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis . Lung Cancer 2018. ; 125 : 212 – 217 . [DOI] [PubMed] [Google Scholar]
- 66. Nakanishi Y , Masuda T , Yamaguchi K , et al . Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer . Respir Investig 2019. ; 57 ( 5 ): 451 – 459 . [DOI] [PubMed] [Google Scholar]
- 67. Shimoji K , Masuda T , Yamaguchi K , et al . Association of Preexisting Interstitial Lung Abnormalities With Immune Checkpoint Inhibitor-Induced Interstitial Lung Disease Among Patients With Nonlung Cancers . JAMA Netw Open 2020. ; 3 ( 11 ): e2022906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hung YP , Hunninghake GM , Miller ER , et al . Incidental nonneoplastic parenchymal findings in patients undergoing lung resection for mass lesions . Hum Pathol 2019. ; 86 : 93 – 101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chae KJ , Chung MJ , Jin GY , et al . Radiologic-pathologic correlation of interstitial lung abnormalities and predictors for progression and survival . Eur Radiol 2022. ; 32 ( 4 ): 2713 – 2723 . [DOI] [PubMed] [Google Scholar]
- 70. Vancheri C . Common pathways in idiopathic pulmonary fibrosis and cancer . Eur Respir Rev 2013. ; 22 ( 129 ): 265 – 272 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Putman RK , Axelsson GT , Ash SY , et al . Interstitial lung abnormalities are associated with decreased mean telomere length . Eur Respir J 2022. . doi: 10.1183/13993003.01814-2021. Published online February 3, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Blasco MA . Telomeres and human disease: ageing, cancer and beyond . Nat Rev Genet 2005. ; 6 ( 8 ): 611 – 622 . [DOI] [PubMed] [Google Scholar]
- 73. Djojosubroto MW , Choi YS , Lee HW , Rudolph KL . Telomeres and telomerase in aging, regeneration and cancer . Mol Cells 2003. ; 15 ( 2 ): 164 – 175 . [PubMed] [Google Scholar]
- 74. Jang JS , Choi YY , Lee WK , et al . Telomere length and the risk of lung cancer . Cancer Sci 2008. ; 99 ( 7 ): 1385 – 1389 . [DOI] [PMC free article] [PubMed] [Google Scholar]