Abstract
Sleep abnormalities are prevalent in Alzheimer’s disease, with sleep quality already impaired at its preclinical stage. Epidemiological and experimental data point to sleep abnormalities contributing to the risk of Alzheimer’s disease. However, previous studies are limited by either a lack of Alzheimer’s disease biomarkers, reduced sample size or cross-sectional design. Understanding if, when, and how poor sleep contributes to Alzheimer’s disease progression is important so that therapies can be targeted to the right phase of the disease. Using the largest cohort to date, the European Prevention of Alzheimer’s Dementia Longitudinal Cohort Study, we test the hypotheses that poor sleep is associated with core Alzheimer’s disease CSF biomarkers cross-sectionally and predicts future increments of Alzheimer’s disease pathology in people without identifiable symptoms of Alzheimer’s disease at baseline. This study included 1168 adults aged over 50 years with CSF core Alzheimer’s disease biomarkers (total tau, phosphorylated tau and amyloid-beta), cognitive performance, and sleep quality (Pittsburgh sleep quality index questionnaire) data. We used multivariate linear regressions to analyse associations between core Alzheimer’s disease biomarkers and the following Pittsburgh sleep quality index measures: total score of sleep quality, binarized score (poor sleep categorized as Pittsburgh sleep quality index > 5), sleep latency, duration, efficiency and disturbance. On a subsample of 332 participants with CSF taken at baseline and after an average period of 1.5 years, we assessed the effect of baseline sleep quality on change in Alzheimer’s disease biomarkers over time. Cross-sectional analyses revealed that poor sleep quality (Pittsburgh sleep quality index total > 5) was significantly associated with higher CSF t-tau; shorter sleep duration (<7 h) was associated with higher CSF p-tau and t-tau; and a higher degree of sleep disturbance (1–9 versus 0 and >9 versus 0) was associated with lower CSF amyloid-beta. Longitudinal analyses showed that greater sleep disturbances (1–9 versus 0 and >9 versus 0) were associated with a decrease in CSF Aβ42 over time. This study demonstrates that self-reported poor sleep quality is associated with greater Alzheimer’s disease-related pathology in cognitively unimpaired individuals, with longitudinal results further strengthening the hypothesis that disrupted sleep may represent a risk factor for Alzheimer’s disease. This highlights the need for future work to test the efficacy of preventive practices, designed to improve sleep at pre-symptomatic stages of disease, on reducing Alzheimer’s disease pathology.
Keywords: Alzheimer’s disease, biomarkers, sleep, preclinical
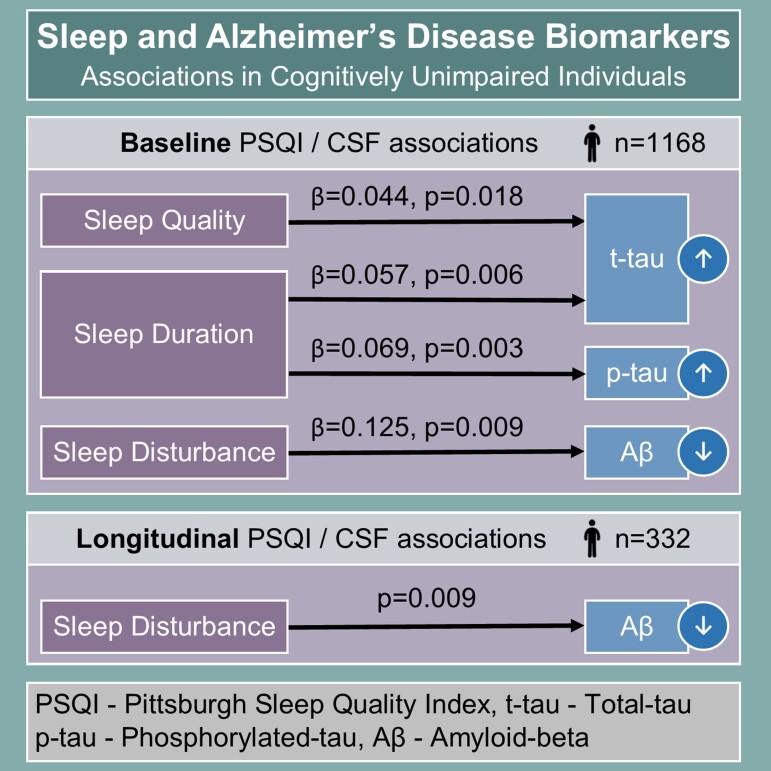
This study tested associations between self-reported sleep abnormalities and CSF biomarkers of Alzheimer’s Disease in a large cohort of cognitively unimpaired individuals. Cross-sectionally, higher CSF tau was associated with overall sleep quality/duration with CSF amyloid-beta associated with sleep disturbances. Further, sleep disturbances predicted decreasing CSF amyloid-beta longitudinally.
Graphical Abstract
Graphical abstract.
Introduction
Sleep disturbance and circadian rhythm disorders are well recognized as intrinsic symptoms of established Alzheimer’s Disease.1–6 Alzheimer’s disease dementia is associated with a broad range of sleep macro-architectural changes, including reduced total sleep time, excessive daytime sleepiness, decreased sleep efficiency and increased sleep fragmentation,7 with the extent of abnormalities correlating with dementia severity.3,7–10 Sleep abnormalities are also well described earlier in the natural history of Alzheimer’s disease, during and even preceding the mild cognitive impairment (MCI) stage.3,11–16 In addition, a growing body of literature recognizes insomnia and conditions associated with fragmented sleep as independent risk factors for Alzheimer’s disease dementia.13–15,17
Abnormalities in sleep may reflect early symptomatic manifestations of Alzheimer’s disease pathology, however, there are also plausible mechanisms by which sleep disturbances could hasten pathophysiology, specifically through the loss of sleep’s modulatory role in governing concentrations of the key metabolites in the pathognomonic changes of Alzheimer’s disease.18
CSF biomarkers, including amyloid-β 42 (Aβ42), total tau (t-tau) and phosphorylated tau (p-tau), reflect key aspects of Alzheimer’s disease pathophysiology, correlating well with amyloid PET,19 and have been validated in providing early high diagnostic accuracy.20,21 Sleep-wake activity has been shown to affect their production, release, clearance (via the glymphatic system) and metabolism.22,23 However, the precise nature of sleep abnormalities and even the direction of its correlation with Alzheimer’s disease CSF biomarkers has not been consistently reported in the literature.
Experimental studies have shown that acute sleep deprivation increases interstitial fluid (ISF) and CSF levels of Aβ in humans and animal models.24–26 However, cross-sectional observational studies have yielded mixed results. Lower actigraphy-measured sleep efficiency and self-reported increased daytime napping have been associated with lower CSF Aβ42 levels in cognitively unimpaired middle-aged adults.27 Similarly, lower CSF Aβ42/Aβ40, higher t-tau/Aβ42 and p-tau/Aβ42 levels have been associated with worse subjective sleep quality and daytime somnolence,28,29 as well as both reduced and excessive sleep duration in cognitively unimpaired adults.29 Yet, higher levels of CSF Aβ42 have been found to be associated with self-reported insomnia,30 and also with reduced slow-wave activity and more fragmented slow-wave sleep in cognitively unimpaired adults.31
The reported relationship between CSF tau and sleep disturbance has also been inconsistent. Previous studies have shown that sleep restriction increases CSF and ISF tau levels in mouse models and humans,32,33 potentially through compromised glymphatic system activity.34 However, others have not reported this association, possibly due to the longer turnover time of tau when compared to Aβ.25,35–37 In cross-sectional observational studies, one study found no differences in CSF tau levels when comparing patients with insomnia to controls.30 Conversely, poor sleep quality over several days has been associated with increased CSF tau in healthy adults25 and a faster rate of CSF tau accumulation has been reported in adults with obstructive sleep apnoea (OSA) compared with controls.38
Several reasons may explain these inconsistencies across published findings. First, most studies are cross-sectional, thereby restricting inferences regarding important dynamic effects of sleep on CSF biomarkers over time. Second, few studies have explored sleep quality in preclinical Alzheimer’s disease, despite this intuitively reflecting the optimum stage for intervention, before architectural changes associated with neurodegeneration have become established. Third, there is significant methodological heterogeneity between studies. Specifically, the use of objective versus subjective sleep measures makes comparison difficult, due to the lack of perfect correlation in these measures more apparent at the earliest disease stages.39 Last, aside from one larger cross-sectional study of 736 participants,29 studies exploring this relationship have been small in sample size.
This study tests the hypotheses that baseline self-reported poor sleep quality in cognitively unimpaired individuals is associated cross-sectionally with a higher burden of Alzheimer’s disease pathology, and with its accumulation over time. These hypotheses are tested in the largest cohort to date using both cross-sectional and longitudinal data to assess the association between subjective sleep quality and Alzheimer’s disease CSF biomarkers. Given the high prevalence associated with both sleep disturbances in the elderly population and MCI/Alzheimer’s disease groups, investigating plausible neurobiological underpinnings of this relationship may enhance understanding of the neurodegenerative processes and clinical trajectory. Disentangling this link may reveal sleep as a target for treatment and prevention strategies. As effective treatments for sleep disturbances exist, they could be rapidly implemented to mitigate cognitive decline when targeted to an appropriate stage of the Alzheimer’s disease continuum.
Materials and methods
Participants and study design
This cross-sectional and longitudinal study includes participants from The European Prevention of Alzheimer’s Dementia Longitudinal Cohort Study (EPAD-LCS) registered at www.clinicaltrials.gov identifier NCT02804789. The primary research goal of the EPAD-LCS is to provide a well-phenotyped probability-spectrum population for developing and continuously improving disease models for Alzheimer’s disease in individuals without dementia.
The cohort comprises over 2000 adults aged 50 years or older without a diagnosis of dementia. Key exclusion criteria included severe medical co-morbidity or major neurological disorders (for full criteria see Supplementary Table 1). Research participants were characterized with MRI, CSF Alzheimer’s disease biomarkers, standard cognitive assessment and genetic data. Additionally, information was collected regarding lifestyle factors including sleep habits [Pittsburgh Sleep Quality Index (PSQI)], smoking habits, alcohol consumption, diet and physical activity variables. Full details of participant selection and methods are described within its study protocol.40,41
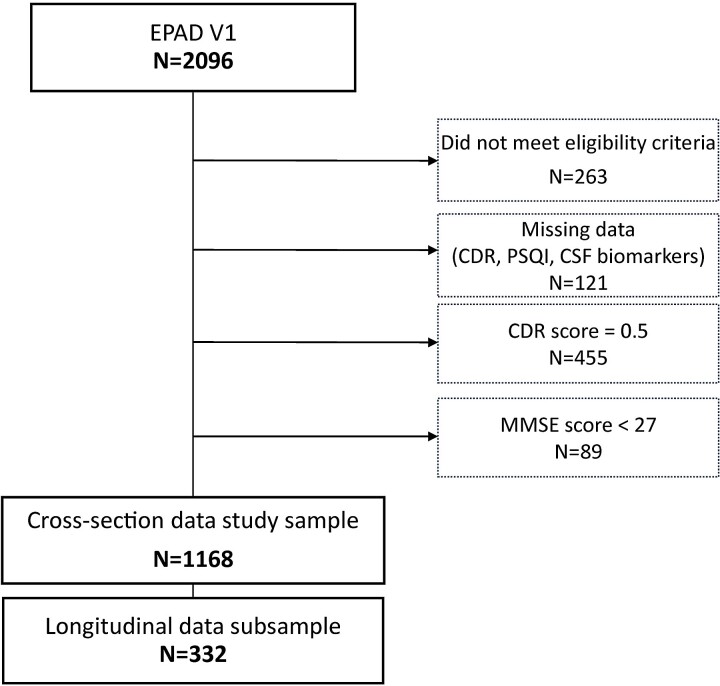
Data used in preparation of this article were obtained from the EPAD-LCS data set V.IMI (doi:10.34688/epadlcs_v.imi_20.10.30) comprising 2096 EPAD participants enrolled from 2016 to 2020 (see Fig. 1).
Figure 1.
The flow-chart illustrating stepwise exclusion process of participants used in this study.
Participants were excluded as a result of ineligibility for full EPAD participation, n = 263; missing data [Clinical Dementia Rating (CDR) score, PSQI or Alzheimer’s disease CSF biomarkers], n = 121; CDR score = 0.5, n = 455; and MMSE < 27, n = 89, leaving a final sample consisting of 1168 cognitively unimpaired individuals (CDR: 0).
We analysed cross-sectional effects of self-reported sleep measures in the whole sample but also investigated longitudinal changes in CSF biomarkers, where this data was available in a subsample of 332 individuals.
Sleep assessment
The Pittsburgh Sleep Quality Index (PSQI), a brief, 19-item, self-rated questionnaire assessing sleep quality over the preceding month, provides the measure of subjective sleep quality for this study. PSQI scoring is based on seven components that assess different sleep-related domains: (i) subjective sleep quality, (ii) latency of sleep, (iii) length of sleep, (iv) sleep efficiency, (v) sleep disturbances, (vi) use of sleep medicines, and (vii) daytime dysfunction. Each component is scored on a scale from 0 to 3, with 3 indicating the extreme negative evaluation—severe difficulty. Finally, all component scores are summated yielding a global score (0–21). A total score above 5 is indicative of poor quality of sleep.42 The PSQI was repeated at each follow-up visit.
CSF samples
All participants underwent lumbar puncture at baseline and CSF samples were obtained following a harmonised protocol.41 In 332 participants, more than one CSF sample was collected during follow-up. Among these, 268 (80.7%) had CSF samples from two separated time points, 63 (19.0%) from three time points and one (0.3%) from four time points. The interval of time between the first and last CSF sample collection was on average 1.5 years (SD 0.5). Total tau (t-tau), p-tau, and Aβ42 levels were measured with fully automatised ElectroChemiLuminescence Roche Elecsys® System immunoassays at the University of Gothenburg from CSF samples obtained using a standard protocol.43
Neuropsychological evaluation
EPAD participants underwent a standardised neuropsychological examination battery that included screening tests such as the Mini-Mental State Examination (MMSE)44 and the CDR scale.45 The Geriatric Depression Scale (GDS) and State-Trait Anxiety Inventory (STAI) were used for the assessment of psychological status.46,47
Statistical analysis
Outliers were excluded utilising Tukey’s criteria set at three times the interquartile range. Normality was assessed visually and by the Shapiro-Wilk test. Non-parametrically distributed variables CSF Aβ42, p-tau and t-tau levels were log10-transformed. For all analyses, a 2-tailed P < 0.05 was considered significant.
Multivariate linear regression analyses were used to assess the relationships between sleep variables yielded by the PSQI questionnaire as predictors, and continuous CSF biomarkers (Aβ42, t-tau and p-tau) as outcomes. The following PSQI measures were used: total score of sleep quality, binarized score (poor sleep quality categorised as PSQI >5), sleep latency, sleep efficiency, sleep duration, sleep disturbances, and daytime dysfunction. Reference categories for categorical variables reflected optimum sleep quality/duration or daytime function (PSQI ≤ 5 for binarized PSQI, sleep latency ≤ 15 min, sleep duration > 7 h, sleep efficiency > 85%, sleep disturbances score of 0, and daytime dysfunction score of 0). For each PSQI component, adjacent categories were collapsed whenever the number of observations in any category was less than 20 [e.g. baseline sleep disturbances score of 10–18 (n = 250) was merged with a score of 19–27 (n = 6)]. Separate models defined each biomarker as the dependent variable with each sleep measure as the predictor. All models were adjusted by core covariates—age, sex, research site and APOE-ɛ4 status (carriers versus non-carriers). In order to adjust by additional potential confounders but minimise data overfitting, we adjusted by additional confounders only if found to be significant (P-value (P < 0.05) in a saturated model. Potential confounders assessed in this model included depression (GDS), anxiety (STAI), physical activity, body mass index (BMI) and sleep medication (dichotomized PSQI component 6 variable—use of sleep medication less than once a week versus at least once a week). To see if the effect of sleep measures on each biomarker was independent from other biomarkers, we further adjusted all models by other biomarkers’ baseline levels. Following this procedure, models with CSF Aβ42 were adjusted by core covariates, anxiety (STAI) and log10(CSF p-tau), and models with CSF t-tau or p-tau as outcomes, were adjusted by core covariates, physical activity, BMI and log10(CSF Aβ42).
For cross-sectional analyses we also performed binary logistic regression models where our outcome measures were dichotomic variables of CSF biomarkers based on established cut-offs: Aβ-positive: CSF Aβ42 < 1000 pg/ml, p-tau-positive: CSF p-tau > 27 pg/ml, t-tau-positive: CSF p-tau > 300 pg/ml.48,49 All models were adjusted following the same procedure outlined previously, resulting in models with dichotomic CSF Aβ42 being adjusted by core covariates and log10(CSF p-tau), models with dichotomic CSF t-tau being adjusted by core covariates and log10(CSF Aβ42), and models with dichotomic CSF p-tau being adjusted by core covariates, physical activity, and log10(CSF Aβ42).
Linear mixed model analysis (LMM) was performed for longitudinal data, using the lme function in the lme package implemented in R v4.0.3. Levels of log(Aβ42), log(p-tau) and log(t-tau) were dependent variables; each sleep variable, age, sex, APOE-ɛ4 status and their interaction with time (operationalised as interval between the first and the last CSF sampling) were included as fixed effects; and patient identity as a random effect in all models. All models were adjusted by the previously mentioned covariate selection procedure so that analyses with CSF Aβ42 as outcome were adjusted by core covariates and log10(CSF p-tau), and models with CSF t-tau or p-tau as outcomes, were adjusted by core covariates and log10(CSF Aβ42). For example, the model specification for CSF Aβ42 levels as outcome and PSQI Total score as sleep variable was: log(Aβ42) ∼ PSQI Total score + age + sex + APOE-ɛ4 status + research site + log(p-tau) PSQI Total score*time + age*time + sex*time + APOE-ɛ4 status*time + (1|Participant). Statistical analyses were performed using the Stata 15 software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and R statistical software (R Core Team 2014. R: A Language and Environment for Statistical Computing, version v4.0.3. Available at: http://www.r-project.org).
Results
Subjects characteristics
Demographic and clinical characteristics of the study population are shown in Table 1.
Table 1.
Demographic, genetic data, CSF, cognitive and clinical data of the sample
| Variable | Entire sample (N = 1168) | Subsample with longitudinal data (N = 332) | |
|---|---|---|---|
| Mean (SD)/count (%) | Mean (SD)/count (%) | P a | |
| Demographic | |||
| Age (years) | 64.7 (7.1) | 65.5 (6.4) | 0.058 |
| Female, n (%) | 678 (58.1) | 176 (51.6) | 0.034 |
| Education (years) | 14.8 (3.5) | 14.4 (3.8) | 0.066 |
| Cognitive and clinical data | |||
| MMSE score | 29.1 (1.0) | 29.0 (1.0) | 0.583 |
| Depression (GDS total score) | 4.4 (4.4) | 4.4 (4.5) | 0.92 |
| Anxiety (STAI total score) | 62.5 (15.0) | 63.1 (14.7) | 0.468 |
| BMI (kg/m2) | 26.3 (4.4) | 26.5 (4.1) | 0.368 |
| Physical activity, n (%) | |||
| Not at all | 123 (10.6) | 36 (10.6) | 0.995 |
| Few times/year | 87 (7.5) | 28 (8.3) | 0.552 |
| 2–3/month | 79 (6.8) | 29 (8.6) | 0.297 |
| Once a week | 197 (16.9) | 44 (13.0) | 0.034 |
| 2–3/week | 473 (40.6) | 133 (39.3) | 0.66 |
| Daily | 204 (17.5) | 68 (20.1) | 0.208 |
| Genetic and CSF biomarkers data | |||
| APOE-e4 carriers, n (%) | 424 (36.8) | 134 (39.8) | 0.278 |
| CSF Aβ42 (pg/mL) | 1452.7 (708.9) | 1338.7 (617.1) | 0.008 |
| CSF p-tau (pg/mL) | 17.8 (8.6) | 18.5 (9.5) | 0.228 |
| CSF t-tau (pg/mL) | 207.9 (83.6) | 213.6 (89.0) | 0.279 |
| Interval between CSF collection (years) | - | 1.5 (0.5) | - |
P-values from two-sample t-test (continuous variables) or two-sample test of proportions (categorical variables). GDS, Geriatric Depression Scale, STAI, State-Trait Anxiety Inventory, MMSE, Mini-mental State Examination, BMI, body mass index.
In summary, the mean age for the entire sample was 64.7 (SD = 7.1) and for the subsample with longitudinal data 65.5 (SD = 6.4). Among the full study population, 58.1% were female, whereas there was a slightly smaller percentage of females in the longitudinal analyses (51.6% female). Participants with longitudinal CSF data displayed significantly lower CSF Aβ42 levels (P = 0.008) compared with the entire sample. Table 2 reports sleep characteristics for the entire sample and for the subgroup with longitudinal data. In the whole study sample, 38.8% of individuals were characterised as poor sleepers based on the PSQI Total score cut off of > 5, compared with 37.4% of those with longitudinal data.
Table 2.
Sleep quality characteristics at baseline
| Variable | Entire sample (N = 1168) | Subsample with longitudinal data (N = 332) | P a |
|---|---|---|---|
| Mean (SD)/count (%) | Mean (SD)/count (%) | ||
| Total PSQI score | 5.2 (3.3) | 5.0 (3.1) | 0.286 |
| Poor sleepers (Total PSQI > 5), n (%) | 453 (38.8) | 124 (37.4) | 0.635 |
| Sleep latency, n (%) | |||
| ≤ 15 min | 445 (38.1) | 138 (41.6) | 0.253 |
| 16–30 min | 478 (40.9) | 134 (40.4) | 0.854 |
| 31–60 min | 176 (15.1) | 41 (12.4) | 0.214 |
| >60 min | 69 (5.9) | 19 (5.7) | 0.899 |
| Sleep duration, n (%) | |||
| >7 h | 713 (61.0) | 203 (61.1) | 0.974 |
| 6–7 h | 323 (27.7) | 94 (28.3) | 0.813 |
| 5–6 h | 108 (9.3) | 29 (8.7) | 0.775 |
| <5 h | 24 (2.1) | 6 (1.8) | 0.776 |
| Sleep efficiency, n (%) | |||
| >85% | 583 (49.9) | 179 (53.6) | 0.234 |
| 75–84% | 326 (27.9) | 90 (27.1) | 0.773 |
| 65–74% | 137 (11.7) | 34 (10.2) | 0.451 |
| <65% | 122 (10.5) | 30 (9.0) | 0.453 |
| Sleep disturbance, n (%) | |||
| 0 | 89 (7.6) | 19 (5.7) | 0.238 |
| 1–9 | 844 (72.3) | 261 (78.6) | 0.020 |
| 10–18 | 229 (19.6) | 51 (15.4) | 0.080 |
| 19–27 | 6 (0.5) | 1 (0.3) | 0.616 |
| Use of sleep medication, n (%) | |||
| Not during past month | 953 (81.6) | 263 (79.2) | 0.330 |
| Less than once a week | 67 (5.7) | 27 (8.1) | 0.112 |
| Once or twice a week | 41 (3.5) | 7 (2.1) | 0.200 |
| Three or more times a week | 107 (9.2) | 35 (10.5) | 0.448 |
| Daytime dysfunction, n (%) | |||
| 0 | 661 (56.6) | 185 (55.7) | 0.778 |
| 1–2 | 449 (38.4) | 132 (39.8) | 0.664 |
| 3–4 | 54 (4.6) | 13 (3.9) | 0.582 |
| 5–6 | 4 (0.3) | 2 (0.6) | 0.508 |
P-values from a two-sample t-test (continuous variables) or two-sample test of proportions (categorical variables).
Cross-sectional analyses
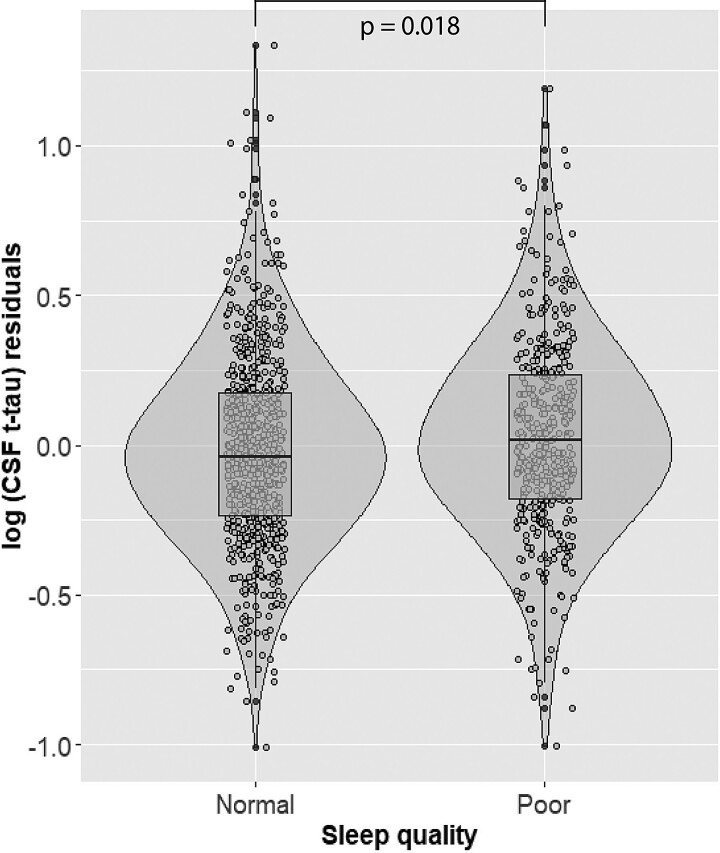
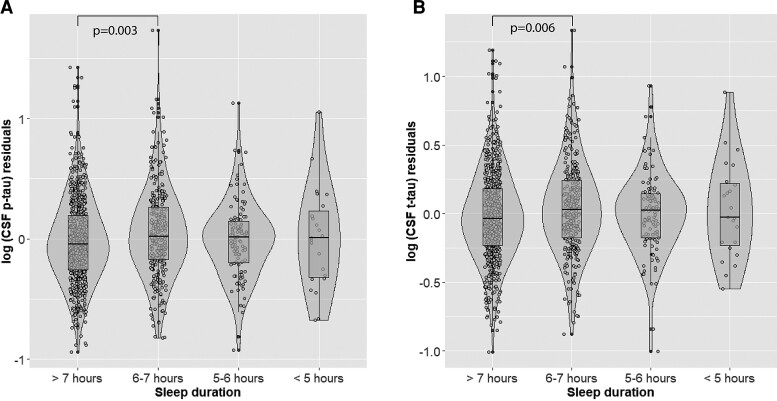
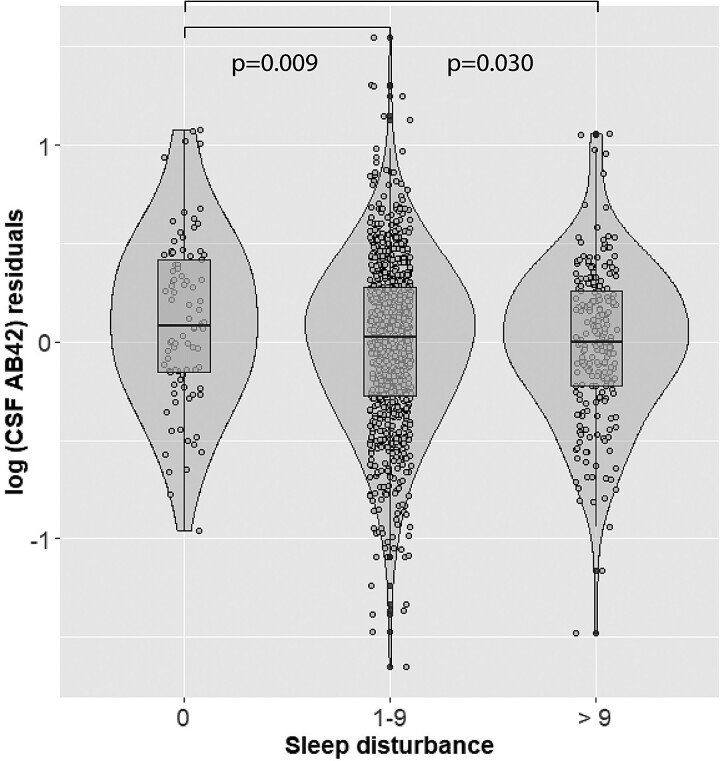
Poor sleep quality (PSQI total > 5) was significantly associated with higher log10(CSF t-tau) (hereinafter CSF t-tau) (β= 0.044, P = 0.018) (Table 3, Fig. 2). Participants who reported sleeping 6–7 h displayed higher CSF p-tau levels than those with >7 h of sleep (β = 0.054, P = 0.028) (Table 3, Fig. 3). Shorter sleep duration was also significantly associated with higher CSF p-tau after dichotomizing sleep duration to > 7 h versus < 7 h (β = 0.069, P = 0.003) and higher log10(CSF t-tau) (β = 0.057, P = 0.006). A higher degree of sleep disturbance (1–9 versus 0 and >9 versus 0) was associated with lower log10(CSF Aβ42) (hereinafter (CSF Aβ42) (β=−0.125, P = 0.009; β=−0.121, P = 0.030) (Table 3, Fig. 4). No significant associations between the remaining PSQI components or total score and CSF Alzheimer’s disease biomarkers were found (Table 3).
Table 3.
Effect of PSQI measures on CSF biomarkers at baseline
| Variables | log(CSF Aβ42) | log(CSF P-tau) | log(CSF t-tau) | |||
|---|---|---|---|---|---|---|
| β Coefficient (95% CI) | P-value | β Coefficient (95% CI) | P-value | β Coefficient (95% CI) | P-value | |
| Total PSQI score | −0.003 (−0.012 0.006) | 0.809 | 0.002 (−0.005 0.008) | 0.609 | 0.003 (−0.003 0.008) | 0.344 |
| Dichotomized PSQI score (ref. Total PSQI ≤5) | −0.013 (−0.064 0.039) | 0.63 | 0.039 (−0.002 0.081) | 0.062 | 0.044 (0.007 0.08) | 0.018 |
| Sleep latency (ref. ≤ 15 min) | ||||||
| 16–30 min | −0.039 (−0.094 0.015) | 0.153 | 0.007 (−0.038 0.053) | 0.759 | 0.001 (−0.039 0.041) | 0.946 |
| 31–60 min | 0.033 (−0.041 0.107) | 0.382 | −0.007 (−0.068 0.054) | 0.824 | −0.008 (−0.062 0.046) | 0.767 |
| >60 min | 0.058 (−0.05 0.165) | 0.29 | −0.02 (−0.108 0.069) | 0.665 | −0.014 (−0.092 0.064) | 0.728 |
| Sleep duration (ref. > 7 h) | ||||||
| 6–7 h | −0.034 (−0.089 0.022) | 0.232 | 0.069 (0.023 0.115) | 0.003 | 0.057 (0.016 0.097) | 0.006 |
| 5–6 h | −0.04 (−0.125 0.045) | 0.356 | 0.019 (−0.052 0.09) | 0.597 | 0.036 (−0.026 0.098) | 0.259 |
| <5 h | 0.097 (−0.076 0.27) | 0.272 | −0.01 (−0.151 0.131) | 0.89 | 0.014 (−0.11 0.138) | 0.824 |
| Sleep efficiency (ref. > 85%) | ||||||
| 75–84% | −0.036 (−0.093 0.021) | 0.212 | 0.015 (−0.033 0.062) | 0.545 | 0.015 (−0.027 0.057) | 0.481 |
| 65–74% | −0.039 (−0.118 0.04) | 0.335 | 0.029 (−0.036 0.094) | 0.385 | 0.024 (−0.034 0.081) | 0.418 |
| <65% | −0.002 (−0.086 0.082) | 0.961 | 0.039 (−0.03 0.108) | 0.267 | 0.046 (−0.015 0.107) | 0.138 |
| Sleep disturbance (ref. 0)a | ||||||
| 1–9 | −0.125 (−0.219 −0.031) | 0.009 | 0.016 (−0.063 0.094) | 0.695 | 0.034 (−0.035 0.104) | 0.334 |
| >9 | −0.121 (−0.23 -0.012) | 0.03 | 0.016 (−0.074 0.107) | 0.722 | 0.028 (−0.052 0.108) | 0.49 |
| Daytime dysfunction (ref. 0)b | ||||||
| 1–2 | −0.037 (−0.09 0.016) | 0.173 | 0.018 (−0.025 0.06) | 0.411 | 0.014 (−0.023 0.052) | 0.448 |
| >2 | −0.032 (−0.15 0.087) | 0.6 | −0.085 (−0.182 0.011) | 0.084 | −0.06 (−0.145 0.025) | 0.165 |
ref.: Level of reference. aCategories corresponding to scores of ‘10–18’ and ‘19–27’ have been collapsed due to <20 observations in one category. bCategories corresponding to scores of ‘3–4’ and ‘5–6’ have been collapsed due to <20 observations in one category. All models with log(CSF Aβ42) as an outcome are adjusted by age, sex, site of data collection, APOE-ɛ4 carriership, anxiety (State-Trait Anxiety Inventory) and log(CSF p-tau) levels. All models with log(p-tau) and log(CSF t-tau) levels as outcomes are adjusted by age, sex, site of data collection, APOE-ɛ4 carriership, body mass index, physical activity and log(CSF Aβ42) levels.
Figure 2.
Main effect of PSQI binary sleep category on CSF t-tau levels. On the X-axis are represented participants’ groups categorized as normal sleep group (PSQI ≤ 5) or poor sleep group (PSQI >5). On the Y-axis are represented the residuals of log-transformed t-tau levels, after regressing out the effect of age, sex, site of data collection, APOE-ɛ4 carriership, body mass index, physical activity and CSF Aβ42 levels. Presented P-values are derived from multivariate linear regression analyses.
Figure 3.
Main effects of sleep duration on CSF p-tau and t-tau levels. On the X-axis are represented participants’ groups categorised based on sleep duration >7 h, 6–7 h, 5–6 h and < 5 h of sleep. On the Y-axis are represented the residuals of log-transformed CSF p-tau (A) and t-tau (B) levels, after regressing out the effect of age, sex, site of data collection, APOE-ɛ4 carriership, body mass index, physical activity, and CSF Aβ42 levels. Presented p-values are derived from multivariate linear regression analyses.
Figure 4.
Main effect of sleep disturbance on CSF Aβ42 levels. On the X-axis are represented participants with sleep disturbance scores (PSQI component 5) of 0, 1–9 or >9. On the Y-axis are represented the residuals of log-transformed CSF Aβ42 levels, after regressing out the effect of age, sex, site, APOE-ε4 carriership, anxiety (State-Trait Anxiety Inventory), and CSF p-tau levels. Presented p-values are derived from multivariate linear regression analyses.
Table 4.
Effect of baseline PSQI measures on longitudinal change in CSF biomarkers
| Variables | log(CSF Aβ42) | log(CSF p-tau) | log(CSF t-tau) | |||
|---|---|---|---|---|---|---|
| β Coefficient (95% CI) | P-value | β Coefficient (95% CI) | P-value | β Coefficient (95% CI) | P-value | |
| Time × total PSQI score | 0.000 (−0.00002 0.000004) | 0.227 | 0.000 (−0.00001 0.00001) | 0.703 | 0.000 (−0.00001 0.00001) | 0.957 |
| Time × dichotomized PSQI score (ref. Total PSQI ≤5) | 0.000 (−0.0001 0.00003) | 0.295 | 0.000 (−0.00004 0.0001) | 0.534 | 0.000 (−0.00005 0.0001) | 0.868 |
| Time × sleep latency (ref. ≤ 15 min)a | ||||||
| 16–30 min | 0.000 (−0.0001 0.00005) | 0.471 | 0.000 (−0.00005 0.00007) | 0.749 | 0.000 (−0.0001 0.00003) | 0.291 |
| >30 min | 0.000 (−0.0001 0.0001) | 0.727 | 0.000 (−0.00007 0.0001) | 0.699 | 0.000 (−0.0001 0.00005) | 0.473 |
| Time × sleep duration (ref. > 7 h)b | ||||||
| 6–7 h | −0.0001 (−0.0001 0.00001) | 0.09 | 0.000 (−0.0001 0.00003) | 0.358 | 0.000 (−0.0001 0.0001) | 0.838 |
| <6 h | 0.000 (−0.0001 0.0001) | 0.57 | 0.000 (−0.0001 0.0001) | 0.868 | 0.000 (−0.0001 0.0001) | 0.902 |
| Time × sleep efficiency (ref. > 85%) | ||||||
| 75–84% | 0.000 (−0.0001 0.00003) | 0.269 | 0.000 (−0.00004 0.0001) | 0.435 | 0.000 (−0.0001 0.0001) | 0.76 |
| 65–74% | 0.000 (−0.0001 0.0001) | 0.529 | 0.000 (−0.0001 0.0001) | 0.828 | 0.000 (−0.0001 0.0001) | 0.988 |
| <65% | 0.000 (−0.0001 0.0001) | 0.687 | 0.000 (−0.0001 0.0001) | 0.48 | 0.000 (−0.0001 0.0001) | 0.808 |
| Time × sleep disturbance (ref. ≤ 9)c | ||||||
| 1–9 | −0.0002 (−0.0004−0.0001) | 0.006 | 0.000 (−0.0001 0.0001) | 0.771 | 0.000 (−0.0001 0.0001) | 0.712 |
| >9 | −0.0002 (−0.0004−0.0001) | 0.005 | 0.000 (−0.0001 0.0002) | 0.527 | 0.000 (−0.0001 0.0002) | 0.742 |
| Time × daytime dysfunction (ref. 0)d | ||||||
| ≥1 | −0.0001 (−0.00012 0.00001) | 0.125 | 0.000 (−0.00005 0.0001) | 0.888 | 0.000 (−0.0001 0.00005) | 0.959 |
ref.: Level of reference. aCategories corresponding to ‘31–60 min’ and ‘>60 min’ have been collapsed due to <20 observations in one category. bCategories corresponding to ‘5–6 h’ and ‘<5 h’ have been collapsed due to <20 observations in two categories. cCategories corresponding to scores ≤ 9 and > 9 have been collapsed due to <20 observations in two categories. dCategories corresponding to scores of ‘1–2’, ‘3–4’ and ‘5–6’ have been collapsed due to <20 observations in two categories. All models are adjusted by age, sex, APOE-ɛ4 carriership (and their interactions with time) and site of data collection (fixed effects). Additionally, models with log(CSF Aβ42) as outcome are adjusted by log(CSF p-tau), and models with log(CSF t-tau) or log(CSF p-tau) as outcomes, are adjusted by log(CSF Aβ42). A random intercept for each CSF biomarker and change over time (slope) are included as random effects.
Results with dichotomised CSF biomarkers’ levels as outcomes, closely resemble those of cross-sectional using continuous measures of CSF biomarkers. Specifically, shorter sleep duration (6–7 h of sleep as compared with >7 h) was associated with an increased odds ratio of abnormal CSF p-tau (OR = 1.948, CI [1.226, 3.097], P = 0.005) and CSF t-tau levels (OR = 1.839, CI [1.169, 2.894], P = 0.008) (Supplementary Table 2). A higher frequency of sleep disturbance (1–9 versus 0 and >9 versus 0) was associated with increased odds ratio of abnormal CSF Aβ42 (OR = 1.821, CI [1.031, 3.217], P = 0.039; and OR = 2.142, CI [1.111, 4.130], P = 0.023) respectively (Supplementary Table 2). No significant associations between the remaining PSQI components or total score and dichotomised CSF Alzheimer’s disease biomarkers were found (Supplementary Table 2).
Results from the analyses stratified by amyloid status revealed that in the amyloid negative group (N = 839), poor sleep quality (PSQI total > 5) was significantly associated with higher CSF p-tau (β = 0.042, P = 0.029) and higher CSF t-tau [(β = 0.037, P = 0.031) (Supplementary Table 3)]. Participants who reported sleep latency between 16–30 min as compared to <15 min, demonstrated lower CSF Aβ42 levels (β = −0.049, P = 0.015). Shorter reported sleep duration of 6–7 h as compared with >7 h was significantly associated with lower CSF Aβ42 levels (β = −0.041, P = 0.046) and higher CSF p-tau levels (β = 0.048, P = 0.025). Increased daytime dysfunction of (1–2 versus none), was also associated with lower CSF Aβ42 (β = −0.051, P = 0.008), higher CSF P-tau (β =0.046, P = 0.019) and t-tau (β = 0.037, P = 0.034) (Supplementary Table 3). In contrast, in the amyloid positive group (N = 329), no significant associations between PSQI components or total score and CSF Alzheimer’s disease biomarkers were found (Supplementary Table 3).
Longitudinal analyses
In all LMM analyses, time was a significant main effect, reflecting that CSF sampling interval was sufficient to capture changes in CSF biomarker levels. There was a significant interaction between sleep disturbances and time, with greater sleep disturbances at baseline (1–9 versus 0 and >9 versus 0) being associated with a decrease in CSF Aβ42 over time (β = −0.0002, P = 0.006; β=−0.002, P = 0.005). There were no other significant interactions between other sleep measures and time for CSF t-tau or p-tau levels (Table 4).
Discussion
This study shows that, in cognitively unimpaired adults, self-reported indicators of poor sleep quality are associated with CSF signatures of Alzheimer’s disease (namely, decreased CSF Aβ42 and increased CSF t-tau and p-tau levels) at baseline. Longitudinally, increased sleep disturbance at baseline predicted a steeper decrease in CSF Aβ42, after an average follow-up of 1.5 years. Understanding longitudinal predictors of the Alzheimer’s disease CSF signature provides potential biomarkers for progression and specific targets for intervention.
Self-reported sleep disturbances are associated with both lower baseline CSF Aβ42 and decreasing CSF Aβ42 over time
The sleep disturbance component of the PSQI was most robustly related to CSF Aβ42, both at cross-sectional and longitudinal levels. This component incorporates a range of factors united in their tendency to interrupt sleep, including snoring, nocturia and uncomfortable breathing. There area range of possible explanations for this finding. Firstly, those reporting increased sleep disturbances may reflect a cohort within the study more likely to have sleep-disordered breathing, itself associated with a pathological beta-amyloid profile.38,50 Alternatively it is possible that sleep interruptions may impede initiation and duration of slow-wave sleep51 distorting sleep-dependent amyloid production/clearance mechanisms,52–56 with such abnormalities contributing to or even driving this CSF profile.
Exploring the extent of sleep disturbance may hold future promise clinically as a marker of abnormal CSF Aβ42 given that the risk for this profile was approximately two-fold in participants reporting any sleep disturbances overnight. Given the multiple underlying causes for sleep disturbances, future work identifying exact underlying aetiologies most connected with this profile would help to shed further light on the mechanism.
When stratified by amyloid status, further self-reported sleep metrics were associated with a baseline reduced CSF Aβ42 but only within the CSF Aβ42 negative group. These include a mid-level increase in sleep latency (15–30mins), a mid-level reduction in sleep duration (6–7 hrs) and the presence of a mid-level degree of daytime dysfunction score (1–2). This finding points tentatively towards these sleep abnormalities as causative factors or that different sleep abnormalities may influence the CSF profile at different stages of the disease continuum. However, more severe scores within these categories were statistically non-significant, showing smaller or even reversed effect sizes. Although this could reflect reduced power to detect change within these categories populated by lower numbers of participants, overall we suggest these specific results be interpreted with caution.
Self-reported measures of sleep abnormality are associated with higher baseline CSF t-tau and p-tau
Impaired sleep quality as defined by Total PSQI > 5 was associated with increased t-tau at baseline. Shorter sleep duration was also related to higher levels of CSF p-tau and t-tau biomarkers, demonstrating that, on average, even 1 hour of sleep loss is related to the accumulation of pathological tau proteins. Although this statistically significant association was observed in only one of the sleep duration groups (6–7 h), it remained in an analysis using dichotomic sleep duration (> 7 h versus < 7 h). While these associations were not present for other more severe categories, this could be explained by loss of power in the context of a smaller group membership.
This is in line with recent evidence showing that short sleep duration is associated with increased dementia risk.57 Like Aβ, ISF levels of tau also fluctuate diurnally,33 with studies supporting the hypothesis that this is driven by increased neuronal activity during wakefulness versus sleep.58,59 Lower sleep efficiency has been associated with higher CSF levels of tau in cognitively unimpaired adults.25 Additionally, evidence has shown a faster rate of tau increase to be present in patients with OSA as compared to controls.38 We hypothesise that those participants in our study with shorter sleep duration would be expected to have commensurate reduced time within a low neuronal/synaptic activity state, leading to a detectable increased tau CSF level. Indeed, shorter sleep duration (6–7 hrs) was associated with an approximately two-fold risk of abnormal CSF p-tau and t-tau, raising the possibility that this could be a marker of clinical interest.
Self-reported measures of sleep abnormality are not associated with longitudinal change in CSF t-tau or p-tau
In contrast, no significant associations were found involving baseline sleep abnormalities and longitudinal change in CSF tau levels. Whilst the absence of a relationship is possible, there are several alternative explanations. Firstly, whilst the study time frame may be sufficient to capture longitudinal change in CSF Aβ, it may be of inadequate length for CSF tau, as tau changes may be more prominent in the later stages of the disease continuum, especially, since this cohort is comprised of cognitively unimpaired individuals at the inception of the pathological events’ cascade.60 Secondly, CSF tau has been shown to follow a non-linear pattern during the preclinical phase of Alzheimer’s disease and this could mask potential longitudinal associations with sleep quality.61 Thirdly, bidirectional causality between tau pathology and sleep abnormalities may be implicated. For example, cerebral tau deposition has been associated with increased total sleep time observed in cognitively unimpaired adults and patients with Alzheimer’s disease.62,63 Hence, whilst initial shorter total sleep duration may be cross-sectionally associated with higher CSF tau, its cerebral deposition could contribute to the opposite clinical effect, nullifying longitudinal relationships.
Other research findings
The largest previous cross-sectional study, in a cohort of 736 cognitively unimpaired individuals, revealed associations of reduced Aβ42 and increased ratio of t-tau/Aβ42 and p-tau/Aβ42 ratio with both reduced and excessive total sleep time, daytime dysfunction and a later bedtime, but only in female or APOE-ε4 carrying participants.29 In agreement, we also found associations between shorter total sleep time and higher CSF p-tau, but not lower CSF Aβ42; findings which extended to our whole population. Decreased sleep efficiency and increased wake time after sleep onset, as measured by actigraph,27 have been associated with low CSF Aβ42, and future amyloid deposition has been associated with decreased sleep efficiency, as measured by polysomnography.64 Whilst no statistically significant relationship in terms of self-reported sleep efficiency was found here, it is reasonable to suppose that increased sleep disturbances overnight will adversely impact on overall sleep efficiency and as such these findings share similarities.
In summary, our strongest findings were in cross-sectional and longitudinal associations with sleep disturbance, which is in line with another study demonstrating the relationship between ‘Sleep problems’ according to the Medical Outcomes Study Sleep Scale (MOSSS) in cognitively unimpaired individuals and low CSF Aβ42 and raised p-tau/t-tau.28 This finding in a large cohort, suggests that sleep disturbances, alongside representing a candidate biomarker plausibly able to predict future amyloid accumulation easily and non-invasively, could offer a target for intervention.
Strengths and limitations
This study has several strengths. Firstly, to our knowledge, it utilises the largest cross-sectional and longitudinal population to date to investigate the relationship between sleep and Alzheimer’s disease biomarkers, with study procedures coordinated and harmonised across multiple sites. Secondly, it is amongst a limited group of studies focussing on the preclinical stage of the disease using the CSF biomarkers to capture the underlying pathology.
Nonetheless, this study is not without weaknesses. The use of sleep monitoring devices (e.g. actigraphy or polysomnography), as opposed to self-reported questionnaires, would have enhanced objectivity and allowed for more sensitive detection of sleep abnormalities. Moreover, the categorical nature of the PSQI dataset available hinders hypotheses testing of potential non-linear associations between sleep quality and Alzheimer’s disease pathological indicators. For example, within this dataset, total sleep time was unavailable as a continuous variable precluding assessment of the effects of excessive sleep (sleep duration > 7 h is the longest category). Nevertheless, PSQI is a validated tool, widely used and relationships between self-reported measures and early Alzheimer’s disease change are of substantial clinical interest.
Other limitations relating to CSF sampling and biomarkers include the lack of CSF Aβ40, which prevented the use of the more sensitive Aβ42/40 ratio as a biomarker for Alzheimer’s disease pathology65 and the fact that, even though CSF was collected before noon, specific times were not provided preventing adjustment to approximate true peptide concentrations. This may be relevant, since CSF metabolism highly depends on circadian rhythm, with well demonstrated cyclic patterns of amyloid levels.66,67 Additionally, slight differences between the composition of cross-sectional and longitudinal samples were found. Specifically, CSF Aβ within the follow-up cohort was lower than in the group providing only baseline data. This may have been due to corresponding differences in age and APOE-ε4 status which we do not believe adversely impacts on analysis or results interpretation.
We also must acknowledge the drawbacks associated with the external validity of our study. The EPAD study population is comparatively highly educated and this may influence CDR score and speed of diagnosis compared to the general population. This, in combination with selection bias universally common to cohort studies of this type, may compromise real-world applicability. However, overall PSQI score and the proportion of poor sleepers (PSQI) across the included population were in keeping with large community samples.68,69 In a similar vein, individuals concurrently utilizing sleep medications were included in the analysis. The sub-cohort taking sleep medications may well be the most significantly affected by sleep disturbance and as such, their exclusion was not felt to be appropriate. Models were adjusted to account for sleep medication use to minimize the potential confounding effect.
Finally, statistically, no correction for multiple comparisons was made and findings should be interpreted accordingly. However, the hypotheses and the primary data analytical approach were clearly determined prior to analysis and in this context correction increases the risk of Type 2 Error.
Conclusion
This study demonstrates that self-reported sleep quality is associated with Alzheimer’s disease biomarkers in a cognitively unimpaired population. Baseline self-reported indicators of poor sleep quality were associated with lower Aβ42 and higher p-tau and t-tau CSF levels, and predicted CSF Aβ42 reduction over time.
Together, whilst warranting further investigation, these results support sleep impairment prior to cognitive symptom onset in Alzheimer’s disease and underline the importance of investigating the links between sleep and Alzheimer’s disease pathology. Effective treatments for sleep disorders and interventions for sleep quality exist and their early implementation may therefore potentially mitigate the progression of cognitive decline.
Supplementary Material
Acknowledgements
Data used in preparation of this article were obtained from the Longitudinal Cohort Study (LCS), delivered by the European Prevention of Alzheimer’s Disease (EPAD) Consortium. As such investigators within the EPAD-LCS and EPAD Consortium contributed to the design and implementation of EPAD and/or provided data but did not participate in analysis or writing of this report. A complete list of EPAD Investigators can be found at: http://ep-ad.org/wp-content/uploads/2020/12/202010_List-of-epadistas.pdf
The EPAD-LCS was launched in 2015 as a public–private partnership, led by Chief Investigator Professor Craig Ritchie MB BS. This work used data and/or samples from the EPAD project which received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking EPAD grant agreement n° 115736 and an Alzheimer’s Association Grant (SG21-818099-EPAD).
Abbreviations
- Aβ =
amyloid-β
- BMI =
body mass index
- EPAD-LCS =
The European Prevention of Alzheimer’s Dementia Longitudinal Cohort Study
- GDS =
Geriatric Depression Scale
- ISF =
interstitial fluid
- MCI =
mild cognitive impairment
- MMSE =
Mini-Mental State Examination
- OSA =
obstructive sleep apnoea
- p-tau =
phosphorylated tau
- PSQI =
Pittsburgh Sleep Quality Index
- t-tau =
total tau
- STAI =
State-Trait Anxiety Inventory
Contributor Information
Jonathan Blackman, North Bristol NHS Trust, Bristol BS10 5NB, UK; Bristol Medical School, University of Bristol, Bristol BS8 1UD, UK.
Laura Stankeviciute, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; Universitat Pompeu Fabra, Barcelona 08005, Spain.
Eider M Arenaza-Urquijo, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona 08003, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Madrid 28029, Spain.
Marc Suárez-Calvet, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona 08003, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Madrid 28029, Spain; Servei de Neurologia, Hospital del Mar, Barcelona 08003, Spain.
Gonzalo Sánchez-Benavides, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona 08003, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Madrid 28029, Spain.
Natalia Vilor-Tejedor, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; Universitat Pompeu Fabra, Barcelona 08005, Spain; Centre for Genomic Regulation (CRG), The Barcelona Institute for Science and Technology, Barcelona 08003, Spain; Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam 3015 GD, The Netherlands.
Alejandro Iranzo, Neurology Service, Hospital Clínic de Barcelona and Institut D'Investigacions Biomèdiques, Barcelona 08036, Spain; Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Hospital Clínic de Barcelona, Barcelona 28029, Spain.
José Luis Molinuevo, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain.
Juan Domingo Gispert, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona 08003, Spain; Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Madrid 28029, Spain.
Elizabeth Coulthard, North Bristol NHS Trust, Bristol BS10 5NB, UK; Bristol Medical School, University of Bristol, Bristol BS8 1UD, UK.
Oriol Grau-Rivera, Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona 08005, Spain; IMIM (Hospital del Mar Medical Research Institute), Barcelona 08003, Spain; Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBERFES), Madrid 28029, Spain; Servei de Neurologia, Hospital del Mar, Barcelona 08003, Spain.
Funding
O.G.-R. receives funding from the Alzheimer’s Association Research Fellowship Program (2019-AARF-644568). J.D.G. is supported by the Spanish Ministry of Science and Innovation (RYC-2013-13054). M.S.-C. receives funding from Instituto de Salud Carlos III (PI19/00155) and from the Spanish Ministry of Science, Innovation and Universities (Juan de la Cierva programme grant IJC2018-037478-I). N.V.-T. is funded by a Spanish Ministry of Science, Innovation and Universities postdoctoral grant (Juan de la Cierva programmegrant FJC2018-038085-I). E.M.A.-U. holds a ‘Ramón y Cajal’ fellowship (RYC2018-026053-I) and a grant of the Ministry of Science and Innovation (PID2019-111514RA-I00). J.B. receives funding from Alzheimer’s Research UK (supported by the Margaret Jost Fellowship and the Don Thoburn Memorial Scholarship) and the David Telling Charitable Trust, and E.C. has received funding from BRACE and ARUK (Bristol & Bath Network).
Competing interests
J.L.M. has served/serves as a consultant or at advisory boards for the following for-profit companies or has given lectures in symposia sponsored by the following for-profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare, ProMIS Neurosciences, NovoNordisk, Zambón, Cytox and Nutricia. M.S.-C. has given lectures in symposia sponsored by ROCHE DIAGNOSTICS, S.L.U. GK and IS are full-time employees of Roche Diagnostics GmbH. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). The rest of the authors have no conflict of interest to declare.
Supplementary material
Supplementary material is available at Brain Communications online.
Data availability
The dataset used for the present study can be found in an online repository (http://epad.org/erap/).
References
- 1. Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Cur Opin Psychiatry. 2014;27(6):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vitiello M V, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol. 1990;45(4):M131–M138. [DOI] [PubMed] [Google Scholar]
- 3. Prinz PN, Vitaliano PP, Vitiello MV, et al. . Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3(4):361–370. [DOI] [PubMed] [Google Scholar]
- 4. Hoch CC, Reynolds CF, Kupfer DJ, Houck PR, Berman SR, Stack JA. Sleep-disordered breathing in Normal and pathologic aging. J Clin Psychiatry. 1986;47(10):499–503. [PubMed] [Google Scholar]
- 5. Mant A, Saunders NA, Eyland AE, Pond CD, Chancellor AH, Webster IW. Sleep-related respiratory disturbance and dementia in elderly females. J Gerontol. 1988;43(5):M140–M144. [DOI] [PubMed] [Google Scholar]
- 6. Grace JB, Walker MP, McKeith IG. A comparison of sleep profiles in patients with dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2000;15(11):1028–1033. [DOI] [PubMed] [Google Scholar]
- 7. Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders. A meta-analysis. Arch Gen Psychiatry. 1992;49(8):651–668; discussion 669-70. [DOI] [PubMed] [Google Scholar]
- 8. Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer’s disease: Further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep. 1995;18(3):145–148. [DOI] [PubMed] [Google Scholar]
- 9. Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S. Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging Clin Exp Res. 1998;10(4):308–315. [DOI] [PubMed] [Google Scholar]
- 10. Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer’s disease: A review. Int J Alzheimer’s Dis. 2010;2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hita-Yañez E, Atienza M, Cantero JL. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep. 2013;36(9):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol. 2013;70(12):1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osorio RS, Pirraglia E, Agüera-Ortiz LF, et al. . Greater risk of Alzheimer’s disease in older adults with insomnia. J Am Geriatr Soc. 2011;59(3):559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yaffe K, Laffan AM, Harrison SL, et al. . Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D’Rozario AL, Chapman JL, Phillips CL, et al. . Objective measurement of sleep in mild cognitive impairment: A systematic review and meta-analysis. Sleep Med Rev. 2020;52(101308):101308. [DOI] [PubMed] [Google Scholar]
- 17. Osorio RS, Gumb T, Pirraglia E, et al. . Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8):552–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg H. Amyloid biomarkers in Alzheimer’s disease. Trends Pharmacol Sci. 2015;36(5):297–309. [DOI] [PubMed] [Google Scholar]
- 20. Blennow K. A review of fluid biomarkers for Alzheimer’s disease: Moving from CSF to blood. Neurol Ther. 2017;6(S1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5(3):228–234. [DOI] [PubMed] [Google Scholar]
- 22. Lucey BP. It’s complicated: The relationship between sleep and Alzheimer’s disease in humans. Neurobiol Dis. 2020;144:105031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shokri-Kojori E, Wang GJ, Wiers CE, et al. . β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci U S A. 2018;115(17):4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang JE, Lim MM, Bateman RJ, et al. . Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ju YES, Ooms SJ, Sutphen C, et al. . Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140(8):2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lucey BP, Hicks TJ, McLeland JS, et al. . Effect of sleep on overnight cerebrospinal fluid amyloid β kinetics. Ann Neurol. 2018;83(1):197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ju YES, McLeland JS, Toedebusch CD, et al. . Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70(5):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sprecher KE, Koscik RL, Carlsson CM, et al. . Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively Normal adults. Neurology. 2017;89(5):445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu W, Tan L, Su B, et al. . Sleep characteristics and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology in cognitively intact older adults: The CABLE study. Alzheimer’s Dement. 2020;16(8):1146–1152. [DOI] [PubMed] [Google Scholar]
- 30. Chen DW, Wang J, Zhang LL, Wang YJ, Gao CY. Cerebrospinal fluid amyloid-β levels are increased in patients with insomnia. J Alzheimer’s Dis. 2017;61(2):645–651. [DOI] [PubMed] [Google Scholar]
- 31. Varga AW, Wohlleber ME, Giménez S, et al. . Reduced slow-wave sleep is associated with high cerebrospinal fluid aβ42 levels in cognitively Normal elderly. Sleep. 2016;39(11):2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Meco A, Joshi YB, Praticò D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol Aging. 2014;35(8):1813–1820. [DOI] [PubMed] [Google Scholar]
- 33. Holth JK, Fritschi SK, Wang C, et al. . The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. 2019;2546:eaav2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iliff JJ, Chen MJ, Plog BA, et al. . Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JAHR. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men. JAMA Neurol. 2014;71(8):971. [DOI] [PubMed] [Google Scholar]
- 36. Olsson M, Ärlig J, Hedner J, Blennow K, Zetterberg H. Sleep deprivation and cerebrospinal fluid biomarkers for Alzheimer’s disease. Sleep. 2018;41(5):1–8. [DOI] [PubMed] [Google Scholar]
- 37. Kent BA, Feldman HH, Nygaard HB. Sleep and its regulation: An emerging pathogenic and treatment frontier in Alzheimer’s disease. Prog Neurobiol. 2021;197:101902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bubu OM, Pirraglia E, Andrade AG, et al. . Obstructive sleep apnea and longitudinal Alzheimer’s disease biomarker changes. Sleep. 2019;42(6):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Most EIS, Aboudan S, Scheltens P, van Someren EJW. Discrepancy between subjective and objective sleep disturbances in early- and moderate-stage Alzheimer disease. Am J Geriatr Psychiatry. 2012;20(6):460–467. [DOI] [PubMed] [Google Scholar]
- 40. Ritchie CW, Molinuevo JL, Truyen L, Satlin A, Van der Geyten S, Lovestone S. Development of interventions for the secondary prevention of Alzheimer’s dementia: The European prevention of Alzheimer’s dementia (EPAD) project. Lancet Psychiatry. 2016;3(2):179–186. [DOI] [PubMed] [Google Scholar]
- 41. Solomon A, Kivipelto M, Molinuevo JL, Tom B, Ritchie CW. European Prevention of Alzheimer’s dementia longitudinal cohort study (EPAD LCS): Study protocol. BMJ Open. 2018;8(12):e021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 43. Teunissen CE, Tumani H, Engelborghs S, Mollenhauer B. Biobanking of CSF: International standardization to optimize biomarker development. Clin Biochem. 2014;47(4-5):288–292. [DOI] [PubMed] [Google Scholar]
- 44. Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 45. Morris JC. The clinical dementia rating (CDR). Neurology. 1993;43(11):2412.2–2412-a. [DOI] [PubMed] [Google Scholar]
- 46. Yesavage JA, Brink TL, Rose TL, et al. . Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatric Res. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 47. Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; 1970. [Google Scholar]
- 48. Lifke V, Kollmorgen G, Manuilova E, et al. . Elecsys® total-tau and phospho-tau (181P) CSF assays: Analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem. 2019;72:30–38. [DOI] [PubMed] [Google Scholar]
- 49. Ingala S, de Boer C, Masselink LA, et al. . Application of the ATN classification scheme in a population without dementia: Findings from the EPAD cohort. Alzheimer’s Dement. 2021;17(7):1189–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma RA, Varga AW, Bubu OM, et al. . Obstructive sleep apnea severity affects amyloid burden in cognitively Normal elderly: A longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184(1):71–78. [DOI] [PubMed] [Google Scholar]
- 52. Nedergaard M. Garbage truck of the brain. Science. 2013;340(6140):1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iliff JJ, Wang M, Liao Y, et al. . A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Iliff JJ, Lee H, Yu M, et al. . Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123(3):1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kastanenka KV, Hou SS, Shakerdge N, et al. . Optogenetic restoration of disrupted slow oscillations halts amyloid deposition and restores calcium homeostasis in an animal model of Alzheimer’s disease. PLoS One. 2017;12(1):e0170275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kastanenka KV, Calvo-Rodriguez M, Hou SS, et al. . Frequency-dependent exacerbation of Alzheimer’s disease neuropathophysiology. Sci Rep. 2019;9(1):8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sabia S, Fayosse A, Dumurgier J, et al. . Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamada K, Holth JK, Liao F, et al. . Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211(3):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pooler AM, Phillips EC, Lau DHW, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14(4):389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jack CR, Knopman DS, Jagust WJ, et al. . Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McDade E, Wang G, Gordon BA, et al. . Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91(14):e1295–e1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lucey BP, McCullough A, Landsness EC, et al. . Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med. 2019;11(474):eaau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liguori C, Romigi A, Nuccetelli M, et al. . Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71(12):1498–1505. [DOI] [PubMed] [Google Scholar]
- 64. Winer JR, Mander BA, Kumar S, et al. . Sleep disturbance forecasts β-amyloid accumulation across subsequent years. Curr Biol. 2020;30(21):4291–4298. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Janelidze S, Zetterberg H, Mattsson N, et al. . CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: Better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol. 2016;3(3):154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-ss levels: Implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68(9):666–669. [DOI] [PubMed] [Google Scholar]
- 67. Lucey BP, Fagan AM, Holtzman DM, Morris JC, Bateman RJ. Diurnal oscillation of CSF aβ and other AD biomarkers. Mol Neurodeg. 2017;12(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hinz A, Glaesmer H, Brähler E, et al. . Sleep quality in the general population: Psychometric properties of the Pittsburgh sleep quality index, derived from a German community sample of 9284 people. Sleep Med. 2017;30:57–63. [DOI] [PubMed] [Google Scholar]
- 69. Sun XH, Ma T, Yao S, et al. . Associations of sleep quality and sleep duration with frailty and pre-frailty in an elderly population rugao longevity and ageing study. BMC Geriatr. 2020;20(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used for the present study can be found in an online repository (http://epad.org/erap/).