Abstract
Objective
To investigate whether oral antimicrobial prophylaxis as an adjunct to intravenous antibiotic prophylaxis reduces surgical site infections after elective colorectal surgery.
Design
Multicentre, randomised, double blind, placebo controlled trial.
Setting
11 university and non-university hospitals in France between 25 May 2016 and 8 August 2019.
Participants
926 adults scheduled for elective colorectal surgery.
Intervention
Patients were randomised to receive either a single 1 g dose of ornidazole (n=463) or placebo (n=463) orally 12 hours before surgery, in addition to intravenous antimicrobial prophylaxis before surgical incision.
Main outcome measures
The primary outcome was the proportion of patients with surgical site infection within 30 days after surgery. Secondary outcomes included individual types of surgical site infections and major postoperative complications (Clavien-Dindo classification grade 3 or higher) within 30 days after surgery.
Results
Of the 960 patients who were enrolled, 926 (96%) were included in the analysis. The mean age of participants was 63 years and 554 (60%) were men. Surgical site infection within 30 days after surgery occurred in 60 of 463 patients (13%) in the oral prophylaxis group and 100 of 463 (22%) in the placebo group (absolute difference −8.6%, 95% confidence interval −13.5% to −3.8%; relative risk 0.60, 95% confidence interval 0.45 to 0.80). The proportion of patients with deep infections was 4.8% in the oral prophylaxis group and 8.0% in the placebo group (absolute difference −3.2%, 95% confidence interval −6.4% to −0.1%). The proportion of patients with organ space infections was 5.0% in the oral prophylaxis group and 8.4% in the placebo group (absolute difference −3.4%, −6.7% to −0.2%). Major postoperative complications occurred in 9.1% patients in the oral prophylaxis group and 13.6% in the placebo group (absolute difference −4.5%, −8.6% to −0.5%).
Conclusion
Among adults undergoing elective colorectal surgery, the addition of a single 1 g dose of ornidazole compared with placebo before surgery significantly reduced surgical site infections.
Trial registration
ClinicalTrials.gov NCT02618720.
Introduction
Surgical site infection is among the most common healthcare related infections1 and is associated with increases in morbidity, readmission rates, mortality, and attributable healthcare costs.2 3 4 Patients who undergo colorectal surgery are particularly at risk of surgical site infection, with reported incidence rates of up to 26%.5 6
Intravenous antimicrobial prophylaxis against both aerobic and anaerobic bacteria is recommended to prevent surgical site infection after colorectal surgery.7 8 9 Several large retrospective database studies10 11 12 and meta-analyses13 14 reported that oral antimicrobial prophylaxis as an adjunct to intravenous antibiotic prophylaxis may help to further reduce surgical site infections after elective colorectal surgery. The results, however, have been controversial because many of these studies used oral antibiotics in addition to mechanical bowel preparation, making it unclear if the reduced infection was related to the oral antibiotics or the additional use of bowel preparation. Moreover, absence of benefit of mechanical bowel preparation compared with no bowel preparation, and concerns about possible harmful effects and discomfort for patients, led to recommendations against the routine use of bowel preparation for colonic surgery.15 16 Furthermore, one randomised trial of 417 patients who had colonic surgery identified no benefit from combined oral antibiotic prophylaxis and mechanical bowel preparation over no bowel preparation.17
Whether oral antimicrobial prophylaxis effectively prevents surgical site infection after colorectal surgery remains unclear, with differences in recommendations between organisations.7 8 A recently published network meta-analysis of randomised trials showed that the addition of oral antimicrobial prophylaxis to intravenous antibiotics was the best option to prevent surgical site infections after elective colorectal surgery, resulting in a reduction by more than 50%; suggesting that this issue is not definitively resolved.18 However, the supporting evidence is limited by the number of studies and patients included. Because evidence of a clear clinical benefit is lacking, we conducted the Comparison of Intravenous versus Combined Oral and Intravenous Antimicrobial Prophylaxis (COMBINE) trial to assess the effectiveness of oral antimicrobial prophylaxis as an adjunct to intravenous antibiotic prophylaxis to reduce surgical site infections after colorectal surgery compared with placebo.
Methods
Trial design and setting
We conducted a pragmatic, multicentre, randomised, double blind, placebo controlled trial in 11 academic teaching and non-academic hospitals in France from May 2016 to August 2019. The study protocol and statistical analysis plan were published before the end of enrolment.19 Written informed consent was obtained from all eligible patients before inclusion in the study. An independent data and safety monitoring board oversaw the conduct of the study and reviewed the interim analysis results so that the trial investigators were blinded. This study followed the consolidated standards of reporting trials (CONSORT) guideline.
Adults who were scheduled for elective laparoscopic or open colorectal surgery were eligible for participation. Patients were excluded if they were to undergo a concomitant surgical procedure (eg, hepatic resection for liver metastasis), had active bacterial infection at the time of surgery or had received antimicrobial treatment within two weeks before surgery, had inflammatory bowel disease, had a body mass index of 35 or higher, had known allergy to β lactams or imidazole antibiotics, had chronic kidney disease (glomerular filtration rate <30 mL/min/1.73 m2), had a known allergy or intolerance to lactose or galactose, and were pregnant or breastfeeding. The supplementary file provides the complete list of exclusion criteria.
Randomisation and trial procedures
Eligible patients were randomly assigned (1:1) to receive a preoperative oral dose of either ornidazole—a nitroimidazole with activity against anaerobic bacteria, or placebo. Randomisation was performed centrally through a dedicated, password protected web based system. Treatment assignments were stratified with the use of a minimisation algorithm according to trial site, surgical technique (laparoscopic or open surgery), and skin antisepsis (chlorhexidine-alcohol or povidone-iodine alcoholic solution). Study pharmacists prepared the ornidazole and placebo in identical opaque blister packs. All other staff, including investigators and research staff, clinical staff, surgeons, and patients were unaware of the trial group assignments.
Patients received a single 1 g dose of ornidazole or placebo orally 12 hours before surgery. A nurse controlled the receipt of the trial drug. All patients received the same intravenous antimicrobial prophylaxis, using a second generation cephalosporin with anaerobic activity (cefoxitin 2 g) as recommended,20 21 administered to all patients at least 30 minutes before skin incision, and readministered intraoperatively if the procedure lasted two hours or more. On 21 June 2018, after 629 patients had been enrolled, an update to French national clinical practice guidelines on antibiotic prophylaxis, external to the trial, substantially modified previous recommendations suggesting the addition of a single intravenous dose of 1g metronidazole to cefoxitin.22 The data and safety monitoring board, the members of which were unaware of treatment assignments and outcome data, did not recommend stopping the trial or changing its conduct.
Site staff were instructed not to prepare the bowel before colonic surgery; however, given the pragmatic nature of the protocol, the operating surgeon ultimately made the decision about whether or not to perform bowel preparation. For rectal surgery, bowel preparation and retrograde rectal enema were recommended the day before surgery,23 and done according to the expertise of the staff at each study site and to routine clinical practice. All patients received skin preparation with an alcohol based antiseptic agent before surgical incision. All centres followed the enhanced recovery after surgery protocol.24 Laparoscopic and open surgery were allowed; the technique was not standardised. Additional patient care followed local protocols and established guidelines.
Outcome measures
The primary outcome was the proportion of patients with any surgical site infection within 30 days after surgery, as defined by the US Centers for Disease Control and Prevention criteria,25 and classified as superficial incisional, deep incisional, or organ space infection. The operating surgeons assessed surgical site infection during the hospital stay and at each follow-up visit. For patients discharged before day 30, trial staff preformed weekly assessments during structured telephone interviews and arranged for in-person clinical evaluations if infection was suspected (see supplementary material for further details). Whenever possible, clinically relevant microbiological samples were cultured.
The secondary outcomes included the proportion of patients with individual types of surgical site infection; the proportion of patients with postoperative complications, defined as Clavien-Dindo classification26; the proportion of patients with postoperative systemic inflammatory response syndrome or sepsis or septic shock within 30 days; postoperative cardiovascular complications within 30 days; postoperative respiratory complications within 30 days; postoperative acute kidney injury within 30 days, defined according to the Kidney Disease: Improving Global Outcomes criteria; postoperative cardiovascular complications (arrhythmia, myocardial infarction, and acute cardiac failure) within 30 days; postoperative anastomotic leakage, reoperation, and surgical or endoscopic drainage within 30 days; the time to initiation of adjuvant chemotherapy; the need for unplanned hospital readmissions; unexpected admission to the intensive care unit; duration of hospital stay; hospital-free days to day 30; and all cause mortality within 30 and 90 days after surgery (see supplementary material for definitions of end points). Clinicians and research staff, who were unaware of the trial group assignments, obtained the data for outcome measures.
Statistical analysis
Assuming a 15% rate of surgical site infections with placebo,5 6 13 we estimated that enrolling 920 patients would provide 80% power to detect a 40% relative between group difference in the incidence of the primary outcome (ie, 15% in the placebo group and 9% in the oral ornidazole group),13 with a 5% two sided type I error. We inflated the sample size to 960 patients to account for a 5% loss to follow-up. As prespecified in the study protocol, one interim analysis was planned after the enrolment of the first 460 patients. The data and safety monitoring board did not recommend stopping the trial, and 960 patients were therefore included.
The planned approach to statistical analysis is published elsewhere.19 We analysed data in the modified intention-to-treat population, which was prespecified as all randomised patients who received a trial drug plus intravenous antimicrobial prophylaxis, with the exception of those who withdrew consent. We also analysed one per protocol population, which included patients from the modified intention-to-treat population except those with one or more major protocol violations.
An unadjusted χ2 test was used to compare the primary outcome between the two groups. Other binary outcomes were tested using an unadjusted χ2 test or Fisher’s exact test as appropriate. Results are reported as absolute differences and relative risks with 95% confidence intervals. Multivariable logistic mixed regression was used to identify prespecified covariates with a known association with the primary outcome (selected if the P value was <0.10 in the bivariable analysis) in addition to the stratification variables. We assessed multicollinearity between variables by computing the variance inflation factor and using the Farrar-Glauber test. The Akaike information criterion and bayesian information criterion were calculated and used as model diagnostics to determine how well the model fit improved after the addition of covariates. Adjusted analyses were performed with the use of robust random effect Poisson generalised linear mixed model regression with robust variance for binary outcomes,27 multinomial logistic mixed model for categorical outcomes, and linear mixed regression for continuous outcomes, with study site as a random effect. Time to event was compared between the two groups using the Kaplan-Meier method. A marginal Cox proportional hazards model was used to estimate hazard ratios and corresponding 95% confidence intervals. The proportional hazard hypothesis was evaluated using the Schoenfeld test and plotting residuals.
We conducted two prespecified subgroup analyses of the primary outcome in subgroups with mechanical bowel preparation versus without and with colonic surgery versus rectal surgery. Interaction terms in the random effect regression model were used to test for heterogeneity of effect between subgroups.
A post hoc analysis was conducted to test for a difference in treatment effect during the conduct of the trial in relation to publication of the update to French guidelines (before versus after publication update). We also conducted a post hoc analysis to investigate a potential treatment effect resulting from non-compliance with bowel preparation. No correction for multiple testing was applied in the analyses of secondary outcomes or subgroups. Complete case analysis was performed for all outcomes. We did not compensate for dropouts. A two sided P value of <0.05 was considered to indicate statistical significance. All analyses were generated with the use of Stata software, version 15.0 (StataCorp).
Patient and public involvement
Except for providing written informed consent before participation, patients and members of the public were not involved in the design or conduct of this study, because it was not customary for them to be involved in the design of scientific studies when the study was started. Patients will be consulted to assist with the dissemination of the study findings.
Results
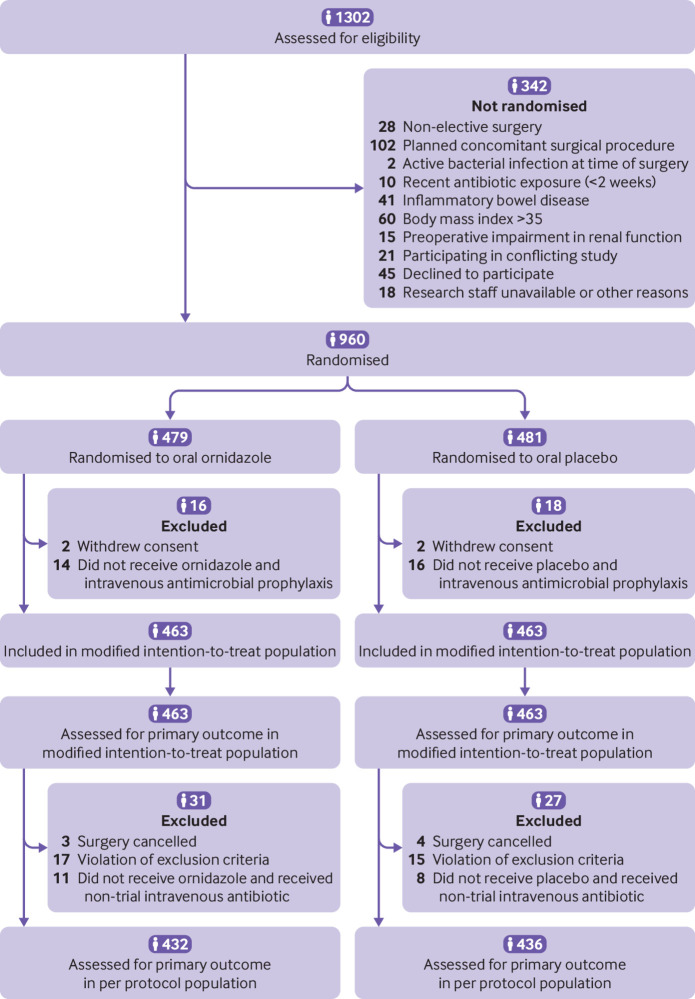
From 25 May 2016 to 8 August 2019, 960 patients provided informed consent and were enrolled in the trial: 479 were randomly assigned to oral prophylaxis and 481 to placebo. After withdrawals (16 patients in the oral prophylaxis group and 18 in the placebo group), 926 patients (463 assigned to oral prophylaxis and 463 assigned to placebo) were included in the analysis (fig 1). Baseline characteristics of both groups, with the exception of men and coronary artery disease both of which were more frequent in the placebo group, and other aspects of perioperative management were comparable (table 1 and table 2, also see supplementary table S1). In the overall population, 64% of patients underwent colon resection and 36% underwent rectal resection; 74% of the surgical procedures were performed laparoscopically. Among the 597 patients (301 in the oral prophylaxis group and 296 in the placebo group) who underwent colon surgery, 103 (17.3%) received bowel preparation (53 patients in the oral prophylaxis group and 50 patients in the placebo group). The mean time from oral prophylaxis or placebo to skin incision was 13 hours and from intravenous antimicrobial prophylaxis to skin incision was 37 minutes, without significant differences between the groups.
Fig 1.
Flow of participants through study. The per protocol population included patients from the modified intention-to-treat population, except those with one or more major protocol violations
Table 1.
Baseline characteristics of participants in modified intention-to-treat population. Values are numbers (percentages) unless stated otherwise
| Characteristics | Oral prophylaxis group (n=463) | Placebo group (n=463) |
|---|---|---|
| Mean (SD) age (years) | 63 (13) | 63 (13) |
| Men | 262 (57) | 292 (63) |
| Mean (SD) weight (kg) | 74 (15) | 75 (15) |
| Mean (SD) body mass index | 26 (4) | 26 (4) |
| ASA physical status classification*: | ||
| 1 | 101/462 (22) | 101/461 (22) |
| 2 | 272/462 (59) | 260/461 (56) |
| 3 | 86/462 (18) | 94/461 (20) |
| 4 | 3/462 (1) | 6/461 (2) |
| Medical history: | ||
| Hypertension | 199 (43) | 198 (43) |
| Diabetes mellitus | 69 (15) | 71 (15) |
| Coronary artery disease | 32 (7) | 56 (12) |
| Chronic pulmonary disease | 22 (5) | 33 (7) |
| Current smoker | 71 (15) | 78 (17) |
| Any alcohol intake | 34 (7) | 39 (8) |
| Disease related malnutrition | 31 (7) | 20 (4) |
| History of multidrug resistant bacteria | 2 (1) | 3 (1) |
| Antibiotic use within 3 months before surgery | 53 (11) | 56 (12) |
| Adjuvant chemotherapy or radiotherapy | 113 (24) | 110 (24) |
| Drugs used at time of surgery: | ||
| Corticosteroid | 11 (2) | 15 (3) |
| Non-steroidal anti-inflammatory drug | 5 (1) | 13 (3) |
| Indication for surgery: | ||
| Colorectal cancer | 351 (76) | 356 (77) |
| Diverticulitis | 78 (17) | 70 (15) |
| Other condition | 34 (7) | 37 (8) |
| Skin preparation: | ||
| Chlorhexidine-alcohol | 43 (9) | 48 (10) |
| Povidone-iodine alcohol | 420 (91) | 415 (90) |
| Mechanical bowel preparation: | 153 (33) | 160 (35) |
| Polyethylene glycol | 64/151 (42) | 57/157 (36) |
| Senna solution | 87/151 (58) | 100/157 (64) |
| Retrograde enema | 199 (43) | 210 (45) |
ASA=American Society of Anesthesiology.
Only male sex (P=0.04) and coronary artery disease (P=0.007) differed significantly.
Percentages may not total 100 because of rounding.
Data on the ASA physical status class were missing for two participants in the placebo group and one participant in the oral prophylaxis (ornidazole) group.
Table 2.
Surgical and other perioperative characteristics of participants in modified intention-to-treat population. Values are numbers (percentages) unless stated otherwise
| Characteristics | Oral prophylaxis group (n=463) | Placebo group (n=463) |
|---|---|---|
| Mean (SD) time from intervention dose to skin incision (hours) | 13.0 (1.3) | 13.1 (1.2) |
| Mean (SD) time from intravenous antimicrobial prophylaxis to skin incision (minutes)* | 36.8 (22.0) | 37.7 (24.2) |
| Type of surgery: | ||
| Colectomy | 301/463 (65) | 296/463 (64) |
| Right hemicolectomy | 121/301 (40) | 110/296 (37) |
| Left hemicolectomy | 164/301 (54) | 169/296 (57) |
| Transverse colectomy | 28/301 (9) | 23/296 (8) |
| Total colectomy | 8/301 (3) | 12/296 (4) |
| Rectal resection | 162/463 (35) | 167/463 (36) |
| Surgical method: | ||
| Open | 60 (13) | 57 (12) |
| Laparoscopic assisted | 337 (73) | 344 (74) |
| Laparoscopy converted to open | 66 (14) | 62 (14) |
| Type of anastomosis: | ||
| Stapled | 326/437 (75) | 331/430 (77) |
| Handsewn | 111/437 (25) | 99/430 (23) |
| Ileostomy or colostomy performed | 154 (33) | 153 (33) |
| Abdominal surgical drain used | 200 (43) | 192 (41) |
| Median (IQR) duration of surgery (minutes)† | 211 (160-280) | 201 (159-270) |
| Median (IQR) volume of intravenous fluid (mL): | ||
| Crystalloid | 2000 (1500-2500) | 2000 (1500-2500) |
| Colloid | 500 (500-1000) | 500 (500-1000) |
| Median (IQR) infusion rate (mL/kg/h) | 7.6 (6.0-10.3) | 8.0 (6.0-11.0) |
| Cardiac output monitoring used | 94/463 (20) | 112/463 (24) |
| Median (IQR) FiO2 (%): | ||
| Start of surgery | 44 (40-50) | 45 (40-50) |
| End of surgery | 45 (40-51) | 45 (39-51) |
| Mean (SD) core temperature at end of surgery (°C) | 36 (1) | 36 (1) |
| Received dexamethasone | 314 (68) | 320 (69) |
| Received intravenous lidocaine | 351 (76) | 330 (71) |
| Median (IQR) intraoperative blood loss (mL) | 150 (100-300) | 150 (100-300) |
| Red blood cell transfusion during surgery | 17 (4) | 14 (3) |
| Postoperative lidocaine used | 150 (32) | 133 (29) |
| Postoperative epidural analgesia used | 57 (12) | 60 (13) |
| Planned postoperative care in HDU or ICU | 117 (25) | 110 (24) |
FiO2=fraction of inspired oxygen; HDU=high dependency unit; ICU=intensive care unit; IQR=interquartile range; SD=standard deviation.
Percentages may not total 100 because of rounding.
Data were missing for 25 patients in the oral prophylaxis group and 20 patients in the placebo group.
Data were missing for three patients in the placebo group.
Primary outcome
Surgical site infections within 30 days after surgery occurred in 60 of 463 patients (13.0%) with oral prophylaxis and in 100 of 463 patients (21.6%) with placebo (absolute difference −8.6%, 95% confidence interval −13.5% to −3.8%; relative risk 0.60, 95% confidence interval 0.45 to 0.80). Supplementary table S2 shows the results of associated univariable and multivariable analyses. The result was unaffected by adjustment for stratification variables and covariates (adjusted relative risk 0.62, 95% confidence interval 0.44 to 0.46) (see supplementary table S3). Similar results were obtained in the per protocol population (see supplementary tables S4-S6). Figure 2 shows the times to surgical site infection.
Fig 2.
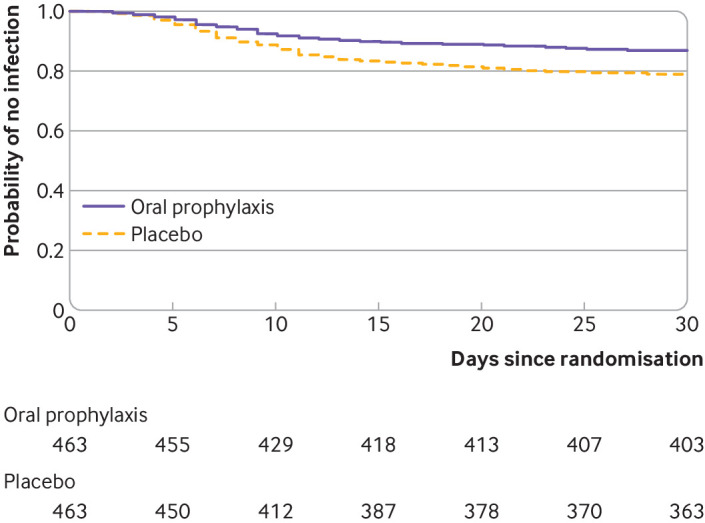
Kaplan-Meier probability of surgical site infection (modified intention-to-treat population). Raw data for the Kaplan-Meier probability of surgical site infection were censored at 30 days after surgery (hazard ratio with oral prophylaxis versus placebo 0.57, 95% confidence interval 0.43 to 0.78). The Cox proportional-hazards model was unadjusted
Secondary outcomes
Table 3 shows predefined secondary outcomes. Significant differences were found between the oral prophylaxis and placebo groups for occurrence of deep infections (4.8% v 8.0%; relative risk 0.54, 95% confidence interval 0.31 to 0.92) and organ space infections (5.0% v 8.4%; 0.53, 0.31 to 0.91). Adjusted analyses yielded similar results (see supplementary table S3). Fewer patients in the oral prophylaxis group than placebo group developed major (Clavien-Dindo grade ≥3) complications within 30 days after surgery (relative risk 0.67, 0.46 to 0.96). Similarly, clinically meaningful between group differences were found for anastomotic leakage and sepsis or septic shock (table 3). Death within 30 days did not differ between the two groups. No serious adverse event from trial drugs was reported by the investigator in either group. Supplementary table S7 shows the spectrum of pathogens isolated from patients with surgical site infection.
Table 3.
Primary and secondary outcomes of participants in modified intention-to-treat population. Values are numbers (percentages) unless stated otherwise
| Outcomes | Oral prophylaxis group (n=463) | Placebo group (n=463) | Relative risk (95% CI)* | P value |
|---|---|---|---|---|
| Primary outcome | ||||
| Any surgical site infection within 30 postoperative days | 60 (13.0) | 100 (21.6) | 0.60 (0.45 to 0.80) | 0.001 |
| Secondary outcomes† | ||||
| Superficial incisional infection | 15 (3.2) | 24 (5.2) | 0.56 (0.29 to 1.09) | 0.09 |
| Deep incisional infection | 22 (4.8) | 37 (8.0) | 0.54 (0.31 to 0.92) | 0.03 |
| Organ space infection | 23 (5.0) | 39 (8.4) | 0.53 (0.31 to 0.91) | 0.02 |
| SIRS | 96 (20.7) | 122 (26.4) | 0.79 (0.62 to 0.99) | 0.045 |
| Sepsis or septic shock | 26 (5.6) | 42 (9.1) | 0.62 (0.39 to 0.99) | 0.046 |
| Arrhythmia | 84 (18.1) | 76 (16.4) | 1.11 (0.83 to 1.47) | 0.49 |
| Acute heart failure | 1 (0.2) | 0 (0.0) | NA | 1.00 |
| Myocardial infarction | 0 (0.0) | 1 (0.2) | NA | 1.00 |
| Pneumonia | 13 (2.8) | 6 (1.3) | 2.17 (0.83 to 5. 65) | 0.11 |
| Postoperative mechanical ventilation | 9 (1.9) | 15 (3.2) | 0.60 (0.27 to 1.36) | 0.22 |
| Acute kidney injury | 61 (13.2) | 63 (13.6) | 0.97 (0.70 to 1.34) | 0.85 |
| Clavien-Dindo classification grade: | ||||
| 1 or 2 | 188 (40.6) | 181 (39.1) | 0.98 (0.74 to 1.29) | 0.86 |
| ≥3 | 42 (9.1) | 63 (13.6) | 0.63 (0.41 to 0.97) | 0.03 |
| Anastomotic leakage | 22 (4.8) | 37 (8.0) | 0.59 (0.36 to 0.99) | 0.046 |
| Reoperation | 35 (7.6) | 49 (10.6) | 0.71 (0.47 to 1.08) | 0.11 |
| Surgical or endoscopic drainage | 3 (0.7) | 7 (1.5) | 0.43 (0.11 to 1.65) | 0.20 |
| Mean (SD) time from randomisation to adjuvant chemotherapy initiation (days) | 20 (5) | 22 (6) | NA | 0.47 |
| Unplanned hospital readmission | 30 (6.5) | 30 (6.5) | 1 (0.61 to 1.63) | 1.00 |
| Unplanned admission to ICU | 1 (0.2) | 5 (1.1) | 0.20 (0.02 to 1.71) | 0.14 |
| Median (IQR) duration of hospital stay (days) | 6 (5-10) | 7 (5-11) | NA | 0.48 |
| Median (IQR) hospital-free days at 30 days (days) | 24 (20-25) | 23 (19-25) | NA | 0.48 |
| Death: | ||||
| At 30 days | 2 (0.4) | 5 (1.1) | 0.40 (0.08 to 2.05) | 0.27 |
| At 90 days | 5 (1.1) | 10 (2.2) | 0.50 (0.17 to 1.45) | 0.20 |
CI=confidence interval; ICU=intensive care unit; IQR=interquartile range; NA=not applicable; SIRS=systemic inflammatory response syndrome.
Relative risk is for oral prophylaxis group compared with placebo group. Confidence intervals were not adjusted for multiple comparisons of other secondary outcomes; thus these analyses are exploratory and should not be used to infer definitive treatment effects. Supplementary table S3 shows the results of adjusted outcome analyses.
All secondary outcomes, except 90 day mortality, were assessed up to 30 days after surgery.
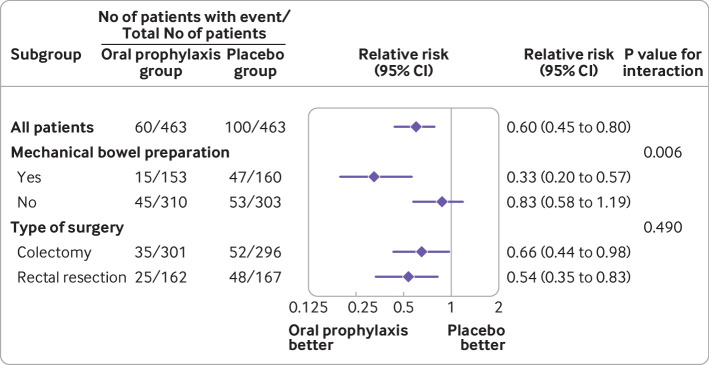
Heterogeneity existed between the oral prophylaxis and placebo groups and bowel preparation for surgical site infection (9.8% v 29.3%; difference −19.6% (95% confidence interval −28.1% to −11.1%) in patients who received mechanical bowel preparation and 14.5% v 17.5%; difference −2.9% (−8.8% to 2.8%) in patients who did not receive mechanical bowel preparation; P for interaction=0.006) (fig 3). We found no evidence of a differential effect according to type of surgery (colon versus rectum resection; P for interaction=0.49).
Fig 3.
Relative risks with 95% confidence intervals for the primary outcome of surgical site infection within 30 days after surgery in the oral prophylaxis group and placebo group, among all patients and in the two predefined subgroups. The widths of the confidence intervals for subgroup analyses were not adjusted for multiplicity and should not be used to infer definitive treatment effects. Mechanical bowel preparation consisted of polyethylene glycol or senna solution
Post hoc analysis
We found no significant interaction between treatment groups in relation to update of the French guidelines (before versus after publication update) for surgical site infection (P for interaction=0.87) (see supplementary materials).
We also conducted a post hoc analysis to investigate a potential treatment effect resulting from non-compliance with bowel preparation. Similar results to the primary analysis were found in a per protocol analysis excluding all patients (30 patients in the oral prophylaxis group and 35 patients in the placebo group) who did not fully comply with bowel preparation (12.7% v 22%; unadjusted relative risk 0.58, 95% confidence interval 0.43 to 0.78; adjusted relative risk 0.59, 95% confidence interval 0.46 to 0.77). Detection of a treatment effect would have suggested uncontrolled confounding.
Discussion
Principal findings
In this multicentre pragmatic double blind randomised trial involving patients undergoing elective colorectal surgery, adding a single dose of 1 g ornidazole 12 hours before surgery as an adjunct to intravenous antibiotic prophylaxis resulted in a significantly lower rate of surgical site infection within 30 days after surgery compared with placebo. Compared with those assigned to placebo, participants assigned to oral prophylaxis had a 40% lower relative risk of surgical site infection; in addition, the oral prophylaxis group had lower rates of other secondary outcomes, including major postoperative surgical complications (33% lower relative risk). The findings suggest that the effect of oral prophylaxis versus placebo was attributed mostly to a reduction in the rates of deep and organ space surgical site infections.
Comparison with other studies
The overall incidence of surgical site infection in our study (17.3%) was slightly higher than hypothesised but consistent with rates reported in previous trials (ranging from 7% to 26%).5 6 17 28 A possible explanation is the 35.5% proportion of rectal procedures in this study. Rectal resection is associated with a higher risk of anastomotic leakage and surgical site infections than would be expected after intraperitoneal colon resection.29
Previous meta-analyses of randomised trials have suggested that use of oral antibiotics, whether alone or in combination with mechanical bowel preparation, is associated with lower rates of surgical site infections.13 14 30 These trials had several limitations however, including the main use of open surgery, which may be associated with a greater risk of surgical site infection than laparoscopic surgery,31 and, in many of these trials, the postoperative continuation of antibiotic prophylaxis—a strategy that is no longer recommended.32 Also, the oral antibiotics, dosages, and timings varied across studies, making the results difficult to translate into clinical practice. Several antibiotic regimens have been previously evaluated for oral prophylaxis.13 Although nitroimidazole derivatives in combination with aminoglycosides were frequently used, no clear evidence exists on which preferred type and dosage of oral antibiotics should be used before colorectal surgery.8 Antibiotics with the narrowest possible spectrum should be used to produce as little inadvertent damage as possible to the endogenous microflora, which provides a natural resistance to colonisation, and to reduce the emergence of resistant organisms. In our study, we assessed the effect of a single preoperative oral dose of 1 g ornidazole, which has a spectrum of activity extended to most anaerobes encountered in the colon and the rectum, has a longer elimination half life than other nitroimidazole derivatives (especially metronidazole)—the pharmacokinetic profile of which allows a single dose to be administered the day before surgery, and is widely available. Additionally, no serious adverse event was recorded in our study.
A major component of the controversy about oral antimicrobial prophylaxis is the use of mechanical bowel preparation. In our pragmatic randomised study, bowel preparation was used in accordance with current practice, which states that bowel preparation should be avoided before colonic surgery but might be beneficial in patients having rectal resections.23 However, the treatment effect showed heterogeneity, suggesting that in patients who received mechanical bowel preparation, an excess risk of surgical site infection might be associated with placebo (versus ornidazole). In patients who did not undergo bowel preparation, we found no evidence of a difference in risk of surgical site infection between placebo and ornidazole. Finally, the combination of ornidazole and mechanical bowel preparation seems to be associated with the lowest risk of surgical site infection. The findings in the present study contrast with those of the recent MOBILE study in patients having elective colon resection, in which mechanical and oral antibiotic bowel preparation was not associated with a reduced rate of surgical site infections or overall morbidity compared with no bowel preparation.17 In another multicentre, single blind, randomised trial of 565 patients who did not receive bowel preparation, oral antibiotic prophylaxis was associated with a reduced rate of surgical site infections; this study did not, however, include rectal surgery.33 In our study, the effects of oral prophylaxis in the prespecified subgroup analysis did not differ between the groups who underwent colonic surgery and rectal surgery. The number of patients who had rectal surgery was, however, too low to draw definite conclusions.8 9
Strengths and limitations of this study
The strengths of our study include the large sample size and the multicentred and pragmatic design with maintenance of usual care, including compliance with the programme for early recovery after surgery. Moreover, unlike previously available published studies, our trial was not restricted to colonic surgeries, allowing the findings to be generalisable to colorectal surgery overall.
Limitations must also be considered. Firstly, protocol deviations could have biased the analyses. Non-adherence to the allocated trial intervention is unlikely to have affected the results, however, because the per protocol analysis, from which these patients were excluded, supported the primary analysis. Mechanical bowel preparation may potentially have biased the estimate of treatment effect in our trial. However, our results were robust in an analysis excluding patients who did not fully comply with bowel preparation. Secondly, we did not assess all cointerventions during the trial that might have influenced the risk of surgical site infection, such as glycaemic control or preoperative bathing during the trial period. The trial was, however, blinded and randomisation was stratified; it is less likely that any imbalance in cointerventions affected the results. Thirdly, although cefoxitin has both aerobic and anaerobic activity, because of increasing resistance of anaerobes (including Bacteroides) to second generation cephalosporins, its use for intravenous antibiotic prophylaxis could have been suboptimal. This may limit external validity, as currently guidelines for preoperative prophylaxis include enhanced anaerobic coverage. Whether the effect for the oral antimicrobial prophylaxis results from improved anaerobic coverage or from an additional decrease in the bioburden from oral antibiotics deserves further evaluation. Fourthly, one third of the participants had rectal surgery. Although the exclusion of participants who were candidates for rectal surgery might have enhanced the study design, we aimed to evaluate whether the intervention effect would be consistent across colorectal procedures. The subgroup analysis suggested that oral antibiotic prophylaxis might be equally beneficial to patients having colon and rectal procedures. A further anticipated limitation, owing to possible pharmacokinetic and pharmacodynamic modifications, was the exclusion of patients with a body mass index of 35 or higher, even though obese patients are at increased risk of surgical site infection. However, the participants in our trial had baseline characteristics, including mean body mass index, that were similar to those of participants in previous studies. Finally, generalisability to populations not included in the trial, such as patients with inflammatory bowel diseases, remains to be evaluated.
Conclusion
In this large pragmatic multicentre trial in patients undergoing elective colorectal surgery, the use of oral antimicrobial prophylaxis with a single oral dose of 1 g ornidazole compared with placebo as an adjunct to intravenous antibiotic prophylaxis resulted in a significantly lower rate of surgical site infection within 30 days after surgery.
What is already known on this topic
Intravenous antimicrobial prophylaxis is recommended in patients who undergo colorectal surgery—a patient population that is particularly at risk of surgical site infections
In a recent network meta-analysis, the use of oral antimicrobial prophylaxis in addition to intravenous antibiotics in patients undergoing colorectal surgery was associated with a statistically significant reduction in surgical site infections
The supporting evidence for that finding, however, remains limited
What this study adds
Compared with placebo, oral antimicrobial prophylaxis using a single dose of 1 g ornidazole 12 hours before surgery as an adjunct to intravenous antibiotic prophylaxis resulted in significantly lower surgical site infections within 30 days
Acknowledgments
A list showing members of the COMBINE study group is in the supplementary materials.
Web extra.
Extra material supplied by authors
Supplementary materials: Additional methods, figure, and tables
Contributors: Ef and SJ are joint first authors. EF obtained funding from the French Ministry of Health to undertake the study. He is the guarantor and accepts full responsibility for the work. EF and CPB conceived the study. EF, SJ, JCL, and CPB designed the study. MG, MV, YP, KS, GL, AO, YEA, PC, AD, SL, ML, and JP acquired the data. EF, SJ, ADJ, and BP analysed and interpreted the data. EF and SJ wrote the initial draft and contributed equally to this manuscript. All authors critically revised the manuscript and approved the final version. The lead author (EF) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Additional contributors: Justine Bourdier (research technician) and Dominique Morand (administrative support, Direction de la recherche clinique Clermont-Ferrand).
Funding: This study was funded by a grant from the French Ministry of Health under its Clinical Research Program (PHRC-14-0060) and by the Clermont-Ferrand University Hospital. The funders had no role in considering the study design or in the collection, analysis, or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: the study was funded by a grant from the French Ministry of Health under its Clinical Research Program and by Clermont-Ferrand University Hospital; EF reports receiving consulting fees from Drager Medical, GE Healthcare, and Edwards Lifesciences, and honorariums for presentation from Baxter outside the submitted work; SJ reports receiving consulting fees from Drager Medical, Fisher & Paykel Healthcare, Fresenius Xenios, Medtronic, Baxter, and Mindray outside the submitted work; SL reports receiving consulting fees from Vifor Pharma and Alexys Sante, and honorariums for lectures from Vifor Pharma, Pharmacosmos, Pfizer, and Masimo outside the submitted work; ML reports receiving consulting fees from Gilead, Ambu, and LFB outside the submitted work; AO reports receiving consulting fees from LFB, Orion Pharma, Vifor Pharma, Nordic Pharma, and iSEP, and honorariums for lectures from Orion Pharma, Nordic Pharma, and LFB outside the submitted work. The authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
The lead author (EF) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained
Dissemination to participants and related patients and public communities: After publication, the findings of this study will be disseminated to appropriate audiences, including participating surgical departments, academia, clinicians, and the general public, through various channels, such as blogs, press releases, and social media. The results were also presented at the French Society of Anaesthesiology and Critical Care Medicine and will serve as a support for the update of French guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This trial was approved for all centres by the South-Est VI Committee for the Protection of Research Subjects (ID: 2015-002559-84) and the French National Agency of Medicine and Health Products Safety.
Data availability statement
Deidentified data about the individual participants will be shared with researchers of further studies on reasonable request. Request for data sharing will be handled in line with the data access and sharing policy of Clermont-Ferrand University Hospital.
References
- 1. Fields AC, Pradarelli JC, Itani KMF. Preventing Surgical Site Infections: Looking Beyond the Current Guidelines. JAMA 2020;323:1087-8. 10.1001/jama.2019.20830. [DOI] [PubMed] [Google Scholar]
- 2. Coello R, Charlett A, Wilson J, Ward V, Pearson A, Borriello P. Adverse impact of surgical site infections in English hospitals. J Hosp Infect 2005;60:93-103. 10.1016/j.jhin.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 3. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387-97. 10.1016/j.ajic.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 4. Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg 2014;149:1045-52. 10.1001/jamasurg.2014.346. [DOI] [PubMed] [Google Scholar]
- 5. Bennett-Guerrero E, Pappas TN, Koltun WA, et al. SWIPE 2 Trial Group . Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med 2010;363:1038-49. 10.1056/NEJMoa1000837. [DOI] [PubMed] [Google Scholar]
- 6. Itani KM, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med 2006;355:2640-51. 10.1056/NEJMoa054408. [DOI] [PubMed] [Google Scholar]
- 7. Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg 2017;152:784-91. 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 8. Allegranzi B, Bischoff P, de Jonge S, et al. WHO Guidelines Development Group . New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e276-87. 10.1016/S1473-3099(16)30398-X. [DOI] [PubMed] [Google Scholar]
- 9. Ban KA, Minei JP, Laronga C, et al. American College of Surgeons and Surgical Infection Society: Surgical Site Infection Guidelines, 2016 Update. J Am Coll Surg 2017;224:59-74. 10.1016/j.jamcollsurg.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 10. Morris MS, Graham LA, Chu DI, Cannon JA, Hawn MT. Oral Antibiotic Bowel Preparation Significantly Reduces Surgical Site Infection Rates and Readmission Rates in Elective Colorectal Surgery. Ann Surg 2015;261:1034-40. 10.1097/SLA.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 11. Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015;262:416-25, discussion 423-5. 10.1097/SLA.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 12. Scarborough JE, Mantyh CR, Sun Z, Migaly J. Combined Mechanical and Oral Antibiotic Bowel Preparation Reduces Incisional Surgical Site Infection and Anastomotic Leak Rates After Elective Colorectal Resection: An Analysis of Colectomy-Targeted ACS NSQIP. Ann Surg 2015;262:331-7. 10.1097/SLA.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 13. Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014;CD001181:CD001181. 10.1002/14651858.CD001181.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rollins KE, Javanmard-Emamghissi H, Acheson AG, Lobo DN. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann Surg 2019;270:43-58. 10.1097/SLA.0000000000003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Slim K, Vicaut E, Launay-Savary MV, Contant C, Chipponi J. Updated systematic review and meta-analysis of randomized clinical trials on the role of mechanical bowel preparation before colorectal surgery. Ann Surg 2009;249:203-9. 10.1097/SLA.0b013e318193425a. [DOI] [PubMed] [Google Scholar]
- 16. Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev 2011;CD001544:CD001544. 10.1002/14651858.CD001544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koskenvuo L, Lehtonen T, Koskensalo S, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. Lancet 2019;394:840-8. 10.1016/S0140-6736(19)31269-3. [DOI] [PubMed] [Google Scholar]
- 18. Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for Antibiotic Administration for Bowel Preparation Among Patients Undergoing Elective Colorectal Surgery: A Network Meta-analysis. JAMA Surg 2022;157:34-41. 10.1001/jamasurg.2021.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vignaud M, Paugam-Burtz C, Garot M, et al. COMBINE trial management committee . Comparison of intravenous versus combined oral and intravenous antimicrobial prophylaxis (COMBINE) for the prevention of surgical site infection in elective colorectal surgery: study protocol for a multicentre, double-blind, randomised controlled clinical trial. BMJ Open 2018;8:e020254. 10.1136/bmjopen-2017-020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin C, Auboyer C, Dupont H, et al. Société française d’anesthésie et de réanimation . Antibioprophylaxis in surgery and interventional medicine (adult patients). Actualization 2010. [In French.] Ann Fr Anesth Reanim 2011;30:168-90. 10.1016/j.annfar.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 21. Bratzler DW, Dellinger EP, Olsen KM, et al. American Society of Health-System Pharmacists. Infectious Disease Society of America. Surgical Infection Society. Society for Healthcare Epidemiology of America . Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013;70:195-283. 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 22. Martin C, Auboyer C, Boisson M, et al. Steering committee of the French Society of Anaesthesia and Intensive Care Medicine (SFAR) responsible for the establishment of the guidelines . Antibioprophylaxis in surgery and interventional medicine (adult patients). Update 2017. Anaesth Crit Care Pain Med 2019;38:549-62. 10.1016/j.accpm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 23. Bretagnol F, Panis Y, Rullier E, et al. French Research Group of Rectal Cancer Surgery (GRECCAR) . Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010;252:863-8. 10.1097/SLA.0b013e3181fd8ea9. [DOI] [PubMed] [Google Scholar]
- 24. Gustafsson UO, Scott MJ, Schwenk W, et al. Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care. European Society for Clinical Nutrition and Metabolism (ESPEN) International Association for Surgical Metabolism and Nutrition (IASMEN) . Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:259-84. 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 25. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309-32. 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28. Darouiche RO, Wall MJ, Jr, Itani KM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med 2010;362:18-26. 10.1056/NEJMoa0810988. [DOI] [PubMed] [Google Scholar]
- 29. Guillou PJ, Quirke P, Thorpe H, et al. MRC CLASICC trial group . Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 30. Toh JWT, Phan K, Hitos K, et al. Association of Mechanical Bowel Preparation and Oral Antibiotics Before Elective Colorectal Surgery With Surgical Site Infection: A Network Meta-analysis. JAMA Netw Open 2018;1:e183226. 10.1001/jamanetworkopen.2018.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caroff DA, Chan C, Kleinman K, et al. Association of Open Approach vs Laparoscopic Approach With Risk of Surgical Site Infection After Colon Surgery. JAMA Netw Open 2019;2:e1913570. 10.1001/jamanetworkopen.2019.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allegranzi B, Zayed B, Bischoff P, et al. WHO Guidelines Development Group . New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis 2016;16:e288-303. 10.1016/S1473-3099(16)30402-9. [DOI] [PubMed] [Google Scholar]
- 33. Espin Basany E, Solís-Peña A, Pellino G, et al. Preoperative oral antibiotics and surgical-site infections in colon surgery (ORALEV): a multicentre, single-blind, pragmatic, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:729-38. 10.1016/S2468-1253(20)30075-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Additional methods, figure, and tables
Data Availability Statement
Deidentified data about the individual participants will be shared with researchers of further studies on reasonable request. Request for data sharing will be handled in line with the data access and sharing policy of Clermont-Ferrand University Hospital.