Abstract
Polycystic ovary syndrome (PCOS) is a complex disease affecting up to 15% of women of reproductive age. Women with PCOS suffer from reproductive dysfunctions with excessive androgen secretion and irregular ovulation, leading to reduced fertility and pregnancy complications. The syndrome is associated with a wide range of comorbidities including type 2 diabetes, obesity, and psychiatric disorders. Despite the high prevalence of PCOS, its etiology remains unclear. To understand the pathophysiology of PCOS, how it is inherited, and how to predict PCOS, and prevent and treat women with the syndrome, animal models provide an important approach to answering these fundamental questions. This minireview summarizes recent investigative efforts on PCOS-like rodent models aiming to define underlying mechanisms of the disease and provide guidance in model selection. The focus is on new genetic rodent models, on a naturally occurring rodent model, and provides an update on prenatal and peripubertal exposure models.
Keywords: androgen excess, gene-modified, metabolic, reproductive, behavior, developmental programming, transgenerational
Polycystic ovary syndrome (PCOS) is an understudied but serious challenge to women's health. Worldwide, ∼15% of women suffer from PCOS, and it is the leading cause of female infertility and is associated with a high number of comorbidities, such as insulin resistance and type 2 diabetes, endometrial cancer, and mental ill health (1-6). Moreover, there are now indications that a male form of PCOS may exist (7). Management of PCOS is currently symptomatic, hindered by a lack of insight into the origin and underlying mechanisms.
The diagnosis of PCOS in adult women requires 2 of 3 criteria: (1) hyperandrogenism, (2) oligo-anovulation, and (3) the PCOS morphology according to the Rotterdam criteria (8, 9). Although >50% of women with PCOS are obese, exacerbating their symptoms (10), the key phenotypic feature of PCOS is hyperandrogenism, with a recent study showing that genetically higher levels of testosterone increase the risks of PCOS, type 2 diabetes, and breast and endometrial cancer (11).
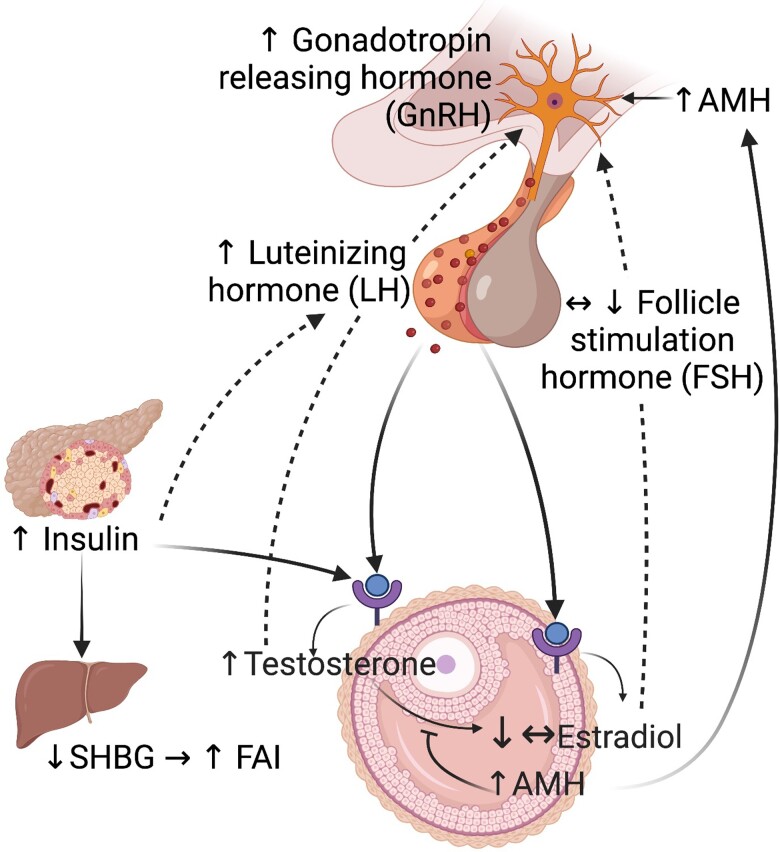
The reproductive phenotype of PCOS could be described as a vicious cycle involving the hypothalamus-pituitary-ovarian axis in which high circulating androgens are the main feature, but it is not known where it starts (Fig. 1). The main source of hyperandrogenism in human PCOS is the intrinsic ovarian hypersecretion of androgen (12-14), which is exacerbated by GnRH-dependent LH secretion that stimulates theca cell androgen synthesis (1, 15-18) and inhibits follicular maturation, resulting in large numbers of small antral follicles and anovulation (19). The exact mechanism of follicle arrest is complex but likely includes excessive secretion of androgens, LH, and insulin (20). The accumulation of small antral follicles results in excessive granulosa-cell production of antimüllerian hormone (AMH), leading to a 2- to 3-fold increase in AMH levels, which in turn increases GnRH and LH secretion (21). AMH could in this way trigger ovarian hyperandrogenemia and play a key role in the pathogenesis of PCOS (22, 23). In addition, androgen excess deriving from subcutaneous abdominal adipose tissue (24, 25) further exacerbates hyperandrogenemia.
Figure 1.
Hypothalamus-pituitary-ovarian axis alterations in polycystic ovary syndrome (PCOS). Women with PCOS have elevated LH to FSH ratios likely because of GnRH-dependent LH secretion. The high LH pulsatility stimulates the theca cell androgen production and impairs the follicle maturation, resulting in the accumulation of small antral follicles that in turn results in excessive granulosa-cell production of antimüllerian hormone (AMH), The high AMH further increases GnRH and LH secretion. In addition, hyperinsulinemia also stimulates theca cells as co-gonadotroph with LH and further contributes to excessive androgen synthesis and reduces hepatic SHBG resulting in elevated free androgen index (FAI). Created with BioRender.com.
The metabolic phenotype of PCOS is characterized by hyperinsulinemia because of insulin resistance in skeletal muscle and adipose tissue, independent of obesity and fat distribution (26-28). Hyperinsulinemia also acts on theca cells as a co-gonadotroph with LH and further stimulates theca cell androgen synthesis and reduces hepatic SHBG, resulting in elevated free testosterone and further aggravating the clinical signs of hyperandrogenism. Hyperinsulinemia is linked to adipose tissue dysfunctions, including suppressed lipolysis and promotes adipocyte lipogenesis (29) and weight gain (30). Indeed, obesity aggravates all metabolic abnormalities, leading to hepatic insulin resistance, dyslipidemia, and aberrant adipose tissue function, as well as reproductive dysfunctions.
The mental ill-health phenotype of PCOS is characterized by an increased prevalence of anxiety and depression symptoms and as many as more than 60% of women with PCOS are diagnosed with at least 1 psychiatric disorder (2, 6). Although hyperandrogenism, insulin resistance, and obesity may contribute to symptoms of anxiety and depression, the exact etiology and pathophysiology remain to be defined.
What we do know is that hyperandrogenemia plays a key pathogenic role and that PCOS runs in families, with an estimated heritability of 70%, but only a small proportion of the heritability in PCOS can be accounted for by the ∼20 identified susceptibility loci (31). It is possible that variants with lower allele frequencies, which cannot be identified by genome-wide association studies arrays, contribute to PCOS. Indeed, whole-genome sequencing and targeted approaches have identified 37 rare AMH2 variants (32) and 32 rare DENND1A variants (33) that are specific to PCOS, implicating the importance of these genes in the pathogenesis of PCOS. Moreover, reproductive and metabolic phenotypes are associated with specific PCOS susceptibility loci, supporting the idea that genetic factors play a role in the PCOS pathogenesis (34-38). Recently, using summary statistics from genome-wide association studies conducted for type 2 diabetes, type 2 diabetes adjusted for body mass index (BMI), fasting glucose, insulin, HbA1c, and PCOS, we found a genetic link underlying type 2 diabetes, glycemic traits, and PCOS, driven by both biological pleiotropy and causal mediation, some of which is independent of BMI (39). Of note, a recent study shows that men who carry high polygenic risk scores for PCOS face an increased risk of obesity, type 2 diabetes, and cardiovascular disease as well as male pattern baldness (7), indicating that not only women are adversely affected by PCOS.
It remains unclear, however, how PCOS and associated comorbidities are inherited in women and men. Although previous studies suggest a genetic association between PCOS and mental health disorders, a recent study performing comprehensive Mendelian randomization analyses found no association between PCOS genetic risk scores and mental health disorders in women with PCOS (40), indicating that other, nongenetic, factors contribute to the inheritance. Indeed, growing evidence suggests that epigenetic processes triggered by an adverse maternal-fetal environment could yield similar phenotypic heritability compared with conventional genetics (41). Thus, it is likely that genetic and epigenetic mechanisms act in concert in a complex trait such as PCOS, as in type 2 diabetes (42).
PCOS-like animal models have increased our understanding of PCOS etiology and pathophysiology. Importantly, no animal model completely resembles the human PCOS. However, many animal models express 2 or 3 PCOS-like traits of the Rotterdam criteria and qualify to be defined as PCOS-like animal models. Because of their relatively short lifespan, PCOS-like rodent models are unique tools to dissect PCOS-specific genetic and nongenetic triggers and molecular pathways of disease development and inheritance. There are several recent detailed reviews on PCOS-like animal models (43-47). This narrative minireview focuses on PCOS-like rodent models and aims to (1) provide a summary of new genetic models, on a naturally occurring rodent model, and an update of already known PCOS-like rodent models addressing both female and male phenotype when possible; and (2) discuss the model selection and translational aspects as well as limitations.
Update on PCOS-Like Rodent Models
Search Strategy
References for this review were identified through PubMed searches starting from January 2020 until August 2022. Terms included: animal models/rodent/androgens/AMH/PCOS/PCOM/polycystic/polycystic ovary/polycystic ovary syndrome/ovary/oocytes/germ cells/adipose tissue/skeletal muscle/genetic/epigenetic/obesity/insulin resistance/type 2 diabetes/anxiety/depression/infertility/ovulation/maternal/pregnancy together with many others. Articles published in English from these searches and relevant references cited in those articles were reviewed and included in this narrative review.
New Genetic PCOS-Like Models
Erbb4-knockout mice (Table 1)
Table 1.
Summary of PCOS-like clinical features of new genetic- and spontaneous PCOS-like mice and rat models
| New genetic PCOS-like mice models | New spontaneous PCOS-like rat model | |||
|---|---|---|---|---|
| Errb4 knockout | Cyp17 overexpression (TC17) | Dennd1a-knock in | Goto-Kakizaki (GK) rats | |
| Errb4Flox/Flox | Cre/LoxP | CMV | Polygenic | |
| Rosa26LacZCre | TetOn | Lhcgr | ||
| Clinical traits | AmhCre | TetOn | ||
| Reproductive phenotype | ||||
| Reduced fertility | √ | √ | ? | ? |
| Less number of pups | ? | √ | ? | ? |
| Prolonged estrus cycle (ie, oligo/anovulation) | √ | √ | ? | √ |
| Multicystic ovary | √ | √ | ? | √ |
| Granulosa cell layer | − | ? | ? | √ (↓) |
| Theca cell layer thickness | √ (↑) | √ (↑) | ? | √ (↑) |
| Endocrine profile | ||||
| Serum (s)-testosterone | √ (↑) | √ (↑) | − | √ (↑) |
| s-estrogen | − | ? | ? | ? |
| s-progesterone | √ (↓) | ? | ? | √ (↓) |
| s-LH | √ (↑) | ? | ? | √ (↑) |
| s-FSH | − | ? | ? | − |
| s-antimüllerian hormone | √ (↑) | ? | ? | √ (↑) |
| Metabolic phenotype | ||||
| Body weight | √ (↑) | √ (↑) | ? | √ (↓) |
| Accumulation of body fat | ? | ? | ? | √ |
| Adipocyte hypertrophy | √ (↑) | ? | ? | √ |
| f-insulin | √ (↑) | ? | ? | √ (↑) |
| f-glucose | ? | ? | ? | √ (↑) |
| Insulin resistance | ? | ? | ? | √ |
| Pancreatic β-cell mass | ? | ? | ? | √ |
| Impaired lipid profile | ? | ? | ? | √ |
| Fulfill 2 or more PCOS-like traits | √ | √ | − | √ |
√, clinical PCOS trait; −, clinical PCOS trait not present; ↑, increased or ↓, decreased; ?, not measured.
Among the ∼20 single-nucleotide polymorphism associated with PCOS is the epidermal growth factor receptor 4 (ERBB4) gene, which is also a risk locus for BMI (31). According to the Human Protein Atlas, Erbb4 is strongly expressed in the brain, endocrine, and female tissues and thus of relevance for the PCOS pathology. The epidermal growth factor receptor (Erbb) family includes Erbb1-4; Erbb4 contrasts with the others because it can produce 4 alternatively spliced isoforms: JMa-Cyt1-2 and JMb-Cyt1-2 with specific signaling assets (48). The ligands neuregulin (NRG1) binds to ERBB4 and is produced and secreted by granulosa cells in response to the ovulatory LH surge and regulates luteinization and oocyte maturation. Moreover, NRG1 activation of Erbb4 also affects glucose metabolism in rodents suggesting that the ERBB4 susceptibility loci contribute to PCOS risk via both reproductive and metabolic pathways (31).
Recently, Erbb4 in granulosa cells was conditionally deleted to define whether it contributes to the development of PCOS-like reproductive and molecular dysfunctions and to define molecular mechanisms (48). Mice with floxed Erbb4 gene were crossbred with AmhCre mice to specifically delete the gene in the ovary. Phenotypic measures show that Erbb4-AmhCre females are subfertile with disrupted estrus cycle, delayed ovulation, and arrested follicles at the secondary stage, demonstrating that Erbb4 signaling regulates follicle progression (48). Moreover, circulating androstenedione, testosterone, LH, and AMH are elevated in Erbb4-deficient mice, the latter also increased intrafollicularly. The Erbb4-deficient mice were slightly heavier than wild-type mice from 6 weeks until 6 months of age and have enlarged adipocytes as well as elevated nonfasting insulin, indicating that Erbb4 mice also develop metabolic dysfunctions. The authors concluded that the Erbb4-deficient mice present with a phenotype similar to a lean PCOS woman and that Erbb4 contributes to oocyte development competence, which is crucial for the normal development of an embryo (48). Although their brief metabolic phenotyping suggests that Erbb4 deficiency may alter metabolic function in female mice, these findings warrant further detailed metabolic phenotypic testing including body composition with dual-energy X-ray absorptiometry and/or an EchoMRI, oral glucose tolerance tests, insulin tolerance tests, metabolic cages that measure oxygen consumption and carbon dioxide production to estimate the respiratory exchange rate, energy expenditure, and substrate utilization, as well as cardiac function with, for example, EchoCardiography measuring heart contractility as the percentage of left ventricular fractional shortening. Whether Erbb4-deficient mice develop anxiety- and/or depression-like behavior remains to be defined. Moreover, detailed molecular analyses of oocytes and target reproductive, metabolic, and brain tissues are warranted to define underlying mechanisms enabling targeted treatment. The Erbb4 mice fulfill 3 PCOS-like traits and can be classified as relevant to PCOS.
Cyp17 theca cell (TC17) overexpression
In human theca cells, the enzyme 17α-hydroxylase/17,20-desmolase (CYP17) converts pregnenolone and progesterone into androgens. In women with PCOS, ovarian hyperandrogenism has been linked to dysfunctional theca cells' Cyp17 enzyme activity (13, 49) (Table 1). However, the physiological and functional role of hyperandrogenism on follicle function remains unknown. To address this knowledge gap, a transgenic mice model overexpressing Cyp17, selectively in theca cells (TC17), created by using the Cre/LoxP and the Tet-dependent expression system, a conditional gene expression system where transcription is turned on (TetOn or TetOff) in the presence of tetracycline (or doxycycline) (50). TC17 mice develop a PCOS-like reproductive phenotype and increased body weight with higher circulating testosterone concentrations, impaired folliculogenesis with a smaller number of antral follicles, and hypertrophic luteinized stromal cells. Moreover, TC17 mice have irregular estrous cycles and a smaller number of pups per litter (2 of 10 compared with 10 of 10 in the controls), indicating impaired fertility. Transcriptional profiling and pathway analyses of TC17 ovaries show many differentially expressed genes involved known as important ovarian markers such as Lhcgr, Fshr, Cyp19, Pgr, Amh, and Foxl2. As for the Erbb4 model, it required further phenotyping, especially metabolically given that the mice are overweight, it is of importance to investigate adipose tissue mass and function, glucose metabolism, and whether they also present with a behavioral phenotype. The TC17 mice fulfill 3 PCOS-like traits and can be classified as relevant to PCOS.
Dennd1a-knockin and knockout mice
The DENND1A is another susceptible locus associated with PCOS and forced expression of DENND1A.V2 in theca cells results in increased synthesis of androgens (51) (Table 1). To create a transgenic mouse model overexpressing human DENND1A.V2 were 3 promotors used: CMV, Lhcgr, and TetOn (52). None of the models resulted in DENND1A.V2 protein overexpression, and the authors conclude that other technologies such as CRISPR/Cas9 to introduce the human DENNDA1A.V2-like gene into the mouse might be more successful. Of note, previously in Dennd1a-knockout mice, it was shown that the gene is crucial for embryonic developmental (53).
Taken together, knock-in and knock-out to define the role of susceptibility loci contributing to PCOS reproductive, metabolic, and behavioral phenotypes is of great value to understand their functional role. Whether Dennd1a-knockin and knockout mice present with PCOS-like traits have not yet been defined and cannot be classified as relevant to PCOS.
New Spontaneous PCOS-like Rat Model
Naturally occurring PCOS has been observed in adult female rhesus monkeys (Macaca Mulatta) (54). Recently, the Goto-Kakizaki (GK) rats, a spontaneous type 2 diabetes model, was shown to present with both reproductive and metabolic PCOS-like phenotypic features (55). The GK-rat strain is polygenic and established by breeding glucose intolerant Wistar rats. Already at embryonic day (E) 16.5, the GK-rat fetus displays a loss of pancreatic β-cell mass, and at adult age, they have impaired insulin secretion, glucose tolerance, insulin resistance, dyslipidemia, hepatic steatosis, accumulation of body fat, and adipocyte hypertrophy, despite lower body weight (55, 56). Whether the fat accumulation is due to decreased lipolysis or other molecular mechanisms requires further investigation. The reproductive phenotyping shows that the GK-rats have longer estrus cycles and 75% are acyclic at 6 months of age. Moreover, they have an increased number of small antral follicles and high circulating testosterone, LH, and AMH, and increased ovarian mRNA expression of Amhr2. Thus, the GK-rat model displays all 3 phenotypic features of human PCOS.
About 50% of daughters of women with PCOS develop phenotypic features at puberty and full-blown PCOS in their 20s. In line with human PCOS adolescents, the first generation (F1) GK-rat female offspring display signs of precocious puberty, delay in the first estrus cycle, and increased circulating testosterone and AMH levels. Moreover, when F1 GK female rats were mated to generate second-generation (F2) GK female offspring, the offspring are born small-for-gestational age (postnatal day 5) and exhibited delayed follicular development and decreased circulating AMH levels, which contrasts with the higher AMH levels in adult GK-rats. Interestingly, the newborn F2 GK female offspring has elongated anogenital distance (AGD), indicating in utero androgen exposure. However, circulating testosterone in pregnant dams remains to be investigated. The gestational AMH levels in GK-rats were lower, which contrasts with the finding in human PCOS. Initial analyses show that placenta mRNA expression of the enzyme responsible for progesterone synthesis (Hsdb31) was increased, whereas Leptin expression was decreased and Amrh2 was unaltered.
Altogether, the GK-rat model needs to be further phenotypically tested, including behavior function, and detailed molecular analyses of oocytes and target reproductive, metabolic, and brain tissues are warranted to define underlying mechanisms enabling targeted treatment and could be a valuable tool in the development of new treatment strategies.
Update on Prenatal Dihydrotestosterone- and AMH-exposure Models in Female Offspring
The observations of fetal growth restriction and longer AGD in F2 GK-rat female offspring are in line with Barker's “developmental origins of health and disease” hypothesis, which proposes that early adaptations to suboptimal conditions in utero translate to adverse health states later in life (57-61). Women with PCOS experience greater weight gain early in pregnancy (62) and retain high levels of circulating androgens and AMH throughout pregnancy (23, 59). These diminish placental aromatase activity (59, 63) and have detrimental effects on fetal development, thereby predisposing the children of PCOS mothers to reproductive, metabolic, and mental disorders (23, 59-61, 64). Moreover, PCOS per se is an independent risk factor for gestational diabetes and hypertension (65). Daughters of women with PCOS have increased sebum production (66) and elongated AGD (67), although 1 study contrasts these findings (68), and elevated circulating levels of androgen (69) and AMH levels (70, 71), as well as PCOS morphology, are seen in girls before the onset of puberty, suggesting that any “in utero programming” occurs at an early stage of ovarian development and oogenesis (72). Indeed, a pilot study investigating global DNA methylation patterns in umbilical cord blood of neonates delivered by women with PCOS points to the existence of a specific PCOS epigenetic signature of differently methylated gene networks (73). These findings support the idea that an adverse intrauterine environment via epigenetic processes could yield the same phenotypic heritability as genetic factors, but to date the underlying mechanism is unknown. An aberrant intrauterine environment would also be expected to have detrimental effects also on male fetuses, but this is less studied. There is no well-established male PCOS phenotype in mouse or man, although early androgenetic alopecia in men is proposed as a sign of hormonal and metabolic abnormalities (ie, a male PCOS equivalent) (74). Indeed, both sons (75-77) and brothers (78-84) of women with PCOS display cardiometabolic and reproductive dysfunctions. Familial studies indicate that elevated androgens are an underlying trait in female as well as male relatives (85).
Prenatal androgen exposure
To understand how maternal androgen excess affects the health of offspring, the prenatal dihydrotestosterone (DHT) exposure mouse model, with and without pregestational diet-induced obesity, has been used. Independent of pregestational and maternal obesity, prenatal DHT exposure causes reproductive dysfunction with elongated AGD, elevated circulating testosterone and LH, and irregular cycles and PCOS-like morphology in female (F1) offspring (86-92) and prenatal testosterone exposure causes lower testes weight, decreased number of Sertoli cells and sperm function in male (F1) offspring (93), whereas DHT exposure in mice showed less effect in male offspring (94). The neuroendocrine consequences of androgen excess have recently been comprehensively reviewed by Silva and Campbell (95). A recent study shows that prenatal androgen (PNA) female mice have increased androgen receptor and decreased progesterone receptor and dynorphin gene expression in neurokinin B and dynorphin cells (ie, the GnRH/LH pulse generator), suggesting impaired negative feedback of progesterone (96). Moreover, both glutamatergic and GABAergic synaptic input to neurokinin B and dynorphin cells is decreased in PNA female mice (96). In line with these observations, PNA female mice have a lower number of total microglia cells, the immune cells of the brain, indicating that microglia may contribute to the neuroendocrine alterations observed in the PCOS-like PNA mice model and could potentially be involved in the developmental alterations observed in the brain (97). These findings warrant further investigation.
Moreover, prenatal DHT exposure causes adverse metabolic programming, including obesity and adipose tissue dysfunction, cardiac programming resulting in left ventricular hypertrophy in F1 female offspring (91), and anxiety-like behavior in female F1 offspring (90, 98). Treating DHT-exposed pregnant mice with flutamide, an androgen receptor antagonist, prevents the development of reproductive and metabolic traits in F1 female (86, 99) and anxiety-like behavior in F1 female and male offspring, the latter with prenatal testosterone exposure plus flutamide (98). This is in line with the observation in a population-based study in which early initiation of antiandrogen treatment increased the likelihood of spontaneous conception and childbirth in women with PCOS (100). Moreover, the global androgen-receptor deficiency protects F1 female offspring from developing a PCOS-like phenotype at adult age (101, 102), supporting the concept that maternal androgen excess, mediated via androgen receptors, is pivotal to the development of PCOS-like traits in female and partly male F1 offspring; the latter warrants further investigation.
Prenatal AMH exposure
Recently it was shown that prenatal AMH (PAMH) exposure triggers maternal GnRH-driven androgen excess, resulting in a PCOS-like reproductive phenotype with delayed vaginal opening and puberty onset, irregular estrus cycles, and elevated LH, testosterone, and AMH in adult F1 female offspring at 3 months (23) and 5 months of age (103). The PAMH F1 male offspring, on the other hand, have no difference in AGD, circulating LH, testosterone, or AMH, despite delayed pubertal onset and longer time to plugging and first litter, indicating sexual dimorphic effects of prenatal AMH exposure (103). Of note, at 10 months of age, circulating LH, testosterone, and AMH was lower in F1 PAMH females (103). No metabolic phenotypic changes are present at 3 months of age (23). Interestingly, a recent study shows that PAMH F1 female mice present with impaired sexual behavior, a symptom that women with PCOS often experience, which was restored by the administration of nitric oxide synthase (104). Treating AMH-exposed pregnant mice with cetrorelix acetate, a GnRH antagonist, prevents reproductive phenotypic changes in F1 female offspring by normalizing circulating testosterone levels and AGD (23). Although these findings imply that the effects of prenatal AMH exposure are mediated by GnRH pathways, they do not preclude a role for androgen receptor pathways. Because GnRH neurons do not express androgen receptors, androgens would act upstream, possibly on hypothalamic kisspeptin neurons, which do express androgen receptors (105). To delineate whether the PCOS-like reproductive phenotype in F1 female and male offspring is a result of direct AMH exposure or the indirect androgen excess has the kisspeptin-specific androgen-receptor deficient mice been used (103). Of note, prenatal AMH-exposed kisspeptin-specific androgen-receptor deficient mice are protected from the development of PCOS-like reproductive features in F1 female and F1 male offspring, demonstrating a close interaction between AMH and androgen in prenatal programming. Whether PAMH-exposed female and male mice develop anxiety-like behavior and whether diet-induced obesity accelerates reproductive and metabolic features remain to be examined. A recent study elegantly showed that PAMH female offspring present with impaired sexual behavior and sexual partner preference (104). Sexual dysfunction in PCOS-like mice was linked to decreased expression of progesterone- sensitive neuronal nitric oxide synthase (nNOS) neurons in the hypothalamus which could be a potential therapeutic target for female sexual dysfunction. Although these findings support the notion of targeting androgen-receptor pathways to prevent PCOS transmission to offspring, they do not define the key trigger or the mechanisms by which DHT and AMH trigger PCOS-like pathology in female and male offspring.
Transgenerational transmission of PCOS-like features because of prenatal DHT or AMH exposure
Because prenatal DHT and AMH exposure results in PCOS-like phenotypic features in F1 female and male offspring, the question is whether PCOS can be transmitted across multiple generations (ie, transgenerational transmission). True transgenerational effects require the transmission of phenotypic traits at least to the F3 generation (ie, through the germline) (41, 106). It is nearly impossible to perform longitudinal studies on humans spanning multiple generations. Studies in mice and rats demonstrate transgenerational effects from environmental factors such as endocrine disruptors (107), nutrition (108), and trauma and stress (109). Recent breakthroughs showed that PCOS-like reproductive and metabolic traits are passed on to the third generation (F3) of female mouse offspring because of prenatal DHT exposure (60) and because of prenatal AMH exposure (64). That transcriptional and mitochondrial perturbations of metaphase II oocytes (60, 110) and of DNA methylation in the ovaries (64) accompany this transgenerational transmission raises the intriguing possibility that epigenetic modifications carried by germ cells and/or somatic cells can transmit PCOS across multiple generations. Importantly, several of the epigenetic and transcriptomic signatures were also detectable in blood from daughters of women with PCOS, supporting a role in epigenetic inheritance of the syndrome in humans (60, 64). Interestingly, treatment with universal methyl group donor S-adenosylmethionine in 6-month-old F3 female offspring for 15 days partially restored reproductive and metabolic traits (64), although methylation of those targeted genes and others was not examined to evaluate the specificity of the treatment. Whether prenatal DHT or prenatal AMH exposure pass on reproductive and metabolic traits in male mouse remains to be investigated.
Finally, prenatal exposure to excess DHT was recently shown to cause transgenerationally inherited anxiety-like behavior in F1–F3 female offspring, whereas among male offspring only the F3 generation displayed anxiety-like behavior because of prenatal DHT exposure or maternal obesity (111). To determine whether anxiety-like behavior in female and male mice is transgenerationally transmitted through the in utero environment or germ cells, in vitro fertilization (IVF) and embryo transfer using metaphase II oocytes from prepubertally DHT-exposed F0 female donors were performed. Interestingly, IVF-generated F1 and F2 female offspring did not develop anxiety-like behavior, whereas the F2 male offspring did (111). These results suggest that germ cells underlie the risk of transgenerational transmission of anxiety disorders in male offspring and that in utero androgen and obesity exposure increase the risk in both female and male offspring.
Altogether, these data imply that an adverse intrauterine environment during pregnancy affects not only the developing fetus's somatic cells, but also the germ cells of that fetus. IVF and surrogacy have been used to understand the germ-cell-mediated influence of parental diet-induced obesity on the development of metabolic disease in the F1 offspring (112, 113). Whether germ cells drive transgenerational transmission of PCOS-like reproductive and metabolic traits in females and males in the absence of direct intrauterine effects remains unexplored.
Update on Peripubertal DHT Exposure PCOS-like Model
The well-established hyperandrogenic PCOS-like mouse model induced by continuous exposure of DHT from a peripubertal time point has been comprehensively characterized by (45, 95). But specific PCOS-like features and mechanisms remain to be defined. Although the model is well characterized, it should be noted that different dose of DHT, different type of pellet/Silastic tubing, a different time point when exposure start, and different timepoint when phenotypic characterization has been performed influence the phenotypic and molecular measures.
Studies published after the comprehensive review (45) have investigated when specific PCOS-like features appear after implantation of a 1-cm DHT containing Silastic implant into 4-week-old female mice. Reproductive (estrus cyclicity and ovarian morphology) and metabolic (adipose tissue morphology, cholesterol and triglycerides, fasting glucose and insulin, and hepatic steatosis) phenotypic measures were performed after 2, 4, and 8 weeks of exposure (114). After 2 weeks of continuous DHT exposure, key reproductive phenotypic traits are present. The metabolic phenotypic traits appear after 8 weeks of DHT exposure with increased visceral adiposity, but no alterations in glucose, insulin, and hepatic morphology (114). Previous studies, however, show that these phenotypic traits occur after 12 weeks of continuous DHT exposure (102, 115, 116). Using the same dose and timepoint of DHT implantation, the pulsatile LH secretion and GABAergic input to GnRH neurons were defined (117). Although androgen receptor-expressing cells in the hypothalamus were significantly increased, there was no difference in LH pulse frequency or the number of estrogen receptor-α-expressing neurons, suggesting that androgen exposure at different developmental time points involves specific and differential mechanisms mediating reproductive dysfunction (95, 117).
Neuron-specific androgen receptor-deficient mice (CamKIIα-Cre) have previously been shown to partly protect from DHT-induced reproductive and adipose tissue dysfunctions, with no protection in granulosa cell androgen-deficient mice (118). Given that both neuroendocrine and also adipose tissue function are involved in the development of PCOS, and to define whether androgen receptor activation is the key to the development of reproductive and metabolic phenotypic features in mice, adipose tissue and brain-specific androgen receptor-deficient mice (the FABP4-Cre) was exposed to continuous DHT from 4 weeks of age (119). Interestingly, the adipose tissue-brain androgen-deficient mice were protected from the DHT-induced reproductive and metabolic features, suggesting that both fat and brain are key organs in the pathophysiology of the PCOS (119). Skeletal muscle-specific androgen-receptor-deficient (HSA-rtTA/TRE-Cre) mice exposed to DHT continuously, on the other hand, are not protected from developing PCOS-like traits (120). DHT-exposed wild-type mice transplanted with white adipose tissue or brown adipose tissue from global androgen receptor-deficient mice showed decreased body and fat depot weight as well as adipocyte size, but no improvement in reproductive function, again highlighting that adipose tissue (both white and brown) and the brain are potential key targets for treatment. However, the FABP4-Cre is widely expressed in various other tissues such as pancreatic Langerhans islet cells, endothelial cells, endometrium, ovaries, and myocardium; thus, the FABP4-Cre may not be regarded as an adipose tissue-brain specific deficient model as silencing of androgen receptors in all these tissues could explain the protective effects of DHT exposure (121). Although the use of tissue-specific androgen receptor-deficient mice could be of value to define the main trigger(s) and pathway(s) resulting in a PCOS-like reproductive and metabolic phenotype, several other variables may affect the outcome such as the site of insertion for silencing, expression of the Cre in organs not previously documented, and also the genetic background of the strain used (121).
When using a commercially available DHT pellet (7.5 mg/90 days) implanted into 4-week-old female mice, fasting glucose, insulin, and Homeostasis Model Assessment of Insulin Resistance were lower after 1 month of DHT exposure. After 3 months of DHT exposure, fat mass (by EchoMRI), as well as fasting insulin, Homeostasis Model Assessment of Insulin Resistance, and proteinuria, a marker of renal injury, were increased, demonstrating that the time of phenotyping is critical (122). Arterial stiffness, as measured by pulse wave velocity, was shown to be increased in DHT-exposed mice (123), strengthening earlier observations of left ventricular remodeling and increased vasocontractile responses in DHT-exposed mice, whereas treatment with flutamide partly alleviated these effects (91). Moreover, challenging the DHT-exposed mice with a high-fat diet exacerbates all metabolic features, whereas the reproductive features were not presented (124). The latter 3 studies (122-124) are abstracts presented at the Experimental Biology meeting and thus not fully and comprehensively presented.
Of note, mice exposed to a continuous low dose of DHT (4-mm Silastic implant) starting at adult age (2 months old) develops impaired glucose tolerance, pyruvate tolerance, and insulin tolerance, without change in fat mass (by EchoMRI) (125). Liver androgen receptor-deficient mice exposed to DHT were protected from these metabolic aberrations, indicating that liver androgen receptors could be a potential target in treating metabolic dysfunction.
Although the peripubertal DHT (and letrozole)-induced PCOS-like mice models are well established and frequently used, they display variable reproductive and metabolic phenotypes, highlighting the need to describe the method used in detail, including dose/length and diameter of Silastic tubing/day of implantation/mouse strain/diet used together with detailed phenotyping in each experiment. Nevertheless, the prenatal and peripubertal mice models are unique tools to investigate androgen-induced pathophysiology and molecular mechanism, as well as when investigating the effect of different interventions.
Model Selection and Translational Aspects
Rodent models have several advantages because they can be used for invasive mechanistic studies that are impossible to perform in humans. But it is also important to be aware of limitations, such as that rodents are multiovulatory species and carry multiple females and male fetuses, which may have an impact on the outcome. Despite this, PCOS-like rodent models serve an important purpose in the studies of the underlying etiology and pathophysiology of PCOS as well as in investigating the effects of new and old therapeutic targets (eg, sodium-glucose cotransporter-2 inhibitor) (126).
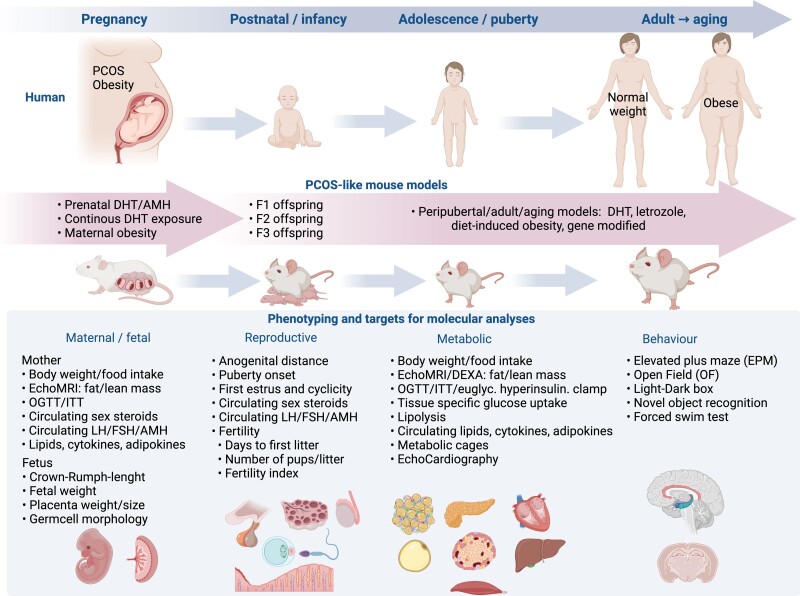
PCOS-like mice models, gene-modified, induced by exposure to androgen, AMH, or letrozole at different developmental windows, consistently associate androgen excess as the trigger of reproductive, metabolic, and behavioral features (Fig. 2). The variable phenotypic features observed in the prenatal DHT and AMH and peripubertal DHT/letrozole exposure models, with or without diet-induced obesity, respectively, highlight the contribution of triggering signals that occur during critical times during development, including fetal, postnatal, and pubertal life. Moreover, when investigating whether and how maternal exposures affect F1 (and beyond) female and male offspring, it is important to define whether the effects depend on in utero environmental effects and/or postnatal maternal care by controlling the maternal environmental care (eg, cross-fostering) as well as diet used.
Figure 2.
Overview of mouse models of PCOS. The selection of animal models is crucial and depends on the specific research question. Always use gold standard methods for phenotypic testing and finalize mice in the same estrus cycle day to avoid hormonal fluctuations affecting the phenotypic and molecular outcomes. For functional and molecular analyses, select state-of-the-art methods although this is out of the scope for this review. DHT, dihydrotestosterone; ITT, insulin tolerance test; OGTT, oral glucose tolerance test. Created with BioRender.com.
Before model selection, it is of utmost importance to define the research question. The PCOS-like model selected fulfill 2 of the 3 PCOS criteria. When starting with a model not previously established in the laboratory, always perform a pilot experiment because the environment, chow diet used in the animal facility, stress, and other factors may affect the phenotypic development. If establishing a new model, perform repeated phenotypic testing at different time points and preferably publish the study protocol. There are few studies following mice for longer than 1 year. From a metabolic, reproductive, and behavioral point of view, this is a field that needs further exploration.
Taken together, developmental programming, genetic and/or environmental through androgen, an letrozole/AMH exposure inducing epigenetic changes or epigenetic augmentation of genotypes, is of importance in the etiology and pathophysiology of PCOS. Androgen and/or AMH exposure models combined with, for example, tissue-specific androgen-receptor knockout mice are key to revealing the strongest factors triggering the development and inheritance of PCOS and associated comorbidities. But they need to be further characterized and compared, both from phenotypic and molecular aspects. Nevertheless, these PCOS-like mice models will significantly improve our ability to diagnose, treat, and prevent the syndrome.
Abbreviations
- AGD
anogenital distance
- AMH
antimüllerian hormone
- BMI
body mass index
- CYP17
17α-hydroxylase/17,20-desmolase
- DHT
dihydrotestosterone
- E
embryonic day
- GK
Goto-Kakizaki rat
- IVF
in vitro fertilization
- PAMH
prenatal antimüllerian hormone
- PCOS
polycystic ovary syndrome
- PNA
prenatal androgen
- TC
theca cell
Funding
Swedish Medical Research Council: Project No. 2018-02435; Novo Nordisk Foundation: NNF22OC0072904; Diabetes Diabetesfonden: DIA2021-633; Strategic Research Program in Diabetes at the Karolinska Institutet; Karolinska Institutet KID funding: 2020-00990.
Disclosures
The author has no conflict of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cesta CE, Mansson M, Palm C, Lichtenstein P, Iliadou AN, Landen M. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 2016;73:196–203. [DOI] [PubMed] [Google Scholar]
- 3. Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(5):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kakoly NS, Earnest A, Teede HJ, Moran LJ, Joham AE. The impact of obesity on the incidence of type 2 diabetes among women with polycystic ovary syndrome. Diabetes Care. 2019;42(4):560–567. [DOI] [PubMed] [Google Scholar]
- 5. Dokras A, Stener-Victorin E, Yildiz BO, et al. . Androgen Excess—Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil Steril. 2018;109(5):888–899. [DOI] [PubMed] [Google Scholar]
- 6. Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2017;32(5):1075–1091. [DOI] [PubMed] [Google Scholar]
- 7. Zhu J, Pujol-Gualdo N, Wittemans LBL, et al. . Evidence from men for ovary-independent effects of genetic risk factors for polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;4(107):e1577–e1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 9. Teede HJ, Misso ML, Costello MF, et al. . Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kataoka J, Larsson I, Bjorkman S, Eliasson B, Schmidt J, Stener-Victorin E. Prevalence of polycystic ovary syndrome in women with severe obesity—effects of a structured weight loss programme. Clin Endocrinol (Oxf). 2019;91(6):750–758. [DOI] [PubMed] [Google Scholar]
- 11. Ruth KS, Day FR, Tyrrell J, et al. . Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med. 2020; 26(2):252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79(4):1158–1165. [DOI] [PubMed] [Google Scholar]
- 13. Gilling-Smith C, Story H, Rogers V, Franks S. Evidence for a primary abnormality of thecal cell steroidogenesis in the polycystic ovary syndrome. Clin Endocrinol (Oxf). 1997;47(1):93–99. [DOI] [PubMed] [Google Scholar]
- 14. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853–861. [DOI] [PubMed] [Google Scholar]
- 15. Taylor AE, McCourt B, Martin KA, et al. . Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82(7):2248–2256. [DOI] [PubMed] [Google Scholar]
- 16. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. [DOI] [PubMed] [Google Scholar]
- 17. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. J Clin Endocrinol Metab. 2005;90(5):2810–2815. [DOI] [PubMed] [Google Scholar]
- 18. Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. 2019;498:110561. [DOI] [PubMed] [Google Scholar]
- 19. Dumesic DA, Abbott DH. Accounting for the follicle population in the polycystic ovary. In: Dunaif ACR, Franks S, Legro RS, eds. Polycystic Ovary Syndrome; Current Controversies, from the Ovary to the Pancreas. Humana Press Inc;2008:9–24. [Google Scholar]
- 20. Franks S, Hardy K. Androgen action in the ovary. Front Endocrinol. 2018;9:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pigny P, Merlen E, Robert Y, et al. . Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. J Clin Endocrinol Metab. 2003;88(12):5957–5962. [DOI] [PubMed] [Google Scholar]
- 22. Cimino I, Casoni F, Liu X, et al. . Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat Commun. 2016;7(1):10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tata B, Mimouni NEH, Barbotin AL, et al. . Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Reilly MW, Kempegowda P, Walsh M, et al. . AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102(9):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiffer L, Arlt W, O'Reilly MW. Understanding the role of androgen action in female adipose tissue. Front Horm Res. 2019;53:33–49. [DOI] [PubMed] [Google Scholar]
- 26. Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 1992;41(10):1257–1266. [DOI] [PubMed] [Google Scholar]
- 27. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38(9):1165–1174. [DOI] [PubMed] [Google Scholar]
- 28. Mannerås-Holm L, Leonhardt H, Kullberg J, et al. . Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. 2011;96(2):E304–E311. [DOI] [PubMed] [Google Scholar]
- 29. Kolb H, Stumvoll M, Kramer W, Kempf K, Martin S. Insulin translates unfavourable lifestyle into obesity. BMC Med. 2018;16(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teede HJ, Joham AE, Paul E, et al. . Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21(8):1526–1532. [DOI] [PubMed] [Google Scholar]
- 31. Dapas M, Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr Rev. Published online January 13, 2022. doi: 10.1210/endrev/bnac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gorsic LK, Dapas M, Legro RS, Hayes MG, Urbanek M. Functional genetic variation in the anti-mullerian hormone pathway in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(7):2855–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dapas M, Sisk R, Legro RS, Urbanek M, Dunaif A, Hayes MG. Family-based quantitative trait meta-analysis implicates rare noncoding variants in DENND1A in polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(9):3835–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dapas M, Lin FTJ, Nadkarni GN, et al. . Distinct subtypes of polycystic ovary syndrome with novel genetic associations: an unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17(6):e1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Day F, Karaderi T, Jones MR, et al. . Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Day FR, Hinds DA, Tung JY, et al. . Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. 2015;6(1):8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brower MA, Hai Y, Jones MR, et al. . Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod. 2019;34(1):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Q, Zhu Z, Kraft P, Deng Q, Stener-Victorin E, Jiang X. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: a large-scale genome-wide cross-trait analysis. BMC Med. 2022;20(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Q, Tang B, Zhu Z, et al. . A genome-wide cross-trait analysis identifies shared loci and causal relationships of type 2 diabetes and glycaemic traits with polycystic ovary syndrome. Diabetologia. 2022;65(9):1483–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jiang X, Deng Q, Stener-Victorin E. Is there a shared genetic basis and causal relationship between polycystic ovary syndrome and psychiatric disorders: evidence from a comprehensive genetic analysis. Hum Reprod. 2021;36(8):2382–2391. [DOI] [PubMed] [Google Scholar]
- 41. Stener-Victorin E, Deng Q. Epigenetic inheritance of polycystic ovary syndrome—challenges and opportunities for treatment. Nat Rev Endocrinol. 2021;17(9):521–533. [DOI] [PubMed] [Google Scholar]
- 42. Xue A, Wu Y, Zhu Z, et al. . Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018; 9(1):2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walters KA, Rodriguez Paris V, Aflatounian A, Handelsman DJ. Androgens and ovarian function: translation from basic discovery research to clinical impact. J Endocrinol. 2019;242(2):R23–R50. [DOI] [PubMed] [Google Scholar]
- 44. Abbott DH, Kraynak M, Dumesic DA, Levine JE. In utero androgen excess: a developmental commonality preceding polycystic ovary syndrome? Front Horm Res. 2019;53:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stener-Victorin E, Padmanabhan V, Walters KA, et al. . Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020;41(4):bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Corrie L, Gulati M, Singh SK, et al. . Recent updates on animal models for understanding the etiopathogenesis of polycystic ovarian syndrome. Life Sci. 2021;280:119753. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez-Garrido MA, Tena-Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Veikkolainen V, Ali N, Doroszko M, et al. . Erbb4 regulates the oocyte microenvironment during folliculogenesis. Hum Mol Genet. 2020;29(17):2813–2830. [DOI] [PubMed] [Google Scholar]
- 49. Nelson VL, Legro RS, StraussJF, 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999; 13(6):946–957. [DOI] [PubMed] [Google Scholar]
- 50. Secchi C, Belli M, Harrison TNH, et al. . Effect of the spatial-temporal specific theca cell Cyp17 overexpression on the reproductive phenotype of the novel TC17 mouse. J Transl Med. 2021;19(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McAllister JM, Modi B, Miller BA, et al. . Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci U S A. 2014; 111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Teves ME, Modi BP, Kulkarni R, et al. . Human DENND1A.V2 drives Cyp17a1 expression and androgen production in mouse ovaries and adrenals. Int J Mol Sci. 2020; 21(7):2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shi J, Gao Q, Cao Y, Fu J. Dennd1a, a susceptibility gene for polycystic ovary syndrome, is essential for mouse embryogenesis. Dev Dyn. 2019;248(5):351–362. [DOI] [PubMed] [Google Scholar]
- 54. Abbott DH, Rayome BH, Dumesic DA, et al. . Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017;32(4):923–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bourgneuf C, Bailbe D, Lamaziere A, et al. . The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome. Nat Commun. 2021;12(1):1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Portha B, Giroix MH, Tourrel-Cuzin C, Le-Stunff H, Movassat J. The GK rat: a prototype for the study of non-overweight type 2 diabetes. Methods Mol Biol. 2012;933:125–159. [DOI] [PubMed] [Google Scholar]
- 57. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. [DOI] [PubMed] [Google Scholar]
- 58. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94(4):1027–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maliqueo M, Sundstrom Poromaa I, Vanky E, et al. . Placental STAT3 signaling is activated in women with polycystic ovary syndrome. Hum Reprod. 2015;30(3):692–700. [DOI] [PubMed] [Google Scholar]
- 60. Risal S, Pei Y, Lu H, et al. . Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med. 2019;25(12):1894–1904. [DOI] [PubMed] [Google Scholar]
- 61. Cesta CE, Oberg AS, Ibrahimson A, et al. . Maternal polycystic ovary syndrome and risk of neuropsychiatric disorders in offspring: prenatal androgen exposure or genetic confounding? Psychol Med. 2010;50(4):616–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kent J, Dodson WC, Kunselman A, et al. Reproductive Medicine Network . Gestational weight gain in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2018; 103(11):4315–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):151–155. [DOI] [PubMed] [Google Scholar]
- 64. Mimouni NEH, Paiva I, Barbotin AL, et al. . Polycystic ovary syndrome is transmitted via a transgenerational epigenetic process. Cell Metab. 2021;33(3):513–530.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum Reprod. 2020;35(7):1666–1674. [DOI] [PubMed] [Google Scholar]
- 66. Homburg R, Gudi A, Shah A AML. A novel method to demonstrate that pregnant women with polycystic ovary syndrome hyper-expose their fetus to androgens as a possible stepping stone for the developmental theory of PCOS. A pilot study. Reprod Biol Endocrinol. 2017;15(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barrett ES, Hoeger KM, Sathyanarayana S, et al. . Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018;9(3):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Glintborg D, Jensen RC, Schmedes AV, et al. . Anogenital distance in children born of mothers with polycystic ovary syndrome: the Odense Child Cohort. Hum Reprod. 2019;34(10):2061–2070. [DOI] [PubMed] [Google Scholar]
- 69. Torchen LC, Legro RS, Dunaif A. Distinctive reproductive phenotypes in peripubertal girls at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(8):3355–3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sir-Petermann T, Codner E, Maliqueo M, et al. . Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(8):3105–3109. [DOI] [PubMed] [Google Scholar]
- 71. Detti L, Christiansen ME, Francillon L, et al. . Serum anti-Mullerian hormone (AMH) in mothers with polycystic ovary syndrome (PCOS) and their term fetuses. Syst Biol Reprod Med. 2019;65(2):147–154. [DOI] [PubMed] [Google Scholar]
- 72. Webber LJ, Stubbs S, Stark J, et al. . Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. [DOI] [PubMed] [Google Scholar]
- 73. Lambertini L, Saul SR, Copperman AB, et al. . Intrauterine reprogramming of the polycystic ovary syndrome: evidence from a pilot study of cord blood global methylation analysis. Front Endocrinol. 2017;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cannarella R, Condorelli RA, Mongioi LM, La Vignera S, Calogero AE. Does a male polycystic ovarian syndrome equivalent exist? J Endocrinol Invest. 2018;41(1):49–57. [DOI] [PubMed] [Google Scholar]
- 75. Crisosto N, Echiburu B, Maliqueo M, et al. . Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr Connect. 2017;6(8):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Recabarren SE, Smith R, Rios R, et al. . Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(5):1820–1826. [DOI] [PubMed] [Google Scholar]
- 77. Recabarren SE, Sir-Petermann T, Rios R, et al. . Pituitary and testicular function in sons of women with polycystic ovary syndrome from infancy to adulthood. J Clin Endocrinol Metab. 2008;93(9):3318–3324. [DOI] [PubMed] [Google Scholar]
- 78. Liu DM, Torchen LC, Sung Y, et al. . Evidence for gonadotrophin secretory and steroidogenic abnormalities in brothers of women with polycystic ovary syndrome. Hum Reprod. 2014;29(12):2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Torchen LC, Kumar A, Kalra B, et al. . Increased antimullerian hormone levels and other reproductive endocrine changes in adult male relatives of women with polycystic ovary syndrome. Fertil Steril. 2016;106(1):50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sam S, Coviello AD, Sung YA, Legro RS, Dunaif A. Metabolic phenotype in the brothers of women with polycystic ovary syndrome. Diabetes Care 2008;31(6):1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sam S, Sung YA, Legro RS, Dunaif A. Evidence for pancreatic beta-cell dysfunction in brothers of women with polycystic ovary syndrome. Metabolism 2008;57(1):84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baillargeon JP, Carpentier AC. Brothers of women with polycystic ovary syndrome are characterised by impaired glucose tolerance, reduced insulin sensitivity and related metabolic defects. Diabetologia 2007;50(12):2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Subramaniam K, Tripathi A, Dabadghao P. Familial clustering of metabolic phenotype in brothers of women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(7):601–603. [DOI] [PubMed] [Google Scholar]
- 84. Karthik S, Vipin VP, Kapoor A, Tripathi A, Shukla M, Dabadghao P. Cardiovascular disease risk in the siblings of women with polycystic ovary syndrome. Hum Reprod. 2019;34(8):1559–1566. [DOI] [PubMed] [Google Scholar]
- 85. Gunning MN, Sir Petermann T, Crisosto N, et al. . Cardiometabolic health in offspring of women with PCOS compared to healthy controls: a systematic review and individual participant data meta-analysis. Hum Reprod Update. 2020;26(1):103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101(18):7129–7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moore AM, Prescott M, Campbell RE. Estradiol negative and positive feedback in a prenatal androgen-induced mouse model of polycystic ovarian syndrome. Endocrinology 2013;154(2):796–806. [DOI] [PubMed] [Google Scholar]
- 88. Fornes R, Maliqueo M, Hu M, et al. . The effect of androgen excess on maternal metabolism, placental function and fetal growth in obese dams. Sci Rep. 2017;7(1):8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fornes R, Manti M, Qi X, et al. . Mice exposed to maternal androgen excess and diet-induced obesity have altered phosphorylation of catechol-O-methyltransferase in the placenta and fetal liver. Int J Obes (Lond). 2019;43(11):2176–2188. [DOI] [PubMed] [Google Scholar]
- 90. Manti M, Fornes R, Qi X, et al. . Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. Faseb J. 2018;32(8):4158–4171. [DOI] [PubMed] [Google Scholar]
- 91. Manti M, Fornes R, Pironti G, et al. . Maternal androgen excess induces cardiac hypertrophy and left ventricular dysfunction in female mice offspring. Cardiovasc Res. 2020;116(3):619–632. [DOI] [PubMed] [Google Scholar]
- 92. Watanabe Y, Prescott M, Campbell RE, Jasoni CL. Prenatal androgenization causes expression changes of progesterone and androgen receptor mRNAs in the arcuate nucleus of female mice across development. J Neuroendocrinol. 2021;33(12):e13058. [DOI] [PubMed] [Google Scholar]
- 93. Ramezani Tehrani F, Noroozzadeh M, Zahediasl S, Ghasemi A, Piryaei A, Azizi F. Prenatal testosterone exposure worsen the reproductive performance of male rat at adulthood. PLoS One 2013;8(8):e71705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Holland S, Prescott M, Pankhurst M, Campbell RE. The influence of maternal androgen excess on the male reproductive axis. Sci Rep. 2019;9(1):18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Silva MSB, Campbell RE. Polycystic ovary syndrome and the neuroendocrine consequences of androgen excess. Compr Physiol. 2022;12(2):3347–3369. [DOI] [PubMed] [Google Scholar]
- 96. Moore AM, Lohr DB, Coolen LM, Lehman MN. Prenatal androgen exposure alters KNDy neurons and their afferent network in a model of polycystic ovarian syndrome. Endocrinology 2021;162(11):bqab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sati A, Prescott M, Holland S, Jasoni CL, Desroziers E, Campbell RE. Morphological evidence indicates a role for microglia in shaping the PCOS-like brain. J Neuroendocrinol. 2021;33(8):e12999. [DOI] [PubMed] [Google Scholar]
- 98. Hu M, Richard JE, Maliqueo M, et al. . Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc Natl Acad Sci U S A. 2015;112(46):14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gong H, Wu W, Xu J, et al. . Flutamide ameliorates uterine decidualization and angiogenesis in the mouse hyperandrogenemia model during mid-pregnancy. PLoS One. 2019;14(5):e0217095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Elenis E, Desroziers E, Persson S, Sundstrom Poromaa I, Campbell RE. Early initiation of anti-androgen treatment is associated with increased probability of spontaneous conception leading to childbirth in women with polycystic ovary syndrome: a population-based multiregistry cohort study in Sweden. Hum Reprod. 2021;36(5):1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Caldwell AS, Eid S, Kay CR, et al. . Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology 2015;156(4):1441–1452. [DOI] [PubMed] [Google Scholar]
- 102. Caldwell AS, Middleton LJ, Jimenez M, et al. . Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014;155(8):3146–3159. [DOI] [PubMed] [Google Scholar]
- 103. Ho EV, Shi C, Cassin J, et al. . Reproductive deficits induced by prenatal antimullerian hormone exposure require androgen receptor in kisspeptin cells. Endocrinology. 2021;162(12):bqab197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Silva MSB, Decoster L, Trova S, et al. . Female sexual behavior is disrupted in a preclinical mouse model of PCOS via an attenuated hypothalamic nitric oxide pathway. Proc Natl Acad Sci U S A. 2022;119(30):e2203503119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tng EL. Kisspeptin signalling and its roles in humans. Singapore Med J. 2015;56(12):649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Perez MF, Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat Cell Biol. 2019;21(2):143–151. [DOI] [PubMed] [Google Scholar]
- 107. Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12(2):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kaspar D, Hastreiter S, Irmler M, de Angelis MH, Beckers J. Nutrition and its role in epigenetic inheritance of obesity and diabetes across generations. Mamm Genome. 2020;2(29):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jawaid A, Roszkowski M, Mansuy IM. Transgenerational epigenetics of traumatic stress. In: Rutten BPF, ed. Progress in Molecular Biology and Translational Science. Vol 158. Elsevier Inc.; 2018:273–298. [DOI] [PubMed] [Google Scholar]
- 110. Chappell NR, Zhou B, Schutt AK, Gibbons WE, Blesson CS. Prenatal androgen induced lean PCOS impairs mitochondria and mRNA profiles in oocytes. Endocr Connect. 2020;9(3):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Risal S, Manti M, Lu H, et al. . Prenatal androgen exposure causes a sexually dimorphic transgenerational increase in offspring susceptibility to anxiety disorders. Transl Psychiatry. 2021;11(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huypens P, Sass S, Wu M, et al. . Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet. 2016;48(5):497–499. [DOI] [PubMed] [Google Scholar]
- 113. Chen B, Du YR, Zhu H, et al. . Maternal inheritance of glucose intolerance via oocyte TET3 insufficiency. Nature 2022;605(7911):761–766. [DOI] [PubMed] [Google Scholar]
- 114. Paris V R, Edwards MC, Aflatounian A, et al. . Pathogenesis of reproductive and metabolic PCOS traits in a mouse model. J Endocr Soc. 2021;5(6):bvab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bertoldo MJ, Caldwell ASL, Riepsamen AH, et al. . A hyperandrogenic environment causes intrinsic defects that are detrimental to follicular dynamics in a PCOS Mouse model. Endocrinology 2019;160(3):699–715. [DOI] [PubMed] [Google Scholar]
- 116. Aflatounian A, Edwards MC, Rodriguez Paris V, et al. . Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J Endocrinol. 2020;245(3):381–395. [DOI] [PubMed] [Google Scholar]
- 117. Coyle CS, Prescott M, Handelsman DJ, Walters KA, Campbell RE. Chronic androgen excess in female mice does not impact luteinizing hormone pulse frequency or putative GABAergic inputs to GnRH neurons. J Neuroendocrinol. 2022;34(4):e13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Caldwell ASL, Edwards MC, Desai R, et al. . Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2017;114(16):E3334–E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cox MJ, Edwards MC, Rodriguez Paris V, et al. . Androgen action in adipose tissue and the brain are key mediators in the development of PCOS traits in a mouse model. Endocrinology 2020;161(7):bqaa061. [DOI] [PubMed] [Google Scholar]
- 120. Xiong T, Rodriguez Paris V, Edwards MC, et al. . Androgen signaling in adipose tissue, but less likely skeletal muscle, mediates development of metabolic traits in a PCOS mouse model. Am J Physiol Endocrinol Metab. 2022;323(2):E145–E158. [DOI] [PubMed] [Google Scholar]
- 121. Heffner CS, Herbert Pratt C, Babiuk RP, et al. . Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat Commun. 2012;3(1):1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pruett JE, Everman S, Romero DG, Cardozo LLY. Temporal development of cardiometabolic complications in a hyperandrogenemic model of polycystic ovary syndrome. Faseb J. 2022;36(S1). [Google Scholar]
- 123. Horton AC, Wilkinson M, Kilanowski-Doroh I, Ogola B, Lindsey S. DHT Induces arterial stiffening in female wild type mice. Faseb J. 2022;36(S1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Basnet J, Rezq S, Huffman AM, Cardozo LLY, Romero DG. High-fat diet exacerbates androgen-mediated obesity and white adipose tissue hypertrophy in a mouse model of polycystic ovary syndrome. Faseb J. 2022;36(S1). [Google Scholar]
- 125. Andrisse S, Feng M, Wang Z, et al. . Androgen-induced insulin resistance is ameliorated by deletion of hepatic androgen receptor in females. Faseb J. 2021;35(10):e21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Pruett JE, Everman SJ, Hoang NH, et al. . Mitochondrial function and oxidative stress in white adipose tissue in a rat model of PCOS: effect of SGLT2 inhibition. Biol Sex Differ. 2022;13(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.